Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #26: Interleukin-6 (IL-6) induced pathology
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
Read in conjunction with:
Although the n-counts were low for this study and there may be a bias towards healthier volunteers the data corroborate the elevated cytokine levels seen in previous studies.
In the discussion the BNT162b2 pathology of IL-6 is considered limited as it declines by day 8, yet for reasons unknown they failed to consider their own 2nd (or 3rd, 4th, 5th…) dose effects out to day 23+ or the effects of immune priming by prior COVID-19 infection. Another example of positive spin to get a paper published?
Other studies have found mRNA + spike protein expression in lymph node germinal centres for at least 60 days, so pathogenic cytokine elevation is far from limited to just 8 days in vivo. This newly published paper also refers to original antigenic sin (OAS) which, as predicted, somewhat limits the efficacy of further boosters to different variants.
Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination (2022)
Summary
During the SARS-CoV-2 pandemic, novel and traditional vaccine strategies have been deployed globally. We investigated whether antibodies stimulated by mRNA vaccination (BNT162b2), including third-dose boosting, differ from those generated by infection or adenoviral (ChAdOx1-S and Gam-COVID-Vac) or inactivated viral (BBIBP-CorV) vaccines. We analyzed human lymph nodes after infection or mRNA vaccination for correlates of serological differences. Antibody breadth against viral variants is lower after infection compared with all vaccines evaluated but improves over several months. Viral variant infection elicits variant-specific antibodies, but prior mRNA vaccination imprints serological responses toward Wuhan-Hu-1 rather than variant antigens. In contrast to disrupted germinal centers (GCs) in lymph nodes during infection, mRNA vaccination stimulates robust GCs containing vaccine mRNA and spike antigen up to 8 weeks postvaccination in some cases. SARS-CoV-2 antibody specificity, breadth, and maturation are affected by imprinting from exposure history and distinct histological and antigenic contexts in infection compared with vaccination.
Full paper:
https://www.sciencedirect.com/science/article/pii/S0092867422000769
SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses (2021)
https://www.nature.com/articles/s41586-021-03738-2
IL-15 & IL-6 induced autoimmunity is discussed, but blink and you would miss it.
Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients (2021)
Inclusion/Exclusion Criteria
Major inclusion criteria for participation in this study included: (i) age above 18 years; (II) ability to sign the informed consent form, and (iii) eligibility for vaccination, according to the national (Greek) program for COVID-19 vaccination (i.e., individuals who had no serious allergy problem and especially they have not been hospitalized due to a serious allergic reaction (anaphylaxis). Major exclusion criteria included the presence of: (i) autoimmune disorder under immunosuppressive therapy; (ii) active malignant disease and (iii) end-stage renal disease, as previously described (Terpos et al., 2021b).
Volunteer donors between the ages of 20-78 (Male = 27 and Female = 36) were tested in the period January 4 to April 1, 2021, in the vaccine center of Alexandra General Hospital in Athens, Greece. All study procedures were carried out in accordance with the declaration of Helsinki (18th World Medical Association Assembly), its subsequent amendments, the Greek regulations and guidelines, as well as the good clinical practice guidelines (GCP) as defined by the International Conference of Harmonization. The study was also approved by the local ethic committee of Alexandra General Hospital (no 15/23 December 2020).
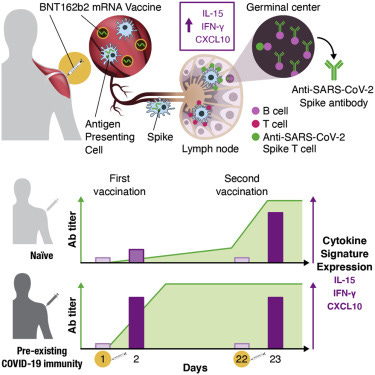
Summary
Early responses to vaccination are important for shaping both humoral and cellular protective immunity. Dissecting innate vaccine signatures may predict immunogenicity to help optimize the efficacy of mRNA and other vaccine strategies. Here, we characterize the cytokine and chemokine responses to the 1st and 2nd dose of the BNT162b2 mRNA (Pfizer/BioNtech) vaccine in antigen-naive and in previously coronavirus disease 2019 (COVID-19)-infected individuals (NCT04743388). Transient increases in interleukin-15 (IL-15) and interferon gamma (IFN-γ) levels early after boost correlate with Spike antibody levels, supporting their use as biomarkers of effective humoral immunity development in response to vaccination. We identify a systemic signature including increases in IL-15, IFN-γ, and IP-10/CXCL10 after the 1st vaccination, which were enriched by tumor necrosis factor alpha (TNF-α) and IL-6 after the 2nd vaccination. In previously COVID-19-infected individuals, a single vaccination results in both strong cytokine induction and antibody titers similar to the ones observed upon booster vaccination in antigen-naive individuals, a result with potential implication for future public health recommendations.
