Ciliary Dysfunction Secondary to COVID-19. Explanation of the Pathogenesis From Analysis of Human Interactome With Sars-Cov-2 Proteome
Something just doesn't smell right
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
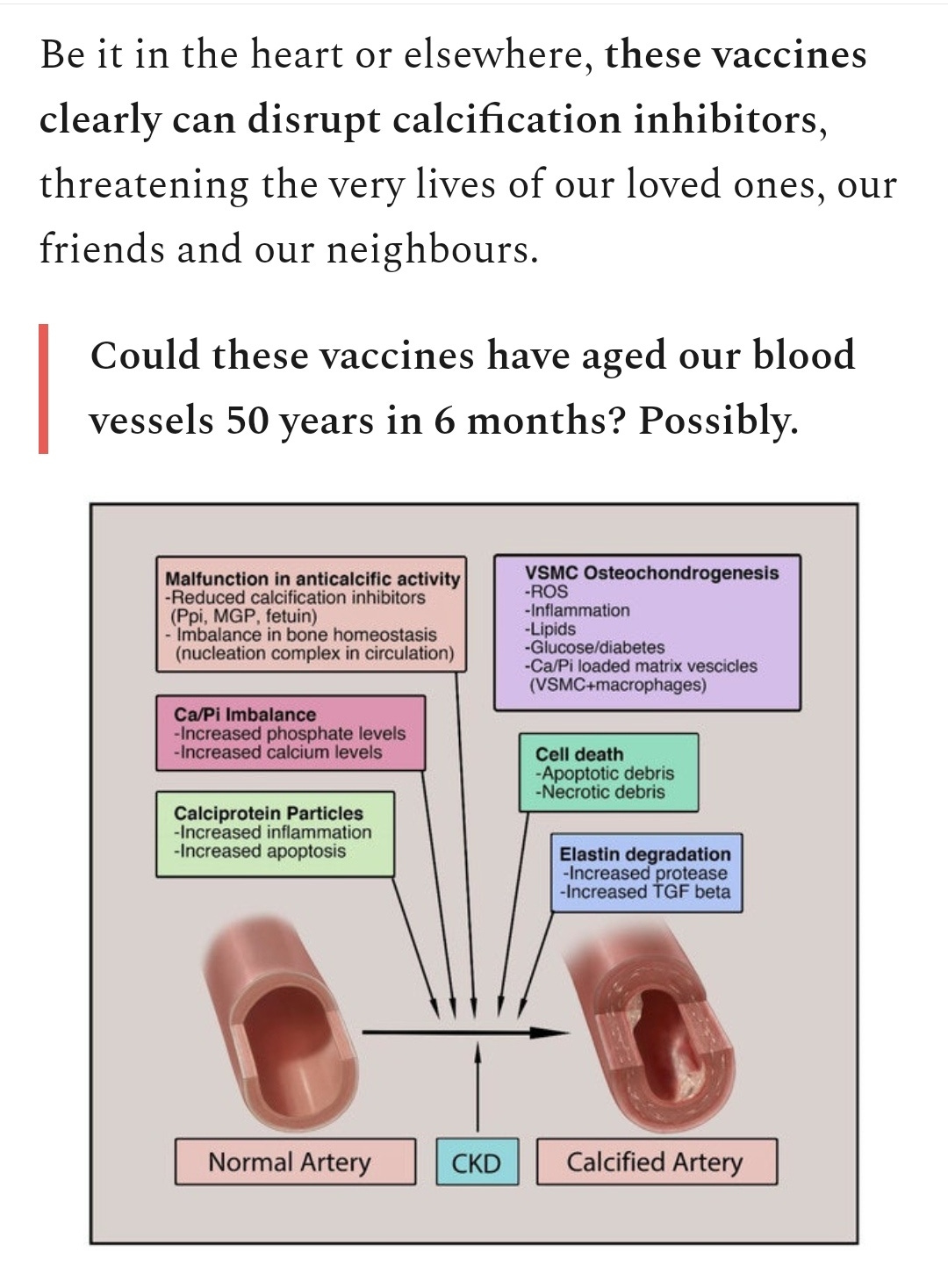
This is an area of SARS-CoV-2 mediated pathology that has received little attention, and indeed even now I was unable to find biopsy slides specifically focused on the effects on vascular primary cilia. These play a crucial role in maintaining homeostasis via signalling & feedback mechanisms.
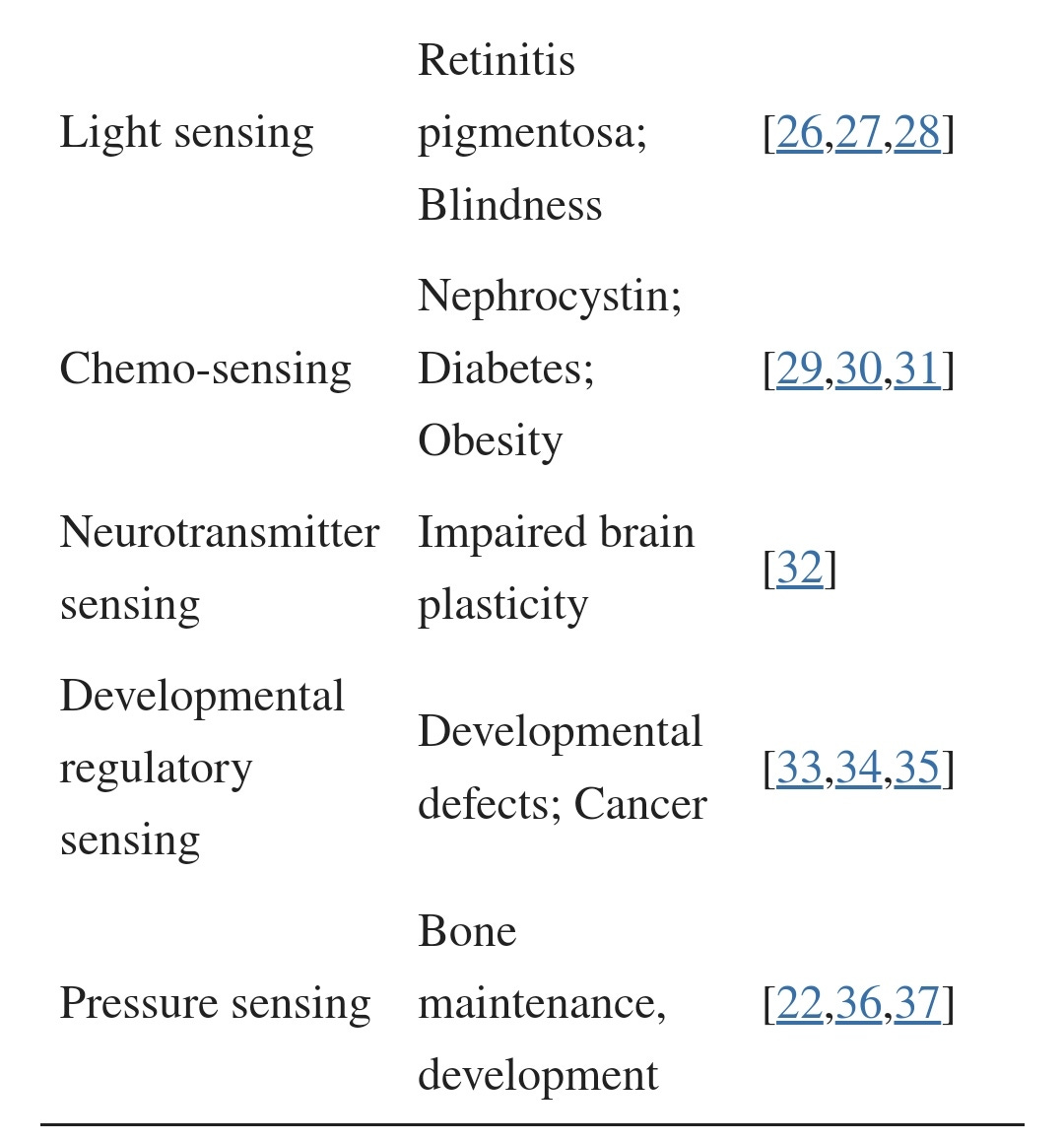
The two main areas of focus are the pulmonary alveoli and the vascular system, but disruption also has implications for multiple organ function, fertility and sense of smell (olfactory).
