Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background
Over to the Reuters fact check post:
Fact Check-SM-102 lipid in Moderna COVID-19 vaccine is not harmful
“SM-102 is listed as one of the lipids in the Moderna COVID-19 vaccine as seen in the U.S. Food and Drug Administration (FDA) fact sheet here . Moderna describes SM-102 here as a “proprietary ionizable lipid” meaning it was produced for use in vaccines by Moderna. This lipid is not in the other COVID-19 vaccines granted an Emergency Use Authorization (EUA) by the FDA, as seen in the Pfizer and Janssen fact sheets here and here .”
“The FDA reviewed safety data from 30,351 participants aged 18 and over in the Moderna vaccine trials and did not identify any specific safety concerns that would prevent them from issuing the EUA (here).”
Verdict
“Partly false. SM-102 is a lipid used in the Moderna COVID-19 vaccine but the Cayman Chemical safety fact sheet in the posts shows the hazards of chloroform not SM-102.
This article was produced by the Reuters Fact Check team.”
https://www.reuters.com/article/factcheck-sm102-moderna-idUSL2N2NE20S
So it doesn't contain toxic or carcinogenic chloroform and it's ok with the FDA, no signals in the early trials so all is well, the evil Anti-Vaxxers got this wrong, right?
Well not so fast, if we look at MedKoo's MSDS for the pure chloroform free compound it says that it cannot be classified based on available data, they just don't know it's safe or not:
“To the best of our knowledge, the chemical, physical and toxicological properties have not been fully investigated”
Medkoo.com/uploads/product/SM-102/safety/SDS-SM102.pdf
Well now thanks to new in vitro research we know it may well not be safe at all, far from it in fact.
The problem? Blocking of potassium ion gradients.
The Khan academy do a great job explaining this for nubies like myself:
Active transport
Electrochemical gradients and the membrane potential. Primary and secondary active transport. Na+/K+ pump.
Introduction
Passive transport is a great strategy for moving molecules into or out of a cell. It's cheap, it's easy, and all the cell has to do is sit there and let the molecules diffuse in. But...it also doesn't work in every situation. For instance, suppose the sugar glucose is more concentrated inside of a cell than outside. If the cell needs more sugar in to meet its metabolic needs, how can it get that sugar in?
Here, the cell can't import glucose for free using diffusion, because the natural tendency of the glucose will be to diffuse out rather than flowing in. Instead, the cell must bring in more glucose molecules via active transport. In active transport, unlike passive transport, the cell expends energy (for example, in the form of ATP) to move a substance against its concentration gradient.
Here, we’ll look in more detail at gradients of molecules that exist across cell membranes, how they can help or hinder transport, and how active transport mechanisms allow molecules to move against their gradients.
Electrochemical gradients
We have already discussed simple concentration gradients, in which a substance is found in different concentrations over a region of space or on opposite sides of a membrane. However, because atoms and molecules can form ions and carry positive or negative electrical charges, there may also be an electrical gradient, or difference in charge, across a plasma membrane. In fact, living cells typically have what’s called a membrane potential, an electrical potential difference (voltage) across their cell membrane.
