N-Acetylcysteine as Adjuvant Therapy for COVID-19 – A Perspective on the Current State of the Evidence (2021)
Disclaimers
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Background:
In reviewing various papers you can dose by mg per kg body mass, but for personal use with non life threatening infections I settled on a minimum 2 x 600 mg/day. Either way it's antioxidant, antiflammatory and immunity optimising action is so potent it's worth focusing on in isolation, and overdosing isn’t an issue.
Needless to say, it's value against transfection related damage must be considered as the pathways are nearly identical due to the toxic ROS inducing spike protein, ACE2 binding, mitochondrial damage, ferroptosis, NFKb, TNFa, IL-6 promotion etc, as covered in depth previously.
The paper:
N-Acetylcysteine as Adjuvant Therapy for COVID-19 – A Perspective on the Current State of the Evidence
Abstract: The looming severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a long-lasting pandemic of coronavirus disease 2019 (COVID-19) around the globe with substantial morbidity and mortality. N-acetylcysteine, being a nutraceutical precursor of an important antioxidant glutathione, can perform several biological functions in mammals and microbes. It has consequently garnered a growing interest as a potential adjunctive therapy for coronavirus disease. Here, we review evidence concerning the effects of N-acetylcysteine in respiratory viral infections based on currently available in vitro, in vivo, and human clinical investigations. The repurposing of a known drug such as N-acetylcysteine may significantly hasten the deployment of a novel approach for COVID-19. Since the drug candidate has already been translated into the clinic for several decades, its established pharmacological properties and safety and side-effect profiles expedite preclinical and clinical assessment for the treatment of COVID-19. In vitro data have depicted that N-acetylcysteine increases antioxidant capacity, interferes with virus replication, and suppresses expression of pro-inflammatory cytokines in cells infected with influenza viruses or respiratory syncytial virus. Furthermore, findings from in vivo studies have displayed that, by virtue of immune modulation and anti-inflammatory mechanism, N-acetylcysteine reduces the mortality rate in influenza-infected mice animal models. The promising in vitro and in vivo results have prompted the initiation of human subject research for the treatment of COVID-19, including severe pneumonia and acute respiratory distress syndrome. Albeit some evidence of benefits has been observed in clinical outcomes of patients, precision nanoparticle design of N-acetylcysteine may allow for greater therapeutic efficacy.
Keywords: N-acetylcysteine, SARS-CoV-2; COVID-19, coronavirus, repurposing approved drugs, engineering nanoparticles, virus infected cells, respiratory viral diseases, antioxidant, glutathione, T lymphocytes, immune modulating activity, anti-inflammatory response, antiviral effect, clinical translation
