Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #22: New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation
+ hepatic & haemoglobin toxicity
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background
Evidence of reverse transcription of vaccinal mRNA into nuclear DNA was explored previously: “Our results indicate a fast up-take of BNT162b2 into human liver cell line Huh7, leading to changes in LINE-1 expression and distribution.” In other words, it stimulates production of LINE-1:
Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line
https://doorlesscarp953.substack.com/p/intracellular-reverse-transcription?utm_source=url
So let's go into more detail about LINE-1. Over to Wiki:
“LINE1 (also L1 and LINE-1) are class I transposable elements in the DNA of some organisms and belong to the group of long interspersed nuclear elements (LINEs). L1 comprise approximately 17% of the human genome.[1] The majority of L1 in the human genome are inactive; however, about 80-100 have retained the ability to retrotranspose, with considerable variation between individuals.[2][3][4] These active L1s can interrupt the genome through insertions, deletions, rearrangements, and copy number variations (CNV).[5] L1 activity has contributed to the instability and evolution of genomes, and is tightly regulated in the germline by DNA methylation, histone modifications, and piRNA.[6] L1s can further impact genome variation by mispairing and unequal crossing-over during meiosis due to its repetitive DNA sequences.”
https://en.m.wikipedia.org/wiki/LINE1
Pathology
Why might inducing expression of LINE-1 be pathogenic, adding to an already long list of potential pathways to harm?
New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation (2020)
Long interspersed nuclear element-1 (LINE-1) retrotransposition is a major hallmark of cancer accompanied by global chromosomal instability, genomic instability, and genetic heterogeneity and has become one indicator for the occurrence, development, and poor prognosis of many diseases. LINE-1 also modulates the immune system and affects the immune microenvironment in a variety of ways. Aberrant expression of LINE-1 retrotransposon can provide strong stimuli for an innate immune response, activate the immune system, and induce autoimmunity and inflammation. Therefore, inhibition the activity of LINE-1 has become a potential treatment strategy for various diseases. In this review, we discussed the components and regulatory mechanisms involved with LINE-1, its correlations with disease and immunity, and multiple inhibitors of LINE-1, providing a new understanding of LINE-1.
Line-1 and Disease
LINE-1 and Cancer
When LINE-1 retrotransposition is out of control, it can lead to diseases. More than 1,000 articles focusing on LINE-1 and cancer are available in the PubMed archive (Rodic, 2018).
LINE-1 Hypomethylation and Cancer
The global hypomethylation of the genome promotes chromosomal instability, genomic instability, and genetic heterogeneity because specific changes in DNA methylation can affect a variety of genome sequences, especially the intergenic and intronic regions of the DNA, resulting in chromosome instability and mutations (Wilson et al., 2007). LINE-1 promoter hypomethylation is a biomarker for genome-wide DNA hypomethylation, which is itself a major hallmark of cancer. Thayer et al. (1993) first demonstrated the methylation status of LINE-1 in cancer cells. Since then, LINE-1 hypomethylation of tumors has attracted widespread attention (Thayer et al., 1993). LINE-1 hypomethylation was reported to be associated with poor survival in more than 200 cases of gastric cancer, suggesting its potential as a prognostic biomarker (Shigaki et al., 2013). This phenomenon was also subsequently found in lung cancer, liver cancer, esophageal cancer, prostate cancer, and endometrial cancer (Iwagami et al., 2013; Kawano et al., 2014; Lavasanifar et al., 2019). Ogino et al. (2008) analyzed 643 colon cancer samples from two independent prospective cohorts, demonstrating a linear correlation between LINE-1 hypomethylation and aggressive tumor behavior. It has been reported that global DNA hypomethylation promotes aggressive tumor behavior by amplifying oncogenes or through abnormal expression of microRNAs (Baba et al., 2014, 2018). In esophageal cancer with high mortality and poor endoscopic screening sensitivity, LINE-1 hypomethylation can serve as a good diagnostic biomarker, thereby improving 5-year survival (Shah et al., 2013). LINE-1 hypomethylation can also be seen in some precancerous lesions. For example, in colorectal cancer, LINE-1 hypomethylation had no significant difference between adenomas and cancerous tissues, but it was significantly lower in adenomas than in normal tissues (Dawwas, 2014). Therefore, LINE-1 hypomethylation also can be used as an early biomarker for cancer.
However, there was no significant difference in the hypomethylation of LINE-1 between the blood samples of patients with leukemia and those of normal subjects (Barchitta et al., 2014).
LINE-1 Integrations and Cancer
Many tumor tissues have been found to present a high level of LINE-1 activity that can rapidly increase their copy number through the “copy-and-paste” mechanism (Dunaeva et al., 2018). LINE-1 can be used as cis-regulatory elements to regulate the expression of host genes (Wanichnopparat et al., 2013). Pan-cancer Analysis of Whole Genomes analysis of 2,954 cancer genomes from 38 histological subtypes revealed that aberrant LINE-1 integrations could lead to gene rearrangement (Rodriguez-Martin et al., 2020). LINE-1-mediated rearrangement can trigger oncogene amplification. In breast cancer, Morse and colleagues first proposed that hypomethylation activates LINE-1 which can utilize the target primed reverse transcription pathway to insert into the oncogene MYC, causing tumor-specific rearrangement and amplification (Morse et al., 1988). LINE-1 was found to induce the amplification of CCND1 oncogene in esophageal tumor by inducing the breakage–fusion–bridge cycles (Rodriguez-Martin et al., 2020). LINE-1 can mediate the deletion of tumor suppressor genes. It may be through X inactivation mechanism that LINE-1 mRNA forms facultative heterochromatin in the inactivated region or LINE-1 mRNA and pre-mRNA form RISC complex to degrade complementary mRNA (Allen et al., 2003; Aporntewan et al., 2011). In colon cancer, Miki et al. reported that LINE-1 insertion disrupts the tumor suppressor gene APC, which can lead to gene inactivation (Miki et al., 1992). In lung squamous cell carcinoma, we found that LINE-1 insertion into tumor suppressor gene FGGY promotes cell proliferation and invasion in vitro, and facilitates tumorigenesis in vivo (Zhang et al., 2019).
