“Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?” - Further reading (Part I)
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Also available with the translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
I was honoured to be invited as a co-author for this review paper. It passed peer review on 5th April, and, so far, has escaped the usual vaporous retraction requests that harass unapproved thoughts.1 The quick take is that we discussed an in vivo study investigating the link between cancer and the degree of nucleoside-modified mRNA used. This research was conducted to improve future mRNA cancer vaccines and treatments.
The authors found that using 100% n1-methyl-pseudouridine (m1Ψ) to replace a ribonucleoside called uridine in mRNA led to more efficient, longer-lasting synthesis of a protein antigen, but this was associated with impaired innate immunity and metastasis to the lungs of the mice, rather than prevention of it.
As many synthetic gene therapy products are in the pipeline, especially to treat or prevent cancer, we urged caution against using 100% m1Ψ modified mRNA, and to conduct extensive, long-term research to confirm the hypothesis - the precautionary principle.
Dr. Robert Malone (the acknowledged inventor of mRNA vaccines) and Jan Jekielek (Senior Editor at the Epoch Times) recently discussed the paper, and we wish to extend our thanks to them for this extended peer review:
Telegram: https://t.me/RWMaloneMD/8893
Epoch Times video: https://theepochtimes.com/epochtv/the-modified-mrna-cancer-link-explained-fallout-5642741
We have come under attack from the so-called “fact checkers”. One of these is called “NewsGuard”, they are here to combat misinformation for you. They also have a talent for parody writing.
(Highlights mine throughout.)
To #####:
My name is #####, ##### at NewsGuard. We're a news organization that reports on and tracks online misinformation.
I'm hoping you will comment on criticism of your article in the International Journal of Biological Macromolecules, titled "Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?"
A FactCheck.org article published today included comment from numerous experts and researchers saying your review "misleads by misinterpreting several studies and the role of N1-methylpseudouridine in vaccines" and ignored the abundant evidence that COVID vaccines cannot cause cancer and do not impair the immune system. https://www.factcheck.org/2024/05/still-no-evidence-covid-19-vaccination-increases-cancer-risk-despite-posts/
What is your response to these researchers' statements that your review article misrepresents the research it cites and that your conclusions are not supported by available evidence about COVID vaccines?
--
Best regards,
########
Judging by the frantic arm-waving I suspect that none of them have taken the time, or had the skill to read the paper, or understand what clearing peer review means. It runs to 12 pages of double-column text, with 151 references, and we certainly don’t have the time or see the point in walking them through all these.
What they could do is read this Substack, the aim of which is to provide background to the paper. I focus on other areas of concern in Part II that were out of the scope of our review.
Turning the lamp the other way, NewsGuard was seeded into existence at a very opportune point in history, just as various biolabs were fine-tuning their products in the US and China.
The UK Column did some fact checking for us, after coming under attack themselves back in 2020. Follow the money:

Another came from “Science Feedback”. Alberto did respond to them and the Epoch Times. Science Feedback’s mission statement:
Our first mission is to help create an Internet where users will have access to scientifically sound and trustworthy information. We also provide feedback to editors and journalists about the credibility of information published by their outlets.
Our mission is pedagogical: we strive to explain whether and why information is or is not consistent with the science. We are nonpartisan and apply the same methodology to claims made in a variety of media outlets, as well as exposing claims that either contradict or over-hype science. We believe it is scientists’ civic duty to better inform our fellow citizens in our area of expertise.
The scientific method is not there to “explain whether and why information is or is not consistent with the science”, whatever that means. It is there to discuss, challenge, create, and test hypotheses.
Their home page takes aim at climate deniers, coconut oil, aspirin, and critics of the HPV vaccine, amongst other things not consistent with the science.
If we follow the money and not the science, one of their biggest sponsors is the “Akosha Fellowship”:
For more than 40 years, Ashoka has built and nurtured the largest network of leading social entrepreneurs in the world by electing them as Fellows.
https://www.ashoka.org/en-gb/program/ashoka-venture-and-fellowship
Without naming names if you look up who supports at least one of their main sponsors you find a familiar list cropping up like a bad penny. These include Bayer, Boehringer Ingelheim, Eli Lilly Canada, Genome Canada, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda Pharmaceuticals, and the Wellcome Trust.
These fact-checks are, once again, almost satirical. We have been put to rights by a team of experts even more expert than our peer review panel. Impressive stuff.
The science, see!




You had an asymptomatic stage 4 recurrence, but didn’t know it until now:
“What we might see is that there are actually more cases of cancer, because people caught up. But it’s not truly an increase. It’s just a catch up of what was there that we didn’t diagnose during 2020.”
- Lisa Richardson, the director of the CDC’s Division of Cancer Prevention and Control.
The EthicalSkeptic does an excellent job at debunking the big pharma-linked “fact checkers”:
This is the 5th-grade level explanation about cancer:
A. More young people are dying of cancer now (left).
B. Fewer older people are dying of cancer now, because they are already dead from Covid (right).
These two effects cancel each other out because one is up and the other is down - This is called an 'age bracket Simpson effect'.
This does not mean that cancer is 'flat...' Even if you force your graph to look flat by a variety of data tricks. We have another whole article listing those tricks and how they work to make flat-looking graphs.
Since cancer is up 25% in younger ages, then because it appears flat overall, likely ALL ages are up in rate as well, but the excess is just temporarily hidden by the Simpson effect.
Application Questions:
1. What if a skilled analyst tried to un-hide these deaths now, so that we knew about them and could examine this issue closer? Would that be wise?
2. What if this made very powerful people very angry - people who don't want any cancer increase to be considered possible. And these people threatened the analyst and his family. Should this analysis still be attempted?
3. Are the people who post graphs hiding the cancer death increase by means of this Simpson effect and the other tricks we cited, telling the truth?
4. See if you can think of some reasons why those powerful people and graph fabricators might want to lie about this issue.
https://twitter.com/EthicalSkeptic/status/1787268565445325274
5th-grade explanation of the cancer death chart:
Now say that 100 people typically die of cancer each week. Then suddenly in early 2021, 150 people start dying of cancer each week, but those new 50 cancer patients also show up with a bunch of other illnesses, possibly caused by the same toxin which also caused their cancer.
They all still die of cancer, but many of these are not listed as 'dying of cancer' because you can only choose one cause of death, and they have a BUNCH of illnesses all at the same time.
Questions in Application:
A. Should all 50 of these extra cancer deaths each week be hidden from the cancer counts? Is that ethical?
B. When families protest that 'early onset Alzheimers' was listed on the death certificate of a person who died of colon cancer (because they were 'confused' for the two days prior to their death), yet cannot get the certificate corrected, is this an ethical circumstance for the family? for society at large?
C. If an analyst had the right information available to correct for such cancer undercounting, ethically should they correct these counts? Do they bear a responsibility to do so - even if health officials ignore the error?
This chart (below) corrects for that error, called in science, categorical fallacy. One cannot use strict criteria in order to deceive, by sorting a threatening signal into different buckets, so that it is more difficult to see. It is akin to playing semantics on what one did wrong in order to avoid getting caught, or a thief hiding the money they stole, in 20 different bank accounts so that no one can easily see that they have extra money all of a sudden.
D. If one claims to be an expert cancer analyst, is aware of this cancer categorical error's existence, and still chooses to show the 'one choice only' stats, are they now using 'facts' in order to lie?
E. Is their choice to use the misleading numbers ethical, given that it does not serve 'informed consent' of those excess 50 cancer victims in future weeks?
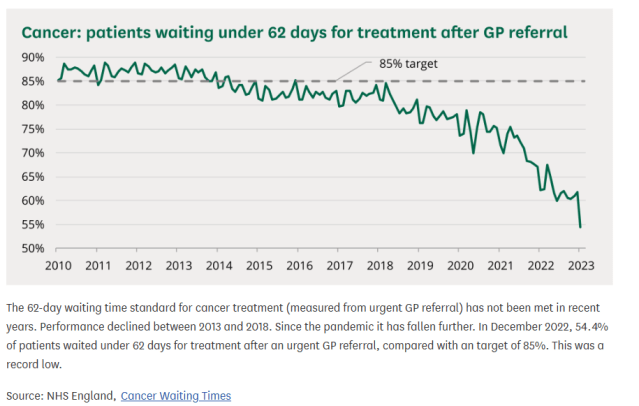
And cancer treatment waiting time targets in the UK are being missed:

Discussion
Most of the following points are adapted from a presentation by myself and Mikolaj to the Corona Investigative Committee on Friday 26th April.
Thank you Viviane Fischer for inviting us along. My accent appeared to confuse the translator at times, but here’s the 2-hour livestream link.
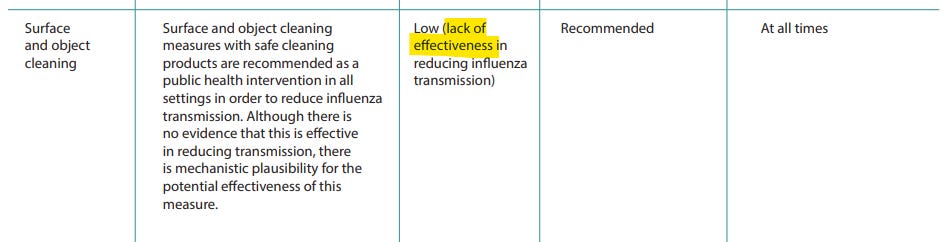
Despite a lack of evidence, non-pharmaceutical interventions (NPI’s) were recommended by the WHO in 2019 for a future influenza pandemic, according to “Non-pharmaceutical public health measures for mitigating the risk and impact of epidemic and pandemic influenza” (2019)
You can’t “see” a typical pandemic, but you can see NPI’s, or “viral theatre”.
Was this to err on the side of caution, or to promote panic and increase vaccine take-up?
This is what a surgical mask looks like to an aerosolised virus. Particles either go straight through it, round it, or both.
1 layer or 10, if you can get enough air through it to breathe then you can get enough aerosols through it to infect you.
Alcohol wipes etc. may remove or denature fomites on surfaces, but “we suggest fomite spread may not be a significant means of transmission for severe acute respiratory syndrome coronavirus 2 in real-world operational scenarios.”2
Contract tracing was never in contention:
Quarantine never had much supporting evidence either, or logic:
Perhaps one of the most despicable measures, “the science” of closing schools, but keeping liquor stores open:
It was never going to work as advertised
Although the HPV vax itself turned into a nightmare of increased cancer risk and lives ruined through side effects3, its inventor Prof. Frazer considered a possible coronavirus vaccine and knew a future lemon when he saw it.
From “We've never made a successful vaccine for a coronavirus before. This is why it's so difficult” (April ‘2020):
The challenge of respiratory infections
There are several reasons why our upper respiratory tract is a hard area to target a vaccine.
‘It's a separate immune system, if you like, which isn't easily accessible by vaccine technology,’ Professor Frazer told the Health Report.
Despite your upper respiratory tract feeling very much like it's inside your body, it's effectively considered an external surface for the purposes of immunisation.
‘It's a bit like trying to get a vaccine to kill a virus on the surface of your skin.’
On the risk of antibody-dependant enhancement (ADE):
It's hard to produce a successful vaccine if the virus isn't activating a strong
immune response.
And if a vaccine elicits an immune response that misses the target cells, the result
could potentially be worse than if no vaccine was given.
‘One of the problems with corona vaccines in the past has been that when the
immune response does cross over to where the virus-infected cells are it actually
increases the pathology rather than reducing it,’ Professor Frazer said.
‘So that immunisation with SARS corona vaccine caused, in animals, inflammation
in the lungs which wouldn't otherwise have been there if the vaccine hadn't been
given.’
On subunit vaccines vs using viral-induced antibodies cloned from serum:
“… Professor Frazer said the narrow, targeted approach is fine, unless you pick the
wrong specific antigen — the substance that stimulates an immune response which
antibodies bind to — in which case you could end up with the same problem.”
Emergency or not, if it’s not broken, don’t fix it
Traditional vaccines take 15 years or longer to develop, not 1.5 years. Note the lack of preclinical and toxicology studies for the synthetic mRNA gene therapy agents.
Phase II trials overlapped with I and II, and production was underway at the end of phase II.
This was a runaway train, and it was not going to be stopped:

They did know well in advance what “adverse outcomes” to expect. This was a slide from a presentation on 22nd October 2020, long before being signed off for mass administration.
Many of these AE’s are autoimmune or involve irreversible pathologies. Caseloads are, as a consequence, tending to go up rather than tail off.
Hat tip to the fact-checkers for confirming the story, and of course they were “not known” as no one had been crazy enough to try it out en masse before:

Leukocytes

Immunisation against respiratory viruses
As for long-term efficacy, parenterally administered vaccines rarely exceed 50% against respiratory infections.
“95% effective” always lacked credibility.

From a study by Tsukinoki et al., “Detection of cross-reactive immunoglobulin A against the severe acute coronavirus-2 spike 1 subunit in saliva” (2021):
Abundant secretory immunoglobulin A (SIgA) in the mucus, breast milk, and saliva provides immunity against infection of mucosal surfaces. Pre-pandemic breast milk samples containing SIgA have been reported to cross-react with SARS-CoV-2
The proportion of IgA-positive individuals aged ≥50 years
lower than that of patients aged ≤49 years (p = 0.008).
Siga was purified from the saliva of patients, which could partially suppress the binding of SARS-CoV-2 spike protein to the angiotensin converting enzyme-2 receptor.
This study demonstrates the presence of SARS-CoV-2 cross-reactive SIgA in the saliva of individuals who had never been infected with the virus, suggesting that SIgA may help prevent SARS-CoV-2 infection.
Adashi & Gruppuso reviewed the importance of mucosal immunity in “SARS-CoV-2 Vaccines: The Mucosal Immunity Imperative” (2022).
We now know that the first point was not correct:
The current parenterally administered SARS-CoV-2 vaccines are remarkable for their effectiveness in reducing the likelihood of hospitalization and in-hospital death.
However, their capacity to prevent breakthrough infections remains limited, a deficiency that appears to be related to the limited mucosal immune (IgA) response elicited by the parenteral vaccines.2
These realities are consistent with the observation that parenterally administered SARS-CoV-2 vaccines give rise to prominent increments in the circulating levels of IgG antibodies against the SARS-CoV-2 receptor-binding domain (RBD) and spike protein (SP) while parallel increments in the circulating levels of IgM and IgA counterparts have proven far more modest.3
Although a modicum of SARS-CoV-2–specific IgA activity was detected in nasal and salivary secretions after the parenteral administration of the mRNA-BNT162b2 SARS-CoV-2 vaccine, it appears that the degree of local mucosal protection afforded by most parenterally administered SARS-CoV-2 vaccines does not rise to a level that is clinically relevant.4
The mRNA vaccines in current use are capable of producing a mucosal IgG response, but current evidence suggests that it is the IgA response that is required to prevent breakthrough infections and their related communal spread.
It follows that vaccine-mediated all-out protection against SARS-CoV-2, one comprising both systemic IgG and mucosal IgA responses, has yet to materialize.
Nevertheless, vaccines that do evoke mucosal immune responses are not without potentially severe drawbacks. At which point, you have to question if this can ever be a safe and effective approach, whether administered parenterally or by targetting mucosa?
Key takes from a guest post from the journal “Mucosal Immunology” (2015):
Hyporesponsive = under-responsive.
The example of formalin-inactivated RSV vaccine illustrates the potential hazards of immunizing humans with the aim of inducing protective immunity at a mucosal surface. Because mucosal surfaces are continuously visited with a large variety of foreign, albeit mostly harmless antigens, the local immune system is predisposed to be hyporesponsive to most of these materials.
The perturbations caused by invading microbes, such as bacteria and viruses, are thought to arm the mucosal surface to a state of responsiveness. Consequently, nasally administered vaccine viruses, which have been carefully designed to exhibit limited virulence, may insufficiently disturb the mucosal surface to generate an effective immune response.
A Th2 response is the allergic type response, for example with asthma:
In addition, even when preparations are sufficiently immunogenic, resident antigen-presenting cells in concert with T lymphocytes can direct individual responses toward Th1 or Th2 dominance (Neurath et al., 2002).
The cytokine profile secreted by activated Th1 cells promotes the development of antigen-specific cytotoxic T lymphocytes, and Th2 cells promote the activation of B cells to produce specific antibodies, including S-IgA antibodies. Both responses make important contributions to the resolution of respiratory infections, but overproduction of STAT6 and GATA-3 can polarize the immune response toward Th2 cell hyperactivity, leading to allergic responses such as asthma (Neurath et al., 2002).
Whereas the Th1 response may promote Crohn’s:
Similarly, activation of the transcription factor T-bet can lead to Th1 hyperresponsiveness, leading to immunopathologic responses such as that seen with Crohn's disease (Ouyang et al., 2000).
Thus, respiratory viral vaccines (including materials coadministered to enhance immunogenicity) must be carefully evaluated to ensure that they lack the capacity to induce harmful immune responses in recipients.
You also need to keep being re-exposed to your vaccinal antigens:
One significant drawback to vaccination strategies for viruses such as influenza that induce homotypic and usually transient immunity is that continual revaccination is required. T-cell immunity, which is primarily directed against nonvariant internal antigens of these viruses (heterotypic immunity), has the potential to provide cross-protection against recurrent disease.
One solution they discuss is DNA vaccination, which takes us back to where we started, with no simple one-size-fits-all solution:
Unfortunately, CD8+ CMI responses to respiratory viruses appear to decline rather rapidly (Woodland, 2003). Recent studies in mice have revealed that long-term CD8+ CMI responses can be generated, particularly with use of DNA vaccination.
These studies, while still relatively early, may eventually lead to vaccine strategies that target the induction of long-term, cross-reactive T-cell immunity that could reduce the requirement for revaccination.
The complexities of the mucosal immune system thus present daunting challenges for vaccinologists. Many of the strategies under study have enormous promise, but they must be examined with great care to guard against the equally great capacity for inadvertent harm.
Gene expression
• Nucleotides form the building blocks of nucleic acids in DNA and RNA.
• In RNA these include: Adenine, guanine, uracil, and cytosine.

