The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Hat tip to Dr. Jon Couey, GigaohmBiological.
https://m.twitch.tv/videos/1277095742
Background
Various pathologies due to endothelial disruption can be caused by spike protein interaction, including to cardiac tissue and due to damage to the blood brain barrier.
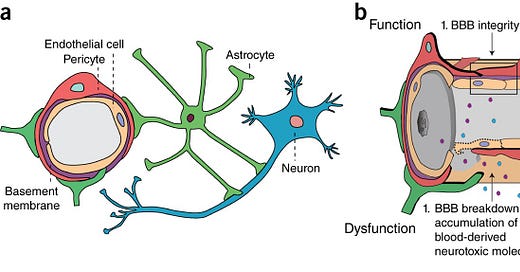
“The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside.”
https://en.m.wikipedia.org/wiki/Blood%E2%80%93brain_barrier
“Pericytes are vascular mural cells embedded in the basement membrane of blood microvessels. They extend their processes along capillaries, pre-capillary arterioles and post-capillary venules. CNS pericytes are uniquely positioned in the neurovascular unit between endothelial cells, astrocytes and neurons.”
https://www.nature.com/articles/nn.4288
“CD147, known as basigin or EMMPRIN, is a transmembrane glycoprotein of the immunoglobulin superfamily,11 which participates in tumor development, Plasmodium invasion, and bacterial and virus infection.”
https://www.nature.com/articles/s41392-020-00426-x
“Within biochemistry, a ligand is defined as any molecule or atom that irreversibly binds to a receiving protein molecule, otherwise known as a receptor. When a ligand binds to its respective receptor, the shape and/or activity of the ligand is altered to initiate several different types of cellular responses.”
https://www.news-medical.net/amp/life-sciences/Ligands-An-Overview.aspx
MMP9
“Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracellular matrix.”
https://en.wikipedia.org/wiki/MMP9
The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease
Elisa Avolio et al. Clin Sci (Lond). 2021.
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a broad range of clinical responses including prominent microvascular damage. The capacity of SARS-CoV-2 to infect vascular cells is still debated. Additionally, the SARS-CoV-2 Spike (S) protein may act as a ligand to induce non-infective cellular stress. We tested this hypothesis in pericytes (PCs), which are reportedly reduced in the heart of patients with severe coronavirus disease-2019 (COVID-19). Here we newly show that the in vitro exposure of primary human cardiac PCs to the SARS-CoV-2 wildtype strain or the α and δ variants caused rare infection events. Exposure to the recombinant S protein alone elicited signalling and functional alterations, including: (1) increased migration, (2) reduced ability to support endothelial cell (EC) network formation on Matrigel, (3) secretion of pro-inflammatory molecules typically involved in the cytokine storm, and (4) production of pro-apoptotic factors causing EC death. Next, adopting a blocking strategy against the S protein receptors angiotensin-converting enzyme 2 (ACE2) and CD147, we discovered that the S protein stimulates the phosphorylation/activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) through the CD147 receptor, but not ACE2, in PCs. The neutralisation of CD147, either using a blocking antibody or mRNA silencing, reduced ERK1/2 activation, and rescued PC function in the presence of the S protein. Immunoreactive S protein was detected in the peripheral blood of infected patients. In conclusion, our findings suggest that the S protein may prompt PC dysfunction, potentially contributing to microvascular injury. This mechanism may have clinical and therapeutic implications.
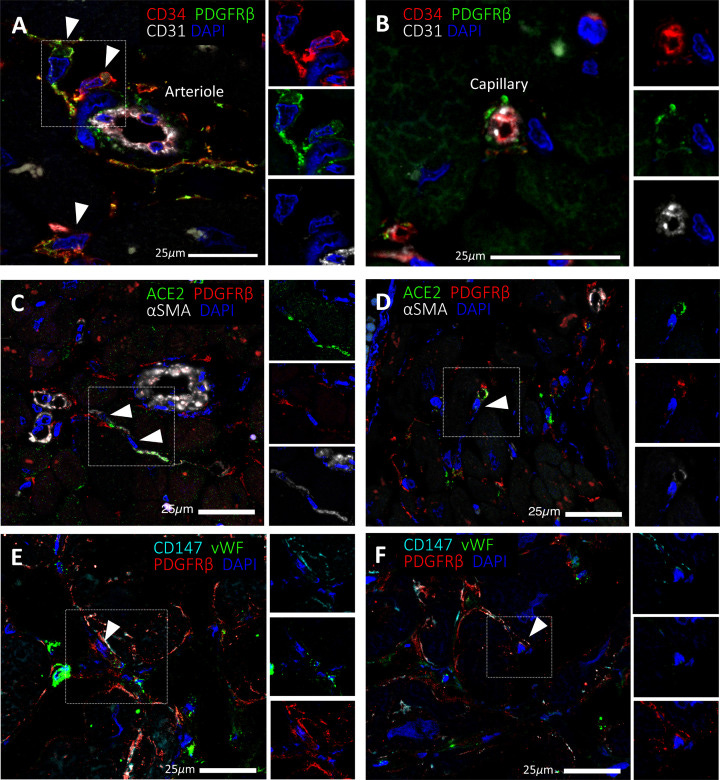
Expression of SARS-CoV-2 receptors in human cardiac PCs in situ
Immunofluorescence stainings showing PCs in the human heart. (A,B) Identification of CD31−CD34+PDGFRβ+ PCs around microvessels. CD31 recognises the vessel lumen, the CD34 labels both the luminal ECs and perivascular PCs, while PDGFRβ labels both PCs and vascular smooth muscle cells (VSMCs) in the arteriole’s tunica media. (C–F) A subset of PDGFRβ+ cardiac PCs express ACE2 (C,D) and CD147 (E,F). α-SMA labels some PCs and arterioles’ VSMCs (C,D), while vWF recognises ECs (E,F). CD147 is also expressed by ECs (E,F). Arrowheads point to PCs.
