Influenza vaccine mediated pneumonia & fibrosis via suppression of MicroRNA-142-3p
- Served with a side salad of tumorigenesis
Updates:
5th June ‘22: Coinfection with SARS-CoV-2 and Influenza A Virus Increases Disease Severity and Impairs Neutralizing Antibody and CD4+ T Cell Responses.
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Vaccine induced amyloidosis and fibrosis is very much the topic of the year, but regarding fibrosis it's really nothing new.
MicroRNA & autoimmune mediated pathology should have been anticipated as it's happened before with influenza vaccines.
Indeed one of my last posts on Twitter before my account was taken down was on this subject, so a revisit is justified.
Background
About influenza A subtype H1N1:
In virology, influenza A virus subtype H1N1 (A/H1N1) is a subtype of Influenza A virus. Well known outbreaks of H1N1 strains in humans include the Spanish flu, the 1977 Russian flu pandemic and the 2009 swine flu pandemic. It is an orthomyxovirus that contains the glycoproteins hemagglutinin and neuraminidase. For this reason, they are described as H1N1, H1N2 etc., depending on the type of H or N antigens they express with metabolic synergy. Hemagglutinin causes red blood cells to clump together and binds the virus to the infected cell. Neuraminidase is a type of glycoside hydrolase enzyme which helps to move the virus particles through the infected cell and assist in budding from the host cells.
MicroRNAs are a recurrent theme in both viral and COVID-19 vaccinal mediated pathologies:
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #28: Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
- A walkthrough of the paper with definitions
https://doorlesscarp953.substack.com/p/innate-immune-suppression-by-sars-315?s=w
Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7
https://doorlesscarp953.substack.com/p/mir-21-promotes-cardiac-fibrosis?s=w
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #21: BNT162b2 derived miR-21
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-880?s=w
Quantum microRNA Assessment of COVID-19 RNA Vaccine: Hidden Potency of BNT162b2 SASR-CoV-2 Spike RNA as MicroRNA Vaccine
Or "would you like rice with that?"
https://doorlesscarp953.substack.com/p/quantum-microrna-assessment-of-covid?s=w
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #20: MiR-200, a new star miRNA in human cancer
Related:
Effect of exosomal miRNA on cancer biology and clinical applications
https://doorlesscarp953.substack.com/p/effect-of-exosomal-mirna-on-cancer?s=w
SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia
https://doorlesscarp953.substack.com/p/sars-cov-2-spike-targets-usp33-irf9?s=w
More microRNA mayhem
Its generally the rule that any vaccine critical papers get blocked from publication unless they have a positive spin to them, but this one got through.
The alarm is sounded on changes to serum microRNA and latent virus reactivation.
Most importantly, it “confirmed downregulation of miR-142-3p in the discovery cohort”. However, they were unable to replicate the findings in a later retest of vaccinated children, and offer several reasons as to why this may be?
What is the potential significance of any miR-142-3p suppression, even if of short duration? We will discuss later.
Their methodology:
Abstract
Background: MicroRNAs (miRNAs) are a class of small regulatory RNAs around 21-25 nucleotides in length which govern many aspects of immunity including the host innate and adaptive responses to infection. RT-qPCR studies of select microRNAs show that vaccination alters the expression circulating microRNAs but the effect of vaccination on the global microRNA population (i.e. micronome) has never been studied.
Aim: To describe vaccine associated changes in the expression of microRNAs 21 days after vaccination in children receiving a pandemic influenza (H1N1) vaccination.
Method: Serum samples were obtained from children aged 6 months to 12 years enrolled in an open label randomised control trial of two pandemic influenza (H1N1) vaccines, in which participants received either ASO3B adjuvanted split virion or a whole virion non-adjuvanted vaccine. MicroRNA expression was profiled in a discovery cohort of participants prior to, and 21 days after vaccination using an Agilent microarray platform. Findings were followed up by RT-qPCR in the original discovery cohort and then in a validation cohort of participants taken from the same study.
Results: 44 samples from 22 children were assayed in a discovery cohort. The microarray results revealed 19 microRNAs were differentially expressed after vaccination after adjustment for multiple testing. The microarray detected ubiquitous expression of several microRNAs which could not be validated by RT-qPCR, many of which have little evidence of existence in publicly available RNA sequencing data. Real time PCR (RT-qPCR) confirmed downregulation of miR-142-3p in the discovery cohort. These findings were not replicated in the subsequent validation cohort (n = 22).
Conclusion: This study is the first study to profile microRNA expression after vaccination. An important feature of this study is many of the differentially expressed microRNAs could not be detected and validated by RT-qPCR. This study highlights the care that should be taken when interpreting omics biomarker discovery, highlighting the need for supplementary methods to validate microRNA microarray findings, and emphasises the importance of validation cohorts. Data from similar studies which do not meet these requirements should be interpreted with caution.
Alarmingly, they found strong evidence for endogenous virus activation in response to vaccination. Reactivation of Kaposi's sarcoma virus in particular should have been detected in earlier trials and trials halted pending investigations. Its a cancer that affects the skin and mouth, sometimes the internal organs and patients with advanced HIV are particularly susceptible.