…N-acetylcysteine is a mucolytic drug which exhibits antioxidant and anti-inflammatory effects.26 The compound has been available in clinical practice for several decades to treat various medical conditions, including bronchitis, acute respiratory distress syndrome, paracetamol intoxication, chemotherapy-related toxicity, doxorubicin cardiotoxicity, heavy metal intoxication, ischemia-reperfusion cardiac injury, human immunodeficiency virus infection or acquired immunodeficiency syndrome, and neuropsychiatric disorders. N-acetylcysteine is also marketed as a dietary supplement that is suggested to possess antioxidant and hepatic-protecting effects. The antioxidant characteristic of N-acetylcysteine has been ascribable to its reactivity with •OH, CO3•−, •NO2, and thiyl radicals, the ability to repair oxidative damaged key cellular molecules, and activity as a precursor for biosynthesis of glutathione.27 There is a growing body of evidence that highlights the intrinsic antimicrobial and antibiofilm activities in many respiratory pathogens, including Escherichia, Pseudomonas, Staphylococcus, Acinetobacter, Haemophilus, and Klebsiella.26,28–30 High concentrations of N-acetylcysteine do not carry the risk of adverse interactions with most commonly used antibiotics and can exert intrinsic antimicrobial activity against Haemophilus influenza.26 Being a precursor for glutathione biosynthesis which is a crucial determinant of antimicrobial activity against bacteria, N-acetylcysteine is often prescribed as a mucolytic agent in conjunction with antibiotic treatment in respiratory tract infections to improve the outcomes of the course of therapy.31
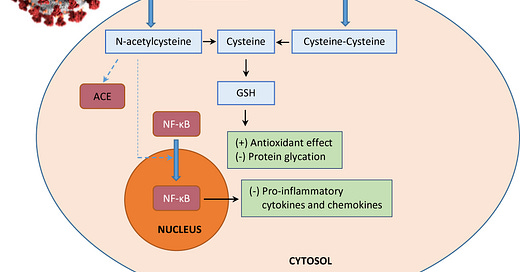
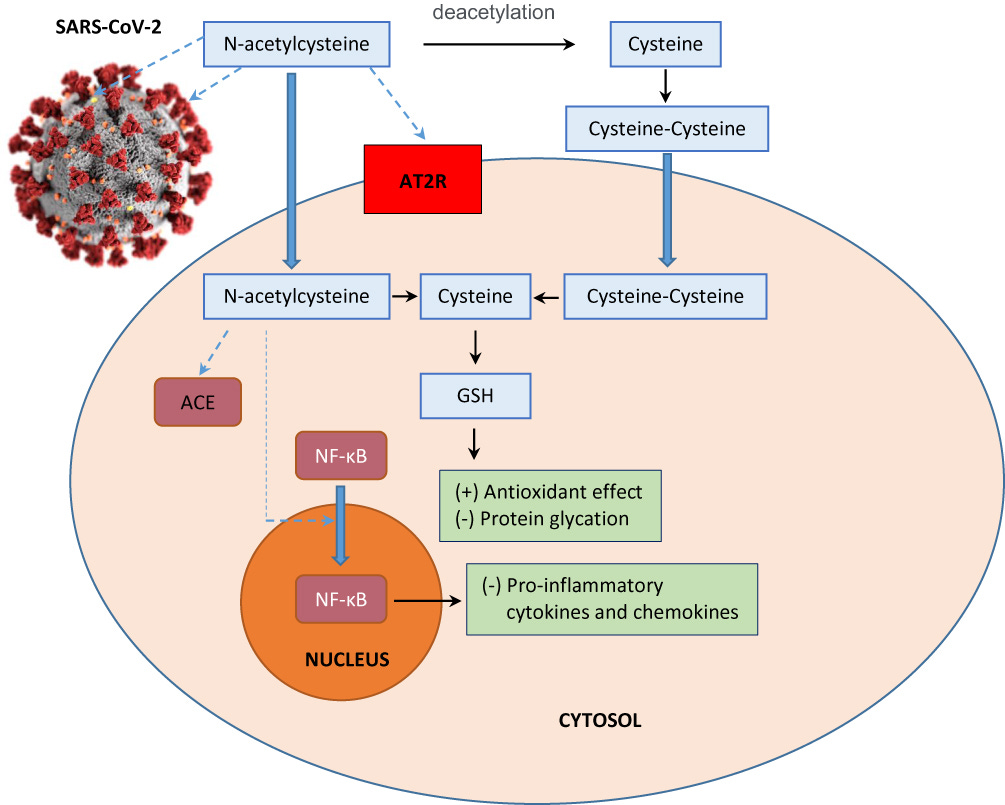
N-acetylcysteine has recently been suggested as an adjunctive therapy to the standard care for SARS-CoV-2 infection considering the favorable risk and benefit ratio and its effects on synthesizing glutathione, improving immune function, and modulating inflammatory response.32–34 It achieves the therapeutic effects through two main activities: 1) mucolytic action conferred by the free sulfhydryl group which reduces disulfide bonds in the cross-linked mucus glycoproteins matrix, thus decreasing the viscosity of mucus; 2) antioxidative action attributable to a direct interaction with free radicals, an indirect effect as a precursor to cysteine which is required for glutathione biosynthesis, and a replenishment of thiol pools that is central to redox regulation and control.35 In light of these properties, we hypothesize that N-acetylcysteine plays a role in the treatment of COVID-19 infection by the following postulated mechanisms of action (Figure 1):
Envelope (E) and spike (S) proteins have a triple cysteine structural motif located directly after the E protein’s transmembrane domain (NH2- … L-Cys-A-Y-Cys-Cys-N … -COOH) and a similar motif located in the carboxy terminus of the S protein (NH2- … S-Cys-G-S-Cys-Cys-K … -COOH). The position, orientation, and composition of these two motifs may serve as a center for the structural link between the E and S proteins which is mediated by the formation of disulfide bonds between the corresponding cysteine residues.36 Previous studies have indicated that the entry of viral glycoprotein is affected by thiol-disulfide balance within the viral surface and the cell-surface of the host.37,38 Any perturbations in the thiol-disulfide interchange equilibrium would deter the entry of the virus into host cells.39,40 Cleavage of disulfide bridges by N-acetylcysteine disrupts the structural components of the interacting proteins, thereby impairing receptor binding affinity and infectivity.