Graphical abstract
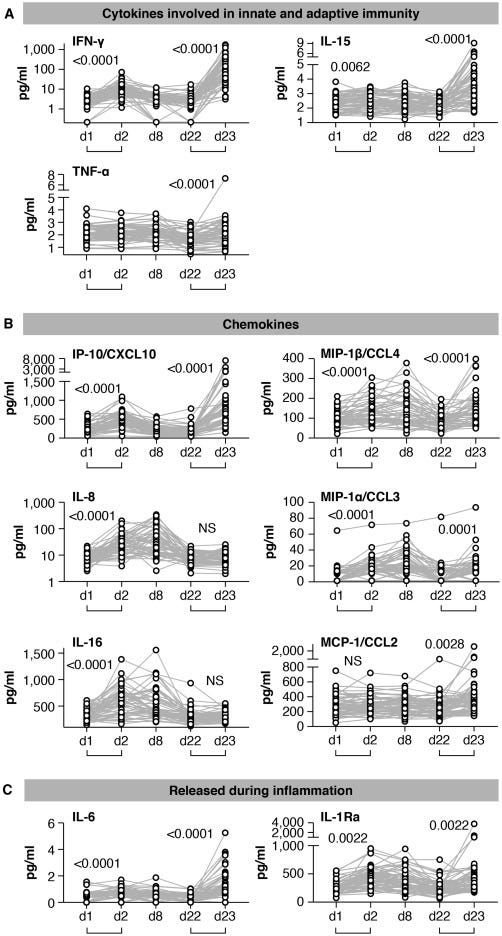
Figure 2. Serum cytokine and chemokine levels after the 1st and 2nd vaccination in COVID-19-naive vaccine recipients
Cytokine and chemokine levels were measured over time using the MSD assay after the 1st vaccination (day 1, day 2, day 8, and day 22) and at 1 day after the 2nd vaccination (day 23) in the 58 COVID-19-naive vaccine recipients.
(A–C) Serum levels of 11 selected analytes among 19 analytes showing changes upon vaccination are plotted over time. (A) Cytokines involved in both innate and adaptive immunity. (B) Chemokines. (C) Molecules released during inflammation. See also Figure S3 and Tables S2 and S3. p values are from paired t test.
Figure 3. Comparison of serum cytokine and chemokine levels
Cytokine and chemokine levels were measured using the MSD assay after the 1st and 2nd vaccination in 58 COVID-19 naive recipients (as described for Figure 2).
(A and D) Heatmaps depicted log2 fold changes in 19 analytes upon the 1st vaccination (green: d2_d1; orange: d8_d1; blue: d22_d1) (A) and after both the 1st (green: d2_d1) and 2nd vaccinations (purple: d23_d22) (D). The comparison of the effects induced by the 2nd vaccination over the 1st (d23_d22 over d2_d1) is shown in gray in (D). Different scales are used in (A) and (D) to better visualize the distinct changes upon the 1st and 2nd vaccination.
(B, C, E, and F) Volcano plots of data shown in (A) and (D) depict differentially expressed analytes upon the 1st vaccination at day 2 in comparison to day 1 (B) and at day 8 in comparison to day 1 (C) and after the 2nd vaccination at day 23 in comparison to day 22 (E). (F) Differentially affected analytes after the 2nd vaccination in comparison to the 1st vaccination (1 day after each vaccine dose). Red dots indicate significant upregulation; blue dots indicate significant downregulation (FDR < 0.05 represented by the broken horizontal line). See also Figure S4 and Tables S2 and S3.