High Expression of ORF1 and ORF2 of LINE-1 and Cancer
The activation of LINE-1 increases the translation of ORF1 and ORF2, which are not expressed in normal somatic tissues. ORF1 encodes an RNA-binding protein, and high expression level of ORF1 was proved to be more common in most of the cancers and therefore as a diagnostic marker. In breast cancer, high expression of nuclear ORF1 is associated with distant metastasis and poor prognosis (Harris et al., 2010). In high-grade ovarian carcinoma, the ORF1 level was high and correlated to the loss of TP53 (Rodic et al., 2014). The expression of both the LINE-1 ORF1 and c-Met protein was significantly increased and peaked in early stage in ovarian cancer, suggesting that LINE-1 ORF1 significantly activates c-Met (Ko et al., 2019). In tumor cell experiments, increased mRNA and protein expression of LINE1-ORF1 can result in significant enhancement in cell proliferation and colony formation (Tang et al., 2018). It is worth noting that the expression of ORF1 was heterogeneous and had histological specificity. Cancers originating in the endometrium, such as biliary tract, esophagus, bladder, head and neck, lung, and colon, exhibit ORF1 overexpression, whereas other cancers, such as renal, liver, and cervical cancer, show little expression of ORF1 (Ardeljan et al., 2017). Recent studies have shown that an ELISA method to measure ORF1 in serum can be better in prostate cancer detection (Hosseinnejad et al., 2018).
ORF2 encodes a protein with reverse transcriptase and endonuclease activities. High expression of endonuclease induces double-strand DNA breakage that can aggravate DNA damage repair and increase genomic instability (Kines et al., 2014). Reverse transcriptase activation can promote cell proliferation and differentiation and also alter the non-coding RNA transcription spectrum and other epigenetic phenotypes, resulting in alterations in cell regulatory networks, tumor development, and other important pathological processes (Rodic and Burns, 2013; Burns, 2017; Christian et al., 2017). ORF2 can express early in the tumorigenesis process, as it can be detected by a highly specific monoclonal antibody (mAb chA1-L1) in both transitional colon mucosa and prostate intraepithelial neoplasias (De Luca et al., 2016). However, studies have shown that chA1-L1 recognizes both ORF2p and the transcriptional regulator BCLAF1, so it is not specific (Briggs et al., 2019). But recently, tumor proteome profiling studies based on mass spectrometry have shown that ORF2p was difficult to be detected, and after affinity capture of ORF1p, ORF2p has not been detected in stem cell LINE-1 proteome analysis (Vuong et al., 2019; Ardeljan et al., 2020). Therefore, the detection and application of ORF2 in tumors are still worth exploring.
LINE-1 and Metabolic Disorders
New research has shown that LINE-1 is also associated with blood sugar and lipid levels (Turcot et al., 2012). LINE-1 methylation is associated with type 2 diabetes mellitus (T2DM). Studies showed that, compared with hypermethylation, LINE-1 hypomethylation was associated with a higher risk of worsening metabolic status, independent of other classic risk factors (Martin-Nunez et al., 2014). This discovery highlights the potential role for LINE-1 DNA methylation as a predictor of the risk of T2DM or other related metabolic disorders. LINE-1 DNA methylation is associated with increased LDL cholesterol and decreased HDL cholesterol levels, and these metabolic changes increase the risk of cardiovascular disease (Pearce et al., 2012). LINE-1 DNA methylation is also associated with many blood-based metabolic biomarkers. In fetal neural tissue with neural tube defects, it was found that the low methylation level of LINE-1 was associated with the significant reduction of vitamin B12 in maternal plasma, as well as lower folate levels and increased concentrations of homocysteine (Wang et al., 2010). Folic acid and other B vitamins play an important role in the biosynthesis of new purines and pyrimidines. Therefore, the methylation status of LINE-1 can be a predictor of some metabolic diseases. Current studies have shown that LINE-1 can also regulate metabolism by inserting metabolic genes. It was reported that LINE-1 insertions in the FGGY gene can upregulate cytochrome P450, arachidonic acid metabolism, and glycerolipid metabolism. These metabolic disorders can lead to the occurrence of a variety of diseases and poor prognosis (Zhang et al., 2019).
LINE-1 and Neurological Disorders
LINE-1 can affect the developing brain at different stages of health and disease (Suarez et al., 2018). Ataxia telangiectasia (AT) is a progressive neurodegenerative disease caused by ataxia telangiectasia mutated (ATM) gene mutation. In 2011, researchers found that in nasopharyngeal carcinomas with ATM deficiency, LINE-1 retrotransposition increased, and ORF2 copy number increased in AT neurons, thus verifying the correlation between LINE-1 retrotransposition and ATM deficiency (Coufal et al., 2011). High expression of LINE-1 was found in Rett syndrome caused by mutation of methyl CpG binding protein 2 (MeCP2) in the X-linked gene, which was caused by the inclusion of LINE-1 5′-UTR sequence in the MeCP2 target, leading to methylation-dependent repression (Muotri et al., 2010). LINE-1 is involved in the aging process. In patients with frontotemporal lobe degeneration, LINE-1 transcripts were found to be elevated (Li et al., 2012). LINE-1 hypomethylation has been observed in most psychiatric studies. Increased copy numbers of LINE-1 as a result of LINE-1 hypomethylation were also found in patients with schizophrenia, bipolar disorder, and major depressive disorder (Liu et al., 2016; Li et al., 2018). The link between LINE-1 methylation levels and Alzheimer’s disease is still being studied.
LINE-1 and Genetic Disorders
LINE-1 is reported to be related to chromosome disorders. The first observation of LINE-1 insertion was in 1988, when Kazazian et al. observed a new exon of F8 LINE-1 insertion in the X-linked gene, which is a gene encoding coagulation factor VIII in a patient with hemophilia A (Kazazian et al., 1988). Then, a LINE-1 insertion was found in the CHM gene of a patient diagnosed with choroideremia. The reverse integration of a LINE-1 element into exon 6 resulted in aberrant splicing of the CHM mRNA (van den Hurk et al., 2003). Furthermore, LINE-1 can also promote mobilization of other RNAs in trans, Alu, and SVA, which can be trans-mobilized, leading to gene insertions (Kemp and Longworth, 2015). Retrotransposon insertions were found to account for up to 0.4% of all NF1 mutations (Wimmer et al., 2011). Neurofibromatosis type I is an autosomal dominant disorder caused by NF1 gene mutations (Messiaen et al., 2011). Alu insertion is located 44 bp upstream of NF1 exon 41, causing the exon 41 to skip and change the open reading frame (Payer and Burns, 2019). Only two cases were thought to be a result of independent SVA insertion in SUZ12P accompanied by 867-kb and 1-Mb deletions that encompassed the NF1 gene (Vogt et al., 2014). In autosomal recessive genetic disease, such as Fanconi anemia caused by SLX4FANCP deficiency and Aicardi–Goutieres syndrome (AGS) of three-prime repair exonuclease 1 mutations, LINE-1 expression was upregulated and pro-inflammatory cytokines were produced through the cGAS–STING pathway (Brégnard et al., 2016; Suarez et al., 2018).
