Last update: 16th February ‘23. Abstract cut down! Contents added.
Also published to preprint server:
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Substack limitations
Once Substacks’ support the use of internal hyperlinks I will add them to the abstract. Otherwise please use a “Find In Page” keyword search to navigate or click on the footnote text numbers.
If received via email I recommend clicking on the hyperlinked title to read the latest correctly formatted version in full, in a browser. Unfortunately it is not possible to email out revised versions.
Contents
Furin inhibition and roles as a tumor suppressor.
Antiviral and neuroprotective properties.
Treatment of ME/Chronic Fatigue Syndrome (CFS).
Treatment of autoimmune disorders, including MS.
Dosing, contraindications and administration in a clinical setting.
Administration in a clinical setting.
And finally...Its over to you!
Abstract
This is a scientific literature review of a large selection of studies drawn from medical research publications databases, including PubMed, bioRxiv and ResearchGate.
Its particular focus concerns the therapeutic benefits and mode of action of baicalin and its associated flavonoids. Broad spectrum in nature, Baicalin can act as tumor suppressor, it has antiviral and neuroprotective properties and is a source of phytomelatonin. It works systemically and quickly breaks down to form metabolites including baicalein and woganin, also with therapeutic properties. It can be used to treat endometriosis, ulcerative colitis, ME, CFS and MS, due its ability to stimulate remyelination. Dosing, contraindications and administration in a clinical setting are then considered to conclude the review.
Discussion
A PubMed search using the keyword “baicalin” returned 2,305 results. This review will consider some of the representative research, with a particular focus on its therapeutic properties. Its aim is to help bridge the gap between this research and its use in traditional medicine, with the aim being to act as a reference for medical professionals, scientists and any interested lay people.
Most literature reviews seek to summarise existing research, perhaps using automated searches or a meta-analysis to reach a conclusion. This review adds an additional step by walking through the papers as well, to make them more accessible and to help form the bigger picture for the reader.
If this is new to you it should build on your understanding and subject knowledge. If you are a specialist I hope it presents something new or puts things in a different light - baicalin is not just another flavonoid!
This isn’t material to skim through but should be seen as a journey of discovery, almost a series of lectures with its aim to do a deep dive into the pathophysiology, biochemistry and signalling pathways being researched.
Not only is it helpful to know that it works as a treatment for condition x or y, but it’s even more important to know why it works this way. Its bringing traditional medicine into the 21st Century, and ironically taking modern allopathic medicine back to its roots as a means to address its many shortcomings - such as cost, availability, lack of or compromised clinical testing, lack of efficacy, resistance, off target effects or toxicity.
Most importantly the profile of baicalin and related therapeutics needs raising so that as many people can benefit as much as possible, as quickly as possible.
And a big thank you must go to Dr Johanna Deinert for highlighting several papers particularly worthy of inclusion, providing clinical data and recommending baicalin as an alternative to ivermectin for use in combination therapies to treat Covid-19 cases and post-acute sequalae of COVID-19 (PASC).
Baicalin1 is a flavone glycoside, a flavonoid and the glucuronide of baicalein.2
A glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond.3
Flavonoids (or bioflavonoids; from the Latin word flavus, meaning yellow, their colour in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6.
Flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides.4
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides.
Glucuronidation, the conversion of chemical compounds to glucuronides, is a method that animals use to assist in the excretion of toxic substances, drugs or other substances that cannot be used as an energy source. Glucuronic acid is attached via a glycosidic bond to the substance, and the resulting glucuronide, which has a much higher water solubility than the original substance, is eventually excreted by the kidneys.
Enzymes that cleave the glycosidic bond of a glucuronide are called glucuronidases.5
Baicalin is found in several species in the genus Scutellaria, including Scutellaria baicalensis, and Scutellaria lateriflora. There are 10 mg/g baicalin in Scutellaria galericulata leaves. It is also present in the bark isolate of the Oroxylum indicum tree.
Scutellaria is a genus of flowering plants in the mint family, Lamiaceae. They are known commonly as skullcaps. The generic name is derived from the Latin scutella, meaning "a small dish, tray or platter", or "little dish", referring to the shape of the calyx. The common name alludes to the resemblance of the same structure to "miniature medieval helmets". The genus has a subcosmopolitan distribution, with species occurring nearly worldwide, mainly in temperate regions.
Skullcaps are common herbal remedies in systems of traditional medicine. In traditional Chinese medicine they are utilized to "clear away the heat-evil and expel superficial evils". Scutellaria baicalensis in particular is a common component of many preparations. Its root, known as Radix Scutellariae, is the source of the Chinese medicine Huang Qin.6
Scutellaria baicalensis, with the common name Baikal skullcap or Chinese skullcap is in the mint family Lamiaceae, and native to China, Korea, Mongolia, and Russia in the Russian Far East and Siberia.
Traditional Chinese medicine
It is one of the 50 fundamental herbs used in traditional Chinese medicine, where it has the name huángqín (Chinese: 黄芩). As a Chinese traditional medicine, huang qin usually refers to the dried root of S. baicalensis Georgi, S. viscidula Bge., S. amoena C.H. Wright, and S. ikoninkovii Ju.
Its use in TCM is for "the prophylaxis and treatment of hepatitis, atherosclerosis, hypertension, hyperlipidemia, type 2 diabetes, dysentery, ulcerative colitis, and respiratory disorders."
Pharmacology
Several chemical compounds have been isolated from the root; baicalein, baicalin, wogonin, norwogonin, oroxylin A and β-sitosterol are the major ones.
Names
It is important to use the Latin name, as the term 'skullcap' is used for over 200 varieties. Sometimes, Scutellaria lateriflora (North American skullcap) is mistaken for S. baicalensis. This confusion can result in the intake of the S. lateriflora variety which can be processed and contaminated with other plants at high enough levels to be of concern.
Adverse effects
There have been several reports and small case series of acute liver injury with jaundice arising 1 to 3 months after starting herbal or dietary supplements containing S. baicalensis.7
Scutellaria baicalensis is a popular traditional plant in Chinese medicine. Scientists report that the Baikal compounds contained in the skullcap extract have a wide range of antitumor activity both in vitro and in vivo (liver cancer, stomach cancer, lung cancer, breast cancer, prostate cancer, bladder cancer, brain cancer, squamous cell carcinoma, mucoepidermoid carcinoma, colorectal cancer, gallbladder cancer, oral cancer, leukemia, lymphoma and myeloma). Widely distributed BAS of skullcap are flavonoids (baicalin, baicalein, wogonin and wogonoside), which are responsible for the antitumor activity of the plant. Their antitumor effect is due to the absorption of oxidative radicals, the weakening of the activity of NF-kB (nuclear factor-kB), the suppression of the expression of the COX-2 gene and the regulation of the cell cycle. In addition, baicalein, baicalin, and wogonin showed strong antioxidant activity.
Ischemic diseases: Diseases caused by diminished blood supply to any tissue or organ of the body, causing a shortage of oxygen.
It is known that baicalein is used in Asian medicine (in China and Japan) for the treatment of ischemic diseases. It has also been proven that this compound has antioxidant activity. In the work of J. Y. Jeong, a study was conducted on the presence of the protective effect of baicalein on DNA damage and apoptosis, as a result of which, scientists proved that baicalein effectively inhibited H2O2-induced cytotoxicity and DNA damage by inhibiting the accumulation of reactive oxygen species (ROS). Wogonin-5,7-dihydroxy-8-methoxyflavone, a flavonoid-like chemical compound, is a flavone that has an antitumor effect, which consists in inhibiting the growth of cancer cells, by stimulating autophagic and apoptotic cell death. Another valuable flavonoid is oroxylin A, which induces apoptosis in human colon cancer cells via the mitochondrial pathway, which has an anti-hepatic effect. In the study of H. Jin, it was proved that oroxylin A relieved alcoholic liver damage by inhibiting the aging of hepatocytes, in addition, it was proved that this compound also has anti-inflammatory, anti-cancer, antibacterial and antiviral effects.
Zhao et al (2019) published a systematic and comprehensive overview on the traditional usages, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Scutellaria baicalensis Georgi. This provides an excellent overview of its effects before going into some of these in greater detail.
MAPK: Mitogen-activated protein kinase.
“The MAPK/ERK pathway (also known as the Ras-Raf-MEK-ERK pathway) is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell.
The signal starts when a signaling molecule binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, such as mitogen-activated protein kinases (MAPKs), originally called extracellular signal-regulated kinases (ERKs), which communicate by adding phosphate groups to a neighboring protein (phosphorylating it), thereby acting as an "on" or "off" switch.
When one of the proteins in the pathway is mutated, it can become stuck in the "on" or "off" position, a necessary step in the development of many cancers. In fact, components of the MAPK/ERK pathway were first discovered in cancer cells, and drugs that reverse the "on" or "off" switch are being investigated as cancer treatments.”8
Excerpts from their paper are presented below:9
Key findings
So far, over 40 compounds have been isolated and identified from Scutellaria baicalensis, including flavonoids, terpenoids, volatile oils and polysaccharides. The compounds and extracts isolated from Scutellaria baicalensis exhibit a wide range of pharmacological activities, including the effects on the nervous system, effects on the immune system, liver protection, antitumour effects, antibacterial and antiviral effects, antioxidant effects and other pharmacological effects.
There are about 360 species of Scutellaria baicalensis in the Labiatae genus. Among them, there are about 98 species and 43 varieties of Scutellaria baicalensis in China. Even though Scutellaria baicalensis is distributed all over the world, it is rare in tropical Africa. In China, Scutellaria baicalensis is commonly named HuangQin and Huang means yellow colour and Qin means a type of herb like a reed. HuangQin is a large-scale Chinese medicine with a long history of economic and medicinal value. Moreover, it is often used as an effective component of traditional Chinese medicine prescriptions.
Phytochemistry
Many chemical constituents of Scutellaria baicalensis have been isolated and identified by various methods since 1973. To date, more than 40 ingredients have been identified, in which flavonoids and their glycosides are considered to be characteristic components of Scutellaria baicalensis. In this part, the chemical constituents of Scutellaria baicalensis are mainly divided into five categories, and the main components and separation methods in each category are introduced and described as below.
Flavonoids
Scutellaria baicalensis contains a wide variety of polyphenols, especially flavonoids, which are the main active substances in Scutellaria baicalensis. Since the late 1970s, more than 40 different polyphenols have been isolated and identified from Scutellaria baicalensis, including flavonoids and their flavonols, dihydroflavones and their dihydroflavonols, chalcones and biflavonoids. Among them, the most representative ingredients are baicalin, baicalein, wogonoside and wogonin.
Volatile oils
The volatile oil in Scutellaria baicalensis has aromatic smell and sweet taste. Furthermore, they exhibit significant antibacterial activity against Gram-positive and Gram-negative bacteria, Bacillus subtilis and Enterococcus faecalis, Klebsiella pneumonia and Salmonella enterica (Pant et al.).
Fukuhara K et al. (1986) identified eight kinds of volatile oil components in Scutellaria baicalensis by GC-MS for the first time. The major constituents of the volatile oils were acetophenone, l-phenyl-1, (E)-4-phenyl-3-buten-2-one, 3-butanedione, palmitic acid and oleic acid. Sixty-four components have been isolated and identified in Scutellaria baicalensis.
Terpenoids
Diterpenoids in Scutellaria baicalensis have a variety of activities such as antitumour effect, insect refusal and antifungal. More than 100 diterpenoids have been isolated from Astragalus plants. However, at present, there are limited reports on terpenoids in Scutellaria baicalensis.
In one report in 1996, Hussein et al. obtained the first detectable diterpene component from the acetone extracts of the aerial part of Scutellaria baicalensis. Then, two new dodecanedioxins contained in the Scutellaria baicalensis plant were also isolated and identified (Bruno M et al., 1998).
Polysaccharides
Polysaccharides from Scutellaria baicalensis exhibit antioxidant, antiviral, immune regulation and other effects.
It was reported that the main polymers of polysaccharides in Scutellaria baicalensis were water-soluble polysaccharides WSPS'-1, WSPS'-2 and WSPS'-3, which were composed of glucose, galactose and arabinose. The secondary components were two highly polyglucans (Olennikov et al., 2008). In addition, the content of polysaccharides in Scutellaria baicalensis is closely related to the origin and processing method.
Other chemical components
In addition to the above chemical compositions, there are some other ingredients that have been isolated and identified from Scutellaria baicalensis including β-sitosterol (64), benzoic acid (65) and benzyl alcohol (66). Furthermore, Tuan et al. (2014) found that the main carotenoids in Scutellaria baicalensis were lutein (67) and β-carotene (68), and the leaves contained a lot of carotene. Lately, it was reported that three lignan glycosides (69–71) from the roots of Scutellaria baicalensis were isolated by spectral detection and qualitative chemical testing.
WESB: water extracts of Scutellaria baicalensis
MESB: methanol extracts of Scutellaria baicalensis
EESB: ethanol extracts of Scutellaria baicalensis
Effects on the nervous system
The pharmacological effects of Scutellaria baicalensis on the nervous system have been completely summarized. Scutellaria baicalensis was widely used in the treatment of stroke in 1999.[45] The potential mechanism of the resistance to stroke in Scutellaria baicalensis was explored by Kim et al. (2001). They reported that the methanol extracts of Scutellaria baicalensis (MESB) at doses of 0.1, 1 and 10 mg/kg could significantly increase the neuronal cell density of ischaemic animals and the mechanism may be related to the inhibition of TNF-α and NO production, and protection of neuronal cells from oxidative stress in vitro.
Furthermore, it was reported that the water extracts of Scutellaria baicalensis (WESB) (10, 30 and 100 mg/kg, p.o.) could significantly improve memory in ibotenic acid-induced model rats via the passive avoidance test and Y-maze test. The mechanism for this effect might be related to that Scutellaria baicalensis could promote the recovery of specific subtypes of neurons. In short, Scutellaria baicalensis is a good natural herb for the treatment of degenerative neuronal diseases with memory loss (Heo et al.)
Effects on the immune system
In 2010, Kim et al. demonstrated that the MESB (0.1 to 1 g/kg, BW) could inhibit compound 48/80 or anti-dinitrobenzene (DNP) IgE-induced allergic reactions in vivo and the mechanism may be associated with a decrease in histamine release from RPMC activated by compound 48/80 or anti-DNP IgE. Additionally, Jung et al. indicated that the ethanol extracts of Scutellaria baicalensis (EESB) had a significant inhibitory effect on allergic inflammation in vivo and in vitro. The mechanism for this effect may be related to downregulation of expression of various inflammatory mediators and decreased the production of inflammatory cytokines and MAPK activation.
Liver protection
Scutellaria baicalensis is a traditional Chinese herbal medicine which is used to treat liver diseases. Furthermore, Scutellaria baicalensis is the main part of Xiao-Chai-Hu-Tang, and it is reported that this prescription is widely used to treat liver necrosis caused by dimethylnitrosamine or pig serum. In 2002, the MESB (150 mg/kg, i.g. for 28 days) was reported to have a significant antibacterial effect on the liver in an animal model of liver fibrosis induced by bile duct ligation and scission (BDL) or carbon tetrachloride (CCl4) in rats.
Antitumour effects
As early as 1992, Konoshima et al. found that the ethyl acetate extracts of Scutellaria baicalensis significantly inhibited the activation of EBV-EA (100% and 33% inhibition at 10 μg/ml and 1 μg/ml, respectively). This provided the basis for finding possible antitumour agents. Small et al. reported the WESB at doses of 0.05 to 0.8 mg/ml had significant growth inhibition on prostate cancer cell lines including LNCaP, androgen-dependent, and PC-3, androgen-independent, and both cell lines had a dose- and time-dependent growth inhibitory effect. PC-SPES is an early herbal mixture for the treatment of prostate cancer. However, their study found that Scutellaria baicalensis was more effective in inhibiting cell growth than PC-SPES (IC50 = 0.38 mg/ml for PC-3 cells). Additionally, the molecular mechanism of Scutellaria baicalensis extracts against prostate cancer activity may be that Scutellaria baicalensis targeted a variety of cell proliferation pathways, including COX-2/PGE2, cyclin D1 and cdk1, leading to inhibition of prostate cancer growth in the G1 or G2 phase.
In addition to these, Lin et al. (2013) revealed that the EESB and its active flavonoids could reversibly inhibit myeloma SP cells, and they also discovered a potential new molecular mechanism possibly associated with the target SP cells by modulating the expression of the ABCG2 protein.
Recently, Tao et al. indicated that baicalin, the main component of Chinese medicine Scutellaria baicalensis, could significantly induce and enhance apoptosis of HT-29 colon cancer cells in a dose- and time-dependent manner, and strongly inhibit tumour growth in xenograft nude mice. Their study confirmed that baicalin induced apoptosis in colon cancer cells through a mechanism possibly associated with the inhibition of c-Myc expression and downregulation of oncomiRs associated with many apoptoses.
AFM1: Aflatoxin M1
AFBO: AFB1-8,9-epoxide
TCDD: 2,3,7,8-Tetrachlorodibenzo-p-dioxin
Antibacterial and antiviral effects
In 2001, Kim et al. showed that the WESB at doses of 5–50 μg/ml could effectively inhibit the production of AFM1 and AFBO with IC50 values of 6.8 and 122.4 μg/ml in TCDD-treated rat microsomes. They also found that the inhibition of AFBO formation by the WESB was weak, probably because the WESB only had an effective inhibitory effect on the formation of AFM1 mediated by CYP1A1/2. Later, it was reported that 80% ethanol extract of Scutellaria baicalensis showed inhibition of Streptococcus mutans at the lower dose of 750 μg/disc (Duan et al.). This study showed that Scutellaria baicalensis had a certain inhibitory effect on oral pathogens and had the potential to prevent dental caries. In addition, at a dose of 1 g/mL (i.g. for 4 days, 0.2 ml/day), the WESB had obvious antibacterial effects against common pathogenic bacteria such as Escherichia coli and Salmonella in vitro and in vivo (Cui et al.).
It is well known that Scutellaria baicalensis extracts display extensive antiviral activity. But the antiviral mechanism is still unclear. Zofia et al. studied the antiviral mechanism of the EESB by using human peripheral blood leucocytes (PBL) that were not infected and infected with vesicular stomatitis virus (VSV). They found that the EESB at dose of 200 μg/ml regulated innate antiviral immunity by regulating cytokine production including IFN-α and IFN-γ, and stimulating human leucocyte resistance that was stimulating the production of TNF-α and IL (IL-12, IL-10).
Apart from these, it was revealed that the acetone extract of Scutellaria baicalensis (AESB) showed potent dose-dependent inhibition on all four DENV serotypes in Vero cells with the values of IC50 ranging from 86.59 to 95.19 μg/ml. An interesting finding was that when cells were treated with extracts of different DENV serotypes during virus adsorption, the IC50 value was reduced to ranging from 56.02 to 77.41 μg/ml (Zandi et al.). Additionally, in one report of 2015, a total of 30 compounds were isolated and identified from Scutellaria baicalensis, and five of them including baicalin, baicalein, wogonin, chrysin and oroxylin A showed significant anti-H1N1 activity with IC50 values of 7.4, 7.5, 2.1, 7.7 and 12.8 μm, respectively. It was worth mentioning that the cytotoxic activity was the strongest at 10 μm, and at this time, the inhibition rate was as high as 61.2%.
Antioxidant effects
In 1997, Gabrielska et al. studied the antioxidation effect of the extracts of Scutellaria baicalensis on UV-induced oxidation and found that Scutellaria baicalensis extracts could mediate various diseases especially skin disease caused by solar radiation through its ability to scavenge free radicals and protect against lipid peroxidation. Additionally, Gao et al. demonstrated that baicalein and baicalin scavenged hydroxyl radicals, DPPH radicals (IC50 = 24, 32 μmol/l) and alkyl groups (IC50 = 10, 20 μmol/l) in a dose-dependent manner, while baicalein and baicalin [ed: wogonin and wogonoside] had no effect on these free radicals. Furthermore, at a dose of 10 μmol/l, baicalein and baicalin could not only effectively inhibit Fe2+-ascorbic acid, AAPH or NADPH-induced mitochondrial lipid peroxidation in rat cortex, but also markedly protect cells from H2O2-induced damage in human neuroblastoma in the SH-SY5Y cell system. Therefore, they suggested that baicalein and baicalin could be used as good free radical scavengers for the treatment of craniocerebral injury associated with free radical attack.
It is well known that microscopic and macrovascular complications of many diabetes are caused by free radical-induced oxidative stress and a decrease in intrinsic antioxidant defence. Scutellaria baicalensis is used to treat many diseases related to oxidative stress such as diabetes because of its powerful antioxidant action and free radical scavenging activity. Moreover, Li et al. demonstrated that baicalin or baicalein was likely to be a candidate for new antidiabetic drugs.
Other pharmacological effects
In addition to the above pharmacological effects, other effects of Scutellaria baicalensis had also been reported. The protease inhibitor is a common class of anti-HIV drugs, especially ritonavir. However, when used by a patient, it can cause significant gastrointestinal disturbances such as nausea or vomiting. Aung et al. (2005) used this rat heterotopic model to assess the effect of Scutellaria baicalensis on nausea caused by ritonavir. They found that the WESB at doses of 0.3 and 3.0 mg/kg with pretreatment could reversibly reduce ritonavir-induced kaolin intake in a dose-dependent manner. The reduction in area under the curves (AUCs) of kaolin intake between ritonavir alone with the WESB 0.3 mg/kg plus ritonavir, ritonavir only and the WESB 3 mg/kg plus ritonavir was 16.0% and 77.7%, respectively. Additionally, it was reported that the Scutellaria baicalensis extracts could protect the auditory function in noise-induced hearing loss (NIHL) and the active ingredient could be baicalein (Kang et al.). However, the protective mechanism of baicalein remained to be studied.
Apart from these, Scutellaria baicalensis extracts have a remarkable effect of absorbing UV radiation. Jin et al. reported that 5% Scutellaria baicalensis extracts BuOH fraction could strongly absorb UV radiation, scavenge free radical activity and attenuate UV-induced HaCaT cell death. At the same time, they first compared the sunscreen performance of sunscreens with or without supplementation of the BuOH portion of the Scutellaria baicalensis extracts in human in-vivo tests, and the sunscreen factor (SPF) values were 22.7 and 17.8, respectively. It was reported that wogonin at a dose of 20 mg/kg significantly reduced the percentage of cell proliferation in endometrial lesions. This mechanism may be related to the inhibition of estrogen receptor expression in T-HESC.
In 2017, Kudo et al. demonstrated that the MESB (35–75 μg/ml) could inhibit the production of melanin in a dose-dependent manner. In the latest study, it was reported that the WESB could cause paralysis and death of leeches in a relatively short time.
Summary of pharmacologic effects
In summary, Scutellaria baicalensis has a wide range of pharmacological activities including effects on the nervous system, effects on the immune system, liver protection, antitumour effects, antibacterial and antiviral effects, and antioxidant effects (Table 4). These pharmacological effects indicate that Scutellaria baicalensis has extensive development prospects in the treatment of liver cancer, depression, diabetes and especially cancer. However, the relationship between its pharmacological activities and specific chemical components is not clear. Therefore, it is urgent and important to research on the structural effect of Scutellaria baicalensis.
Worth pointing out that these are extremely high doses relative to the biologically active dose needed. For comparison, using a mouse model the maximum tolerated dose of ethanol is only 986 mg/kg, the lethal dose (LD50) being 1.6-4.3 g/kg10:
Toxicology
For thousands of years, Scutellaria baicalensis was widely regarded as a safe and nontoxic traditional Chinese medicine in China. However, some studies have pointed out that Scutellaria baicalensis has potential toxicity. In 2013, Kim et al. demonstrated that the aqueous extracts of Scutellaria baicalensis had no significant changes in body weight, clinical symptoms and mortality on rabbits and guinea pigs during the dermal stimulation/corrosion and skin sensitization tests. Liu et al. studied the acute toxicity test of the water extracts and alcohol extracts of Scutellaria baicalensis and then found that maximal tolerated dose (MTD) of the aqueous extracts of Scutellaria baicalensis in mice was 72.0 g/kg, the median lethal concentration (LD50) value of 80% ethanol extracts of Scutellaria baicalensis was 39.60 g/kg. In the latest report, Yi et al. used different doses (300, 1250 and 2500 mg/kg per day, p.o.) of ethanol extracts of Scutellaria baicalensis to explore its toxicity to the liver. They found that when the dose was as high as 2500 mg/kg per day, the liver tissue of the rats showed some reversible inflammatory changes. In addition, there were some changes in GLU, electrolyte and lipid levels.
In recent years, the toxicity of active ingredients isolated from Scutellaria baicalensis has also been investigated. It was reported that baicalin inhibited the proliferation of embryonic stem cells D3 and 3T3 to a certain extent, and their IC50 values were 135.9 and 63.3 mg/l, respectively (Zhang et al.). Thus, they came to the conclusion that baicalin had weak embryotoxicity. In 2017, Do et al. reported that the LD50 value of the mouse was 286.15 mg/kg after intravenous injection (i.v.) of wogonin.
There is no obvious adverse reaction in the oral preparation of Scutellaria baicalensis, but there may be stomach discomfort, diarrhoea, etc., for individual patients, and a large blister-like drug eruption may occur in allergic constitutions. Scutellaria baicalensis may also cause symptoms such as hypothermia, muscle soreness and leucopenia when used in large doses of injectable preparations. In short, Scutellaria baicalensis exhibits low toxicity and can be widely used in clinical practice (Table 5). However, due to its bitter and cold medicinal properties, it is not suitable for people with spleen and stomach deficiency.
Our paper systematically reviews the traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology, and points out several directions worth researching. We hope that this review highlights the important value of Scutellaria baicalensis and promotes its all-round development.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Furin inhibition and roles as a tumor suppressor
Furin is a protease, a proteolytic enzyme that in humans and other animals is encoded by the FURIN gene. Some proteins are inactive when they are first synthesized, and must have sections removed in order to become active. Furin cleaves these sections and activates the proteins. It was named furin because it was in the upstream region of an oncogene known as FES. The gene was known as FUR (FES Upstream Region) and therefore the protein was named furin. Furin is also known as PACE (Paired basic Amino acid Cleaving Enzyme). A member of family S8, furin is a subtilisin-like peptidase.
Clinical significance
Furin is one of the proteases responsible for the proteolytic cleavage of HIV envelope polyprotein precursor gp160 to gp120 and gp41 prior to viral assembly. This protease is thought to play a role in tumor progression. The use of alternate polyadenylation sites has been found for the FURIN gene.
Furin is enriched in the Golgi apparatus, where it functions to cleave other proteins into their mature/active forms. Furin cleaves proteins just downstream of a basic amino acid target sequence (canonically, Arg-X-(Arg/Lys) -Arg'). In addition to processing cellular precursor proteins, furin is also utilized by a number of pathogens. For example, the envelope proteins of viruses such as HIV, influenza, dengue fever, several filoviruses including ebola and marburg virus, and the spike protein of SARS-CoV-2, must be cleaved by furin or furin-like proteases to become fully functional. When SARS-CoV-2 virus is being synthesized in an infected cell, furin or furin-like proteases cleave the spike protein into two portions (S1 and S2), which remain associated.
Anthrax toxin, pseudomonas exotoxin, and papillomaviruses must be processed by furin during their initial entry into host cells. Inhibitors of furin are under consideration as therapeutic agents for treating anthrax infection.
Furin is regulated by cholesterol and substrate presentation. When cholesterol is high, furin traffics to GM1 lipid rafts. When cholesterol is low, furin traffics to the disordered region. This is speculated to contribute to cholesterol and age dependent priming of SARS-CoV.
Expression of furin in T-cells is required for maintenance of peripheral immune tolerance.11
The Ukranians have known for at least 12 years that Baicalin (among other flavonoids) has furin inhibitory effects. Translated from Russian, a paper by Kibirev et al from back in 2010:12
Abstract
Furin, a human subtilisin-related proprotein convertase, is the most important pharmaceutical target because it plays a vital role in development of numerous disease processes. To identify a new class of small non-peptide inhibitors of furin we performed a study of several flavonoids and some natural products. Glycosylated flavonoids: rutin, naringin, baikalin and methylhesperidin were shown to inhibit furin at pH 7.2 reversibly and competitively with Ki- 80-200 microM. The Ki values were derived from Dixon and/or Eadie-Hofstee plots using fluorogenic substrate Boc-Arg-Val-Arg-Arg-AMC. Although studied flavonoids display only a temperate furin inhibition, they may serve as a great potential for the future development of more potent non-peptide inhibitors against furin.
Furine (QF 3.4.21.75) – the most interesting-a certain and characterized enzyme, belonging to the Proprote family-inconvertase (PC), is intracellular dependent serine en Animals. Due to limited splitting peptide bonds, it is able to activate precursors of various proteins: re-receptors, hormones, growth factors and diffe-timing, plasma proteins, sis enzymes-blood clotting topics, etc. Besides Of this furin is involved in the processing of distinctions. Bacterial exotoxins, including anthrax toxins [4, 5] and diphtheria [6], as well as in the activation of glycoproteins distinguish-Viruses, such as fever viruses Ebola, gp 160 HIV-1, avian influenza. The ability of furies to to activate enzyme precursors, involved in the pathogenesis of cancerous diseases-, Alzheimer's disease and others Diseases. Therefore, furin is considered as an important pharmacological target for the synthesis of appropriate inhibitors with the purpose of creating on their basis modern of carbohydrate preparations.
It’s an imperfect translation but we can see that the gycosidic groups on the flavenoids and amino acid residues bind with furin to varying degrees and cause competitive inhibition:
So, the work carried out showed that rutin, naringin and some other glycosi-lated flavonoids that do not have in the mole-Coole positive charge, capable of mutual-act with furin and inhibit it competitive with magnitude Kilocated in ranges of ~ 80–200 μM. Competition research Mature compounds with substrate witness-That flavonoids are direct-but bind to the active center of the enzyme. Although the mechanism of such interaction has not yet quite clear, it can be assumed that the tormo-The pressure is caused by both hydrophobic interchanges-by the actions of the skeleton of the flavonoid molecule, so and the formation of hydrogen bonds between gid-rocky bands glycosylated fragment and residues of amino acids actively The center of the furin. In favor of this assumption-As evidenced by the work, in which shown that glycosylation of andrographo-Lida causes the growth of its inhibitory ac-It's a good thing. Differences in the effectiveness of the tor-Furin in the studied compounds depend, apparently, on the method of orientation in the space of their glycosidic groupings, providing an accurate "fit" to the molek-The flavonoid to the enzyme and its most pronounced ductive interaction with the binding the center of the furin.
Institute of Biological Chemistry and Chemistry Naftokhimii NAS Ukrainy, Kyiv;
Institute of Biokhimii im. O. V. Palladina NAS of Ukraine, Kyiv;
e-mail: kibirev@bpci.kiev.ua
Four years later and in 2016 the Palladin Institute published an updated paper on the latest research into furin inhibitors, their structure and efficiency:13
Data on cancer cell proliferation and migration revealed that baicalein and oroxylin A glycoside were more effective (among tested flavonoids) in suppressing of growth and migration of pathogenic cells. It was also found that baicalein (compound 46), which did not have the carbohydrate moiety in its structure, inhibited more effectively growth and proliferation of cancer cells than its glycoside – baicalin (compound 50).
Majumbar et al. demonstrated that these flavonoids exhibit inhibitory effect against furin and other PCs. Ki and IC50 values were found to be in the range of 5-35 µM. Thus, oroxylin A was the most potent furin inhibitor with Ki = 5.0 µM that is 5- and 7-fold higher than for baicalein and chrysin, respectively. Its anticancer effectiveness was lower than that of baicalein and oroxylin A glycoside. This difference might result from the involvement, be-sides furin, other PCs (particularly PCSK6) in the process of tumor growth. A comparative analysis of the inhibitory effectiveness against furin, PCSK4, PCSK5 and PCSK7 revealed that only baicalein has selectivity for PCSK4.
PCSK9 is ubiquitously expressed in many tissues and cell types. PCSK9 binds to the receptor for low-density lipoprotein particles (LDL), which typically transport 3,000 to 6,000 fat molecules (including cholesterol) per particle, within extracellular fluid. The LDL receptor (LDLR), on liver and other cell membranes, binds and initiates ingestion of LDL-particles from extracellular fluid into cells, thus reducing LDL particle concentrations. If PCSK9 is blocked, more LDLRs are recycled and are present on the surface of cells to remove LDL-particles from the extracellular fluid. Therefore, blocking PCSK9 can lower blood LDL-particle concentrations.14
Of particular interest is that a skullcapflavone (“compound 1”) isolated from root extracts of S. baicalensis was also able to inhibit PCSK9. Nhoek et al investigated flavonoids from Scutellaria baicalensis and their inhibitory activity against PCSK9 expression in a paper from 2018.15
Also note the efficacy of berberine against elevated LDL levels, which I reviewed separately:

Out of the active compounds, compound 1 was further tested for its PCSK9 and LDL-R protein expression and it was found that compound 1 was able to inhibit PCSK9 and increase LDL-R protein expression, respectively (Figure 3C). As mentioned earlier, PCSK9 facilitates LDL-R degradation and prevents LDL-R recycling. Taking into consideration this function of PCSK9, compound 1 may potentially lower cholesterol levels by decreasing PCSK9 expression and concomitantly increasing LDL-R expression.

Note that compounds 1 and 4 were more effective than berberine at downregulating SREBP-1. Indeed, at a concentration of 20uM compound 1 almost totally inhibited expression.
SREBP-1 is a “key transcription factor that regulates expression of genes involved in cholesterol biosynthesis and lipid homeostasis”, ie it promotes sterol biosynthesis16 and it promotes breast cancer.
Further analysis demonstrated that downregulation of SREBP-1 mRNA was detected in compounds 1 and 4, suggesting inhibition of PCSK9 mRNA expression was mediated by SREBP-1 as reported in the literature. Thus, it merits enantioselective synthesis of (2R,3R) compound 1 and its derivatives for further chemical modifications and in vivo studies.
In 2016 Bao et al conducted in vivo studies and found a strong correlation between SREBP-1 levels and breast cancer tumor differentiation and metastasis.17
The potential therapeutic value of S. baicalensis extracts are clear:
Abstract
Re-programming of lipogenic signaling has been previously demonstrated to result in significant alterations in tumor cell pathology. Sterol regulatory element-binding protein 1 (SREBP-1) is a known transcription factor of lipogenic genes. Despite the fact that its functions in proliferation and apoptosis have been elucidated in recent studies, its role in tumor cell migration and invasion, particularly in breast cancer, remains unclear. In present study, the messenger RNA and protein expression levels of SREBP-1 in cancer tissues were observed to be overexpressed compared with those in matched para-cancerous tissues (P<0.01). SREBP-1 level was highly positively correlated with tumor differentiation (P<0.001), tumor-node-metastasis stage (P=0.044) and lymph node metastasis (P<0.001). High expression of SREBP-1 predicted poor prognosis in patients with breast cancer. Additionally, multivariate analysis revealed that SREBP-1 was an independent factor of 5-year overall and disease-specific survival in breast cancer patients (P<0.01). In vitro studies revealed that the suppression of SREBP-1 expression in both MDA-MB-231 and MCF7 cells significantly inhibited cell migration and invasion (P<0.01). The present data indicate that SREBP-1 plays a critical role in breast cancer migration and invasion, and may serve as a prognostic marker of this malignancy.
Keywords: SREBP-1; breast cancer; invasion; migration; prognosis.
Coming back to PCSK9 (proprotein convertase subtilisin/kexin type 9), inhibition by S. baicalensis compounds due to SREBP-1 suppression may enhance inhibition of tumor growth by promoting apoptosis and by limiting cholesterol availability to cancer cells.
PCSK9 is ubiquitously expressed in many tissues and cell types. PCSK9 binds to the receptor for low-density lipoprotein particles (LDL), which typically transport 3,000 to 6,000 fat molecules (including cholesterol) per particle, within extracellular fluid. The LDL receptor (LDLR), on liver and other cell membranes, binds and initiates ingestion of LDL-particles from extracellular fluid into cells, thus reducing LDL particle concentrations. If PCSK9 is blocked, more LDLRs are recycled and are present on the surface of cells to remove LDL-particles from the extracellular fluid. Therefore, blocking PCSK9 can lower blood LDL-particle concentrations.18
Mahboobnia et al performed a literature review in 202119
In contrast to suppressing apoptosis, PCSK9 appears to promote apoptosis of nerve cells in the cerebellum.
Basically, tumor cells rely on cholesterol for membrane and lipid raft biosynthesis, signaling molecules, or other factors in order to meet the fast growth requirements. Cancer cell’s demand for cholesterol is supplied by both uptake from the blood or de novo synthesis (Fig. 1). Therefore, high plasma cholesterol may provide such a high cancer cell requirement.
Sun et al., demonstrated that PCSK9 has an anti-apoptotic effect in mouse liver, and the absence of PCSK9 can be preservative against melanoma invasion in liver, as a result of lower circulating LDL-C. They injected B16F1 melanoma cells into both wild-type and PCSK9 KO mice to induce liver metastasis. They found a lower risk to develop liver metastases in PCSK9 KO mice, a result that was related to cholesterol levels. Thus, the authors proposed that PCSK9 inhibitors which lower LDL-C could be advantageous to control hepatic metastasis. Indeed, these effects arise from lower cholesterol levels in PCSK9 deficiency and augmentation of tumor necrosis factor α-mediated apoptosis, resulting in a less desirable environment for tumor progression. They come into the conclusion that PCSK9 inhibitor, might be effective in melanoma and possibly other types of cancer. On the contrary, pro-apoptotic action of PCSK9 in primary cultures of cerebellar granular neurons has been described via its ability to promote the degradation of the apolipoprotein E receptor 2.
Note that its context sensitive - lung cancer can be inhibited by PCSK9 siRNA (small interfering or silencing RNA), two contrasting examples:
Recently, however, another role for PCSK9 has been identified in lung adenocarcinoma. Xu et al. found that PCSK9 siRNA provides an anti-tumor effect via inducing mitochondrial pathway and ER-related cell death in A549 human lung adenocarcinoma cells.
Another research by Demidyuk et al. was conducted on samples of human lung cancer to investigate the expression profile of PCs genes. Expression analysis revealed dramatic differences between tumor and normal tissues. Among the other PCs, PCSK9 mRNA was found in 29 normal tissue samples versus only in 18 tumor ones. Thus, the variations in expression of PC genes may indicate different pathways involved in tumorigenesis.
4.3. PCSK9 and leukemia
Hypercholesterolemia arising from elevated LDL levels is a common manifestation among leukemia patients. Importantly, the prolonged existence of LDL in blood raises its oxidation. Interestingly, activation of scavenger receptor LOX1 by ox-LDL leads to induction of PCSK9 expression in non-hepatic tissues, highly suggesting the existence of a crosstalk between ox-LDL, LOX1 and PCSK9. It is important to mention that high levels of serum ox-LDL and upregulation of LOX1 can raise also the risk of prostate, lung, and colon cancer. Zia et al. found that PCSK9 expression was significantly induced in leukemia cells. In addition, since eugenol could block LDL oxidation and simultaneously reduce PCSK9 expression through the inactivation of LOX1 receptor, they suggested that PCSK9 could be a promising target in patients with leukemia.
Funding
This study was supported by the Russian Science Foundation (Grant # 18-15-00254).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
PCSK9 has low cancer specificity but moderate to strong cytoplasmic positivity, ie it is common to many types of cancer and its presence correlates strongly with cancer cell protoplasm outside the nucleus:20
In 2016, Moore et al published a paper on how to optimise the extraction efficiency of baicalin, its anticancer effects, bioavailability and nanotechnology.
They found that ultrasound-assisted extraction (UAE), which uses cavitation, is an efficient way to extract the compound using a solvent of 40% ethanol at 60°C, and combing this technique with heat reflux extraction (HRE) increase the yield and efficiency further still.
They then described modes of action at inhibiting cancer cells, which is promising as an alternative to the very common side effects of radiotherapy and chemotherapy:21
Surgery, radiation therapy, chemotherapy, and targeted therapy are the most common types of cancer treatment. However, they might cause severe side effects in patients. Fatigue, a common early and chronic side effect of irradiation, was reported in up to 80% of patients during radiation therapy and 30% of follow-up visits. The incidence of chemotherapy-induced nausea and vomiting (CINV) in patients was 59.7% within 24 hours after treatment. And the incidence of delayed-CINV was 22.9% in patients who experienced neither vomiting nor nausea during the first 24 hours post-chemotherapy. Thus, it is important to find an alternative cancer treatment with fewer adverse side effects.
There is increasing interest in selecting specific dietary flavonoids as potential anticancer agents because of their high efficiency and low toxicity. Flavonoids are a class of plant secondary metabolites, which consist of two phenyl rings and a heterocyclic ring. Some of the plants containing flavonoids have been used in traditional Chinese medicine for thousands of years.
Different extraction methods are employed to isolate baicalin from Scutellaria baicalensis. Among them, heat reflux extraction (HRE), ultrasound-assisted extraction (UAE) and supercritical fluid extraction (SFE) are three most widely used extraction methods. Baicalin displays activities against cancer, bacterial infections, and oxidative stress-related disease. Nevertheless, like other flavonoids, poor bioavailability limits its application. Strategies to improve the bioavailability of baicalin are being explored. The nanonization of bioactives derived from herbal medicines has gained more and more attention in recent years. Nanoformulations, such as nanoparticles, nanoemulsions and liposome, are colloidal systems with particles varying in size from 10 nm to 1000 nm. Nanoformulation of herbal medicines possess benefits, such as improving solubility, enhancing bioavailability, increasing absorbency of the organism, reducing medicinal herb doses, and achieving steady-state therapeutic levels of drugs over an extended period compared with traditional herbal medicine preparations. Though nanotechnology shows a lot of promise, further studies should be conducted to validate this.
Baicalin uses several mechanisms to exert its anticancer effects, including by promoting apoptosis, by inhibiting migration, invasion and metastasis of cancer cells and by inhibiting the growth of new blood vessels (angiogenesis) which aids the growth and progression of the tumor.
Caspase-8 promotes apoptosis and Bcl-2 (B-cell lymphoma 2) is a protein that either inhibits or promotes cell death.
MMPs are matrix metalloproteinases that promote tumorigenesis via several signalling pathways.22
Anticancer Effect
Baicalin exhibits its anticancer activity against various cancers. The inhibitory effect of baicalin was investigated in two ovarian cancer cell lines (OVCAR-3 and A2780/CP-70) and a normal ovarian cell line (IOSE-364). Baicalin significantly and selectively inhibited the viability of ovarian cancer cells. Meanwhile, it had little adverse side effects on normal ovarian cells.
Lacking apoptosis is a hallmark of cancer. Baicalin has been proven to induce apoptosis in human prostate cancer cells and human cervical cancer cells. In baicalin-treated HeLa cells, the expression of Bax, Fas, FasL and Caspase-8 was up-regulated, and the expression of Bcl-2 was down-regulated. It indicated that baicalin triggered apoptosis via activating extrinsic apoptotic pathway.
Baicalin has been reported to retard cancer progression by inhibiting migration, invasion and metastasis of cancer cells. Baicalin suppressed the tumorigenecity of MDA-MB-231 cells by down-regulating the expression of MMP-2, MMP-9, uPA and uPAR through modulating p38MAPK signaling pathway.
Angiogenesis is the process of forming new capillary blood vessels from preexisting vasculature leading to neovascularization. Neovascularization is the development of new blood vessels that typically takes place in tissues where circulation has been impaired, either by disease or trauma. Under proper stimulation, endothelial cells begin to form new capillary vessels in the presence of angiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Angiogenesis is essential for development and other physiologic conditions. Angiogenesis is now believed to be required for the growth and progression of solid cancers. During solid cancer growth, transformed cells undergo clonal expansion in an avascular state when the expanding lesion is small enough to take in nutrients and to expel metabolic wastes by diffusion. Neovascularization is absolutely required for solid tumor expansion, as the proliferation, as well as metastatic spread, of cancer cells depends on an adequate supply of oxygen and nutrients and the removal of waste products. Cancer cells can stimulate adjacent cells or themselves to release angiogenic factors to promote angiogenesis. Because of the critical dependence of tumor growth and metastasis on angiogenesis, it is suggested that the process of angiogenesis might be a target for therapy. Baicalin decreased the expression of VEGF in two ovarian cancer cell lines (OVCAR-3 and A2780/CP-70). Liu et al. carried out chicken chorioallantoic membrane (CAM) assay to assess the anti-angiogenic potential of baicalin in vivo. The result of CAM assay revealed that baicalin blocked basic fibroblast growth factor-induced angiogenesis in a dose-dependent manner.
Bioavailability
The bioavailability of baicalin after intravenous, intraportal venous, intra-gastric, intra-duodenal, and intra-colonic administration to male rats was studied. Baicalin could not be detected in the hepatic extraction of the rats. The incomplete gastrointestinal absorption seemed to be the main barrier to oral bioavailability and the colon was the main absorption organ of baicalin.
The contents of baicalin and its metabolites in the plasma, colon, small intestine, lung, liver, pancreas, kidney, prostate, and in pancreatic tumor were measured in a xenograft animal model. A substantial amount of baicalin (34%–63%) was methylated to oroxylin A and its conjugates in various organs during absorption. Aglycones and conjugates of baicalin were detected in pancreatic tumor and in all tissues investigated except plasma. Plasma contained predominantly conjugates of baicalein, wogonin, and oroxylin A. The result implied that baicalin was potent to be a preventive supplement for pancreatic cancer.
Yu et al. found hydrolysis of flavonoids from Baikal skullcap enhanced anticancer activity. Baicalin, baicalein, wogonoside and wogonin are four main flavonoids in Baikal skullcap. The two glycosides (baicalin and wogonoside) can be transformed into their aglycones (baicalein and wogonin), which possess positive anticancer potential. Yu et al. elucidated that catalyzing flavonoids in Baikal skullcap using glycosidase increased the anticancer activities of this herb. Compared with the untransformed control, 8 h of glycosidase catalysis significantly increased anti-proliferative activity on human colorectal and breast cancer cells. The cancer cell growth inhibition was partially mediated by induction of cell cycle arrest at the S-phase and activation of apoptosis. The result suggested that there was a positive correlation between the aglycone content and the anti-proliferative effects. Using glycosidase to catalyze S. baicalensis might be a promising approach to modify the bioavailability of baicalin.
In 2017, Lin et al summarised which natural compounds from herbs can induce autophagy in cancer cells. I will include the table here as these may be considered for inclusion in combination therapies, subject to further checks for any interactions.23
Abstract
Accumulated evidence indicates that autophagy is a response of cancer cells to various anti-cancer therapies. Autophagy is designated as programmed cell death type II, and is characterized by the formation of autophagic vacuoles in the cytoplasm. Numerous herbs, including Chinese herbs, have been applied to cancer treatments as complementary and alternative medicines, supplements, or nutraceuticals to dampen the side or adverse effects of chemotherapy drugs. Moreover, the tumor suppressive actions of herbs and natural products induced autophagy that may lead to cell senescence, increase apoptosis-independent cell death or complement apoptotic processes. Hereby, the underlying mechanisms of natural autophagy inducers are cautiously reviewed in this article. Additionally, three natural compounds-curcumin, 16-hydroxycleroda-3,13-dien-15,16-olide, and prodigiosin-are presented as candidates for autophagy inducers that can trigger cell death in a supplement or alternative medicine for cancer therapy. Despite recent advancements in therapeutic drugs or agents of natural products in several cancers, it warrants further investigation in preclinical and clinical studies.
Keywords: autophagy inducer; autophagy inhibitor; cancer therapy; natural compound.
Baicalin is the last entry in Table 2. In the case of hepatocarcinoma (liver cancer) they found that it promoted RelB/p52 induced autophagy.

Key:
Heat shock factor 1 (HSF1); Heat shock protein 70 (Hsp70); Hexokinase 2 (HK2); Mammalian target of rapamycin (mTOR); Microtubule-associated protein 1 light chain 3 α (LC3); Mitogen-activated protein kinase kinase (MEK); Nerve growth factor IB (TR3); Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB); p70 ribosomal protein S6 kinase β-1 (p70S6K); Phosphoinositide 3-kinase (PI3K); Protein kinase B (Akt, PKB); Protein kinase RNA-like endoplasmic reticulum kinase (PERK); Reactive oxygen species (ROS); Sequestosome-1 (SQSTM1); Uncoordinated-51-like kinase (ULK); Wingless/Integrated (Wnt).
Transcription factor RelB is a protein that in humans is encoded by the RELB gene. It forms a dimer complex with tumor suppressor p52 and is involved with the pro-tumor NF-kappaB signalling pathway.24
Focusing on reference 88 above, in 2015 Tan et al demonstrated using a mouse model that baicalin changes tumour-associated macrophages into anti-tumor proinflammatory cytokine emitting M1 types whilst elevating RelB/p52 associated autophagy.25
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation, partly through the induction of angiogenesis.26
Abstract
The plasticity of tumour-associated macrophages (TAMs) has implicated an influential role in hepatocellular carcinoma (HCC). Repolarisation of TAM towards M1 phenotype characterises an immune-competent microenvironment that favours tumour regression. To investigate the role and mechanism of TAM repolarisation in suppression of HCC by a natural compound baicalin, Orthotopic HCC implantation model was used to investigate the effect of baicalin on HCC; liposome-clodronate was introduced to suppress macrophage populations in mice; bone marrow-derived monocytes (BMDMs) were induced to unpolarised, M1-like, M2-like macrophages and TAM using different conditioned medium. We observed that oral administration of baicalin (50 mg/kg) completely blocked orthotopic growth of implanted HCC. Suppression of HCC by baicalin was diminished when mice macrophage was removed by clodronate treatment. Baicalin induced repolarisation of TAM to M1-like phenotype without specific toxicity to either phenotype of macrophages. Baicalin initiated TAM reprogramming to M1-like macrophage, and promoted pro-inflammatory cytokines production. Co-culturing of HCC cells with baicalin-treated TAMs resulted in reduced proliferation and motility in HCC. Baicalin had minimal effect on derivation of macrophage polarisation factors by HCC cells, while directly induced repolarisation of TAM and M2-like macrophage. This effect was associated with elevated autophagy, and transcriptional activation of RelB/p52 pathway. Suppression of autophagy or RelB abolished skewing of baicalin-treated TAM. Autophagic degradation of TRAF2 in baicalin-treated TAM might be responsible for RelB/p52 activation. Our findings unveil the essential role of TAM repolarisation in suppressive effect of baicalin on HCC, which requires autophagy-associated activation of RelB/p52.
PBS liposomes were used as a baicalin-free control (column 1).
Baicalin treated macrophages demonstrated HCC tumour inhibition (column 2).
Clodronate liposome was used to deplete macrophage from the mice, but with baicalin (column 3).
(a) Schematic presentation of experimental design. (b) Removal of macrophage from mice completely blocked the anti-tumour effect of baicalin. Orthotopic HCC tumour growth in mice (n=4) with baicalin treatment was not obvious, whereas removal of macrophage by intraperitoneally injecting clodronate liposome resulted in relapse of hepatic tumour. (c) The size calculation of hepatic tumour. **P<0.01, compared with liposome control group without baicalin treatment; ΔΔP<0.01, compared with liposome control group with baicalin treatment. (d) The amount of intrahepatic tumour was slightly increased upon baicalin treatment without significance, while presence of clodronate liposome remarkably reduced hepatic macrophage. (e) Baicalin treatment did not potently induce production of inflammatory monocytes in bone marrow. (f) Baicalin treatment did not elevate inflammatory monocytes populations in circulating system.
To explore the mechanism underlying autophagy-induced repolarisation of TAM by baicalin, we examined the activation of RelB/p52 pathway, which was reported to dominate reprogramming of Th1 cells to Th2 phenotype. Increased RelB expression was observed in baicalin-treated TAMs, which could be attenuated by depletion of Atg5 (Figure 5a). Interestingly, it was observed that in baicalin-treated TAMs, the mRNA expression of RelB was elevated (Supplementary Figure S2). The consistent increase of phosphorylation of p100 and p52 in baicalin-treated TAMs revealed that the transcriptional increase of RelB may be due to the positive feedback loop of RelB/p52 activation.

