Spike protein induced carcinogenesis via interactions with Snail protein and anti-cancer effects of the over-the-counter drug Glucosamine Sulphate
Updated:
3rd June ‘23 (Immunosuppressive activity & psoriasis)
In Spanish:
French:
German:
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Glucosamine, gene expression and signalling pathways
Snail family proteins and their role in carcinogenesis and metastasis
The link between Snail family proteins and glucosamine
Glucosamine and anticancer effects
Glucosamine and ocular disorders
Glucosamine, autoimmunity and psoriasis
Background
The basics of epithelial-mesenchymal transition (2009)
Abstract
The origins of the mesenchymal cells participating in tissue repair and pathological processes, notably tissue fibrosis, tumor invasiveness, and metastasis, are poorly understood. However, emerging evidence suggests that epithelial-mesenchymal transitions (EMTs) represent one important source of these cells. As we discuss here, processes similar to the EMTs associated with embryo implantation, embryogenesis, and organ development are appropriated and subverted by chronically inflamed tissues and neoplasias. The identification of the signaling pathways that lead to activation of EMT programs during these disease processes is providing new insights into the plasticity of cellular phenotypes and possible therapeutic interventions.
What is epithelial-mesenchymal transition?
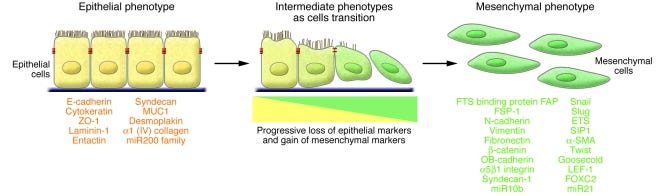
An epithelial-mesenchymal transition (EMT) is a biologic process that allows a polarized epithelial cell, which normally interacts with basement membrane via its basal surface, to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype, which includes enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and greatly increased production of ECM components (1). The completion of an EMT is signaled by the degradation of underlying basement membrane and the formation of a mesenchymal cell that can migrate away from the epithelial layer in which it originated.
A number of distinct molecular processes are engaged in order to initiate an EMT and enable it to reach completion. These include activation of transcription factors, expression of specific cell-surface proteins, reorganization and expression of cytoskeletal proteins, production of ECM-degrading enzymes, and changes in the expression of specific microRNAs. In many cases, the involved factors are also used as biomarkers to demonstrate the passage of a cell through an EMT (Figure (Figure1)
The pioneering work of Elizabeth Hay first described an “epithelial-mesenchymal transformation” using a model of chick primitive streak formation (2). In the intervening time, the term “transformation” has been replaced with “transition,” reflecting in part the reversibility of the process and the fact that it is distinct from neoplastic transformation (1). The phenotypic plasticity afforded by an EMT is revealed by the occurrence of the reverse process — a mesenchymal-epithelial transition (MET), which involves the conversion of mesenchymal cells to epithelial derivatives. Relatively little is known about this process; the best-studied example is the MET associated with kidney formation, which is driven by genes such as paired box 2 (Pax2), bone morphogenetic protein 7 (Bmp7), and Wilms tumor 1 (Wt1) (3–5).
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2689101/#__ffn_sectitle
The aggressive tumour growths & metastasis caused by spike protein upregulating Snail proteins and downregulating tumour suppressing microRNA’s 200b & c in particular were covered previously in a previous article:
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-bcf/comments
Downregulation of microRNA’s including the tumour suppressors miR-200b & c from the cited article:
Snail and the microRNA-200 Family Act in Opposition to Regulate Epithelial-to-Mesenchymal Transition and Germ Layer Fate Restriction in Differentiating ESCs (2011)
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3339404/
Further investigations into the specific molecular pathways involved referred coincidentally to caspases and matrix metalloproteinases (MMP’s) which are powerful protein destroying enzymes, responsible for breaking down cartilage and cause rheumatoid arthritis (RA), of particular note. As an autoimmune disorder it is also a known, fairly common adverse event. Ask a colleague or a friend.
The COVID-19 pandemic: an increased risk of rheumatoid arthritis (2021)
Rheumatoid arthritis (RA) is one of the most prevalent and debilitating autoimmune diseases characterized by the inflammation of the synovium [6,7]. The patients suffer from acute pain and swelling of joints such that they are unable to move, walk, hold objects and thus become dependent on others for their daily activities. Although etiology of RA is unknown yet it is linked to several genetic and environmental factors [8,9]. This disease condition is marked by presence of a dysfunctional immune system that recognizes self antigens as foreign. The preclinical phase is said to be characterized by the generation of auto antibodies and the clinical phase is attained when the body reacts to these auto-antibodies leading to inflammation [8]. The inflammatory condition is driven by the cytokines that are upregulated during the disease condition. Th1cells producing IFN-γ and Th17 cells producing IL-17 are the key regulators for disease progression [10,11]. The release of granzyme and perforin by the activated CD8+ T cells is also known to aggravate the disease condition [12]. The similarities in the cytokine profile, lymphocyte population characteristics and inflammatory mediators interestingly present an intricate relationship between COVID-19 and RA. We thus have tried to find potential reasons of co-existence of COVID-19 and RA as well as the repercussions of COVID-19 pandemic in the increase in population of RA patients (Figure 1) worldwide.
https://www.futuremedicine.com/doi/10.2217/fvl-2020-0393
I was also aware that glucosamine sulphate is a recommended long term treatment on account of the inhibition of inflammatory cytokines and MMPs. It is said to be as effective a painkiller as some corticosteroids, but without the long term risks of immune suppression and osteoporosis or cardiovascular disease. Vascular resistance is not good!