Line-1 and Immune Regulation of Disease
LINE-1 and Autoimmune Disease
Hypomethylated and highly expressed LINE-1 has been found in autoimmune diseases such as systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), and psoriasis (Schulz et al., 2006; Yooyongsatit et al., 2015; Mavragani et al., 2016). LINE-1 RNA is characterized by viral RNA and exists as RNP particles, which can be recognized by RNA sensors and activate innate immune responses (Mavragani et al., 2016). Cell studies demonstrated that LINE-1 activates the production of IFNβ by RNA pathway (Zhao et al., 2018). When LINE-1 retrotransposition was inhibited by RT inhibitors, significant reductions were observed in IFNα, IFNβ, and IFNγ mRNA levels (Brégnard et al., 2016). LINE-1 transcripts and p40 protein (a 40−kDa RNA binding protein) that LINE-1 encodes have been detected in SLE and SS patients. It has been demonstrated that LINE-1 can induce the production of IFN-I in vitro by TLR-dependent and TLR-independent pathways (Mavragani et al., 2016). In MRL autoimmune lymphoproliferative syndrome, LINE-1 ORF2 encoding an RT and its products are associated with an MHC class I molecule on the cell membrane (Benihoud et al., 2002). In Fanconi anemia and AGS, LINE-1 was found to be associated with the activation of the autoimmune system. LINE-1 also regulates immunity by acting as a cis-regulatory element through the mechanism of LINE-1 mRNA and pre-mRNA forming RISC complex to degrade the complementary mRNA (Wanichnopparat et al., 2013).
LINE-1 and Tumor Immunity
In 112 TCGA cancer samples, the scientists measured the transcriptional activity of 1789 pathways and found that 49 of 176 immune pathways were significantly negatively correlated with LINE-1 (Jung et al., 2018). LINE-1 is inversely correlated with the expression of immunologic response genes. Less LINE-1 activity was found in tumors with high immune activity. In esophageal cancer tissues, scientists found that the LINE-1 methylation level in tumors was significantly positively associated with the peritumoral lymphocytic reaction (Kosumi et al., 2019). The activities of regulatory T cells and PD1 signaling as reported in cancer immune evasion and chronic inflammatory conditions also have negative correlations with LINE-1. It is reported that the negative correlation between LINE-1 and immune activity may be caused by the destruction of LINE-1 inhibition, but the specific mechanism is still unclear. LINE-1 may also mediate immune tolerance, which may change from immune stimulation mode to immunosuppression mode through continuous IFN signaling or directly affect lymphocyte signaling.
LINE-1 and Metabolism-Induced Immunity
LINE-1 is also associated with blood sugar and lipid levels. Abnormal glucose and lipid metabolism can lead to metabolic reprogramming in tumor cells. The most classic metabolism of tumor is Warburg effect, where a large amount of glucose is absorbed to fulfill the need for proliferation and produce lactic acid (Lunt and Vander Heiden, 2011). The acidic microenvironment caused by lactic acid leads to impaired T-cell activation and proliferation, prevents NK cell activation, stabilizes HIF1α to stimulate the polarization of anti-inflammatory M2 macrophages, and inhibits the production of IFN-γ in tumor-infiltrating T cells (Husain et al., 2013; Colegio et al., 2014; Brand et al., 2016). Abnormal lipid metabolism in tumor cells also can lead to local immunosuppression in the microenvironment (Hao et al., 2019). LINE-1 can affect local immune homeostasis by inserting elements into metabolism-related genes. FGGY is known to encode a protein that phosphorylates carbohydrates and is associated with obesity and sporadic amyotrophic lateral sclerosis (Zhang et al., 2011). LINE-1 retrotransposons suppress FGGY, leading to lipid metabolism disturbance and diet-induced obesity in mice (Taylor et al., 2018). Lung squamous cell carcinoma patients with L1-FGGY+ tissue have a poor prognosis, have low levels of CD3+ T cells, and have high levels of CD68+ macrophages and CD33+ myeloid-derived cells (Zhang et al., 2019). L1-FGGY+ also regulates the abnormal transcription of cytokines related to the immunosuppressive micromilieu.
Full paper:
https://www.frontiersin.org/articles/10.3389/fcell.2020.00657/full
How can one spike protein expressing mRNA activate so many pathophysiological pathways? We can partly thank gain of function bioweapon work on the original coronavirus, and then taking the pathogenic spike payload from that, wrapping it in a cytotoxic LNP envelope which allows distribution potentially to every cell in your body at various dilutions.
Think of how exposure to ionising radiation affects some people more than others in a myriad of unpredictable ways, with bystander effects. And this is no coincidence as the ROS generation and DNA damage mechanisms bare many similarities, the same pathways, in this case LINE-1 hypomethylation:
LINE-1 in response to exposure to ionizing radiation (2017)
ABSTRACT
It is becoming increasingly recognized that Long Interspersed Nuclear Element, 1 (LINE-1), the most ubiquitous repetitive element in the mammalian genomes, plays an important role in the pathogenesis of disease and in the response to exposure to environmental stressors. Ionizing radiation is a known genotoxic stressor, but it is capable of targeting the cellular epigenome as well. Radiation-induced alterations in LINE-1 DNA methylation are the most frequently observed epigenetic effects of exposure. The extent of this aberrant DNA methylation, however, strongly depends on a number of factors, including the type and dose of radiation. Two other factors are being discussed in this commentary – the evolutionary age and type of the LINE-1 promoter, as well as the type of irradiated cell. This knowledge will further aid in elucidating the mechanisms of response to ionizing radiation exposure, as well in understanding the pathogenesis of the negative health effects associated with exposure.
