Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line
25th February 2022
Updated: 16th May ‘22
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
This is huge, breaking news and the potential public health consequences are horrifying.
“Our results showed that BNT162b2 mRNA readily enters Huh7 cells at a concentration (0.5 µg/mL) corresponding to 0.5% of the local injection site concentration, induce changes in LINE-1 gene and protein expression, and within 6 h, reverse transcription of BNT162b2 can be detected.”
It probably goes some way to explain Long Covid & post vax sequalae and why the germinal centres in lymph nodes were still expressing spike protein over 60 days post transfection, long after the mRNA should have been degraded (the intracellular half life can be measured in hours, though they did use pseudouridine substitutions to extend this, just not for months!).
If later studies confirm it can enter the genome and be replicated during cell division, especially via the ovaries or testes, then these may be passed on to the next generation. Or infertility may be a consequence.
Needless to say all transfections must be stopped immediately.
SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses
(2022)
Abstract
SARS-CoV-2 mRNA-based vaccines are about 95% effective in preventing COVID-191,2,3,4,5. The dynamics of antibody-secreting plasmablasts and germinal centre B cells induced by these vaccines in humans remain unclear. Here we examined antigen-specific B cell responses in peripheral blood (n = 41) and draining lymph nodes in 14 individuals who had received 2 doses of BNT162b2, an mRNA-based vaccine that encodes the full-length SARS-CoV-2 spike (S) gene1. Circulating IgG- and IgA-secreting plasmablasts that target the S protein peaked one week after the second immunization and then declined, becoming undetectable three weeks later. These plasmablast responses preceded maximal levels of serum anti-S binding and neutralizing antibodies to an early circulating SARS-CoV-2 strain as well as emerging variants, especially in individuals who had previously been infected with SARS-CoV-2 (who produced the most robust serological responses). By examining fine needle aspirates of draining axillary lymph nodes, we identified germinal centre B cells that bound S protein in all participants who were sampled after primary immunization. High frequencies of S-binding germinal centre B cells and plasmablasts were sustained in these draining lymph nodes for at least 12 weeks after the booster immunization. S-binding monoclonal antibodies derived from germinal centre B cells predominantly targeted the receptor-binding domain of the S protein, and fewer clones bound to the N-terminal domain or to epitopes shared with the S proteins of the human betacoronaviruses OC43 and HKU1. These latter cross-reactive B cell clones had higher levels of somatic hypermutation as compared to those that recognized only the SARS-CoV-2 S protein, which suggests a memory B cell origin. Our studies demonstrate that SARS-CoV-2 mRNA-based vaccination of humans induces a persistent germinal centre B cell response, which enables the generation of robust humoral immunity.
https://www.nature.com/articles/s41586-021-03738-2
Decay Rates of Human mRNAs: Correlation With Functional Characteristics and Sequence Attributes (2003)
“In our series of experiments, we determined thousands of decay rates for transcripts in human cells. Our estimated median mRNA half-life in human cells is 10 h, a number that scales linearly relative to division time when compared with bacteria and yeast”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC403777/
Hat tip to Walter Chestnut.
It's also a great way to introduce random mutations to your DNA. You think spike protein sequences are toxic, try adding in random changes, make it persistent and see what happens…
HIV and Acquired Immunodeficiency Syndrome
Tak W. Mak, Mary E. Saunders, in The Immune Response, 2006
i) Reverse Transcriptase
“Reverse Transcriptase (RT) is essential for HIV replication because the viral RNA genome on its own is highly susceptible to degradation by intracellular RNases. RT rapidly makes a much more nuclease-resistant double-stranded DNA copy of the RNA template that later integrates to form the proviral DNA.”