Surprisingly, four viral miRNAs expressed by: human cytomegalovirus, herpes simplex 1 virus, herpes simplex 2, and Kaposi’s sarcoma virus were significantly upregulated after vaccination. Multiple studies have identified circulating viral miRNAs in asymptomatic patients, found them to be differential expressed between clinical states[47][48]. It is tempting to speculate that activation of the immune system by pathogens or vaccine antigens could alter the ability of the immune to control latent infections leading to viral reactivation. Unfortunately, we were unable to optimise the RT-qPCR assays to validate upregulation of these four viral miRNAs, and for reasons further discussed below, these viral miRNAs may have been falsely detected by the microarray. Although it is theoretically possible that contamination is the cause of viral miRNAs being detected on the array, we believe that this is highly unlikely in practice because: 1) miRNAs from herpes simplex I, herpes simplex II and Kaposi’s sarcoma virus were detected in 100% of samples, and miRNAs from Epstein–Barr virus and human cytomegalovirus were detected in 90% of samples. Such ubiquitous contamination of samples with RNA from all these viruses is unlikely as we do not work with these viruses in our lab; 2) contamination with viruses being the cause of differential expression of viral miRNAs would require systematic addition of miRNAs from each virus to either pre or post vaccination samples (for up- and downregulated viral miRNAs respectively), which we believe unlikely, given one would expect contamination to happen in a random or universal manner; 3) if contamination with viruses was the cause for differential expression of viral miRNAs, one would expect that all the miRNAs of that virus (detected on the array) would be upregulated/downregulated, yet this is not the case as only specific miRNAs are differentially expressed for each virus; 4) we were unable to detect viral miRNAs by RTPCR.
On the difficulty of validating very strong PCR confirmed signals:
RT-qPCR could confirm differential expression in one out of the three miRNAs that could be detected. This suggests that microarrays can provide some reliable data. Despite this, the significant downregulation of miR-142-3p could not be replicated in an independent validation cohort. A challenge for validating differential expression in the independent cohort is that it is dependent on miR-29c being an effective reference gene in the validation cohort, but as Fig 8 shows, the expression of miR-29c in the validation cohort does not appear to be the reason for the lack of validation.
Lack of validation does not appear to be due to inadequate study power because: 1) the validation cohort was over 90% powered to detect significant downregulation of miR-142-3p at the same fold-change as seen in the discovery cohort and 2) average fold-change was greater than 1 for miR-142-3p post vaccination, which is the opposite direction to the discovery cohort where fold-change was less than 1.
Differences in the findings of the discovery and validation cohort is unlikely to be due to differences in the demographics of the two cohorts as there was no obvious interaction between miR-30b and miR-142-3p expression and age, sex or vaccine administered, and the population characteristics of the two cohorts were similar (S3 and S4 Tables). It is possible that despite trying to control for a type 1 error rate by using an FDR adjusted p-value, chance alone identified differential expression in the discovery cohort. Another possibility is the “winners curse” phenomenon which has been described in genetic association studies, and can lead to an overestimated effect size in the amongst differentially expressed genes, meaning that to achieve enough power, a subsequent validation cohort must be larger than the size which is calculated by a standard power calculation[53]. This study reiterates the importance of confirming results in a second independent cohort.
Finally, we note that investigating miRNA expression 3 weeks post 2nd vaccination may only identify circulating miRNA changes associated with memory responses. We were limited to this timepoint however as this was the only post vaccination timepoint when serum was collected in the trial. An earlier timepoint may have captured changes in the expression of miRNAs related to the early immune response, and this would be interesting to look at in future studies.
Ruth Elizabeth Drury et al. PLoS One, The effect of H1N1 vaccination on serum miRNA expression in children: A tale of caution for microRNA microarray studies, (2019)
https://pubmed.ncbi.nlm.nih.gov/31430297/
It was a small scale study but statistically significant in the first cohort. One mode of action of microRNA-142-3p is to function as a tumor suppressor. Not a microRNA that you would want silencing if you were in remission from cancer or in a clinically vulnerable group.
If silencing is only for a few weeks that can be enough to break the tumor growth-immune response control cycle and change the equilibrium permanently in favour of renewed tumorigenesis.
Reactivation of oncogenic viruses only serves to multiply the risk. This recently published case study comes up with a hypothesis to explain how the BNT162b2 mRNA vaccine can induce this:
Herpes zoster which is the reactivation of varicella-zoster virus, a pathogenic human alpha-herpes virus, following primary infection or chicken pox, is known to occur especially in advanced age and in the immunocompromised among other predisposing factors. COVID-19 vaccination-induced immunomodulation is a novel scenario, hypothesized to be a result of shifting of T-lymphocyte population towards vaccine-induced naïve CD8+ subset, offsetting the balance of varicella-zoster virus responsive T-helper cells, thereby defecting the cell-mediated immunity which suppresses the latent varicella-zoster virus.
BM Munasinghe, UPM Fernando, M Mathurageethan, Durga Sritharan, Reactivation of varicella-zoster virus following mRNA COVID-19 vaccination in a patient with moderately differentiated adenocarcinoma of rectum: A case report, SAGE Open Medical Case Reports, vol. 10, First Published February 26, 2022
https://journals.sagepub.com/doi/full/10.1177/2050313X221077737
Further research into microRNA changes in cancer patients, the elderly or immunosuppressed would have been warranted too.