After the 1st vaccination, the 58 naive vaccine recipients showed a highly significant but transient increase of IFN-γ (∼2.5×; Figure 2A), IP-10/CXCL10 (∼2×; Figure 2B), and IL-6 (1.5×; Figure 2C) at day 2 followed by rapid downregulation close to baseline levels by day 8. IL-15 also showed a small but significant upregulation (Figure 2A). Other analytes including IL-8 (∼3×; Figure 2B), IL-16 (∼1.5×; Figure 2B), MIP-1α/CCL3 (∼2.5×; Figure 2B), MIP-1β/CCL4 (∼1.5×; Figure 2B), and IL-1Ra (∼2×; Figure 2C) were significantly upregulated over baseline both at day 2 and day 8, indicating longer-lasting vaccine effects (1 week after administration). No significant differences after the 1st vaccination were observed for TNF-α (Figure 2A), MCP-1/CCL2 (Figure 2B), and IL-3 (Figure S3). A slight decrease in IL-12/IL-23p40, MDC/CCL22, and IL-7 levels (Figure S3) was also observed, whereas CRP, Eotaxin, SAA, and VEGF-A were all increased (Figure S3) as consequence of the inflammation process. For most of the analytes, serum levels at day 22, prior to the 2nd vaccination, were comparable to pre-vaccination levels, as shown in the heatmap (Figure 3A).
The cytokine/chemokine response pattern was different at day 23 (1 day after the 2nd vaccination; Figures 2, S3, and 3D–3F). IFN-γ, IL-15, IP-10/CXCL10, and IL-6 showed elevated levels at day 23 that were significantly higher than those at day 2 (Figure 2). Remarkably, IFN-γ and IP-10/CXCL10 levels increased up to ∼20× and ∼4× over baseline after the 2nd vaccination, respectively. About 2× higher IL-15 and IL-6 peaks were detected after the 2nd vaccination (Figure 2). A similar effect was also observed for MIP-1β/CCL4 (Figure 2B), CRP, and SAA (Figure S3). IL-16, IL-8 (Figure 2B), MDC/CCL22, and VEGF-A (Figure S3) were not affected, whereas MIP-1α/CCL3 and IL-1Ra behave similarly after each vaccine dose (Figures 2B and 2C). Eotaxin showed a slight downregulation at day 23 (Figure S3). Importantly, TNF-α (Figure 2A), MCP-1/CCL2 (Figure 2B), IL-7 (Figure S3), IL-3 (Figure S3), and IL-12/IL-23p40 (Figure S3) showed significant increases only after the 2nd vaccination (day 23; Figure 2; Figure S3).
Vaccine recipient clustering did not reveal a differential effect of the vaccination on the cytokine/chemokine profile based on age (cutoff age of 50) in this cohort. However, we found a stronger induction in female versus male vaccine recipients (Figure S4) of IFN-γ, IL-15, IL-6, and IP-10/CXCL10 upon the 2nd vaccination. Because the dominant adverse effect (AE) upon the vaccinations (Table S3) was pain at site of injection as reported by 80% and 76% of vaccinees, respectively, this precluded further dissection of AE and cytokine/chemokine changes.
Serum cytokine and chemokine profile induced by the BNT162b2 mRNA vaccine in recipients with pre-existing anti-COVID-19 immunity
A similar chemokine/cytokine analysis was performed in the 5 vaccine recipients with pre-existing SARS-CoV-2 immunity. The cytokine/chemokine signature upon the 1st and 2nd vaccination is depicted in heatmaps (Figures 4A and 4B). The effects of the 1st vaccination were also compared among the 2 vaccine groups (Figures 4C to 4F). In recipients with pre-existing CoV-2 immunity, the 1st vaccination induced a much stronger upregulation of IFN-γ, IP-10/CXCL10, TNF-α, and IL-6. At day 2, high levels of IFN-γ and IP-10/CXCL10 were detected that were comparable to the levels achieved at 1 day after the 2nd vaccination in CoV-2 naive recipients (Figures 4C and 4D). Similarly, a greater increase of TNF-α and IL-6 was found after the 1st vaccination in individuals with pre-existing COVID-19 immunity (Figures 4E and 4F). Both vaccine groups showed similar levels for these analytes after the 2nd vaccination.
