This is the untold and shocking story of Pfizer’s 12-to-15-year-old clinical trial.
Repost from author Jonathan Weissman
I’m unable to contact Jonathan to thank him but I'm sure he'll appreciate the repost. If anyone can on my behalf that would be appreciated.
Reposted here on request for the benefit of those who, worse than myself can not even read twitter posts due to the obscene silencing of any voices that do not comply with the official, corrupted narrative. This post is being updated as new information comes in.
At the end of the day we are here to save lives, and preserve quality of life, by mentioning the unmentionable as a warning and to hold these people to account…
One can only guess at the long term damage, but my research points to impaired immunity, heightened cancer risk, accelerated or accute cardiovascular damage, musculoskeletal disorders, autoimmune disorders and in particular multiple ROS induced neurological and development disorders & delay, especially autistic spectrum disorders, see previous Substack.
These experimental gene therapies must be stopped immediately pending further investigations.
Mar 3 • 9 tweets • 7 min read
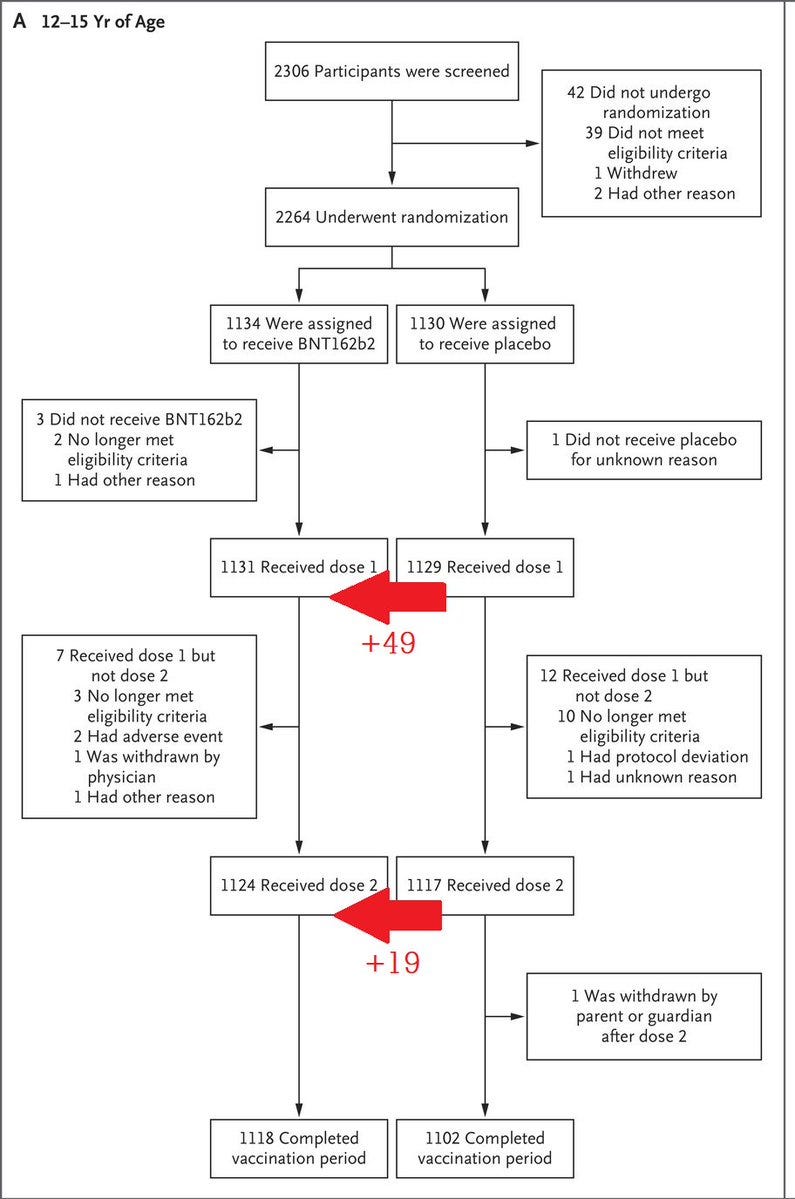
[1/9] This is the untold and shocking story of Pfizer’s 12-to-15-year-old clinical trial. Just 2264 adolescents were randomised between October 2020 and January 2021. I will describe the various related, serious and life-threatening injuries sustained by these youngsters.
[2/9] 1180 of these youngsters received at least one 30μg dose before the data cutoff date, 13 March 2021, comprised of 1131 adolescents during the blinded study and 49 from the placebo group, who turned 16, were unblinded and chose to be treated under the FDA’s recent 16+ EUA.
[3/9] Although the study’s sample size was tiny and its statistical power weak, clinical trial data (however incomplete!) is as gold dust, as it represents a closed cohort. It can detect a crucial safety signal before treating every adolescent on the planet. Injuries included…
[4/9]* 1 related life-threatening fever* 1 related life-threatening anaphylaxis* 1 related with “reasonable possibility” myopericarditis, hospitalised, “limited activity” advised at 2 months* 3 on SSRI medication for depression, each hospitalised with symptom “exacerbation”
[5/9] One more. Maddie de Garay suffered severe abdominal/chest pain and extreme numbness within a day of dose 2. She developed blood in her urine, mobility issues, is now paralysed and uses a nasogastric tube to eat. Mislabelled as “functional abdominal pain” and “neuralgia”.
[6/9] Aside from those 7 youngsters, lymphadenopathy, swollen lymph nodes, occurred at a statistically significantly higher rate in the treatment group (9 vs. 2). Likewise for lymphadenopathy instances judged to be related to treatment (7 vs. 1).
[7/9] The reactogenicity safety data is dramatic. These side effects were likely too obvious for the study blind to be maintained. The study’s administrators were unblinded anyway! Post dose 2, in the treatment group, 51% used antipyretic medication vs. 9% in the placebo group.
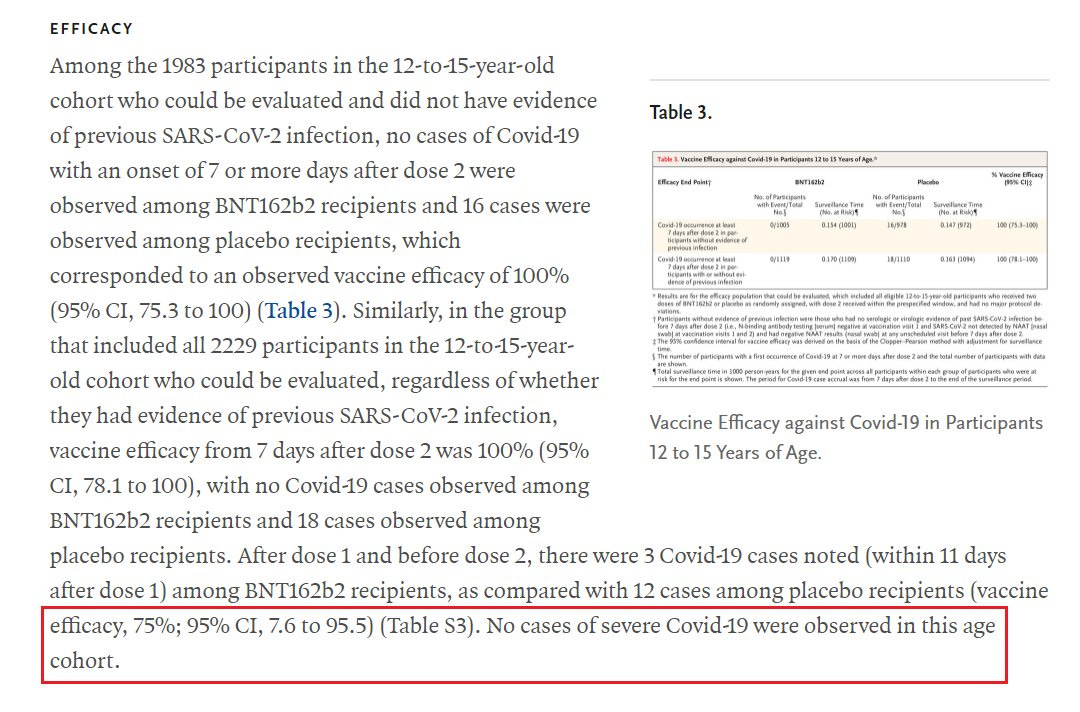
[8/9] There were no severe COVID-19 cases in either group. What was the efficacy “benefit” for all these injuries? The clinical endpoint was symptomatic infection confirmed by an NAAT test. We don’t know the positive PCR Cts, the symptoms, nor whether transmission was reduced.
[9/9] To reiterate, the study’s data cutoff date was 13 March 2021. These injuries were all known by the time:
* Pfizer boasted their drug was “well-tolerated”
* the trial results were published in the NEJM
* the MHRA extended their TUA
* the JCVI advised the UK-wide roll
https://twitter.com/AllTheRisks/status/1500317389170393089?s=19
It is difficult to overstate how scandalous this is.
* * *
Added 10th March ‘22:
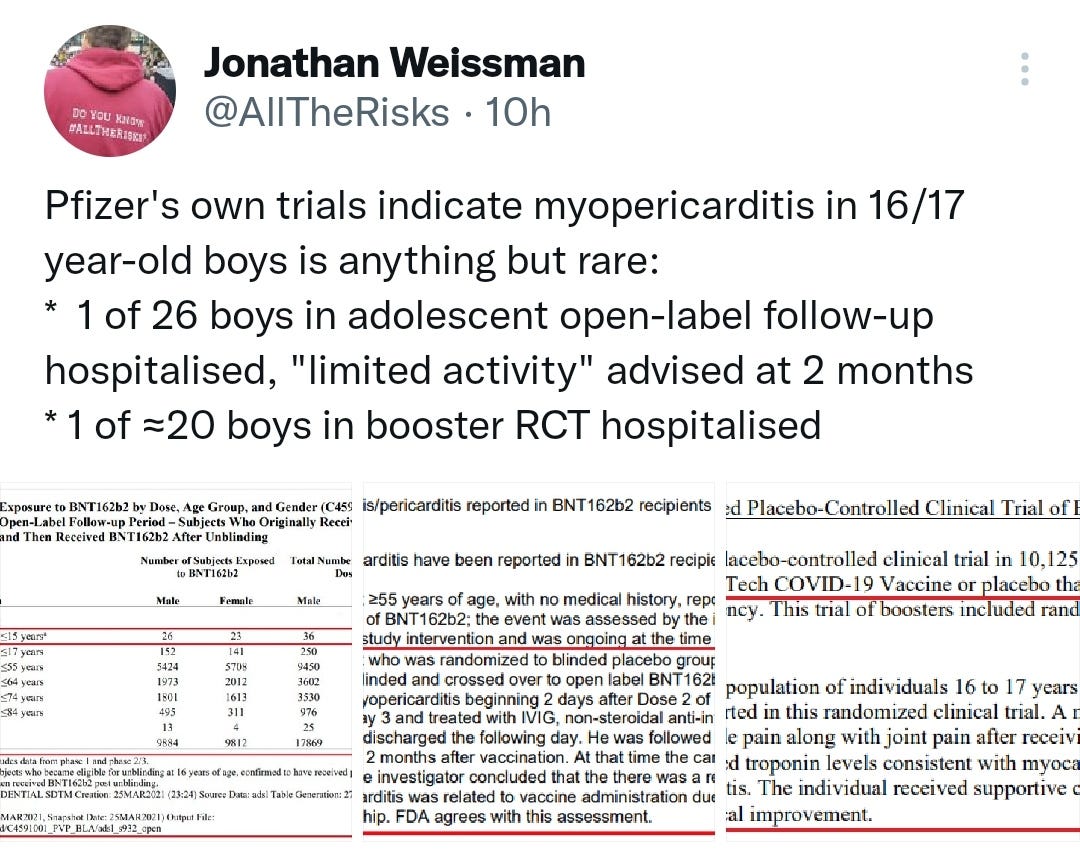
“Pfizer's own trials indicate myopericarditis in 16/17 year-old boys is anything but rare:
* 1 of 26 boys in adolescent open-label follow-up hospitalised, "limited activity" advised at 2 months
* 1 of ≈20 boys in booster RCT hospitalised”
































THANK YOU for getting this information to us all.
Thanks, DC.