One of the papers explores in detail how the coronavirus remodels endothelial airway cells. Another concluded ominously:
“The reduced release of NO resulting from the endothelial dysfunction in regenerated areas creates a locus minoris resistentiae which favors the occurrence of vasospasm and thrombosis as well as the initiation of atherosclerosis.”
The Roles of Primary Cilia in Cardiovascular Diseases (2018)
Abstract
Primary cilia are microtubule-based organelles found in most mammalian cell types. Cilia act as sensory organelles that transmit extracellular clues into intracellular signals for molecular and cellular responses. Biochemical and molecular defects in primary cilia are associated with a wide range of diseases, termed ciliopathies, with phenotypes ranging from polycystic kidney disease, liver disorders, mental retardation, and obesity to cardiovascular diseases. Primary cilia in vascular endothelia protrude into the lumen of blood vessels and function as molecular switches for calcium (Ca2+) and nitric oxide (NO) signaling. As mechanosensory organelles, endothelial cilia are involved in blood flow sensing. Dysfunction in endothelial cilia contributes to aberrant fluid-sensing and thus results in vascular disorders, including hypertension, aneurysm, and atherosclerosis. This review focuses on the most recent findings on the roles of endothelial primary cilia within vascular biology and alludes to the possibility of primary cilium as a therapeutic target for cardiovascular disorders.
Keywords: primary cilia, calcium, nitric oxide, biochemical signaling, hypertension, aneurysm, atherosclerosis
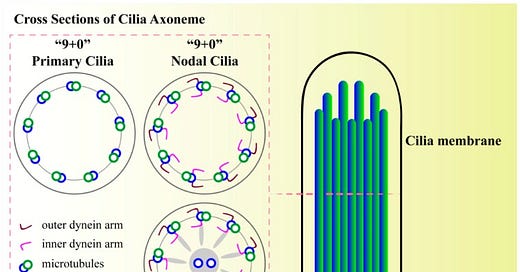
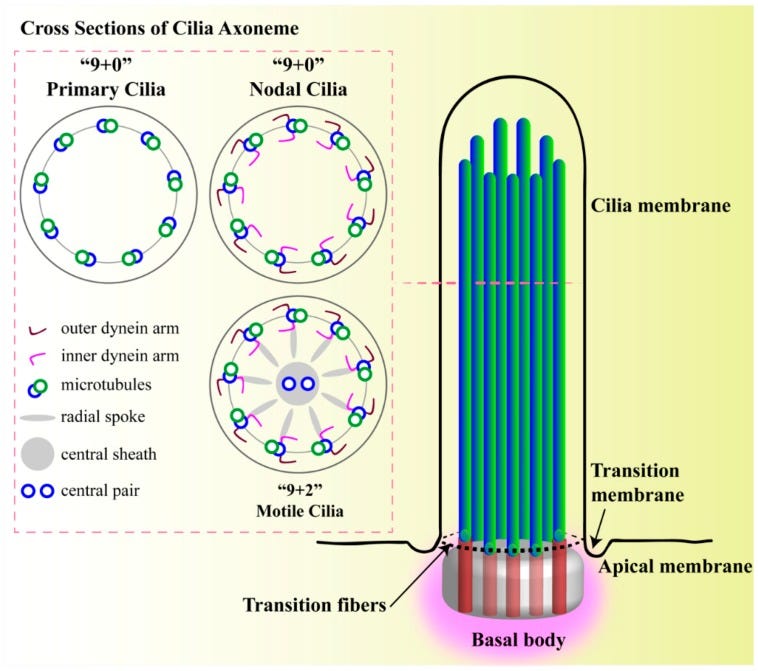
Structure of primary cilium. A cilium is a membrane-bound structure and composed of multiple central pairs of microtubules (axoneme) running from the basal body. A basal body is a microtubule-based structure composed of mother and daughter centrioles. The ciliary membrane and axoneme contributes to the upper part of the cilium. The ciliary membrane is continuous with the cell membrane, but they have their own proteins, ion channels and/or receptors. The ciliary skeleton may have 9 + 0 or 9 + 2 axoneme compositions. Most 9 + 0 cilia lack inner and outer dynein arms, radial spokes, and central sheath and are commonly referred as non-motile primary cilia. Some 9 + 0 cilia lack the central microtubule only and are motile. Between the cell membrane and cilium, there is a transition-membrane at the junction of the basal body acting as a barrier for molecules to enter or exit from the primary cilium.