Glucosamine, gene expression and signalling pathways
Glucosamine Downregulates the IL-1β-Induced Expression of Proinflammatory Cytokine Genes in Human Synovial MH7A Cells by O-GlcNAc Modification-Dependent and -Independent Mechanisms (2016)
Abstract
Osteoarthritis (OA) is one of the major joint diseases, and the synovial inflammation is involved in the pathogenesis and progression of OA. Glucosamine (GlcN) is widely used as a dietary supplement for OA, and is expected to exert the antiinflammatory action in OA. However, the detailed mechanism for the antiinflammatory action of GlcN remains poorly understood. In this study, to elucidate the molecular mechanism involved in the GlcN-medicated regulation of synovial cell activation, we comprehensively analyzed the effect of GlcN on the gene expression using a human synovial cell line MH7A by DNA microarray. The results indicated that GlcN significantly downregulates the expression of 187 genes (≤1/1.5-fold) and upregulates the expression of 194 genes (≥1.5-fold) in IL-1β-stimulated MH7A cells. Interestingly, pathway analysis indicated that among the 10 pathways into which the GlcN-regulated genes are categorized, the 4 pathways are immune-related. Furthermore, GlcN suppressed the expression of proinflammatory cytokine genes (such as IL-6, IL-8, IL-24 and TNF-α genes). In addition, GlcN-mediated O-GlcNAc modification was involved in the downregulation of TNF-α and IL-8 genes but not IL-6 and IL-24 genes, based on the effects of alloxan, an O-GlcNAc transferase inhibitor. Thus, GlcN likely exerts an antiinflammatroy action in OA by suppressing the expression of proinflammatory cytokine genes in synovial MH7A cells by O-GlcNAc modification-dependent and -independent mechanisms.
Snail family proteins and their role in carcinogenesis and metastasis
Why induction of expression of SNAI1 proteins by spike protein, whether viral in orginin or due to repeated exposure to synthetic mRNA gene agents is not good:
Zinc finger protein SNAI1 (sometimes referred to as Snail) is a protein that in humans is encoded by the SNAI1 gene. Snail is a family of transcription factors that promote the repression of the adhesion molecule E-cadherin to regulate epithelial to mesenchymal transition (EMT) during embryonic development.
Clinical significance
Snail gene may show a role in recurrence of breast cancer by downregulating E-cadherin and inducing an epithelial to mesenchymal transition. The process of EMT is also noted as an important and noteworthy process in tumor growth, through the invasion and metastasis of tumor cells due to repression of E-cadherin adhesion molecules. Through knockout models, one study has shown the importance of SNAI1 in the growth of breast cancer cells. Knockout models showed significant reduction in cancer invasiveness and therefore can be used as a therapeutic measure for the treatment of breast cancer before chemotherapy treatment.
Epithelial-mesenchymal transition induced by SARS-CoV-2 required transcriptional upregulation of Snail (2021)
Abstract
The engagement of human angiotensin-converting enzyme 2 (hACE2) and SARS-CoV-2 spike protein facilitate virus spread. Thus far, ACE2 and TMPRSS2 expression is correlated with the epithelial-mesenchymal transition (EMT) gene signature in lung cancer. However, the mechanism for SARS-CoV-2-induced EMT has not been thoroughly explored. Here, we showed that SARS-CoV-2 induces EMT phenotypic change and stemness in breast cancer cell model and subsequently identified Snail as a modulator for this regulation. The in-depth analysis identifies the spike protein (S), but not envelope (E), nucleocapsid (N), or membrane protein (M), of SARS-CoV-2 induces EMT marker changes. Suppression of Snail expression in these cells abrogates S protein-induced invasion, migration, stemness, and lung metastasis, suggesting that Snail is required for SARS-CoV-2-mediated aggressive phenotype in cancer. This study reveals an important oncogenic role of SARS-CoV-2 in triggering breast cancer metastasis through Snail upregulation.
Keywords: SARS-CoV-2, ACE2, TMPRSS2, epithelial-mesenchymal transition, EMT, spike, Snail

Discussion
With the ongoing pandemic of COVID-19, SARS-CoV-2 not only causes severe pneumonia but exacerbates many pre-existing diseases, such as diabetes, stroke, and cancers. COVID-19 patients with cancer have a higher mortality rate compared to those who do not have cancer [28]. A few observational studies have evaluated the positive association with adverse prognosis in cancer patients compared with those without cancer [29,30]. At the same time, the inflammatory factors triggered by the virus in the tumor microenvironment might implicate reactivating dormant breast cancer cells [31]. Thus, our present study could provide a strong scientific base to support a potential risk of cancer metastasis at the early stage of COVID-19 infection. Several lines of evidence suggest that SARS-CoV-2-mediated Snail expression in breast cancer cells contributes to aggressive phenotype. First, we demonstrated that SARS-CoV-2 upregulates many oncogenic pathways, including EMT. Secondly, Ace2 expression is positively correlated with Snail expression in breast cancer cells. Thirdly, many canonical modules in NF-κB signaling were known to induce Snail expression [26]. Lastly, Snail expression is required for the SARS-CoV-2 or spike-mediated EMT changes. Although Snail contains a functional p65 binding motif on the promoter, we propose that SARS-CoV-2 induced breast cancer EMT is coordinately through canonical NF-κB signaling involved in Snail.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8167694/
Reading the following paper prompted me to cross reference glucosamine as I recognised interactions with overlapping signalling pathways.
Regarding the reference to E-cadherin:
E-cadherin is a tumor suppressor protein, and the loss of its expression in association with the epithelial mesenchymal transition (EMT) occurs frequently during tumor metastasis.
Matrix metalloproteinases (MMPs) are associated with cancer metastasis:
MMPs facilitate tumor cell invasion and metastasis by at least three distinct mechanisms. First, proteinase action removes physical barriers to invasion through degradation of ECM macromolecules such as collagens, laminins, and proteoglycans.
