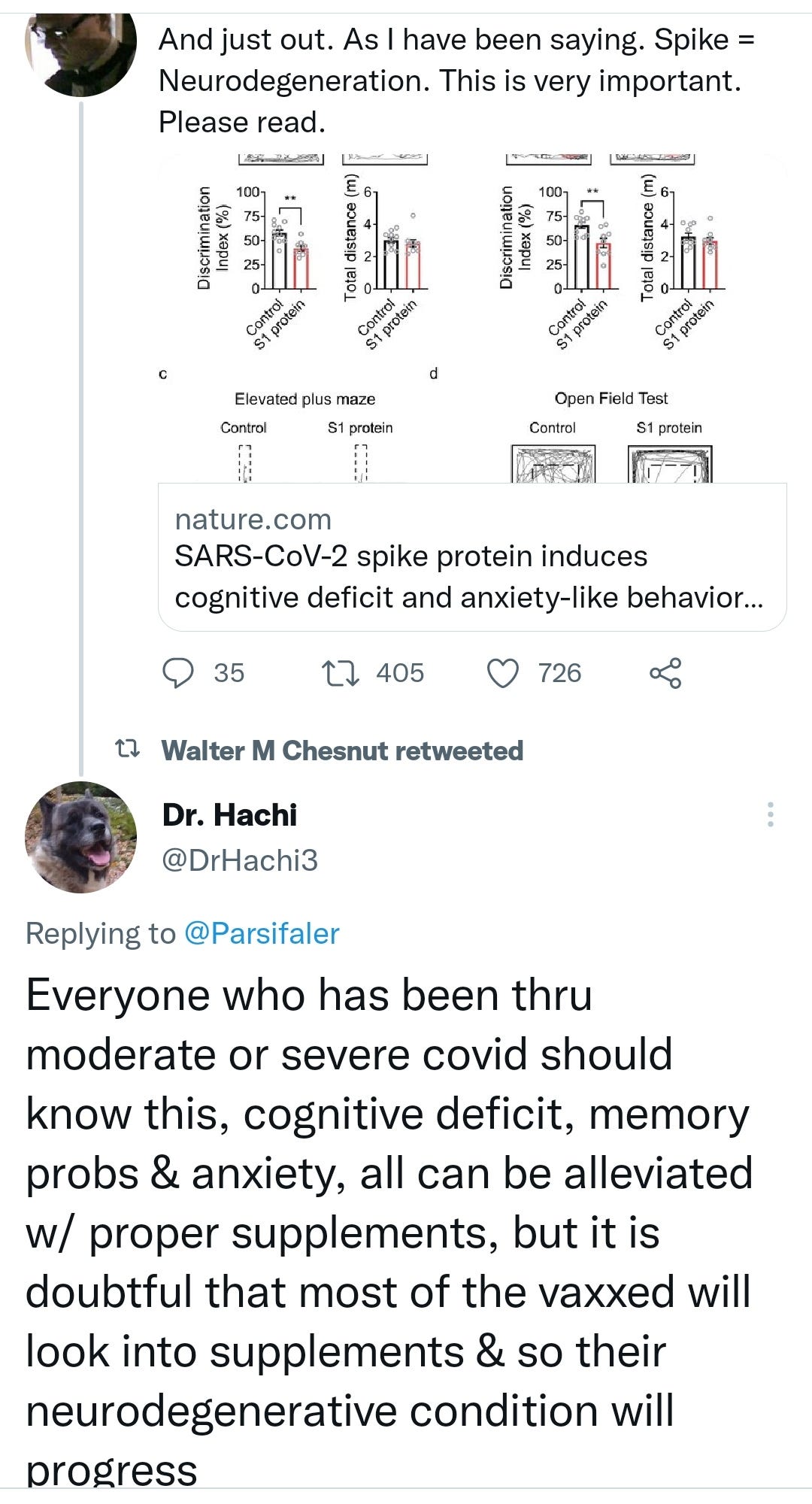
SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death
(31st March 2022)
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Another hat tip to Walter P Chesnut
https://twitter.com/Parsifaler/status/1509736631468797960?s=19
SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is accompanied by chronic neurological sequelae such as cognitive decline and mood disorder, but the underlying mechanisms have not yet been elucidated. We explored the possibility that the brain-infiltrating SARS-CoV-2 spike protein contributes to the development of neurological symptoms observed in COVID-19 patients in this study. Our behavioral study showed that administration of SARS-CoV-2 spike protein S1 subunit (S1 protein) to mouse hippocampus induced cognitive deficit and anxiety-like behavior in vivo. These neurological symptoms were accompanied by neuronal cell death in the dorsal and ventral hippocampus as well as glial cell activation. Interestingly, the S1 protein did not directly induce hippocampal cell death in vitro. Rather, it exerted neurotoxicity via glial cell activation, partially through interleukin-1β induction. In conclusion, our data suggest a novel pathogenic mechanism for the COVID-19-associated neurological symptoms that involves glia activation and non-cell autonomous hippocampal neuronal death by the brain-infiltrating S1 protein.