KEYWORDS: biomarker, environmental stressors, epigenetics, retrotransposon
LINE-1 DNA methylation
Long Interspersed Nuclear Element, 1 (LINE-1) is the most ubiquitous transposable element in the mammalian genomes, accounting for approximately 17% of the human and 20% of the mouse genomes.1 A typical mammalian LINE-1 element contains a 5′-UTR, two open-reading frames – ORF1 and ORF2 – and a 3′-UTR. While both ORFs are AT-rich, the 5′-UTR of LINE-1 elements in mammals is enriched in GC, with an average GC content of 57.2%.2 This high density of GpC dinucleotides forms a heavily methylated CpG island in the promoter region of LINE-1. Methylation of LINE-1 DNA is considered among the primary mechanisms for its silencing and prevention of unwanted retrotransposition.3
Exposures to various environmental stressors have been shown to affect the DNA methylation status of LINE-1.4 Alterations in DNA methylation may result in the loss of the epigenetic control over LINE-1 and lead to its transcriptional reactivation and retrotransposition. The latter event may lead to disruptive insertional mutagenesis when LINE-1 (most frequently – its 5′-truncated transpositionally inactive fragment) can be introduced within the ORF of the functional gene, leading to the aberrant function of the latter. LINE-1 DNA hypomethylation and retrotransposition have been associated with genomic instability and development of numerous pathological states, including cancer.5 Even without retrotransposition, aberrant LINE-1 DNA methylation can substantially affect the tumor landscape. For instance, loss of DNA methylation in the intronic regions of MET, RAB3IP and CHRM3 proto-oncogenes within the fragments that owe to previous LINE-1 insertions leads to inadvertent activation of methylation-silenced genes, and is inversely correlated with metastasis-free survival and response to cancer therapy.6,7
LINE-1 and ionizing radiation
Ionizing radiation (IR) is a ubiquitous genotoxic stressor with recognized ability to alter the cellular epigenome. Exposure to IR often leads to the loss of global DNA methylation, which is attributed primarily to the loss of DNA methylation from repetitive elements and LINE-1 in particular (for a review, see ref. 8). This effect is mostly observed after exposures to doses of 1 Gy and above. At the same time, with the growth of interest in radiation epigenetics, a number of studies have indicated that exposure to IR, especially at doses below 1 Gy, may also result in either an absence of changes in LINE-1 DNA methylation or even in DNA hypermethylation.9–13
To a certain degree, the observed discrepancies could be explained by the utilization of different models (in vitro, in vivo), doses and methods of analysis. Furthermore, it is becoming increasingly recognized that different types of IR may differentially affect LINE-1 DNA methylation. For instance, exposure to high-linear energy transfer (LET) IR, such as protons and heavy ions predominant in the space environment, often results in DNA hypermethylation.14–16 We have previously shown that these effects originate primarily from repetitive elements, including LINE-1.16
More:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC5706599/
Most alarmingly it would indeed appear that we may have succeeded in replicating one of the worst of the pathologies via transfecting mRNA into individuals without conducting in depth long term studies.
Such genetic modification treatments must be stopped immediately pending further investigations, especially in developing children:
Exogenous Coronavirus Interacts With Endogenous Retrotransposon in Human Cells (Feb 2021)
There is an increased global outbreak of diseases caused by coronaviruses affecting respiratory tracts of birds and mammals. Recent dangerous coronaviruses are MERS-CoV, SARS-CoV, and SARS-CoV-2, causing respiratory illness and even failure of several organs. However, profound impact of coronavirus on host cells remains elusive. In this study, we analyzed transcriptome of MERS-CoV, SARS-CoV, and SARS-CoV-2 infected human lung-derived cells, and observed that infection of these coronaviruses all induced increase of retrotransposon expression with upregulation of TET genes. Upregulation of retrotransposon was also observed in SARS-CoV-2 infected human intestinal organoids. Retrotransposon upregulation may lead to increased genome instability and enhanced expression of genes with readthrough from retrotransposons. Therefore, people with higher basal level of retrotransposon such as cancer patients and aged people may have increased risk of symptomatic infection. Additionally, we show evidence supporting long-term epigenetic inheritance of retrotransposon upregulation. We also observed chimeric transcripts of retrotransposon and SARS-CoV-2 RNA for potential human genome invasion of viral fragments, with the front and the rear part of SARS-CoV-2 genome being easier to form chimeric RNA. Thus, we suggest that primers and probes for nucleic acid detection should be designed in the middle of virus genome to identify live virus with higher probability. In summary, we propose our hypothesis that coronavirus invades human cells and interacts with retrotransposon, eliciting more severe symptoms in patients with underlying diseases. In the treatment of patients with coronavirus infection, it may be necessary to pay more attention to the potential harm contributed by retrotransposon dysregulation.
…In our study, we analyzed publicly available transcriptome data of human cells infected with coronavirus MERS-CoV, SARS-CoV, and SARS-CoV-2, and observed enhanced expression of TEs including several retrotransposons, as well as inflammation, immunity, and apoptosis related genes. We further noticed potential fusion of SARS-CoV-2 RNA with retrotransposon transcripts especially LINEs and SINEs. Therefore, further examinations on genome and transcriptome of cells from patients and studying models will be valuable to evaluate potential crosstalk between coronavirus and retrotransposons.
Figure 5 Model of how coronavirus may impact retrotransposons to harm human cells. (A) Generally, entry of coronavirus into human cells enhances ten-eleven translocation (TET) activity for genome-wide DNA hypomethylation to facilitate retrotransposon enhancement. This may lead to increased fusion between viral and retrotransposon transcripts, reduced genome stability, and increased susceptibility of aged people and cancer patients, dysregulated TE-adjacent gene expression, and this influence may be inherited for a long term. Meanwhile, increased retrovirus-like particles of retrotransposons may be induced. (B–G) Endogenous retrotransposon expression and distribution of subfamilies are variable in human tissues and cells. Bar graphs indicate percentages of reads mapped to TE to reads mapped to genes in human tissues and immunocytes (B), human oocytes and early embryos (C), and normal Calu-3, MRC5 and A549 cells (D). Bar charts (E–G) demonstrate distribution of individual subfamilies of TE in tissues or cell types shown above. (H) UCSC genome browser view of an example of retrotransposon-initiated gene expression by readthrough mechanism.