Abstract
An increasing number of studies indicate that microRNAs (miRNAs) may exert an oncogenic or tumor suppressive role in diverse types of cancer. MicroRNA (miR)-142-3p has been detected to be downregulated in a number of cancer types, and it may function as a tumor suppressor. However, the expression profile and potential role of miR-142-3p in gastric cancer remain unknown. In the present study, the expression of miR-142-3p in numerous gastric cancer samples was investigated. It was observed that miR-142-3p was markedly downregulated in cancer tissues compared with normal tissues. Furthermore, a low expression level of miR-142-3p was associated with higher tumor stages. The overexpression of miR-142-3p was able to inhibit the proliferation, invasion and migration of gastric cancer cells. A further investigation into the mechanism underlying the effect of miR-142-3p identified cyclin T2 (CCNT2) as a target of miR-142-3p in gastric cancers. miR-142-3p may exert its tumor suppressor role partially by downregulating CCNT2. These results suggested that the abnormal downregulation of miR-142-3p and the subsequent increase in CCNT2 expression may have an important role in gastric cancer carcinogenesis.
Keywords: cyclin T2; gastric cancer; microRNA-142-3p; tumor suppressor.

Yi Wang et al. Oncol Lett., Downregulation of microRNA-142-3p and its tumor suppressor role in gastric cancer (2018 May).
https://pubmed.ncbi.nlm.nih.gov/29849811/
MicroRNA-142-3p also interacts with P53. Hypermethylation is a means to semi-permanently silence miRNA mediated gene expression, an epigenetic process.
Abstract
Background: The poor prognosis of pancreatic ductal adenocarcinoma (PDAC) is accounted for by the absence of early diagnostic markers and effective treatments. MicroRNAs inhibit the translation of their target mRNAs. The production of microRNAs is strongly altered in cancers, but the causes of these alterations are only partially known. DNA hypermethylation is a major cause of gene inactivation in cancer. Our aims were to identify microRNAs whose gene expression is inactivated by hypermethylation in PDAC and to determine whether this hypermethylation-mediated repression is an early event during pancreatic carcinogenesis. We also sought to investigate whether these differentially methylated regions can serve as a diagnostic marker for PDAC.
Methods: MicroRNA production was measured by microarray hybridization and reverse-transcription quantitative PCR. The level of DNA methylation was measured by bisulfite mapping and semiquantitative methylation-specific PCR.
Results: We identified 29 microRNAs encoded by genes whose expression is potentially inactivated by DNA hypermethylation. We focused our study on microRNA 148a (miR-148a) and found its production to be repressed, not only in PDAC samples but also in preneoplastic pancreatic intraepithelial neoplasia (PanIN) lesions. More importantly, we found that hypermethylation of the DNA region encoding miR-148a is responsible for its repression, which occurs in PanIN preneoplastic lesions. Finally, we show that the hypermethylated DNA region encoding miR-148a can serve as an ancillary marker for the differential diagnosis of PDAC and chronic pancreatitis (CP).
Conclusions: We show that the hypermethylation of the DNA region encoding miR-148a is responsible for its repression in PDAC precursor lesions and can be a useful tool for the differential diagnosis of PDAC and CP.
Hanoun N, Delpu Y, Suriawinata AA, Bournet B, Bureau C, Selves J, Tsongalis GJ, Dufresne M, Buscail L, Cordelier P, Torrisani J. The silencing of microRNA 148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin Chem. 2010 Jul;56(7):1107-18. doi: 10.1373/clinchem.2010.144709. Epub 2010 Apr 29. PMID: 20431052.
https://pubmed.ncbi.nlm.nih.gov/20431052/
And a more recent 2018 study:
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an extremely aggressive disease with poor prognostic implications. This is partly due to a large proportion of PDACs carrying mutations in TP53, which impart gain-of-function characteristics that promote metastasis. There is evidence that microRNAs (miRNAs) may play a role in both gain-of-function TP53 mutations and metastasis, but this has not been fully explored in PDAC. Here we set out to identify miRNAs which are specifically dysregulated in metastatic PDAC. To achieve this, we utilised established mouse models of PDAC to profile miRNA expression in primary tumours expressing the metastasis-inducing mutant p53R172H and compared these to two control models carrying mutations, which promote tumour progression but do not induce metastasis. We show that a subset of miRNAs are dysregulated in mouse PDAC tumour tissues expressing mutant p53R172H, primary cell lines derived from mice with the same mutations and in TP53 null cells with ectopic expression of the orthologous human mutation, p53R175H. Specifically, miR-142-3p is downregulated in all of these experimental models. We found that DNA methyltransferase 1 (Dnmt1) is upregulated in tumour tissue and cell lines, which express p53R172H. Inhibition or depletion of Dnmt1 restores miR-142-3p expression. Overexpression of miR-142-3p attenuates the invasive capacity of p53R172H-expressing tumour cells. MiR-142-3p dysregulation is known to be associated with cancer progression, metastasis and the miRNA is downregulated in patients with PDAC. Here we link TP53 gain-of-function mutations to Dnmt1 expression and in turn miR-142-3p expression. Additionally, we show a correlation between expression of these genes and patient survival, suggesting that they may have potential to be therapeutic targets.
