Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Also available with a translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc:
TL;DR: Jabs are still being pushed by people who should know better. A new paper shows that boosting and breakthrough infections by new variants may significantly boost your IgG4 levels, years later. Engineered mRNA can lead to new onset IgG4-RD organ damage days, weeks or months later, even after just 1 dose. If you had stable IgG4-RD for years it may lead to relapse. Misdiagnosis may mean the wrong treatments are given and further organ damage may result, perhaps fatally, or result in the loss of your pancreas and being insulin dependent for the rest of your life. And once treatments are withdrawn (even if tapered gradually) symptoms may return without warning, perhaps involving new organs.
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Case 1: “IgG4-related Disease Emerging after COVID-19 mRNA Vaccination”
Case 2: “IgG4-Related Membranous Nephropathy After COVID-19 Vaccination: A Case Report”
Case 3: “Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine”
Case 4: “SERONEGATIVE TYPE I AUTOIMMUNE PANCREATITIS WITH IMMUNOGLOBULIN G4-RELATED DISEASE TRIGGERED BY THE PFIZER-BIONTECH COVID-19 VACCINE”
Case 5: “Acute liver injury and IgG4-related autoimmune pancreatitis following mRNA-based COVID-19 vaccination”
Case 6: “IgG4 related pleural disease: Recurrent pleural effusion after COVID-19 vaccination”
Case 7: “Immunoglobulin G4-related Hepatopathy after COVID-19 Vaccination”
Introduction
Normal service resumes after a walkthrough of IgG4-cancer research relevant to this series, - you could almost call it Part Ib. In Part I I gave some background as to what antibodies are, about IgG4 class switching, and why this is a problem. I reproduced some graphs analysing the increases in all-cause mortality in Finland. IgG4-RD may well be contributing to this. Most alarmingly, excess deaths of 0-24 year olds are over 1.5 times the baseline rate and rising.
Iikka’s latest graphs aren’t looking any better either:

I reviewed various case studies of patients admitted with IgG4-related aortitis; an analysis of the survival rates of hospitalised myocarditis patients; how even mild cases can reduce your life expectancy; and a case report of IgG4-related myocarditis.
In Part II I will review seven case reports of IgG4-RD after COVID transfection. Part III and thereafter will review research discussing the mechanisms behind IgG4-RA, and IgG4-RD as a whole.
I need to work from fundamentals as a literature view of various journals failed to present many papers discussing IgG4-related aortitis, or any research that directly investigated causality, beyond discussing immune cell histology.
Discussion
Case reports of IgG4-RD after COVID transfection
Our life would be much easier if, despite the elevated IgG4 levels of the class switched vaccinated, there was no disease association with this, or that it might even be beneficial. Why waste everyone’s time by going into great depth discussing something that’s not going to happen?
Unfortunately, we know from the previous reviews that just the presence of IgG4 antibodies can make pre-existing conditions much worse, with a significantly poorer prognosis. Antigen specificity doesn’t appear to be a prerequisite either, as IgG4-RD is a systemic disease that can involve virtually any organ, although the most common are the pancreas, kidneys, orbita (tissues surrounding the eyeball), salivary glands, and retroperitoneum (the tissue that lines the abdominal wall and covers most of the organs in the abdomen).1 And because the IgG4 molecule is unstable it can undergo Fab-arm exchange (FAE), cleave into two and rehybridize. It can also induce immunosuppressive M2 macrophage phenotypes through FcR interactions.2
Are engineered mRNA-induced Spike-specific IgG4 antibodies also associated with IgG4-RD? I found 7 case studies. As only a fraction of cases get diagnosed correctly, let alone recorded and written-up the true number must be many many times this amount, and potentially increasing with time due to reinfection or boosters still being administered to vulnerable people who cannot give informed consent.
We could play liar’s bingo with this recent post by the NHS, a steaming pile of horse s### with no citations to support it, being pushed out at time of writing. It comes across somewhat with an air of desperation.
See how many lines you get, probably a full house?
(Emphasis theirs, because they are the Experts™, don’t you know. “Experts” in what I’m not too sure, and it’s probably not printable here).
They are even doing a 2-for-1 offer:
2 risks of disease and early death for every 1 application. What’s not to like?
NHS launches final call for people to get their covid jab
27 June 2024
With just days left of this year’s spring covid vaccine programme, the NHS is urging anyone eligible to get their jab by this Sunday [30 June 2024].
More than 4 million people have now received their spring booster jab since the campaign began in April.
The NHS has made it easier than ever before for people to book their vaccination with the rollout of joint booking. By going online and selecting a joint booking, 2 eligible people aged 18 and over can get the covid-19 vaccine in the same location at the same time.
Anyone currently aged 75 and over, or who will be aged 75 by this Sunday [30 June 2024], is able to get the covid-19 vaccine, along with older adult care home residents and people with a weakened immune system aged 6 months and over.
For the next 4 days, there are thousands of appointments available across the country, including at pharmacies and GP practices. Some areas also offer convenient walk-in options, with a full list of walk-in sites available online.
Since the spring covid vaccine campaign launched on 15 April, around two-thirds of care home residents have received their protection.
Covid-19 can still cause severe illness and hospitalisations in some cases, particularly among those most at risk. The vaccine gives the best protection against the virus and its different variants and helps reduce the risk of serious illness.
Steve Russell, NHS National Director for Vaccinations and Screening said: “It is fantastic that since April 4 million people have received their covid spring booster, but there are still thousands more appointments available over the next few days for eligible people to get their all-important protection against covid-19.
“The Joint Committee on Vaccination and Immunisation (JCVI) recommends the vaccination for those who are most at-risk including people aged over 75 or those with compromised immune systems – for these people covid-19 can still have severe consequences, and even if you’ve been vaccinated before, protection does fade over time, so it is important to come forward if you’re eligible.
“It’s now easier than ever to get protected, and with joint booking, you and a loved one can come forward at the same time, you can also book online, download the NHS App or call 119. Some areas also offer convenient walk-in options, with a full list of walk-in sites which are also available online”.
Dr Jamie Lopez Bernal, Consultant Epidemiologist for Immunisation at UK Health Security Agency said: “We are seeing an increase in covid-19 across all indicators, including hospitalisations. Those eligible only have until Sunday to take up the offer of a vaccine, which helps boost your protection against serious illness. So, make sure you book your vaccine as soon as possible, either online at nhs.uk/get-vaccine or call 119 if you don’t have access to the internet.
“If you are 75 years or older, reside in a care home, or have a weakened immune system, you are eligible for your covid-19 vaccine.
“If you are showing symptoms of covid-19 or flu, help protect others by staying at home and avoiding contact with other people, especially those who are more vulnerable. If you do need to leave home, consider wearing a mask”.
Anyone who believes they should be eligible for a vaccine but does not get invited can check online. Alternatively, they can self-declare via the National Booking Service and then speak to a clinician on site.
The offer of 2 covid-19 vaccinations for everyone who was aged 5 on or before 31 August 2022 will also end after 30 June, following advice from the JCVI.
https://www.england.nhs.uk/2024/06/nhs-launches-final-call-for-people-to-get-their-covid-jab/
It’s like 4 years of safety data, and multiple studies showing negative efficacy long-term never happened. With this campaign, the NHS just gave 4 million vulnerable individuals yet another booster dose, if the stats are to be believed. It’s the gift that keeps on giving, albeit mainly to its Pharma sponsors.3
According to Blood Cancer UK, if you are fully boosted, then you will be rolling your sleeves up for your 9th shot this weekend.
Spoiler alert: If you need that many shots to restore immunity it’s not a “vaccine”.
"Blood Cancer UK. Support you can do without”:
How many doses of covid vaccine have people with blood cancer been offered?
The covid vaccine schedule for people with blood cancer up to now has been:
1st primary dose – from around Jan 2021
2nd primary dose – from around April 2021
3rd primary dose – from around September 2021
4th dose (1st booster) – from around January 2022
5th dose (Spring 2022 booster) – from around April 2022
6th dose (Autumn 2022 booster) – from around September 2022
7th dose (Spring 2023 booster) – from around April 2023
8th dose (Autumn 2023 booster) – from around October 2023
Regardless of which doses you've had so far, you should get your booster doses when they are offered. Read the latest evidence about the effectiveness and safety of covid vaccination for people with blood cancer.
https://bloodcancer.org.uk/support-for-you/coronavirus-covid-19/covid-vaccine-blood-cancer/
As long as the vulnerable keep being preyed on, and those we trust and turn to for advice are happy to disregard all safety and efficacy data, and lie to your face, the risk of IgG4-RD can only go one way.
