SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia
Front. Immunol., 14 April 2021
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
I was looking into the SARS-CoV-2 & inhibition of microRNA miR-24 which moderates neuropilin-1, which is linked to vascular disease and tumor growth, bad enough in itself:
The Role of Neuropilin-1 (NRP-1) in SARS-CoV-2 Infection: Review (2021)
https://www.mdpi.com/2077-0383/10/13/2772/htm
but it led me to this research that somehow stayed below the radar and is far more disturbing in its implications. Even with the viral infection itself its not the viral replication that causes most of the pathology, its due to the spike protein and exosome bystander-spread microRNA’s.
Incidentally later stage tumors also use exosomes for signalling purposes to USP33-IRF9 , causing bystander pathology, more on this later.
The spike protein triggers the release into the circulation of exosomes charged with a payload of miRNA that go everywhere, including across the blood brain barrier where it can trigger the damaging inflammatory response, at a minimum.
This ground-breaking research also explains how the inflammatory cytokine levels are elevated, as per previous posts.
SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia (2021)
Key excerpts:
SARS-CoV-2 Spike Activates Cytokine Expressions
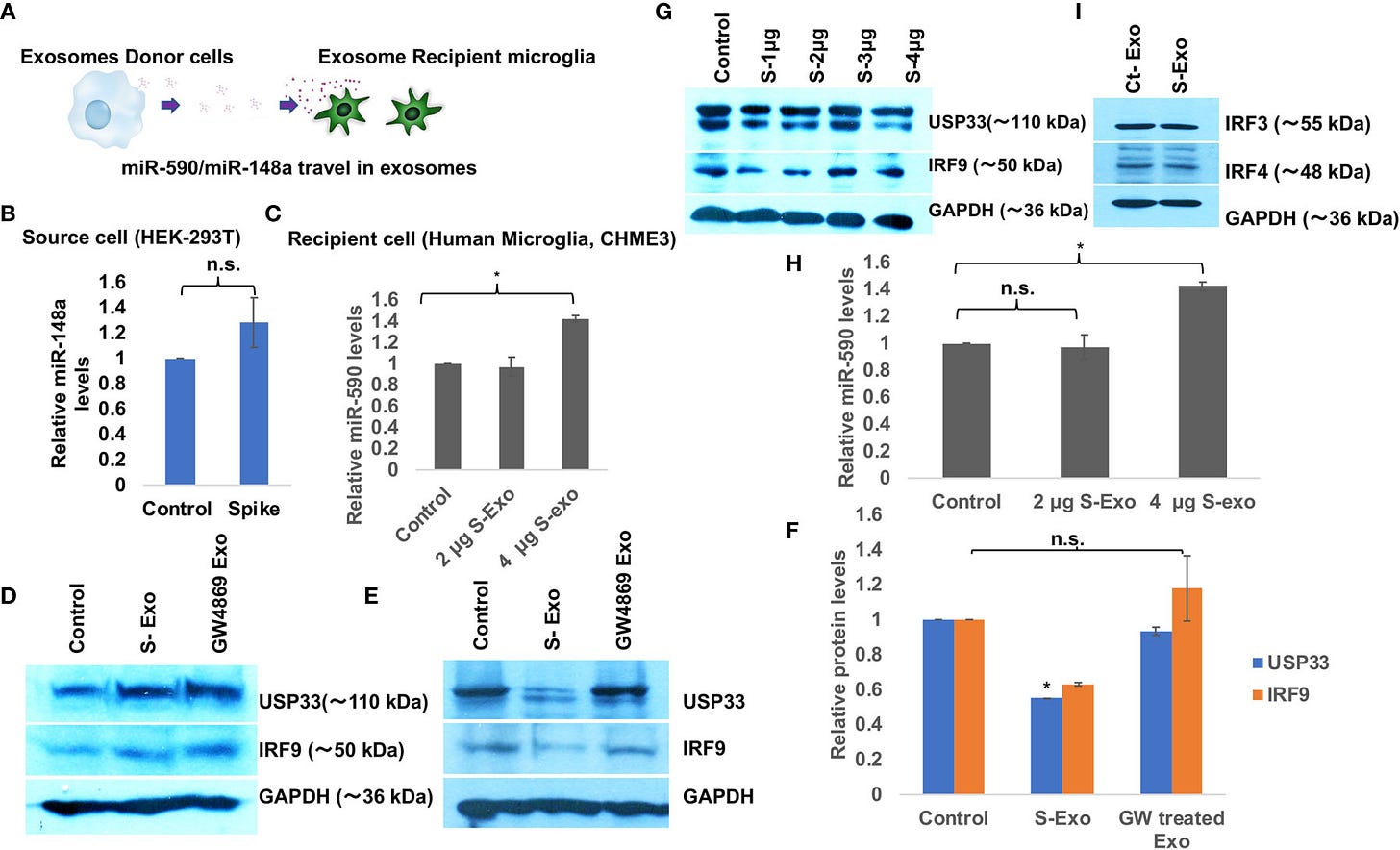
IRF9 expression levels have been considered as an important determinant of viral disease severity (49–51). Previous studies have demonstrated the crucial role of IRF9 in inflammation (62), autoimmune diseases like SLE (63), cardiovascular diseases (64), cell proliferation and immune cell regulation (65). In our experiments, we observed a sharp decline in IRF9 expression levels upon S-exo treatment in human microglia. Considering its multifunctional roles especially for inflammation and autoimmune regulation, we were interested to resolve its specific role in controlling inflammatory gene expression pathways such as NF-κB, TNFα and IFNβ. Firstly, we performed Spike gene transfection along with three major promoter luciferase plasmids of TNFα, IFNβ and NF-κB in a co-transfection experiment and checked the reporter luciferase expression levels as an indicator of these promoter activities. SARS-CoV-2 Spike gene transfection were able to induce all the three cytokine regulatory promoter activities of NF-κB, TNFα and IFNβ (Figures 6A–C). We mimicked our experimental set up of treatment with S-exo on cells and checked the promoter activities of these cytokine pathways. We could observe a significant elevation in promoter activity of all three pathways namely NF-κB, TNFα and IFNβ…
Discussion
Majority of clinical reports from COVID-19 patients suggest that patients deterioration happens 7-10 days after the onset of disease, which is accompanied by decrease in viral load (66). This suggests that pathological manifestations of COVID-19 are primarily driven by hyperinflammation leading to multi-organ dysfunctions rather than direct viral injury. Previous episodes of coronavirus infections such as SARS-CoV and MERS-CoV have been shown to cause an uncontrolled, tissue-damaging inflammatory phenomenon, also known as ‘Cytokine storm’ (67, 68). The ‘Cytokine storm’ phenomenon have been linked with severity of many viral diseases such as Influenza (IAV) (69), Dengue hemorrhagic fever (70) and Ebola viruses (71) etc.