mRNA gene therapies: For them to work, they have to fail.
100% N1-methyl-pseudouridine (m1Ψ) helps to prevent nucleases from breaking down mRNA and suppresses innate immune responses by pattern recognition receptors (PRRs). These include:
• Toll-like receptors (TLRs).
• Nucleotide oligomerization domain (NOD)-like receptors (NLRs).
• Retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs).
• C-type lectin receptors (CLRs).
• Absent in melanoma-2 (AIM2)-like receptors (ALRs).
Membrane-associated PRRs may be located on the cell surface, to detect intracellular pathogens. TLR’s are an example of these.
From a review by El-Zayat et al. “Toll-like receptors activation, signaling, and targeting: an overview“ (2019):
To date, 10 human and 13 murine subtypes of TLR have been identified, although TLR10 is non-functional in the mouse (Wang et al., 2015). They can recognize both the external pathogen-associated molecular patterns (PAMPs) (Zhang & Liang, 2016) and the internal damage-associated molecular patterns (DAMPs) (Yu & Feng, 2018).
They are expressed on all innate immune cells such as macrophage, neutrophils, dendritic cells (DCs), natural killer (NK) cells, mast cells, basophils, and eosinophil (Delneste et al., 2007).
TLR activation stimulates signaling cascades by the host as a defense mechanism against invaders and to repair the damaged tissue (Wang et al., 2015), leading to the release of various inflammatory cytokines and immune modulators (Wong et al., 2009).
Excessive TLR activation disrupts the immune homeostasis by sustained pro-inflammatory cytokines and chemokine production and consequently contributes to the development and progression of many diseases (Komurcu et al., 2016), such as autoimmune diseases including lupus erythematous (Subramanian et al., 2006) and rheumatoid arthritis (Huang & Pope, 2009), cancer (So & Ouchi, 2010), sepsis (Tsujimoto et al., 2008), Alzheimer’s disease (Zhang et al., 2011), and diabetes type 1 (Jialal et al., 2015).
Kariko´ et al. reported that adding modified nucleosides, such as m1Ψ, decreased TLR activity, and received a Nobel for this work.4
Key takes from “Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines” (2021) by Nance & Meier:




“Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules.”
m1Ψ stops the TLR (TLR7 forms an m-shape symmetrical homodimer) from binding with single-stranded RNA, which is normally a ligand for TLR7 (ie binds and activates):


Modified nucleotides have also been found to reduce the ability of mRNAs to propagate immune signaling through RIG-I, indicative of their ability to influence protein–RNA interactions. (48)
It is important to note that in many studies, the specific contributions of each of these mechanisms to mRNA immunogenicity have not been explicitly defined. In such cases, an mRNA modification may be exerting its activity by altering antisense transcript synthesis, mRNA structure, immune recognition, or some combination thereof.
Consistent with this, in the initial report where m1Ψ-containing mRNA was found to drive high levels of protein production, this was attributed in part to its ability to blunt TLR3 activation. (39) To decouple translation and immune activation, Svitkin and co-workers analyzed the translation of m1Ψ mRNAs in a cell-free translation system. (58)
They observed that incorporation of m1Ψ increases the size and abundance of polysomes, leading them to propose that the more rapid translation initiation and slower elongation of m1Ψ mRNAs may coordinately increase their half-life as well as induce productive interactions with the ribosome. These studies provided the first evidence that m1Ψ may directly impact mRNA translation.

The efficient translation of many different m1Ψ-containing mRNAs suggests that the secondary structures induced by this modification do not activate immune sensors. This may reflect their small size or greater dynamics relative to the stable duplexes found in classic TLR3 agonists such as poly(I:C) or the intrinsic ability of m1Ψ to impede the protein–mRNA interactions responsible for immune activation.
You could also say how rushing out a largely untested “modification in an emergency” on a huge scale has led to a “global health catastrophe”:
The nucleobase m1Ψ, a “modification in an emergency”, provides an example of how contemplation can also lead to intervention, offering hope and rest in a time of crisis.
Durbin et al. warned of this back in 2016, in “RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling”:
…the presence of nucleotide modifications in RNA is known to correlate with diminished innate immune signaling… These data contribute to defining the molecular basis for innate immune signaling suppression by RNAs containing modified nucleotides. The results have important implications for designing therapeutic RNAs that evade innate immune detection.
The effect was so pronounced that Mokuda et al. investigated the incorporation of m1ψ in gene therapies for joint diseases, due to having low immunogenicity.
Inflammation exacerbates the pathophysiology of joint diseases, and human RA-derived FLS reportedly produce high levels of IL-6 and CXCL10 (Bartok and Firestein 2010; Kuranobu et al. 2020; Yukawa et al. 2020). Therefore, it is desirable that IVT mRNA has low immunogenicity when developing a therapeutic strategy involving gene replacement for arthritis.
To avoid immune responses induced by IVT mRNA, it is necessary to block the interaction between these ssRNAs, which partially form double strands, and PRRs, such as TLR3, TLR7, TLR8, RIG-I, PKR, OAS/ribonuclease L, and MDA5.