Jack D. Godfrey, Jennifer P. Morton, …Martin D. Bushell, MiR-142-3p is downregulated in aggressive p53 mutant mouse models of pancreatic ductal adenocarcinoma by hypermethylation of its locus, Cell Death & Disease volume 9, Article number: 644 (2018)
https://www.nature.com/articles/s41419-018-0628-4
Of particular interest, MicroRNA-142-3p suppression in heart tissue can help mediate myocardial fibrosis.
“Transforming growth factor beta 1 or TGF-β1 is a polypeptide member of the transforming growth factor beta superfamily of cytokines. It is a secreted protein that performs many cellular functions, including the control of cell growth, cell proliferation, cell differentiation, and apoptosis.”
https://en.wikipedia.org/wiki/TGF_beta_1
Abstract
Myocardial ischemia/reperfusion (I/R) injury may cause the apoptosis of cardiomyocytes as well as cardiac fibrosis, which is characterized as the transdifferentiation of fibroblasts to myofibroblasts and collagen deposition. MicroRNAs (miRNAs or miRs) have been demonstrated to be involved in myocardial I/R injury. However, the underlying molecular mechanism remains largely unclear. In the present study, mouse cardiomyocyte M6200 cells were treated with hypoxia/reoxygenation (H/R). Our data indicated that H/R treatment led to cell apoptosis, the increased expression of fibrosis-related proteins, namely collagen I, II, III, and fibronectin, as well as the downregulation of miR-142-3p in M6200 cells. Overexpression of miR-142-3p suppressed the H/R-induced apoptosis and fibrosis of M6200 cells. Bioinformatics analysis and a Dual-Luciferase reporter assay further identified high mobility group box 1 (HMGB1) as a direct target gene of miR-142-3p, and miR-142-3p negatively regulated the protein level of HMGB1 in M6200 cells. Furthermore, knockdown of HMGB1 enhanced cell proliferation whereas it inhibited the apoptosis and fibrosis of M6200 cells. In addition, TGF-β1/Smad3 signaling was suggested to be involved in the miR-142-3p/HMGB1-mediated apoptosis and fibrosis of M6200 cells treated with H/R. Taken together, the findings of the present study demonstrate that miR-142-3p inhibits H/R-induced apoptosis and fibrosis of cardiomyocytes, partly at least, by the direct inhibition of HMGB1 expression. Therefore, these findings have increased our understanding of the pathogenesis of H/R-induced myocardial injury.
Yi Wang, Min Ouyang, [...], and Zaijin Jian, MicroRNA-142-3p inhibits hypoxia/reoxygenation-induced apoptosis and fibrosis of cardiomyocytes by targeting high mobility group box 1, (2016).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5065300/#__ffn_sectitle
And again with pulmonary fibrosis. This Substack concerns influenza vaccines, but the pathology of systemic fibrosis has a certain familiarity to it:
Abstract
Introduction Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing interstitial lung disease of unknown aetiology and cure. Recent studies have reported a dysregulation of exosomal microRNAs (miRs) in the IPF context. However, the impact of IPF-related exosomal miRs on the progression of pulmonary fibrosis is unknown.
Methods
Two independent cohorts were enrolled at the ambulatory care polyclinic of Liège University. Exosomes from sputum were obtained from 19 patients with IPF and 23 healthy subjects (HSs) (cohort 1), and the ones from plasma derived from 14 patients with IPF and 14 HSs (cohort 2). Exosomal miR expression was performed by quantitative reverse transcription–PCR. The functional role of exosomal miRs was assessed in vitro by transfecting miR mimics in human alveolar epithelial cells and lung fibroblasts.
Results
Exosomal miR analysis showed that miR-142-3p was significantly upregulated in sputum and plasma of patients with IPF (8.06-fold, p<0.0001; 1.64 fold, p=0.008, respectively). Correlation analysis revealed a positive association between exosomal miR-142-3p and the percentage of macrophages from sputum of patients with IPF (r=0.576, p=0.012), suggesting macrophage origin of exosomal miR-142-3p upregulation. The overexpression of miR-142-3p in alveolar epithelial cells and lung fibroblasts was able to reduce the expression of transforming growth factor β receptor 1 (TGFβ-R1) and profibrotic genes. Furthermore, exosomes isolated from macrophages present antifibrotic properties due in part to the repression of TGFβ-R1 by miR-142-3p transfer in target cells.
Discussion
Our results suggest that macrophage-derived exosomes may fight against pulmonary fibrosis progression via the delivery of antifibrotic miR-142–3 p to alveolar epithelial cells and lung fibroblasts.