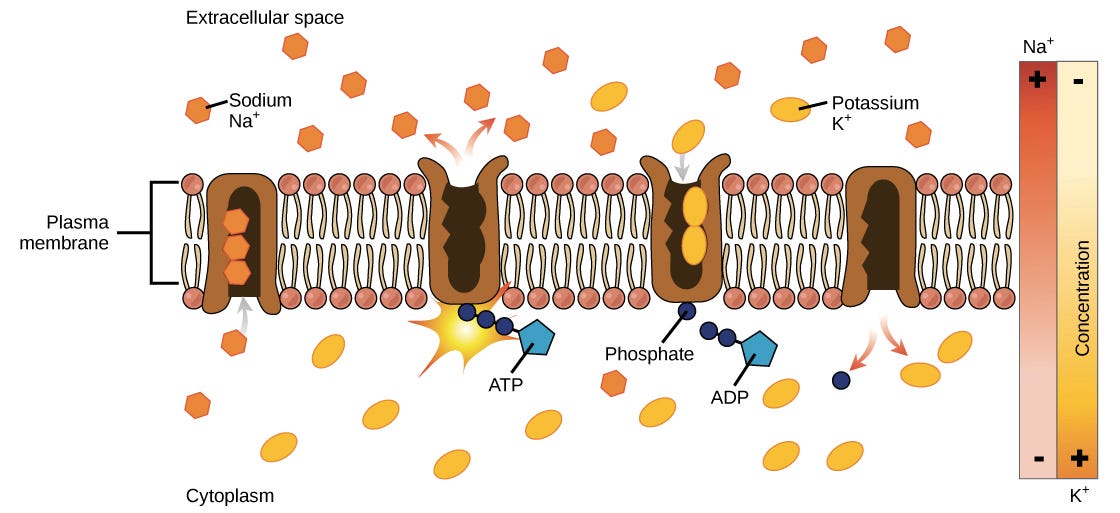
An electrical potential difference exists whenever there is a net separation of charges in space. In the case of a cell, positive and negative charges are separated by the barrier of the cell membrane, with the inside of the cell having extra negative charges relative to the outside. The membrane potential of a typical cell is -40 to -80 millivolts, with the minus sign meaning that inside of the cell is more negative than the outside. The cell actively maintains this membrane potential, and we’ll see how it forms in the section on the sodium-potassium pump (below).As an example of how the membrane potential can affect ion movement, let’s look at sodium and potassium ions. In general, the inside of a cell has a higher concentration of potassium (K^+ +) and a lower concentration of sodium (Na^+ +) than the extracellular fluid around it.If sodium ions are outside of a cell, they will tend to move into the cell based on both their concentration gradient (the lower concentration of Na^+ + in the cell) and the voltage across the membrane (the more negative charge on the inside of the membrane).Because K^+ + is positive, the voltage across the membrane will encourage its movement into the cell, but its concentration gradient will tend to drive it out of the cell (towards the region of lower concentration). The final concentrations of potassium on the two sides of the membrane will be a balance between these opposing forces.The combination of concentration gradient and voltage that affects an ion’s movement is called the electrochemical gradient.
The sodium-potassium pump cycle:
The sodium-potassium pump transports sodium out of and potassium into the cell in a repeating cycle of conformational (shape) changes. In each cycle, three sodium ions exit the cell, while two potassium ions enter. This process takes place in the following steps:To begin, the pump is open to the inside of the cell. In this form, the pump really likes to bind (has a high affinity for) sodium ions, and will take up three of them.When the sodium ions bind, they trigger the pump to hydrolyze (break down) ATP. One phosphate group from ATP is attached to the pump, which is then said to be phosphorylated. ADP is released as a by-product.Phosphorylation makes the pump change shape, re-orienting itself so it opens towards the extracellular space. In this conformation, the pump no longer likes to bind to sodium ions (has a low affinity for them), so the three sodium ions are released outside the cell.In its outward-facing form, the pump switches allegiances and now really likes to bind to (has a high affinity for) potassium ions. It will bind two of them, and this triggers removal of the phosphate group attached to the pump in step 2.With the phosphate group gone, the pump will change back to its original form, opening towards the interior of the cell.In its inward-facing shape, the pump loses its interest in (has a low affinity for) potassium ions, so the two potassium ions will be released into the cytoplasm. The pump is now back to where it was in step 1, and the cycle can begin again.
More:
So why is this a problem?
Potassium gating disruption can cause apotosis of neurons, disrupt T cell proliferation, cause cardiac cell misfiring and tumorogenesis: pathology and potentially sudden death. So that's ok then.