Even as at 2022, 1-2 years after the rollout, mechanisms were not well understood:
One strategy is using modified nucleic acids, such as m1ψ. However, the biological functions of m1ψ-incorporated IVT mRNA in cultured human cells are not well understood.
Most studies have indicated that this mRNA increases expression efficiency; however, its ability to suppress innate immune responses has not been evaluated in detail.
Particularly, there are only a few comprehensive studies on cytokine production induced by IVT mRNA-stimulated cells at the protein level.
Immunosuppression and impaired chemoattraction of lymphocytes by CCL5 (RANTES) & CXCL10. This is an example of impaired paracrine interactions caused by m1ψ:
The following findings of in vitro experiments have been reported:
IFN-β and C-C motif chemokine ligand 5 (CCL5) expression is suppressed in IVT mRNA (m1ψ)-transfected human A549 cells;
We will return to the possible implications of this in Part II:
both IFN-α and IFN-β expression is downregulated in IVT mRNA (m1ψ)-transfected rat cardiomyocytes;
and CXCL10 expression is downregulated in IVT mRNA (m1ψ)-transfected human monocyte-derived macrophages in vitro 20,24,26. These results are consistent with our results.
Evolution appears to have selected this effect to minimise the risk of autoimmune disease, according to a paper from 2005 by Karikó, Buckstein, Ni & Weissman called “Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA” (2005):
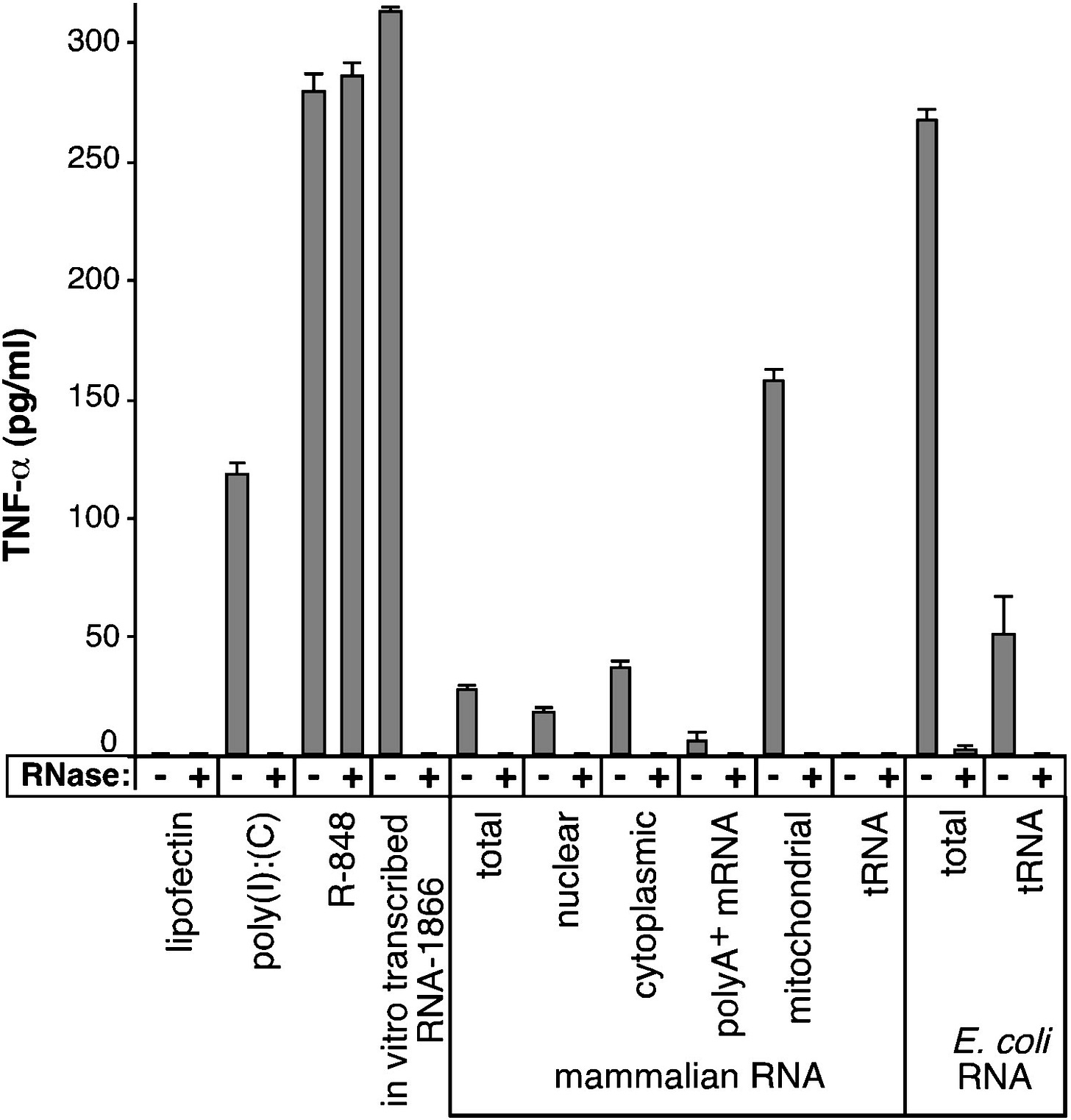
Mitochondrial RNA isolated from human platelets also stimulated human TLR8, but not TLR3 or TLR7 (Figure 2C). Collectively, these data directly demonstrate that RNA that is scarce in modified nucleosides, such as those isolated from bacteria or mitochondria, stimulate selected human TLRs, whereas total mammalian RNA abundant in nucleoside modifications are non- or minimally stimulatory.
Treatment of myeloid human monocyte-derived dendritic cells (MDDCs) with nucleoside-modified Long Intergenic Non-Protein Coding RNA-1571 caused them to irreversibly mature, and decreased their ability to express proteins needed to signal and activate other T cells:
FACS analysis of MDDCs treated with RNA-1571 and its modified versions revealed that modified nucleosides such as m5C, m6A, Ψ, s2U, and m6A/Ψ decrease the ability of RNAs to induce cell surface expression of CD80, CD83, CD86, and MHC class II (Figure 4).
Collectively, these results demonstrate that the capacity of RNA to induce DCs to mature and secrete cytokines depends on the subtype of DC as well as on the characteristics of nucleoside modification present in the RNA with the general tendency of modifications blocking stimulation.
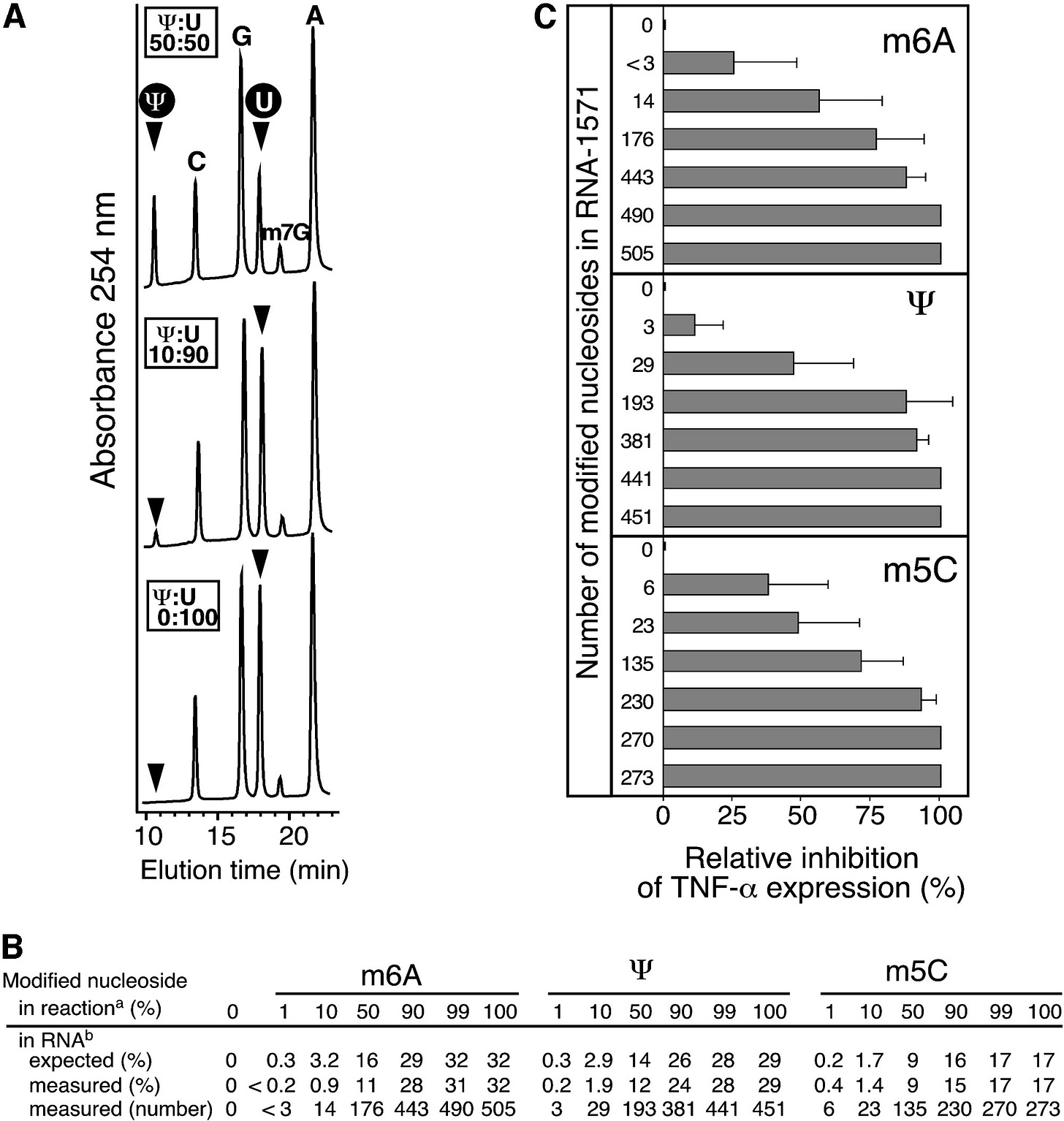
How many nucleosides need to be modified to suppress the immune response by TLRs? Not many - even just 3 modified uridines can suppress TNF-a expression by up to 25%.
If 193 out of 451, or just over 40% are modified, then Kariko et al. found that inhibition of this pro-inflammatory cytokine approached 100%:
When RNA-1571 with increasing amounts of modified nucleoside content were transfected into MDDCs, we detected that the presence of an increasing amount of modified nucleosides proportionally inhibited the capacity of RNA to induce TNF-α (Figure 5B).
The presence of 0.2%–0.4% m6A, Ψ, or m5C in the RNA, which corresponds to approximately three to six modified nucleosides per one molecule of the 1571 nt-long RNA, was sufficient to cause detectable inhibition of cytokine secretion (Figure 5B).
When RNAs with modified nucleoside levels of 1.7%–3.2%, which correspond to 14–29 modifications per molecule, were tested, the RNA could maintain only half of its capacity to induce expression of TNF-α.
In summary:
Pseudouridine is the most abundant modified nucleoside in RNA. It is generated by isomerization of uridines. We have demonstrated here that pseudouridine along with the other uridine modifications m5U and s2U uniquely suppress the capacity of RNA to activate primary DCs (Figure 3D). This finding implies that unmodified uridine probably contributes to the immune stimulatory action of RNA.
Recently published, Drzeniek et al. also explained why it may be a particularly bad idea to transfect cancer patients with m1ψ modified mRNA gene therapies. Unfortunately, this went to print too late for our review, but I highlight their findings here from “In Vitro Transcribed mRNA Immunogenicity Induces Chemokine-Mediated Lymphocyte Recruitment and Can Be Gradually Tailored by Uridine Modification” (2024):
Targeted proteomics analyses reveal that unmodified mRNA induces a pro-inflammatory paracrine pattern marked by the secretion of chemokines, which recruit T and B lymphocytes toward transfected cells.
Importantly, the magnitude of mRNA-induced changes in cell function varies quantitatively between unmodified, Ψ-, m1Ψ-, and 5moU-modified mRNA and can be gradually tailored, with implications for deliberately exploiting this effect in mRNA drug design.
Indeed, both the immunosuppressive effect of stromal cells on T-cell proliferation, and the anti-inflammatory effect of IL-10 mRNA are enhanced by appropriate uridine modification. The results provide new insights into the effects of mRNA drugs on cell function and cell-cell communication and open new possibilities to tailor mRNA-triggered immune activation to the desired pro- or anti-inflammatory application.
Importantly, we show that the extent of these mRNA-triggered changes in cell function varies quantitatively between unmodified, Ψ-, m1Ψ-, and 5moU-modified IVT mRNA (from highest immune activation to lowest).
Thus, the intensity of mRNA's immune stimulatory function can be gradually tailored to the desired inflammatory (e.g., cancer or viral vaccination) or non/anti-inflammatory (e.g., tolerance induction, protein replacement, regeneration) application.
BMSCs: Bone marrow stromal cells, or mesenchymal stem cells (MSCs): “BMSCs are clonogenic, fibroblastic in shape, and can differentiate along multiple lineages such as osteoblasts, chondrocytes, adipocytes, and hematopoiesis-supportive stroma.”5 They are important for the generation of new or replacement bone, cartilage, and other skeletal tissues.
One of the most important roles for BMSCs is to protect self-renewing hematopoietic stem cells (HSCs), which give rise to all blood lineages following a multistep differentiation process.
And that’s not all, they also help to modulate the immune system and prevent autoimmune diseases.
Alarmingly, the authors found that after transfection with modified nucleosides they failed to proliferate for at least 5 days, or to activate interferon type 1 immune responses:
To illustrate this, we show that both the anti-proliferative effect of BMSCs on T cell proliferation, and the immunosuppressive effect of IL-10 mRNA could be reinforced through uridine modification.
Downstream immune effects show that systemic immunosuppression is occurring - the effects of m1ψ are not just localised, but paracrine:

“T cell suppression”. This isn’t some tinfoil hat conspiracy theory, it’s what you need to do as part of the immune suppression to optimise protein overexpression. Spike protein overexpression is a case in point.
An excellent study, they wanted to know if the effects of transfecting m1ψ modified mRNA were short-lived or not:
To understand if mRNA transfection causes a lasting impairment of cell proliferation, metabolic activity of BMSCs was measured on days 1, 3 and 5 post-transfection (Figure 2b).
Although no differences in metabolic activity could be discerned 24 h after transfection, cells transfected with U or Ψ-mRNA failed to proliferate, indicating a lasting impairment of cell fitness.
At day 5, metabolic activity in the m1Ψ and 5moU groups was significantly higher than in the U and Ψ groups. Remarkably, 5moU-transfected BMSCs proliferated to a similar extent as untransfected cells or the LMM control group, implying a minimal impact of this IVT-mRNA on cell fitness.
The cells were hyporesponsive, but not senescent:
On day 5 post-transfection, cells were stained for senescence-associated β-galactosidase activity. Similar levels of senescence could be observed in all tested groups, indicating that this parameter is not affected by mRNA transfection, regardless of its immunogenicity (Figure 2c).
m1ψ modified mRNA is in dark blue throughout:
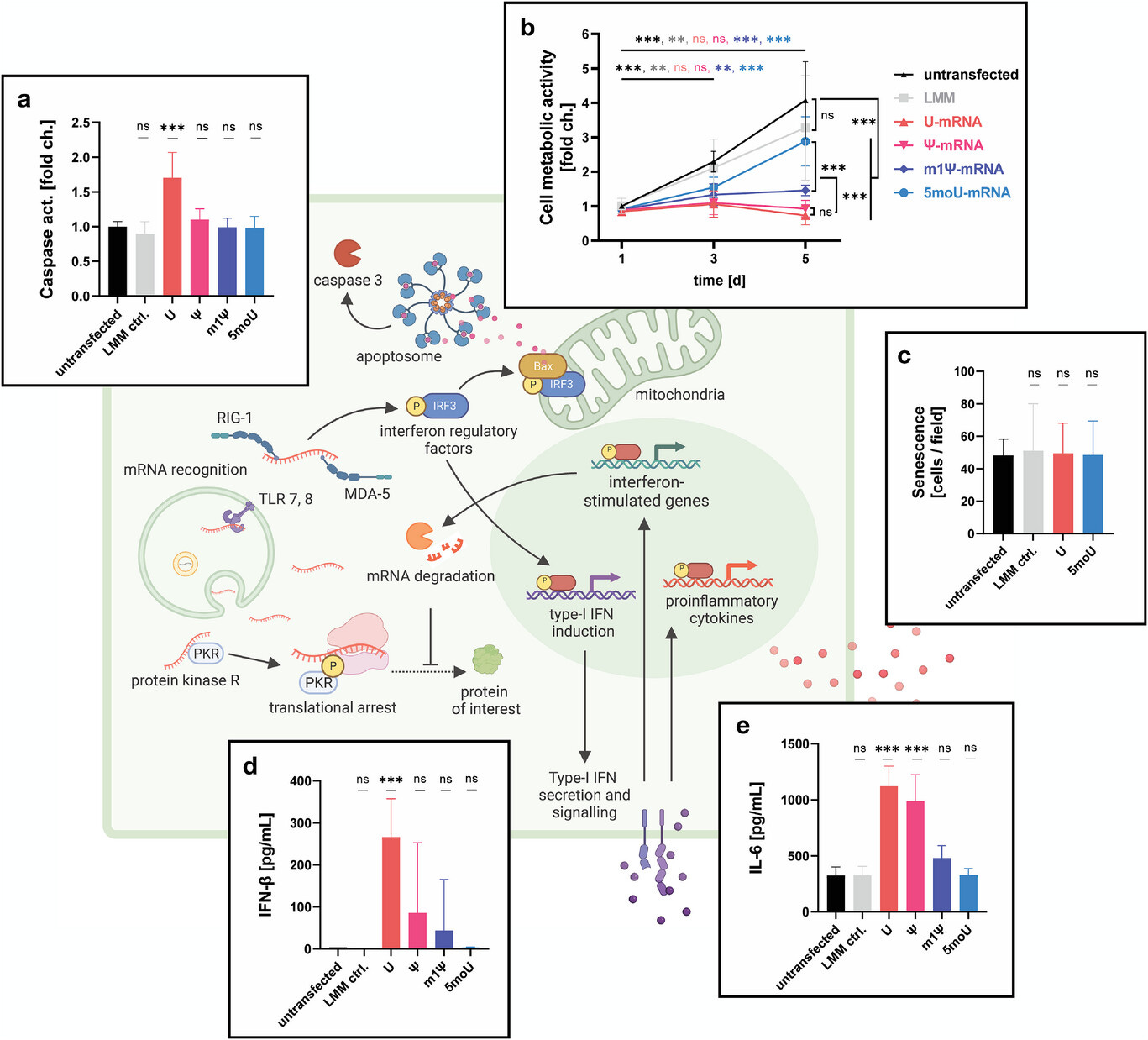
Unfortunately, even after 5 days, metabolic activity remained significantly suppressed, showing that the T cells were not activated or proliferating. It would have been instructive to have a study cutoff date well beyond 5 days.
Extracting b for clarity:

To model sepsis, they treated IL-10 mRNA transfected cells with lipopolysaccharides (LPS, endotoxin) to stimulate TNFa and IL-6, and studied the effects of m1Ψ on IL-10 expression. It led to vastly increased expression of the cytokine compared to unmodified uridine (b).
INF-β and CXCL10 secretion in C and D confirmed impaired type I interferon responses. INF-β from unmodified uridine peaked at over 600 pg/ml, whereas with m1Ψ the maximum was only around 100 pg/ml.
Chemokine CXCL10 levels with m1Ψ were around half that of the unmodified uridine.
When you read about severe COVID infections after transfection, this is partly why.

From the discussion, its notable that immune responses were suppressed by modified nucleosides regardless of the protein being expressed by the mRNAs they used (GFP-mRNA, mCherry-mRNA, and IL-10-mRNA).
Thus the findings from this paper should be relevant to the study we reviewed by Sittplangkoon et al., “mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model”6 (2024), further supporting our hypothesis.
Considering the potentially endless variety of target proteins that may be encoded by mRNA drugs, it was important to understand whether the characterized cellular responses toward EGFP-encoding mRNA are specific for this mRNA sequence or could be generalized for all mRNAs, regardless of which protein they encode.
To answer this, we tested mCherry-encoding and IL-10-encoding mRNA alongside the EGFP-encoding mRNA (Figure 6). For all three mRNAs the relationships between mRNA chemistry and protein expression and between mRNA chemistry and immune activation matched closely.
Even raw values of induced IFN-β and CXCL10 secretion were in the same range, demonstrating that an mRNA's function as an immune receptor ligand is largely independent from the protein it encodes.