Guiot J, Cambier M, Boeckx A, et al, Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p, Thorax 2020;75:870-881.
https://thorax.bmj.com/content/75/10/870
Going further down this rabbit hole, interfering with microRNA’s can have unexpected consequences, including triggering autoimmunity:
Tregs play a fundamental role in immune tolerance via control of self-reactive effector T cells (Teffs). This function is dependent on maintenance of a high intracellular cAMP concentration. A number of microRNAs are implicated in the maintenance of Tregs. In this study, we demonstrate that peripheral immune tolerance is critically dependent on posttranscriptional repression of the cAMP-hydrolyzing enzyme phosphodiesterase-3b (Pde3b) by microRNA-142-5p (miR-142-5p). In this manner, miR-142-5p acts as an immunometabolic regulator of intracellular cAMP, controlling Treg suppressive function. Mir142 was associated with a super enhancer bound by the Treg lineage–determining transcription factor forkhead box P3 (FOXP3), and Treg-specific deletion of miR-142 in mice (TregΔ142) resulted in spontaneous, lethal, multisystem autoimmunity, despite preserved numbers of phenotypically normal Tregs. Pharmacological inhibition and genetic ablation of PDE3B prevented autoimmune disease and reversed the impaired suppressive function of Tregs in TregΔ142 animals. These findings reveal a critical molecular switch, specifying Treg function through the modulation of a highly conserved, cell-intrinsic metabolic pathway. Modulation of this pathway has direct relevance to the pathogenesis and treatment of autoimmunity and cancer.
Nelomi Anandagoda, Joanna C.D. Willis, Arnulf Hertweck, Luke B. Roberts, Ian Jackson, M. Refik Gökmen, Richard G. Jenner, Jane K. Howard, Graham M. Lord, J, microRNA-142–mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance, Clin Invest. 2019;129(3):1257-1271, (February 11, 2019) https://doi.org/10.1172/JCI124725.,
The research as discussed has been theoretical, conflicting or in vitro up to now, but did these signalling pathways actually lead to observed cases in the vaccinated? Indeed it did.
It should also be noted that again we see recurrence or exacerbation of a pre-existing condition post-vaccination, which makes case specific proof of cause and effect challenging apart from via larger population level studies.
Legal redress & compensation claims would be difficult, especially if there is any significant time lag before presenting symptoms.
Abstract
We report a case of acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) after pandemic influenza (H1N1) vaccination. A 57-year-old man, who had been diagnosed with IPF in September 2008, was admitted to our hospital in December 2009 because of aggravation of dyspnea and fever two days after H1N1 vaccination. Chest computed tomography showed diffuse bilateral ground-glass opacities superimposed on preceding reticular opacities. We diagnosed AE-IPF. Corticosteroid and cyclophosphamide were effective. Although the efficacy of influenza vaccination in patients with chronic lung diseases is well established, physicians should keep in mind that influenza vaccination has the potential to cause AE-IPF.
Yukihiro Umeda et al, Acute exacerbation of idiopathic pulmonary fibrosis after pandemic influenza A (H1N1) vaccination, Intern Med. 2010.
https://pubmed.ncbi.nlm.nih.gov/21048370/
Another case report from 2013:
Transbronchial lung biopsy (TBLB) specimens revealed interstitial inflammation with lymphocytes, mild interstitial fibrosis, and reactive hyperplastic type II pneumocytes. There were no findings of infection, granuloma or malignancy. These findings were consistent with a diagnosis of ILD.
Based on the clinical course, a possible cause was influenza vaccination. The temporal relationship between influenza vaccination and clinical symptoms argued strongly for a causative role of this agent. Several evaluations support the assessment that the ILD could not be explained by other causes, including infections, collagen vascular disease, granulomatous or pulmonary oedema. A drug lymphocyte stimulation test (DLST) on her peripheral lymphocytes gave a positive reaction to the influenza vaccine with a stimulation index of 296% (normal range <180%).