…Second, MMPs have the ability to modulate cell adhesion. For cells to move through the ECM, they must be able to form new cell-matrix and cell-cell attachments and break existing ones.
…Finally, MMPs may act on ECM components or other proteins to uncover hidden biologic activities. For example, the angiogenesis inhibitor angiostatin may be produced from plasminogen by MMP action and laminin-5 is specifically degraded by MMP-2 to produce a soluble chemotactic fragment. Thus MMPs play multiple key roles in facilitating the metastasis of tumor cells. Therapies designed to interfere with specific MMP actions may be useful in the control of metastatic disease.
You could write a thesis about this, let alone a dedicated Substack:
Snail-Family Proteins: Role in Carcinogenesis and Prospects for Antitumor Therapy (2021)
Abstract
The review analyzes Snail family proteins, which are transcription factors involved in the regulation of the epithelial-mesenchymal transition (EMT) of tumor cells. We describe the structure of these proteins, their post-translational modification, and the mechanisms of Snail-dependent regulation of genes. The role of Snail proteins in carcinogenesis, invasion, and metastasis is analyzed. Furthermore, we focus on EMT signaling mechanisms involving Snail proteins. Next, we dissect Snail signaling in hypoxia, a condition that complicates anticancer treatment. Finally, we offer classes of chemical compounds capable of down-regulating the transcriptional activity of Snails. Given the important role of Snail proteins in cancer biology and the potential for pharmacological inhibition, Snail family proteins may be considered promising as therapeutic targets.
Keywords
transcription factors, Snail, Slug, epithelial-mesenchymal transition, mesenchymal-epithelial transition, breast cancer
Snail/Slug-dependent transcription leads not only to the repression of E-cadherin but also to the disassembly of desmosomes and tight intercellular junctions due to repression of occludin, claudin 3, 4, and 7, and desmoplakin genes [43, 44]. Snail and Slug also increase synthesis of matrix metalloproteinases (MMPs), thereby promoting degradation of ECM components.
Snail plays an important role in the cell cycle and in cell survival. During embryonic development, Snail represses the transcription of the cyclin D2 gene and increases the expression of the p21Cip1/WAF1 gene in order to regulate early-to-late G1 phase transition. An increase in the expression of cyclin-dependent kinases CDK4/6 promotes Snail stabilization through DUB3-mediated deubiquitination [27]. In renal epithelial cells (MDCK line) stably expressing exogenous Snail, about 90% of the cells remain in the G0/G1 phase after 72-h incubation. Overexpression of Snail decreases CDK4, and phosphorylation of Rb and increases the p21Cip1/WAF1 level [52]. Thus, Snail can be used to delay or stop the transition of cells in the cell cycle.
Snail regulates cell survival through decreasing the serum concentration in the culture medium by activating the MAPK (Mek/Erk) and PI3K signaling pathways. Snail and Slug suppress the expression of several pro-apoptotic factors at the transcriptional level; in particular p53, BID, caspase 6, PUMA/BBC3, ATM, DFF40 (DNA fragmentation factor), and PTEN (phosphatase in the PI3K cascade) [52, 55–57]. Interestingly, the Snail protein can directly interact with the tumor suppressor p53, blocking its DNA-binding domain.
REGULATION OF Snail-FAMILY PROTEINS DURING EMT
EMT is a dynamic process that can be initiated by ECM proteins and secreted, soluble growth factors, such as the epidermal growth factor (EGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs), TGF-β, Wnt, Notch, tumor necrosis factor α (TNF-α), and cytokines [60, 61]. Many of these signaling molecules from the tumor cell microenvironment induce the expression of Snail-family proteins (Fig. 3).
CONCLUSION
Snail family proteins are key EMT regulators that modulate many ontogenetic and neurobiological processes. A detailed investigation of EMT in tumor cells has revealed the important role played by this process in invasion and metastasis. Snail transcription factors are specific “switches” of the epithelial, more favorable, phenotype of cells to an aggressive prometastatic one. That is why molecular events mediated by these proteins are of interest as targets for therapy of, in particular, resistant metastatic tumors. The development of pharmacological approaches to Snail inhibition is in its infancy. However, chemical classes of synthetic and natural compounds affecting the transcriptional activity and expression of Snail and initiating MEP have already been characterized. Further investigation of EMT and its regulators appears promising for a personalized therapy of tumors.
The authors are grateful to A.A. Shtil for discussion of the study and E.A. Varlamova for assistance in preparing the manuscript.
Illustrations were prepared using Servier Medical Art templates (Creative Commons Attribution 3.0 Unported License);
https://actanaturae.ru/2075-8251/article/view/11062
The link between Snail family proteins and glucosamine
MMP’s, caspases, inflammation etc. Could glucosamine be disrupting the Snail pathway in its anti-inflammatory action? Read on…and one hell of a twist in the tail.