These information laid the foundation for our hypothesis, where we wanted to investigate the triggering factor for cytokine storm even when whole virus count is declined or even disappeared from host circulation. We specially chose to investigate the immune-modulatory functions of the Spike protein of SARS-CoV-2 since it is the outermost structural protein that interacts with host cell while infecting it. Another reason was its prominent use as effective epitope for vaccine development, which demands the more detail dissection of host inflammatory responses against Spike gene. Apart from acting as instrument for virus entry, Spike has been regarded as critical determinant of host immune responses, tissue tropism and influencing host range for viral transmission (56).
Recent report by Ramani et al. (20), were suggesting that SARS-CoV-2 replication might not be equally potentially supported in all tissues as in lungs despite the presence of ACE2 receptors (20). In this study, authors showed in a 3D brain organoid model that SARS-CoV-2 were infecting neurons but were not replicating efficiently, yet there were enough neuronal damage similar to neurodegenerative phenotype (20). Even some clinical reports are indicating multiple signs of neurological damages in otherwise asymptomatic COVID-19 patients (72, 73). These reports strongly indicate that not just SARS-CoV-2 viral particle but shed viral proteins or ‘toxic trails’ after SARS-CoV-2 can induce a cascade of strong host immune response. These leftover ‘toxic trail’ after viral reclining phase often includes cellular transcription factors, microRNAs and other circulating factors in host plasma. These reports influenced our experimental design and we were curious to look into the role of exosomes for transmitting and transferring the cellular and viral signals during the course of SARS-CoV-2 neuropathogenesis.
Our results (Figures 2 and 3) are clearly indicating that Spike transfected cells release a significant amount of exosomes (S-exo), actively loaded with inflammation promoting microRNAs such as miR-590, miR-148a etc. We have chosen human microglia to study the impact of SARS-CoV-2 spike protein induced disruption of CNS innate immune responses. Human microglia, the ‘brain-resident macrophages’ are rightly considered the real executor of neuroinflammation since their role in causing neuroinflammation in various viral diseases (HIV-1, JEV, Dengue etc) are well established (29, 37, 74, 75). Upon exposure with S-exo cargo, human microglia internalizes its cargo such as microRNAs, which ultimately results in suppression of its target genes. In our results, we could show that cellular USP33 levels (a potential target of miR-148a) gets significantly diminished (Figure 2E). We have previously established a regulatory axis run by miR-148a mediated targeting of USP33 and downstream regulation of ATF3 turnover during DENV neuropathogenesis (29). Role of miR-148a in congenital ZIKV infection, targeting TLR3 during Duck tembusu virus (DTMUV) and playing their role in tumor invasion and migration are well known (76–78). Similarly diverse roles of USP33 in deubiquitinating Parkin gene, HERC2, centrosome biogenesis, tumor progression of gastric carcinoma as well as DENV neuropathogenesis are well established (29, 79–81).
…Predominant use of Spike gene as candidate epitope in vaccine development also warrants some detail investigation regarding its impact on host immune response and other safety concerns since few episodes of vaccine administration have reported some unexpected negative outcomes on host bodies.
More:
https://www.frontiersin.org/articles/10.3389/fimmu.2021.656700/full