(a) The induced expression of RelB in baicalin-treated TAM is associated with autophagy induction. RNA interference against Atg5 was conducted as described and TAM was treated with 20 μM baicalin for 48 h. Expression of RelB, p-p100 and p52 was detected by immunoblotting. It was shown that baicalin treatment resulted in increase of RelB and p52 expression, as well as phosphorylation of p100, and this effect of baicalin could be blocked by inhibition of autophagy by RNA interference against Atg5. (b) Baicalin treatment induced association of p52 with RelB. TAM was treated and co-immunoprecipitation assay was conducted as described. Increased association of RelB with p52 was observed in baicalin-treated TAM. Reduced IκBα with RelB was also observed. Expression of protein level was normalised by GAPDH as INPUT samples and by RelB as IPed-samples. (c) Baicalin treatment induced nuclear localisation of RelB and p52. DAPI was used to stain nuclei of the cells. TAM with or without baicalin treatment was fixed and stained with antibodies against RelB and p52, followed by observation under confocal microscope. Increased RelB and p52 presentation in nuclear area was found in baicalin-treated cells. (d) Elevation of RelB/p52-specific target gene expression CCL19 and CXCL12 was observed in TAM in the presence of baicalin (magnification: x60). (e) Suppression of RelB by RNA interference induced re-skewing of baicalin-treated TAMs to M2 phenotype. RNA interference was conducted as described and TAM was subjected to baicalin treatment. Suppression of RelB expression significantly blocked change of CD86 and CD206 expression induced by baicalin. This was further evidenced by observation in cytokine expression profile (f) of baicalin-treated TAMs with or without RNA interference against RelB. (g) Expression of RelB and p52 in different phenotypes of macrophages. BMDMs were cultured and differentiated as described and protein was collected for analysis. It was observed that RelB and p52 were highly expressed in M1-like macrophage but not in either unpolarised or M2-like macrophage

Dicer cleaves double-stranded RNA (dsRNA) into small interfering RNA (siRNAs).27 Also known as silencing RNA, this can interfere with the expression of specific genes with complementary nucleotide sequences by degrading mRNA after transcription, preventing translation.28
In this case they used RNA interference to demonstrate that baicalin was inducing autophagy by silencing expression of IκB kinase α (IKKα), a critical negative regulator of apoptosis-associated specklike protein.29
(a) Induced expression of IKKα in baicalin-treated TAMs is associated with autophagy induction. RNA interference against Atg5 was conducted as described and TAM was treated with 20 μM baicalin for 48 h. Expression of IKKα was detected by immunoblotting. It was shown that baicalin treatment resulted in increase of IKKα expression, which could be blocked by inhibition of autophagy by RNA interference against Atg5. (b) Silencing of IKKα expression attenuated RelB overexpression in baicalin-treated TAM. RNA interference against IKKα was conducted as described and TAM was treated with 20 μM baicalin for 48 h. It was shown that increased expression of RelB by baicalin was attenuated upon IKKα suppression. (c) Autophagy induction is independent to IKKα overexpression. (d) Suppression of IKKα by RNA interference induced re-skewing of baicalin-treated TAM to M2 phenotype. RNA interference was conducted as described and TAM was subjected to baicalin treatment. Suppression of IKKα expression significantly blocked change of CD86 and CD206 expression induced by baicalin. This was further evidenced by observation in cytokine expression profile (e) of baicalin-treated TAMs with or without RNA interference against IKKα. (f) RelA pathway may not be activated upon baicalin treatment. TAM was treated with baicalin in the presence of soluble TNF-α. Expression of RelA and IκBα was determined. (g) Baicalin treatment induced reduction of TRAF2 and TRAF3 expression. (h) Baicalin-suppressed TRAF2 expression may be blocked when autophagy was blocked by either Bafilomycin A1 or RNA interference against Atg5. (i) Baicalin induced TRAF2 degradation in M2-like macrophage but not in M1 phenotype. (j) Degradation of TRAF2 induced by baicalin may be dependent on autophagy-associated lysosomal pathway. TAM treated with or without baicalin was fixed and stained with TRAF2 antibody. Lysosome was stained with Lysotracker Green for 30 min. It was observed that TRAF2 was translocated to lysosome after treatment of baicalin in TAM (magnification: x60). (k) p62 cargo protein may dominate selective autophagic degradation of TRAF2 in baicalin-treated TAM. TAM was treated with or without baicalin in the presence of lysosome inhibitor leupeptin. Co-immunoprecipitation assay was conducted with TRAF2 antibody and association of p62 with TRAF2 was detected by immunoblotting. Recruitment of p62 to TRAF2 upon baicalin treatment was observed.
In our study, baicalin-mediated downregulation of TRAF2 promotes sustained activation of IKKα and RelB. IKKα is a tumour promoter in the context of colorectal cancer that is responsible in blocking the recruitment of M1-like myeloid cells. IKKα and RelB are important regulators of non-canonical NF-кB signalling and p100 processing contributes to the activation of RelB/p52 complex, while TRAF2 negatively regulates the pathway. Inactive form of RelB is associated with IкBα and it mobilises to nucleus followed by IкBα degradation. RelB is associated with p52 to activate transcription of target genes. This non-canonical activation of NF-кB pathway has been reported to dominate the reprogramming of Th1 cells into Th2 phenotype in cells other than macrophage.
In conclusion, our data postulate that the tumour suppressive effect of baicalin was mediated by re-education of TAMs away from M2-like, towards tumour inhibiting M1-like phenotype. This effect was regulated by activation of RelB/p52 pathway via TRAF2 lysosomal degradation-dependent pathway. Baicalin-induced autophagy was responsible for lysosomal degradation of TRAF2 as well as TAM repolarisation. This study proposes baicalin as a potential immune therapeutic candidate for the treatment of hepatocellular carcinoma.
Notes
The authors declare no conflict of interest.
TNF receptor-associated factor 2 (TRAF2) is a protein that in humans is encoded by the TRAF2 gene and is required for TNF-alpha-mediated activation of MAPK8/JNK and NF-κB.30
Shen et al (2013) describe how TRAF2 is an NF-κB-activating oncogene in epithelial cancers, which is why induced degradation by baicalin can provide us with another anti-cancer signalling pathway.
This is in addition to the tumour associated repolarisation to M1 phenotypes as discussed earlier:31
Abstract
Aberrant nuclear factor (NF)-κB activation is frequently observed in human cancers. Genome characterization efforts have identified genetic alterations in multiple components of the NF-κB pathway, some of which have been shown to be essential for cancer initiation and tumor maintenance. Here, using patient tumors and cancer cell lines, we identify the NF-κB regulator, TRAF2 (tumor necrosis factor (TNF) receptor-associated factor 2), as an oncogene that is recurrently amplified and rearranged in 15% of human epithelial cancers. Suppression of TRAF2 in cancer cells harboring TRAF2 copy number gain inhibits proliferation, NF-κB activation, anchorage-independent growth and tumorigenesis. Cancer cells that are dependent on TRAF2 also require NF-κB for survival. The phosphorylation of TRAF2 at serine 11 is essential for the survival of cancer cells harboring TRAF2 amplification. Together, these observations identify TRAF2 as a frequently amplified oncogene.
Hua et al (2019) describe RelB/p52 associated autophagy in detail, with reference to cervical cancer:32
Discussion
In this study, the expression levels and binding affinities of NF-κΒ complex proteins were examined in cervical cancer tissues, and the results obtained are very interesting. NF-κΒ complex proteins, p52, RelB, p50, RelA and c-Rel were examined. In normal cells, NF-κΒ remains in a latent state and resides in the cytoplasm; however, upon activation, NF-κΒ undergoes degradation by means of an inhibitory protein, IkB, and the subunits are translocated to the nucleus, where they bind to specific DNA sequences and initiate transcription, followed by protein translation. However, if NF-κΒ activation is caused by chronic inflammation, the expression of NF-κΒ subunits is further enhanced, which results in uncontrolled protein translation, giving rise to chronic infection, especially in cancer.
A side effect of radiotherapy to treat cervical cancer may be…recurrence of cervical cancer:
Generally after cancer treatment, the rate of recurrence is high, and this is also true for cervical cancer. One cause of recurrence is radiation. High levels of radiation activate the alternative NF-κΒ pathway, in which phosphorylation and independent degradation of IκB occur, causing translocation of the p52 and RelB subunits of NF-κΒ to the nucleus where they are aberrantly expressed. Some inflammatory agents, such as cytokines, also induce abnormal expression of NF-κΒ in cervical cancer.
Our findings suggest that high nuclear expression of the NF-κΒ p52/RelB complex may contribute to malignancy.
Similar to the gene silencing by small interfering RNAs as discussed earlier, “microRNAs (miRNAs) are short (20-24 nt) non-coding RNAs that are involved in post-transcriptional regulation of gene expression in multicellular organisms by affecting both the stability and translation of mRNAs.
miRNAs are transcribed by RNA polymerase II as part of capped and polyadenylated primary transcripts (pri-miRNAs) that can be either protein-coding or non-coding.
The primary transcript is cleaved by the Drosha ribonuclease III enzyme to produce an approximately 70-nt stem-loop precursor miRNA (pre-miRNA), which is further cleaved by the cytoplasmic Dicer ribonuclease to generate the mature miRNA and antisense miRNA star (miRNA*) products.
The mature miRNA is incorporated into a RNA-induced silencing complex (RISC), which recognizes target mRNAs through imperfect base pairing with the miRNA and most commonly results in translational inhibition or destabilization of the target mRNA. The RefSeq represents the predicted microRNA stem-loop."33
Some miRNAs have oncogenic or tumor suppressive properties. In 2021 Ge et al performed in vitro based microarray analysis of the effects of baicalin on miRNAs involved with breast cancer, which is another of the signalling pathways that it uses to inhibit tumorigenesis.34
A brief role of the miRNAs showing significant upregulation in response to baicalin:
hsa-miR-15a is a tumor suppressor that regulates the stemness maintenance, epithelial–mesenchymal transition, and chemosensitivity of gastric cancer stem cells (GCSCs) through targeting ONECUT2. Also, hsa-miR-15a-5p inhibits G0 phase block of GCSCs by regulating ONECUT2/β-catenin signaling pathway.35
hsa-miR-100 can act as either a tumor suppressor gene or an oncogene, depending on the tumor type in different cancers. For example, hsa-miR-100-5p overexpression has been demonstrated in nasopharyngeal cancer, esophageal squamous cell carcinoma, colon cancer, and gastric cancer. In these tumors, this miRNA contributes to tumor progression. In contrast, hsa-miR-100-5p expression is suppressed in epithelial ovarian cancer, endometrial cancer, bladder carcinoma, renal cell carcinoma, prostate cancer, breast carcinoma, hepatocellular carcinoma, and non-small cell lung cancer. In these tumors, this miRNA behaves as a tumor suppressor.36
hsa-miR-16. Although first discovered in chronic lymphocytic leukaemia, miR-15a and 16 have been found to be tumor-suppressors and are downregulated in many types of cancer, including human brain glioma, colorectal cancer, bladder cancer, hepatoma, prostate cancer and different kinds of lymphoma or leukemia. Restoring their level helped to enhance apoptosis and retard the cell-cycle of the cancer cells. There have been researches aimed at enhancing chemosensitivity of tumor by upregulating miR-15a/16. These two miRNAs are also reported to regulate angiogenesis and immune response.37
hsa-let-7c is one member of the let-7 family; it maps to human chromosome 21q11-21 and is known as a putative tumor suppressor in several cancer cell lines such as prostate, lung, Burkitt lymphoma and primary pigmented nodular adrenocortical disease (PPNAD).38
Abstract
Objective
To explore the mechanism of baicalin intervention in breast cancer based on microRNA microarrays.
Methods
The inhibitory rate of baicalin intervention in MCF-7 breast cancer cells was determined by MTT. Then, the miRNA microarrays were used to validate the key microRNAs. After that, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to validate microRNA, hsa-miR-15a, hsa-miR-100, hsa-miR-16, and hsa-miR-7t. Finally, the potential targets of these key microRNAs are predicted by miRWalk, and DAVID was utilized for gene ontology (GO) enrichment analysis and pathway enrichment analysis.
Results
Baicalin may inhibit the proliferation of MCF-7 cells in a dose-dependent and time-dependent manner. The concentration of baicalin 150 μmol/L was determined for the subsequent miRNA chip research. A total of 92 upregulated microRNAs and 35 downregulated microRNAs were obtained. The upregulated miRNAs include hsa-miR-6799-5p, hsa-miR-6126, hsa-miR-4792, hsa-miR-6848-5p, hsa-miR-3197, hsa-miR-6779-5p, and hsa-miR -654-5p. The downregulated miRNAs include hsa-miR-3911, hsa-miR-504-5p, hsa-miR-30a-3p, hsa-miR-193b-3p, and hsa-miR-181b-5p. Then, differentially expressed miRNA was verified by qRT-PCR. The results showed that the expression of hsa-miR-15a, hsa-miR-100, hsa-miR-16, and hsa-let-7c was upregulated (P < 0.05), which was consistent with the results of the miRNA microarray. The enrichment analysis showed that baicalin might regulate the DNA-templated proliferation, DNA-templated transcription, p53 signaling pathway, etc., of MCF-7 breast cancer cells through miRNA.
Conclusion
Baicalin inhibits the proliferation of breast cancer cells. It may achieve antitumor effects through regulating microRNAs so as to affect the DNA replication (such as cellular response to DNA damage stimulus and DNA binding), RNA transcription (such as regulation of transcription, DNA-templated, transcription from RNA polymerase II promoter, and transcription factor binding), protein synthesis (such as mRNA binding, Golgi apparatus, and protein complex), endocytosis, pathways in cancer, p53 signaling pathway, and so on.
3. Results
3.1. Inhibition of Baicalin on the Proliferation of Breast Cancer Cell MCF-7
Baicalin at concentrations of 0, 50, 100, 150, and 200 μmol/L was added and interfered with breast cancer cell MCF-7 for 24, 48, and 72 hours. The growth of breast cancer cell MCF-7 was significantly inhibited and was time and concentration dependent within a certain time and concentration range. The differences between the baicalin groups and the negative control group were statistically significant (P < 0.05) (Figure 1).
Higher is better:

3.2. Differentially Expressed MicroRNA
The concentration of baicalin 150 μmol/L was determined for the subsequent miRNA chip research. Log2FC ≥ 1 or ≤−1 and P < 0.05 were used as the standard to screen for differentially expressed microRNA. Finally, a total of 92 upregulated microRNAs and 35 downregulated microRNAs were obtained (Figure 2). The most upregulated miRNAs include hsa-miR-6799-5p, hsa-miR-6126, hsa-miR-4792, hsa-miR-6848-5p, hsa-miR-3197, hsa-miR-6779-5p, and hsa-miR -654-5p. The most downregulated miRNAs include hsa-miR-3911, hsa-miR-504-5p, hsa-miR-30a-3p, hsa-miR-193b-3p, and hsa-miR-181b-5p.
Lightly edited to highlight the miRNAs associated with tumor suppressor roles:
The signaling pathways include pancreatic cancer, endocytosis, pathways in cancer, p53 signaling pathway, prostate cancer, glioma, colorectal cancer, Hippo signaling pathway, FoxO signaling pathway, chronic myeloid leukemia, and signaling pathways regulating pluripotency of stem cells (Figure 3(d)).
“Fold change is so called because it is common to describe an increase of multiple X as an "X-fold increase". As such, several dictionaries, including the Oxford English Dictionary and Merriam-Webster Dictionary, as well as Collins's Dictionary of Mathematics, define "-fold" to mean "times", as in "2-fold" = "2 times" = "double". Likely because of this definition, many scientists use not only "fold", but also "fold change" to be synonymous with "times", as in "3-fold larger" = "3 times larger".
Fold change is often used in analysis of gene expression data from microarray and RNA-Seq experiments for measuring change in the expression level of a gene. A disadvantage and serious risk of using fold change in this setting is that it is biased and may misclassify differentially expressed genes with large differences (B − A) but small ratios (B/A), leading to poor identification of changes at high expression levels. Furthermore, when the denominator is close to zero, the ratio is not stable, and the fold change value can be disproportionately affected by measurement noise."39
With that in mind, we can see a small number of baicalin upregulated miRNAs strongly correlating with pancreatic cancer suppression at one extreme, and a large number of miRNAs correlating less strongly with inhibition of signalling pathways in cancer at the other:


In breast cancer cells, miRNAs can bind to the mRNA 3′-UTR of a specific gene through complementary pairing, resulting in inhibition of translation of the target gene, thereby regulating gene expression and function. As an endogenous inhibitor of gene expression, miRNA forms a complex intracellular network with its upstream and downstream effector molecules, regulating the process of cell life activities, such as the occurrence and development of tumor cells.
Recent studies have shown that dysregulation of miRNAs not only affects cellular processes associated with carcinogenesis, tumor resistance, invasion, and metastasis but may also have a direct impact on the effectiveness of treatment. Among them, there are oncogenic miRNA and tumor suppressor miRNA, which act on tumor suppressor genes and proto-oncogenes, respectively.
miRNAs also play an important role in the regulation of breast cancer stem cells. Shimono et al. showed a decrease in the expression of the miR-200 family (miR-200a/-200b/-200c, miR-101, miR-182miR-183, and miR-96) in CD44+CD24-/low lineage-primary breast cancer stem cells. miR-200c also strongly inhibits the formation of mammary ducts and the ability of breast cancer stem cells to form tumors in normal breast stem cells. In the cancer cells of breast cancer with bone metastasis and lung metastasis, the expression of 8 miRNAs decreased, including miR-126, miR-206, and miR-335; these miRNAs also decreased in distant metastatic cancer cells in other sites.
Recent studies indicate that miRNAs are key regulators of these drug transporters, such as miRNAs targeting MDR1 including miR-451, miR-7, miR-345, and miR-326. Increased expression of miRNAs associated with response to radiation therapy results in increased resistance of the tumor to treatment. Many miRNAs that have been found to be involved in drug resistance of breast cancer include the miRNA-let-7 family, miR-451, miR-21, miR-101, and miR-221/222 [44, 45]. miRNAs associated with resistance to endocrine therapy include miR-221, -222, and -181b, miR-30c, and miR-301.
In summary, miRNA dysregulation in breast cancer, whether through polymorphisms in miRNA sequences, binding sites in target genes, or epigenetic mechanisms, has been shown to play a key role in the entire carcinogenesis process. Meanwhile, this study shows that baicalin can interfere with these miRNAs, suggesting that baicalin may exert antitumor effects on breast cancer at miRNA levels.
5. Conclusion
Baicalin inhibits the proliferation of breast cancer cells. It may achieve antitumor effects through regulating microRNAs so as to affect the DNA replication (such as cellular response to DNA damage stimulus and DNA binding), RNA transcription (such as regulation of transcription, DNA-templated, transcription from RNA polymerase II promoter, and transcription factor binding), protein synthesis (such as mRNA binding, Golgi apparatus, and protein complex), endocytosis, pathways in cancer, p53 signaling pathway, and so on.
2016, and Liu et al conducted a review of research into the effects of baicalein on different cancer types and the possible mechanisms involved. The abstract and diagram alone are extremely informative. It’s also worth bearing in mind that even though bioavailability was relatively low most of the animals in the study were administered baicalein or baicalin by feeding and not intravenously and yet still demonstrated varying degrees of tumor suppression.40
Abstract
Cancer is one of the leading causes of death worldwide and a major global health problem. In recent decades, the rates of both mortality and morbidity of cancer have rapidly increased for a variety of reasons. Despite treatment options, there are serious side effects associated with chemotherapy drugs and multiple forms of drug resistance that significantly reduce their effects. There is an accumulating amount of evidence on the pharmacological activities of baicalein (e.g., anti-inflammatory, antioxidant, antiviral, and antitumor effects). Furthermore, there has been great progress in elucidating the target mechanisms and signaling pathways of baicalein’s anti-cancer potential. The anti-tumor functions of baicalein are mainly due to its capacities to inhibit complexes of cyclins to regulate the cell cycle, to scavenge oxidative radicals, to attenuate mitogen activated protein kinase (MAPK), protein kinase B (Akt) or mammalian target of rapamycin (mTOR) activities, to induce apoptosis by activating caspase-9/-3 and to inhibit tumorinvasion and metastasis by reducing the expression of matrix metalloproteinase-2/-9 (MMP-2/-9). In this review, we focused on the relevant biological mechanisms of baicalein involved in inhibiting various cancers, such as bladder cancer, breast cancer, and ovarian cancer. Moreover, we also summarized the specific mechanisms by which baicalein inhibited the growth of various tumors in vivo. Taken together, baicalein may be developed as a potential, novel anticancer drug to treat tumors.
Keywords: baicalein, flavonoids, MAPK, Akt, reactive oxygen species (ROS), cancer, therapy
Emerging evidence has demonstrated that baicalein exerts multiple pharmacological effects including anti-inflammatory, antioxidant, and antiviral properties, as well as protection against cardiovascular illness. In the past decade, there has been great progress in exploring the target mechanisms and signaling pathways of baicalein’s anti-cancer potential. The main molecular mechanisms of the anti-tumor effects of baicalein include inhibiting several cyclins or cyclin-dependent kinases (CDKs) to regulate the cell cycle, scavenging oxidative radicals, attenuating MAPK, Akt or mTOR activities, inducing apoptosis through activating caspase-9/-3 and inhibiting tumor invasion and metastasis by reducing the expression of MMP-2/-9. As shown in Figure 2, we summarized the relevant biological mechanisms of baicalein involved in inhibiting various types of cancer (e.g., bladder cancer, breast cancer, cervical cancer, colorectal cancer, gastric cancer, hepatocellular carcinoma, osteosarcoma, multiple myeloma, melanoma/skin cancer, ovarian cancer, pancreatic cancer, prostate cancer, and lung cancer).

16. Potential Clinical Implication and Discussion
Multitudinous preclinical studies have proved that baicalein could be developed as a tremendous potential antitumor drug against cancer by targeting multiple molecular mechanisms and signaling pathways. In addition, accumulating evidence from animal studies demonstrates that baicalein significantly inhibited tumor volume and tumor weight, such as in hepatocellular cancer and lung cancer. Furthermore, it is worth noting that the development of appropriate delivery systems and chemical modification of baicalein can enhance its efficacy in preclinical studies. Unfortunately, very few clinical trials of baicalein were conducted to study its efficacy for the treatment of tumors in clinics. In order to promote the anti-tumor potential of baicalein to clinical use, in addition to some conventional animal experimental data, some issues, such as bioavailability and toxicological profiles of baicalein, have to be comprehensively investigated before clinical trials are initiated. In some tumors, such as breast cancer, colorectal cancer and hepatocellular cancer, animal experiments suggested that baicalein significantly decreased tumor weights and volumes without toxicity. Therefore, it is of great significance to select some tumors such as breast cancer or coloreactal cancer to initiate the phase I and II trials.
17. Conclusions and Future Perspectives
As is well known, cancer is a multifactorial disease. In recent decades, the mortality and morbidity rates of cancer have rapidly increased due to environmental pollution, various carcinogens, unhealthy lifestyles, high stress levels, and pressures of work. Chemotherapy plays a vital role in the treatment of cancer patients by killing tumor cells. However, chemotherapy agents can also cause toxicity to surrounding normal tissue cells, resulting in a variety of side effects. Moreover, the effectiveness of chemotherapy is seriously limited by the occurrence of resistance. Therefore, finding a novel drug with few side effects and high efficacy has become an urgent trend in cancer therapy. Baicalein has garnered increasing attention in recent years due to its strong antitumor activity and low toxicity. In this review, we introduced various molecular mechanisms and signaling pathways to elucidate its anticancer potential, including blockage of the cell cycle, inducing apoptosis, inhibiting tumor cell invasion and metastasis, potentiating the actions of chemotherapeutic agents, triggering autophagic cell death, and so on. Moreover, we also summarized specific mechanisms by which baicalein inhibited various tumor growths in vivo in Table 1. It is worth noting that the bioavailability of baicalein in vivo remains low. Despite the promising results of preclinical studies on compounds derived from baicalein, additional research is required to improve their biological effects on various cancers. Nevertheless, based on the great number of studies, we have reason to believe that baicalein may potentially be developed as a novel anticancer drug, to be administered alone or applied with current popular chemotherapeutic drugs, thus improving the future treatment of cancer.
Acknowledgments
This research was supported by grants from the National Nature Science Foundation of China (No. 81472082).
Conflicts of Interest
The authors declare no conflict of interest.
Prostate cancer is one of the most common types of cancer in men. Usually prostate cancer grows slowly and is initially confined to the prostate gland, where it may not cause serious harm. However, while some types of prostate cancer grow slowly and may need minimal or even no treatment, other types are aggressive and can spread quickly. Prostate cancer that's detected early — when it's still confined to the prostate gland — has the best chance for successful treatment.41
In 2000, Chan et al conducted an in vitro study into the effects of baicalin on several prostate cancer cell lines. They found that the responses to baicalin were different among different cell lines, and that 50% inhibition of DU145 cells (considered a standard prostate cancer cell line) occurred at concentrations of 150μmol or above. LNCaP cells were the most resistant (androgen-sensitive human prostate adenocarcinoma cells):42
Abstract
The flavonoid baicalin (baicalein 7-D-beta-glucuronate), isolated from the dried root of Scutellaria baicalensis Georgi (Huang Qin), is widely used in the traditional Chinese herbal medicine for its anti-inflammatory, anti-pyretic and anti-hypersensitivity effects. In the present study, we investigated the in vitro effects of baicalin on the growth, viability, and induction of apoptosis in several human prostate cancer cell lines, including DU145, PC-3, LNCaP and CA-HPV-10. The cell viability after treating with baicalin for 2-4 days was quantified by a colorimetric 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-s ulfophenyl)- 2H-tetrazolium (MTS) assay. The results showed that baicalin could inhibit the proliferation of prostate cancer cells. The responses to baicalin were different among different cell lines, with DU145 cells being the most sensitive and LNCaP cells the most resistant. Baicalin caused a 50% inhibition of DU145 cells at concentrations of 150 microM or above. The inhibition of proliferation of prostate cancer cells after a short period of exposure to baicalin was associated with induction by apoptosis, as evidenced by the typical nuclear fragmentation using Hoechst 33258 staining, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) labeling, DNA fragmentation, activation of caspase-3 and cleavage of poly-ADP-ribose polymerase (PARP). The results indicate that baicalin has direct anti-tumor effects on human prostate cancer cells.
The absorbance of the baicalin-treated cells was expressed as a percentage of the control untreated cells in order to show the extent of inhibition on cell growth (Fig. 2).