“HIV RT is responsible for much of the antigenic variation of HIV that confounds both the natural immune response and vaccine development. HIV RT lacks the proof-reading capabilities inherent in cellular polymerases, meaning that its duplication of the HIV genome is highly error-prone. Mutations due to uncorrected RT activity appear in the HIV genome at a rate of about 1 in every 1500–4000 nucleotides per replication cycle. As a point of comparison, the average rate of mutation of the human cellular genome is 1 in 107–108 base pairs.”
https://www.sciencedirect.com/science/article/pii/B9780120884513500272
The paper:
Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line
By Markus Aldén, Francisko Olofsson Falla, Daowei Yang, Mohammad Barghouth, Cheng Luan, Magnus Rasmussen and Yang De Marinis
Abstract
Preclinical studies of COVID-19 mRNA vaccine BNT162b2, developed by Pfizer and BioNTech, showed reversible hepatic effects in animals that received the BNT162b2 injection. Furthermore, a recent study showed that SARS-CoV-2 RNA can be reverse-transcribed and integrated into the genome of human cells. In this study, we investigated the effect of BNT162b2 on the human liver cell line Huh7 in vitro. Huh7 cells were exposed to BNT162b2, and quantitative PCR was performed on RNA extracted from the cells. We detected high levels of BNT162b2 in Huh7 cells and changes in gene expression of long interspersed nuclear element-1 (LINE-1), which is an endogenous reverse transcriptase. Immunohistochemistry using antibody binding to LINE-1 open reading frame-1 RNA-binding protein (ORFp1) on Huh7 cells treated with BNT162b2 indicated increased nucleus distribution of LINE-1. PCR on genomic DNA of Huh7 cells exposed to BNT162b2 amplified the DNA sequence unique to BNT162b2. Our results indicate a fast up-take of BNT162b2 into human liver cell line Huh7, leading to changes in LINE-1 expression and distribution. We also show that BNT162b2 mRNA is reverse transcribed intracellularly into DNA in as fast as 6 h upon BNT162b2 exposure. View Full-Text
Keywords: COVID-19 mRNA vaccine; BNT162b2; liver; reverse transcription; LINE-1; Huh7
3.2. Effect of BNT162b2 on Human Endogenous Reverse Transcriptase Long Interspersed Nuclear Element-1 (LINE-1)
Here we examined the effect of BNT162b2 on LINE-1 gene expression. RT-qPCR was performed on RNA purified from Huh7 cells treated with BNT162b2 (0, 0.5, 1.0, and 2.0 µg/mL) for 6, 24, and 48 h, using primers targeting LINE-1. Significantly increased LINE-1 expression compared to control was observed at 6 h by 2.0 µg/mL BNT162b2, while lower BNT162b2 concentrations decreased LINE-1 expression at all time points (Figure 3).
Next, we studied the effect of BNT162b2 on LINE-1 protein level. The full-length LINE-1 consists of a 5′ untranslated region (UTR), two open reading frames (ORFs), ORF1 and ORF2, and a 3′UTR, of which ORF1 is an RNA binding protein with chaperone activity. The retrotransposition activity of LINE-1 has been demonstrated to involve ORF1 translocation to the nucleus [35]. Huh7 cells treated with or without BNT162b2 (0.5, 1.0 and 2.0 µg/mL) for 6 h were fixed and stained with antibodies binding to LINE-1 ORF1p, and DNA-specific probe Hoechst for visualization of cell nucleus (Figure 4a). Quantification of immunofluorescence staining intensity showed that BNT162b2 increased LINE-1 ORF1p protein levels in both the whole cell area and nucleus at all concentrations tested (Figure 4b–d).