IgG4 after booster vaccination
Hat tip to Mikolaj for a walkthrough of a new preprint on the Merogenomics channel:
“XBB.1.5 monovalent booster improves antibody binding and neutralization against emerging SARS-CoV-2 Omicron variants” (2024) by Jain et al.
Their findings aren’t good news, as they found that breakthrough infections, even with highly mutated variants such as XBB.1.5, are not breaking the recall of IgG4 Abs from memory B cells. This is otherwise known as original antigenic sin (OAS), immune imprinting, primary addiction or the Hoskins effect.
Engineered mRNA-related IgG4-RD is therefore less likely to dissipate on its own in affected individuals.
Key takes:
The rapid emergence of divergent SARS-CoV-2 variants has led to an update of the COVID-19 booster vaccine to a monovalent version containing the XBB.1.5 spike.
To determine the neutralization breadth following booster immunization, we collected blood samples from 24 individuals pre- and post-XBB.1.5 mRNA booster vaccination (∼1 month).
The XBB.1.5 booster improved both neutralizing activity against the ancestral SARS-CoV-2 strain (WA1) and the circulating Omicron variants, including EG.5.1, HK.3, HV.1, XBB.1.5 and JN.1.
Relative to the pre-boost titers, the XBB.1.5 monovalent booster induced greater total IgG and IgG subclass binding, particular IgG4, to the XBB.1.5 spike as compared to the WA1 spike.
Evidence for immune refocusing to different antigens. This can increase the risk of other autoimmune disorders if the new epitopes being targeted are homologous to those specific to certain tissues:
We evaluated antigen-specific memory B cells (MBCs) using either spike or receptor binding domain (RBD) probes and found that the monovalent booster largely increases non-RBD cross-reactive MBCs. These data suggest that the XBB.1.5 monovalent booster induces cross-reactive antibodies that neutralize XBB.1.5 and related Omicron variants.
OAS in action. Here we see a strong antibody recall response to now long-extinct variants, such as ancestral Wunan WA.1 from 2020. The y-axis is logarithmic too, so it’s worse than it looks:
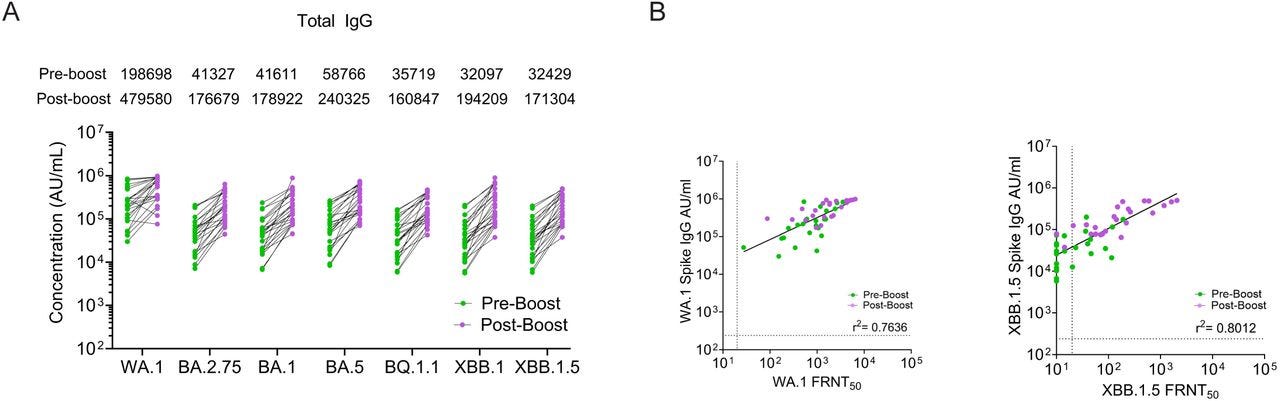
We next evaluated IgG subclass binding to the WA1, XBB.1.5 and related Omicron spike proteins. Prior to the XBB.1.5 monovalent booster, we observed IgG1 (GMT: 12.87; range: 0.52-114), IgG2 (GMT: 0.459; range: 0.05–6.27), IgG3 (GMT: 0.109; range: 0.04–5.85), and IgG4 (GMT: 3.346; range: 0.05–70.64) binding to the WA1 spike protein.
In comparison, IgG subclass binding to the XBB.1.5 spike protein was substantially lower for IgG1 (GMT: 2.192; range: 0.07-24), IgG2 (GMT: 0.459; range: 0.02–0.81), IgG3 (GMT: 0.103; range: 0.01–0.18), and IgG4 (GMT: 0.057; range: <0.00006–24).
Following the XBB.1.5 monovalent booster, IgG1, IgG2, IgG3, and IgG4 binding to the WA1 spike protein increased by 1.8-fold, 2.4-fold, 3-fold, 3.2-fold, respectively (Fig. 3).
On the other hand, IgG1, IgG2, and IgG3 binding to the XBB.1.5 spike increased by 3.7-fold, 4-fold, and 8.6-fold, respectively. In the case of IgG4 subclass antibodies, we observed no detectable IgG4 binding titer against the XBB.1.5 spike in 7 out of 24 participants (29%) before the booster immunization. In these 7 individuals, after the administration of XBB.1.5 monovalent booster, IgG4 binding titers (GMT: 0.22; range: 0.05-5.86) increased robustly against the XBB.1.5 spike protein. For the other 17 individuals, we observed an approximately 7-fold increase in binding to the XBB.1.5 spike after the XBB.1.5 booster dose.
This is disastrous, especially when you consider that the FDA approved the updated monovalent XBB.1.5 engineered mRNA in September 2023, with a rollout almost 2 years after the first-generation products were administered.
Even so, with some patients, IgG4 concentrations here equal or exceed the other isotypes. Regardless of the total concentration, IgG4 exerts outsize effects:
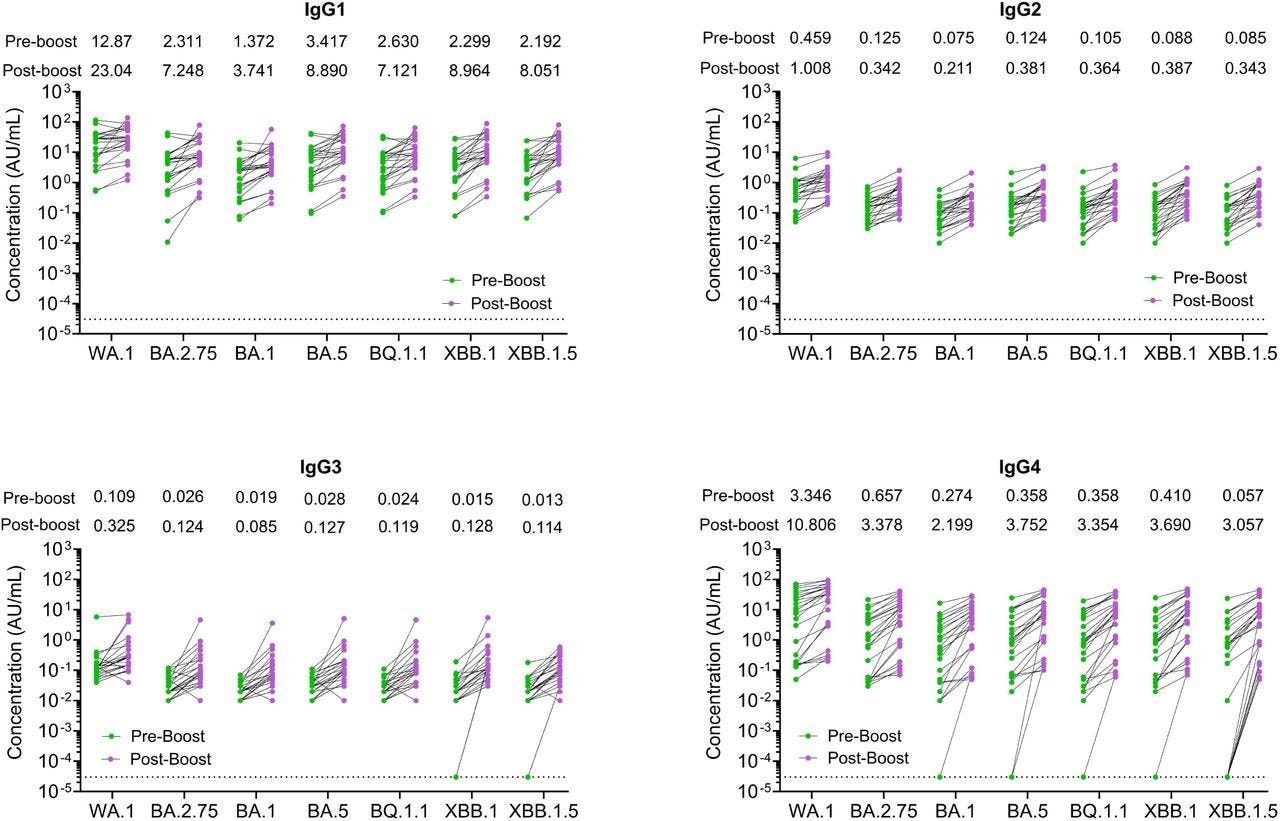
This is where we should be; not matching the other classes 1-1 or even 2-1 as its a log scale:

Nowhere in this study do they mention “cancer”, let alone the risks of IgG4-RD:
We report that the XBB.1.5 monovalent mRNA booster results in a greater increase across all IgG subclasses, including IgG4. Early in the pandemic, high levels of anti-spike IgG4 antibodies were associated with poor clinical outcomes16,17.