The cytotoxicity of baicalin on different cell lines was also assessed by fluorescent labeling of viable and dead cells with the calcein AM and EthD-1 (Fig. 3).

The characteristic fragmented condensed nuclei or apoptotic bodies were clearly shown in apoptotic cells after staining with Hoechst 33258 (Fig. 4):

Apoptotic DU145 and LNCaP cells were also demonstrated by fluorescent TUNEL labeling and the positive apoptotic cells clearly exhibited the fluorescent labeled fragmented nuclei (Fig. 5a,b):

There have been several in vitro studies showing that purified baicalin, or an extract from a Japanese medicinal herbal preparation (Sho-saiko-to for treating various chronic liver diseases) which is rich in both baicalin and baicalein, has demonstrated some anti-proliferative or anti-tumor effects on several human hepatoma cell lines, a pancreatic cancer cell line and a cholangiocarcinoma cancer line, rat glioma cells, a transgenic mouse model of melanoma and a derived melanoma cell line from this transgene. It has also been shown that baicalin is more potent than baicalein (which does not carry a glucuronate group) on the human hepatoma cells, and it takes 20 μg/ml of baicalin to inhibit growth of PLC/PRF/5 hepatoma cells by 50%. On the other hand, the herbal medicine, Sho-saiko-to, was shown to have no suppressive effects on the normal human peripheral blood lymphocytes and normal rat hepatocytes. It was also demonstrated that baicalin can prevent the formation of aberrant crypt foci induced by azoxymethane in the rat colon by inhibiting the cyclooxygenase activity, suggesting that this drug could be used as a chemopreventive agent for colon cancer. Recently, a herbal preparation, named PC-SPES, has been sold in the US as a dietary supplement for prostate cancer patients. This preparation, which contains extract from the S. baicalensis Georgi, has been claimed to be cytotoxic against PC-3 and LNCaP cells by the induction of apoptosis. In animal studies, this herbal preparation can also inhibit the growth and metastasis of a Dunning rat prostate cancer cell line, MAT-LyLu, grown in Copenhagen rats. In a recent clinical study, PC-SPES was shown to decrease the serum levels of testosterone and prostate-specific antigen in patients with hormone-sensitive prostate cancer.
In summary, our present study has shown that baicalin is cytotoxic for several human prostatic cancer cell lines, particularly the androgen-independent DU145 cells, and its inhibitory effect on these cells is mediated through the induction of apoptosis. Although its exact mechanisms on the induction of apoptosis on these cancer cells are not fully understood, baicalin could be promising as a chemopreventive agent or an adjuvant to the conventional therapeutic modalities for prostate cancers.
Acknowledgements
This study was supported partially by RGC Earmarked Research Grants, Hong Kong. The authors gratefully acknowledge Mrs HJ Chan-Hou for the confocal microscopy.
“Silibinin, also known as silybin (both from Silybum, the generic name of the plant from which it is extracted), is the major active constituent of silymarin, a standardized extract of the milk thistle seeds, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silychristin, silidianin, and others. Silibinin itself is a mixture of two diastereomers, silybin A and silybin B, in approximately equimolar ratio. The mixture exhibits a number of pharmacological effects, particularly in the fatty liver, non-alcoholic fatty liver, non-alcoholic steatohepatitis, and there is great clinical evidence for the use of silibinin as a supportive element in alcoholic and Child–Pugh grade 'A' liver cirrhosis.”43
“Silymarin has also been shown to sensitize tumors to chemotherapeutic agents through down-regulation of the MDR protein and other mechanisms. It binds to both estrogen and androgen receptors, and down-regulates PSA. In addition to its chemopreventive effects, silymarin exhibits antitumor activity against human tumors (e.g., prostate and ovary) in rodents. Various clinical trials have indicated that silymarin is bioavailable and pharmacologically safe. Studies are now in progress to demonstrate the clinical efficacy of silymarin against various cancers.”44
It also improves the bioavailability of other therapeutics including baicalein and berberine, which has similar broad-spectrum properties.
Xu et al (2018) conducted in vitro and in vivo investigations into the hepatoprotective effects of silybin when co-administered with baicalein, using rats as a model. They found that baicalein improved the oral bioavailability of silybin with enhanced liver protective effects, inhibition of breast cancer resistance protein (BCRP) and suppression of multidrug resistance protein 2 (MRP2):45
Abstract
Although hepatoprotective properties of silybin are well documented, the clinical therapeutic efficacy is limited by its low bioavailability due to absorption rates, extensive phase II metabolism, and biliary excretion. As our previous study indicated that metabolic enzymes may have limited effects on the pharmacokinetic (PK) behavior of silymarin, here, we intended to increase the oral bioavailability and bio-efficacy of silybin through the inhibition of active efflux. In Caco-2 and transfected MDCKII cell models, flavone baicalein significantly inhibited the efflux of silybin as a BCRP and MRP2 inhibitor. In addition, baicalein reduced the biliary excretion index (BEI) and biliary clearance of silybin conjugates in the sandwich-cultured rat hepatocyte (SCH) model, indicating the inhibition of baicalein in biliary excretion of conjugated silybin metabolites. PK study demonstrated that baicalein significantly increased the area under the curve (AUC) and Cmax of silybin and its conjugates, suggesting enhanced absorption in vivo. Moreover, coadministration of silybin with baicalein boosted the liver protective, antioxidant, and anti-inflammatory effects of silybin in the carbon tetrachloride (CCl4)-induced liver injury model in comparison with silybin given alone. In summary, efflux transporters play a critical role in the low bioavailability of silybin, while inhibition of breast cancer resistance protein (BCRP) and multi-drug resistance protein 2 (MRP2) by baicalein can significantly increase the absorption and bio-efficacy of silybin, which provides a new combination therapeutic approach for the treatment of chronic liver diseases.
Keywords: ABCC2 (MRP2); ABCG2 (BCRP); baicalein; liver injury; pharmacokinetics; silybin.
Baicalein (5,6,7-trihydroxyflavone), one of the primary active components of Scutellaria species, is a well-known compound that possesses multiple pharmacological functions such as anticancer, antioxidant, anti-inflammatory, cardio-protective, and neuro-protective properties (Woo et al., 2005; Liu et al., 2010; Bie et al., 2017). Baicalein is well absorbed by passive diffusion (40% absolute absorption) and extensively glucuronidated to baicalin and baicalein 6-O-glucuronide by UGT in either the small intestine or the liver in vivo (Lai et al., 2003). Notably, baicalein is a p-glycoprotein (P-gp) inhibitor (Kitagawa et al., 2005), increasing the area under the curve (AUC) of nimodipine (a P-gp substrate) in rats because of the inhibition of P-gp efflux and/or CYP3A4-mediated metabolism (Cho et al., 2011). Moreover, baicalin, the glucuronide of baicalein, has also been reported for its interaction with ABC transporters, including BCRP, MDR1, MRP2, MRP3, and MRP4 (Zhang et al., 2007; Kalapos-Kovacs et al., 2015). However, limited studies have been carried out exploring baicalein as an enhancer of bioavailability and therapeutic effects based on its action on efflux transporters.
In this study, we wanted to get an insight into the key role of efflux transporters in affecting the absorption of silybin based on in vitro cell model and PK and pharmacodynamic studies by coadministration with baicalein. This might be of particular interest in explaining the activation of baicalein as a potential efflux transporter inhibitor and in providing a therapeutic method for the clinical use of drugs with low-bioavailability.
The SD rats were randomly divided into three groups with six rats per group and were given oral administration as follows: (1) silybin at a dosage of 50 mg/kg bodyweight, (2) silybin and baicalein at a dosage of 50 mg/kg each, and (3) baicalein at a dosage of 50 mg/kg body weight. Before the experiment, rats were pre-fasted for 24 h with water ad libitum and were maintained at a temperature of 21°C with 70% relative humidity and a 12 h light/dark cycle. Blood samples were collected via the tail vein (about 0.1 ml) at time intervals of 0, 15, 30, 45, 60, 90, 120, 240, 360, 480, and 720 min after oral administration with a temporary cannula.
Baicalein Inhibited Efflux Transport of Silybin in Caco-2 Cells
Baicalein was selected based on primary screening experiments in Caco-2 cells. The mean efflux ratios of silybin A and B in Caco-2 model were 5.62 and 5.13, respectively, indicating active transport of efflux, which is consistent with the literature (Yuan et al., 2017). As shown in Figures 1A–C, baicalein could inhibit efflux transport of silybin (10 μM) in the Caco-2 cell monolayers with the lowest effective dosage at 0.5 μM. Co-treatment with baicalein (5 μM) could reduce the efflux ratios of silybin A and B to 2.38 ± 0.27 and 2.33 ± 0.09, respectively. We noted that the highest dosage of baicalein (15.8 μM) used in this study revealed a similar effect as that of the dosage at 5 μM, indicating that efflux inhibition of baicalein could be saturated.

Coadministration of Baicalein Enhances the Absorption of Silybin in Rats
Next, we wanted to investigate the effect of baicalein on the PKs of silybin in vivo.
In comparison with silybin given alone (50 mg/kg), coadministration with baicalein (50 mg/kg) effectively enhanced the plasma concentration of free silybin (Figure 5) and increased the maximum concentrations (Cmax) of silybin A and B increased from 508 ± 114 to 1526 ± 126 and 413 ± 69 ng/ml to 915 ± 150 ng/ml, respectively Homogeneously, AUC(0-t) of free silybin A and B were increased from 1292 ± 341 to 3534 ± 344 and 1195 ± 302 to 1808 ± 225 ng∗h/ml, respectively. No significant difference in Tmax was found among the groups studied.

A 2.4 to 2.8 fold increase in bioavailability and area under the curve (AUC) almost doubled:
Absorption of total silybin, including all glucuronide and sulfate conjugates and free silybin, were also evaluated at the same time. Oral coadministration of silybin with baicalein resulted in a significant increase in Cmax of total silybin A and B by 283% and 243%, respectively, while AUC(0-t) of total silybin A and B increased from 12450 ± 2553 to 25921 ± 2277 and 53184 ± 7340 to 97584 ± 23856 ng∗h/ml, respectively. Considering the wide therapeutic window of silybin (Fried et al., 2012), increased bioavailability of silybin might lead to enhanced therapeutic efficacy without side effects.
Discussion
Based on literature reports, silybin seems to be a promising drug for the treatment of chronic liver disease with clearly demonstrated anti-fibrotic, antioxidant, and metabolic effects in experimental studies. The good safety profile (doses <10 g/d have no significant side effects), standardization, and easy availability are added advantages to its promising therapeutic effects (Pradhan and Girish, 2006). However, previous human studies of silybin were insufficient to confirm the clinical efficacy in chronic liver disease because of no definitive results in terms of clinical efficacy, which might be caused by its poor absorption and low bioavailability (Loguercio and Festi, 2011).
The present PK study clearly demonstrates that the coadministration of baicalein markedly increased the absorption of silybin that was quantified with AUC and Cmax in rats. Moreover, the boosted bio-efficacy of silybin was observed by coadministration with baicalein in the CCl4-induced acute liver injury model with enhanced anti-inflammatory and antioxidant effects by comparing with silybin given alone. Baicalein itself failed to show significant benefits in the protection of live injury. These findings suggested that baicalein increased the bioavailability of silybin leading to enhanced hepatoprotective effects via the inhibition of the efflux transporters MRP2 and BCRP. However, synergistic effects observed in our study for silybin and baicalein could not only be related with the enhanced absorption but also caused by the direct pharmacological synergistic effects as both baicalein and silybin have anti-inflammatory and antioxidant activities.
In this study, our results demonstrated that baicalein inhibits the efflux of silybin and improves the intestinal absorption. Therefore, both free and conjugated silybin glucuronides increased in plasma, which is consistent with the observations from the in vitro experiment. But the enhanced absorption might also be contributed by the metabolism, although previous studies carried out with UGT1A1∗28 polymorphism with reduced UGT1A1 activity did not appear to affect silymarin exposures. Baicalein undergoes quick phase II metabolism, which is similar to that observed with silybin after absorption. After coadministration, baicalein might inhibit the conjugation of silybin by competing with UGT or/and sulfotranferase (SULT). We are currently working on this hypothesis. Moreover, baicalein dramatically decreased biliary clearance and BEI of silybin conjugates in primary liver cells. As glucuronide of silybin is deconjugated readily by rat liver β-glucuronidase, inhibition of biliary excretion of silybin conjugates could increase the concentration of conjugates as well as free silybin, leading to increased circulation concentration of silybin in addition to its therapeutic effects.
Both in vivo and in vitro studies demonstrated that baicalein improves the bioavailability and therapeutic effects of silybin via the inhibition of the efflux transporters, including MRP2 and BCRP. Combination therapy with silybin and baicalein may be a good choice for the clinical treatment of chronic liver diseases.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
In future clinical trials it would be interesting to combine baicalin with zinc, berberine and silybin as all four are complementary and likely work synergistically with each other.
Antiviral and neuroprotective properties
In 2000, Li et al performed an in vitro investigation into how baicalin flavonoids can inhibit HIV-1 infection at the level of viral entry.46
They used 96-well tissue culture plates in the presence or absence of BA (Baicalin, 7-glucuronic acid,5,6-dihydroxyflavone) at designated concentrations, and repeated the experiments at least 3 times before analysing the group data using a Student’s t test, and found that baicalin can inhibit viral entry and replication, but not block binding to CD4 T-cells via glycoprotein 120 (gp120).
What is the function of CCR5 in HIV? CCR5 is the primary co-receptor used by gp120 sequentially with CD4. This bind results in gp41, the other protein product of gp160, to be released from its metastable conformation and insert itself into the membrane of the host cell. CCR5 is essential for the spread of the R5-strain of the HIV-1 virus.47
C-X-C chemokine receptor type 4 (CXCR-4) also known as fusin or CD184 (cluster of differentiation 184) is a protein that in humans is encoded by the CXCR4 gene. The protein is a CXC chemokine receptor. CXCR-4 is an alpha-chemokine receptor specific for stromal-derived-factor-1 (SDF-1 also called CXCL12), a molecule endowed with potent chemotactic activity for lymphocytes. CXCR4 is one of several chemokine co-receptors that HIV can use to infect CD4+ T cells. HIV isolates that use CXCR4 are traditionally known as T-cell tropic isolates. Typically, these viruses are found late in infection. It is unclear as to whether the emergence of CXCR4-using HIV is a consequence or a cause of immunodeficiency.48
Abstract
Baicalin (BA) is a flavonoid compound purified from medicinal plant Scutellaria baicalensis Georgi and has been shown to possess anti-inflammatory and anti-HIV-1 activities. In an effort to elucidate the mechanism of the anti-inflammatory effect of BA, we recently found that this flavonoid compound was able to form complexes with selected chemokines and attenuated their capacity to bind and activate receptors on the cell surface. These observations prompted us to investigate whether BA could inhibit HIV-1 infection by interfering with viral entry, a process known to involve interaction between HIV-1 envelope proteins and the cellular CD4 and chemokine receptors. We found that BA at the noncytotoxic concentrations, inhibited both T cell tropic (X4) and monocyte tropic (R5) HIV-1 Env protein mediated fusion with cells expressing CD4/CXCR4 or CD4/CCR5. Furthermore, presence of BA at the initial stage of HIV-1 viral adsorption blocked the replication of HIV-1 early strong stop DNA in cells. Since BA did not inhibit binding of HIV-1 gp120 to CD4, we propose that BA may interfere with the interaction of HIV-1 Env with chemokine coreceptors and block HIV-1 entry of target cells. Therefore, BA can be used as a basis for developing novel anti-HIV-1 agents.
Dose dependant inhibition of HIV-1 envelope proteins with T cells:
The same assessment, but this time by assaying the CAT reporter gene:
HIV-1 envelope mediated cell fusion is marked by the induction of syncytia, D demonstrates the degree of inhibition:
If cellular fusion has been blocked then synthesis of HIV-1 strong stop DNA should also be inhibited, a useful marker from early stage infections:
We have shown that BA, a flavonoid compound purified from Scutellaria baicalensis Georgi was able to inhibit HIV-1 Env-mediated fusion thereby block viral entry of cells expressing CD4 and chemokine coreceptors. Previously, we and other investigators have shown that BA inhibited the infection of human PBMC by HIV-1. Although it was suggested that most flavonoid compounds including BA might inhibit the HIV-1 reverse transcriptase, the mechanisms of its anti-HIV-1 activity remain to be determined. Our recent study demonstrated that BA was able to conjugate with selected chemokines and interfere with their capacity to activate cellular receptors. Among the chemokines affected by BA are MIP-1b and SDF-1a that use CCR5 or CXCR4 as the functional receptors. CCR5 and CXCR4 are two essential coreceptors used by HIV-1 for fusion and infection of host cells. HIV-1 Env has been suggested to contain domains that interact with CCR5 or CXCR4 after binding to CD4. Since BA did not inhibit the binding of HIV-1 Env gp120 to CD4 (data not shown), it may interact with HIV-1 Env domains and interfere with their interaction with chemokine co-receptors and block HIV-1 at the early stage of infection.
Flavonoids are major components contained in fruits, nuts and certain vegetables. In addition to being one of the essential nutrients, flavonoids have been shown to display a remarkable array of biochemical and pharmacological actions, some of which significantly affect the function of immune system. BA is a major active component of the medicinal plant Scullateria baicallensis Georgi, which has been widely used in oriental medicine and shown to alleviate the progress of a number of inflammatory and infectious diseases. The antibacterial and antifungal activity of BA and its derivative have been confirmed by in vitro experiments. BA has also been observed to inhibit tumor growth and metastasis in vivo and in vitro, suggesting that BA has multiple beneficial effects on host defense. However, the mechanism(s) of action of BA may involve diverse biological and biochemical process including upregulation of Fas ligand mediated tumor cells apoptosis and downregulation of cyclin-dependent-kinase-mediated cell cycle, inhibition of cellular matrix metalloproteinase activity and DNA synthesis in cancer cells. The anti-inflammatory activity of BA has been attributed to its antioxidant ability based on its inhibition of reactive oxygen intermediates through an enhanced auto-oxidation of Fe2+ chelation with iron ion. BA has also been shown to inhibit the activity of several metabolic enzymes such as xanthine oxidase, and sialidase. These observations suggest that by interacting with diverse biological molecules, BA and its analogues are potentially very useful anti-inflammatory and anti-HIV-1 agents.
No conflict of interest statement was provided.
It has been confirmed by replicable BLAST analysis49 that the spike protein trimer includes HIV inserts homologous with gp4150 and the cytotoxic & neurotoxic glycoprotein 120 (gp120)51.
It is also known that spike S1 has binding affinity for neuropilin 1 (Nrp1) receptors52 and nicotinic acetylcholine receptors53, thus providing mechanisms to facilitate endocytosis into several cell types including neurons54, much as the HIV virus does.
This may contribute to the symptomology of several long covid/vaccine sequalae cases as discussed in depth here:
A paper by Lee et al also makes the connection between gp120 and amyloidosis via signalling pathways including iNOS and COX-2.55 All this is of potential relevance to the diagnosis and treatment of the condition and as COX-2 is linked to tumorigenesis and amyloidosis is linked to Alzheimer's disease, although cause and effect is controversial.
iNOS: “Inducible nitric oxide synthase (iNOS, also named NOSII or NOS2) is a high-output Ca++-independent NOS whose expression can be induced in a wide range of cells and tissues by cytokines and other agents (for a review see Kleinert et al (2000)). After expressional induction, iNOS continuously produces NO until the enzyme is degraded MacMicking et al (1997). The high amounts of NO produced by iNOS can have beneficial microbicidal, antiviral, antiparasital, and antitumoral actions. On the other hand, aberrant iNOS induction may have detrimental consequences and seems to be involved in the pathophysiology of human diseases such as asthma, arthritis, multiple sclerosis, colitis, psoriasis, neurodegenerative diseases, tumor development, transplant rejection, or septic shock”.56
COX-2: “Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) (The HUGO official symbol is PTGS2; HGNC ID, HGNC:9605), also known as cyclooxygenase-2 or COX-2, is an enzyme that in humans is encoded by the PTGS2 gene. In humans it is one of two cyclooxygenases. It is involved in the conversion of arachidonic acid to prostaglandin H2, an important precursor of prostacyclin, which is expressed in inflammation…The expression of PTGS2 (COX-2) is upregulated in many cancers.”57
Arachidonic acid: (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter.
Abstract
Human immunodeficiency virus 1 (HIV-1) infection can cause several HIV-associated neurocognitive disorders a variety of neurological impairments characterized by the loss of cortical and subcortical neurons and decreased cognitive and motor function. HIV-1 gp120, the major envelope glycoprotein on viral particles, acts as a binding protein for viral entry and is known to be an agent of neuronal cell death. To determine the mechanism of HIV-1 gp120-induced memory dysfunction, we performed mouse intracerebroventricular (i.c.v.) infusion with HIV-1 gp120 protein (300 ng per mouse) and investigated memory impairment and amyloidogenesis. Infusion of the HIV-1 gp120 protein induced memory dysfunction, which was evaluated using passive avoidance and water maze tests. Infusion of HIV-1 gp120 induced neuroinflammation, such as the release of iNOS and COX-2 and the activation of astrocytes and microglia and increased the mRNA and protein levels of IL-6, ICAM-1, M-CSF, TIM, and IL-2. In particular, we found that the infusion of HIV-1 gp120 induced the accumulation of amyloid plaques and signs of elevated amyloidogenesis, such as increased expression of amyloid precursor protein and BACE1 and increased β-secretase activity. Therefore, these studies suggest that HIV-1 gp120 may induce memory impairment through Aβ accumulation and neuroinflammation.
Keywords: Amyloid beta; HIV-1; Neuroinflammation; gp120.
…the envelope protein gp120 has been demonstrated to trigger the release of TNF-α and IL-1β which elicit neuronal apoptosis in numerous ex vivo and in vivo studies (Garden et al. 2002; Shah et al. 2011). Gp120-injected primary hippocampal cells have demonstrated the promotion of Aβ1–42 secretion (Aksenov et al. 2010). The brain of HIV+ patients showed increased expression of BACE1 (commonly observed with AD) (Stern et al. 2018). However, there are no definite data demonstrating a causal relationship between HIV-1 infection and neuronal damage or the onset of AD. Therefore, in the present study, we examined whether injection of the HIV-1 gp120 protein affects the development of AD and whether this effect is related to neuroinflammation-mediated Aβ-accumulation in the brain.
HIV-1 gp120 in mice brains significantly impaired the memory ability of mice. Statistical analysis of data from days three to five showed the significance of the memory impairment effect of HIV-1 gp120 injection. The escape latency among the HIV-1 gp120-injected group (35.4 ± 5.6 s) on day five was greater than that of the saline-injected group (18.2 ± 3.7 s, p < 0.001, Fig. 2A). The swimming distance in the HIV-1 gp120-injected group (979.4 ± 167.9 cm) on day five was also longer than that of the saline-injected group (298.7 ± 67.5 cm, p < 0.001) (Fig. 2B).
Immunohistochemical analysis using an Aβ1–42 specific antibody showed Aβ deposition in the cortex and hippocampus regions of HIV-1 gp120-injected mouse brains but not in saline-injected mouse brains (Fig. 3A).