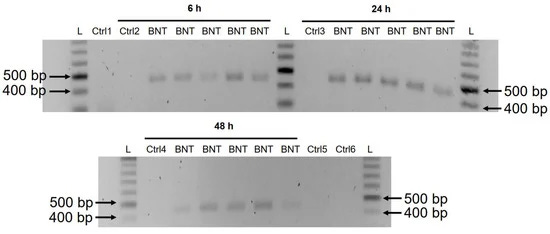
3.3. Detection of Reverse Transcribed BNT162b2 DNA in Huh7 Cells
A previous study has shown that entry of LINE-1 protein into the nucleus is associated with retrotransposition [35]. In the immunofluorescence staining experiment described above, increased levels of LINE-1 in the nucleus were observed already at the lowest concentration of BNT162b2 (0.5 µg/mL). To examine if BNT162b2 is reversely transcribed into DNA when LINE-1 is elevated, we purified genomic DNA from Huh7 cells treated with 0.5 µg/mL of BNT162b2 for 6, 24, and 48 h. Purified DNA was treated with RNase to remove RNA and subjected to PCR using primers targeting BNT162b2, as illustrated in Figure 1. Amplified DNA fragments were then visualized by electrophoresis and gel-purified (Figure 5). BNT162b2 DNA amplicons were detected in all three time points (6, 24, and 48 h). Sanger sequencing confirmed that the DNA amplicons were identical to the BNT162b2 sequence flanked by the primers (Table 2). To ensure that the DNA amplicons were derived from DNA but not BNT162b2 RNA, we also performed PCR on RNA purified from Huh7 cells treated with 0.5 µg/mL BNT162b2 for 6 h, with or without RNase treatment (Ctrl 5 and 6 in Figure 5), and no amplicon was detected in the RNA samples subjected to PCR.
4. Discussion
In this study we present evidence that COVID-19 mRNA vaccine BNT162b2 is able to enter the human liver cell line Huh7 in vitro. BNT162b2 mRNA is reverse transcribed intracellularly into DNA as fast as 6 h after BNT162b2 exposure. A possible mechanism for reverse transcription is through endogenous reverse transcriptase LINE-1, and the nucleus protein distribution of LINE-1 is elevated by BNT162b2.
Intracellular accumulation of LNP in hepatocytes has been demonstrated in vivo [36]. A preclinical study on BNT162b2 showed that BNT162b2 enters the human cell line HEK293T cells and leads to robust expression of BNT162b2 antigen [37]. Therefore, in this study, we first investigated the entry of BNT162b2 in the human liver cell line Huh7 cells. The choice of BNT162b2 concentrations used in this study warrants explanation. BNT162b2 is administered as a series of two doses three weeks apart, and each dose contains 30 µg of BNT162b2 in a volume of 0.3 mL, which makes the local concentration at the injection site at the highest 100 µg/mL [31]. A previous study on mRNA vaccines against H10N8 and H7N9 influenza viruses using a similar LNP delivery system showed that the mRNA vaccine can distribute rather nonspecifically to several organs such as liver, spleen, heart, kidney, lung, and brain, and the concentration in the liver is roughly 100 times lower than that of the intra-muscular injection site [38]. In the assessment report on BNT162b2 provided to EMA by Pfizer, the pharmacokinetic distribution studies in rats demonstrated that a relatively large proportion (up to 18%) of the total dose distributes to the liver [26]. We therefore chose to use 0.5, 1, and 2 μg/mL of vaccine in our experiments on the liver cells. However, the effect of a broader range of lower and higher concentrations of BNT162b2 should also be verified in future studies.
In the current study, we employed a human liver cell line for in vitro investigation. It is worth investigating if the liver cells also present the vaccine-derived SARS-CoV-2 spike protein, which could potentially make the liver cells targets for previously primed spike protein reactive cytotoxic T cells. There has been case reports on individuals who developed autoimmune hepatitis [39] after BNT162b2 vaccination. To obtain better understanding of the potential effects of BNT162b2 on liver function, in vivo models are desired for future studies.
In the BNT162b2 toxicity report, no genotoxicity nor carcinogenicity studies have been provided [26]. Our study shows that BNT162b2 can be reverse transcribed to DNA in liver cell line Huh7, and this may give rise to the concern if BNT162b2-derived DNA may be integrated into the host genome and affect the integrity of genomic DNA, which may potentially mediate genotoxic side effects. At this stage, we do not know if DNA reverse transcribed from BNT162b2 is integrated into the cell genome. Further studies are needed to demonstrate the effect of BNT162b2 on genomic integrity, including whole genome sequencing of cells exposed to BNT162b2, as well as tissues from human subjects who received BNT162b2 vaccination.