More:
https://www.frontiersin.org/articles/10.3389/fcimb.2021.609160/full
And just in, huge credit to Igor for doing a BLAST analysis on the BNT162B2 sequence that was transfected into the liver cells. He does caution that these mutations maybe harmless. If not then we can expect to see a spike in cases of liver cancer…see later.
DNA Transcribed from Pfizer mRNA Vaccine Contains MUTANT gp130 Tumor Gene
Having fun BLASTing DNA Reverse Transcribed from Pfizer Vax
“The first few results are from the usual suspects such as chimeric viruses, Sars-Cov-2 sequences, etc. It is understandable why we should ignore them — the chimeric viruses are pure lab constructs of unknown significance, and Sars-Cov-2 sequences are obviously there because the vaccine encodes Sars-Cov-2 spike protein. Those are “expected matches”.
What is interesting — and I am not saying it is the only thing — is the gp130 glycoprotein gene that is 97% similar to the human gp130 glycoprotein gene. The chance of that being a random coincidence, per BLAST tool, is 0.000000000000000000000000000000000000000000000000000000000002.”
“So, we have Pfizer Covid mRNA vaccine reverse transcribe to a MUTATED gp130 gene. Remember the 97% match? The 3% non-matches, the four red dots, are the mutations of the gp130 gene.
So, without looking at these specific mutations, what happens when gp130 gene mutates in general? Nothing good comes up and you can search for it too.”
Clarifications
“The sequence of mutated gp130 genes, found in the DNA reverse transcribed from Pfizer Covid vaccine,
Does not mean that it will NECESSARILY cause cancer
The four “red dot” mutations listed, may be harmless
Does not mean that it was added to Pfizer vaccine intentionally to cause harm
Does not mean that it is even expressed as a gp130 protein, as expression of genes depends on cellular context
What it DOES mean is that we need to look at this closely.”
Full article:
https://igorchudov.substack.com/p/dna-transcribed-from-pfizer-mrna?utm_source=url
Supporting Evidence
It may be too early yet to be symptomatic enough for presentation to physicians for investigation:
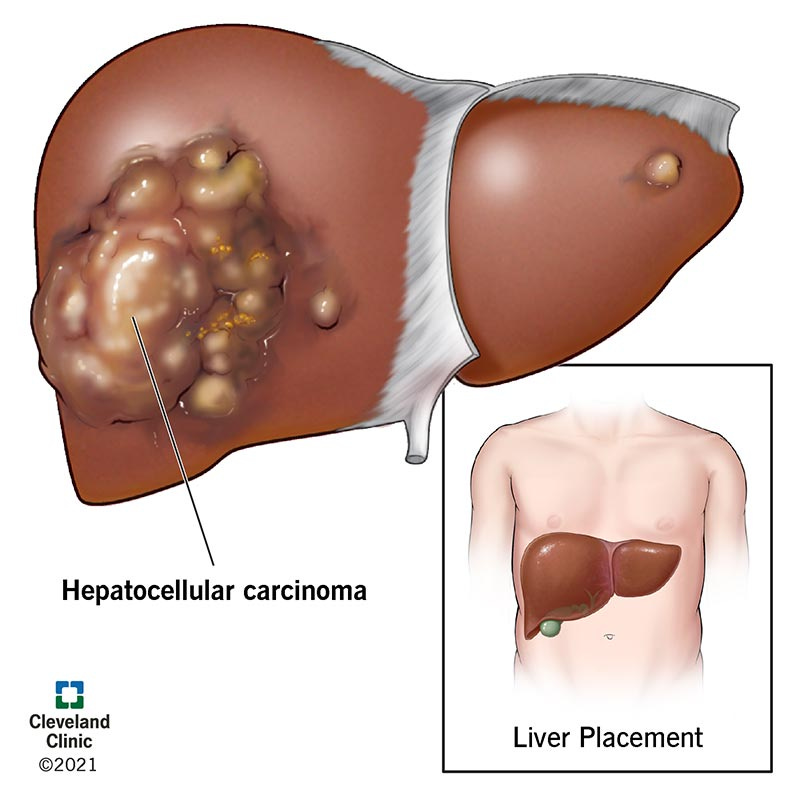
What is hepatocellular carcinoma (HCC)?
Hepatocellular carcinoma is the most common form of liver cancer. It is a serious illness that can be life-threatening. If it diagnosed early, hepatocellular carcinoma can be treated with surgery to remove the cancerous tumor or with a liver transplant. Other treatments can shrink the tumor or slow its growth and relieve your symptoms. Hepatocellular carcinoma is linked to cirrhosis of the liver and non-alcoholic fatty liver disease (NAFLD). People who have cirrhosis or NAFLD should be regularly checked for signs of hepatocellular carcinoma.
How does hepatocellular carcinoma affect my body?
Over time, hepatocellular carcinoma can cause liver failure. Before that happens, however, hepatocellular carcinoma can keep your liver from managing your body’s vital functions. Among other things, your liver:
Keeps track of your body’s nutrients, converting them into substances your body can use, storing and delivering them to your cells as needed.
Gathers toxic substances, making sure they are harmless or released from your body.
Supports healthy blood flow, producing substances that help your blood to clot and removing bacteria that cause infection.
Who does it affect?
Men ages 60 and older are more likely to develop hepatocellular carcinoma than women and younger men.
How common is hepatocellular carcinoma?
With approximately six new cases a year per 100,000 people in the United States, hepatocellular carcinoma is considered a relatively rare form of cancer. Hepatocellular carcinoma accounts for about 85%-90% of all primary liver cancers, meaning cancers that start in your liver and not another area of your body.
Is hepatocellular carcinoma the same as liver cancer?
There are several types of liver cancer. Hepatocellular carcinoma is the most common form of liver cancer.
Can hepatocellular carcinoma be cured?
Surgery to remove your tumor or a liver transplant are the best options for a cure. If surgery is not an option, there are other treatments to ease your symptoms, slow the tumor’s growth and help you to live longer.
Is hepatocellular carcinoma a fast-growing cancer?
In the beginning, hepatocellular carcinoma grows very slowly. It can take years before you notice any symptoms. Hepatocellular carcinoma growth speeds up as it progresses.
What is the life expectancy of a person with hepatocellular carcinoma?
Every case of hepatocellular carcinoma is different. Your prognosis — or expected outcome — depends on several factors. Talk to your healthcare provider about your individual situation. They’ll have specific insight into your condition and what you might expect.