Considering local application of mRNA drugs in patients these findings point toward an opportunity to deliberately exploit the immune stimulatory and lymphocyte recruiting effects of IVT mRNA in redirecting the immune system. As mentioned above, the classification of mRNA immune activation as favorable or unfavorable depends on the intended application.[3, 40]
Importantly however, we show that the intensity of this effect can be gradually tailored by replacing uridine in the IVT reaction with the appropriate derivate: Ψ for moderate immune activation and m1Ψ or 5moU for low or very low immune activation.
CXCL10 is a ligand for CXCR3, and secretion was significantly impaired by m1ψ modified nucleosides, along with INF-β and TNF-α:
We have previously shown that activation of the CXCR3 chemokine system plays a role in the immune response toward solid tumors and is predictive of therapy response.[35] Thus, the ability to engineer an immune activation of bespoke intensity paves a way toward exploiting mRNA-induced CXCR3 ligand secretion in the context of tumor therapy, possibly to boost other regimens, such as CXCR3-expressing CAR-T cells against solid tumors.
To avoid the risk of cancer recurrence or proliferation we might conclude that using unmodified uridine in IVT mRNA might be the logical way forward, as even just very low levels of modified nucleosides (0.2% - 0.4% m6A, Ψ, or m5C, according to the 2005 paper by Karikó et al.) may significantly inhibit cytokine expression.
However, this has serious drawbacks too, meaning that it may simply not be possible to have both a safe and effective dose of any mRNA gene therapy. This applies at least to cancer patients, given the current state of the technology:
Despite its potential for clinical applications, IVT mRNA technology also faces some challenges that have dampened the enthusiasm toward its therapeutic use. One challenge is the rather short-lived effective expression window, usually lasting only one or two days, due to the low stability and rapid degradation of the mRNA.[13, 14]
Another major limitation of IVT mRNA is the recognition of IVT mRNA as foreign by intracellular pattern recognition receptors such as TLR 7 and 8, RIG-1 or MDA-5, which triggers an inflammatory type I interferon response in transfected cells, similar to viral RNA (Figure 2 schematic).
This is good if it is specific to the tumour, but off-target effects may lead to serious complications. Your immune system may attack any transfected cell, as if it was infected by a virus. Caspase levels are elevated by uridine and lead to apoptosis:

This triggers several cellular defense mechanisms, such as faster degradation of mRNA, translational arrest and even apoptosis to inhibit viral replication and prevent the spread of infection.[15]
The extent to which these endogenous defense mechanisms also affect the functionality of IVT mRNA-engineered cells is still incompletely understood, e.g., in the case of ex vivo modified cell therapeutics[6, 16, 17] or in transfected tissues upon in situ delivery of mRNA drugs.[4, 18]
The previous study transfected IL-10-mRNA to mimic the immunosuppressive effects of sepsis. Of relevance to this, there are some studies that showing that Pfizer’s BNT162b2 also upregulates interleukin-10 for months.
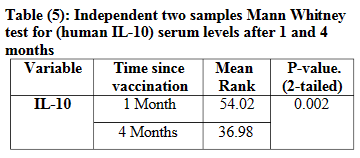
In 2023, Kadhim et al. conducted a clinical trial to measure the serum level of IL-10 in students, both one month and four months after administration of their 2nd dose of Pfizer-BioNTech mRNA vaccine. 90 blood samples were taken at random. 45 after one month, and the other 45 after four months.
It should be noted that 1 month can be a long time in terms of cancer progression.7
Key takes from “Reduction of IL-10 Serum Level after 4 Months of Pfizer-BioNTech Vaccine Administration of 2 nd Dose in Students at Al-Iraqia University” (2023):
After ELISA results of IL-10 serum levels for both groups were obtained, the data were analyzed as they were non-parametric.

Because all data were not normally distributed, non-parametric tests were used to test the research hypothesis using Mann-Whitney Test. The data revealed a significant reduction (P<0.05) in levels of IL-10 post 4 months in comparison to 1-month post the 2nd dose of Pfizer-BioNTech vaccination (Table 5).
DISCUSSION
IL-10 is one of the important cytokine types in the case of COVID-19 infection, which can inhibit proinflammatory production such as IL-1, 6, TNF (Tumour Necrosis Factor), and INF (Interferons) in different types of cells. Furthermore, IL-10 can block IL-12 (8), which in turn inhibits the maturation of DCs (dendritic cells), (9).
CONCLUSION
Our study showed that IL-10 serum levels significantly drop (P<0.05) after 120 days (4 months) of complete vaccination. The increase in IL-10 at the beginning of vaccination could be explained as an attempt to temper hyper-inflammation and protect tissue. This might be due to the anti-inflammatory property that IL-10 levels were expected to be raised as soon as the vaccine is administered to act to prevent any inflammatory reaction against local vaccine lesions.
Noé et al. analysed cytokine responses to whole‐blood stimulations 28 days after BNT162b2 vaccination in children: “BNT162b2 COVID-19 vaccination in children alters cytokine responses to heterologous pathogens and Toll-like receptor agonists” (2023). They also found a multifold increase in IL-10. The impaired IFN-γ responses are notable too:

Transfection of leukocytes
Both macrophages and dendritic cells, but not T-cells, get transfected by BNT162b2, according to a 2022 murine study by Li et al., “Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine” (2022)
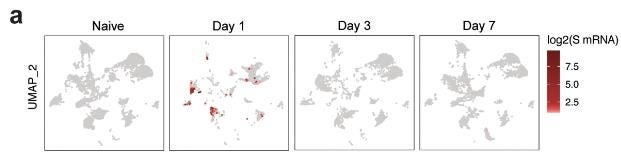
mRNA uptake by lymph node DCs and macrophages
Using the scRNAseq data, we investigated the cells containing spike mRNA (S mRNA) in the dLNs. We observed high levels of spike mRNA reads on day 1 that decreased sharply by day 3 (Fig. 6a).
The reads were primarily restricted to Clusters 2 and 4, which represent monocyte/macrophages and migratory DCs, respectively (Fig. 6a).
Quantification of cells with at least one read per cell demonstrated that 40% of monocyte/macrophages and 20% of migratory DCs contained spike mRNA on day 1 (Fig. 6b). The frequency of these cells increased after vaccination (Fig. 6c).
Extracted from Figure 6:

Mouse responses in the study were not directly comparable and indicated a greater degree of immunosuppression in humans than in mice:
Strikingly, there was a significant induction of IFNα ( IFNα2 and IFNα4) in mice at 6 h, which was not observed in humans at the 1-day time point we examined9. Of note, the lower dose (0.2 μg) immunization did not induce IFNα as seen in humans; however, there was also an absence of IFNγ response (Extended Data Fig.1f), suggesting that there was not a human equivalent dose of BNT162b2 in mice.