Human autonomous retrotransposon LINE-1 is a cellular endogenous reverse transcriptase and the only remaining active transposon in humans, able to retrotranspose itself and other nonautonomous elements [40,41], and ~17% of the human genome are comprised of LINE-1 sequences [42]. The nonautonomous Alu elements, short, interspersed nucleotide elements (SINEs), variable-number-of-tandem-repeats (VNTR), as well as cellular mRNA-processed pseudogenes, are retrotransposed by the LINE-1 retrotransposition proteins working in trans [43,44]. A recent study showed that endogenous LINE-1 mediates reverse transcription and integration of SARS-CoV-2 sequences in the genomes of infected human cells [25]. Furthermore, expression of endogenous LINE-1 is often increased upon viral infection, including SARS-CoV-2 infection [45,46,47]. Previous studies showed that LINE-1 retrotransposition activity is regulated by RNA metabolism [48,49], DNA damage response [50], and autophagy [51]. Efficient retrotransposition of LINE-1 is often associated with cell cycle and nuclear envelope breakdown during mitosis [52,53], as well as exogenous retroviruses [54,55], which promotes entrance of LINE-1 into the nucleus. In our study, we observed increased LINE-1 ORF1p distribution as determined by immunohistochemistry in the nucleus by BNT162b2 at all concentrations tested (0.5, 1, and 2 μg/mL), while elevated LINE-1 gene expression was detected at the highest BNT162b2 concentration (2 μg/mL). It is worth noting that gene transcription is regulated by chromatin modifications, transcription factor regulation, and the rate of RNA degradation, while translational regulation of protein involves ribosome recruitment on the initiation codon, modulation of peptide elongation, termination of protein synthesis, or ribosome biogenesis. These two processes are controlled by different mechanisms, and therefore they may not always show the same change patterns in response to external challenges. The exact regulation of LINE-1 activity in response to BNT162b2 merits further study.
The cell model that we used in this study is a carcinoma cell line, with active DNA replication which differs from non-dividing somatic cells. It has also been shown that Huh7 cells display significant different gene and protein expression including upregulated proteins involved in RNA metabolism [56]. However, cell proliferation is also active in several human tissues such as the bone marrow or basal layers of epithelia as well as during embryogenesis, and it is therefore necessary to examine the effect of BNT162b2 on genomic integrity under such conditions. Furthermore, effective retrotransposition of LINE-1 has also been reported in non-dividing and terminally differentiated cells, such as human neurons [57,58].
The Pfizer EMA assessment report also showed that BNT162b2 distributes in the spleen (<1.1%), adrenal glands (<0.1%), as well as low and measurable radioactivity in the ovaries and testes (<0.1%) [26]. Furthermore, no data on placental transfer of BNT162b2 is available from Pfizer EMA assessment report. Our results showed that BNT162b2 mRNA readily enters Huh7 cells at a concentration (0.5 µg/mL) corresponding to 0.5% of the local injection site concentration, induce changes in LINE-1 gene and protein expression, and within 6 h, reverse transcription of BNT162b2 can be detected. It is therefore important to investigate further the effect of BNT162b2 on other cell types and tissues both in vitro and in vivo.
5. Conclusions
Our study is the first in vitro study on the effect of COVID-19 mRNA vaccine BNT162b2 on human liver cell line. We present evidence on fast entry of BNT162b2 into the cells and subsequent intracellular reverse transcription of BNT162b2 mRNA into DNA.
Full paper:
https://www.mdpi.com/1467-3045/44/3/73/htm
Added 16th May ‘22.
Guess how long LNP transfected & reverse transcripted cells could live for, expressing spike protein?
I'm not saying all of these cell types are susceptible, won't undergo apoptosis or get targeted by the immune system, but it could be for years:
What cells in the human body live the longest?
https://www.sciencefocus.com/the-human-body/what-cells-in-the-human-body-live-the-longest/