Within the arteries, primary cilia play an important role in the structure and the function of endothelial cells [12,60]. Therefore, the absence or dysfunction of primary cilia can induce aneurysm formation and progression during vascular injuries [10,155]. Vascular aneurysms are associated with tissue remodeling due to unusual proliferation of the endothelial cell layers through the hemodynamic fluctuations in fluid-shear forces [156]. Endothelial cilia are required for shear stress-induced Ca2+ influx and NO signaling [11], and eNOS deficiency is the hallmark of endothelial dysfunction and associated with cardiovascular complications including aneurysm, indicating the protective role of eNOS [157]. Primary cilia regulate endothelial actin organization and focal adhesion assembly that can affect directional migration and cell permeability through hsp27 and Notch/foxc1b signaling [158,159]. It is therefore thought that the mechano-sensation of primary cilia is essential in promoting proper vascular development.
Full paper:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC6315816/#__ffn_sectitle
Role of endothelial primary cilia as fluid mechanosensors on vascular health (2018)
Abstract
Primary cilia are microtubule-based organelles that protrude from the cell surface of many mammalian cell types, including endothelial and epithelial cells, osteoblasts, and neurons. These antennal-like projections enable cells to detect extracellular stimuli and elicit responses via intracellular signaling mechanisms. Primary cilia on endothelial cells lining blood vessels function as calcium-dependent mechanosensors that sense blood flow. In doing so, they facilitate the regulation of hemodynamic parameters within the vascular system. Defects in endothelial primary cilia result in inappropriate blood flow-induced responses and contribute to the development of vascular dysfunctions, including atherosclerosis, hypertension, and aneurysms. This review examines the current understanding of vascular endothelial cilia structure and function and their role in the vascular system. Future directions for primary cilia research and treatments for ciliary-based pathologies are discussed.
Keywords: Cardiovascular disease; Endothelial cell; Mechanosensor; Primary cilia.
https://pubmed.ncbi.nlm.nih.gov/29945035/
Pathology:
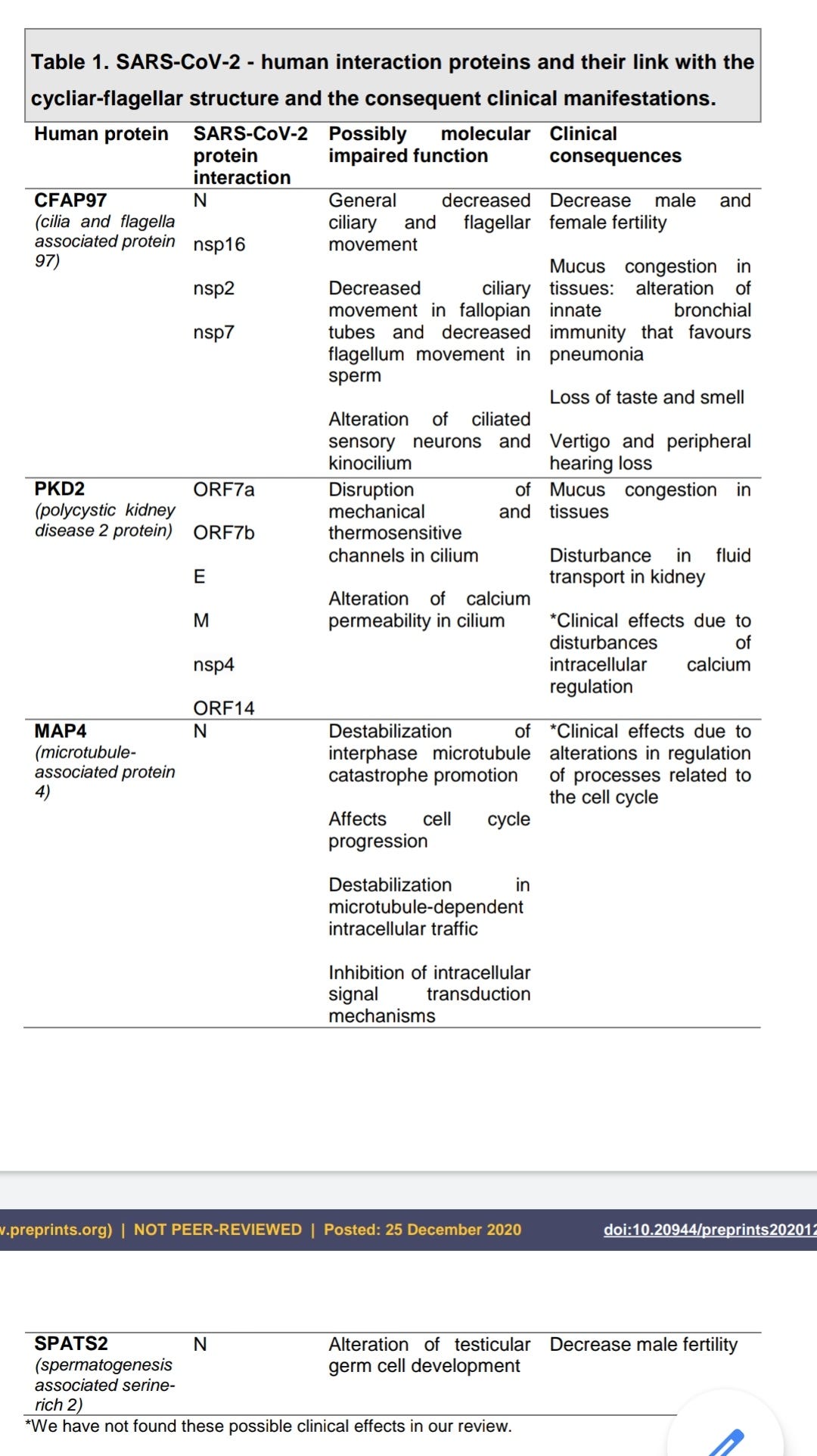
Ciliary Dysfunction Secondary to COVID-19. Explanation of the Pathogenesis From Analysis of Human Interactome With Sars-Cov-2 Proteome (preprint, 2020)
Link to full paper Pdf download:
https://www.preprints.org/manuscript/202012.0663/v1
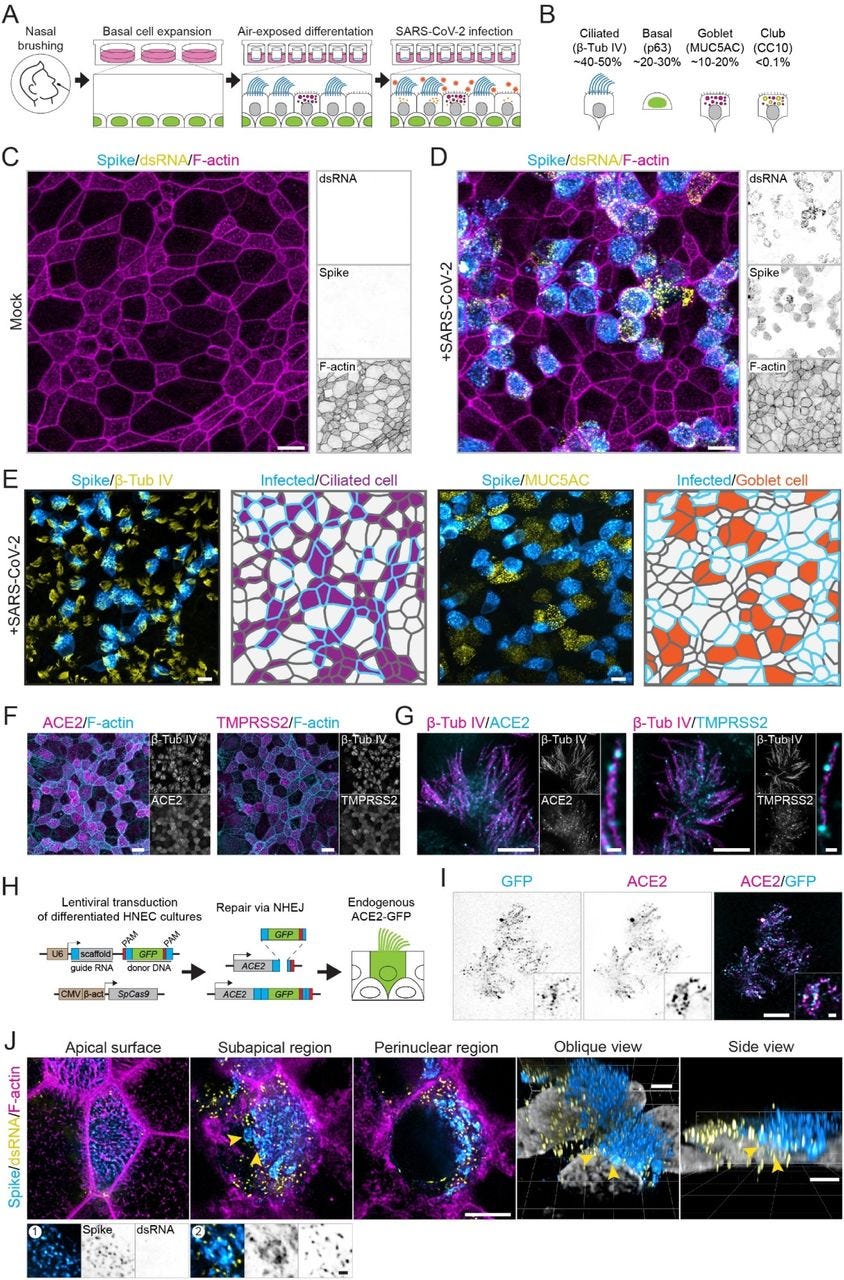
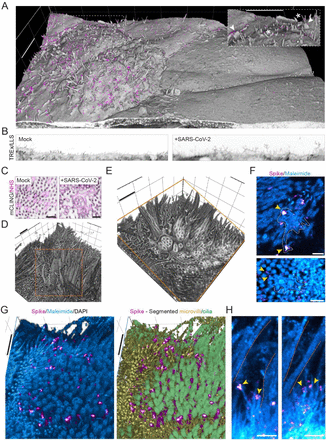
Optical nanoscopy reveals SARS-CoV-2-induced remodeling of human airway cells (August, 2021)
Abstract
A better understanding of host cell remodeling by the coronavirus SARS-CoV-2 is urgently needed to understand viral pathogenesis and guide drug development. Expression profiling and electron microscopy have frequently been used to study virus-host interactions, but these techniques do not readily enable spatial, sub-cellular and molecular analysis of specific cellular compartments. Here, we use diffraction-unlimited fluorescence microscopy to analyze how SARS-CoV-2 infection exploits and repurposes the subcellular architecture of primary human airway cells. Using STED nanoscopy, we detect viral entry factors along the motile cilia of ciliated cells and visualize key aspects of the viral life cycle. Using Tenfold