https://www.nature.com/articles/s41598-022-09410-7
I wrote a long Substack on gp120 & brain fog back in January, followed up a hypothesis and well, correlation isn't causation but see for yourself…
Pathophysiology of spike protein expressed GP120 mediated "brain fog" & dementia
https://doorlesscarp953.substack.com/p/pathophysiology-of-spike-protein?s=w
https://twitter.com/Jikkyleaks/status/1509635976397668385?s=19
HIV-1 coat protein gp120 stimulates interleukin-1β secretion from human neuroblastoma cells: evidence for a role in the mechanism of cell death
(2001)
Abstract
The role of the pro-inflammatory cytokine interleukin-1β (IL-1β) in the mechanism of cell death induced by the human immunodeficiency virus type 1 (HIV-1) recombinant coat glycoprotein, gp120 IIIB, has been studied in the human CHP100 neuroblastoma cell line maintained in culture.
Death of neuroblastoma cells typically elicited by 10 pm gp120 or by human recombinant IL-1β (10 ng ml−1) has been minimized by the antagonist of IL-1 receptor, i.e. IL-1ra (0.5 and 50 ng ml−1, respectively), an endogenous molecule that antagonizes most of the biological actions of IL-1β, or by an antibody (5 and 50 ng ml−1) which blocks the human IL-1 receptor type I (IL-1RI).
ELISA experiments have established that gp120 enhances immunoreactive IL-1β levels in the culture medium and this is prevented by exposure to the IL-1 converting enzyme (ICE) inhibitor t-butoxycarbonyl-l-aspartic acid benzyl ester-chloromethylketone [Boc-Asp(OBzl)-CMK] used at a concentration (2.5 μm) which significantly (P<0.001) reduces cell death.
Death of CHP100 cells induced by gp120 is also prevented by acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK; 10 – 100 μm), a second inhibitor of ICE, supporting the concept that the viral protein stimulates the conversion of the 31 kDa pro-IL-1β in to the 17 kDa mature cytokine which is then secreted to cause death.
In conclusion, our present data demonstrate that gp120 stimulates the secretion of IL-1β which then triggers CHP100 neuroblastoma cell death via stimulation of IL-1 receptor type I.
Keywords: HIV-1 recombinant gp120 IIIB, CHP100 neuroblastoma cells, IL-1β secretion, ICE inhibitors, Ac-YVAD-CMK, Boc-Asp(OBzl)-CMK, IL-1ra, IL-1 receptor type I, cell death
"In particular, double-labelling immunofluorescence experiments have established that neuronal as well as non-neuronal cells, very likely microglial cells, represent the main source for enhanced IL-1β expression which then is secreted in the extracellular space"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1573068/
Vs this from the newly published paper:
“As mechanisms, we found that SARS-CoV-2 S1 protein exerted non-cell autonomous hippocampal neuronal cell death by inducing interleukin-1 beta (IL-1β) expression from glial cells.”







HMGB1- is associated with Amyloidosis, impaired cognition and neurodegeneration.