Glucosamine-induced reduction of integrin β4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells (2013)
Abstract
“We investigated the role of glucosamine (GlcN) on the integrin β4/plectin complex and its role in the regulation of mouse embryonic stem cell (mESC) migration and proliferation. GlcN significantly decreased integrin β4 mRNA/protein expression, whereas plectin protein expression did not change. Also, decrease of integrin β4 expression caused reduction of integrin β4/plectin complex formation, and then increased cell migration. GlcN increased intracellular calcium influx and protein kinase C (PKC) phosphorylation followed by integrin β4 serine phosphorylation and reduction of the integrin β4/plectin complex. GlcN entered into the cell through glucose transporter 1 and then increased O-GlcNAc transferase (OGT) and the level of glycosylation (CTD110.6). Inhibition of OGT (OGT inhibitor; ST045849) increased integrin β4/plectin complex opposite with decreased cell migration. Moreover, GlcN increased O-GlcNAc-specificity protein 1 (Sp1) and nuclear translocated p-Sp1 stimulated calmodulin (CaM) expression, which combined with plectin. In addition, GlcN increased Akt glycosylation and glycogen synthase kinase-3β (GSK-3β) phosphorylation, and then Snail1 glycosylation. Snail small interfering ribonucleic acid (siRNA) reversed the reduction of integrin β4/plectin complex and dissociation of cell junctions (tight and adherent junction). GlcN increased cell migration, cell cycle regulatory proteins [cyclinD1, cyclin-dependent kinase 4 (CDK4), cyclinE, and CDK2], and the percentage of S phase cells, which were inhibited by a PKC inhibitor, CaM siRNA, or Snail1 siRNA. Additionally, GlcN maintained the undifferentiation status of ESCs. In conclusion, GlcN contributed to migration and proliferation of mESCs through integrin β4/plectin complex reduction via Ca²⁺/PKC, as well as the Sp1/CaM and Akt/GSK-3β/Snail1 signaling pathway.”
https://pubmed.ncbi.nlm.nih.gov/23815613/
ESCs are embryonic stem cells. Cancers can have stem cells and blood cancers are associated with uncontrolled proliferation and differentiation.
Hi-lighted in bold, is it saying what I think it is saying? Hiding in plain sight all along in my med cabinet, typically 3 x 500 mg per day for up to 3 years and cheap as chips, but note cautions at the end, discontinue if it makes a condition worse:
Glucosamine - Uses, Side Effects, And More
https://www.webmd.com/vitamins/ai/ingredientmono-807/glucosamine-sulfate
The Truth About Glucosamine and Chondroitin Sulfate
https://www.oaph.com/patient-resources/education/truth-about-glucosamine-and-chondroitin-sulfate
Glucosamine and anticancer effects
Molecular mechanisms of anticancer effects of Glucosamine (2017)
Abstract
Glucosamine is an amino sugar that is produced naturally in human body. It is an essential carbohydrate component of many cellular glycoproteins, glycolipids, and glycosaminoglycans (GAGs). This popular over-the-counter supplement is also found in the exoskeleton of crustaceans. Glucosamine and its derivatives have a long history in medicine for inflammatory conditions specially to relieve arthritis. This dietary supplement has numerous biological and pharmacological properties, including anti-inflammatory, antioxidant, anti-aging, anti-fibrotic, neuroprotective and cardioprotective activities. Many studies have shown that glucosamine has anti-cancer activity through influence on biological pathways involved in cell death, apoptosis, cell proliferation, and angiogenesis. Accordingly, this comprehensive review summarizes anti-cancer molecular mechanisms of glucosamine in details.
4. Conclusion
Cancer is the main problem of the healthcare system due to not only biological complexity of cancer but also high cost of medical treatment and serious side effects of chemotherapy. Therefore, developing of therapeutic agents with lower side effects and more cost effective is clear. Experimental evidences indicate that glucosamine could be effective against a wide range of cancer cells. Glucosamine exerts anti-cancer activity through targeting multiple signaling pathways and genes responsible in cancer development (Fig. 1). Generally, the main functions of GlcN are changing in the free nucleotide contents, disruption of cellular membrane systems, suppression of ubiquitin proteasome and STAT-3 signaling pathway, inhibition of HIF-1, antioxidant and anti-inflammatory activities. Altogether, glucosamine can be a novel and prospective anti-cancer candidate due to its encouraging results in inhibition of molecular pathways deregulated in broad spectrum cancer cells and no toxic effects in normal cells as well. Nevertheless, the lack of strong clinical studies is the main hindrance of its commercial development.
Full version:
https://www.sciencedirect.com/science/article/pii/S0753332217328111?via%3Dihub
The paper does caution that once the tumor has formed do not expect it to magically shrink away, the damage is done by then, although metastasis may be inhibited.
Glucosamine is largely about regulating the epithelial-mesenchymal transition in the first place so that you don't get yourself in that position.
Why won't Pharma sponsor large scale clinical studies of a generic you can buy for pennies from the vitamin shelf in every supermarket? The question kind of answers itself.
TGF: Transforming growth factor beta.
https://twitter.com/TheoNNN22/status/1488852452107595777?s=19
Glucosamine and ocular disorders
EMT is involved with cataract formation and detached retinas. Glucosamine may act as a prophylactic:
Natural therapies for ocular disorders, part two: cataracts and glaucoma (2001)
Abstract
Pathophysiological mechanisms of cataract formation include deficient glutathione levels contributing to a faulty antioxidant defense system within the lens of the eye. Nutrients to increase glutathione levels and activity include lipoic acid, vitamins E and C, and selenium. Cataract patients also tend to be deficient in vitamin A and the carotenes, lutein and zeaxanthin. The B vitamin riboflavin appears to play an essential role as a precursor to flavin adenine dinucleotide (FAD), a co-factor for glutathione reductase activity. Other nutrients and botanicals, which may benefit cataract patients or help prevent cataracts, include pantethine, folic acid, melatonin, and bilberry. Diabetic cataracts are caused by an elevation of polyols within the lens of the eye catalyzed by the enzyme aldose reductase. Flavonoids, particularly quercetin and its derivatives, are potent inhibitors of aldose reductase. Glaucoma is characterized by increased intraocular pressure (IOP) in some but not all cases. Some patients with glaucoma have normal IOP but poor circulation, resulting in damage to the optic nerve. Faulty glycosaminoglycan (GAG) synthesis or breakdown in the trabecular meshwork associated with aqueous outflow has also been implicated. Similar to patients with cataracts, those with glaucoma typically have compromised antioxidant defense systems as well. Nutrients that can impact GAGs such as vitamin C and glucosamine sulfate may hold promise for glaucoma treatment. Vitamin C in high doses has been found to lower IOP via its osmotic effect. Other nutrients holding some potential benefit for glaucoma include lipoic acid, vitamin B12, magnesium, and melatonin. Botanicals may offer some therapeutic potential. Ginkgo biloba increases circulation to the optic nerve; forskolin (an extract from Coleus forskohlii) has been used successfully as a topical agent to lower IOP; and intramuscular injections of Salvia miltiorrhiza have shown benefit in improving visual acuity and peripheral vision in people with glaucoma.