Robust Expansion (TREx) microscopy, we analyze the extensively remodeled three-dimensional ultrastructure of SARS-CoV-2-infected ciliated cells and uncover Golgi fragmentation, emergence of large and atypical multivesicular bodies enclosing viral proteins, ciliary clustering, and remodeling of the apical surface. These results demonstrate a broadly applicable strategy to study how viruses reorganize host cells with spatial and molecular specificity and provide new insights into SARS-CoV-2 infection in primary human cell models.
To achieve coordinated ciliary beating, cilia positioning is organized through the positioning of basal bodies, centriole-like structures at the base of each cilium [55]. To test if ciliary clustering was related to basal body reorganization, we analyzed basal body organization in mock-infected and SARS-CoV-2-infected cells. During development, ciliated cells undergo three stages of basal body arrangement – floret, scattered and aligned – to achieve a uniform beating direction [55]. These stages are distinguished by the alignment of the basal bodies in combination with the orientation of the basal foot relative to the basal body, which indicates the ciliary beating direction (Figure 6F). To analyze basal body alignment and polarization, we stained for ODF2 and centriolin, markers for the basal body and basal foot, respectively (Figure 6G-I). As ciliary clustering was observed throughout the infected culture, independent of spike signal (Figure 6C), we included all ciliated cells in infected HNEC cultures in our analysis. This analysis revealed that infection did not affect basal body alignment or polarization, even in highly infected cells (Figure 6G). Thus, SARS-CoV-2 infection induces ciliary clustering and disorganization, but this is not a result of basal body disorganization.
In addition to intracellular reorganizations, we also observed severe morphological rearrangements at the cell surface. In Vero E6 cells, we observed overall surface roughening and the emergence of spike-positive filopodia. Smooth Vero E6 cells without filopodia were almost devoid of spike labeling both on the surface and in the cytoplasm. This indicates that these virus-induced filopodia result from viral replication or egress and are not related to viral entry. These findings are consistent with recent reports that SARS-CoV-2-infected Vero E6 and Caco-2 cells form an increased number of actin-rich filopodial protrusions that are branched and elongated compared to those on uninfected cells. These protrusions were decorated by viral particles and were postulated to be involved with viral egress [56, 64]. Indeed, other viruses are known to induce actin extensions, such as elongated microvilli or filopodia, for efficient cell-to-cell spread of virions [65]. Importantly, we recapitulated these findings in human ciliated airway cells, where we observed severe apical remodeling and spike accumulation on actin-rich microvilli. In these infected HNECs, we also observed clustering of ciliary tips in both infected and neighboring uninfected cells. This was not caused by basal body disruption nor by direct crosslinking of cilia by viral particles, as we did not observe a clear accumulation of spike on cilia. Ciliary clustering could be caused by a more global antiviral response, potentially related to interferon signaling. Elucidating how innate immune responses prevent the spread of viral infection in airway cultures will be an important direction for future work.