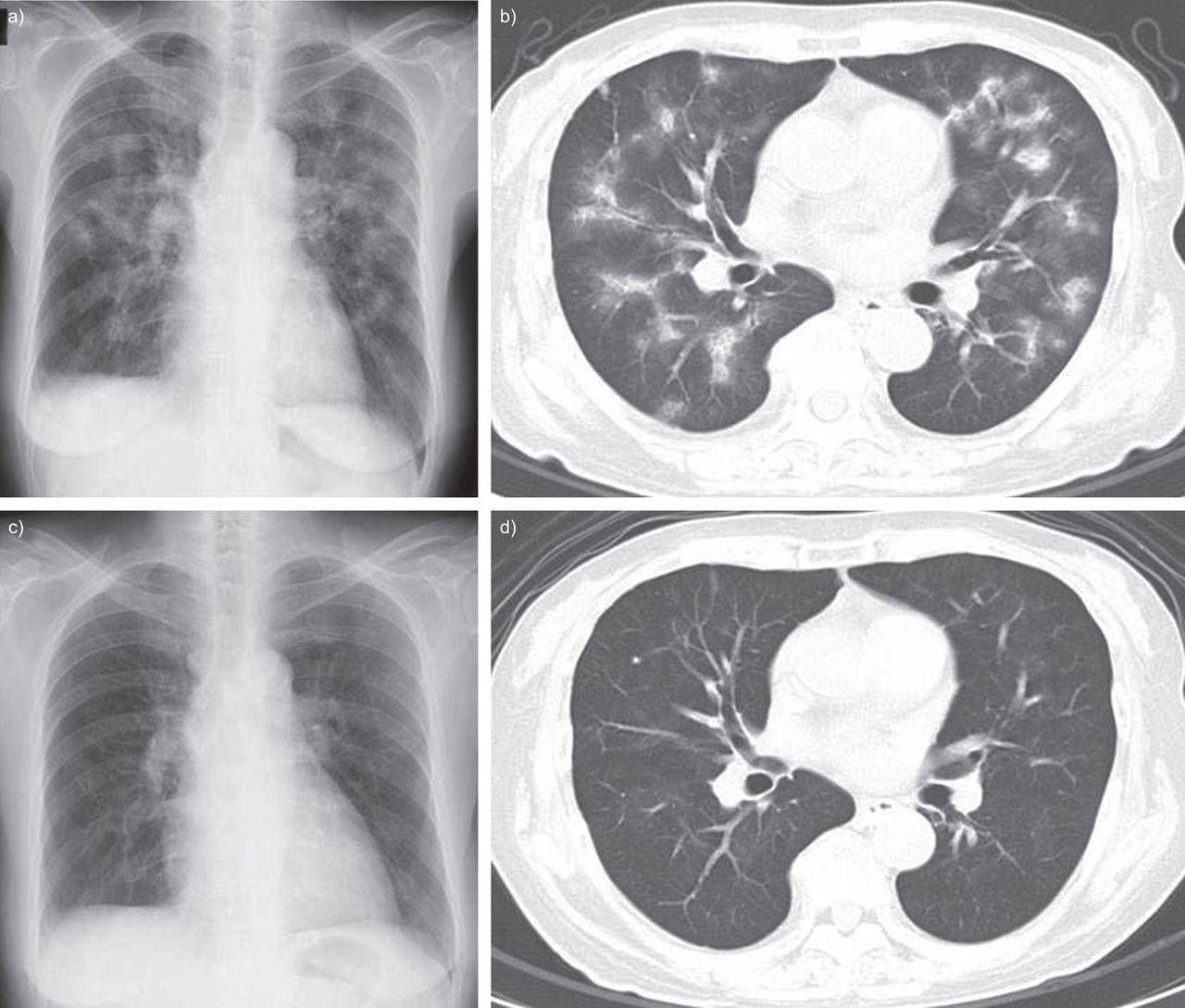
Satoshi Watanabe, Yuko Waseda, Hazuki Takato, Kanako Inuzuka, Nobuyuki Katayama, Kazuo Kasahara, Masaki Fujimura, Influenza vaccine-induced interstitial lung disease, European Respiratory Journal 2013 41: 474-477; DOI: 10.1183/09031936.00146912
https://erj.ersjournals.com/content/41/2/474
Although not fibrosis related (pre-existing), the following year there were several accounts of influenza vaccine mediated pneumonia:
Abstract
Although the influenza vaccine is relatively safe and effective, serious complications can develop in rare cases. We encountered two cases of interstitial pneumonia that developed after vaccination during the 2014-2015 influenza season. Overall, nine cases, including the two presented here, have been recorded in PubMed and the Cochrane library; eight patients were treated with corticosteroids, and all nine survived, suggesting a good prognosis. Interstitial pneumonia is rare; however, we found an increase in its incidence after 2009. Therefore, clinicians must be aware of the possibility of this complication and duly educate all patients in advance.
Keywords: bronchiolitis obliterans organizing pneumonia, influenza A virus, H1N1 subtype, interstitial pneumonia
Oral prednisolone at 35 mg (0.8 mg/kg/day) was administered daily, and within a week, our patient's symptoms and abnormal radiography and laboratory findings began resolving. We then performed pulmonary function tests and found a forced vital capacity (FVC) of 2.07 L (normal, 79.6% predicted), forced expiratory volume in 1 s (FEV1) of 1.68 L (normal, 88.0% predicted), and an FEV1/FVC ratio of 81.1%. Oral steroid therapy was tapered on an outpatient basis. Ten months after the treatment, her prednisolone dose was tapered. The patient remains healthy with no further symptoms or radiographic abnormalities (Fig. 1D), and she did not receive the annual influenza vaccine the following year.
Intravenous methylprednisolone was administered at 1,000 mg daily for 3 days, followed by oral prednisolone at 40 mg (0.6 mg/kg/day) daily. His symptoms and radiographic and laboratory abnormalities gradually resolved, and oral steroid therapy was tapered on an outpatient basis. Thirteen months after treatment, his steroid dose was tapered off. The patient remains healthy with no further symptoms or radiographic abnormalities (Fig. 2D), and he did not get the annual influenza vaccine the following year.
In the two cases described above, the patients were never smokers and had no history of allergy, recent visits abroad, or inhalation exposure. In addition, they reported no family history of pulmonary, allergic, or connective tissue diseases. Their serological tests were negative for several autoimmune markers such as antinuclear antibody, anti-double stranded DNA, anti-SS-A antibody, anti-SS-B antibody, anti-Scl-70 antibody, anti-centromere antibody, anti U1-ribonucleoprotein (RNP) antibody, anti-aminoacyl tRNA synthetase antibodies (including anti-Jo1 antibody), rheumatoid factor, anti-cyclic citrullinated peptide antibody, and anti-neutrophil cytoplasmic antibodies (proteinase-3 and myeloperoxidase). Paired serum tests were negative for current infection of Mycoplasma pneumoniae, Chlamydophila pneumoniae and psittaci, and Legionella pneumophila. Urine antigen tests were negative for Streptococcus pneumoniae and L. pneumophila. No pathogen was detected from the BALF by culture of general bacteria, acid-fast bacteria, and fungi; by loop-mediated isothermal amplification tests for M. pneumoniae and L. pneumophila; or by Luminex xTAGⓇ respiratory viral panel for Respiratory syncytial virus, Influenza virus A or B, Parainfluenza virus, Metapneumovirus, Adenovirus, Entero-Rhinovirus, Corona virus or Bocavirus.