https://pubmed.ncbi.nlm.nih.gov/11302779/
Proliferative vitreoretinopathy (PVR), a major complication of rhegmatogenous retinal detachment (RRD), is an abnormal process whereby proliferative, contractile cellular membranes form in the vitreous and on both sides of the retina, resulting in tractional retinal detachment with fixed retinal folds. However, it is increasingly being recognised that PVR may be intraretinal also, which causes retinal shortening. Research suggests that membranes form in response to cytokines and inflammatory mediators that arise following anatomic disruption and tissue damage caused by rhegmatogenous retinal detachment (RRD) and resultant inflammation. Treatment is principally surgical and often requires multiple procedures that, in fact, yield a high rate of retinal reattachment; nevertheless, many anatomically successful eyes do not recover good visual function likely due to the long standing macular detachment.
Glucosamine inhibits epithelial-to-mesenchymal transition and migration of retinal pigment epithelium cells in culture and morphologic changes in a mouse model of proliferative vitreoretinopathy (2011)
Abstract
Purpose: To explore the effect of glucosamine (GlcN) on transforming growth factor (TGF)-β signalling and several processes involved in proliferative vitreoretinopathy (PVR).
Methods: We evaluated the surface levels of TGF-β receptor and its binding of TGF-β in ARPE-19 cells. Release of cytokines and collagen, and expression of signalling intermediates were quantified. Migration was qualitatively and quantitatively examined. The morphology of cells undergoing PVR in vitro and in a mouse PVR model was observed.
Results: Glucosamine reduced the surface levels of TGF-β receptor and the ability of ARPE-19 cells to bind TGF-β. In ARPE-19 cells, TGF-β1 plus epidermal growth factor (EGF) or TGF-β2 increased the expression of alpha-smooth muscle actin (α-SMA) and decreased the expression of zona occludens protein (ZO-1). Transforming growth factor-(β2) also caused the release of platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF) and type 1 collagen and increased the phosphorylation of SMAD2 and SMAD3. Platelet-derived growth factor and CTGF stimulated cell migration, and TGF-β2 stimulated wound closure, contraction of collagen and changes in cell morphology.
Conclusions: Treatment with GlcN counteracted all of these effects, and its administration in the mouse model reduced the morphologic appearance of PVR. Glucosamine could inhibit the TGF-β signalling pathway in retinal pigment epithelium cells and several of the downstream events associated with epithelial-mesenchymal transition and PVR.
https://pubmed.ncbi.nlm.nih.gov/21457483/
Glucosamine, autoimmunity and psoriasis
Glucosamine can suppress autoimmune reactivity via multiple mechanisms.
Glucosamine Interferes With Myelopoiesis and Enhances the Immunosuppressive Activity of Myeloid-Derived Suppressor Cells (2021)
Abstract
Glucosamine (GlcN) is the most widely consumed dietary supplement and exhibits anti-inflammatory effects. However, the influence of GlcN on immune cell generation and function is largely unclear. In this study, GlcN was delivered into mice to examine its biological function in hematopoiesis. We found that GlcN promoted the production of immature myeloid cells, known as myeloid-derived suppressor cells (MDSCs), both in vivo and in vitro. Additionally, GlcN upregulated the expression of glucose transporter 1 in hematopoietic stem and progenitor cells (HSPCs), influenced HSPC functions, and downregulated key genes involved in myelopoiesis. Furthermore, GlcN increased the expression of arginase 1 and inducible nitric oxide synthase to produce high levels of reactive oxygen species, which was regulated by the STAT3 and ERK1/2 pathways, to increase the immunosuppressive ability of MDSCs. We revealed a novel role for GlcN in myelopoiesis and MDSC activity involving a potential link between GlcN and immune system, as well as the new therapeutic benefit.
Keywords: d-Glucosamine hydrochloride (PubChem CID: 91431); hematopoietic stem cell (HSC); immunosuppression; myeloid-derived suppressive cell; myelopoiesis.
https://pubmed.ncbi.nlm.nih.gov/34901113/
Interleukin-2 receptor alpha chain (also called TAC antigen, P55 and mainly CD25) is a protein involved in assembly of high-affinity Interleukin-2 receptor, consisting of alpha (IL2RA), beta (IL2RB) and the common gamma chain (IL2RG). As the name indicates, this receptor interacts with pleiotropic cytokine called Interleukin-2, which effect is mainly important for immune homeostasis.

Interleukin-2 (IL-2) is an interleukin, a type of cytokine signaling molecule in the immune system. It is a 15.5–16 kDa protein that regulates the activities of white blood cells (leukocytes, often lymphocytes) that are responsible for immunity. IL-2 is part of the body's natural response to microbial infection, and in discriminating between foreign ("non-self") and "self". IL-2 mediates its effects by binding to IL-2 receptors, which are expressed by lymphocytes. The major sources of IL-2 are activated CD4+ T cells and activated CD8+ T cells. Put shortly the function of IL-2 is to stimulate the growth of helper, cytotoxic and regulatory T cells.
Th1/Th2 model
Proliferating helper T cells that develop into effector T cells differentiate into two major subtypes of cells known as Th1 and Th2 cells (also known as Type 1 and Type 2 helper T cells, respectively).