Interferons and cancer immunity
Impaired dendritic cell signaling can lead to reduced cancer according to this review by Gardner et al., “Dendritic Cells and Their Role in Immunotherapy” (2020) :
… cross-presentation by cDC1 is enhanced by type I interferon (IFN) signaling (40). The absence of type I IFN in the tumor microenvironment, or the inability of cDC1 to sense type I IFN, are sufficient to impair the development of a CD8+ T cell response (34, 40).
Sittplangkoon’s et al. paper
Dr. Rubio Casillas summary of “mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model” (2022):
In an experiment, the experimental group receives the variable being tested (OVA-LNP), while the control group does not. The experimental group is also known as the treatment group. The control group may receive no treatment, a standard treatment, or a placebo. In all other ways, the experimental and control groups should be identical.
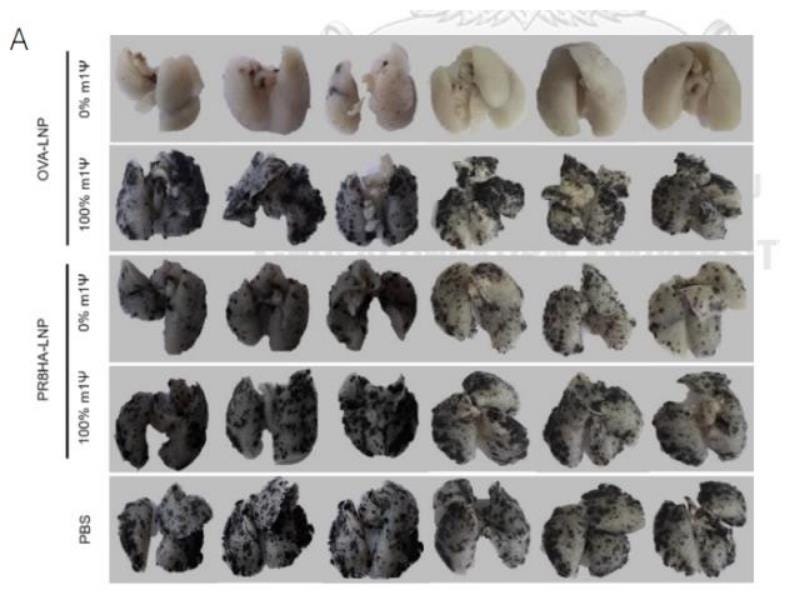
Sittplangkoon et al. wanted to see if a certain type of vaccine made from genetic material could help stop cancer from spreading to the lungs in a melanoma model. Here's what they did:
1. They injected melanoma cells into the bloodstream of mice to make them develop lung metastasis, which means the cancer spread to their lungs.
2. Then, they gave the mice two doses of a vaccine made from mRNA called OVA-LNP. Some mice got the vaccine without any modifications, while others got a version of the vaccine with a specific modification (100 % of m1Ψ).
3. They also had some mice that didn't get any vaccine (PBS= phosphate buffered saline) or got a different type of vaccine (PR8HA-LNP).
4. After 18 days, they looked at the lungs of the mice to count how many cancer nodules had formed.
5.They found that only the unmodified vaccine clearly stopped the cancer from forming nodules in the lungs. The modified vaccine 100 % of m1Ψ) and the ones that received a vaccine with an unrelated antigen (PR8HA-LNP) had just as many nodules as the mice that didn't get any vaccine at all (PBS).
So, in simple terms, they found that the unmodified vaccine was effective at preventing the spread of cancer to the lungs in this melanoma model, while the modified vaccine with 100 % of m1Ψ and other vaccine (PR8HA-LNP ) didn't prevent metastasis.
This mRNA vaccine containing PR8HA was used as a control. Why? As it does not contain cancer antigens, it should not induce an anti-tumor immune response. This would result in an inability to prevent metastasis and the formation of cancerous nodules in the lungs. The same for PBS (Phosphate buffered saline) which is a non-toxic solution of water and salts that's commonly used in biological research.
As you can observe in the figure, lungs from animals who received a PR8HA-LNP vaccine show multiple cancerous nodules, as PBS injected animals.
He gives us the answer to why the mice that received placebo (PBS) and the group that received the mRNA with 100% m1Ψ did not produce an effective anti-tumor response:
“The mRNA vaccine modified with 100 % m1Ψ did not confer protection against tumor model in our study. By using unmodified mRNA, a robust anti-tumor immunity was observed, and the mechanism is linked to the production of interferon type I, a key cytokine for anti-tumor immunity.”
The mRNA vaccine modified with 100 % m1Ψ did not confer protection against metastasis of cancer cells because due to such m1Ψ modification, the immune sensors (RIG-I and TLRs) were not activated, they did not detect the cancer cells, interferon type I was not produced and such immune suppression allowed metastasis to the lungs. The same can be said for the animals receiving PBS and the PR8HA-LNP (Influenza) vaccine.
I previously discussed cancer signals from flu vaccine-induced oncogenic cytokines, and we know that m1Ψ suppresses interferon type 1 signalling regardless of the protein being expressed. This could have even worse outcomes:
Finally, as the PR8HA-LNP vaccine is made from the hemagglutinin (HA) gene of influenza A, they should have indicated it clearly. The implications are worrying. An mRNA vaccine against influenza (or other diseases) can induce an adequate immune response against said pathogen, but, by not preventing metastasis due to the suppression of type I Interferon synthesis, people who have early-stage cancer or those who are undergoing treatment for cancer could experience an exacerbation in cancer development. This is what has been called “ turbo cancer”
In their work, Sittplangkoon et al reported that “Mice immunized with unmodified OVA-LNP survived until the end of the experimental period of 31 days while all mice in the PBS or Luc-LNP control group were dead (Figure 4B). For OVA-LNP with m1Ψ modification of 100%, half of the mice survived.”
The fact that half of the mice who received the OVA-LNP with m1Ψ modification of 100 % died suggests that the immune suppression was not total, but 50% mortality is an extremely high number.
This is why we encourage researchers that it is urgent to investigate if this could occur, for example, performing a similar experiment, but using the 100% m1Ψ modified mRNA that encodes the spike protein of SARS-CoV-2 in the control group, and compare the effects on metastasis using the same unmodified OVA-LNP vaccine in the experimental group.
Key:
MDA5 (melanoma differentiation-associated protein 5)
RIG-I (retinoic acid-inducible gene-I)
LGP2 (Laboratory of Genetics and Physiology 2, encoded by DXH58)
NF-kB (Nuclear factor kappa-light-chain-enhancer of activated B cells)
IRF-3 (Interferon regulatory factor 3)


Is immune suppression vaccine-specific to transfected cells?
No, due to macrophage polarization to the M-2 phenotype, impairment of IFN signaling, LGP2 upregulation, and exhaustion associated PD-1+ CD8+ T cells.
It also becomes a moot point as many as 40% of monocytes/macrophages and 20% of migratory dendritic cells get transfected and deactivated, along with stromal cells.
Key takes from Chutamath Sittplangkoon’s PhD thesis from 2021: “mRNA vaccine encoding neoantigen for cancer immunotherapy using mouse melanoma as a model.” The 2022 paper was a summary of this research.
We demonstrated that the maturation of cDC1 and cDC2 upon delivery of unmodified and 40% substitution with m1Ψ mRNA-LNP was evident compared with 100% substitution with m1Ψ mRNA-LNP. While unmodified mRNA-LNP compromises the translation efficiency of mRNA into protein antigen, its superior impact on DC maturation is beneficial for anti-tumor immunity.
We have been accused of misrepresenting these findings, but this thesis (and the resulting paper) are quite explicit about how high levels of m1Ψ substitution inhibit robust anti-tumour immune responses to tumour-associated antigens. This is in keeping with other research.
“Serum” means type 1 interferon measured in the blood, ie circulating systemically:
Based on the result in Figure 4.3A, substitutions of 0% and 40%, but not 70% and 100% m1Ψ in mRNA-LNPs could induce serum IFN-I secretion 6 hrs after the prime and booster doses.
In the second part of this study, we investigated the anti-tumor effects of the mRNA-LNP platform in localized and metastatic B16 melanoma models. When challenged with the highly aggressive B16 melanoma model, unmodified OVA-LNP vaccinated mice showed outstanding anti-tumor effect by extending survival time, controlling tumor growth and lung metastasis inhibition as compared to modified OVA-LNP and control treated mice.
In our studies, we consistenly observed higher frequency of PD-1 + cells in tumor infiltrating T cells when IFNAR1 was blocked. This result may imply that unmodified mRNA via IFN-I help alleviates T cell exhaustion that allows anti-tumor T cells to be fully functional.
“M2-like macrophages release immunosuppressive chemicals to block the Th1-type immune activity as well as boost the Th2-type immune activity. This activity reduces the control over inflammatory reactions while promoting tumor cell growth, drug resistance, angiogenesis, and tissue healing”.8
Consistent with this observation, more M2-like tumor-associated macrophages were observed when IFN-I is blocked in the mRNA/LNP vaccinated group.
Taken together, we provide strong evidence that IFN-I plays an indispensable role in inducing anti-tumor response by mRNA vaccine.
The prominent therapeutic efficacy of unmodified mRNA is possibly due to activation of endosomal toll-like receptor 7/8 (TLR7/8) and subsequently causes pro-inflammatory cytokine secretion via MyD88-dependent IRF-5 phosphorylation (257).
Taken together, we provide strong evidence for the anti-tumor immune response by unmodified mRNA vaccines encoding dominant and neoantigens.
In summary, the present study reveals that unmodified mRNA-LNP induces substantial IFN-I production and the maturation of DCs. In B16F0-OVA murine 88 melanoma model, unmodified OVA-LNP significantly reduced tumor growth and prolonged survival, compared to OVA-LNP with m1Ψ substitution. This robust anti-tumor effect correlated with the increase in intratumoral CD40+ DCs and the frequency of granzyme B+ /IFN-γ + /TNF-α+ polyfunctional antigen-specific CD8+ T cells.
The robust anti-tumor effect of unmodified OVA-LNP was also observed in the lung metastatic tumor model. Finally, this mRNA vaccine was tested using B16F10 melanoma neoantigens (Pbk-Actn4) which resulted in delayed B16F10 tumor growth.
Taken together, our findings demonstrated that an unmodified mRNA vaccine may be a better tumor vaccine strategy that induces robust antitumor immunity in IFN-I dependent manner.
OVA-LNP is the ovalbumin anti-tumour vaccine.
Luc-LNP encodes luciferase and was used as an unrelated antigen control.
PBS, Phosphate buffer saline is the negative control for ovalbumin vaccine.