SYMPTOMS AND CAUSES
What are symptoms of hepatocellular carcinoma?
There are many conditions with the same symptoms as hepatocellular carcinoma. Having one or more of these symptoms doesn’t mean you have hepatocellular carcinoma. But talk to your healthcare provider if you have these symptoms. They’ll identify and treat the condition that caused your symptoms. Potential hepatocellular symptoms include:
You’re losing weight without trying.
You feel very full after a small meal, or you don’t have much appetite.
You’re nauseous and vomiting.
You notice a fullness or knot under your ribs on your right side. This might indicate your liver is enlarged.
You notice fullness under your ribs on your left side. This might be a sign your spleen is enlarged.
You have stomach pain or pain near your right shoulder blade.
Your stomach feels swollen, as if it’s filling up with fluid.
Your skin itches.
Your eyes and skin are turning sallow or yellow. This might be a sign you have jaundice.
What causes hepatocellular carcinoma?
Cirrhosis of the liver is the most common cause of hepatocellular carcinoma. Increasingly, healthcare providers are seeing hepatocellular carcinoma cases in people who have non-alcoholic fatty liver disease (NAFLD). There are other medical conditions and activities that increase your risk of developing hepatocellular carcinoma.
If you have or have had any of these illnesses, talk to your healthcare provider about being screened for hepatocellular cancer. If you smoke, have obesity or drink a lot of alcohol, your provider can help you improve your health and decrease your risk of developing hepatocellular carcinoma.
More:
https://my.clevelandclinic.org/health/diseases/21709-hepatocellular-carcinoma-hcc
But what doctors are seeing are cases of vaccine induced liver toxicity. These may be, in part, also due to autoimmune T-lymphocyte attack (M2 liver mitochondria are known targets due to pathogenic priming) and highly cytotoxic lipid nanoparticles also accumulate in the liver for months due to a long half life:
Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases (2021)
“Using this 0.34 OD as a cutoff, we found that human anti-SARS-Cov-2 spike protein antibody reacted strongest with neurofilament protein or NFP (OD 1.98), followed by strong reactions with M2 (OD 1.52), GAD-65 (OD 1.35), and nuclear antigen or NA (OD 1.34). The reaction of this spike protein antibody with TPO and liver microsome was moderate (ODs 0.98, 1.0). With another 19 antigens the spike antibody’s reaction was weak with ODs ranging from 0.41 to 0.85 (see Figure 1).”
More:
https://www.frontiersin.org/articles/10.3389/fimmu.2020.617089/full#T1
The EMA covid-19 data leak, and what it tells us about mRNA instability
(Published 10 March 2021)
Lipid nanoparticles—where do they go and what do they do?
Conceived three decades ago, RNA based therapeutics11 have long inspired imaginations for their theoretical potential to transform cells of the body into “an on-demand drug factory.”12 But despite heavy investment by the biotech industry, bench-to-bedside translation was constantly hindered by the fragility of mRNA.
Over the years, researchers attempted to resolve intrinsic instability by encapsulating mRNA in nanocarriers made of polymers, lipids, or inorganic materials. Lipid nanoparticles (LNPs) were chosen by Moderna, Pfizer-BioNTech, CureVac, and Imperial College London for their covid-19 vaccines. This has attracted the attention of specialists in the field of pharmaceutical biotechnology, some of whom have raised concerns about further unknowns.
In a rapid response posted on bmj.com, JW Ulm, a gene therapy specialist who has published on tissue targeting of therapeutic vectors,13 raised concerns about the biodistribution of LNPs: “At present, relatively little has been reported on the tissue localisation of the LNPs used to encase the SARS-CoV-2 spike protein-encoding messenger RNA, and it is vital to have more specific information on precisely where the liposomal nanoparticles are going after injection.”14
It is an unknown that Ulm worries could have implications for vaccine safety.
Ulm told The BMJ: “Pfizer-BioNTech and Moderna did a remarkable job of rapidly scaling up manufacturing of such a novel system in swift fashion, which is genuinely a landmark technological achievement. However, pharmacokinetic studies, with independent laboratory confirmation, are essential to ascertain potential cytotoxicity and macroscopic toxicity, especially given the likelihood of booster injections over months or years, since the tissue trafficking patterns of the mRNA vaccine payload will determine which cells and tissues are killed by cytotoxic T-cells in each round.” Given the variation in LNP formulations, it is unclear how relevant previous animal experiments are to answering this question.
Regulators and manufacturers contacted by The BMJ for this article did not wish to address any of the questions raised by Ulm’s rapid response.
More:
https://www.bmj.com/content/372/bmj.n627
Interview with Dr. Vanessa Schmidt-Kruger
Hearing # 37 of German Corona Extra-Parliamentary Inquiry Committee 30 January, 2021
…So what is the distribution of the lipid nanoparticles (LNPs) in the animal trial? This is similar to that described in other publications and by other scientists. They used LNPs with mRNA, not with the spike protein but with Luciferase. Luciferase has the advantage that you can make things visible. Useful for this trial as they they gave the lipids a radioactive marker.
1.16.57
If you can use the radioactivity as a marker, you can use a technique whereby can can see the organs and whether the lipids were in them or not to see. They injected the whole muscle and watched how the lipids spread out throughout the body, and found that these lipids were in many organs after just 15 minutes. Most were at the injection site, in this case it was the muscle, but a lot in the plasma, too. Logical because it’s transported in the plasma, but also 22% in the liver. And if you inject it into the veins then 60% of the cationic lipids can be found in the liver, and 20% of the PEG lipids. They were also found in the spleen, the adrenals, and in both sexual organs. Further organs were not described. So I assume that it spread out throughout all organs. 1.18.02 It is basically absorbed everywhere where blood flows. The description focuses most on the injection site, the plasma, and the liver.
Then they looked at how the lipids were degraded. They found evidence of the cationic lipid in the plasma for 12 days, and evidence of the PEG lipid for 6 days. So they remained for quite some time. There isn’t any more information, so I don’t know whether the lipids could be evidenced for longer or not. 50% of the PEG is degraded via excretion, i.e., it is excreted from the body. It goes into our “sewer system”, as it were. The cationic lipids are exclusively degraded in the cells, only 1% was found in the stool. This means the cells take the full hit of the toxicity. Then they analysed the half life of this cationic lipid in the liver, they say it is 3 weeks. With half life at the beginning the substance always degrades faster, and then it gets less, the curve gets flatter. This half life at the outset is already 3 weeks, which is relatively long. And how long does the elimination take? One can still find 5% of the lipid in the liver after 4 - 6 weeks – that is incredibly long, and with the PEG the half life is 1 week. So it is shorter, but because a large proportion, i.e., 50%, is excreted. That is not the case with the cationic lipid.