Th1 helper cells lead to an increased cell-mediated response (primarily by macrophages and cytotoxic T cells), typically against intracellular bacteria and protozoa. They are triggered by the polarising cytokine IL-12 and their effector cytokines are IFN-γ and IL-2. The main effector cells of Th1 immunity are macrophages as well as CD8 T cells, IgG B cells, and IFN-γ CD4 T cells. The key Th1 transcription factors are STAT4 and T-bet. IFN-γ secreted by CD4 T cells can activate macrophages to phagocytose and digest intracellular bacteria and protozoa. In addition, IFN-γ can activate iNOS (inducible nitric oxide synthase) to produce nitric oxide free radicals to directly kill intracellular bacteria and protozoa. Th1 overactivation against autoantigens will cause Type IV or delayed-type hypersensitivity reaction. Tuberculin reaction and Type 1 diabetes belong to this category of autoimmunity.
Th2 helper cells lead to a humoral immune response, typically against extracellular parasites such as helminths. They are triggered by the polarising cytokines IL-4 and IL-2, and their effector cytokines are IL-4, IL-5, IL-9, IL-10, IL-13 and IL-25. The main effector cells are eosinophils, basophils, and mast cells as well as B cells, and IL-4/IL-5 CD4 T cells. The key Th2 transcription factors are STAT6 and GATA3. IL-4 is the positive feedback cytokine for Th2 cells differentiation. Besides, IL-4 stimulates B-cells to produce IgE antibodies, which in turn stimulate mast cells to release histamine, serotonin, and leukotriene to cause broncho-constriction, intestinal peristalsis, gastric fluid acidification to expel helminths. IL-5 from CD4 T cells will activate eosinophils to attack helminths. IL-10 suppresses Th1 cells differentiation and function of dendritic cells. Th2 overactivation against antigen will cause Type I hypersensitivity which is an allergic reaction mediated by IgE. Allergic rhinitis, atopic dermatitis, and asthma belong to this category of overactivation. In addition to expressing different cytokines, Th2 cells also differ from Th1 cells in their cell surface glycans (oligosaccharides), which makes them less susceptible to some inducers of cell death.
The Th1 response is more associated with autoimmune disorders:
The Type 1 cytokine IFNγ increases the production of interleukin 12 by dendritic cells and macrophages, and via positive feedback, IL-12 stimulates the production of IFNγ in helper T cells, thereby promoting the Th1 profile. IFNγ also inhibits the production of cytokines such as interleukin 4, an important cytokine associated with the Type 2 response, and thus it also acts to preserve its own response.
The Type 2 response promotes its own profile using two different cytokines. Interleukin 4 acts on helper T cells to promote the production of Th2 cytokines (including itself; it is auto-regulatory), while interleukin 10 (IL-10) inhibits a variety of cytokines including interleukin 2 and IFNγ in helper T cells and IL-12 in dendritic cells and macrophages. The combined action of these two cytokines suggests that once the T cell has decided to produce these cytokines, that decision is preserved (and also encourages other T cells to do the same).
https://en.wikipedia.org/wiki/T_helper_cell#Th1/Th2_model
Th2 is more associated with allergic type responses:
Atopy is the tendency to produce an exaggerated immunoglobulin E (IgE) immune response to otherwise harmless substances in the environment. Allergic diseases are clinical manifestations of such inappropriate, atopic responses.
Th1-type cytokines tend to produce the proinflammatory responses responsible for killing intracellular parasites and for perpetuating autoimmune responses. Interferon gamma is the main Th1 cytokine. Excessive proinflammatory responses can lead to uncontrolled tissue damage, so there needs to be a mechanism to counteract this. The Th2-type cytokines include interleukins 4, 5, and 13, which are associated with the promotion of IgE and eosinophilic responses in atopy, and also interleukin-10, which has more of an anti-inflammatory response. In excess, Th2 responses will counteract the Th1 mediated microbicidal action. The optimal scenario would therefore seem to be that humans should produce a well balanced Th1 and Th2 response, suited to the immune challenge.
Glucosamine can suppress pro-inflammatory cytokines linked to both Th1 and Th2:
Glucosamine Modulates T Cell Differentiation through Down-regulating N-Linked Glycosylation of CD25 (2015)
Abstract
Glucosamine has immunomodulatory effects on autoimmune diseases. However, the mechanism(s) through which glucosamine modulates different T cell subsets and diseases remain unclear. We demonstrate that glucosamine impedes Th1, Th2, and iTreg but promotes Th17 differentiation through down-regulating N-linked glycosylation of CD25 and subsequently inhibiting its downstream Stat5 signaling in a dose-dependent manner. The effect of glucosamine on T helper cell differentiation was similar to that induced by anti-IL-2 treatment, further supporting an IL-2 signaling-dependent modulation. Interestingly, excess glucose rescued this glucosamine-mediated regulation, suggesting a functional competition between glucose and glucosamine. High-dose glucosamine significantly decreased Glut1 N-glycosylation in Th1-polarized cells. This finding suggests that both down-regulated IL-2 signaling and Glut1-dependent glycolytic metabolism contribute to the inhibition of Th1 differentiation by glucosamine. Finally, glucosamine treatment inhibited Th1 cells in vivo, prolonged the survival of islet grafts in diabetic recipients, and exacerbated the severity of EAE. Taken together, our results indicate that glucosamine interferes with N-glycosylation of CD25, and thereby attenuates IL-2 downstream signaling. These effects suggest that glucosamine may be an important modulator of T cell differentiation and immune homeostasis.