Discussion
The most important finding in this study was that administration of the HIV-1 gp120 protein induced memory impairment as well as amyloidogenesis and neuroinflammation in the mouse brain. It has been reported that HIV-1 proteins, including env, can directly influence the CNS and activate neuroinflammatory pathways, leading to neuronal injury and dysfunction. In addition, abnormal Aβ, a pathological hallmark of AD, was found to be deposited in individuals suffering from HIV-1 infection (Anderson et al. 2002; Zhang et al. 2015).
HIV-associated neurocognitive disorder (HAND) is a common primary neurological disorder associated with HIV infection. It is known that HAND patients often develop cognitive impairment, motor dysfunction and speech problems. Clinical severity of HAND ranges from asymptomatic neurocognitive impairment and mild neurocognitive disorder to HIV-associated dementia (HAD) (Ru and Tang 2017). Our study showed that injection of HIV-1 gp120 protein in mouse brain induced learning and memory impairment through water maze, probe and step-through tests. The impairment on the learning and memory capability is suggested to be caused by neuropathological change in HIV-1 gp120 injection.
Several viruses, such as cytomegalovirus, herpes simplex virus, HIV-1, severe acute respiratory syndrome (SARS) coronavirus, and SARS-CoV-2, have been found to be related to the cause of neurodegenerative diseases, such as AD (Lurain et al. 2013; Mawanda and Wallace 2013; Barnes et al. 2015; Ardura-Fabregat et al. 2017; Harris and Harris 2018; Lewczuk et al. 2018; Verkhratsky et al. 2019; Devanand et al. 2020; Hampel et al. 2020; Uddin et al. 2020), and clinical associations among viral infections and β-amyloid accumulation and neuroinflammation have also been observed. Therefore, the repurposing of anti-viral drugs as AD-treatment drugs could be possible. This present study demonstrated that viral infection could affect AD development and there may be an association between the amyloidogenic and neuroinflammatory properties of viruses.
Declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
The relevance of this study to a review of the therapeutic properties of baicalein for treating long covid/vaccine sequalae is that baicalin demonstrates neuroprotective properties, including by targeting the above iNOS and COX-2 inflammatory signalling pathways. Woo et al conducted an in vitro investigation (2006)58 and found that it mediates this by inhibiting COX-2 gene expression.
Alvarez et al (2007) also conducted an in vitro study of gp120 and found that it induces COX-2 promoter transcription through NF-kappaB activation in astrocytoma cells.59
Abstract
To evaluate the possible mechanisms responsible for the anti-inflammatory effects of baicalein or baicalin, lipopolysaccharide (LPS)-induced inflammatory responses in cultured Raw 264.7 cells were studied. In the present study, baicalein and baicalin, a flavonoid present in the root of Scutellaria baicalensis Georgi, were examined for their effects on LPS-induced cyclooxygenase-2 (COX-2) gene expression in Raw 264.7 macrophages. Baicalein, but not baicalin, inhibited COX-2 gene expression in LPS-induced Raw 264.7 cells. However, both polyphenolic compounds inhibited LPS-induced inducible nitric oxide synthase (iNOS) protein expression, iNOS mRNA expression, and NO production in a dose-dependent manner. To investigate the mechanism by which baicalein inhibits COX-2 gene expression, we examined activation of mitogen-activated protein kinases (MAPKs) in Raw 264.7 cells. We did not observe any significant change in the phosphorylation of MAPKs between baicalein- and baicalin-treated cells. Baicalein and baicalin had no effect on LPS-induced nuclear factor-kappaB (NF-kappaB) and cAMP response element binding protein (CREB) DNA binding activity. Baicalein, but not baicalin, significantly inhibited the DNA binding activity of CCAAT/enhancer binding protein beta (C/EBPbeta) These results indicated that differential effects of baicalein and baicalin on COX-2 gene expression in LPS-induced Raw 264.7 cells were mediated through inhibition of C/EBPbeta DNA binding activity. Taken together, these results suggest that baicalein acts to inhibit inflammation through inhibition of COX-2 gene expression through blockade of C/EBPbeta DNA binding activity.
COX-2 is thought to be the predominant isoform involved in the inflammatory responses (Smith et al., 1996; Herschman, 1996; Griswold and Adams, 1996). Multiple lines of evidence suggest that COX-2 has a significant role in carcinogenesis. COX-2 is over-expressed in transformed cells as well as in various forms of cancer (Chan et al., 1999; Kutchera et al., 1996; Subbaramaiah and Dannenberg, 2003). Consequently, the targeted inhibition of COX-2 is a promising approach to inhibiting inflammation and carcinogenesis as well as to preventing cancer.


One caution is that they used a comparatively high concentration of baicalein flavonoids in comparison to earlier studies:
Chen et al. (2001) reported that baicalein and baicalin inhibit LPS-induced nitric oxide production and nitric oxide synthase gene expression, but not COX-2 gene expression. One of the possible reasons for this discrepancy is that our flavonoids have been used a high concentrations (50–100 μM) compared with Chen et al. (40 μM maximum). The treatment of cells with 100 μM baicalein neither induced morphological changes nor cytotoxicity (data not shown). Here, we demonstrate that baicalein inhibits COX-2 gene expression in LPS-stimulated cultured macrophages, and we propose that the actions of baicalein are mediated by inhibition of C/EBPβ binding activity. It is possible that baicalein derivatives are lead compounds for novel anti-inflammatory drugs having inhibitory activity on the production of various inflammatory mediators such as PGE2.
Next I did a search to get some more guidance on measured, in vivo serum baicalein concentrations and interactions. Bear in mind this first study was on rats not humans and bioavailability can vary due to many factors including genetic makeup, gut biome, diet and other therapeutics being administered.
In 2015, Noh et al were studying the effects of baicalin on oral pharmacokinetics of caffeine in rats, specifically for interactions with CYP3A4 activity as marked by ethoxyresorufin O-deethylase (EROD), methoxyresorufin O-demethylase (MROD), benzyloxyresorufin O-debenzylase (BROD) and p-nitrophenol hydroxylase and erythromycin N-demethylase.
Baicalin was suspended in corn oil and fed to the rats with oral baicalin reaching its maximum concentration in plasma 8 hr after administration. Plasma concentration was then determined and found to be well below the concentrations used in the previous study:60
RESULTS
Inhibitory effects of baicalin and baicalein on monooxygenase activities
The half maximal inhibitory concentration (IC50) of baicalin and baicalein on CYP enzymes were shown in Table 1. Baicalin inhibited EROD, MROD and BROD activities in rat liver microsomes with the IC50 values of 24.2, 9.3 and 22.9 μM, respectively. Baicalein also showed inhibitory effects on EROD, MROD and BROD activities. Especially, the inhibition was much more potent on EROD and MROD, when compared to the inhibition by baicalin. There were no inhibitory effects on PNPH and ERDM activities by neither baicalin nor baicalein
Plasma concentrations were in fact very low because it is converted quickly to other metabolites, meaning that certain CYP drug interactions would not be contraindicative:
Pharmacokinetics of baicalin
Because baicalin at 200 mg/kg did not show any drug interaction with caffeine, the concentration of plasma baicalin was determined to explain the reason for inconsistency. Based on the dosing schedule for drug interaction study in Fig. 2, time-concentration profile of baicalin from 8 hr after oral administration of rats with 200 mg/kg baicalin were obtained, as depicted in Fig. 3. The plasma concentrations of baicalin were highly variable. The mean maximum concentration of baicalin was calculated to be 3.7 μM, which was below IC50 values for EROD and MROD for baicalin that showed inhibition. The results clearly explained the reason why baicalin might not interact with caffeine in rats, and indicated that baicalin at the dose tested in the present study would be safe in terms of the possible drug interaction with certain drugs that are CYP1A2 and 2E1 substrates.

Orally administered baicalin was reportedly to be metabolized to baicalein by intestinal microbiota for absorption (Kim et al., 2008). In addition, baicalein more strongly inhibited CYP1A activities than baicalin (Table 1). Nevertheless, baicalin was selected to evaluate the drug interaction with caffeine in the present study, because it was predominantly present in the blood when even baicalein was administered (Lai et al., 2003; Kim et al., 2007), and because baicalein was hardly detected in the blood due to the biotransformation (Kang et al., 2014). In fact, most baicalein absorbed could be metabolized to baicalin, a glucuronate on 7-hydroxy position, and to baicalin-6-glucuronide in our recent report (Kang et al., 2014). In a previous report on oral pharmacokinetics, baicalin showed not only a double-peak phenomenon but also the second peak as high as the first peak at 8 hr after drug administration in rats (Lu et al., 2007). Based on this time-concentration profile in rat plasma, baicalin was orally administered 8 hr prior to caffeine administration in the present study. As results, although the activity of CYP1A2, responsible for caffeine metabolism, was inhibited by baicalin in vitro, there were no significant differences in the pharmacokinetic parameters of caffeine and its three metabolites, regardless of pretreatment of rats with baicalin (Table 2). It suggested that the systemic exposure to baicalin following a single oral administration was not enough to affect caffeine pharmacokinetics. In fact, the observed plasma concentration of baicalin determined in the present study was below the IC50 value for MROD (9.3 μM). The extensive metabolism of baicalin to other metabolite might also contribute to this result (Wang et al., 2012). Taken together, it could be concluded that the drug interaction of baicalin with caffeine would be clinically marginal. The dosage of baicalin in this study was to be reasonable in clinical situation, based on the human dosage of 1.5 g/day in the previous report (Fan et al., 2009), because the recalculated dose of baicalin equivalent to animal was approximately to be 155 mg/kg in rats according to the FDA guideline (FDA, 2005).
In conclusion, we evaluated the effects of baicalin and baicalein on CYP enzymes in rat liver microsomes, and found that CYP1A and 2B activities were significantly inhibited by these compounds. However, baicalin had no effects on the pharmacokinetics of caffeine and its metabolites, paraxanthine, theobromine and theophylline, following a single oral administration with baicalin in rats, possibly because the plasma concentration of baicalin was not higher enough to inhibit CYP enzymes at the dose tested.
So does this low plasma concentration mean that baicalin fails as a therapeutic for the treatment of amyloidosis related pathologies in conditions such as Alzheimer’s disease in vivo vs in vitro?
A study by Yu et al in 2022 found quite the opposite using a mouse model as it attenuates amyloid β oligomers induced memory deficits and mitochondria fragmentation through regulation of PDE-PKA-Drp1 signalling.
They injected amyloid β oligomers (AβOs) into mice and 3 days later baicalin (30 and 60 mg/kg) was administered intragastrically once daily for 14 days. The doses of baicalin were chosen based on previous studies.61
“What is mitochondrial fragmentation? Mitochondrial fragmentation is the result of decreased fusion and increased fission in mitochondria. It is characterized by a large number of smaller mitochondria—as opposed to a network of highly interconnected and elongated mitochondria, which is the product of increased fusion.
Mitochondrial fragmentation is necessary for mitophagy, since smaller mitochondria are more easily engulfed by autophagosomes than larger ones and require less energy to be autophagocytosed. The fragmented state predominates during periods of high stress as well as before and after the release of apoptogenic factors, which signal for cell death.”62
“Mitochondria are symbiotic intracellular organelles with a variety of functions that are vital for neurons. They “fuel” neuronal metabolism by generating ATP, ensure neuroprotection under traumatic conditions by taking up excessive calcium, and ultimately can trigger apoptosis by opening their “megapore” and releasing the stored calcium and cytochrome c.
Mitochondrial function is tightly linked to their morphology: healthy, charged and neuroprotective mitochondria are fused together and appear as long tubular network; in contrast, damaged, calcium-overloaded, and “neurotoxic” mitochodria are fragmented and have an appearance of dissociated vesicular structures.”63
“Phosphodiesterase-4 inhibitor, commonly referred to as a PDE4 inhibitor, is a drug used to block the degradative action of phosphodiesterase 4 (PDE4) on cyclic adenosine monophosphate (cAMP). It is a member of the larger family of PDE inhibitors. The PDE4 family of enzymes are the most prevalent PDE in immune cells. They are predominantly responsible for hydrolyzing cAMP within both immune cells and cells in the central nervous system.”
“…PDE4 inhibitors are known to possess procognitive (including long term memory-improving), wakefulness-promoting, neuroprotective, and anti-inflammatory effects. Consequently, PDE4 inhibitors have been investigated as treatments for a diverse group of different diseases, including central nervous system disorders such as major depressive disorder (clinical depression), anxiety disorders, schizophrenia, Parkinson's disease, Alzheimer's disease, multiple sclerosis, attention deficit-hyperactivity disorder, Huntington's disease, stroke, autism and inflammatory conditions such as chronic obstructive pulmonary disease (COPD), asthma and rheumatoid arthritis.”64
“In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory (see Hebbian theory).”65
“Postsynaptic density protein-95 (PSD-95) is a major regulator of synaptic maturation by interacting, stabilizing and trafficking N-methyl-d-aspartic acid receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isox-azoleproprionic acid receptors (AMPARs) to the postsynaptic membrane. Recently, there has been overwhelming evidence that associates PSD-95 disruption with cognitive and learning deficits observed in SCZ [ed: schizophrenia] and autism.”66
Abstract
Rationale: Mitochondrial fragmentation contributes to the initiation of Alzheimer's disease (AD) pathology. Baicalin plays a significant role in rescuing mitochondrial dysfunction. However, the effect of baicalin treatment on the modulation of mitochondrial fragmentation has not yet been assessed.
Objectives: The present study was designed to evaluate the effect of baicalin on memory and understand its mechanism of action.
Results: Baicalin treatment significantly reversed the altered learning and memory behaviours in AD mouse model. We found that baicalin treatment significantly improved the levels of microtubule association protein-2 and enhanced the expression of synaptophysin and postsynaptic density protein 95 (PSD95). Moreover, treatment with baicalin reversed amyloid-β oligomer (AβO)-induced abnormalities in the succinate dehydrogenase complex iron sulphur subunit B (SDHB) and cytochrome c oxidase components I (COXI) and mitochondrial fragmentation in the hippocampus. Further, we found that baicalin decreased the PDE4 levels and upregulated the levels of phosphorylated Ser157 site of vasodilator-stimulated phosphoprotein (pVASPs157) and phosphorylated Ser637 site of mitochondrial dynamin-related protein 1 (pDrp1S637). Moreover, in AβO-treated HT-22 cells, H89 inhibited the effect of baicalin on PSD95, mitochondrial fragmentation, SDHB and COXI, PDE4, pVASPs157, and pDrp1S637.
Conclusion: The effect of baicalin on memory improvement may be due to improved synaptic plasticity, mitochondrial fragmentation, and rescue of dysfunction via the inhibition of PDE4, which leads to activation of pDrp1S637 in the AβO-induced model.
Keywords: Alzheimer’s disease; Baicalin; Mitochondria fragmentation; PDE; PKA-pDrp1S637.
To evaluate the neuroprotective effects of baicalin on AβO-induced memory deficits, behavioural changes were examined 1 h after the final treatment.
As shown in Fig. 1B, AβO model mice had significantly reduced number of spontaneous alternations in comparison with the control mice in the Y-maze test (P < 0.01), and this was reversed by treatment with baicalin at 30 and 60 mg/kg (P < 0.01). Similarly, treatment with baicalin at 30 and 60 mg/kg reversed the AβO-induced decrease in discrimination index in the NOR test (P < 0.01, Fig. 1C), as shown in Fig. 1D, and baicalin at 30 and 60 mg/kg do not affected shuttle times. These results demonstrate the ability of baicalin to improve memory deficits in AβO-induced mouse models.

Neuronal synaptic plasticity plays an important role in learning and memory. To determine whether baicalin improves hippocampal synaptic plasticity, we measured the MAP2 and synaptic protein levels. We found that, AβOs decreased the mean density of MAP2 (P < 0.01, Fig. 2A) and levels of postsynaptic density protein 95 (PSD95) and synaptophysin synaptic proteins in the hippocampus (P < 0.01; Fig. 2B, C). These effects were reversed by the administration of baicalin at 30 and 60 mg/kg (P < 0.01, Fig. 2), demonstrating that baicalin treatment improves neuronal synaptic plasticity in the hippocampus.

AβOs cause rapid and severe impairment of mitochondrial function. Thus, we determined whether baicalin-mediated improvement in synaptic plasticity is associated with improved mitochondrial function in the hippocampus. We measured the levels of mitochondrial functional proteins, succinate dehydrogenase complex iron sulphur subunit B (SDHB), and cytochrome c oxidase components I (COXI), in the hippocampus. As shown in Fig. 3, AβOs decreased COXI and SDHB expression in the hippocampus (P < 0.01), which was reversed by baicalin treatment at 30 and 60 mg/kg (P < 0.05, P < 0.01).

To determine whether baicalin improves mitochondrial dysfunction, we examined the mitochondrial morphology using electron microscopy (EM). We noted changes in the distribution of the mitochondria in the neuronal processes in AβO-induced mice, such that mitochondrial density was significantly reduced in the neurons of AβO-induced mice (P < 0.01, Fig. 4A), and this was reversed by baicalin treatment at 30 and 60 mg/kg (P < 0.05, P < 0.01, Fig. 4A). Furthermore, long and thin mitochondria were abundant in both the soma and the neuronal processes of control mice, while fewer mitochondria that were usually shorter and rounder were found in the cytoplasm of neurons in AβO-induced mice (P < 0.01, Fig. 4B). This was also reversed by the administration of 30 and 60 mg/kg of baicalin (P < 0.001, Fig. 4B).

To determine whether baicalin improves the mitochondrial fragmentation associated with mitochondrial protein signalling activation, we assessed the phosphorylation of VASP at Ser157 and Drp1 at Ser637 using western blotting. We found that, AβOs altered the expression of pVASPs157 and pDrp1s637 (P < 0.01, Fig. 5A, B), and these effects were reversed by baicalin treatment at 30 and 60 mg/kg (P < 0.05, and P < 0.01, respectively, Fig. 5A, B). These results show that baicalin improves mitochondrial fragmentation and is associated with abnormal mitochondrial dynamic protein signalling.

HT22 is an immortalized mouse hippocampal cell line.
H-89 is a protein kinase inhibitor that competitively inhibits the ATP site on the protein kinase A (PKA) catalytic subunit, and is used in studying mechanisms of cell signalling.67
To confirm the effect of baicalin on synaptic plasticity, we measured the mechanism of action of baicalin on AβO-induced HT-22 cells in the presence or absence of H89. As shown in Fig. 8, AβOs decreased the expression of PSD95 (P < 0.01; P < 0.01). This effect was reversed by the administration of baicalin 0.5 and 5 μM (P < 0.01), and the effects of baicalin were blocked by H89 (P < 0.01). Furthermore, none of these effects were observed with administration of H89 alone.
Note that even at a very low molar concentration baicalin increased the expression of PSD95, more than cancelling out the effects of the amyloid β oligomer injections (AβOs).
Beta-actin is a highly conserved protein (ie doesn’t change much through evolution) with several roles in cellular biology, including building the cytoskeletal components of nerve cells. Beta-actin is found to be primarily localized in dendritic spines, structures which are involved in plasticity in mature cells.68

To confirm the mechanism of action of baicalin on synaptic plasticity and its relationship with mitochondrial function, we next measured the mitochondrial proteins following baicalin treatment on AβO-induced HT-22 cells in the presence or absence of H89. We found that AβOs decreased the expression of COXI and SDHB (P < 0.01 and P < 0.01, respectively, Fig. 9A, B). This effect was reversed by the administration of 0.5 and 5 μM of baicalin (P < 0.01), and the effects of baicalin were blocked by H89 (P < 0.01). Furthermore, none of these effects were observed with administration of H89 alone.
To further confirm the mechanism of action of baicalin on mitochondrial fragmentation, we examined the mitochondrial morphology upon baicalin administration on AβO-induced HT-22 cells in the presence or absence of H89. While control HT-22 cells had abundant long and thin mitochondria, AβO-induced HT-22 cells had shorter and rounder mitochondria (P < 0.01, Fig. 10). However, this was reversed by the administration of 0.5 and 5 μM of baicalin (P < 0.01). Moreover, these effects of baicalin were also blocked by H89 (P < 0.01). None of these effects were observed with administration of H89 alone.

To further determine the effect of baicalin on the activation of the PKA-pDrp1s637 signalling, we analysed the phosphorylation of these proteins upon baicalin treatment on AβO-induced HT-22 cells in the presence or absence of H89. As shown in Fig. 11 A and B, the expression of pVASPs157 and pDrp1s637 was significantly decreased (P < 0.01, P < 0.01, respectively) in AβO-treated HT-22 cells. Baicalin 0.5, and 5 μM dramatically reversed the pVASPs157 and pDrp1s637 levels (P < 0.01, P < 0.05, respectively). These effects of baicalin were blocked by H89 (P < 0.01), and none of these effects were observed with the administration of H89 alone. These results demonstrate that baicalin may be involved in inhibiting PDE4 and activating the cAMP-PKA-pDrp1s637 signal.

In summary, our study demonstrated that baicalin improved AβO-induced learning and cognitive deficits and restored neuronal synaptic plasticity. These results strongly suggest that baicalin might be a potential therapeutic option for AD and related neurodegeneration. Moreover, baicalin administration restored the levels of COXI and SDHB. Mechanistically, baicalin also improved mitochondrial fragmentation and abnormal phosphorylation of Drp1 at S637, possibly through regulation of the PDE4-cAMP-PKA-pDrp1s637 signalling pathway. However, additional studies are required to further understand baicalin’s mechanism of PDE4 inhibition in order to verify the interaction of baicalin and PDE4 in animal models and establish its clinical effectiveness in patients. We believe that the administration of baicalin may be a useful natural adjuvant in the field of AD therapy.
No conflict of interest statement was provided.
Parkinson’s disease. In 2019 Tu et al used a compound called 6-OHDA to induce the disease in rats. 6-hydroxydopamine is a kind of nerve agent that induces lesions in the brain, the resulting loss of dopamine produces Parkinson’s like symptoms which can be used as control against rats also administered baicalin.
They concluded that baicalin has significant protective effect on 6-OHDA-induced PD rats, which may play a protective role through an antioxidant, promoting the release of neurotransmitters and regulating the metabolism of N-acetyl aspartate and glutamate.
When using rat models, baicalin is usually administered with their food or directly to the stomach, but the paper doesn't actually tell us this:69
Abstract
Objective
This research was aimed to investigate the effects of baicalin on 6-hydroxydopamine (6-OHDA)-induced rat model of Parkinson’s disease (PD) and the main mechanism of baicalin based on metabolomics.
Methods
The rat model of PD was induced by 6-OHDA. The protective effects of baicalin on rat model of PD were evaluated by open field test and rotarod test. The anti-PD efficacy of baicalin was evaluated by examining the morphologic changes of neurons and the level of monoamine neurotransmitters in the striatum, the number and morphology of tyrosine hydroxylase (TH)-positive neurons, and oxidative stress. Combined with metabolomics methods, the pharmacodynamic mechanism of baicalin on PD pathogenesis was also explored.
Results
Baicalin treatment improved the rod time and voluntary movement in rat model of PD (P<0.05) by the open field test and rotarod test. In addition, baicalin also protected from oxidative stress injury (P<0.05), and regulated the content of monoamine neurotransmitters dopamine, 3,4-dihydroxyphenylacetic acid, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid (P<0.05) and the number and morphology of TH-positive cells in 6-OHDA-induced PD model rats. By metabolomics, multivariate statistical analysis, and receiver operating characteristic curve analysis, we found that two metabolites N-acetyl aspartic acid and glutamic acid had a good diagnostic value. Quantitative analysis of metabolites showed a regulatory function of baicalin.
Conclusion
Baicalin has significant protective effect on 6-OHDA-induced PD rats, which may play a protective role through an antioxidant, promoting the release of neurotransmitters and regulating the metabolism of N-acetyl aspartate and glutamate.
Keywords: Parkinson’s disease, neurotransmitter, baicalin, metabolomics
A total of 60 rats had PD model successfully established in our experiment. The model rats were randomly divided into four groups: PD group, PD + baicalin low-dose group (50 mg/kg), PD + baicalin middle-dose group (100 mg/kg), PD + baicalin high-dose group (150 mg/kg). Rats were continuously administered with baicalin for 8 weeks.
At present, the existing anti-Parkinson drugs can only improve the effect of symptoms, however not delay the process of disease, and not prevent the degeneration of DA neurons. In recent years, the treatment of PD is mainly concentrated on neuroprotective factors, neurotrophic factors, and growth factors such as dopamine. A large number of studies showed increased oxidative stress and iron in substantia nigra (SN) in PD patients.
Elevated activity of iron can promote oxidative stress, leading to a large number of oxygen free radicals. Excessive active iron can promote the occurrence of oxidative stress, resulting in a large number of oxygen free radicals, resulting in cell death.
Baicalin is isolated from Labiatae Scutellaria Linn Scutellaria baicalensis Georgi dry roots and extracted from flavonoids. Baicalin has antibacterial, antiviral, anti-inflammatory, antitumor, cardiovascular, and neuroprotective activities. Studies show that baicalin is protective on rotenone-induced and MPTP-induced dopaminergic neuron damage in PD model rats. Baicalin downregulated iron concentration, which positively regulated divalent metal transporter 1 expression and negatively regulated ferroportin 1 expression, and decreased iron accumulation in the SN. Baicalin and deferoxamine alleviate iron accumulation in different brain regions of PD rats. Preventive medication of baicalin shows a protective effect on C57 BL mouse with PD. However, MPTP-induced motor dysfunction in model mouse was not significantly improved by a short-time medication.
Results from a balance test on a rotating rod, higher is better:
Notes: Values are expressed as mean ± SEM. n=15. *P<0.05, **P<0.01 compared to that after administration appropriately.
Abbreviations: PD, Parkinson’s disease; SEM, standard error of the mean.
Voluntary movements, higher is better:
Notes: (A) The analysis of number of rearing; (B) the analysis of number of crossing. Values are expressed as mean ± SEM. n=10. *P<0.05, **P<0.01 compared to the sham group; #P<0.05, ##P<0.01 compared to the PD group.
Abbreviations: PD, Parkinson’s disease; SEM, standard error of the mean.
Oxidative stress markers induced by loss of DA cells, higher is better (A-C):