However, in cohorts of vaccinated healthcare workers, an increase in anti-spike IgG4 antibodies has been reported months after the second booster immunization with the ancestral monovalent mRNA vaccines18.
The IgG4 class switching occurred after repeated mRNA vaccinations but not after viral vector-based immunization19,20.
Preferential induction of IgG4 antibodies by vaccination has been reported to be associated with a high dose of vaccine or repeated immunization with the same antigen21 that resulted in the induction of specific T cell tolerance22,23.
They mandated or coerced an experimental gene therapy that went into the arms of billions under Emergency Use Authorisation (EUA) without the research being done. And it still hasn’t, four years later.
Until then, what we can do is use existing research to write reviews, form hypotheses as to specific signalling pathways, immunity mechanisms and therefore predict potential clinical outcomes.
It is one of the reasons we were able to predict an epidemic of cardiovascular, neurological, autoimmune diseases and cancers long before the data started to be presented.
Interestingly, repeated immunization with a SARS-CoV-2 RBD-based vaccine induced tolerance in mice24. Further research is therefore needed to evaluate the mechanisms of the preferential IgG4 class switching induced by the XBB.1.5 monovalent mRNA vaccine, which is relevant for designing future seasonal SARS-CoV-2 booster immunizations.
It has been speculated that the emergence of isotype-switched memory B cells is consistent with a more robust and durable germinal center (GC) response induced by mRNA-based vaccines25.
These findings contrast with the predominant induction of IgG1 and IgG3 after natural SARS-CoV-2 infections26.
IgG4 has limited functional activity due to structural features that impair its binding to C1q and Fc receptors that mediate opsonization and antibody-depending cellular cytotoxicity27.
These features likely make IgG4 a blocking or suppressing antibody28. Further investigations are therefore required to define the role of anti-spike IgG4 induced by XBB.1.5 monovalent booster vaccine in protection or its impact on the response to future booster immunizations.
The paper as a whole is fairly dismissive of the class-switching disaster and puts a high emphasis on booster-induced neutralising Abs to XBB.1.5.
They also neglected to mention that chasing variants with vaccines is a fool’s errand. It’s gold at the end of a rainbow you can never reach. By the end of 2023, XBB.1.5 had already declined to about 4% of variants. The virus had quickly moved on and mutated further away from neutralisation by engineered mRNA-primed antibodies:

The COI declarations help to explain the positive spin:
Declarations of Interest
C.A.R. has received institutional research support from Pfizer Inc., BioFire Inc., GSK plc, Janssen Pharmaceuticals, MedImmune, Micron Technology Inc., ModernaTX, Inc., Merck & Co., Inc., Novavax, PaxVax, Regeneron, Sanofi Pasteur, and from the Centers for Disease Control and Prevention and the National Institutes of Health. She is coinventor of patented RSV vaccine technology which has been licensed to Meissa Vaccines, Inc. N.R. serves as a paid consultant for ICON, CyanVac and EMMES, as a safety consultant for clinical trials and served on the advisory boards for Sanofi, Seqirus, Pfizer and Moderna. Emory receives funds for N.R. to conduct research from Sanofi, Lilly, Merck, Quidel, Immorna, Vaccine Company and Pfizer. M.S.S receives funds to conduct research from Ocugen, Inc.
The following case studies show that IgG4-RD is indeed a possible consequence of engineered mRNA-induced class switching to IgG4.
IgG4-RA is the next logical step. Just because post-vax case reports aren’t published yet doesn’t mean it isn’t happening. It almost certainly is, and at scale, but it just isn’t being diagnosed or reported.
Case 1
“IgG4-related Disease Emerging after COVID-19 mRNA Vaccination”4 (2023) by Aochi et al.
A 78-year-old Japanese woman with no history of rheumatic disease received 2 doses of the BNT162b2 COVID-19 mRNA vaccine. Two weeks later, she noticed bilateral swelling in the submandibular region.
Blood tests showed hyper-immunoglobulin (Ig)G4emia, and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) revealed the strong accumulation of FDG in the enlarged pancreas.
She was diagnosed with IgG4-related disease (IgG4-RD) according to the American College of Rheumatology (ACR)/the European League Against Rheumatism (EULAR) classification criteria.
Treatment was started with prednisolone at 30 mg/day, and the organ enlargement improved.
We herein report a case of IgG4-RD that may have been associated with an mRNA vaccine.

Case 2
“IgG4-Related Membranous Nephropathy After COVID-19 Vaccination: A Case Report”5 (2024) by Mizuno et al.
A kidney disease: “Membranous nephropathy (MN), also known as membranous glomerulopathy, is one of the many glomerular diseases causing nephrotic syndrome. It is characterized by massive proteinuria (>3.5 g/day) and clinically presents with peripheral edema, hypertension, frothy urine, and manifestations of thromboembolic phenomena.”6
Although immunoglobulin G4 (IgG4)-related kidney diseases are typically characterized by tubulointerstitial nephritis with abundant infiltration of IgG4-positive plasma cells and fibrosis, there have been relatively rare cases of IgG4-related glomerulonephritis.
Several cases of IgG4-related disease (IgG4-RD) following coronavirus disease 2019 (COVID-19) mRNA vaccination have been reported. However, there are no reports of IgG4-related glomerulonephritis following COVID-19 vaccination. Herein, we present a case of IgG4-related membranous nephropathy (MN) occurring after COVID-19 vaccination.
A 69-year-old Japanese male presented to our hospital with edema that started the day after his second COVID-19 vaccination. The patient exhibited nephrotic syndrome and was diagnosed with MN based on the results of a kidney biopsy.
Although serum IgG4 levels were elevated to 946 mg/dL, no evidence of organ involvement suggestive of IgG4-RD was observed. Treatment with prednisolone and cyclosporine resulted in complete remission, and immunosuppressive agents were tapered.
Symptoms returned once the drugs were withdrawn:
However, one month after discontinuing the immunosuppressive agents, the patient was readmitted with swelling around the submandibular glands and exertional dyspnea.
Serum IgG4 level was markedly elevated at 2,320 mg/dL, and computed tomography revealed submandibular gland swelling and thickening of the interlobular septum and bronchovascular bundles in both lungs.
The patient was diagnosed with IgG4-RD based on elevated serum IgG4 levels and infiltration of IgG4-positive plasma cells in the submandibular gland biopsy.
Upon resuming treatment with prednisolone, the symptoms attributed to IgG4-RD improved within a few days.
In cases of nephrotic syndrome following COVID-19 vaccination, it may be advisable to conduct detailed examinations to assess the possibility of the development of IgG4-RDs.
Although serum IgG4 levels were elevated, lymphoplasmacytic infiltrates rich in IgG4-positive plasma cells, which are characteristic of IgG4-RKD, were not observed. Serum phospholipase A2 receptor (PLA2R) antibodies tested negative.
