Oncology Meets Immunology: The Cancer-Immunity Cycle (2013): Beware treatments that can disrupt this cycle
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
The cancer-immunity cycle. It's a fine line between your immune system getting the upper hand, killing cancer cells which triggers a greater immune response and so on and the inhibitory cycle that works the other way, if a tumor(s) has advanced far enough to suppress your immune response.
What do you think can happen during the 2 weeks or so of spike protein expression? This cycle may be disrupted for a time eg CD8+ T cell counts can plunge. Tumor suppressors are downregulating. Toll like receptors stop signalling. DNA double strand breaks occur due to ROS and go unrepaired. And so on, as per previous posts. Your immune response has effectively come to a halt for a time.
Your microtumor or cancer may be able to advance a stage until normal immune function is restored.
And what if during that stage it has advanced far enough to hijack your immune system, as tumors do? Your immune system doesn't come back strong enough, the cancer goes metastatic, you die, to put it bluntly.
Apparently this is what clinicians are now seeing. Frequently.
Excerpts:
“As demonstrated by elegant analyses of cancer in mice, the continued deletion of cancer cells expressing T cell targets (immune editing) may enable cancers to evolve to avoid attack (Dunn et al., 2002). Despite these findings, recent results from human cancer have demonstrated that overcoming negative regulators to T cell responses in lymphoid organs (checkpoints) and in the tumor bed (immunostat function) are likely to explain the failure of immune protection in many patients (Mullard, 2013). Factors in the tumor microenvironment can act to modulate the existing activated antitumor T cell immune response, acting as an immune rheostat or “immunostat.” This class of molecules, including PD-L1:PD-1 (reviewed in Chen et al., 2012, Topalian et al., 2012a), emphasizes that the immune response in cancer reflects a series of carefully regulated events that may be optimally addressed not singly but as a group.”
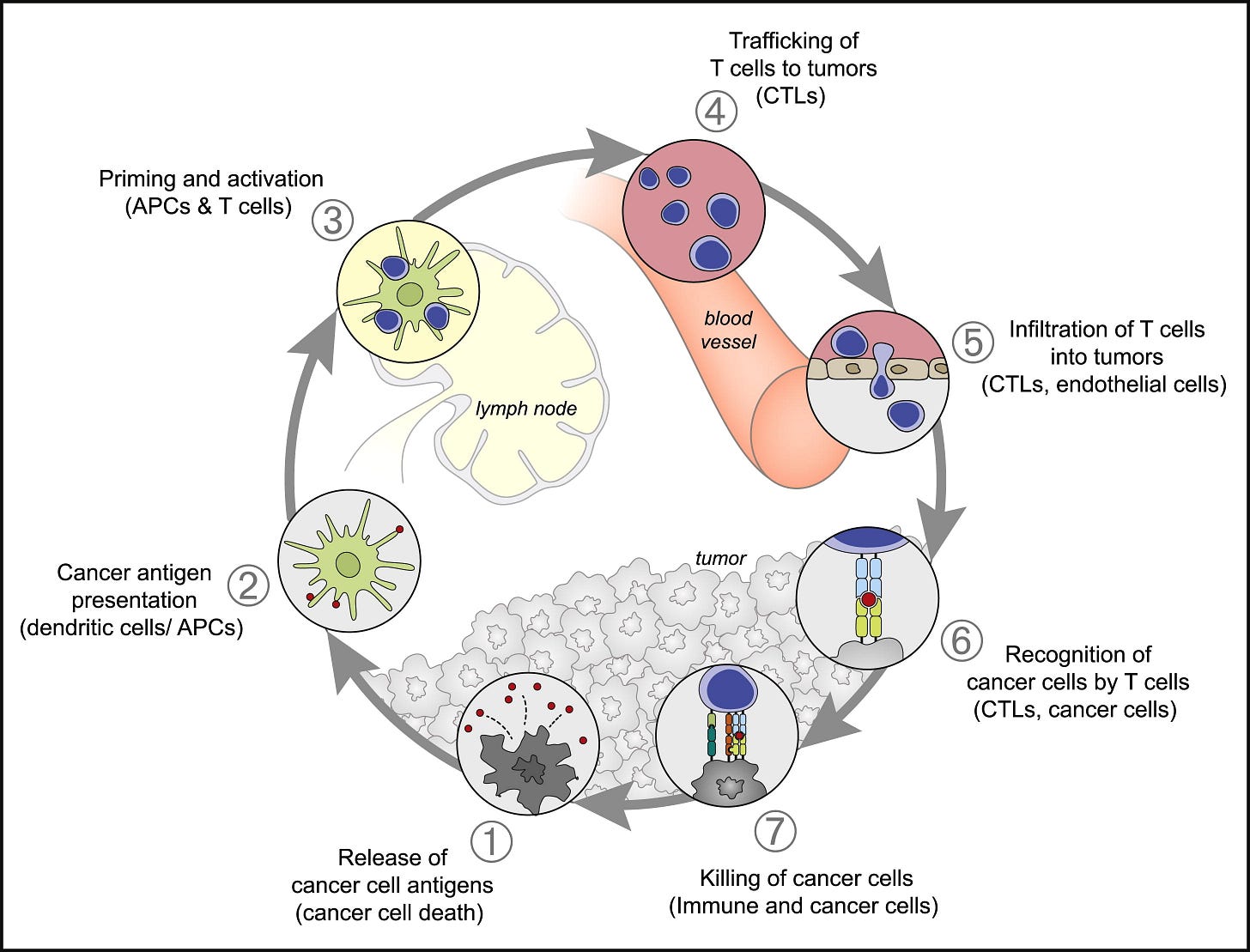
Figure 1. The Cancer-Immunity Cycle
The generation of immunity to cancer is a cyclic process that can be self propagating, leading to an accumulation of immune-stimulatory factors that in principle should amplify and broaden T cell responses. The cycle is also characterized by inhibitory factors that lead to immune regulatory feedback mechanisms, which can halt the development or limit the immunity. This cycle can be divided into seven major steps, starting with the release of antigens from the cancer cell and ending with the killing of cancer cells. Each step is described above, with the primary cell types involved and the anatomic location of the activity listed. Abbreviations are as follows: APCs, antigen presenting cells; CTLs, cytotoxic T lymphocytes.
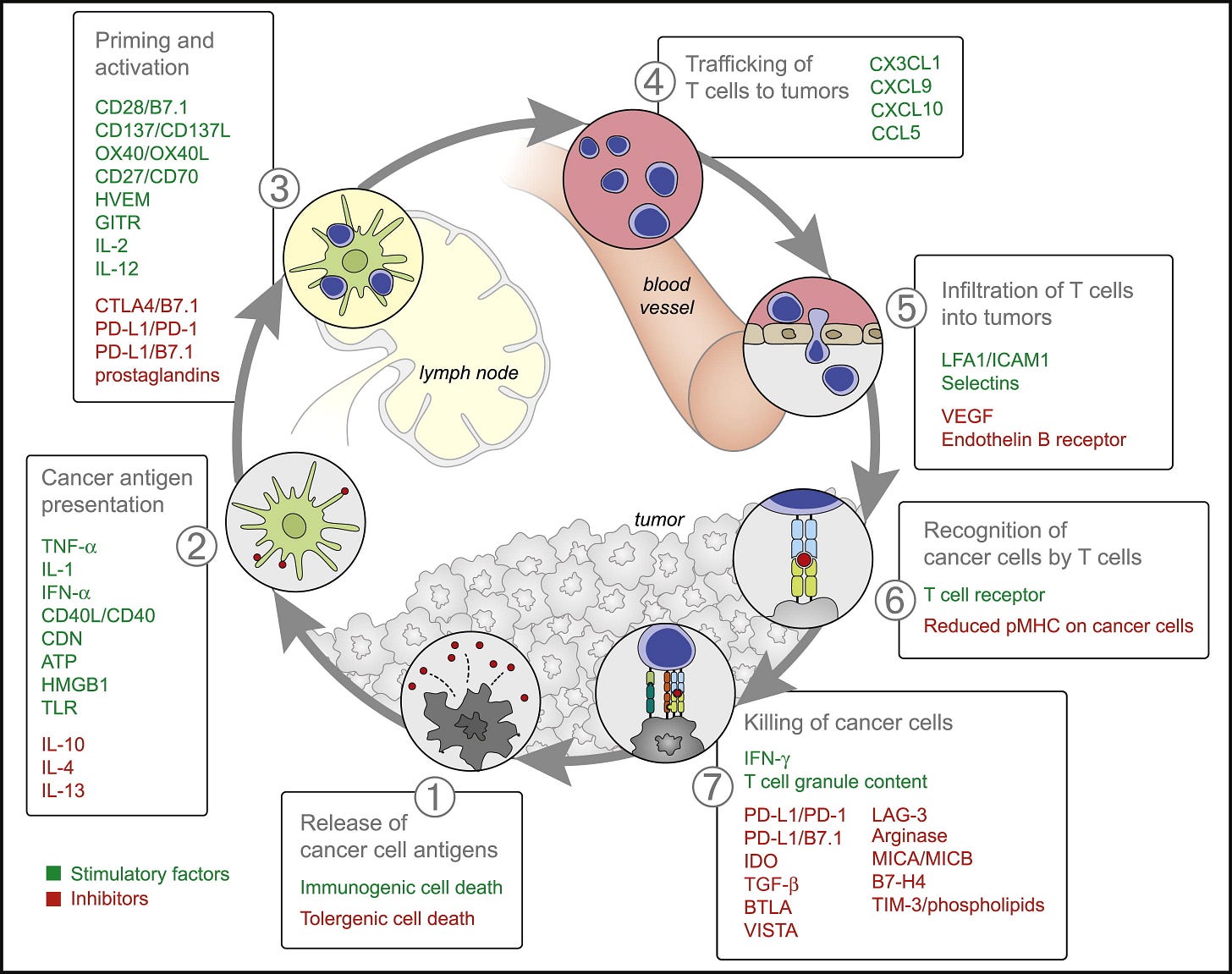