Notes: (A) SOD activity in different groups; (B) CAT activity in different groups; (C) GPx activity in different groups; (D) MDAin different groups. Values are expressed as mean ± SEM. n=10. *P<0.05, **P<0.01 compared to the sham group; #P,0.05, ##P,0.01 compared to the PD group.
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; PD, Parkinson’s disease; SEM, standard error of the mean; SOD, superoxide dismutase.
Effects of baicalin on monoamine neurotransmitter in the striatum of 6-OHDA-induced PD rat model
DA is a key neurotransmitter controlling movements. When 80% dopaminergic neurons are lost in the nigrostriatal nucleus and DA levels are reduced in the striatum, symptoms of PD appear. Previous studies showed that the occurrence of PD is associated with decrease in neurotransmitter levels. When striatum is damaged in patients and DA levels reduced, syndrome of tremor and paralysis gets aggravated. Decreases in the DA metabolite DOPAC and the content of 5-HT and 5-HIAA were accompanied with that. The results showed that the contents of DA, DOPAC, 5-HT, and 5-HIAA in the model group were lower than those in the sham-operated group (Table 1). After administration of baicalin, the levels of monoamine neurotransmitters were reversed, which indicate that baicalin can promote the release of monoamine neurotransmitters to improve movements.
“Tyrosine hydroxylase (TyrH) is the rate-limiting enzyme of catecholamine synthesis. It catalyzes the hydroxylation of tyrosine to L-DOPA. The catecholamines dopamine, epinephrine and norepinephrine are the products of the pathway, important as hormones and neurotransmitters in both the central and peripheral nervous systems.”70
Effect of baicalin on neurons in 6-OHDA-induced PD rat model
TH immunohistochemistry (Figure 4) showed that TH-positive neurons had a clear shape, high TH expression, and strong immunocompetence in the SN of rats in the sham-operated group. However, in PD model group, the number of TH-positive neurons in the brain was significantly decreased, TH levels were poor, the cytoplasm color was light, and the cell morphology blurred compared to the sham-operated group. After administration of baicalin, the number of neurons was increased. The effect of medium- and high-dose groups was obviously better than that in the low-dose group. Number of TH-positive cells increased with darker cytoplasm and clear cell morphology.
Note: Effect of baicalin on neurons in the brain of 6-OHDA-induced PD rat model.
Abbreviations: 6-OHDA, 6-hydroxydopamine; TEM, transmission electron microscopy; TH, tyrosine hydroxylase; PD, Parkinson’s disease.
Determination of NAA and Glu levels
To further evaluate the role of NAA and Glu in the pathogenesis of PD, quantitative analysis by MRS showed that NAA was decreased and Glu was increased. After administration of baicalin, the levels of NAA and Glu significantly reversed (Table 2).
NAA is a hallmark of neuronal changes in the brain, and a decreased level suggests a loss or dysfunction of neurons. Excitatory amino acid Glu is mainly involved in synaptic excitability transmission, learning and memory formation, and neurodegenerative diseases. When Glu is abnormally elevated, DA receptors on DA neurons are activated. These result in an increase in intracellular Ca2+ levels, and lead to the destruction of cytoskeleton and degenerate neurons, which is also an important pathologic feature of PD.
Conclusion
In conclusion, our results showed that baicalin can prevent neurodegeneration in 6-OHDA-induced PD rats through antioxidative stress, inhibition of apoptosis of dopaminergic neurons in SN, and regulation of monoamine neurotransmitter release. The main mechanism of baicalin may be by the regulation of NAA and Glu metabolism. This study explored the pathogenesis of PD and the mechanism of baicalin against PD by metabonomics technology, which provided a basis for subsequent researchers to understand the pathogenesis of PD and explore the mechanism of action of drugs.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81560201) and Doctor Foundation of Guizhou Provincial People’s Hospital (GZSYBS[2015]03).
Disclosure
The authors report no conflicts of interest in this work.
From the previous studies we know that baicalein and baicalin are quickly metabolised. But what are these and do they also have any therapeutic properties?
In 2012, Lu et al came up with an answer to the first part of the question - using mice bearing pancreatic tumor grafts which had been fed to their stomachs with a supplemented diet they found that some of the baicalin gets methylated to oroxylin A (OA) and its conjugates in various organs during absorption, and some of these also have anti-tumor properties. In fact the anti-tumor effects in this study were so pronounced that “only 3 out of 6 mice treated with SB extract grew tumors large enough to be analyzed”:71
Abstract
Objectives
Scutellaria baicalensis has been a subject of research interests due to its potential multiple therapeutic benefits. This study was to examine the distribution of baicalein, wogonin, oroxylin A and their glucuronide/sulfate conjugated metabolites in plasma, colon, small intestine, lung, liver, pancreas, kidney, and prostate tissues and in pancreatic tumor in a xenograft animal model. In addition, we examined metabolic stability of baicalin in these tissues.
Methods
A mouse xenograft model was prepared by injection of 3×106 human pancreatic cancer MiaPaCa-2 cells subcutaneously into nude mice. Mice were randomly allocated to control diet (AIN76A) and 1% SB diet (n=8 per group) for 13 weeks. Levels of baicalein, wogonin, oroxylin A, and their conjugates in mouce tissues were measured by high-pressure liquid chromatography following enzymatic hydrolysis and then extraction.
Results
A substantial amount of baicalin (34–63%) was methylated to oroxylin A and its conjugates in various organs during absorption. While plasma contained predominantly conjugates of baicalein, wogonin, and oroxylin A, both aglycones and conjugates were found in all other tissues investigated and in tumor.
Conclusions
Substantial accumulation of bioactive metabolites are found in target tissues, suggesting strong potential for SB use as a preventive or adjuvant supplement for pancreatic cancer.
Keywords: Scutelleria baicalensis, baicalin, baicalein, oroxylin A, pancreatic cancer, in vivo
An in situ absorption experiment revealed the existence of double-site absorption of BG. The first absorption site was in upper intestine and might be due to the directly absorption of BG and the second site was in colon in the form of aglygone. In rat plasma glucuronic acid conjugates were the predominant forms, the result of first-pass metabolism. A few recent pharmacokinetics studies identified and quantified rat plasma OA, a 6-O-methylated B.Hou et al reported the distribution of B and W and their conjugates in the lung, liver, kidney, and brain of rats to which a decoction of SB roots at 2.0 g/4 mL/kg three times daily for 7 doses was administered via gastric gavage. We are unaware of any report of tissue absorption and distribution after long-term oral administration of SB in animal models.
Our research team has demonstrated that B induces apoptosis in human pancreatic cancer BxPC-3, HPAF-II, Panc-1 and MicPaCa-2 cell lines which is mediated through the inhibition of Mcl-1 activity. Furthermore, we have shown that a diet containing an extract from the root of SB inhibited the growth of pancreatic tumor xenograft in a mouse in vivo model after a long-term oral administration (unpublished data). To exert its biologic activity, BG must be sufficiently absorbed in the gastrointestinal tract and reach pharmacological concentrations in the target tissue. In order to understand how BG is absorbed and metabolized in mice, we investigated uptake of BG and presence of metabolites in pancreas and tumor tissue, and in the respiratory and gastrointestinal organs including lung, colon, small intestine, as well as in liver, kidney, and prostate in xenograft-bearing mice. In addition, we examined metabolic stability of BG in the tissues sampled.

Tissue levels of baicalin and its metabolites in xenograft mice after oral administration of SB diet
To investigate the absorption of SB extract in pancreas and tumor as target tissues, we also evaluated concentrations of metabolites in lung and liver, as lung and liver are common sites of metastases, especially of pancreatic tumors. In addition, we also investigated metabolites in the tissues of small intestine, colon, kidney, and prostate. Generally, all tissues from mice fed with 1% of SB extract diet accumulated B, W, and OA, as well as their glucuronidates/sulfates. Table 1 shows that levels of total B, W, and OA are highest in colon, followed by small intestine, liver, kidney, prostate, pancreas, lung, and tumor. Moreover, except for in prostate, about half of the BG is metabolized to the 6-O-methylated OA compound. The percentage of OA was highest in pancreas (63%), followed by tumor (54%), small intestine (51%), liver (47%), lung (45%), colon and kidney (both 43%), and prostate (34%). Note that only 3 out of 6 mice treated with SB extract grew tumors large enough to be analyzed.
Discussion
Oral bioavailability of SB has been a subject of research interests due to its potential multiple therapeutic benefits. However, accumulation of the SB flavones in plasma and tissues of animals fed SB diet for a relatively long term has not been reported. In this study, we reported that flavones from SB and their metabolites accumulate in tumor xenograft, pancreas, and other tissues in mice after administered with 1% SB diet for 13 weeks. B, W, OA, and their conjugates were found in all organs investigated and in tumor, but levels varied on the order of 100-fold from organ to organ. Generally, tissues from the GI tract accumulated highest levels of B, W, OR, and their conjugates with the highest level observed in colon and small intestine, followed by liver, kidney, prostate, pancreas, lung, and tumor. Levels of W and OA in tissues followed the same order. Furthermore, we found that all investigated organs contained a considerable proportion of deconjugated B, W, and OA, ranging from 38–97% of the total concentration. In general, higher proportions of O-methylated aglycones W and OA were found in tissues compared with non-methylated B. In tumor, 71% of B, 79% of W and 93% of OA are in aglycone forms.
Most of the flavonoids from plant origin are present in the form of β-glycosides. The flavones are mainly glycosylated in the 7 position. It is generally accepted that the mechanism of absorption of these glycosides involves hydrolysis of the glucosides in the intestinal lumen by lactase phlorizin hydrolase followed by the diffusion of the released aglycones and/or active transport by the sodium-dependant glucose transporter into enterocytes, with subsequent deglycosylation within the enterocyte by cytosolic β-glucosidase. It has been reported that human and rat tissues possess β-glucuronidase enzymes, which can be released under certain physiological conditions such as inflammation. Mouse tumor and human pancreatic cancer tissue contain large amounts of β-glucuronidase as well. The deglycosylation of flavonoids and isoflavonoids by β-glucuronidase is an important first step in their uptake, metabolism, excretion, and biological activity.
In summary, this study showed that BG can accumulate in plasma, tumor xenografts, and pancreas, liver, lung, and other tissues in vivo. Substantial amount of BG (34–63%) is methylated to OA in various organs during absorption. While plasma contains predominantly conjugates of B, W, and OA, in all other organs investigated and in tumor, both aglycones and conjugates were found. Enzymes such as glucuronidase and sulfatase present in the cell membrane in mouse organs contribute to the aglycones found in tissues. Our data can be used to predict the organ site(s) most likely to benefit from SB treatment, and to provide important information on predicting chemopreventive or therapeutic efficacy of SB.
Acknowledgments
This work was supported by the National Institutes of Health (P01AT003960) and the Hirshberg Foundation for Pancreatic Cancer Research.
This paper by Ha et al (2012) is paywalled but also found that the breakdown product of baicalin, oroxylin A (OA) inhibits COX-2 as well as decreasing PGE2 levels in HT-29 human colon cancer cells.
Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties. PGE2 supports tumor growth by promoting angiogenesis, stimulating tumor-cell proliferation, and protecting tumor cells from apoptosis.
Bcl-2 (B-cell lymphoma 2) is a regulator protein that regulates cell death through apoptosis.
P53 or “the guardian of the genome” is a key tumor suppressor,
BAX is an apoptosis regulator in the Bcl-2 gene family.
Poly (ADP-ribose) polymerase (PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death.
Procaspase-3 is an “executioner procaspase” which promotes cell death.
Oroxylin A improves the sensitivity of HT-29 human colon cancer cells to 5-FU through modulation of the COX-2 signaling pathway72
Abstract
5-Fluorouracil (5-FU) is a principal drug for the treatment of colorectal cancer. Due to its low response and high toxicity, synergistic effects of 5-FU in combination with other drugs have been widely researched. This study investigated whether oroxylin A improved the sensitivity of HT-29 human colon cancer cells to 5-FU. A correlation between COX-2 inhibition by oroxylin A and a synergistic effect of 5-FU on the growth of HT-29 cells was observed, and a COX-2 pathway for this effect was recognized; oroxylin A evidently elevated the level of reactive oxygen species in HT-29 cells, which subsequently inhibited COX-2 expression and enhanced the susceptibility of HT-29 cells to 5-FU. Likely also related to COX-2 inhibition, oroxylin A decreased PGE(2) levels in HT-29 cells. The synergistic effect of 5-FU induced by oroxylin A was also found in the suppression of Bcl-2 and in the activation of P53, Bax, PARP, and procaspase-3 proteins in HT-29 cells. Ultimately, a combination of 5-FU with oroxylin A significantly reduced the growth of HT-29 tumors in nude mice compared with treatment with 5-FU or oroxylin A alone. In conclusion, a combination of 5-FU and oroxylin A has a significant synergistic effect in the inhibition of HT-29 cell proliferation in vitro and controls HT-29 tumor growth in vivo. This synergistic effect may be mainly related to COX-2 inhibition by oroxylin A in HT-29 cells.
In addition to the above, research by Chen, Yang & Lee (2000) added the following to our knowledge about the effects of oroxylin A:73
LPS: a lipopolysaccharide.
p65: RELA, also known as p65, is a REL-associated protein involved in NF-κB heterodimer formation, nuclear translocation and activation. NF-κB is an essential transcription factor complex involved in all types of cellular processes, including cellular metabolism, chemotaxis, etc. Phosphorylation and acetylation of RELA are crucial post-translational modifications required for NF-κB activation. RELA has also been shown to modulate immune responses, and activation of RELA is positively associated with multiple types of cancer.74
This was consistent with the findings that oroxylin A but not emodin or physcion inhibited prostaglandin E2 synthesis induced by LPS. The inhibitory effects of oroxylin A on LPS-induced iNOS and COX-2 gene expression were also demonstrated in Bcl-2-overexpressing RAW264.7 macrophages, suggesting that oroxylin A inhibition of iNOS and COX-2 expression was not due to its antioxidant effect. Furthermore, oroxylin A but not emodin blocked nuclear factor-κB (NF-κB) binding and transcriptional activation associated with decreased p65 proteins in the nucleus induced by LPS. These results indicated that oroxylin A, an active component in Huang Qin, inhibited LPS-induced iNOS and COX-2 gene expression by blocking NF-κB activation, whereas emodin inhibition of LPS-induced iNOS expression may be mediated by a different transcription factor.
Returning to antiviral properties, a patent was filed in 2009 for Baicalin as a treatment for SARS infection.75
Abstract
The present invention relates to a pharmaceutical composition useful for the treatment of diseases associated with viruses of the order Nidovirales of the family Coronaviradae, such as Severe Acute Respiratory Syndrome (SARS) viruses in humans and other animals. In particular, this invention relates to a naturally occurring compound, namely, baicalin, extracted and purified from the Chinese medicinal plant Scutellaria baicalensis Georgi (Chinese name: Huang Qin), that exhibits potent antiviral activity against members of the order Nidovirales of the family Coronaviradae that infects humans and other animals; in particular, SARS viruses in humans (“hSARS virus”). The invention also relates to a therapeutic method, using pharmaceutical compositions comprising baicalin compounds, for the treatment, amelioration, management or prevention of diseases associated with members of the order Nidovirales of the family Coronaviradae, such as hSARS.
And in 2020 Su et al published a preprint of their research findings from investigating the protease inhibition of SARS-CoV-2 by baicalin and baicalein:76
Abstract
Human infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cause coronavirus disease 19 (COVID-19) and there is currently no cure. The 3C-like protease (3CLpro), a highly conserved protease indispensable for replication of coronaviruses, is a promising target for development of broad-spectrum antiviral drugs. To advance the speed of drug discovery and development, we investigated the inhibition of SARS-CoV-2 3CLpro by natural products derived from Chinese traditional medicines. Baicalin and baicalein were identified as the first non-covalent, non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro and exhibited potent antiviral activities in a cell-based system. Remarkably, the binding mode of baicalein with SARS-CoV-2 3CLpro determined by X-ray protein crystallography is distinctly different from those of known inhibitors. Baicalein is perfectly ensconced in the core of the substrate-binding pocket by interacting with two catalytic residues, the crucial S1/S2 subsites and the oxyanion loop, acting as a “shield” in front of the catalytic dyad to prevent the peptide substrate approaching the active site. The simple chemical structure, unique mode of action, and potent antiviral activities in vitro, coupled with the favorable safety data from clinical trials, emphasize that baicalein provides a great opportunity for the development of critically needed anti-coronaviral drugs.
As the result, two fractions from S. baicalensis showed significant inhibition on SARS-CoV-2 3CLpro at 10.0 μg/mL (Table S1). Surprisingly, baicalin, the major component in fraction 8, shows an IC50 of 6.41 μM against the protease, while baicalein, the major component in fraction 12, has an IC50 of 0.94 μM (Fig. S2; Table 1). Accordingly, baicalin and baicalein are identified as novel non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro with single-digit micromolar potency.

The corresponding selectivity index (SI = CC50/EC50) values are >19 and >118 for baicalin and baicalein, respectively. Therefore, the cell-based antiviral activity of baicalin or baicalein is superior to most of the reported compounds and that of baicalein is close to those of chloroquine (EC50: 1.13 μM; SI > 88) and remdesivir (EC50: 0.77 μM; SI > 129).
Even at concentrations comparable to those seen in the rat plasma profiles earlier there is significant inhibition:

In view of the long history of TCMs in treating viral infections and the urgent need of drug development against COVID-19, we investigated the anti-SARS-CoV-2 potential of natural products isolated from S. baicalensis, a long-term used TCM. S. baicalensis is also the major component of Chinese traditional patent medicines such as “Shuanghuanglian” and “Qingfei Paidutang” which have proven effective in curing patients of COVID-19 in China. As a result, we identified two small natural products, baicalin and baicalein, as the first class of non-covalent, non-peptidomimetic inhibitors of SARS-CoV-2 3CLpro by the enzymatic assay in combination with the ITC, ESI-MS, and X-ray protein crystallography studies. In contrast to most available covalent, peptidomimetic inhibitors of the 3CL proteases, the ITC measurement also revealed that these two compounds are non-covalent inhibitors with a high ligand-binding efficiency. The binding mode of baicalein with the protease revealed by the crystal structure demonstrates that a unique protein-ligand interaction pattern is utilized by baicalein to block the proteolytic activity of SARS-CoV-2 3CLpro. The crystal structure of baicalein-bound SARS-CoV-2 3CLpro thus reported the first demonstration, to the best of our knowledge, of the 3CLpro reversibly inhibited by a small molecule derived from TCM. With a molecular weight of 270.24 Da and a Kd of 4.03 μM, baicalein serves as the most efficient binder of 3CLpros known to date. Moreover, it possesses an antiviral activity in the cells with a low cytotoxicity. Given the high conservation of 3CLpro among various CoVs, baicalein is expected to inhibit other 3CL proteases of CoVs, providing a new candidate for development of broad-spectrum antiviral drugs.
In addition, baicalin tablets have been used as an adjuvant therapy for the treatment of acute, chronic, or persistent hepatitis in China. Single oral doses of 100–2800 mg of baicalein were safe and well tolerated by healthy subjects (clinical trials registration number CTR20132944) (23). Furthermore, a randomized, double-blind, placebo-controlled, multicenter, and phase IIa clinical trial for the effectiveness and safety of baicalein tablets in the treatment of improving other aspects of healthy adults with influenza fever is under way (clinical trials registration numbers CTR20182427 and NCT03830684). These favorable safety data together with the potent inhibitory activities in enzyme and cell-based assays show a strong preference for in vivo and clinical trials studies of baicalin and baicalein for COVID-19 treatment. Overall, the present study provides a good example for exploring the in vitro potency of TCMs and effectively identifying bioactive ingredients toward a specific target, especially when Chinese traditional patent medicines have been proven effective in curing patients of COVID-19 in China.
Funding
This work was supported by the National Key R&D Program of China (Nos. 2017YFB0202604 and 2016YFA0502301), Chinese Academy of Engineering and Ma Yun Foundation (No. 2020-CMKYGG-05), and Science and Technology Commission of Shanghai Municipality (No. 20431900200).
Competing interests
The authors declare no competing interests.
“Shuanghuanglian (Chinese: 双黄连) is a traditional Chinese medicine with a long history for treating respiratory tract infection in China. Some of its active ingredients are Wogonin, Baicalin and baicalein. It is derived from three Chinese herbal medicines, namely, Lonicera japonica Thunb., Scutellaria baicalensis Georgi, and Forsythia suspense (Thunb.) Vahl. It has been used for the treatment of acute respiratory tract infections since 1973. It is mentioned in the Chinese Pharmacopoeia.
The substance has been shown in vitro to be cytotoxic "against a clinical isolate of SARS-CoV-2".”77
Zhang et al analysed Shuang-Huang-Lian oral liquid (SHL) in 2015. It’s mostly sugar to hide the bitterness, almost like cough medicine, which may explain the large doses needed to get therapeutic benefits. SHL is very popular in China:78
The results indicated that 57.52-78.11% (w/w) of SHL could be quantitatively determined (non-saccharide small molecules: 1.77-3.75%, monosaccharides: 0.93-20.93%, macromolecules: 2.63-5.76% and sucrose: 49.20-65.94%). This study may provide a useful way to comprehensively evaluate the quality of SHL.
Since the outbreak of Severe Acute Respiratory Syndromes in 2003, commercial demand for SHL has steadily increased. The drug is produced by several manufacturers. The annual output of SHL by one of these manufacturers has reached 600 million bottles, with annual revenue of USD 500 million and an annual growth rate of 13.93%. SHL has become the leading choice in preventing and treating the “wind-heat” type of common cold in China.
Chinese patent drugs may consist of hundreds or even thousands of components which are responsible for the therapeutic effects by synergistic and/or antagonistic interaction. And SHL has a complex chemical profile with a wide range of components, including phenylethanoid glycosides, lignans, quinic acids, saponins and flavonoids.
Note the significant amount of oroxylin A-7-0-glucuronide, batch variability and that the units of weight of fructose, glucose, sucrose, macromolecules and freeze-dried powder are mg/ml, all the other analytes are measured in μg/ml:
In 2021, Ni et al published their findings from a randomized clinical trial of the effects of Shuanghuanglian against COVID-19.79
They found that although the time to disease recovery was not reduced the viral load was significantly lower and pneumonia associated inflammation was significantly reduced. They do suggest that time to recovery may have been skewed upwards due to patient transfers and waiting times before being randomly selected for trial.
Abstract
We conducted a randomized, open-label, parallel-controlled, multicenter trial on the use of Shuanghuanglian (SHL), a traditional Chinese patent medicine, in treating cases of COVID-19. A total of 176 patients received SHL by three doses (56 in low dose, 61 in middle dose, and 59 in high dose) in addition to standard care. The control group was composed of 59 patients who received standard therapy alone. Treatment with SHL was not associated with a difference from standard care in the time to disease recovery. Patients with 14-day SHL treatment had significantly higher rate in negative conversion of SARS-CoV-2 in nucleic acid swab tests than the patients from the control group (93.4% vs. 73.9%, P = 0.006). Analysis of chest computed tomography images showed that treatment with high-dose SHL significantly promoted absorption of inflammatory focus of pneumonia, which was evaluated by density reduction of inflammatory focus from baseline, at day 7 (mean difference (95% CI), -46.39 (-86.83 to -5.94) HU; P = 0.025) and day 14 (mean difference (95% CI), -74.21 (-133.35 to -15.08) HU; P = 0.014). No serious adverse events occurred in the SHL groups. This study illustrated that SHL in combination with standard care was safe and partially effective for the treatment of COVID-19.
Keywords: COVID-19; SARS-CoV-2; Shuanghuanglian oral liquid; clinical trial.
Traditional Chinese medicines (TCMs) have attracted the attention of clinicians and researchers in China during the COVID-19 pandemic, but the use of TCMs for treating COVID-19 is controversial because of lack of data on the efficacy and safety of TCMs for COVID-19. Accordingly, well-controlled clinical trials are necessary to provide the proof of evidence-based medicine by showing the efficacy and safety of TCMs on COVID-19. Recently, the result of a multicenter, prospective, randomized controlled trial on the efficacy and safety of Lianhuaqingwen (LH) capsules, a repurposed Chinese herb, in patients with COVID-19, has been published by Hu et al. This clinical trial shows the application of TCM in the treatment of COVID-19. In the present study, we report the outcomes of a randomized, open-label, parallel-controlled, multicenter clinical trial of Shuanghuanglian (SHL) oral liquid for the treatment of COVID-19. SHL oral liquid, a traditional Chinese patent medicine containing extracts of three herbs, including Lonicera japonica Thunb., Scutellaria baicalensis Georgi, and Forsythia suspense (Thunb.), has been used in clinical practice for a long time in China, particularly for patients with symptoms of colds, sore throat, and cough with fever. SHL has been viewed as an effective broad-spectrum antiviral drugs, which plays an important role in preventing and controlling the epidemic of SARS, Ebola hemorrhagic fever, influenza A, and avian influenza in China. For more than 17 years, a team led by Prof. Jianping Zuo (one of the co-authors of this paper) at Shanghai Institute of Materia Medica, Chinese Academy of Sciences (CAS), has been studying the antiviral effects of SHL against SARS coronavirus, Middle East respiratory syndrome coronavirus, and influenza virus (H7N9, H1N1, and H5N1). Another team at CAS found that SHL and some of its ingredients have antiviral activities against SARS-CoV-2 in cultured cells with high potency. Considering that no specific agents have been recommended for COVID-19 at the beginning of the COVID-19 pandemic, we conducted compassionate use of SHL in a family case of three patients (daughter and her parents) with COVID-19. We observed a positive result of SHL in treating all three patients who were fully recovered from COVID-19 after using SHL in combination with standard care. Based on the abovementioned findings, we conducted a randomized, open-label, parallel-controlled, multicenter trial to evaluate the efficacy and safety of SHL (provided by Sanchine Phamaceutical, Harbin Pharmaceutical Group Co., Ltd.) for COVID-19 (registration number of Chinese clinical trial registration: ChiCTR2000029605).
The standard care protocols, as used for comparative purposes, also included de facto antivirals including azithromycin:80
A total of 235 patients were randomized in a 1:1:1:1 ratio to receive either SHL (20 mL for the low-dose group, 40 mL for the middle-dose group, and 60 mL for the high-dose group, three times daily) in addition to standard therapy or standard therapy alone for 14 days. All patients received standard care, which consisted of supportive treatments, including supplemental oxygen therapy, daily symptom and vital sign monitoring, clinical laboratory testing, correction of water, electrolyte and acid base imbalances, and administration of antiviral agents and antibiotic agents if bacterial infection was found. The antiviral agents used in the standard care included lopinavir/ritonavir, ganciclovir, arbidol hydrochloride, oseltamivir phosphate, ribavirin, entecavir, and interferon. The antibiotics used in the standard care included cephalosporin, moxifloxacin, lavoofloxacin, and azithromycin. Standard care was used according to the “COVID-19 Diagnosis and Treatment Protocol (Trial Fifth Version or later updated versions)” released by the General Offices of National Health Committee and National Administration of Traditional Medicine of the People’s Republic of China.
They complied with ethical best practices for conducting experimental clinical trials, including obtaining written informed consent:
The study was approved by respective local ethics committees and performed according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients in this study signed a written informed consent.
To avoid false negatives they performed antibody tests as well as PCR:
Notably, in early February 2020, given the high false-negative rate of nucleic acid test, a large number of patients in Hubei met the clinical diagnosis but were negative for multiple nucleic acid tests. Therefore, in the “COVID-19 Diagnosis and Treatment Protocol (Trial Fifth Version),” Hubei Province added the classification of “clinical diagnosis,” in particular antibody test and CT scan, to confirm the diagnosis of patients with COVID-19. Almost all the patients in this clinical trial received antibody tests and CT scan to confirm the diagnosis (Fig. S1 and Table S7). These procedures are in line with the policy of this special case in Hubei Province at that time.
Lung inflammation, a marker for pneumonia, was reduced in a dose dependent manner:
Pneumonia is a major manifestation in patients with COVID-19, where the decreased density of chest CT imaging in infection focus can be considered as a sign of symptom improvement. In obtaining a sophisticated analysis result, we used AI software to quantitatively calculate the infection density of inflammatory focus of the lungs rather than to evaluate the infection area. The results indicated that the reduction in density of pneumonia infection focus from the baseline after treatment in the SHL groups was more than that in the control group. In particular, the reduction in imaging density of infection focus in the high-dose SHL group was significantly more than that in the control group on day 7 and day 14. In addition, the reduction of lung inflammation enhanced by SHL showed a dose–effect relationship, in which the high dose SHL treatment achieved better pneumonia recovery than the low- and middle-dose treatments. As for the detailed mechanism of SHL to speed up the absorption of pneumonia focus, further pharmacological research and clinical experiments are necessary.
Heart injury was also considered:
The criteria for scoring primary symptoms (fever, fatigue, and cough) and secondary symptoms (diarrhea, nausea or vomiting, feeling cold, chest pain, polydipsia, hypohidrosis, chest tightness, and shortness of breath) are shown in Tables S2 and S3. Other outcomes included markers of inflammation and heart injury (NT-proBNP, cTNI). Pneumonia is the major damage in patients with COVID-19; thus, the quantification of infection degree on chest CT could be considered as an important outcome to evaluate the progress of pneumonia and effects of treatment according to the guideline in “COVID-19 Diagnosis and Treatment Protocol (Trial Fifth Version or later updated versions).
This were more like a positive control due to their antiviral, antibacterial an anti-inflammatory action, but even so they were still bettered by both mid and high dose SHL:
Higher is better:
As previously mentioned, in the middle phase of our clinical trial, the role of inflammatory factors in COVID-19 had attracted considerable attention. Accordingly, we only investigated inflammatory cytokines for a portion of participants, including 117 from the SHL groups and 43 from the control group on day 0, 52 from the SHL groups and 15 from the control group on day 7, and 57 from the SHL groups and 20 from the control group on day 14. Therefore, we did not observe the effects of SHL on inhibiting inflammatory cytokines in the trial (Table S4), although other studies indicated that SHL and some of its ingredients such as baicalin could inhibit the expression levels of TNF-α, IL-6, and IL-8 in MRC5 cells induced by LPS.
Cardiac injury also contributed to the system damage in patients with COVID-19. Our clinical trial revealed a phenomenon that SHL might affect the hearts of patients with COVID-19 because the trial observed a trend that N-terminal brain natriuretic peptide (NT-proBNP), a biomarker for heart failure, was lower in the SHL groups compared with the control group (Table S5). Similar to the absorption of pneumonia focus, the mechanism of SHL lowering the NT-proBNP of patients should be further studied.
There were several confounding factors, mainly due to false positives and negatives, but the antibody tests helped correct for this. Late presentation before treatment could not be allowed for though:
The false-positive rate was low because of the additional antibody test, and the false-negative rates dropped in the later testing. Therefore, the statistical analysis of the rate in the negative conversion of SARS-CoV-2 in nucleic acid swab tests was not affected by the number of negative patients before randomization. Third, about half of the patients at randomization had no any symptoms, and the time of illness onset to randomization was long. Considering that Tongji Hospital was a designated hospital for severe and critical cases of COVID-19 in Hubei Province, most of the patients were transferred from other general hospitals or mobile cabin hospitals; thus, a considerable number of patients in this clinical trial had already waited for a long time before randomization in this trial. This limitation is possibly one of the reasons that SHL did not improve the symptom and shorten the time of recovery. Therefore, a further clinical study with larger samples and appropriate clinical design is necessary to investigate the detailed effects of SHL on patients with COVID-19. Treatment with SHL oral liquids could accelerate Li Ni et al. negative conversion rate in SARS-CoV-2 nucleic acid tests and promote the absorption of inflammatory focus of pneumonia in mild, moderate, and severe patients with COVID-19. These findings indicated that combining SHL with standard care could enhance antiviral effects and improve clinical outcomes in patients with COVID-19. This trial provided direct evidence for the efficacy and safety of SHL for COVID-19 by using evidence-based medicine.
Phytomelatonin
In 2018 Bhattacharjee & Dey published a literature review discussing phytomelatonin or plant based sources of melatonin, and S. baicalensis is a source of the hormone.81
Abstract
Melatonin (N-acetyl-5-methoxytryptamine), a well-known pineal gland hormone, was discovered in plants in 1995 but till then very little research into it has been carried out in this arena. It is present in different parts of all the plant species studied, including leaves, stems, roots, fruits and seeds. Based on the ubiquitous distribution of melatonin in all kingdoms, melatonin was even suggested as the nature's most versatile biological signal molecule. Since the identification of melatonin in plants by Hattori. Several reports have published and opened up a new area in the field of plant derived melatonin i.e. phytomelatonin. Phytomelatonin is biosynthesized in plants from tryptophan precursor. Majority of the herbs containing high levels of melatonin have been used traditionally to treat neurological disorders associated with the generation of free radicals which might be associated with its potent antioxidant activity. This concise survey will endeavor to provide an overview phytomelatonin along with its distribution, biosynthesis and probable role in plant growth and regulation.
Keywords: phytomelatonin, tryptophan, medicinal plants
Introduction
Melatonin is old and understood companion in human and creature physiology however novel to plant physiology. Melatonin was first isolated from the bovine pineal gland and identified as N-acetyl-5-methoxy tryptamine by Lerner and co-workers in 1958. It was named melatonin because of its capacity to whiten the skin in certain fish, reptiles and amphibians. In mammals, melatonin plays a key role to regulate circadian rhythm. This molecule is an powerful antioxidant and preserves mitochondrial homeostasis, increases gene expression for antioxidant enzymes and thereby extremely beneficial in neurodegenerative disorders like Alzheimer's, Parkinson's disease whose pathogenesis is associated with the cytotoxic effect of reactive oxygen species.
Melatonin also enhances the rate of germination and growth and plant productivity. It acts as a retardant in stress-induced leaf senescence. These cumulative observations bring forward the idea that exogenous melatonin treatment of cultivated plants or overproducing higher melatonin containing plants might help crops resist more easily against many adverse environmental conditions from which they normally suffer throughout their development. The later aspects refer to the possibility of introducing melatonin-rich plants foods or food supplements due to its immense health benefits particularly against neurodegenerative disorders like Alzheimer’s. Studies revealed that oral dose of melatonin of up to 1 gram/day produce no adverse effects in humans. In addition, melatonin is easily absorbed via the gastrointestinal tract. So, the utility of melatonin as a nutraceutical seems to have a promising future to promote healthier life.
Acknowledgements
None.
Conflict of interest
The author declares there is no conflict of interest.
At up to 7µg/g S. baicalensis is not a rich source of melatonin but it is worth considering as 3 grams/day could be enough to reach the physiological dose threshold for some people and synergism tends to amplify any effects.
In 1994 Zaidan et al found that only 20 micrograms was enough to have an effect:82
Abstract
A single physiological dose of melatonin (20 micrograms for 3 h given intravenously at different times of the day (04.00-12.00, 16.00 and 20.00 h) was able to shift the endogenous plasma melatonin profile of healthy volunteers under entrained conditions according to a phase-response curve (PRC). ANOVA showed an effect of the time of administration on the onset, the acrophase or the offset of the melatonin profiles. These profiles were significantly delayed when the infusion was administered at 12.00 h and advanced when the infusion was given at 20.00 h. Further, the AUCs evaluated on the nocturnal melatonin profiles were increased after the 04.00 h infusion (+20.5%, p < 0.05), whereas they were decreased after the 12.00 h infusion (-20%, p < 0.05). Lastly, no alteration was observed for cortisol rhythm, whatever the time of melatonin administration. These results, which show that according to a PRC the system regulating melatonin secretion is sensitive to a single short-term administration of the hormone given at a low dose, support the paradigm of the endogenous synchronizer melatonin.
Endometriosis
As baicalein and baicalin suppress various inflammatory signalling pathways it follows that many conditions that are related to these may also stand to benefit due its beneficial effects. One example of a condition sharing a common pathway is endometriosis. In 2017 Jin, Huang and Zhu collected tissue samples from 6 female patients with endometriosis, cultured the endometrial stromal cells and exposed some of them to baicalein, with control cells for comparison. They found that baicalein may suppress the viability of human endometrial stromal cells through the NF-κB signalling pathway in vitro, and may induce apoptosis and promote cell cycle arrest at the G0/G1 phase. Thus, baicalein may provide a novel treatment option for endometriosis:83
Abstract
The aim of the present study was to evaluate the effects of baicalein on human endometrial stromal cells in vitro. Ectopic endometrium samples were obtained from 6 female patients with ovarian endometriosis who underwent laparoscopic surgical procedures from July to September 2015. After culturing the cells, immunocytochemistry was performed to verify the purity and homogeneity of the endometrial stromal cells, and a Cell Counting Kit-8 assay was used to evaluate cell viability. In addition, cell cycle progression was analyzed using flow cytometry, and the effects of baicalein on the expression of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), proliferating cell nuclear antigen (PCNA) and cyclin D1 in endometrial stromal cells were evaluated using western blot analysis. The related signaling pathways were also investigated by incubating cells with inhibitors of signaling pathways, prior to adding 40 µM baicalein for 48 h, followed by analysis of cell viability using a Cell Counting Kit-8 assay. The results indicated that treatment with baicalein significantly induced a dose-dependent decrease (P<0.05) in the viability of human endometrial stromal cells, which was abolished by inhibition of the nuclear factor (NF)-κB signaling pathway. However, baicalein treatment did not induce a time-dependent decrease in viability, as cell viabilities between the 24, 48 and 72 h treatment groups did not differ significantly. The number of cells in the G0/G1 phase significantly increased following treatment with baicalein (P<0.05), while the number of cells in the S and G2/M phases significantly decreased (P<0.05). Baicalein-treated cells also exhibited significantly reduced expression of Bcl-2, PCNA and cyclin D1 compared with control cells (P<0.05). These results suggested that baicalein may suppress the viability of human endometrial stromal cells through the NF-κB signaling pathway in vitro, and may induce apoptosis and promote cell cycle arrest at the G0/G1 phase. Thus, baicalein may provide a novel treatment option for endometriosis.
Keywords: baicalein, endometriosis, cell viability, nuclear factor-κB pathway
Surgery and medicine are current treatment options for endometriosis. Although endometriotic lesions may be removed during surgery, complete elimination of the lesions is rarely achieved, and post-operative relapse typically occurs. The hypo-estrogenic status caused by pseudopregnancy therapy, such as oral contraceptives, and pseudomenopause therapy, such as gonadotropin-releasing hormone-analogs, are often effective in the short-term treatment of endometriosis; however, associated side effects limit the use of these therapies in the long-term. Therefore, safe and effective treatments for endometriosis in the long-term are required.
Although endometriosis is a benign disease, previous results have suggested that it represents the initial stages of neoplastic processes. In particular, atypical endometriosis may represent a transitional stage between benign disease and cancer. Common features shared by endometriosis and cancer include the ability to evade apoptosis, adult stem cell-like dysregulation, neovascularization, a progressive course, implantation at distal sites, the creation of a microenvironment that enables cells to become anchorage-independent, and mobilization of the immune system
As baicalein exhibits a wide range of anti-tumor effects, is easily obtained, produces little toxicity, causes few side effects, and has demonstrated potential in treating cancer, it may be a promising therapeutic agent in the treatment of endometriosis. In the current study, the effect of baicalein on human endometrial stromal cells was evaluated in vitro.
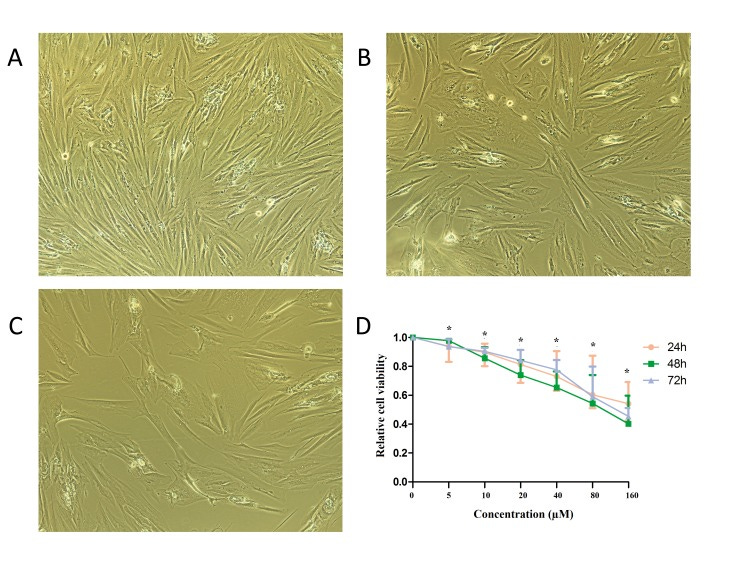
Bcl-2 (B-cell lymphoma 2), encoded in humans by the BCL2 gene, is the founding member of the Bcl-2 family of regulator proteins that regulate cell death (apoptosis), by either inhibiting (anti-apoptotic) or inducing (pro-apoptotic) apoptosis. The over-expression of the anti-apoptotic Bcl-2 protein in lymphocytes alone does not cause cancer. But simultaneous over-expression of Bcl-2 and the proto-oncogene myc may produce aggressive B-cell malignancies including lymphoma.84
Effect of baicalein on the protein expression level of Bax, Bcl-2, PCNA and Cyclin D1
The effect of baicalein on the expression of apoptotic proteins was evaluated. As depicted in Fig. 3 and Table III, the level of Bax protein did not change significantly, while the level of Bcl-2 protein significantly decreased following baicalein treatment, relative to the control (P<0.05). The expression of PCNA was also used to evaluate cell proliferation following treatment with baicalein. It was observed that treatment with baicalein caused a significant decrease in the level of PCNA protein compared with the control (P<0.05; Fig. 3 and Table III). In addition, levels of Cyclin D1, as a marker of the M to G1 transition of the cell cycle, were significantly reduced by baicalein treatment when compared with the control (P<0.05;

Due to the reported anti-tumor properties of baicalein and the relationship between endometriosis and cancer, the current study aimed to evaluate the therapeutic potential of baicalein in endometriosis. The effect of baicalein on endometrial stromal cell viability was assessed in vitro to provide a theoretical foundation for its use in the treatment of endometriosis, and to gain insight into the pathogenesis of this disease. It was observed that 5 µM baicalein significantly reduced the viability of human endometrial stromal cells, and that this effect was more pronounced with increasing doses of baicalein. In turn, the suppressive effects of baicalein were abolished by treatment with an inhibitor of the NF-κB signaling pathway. Treatment with baicalein also reduced the expression of the anti-apoptosis protein Bcl-2, indicating that baicalein may induce apoptosis in human endometrial stromal cells. Furthermore, results obtained from cell cycle analysis indicated that the number of cells in the G1 phase increased following treatment with baicalein when compared with control cells, while the number of cells in the S and G2/M phases decreased. Therefore, baicalein effectively caused G1 phase cell cycle arrest in endometrial stromal cells. Future studies are now required to further evaluate the activity of baicalein, particularly regarding its potential effects on angiogenesis, inflammation and immunity.
In conclusion, the present study observed that baicalein significantly reduced the viability of endometrial stromal cells in vitro, potentially through mediation of the NF-κB signaling pathway. Baicalein also induced apoptosis and promoted G0/G1 phase cell cycle arrest of endometrial stromal cells.
Acknowledgements
The present study was supported by the Science and Technology Commission of Shanghai Municipality, China (grant no. 12401902200).
Ulcerative colitis (UC)
Liang et al (2019) induced UC in rats and treated it using baicalin, baicalein, or a combination of the two. They found that the high baicalin content of YSR (Young Scutellaria baicalensis ratio of baicalin and baicalein) exhibited the best treatment effects.
Once again, if untreated then lesions caused by persistent inflammation may become cancerous, and IL-6, TH17 T helper cells and tumor growth factor TGF-β are involved in the signalling pathway:85
Abstract
Introduction: Ulcerative colitis (UC) is an inflammatory bowel disease with a high incidence rate and a difficult treatment regimen. Recently, significant advances in the treatment of intestinal diseases, particularly UC, have been made with the use of the drugs baicalin and baicalein, separately or in combination. However, the therapeutic efficacy and mechanism of action of baicalin, baicalein, and their combination therapy, in the treatment of UC has not been fully elucidated. Materials and Methods: we constructed a UC rat model that encompassed a variety of complex factors, including a high-sugar and high-fat diet, a high temperature and humidity environment (HTHE), excess drinking, and infection of Escherichia coli. Model rats were then treated with baicalin, baicalein, or a combination of the two. Results: The results showed significant differences in the therapeutic effects of baicalin, baicalein, and the combination therapy, in the treatment of UC, as well as differences in the inhibition of inflammation via the nuclear factor-κB and MAPK pathways. The rat model of UC was established as described above. Then, the rats were treated for 7 days with baicalin (100 mg kg-1), baicalein (100 mg kg-1), or both (100 mg kg-1, baicalin: baicalein = 4:1/1:1). Clinical symptoms and signs, body temperature, organ indices, histopathology, blood biochemistry, and metabolites were examined to compare treatment effects and indicators of UC. Baicalin, YSR (Young Scutellaria baicalensis ratio of baicalin and baicalein), baicalein, and WSR (Withered Scutellaria baicalensis ratio of baicalin and baicalein) had significantly different effects in terms of clinical symptoms and signs, body temperature, organ indices, serum inflammatory cytokine levels, blood biochemistry, and histopathology changes in the main organs; YSR exhibited the best treatment effects. LC-MS/MS was used to detect the conversion of baicalin, baicalein, or both, into the six types of metabolites: baicalin, wogonoside, oroxin A, baicalein, wogonin, and oroxylin A. The levels of the six metabolites under the different treatment conditions were significantly different in the large intestine, small intestine, and lungs, but not in the blood. The levels of the six metabolites were significantly different in the large intestine, small intestine, and lung, but not in the serum. Conclusion: All these results indicate that baicalin and baicalein should be used more accurately in specific diseases, especially baicalin or high content of baicalin in Scutellaria baicalensis (Tiaoqin) should be preferred in treatment of UC.
Keywords: baicalein and combination; baicalin; mechanisms comparison; metabolism distribution; therapeutic effects; ulcerative colitis; withered Scutellaria baicalensis; young Scutellaria baicalensis.
Currently, UC cannot be completely cured and life-long treatment is needed in most cases (Nowarski et al., 2015). Further, 20–30% of patients with severe UC require surgery as a part of their treatment (Lanzoni et al., 2008). Anti-inflammatory treatment with drugs such as antibiotics, aminosalicylic acid, and glucocorticoids, as well as immunosuppression treatment via hormone therapy, are the primary treatment approaches. However, adverse side-effects and limited efficacy have been reported (Ishiguro et al., 2006). There is also doubt as to whether hormones should be used continuously in the chronic phase of UC (Caprilli et al., 2007). Thus, more rational treatment of this disease is needed.
It is believed that abnormality of the intestinal mucosal immune system plays an important role in the pathogenesis of UC. Diffuse inflammation of the intestinal mucosa and crypt abscesses are the typical symptoms observed during the active period of UC. When crypt abscesses collapse, the mucosa appears presents with a large number of small ulcers that gradually merge into large ulcers. These large ulcers continue to destroy the intestinal mucosa causing tissue damage, and can even result in the formation of cancerous lesions (Xavier and Podolsky, 2007). The use of anti-inflammatory and immunosuppressive drugs for treatment of UC results in a cycle of remission and recurrence (Sha et al., 2013).
Th17 cells are a subset of Th cells that produce interleukin (IL)-17. These cells are involved in the development and progression of many inflammatory and autoimmune diseases (Machino-Ohtsuka et al., 2014). Recently, scientists have discovered the proliferation of Th17 T helper cells (Th17) in UC, and this appears to be of great significance to autoimmune diseases and the body’s defense response (Kuwabara et al., 2017)
Given the difficulty in treating UC, it is important to look for alternative therapeutic strategies. Traditional Chinese Medicines (TCMs) such as Scutellaria baicalensis have been widely used to treat UC. These medicines have multi-component and multi-target characteristics. In recent years, a large number of clinical and experimental studies have shown that TCM can regulate the Th17/Treg balance, which is an important mechanism in the treatment of UC (Fan et al., 2009).
As shown in Figures 6A, B, there was severe tissue damage in the colon and small intestine of the UC model rats. Based on all indices of intestinal change, baicalin was superior to baicalein on the colon, and YSR was superior to WSR on the small intestine, indicating that baicalin is a more potent regular of colon damage.