The multihit model of carcinogenesis
In this model, malignancy results from a series of rate-limiting mutation steps. These “hits” are induced by DNA damage caused by radiation, chemicals, viruses, or other factors. DNA repair may slow the process, whereas tumour-promoting agents accelerate it once underway.
From: “The multihit model of carcinogenesis: etiologic implications for colon cancer” (1984)
Farber [ 15, 16] suggested that a malignant cell is the result of an evolutionary process in which a normal cell undergoes several mutation-like events, each followed by selection as the altered cell reproduces itself. These events were considered rate-limiting steps, which could be viewed as “hits” in the multihit model of carcinogenesis. Since either mutation-like events or actual mutations have the same mathematical implications in the multihit model, these terms will be used interchangeably.
… A heuristic model of this process has been proposed [13. 14], in which a malignant cell is induced through alteration of cellular DNA by radiation, chemicals, viruses, or other factors. This process may be retarded by phenomena such as DNA repair, or accelerated by promoting agents. A single malignant cell replicates to form a tumor at a rate dependent on numerous stimulating and inhibiting factors.
… Findings are generally consistent with the adenoma-carcinoma etiologic sequence postulated by Hill, Morson and Bussey with one exception. A large proportion of the population may be at risk of four-hit colon tumors following a non-adenoma etiologic sequence.
Carcinogen groups
From “Known and Probable Human Carcinogens”:
What you should know
The IARC and NTP act independently. Many known or suspected carcinogens appear on both organization’s lists; however, if a substance or exposure is only on one agency’s list, this it does not necessarily mean there is a controversy, as one agency may not have evaluated it.
These lists are alphabetical, but many of the substances and exposures here can go by different names. This can make it hard to find a particular substance on one or both of these lists.
These lists include only those agents that have been evaluated by the agencies. These agencies tend to focus on substances and exposures most likely to cause cancer, but there are many others that have not been fully studied yet.
These lists include agents that have been classified as known or probable human carcinogens. The lists do not include substances that have been classified as possible carcinogens, for which the evidence is not as strong. These lists also do not include substances evaluated as “not classifiable as to its carcinogenicity in humans.”
Most of the agents on the lists have been linked only with certain kinds of cancer, not all cancer types. See each agency’s website for more details about the substances and exposures on their lists.
The lists describe the level of evidence that something can cause cancer, not how likely it is that something will cause cancer in any person (or how much it might raise your risk). For example, IARC considers there to be strong evidence that both tobacco smoking and eating processed meat can cause cancer, so both are listed as “carcinogenic to humans.” But smoking is much more likely to cause cancer than eating processed meat, even though both are in the same category.
Carcinogens do not cause cancer at all times, under all circumstances. In other words, a carcinogen does not always cause cancer in every person, every time there is any kind of exposure. Some may only be carcinogenic if a person is exposed in a certain way (for example, swallowing it as opposed to touching it). Some may only cause cancer in people who have a certain genetic makeup. Some of these agents may lead to cancer after only a very small exposure, while others might require intense exposure over many years. Again, refer to the agencies’ reports for specifics.
Even if a substance or exposure is known or suspected to cause cancer, this does not necessarily mean that it can or should be avoided at all costs. For example, sunlight is a major source of ultraviolet (UV) rays, which are a known cause of skin cancer, but it’s not practical (or advisable) to completely avoid the sun. (See How to Interpret News About Cancer Causes for more about this.)
These lists also include many commonly used medicines, particularly some hormones and drugs used to treat cancer. For example, tamoxifen increases the risk of certain kinds of uterine cancer, but it can be very useful in treating some breast cancers, which may be more important for some women. If you have questions about a medicine that appears on one of these lists, be sure to ask your doctor.
Group 1 also includes “human immunodeficiency virus type 1 (HIV-1) (infection with)”.
I would not include Aloe vera, tea or coffee in Group 3 as an abundance of research demonstrates anti-cancer properties. Contamination might change this (eg tea and heavy metals), but then the contaminant in question becomes the focus:
From Figure 1 of “Resveratrol nanoformulation for cancer prevention and therapy” (2015) by Siddiqui et al.
(A) Initiation involves the alteration, change,or mutation of genes arising spontaneously or induced by exposure to a carcinogenic agent. Genetic alterations can result in dysregulation of biochemical signaling pathways associated with cellular proliferation, survival, and differentiation, which can be influenced by a number of factors, including the rate and type of carcinogenic metabolism and the response of the DNA repair function.
(B) The promotion stage is considered to be a relatively lengthy and reversible process in which actively proliferating preneoplastic cells accumulate. Within this period, the process can be altered by chemopreventive agents and affect growth rates. Progression is the phase between a premalignant lesion and the development of invasive cancer.
(C) Progression is the final stage of neoplastic transformation, where genetic and phenotypic changes and cell proliferation occur. This involves a fast increase in the tumor size, where the cells may undergo further mutations with invasive and metastatic potential. Chemopreventive agents should be able to preferentially act within the initiation and promotion processes of carcinogenesis.
(D) Metastasis involves the spread of cancer cells from the primary site to other parts of the body through the bloodstream or the lymph system. Chemopreventive agents are known to inhibit angiogenesis and invasion of primary tumors, and thus could be utilized to inhibit the metastasis of cancer.
Multiple papers show that Spike S1 and S2 inhibit DNA repair, but promotion and progression are the focus of our reviews.
The second of these papers focuses on igG4 and cancer, and includes references from this Substack:
Our review passed peer review and was published on 26th April in “Exploration of Immunology”. This has been much less controversial, even though in some ways it’s worse than m1Ψ associated immunosuppression due to the possible longevity of class switching - at least 8 years with some experimental HIV vaccines.
Causes include repeated exposure to the antigen through boosting, infections, or persistent expression:
Conclusion
Thank you to my colleagues, and for your ongoing support for this series of Substack reviews. Without this, it is unlikely I would have been in a position to contribute to these two review papers.
Just because their findings may not align with the science does not make their conclusions wrong (or right, for that matter).
Research needs to be evaluated on its own merit, without emotion or bias, and that is the value of the high bar set by a working peer review process. It should be pointed out that we weren’t given an easy ride either, as more citations were requested, passages needed redrafting for greater clarity, and some points needed rebutting. Additional figures were also requested.
A suggestion from the panels for both papers was to conduct further research and in both cases we have to agree, and concluded our work with proposals for this.
Sittplangkoon’s doctoral paper has obviously been carefully worded to try to avoid controversy. However, it is difficult to conduct research to improve (and promote) future therapeutics without casting light on any serious shortcomings of existing products. In this respect, the conclusions are inescapable and supported by other research, as discussed, but more is needed - especially with spike protein expression.
In part II I will review case studies, and go further into LNP/mRNA-associated signaling pathways and pathologies.

References
Rubio-Casillas A, Cowley D, Raszek M, Uversky VN, Redwan EM. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? Int J Biol Macromol. 2024;267(Pt 1):131427. doi:10.1016/j.ijbiomac.2024.131427
Horoho S, Musik S, Bryant D, Brooks W, Porter IM. Questioning COVID-19 Surface Stability and Fomite Spreading in Three Aeromedical Cases: A Case Series. Mil Med. 2021;186(7-8):e832-e835. doi:10.1093/milmed/usaa548
Doctor AM. The Forgotten Science of Vaccine Disease Provocation. Published March 24, 2024. Accessed May 11, 2024.
https://www.midwesterndoctor.com/p/the-forgotten-science-of-vaccine
Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165-175. doi:10.1016/j.immuni.2005.06.008
Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32(1):160-165. doi:10.1023/b:abme.0000007800.89194.95
Sittplangkoon C, Alameh MG, Weissman D, et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front Immunol. 2022;13:983000. doi:10.3389/fimmu.2022.983000
Every month delayed in cancer treatment can raise risk of death by around 10% | BMJ. Accessed May 11, 2024. https://www.bmj.com/company/newsroom/every-month-delayed-in-cancer-treatment-can-raise-risk-of-death-by-around-10/
Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance | npj Precision Oncology. Accessed May 11, 2024. https://www.nature.com/articles/s41698-024-00522-z































Open access link to a pdf of the full paper (posted by others):
Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?
https://www.sabinopaciolla.com/wp-content/uploads/2024/04/Rubio_Casillas_Methylpseudouridine_Promotes_Cancer_Wow_2024.pdf
Congratulations on your publication!! That’s amazing! Now post-peer review, they will still try to attack it, I’m sure.
Excellent write up. I had a very rare form of melanoma 2 years ago. In my follow up, post surgery, where luckily (and so far, knock on wood), they got everything out, which was really lucky, as chemo and radiation would not have worked, I met up with a very tired Head of Oncology at Yale. She was in charge of monitoring and keeping tabs on all of the cancer and giving info and advice. She was a smart woman, you could see the gears turning and she kept staring at me as I asked my detailed questions. What saved me was my low mitotic rate (so far) and she asked me why did I think I had a low mitotic rate? I said I guessed that I had unwittingly started an intermittent fasting diet AND I did not get a covid jab. Then s he asked, why did I not get one- I responded, because it was a p53 suppressor. It just so happened that Arkmedic’s article about the Jiang and Mei study was something that came out a couple of weeks before my meeting and I was able to say a couple reasonably well founded sentences based on that article. She visibly blanched. I’m telling you she KNEW that the jabs were killing her patients,, I was probably just one of the first to openly admit I didn’t get one and that I knew that it was a huge factor. Oddly enough, that hospital did not require me to get one to receive treatment- I know there were hospitals that did. Maybe she was responsible for that, I don’t know. I didn’t know enough about the pseudouridine at that point to say much, but I think I did mention that the LNP model was dangerous. I still cannot fathom how they recommended cancer patients to get these jabs when before, those with depressed immune systems were recommended to stay away from all vaccines. It simply beggars belief.