Full paper:
https://www.biorxiv.org/content/10.1101/2021.08.05.455126v1.full
COVID‐19, cilia, and smell (2022)
Abstract
The novel coronavirus SARS‐CoV‐2 is the causative agent of the global coronavirus disease 2019 (COVID‐19) outbreak. In addition to pneumonia, other COVID‐19‐associated symptoms have been reported, including loss of smell (anosmia). However, the connection between infection with coronavirus and anosmia remains enigmatic. It has been reported that defects in olfactory cilia lead to anosmia. In this Viewpoint, we summarize transmission electron microscopic studies of cilia in virus‐infected cells. In the human nasal epithelium, coronavirus infects the ciliated cells and causes deciliation. Research has shown that viruses such as influenza and Sendai attach to the ciliary membrane. The Sendai virus enters cilia by fusing its viral membrane with the ciliary membrane. A recent study on SARS‐CoV‐2–human protein–protein interactions revealed that the viral nonstructural protein Nsp13 interacts with the centrosome components, providing a potential molecular link. The mucociliary escalator removes inhaled pathogenic particles and functions as the first line of protection mechanism against viral infection in the human airway. Thus, future investigation into the virus–cilium interface will help further the battle against COVID‐19.
Keywords: COVID‐19, SARS‐CoV‐2, smell loss, cilia
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7426555/
Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells? (2021)
Abstract
A worldwide coronavirus pandemic is in full swing and, at the time of writing, there are only few treatments that have been successful in clinical trials, but no effective antiviral treatment has been approved. Because of its lethality, it is important to understand the current strain’s effects and mechanisms not only in the respiratory system but also in other affected organ systems as well. Past coronavirus outbreaks caused by SARS-CoV and MERS-CoV inflicted life-threatening acute kidney injuries (AKI) on their hosts leading to significant mortality rates, which went somewhat overlooked in the face of the severe respiratory effects. Recent evidence has emphasized renal involvement in SARS-CoV-2, stressing that kidneys are damaged in patients with COVID-19. The mechanism by which this virus inflicts AKI is still unclear, but evidence from other coronavirus strains may hold some clues. Two theories exist for the proposed mechanism of AKI: 1) the AKI is a secondary effect to reduced blood and oxygen levels causing hyperinflammation and 2) the AKI is due to cytotoxic effects. Kidneys express angiotensin-converting enzyme-2 (ACE2), the confirmed SARS-CoV-2 target receptor as well as collectrin, an ACE2 homologue that localizes to the primary cilium, an organelle historically targeted by coronaviruses. Although the available literature suggests that kidney damage is leading to higher mortality rates in patients with COVID-19, especially in those with preexisting kidney and cardiovascular diseases, the pathogenesis of COVID-19 is still being investigated. Here, we present brief literature review supporting our proposed hypothesis of a possible link between SARS-CoV-2 cellular infection and cilia.
https://journals.physiology.org/doi/full/10.1152/physiolgenomics.00015.2021
SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance (2021)
Abstract
Understanding how SARS-CoV-2 spreads within the respiratory tract is important to define the parameters controlling the severity of COVID-19. Here we examine the functional and structural consequences of SARS-CoV-2 infection in a reconstructed human bronchial epithelium model. SARS-CoV-2 replication causes a transient decrease in epithelial barrier function and disruption of tight junctions, though viral particle crossing remains limited. Rather, SARS-CoV-2 replication leads to a rapid loss of the ciliary layer, characterized at the ultrastructural level by axoneme loss and misorientation of remaining basal bodies. Downregulation of the master regulator of ciliogenesis Foxj1 occurs prior to extensive cilia loss, implicating this transcription factor in the dedifferentiation of ciliated cells. Motile cilia function is compromised by SARS-CoV-2 infection, as measured in a mucociliary clearance assay. Epithelial defense mechanisms, including basal cell mobilization and interferon-lambda induction, ramp up only after the initiation of cilia damage. Analysis of SARS-CoV-2 infection in Syrian hamsters further demonstrates the loss of motile cilia in vivo. This study identifies cilia damage as a pathogenic mechanism that could facilitate SARS-CoV-2 spread to the deeper lung parenchyma.
“Ultrastructural analysis by SEM revealed a loss of motile cilia in productively infected human bronchial cells. Misshapen and shortened cilia were also detected, reminiscent of abnormalities observed in certain cases of primary ciliopathies17,49. We documented by immunofluorescence a thinning of the ciliary layer as early as 2 dpi, associated to a layer of viral spike protein at the base of motile cilia. SARS-CoV-2 particles were not released from cilia, but rather from deciliated areas, as indicated by the minimal colocalization between the spike and β-tubulin markers. Clusters of viral particles could be detected on microvilli, consistent with findings suggesting that SARS-CoV-2 could induce the formation of actin-based filaments in transformed cells50. The absence of viral particles within cilia may result from the restricted protein access imposed by the transition zone at the base of motile cilia51. Access of cellular proteins into the ciliary axoneme is limited to those bound by the intraflagellar transport machinery, which likely prevents the import of viral proteins. Therefore, the destruction of motile cilia by SARS-CoV-2 seems mediated by an indirect mechanism, rather than by direct viral production within these structures.”
Full paper:
https://www.nature.com/articles/s41467-021-24521-x
As noted earlier, although vascular damage from both severe viral infection or artificial transfection is now well established ciliopathy in this context has received little focus, but is strongly implied and possibly contributes to vascular rupture or cardiovascular disease as discussed previously. We know the virus readily binds to ACE2 receptors on endothelial cells lining blood vessels but confirmation of subsequent damage to primary cilia remains speculative until we have more data. The role of autoimmune attack also needs consideration.
Although not yet proven to be directly damaging to cilia, spike protein transfections are implicated in ciliopathy due to them mediating endothelial damage in general (vasculitis, fibrosis) and secondarily by promoting more serious infections due to immunosuppression and immune escape.