N-acetylcysteine is a chemical reducer of disulfide bonds via its free sulfhydryl groups may interact with the extracellular disulfide bridges of angiotensin II receptor, alter its tertiary structure, and inhibit the binding of angiotensin II to its surface receptors (AT1a receptors) with subsequent attenuation of signal transduction and cell action. The AT1a receptors possess two sets of disulfide bridges at the extracellular domain of the receptors: C18-C274 and C101-C180. N-acetylcysteine can reduce the disulfide bonds in a dose-dependent manner,41,42 decreasing angiotensin II and increasing angiotensin 1–7 (a biologically active peptide exerting many opposing actions to angiotensin II), thus protecting against lung inflammation and fibrosis.43
The sulfhydryl group of N-acetylcysteine inhibits angiotensin converting enzyme, reducing production of angiotensin II.44 In human lungs, angiotensin converting enzyme is expressed in lower lungs on type I and II alveolar epithelial cells. Following infection, viral entry begins with the attachment of spike (S) protein expressed on the viral envelope to angiotensin converting enzyme on the alveolar surface. Hence, N-acetylcysteine may prevent viral entry by limiting viral protein angiotensin converting enzyme interaction and internalization of the receptor-ligand complex.45 It also protects against oxidative stress and prevents glycosylation of proteins which may confer protection against respiratory disease syndrome and lung failure.
The antioxidant effect of N-acetylcysteine ameliorates oxidative stress and inflammatory response in COVID-19.46 It amplifies the signaling functions of toll-like receptor 7 protein and mitochondrial antiviral-signaling protein for boosting type 1 interferon production.47 Type I interferon functions to induce expression of various interferon-stimulated genes that exert antiviral activities to host cells.48
The receptor for advanced glycation end products (RAGE) and its ligands have a crucial role in the pathogenesis of COVID-19 pneumonia and acute respiratory distress syndrome as well as lung inflammation. Circulating levels of soluble RAGE (sRAGE, a decoy receptor) are positively associated with acute respiratory distress syndrome severity and mortality risk, whereas reduction in circulating levels of sRAGE drop results in disease resolution.49 Advanced glycation end products are formed by a reaction of the dicarbonyl compounds methylglyoxal and glyoxal with amino acids in proteins during glycolysis. Methylglyoxal and methylglyoxal-derived AGE can further activate inflammatory cells by binding to RAGE.50 N-acetylcysteine induces endogenous glutathione and hydrogen sulfide synthesis, thus attenuating methylglyoxal-induced protein glycation and additional glycosylation events in SARS-CoV-2 which may then inhibit the virus’s infectivity and associated pathologies.51
N-acetylcysteine inhibits NF-κB activation by suppressing TNF-induced IκB kinases, followed by impediment of proteasome-dependent degradation.52 This prevents translocation of NF-κB from cytoplasm to the nucleus and block expression of pro-inflammatory cytokines and chemokines which have been correlated with severity and lethality in various acute respiratory viral infections, including Influenza A H5N1, highly pathogenic H1N1, SARS-CoV, MERS-CoV, and SARS-CoV-2.53
Figure 1 Schematic representation of the possible effects of N-acetylcysteine on SARS-CoV-2 infection. N-acetylcysteine may inhibit envelope (E) protein and spike (S) protein of the virus, decrease angiotensin II receptor binding (AT2R), inhibit angiotensin converting enzyme (ACE), induce endogenous glutathione (GSH) synthesis which is associated with increased antioxidant effect and decreased glycation of intracellular proteins, and prevent nuclear translocation of NF-κB which suppresses the production of pro-inflammatory mediators and cytokines.
More:



Is this the paper which led FDA to reclassify NAC as a "drug," (since they use it to detox the liver in paracetamol / acetaminophen overdose) and remove it from supplement shelves? That happened in late 2020, and I stocked up then - but haven't had trouble sourcing since - as if they tried, and failed?