Voltage-Gated Potassium Channels as Regulators of Cell Death (2020)
“Figure 2. Kv1.3 regulates T lymphocyte proliferation: the «membrane potential model ». Upon activation of T cell receptors (TCR) by antigen-presenting cells, phospholipase C (PLC) cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 activates the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), which releases Ca2+ into the cytosol. Ca2+ depletion from the ER lumen leads to conformational changes of the ER-resident protein STIM1, which couples the ER to the plasma membrane and activates Orai1, a calcium release-activated Ca2+ channel (CRAC). The following increased Ca2+ concentration in the cytosol activates the phosphatase Calcineurin, which dephosphorylates the Nuclear Factor of Activated T cells (NFAT). This transcription factor translocates to the nucleus and activates the transcription of interleukin-2 (IL-2), thus inducing T cell proliferation. “
https://www.frontiersin.org/articles/10.3389/fcell.2020.611853/full
It was discussed previously as a contributor to brain fog & dementia:
“Pathophysiology of spike protein expressed GP120 mediated "brain fog" & dementia”
https://doorlesscarp953.substack.com/p/pathophysiology-of-spike-protein
* * *
Effective Perturbations on the Amplitude and Hysteresis of Erg-Mediated Potassium Current Caused by 1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (SM-102), a Cationic Lipid (Oct, 2021)
Abstract
“SM-102 (1-octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino]-octanoate) is an amino cationic lipid that has been tailored for the formation of lipid nanoparticles and it is one of the essential ingredients present in the ModernaTM COVID-19 vaccine. However, to what extent it may modify varying types of plasmalemmal ionic currents remains largely uncertain. In this study, we investigate the effects of SM-102 on ionic currents either in two types of endocrine cells (e.g., rat pituitary tumor (GH3) cells and mouse Leydig tumor (MA-10) cells) or in microglial (BV2) cells. Hyperpolarization-activated K+ currents in these cells bathed in high-K+, Ca2+-free extracellular solution were examined to assess the effects of SM-102 on the amplitude and hysteresis of the erg-mediated K+ current (IK(erg)). The SM-102 addition was effective at blocking IK(erg) in a concentration-dependent fashion with a half-maximal concentration (IC50) of 108 μM, a value which is similar to the KD value (i.e., 134 μM) required for its accentuation of deactivation time constant of the current. The hysteretic strength of IK(erg) in response to the long-lasting isosceles-triangular ramp pulse was effectively decreased in the presence of SM-102. Cell exposure to TurboFectinTM 8.0 (0.1%, v/v), a transfection reagent, was able to inhibit hyperpolarization-activated IK(erg) effectively with an increase in the deactivation time course of the current. Additionally, in GH3 cells dialyzed with spermine (30 μM), the IK(erg) amplitude progressively decreased; moreover, a further bath application of SM-102 (100 μM) or TurboFectin (0.1%) diminished the current magnitude further. In MA-10 Leydig cells, the IK(erg) was also blocked by the presence of SM-102 or TurboFectin. The IC50 value for SM-102-induced inhibition of IK(erg) in MA-10 cells was 98 μM. In BV2 microglial cells, the amplitude of the inwardly rectifying K+ current was inhibited by SM-102. Taken together, the presence of SM-102 concentration-dependently inhibited IK(erg) in endocrine cells (e.g., GH3 or MA-10 cells), and such action may contribute to their functional activities, assuming that similar in vivo findings exist.”
“These polycationic molecules (e.g., spermine and spermidine) enter the K ir or K erg channel pore from the intracellular side and block K+ion movement through the channel at depolarized potentials, thereby ensuring the longer plateau phase of the cardiac action potential [39]. However, to what extent SM-102-mediated perturbations on membrane ionic currents confer their effectiveness in the adverse effects of mRNA-based vaccines (e.g., Moderna TM) has yet to be further delineated. Whether the working concentrations of SM-102 or TurboFectin TM used for their direct adjustments on ionic currents could be achieved in vitro or in vivo also remains to be determined.”
“The endocrine system is a complex network of glands and organs. It uses hormones to control and coordinate your body's metabolism, energy level, reproduction, growth and development, and response to injury, stress, and mood. The following are integral parts of the endocrine system: Hypothalamus.”
“GH3 cells produce growth hormone faster than the GH1 cell line and also produce prolactin. Hydrocortisone stimulates growth hormone production and inhibits prolactin production.”
“MA-10 cells are a clonal strain of mouse Leydig tumor cells that retain many of the properties of Leydig cells including expression of the endogenous lutropin/choriogonadotropin receptor (LHR) and the ability to respond to LH/CG with increased steroidogenesis.”
Big hat tip to MW for flagging this up:
https://twitter.com/Spyder550a/status/1489522965519556614?s=19
And as ever we come to the potential pathology. Toxicology is a complex subject and varies with so many factors including age, sex, weight, genetics, epigenetics, dose, co-factors also causing toxicity eg ACE2 binding, cellular fusion, T-lymphocyte attack and so on as per the many other papers reviewed previously.
Then there are potential drug interactions which can take years to investigate fully when you do it properly. The authors of the paper are sufficiently concerned, so should we and in particular so should the regulators. But we are where we are with that.
An example of a poison that works by blocking potassium channels as SM-102 does? Scorpion venom.
And the ERG channels referred to have a great name to join our list including slug, snail and hedgehog: ether-a-go-go-related genes.
A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centruroides noxius scorpion venom
G B Gurrola et al. FASEB J. 1999 May.