…Overall, these data showed that BNT162b2 mRNA vaccination is accompanied by the rapid release in the blood of inflammatory markers, chemokines, and cytokines. In particular, the vaccination resulted in a strong response driven by IL-15, IFN-γ, and IP-10/CXCL10. Booster vaccination in naive individuals or one single vaccine dose in previously SARS-CoV-2-infected individuals induced anamnestic responses and high levels of cytokines critical for the rapid recruitment and stimulation of virus-specific effector immune cells.
…The cytokine signature at day 2 suggested a rapid co-expression of molecules promoting inflammation and priming of adaptive immunity. Indeed, a role of IL-15 in inducing both IFN-γ directly and IP-10/CXCL10 through the IFN type 2 pathway has been previously reported (Bergamaschi et al., 2020). Additionally, in autoimmune conditions such as rheumatoid arthritis, localized inflammatory responses are characterized by the concerted release of both IL-15 and IL-6 that are maintained by positive feedback loops and result in systemic disorders.
Figure 5. Correlation of chemokine and cytokine changes
Pairwise correlations were calculated among the log2 fold changes at day 23 (after the 2nd vaccination) for the 19 biomarkers that were affected by the vaccination by using the Spearman correlation coefficient (adjusted p < 0.05). The analysis was performed for the 58 COVID-19-naive vaccine recipients.
(A) Correlation matrix for the 2nd vaccination is plotted as a heatmap. Spearman r values of correlations are indicated in the grid cells, and ellipses identified significant correlations. The color and shape of ellipses correspond to the value of the Spearman correlation coefficient, with red color indicating a positive correlation. The red box identifies the cluster of positive associations featuring IFN-γ, IL-15, TNF-α, IL-6, and IP-10/CXCL10. The gray box data are described in Figure S6.
(B) Correlation plots for the selected analytes from (A) (red box). Each dot represents a single vaccine recipient response. r is shown in plots; all correlations are characterized by an adjusted p < 0.05. See also Figure S5 and Tables S2 and S3.
Discussion
…Many studies have shown that uncontrolled inflammation and cytokine storm syndrome contribute to the severity of COVID-19 disease. Patients with severe disease are characterized by high levels of inflammatory markers, including CRP, ferritin, and D-dimer and high levels of chemokines, such as granulocyte colony-stimulating factor (G-CSF), MCP-1/CCL2, MIP-1α/CCL3, IL-8, and IP-10/CXCL10, resulting in inflammatory cell infiltration and tissue damage in the lungs and in a high neutrophil-to-lymphocyte ratio (Mehta et al., 2020; Merad and Martin, 2020). A systemic increase in the levels of IL-2, IL-7, IL-10, IL-6, and TNF-α has also been reported (Huang et al., 2020). In particular, IL-6, IL-8, and TNF-α serum levels are significant predictors of disease severity and death (Del Valle et al., 2020). In contrast, early activation of the IFN type I pathway was associated with the prevention of disease progression (Bastard et al., 2020; Zhang et al., 2020). Although several of the cytokines and chemokines induced by viral infection were also elevated after mRNA vaccination, important differences are to be highlighted. Upon vaccination, we observed an early but transient inflammatory cytokine response. IFN-γ, IP-10/CXCL10, IL-6, and CRP increased acutely at day 2 and returned to baseline levels by day 8 after vaccine administration.
Full paper:
https://www.sciencedirect.com/science/article/pii/S2211124721009323
As an aside, CXCL10 is elevated too for weeks, is neurotoxic and associated with HIV.
“C-X-C motif chemokine ligand 10 (CXCL10) also known as Interferon gamma-induced protein 10 (IP-10) or small-inducible cytokine B10 is an 8.7 kDa protein that in humans is encoded by the CXCL10 gene.[5][6] C-X-C motif chemokine 10 is a small cytokine belonging to the CXC chemokine family.”
CXCL10-induced cell death in neurons: role of calcium dysregulation
Yongjun Sui et al. Eur J Neurosci. 2006 Feb.