Keywords: autoimmune disease, cytokine, diabetes, multiple sclerosis, N-linked glycosylation, T helper cells, CD25, Glucosamine
Take both omega 3 rich fish oils and glucosamine to slow down the growth rate of the keratinocytes and to inhibit the proinflammatory & autoimmune pathways:
Glucosamine for psoriasis? (1997)
Abstract
Amphiregulin and transforming growth factor-alpha, agonists for the epidermal growth factor receptor, are the major autocrine growth factors for cultured keratinocytes, and their substantial overexpression in psoriatic lesions suggests that they are crucial to the basal hyperplasia that characterizes psoriasis. Amphiregulin binds to heparin and related highly sulfated polysaccharides, and exogenous heparin blocks its growth factor activity, rationalizing previous reports that psoriasis responds to heparin therapy. Differentiating keratinocytes produce increased amounts of protein-bound as well as free-chain heparan sulfates, which may function physiologically as amphiregulin antagonists. By promoting keratinocyte synthesis of these heparan sulfates, glucosamine administration may inhibit amphiregulin function and thus provide therapeutic benefit in psoriasis. Concurrent ingestion of fish oil, by impeding the excessive activation of protein kinase C, may decrease keratinocyte production of amphiregulin and other autocrine growth factors, thus complementing the postulated benefits of glucosamine.
These speculations were prompted by an anecdotal report of substantial resolution of long-standing psoriasis in a woman who was using a relatively high dose of glucosamine hydrochloride (1.5 g t.i.d.) to treat spinal osteoarthritis (Evans E, personal communication). Subsequent to receiving this report, I obtained a copy of a US patent, authored by Burton and Freeman, disclosing the use of N-acetyl-glucosamine (another effective precursor for mucopolysaccharide production) to treat inflammatory bowel disorders. The authors offer twelve case histories of patients who had responded to such therapy; in two of the cited cases, the patients concurrently suffered from psoriasis, which resolved during the successful treatment of their bowel disorders with N-acetylglucosamine (29). In light of these intriguing anecdotal observations and the theoretical considerations cited above, an informal pilot trial to evaluate glucosamine supplementation in psoriasis is now being planned.
Complementary benefits of fish oil
If glucosamine proves to have some utility in the management of psoriasis, perhaps it will work in a complementary or synergistic fashion with fish oil. The use of omega-3-rich fish oil to treat psoriasis has been prompted by a report that psoriasis was quite uncommon among Eskimos following a traditional lifestyle (30), as well as by evidence that eicosapentaenoic acid (EPA) can inhibit the production and activity of leukotrienes (31,32), believed to play a role in the inflammatory aspects of psoriasis. (Increased production of LTB4, a potent chemotactic agent which has mitogenic activity for keratinocytes (33,34), has been documented in psoriatic epidermis (35), and excellent clinical response to the 5-1ipoxygenase inhibitor benoxaprofen (36) - unfortunately too toxic for standard use - suggests an important role for leukotrienes in this disorder.) Although an initial clinical report, in which very large amounts of fish oil were ingested in the context of a low-fat diet, was quite encouraging (37), subsequent studies involving less intensive regimens have generally seen more limited responses (38-40). For optimal benefit, it may be necessary to reproduce the relative omega-3 content of an Eskimo diet. This may be feasible if a very-low-fat diet is consumed (10-15% fat calories) in conjunction with an ample supplemental intake of a fish oil preparation highly enriched in EPA. While fish oil as a monotherapy for psoriasis seems unlikely to achieve a fully adequate clinical response in the majority of patients, its use as a complement to other therapies has promise. Complementarity or synergism of glucosamine and fish oil in the management of psoriasis, can be predicted on the basis of the following considerations:
Overactivity of membrane phospholipases - as indicated by increased epidermal levels of diacylglycerol (DAG) and free arachidonic acid - appears likely to be key to the pathophysiology of psoriasis (21,41,42). Receptor-mediated stimulation of phospholipase C activity may play a triggering role in this regard (41), as the resulting increase in levels of DAG and free intracellular calcium (and thus of PKC activity) can be expected to activate both phospholipase D (43) and phospholipase A2 (44). The former activity should maintain increased DAG levels despite the anticipated PKC-mediated downregulation of phospholipase C; the latter activity is responsible for the increased liberation of free arachidonic acid that promotes the observed excessive production of LTB4 and other eicosanoids. The persistent elevation of DAG provides a continual activating stimulus to PKC; increased activation of PKC in psoriasis is suggested by characteristic patterns of protein induction (21,22), as well as by reduced epidermal levels of PKC protein (45) (presumably indicative of proteolytic down-regulation of the activated enzyme). PKC has been shown to have an inductive effect on TGF-ot (8); since PKC induces AR production in mammary epithelium (46), it may have an analogous effect in keratinocytes. PKC also promotes keratinocyte production of interleukin-8 (47), which also has growth factor activity for keratinocytes and appears to be the chief protein chemotactic factor released by psoriatic epidermis (48,49). As suggested by Pittelkow and colleagues (8), the ability of PKC to induce autocrine growth factors for keratinocytes may explain the hyperplastic effects of phorbol esters on the epidermis. Also, independent of this inductive effect, it is conceivable that PKC plays a co-mitogenic role in abetting the mitogenic effects of the EGFR and other tyrosine kinases. The therapeutic activity of PKC inhibitors in psoriasis (50,51), suggests a key role for PKC overactivity in this syndrome, and it has frequently been noted that phorbol ester treatment of epidermis produces a histological picture analogous to psoriasis (21,23,41,45). Thus, excessive activation of PKC may be crucial to the overproduction of autocrine growth factors and chemotactic agents that engender and sustain psoriatic lesions.