We considered secondary MN based on renal histological findings and the negative result for PLA2R antibodies; however, there was no evidence of secondary MN causes such as infections or malignancy.

Cyclosporine is a calcineurin inhibitor used as an immune suppressant:
The patient was initiated on treatment with an initial dose of 0.8 mg/kg/day (50 mg/day) of prednisolone. Cyclosporine was added to the prednisolone regimen after four weeks because proteinuria persisted despite prednisolone therapy. Subsequently, proteinuria gradually decreased, leading to complete remission (Figure 2).
IgG4 levels were trending up as the steroids were tapered and then surged after around six months:

Every time they cut the steroids, IgG4-RD symptoms returned:
Approximately one year after the initiation of treatment, the patient experienced a single relapse of nephrotic syndrome while tapering prednisolone. Prompt improvement was achieved by increasing the prednisolone dose to 30 mg/day.
Subsequently, there was no further relapse and the patient maintained complete remission. Therefore, immunosuppressive therapy with prednisolone and cyclosporine was discontinued approximately two years after the start of treatment.
Not so fast! But next time it wasn’t the kidneys affected:
Approximately two weeks after discontinuing the immunosuppressive therapy, the patient experienced exertional dyspnea and swelling around the jaw, which gradually worsened and led to readmission to our hospital four weeks later.
Similar to Case 1, it was submandibular (in the jaws):
Physical examination upon readmission revealed swelling in the bilateral submandibular regions. Laboratory data on the second admission are shown in Table 1. Significant elevations in serum IgG and IgG4 levels along with hypocomplementemia were observed.
Although the serum creatinine level was mildly elevated to 1.37 mg/dL, there was no evidence of urinary protein or hematuria, and urine sediment examination revealed no abnormalities.
Tests for autoimmune antibodies, such as MPO-ANCA, PR3-ANCA, antinuclear antibodies, anti-SS-A antibodies, and anti-SS-B antibodies, were negative.
Whole-body computed tomography (CT) revealed bilateral swelling of the submandibular glands and thickening of the interlobular septum and bronchovascular bundles in both lungs (Figures 3A-3F).
This is not good: “Interstitial lung disease (ILD) is an umbrella term used for a large group of diseases that cause scarring (fibrosis) of the lungs.”
A submandibular gland biopsy revealed significant plasma cell infiltration, interstitial fibrosis, and acinar and ductal destruction. Immunohistochemical staining revealed IgG4-positive/CD138-positive cell ratios exceeding 40% (Figures 4A-4C), leading to the diagnosis of IgG4-RD.

Translation: Salivary gland and duct destruction.

The plan was to taper the prednisolone down to a maintenance dose.
Conclusions
Here, we report a case of IgG4-RD presenting with MN following COVID-19 vaccination, with subsequent relapse in the lungs and salivary glands after tapering prednisolone and cyclosporine.
Even with a biopsy, they missed the diagnosis of IgG4-RD.
You can imagine our health services getting bogged down with patients presenting with unexplained symptoms that aren’t responding to treatment, or keep relapsing. This applies to many complex vax-induced autoimmune diseases with latent virus reactivation complicating matters further still.
Kidney biopsy is a crucial diagnostic tool for kidney diseases; however, in IgG4-RKDs, there are rare cases where the diagnosis remains challenging even after the biopsy.
Therefore, in cases of nephrotic syndrome following COVID-19 vaccination, particularly when accompanied by symptoms unrelated to renal involvement, considering the possibility of IgG4-RDs and pursuing further investigations, including serum IgG4 measurements and whole-body CT, may be warranted.
Case 3
Also a kidney disease: “Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine”7(2021) by Masset et al.
Several reports have highlighted the increased risk of immune disease recurrence following mRNA vaccination, including minimal change disease, membranous nephropathy, or even acute allograft rejection.
In 2019 he had IgG4-RD with kidney disease, lupus-associated antibodies and generalised weakness (asthenia) that was brought under control with steroids, until he was vaccinated:
We report the case of a 66-year-old man who was diagnosed with IgG4-related disease (IgG4-RD) nephritis in December 2019. At this time, he presented with asthenia associated with acute kidney injury, evaluated by serum creatininemia (SCr) at 450 μmol/L and aseptic leukocyturia (120/mm3) without proteinuria or hematuria.
“SSA-52 (Ro52) and/or SSA-60 (Ro60) antibodies are associated with a diagnosis of Sjögren syndrome, systemic lupus erythematosus (SLE), and systemic sclerosis. SSA-52 antibody overlaps significantly with the major SSc-related antibodies.”8
The patient was vaccinated with an mRNA vaccine (BNT162b2 mRNA; Pfizer BioNTech) on January 28, 2021, and February 17, 2021.
Two weeks later, he presented with intense asthenia with arthralgias and myalgias. SCr was elevated at 210 μmol/L on March 5, 2021, increased to 250 μmol/L on March 22, 2021, and was associated with recurrence of aseptic leukocyturia.
Anti–SSA-52 levels increased from 4 to 17 UI/L. SARS-CoV-2 serology was positive for anti–spike protein at 177 UI/L (electrochemiluminescence immunoassay, Roche Elecsys).
Steroid therapy was initiated at 0.5 mg/kg and associated with rituximab perfusion (500 mg), allowing a quick improvement of the general symptoms and resolution of acute kidney injury. Anti–SSA-52 levels also decreased to 12 UI/L by May 2021 (Figure 2).
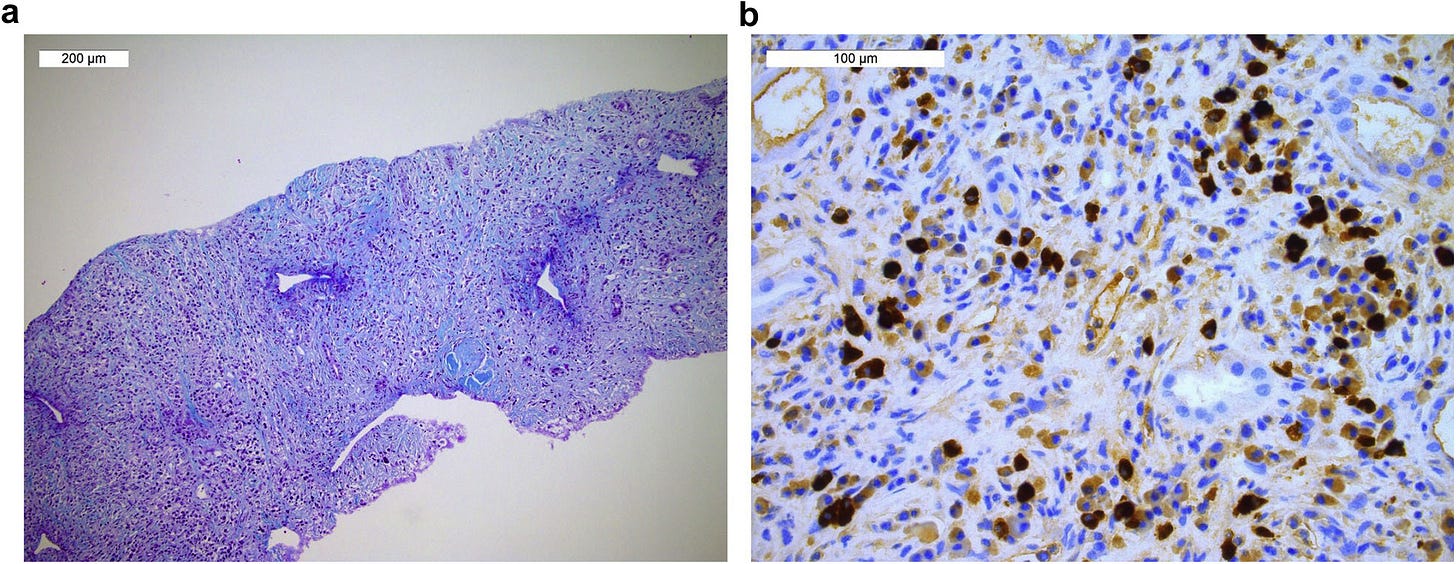
Rituximab is a MaB that targets CD20 protein on leukocytes, causing them to become a target for destruction. It’s a treatment for blood cancers, autoimmune disorders and IgG4-RD:
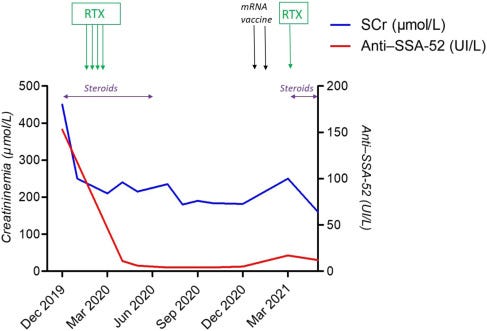
Our report highlights the possibility of immune disease relapse following mRNA vaccine, a situation previously described by others. It is currently unknown if immune disease recurrence is linked to direct immune activation following vaccination, chronic immune activation following a paucisymptomatic allergic reaction, or both. Indeed, IgG4-RD pathogenesis has been linked to IgE production, as seen in late immune-mediated allergic reactions.