Abstract
Chemokines play a key role in the regulation of central nervous system disease. CXCL10 over-expression has been observed in several neurodegenerative diseases, including multiple sclerosis, Alzheimer's disease and HIV-associated dementia. More recent studies by others and us have shown that CXCL10 elicits apoptosis in fetal neurons. The mechanism of CXCL10-mediated neurotoxicity, however, remains unclear. In this study, we provide evidence for the direct role of Ca(2+) dysregulation in CXCL10-mediated apoptosis. We demonstrate that treatment of fetal neuronal cultures with exogenous CXCL10 produced elevations in intracellular Ca(2+) and that this effect was modulated via the binding of CXCL10 to its cognate receptor, CXCR3. We further explored the association of intracellular Ca(2+) elevations with the caspases that are involved in CXC10-induced neuronal apoptosis. Our data showed that increased Ca(2+), which is available for uptake by the mitochondria, is associated with membrane permeabilization and cytochrome c release from this compartment. The released cytochrome c then activates the initiator active caspase-9. This initiator caspase sequentially activates the effector caspase-3, ultimately leading to apoptosis. This study identifies the temporal signaling cascade involved in CXCL10-mediated neuronal apoptosis and provides putative targets for pharmaceutical intervention of neurological disorders associated with CXCL10 up-regulation.
https://pubmed.ncbi.nlm.nih.gov/16519660/
Elevated Basal Pre-infection CXCL10 in Plasma and in the Small Intestine after Infection Are Associated with More Rapid HIV/SIV Disease Onset (2016)
"Elevated blood CXCL10/IP-10 levels during primary HIV-1 infection (PHI) were described as an independent marker of rapid disease onset, more robust than peak viremia or CD4 cell nadir."
How BNT162b2 induced cytokines may paradoxically promote viral survival (and therefore replication & viral count), thus delaying recovery rather than accelerating it:
Interleukin-6 (IL-6) and IL-17 Synergistically Promote Viral Persistence by Inhibiting Cellular Apoptosis and Cytotoxic T Cell Function (2014)
ABSTRACT
Interleukin-6 (IL-6) plays an important role in the development and progression of inflammatory responses, autoimmune diseases, and cancers. Many viral infections, including Theiler's murine encephalomyelitis virus (TMEV), result in the vigorous production of IL-6. However, the role of IL-6 in the development of virus-induced inflammatory responses is unclear. The infection of susceptible mice with TMEV induces the development of chronic demyelinating disease, which is considered a relevant infectious model for multiple sclerosis. In this study, we demonstrate that resistant C57BL/6 mice carrying an IL-6 transgene (IL-6 Tg) develop a TMEV-induced demyelinating disease accompanied by an increase in viral persistence and an elevated Th17 cell response in the central nervous system. Either IL-6 or IL-17 induced the expression of Bcl-2 and Bcl-xL at a high concentration. The upregulated expression of prosurvival molecules in turn inhibited target cell destruction by virus-specific CD8+ T cells. More interestingly, IL-6 and IL-17 synergistically promoted the expression of these prosurvival molecules, preventing cellular apoptosis at a much lower (<5-fold) concentration. The signals involved in the synergy appear to include the activation of both STAT3 and NF-κB via distinct cytokine-dependent pathways. Thus, the excessive IL-6 promotes the generation of Th17 cells, and the resulting IL-6 and IL-17 synergistically promote viral persistence by protecting virus-infected cells from apoptosis and CD8+ T cell-mediated target destruction. These results suggest that blocking both IL-6 and IL-17 functions are important considerations for therapies of chronic viral diseases, autoimmune diseases, and cancers.
IMPORTANCE This study indicates that an excessive level of IL-6 cytokine produced following viral infection promotes the development of IL-17-producing pathogenic helper T cells. We demonstrate here for the first time that IL-6 together with IL-17 synergistically enhances the expression of survival molecules to hinder critical host defense mechanisms removing virus-infected cells. This finding has an important implication in controlling not only chronic viral infections but also autoimmune diseases and cancers, which are associated with prolonged cell survival.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4135960/
On how not just IL-17 or VEGF but IL-6 itself is just as effective at promoting angiogenesis (blood vessel growth) in tumors, and the effect is seen within days of exposure. = Implications for those with microtumors or in remission.