Figure 2. Stimulatory and Inhibitory Factors in the Cancer-Immunity CycleEach step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, whereas inhibitors shown in red help keep the process in check and reduce immune activity and/or prevent autoimmunity. Immune checkpoint proteins, such as CTLA4, can inhibit the development of an active immune response by acting primarily at the level of T cell development and proliferation (step 3). We distinguish these from immune rheostat (“immunostat”) factors, such as PD-L1, can have an inhibitory function that primarily acts to modulate active immune responses in the tumor bed (step 7). Examples of such factors and the primary steps at which they can act are shown. Abbreviations are as follows: IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; CDN, cyclic dinucleotide; ATP, adenosine triphosphate; HMGB1, high-mobility group protein B1; TLR, Toll-like receptor; HVEM, herpes virus entry mediator; GITR, glucocorticoid-induced TNFR family-related gene; CTLA4, cytotoxic T-lympocyte antigen-4; PD-L1, programmed death-ligand 1; CXCL/CCL, chemokine motif ligands; LFA1, lymphocyte function-associated antigen-1; ICAM1, intracellular adhesion molecule 1; VEGF, vascular endothelial growth factor; IDO, indoleamine 2,3-dioxygenase; TGF, transforming growth factor; BTLA, B- and T-lymphocyte attenuator; VISTA, V-domain Ig suppressor of T cell activation; LAG-3, lymphocyte-activation gene 3 protein; MIC, MHC class I polypeptide-related sequence protein; TIM-3, T cell immunoglobulin domain and mucin domain-3. Although not illustrated, it is important to note that intratumoral T regulatory cells, macrophages, and myeloid-derived suppressor cells are key sources of many of these inhibitory factors. See text and Table 1 for details.
More:
https://doi.org/10.1016/j.immuni.2013.07.012