Spoiler. It never did:
If the benefit-to-risk ratio indisputably favors vaccination of this at-risk population, physicians should be aware of the possibility of immune disease recurrence to provide close monitoring of these patients and fast treatment of relapses to avoid long-term consequences and progression to end-stage renal disease.
Case 4
“SERONEGATIVE TYPE I AUTOIMMUNE PANCREATITIS WITH IMMUNOGLOBULIN G4-RELATED DISEASE TRIGGERED BY THE PFIZER-BIONTECH COVID-19 VACCINE”9 (2022) by Rodrigues & Komanduri.
Case report: A previously healthy 65-year-old male presented to a community hospital with one week of jaundice. Two weeks prior, he had received his first dose of the COVID-19 vaccine.
Cholestatic: A stagnation or marked reduction in bile secretion and flow.
Admission labs demonstrated a cholestatic liver injury without eosinophilia. After initial imaging, an endoscopic ultrasound demonstrated a diffusely heterogenic pancreas with features of pancreatitis (lobularity, stranding).
Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a 2cm distal common bile duct stricture. A sphincterotomy was performed and fully covered metal stent was placed (due to minor post-sphincterotomy bleeding).
The patient’s jaundice resolved, and he was discharged. He received his second dose of the COVID-19 vaccine shortly after hospitalization.
Serum CA19-9 is a tumour-associated antigen, tested for to diagnose pancreatic cancer.
One month later, repeat ERCP showed resolution of the stricture and his stent was removed. Outpatient laboratory evaluation revealed normal IgG4 and CA 19-9 levels.
After removal of treatment, you guessed it, symptoms returned.
I think they mean “extrahepatic stricturing” of the biliary tree; either the hepatic ducts or common bile duct:
The patient was readmitted two months after initial presentation with worsening jaundice. Repeat ERCP demonstrated multifocal intrahepatic and extrahepatic structuring.
The vaccine also significantly elevated the tumour-associated antigen CA19-9.
AIP: Autoimmune pancreatitis.
A CA 19-9 was elevated from prior to 225 unit/mL [<34 unit/mL]. The patient was referred to our center and had a repeat ERCP confirming multifocal stricturing and a magnetic resonance imaging (MRI) cholangiogram that showed intrahepatic biliary ductal dilatation with an irregular, beaded appearance, regions of restricted diffusion/hypoenhancement within both kidneys, and irregularity and beading of the main pancreatic duct, consistent with AIP with extrapancreatic IgG4-related disease.
Steroids resolved the symptoms, and quite rightly he declined his next offer of a booster. Thanks for offering though:
The patient was treated with prednisone 40mg daily with complete normalization of all liver tests [Figure 1]. Repeat MRI [Figure 2] after eight weeks of therapy showed complete resolution of all biliary dilation and pancreatic/renal abnormalities.
The patient remains asymptomatic 3 months after treatment and has chosen not to obtain his COVID-19 booster.
Case 5
“Acute liver injury and IgG4-related autoimmune pancreatitis following mRNA-based COVID-19 vaccination”10 (2022) by Patel et al.
The previous case report was also from 2022:
Currently, there are no cases in which IgG4-RD involving the hepatobiliary system has been reported following the COVID-19 vaccination. We present the first case of IgG4-RD and AIP type 1 to be associated with the mRNA-based COVID-19 vaccination.
We report a case of a 63-year-old male who we believe developed the first case of IgG4-RD involving the pancreas (AIP type 1) shortly after receiving the mRNA-based COVID-19 vaccine.
A 63-year-old African American male was in his normal state without any significant medical issues until 2021. He had received two doses of the mRNA-based COVID-19 vaccine in March/April 2021.
In June 2021, he presented with complaints of fatigue and a rapid 20 lb weight loss. At that time, he was found to be severely hyperglycemic, diagnosed with diabetes mellitus, and started on metformin. Liver enzymes were normal at that time. He denied a history of alcohol intake, illicit drug use or hepatotoxic medications, preexisting liver disease, or prior diagnosis of COVID-19. Bloodwork from 2020 showed a normal fasting blood sugar. The physical examination was unremarkable.
They administered the usual meds for these symptoms, but of course, nothing was working:
He was started on metformin and repaglinide, but his blood sugar remained uncontrolled. In September 2021, he was found to have liver enzyme abnormalities, and therefore repaglinide was stopped. However, he developed worsening jaundice and pruritus and was then referred to gastroenterology.
Eventually, they diagnosed IgG4-RD-associated AIP and administered a treatment that worked. Medics just weren’t aware of synthetic mRNA-induced IgG4-RD, and I suspect they still aren’t, especially if this is where we are at:

They are stuck in a time warp from 2020, or as Tucker Carlson puts it, like a Japanese soldier on Okinawa, still fighting years after the war ended.
CT abdomen/pelvis revealed a hypervascular arterial enhancing pancreatic head mass measuring up to 5.5 cm with moderate intrahepatic biliary dilatation and mild common bile duct dilatation to the pancreatic head (Fig. 1a).
Triple phase liver protocol showing a hypervascular arterial enhancing mass at the pancreatic head measuring up to 3.6 × 2.6 × 5.5 cm in the largest dimension (Fig. 1b, c). CT chest and neck did not demonstrate mass or adenopathy.

My emergency doctor friend also noticed the enlarged gallbladder in the CT scan (the dark egg shape above the arrow in a, b and c).
He underwent an ERCP with biliary stent placement and endoscopic ultrasound-guided pancreas biopsy. The biopsy specimen was diffusely positive for IgG4 but was “overstained” and nondiagnostic. A liver biopsy was not obtained.
AIP was suspected given the absence of malignancy on biopsy, normal Ca 19-9 and CEA levels, and the very elevated IgG4 level. He was started on prednisone 40 mg daily with a tapering schedule.
Within 4 weeks, liver enzymes became normal (Table 1). At 6 weeks, his prednisone was reduced to 15 mg daily, and imaging obtained at that time showed resolution of the pancreatic mass with continued normal liver transaminases (Fig. 1d). The patient felt well and had gained 10 lb. Biliary stent removal was planned.
The temporal association with the vaccine could be coincidental, but the patient had completely normal blood sugar and liver tests 1 year earlier. The significantly elevated IgG4 level supports the diagnosis. The stain on the biopsy was compromised by “overstain” but was not negative.
Insulin-dependant type 1 diabetes, or T1D, is due to T cell-mediated autoimmune destruction of insulin-secreting beta cells:
The patient developed insulin-dependent diabetes shortly after the COVID-19 vaccination. Abnormalities in endocrine function of the pancreas and diabetes mellitus are seen up to 78% in patients with AIP.[10] Finally, a complete response following initiation of prednisone therapy further supports a diagnosis of IgG4 RD.
In summary, we suggest that patients who present with new-onset diabetes mellitus or cholestasis with or without signs of obstructive jaundice in the era of widespread COVID-19 vaccination should be screened for IgG4-RD.
Case 6
“IgG4 related pleural disease: Recurrent pleural effusion after COVID-19 vaccination”11 (2022) by Tasnim et al.
“Pleural effusion occurs when fluid builds up in the space between the lung and the chest wall.”
Our patient presented with pleural effusion, and was diagnosed with IgG4-related lung disease (IgG4-RLD) after he received two doses of the Pfizer COVID-19 vaccine.
Note that most of these cases were logged medically within a week or two of vaccination. Causality would be harder to demonstrate the greater the time lag before becoming symptomatic, i.e. many cases won’t be recorded as vaccine injuries.
The patient developed dyspnea and hypoxia 2 weeks after receiving the second dose of the Pfizer COVID-19 vaccine. CT scan revealed left pleural effusion which was drained. However, the effusion recurred requiring thoracoscopic drainage, placement of an indwelling catheter, and decortication with biopsy.
IgG4 serum level was 268 mg/dl and pathology revealed pleural fibrosis, lymphoplasmacytic infiltrates, and increased IgG4-positive plasma cells with no malignant cells leading to a diagnosis of IgG4-RLD.
Although COVID vaccine-related IgG4-RLD is a novel finding, having a high degree of suspicion following vaccination is always important for early diagnosis and effective treatment.
Our patient is a 71-year-old male with hypertension, coronary artery disease, prostate cancer and chronic obstructive pulmonary disease with a 50-pack year smoking history.
These are almost always benign:12
The patient started being followed in the pulmonology department after a screening chest CT revealed left hilar fullness and peri-fissural nodules, the largest of which measured 0.4 cm (Figure 1).
Repeat CT 2 years later showed unchanged nodules, with two new nodules measuring 0.4 cm. At this point, he elected not to continue surveillance.
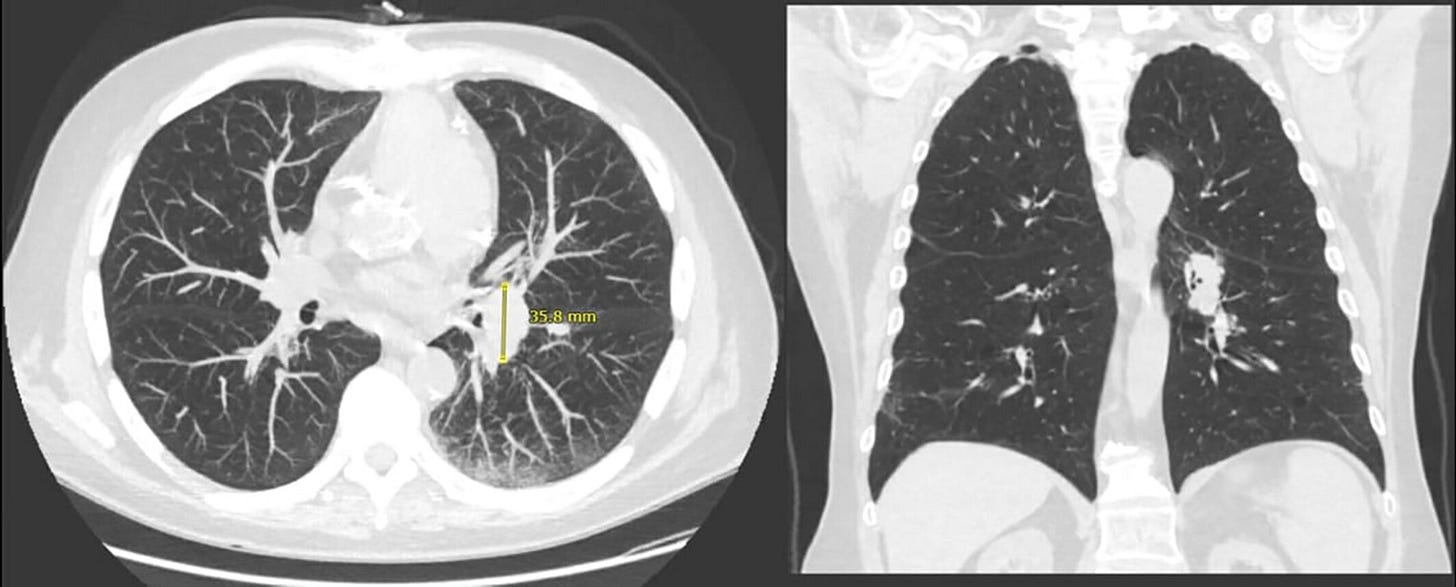
Two years later, he received a first dose of the Pfizer COVID-19 vaccine and visited urgent care after a week with wheezing and dyspnea which improved with albuterol.
Having survived the first dose he went back for more.
DLCO: A lung diffusion test that shows how well your lungs are working, passing oxygen from the air sacks into the blood.
However, he returned to clinic with dyspnea 2 weeks after receiving the second dose of the vaccine. He underwent pulmonary function testing which revealed a mixed obstructive and restrictive pattern with reduced DLCO. Six-minute walk test revealed hypoxia requiring 3 L oxygen.
Chest CT revealed new left pleural effusion with atelectasis (Figure 2). 1.4 L straw-coloured exudative fluid was removed by thoracentesis which was negative for malignant cells. Summary of the laboratory, pulmonary function test and pleural fluid analysis of our case are shown in Table 1.
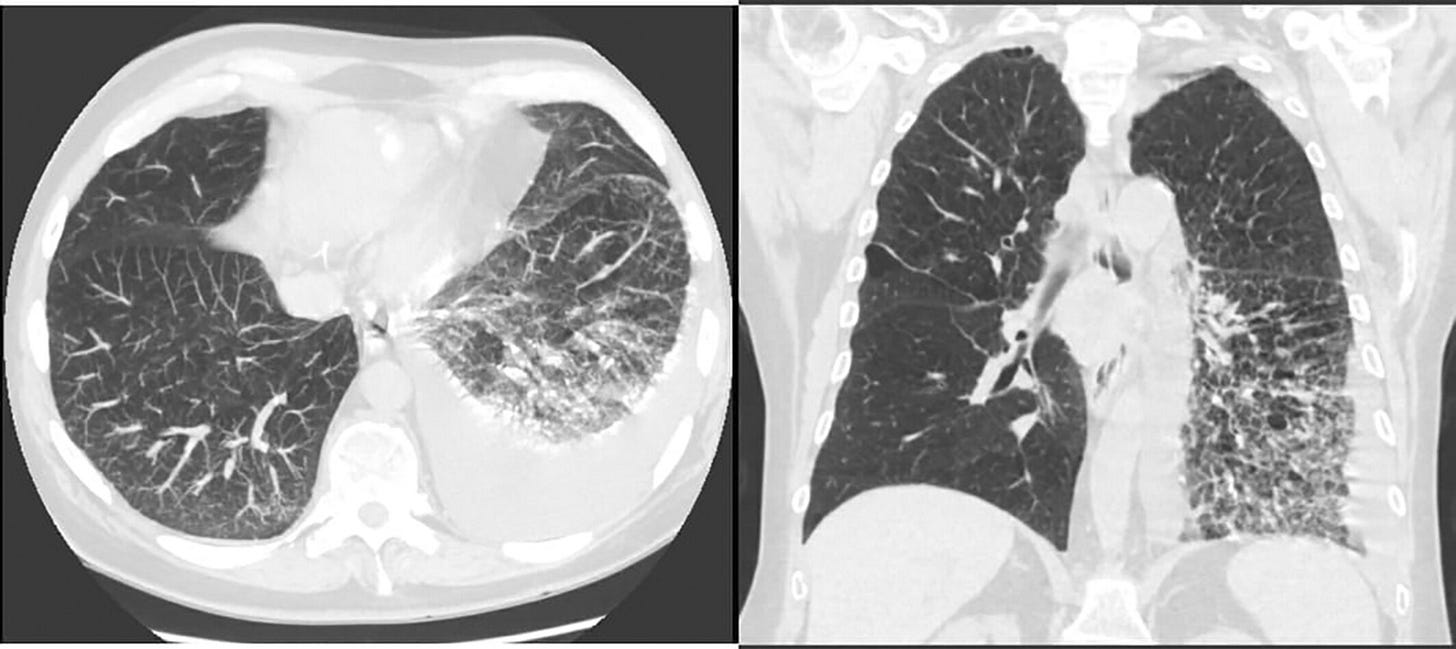
Lymphoid hyperplasia, or an increase in the number of normal cells contained in the lymph nodes, sometimes progresses to malignant lymphoma (Leibel and Phillips Textbook of Radiation Oncology (Third Edition), 2010).
His symptoms initially improved but worsened again. Repeat CT scan 10 days later revealed large left pleural effusion. He was referred to thoracic surgery and underwent left thoracoscopy with drainage of 2.5 L of pleural fluid followed by pleural biopsy and chemical pleurodesis with insertion of an indwelling tunnelled pleural catheter.