VEGF:
Vascular endothelial growth factor, originally known as vascular permeability factor, is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. Wikipedia
Pericyte:
Pericytes (previously known as Rouget cells) are multi-functional mural cells of the microcirculation that wrap around the endothelial cells that line the capillaries throughout the body. Wikipedia
Interleukin-6 stimulates defective angiogenesis (2016)
Abstract
The cytokine interleukin-6 (IL-6) has a number of tumor-promoting activities in human and experimental cancers, but its potential as an angiogenic agent has not been fully investigated. Here we show that IL-6 can directly induce vessel sprouting in the ex vivo aortic ring model, as well as endothelial cell proliferation and migration, with similar potency to VEGF. However, IL-6-stimulated aortic ring vessel sprouts had defective pericyte coverage compared to VEGF-stimulated vessels. The mechanism of IL-6 action on pericytes involved stimulation of the Notch ligand Jagged1 as well as Angiopoietin2 (Ang2). When peritoneal xenografts of ovarian cancer were treated with an anti-IL-6 antibody, pericyte coverage of vessels was restored. In addition, in human ovarian cancer biopsies there was an association between levels of IL-6mRNA, Jagged1 and Ang2. Our findings have implications for the use of cancer therapies that target VEGF or IL-6 and for understanding abnormal angiogenesis in cancers, chronic inflammatory disease and stroke.
Introduction
Interleukin-6, IL-6, is a major tumor-promoting cytokine produced by both malignant and host cells in the tumor microenvironment 1. It is also a downstream product of oncogenic mutations, e.g., ras and TP53 2,3. Typically via its major downstream signal transducer STAT3, IL-6 has both local and systemic pro-tumor actions in experimental and human cancers. In the tumor microenvironment, these include stimulation of malignant cell growth and survival 4, promotion of invasion and metastasis 5, modulation of tumor-promoting T cell subtypes, involvement in autocrine tumor cell cytokine networks 6, and regulation of the myeloid cell infiltrate 7. Systemic effects of excess IL-6 production include induction of acute phase reactants and involvement in the elevated platelet count (paraneoplastic thrombocytosis) 8 that is a complication of several common human cancers.
To add to this catalogue of tumor-promoting actions, there are reports that IL-6 stimulates angiogenesis in the tumor microenvironment 9 with evidence that STAT3 signaling induces HIF-1 mediated VEGF-A transcription 10. IL-6 is also reported to have direct effects on endothelial cell proliferation and migration 9,11,12 and has been implicated in resistance to anti-VEGF antibody treatment in patients 13,14. In preclinical and clinical studies we found that a therapeutic neutralizing anti-IL-6 antibody reduced systemic VEGF levels in ovarian cancer patients, and that in peritoneal ovarian cancer xenografts, blood vessels were reduced, with a concomitant inhibition of the Notch ligand Jagged 1 7.
This led us to study further the actions of IL-6 in normal and cancer angiogenesis. In this paper we present novel evidence that IL-6 directly stimulates angiogenesis, but in contrast to VEGF, IL-6 stimulated vessels have defective pericyte coverage. We show that this may be due to differential regulation of Notch ligands and Ang2 by these two mediators. Our findings have implications for the use of cancer therapies that target VEGF or IL-6.
…Rat aortic ring wells were treated with OptiMEM with 1% FBS and 10ng/ml VEGF, 10ng/ml rat IL-6 or 10nM VEGFRi (Cediranib, VEGFR2 inhibitor) and incubated at 37°C, 10% CO2. Angiogenic sprouts were counted after 7 days of culture for mouse aortic ring and after 4 days of culture for rat aortic rings.