We don’t have any other information or investigations regarding other organs, they just investigated liver, plasma, urine and stool. They should definitely have looked at other organs. Perhaps they did, but there’s nothing in the publication about that.
And then they looked at how fast the RNA is degraded. This is where the Luciferase comes into its own. The Luciferase can transform a substrate so that one sees it in colour, it fluoresces. You can detect it. But it’s not a very sensitive method. And they only injected 2 micrograms of RNA. 30 micrograms are being used for us. This means what you are seeing is probably a lot stronger in the case of the actual vaccine being used. So in the muscle where it was injected there was a peak after 6 hours. First the LNPs have to be taken up into the cell, the protein has to be formed, this Luciferase, and only then does the reaction take place. You see this after a max. of 6 hours, it is taken up by the cells extremely fast, and the protein is also expressed very fast. You can still see the protein after 9 days. There are publications – there is one from 2016 for example – where they say that one can see the Luciferase for 35 days, but that always depends on how stable the RNA is, and they didn’t do it with the spike RNA but just with the luminescence, and the spike RNA may very well have a different stability. So they didn’t investigate it properly for our vaccine, I would say.
In the liver they saw a peak after 6 hours, and after two days it was gone. This is because the liver has a very high metabolic rate.
So to summarise, both the RNA and the LNP are taken up relatively fast. And the cationic lipids remain in our bodies for a very long time. This was also interesting. There seems to have been a discussion of the EMA with BioNTech about the period that it remains in the body: how long is it in the case of human beings, they asked, because the study wasn’t done. BioNTech referred to a study from 2010, by Mamoth et al. I have not been able to find this in the publications database, and there is no list of references below the EMA report, so I don’t know whether this is true at all and whether that article exists, but they say they have used similar lipids, and when they calculate the conversion from this mouse or rat study to human beings, that cationic lipids have a half life of 20 to 30 days in human beings, and the elimination to 5%, so not really eliminated, takes 4 - 5 months. They assume 4 - 5 months, and the EMA Committee just said “That’s a long time”. 1.22.54
Dr. H: The second vaccination comes on top of that after 30 days …
VSK: Yes exactly: none of that has been investigated. Basically they haven’t conducted any kinetics with this vaccine. (1.23.12) Not on the mice either. The LNPs were the same [die Zusammenrechnung – in the calculation? Inaudible], but the RNA was different. They should really have done it with the actual vaccine. They should have marked it and then carried out the whole study again. They didn’t do that.
…VSK: So that’s what happens locally, at the site of the muscle.\ We have heard that a great deal goes to the liver, and that is a bit more serious. This leads to hepatocellular periportal vacuolisation. (1.34.30). On the day of the autopsy, where they found it, and probably a lot earlier, because it gets into the liver relatively fast and then that takes place relatively quickly. So what does that mean? Hepatocellular means relating to the cells of the liver. Periportal means the liver cells near the portal vein. That is the place where the blood enters the liver. I.e., this damage will not be caused by anything else in the rat. If the rats drank alcohol, ok, then this damage would also occur, but it would be across the entire liver. But this is something which is entering via the blood flow, and only in the proximity of this vein, and there one particularly sees the damage. And they are so damaged that they are vacuolising: that is always an indication that the liver cells are dying. I’m loathe to use the word poisons, but the liver is trying to sequester away the substance that is damaging to it; it doesn’t manage, and the the cationic lipids are the perpetrator, BioNTech admits that themselves, that’s in the report, it’s the cationic lipids. The liver tries to eliminate these cationic lipids, to metabolise them, but doesn’t manage because there are too many of them. The volume is too great. So it tries to ferret them away in an area of the cell, and that is when vacuoles arise in the cell: water streams in, it’s simply an area where they no longer do any harm. But when these vacuoles arise then the function of the liver cell is massively disrupted, many of them die, they lose their function. They self-destruct, commit apoptosis. So that’s what happens in the liver.
RF: If this is found during the autopsy then it seems to me to be a clear indication that it has been caused by the vaccination. Or can there be other causes for it? You have just said that if you drink alcohol this occurs too, but not like that. What is found there seems to be a sure sign that it was the vaccine that led to the death.
VSK: Yes, one can also investigate what there exactly is in these vacuoles. One can look and see whether the cationic lipids are there. If you have a vacuole caused by alcohol, you have a fatty liver; the alcohol is made into fat, it is stored in fat. That’s this steatosis that one hears about.\ \ RF: I just wanted to ask – independently of the severe medical malpractice that was just described previously: you can find out what the cause is via an autopsy can’t you?
VSK: Yes, you can I’d say. And you can also see that the liver is severely damaged from the blood parameters, doctors should know this really. These are standard values: an elevation in GGT, an enzyme, can have various causes. It’s definitely an indication of liver damage from medications or poison, for example. It is an indication that the liver cells are dying, that is when increased GGT is secreted.
And then we have elevated AST. This is a metabolic enzyme that goes up in liver inflammation and cardiac damage. There’s elevated alkaline phosphotase – this is produced by the bones and liver, for example, and one or two other organs; an increase points to liver and bone injury. And then we have a decrease in the ratio of albumin to globulin. This ratio is always measured to see whether the volume of protein in blood is constant. If not, it is a sign of disease: too much protein is being eliminated. If a decline is noticed, this is a sign of severe liver damage, inflammation, a digestive disorder, etc. The rat is displaying a loss of protein.
To summarise, one can say that the liver is massively damaged, and the liver cells are dying.\ \ They did say that after the autopsy, three weeks later, the liver had regenerated. But the EMA didn’t discuss what the situation might be with people who have a liver disorder, who don’t have this regenerative capacity. What of those who have hepatitis or an alcoholic liver or whatever? Who had been living an unhealthy lifestyle? If something comes ontop of that, you can very quickly get organ failure. This shouldn’t be forgotten, it needs to be discussed, but it’s being completely swept under the carpet.