Abstract
Toxins isolated from a variety of venoms are tools for probing the physiological function and structure of ion channels. The ether-a-go-go-related genes (erg) codify for the K+ channels (ERG), which are crucial in neurons and are impaired in human long-QT syndrome and Drosophila 'seizure' mutants. We have isolated a peptide from the scorpion Centruroides noxius Hoffmann that has no sequence homologies with other toxins, and demonstrate that it specifically inhibits (IC50=16+/-1 nM) only ERG channels of different species and distinct histogenesis. These results open up the possibility of investigating ERG channel structure-function relationships and novel pharmacological tools with potential therapeutic efficacy.
https://pubmed.ncbi.nlm.nih.gov/10224238/
And:
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels,[3] and Transient Receptor Potential (TRP) channels.[4][5] The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants (MeuNaTxα-12 and MeuNaTxα-13) exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs.[6] Ultimately, these actions can serve the purpose of warding off predators by causing pain (e.g., through the activation of sodium channels or TRP channels in sensory neurons)[7] or to subdue predators (e.g., in the case of inhibition of cardiac ion channels).[8]
https://en.m.wikipedia.org/wiki/Scorpion_toxin
There is potentially a benefit to blocking potassium channels: it can inhibit tumor growth. But the downside is the risk of many pathologies, including cardiac toxicity and sudden death.
It's not something you would want to risk dysregulating without high quality statistically significant data from double blind trials conducted first over long periods and only after prior in vitro animal tests as well as in vivo. Or perhaps design it out of the product in the first place?
Antibodies Targeting KV Potassium Channels: A Promising Treatment for Cancer (2019)
Abstract
‘Voltage-gated potassium channels are transmembrane proteins that allow flow of potassium across the membrane to regulate ion homeostasis, cell proliferation, migration, cell volume, and specific processes such as muscular contraction. Aberrant function or expression of potassium channels can underlie pathologies ranging from heart arrhythmia to cancer; the expression of potassium channels is altered in many types of cancer and that alteration correlates with malignancy and poor prognosis. Targeting potassium channels therefore constitutes a promising approach for cancer therapy. In this review, we discuss strategies to target a particular family of potassium channels, the voltage-gated potassium channels (KV) where a reasonable structural understanding is available. We also discuss the possible obstacles and advantages of such a strategy.”
Keywords: potassium channels, immunotherapy, antibodies, cancer
“As anticipated, due to the canonical roles of potassium channels, most identified mutations in KV channels lead to altered excitability of neurons and muscle cells. Loss-of-function mutations can cause hyperexcitability leading to epilepsy or to cardiac arrhythmias such as long QT syndrome (LQT; e.g., KV7.113). Mutations in KV7.2 or KV7.3 channels are correlated with neonatal epilepsy and benign familial neonatal convulsions,14,15 whereas KV7.4 is correlated with deafness16 (autosomal dominant 2a type). Aberrant function of members of the Kv1 family, in particular KV1.3 and KV1.5, correlates with central neural system disorders,17 atrial fibrillation,18 and immune abnormalities19–21; whereas loss of function or mutations in KV1.1 and KV1.2 induce convulsions, ataxia episodes, and myokymia disorders,22,23 and mutations in KV11.1 cause Type 2 LQT.24,25 The exquisite equilibrium between ion channels required for proper function of excitable cells is also reflected in the pathological effects of gain-of-function mutations. For example, such mutations in KV7.1 cause familial atrial fibrillation, short QT syndrome, or type 2 diabetes mellitus.26 Similarly, excessive activity of KV10.1 underlies developmental defects that occur with seizures and cognitive impairment, such as Temple–Baraitser and Zimmermann–Laband syndromes27–31; interestingly, excess of function of other potassium channels (SK3, KCa2.3) has been identified in other patients with Zimmermann–Laband syndrome.32
In some cases, a loss of ion selectivity as a result of a mutation can be the basis for pathological phenotypes. Although not reported for KV channels, this mechanism has been described for Kir3.4. It has been shown that a mutation in the structure of this channel results in loss of selectivity for K+ and a higher permeability for Na+, inducing severe aldosteronism33; nevertheless, the intimate molecular mechanism is still unclear.”
“Its inhibition reduces tumor growth in vivo, highlighting its potential as a therapeutic target in cancer. We therefore set out to produce a monoclonal antibody able to inhibit KV10.1 in intact cells while preserving KV10.2 and KV11 channels. The latter (most frequently termed HERG) poses a significant safety concern because its inhibition underlies a majority of drug-induced long QT events, which can produce malignant arrhythmia and sudden death. “