Results
IL-6 stimulates angiogenesis in the aortic ring assay
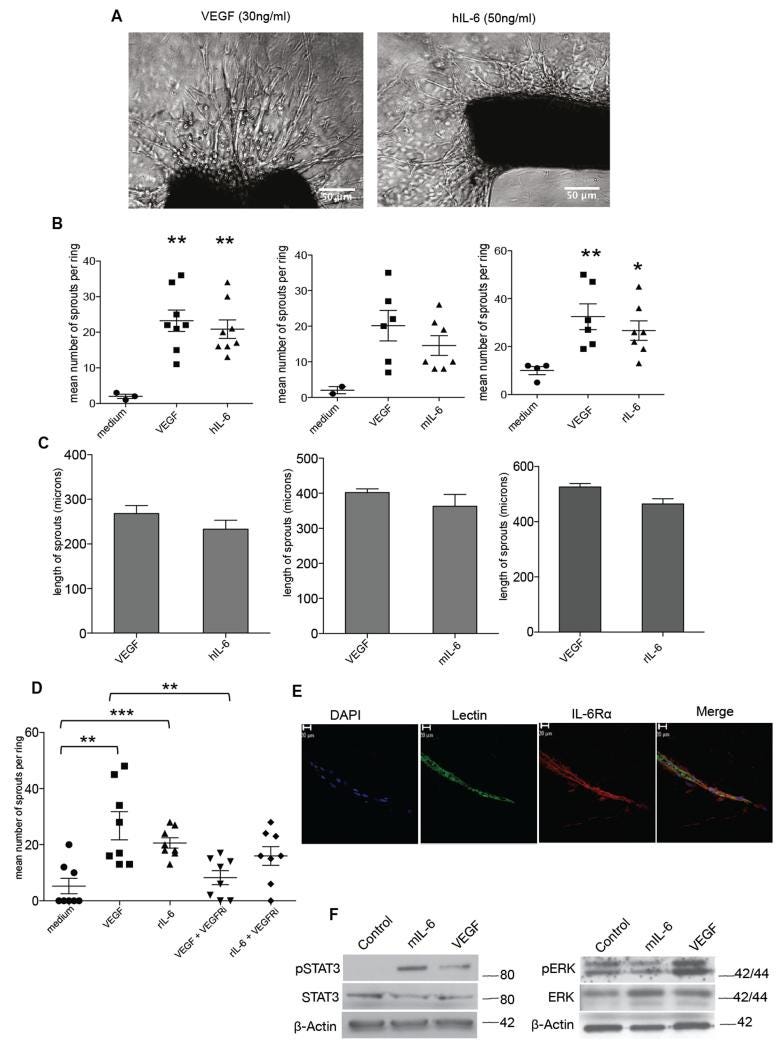
As we had found that anti-human IL-6 reduced tumor blood vessel density in human tumor xenografts 7, we tested the activity of human IL-6 in the aortic ring assay. This is an ex vivo model of angiogenesis that studies the effects of mediators on normal vessel sprouting. Optimal vessel sprouting was observed in the mouse aortic ring assay 7-10 days after treatment with 30ng/ml VEGF or 50ng/ml hIL-6 (Figure 1A). Mouse IL-6 (30ng/ml) in mouse aortas and rat IL-6 (10ng/ml) in rat aortas also stimulated vessel sprouting and there was no significant difference in the number of sprouts between the VEGF and IL-6 treatments (Figure 1B). VEGF and IL-6 treatments also gave similar results in terms of length of vessel sprouts (Figure 1C).
IL-6 stimulates angiogenesis in the aortic ring assay
A. Phase contrast images of aortic rings embedded in collagen I with the indicated concentrations of VEGF or human IL-6. Human and mouse IL-6 experiments were carried out with aortas isolated from wild-type C57BL/6 mice (8–12 weeks) and rat IL-6 experiments were carried out with aortas isolated from Wistar rats weighing 180-200g. B. Angiogenic sprouts were counted after a week in culture for mouse aortic rings or after 4 days in culture for the rat aortic rings following treatment with VEGF, hIL-6 or mIL-6 or rIL-6. Significant increases in microvessel sprouting are observed in VEGF and IL-6 treated rings (n=9 per group) compared with PBS-treated controls, however no difference in number of sprouts is observed between VEGF and IL-6 treated rings. The p value for medium vs VEGF (p<0.058) and medium vs VEGF (0.054) just failed to reach significance. Statistical analysis carried out using student T-test is shown as (**) p ≤ 0.01; (***) p ≤ 0.001. C. Length of sprouts was measured using ImageJ analysis. Mean length of sprouts (n=9 per group) shows no significant difference between the VEGF and IL-6 treated sprouts. D. 10nM of VEGFRi inhibited vessel sprouting in VEGF (10ng/ml) treated rings but not in rat IL-6 (10ng/ml) treated rings. Statistical analysis carried out using student T-test is shown as (**) p ≤ 0.01; (***) p ≤ 0.001 E. Aortic ring vessel stained for endothelial cells using BS1 lectin (green) and for IL-6Rα (red). F. Western blot analysis of protein extracted from mouse aortas treated with VEGF (30ng/ml) or mouse IL-6 (30ng/ml). Mouse IL-6 activated downstream pSTAT3 and VEGF induced downstream pERK levels.