In contrast, they met all of the following diagnostic criteria for drug-induced interstitial lung disease: correct identification of the drug; singularity of the drug; temporal eligibility; characteristic clinical, imaging, BALF, and pathological patterns of the reaction to the specific drug; and exclusion of other causes (5). Based on these findings, interstitial pneumonia associated with the influenza vaccine was diagnosed after excluding other causes of secondary interstitial pneumonia, such as infectious and connective tissue disease. The pathophysiology of drug-induced interstitial pneumonia, although not obvious, is believed to be cytotoxic or immune-mediated lung injury. The present two cases of interstitial pneumonia showed immune-mediated lung injury, as indicated by the lymphocytic and eosinophilic BALF and pathological findings of organizing pneumonia with alveolitis, positive DLST results for the influenza vaccine, and a good response to glucocorticoid therapy.
Conclusions
Our findings suggest that clinicians should be aware of the possibility of interstitial pneumonia as a complication of the influenza vaccine, ask closed questions about vaccination in medical interviews, and educate patients about this complication, as these will facilitate early detection and treatment. Although the safety of this vaccine has been confirmed, relatively newer drugs warrant further investigation to confirm their association with interstitial pneumonia.
Hibino, M., & Kondo, T. (2017). Interstitial Pneumonia Associated with the Influenza Vaccine: A Report of Two Cases. Internal medicine (Tokyo, Japan), 56(2), 197–201. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337467/
Related to the above, a 2018 peer reviewed paper on influenza vaccine mediated carcinogenesis. And we know that COVID-19 infection or mRNA transfection also activates the bradykinin receptor B2-associated signalling pathway via spike protein binding to ACE2 and angiotensin II upregulation.
Abstract
Seasonal flu vaccine is recommended as the best protection for cancer patients against influenza infection. Recent in silico and experimental data suggest that antibodies elicited with influenza vaccine could activate bradykinin receptor B2-associated signaling pathway, which is also involved in cell proliferation and migration of tumor cells. These results point to an urgent need for the reexamination of safety of influenza vaccine(s) in cancer patients.
Recently, it was suggested that the vaccine against influenza A viruses could elicit agonistic antibodies for bradykinin receptor B2 (BKB2R), which activates a BRB2R-associated signaling pathway that may contribute to the protection against cardiovascular diseases4. Moreover, it has been established that antibody activation of BKBR2 is possible, with all biological activities associated with it5. Recently, a monoclonal antibody with agonistic BKBR2 activity as well as anti-influenza A activity has been patented for multiple purposes6. Taken together, these data support the hypothesis of “molecular mimicry” between BKBR2 and hemagglutinin (HA) of influenza A viruses that may allow for generation of cross reactive antibodies.
In addition, it was found that levels of kinins in biological fluids of cancer patients are increased and that activation of kinin receptors expressed on cancer cells produces an increase in cell proliferation and migration of tumor cells [reviewed in Ref. 7]. Additionally, it has been demonstrated that tumor growth is increased by stimulation of kinin receptors expressed on other cells within the tumor microenvironment7, and that bradykinin and its receptors are involved in pathogenesis of numerous common cancers (gastric8, hepatocellular9, brain10, bladder11, renal12, prostate13 and breast14). These data point out that, because of possible activation of BKB2R with antibodies elicited by influenza vaccine, safety of this vaccine in cancer patients is an important issue.
Not many influenza safety studies of cancer patients were conducted and they were short term in nature, not long enough to detect cancer relapses and quickly signed off as “effective and safe”:
Screening of the clinical trials database (clinicaltrial.gov) for trials that investigated safety of the influenza vaccine in cancer patients with solid tumors (literature data connect pathogenesis of this type of tumors with BKB2R-pathway) revealed only five completed studies (Table 1). Patients were monitored in the period between 21 days and 6 months following vaccination and results were released only for one study (NCT01666782 in Table 1) for the monitoring time frame of 21 days. These short-term studies suggest that influenza vaccination is effective and safe in cancer patients in general1,15–17. However, long-term studies might be needed to test the hypothesis that if antibodies elicited by the seasonal flu vaccine may contribute to activation of BKB2R in cancer patients with potentially far-reaching consequences.
It concludes that vaccines should be screened for cross reactive antibodies to bradykinin receptor B2 to help prevent tumor pathogenesis in cancer patients.
Why just for this subset of the population, as we are all potentially at risk, and why not for COVID-19 gene therapy transfection agents too?