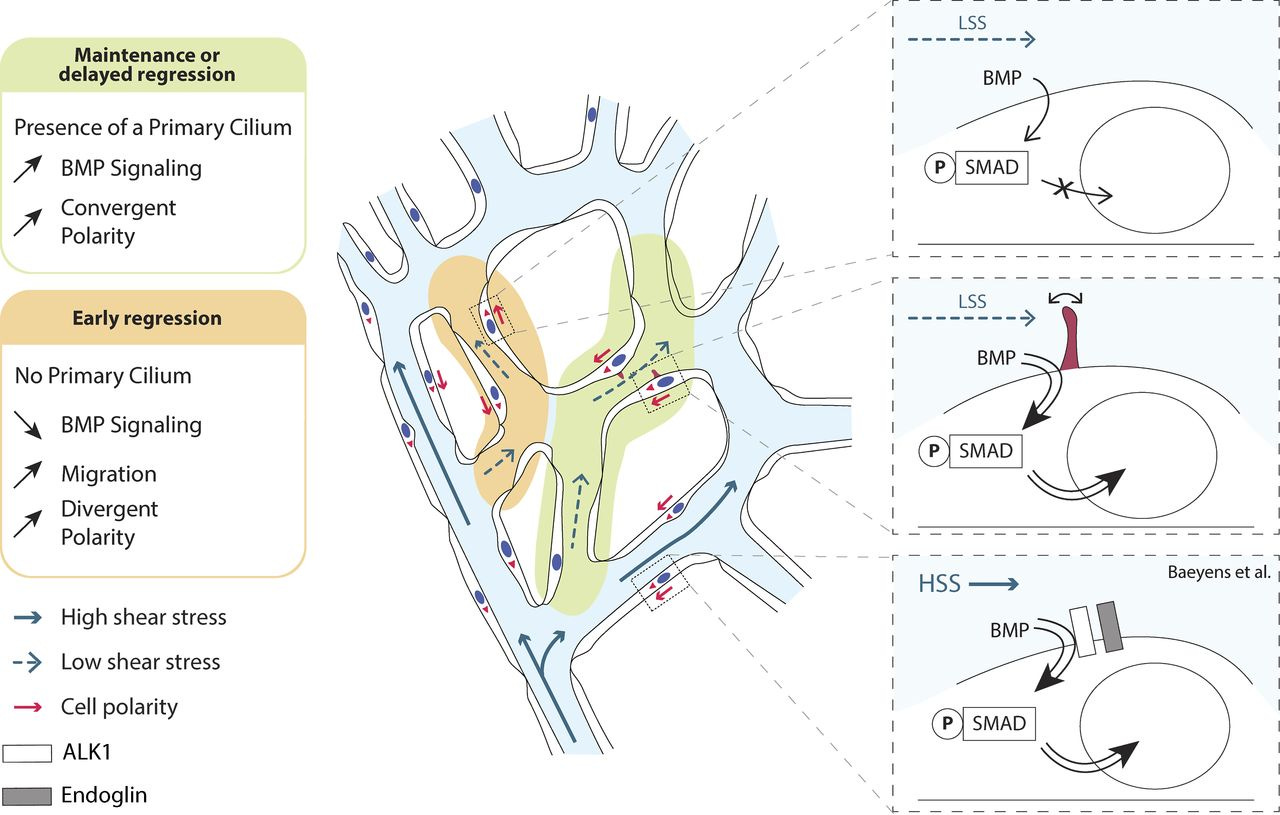
Primary cilia sensitize endothelial cells to BMP and prevent excessive vascular regression (2018)
Blood flow shapes vascular networks by orchestrating endothelial cell behavior and function. How endothelial cells read and interpret flow-derived signals is poorly understood. Here, we show that endothelial cells in the developing mouse retina form and use luminal primary cilia to stabilize vessel connections selectively in parts of the remodeling vascular plexus experiencing low and intermediate shear stress. Inducible genetic deletion of the essential cilia component intraflagellar transport protein 88 (IFT88) in endothelial cells caused premature and random vessel regression without affecting proliferation, cell cycle progression, or apoptosis. IFT88 mutant cells lacking primary cilia displayed reduced polarization against blood flow, selectively at low and intermediate flow levels, and have a stronger migratory behavior. Molecularly, we identify that primary cilia endow endothelial cells with strongly enhanced sensitivity to bone morphogenic protein 9 (BMP9), selectively under low flow. We propose that BMP9 signaling cooperates with the primary cilia at low flow to keep immature vessels open before high shear stress–mediated remodeling.
More:
https://rupress.org/jcb/article/217/5/1651/38912/Primary-cilia-sensitize-endothelial-cells-to-BMP
Endothelial dysfunction in rheumatic autoimmune diseases
Giuseppe Murdaca et al. Atherosclerosis. 2012 Oct.
Abstract
Rheumatic autoimmune diseases have been associated with accelerated atherosclerosis and various types of vasculopathies. Atherosclerosis is an inflammatory condition which starts as a "response to injury" favoring endothelial dysfunction which is associated with increased expression of adhesion molecules, pro-inflammatory cytokines, pro-thrombotic factors, oxidative stress upregulation and abnormal vascular tone modulation. Endothelial dysfunction in rheumatic autoimmune diseases involves innate immune responses, including macrophages and dendritic cells expression of scavenger and toll-like receptors for modified or native LDL as well as neutrophil and complement activation, and dysregulation of adaptive immune responses, including proliferation of autoreactive T-helper-1 lymphocytes and defective function of dendritic and regulatory T cells. Specific differences for endothelial function among different disorders include: a) increased amounts of pro-atherogenic hormones, decreased amounts of anti-atherogenic hormones and increased insulin resistance in rheumatoid arthritis; b) autoantibodies production in systemic lupus erythematosus and antiphospholipid syndrome; c) smooth muscle cells proliferation, destruction of internal elastic lamina, fibrosis and coagulation and fibrinolytic system dysfunction in systemic sclerosis. Several self-antigens (i.e. high density lipoproteins, heat shock proteins, β2-glycoprotein1) and self-molecules modified by oxidative events (i.e. low density lipoproteins and oxidized hemoglobin) have been identified as targets of autoimmune responses. Endothelial dysfunction leads to accelerated atherosclerosis in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropaties whereas obliterative vasculopathy is associated with systemic sclerosis. In this paper, we will briefly review the most relevant information upon endothelial dysfunction and inflammatory mechanisms in atherosclerosis and we will summarize the similarities and differences in vascular disease patterns underlying different rheumatic autoimmune diseases.
More:
https://pubmed.ncbi.nlm.nih.gov/22673743/
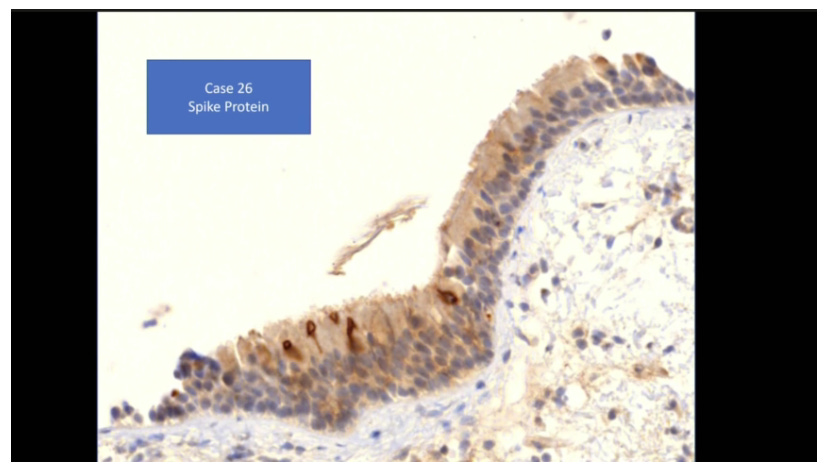
Credits go to Prof Arne Burkhardt for providing these slides showing spike protein expression and T cell proliferation in vascular tissues:
So how long does it take damage to the endothelial cells to recover (rendotheliation)?
In balloon angioplasty up to around 5 months, if at all, and this repair tissue appears to be a poor replacement, perhaps not quite fibrosis but lacking a healthy structure (or cilia?).
That's not to say definitely that covid-19 or spike protein induced endothelial damage and ciliopathy is as severe, but as microthrombi & hypoxia induced fibrosis is a commonplace there is arguably a high likelihood of this. Cumulative dose dependent ciliopathy and vasculitis & fibrosis must also be considered.
Endothelial regrowth after arterial injury: from vascular repair to therapeutics (1998)
2.2 The natural history of endothelial regrowth after arterial injury
In the hours following experimental angioplasty, ECs rapidly enter the replication cycle to restore endothelial continuity. Proliferation and migration can be initiated either by loss of contact inhibition, stretch or growth factors secreted by ECs, VSMCs and circulating cells [64, 65]. Endothelial regeneration starts from the leading edge of the denuded area and from the ostia of collateral arteries [66]. This process begins within the first 24 hours after arterial denudation and ceases 6 or 10 weeks later, depending upon animal species [67]. The rate of endothelial regrowth from the leading edges is related to animal models and experimental conditions; for example in the common carotid artery, the endothelial outgrowth is 8–10 mm in the rat and 5–6 mm in the rabbit [67]. However, the most important factors determining the final reendothelialized area seems to be the extent of the initial deendothelialization and the number of side branches within the denuded area. Indeed, while complete reendothelialization can be achieved after minor degrees of denudation [68, 69]or in vessels with numerous side branches [70, 71], ECs have been found to be incapable of sustained regrowth after widespread denudation [67, 72]. Even if complete reendothelialization occurs, the regenerated endothelium shows abnormal morphologic characteristics. In contrast to normal ECs, regenerating ECs grow as a sheet with close cell-to-cell contacts, no longer aligned with blood flow and are polygonal-shaped and irregular-sized with cytoplasm bulging toward the lumen [66, 67, 69, 70, 73].
In humans, there is limited information on reendothelialization following percutaneous transluminal coronary angioplasty (PTCA) [1, 2]. As in animal models, findings in specimens retrieved early after PTCA demonstrate that balloon injury is associated with a complete loss of the EC lining. In addition, examination of specimens retrieved at later time-points suggests that there is limited reendothelialization at the injured site within 1 month of balloon angioplasty while extensive reendothelialization occurs between 1 and 5 months. Reendothelialization is typically completed 5 months after PTCA.
More:
https://academic.oup.com/cardiovascres/article/38/1/54/330562
Regeneration of the endothelium in vascular injury
Paul M Vanhoutte. Cardiovasc Drugs Ther. 2010 Aug.
Abstract
The endothelium mediates relaxations (dilatations) of the underlying vascular smooth muscle cells. The endothelium-dependent relaxations are due to the release of non-prostanoid vasodilator substances. The best characterized endothelium-derived relaxing factor (EDRF) is nitric oxide (NO). The endothelial cells also release substances (endothelium-derived hyperpolarizing factor, EDHF) that cause hyperpolarization of the cell membrane of the underlying vascular smooth muscle. The release of EDRF from the endothelium can be mediated by both pertussis toxin-sensitive G(i) (alpha(2)-adrenergic activation, serotonin, thrombin) and insensitive G(q) (adenosine diphosphate, bradykinin) coupling proteins. The ability of the endothelial cell to release relaxing factors can be upregulated by impregnation with estrogens, exercise and antioxidants, and down-regulated by oxidative stress and increased presence of oxidized LDL. Following injury or apoptotic death, the endothelium regenerates. However, in regenerated endothelial cells, there is an early selective loss of the pertussis-toxin sensitive mechanisms of EDRF-release. Functional studies suggest that abnormal handling of LDL because of increased oxidative stress play a key role in this selective loss. Genomic analysis demonstrates the emergence of fatty acid binding protein-A (A-FBP) and metalloproteinase-7 (MMP7) in regenerated endothelial cells. The reduced release of NO resulting from the endothelial dysfunction in regenerated areas creates a locus minoris resistentiae which favors the occurrence of vasospasm and thrombosis as well as the initiation of atherosclerosis.
https://pubmed.ncbi.nlm.nih.gov/20689986/
And as for damaged cilia in the lungs/mucosa?
“A critical aspect of lung health is healthy cilia. Cilia are tiny hairlike organelles that are found all throughout your body. Cilia in the lungs sweep out debris, mucus, and other pollutants.
Lung improvement begins after 2 weeks to 3 months. The cilia in your lungs take 1 to 9 months to repair.”
https://plushcare.com/blog/learn-how-to-clean-lungs-after-quitting-smoking/
An important footnote here is that more permanent damage may also be present, fibrotic cells will not clear and can contribute to other pathologies like pulmonary arterial hypertension.
There are further implications for cardiac health, development and repair mechanisms:
A change of heart: new roles for cilia in cardiac development and disease (2021)
“Furthermore, we also highlight research from the past 10 years demonstrating the role of cilia function in common cardiac valve disorders, including mitral valve prolapse and aortic valve disease, and describe findings that implicate cardiac cilia in mechanosensation potentially linking haemodynamic and contractile forces with genetic regulation of cardiac development and function. Finally, given the presence of cilia on cardiac fibroblasts, we also explore the potential role of cilia in fibrotic growth and summarize the evidence implicating cardiac cilia in heart regeneration.”
https://www.nature.com/articles/s41569-021-00635-z
Cytokines are known to be significantly upregulated due to spike protein transfections:
Cytokine Signature Induced by SARS-CoV-2 Spike Protein in a Mouse Model (2021)
“High levels of TNF-α and nearly 100 times increased IL-6 were detected at 6 h, but disappeared by 24 h in bronchoalveolar lavage fluid (BALF) following immunostimulant challenge.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7876321/
Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation (2018)
Abstract
Pulmonary arterial hypertension (PAH) is a syndrome characterized by progressive lung vascular remodelling, endothelial cell (EC) dysfunction, and excessive inflammation. The primary cilium is a sensory antenna that integrates signalling and fine tunes EC responses to various stimuli. Yet, cilia function in the context of deregulated immunity in PAH remains obscure. We hypothesized that cilia function is impaired in ECs from patients with PAH due to their inflammatory status and tested whether cilia length changes in response to cytokines. Primary human pulmonary and mouse embryonic EC were exposed to pro- (TNFα, IL1β, and IFNγ) and/or anti-inflammatory (IL-10) cytokines and cilia length was quantified. Chronic treatment with all tested inflammatory cytokines led to a significant elongation of cilia in both control human and mouse EC (by ∼1 µm, P < 0.001). This structural response was PKA/PKC dependent. Intriguingly, withdrawal of the inflammatory stimulus did not reduce cilia length. IL-10, on the other hand, blocked and reversed the pro-inflammatory cytokine-induced cilia elongation in healthy ECs, but did not influence basal length. Conversely, primary cilia of ECs from PAH patients were significantly longer under basal conditions compared to controls (1.86 ± 0.02 vs. 2.43 ± 0.08 µm, P = 0.002). These cilia did not elongate further upon pro-inflammatory stimulation and anti-inflammatory treatment did not impact cilia length. The missing length modulation was specific to cytokine stimulation, as application of fluid shear stress led to increased cilia length in the PAH endothelium. We identified loss of cilia length regulation upon cytokine stimulation as part of the endothelial dysfunction in PAH.
Hat tip to Dr Jon Couey:
Endothelial cell-activating antibodies in COVID-19
Hui Shi et al. medRxiv. 2022.
Abstract
Objective: While endothelial dysfunction has been implicated in the widespread thrombo-inflammatory complications of coronavirus disease-19 ( COVID-19 ), the upstream mediators of endotheliopathy remain for the most part cryptic. Our aim was to identify circulating factors contributing to endothelial cell activation and dysfunction in COVID-19.
Methods: Human endothelial cells were cultured in the presence of serum or plasma from 244 patients hospitalized with COVID-19 and plasma from 100 patients with non-COVID sepsis. Cell adhesion molecules (E-selectin, VCAM-1, and ICAM-1) were quantified by in-cell ELISA.
Results: Serum and plasma from patients with COVID-19 increased surface expression of cell adhesion molecules. Furthermore, levels of soluble ICAM-1 and E-selectin were elevated in patient serum and tracked with disease severity. The presence of circulating antiphospholipid antibodies was a strong marker of the ability of COVID-19 serum to activate endothelium. Depletion of total IgG from antiphospholipid antibody-positive serum markedly restrained upregulation of cell adhesion molecules. Conversely, supplementation of control serum with patient IgG was sufficient to trigger endothelial activation.
Conclusion: These data are the first to suggest that some patients with COVID-19 have potentially diverse antibodies that drive endotheliopathy, adding important context regarding thrombo-inflammatory effects of autoantibodies in severe COVID-19.
https://pubmed.ncbi.nlm.nih.gov/33501469/
In case you were wondering, it appears quite plausible that transfection can indeed mediate generation of the same autoimmune antibodies:
Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: The straw that breaks the camel's back? (2021)
Hypothetical immunothrombotic pathways triggered by COVID-19 vaccines.
The left side of the picture depicts the potential scenario occurring after the administration of adenoviral vector-based vaccines. The latter may induce the aggregation of platelets and their sequestration into the reticuloendothelial system, hence contributing to thrombocytopenia and platelet activation. Furthermore, adenoviral epitopes may cross-react with epitopes within self-complexes of proteins and anionic lipids, giving rise to aPLs. Anti-spike antibodies may elicit after several weeks the generation of anti-idiotype antibodies, able to promote platelet activation through the interaction with platelet ACE2. The latter mechanism could potentially take place also after immunization with mRNA-based vaccines.
The right side of the picture instead represents the scenario which may occur with the mRNA-based vaccine formulations. Minimal amounts of mRNA unwantedly released from liposomal capsules may activate Toll-like receptors in plasmacytoid dendritic cells and contribute to the synthesis of type I IFN-related cytokines. These may foment the production of aPLs from plasma cells and NETosis. Furthermore, mRNA may promote the initiation of the coagulation cascade by activating the coagulation factors VII, XI and XII. Finally, liposomal formulations may interfere with the physiological cross-talk occurring between platelets and endothelial cells and favour the activation of the inflammasome platforms into endothelial cells and thus the secretion of pro-inflammatory cytokines.
Abbreviations: Ab, antibody; aPL, antiphospholipid antibody; ACE2, angiotensin-converting enzime 2; TLR, Toll-like receptor; pDC, plasmacytoid dendritic cells; NET, neutrophil extracellular trap; IFN, interferon.
Full paper:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8159713/#__ffn_sectitle
Related:
Embalmer Conundrum "AHA" Moment
Vaccine Safety Myth - Part 2 - "What happens to those billions of NanoParticles you've become host to?"
Marc Girardot
Mar 12
https://covidmythbuster.substack.com/p/embalmer-conundrum-aha-moment?s=r
To conclude, are there any specific drugs to treat ciliopathy? Quick answer is no, apart from potentially to treat inherited conditions.
Look after your immune system, endothelia & cilia. Limit exposure to any potentially pathogenic agents.
Potential drug to cure cilio-pathies (2018)
“Currently, there are no approved drugs available for treating most ciliopathies. In fact, this is the case for most of the rare genetic disorders involving functional abnormalities through genetic mutation, and gene therapy is usually the only treatment available.”
https://www.drugtargetreview.com/news/34707/potential-drug-to-cure-ciliopathies/