Full paper:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8674568/
Cyclophilin a signaling induces pericyte-associated blood-brain barrier disruption after subarachnoid hemorrhage (2020)
Results
Our data demonstrated both intracellular CypA and CypA secretion increased after SAH and could activate CD147 receptor and downstream NF-κB pathway to induce MMP9 expression and proteolytic functions for degradation of endothelium tight junction proteins and basal membranes. CypA served as autocrine or paracrine ligand for its receptor, CD147. Although CypA could be endocytosed by pericytes, specific endocytosis inhibitor chlorpromazine did not have any effect on MMP9 activation. However, specific knockdown of CD147 could reverse the harmful effects of CypA expression in pericytes on the management of SAH patients.
Neurological outcomes and blood brain barrier permeability after subarachnoid hemorrhage in CypA−/− mice. a Modified Garcia test results of each group at 24 h after SAH. b Beam balance test results of each group at 24 h after SAH. c Evans blue fluorescence evaluation. d extravasation at 24 h after SAH. e Cadaverine-Alexa Flour 555 fluorescence evaluation. f Fluorescent efficiency at 24 h after SAH. g Representative immunohistochemistry staining slices of Collagen IV (Green) and Lectin (White) at 24 h after SAH. n = 6 for each group @: vs. flox/flox + sham P < 0.05, #: vs. flox/flox + SAH P < 0.05, &: vs. KO + sham, P < 0.05. Arrow indicates the breakdown of continuous endothelia cell layer.
Full paper:
https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-020-1699-6
Endothelial genetic deletion of CD147 induces changes in the dual function of the blood-brain barrier and is implicated in Alzheimer’s disease (2021)
Abstract
Aims
The blood-brain barrier (BBB) is a specialized and indispensable structure in brain blood vessels that is damaged during Alzheimer's disease (AD). CD147 is expressed on the BBB and deeply engaged in the AD pathological process. In this study, we aimed to provide a better understanding of the roles of CD147 in BBB function in health and neurodegenerative disease.
Methods and Results
We measured CD147 expression in mouse brains and demonstrated that CD147 is exclusively expressed in brain endothelial cells (BECs), and its expression decreases with age. After constructing endothelial-specific CD147 knockout mice, we performed RNA-sequencing on BECs isolated from mice of different ages as well as a range of database analyses. We found that endothelial CD147 is essential for the dual functions of the BBB, including barrier maintenance and transporter regulation. This study also shows that CD147 plays a pivotal role in neurodegenerative diseases, particularly in AD.
Conclusions
Our findings suggested that targeting CD147 in BECs may represent a novel therapeutic strategy, which promoted the design of future experimental investigations and the mechanistic understanding of neurodegenerative diseases.
Effects of CD147 deficiency on BBB integrity in vivo and in vitro. (A) WT and EC-KO mice at 8–12 weeks or 6–7 months of ages were given an i.v. injection of sodium fluorescein (Na-F), and the absorption of Na-F in the mouse brain was measured by a microplate reader at 485 nm, n = 5. (B) Brain water content of WT and EC-KO mice at 8–12 weeks or 6–7 months of age, n = 6. (C) Heatmap showing the 82 DEGs (see Table S1) from the following five KEGG pathways: Tight junction (ko 04530), Adherens junction (ko 04520), Focal adhesion (ko 04510), Cell adhesion molecules (CAMs; ko 04514) and Gap junction (ko 04540). All these enrichment pathways were p < 0.05 by hypergeometric tests. (D) Expression changes of selected genes (Ocln, Cldn5, Sparcl1) from the above dataset by RT-qPCR. Data are presented as the mean ± SEM of 3 independent experiments. (E) Western blot of DEGs of interest (OCLN, TJP1, SPARCL1, JAM-1). (F) Quantification of protein levels of molecules in (E) relative to GAPDH. (G) TEER measurement at 7 days after seeding BECs in the upper chamber via an EVOM Volt-Ohm resistance meter with STX2 chopstick electrodes. A schematic of the in vitro monocellular BBB model and TEER measurement is shown (upper right), n = 3 per group. (H) The paracellular permeability of the BBB model was assessed by 40 kDa FITC-dextran at different timepoints, including 0, 0.5, 1, 2, 4, 8, and 24 h. The fluorescence was measured using a Cytation Imaging Reader, n = 3 per group. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared to WT at the same ages; #p < 0.05 and ##p < 0.01 compared to 8–12 weeks with the same genotype
Full paper: