Reading time: about 50 minutes.
Also available in other languages:
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
If received via email I recommend clicking on the hyperlinked title to read the latest version in full, in a browser.
Contents
Spike protein and class switching
Gal-3 and class switching to IgG4
Long term expression of spike protein in lymph node germinal centres (GCs)
A pandemic of mystery illnesses
Background
Dr Jessica Rose posted an excellent introduction to IgG4 and early experimental findings of significantly elevated plasma levels following administration of experimental gene transfection agents.
Please read through it if you require an introduction or a refresher, but the substance of it is clear enough in the figure below:
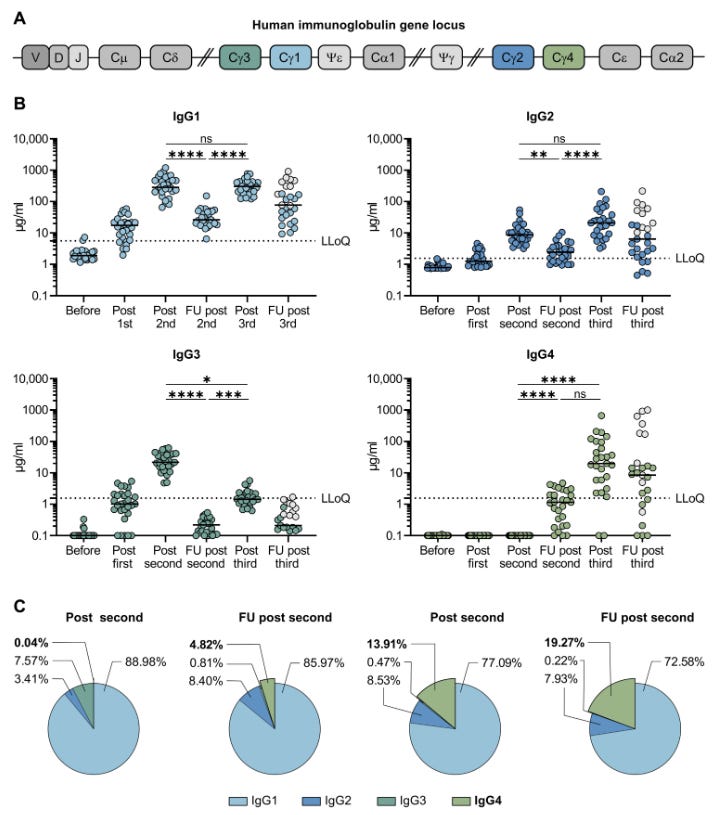
Also see the latest paper (preprint) by Rubio-Casillas, Redwan & Uversky.
Some cancers use non-specific IgG4 for immune evasion, which must also be considered within the context of systemically elevated levels - the body is in effect becoming tolerant to its own cancer cells:
“Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system?”1 (2023):
Abstract
SARS-CoV-2, the virus that causes the COVID-19 disease, has been demonstrated to cause immune suppression in certain individuals. This can manifest as a reduced ability for the host's immune system to effectively control the infection. Studies have reported that patients with COVID-19 can exhibit a decline in white blood cell counts, including natural killer cells and T cells, which are integral components of the immune system's response to viral pathogens. These cells play critical roles in the immune response to viral infections, and their depletion can make it harder for the body to mount an effective defense against the virus. Additionally, the virus can also directly infect immune cells, further compromising their ability to function. Some individuals with severe COVID-19 pneumonia may develop a "cytokine storm," an overactive immune response that may result in tissue damage and organ malfunction. The underlying mechanisms of immune suppression in SARS-CoV-2 are not entirely comprehended at this time, and ongoing research is being conducted to gain a more comprehensive understanding. Research has shown that severe SARS-CoV-2 infection promotes the synthesis of IgG4 antibodies. In this work, we propose the hypothesis that the IgG4 antibody produced by B cells in response to infection by SARS-CoV2 generates immunological tolerance that prevents its elimination, and leads to persistence and chronic infection. In sum, we believe that this constitutes another immune evasion mechanism that bears striking similarities to that developed by cancer cells to evade immune surveillance.

I reviewed viral interactions with T-cells in the last Substack:
With elevated igG4 the cancer risk is not just theoretical, a systematic review and meta-analysis of patients with IgG4 related disease (RD) found that standardized incidence ratios (SIRs, ie an estimate of the number of cancer cases in a given population compared to what might be “expected” based on a comparison with the cancer experience in a larger population2) were significantly increased, eg 4x fold for pancreatic cancer and 69x fold for lymphoma, the latter is certainly the sentinel condition to be monitoring closely at population level.
“The risk of malignancy in patients with IgG4-related disease: a systematic review and meta-analysis” (2022) by Yu et al3:
Results
A total of 10 studies were included in the article. The overall SIR estimates suggested an increased risk of overall cancer in IgG4-RD patients (SIR 2.57 95% CI 1.72–3.84) compared with the general population. The specific SIRs for pancreas and lymphoma were higher than those of the general population in IgG4-RD patients (SIR 4.07 95% CI 1.04–15.92, SIR 69.17 95% CI 3.91–1223.04, respectively). No significant associations were revealed in respiratory and gastric cancer (SIR 2.14 95% CI 0.97–4.75, SIR 0.95 95% CI 0.24–3.95, respectively). Four studies were found to be the major sources of heterogeneity by sensitivity analysis. There was no evidence of publication bias via Egger’s test.
Conclusion
Compared with the general population, patients with IgG4-RD appear to have a higher risk of overall cancer, especially pancreatic and lymphoma. The risk of lung and gastric cancer was not different between IgG4-RD patients and the general population.
Spike protein and class switching
IgG4 class switching is rarely associated with immunisation or even repeated infections:
Generally, IgG4 responses have been rarely observed even after repeated immunizations or infections. To corroborate this, we analyzed tetanus-specific antibody responses in 23 volunteers who had received several doses (2–16, median 6) of a tetanus toxoid (TT) vaccine (Table S3). Sera were tested for TT-specific total IgG or IgG4 antibodies using an ELISA format. TT-specific IgG4 were detectable in 9 of 23 sera, albeit at very low levels, and no correlation was found with the number of vaccinations received (Fig. S5A, B). Additionally, we tested ten individuals from our cohort 2 (Table 1) for the presence of antibodies against the respiratory syncytial virus (RSV), a respiratory pathogen that regularly causes re-infections in humans. While we found RSV-F protein-specific IgG1 antibodies in all tested sera, IgG4 was not detected (Fig. S5C). These findings support the notion that class-switching to IgG4 is not a general consequence of repeated antigen exposure in form of vaccinations or infections.
“Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination” (2022)
The gp120 like homologous inserts on the S1 spike protein are certainly strongly implicated as igG4 class switching was also associated with the V2 variable loop of gp120 used in the VAX003 HIV vaccine trial4.
Repeated exposure to specific epitopes rather than whole or attenuated virus appears to be one of the conditions for this.
Also see:
Galectin-3 (Gal-3)
This will not be a literature review of Gal-3, suffice to say that over 10,000 papers have been published on Gal-3 (or “IgE binding protein”) alone since 1982, so its not something to be undertaken lightly.
But I can give an overview as background to its relation to spike protein, which is big problem from the patho viewpoint. It is associated with so many conditions that the authors of this review were literally able to create an A-Z of diseases.
Apart from cytotoxic and neurotoxic gp120, if you were creating a bioweapon its certainly one you would consider for your shortlist, and of course a homologous version is indeed there on our friendly spike protein, which I will come to later.
“Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z”5 (2018), key highlights:
Galectin-3 (Gal-3) regulates basic cellular functions such as cell–cell and cell–matrix interactions, growth, proliferation, differentiation, and inflammation. It is not surprising, therefore, that this protein is involved in the pathogenesis of many relevant human diseases, including cancer, fibrosis, chronic inflammation and scarring affecting many different tissues.
Considering the crucial role exerted by Gal-3 in many different clinical conditions, Gal-3 is emerging as a new diagnostic, prognostic biomarker and as a new promising therapeutic target.
Galectins were initially isolated as β-galactoside-binding proteins. They represent a family of widely expressed lectins in metazoans. In mammalians a total of 15 galectins have been identified so far, containing either one or two ∼130 amino-acid-long conserved carbohydrate recognition domains (CRD)s [1,2,3].
Galectins are synthesized in the cytoplasm and interact with cell surface glycans following their secretion by a non-classical exocytic pathway (i.e., not via the ER/Golgi secretory route), that is likely to be an exosome-mediated secretory route [4,5]. Their ability to crosslink glycosylated ligands allows them to form a dynamic lattice. The galectin lattice regulates many different functions such as the diffusion, compartmentalization and endocytosis of plasma membrane glycoproteins and glycolipids, the selection, activation and arrest of T cells, receptor kinase signaling and the functionality of membrane receptors, (including the glucagon receptor, glucose and amino acid transporters, cadherins and integrins) [6].
Gal-3 is a member of a growing family of β-galactoside binding lectins [8], with an approximate molecular weight of 30,000.
Gal-3 is encoded by the LGALS3 Gene, which has previously been known under different names: IgE-Binding Protein [18], MAC-2 [9], Lectin L-29 [19], L-34 [20] CBP-30 [21], Galectin 3 (Gal-3), Advanced Glycation End-Product Receptor 3, Lectin, Galactoside-Binding, Soluble, 3, Carbohydrate-Binding Protein 35 (CBP 35) [22], Galactose-Specific Lectin 3, Laminin-Binding Protein [23], 35 KDa Lectin, Galactoside-Binding Protein, GALBP [12].
In adults, Gal-3 is ubiquitously expressed, while experiments performed in mice demonstrated during embryogenesis its expression is tissue- and time-dependent. Although its expression is mainly related to the epithelial cells and myeloid/amoeboid cells, Gal-3 expression was detected in many different types of cells, including: Small intestinal epithelial cells, colonic epithelia, corneal and conjuctival epithelia, olfactory epithelium, epithelial cells of kidney, lung, thymus, breast, and prostate. It was also detected in ductal cells of salivary glands, pancreas, kidney, eye, in intrahepatic bile ducts, in fibroblasts, chondrocytes and osteoblasts, osteoclasts, keratinocytes, Schwann cells and gastric mucosa, as well as in the endothelial cells from various tissues and organs [24].
…Gal-3 displays pathological expression in many tumors, such as those affecting the pancreas, the liver, the colonic mucosa, the breast, the lung, the prostate, the head and neck, the nervous system and the thyroid [24,25,26].
The current review represents an attempt to examine the many clinical implications of Gal-3 protein, based on a classification of clinical diseases, listed in alphabetical order (Figure 3).

“Conditions and diseases in which a role for Gal-3 has been postulated, listed in alphabetical order.” from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5855601/
A ribbon diagram, or Richardson diagram for Gal-3. The direction of the arrow is the direction of the amino acid sequence (polypeptide chain) in the β-strand.
If you see a coiled ribbon or thick tube then this depicts α-helices.
The thin wires have no regular structure6:

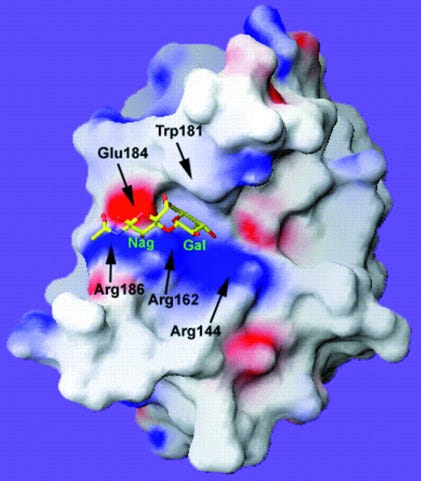
As for cancer implications, its impossible to generalise:
Gal-3 has been shown to influence many significant biological processes linked to cancer development and progression, including cell adhesion, proliferation, differentiation, mRNA splicing, cell-cycle progression, immune system evasion, inflammation, angiogenesis, apoptosis and metastasis [25,38,106,107,108,109,110].
In addition, Gal-3 exerts a role as a pro-tumor factor by acting within the tumor microenvironment, the so-called “tumor niche”, to suppress immune surveillance. Its ability to promote tumor cell proliferation and survival of the cancer cell both directly and indirectly, by acting on cell adhesion and cell contact with mesenchymal stromal cells, has lead to consider Gal-3 as a guardian of the tumor microenvironment [111].
Gal-3 influences oncogenesis, cancer progression and metastasis through a variety of pathways both inside and outside the cell [112,113,114,115,116,117,118]. The studies on the role of Gal-3 in different types of cancers indicate that such effects may be specific for the different types of cancer. In fact, Gal-3 may have both positive and negative effects on cancer cell survival depending of the type of tumor considered and its subcellular localization likely influences the role of Gal-3 in different cells [24,25,26].

Secondary effects of spike protein on p53 leading to Gal-3 overexpression are just as relevant from a patho point of view:
Gal-3 exerts its effect on apoptosis has led to identify a new apoptotic pathway in which different types of DNA damage triggers the HIPK2 activation and induces p53 mediated repression of Gal-3 expression [128]. Dysregulation of this pathway due to the occurrence of p53 mutations or due to HIPK2 loss may alter such pathway thus leading to Gal-3 overexpression and [129]. Gal-3 influences also many other signal transduction cascades and pro-survival processes that include major oncogenes such as RAS, BCL2, and MYC [130,131,132,133,134,135,136,137].
I discussed spike protein binding to integrins α5β1 and αvβ3 via the Tat-like motif in the last Substack:
Gal-3 can regulate vascular signaling programs through binding to integrin avb3 or by sustaining the pro-angiogenic capacity of tumor-associated macrophages. Therefore,Gal-3 serves as a bifunctional messenger that has emerged as a novel regulatory checkpoint able to couple immunosuppression and angiogenesis, two events that occur simultaneously during tumor growth and are often mediated by the same cytokines and the same cell types [144].
Spike protein and Galectin-3
The N-terminal domain of spike S1 possesses a “galectin-fold” or pocket with a structural homology that is almost identical to human Gal-3 and a study comparing interactions of Gal-3 with monocytes and S1-NTD with monocytes found that a near identical pattern of cytokines was being signalled in response.
Without further experimental data we cannot extrapolate this to other conditions in the A-Z of Gal-3 pathologies but it is strong evidence that the results might be replicated with some of these too, as we keep seeing with homologous gp120 & Tat motif associated pathologies. And viral mutations and co-infections (eg influenza) can also lead to binding to galectin ligands not usually associated with coronaviruses.
A Russian study used ELISA to analyse the structure of glycans which bind to S protein, and intriguingly discussed how co-infection with influenza could expose pulmonary epithelial cells to the galectin-fold, thus increasing pathogenicity further still:
The revealed ability to recognize glycans with the terminal glucosamine moiety, for instance GlcNAcα1-3GalNAcβ, as well as other glycans that do not belong to the lactosamine family (see above), at first glance does not fit the hypothesis of the galectin-like binding site in the S protein. Nevertheless, there are examples when replacing just one or two amino acids in a lectin not only abolished the binding of carbohydrates, but led to the ability to recognize another glycan [25]. In our case, the difference in the amino acid sequence is very significant (data not shown), so we are talking only about the similarity of their folds. Hence, the difference between the S protein and glycan-recognizing galectin is not surprising in itself. More surprising is the “restoration” of the ability of the galectin fold in the S protein of the latest coronavirus to bind typical galectin ligands, which has been lost by other coronaviruses.
…Most lactosamine motifs on the surface of the lung epithelium cells are masked by sialic acid attached to them; therefore, in a healthy person, an additional affinity of the virus (for lactosamines) is unlikely to make a significant contribution to the primary virus adhesion. However, neuraminidase, which is present in many pathogens (first of all, in the influenza virus), may release sialic residues, thus exposing lactosamine residues. Hence, we hypothesize that parallel infection of a pathogen with strong neuraminidase activity will promote adhesion of SARS-CoV-2 and, therefore, increase its virulence.
“Recombinant SARS-CoV-2 S Protein Binds to Glycans of the Lactosamine Family in vitro” (2020)
From “The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3”7 (2022) by Schroeder & Bieneman:
It is further known that the spike protein (S) of SARS-CoV-2 (as first reported for other β-coronaviruses) possesses a so-called galectin-fold within the N-terminal domain of the S1 subunit (S1-NTD). This fold (or pocket) shows structural homology nearly identical to that of human galectin-3 (Gal-3). In this respect, we have recently shown that Gal-3, when associated with epithelial cells or anchored to a solid phase matrix, facilitates the activation of innate immune cells, including basophils, DC, and monocytes.
CRS: cytokine release syndrome.
A synthesis of these findings prompted us to test whether segments of the SARS-CoV-2 spike protein might also activate innate immune cells in a manner similar to that observed in our Gal-3 studies. Indeed, by immobilizing S components onto microtiter wells, we show that only the S1 subunit (with the NTD) activates human monocytes to produce a near identical pattern of cytokines as those reported in COVID-19-related CRS.
Other parts of the spike protein did not recreate the same cytokine pattern:
In contrast, both the S1-CTD/RBD, which binds ACE2, and the S2 subunit (stalk), failed to mediate the same effect. Overall, these findings provide evidence that the SARS-CoV-2 spike protein can activate monocytes for cytokines central to COVID-19, thus providing insight into the innate immune mechanisms underlying the CRS and the potential for therapeutic interventions.
This is not good, especially if synthetic, stabilised forms of spike protein have the same galectin-fold conformation. Along with the many other immunosuppressive pathways associated with LNP-mRNA gene transfection discussed in these Substacks, a glance at the Gal-3 A-Z is almost a glance at the VAERS reports, although correlation isn’t causation:
Within the NTD of SARS-CoV-2 (and other β-coronaviruses) is a region often referred to as the “galectin-fold”, given its high degree of structural homology to that of human galectin-3 (Gal-3) (20, 21). Because of this remarkable similarity, it has been proposed that the S1-NTD of SARS-CoV-2 may very well act like Gal-3 and that this might explain, in part, the immunological sequelae observed in COVID-19 (22, 23).
Indeed, intracellular Gal-3 has been linked to immune cell activation, namely that of monocytes/macrophages (24). We also recently reported evidence that epithelial cell-associated Gal-3 (EC-Gal-3) can activate a variety of innate immune cells to produce pro-inflammatory cytokines (25–27). In particular, we showed the activation of human dendritic cells (DC) and monocytes, demonstrating that these cells produced high levels of IL-6 and TNF-α –two hallmark cytokines in COVID-19-associated CRS (27).
Overall, these findings provide novel evidence that the S1 subunit of the SARS-CoV-2 spike protein directly activates monocytes for cytokines central to COVID-19-related CRS, with mechanistic implications fundamental to the pathogenesis of the disease.
In testing whether recombinant components of the SARS-CoV-2 spike protein activate innate immune cells for cytokine production, we focused on the effects potentially seen with basophils, monocytes, and dendritic cell subtypes (pDC and mDC) –all freshly isolated from blood. These cell types were chosen because we have shown that all are activated by EC-Gal-3.
And, since the S1-NTD of the spike protein expresses a “galectin-fold”, we hypothesized that each might likewise be stimulated. Two additional approaches were done for these experiments: 1) cultures were performed in microtiter plates pre-coated with spike protein components, since preliminary results indicated that proteins used in solution showed no to little capacity to stimulate cells (data not shown); and 2) we investigated the effects of co-stimulation with IL-3. Importantly, both in vitro culture strategies had proved instrumental is establishing the role of Gal-3 in activating these cells types (26, 27).
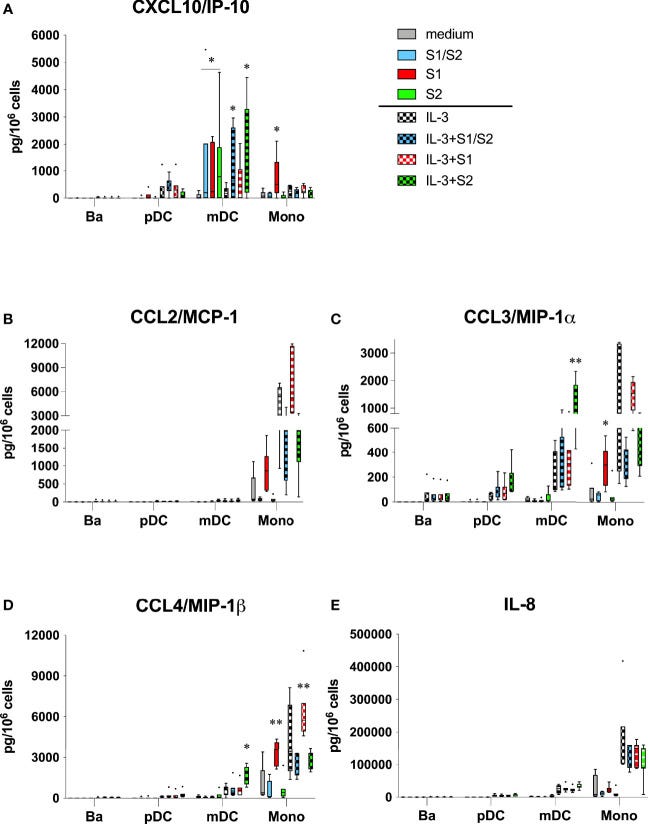
Relating to my last Substack, chemokines including CCL4 were induced by S1, which also has implications for cancer as CCL4 can promote tumour development and progression. Spike induced cancer pathways are certainly a recurring theme8:
The S1 subunit also acted on monocytes to produce several chemokines that are prominent in severe COVID-19 (Figures 2A–E). In particular, CXCL10/IP-10, CCL3/MIP-1α, and CCL4/MIP-1β were all significantly induced in culture wells coated with S1, but not in culture wells containing S2 or the S1/S2 component. IL-3 augmented these responses for the latter two chemokines, although this was only significant for CCL4/MIP-1β.
When used alone, the S1 subunit showed no capacity to induce any of these chemokines from the other cell types (basophils, pDC, or mDC). However, when combined with IL-3, the S2 subunit significantly induced both CCL3/MIP-1α and CCL/MIP-1β from mDC, but not from any other cell type.
We know that S1 binds with CCL5 (RANTES) but its expression wasn’t increased (not shown):
We know that pro-inflammatory and oncogenic IL-6 is upregulated by synthetic mRNA transfection, which directly implicates structural and functional homology to Gal-3. Again, this has serious implications due to positive correlations with Gal-3 or Gal-3-like levels:
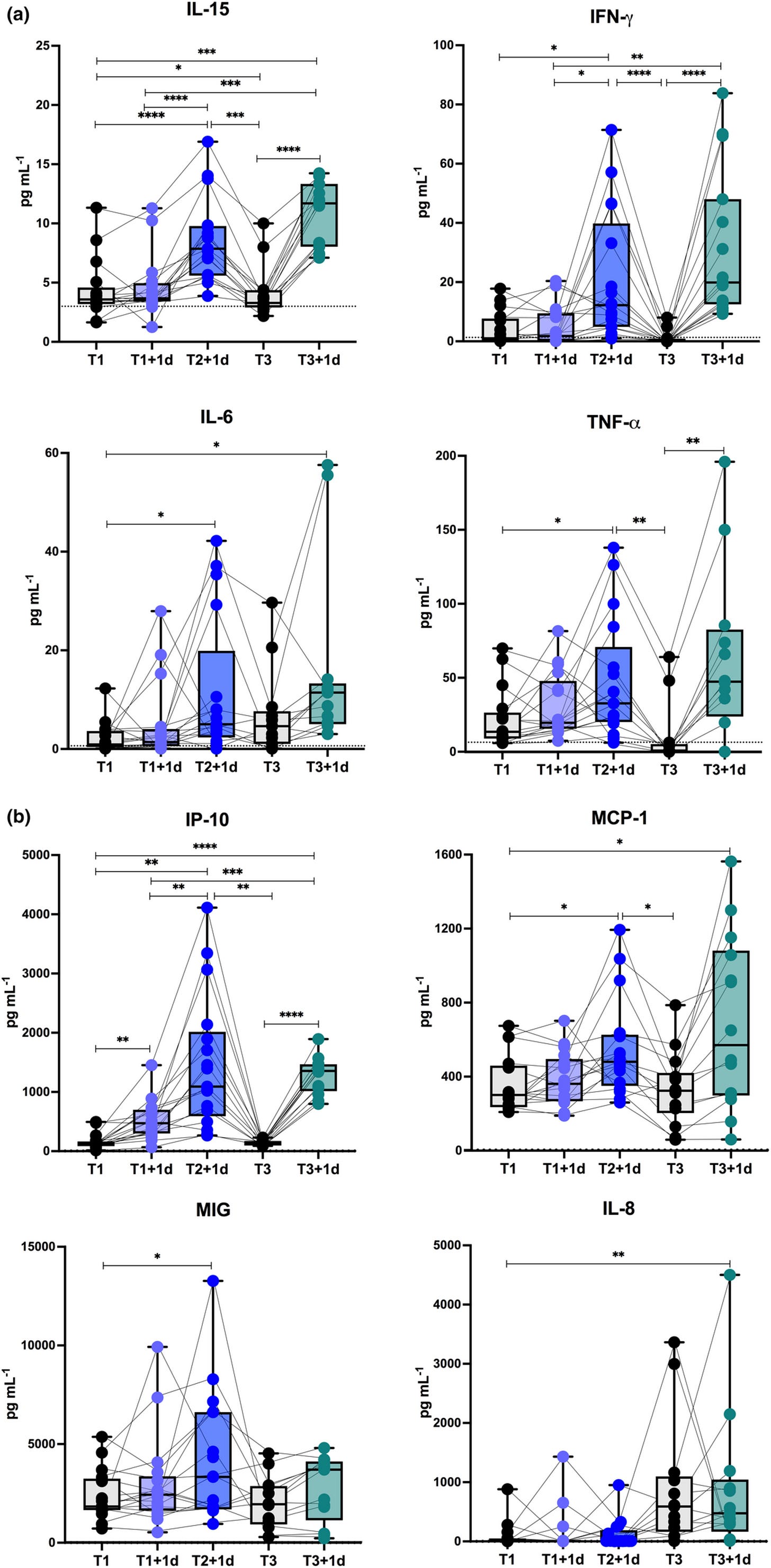
Use of a Gal-3 inhibitor suppressed spike S1 from activating monocytes for IL-6 secretion. It didn’t stop it completely, but again is strong evidence as demonstrated by the multi-fold increase in vaccinees:
As shown in Figure 4, a consistent dose response suppression of the IL-6 produced by monocytes was observed with increasing amounts of LGALS3BP for an average inhibition of 59% (range 50-70%) observed at the 1μg/ml concentration (P=0.012).
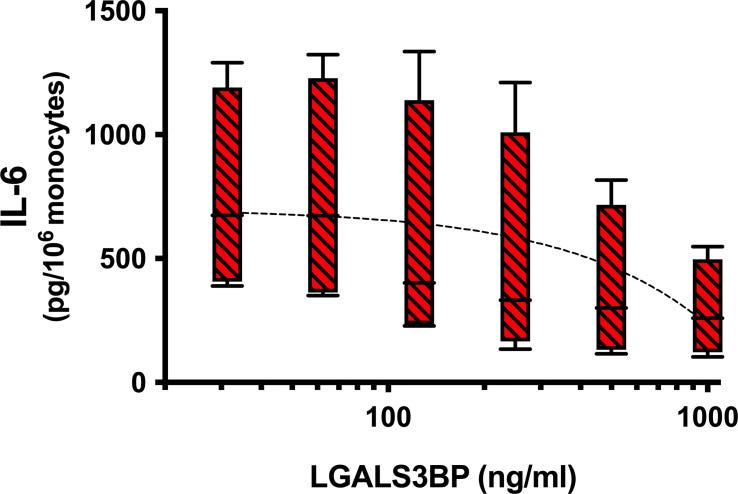
The Gal-3>monocyte>IL-6 and Gal-3>bone marrow mesenchymal stem cells (BMMSC)>IL-6 pathway is also associated with protumourigenic effects on solid neuroblastoma tumours in children9.
As for tumour necrosis factor alpha (TNF-α), Gal-3 knockdown also led to its secretion being eliminated, not just reduced. If you see it in vaccinees then it is highly likely that functionally homologous Gal-3 or Gal-3 itself is also being expressed:
For further context, we have recently demonstrated that monocytes, and to a lesser extent DC subtypes, secrete IL-6 and TNF-α when co-cultured with A549 epithelial cells. However, these cytokine responses were eliminated upon knocking down Gal-3 expression in this adenocarcinoma cell line (27). We had also shown in earlier reports that IgE-expressing basophils produced IL-4 and IL-13 when co-cultured with EC-Gal-3 (26).
IL-3 is a necessary co-factor:
Moreover, many of these EC-Gal-3-dependent cytokine responses were similarly replicated by culturing basophils, monocytes, and DC with microspheres coupled with rhGal-3 (MS-Gal-3). And, that IL-3 augmented Gal-3 dependent cytokine production by several of the innate immune cells, in particular basophils and pDC –those that bear the highest levels of IL-3R (CD123).
They further tested their hypothesis by using recombinant and endotoxin-free proteins encompassing the full length spike protein, and recombinant S1 proteins made by two different companies. In each case the results were replicated, but only with S1-NTD:
To address the belief that S1-NTD acts similarly to Gal-3 in promoting cytokine responses, we took the approach of using recombinant and endotoxin-free proteins that encompass various regions of the SARS-CoV-2 spike protein and that collectively span the entire ~1211 amino acid sequence. Importantly, with the exception of the S2 subunit (stalk), all of the recombinant proteins investigated possess the CTD/RBD, which, according to data sheets supplied by R&D Systems, enables functional binding to ACE2.
Unfortunately, none of the proteins consisted only of the NTD. Nonetheless, of those investigated, only the S1 subunit, comprising the first ~615 amino acids and containing both the NTD and CTD, showed the capacity to induce cytokines from monocytes. In fact, this activity was observed using recombinant S1 proteins made by two different companies (see Materials & Methods). In contrast, both the CTD/RBD alone (a.a. 319-541) and the S2 subunit (a.a. 686-1211) failed to activate monocytes, thus implying the importance of the NTD –the region showing structural homology to Gal-3 (20, 21).
The stabilized prefusion conformation of synthetic mRNA derived spike protein ensures that S1-NTD/Gal-3 remains exposed. If it didn’t then immunological responses to the product would have been greatly impaired10:
Most unexpectantly, the so-called S1/S2 “active trimer”, which encompasses nearly the entire spike protein sequence (a.a. 16-1211), failed to induce significant cytokine levels, despite including the S1-NTD. Exactly why this full-length spike protein lacked the capacity to activate monocytes remains unknown. However, we propose that in this form, the S1-NTD may not be properly exposed, perhaps even hidden, and thus unable to activate monocytes, as did the S1 subunit.
As ACE2 is not expressed by human immune cells, TMPRSS2 can act to cleave full length spike protein. Synthetic spike protein is also quickly cleaved to S1 and S2 in vivo by cellular proteases, if not by TMPRSS211 and then binds with many different receptors in the body (including in the myocardium) or gets cleared by the kidneys12.
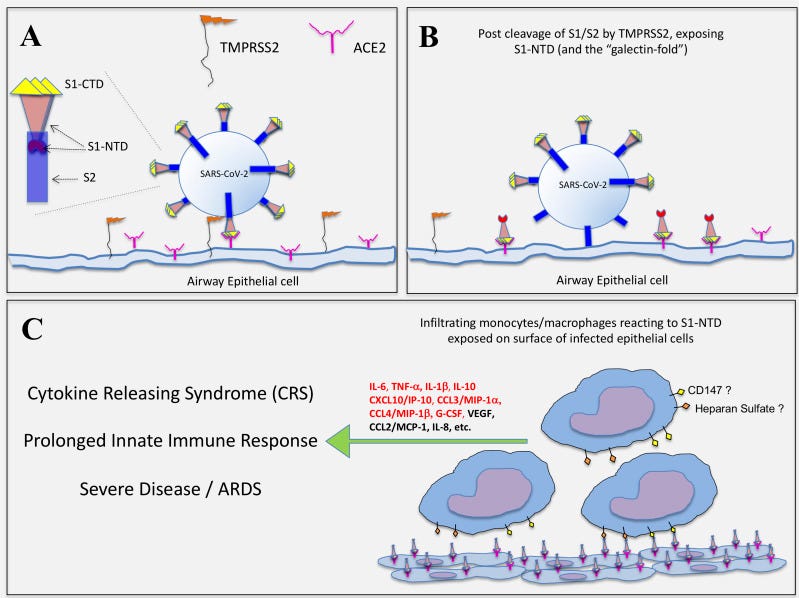
Here, the authors hypothesise that as human Gal-3 exists in many forms and only monocytes were reactive in their study then S1-NTD/Gal-3 may not be as pathogenic:
Interestingly, of the innate immune cells co-cultured with plate bound S1 protein, only monocytes reacted by producing relevant cytokines. Basophils, pDC and mDC did not react to this spike protein subunit, as was predicted based on our prior work showing EC-Gal-3-dependent activation. While there is currently no precise explanation for these findings, one could argue that the S1-NTD, while closely resembling Gal-3, may not function to the full range of this lectin, especially given that we only tested recombinant protein. Moreover, Gal-3 is somewhat unique among the known mammalian galectins in that it can exist in many forms, ranging in structure from monomers to multivalent pentamers, each potentially having different binding affinities for many kinds of glycoproteins that contain the β-galactosides required for binding this lectin (35).
However there are studies showing that plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs) are indeed activated by BNT162b2, with an impaired response in the elderly:
…we showed that BNT162b2 mRNA induces a short-term in vivo activation of the pDCs that contributes to the immune response against SARS-CoV-2 vaccine.
“Impact of BNT162b2 mRNA anti-SARS-CoV-2 vaccine on interferon-alpha production by plasmacytoid dendritic cells and autoreactive T cells in patients with systemic lupus erythematosus: The COVALUS project” (2023)
pDC’s remained elevated for two months after the second dose:
In addition to the alteration of the adaptive immunity associated with lower response to the vaccine in the elderly, there is a remodeling of the innate immune system with aging (Shaw et al., 2010). Thus, we next studied plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs) (Figure S4A), innate immune cells with a key role in the modulation of T cell response (Guermonprez et al., 2002). We first observed a decrease in pDC percentages two months after the second dose (Figure 5A). Although we did not find differences between young and elderly people in pDC levels (Figure 5A), we observed a considerable difference in pDC functional capacity (Figure 5B). When cells were stimulated in a toll like receptor (TLR)-9-dependent manner by CpG-A, a lower IFN-α production was observed in elderly people comparing with young subjects, both after the first and second dose of the SARS-CoV-2 vaccine (Figure 5B, left panel). Interestingly, we also observed how this functional capacity of the pDCs was associated with anti-RBD IgG levels after the first dose
…In response to TLR-3, TLR-7/8, and TLR-9 ligand stimulation, CD141+ mDCs and pDCs from old subjects secreted lower levels of IL-6, IL-12, and TNF-α (Panda et al., 2010).
“Innate and adaptive immune defects associated with lower SARS-CoV-2 BNT162b2 mRNA vaccine response in elderly people” (2022)
https://www.medrxiv.org/content/10.1101/2022.01.07.22268806v1.full
As for the toll-like receptor ligand stimulation referred to above, Gal-3 knockdown again inhibits TLR-induced IL-6 secretion.
Its ironic to see some studies referencing Gal-3 associated cytokines as the target, whilst many other studies are investigating and suggesting therapeutics to treat conditions which are contributed to by Gal-3, ie no joined-up thinking, especially where cancer patients are being coerced:
We investigated the role of galectin-3 in TLR-induced cell activation in human synovial fibroblasts (SF) in order to better understand the mechanism(s) of the proinflammatory function of galectin-3 in arthritis.
Galectin-3 expression in SF obtained from rheumatoid arthritis and osteoarthritis patients was inhibited by siRNA mediated gene-knockdown. Galectin-3 was also inhibited with modified citrus pectin (MCP), a polysaccharide galectin-3 ligand. Galectin-3 knockdown inhibited TLR-2, -3 and -4-induced IL-6 secretion, but not TLR-2, -3 and -4-mediated matrix metalloproteinase-3 or CC chemokine ligand-5 secretion. When the SF were stimulated with phorbol 12-myristate 13-acetate, a protein kinase C activator that bypasses the membranal receptors, galectin-3 knockdown no longer influenced IL-6 secretion. MCP reduced IL-6 levels in a dose-dependent manner.
Our results indicate that galectin-3 is a positive sensor-regulator of TLR-induced IL-6 secretion in human synovial fibroblasts, thus adding new insights into the mechanisms by which galectin-3 augments synovial inflammation. These findings corroborate the potential role of glycan inhibitors of galectin-3 as a therapeutic approach for the treatment of inflammatory arthritis.
“Galectin-3 is a sensor-regulator of toll-like receptor pathways in synovial fibroblasts” (2015)
https://www.sciencedirect.com/science/article/abs/pii/S1043466615000204
Galectin-3 (Gal3) is one of the most studied members of the galectin family that mediate various biological processes such as growth regulation, immune function, cancer metastasis, and apoptosis. Since Gal3 is pro-inflammatory, it is involved in many diseases that are associated with chronic inflammation such as cancer, organ fibrosis, and type 2 diabetes. As a multifunctional protein involved in multiple pathways of many diseases, Gal3 has generated significant interest in pharmaceutical industries. As a result, several Gal3-targeting therapeutic drugs are being developed to address unmet medical needs.
“Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases” (2023)
From their concluding discussions:
The pattern of monocyte-derived cytokines induced by the S1 subunit is among the more striking observations revealed in this study because the profile is remarkably similar to that implicated in the CRS associated with severe COVID-19. Again, IL-6 was the cytokine most consistently induced by the S1 subunit, which occurred regardless of whether IL-3 was added to augment the response. Likewise, IL-6 is perhaps the most consistently elevated cytokine associated with COVID-19
Several chemokines linked to COVID-19 were also significantly induced by the S1 subunit, including CCL3/MIP-1α, CCL4MIP-1β, and CXCL10/IP-10. All are implicated in playing a role in monocyte recruitment and are reportedly secreted by monocytes. Several studies, in fact, have reported CXCL10/IP-10 as a key marker of severe disease (9, 12).
Therefore, we propose that the findings presented herein provide insight into a potentially relevant mechanism –one in which the S1-NTD of the viruses’ spike protein (and likely that of other β-coronaviruses) mimics Gal-3 and the capacity of this lectin to modulate activation of innate immune cells, namely monocytes. Therefore, the development of therapeutics, such as Gal-3-like antagonists or neutralizing antibodies that target the S1-NTD of the spike protein, cannot be overstated in that they could prove efficacious in preventing prolonged innate immune dysfunction and onset of CRS leading to ARDS.
Perhaps not repeatedly expressing large amounts of reactive synthetic spike protein with a galectin-fold in ~85% of the population was not the best way to target Gal-3 induced immune dysfunction?
Gal-3 and class switching to IgG4
Although quite comprehensive, the previous authors did not discuss Gal-3, IgG4 or IgG4-RD.
Fortunately in 2020 Perugino et al did perform this study. They sequenced Ig genes from single cell clones of dominantly-expanded plasmablasts and generated human monoclonal antibodies to identify relevant antigens in IgG4-RD using mass spectrometry.
What they found was that Gal-3 was identified as the autoantigen specifically recognised by two dominant plasmablast clones from a patient with multi-organ IgG4-RD.
From “Identification of Galectin-3 as an Auto-Antigen in IgG4-Related Disease”13:
Conclusion
Affinity chromatography using patient-derived monoclonal antibodies identifies relevant auto-antigens in IgG4-RD. IgG4 galectin-3 autoantibodies are present in a subset of IgG4-RD patients and correlate with galectin-3 plasma levels. The marked elevations of circulating IgG4 and IgE observed clinically are, at least in part, due to the development of IgG4 and IgE specific autoantibody responses.
In fact their heatmap is lit up like a red beacon by Gal-3.
We can therefore, given the weight of evidence probably add S1-NTD/Gal-3 as a mediator of spike protein induced class switching to IgG4, alongside gp120-like motifs.
Our group has shown that B cell depletion mediates prompt clinical improvement15 and also leads to a corresponding decline in CD4+CTLs.5 One possibility with regard to pathophysiology, therefore, is that dominantly-expanded B cells maintain or present some unidentified antigen(s) to expanded CD4+CTLs in IgG4-RD tissues.
In order to identify the antigen expressed by PaCa2 cells that was recognized by both patient-derived mAbs, PaCa2 cells were lysed to generate a protein source for immunoprecipitation. Both mAbs bound a protein of approximately 30 kDa, which was not recognized by the isotype control (Supplemental Figure 2). Galectin-3 was then identified by multiplexed quantitative mass spectrometry-based proteomics as the protein with the highest differential binding affinity for both mAbs (Figure 4).
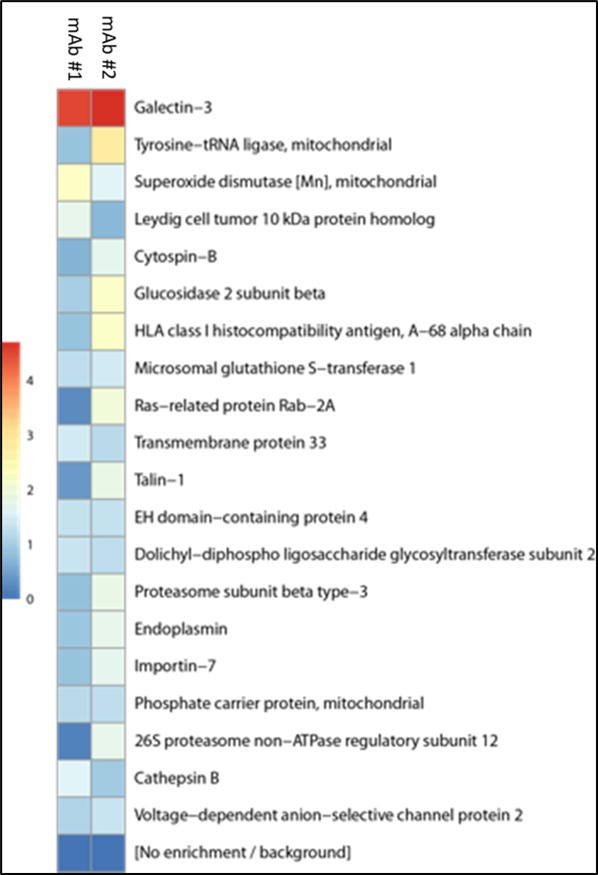
To validate the finding of galectin-3 as an auto-antigen in IgG4-RD, we quantified anti-galectin-3 antibodies by ELISA in a large, clinically-diverse cohort of IgG4-RD patients.
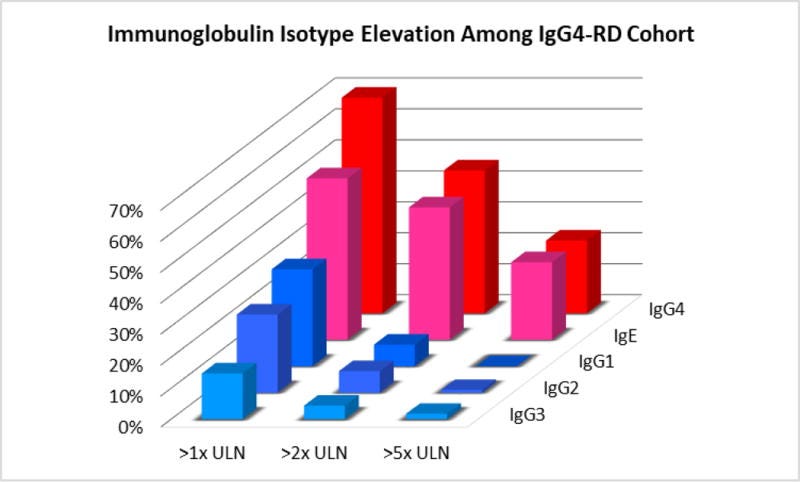
Because dominant plasmablast clones have been shown to express IgG42 and IgG4 is the most robustly elevated IgG subclass observed in the plasma of IgG4-RD patients, we first measured IgG4-specific anti-galectin-3 antibody responses. This revealed a reactive frequency of 28% among the IgG4-RD cohort with little to no reactivity in the control groups (Figure 5). We next tested the cohort for galectin-3 reactivity by IgG1, IgG2, IgG3 and IgE isotypes. IgE isotype anti-galectin-3 antibodies were also detected in these experiments (Figure 5), but there was little to no reactivity by other IgG subclasses (Supplemental Figure 3).
The presence of anti-galectin-3 antibodies of only IgG4 and IgE isotypes is consistent with the clinical observation of predominant IgG4 and IgE elevations in the blood of IgG4-RD patients compared to other IgG subclasses, which tend to be only modestly elevated and at a lower overall frequency (Figure 6). These results support the notion that at least a portion of the IgG4 and IgE expansion in the blood of IgG4-RD patients is due to the development of autoantibodies of these isotypes.
The authors also considered the importance of cytokine combinations, as we saw earlier with Gal-3 and IL-3:
One potential explanation for the observation of concordant IgG4 and IgE isotype responses is the effect of certain cytokine combinations such as interleukin-4 plus interleukin-10. This combination is reported to drive isotype switching to both IgG4 and IgE, with some skewing towards IgG4.21
Gal-3 demonstrates a positive correlation with IL-4:
Although Gal-3 is not expressed in resting B cells from normal mice, its expression is markedly induced after activation with stimuli such as IL-4 and CD40 cross-linking. These signals promote survival and block the final differentiation of these cells, thus allowing the rising of a memory B cell phenotype.
“Galectin-3 Mediates IL-4-Induced Survival and Differentiation of B Cells: Functional Cross-Talk and Implications during Trypanosoma cruzi Infection” (2004)
Gal-3 has an inverse correlation with anti-inflammatory IL-10:
The amount of circulating galectin-3 inversely correlated with the production of IL-10 (r=−0.59, P<0.001) and IL-12 (r=−0.69, P<0.001).
“Association between circulating galectin‑3 levels and the immunological, inflammatory and nutritional parameters in patients with colorectal cancer” (2016)
We next asked if the occurrence of IgG4-specific anti-galectin-3 antibodies was associated with over-expression of this protein, in accordance with the notion that accumulation of proteins within tumors of various types has previously been associated with the development of auto-antibodies directed against the over-expressed protein.22
Galectin-3 was observed as one of the highest differentially expressed proteins in IgG4-RD pancreata, present at a 13-fold higher amount compared to controls.24
After segregating the IgG4-RD cohort based on elevated as opposed to normal plasma galectin-3 levels, IgG4 anti-galectin-3 antibodies were detected in 64% of those with elevated levels compared to only 23% in those with normal levels (p = 0.0032) (Figure 7B).
IgG4-RD response to rituximab is associated with a decline of anti-galectin 3 autoantibodies.
Class switching to IgG4 as an anti-inflammatory, antifibrotic homeostatic response:
IgG4 anti-galectin-3 antibodies could potentially reflect an attempt to mitigate the pro-fibrotic function of galectin-330 These autoantibodies may sterically interfere with galectin-3 induced myofibroblast proliferation and collagen secretion, thereby tempering the fibrotic process.
In the same way, is elevated IgG4 in vaccinees caused by anti-fibrotic antibodies to the galectin-fold and/or gp120 motifs?
As an aside, I discussed spike-induced expression of BRD4 previously, which is also linked to cancer, SASP and fibrosis:
The authors next considered IgG4 anti-galectin-3 antibodies, Gal-3, lymph node germinal centres and autoimmune implications. We will revisit this later:
The germinal center is a specialized structure within secondary lymphoid organs in which responding B cells undergo somatic hypermutation and selection for increased antigen affinity (affinity maturation).
In contrast, IgG4 anti-galectin-3 antibodies could be of direct pathogenic significance in IgG4-RD. Spontaneous germinal center formation and accentuated autoreactivity have recently been observed in Gal3−/− mice suggesting that galectin-3 negatively regulates germinal center formation.35 IgG4 anti-galectin-3 autoantibodies may enhance inflammation and autoimmunity by interfering with the inhibitory role of galectin-3 in germinal center responses. These anti-galectin-3 autoantibodies may thus accelerate plasmablast differentiation, immunoglobulin production, follicular helper T cell expansion and further autoantibody development.
We know from the HIV vaccine trials that class switched memory B cells can be recalled at least 8 years later:
Activated B cells and plasmablasts that arise in germinal centers and infiltrate tissue sites are likely to be important for the re-activation of CD4+CTLs and may thus contribute to the inflammatory pathology observed in this disease.
In the case of galectin-3 as an auto-antigen, the association of auto-antibodies with over-expression of this protein, suggests that this specific immune response and the clonal expansion of plasmablasts in the index subject occurred secondary to rather than being the cause of the underlying disease.
In the microenvironment of tumefactive lesions as in IgG-4RD, similar to malignant tumors, many proteins may accumulate to pathologic levels. Therefore, over time and without treatment, IgG4-RD may beget additional antigenic triggers, which could either amplify or attempt to quell the fibro-inflammatory process depending on the predominant autoantibody subclass response.
Long term expression of spike protein in lymph node germinal centres (GCs)
This is quite relevant as persistent presentation of Gal-3 with IL-3, IL-4 & IL-10 is associated with class switching to IgG4.
An analysis of plasma samples from three-times BNT162B2 boosted vaccine recipients found that spike-specific immunoglobulins (not separated by subclass) remained elevated for 9 months, and elevated further still after boosting.
From “Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination”14 (2022) by Roltgen et al:

Histological analysis of draining LN shows marked impairment of GCs in severe COVID-19 compared with mRNA vaccination, higher quantities, and persistence of spike antigen accumulated in the GCs of mRNA vaccinees and detectable vaccine RNA in GCs for up to 2 months post-second dose.
And yet the fact-checker “experts” assured us it stayed in the arm for a couple of days.
This never made much sense as the antigen presenting cells migrate from the tissues to present antigens to the T and B cells in the lymph nodes.
“Science”, see:
Dr. Zaher Merhi MD, OBGYN and founder of the Rejuvenating Fertility Center, shed some light on the evidence available to dispute the claim that the COVID-19 vaccine may damage male reproductive organs.
“For those who are skeptical: the mechanism by which mRNA vaccines work could not physiologically cause any fertility problems since the vaccine itself does NOT circulate into the blood; rather, the vaccine stays in the arm (site of injection) and the body develops immunity to it. This natural immunity is what circulates in the blood and cannot be harmful,” Merhi said.
“With All Due Respect to Nicki Minaj, There’s Still Zero Evidence COVID Vaccines Cause Swollen Testicles (Updated)” (2021)
https://www.yahoo.com/lifestyle/according-experts-zero-evidence-covid-230004784.html
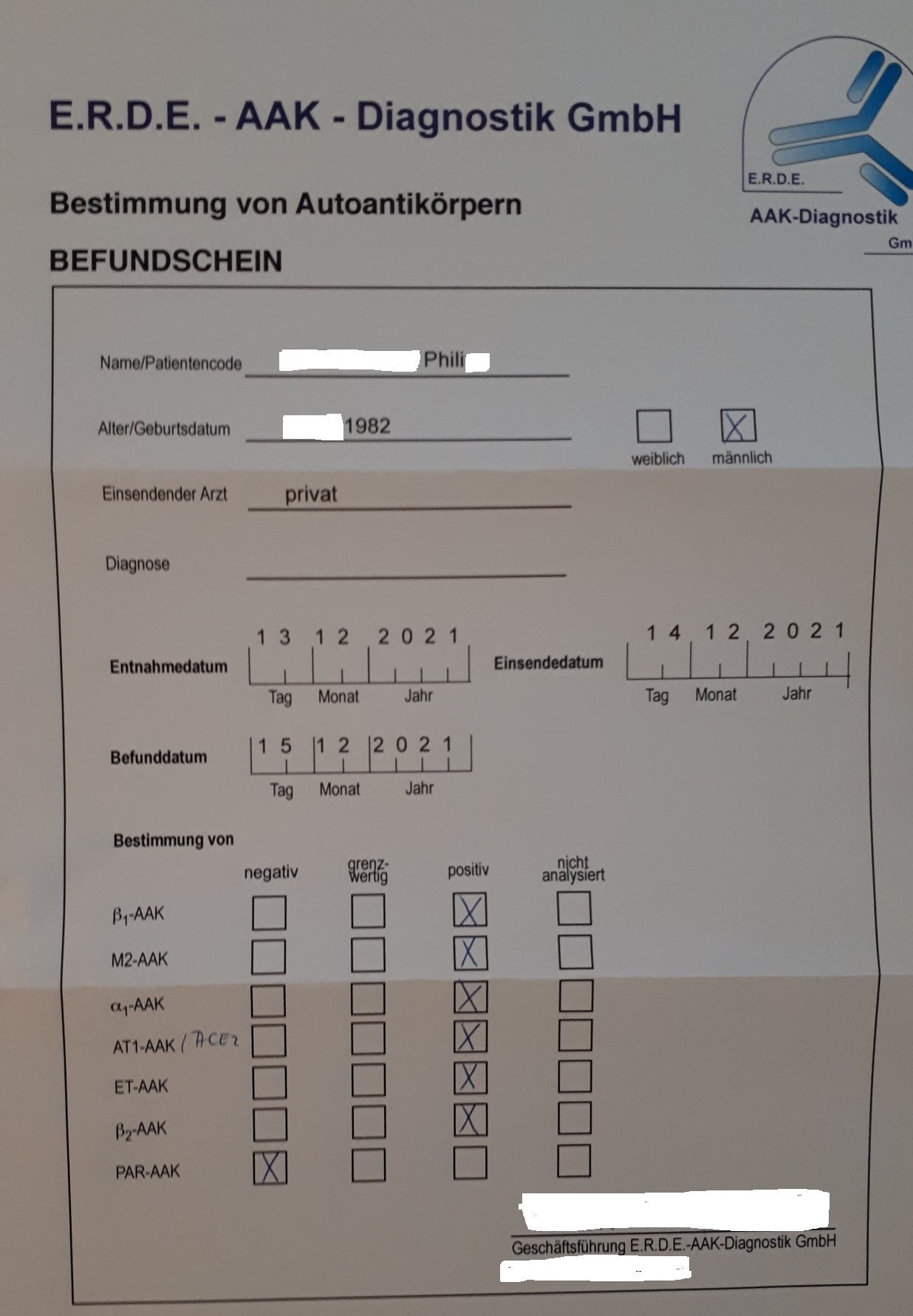
And a patient who published his results goes one better than this. He finally found competent physicians who checked his bloods and they confirmed non-viral spike protein (ie synthetic) 2 years after transfection. Unfortunately this also led to a host of autoimmune antibodies, but they didn’t test for IgG4 or anti-Gal-3 aa’s.
Its in German so I include a translation:
Many people ask me: How do you actually know that you have a #Impfschaden #postvac and not #LongCovid ? Well, almost 2 years after my @BioNTech_Group #Impfung the University of Tübingen still found spike protein in my blood. But no evidence of #Corona infection.
https://twitter.com/Zero_Long_Covid/status/1694013042596446640?s=20
Yes, the only problem is that the spike protein is very similar to our body's own cells and the immune system "confuses" spike-infested and healthy cells to a certain extent. Then the body turns against itself with so-called autoantibodies.
Beta1 aa’s are adrenergic receptor autoantibodies and these are linked to Postural orthostatic tachycardia syndrome (POTS)15.
M2 aa’s are antimitochondrial autoimmune antibodies, and are linked to liver disorders16.
Both Alpha1 adrenergic receptor and Beta1 aa’s are linked to Alzheimer’s disease pathology and vascular dementia17.
AT1/ACE2 aa’s are linked to PASC18, Parkinson’s Disease19, hypertension and cardiovascular disease. ACE2 also acts as an anti-oxidant too. Think of accelerated aging and you get the idea.
ET aa’s relate to endothelial cells, and are linked to autoimmune rheumatic diseases, lupus, vasculitis, sarcoidosis20 and vascular injury in scleroderma renal crisis21.
Beta2 aa’s are associated with glaucoma22, POTS and associated autonomic neuropathies (ie damage to the nerves that control automatic body functions including blood pressure, temperature control, digestion, bladder function and sexual function)23.
found the answer on the lab's website, et = endothelin, which i'll assume is a part of the endothelium
https://twitter.com/a_nineties/status/1694337028903645680?s=20
Some people react to a corona infection or to vaccination with the formation of agonistic autoantibodies. The reason for this may be the reaction to the spike protein, which also controls a G protein-coupled receptor to enter the cell.
The reaction of those affected depends on how strongly the immune system has reacted. There is no specific constellation for this.
The receptors for the B-1 AR, B-2 AR and M2 receptors are located in the smooth muscle cells, especially in the heart but also in the first stem, in the adipose tissue and in the nerve cells.
The receptors for the other agonistic autoantibodies (alpha, AT-1, ACE2 and endothelin) are mainly located in the vessels but also in the heart, brain, liver, intestines, kidneys and bronchi.
In the diagnosis of agonistic autoantibodies, only the circulating autoantibodies can be determined, but these are not in any quantity equilibrium to the bound autoantibodies. For this reason, we do not give titers, as we cannot measure the bound autoantibodies because they are firmly bound in the tissue. The autoantibodies are not part of the healthy immune system. For this reason, no reference ranges are given.
Due to the long-lasting binding of the autoantibodies ( 7-21 days ), the physiological agonist can no longer reach the receptor and the various signaling cascades no longer run correctly. This lack of nutrients often leads to mitochondriopathy.
For the reasons mentioned, patients suffer from various complaints.
The agonistic autoantibodies do not respond to immunosuppression, but only to the respective antagonists. The administration of antagonists is problematic if the patient has formed autoantibodies against several receptors because they lower the pulse and blood pressure.
For many decades, agonistic autoantibodies have been used for diseases of the heart muscle, high blood pressure, TAO, etc. removed with the help of immunoadsorption. This effective treatment has been published in numerous publications. If the agonistic autoantibodies are not removed, the consequences can be vascular and end-organ damage.
September 2022 Marion Bimmler
A pandemic of mystery illnesses
Reading the above alongside all the other pathologies I have reviewed in these Substacks since December 2021 and you get an idea of the complicated feedback processes in play and multiple conditions arising in consequence, perhaps decades later or due to future viral mutations.
And this is borne out daily in cases of “mystery illnesses” that are a challenge to even diagnose, let alone treat appropriately. You will also be joining an ever lengthening queue waiting for treatment.
I suspect that many influencers, some of whom were paid to promote vaccination to their followers24, may be all too aware of the likely causative factor due to multiple reports in the media and their own test results, or they might be post-viral, or they might be completely unrelated. But non-disclosure does not help others affected similarly.
Are these cases normal, after all we all get ill at some point?
Analysis by @EthicalSkeptic would tend to say not, and these will be the tip of the iceberg - your family friend, neighbour or colleague’s illnesses won’t be leading tonight’s headlines:
Bruce Springsteen concert postponed: Mystery illness leaves fans concerned
While specific details about Springsteen's illness have not been disclosed, his representatives declined to provide further comments, referencing his X post.
19 Jun 2021:
Bruce Springsteen will return to Broadway as the first show to open since March last year, but on the condition, attendees show proof of a COVID vaccine - approved by the US medical regulator.
Tori Spelling seen with bruising on her face while leaving hospital following mystery illness
The actress was discharged after a four-day stay.
…Although Spelling revealed she was in hospital, she didn't share any details on what caused her to be admitted. https://www.marca.com/en/lifestyle/celebrities/2023/08/22/64e4cffb22601d69178b456d.html
Sex/Life star Wallis Day reveals she was hospitalised with mystery illness as she issues health update
Sex/Life star Wallis Day has revealed she was admitted to St John & St Elizabeth Hospital in London during a mystery health battle.
The actress, 28, took to Instagram on Monday to share a series of snaps from her time in the ward as she thanked her loved ones for their support.
Wallis did not reveal why she was hospitalised but added a link to a leading kidney research charity in her bio.
‘IRREPARABLE LOSS’ Raju Punjabi dead – singer dies aged 40 after mystery illness left him hospitalised
SINGER Raju Punjabi has tragically passed away at the age of 40 after being hospitalised with a mystery illness.
The star is said to have started receiving treatment at a private facility in the Indian state of Haryana ten days ago.
GBS? Hmm, what could it be?
Jenna Jameson is ‘off all medication’ after mystery illness, addresses weight loss
Although she did not specify what medication she was no longer taking, she previously documented her health struggles amid a mystery illness battle for most of 2022.
The “Love Service” author first checked into a hospital when she lost her ability to walk and was initially diagnosed with Guillain-Barré syndrome in January 2022.
Jackie 'O' Henderson struck down by mystery illness as she reveals her bizarre symptoms
Jackie 'O' Henderson is battling some very unfortunate symptoms after falling ill with a mystery illness this week.
The radio host, 48, complained about her health condition to Dr Sam Hay, also known as Dr KIIS, on Tuesday's radio show, detailing symptoms such as a blocked nose and a loss of smell and taste.
…However, Dr Hay pointed out that Jackie's other symptoms didn't match up with the diagnosis of menopause.
'It's not necessarily [relevant]. We don't lose our sense of smell when it comes to the menopausal stuff,' he explained.
Amy Shark hospitalised and is set to undergo surgery for mystery illness - as singer is forced to cancel her US tour
Amy Shark has been forced to cancel her North American tour after being hospitalised with a mystery illness.
The singer, 37, is set to undergo surgery in the next few days.
The hitmaker announced the news on Instagram on Wednesday, and explained she's been suffering 'uncomfortable and painful moments'.
…'In recent days I've had some very uncomfortable painful moments and have just found out that I need surgery ASAP,' Amy's post read along with a photo of herself wearing a blue hospital gown.
'I am choosing to keep the details private but I'm all good DW. I will need a month to recover, meaning I have to cancel the North American tour next month.
Strictly's Greg Rutherford rushed to hospital with mystery illness
Strictly Come Dancing star Greg Rutherford dashed to A&E by fiancé after a mystery illness left him 'clawing at his skin and screaming in pain'
Greg Rutherford, the former Strictly Come Dancing star and track and field athlete, was rushed to the hospital after a suspected allergic reaction that left him in distress.
Super gym-fit, yet contracted sepsis:
GRATITUDE | Madonna ‘lucky to be alive’ after mystery illness that left her hospitalised
“I sobbed when I opened this gift because I realized how lucky I am to be alive.”
Madonna has thanked her fans and loved ones as she continues to recover from a mystery illness that left her hospitalised last month.
The star took to social media to express her gratitude following a bacterial infection that left her hospitalised for several days.
2020:
Madonna says she's had COVID-19; donates $1Million for vaccine
Madonna also clarified that she is currently not sick from the coronavirus.
Queen of Pop Madonna shared on Thursday evening, May 7, that she's probably had the coronavirus that's why she had to cancel her concert in Paris back in February early this year.
'America’s Got Talent' Winner Michael Grimm Is 'Sedated' and on a Ventilator Due to Mystery Illness
"We don't know what's wrong," the singer’s wife Lucie Zolcerva-Grimm told fans as he remains hospitalized after over a week
…She explained that no doctors could figure out what was wrong with the singer.
“He was looking increasingly sickly, all of a sudden he could barely walk, couldn’t lift his head, he couldn’t respond right away when I would ask him things, he would be really fussy,” she recalled.
https://people.com/americas-got-talent-michael-grimm-sedated-mystery-illness-7509849
The Chicks cancel multiple shows due to mystery illness
Formerly known as The Dixie Chicks, the female trio announced they have rescheduled shows amid 'ongoing illness'
https://www.foxnews.com/entertainment/the-chicks-cancel-multiple-shows-mystery-illness
Shane MacGowan looks frail as Clannad's Moya Brennan latest celebrity to visit his hospital bedside
The Pogues frontman was admitted back in July for a mystery illness
Hellraiser Shane MacGowan appears frail as the singer as celebrity pals continue to visit him while he remains in hospital.
The Pogues frontman was admitted to hospital in July with a mystery illness. The star had previously been treated for viral encephalitis last December.
https://www.irishmirror.ie/showbiz/irish-showbiz/shane-macgowan-looks-frail-clannads-30768854
Florence Welch rushed to hospital after needing life-saving surgery as gigs cancelled
Florence and the Machine singer Florence Welch has revealed she has had to pull out of the band's upcoming shows after needing emergency surgery.
The star took to Instagram on Sunday to inform fans about why the group could no longer perform several of their scheduled European concert dates.
In the post, Florence explained she didn't feel "strong enough" to go into details about the reason behind her hospitalisation.
Dina Asher-Smith upbeat about Olympics after worlds bid hit by mystery problem
…She said: “I was going great and then I just couldn’t feel anything below my waist.
“That’s why I was able to go and run the (100m) final because I wasn’t in pain but neurally I didn’t have any control. I was still dealing with that.
“It was about still coming back and just making everything work. I’m grateful to have got through it all in one piece after just not being able to feel from here (waist) downwards during that 100m.
Liam Payne rushed to hospital with 'serious' condition and forced to cancel tour dates
Liam Payne has cancelled his upcoming tour after suffering a 'serious kidney infection'. The One Direction star has been forced to call off his performances in South America after being rushed to hospital.
The 29-year-old is now following strict doctors orders to 'rest and recover' after his health scare. Announcing the news on social media, Liam said he was 'sorry' to anyone who had bought tickets and that refunds were being processed.
Tony Hadley rushed to hospital as he's forced to pull out of gig at the last minute
Spandau Ballet's Tony Hadley was rushed to hospital moments before he was due to take to the stage, forcing him to cancel the gig at the last minute.
The singer, who is best known for his 80s band with hits True, fell ill and needed medical care. He was set to perform at the Clearer Water Antrim Coast Half Marathon Live Lounge in Northern Ireland. But during soundcheck, it became clear to onlookers that Tony, 63, was unwell. He met some of his fans for a meet and greet on Sunday evening after the soundcheck, but appeared uncomfortable. When he returned to take to the stage, Tony told organisers he wasn't well. He was then taken to hospital.
Michelle McTernan, who was in charge of PR for the event told the Belfast Telegraph : "We had to do what was right by him and he was taken to hospital, and his crew have all gone with him, to stick together. We hope he gets better soon."
Therapeutics
Ahmed et al (2023) reviewed the key pathologies associated just with Gal-3 and included some great slides to illustrate pro-tumour effects and fibrosis. Gal-3 looks like a red and blue dart25:
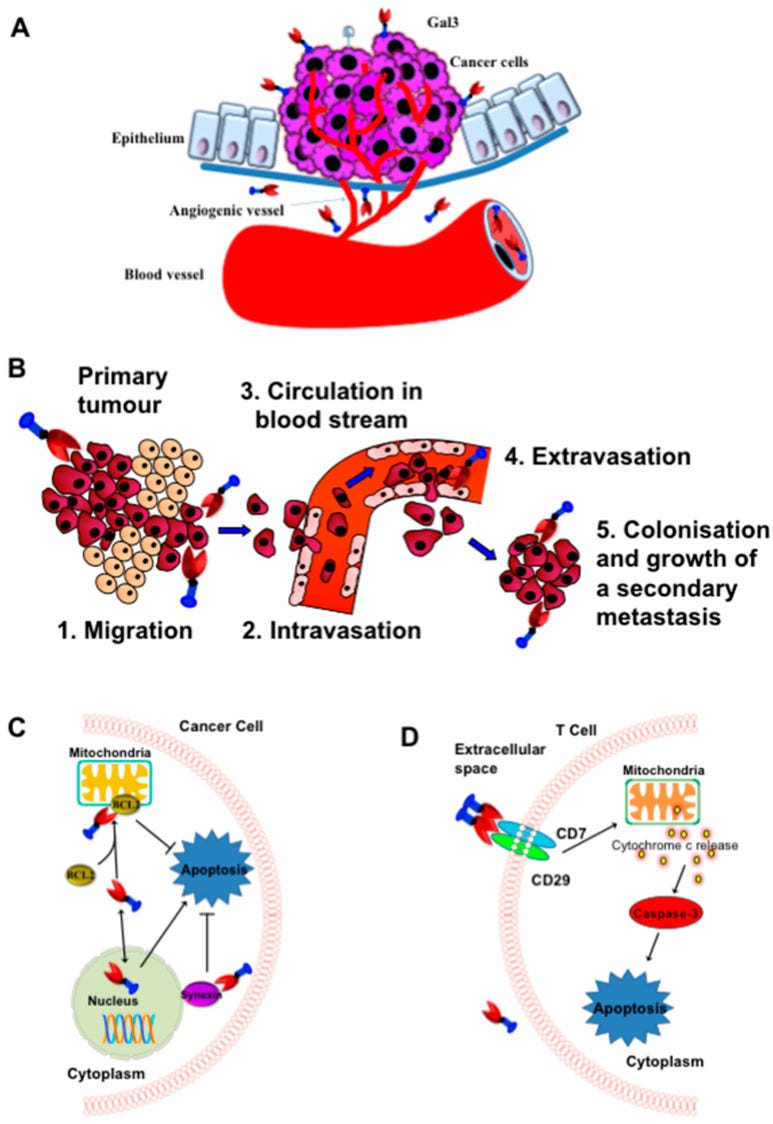
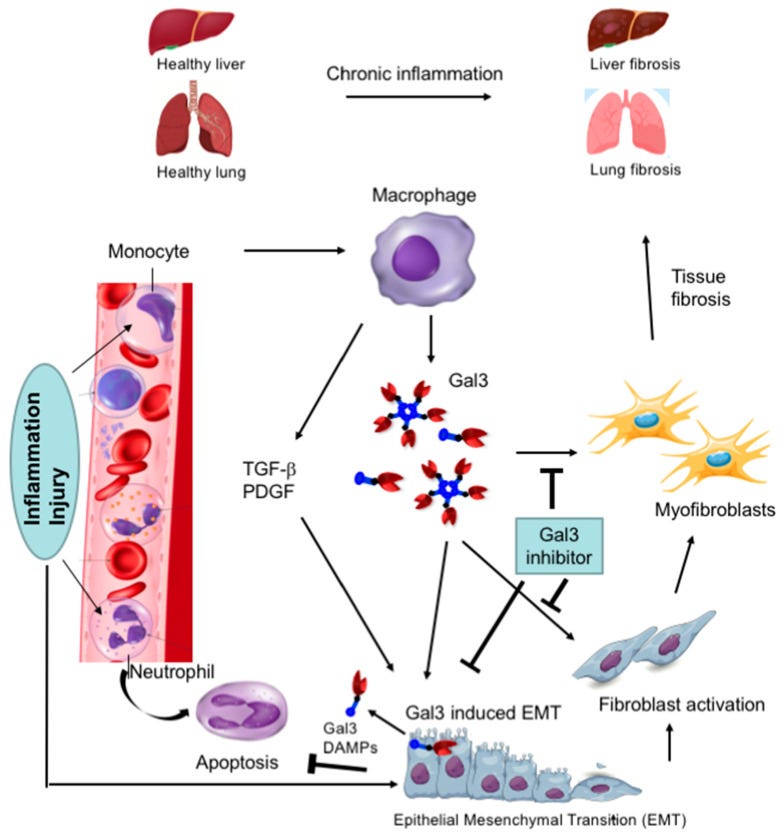
Research and clinical trials are mainly focused on synthetic small and large molecule Gal-3 inhibitors. Small synthetic molecules have the potential to be toxic at higher doses, whereas larger molecules don’t tend to bind so well. Research is still progressing into these.
Of particular note, they also reviewed modified citrus pectin (MCP):
Modified citrus pectins (MCP) are produced from citrus pectin by sequential alkali and acidic hydrolytic processes to enhance absorbability. Studies in cell lines and animal models suggested that MCP could be an effective anti-metastatic drug for many cancers [185,186,187,188]. The MCP was shown to inhibit in vitro tumor cell adhesion to endothelium [186] and homotypic aggregation and the formation of metastatic deposits of human breast and prostate carcinoma cells in lungs and bones in xenograft rodent models [185,187].
Recently, Gal3-targeting multifunctional core-shell glyconanoparticles based on the low molecular weight dialdehyde oligomers of citrus pectin (CPDA) have been described [188]. These CPDA-based core-shell nanoparticles have been shown to reduce homotypic cellular aggregation, tumor-endothelial cell interactions, and endothelial tube formation—the significant steps of cancer progression [188].
The great thing about MCP is that it is available cheaply, almost off the shelf, and it is being trialled. Unfortunately I would not expect it to be allowed to progress further exactly for this reason:
MCP is commercially available as a food supplement, and at least two clinical investigations of MCP have been completed so far on prostatic neoplasm (FDA-regulated, NCT01681823, Phase 2) and hypertension (non-regulated, NCT01960946). The former study of MCP on prostatic neoplasm assessed its effect on prostate-specific antigen (PSA) kinetics in biochemically relapsed prostate cancer with serial increases in PSA. Moreover, Massachusetts General Hospital (MGH) initiated a Phase 3 randomized, double-blind clinical trial of MCP on knee osteoarthritis (n = 50) (NCT02800629), but the recruitment status is “unknown” on clinicaltrials.gov (accessed on 20 February 2023).
Optimal therapy of biochemically relapsed prostate cancer (BRPC) after local treatment is elusive. An established modified citrus pectin (PectaSol®, P-MCP), a dietary polysaccharide, is an established antagonist of galectin-3, a carbohydrate-binding protein involved in cancer pathogenesis.
…Sixty patients were enrolled, and one patient withdrew after a month. Patients (n = 59) were given P-MCP, 4.8 grams X 3/day, for six months.
…After six months, 78% (n = 46) responded to therapy, with a decreased/stable PSA in 58% (n = 34), or improvement of PSADT in 75% (n = 44), and with negative scans, and entered the second twelve months treatment phase. Median PSADT improved significantly (p = 0.003). Disease progression during the first 6 months was noted in only 22% (n = 13), with PSA progression in 17% (n = 10), and PSA and radiologic progression in 5% (n = 3). No patients developed grade 3 or 4 toxicity.
Keywords: PSA doubling time; PectaSol; modified citrus pectin; non-metastatic biochemically relapsed prostate cancer.
From: “Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Results of a Prospective Phase II Study” (2021)
I conducted a PubMed keyword search for efficacy of therapeutics listed in the therapeutics Substack, and also for ellagic acid (pomegranate) and Nigella sativa (black seed):
Its challenging to inhibit its action due to poor druggability of the Gal-3 CBD site.
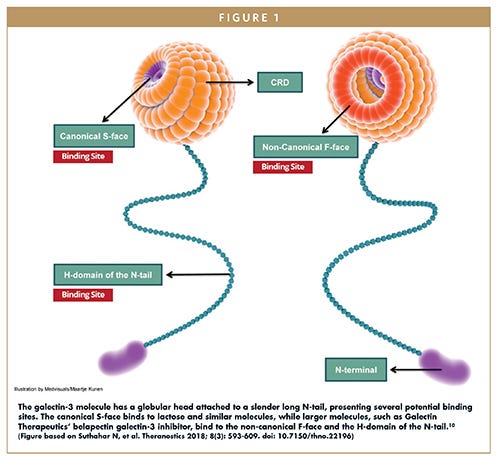
Note the potential binding sites from “GALECTIN INHIBITORS – Is a Galectin-3 Inhibitor the Answer for Millions of Patients With Cirrhosis & Cancer?”26 and the spiralling β-strands we’ve seen depicted in different ways:
Apart from synthetic inhibitors and MCP, I found supporting research for resveratrol via an indirect pathway:
RES has no effect on mRNA of galectin‐3 (GAL‐3, A) but increases mRNA of miR‐424 (B) in both SKOV3 and OVCAR‐3 cell lines. C and D: demonstrate negative correlation between mRNA of miR‐242p [ed: miR-424] levels and protein levels of GAL‐3 in both SKOV3 and OVCAR‐3 cell lines, respectively. Notes: Data are presented as mean ± SD of n = 6 samples/group
From: “The apoptotic effect of resveratrol in ovarian cancer cells is associated with downregulation of galectin‐3 and stimulating miR‐424‐3p transcription” (2019)
Berberine:
BBR suppressed ox-LDL-induced upregulation of galectin-3 and macrophage activation. Overexpression of galectin-3 intervened the inhibitory effect of BBR on macrophage activation. BBR activated phospho-AMPK and inhibited phospho-NF-κB p65 nuclear translocation. AMPK inhibition and NF-κB activation abolished the inhibitory effects of BBR on galectin-3 expression and macrophage activation. Combination of BBR and rosuvastatin exerted greater effects than BBR or rosuvastatin alone. However, BBR treatment did not further reduce plasma galectin-3 after PCI in patients receiving standard therapy. In conclusion, BBR alleviates ox-LDL-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways.
From: “Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways” (2018)
Quercetin:
Plaques from ApoE-/- mice fed by high-fat diet (HFD) with or without quercetin (100 mg (kg·bw)-1 ) for 16 weeks, and carotid plaques from patients with carotid stenosis are collected for histopathological examinations and molecular mechanism assays. Quercetin significantly alleviates atherosclerotic lesions and reduces lipid retention caused by HFD. Proteomic technology identified Gal--3 increased by HFD but lowered by quercetin. Furthermore, immunofluorescence and immunohistochemistry exhibit higher expressions of Gal-3 and NLRP3 in carotid plaques and plaques from HFD-fed mice, which are concurrently down-regulated by quercetin. Similar to TD139, quercetin dramatically suppresses NLRP3 inflammasome activation in oxidized low-density lipoprotein-laden macrophages, and accordingly alleviates cellular steatosis and IL-1β secretion, which is abolished by recombinant Gal-3. Co-immunoprecipitation shows Gal-3 binding to NLRP3 promotes inflammasome activation.
From: “Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway” (2021)
Nigella sativa seed (black seed) & silymarin:
…We investigated the effect of Nigella sativa seed (black seed), a common hepatoprotective natural remedy, on Gal-3 level and progression of liver fibrosis in thioacetamide (TA)-induced liver injury in rats.
Treatment with silymarin and black seed resulted in decreasing levels of liver Gal-3 and TGF-β1 and marked improvement in liver functions, as well as reducing the fibrotic areas in the liver. Gal-3 exhibited positive correlation with TGF-β1, malondialdehyde, nitric oxide, alanine transaminase and aspartate transaminase, while it negatively correlated with total antioxidant capacity and catalase.
Conclusion
Black seed reduced liver Gal-3 level and ameliorated fibrogenesis in liver due to TA administration.
From: “Nigella sativa seed reduced galectin-3 level and liver fibrosis in thioacetamide-induced liver injury in rats” (2017)
Some names are regulars to this slot, such as quercetin and resveratrol.
If you have others by all means share them in the comments? Thanks.
As for IgG4, if you can keep spike protein levels as low as possible and block the binding affinity of the galectin fold then this should be moderated as much as possible, but prevention is much better than cure:

Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References
Rubio-Casillas A, Redwan EM, Uversky VN. Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system? Published online August 9, 2023. doi:10.20944/preprints202308.0776.v1
Standardized Incidence Ratio (SIR), A Math-based Approach to Evaluating Unusual Patterns of Cancer.
https://www.cdc.gov/nceh/cancer-environment/pdfs/standardized-incidence-ratio-fact-sheet-508.pdf
Yu T, Wu Y, Liu J, Zhuang Y, Jin X, Wang L. The risk of malignancy in patients with IgG4-related disease: a systematic review and meta-analysis. Arthritis Research & Therapy. 2022;24(1):14. doi:10.1186/s13075-021-02652-2
Chung AW, Ghebremichael M, Robinson H, et al. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Science Translational Medicine. 2014;6(228):228ra38-228ra38. doi:10.1126/scitranslmed.3007736
Sciacchitano S, Lavra L, Morgante A, et al. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int J Mol Sci. 2018;19(2):379. doi:10.3390/ijms19020379
Ribbon diagram - Wikipedia
Schroeder JT, Bieneman AP. The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3. Front Immunol. 2022;13:831763. doi:10.3389/fimmu.2022.831763
Mukaida N, Sasaki S ichiro, Baba T. CCL4 Signaling in the Tumor Microenvironment. In: Birbrair A, ed. Tumor Microenvironment: The Role of Chemokines – Part A. Advances in Experimental Medicine and Biology. Springer International Publishing; 2020:23-32. doi:10.1007/978-3-030-36667-4_3
Silverman AM, Nakata R, Shimada H, Sposto R, DeClerck YA. A galectin-3-dependent pathway upregulates interleukin-6 in the microenvironment of human neuroblastoma. Cancer Res. 2012;72(9):2228-2238. doi:10.1158/0008-5472.CAN-11-2165
Lorenz P, Steinbeck F, Mai F, Reisinger EC, Müller-Hilke B. A linear B-cell epitope close to the furin cleavage site within the S1 domain of SARS-CoV-2 Spike protein discriminates the humoral immune response of nucleic acid- and protein-based vaccine cohorts. Front Immunol. 2023;14:1192395. doi:10.3389/fimmu.2023.1192395
Cosentino M, Marino F. The spike hypothesis in vaccine-induced adverse effects: questions and answers. Trends Mol Med. 2022;28(10):797-799. doi:10.1016/j.molmed.2022.07.009
Yonker LM, Swank Z, Bartsch YC, et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147(11):867-876. doi:10.1161/CIRCULATIONAHA.122.061025
Perugino CA, AlSalem SB, Mattoo H, et al. Identification of Galectin-3 as an Auto-Antigen in IgG4-Related Disease. The Journal of allergy and clinical immunology. 2019;143(2):736. doi:10.1016/j.jaci.2018.05.011
Röltgen K, Nielsen SCA, Silva O, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185(6):1025-1040.e14. doi:10.1016/j.cell.2022.01.018
Adrenergic receptor autoantibodies - Wikipedia
https://en.m.wikipedia.org/wiki/Adrenergic_receptor_autoantibodies
Antimitochondrial Antibody and AMA M2 (2021)
https://www.testing.com/tests/antimitochondrial-antibody-and-ama-m2/
Karczewski P, Hempel P, Kunze R, Bimmler M. Agonistic Autoantibodies to the α1-Adrenergic Receptor and the β2-Adrenergic Receptor in Alzheimer’s and Vascular Dementia. Scandinavian Journal of Immunology. 2012;75(5):524-530. doi:10.1111/j.1365-3083.2012.02684.x
Arthur JM, Forrest JC, Boehme KW, et al. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLOS ONE. 2021;16(9):e0257016. doi:10.1371/journal.pone.0257016
Labandeira CM, Pedrosa MA, Quijano A, et al. Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson´s disease. npj Parkinsons Dis. 2022;8(1):1-12. doi:10.1038/s41531-022-00340-9
Domiciano DS, Carvalho JF, Shoenfeld Y. Pathogenic role of anti-endothelial cell antibodies in autoimmune rheumatic diseases. Lupus. 2009;18(13):1233-1238. doi:10.1177/0961203309346654
Hegner B, Kretzschmar T, Zhu N, et al. Autoimmune activation and hypersensitization of the AT1 and ETA receptors contributes to vascular injury in scleroderma renal crisis. Rheumatology (Oxford). 2023;62(6):2284-2293. doi:10.1093/rheumatology/keac594
Hohberger B, Kunze R, Wallukat G, et al. Autoantibodies Activating the β2-Adrenergic Receptor Characterize Patients With Primary and Secondary Glaucoma. Frontiers in Immunology. 2019;10. Accessed August 28, 2023. https://www.frontiersin.org/articles/10.3389/fimmu.2019.02112
Li H, Kem DC, Reim S, et al. AGONISTIC AUTOANTIBODIES AS VASODILATORS IN ORTHOSTATIC HYPOTENSION: A NEW MECHANISM. Hypertension. 2012;59(2):402-408. doi:10.1161/HYPERTENSIONAHA.111.184937
Kelly J. Thousand-Dollar Cash Payments And A TikTok ‘Influencer Army’ Are Part Of The Campaign To Get People Vaccinated. Forbes. Accessed August 28, 2023. https://www.forbes.com/sites/jackkelly/2021/08/04/thousand-dollar-cash-payments-and-tiktok-influencer-army-are-part-of-the-campaign-to-get-people-vaccinated/
Ahmed R, Anam K, Ahmed H. Development of Galectin-3 Targeting Drugs for Therapeutic Applications in Various Diseases. Int J Mol Sci. 2023;24(9):8116. doi:10.3390/ijms24098116
Marino D. GALECTIN INHIBITORS - Is a Galectin-3 Inhibitor the Answer for Millions of Patients With Cirrhosis & Cancer? Drug Development and Delivery. Published January 14, 2022. Accessed August 29, 2023. https://drug-dev.com/galectin-inhibitors-is-a-galectin-3-inhibitor-the-answer-for-millions-of-patients-with-cirrhosis-cancer/


















And the difference is what exactly?
The nurse must be seeing lots of lumps.
***
Newly-wed mum, 37, dies after cancer symptoms 'dismissed as Covid jab side-effects'
...The NHS nurse manager claimed that medical professionals initially misdiagnosed her symptoms as side-effects from the Covid vaccine. She was reportedly told twice that there was 'nothing to worry about' after discovering a lump, attributing her symptoms to the Pfizer vaccine.
https://www.mirror.co.uk/news/uk-news/newly-wed-mum-37-dies-30821493
Breaking news, its happening:
Influencer Larissa Borges dies aged 33 after double cardiac arrest
The influencer passed away on Monday August 28 following a week-long coma
Larissa's family shared the devastating update on her Instagram account
Local police are reportedly investigating the 33-year-old's death
Instagram influencer, Larissa Borges, has tragically died aged 33 following a double cardiac arrest.
She passed away on Monday August 28 after being hospitalised in a coma for over a week.
Larissa was first admitted to hospital on August 20, after suffering cardiac arrest in the city of Gramado, according to posts from her family on social media.
After suffering a second cardiac arrest, the Brazilian influencer and physical education student sadly passed away.
https://www.msn.com/en-gb/health/other/influencer-larissa-borges-dies-aged-33-after-double-cardiac-arrest/ar-AA1fYX6U?ocid=entnewsntp&cvid=c903be19a96f43cd86162f60aa5178bf&ei=7