In conclusion, previously published results suggest that influenza vaccines could produce antibodies with BKB2R-agonistic activity. On the other hand, experimental and clinical data showed that activation of the bradykinin pathways plays an important role in pathogenesis of several common solid tumors. All these data suggest that until the role of the influenza vaccine in activation of BKB2R is clarified, vaccination of cancer patients against flu should be taken with some caution, and vaccines need to be monitored beyond the flu season. This especially concerns children with cancer, who represent the most vulnerable population of oncology patients. In addition, previous in silico analysis of informational properties of BKB2R and HAs from different influenza A viruses suggested that flu vaccines are not equally efficient in production of agonistic antibodies for BKB2R4. This opens the possibility for selection of antigens with low crossreactivity with BKB2R and design influenza vaccines incapable of inducing production of cross-reactive antibodies for safer use in cancer patients.
Slobodan Paessler1,2, Veljko Veljkovic, Should safety of the flu vaccine for cancer patients be reexamined? [version 1; peer review: 2 approved with reservations], PUBLISHED 02 Jan 2018,
https://orcid.org/0000-0002-1980-09273
https://f1000research.com/articles/7-1
The same pathway is activated via spike protein binding & COVID-19:
The leaky blood vessels and lung fluid build-up in some COVID-19 patients might be explained by the virus’s corruption of an inflammation safeguard.
Interplay between the kinin pathway, which mediates acute inflammation; the renin-angiotensin system (RAS), which regulates blood pressure and fluid balance; and macrophages, immune cells that are activated in infection, leads to leaky blood vessels and inflammation in some cases of SARS-CoV-2 infection.
Researchers propose that the kinin cascade—in which bradykinin and des-Arg9-bradykinin (DABK) are major proteins—goes into overdrive to cause these effects during COVID-19. Ordinarily, the RAS—in which angiotensin-converting enzyme (ACE) and ACE2 are key enzymes—keeps the kinin cascade under control. ACE breaks down the protein bradykinin, preventing it from binding to its receptor B2R, while ACE2 degrades DABK and stops it from binding to B1R. When SARS-CoV-2 hooks up with ACE2 as a means of entering cells, some of the brakes are removed, according to the model, thereby permitting DABK to bind to its receptor and trigger blood vessel leakage and inflammation.
Alakananda Dasgupta, The leaky blood vessels and lung fluid build-up in some COVID-19 patients might be explained by the virus’s corruption of an inflammation safeguard, (Aug 26, 2020)
Updated 5th June, ‘22
Hat tip to John Paul. Within the context of amyloidosis he says:
https://twitter.com/ThingsHiddenn/status/1533090247009087488?s=19
This is the paper:
ABSTRACT
Given the current coronavirus disease 2019 (COVID-19) pandemic, coinfection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza A virus (IAV) is a major concern for public health. However, the immunopathogenic events occurring with coinfections of SARS-CoV-2 and IAV remain unclear. Here, we report the pathogenic and immunological consequences of SARS-CoV-2 and IAV H1N1 coinfection in the K18-hACE2 transgenic mouse model. Compared with a single infection with SARS-CoV-2 or IAV, coinfections not only prolonged the primary virus infection period but also increased immune cell infiltration and inflammatory cytokine levels in bronchoalveolar lavage fluid leading to severe pneumonia and lung damage. Moreover, coinfections caused severe lymphopenia in peripheral blood, resulting in reduced total IgG, neutralizing antibody titers, and CD4+ T cell responses against each virus. This study sheds light on the immunopathogenesis of SARS-CoV-2 and IAV coinfection, which may guide the development of effective therapeutic strategies for the treatment of patients coinfected with these viruses.
IMPORTANCE The cocirculation of influenza virus merging with the COVID-19 pandemic raises a potentially severe threat to public health. Recently, increasing numbers of SARS-CoV-2 and influenza virus coinfection have been reported from many countries. It is a worrisome issue that SARS-CoV-2 coinfection with other pathogens may worsen the clinical outcome and severity of COVID-19 and increase fatality. Here, we evaluated SARS-CoV-2 and IAV coinfection using the K18-hACE2 mouse model. Coinfected mice exhibited increased mortality with prolonged IAV shedding. Furthermore, coinfected mice showed a higher level of cytokines and chemokines than a single infection condition. Interestingly, our data show that coinfected mice showed significantly fewer virus-specific and neutralizing antibodies than the mice with a single infection. Overall, this study suggests that coinfection aggravates viral pathology by impaired neutralizing antibody response.
Conclusion
Currently administered influenza vaccines should also be investigated for microRNA changes and for reactivation of latent viruses, many of which may be oncogenic in nature.
There is no evidence that microRNA-142-3p itself is suppressed by COVID-19 transfection, but many other microRNA’s are disrupted.
There is a growing body of evidence of fibrosis in multiple organs, amyloidosis, Treg disruption, cardiovascular, neurological, immunosuppression & autoimmune disorders and tumorigenesis in those in remission and the previously healthy.
The current large scale administration of Covid-19 vaccines should be suspended immediately pending further safety investigations.
Thank you for reading.












I was just listening to a summit on peptides and a Dr Isaac Eliaz discusses galectin-3 (pro- inflammatory) elevation in fibrosis n cancer. Treatment modified citrus pectin. Going to test my levels and finally read his book
Thorough. Wow. They knew, and they know what they are doing with the fauXi virus and jabs. What Bastards