So why exactly is the liver being damaged? It’s because the liver is the organ that takes up the most lipoproteins. And why does it take up the most? Because one of its functions is to break down cholesterol; I’ve explained that the nanoparticles are bound to ApoE proteins. These make their way directly back to the liver where the cholesterol is broken down, and that’s why the liver comes into contact with a huge amount of this.
RF: I just have to reiterate: how can they be vaccinating against this backdrop?
1.40.44
VSK: That’s not the whole story. You get inflammation of the perineural tissue of the iscias nerve, the strongest nerve in the body. And then inflammation in the extracapsular tissue was found, I don’t know exactly what capsules they mean, they didn’t specify that, but I assume that’s the joint capsules. What about people with arthritis for example? And then this is particularly important, very dangerous: they found a moderate to strong reduction in red blood cells and reticulocytes in the bloodcount. That accounts for the hypoxia. They are massively damaged by the lipid nanoparticles. Why is that? Because it is exactly these red blood cells that are used as a cell model for oxidative stress, they are particularly sensitive to oxidative stress. Because they carry the haemoglobin. All cells that carry oxygen are always sensitive to oxidative stress. And when the LNPs get into them and cause this massive oxidative stress, they die very quickly. So the rats would have to be suffering from hypoxia or at least they found that they had less haemoglobin because obviously that is gone when the cell is gone, and lower haematocrit. These are very clear signs of hypoxia, and I have to say this needs to be looked at very critically, because what about people with cardiac disorders for example. A cardiac muscle, for instance, if it is undersupplied with oxygen, this can very quickly turn into a heart attack. And as far as I know there is someone who had a heart attack after vaccination. I’m not saying that person died of it, but one should at least look into it.
More:
http://enformtk.u-aizu.ac.jp/howard/gcep_dr_vanessa_schmidt_krueger/
Although not directly relevant to the liver I left in the last paragraph as it relates to the microscopy slide findings of Dr Richard M Fleming and Dr Kevin McCairn: BNT162b2 causes breakdown of erythrocytes. Starts around 1h30m:
Latest SARS News & Multiple Corporate Jabberwockies Tested Against Blood
(26th Feb 2022)
https://www.mccairndojo.com/past-episodes/#boxcast:fo9lvpsdmztpcfpq4yo5@00:12:40
Drug-Induced Liver Injury After COVID-19 Vaccine
Published: July 19, 2021
Abstract
The first case of coronavirus disease 2019 (COVID-19) was reported in December 2019 in China. World Health Organization declared it a pandemic on March 11, 2020. It has caused significant morbidity and mortality worldwide. Persistent symptoms and serious complications are being reported in patients who survived COVID-19 infection, but long-term sequelae are still unknown. Several vaccines against COVID-19 have been approved for emergency use around the globe. These vaccines have excellent safety profiles with few reported side effects.
Drug-induced hepatotoxicity is mainly seen with different drugs or chemicals. There are only a few reported cases of hepatotoxicity with vaccines. We present a case of liver injury after administration of the vaccine against the COVID-19 infection.
…Given that all work-up for infection, autoimmune diseases, and any obstruction came back negative, the patient's clinical picture and laboratory findings were attributed as a liver injury due to the COVID-19 vaccine. Her liver function levels continued to trend down, and she was discharged from the hospital after a week of hospitalization. On the patient's follow-up with a gastroenterologist, abdominal pain was resolved, and her liver function test values normalized (Table 1 and Figure 1).
Discussion
Drug-induced hepatotoxicity leads to nearly 10% of all cases of acute hepatitis and more than 50% cases of liver failure [18]. It is one of the common reasons for the withdrawal of medications from the market and modification of use [19]. It can be either type A (predictable), dose-related and short latent period in days, or type B (idiosyncratic), dose-independent, unpredictable, and variable latency [20,21]. Based on population-based studies, drug-induced liver injury incidence varies between 13.9 and 19.1 cases per 100,000 people per year [22,23]. Patients have either hepatocellular injury (three times upper limit of transaminase in comparison to ALP), cholestatic injury (three times increase in ALP comparison to transaminase), or mixed pattern (where both ALP and aminotransferase are three times upper limit) [24-26]. Most patients improve spontaneously after the removal of the offending drug. If acute liver failure (ALF) is suspected, early liver transplant referral is important due to high ALF mortality [25,27].
From the spontaneous reports from patients who received Pfizer/BioNTech BNT162b2 mRNA in the UK between 9/12/20 and 26/05/2021, there are reports of 45 patients having abnormal liver function analysis and three patients having drug-induced liver injury [28].
In this case, the review of medications and history did not reveal any other reason for hepatotoxicity. She also denied the use of any over-the-counter medications or supplements. Although it is rare with vaccination, the COVID-19 vaccine is likely the cause of hepatotoxicity in our patient based on a diagnosis of exclusion. In this case, the patient had a cholestatic pattern with elevated ALP and bilirubin with mild elevation in the transaminases.
Pfizer/BioNTech BNT162b2 mRNA trial included only 0.6% (217/37,706) patients with liver disease. Among patients with liver disease, 214 were with mild liver disease and only three with moderate to severe liver disease. This patient has underlying fatty liver disease. It is unclear if that was a likely risk factor for hepatotoxicity in this case [5].
Although only a small number were included in trials for Pfizer/BioNTech BNT162b2 mRNA, Moderna mRNA-1273, and the AstraZeneca/University of Oxford ChAdOx1-nCoV-19 chimpanzee adenovirus vector vaccine, both the American Association for Study of Liver Diseases and European Association for the Study of Liver recommend vaccination against SARS-COV-2 with these highly effective and safe vaccines, given a greater risk of health consequences from SARS-COV-2 infection in these patients [29,30].
Hepatotoxicity can occur with vaccines, even though it is more common with prescription and nonprescription drugs. So, the clinician should be watchful in patients showing clinical signs and symptoms after a vaccine.
Conclusions
In summary, we presented a case of liver injury after the COVID-19 vaccine. We attributed the cause of liver injury to the COVID-19 vaccine, given no other cause in our patient after extensive work-up. There are reports of drug-induced liver injury and abnormal liver function analysis from the spontaneous reports from patients who received Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine in the UK. The purpose of this manuscript is to raise awareness of potential side effects; it should not alter the recommendation of healthcare providers regarding vaccinations.
Full paper:
https://www.cureus.com/articles/63877-drug-induced-liver-injury-after-covid-19-vaccine