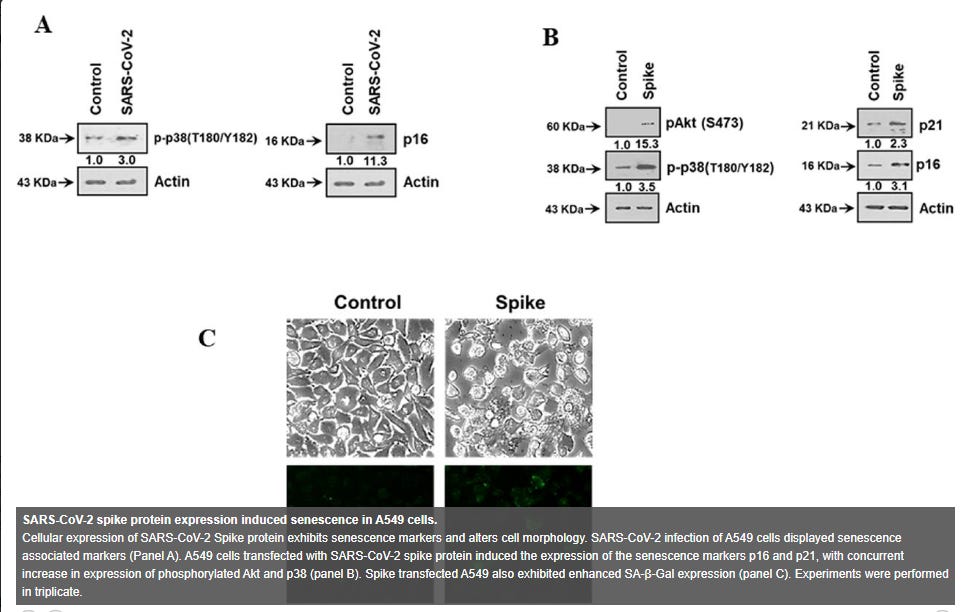
SARS-CoV-2 spike protein expressing epithelial cells promotes senescence associated secretory phenotype in endothelial cells and increased inflammatory response (2021)
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Endothelial cell senescence = vascular disease.
One of the reasons we have strict legal limits on exposure to ionising radiation is to limit the damage caused by generation of reactive oxygen species (ROS) and bystander effects.
The damage from one experimental transfection dose is the equivalent to many thousands of chest x-rays. Yet instead of strict limits we have up to 3 monthly mandates.
ROS induced damage is reason enough alone to put your health above your job for a time, if you are able. Regardless, you may not be able to work after several years of this due to cardiovascular disease etc etc.
https://www.bfs.de/EN/topics/ion/radiation-protection/limit-values/limit-values_node.html
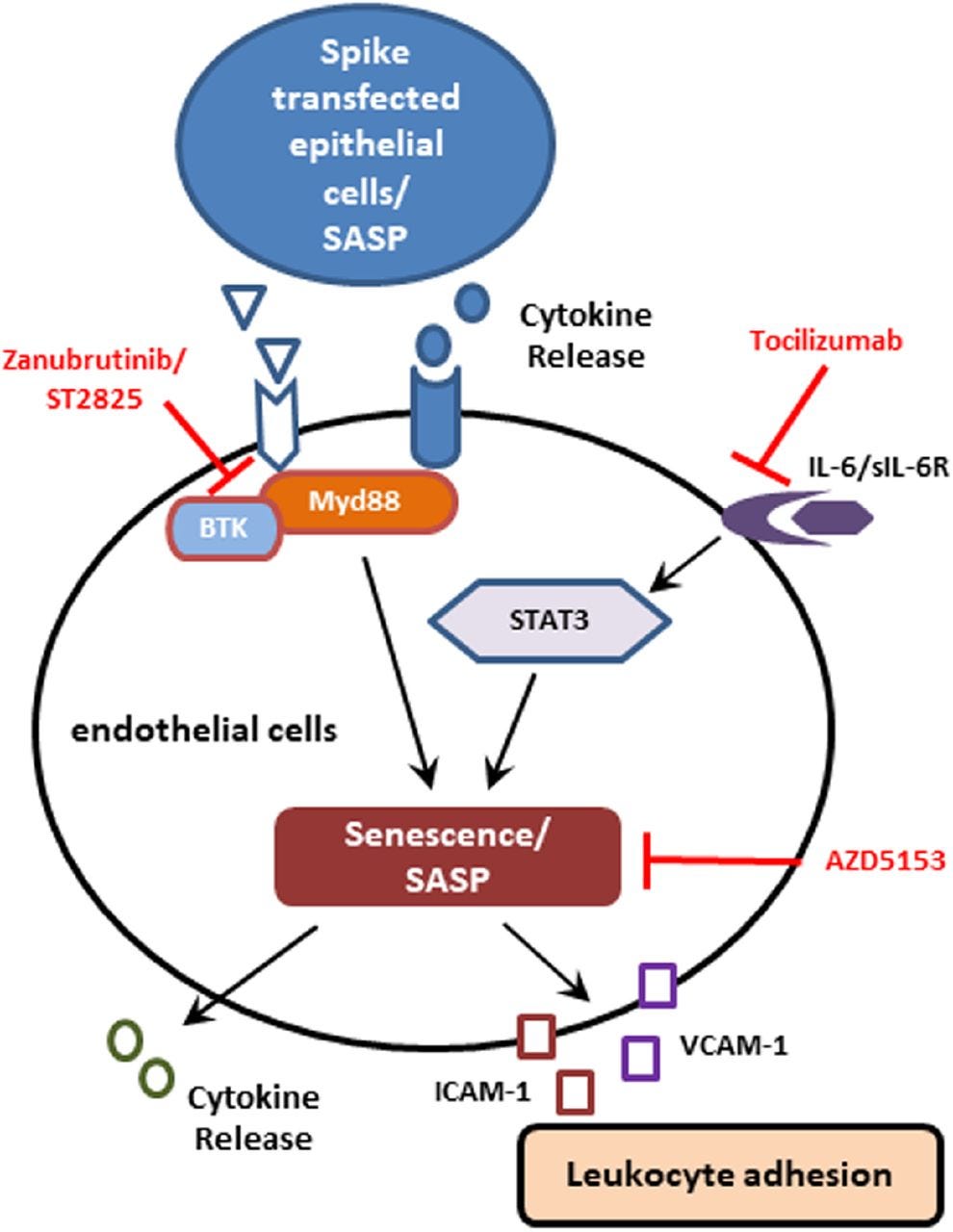
"Taken together, we identified that the exposure of human endothelial cells to cell culture supernatant derived from SARS-CoV-2 spike protein expression displayed cellular senescence markers leading to enhanced leukocyte adhesion with coronary blockade potential."
"Our study reveals that virus infected endothelial cells undergo senescence. The SASP release from the senescent cells serve as a paracrine signal to other tissues or cells, inducing senescence in distant organs or tissues. The reversal of senescence demonstrated in this study may be extended to clinical applications to alleviate disease severity and as a potential adjunct therapy to reduce COVID-19 mortality."