Pleural biopsy revealed chronic organizing pleuritis with lymphoid hyperplasia and reactive mesothelial hyperplasia.
Thoracoscopy revealed multiloculated pleural effusions with significant visceral pleural thickening and the patient underwent partial decortication.
Pathology report revealed pleural thickening and fibrosis with surrounding pulmonary parenchyma revealing diffuse alveolar damage, lymphoplasmacytic infiltrates, and increased IgG4-positive plasma cells with fibrinous adhesions.
IgG4-RD was restricted to the lungs, which is bad enough in itself as it involved fibrosis (scar tissue leading to impaired blood flow and additional strain on the heart).
As the case was lost to follow-up we don’t know how the disease progressed:
At this time, blood IgG4 level was 268 mg/dl. Autoimmune, malignant and genetic conditions with similar presentation were excluded. The patient was diagnosed with IgG4-RD affecting the lungs and pleura, non-pulmonary involvement was not found. He was referred to the pulmonology clinic for further management but he was lost to follow-up.
The definite diagnosis of IgG4-RLD relies on histopathological analysis. The key morphological features are dense lymphoplasmacytic infiltrates with collagenised fibrosis and active fibroblastic proliferation.4
Patients who fulfil the following three criteria are diagnosed as having IgG4-RD: consistent organ involvement or damage; serum IgG4 level of >135 mg/dl; and histopathology showing marked lymphoplasmacytic infiltration, fibrosis. Our case fulfilled all three comprehensive diagnostic criteria and involved lung; therefore, the diagnosis of IgG4-RLD was made.
The mainstay treatment for IgG4-RLD is corticosteroid therapy, and most patients have favourable response in 2 weeks. The usual dose for oral prednisone is between 0.6 and 1 mg/kg/day, and then gradually tapered every 2–4 weeks to maintain a dose of 2.5–5 mg/day and then discontinued within 3 years. For steroid-refractory patients, immunosuppressants or anti CD20 antibody can be considered.5
A profound warning by the authors for a paper that passed peer review:
As the worldwide COVID-19 vaccination continues to accelerate, it is probable to see more autoimmune events. However, due to reporting bias and pre-existing undiagnosed autoimmune lung disease, true incidence of IgG4-RLD will be difficult to determine. As multiple doses of vaccines are now being offered, close observation will be needed. Anyone with rapidly progressive lung or pleural disease after COVID 19 vaccine, more aggressive approach for diagnosis is recommended so diseases such as IgG4-RLD cannot be missed and prompt treatment can be started to mitigate this disease.
IgG4 related pleural disease: Recurrent pleural effusion after COVID-19 vaccination (2022)
Case 7
Also a liver disease: “Immunoglobulin G4-related Hepatopathy after COVID-19 Vaccination”13 (2023) by Kuno et al.
An 84-year-old woman with IgG4-related disease presented with jaundice and liver dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Serum IgG4 levels were elevated.
Diagnostic imaging showed no stenotic lesions in the bile ducts. A liver biopsy was performed because of the enlarged liver. Infiltration of IgG4-positive plasma cells, which accounted for approximately 74% of total plasma cells, was found in the portal area, but there was no evidence of periportal hepatitis, and inflammatory cell infiltration into the lobular space was minimal.
IgG4-related hepatopathy was diagnosed. The patient achieved spontaneous remission with no treatment and only follow-up and remains under observation at the time of writing.
Before vaccination:
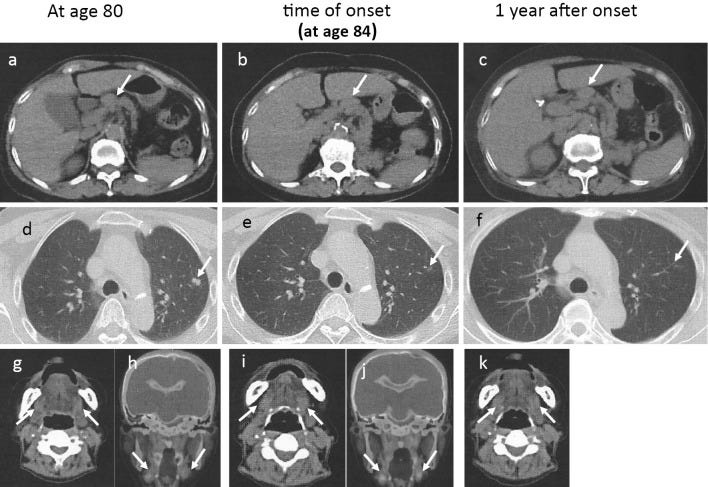
An 80-year-old woman who had a history of left nephrectomy for left kidney cancer at 75 years old with multiple nodules in the lungs presented to our hospital with enlarged generalized lymph nodes and mild enlargement of submandibular glands on computed tomography (Fig. 1a, d, g, h).
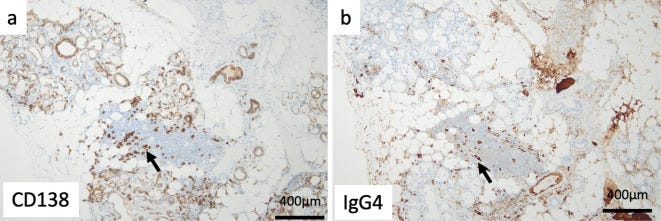
The serum IgG4 level was high at 2,540 mg/dL. A lip biopsy showed a positive IgG4:CD138 ratio of more than 70% (Fig. 2a, b). IgG4-related disease was diagnosed. However, the patient's symptoms were mild, so she was followed up without treatment.
“… and then you go and spoil it all…”
She got dose one 4 years after the previous symptoms self-resolved, and her markers exploded.
The diagonal lines in Figure 3 may be misleading as they imply pre-vaccinal elevation, but we don’t see the intermediate data points.
We do see more points after being admitted, which is what you would expect.
At 84 years old, the patient developed generalized itching, anorexia, and nausea 1 day after receiving the first dose of the COVID-19 vaccine. Her symptoms worsened, and she was hospitalized on day 7 after vaccination.
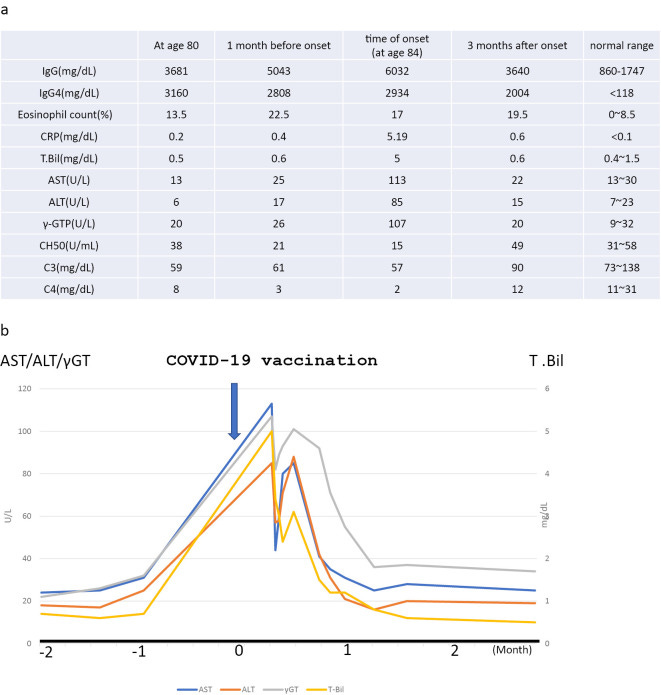
Computed tomography showed an enlarged liver and spleen and enlarged bilateral axillary, mediastinal, hilar, periaortic and mesenteric lymph nodes (Fig. 1b, e, i, j). No pancreatic enlargement was seen. Magnetic resonance cholangiopancreatography showed no abnormalities in the bile duct system.
An ultrasound-guided liver biopsy was performed.
The portal areas were enlarged and showed numerous lymphocytes and plasma cells and a small number of eosinophils. The portal areas were well demarcated, and there was no obvious evidence of periportal hepatitis.
The bile ducts were normal and free from inflammation, and no bile duct lesions suggestive of IgG4-related sclerosing cholangitis were identified (Fig. 4a, b).