Relevance of these findings to malignant disease
Our results so far would suggest that anti-IL-6 treatment would increase pericyte coverage of tumor blood vessels. We previously reported that when peritoneal xenografts of IGROV-1 ovarian cancer cells, that constitutively produce IL-6, were treated with anti-human IL-6 antibodies, vessel density was reduced, as was Jagged1 mRNA and protein 7. We repeated these experiments with the anti-human IL-6 neutralizing antibody MEDI5117, this time assessing pericyte coverage of the vessels. Using α-SMA (Figure 5A) and NG2 (Figure 5B) as pericyte markers, we found that anti-IL-6 treatment increased pericyte coverage…
Discussion
A role for IL-6 in pathogenic angiogenesis has been suggested in diseases such as stroke, rheumatoid arthritis and various cancers 29,1,30. In a previous publication we showed that treatment of ovarian cancer xenografts with an anti-IL-6 antibody reduced the tumor vasculature with concomitant inhibition of the NOTCH ligand Jagged1, which has been implicated in vessel sprouting 7. Collectively, the published literature suggested that IL-6 could drive abnormal angiogenesis and that the anti-IL-6 antibody had a potential as anti-angiogenic agent. Thus we investigated the direct effects of IL-6 on normal angiogenesis using endothelial in vitro and ex vivo studies, and used the findings from those studies to explore its importance in tumor angiogenesis using peritoneal models of ovarian cancer and ovarian cancer biopsies.
We found that IL-6 is as potent as VEGF in inducing vessel sprouting in the aortic ring assay and was also able to stimulate endothelial cell migration and proliferation in MLEC cells. VEGFR inhibition studies in the aortic ring model and protein analysis of downstream VEGF and IL-6 signaling indicate that the angiogenic effects observed with IL-6 may not depend on VEGF in endothelial cells. The effects on IL-6 on malignant cells may be different because of the complex autocrine signaling networks generated in cancer cells. Preliminary experiments on malignant cell lines showed that, in contrast to endothelial cells, IL-6 can stimulate VEGF signaling and vice versa. Additionally, in the RNAseq experiments of Figure 6, we found that there was a positive association between IL-6 and VEGF mRNA levels, but this would be expected when studying isolates from a complex multi-cellular tumor microenvironment. Hence we suspect that IL-6 may have different effects on malignant cells and endothelial cells.
We found that the angiogenesis stimulated by IL-6 leads to formation of vasculature with defective pericyte coverage, and that anti-IL-6 treatment of the peritoneal ovarian cancer xenografts leads to restoration of pericytes on the blood vessels. Investigating the mechanism of action of IL-6 and VEGF in inducing this different phenotype of vessel maturation has shown roles for Notch ligands and Ang2.
This is, to our knowledge, the first study showing that IL-6 can induce a type of vessel sprouting with abnormal pericyte coverage compared to VEGF. These observations have clinical implications for malignant and other diseases, especially as studies in various cancers suggest that pericyte depletion leads to increased metastasis 22,31,32. The ability of anti-IL-6 treatment to improve pericyte coverage of vessels in xenograft models suggests that in the tumor microenvironment, defective pericyte coverage may be due to the action of IL-6 in those tumors with high levels of this cytokine.
The regulation of Ang2 by IL-6 is another interesting finding as Ang2 expression has been shown to correlate with lymph node metastasis in various cancers 33, 34, 35. Moreover, the angiopoietin inhibitor Trebananib increased progression-free survival in patients with recurrent ovarian cancer 36.
As STAT3 signaling is implicated in the treatment failure of various anti-angiogenic agents 13,37,38,39, combinations of IL-6 and angiogenesis antagonists may be worthy of further study.
Full paper:







good work. i'm going back and reading every single entry in this series of yours, i'll even pay money. signing up to too many substacks can really crowd the ole inbox!