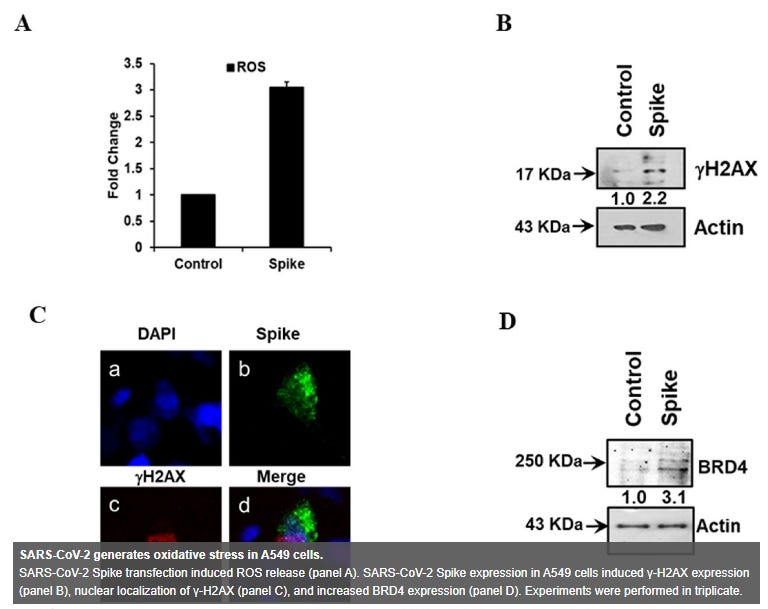
"Oxidative stress has been associated with the pathology of SARS-CoV-2 infection, including its amplification of cytokine storm and coagulopathy (14). ROS generation has been associated with the induction of senescence and maintenance of a viable senescent state in A549 cells was dependent upon ROS (15, 16). Here, we measured intracellular ROS, and observed an approximate 3-fold increase in ROS in A549 Spike transfected cells compared to non-transfected control cells (Fig. 2, panel A). Free radicals are known to cause DNA damage. The increased production of intracellular ROS plausibly contributes to DNA damage leading to cellular senescence. We observed an increase in the DNA damage response (DDR) marker, γ-H2AX, in cells transfected with viral spike (Fig. 2, panel B) and γ-H2AX was found to be localized to the nucleus in A549 cells expressing SARS-CoV-2 Spike protein (Fig. 2, panel C). Brd4 has been observed to be required in senescence immune surveillance and SASP associated paracrine signaling (17). Spike gene transfection of A549 cells led to an enhanced expression of Brd4 (Fig. 2, panel D). Our results indicated that the expression of SARS-CoV-2 Spike protein induces a senescent state in infected or Spike transfected A549 cells, potentially leading to enhanced SASP associated paracrine signaling.”
https://www.biorxiv.org/content/10.1101/2021.04.16.440215v1.full