Well above the commonly adopted threshold of 40%:
Immunostaining revealed conspicuous infiltration of IgG4-positive cells in the portal areas, and the ratio of IgG4- to CD38-positive cells (indicating the plasma cell ratio) was elevated at 74% (Fig. 4c, d), consistent with IgG4-related hepatopathy.
There was an overall poor inflammatory response and some necrosis in the lobules (Fig. 4e). Within the sinusoids, lymphocytes were mildly increased, and aggregates of macrophages positive for periodic acid-Schiff with diastase stain were seen (Fig. 4f), suggesting focal necrosis.
This part of the lesion was considered to be related to recovery from mild acute hepatitis. The absence of characteristic centrilobular hepatitis did not support a diagnosis of autoimmune hepatitis.
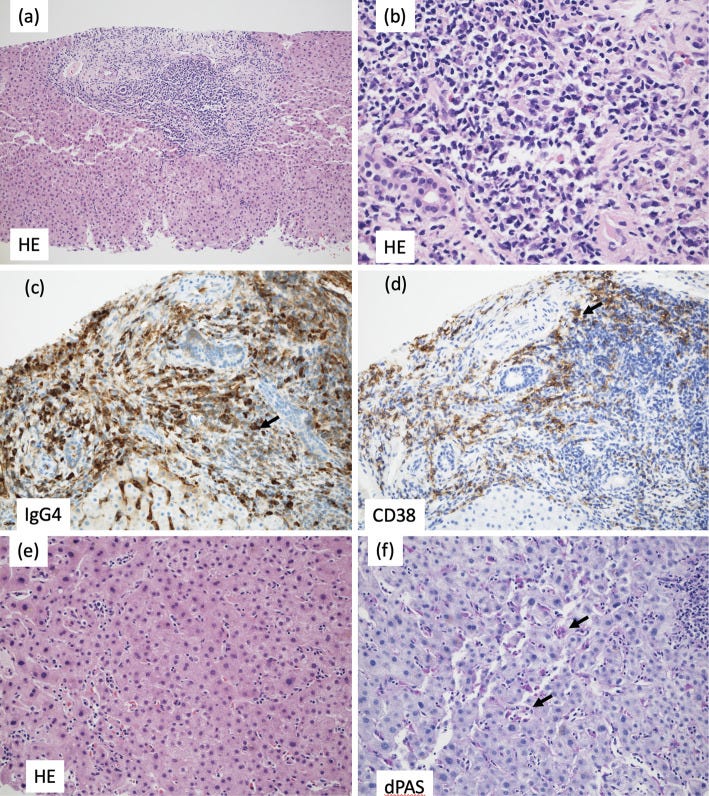
The diagnosis
The findings did not meet the criteria for IgG4-related autoimmune hepatitis proposed by Umemura et al. (3,4) or the criteria for IgG4-related sclerosing cholangitis on imaging (5). Therefore, IgG4-related hepatopathy was diagnosed.
Clinical course
After admission, the jaundice resolved without treatment, and liver function tests normalized after four weeks (Fig. 3b). One year and six months later, there was no recurrence of liver function abnormalities and no recurrence of IgG4-related disease (Fig. 1c, f, k).
Among IgG-related diseases, liver involvement is classified into three categories: IgG4-related sclerosing cholangitis, IgG4-related autoimmune hepatitis and IgG4-related hepatopathy. Common findings in these three diseases are high serum IgG4 levels and characteristic histological findings with IgG4-positive plasma cell infiltration and being highly responsive to steroid therapy.
For the second time, symptoms resolved on their own:
Although corticosteroid therapy was initially considered for this patient, no aggressive treatment was eventually given because the liver function improved spontaneously after hospitalization and follow-up observation.
A common sense approach, finally, that treats the jab as the poison it is. I admire their candour:
If the disease had been induced by drugs, it might have spontaneously resolved if the drugs were discontinued and were not administered again. Based on this idea, a second dose of the COVID-19 vaccine was not administered in this case.
Immunoglobulin G4-related Hepatopathy after COVID-19 Vaccination (2023)
Conclusion
I hope these case reports, and the new research into post-vax IgG4, can help act as a wake-up call to the size of the problem. I’m hoping these will be of use to medical professionals too, to speed up and improve the quality of diagnoses. As we have seen, misdiagnosis may mean the condition worsens significantly for months or even years, sometimes with fatal consequences.
I’ve had to serialize engineered mRNA-induced class switching and its mechanisms as the first draft was approaching thesis length. A thesis keeps all the discussion in one publication but is far too long to email and for our concentration spans.
I find myself scrolling quickly through long, drawn-out diatribes, having no end in sight, no matter how good they are. It’s like writing a blank check with your valuable reading time. I don’t want my reviews to fall into this trap!
The next Substack will be a little different, and it has nothing to do with Biomed - it’s a travelogue from a hike this week through stunning countryside in the UK’s Yorkshire Dales.
DoorlessCarp’s Scientific Literature Reviews is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
References
Nambiar S, Oliver TI. IgG4-Related Disease. In: StatPearls. StatPearls Publishing; 2024. Accessed June 28, 2024. http://www.ncbi.nlm.nih.gov/books/NBK499825/
Zhang W, Chen X, Chen X, et al. Fc–Fc interactions and immune inhibitory effects of IgG4: implications for anti-PD-1 immunotherapies. J Immunother Cancer. 2024;12(6):e009034. doi:10.1136/jitc-2024-009034
NHS England » Managing conflicts of interest in the NHS. Accessed June 28, 2024. https://www.england.nhs.uk/blog/mike-thompson/
Aochi S, Uehara M, Yamamoto M. IgG4-related Disease Emerging after COVID-19 mRNA Vaccination. Intern Med. 2023;62(10):1547-1551. doi:10.2169/internalmedicine.1125-22
Mizuno T, Endo Y, Suzuki A, et al. IgG4-Related Membranous Nephropathy After COVID-19 Vaccination: A Case Report. Cureus. 2024;16(3). doi:10.7759/cureus.56028
Alok A, Yadav A. Membranous Nephropathy. In: StatPearls. StatPearls Publishing; 2024. Accessed July 3, 2024. http://www.ncbi.nlm.nih.gov/books/NBK559169/
Masset C, Kervella D, Kandel-Aznar C, Fantou A, Blancho G, Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney International. 2021;100(2):465-466. doi:10.1016/j.kint.2021.06.002
SSA 52 and 60 (Ro) (ENA) Antibodies, IgG | ARUP Laboratories Test Directory. Accessed July 3, 2024. https://ltd.aruplab.com/Tests/Pub/2012074
Rodrigues T, Komanduri S. SERONEGATIVE TYPE I AUTOIMMUNE PANCREATITIS WITH IMMUNOGLOBULIN G4-RELATED DISEASE TRIGGERED BY THE PFIZER-BIONTECH COVID-19 VACCINE. Gastrointestinal Endoscopy. 2022;95(6):AB36. doi:10.1016/j.gie.2022.04.130
H Patel A, Amin R, Lalos AT. Acute liver injury and IgG4-related autoimmune pancreatitis following mRNA-based COVID-19 vaccination. Hepatology Forum. 2022;3(3):97. doi:10.14744/hf.2022.2022.0019
Tasnim S, Al-Jobory O, Hallak A, Bharadwaj T, Patel M. IgG4 related pleural disease: Recurrent pleural effusion after COVID-19 vaccination. Respirology Case Reports. 2022;10(10):e01026. doi:10.1002/rcr2.1026
Hoop B de, Ginneken B van, Gietema H, Prokop M. Pulmonary Perifissural Nodules on CT Scans: Rapid Growth Is Not a Predictor of Malignancy. Radiology. Published online November 1, 2012. doi:10.1148/radiol.12112351
Kuno M, Sawa N, Mizuno H, et al. Immunoglobulin G4-related Hepatopathy after COVID-19 Vaccination. Internal Medicine. 2023;62(14):2139-2143. doi:10.2169/internalmedicine.1634-23












I feel very sorry for the people who have blind faith in the NHS and will continue to receive the mRNA injections for as long as this institution recommends them. You can sense that there is a ticking time bomb of ill health ahead and for older people it will be put down to the ageing process because at the best of times investigation for this age group in the UK can be minimal.
Great post - I was able to follow through to the end...the order/structure of this article is excellent , thank you!