As noted, activation of phospholipase C seems likely to play a triggering role in the chain of events that activates PKC and other phospholipases (41). If the effects of LTB4 on keratinocytes are similar to its effects in other tissues, LTB4 can be expected to stimulate phospholipase C-[~ activity in keratinocytes via a G protein-linked receptor (52). (Whether the EGFR activates phospholipase C-'f in keratinocytes - as it does in various other tissues - is questionable (53), although it does tend to increase PKC activity to some degree (53-55).) In a number of other tissues, membrane enrichment with omega-3 fish oils appears to down-regulate the activation of phospholipase C-~ via G protein-linked receptors (56); this suggests that fish oil may impede the ability of LTB4 (and perhaps of other cytokines and inflammatory mediators produced by leukocytes in psoriatic lesions) (57) to activate PKC and thereby stimulate induction of autocrine growth factors.
Current evidence suggests that AR is the most crucial of the autocrine growth factors produced by normal and psoriatic keratinocytes. If, as predicted above, fish oil therapy can reduce keratinocyte production of AR, while glucosamine can inhibit the activity of this growth factor, it would be reasonable to expect complementarity or synergism when these two measures are used conjointly in the treatment of psoriasis. This possibility merits clinical evaluation; obviously, a safe and effective nutritional regimen for managing psoriasis would be a most worthwhile development. It is intriguing to speculate that modulation of the activities of AR (or of other heparin-binding growth factors) by endogenously produced HS proteoglycans, may be a more general phenomenon, occurring in tissues other than just the epidermis. In such tissues, glucosamine administration may help to control hyperproliferative disorders and, in the long term, may reduce cancer risk. It should be noted that AR was first isolated from cultured mammary epithelium, and functions as an autocrine growth factor for this tissue (46).
https://pubmed.ncbi.nlm.nih.gov/9185133/
Footnote
Integrins were touched on earlier and are another rabbit hole to go down. Spike protein can bind with these too and possibly trigger yet another route to immunosuppression, osteoporosis or carcinogenesis by disrupting hematopoietic stem cell differentiation.
Can the SARS-CoV-2 Spike Protein Bind Integrins Independent of the RGD Sequence? (2021)
Abstract
The RGD motif in the Severe Acute Syndrome Coronavirus 2 (SARS-CoV-2) spike protein has been predicted to bind RGD-recognizing integrins. Recent studies have shown that the spike protein does, indeed, interact with αVβ3 and α5β1 integrins, both of which bind to RGD-containing ligands. However, computational studies have suggested that binding between the spike RGD motif and integrins is not favourable, even when unfolding occurs after conformational changes induced by binding to the canonical host entry receptor, angiotensin-converting enzyme 2 (ACE2). Furthermore, non-RGD-binding integrins, such as αx, have been suggested to interact with the SARS-CoV-2 spike protein. Other viral pathogens, such as rotaviruses, have been recorded to bind integrins in an RGD-independent manner to initiate host cell entry. Thus, in order to consider the potential for the SARS-CoV-2 spike protein to bind integrins independent of the RGD sequence, we investigate several factors related to the involvement of integrins in SARS-CoV-2 infection. First, we review changes in integrin expression during SARS-CoV-2 infection to identify which integrins might be of interest. Then, all known non-RGD integrin-binding motifs are collected and mapped to the spike protein receptor-binding domain and analyzed for their 3D availability. Several integrin-binding motifs are shown to exhibit high sequence similarity with solvent accessible regions of the spike receptor-binding domain. Comparisons of these motifs with other betacoronavirus spike proteins, such as SARS-CoV and RaTG13, reveal that some have recently evolved while others are more conserved throughout phylogenetically similar betacoronaviruses. Interestingly, all of the potential integrin-binding motifs, including the RGD sequence, are conserved in one of the known pangolin coronavirus strains. Of note, the most recently recorded mutations in the spike protein receptor-binding domain were found outside of the putative integrin-binding sequences, although several mutations formed inside and close to one motif, in particular, may potentially enhance binding. These data suggest that the SARS-CoV-2 spike protein may interact with integrins independent of the RGD sequence and may help further explain how SARS-CoV-2 and other viruses can evolve to bind to integrins.
https://www.frontiersin.org/articles/10.3389/fcimb.2021.765300/full
Dysregulated hematopoiesis in bone marrow marks severe COVID-19 (2021)
Abstract
Severe coronavirus disease 2019 (COVID-19) is often indicated by lymphopenia and increased myelopoiesis; however, the underlying mechanism is still unclear, especially the alteration of hematopoiesis. It is important to explore to what extent and how hematopoietic stem cells contribute to the impairment of peripheral lymphoid and myeloid compartments in COVID-19 patients. In this study, we used single-cell RNA sequencing to assess bone marrow mononuclear cells from COVID-19 patients with peripheral blood mononuclear cells as control. The results showed that the hematopoietic stem cells in these patients were mainly in the G1 phase and prone to apoptosis, with immune activation and anti-viral responses. Importantly, a significant accumulation of immature myeloid progenitors and a dramatic reduction of lymphoid progenitors in severe cases were identified, along with the up-regulation of transcription factors (such as SPI1, LMO4, ETS2, FLI1, and GATA2) that are important for the hematopoietic stem cell or multipotent progenitor to differentiate into downstream progenitors. Our results indicate a dysregulated hematopoiesis in patients with severe COVID-19.
https://www.nature.com/articles/s41421-021-00296-9
Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.










Great write up! i do read it somewhere in twitter about glucosamine months back, while searching something or other, also used glucosamine sulphate tablets for few months...still have the combination of glucosamine and MSM( without chandrotin), and i take it from time to time, for my mom (vaxed with 2 doses) she was having back pain and some leg pain issues, which disappeared after i gave her bottle ,she still takes them daily or at least 5 days a week.. ..some people knew a lot from the beginning and they actually wrote stuffs on twitter posts, we just need to dig up well and then analyse...great work, many thanks!
Wow. Excellent post. Thank you. I am subscribing to your Substack. I am a retired engineer but I was able to follow the logic and concepts. Peace.