Effect of Baicalin and Baicalein on the Nuclear Factor κB and MAPK Pathway
Activation of the nuclear factor-κB (NF-κB) and MAPK pathways increases the production of inflammatory cytokines and chemokines (Figure 6D). Compared with the NC group, NF-κB (STAT3) and MAPK (P38) protein expression in the UC group was significantly increased. After drug treatment, the expression levels of NF-κB and MAPK proteins were significantly decreased compared to those in the UC model group. Baicalin was found to exert a superior effect to baicalein, and YSR was superior to WSR, indicating that baicalin is a more potent regulator of inflammatory pathways.
We found that both baicalin and baicalein were converted into four new metabolites: wogonin, wogonoside, oroxylin A, and oroxin A. The concentrations of these metabolites were significantly different in the lungs, small intestine, and colon. All four metabolites were found to have anti-inflammatory activity. The content differences of the six metabolites may work together to produce differences in efficacy. This may explain why baicalin is effective for treating colon diseases while baicalein is effective for treating lung diseases. Furthermore, this may be the basis for the effects of Tiaoqin and Kuqin on the large intestine and lungs. The above results indicate that baicalin and baicalein have a differing distribution tendency, which is consistent with the traditional ideas on the use of young Scutellaria baicalensis (Tiaoqin in Chinese) and withered Scutellaria baicalensis (Kuqin in Chinese) in TCM.
In result, baicalin, baicalein, and the two drugs in combination, have significantly different in the anti-inflammatory, liver protection, lipid-lowering, anti-oxidation, and immunomodulation effects. Furthermore, there are significant differences in the drug-induced expression of the NF-κB and MAPK signaling pathways. These differences may cause the differences in the distribution of metabolites.
The ratio of baicalin to baicalein that was most efficacious was also consistent with the ratio found in extracts prepared from young S. baicalensis plants, which is somewhat fortuitous:
Conclusion
Scutellaria baicalensis is a herbal medicine traditionally used for the treatment of UC. The main components of Scutellaria baicalensis are baicalin and baicalein. In this study, we tested each component separately and in combination for the treatment of UC. Baicalin, baicalein, and a combination of the two drugs, had significantly different effects on UC. We found that baicalin and baicalein have different advantages in treating UC, and a combination of the two drugs provides more comprehensive treatment; specifically, YSR was the most effective treatment. The proportion of baicalin in YSR is higher than the proportion of baicalein, consistent with the natural proportion of baicalin and baicalein in young Scutellaria baicalensis. This study compared the mechanisms of action of baicalin and baicalein based on pharmacodynamic indicators and evaluation of chemical composition using metabolomics techniques, UPLC-MS/MS, and traditional pharmacological indicators. It was found that compared with baicalein, baicalin was more potent for the treatment of large intestine disease. The mechanism underlying its effects may be related to the slower absorption of its metabolites in the large intensity as compared to baicalein. This increased retention time may aid in regulating UC. These results indicate that baicalin and baicalein should be used to target more specific diseases and may prove useful as new alternatives or supplements for clinical drug development.
Funding
This work was supported by National Natural Science Foundation of China grants (No. 81773893, No. 31600272 and No.81503203), National Major Scientific and Technological Special Project for “Significant New Drugs Development” (No. 2017ZX09301060-001), National Key R&D Program of China (No.2017YFC171004), Hubei Province Natural Science Foundation of China (No. 2015CFB302), and Fundamental Research Funds for the Central Universities “South-Central University for Nationalities” (No. CZP17074).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Treatment of ME/Chronic Fatigue Syndrome (CFS)
Various sufferers of the syndrome have reported increased energy levels after taking extracts of S. baicalensis, which was attributed to Baicalin:
“Rationale: I started taking Chinese skullcap on the basis of the recent article that showed that people with ME only have a problem with the fifth step in the energy chain, and discovered that ALA only affects the downstream ones, while Chinese skullcap exerts its effect on the fifth. The analogy I came up with for this is that ALA in ME is like putting higher-grade fuel in a faulty engine: it will increase the performance but the engine is still inefficient; while Chinese skullcap basically corrects the problem with the engine itself.”
“I have taken skullcap for over a month so far, and this has pretty much made me functionally normal, which has always been my goal in terms of pursuing treatment; I like to think that I am the normal person version of Henry Slade, the diabetic in the England rugby team who is able to play one of the most physical sports at an elite level, as long as he takes his insulin before the game. As a 35-year old with ME though, I am just happy if I can stay in shape and enjoy exercise again.”
“Baicalin (a chemical in Chinese skullcap) restored the infection-triggered decrease in ATPase (the enzyme for Complex V, or the fifth step in the energy chain), thereby restoring normal energy metabolism.”86
As referred to above, in 2019 Ishfaq et al investigated the antagonistic effects of baicalin on infected chicken lungs by the restoration of energy metabolism and found a significant improvement.87
“Mycoplasma gallisepticum (MG) is a bacterium belonging to the class Mollicutes and the family Mycoplasmataceae. It is the causative agent of chronic respiratory disease (CRD) in chickens and infectious sinusitis in turkeys, chickens, game birds, pigeons, and passerine birds of all ages."88
Abstract
Background
Baicalin possesses potential anti-inflammatory, anti-tumor and anti-oxidant activities. In the present study, we attempted to investigate the preventive effects of baicalin against Mycoplasma gallisepticum (MG)-induced inflammation, apoptosis and energy metabolism dysfunction in chicken lungs.
Methods
Experimental chickens were randomly divided into 1) control group, 2) MG infection group, 3) MG-infected group treated with baicalin at a dose of 450 mg/kg and 4) baicalin alone treated group (450 mg/kg). After 7 days of post-treatment, serum and lung tissues were collected for different experimental analyses. The hallmarks of inflammation, apoptosis and energy metabolism dysfunction were detected by histological and ultrastructural examination, qRT-PCR, Western blotting and terminal deoxynucleotidyl transferase-mediated dUTP nick endlabeling (TUNEL) assay.
Results
The level of serum inflammatory markers were increased with MG infection. Histological and ultrastructural analysis showed excessive inflammatory cells infiltrates, alveolar wall thickening, hemorrhages, mitochondrial and nuclear damage, including mitochondrial swelling and condensation of DNA in the lungs of chickens infected with MG. TUNEL assay positive-stained nuclei were significantly increased in MG infection group. In addition, the mRNA and protein expression level of energy metabolism-related genes and ATPase activities were significantly reduced. Meanwhile, MG-induced morphological and ultrastructural changes were partially disappeared with baicalin-treatment, and the level of serum inflammatory markers were significantly reduced. It has been noted that baicalin significantly attenuated MG-induced inflammation and apoptosis in the chicken lungs through the suppression of nuclear factor-kappa B and reduced extensive positive-stained apoptotic nuclei. More importantly, ATPase activities and mRNA and protein expression level of energy metabolism-related genes were significantly improved with baicalin-treatment in the lungs of chickens infected with MG.
Conclusion
Conclusively, it has been suggested from these results that baicalin-treatment efficiently prevented MG-induced inflammation, apoptosis and energy metabolism dysfunction in the chicken lungs and provide basis for new therapeutic targets to control MG infection.
Keywords: baicalin, inflammation, Mycoplasma gallisepticum, lungs, energy metabolism
Baicalin (purity ≥98.0%, Huifeng Animal Health Co., Ltd. Heilongjiang, People’s Republic of China) was dissolved in distilled water (0.5 mL) and given orally, once a day after 3 days of post-infection.
Higher is better:

Higher is better:

Conclusion
In conclusion, MG infection induced inflammation and apoptosis in chicken lungs. ATPase activities and the expression of energy metabolism-related genes were significantly reduced in the lungs of MG-infected chickens, while baicalin-treatment significantly ameliorated MG-induced inflammation, apoptosis and energy metabolism dysfunction in chicken lungs. Nevertheless, higher studies are needed to scrutinize the inhibitory mechanisms of baicalin and the complex relationship among energy metabolism dysfunction, inflammatory responses and apoptosis during MG infection.
Disclosure
All the authors have no potential conflicts of interests regarding the publication of this manuscript.
Treatment of autoimmune disorders, including MS
Zhang et al (2015) found that baicalin has significant potential as an anti-inflammatory agent for the treatment of autoimmune diseases such as multiple sclerosis (MS) by preventing Th1 and Th17 cell differentiation via the STAT/NFκB signalling pathway:89
Abstract
Natural compounds derived from medicinal plants have long been considered a rich source of novel therapeutic agents. Baicalin (Ba) is a bioactive flavonoid compound derived from the root of Scutellaria baicalensis, an herb widely used in traditional medicine for the treatment of various inflammatory diseases. In this study, we investigate the effects and mechanism of action of Ba in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS). Ba treatment effectively ameliorated clinical disease severity in myelin oligodendrocyte glycoprotein (MOG)35–55 peptide-induced EAE, and reduced inflammation and demyelination of the central nervous system (CNS). Ba reduced infiltration of immune cells into the CNS, inhibited expression of proinflammatory molecules and chemokines, and prevented Th1 and Th17 cell differentiation via STAT/NFκB signaling pathways. Further, we showed that SOCS3 induction is essential to the effects of Ba, given that the inhibitory effect of Ba on pathogenic Th17 responses was largely abolished when SOCS3 signaling was knocked down. Taken together, our findings demonstrate that Ba has significant potential as a novel anti-inflammatory agent for therapy of autoimmune diseases such as MS.
C57BL/6 mice were injected i.p. with PBS (●) or Ba (100 mg/kg, ○) daily (a) at the day of EAE induction, (b) day 10 p.i. (disease onset), (c) day 15 p.i. (disease peak), or (d) during the indicated time points. Results are shown as mean ± SEM (n = 5 each group). (e) Disease distribution at the end points of experiment, and (f) incidence of disease severity at the end points of experiment when treatment started at day 10 p.i. Disease severity is graded as severe (clinical score: > 3), moderate (clinical score: 1.5–3), mild (clinical score: <1.5) or none (no clinical signs). One representative of three independent experiments is shown in a, b, and c. The data in d, e, and f came from three independent experiments when Ba was injected from day 10 p.i. (n = 5–6 each group). **P < 0.01; ***P < 0.001.
Ba- or PBS-treated control EAE mice described in Fig. 1c (treatment protocol) were sacrificed at day 30 p.i., and spinal cords were harvested. Sections at lumbar level (L3) were analyzed (a) by H&E (for inflammation; left) and Luxol fast blue (LFB) (for degree of demyelination; right), and (b) pathology scores of inflammation and demyelination are expressed as mean ± SD (n = 6 each group). The absolute numbers of MNCs in the CNS of above mice were counted (n = 3 each group) (c), and expression of cytokine (d) and chemokine (e) genes was determined using real-time RT-PCR analysis, and their relative expression was calculated by log2 of −ΔΔCt values from triplicate of PCR. More than two fold changes (log2 < −1 or log2 > 1) were considered significant between groups (red dotted line). (f) The percentage of CD4+ or CD8+ in the lymphocyte gate of the CNS of the above mice was analyzed by flow cytometry. (g) Absolute numbers of CD4+ or CD8+ cells in spinal cord were calculated (n = 5 each group). (h) Splenocytes of Ba- or PBS-treated EAE mice were isolated, stimulated with MOG35–55 (25 μg/ml) or Con A (5 μg/ml) and examined for proliferation at 72 h culture using BrdU incorporation assay (n = 6 each group). Data are expressed as mean ± SEM. **P < 0.01; ***P < 0.001. One representative of three experiments is shown.
In the present study we demonstrate that Ba can regulate differentiation and activity of Th17 and Th1 cells without affecting Th2 and regulatory T cells. This was first demonstrated by diminished Th1/Th17 response in EAE mice treated with Ba and further substantiated by adoptive-transfer experiments showing the loss of encephalitogenicity in Ba-treated, MOG-specific Th17 cells. The STAT signaling pathway is known to be a major signaling network involved in Th cell differentiation. We found that Ba selectively acts on pathogenic Th1 and Th17 cells, while sparing Th2 and Treg cells. Different Th lineages rely on distinct signaling pathway(s) for their development, providing a mechanistic basis for differential effects of Ba on various Th lineages. In this way, Ba inhibited STAT3 phosphorylation and RORγt expression in differentiating Th17 cells and reduced STAT4 and STAT1 phosphorylation and T-bet expression during Th1 differentiation. To our knowledge, this is the first demonstration that Ba regulates T cell differentiation and function through the STAT pathway.
It is known that pathogenesis of autoimmune diseases involves the breakdown of multiple regulatory pathways. Thus, anti-inflammatory drugs obtained from a single, target-based drug discovery process may be unlikely to achieve adequate efficacy in the treatment of autoimmune conditions. In comparison, multiple compounds from traditional herbal medicine that target distinct immune/inflammation pathways may be more successful at reining in the immune system. The regulatory effects of Ba on the immune response include suppression of DC maturation, inhibition of Th1/Th17 cell development and proliferation, and re-ordering the cytokine microenvironment. The powerful therapeutic effects of natural compounds have motivated search for new drugs. For example, fingolimod (FTY720), the first oral treatment for MS, which was approved in 2010, was discovered by chemical modification of a natural product, myriocin, which is isolated from Cordyceps sinensis, a fungus used in Chinese traditional medicine. Likewise, the novel anti-inflammatory properties of Ba that we discovered through mechanistic studies in EAE show its potential for the development of pathway-based immunomodulatory therapeutics.
Acknowledgments
This study was supported by the NIH and the Groff Foundation. Y.Z. and X.L. are partly supported by the Chinese National Natural Science Foundation (grant no. 31300256) and the Overseas Scholarship Program of Shaanxi Normal University. We thank Katherine Regan for editorial assistance.
Building on previous research, in 2018 another team of Chinese researchers, Xu et al, used dextran sodium sulfate (DSS) to induce colitis in mice and found that baicalein and baicalin downregulated STAT4 transcription in colon epithelial cells, thus exhibiting therapeutic effects on autoimmune diseases by regulating cell proliferation.90
The JAK-STAT (Janus kinase/signal transducers and activators of transcription) signalling pathway visualised:91

Phosphorylation is a particularly common mechanism for regulating enzyme activity; the addition of phosphate groups either stimulates or inhibits the activities of many different enzymes (Figure 2.30):92
Abstract
A series of natural compounds have been implicated to be useful in regulating the pathogenesis of various autoimmune diseases. The present study demonstrated that the Scutellariae radix compounds baicalein and baicalin may serve as drugs for the treatment of autoimmune diseases, including rheumatoid arthritis and inflammatory bowel disease. Following the administration of baicalein and baicalin in vivo, T cell‑mediated autoimmune diseases in the mouse model were profoundly ameliorated: In the collagen‑induced arthritis model (CIA), the severity of the disease was reduced by baicalein and, consistently, baicalein was demonstrated to suppress T cell proliferation in CIA mice. In the dextran sodium sulfate (DSS)‑induced colitis model, the disease was attenuated by baicalin, and baicalin promoted colon epithelial cell (CEC) proliferation in vitro. The present study further revealed that the mRNA expression of signal transducer and activator of transcription (STAT)3 and STAT4 in the tyrosine‑protein kinase JAK‑STAT signaling pathway in T cells was downregulated by baicalein, contributing to its regulation of T cell proliferation. However, in the DSS model, the STAT4 transcription in CECs, which are the target cells of activated T cells in the gut, was downregulated by baicalin, suggesting that baicalein and baicalin mediated similar STAT expression in different cell types in autoimmune diseases. In conclusion, the similarly structured compounds baicalein and baicalin selectively exhibited therapeutic effects on autoimmune diseases by regulating cell proliferation and STAT gene expression, albeit in different cell types.


Discussion
The results of the present study demonstrated that the components of Scutellariae radix (termed Huang-Qin in Chinese), baicalein and baicalin, regulate T cell and epithelial cell function to exert a therapeutic effect on autoimmune disease. The distinct biological activities of baicalein and baicalin were assessed in diverse autoimmune diseases. Baicalein ameliorated the severity of disease in the CIA model, while baicalin attenuated DSS-induced colitis; the in vitro study demonstrated that the two compounds mediated cell activation via the JAK-STAT pathway and cell proliferation. It has been reported that baicalein and baicalin exert multiple physiological activities. Baicalin ameliorates camptothecin-induced intestinal toxicity in rats. In vitro, baicalin exhibited a protective role in renal cell injury. It has additionally been reported that baicalein and baicalin are potent inhibitors of reverse transcriptase, and that they suppress the human T-cell leukemia virus and promote the apoptosis of human immunodeficiency virus-infected CEM cells. Furthermore, the antitumor effects of baicalein and baicalin on human hepatoma cell lines have been reported.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81401230).
Competing interests
The authors declare that they have no competing interests.
Moving on to 2021, and Wang et al reported on how baicalin protects the blood-brain barrier from injury mediated by lipopolysaccharide (LPS) using a mouse model.93
In this study, we revealed that baicalin can protect BBB under LPS attack. This process may be mediated by inhibiting the ROS production of the Nrf2 antioxidant pathway and inhibiting the inflammatory response of BBB endothelial cells. These findings indicate that baicalin has a good protective effect on brain diseases caused by LPS and provides a choice for clinical medication.
Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the NFE2L2 gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research.94
Abstract
The integrity of the BBB is closely related to brain microvascular endothelial cells and TJs, and its dysfunction can lead to stroke, multiple sclerosis, extracranial injury and neurodegenerative diseases. Baicalin is one of the main bioactive extracts from Scutellaria Baicalensis Georgi, which has anti-inflammatory and anti-oxidation pharmacological functions. Preventive protection with baicalin for seven consecutive days can significantly improve the appearance of cell apoptosis and Fluorescein sodium infiltration in the brain tissue of BALB/C mice. In addition, baicalin can inhibit the production of pro-inflammatory cytokines induced by LPS in mice and bEnd.3 cells, including IL-1β and TNF-α. At the same time, LPS caused a decrease in tight junction proteins in the blood-brain barrier, but baicalin can alleviate the damage of the blood-brain barrier by up-regulating Claudin-5 and ZO-1 protein expression. In addition, the results showed that baicalin reduced the production of ROS and MDA in bEnd.3 cells and promoted the production of SOD, and up-regulated the expression of Nrf2, HO-1 and NQO1. The mechanism of this change was mediated by activating the Nrf2 signaling pathway. All in all, Baicalin protected LPS-induced blood-brain barrier damage and activated Nrf2-mediated antioxidant stress pathway.
Keywords: Baicalin; Blood-brain barrier; Nrf2; Oxidative stress; Tight junction.
As the most selective biological barrier in mammals, the blood–brain barrier (BBB) helps to establish and maintain the central nervous system (CNS) microenvironment. It plays a key role in reflecting brain cognition, regulating metabolism and coordinating the function of surrounding organs. Therefore, when the BBB is dysfunctional, it can lead to neuronal dysfunction and degeneration, which can induce neurological diseases, including stroke, multiple sclerosis (MS), head trauma and neurodegenerative diseases. The basic components of BBB are cerebral vascular endothelial cells, astrocytes and pericytes. They are connected by tight junction proteins, so tight junction proteins play an important role. Tight junction proteins are formed mainly by claudins and occludins, and these proteins are connected to the cytoskeleton by members of the ZO family. In the blood–brain barrier, Claudin-5 is considered a dominant tight junction protein (TJ), and the contribution of Occludin is small. Relatedly, ZO-1 acts as a bridge between transmembrane proteins and skeletal proteins, so when ZO-1 dissociates from the complex, it will cause the permeability of the blood–brain barrier to increase.
Lower is better:

Superoxide dismutase (SOD) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O−2) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H2O2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen.95

According to reports, damage to the blood–brain barrier and changes in permeability occurred in subarachnoid brain injury and TBI. However, baicalin can effectively protect the blood–brain barrier through its anti-inflammatory and anti-oxidant signaling pathway Nrf2. This implied that baicalin activated the Nrf2 signaling pathway to regulate oxidative stress to protect the blood–brain barrier from damage by LPS.
In conclusion, our results demonstrated that baicalin might attenuate LPS-induced blood–brain barrier damage by inhibiting inflammation and oxidative stress, which may be related to the Nrf2 signaling pathway.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Of particular interest for the treatment of demyelinating diseases like MS or peripheral neuropathy, in 2022 Ai et al found that, using a neonatal mouse model, baicalin promotes myelin production and regeneration by activating the PPARγ signal pathway and also confirmed that BA is an effective natural product for the treatment of demyelinating diseases.96
In the field of molecular biology, the peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins that function as transcription factors regulating the expression of genes. PPARs play essential roles in the regulation of cellular differentiation, development, and metabolism (carbohydrate, lipid, protein), and tumorigenesis of higher organisms.97
Abstract
Background and objectives: Demyelinating diseases in the CNS are characterized by myelin sheath destruction or formation disorder that leads to severe neurologic dysfunction. Remission of such diseases is largely dependent on the differentiation of oligodendrocytes precursor cells (OPCs) into mature myelin-forming OLGs at the demyelinated lesions, which is defined as remyelination. We discover that baicalin (BA), a natural flavonoid, in addition to its well-known antiinflammatory effects, directly stimulates OLG maturation and CNS myelin repair.
Methods: To investigate the function of BA on CNS remyelination, we develop the complementary in vivo and in vitro models, including physiologic neonatal mouse CNS myelinogenesis model, pathologic cuprizone-induced (CPZ-induced) toxic demyelination model, and postnatal OLG maturation assay. Furthermore, molecular docking, pharmacologic regulation, and transgenic heterozygous mice were used to clarify the target and action of the mechanism of BA on myelin repair promotion.
Results: Administration of BA was not only merely effectively enhanced CNS myelinogenesis during postnatal development but also promoted remyelination and reversed the coordination movement disorder in the CPZ-induced toxic demyelination model. Of note, myelin-promoting effects of BA on myelination or regeneration is peroxisome proliferator-activated receptor γ (PPARγ) signaling-dependent.
Discussion: Our work demonstrated that BA promotes myelin production and regeneration by activating the PPARγ signal pathway and also confirmed that BA is an effective natural product for the treatment of demyelinating diseases.
Our previous study demonstrated that oral BA effectively ameliorated clinical disease severity of experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and reduced the inflammation and demyelination of the CNS, indicating its potential application on demyelinating disease. However, the efficacy of BA for inhibiting demyelination might be the secondary effects of its well-known anti-inflammatory activity. Whether BA functions on OPC differentiation and remyelination directly is hardly known.
The stock solution of BA (Sigma-Aldrich, St. Louis, MO) was prepared in DMSO (1 mg/mL). BA treatment was applied by consecutive 12 daily subcutaneous injections, 100 mg/kg/d according to our previous optimal results, starting at postnatal days 3–14. Littermate mice were treated by phosphate-buffered saline (PBS) as sham control.
BA Enhances CNS Myelinogenesis During Postnatal Development
Previous studies have demonstrated that the intensive myelination of C57BL/6 mice occurs postnatally during the first 3 weeks of their life, when the myelin sheath develops significantly. To understand whether BA influenced myelination during postnatal development, we first detected the expression of specific proteins at different stages of OLG maturation and then investigated its effect on the OPC differentiation in the CNS (Figure 1A).
(A) Treatment paradigms. (B) Representative sections stained for Olig2, NG2, APC, and DAPI of spinal cord segment harvested on postnatal day 14 (Scale bar = 50 μm). Quantifications were performed by counting the Olig2, NG2, and APC positive nucleated cells at random areas. (C) Ventral roots of peripheral motor nerve at the level of the lumbar spinal cord segment and corpus callosum harvested on postnatal day 14 were stained for myelin with antibodies to MBP and for axonal fibers with antibodies for neurofilament H (Scale bar, 50 μm). MBP intensity was measured in the ventral roots of spinal cord or corpus callosum using Image-Pro. (D.a-c) D.a: Beam walking test was performed on postnatal day 18, and the latency to traverse each beam and the time the hind feet slip off each beam are recorded for each trial. D.b: The wire hang test was performed on postnatal day 19. Mice received 2 consecutive trials, and the latency to traverse the beam was recorded for each trial (cutoff time 60 seconds). D.c: The rotarod test was performed on postnatal day 21, and the latency to fall off the rod and the actual rotating speed level was measured. The average latency of falling off the rod and the average actual rotating speed was recorded. Data of (B, C, D) are shown as mean values ±SEM and analyzed by 2-tailed Student t-test (n = 5 each group). *p < 0.05, **p < 0.01, and ***p < 0.001. BA = baicalin.
BA Enhances Remyelination in CPZ-Induced Demyelination
To further investigate the direct effects of BA on remyelination in vivo, we used the toxic CPZ-induced demyelination model. This model is widely accepted for studying the effects of drugs on demyelination and remyelination processes in the CNS. In contrast to the immune-induced demyelination animal model-like EAE, the blood–brain barrier of CPZ-induced mice occurs intact, which allows us to analyze the pathomechanisms of remyelination bypassing possible interference of peripheral immune cells.
Mice were fed with CPZ-containing chow for 6 weeks to achieve complete demyelination in the corpus callosum. CPZ was then withdrawn, and mice were fed with normal chow, allowing for spontaneous remyelination to take place within the next 2 weeks. Mice were administrated with either BA (100 mg/kg/d) or PBS along with CPZ-containing chow beginning at 0 weeks and continuing treatment until 6 + 3 weeks (Figure 2A). Remarkably, BA treatment enhanced remyelination as evaluated by LFB and FluoroMyelin staining (Figure 2B, p < 0.05). During 3–6 weeks, CPZ-induced demyelination in the corpus callosum of mice was not altered under BA treatment. However, in 6 + 1 week and 6 + 2 weeks, BA promoted remyelination remarkably compared with the PBS-treated control. As shown in LFB staining test (Figure 2B), BA-treated mice showed a certain recovery of demyelination and a significantly larger remyelination area than that of PBS-treated control at 6 + 1 week and 6 + 2 weeks. In agreement with these findings, the postcuprizone recovery of mature myelin in the body of the corpus callosum of BA-treated group, but not in PBS-treated control, was accelerated by BA supplementation.
(A) A schematic drawing of demyelination/remyelination mice model. Male, 8- to 10-wk-old C57BL/6 mice were fed with cuprizone for 6 weeks to achieve complete demyelination, followed by feeding normal chow in another 2 weeks. Mice were randomly divided into normal chow + PBS groups (NC, n = 6 and PBS-treated groups (CPZ + PBS, n = 12) and BA-treated (CPZ + BA, n = 12), all groups receive BA or PBS treatment starting at CPZ model beginning until the 6 + 3 weeks. A schematic diagram of corpus callosum lateral to lateral ventricle and location observed by paraffin section staining (LFB). (B) LFB and FluoroMyelin staining at different time point (Scale bar, 200 μm). Slices were assessed and scored in a blinded fashion for demyelination: 3, large (confluent); 2, a few areas of demyelination; 1, rare foci; 0, none. The quantification of the mean density of FluoroMyelin staining was performed using ImageJ. (C) Representative electron micrographs at 6 + 2 weeks (Scale bar, 2 μm for the upper row, and 1 μm for the insets in the lower row, rad arrows indicate typical myelin structure in each group). (D) The quantification of myelin G-ratio value (axon diameter/fiber diameter) of NC, CPZ + PBS, and CPZ + BA group. Scatter plot of G-ratio value. Data are shown as mean values ±SEM (n = 6 each group). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control group, one-way analysis of variance with Tukey multiple comparisons test. BA = baicalin; NC = naïve chow; PBS = phosphate-buffered saline.
BA Directly Induced OPC Maturation via PPARγ Signaling
Although our data indicated that BA promoted OLGs differentiation and enhanced remyelination in CPZ-induced demyelination model, there is still a lack of in-depth studies on its target and mechanism of action. Thus, we obtained the molecular docking map of BA into the PPARγ active pocket, which showed that BA could form hydrogen bond interactions with the protein structure of PPARγ at multiple positions (Figure 4A). Subsequently, we performed MST affinity assay experiments, and the results showed that the dissociation constant of BA and PPARγ was 1.35 μM, indicating that the 2 moleculars had affinity cooperation, which was consistent with the results of molecular docking (Figure 4B).
OPCs: oligodendrocyte precursor cells.
OLGs: myelin forming oligodendrocytes.
(A) Three-dimensional structure diagram of PPARγ docked with BA. (B) MST affinity determination of BA and PPARγ. (C) HEK293T cells were transfected with PPRE×3-TK-luciferase vector, a PPRE-dependent luciferase reporter constructs. After 24 hours of transfection, cells were cultured with BA of 10 μg/mL for 6 hours, and the activity of luciferase was monitored in cell lysates by a ONE-Glo EX Luciferase Assay System kit (Promega). (D) BA enhanced oligodendrocyte differentiation in primary OPC cultures. Primary OPCs, prepared from brains of newborn C57BL/6 mice, were cultured in the differentiation medium with or without BA (1, 5, or 10 μg/mL) for 5 days, followed by MBP immunofluorescence staining. Primary OPCs were cultured in a differentiation medium with BA (10 μg/mL) alone or pretreated for 30 minutes with GW-9662 (PPARγ-specific antagonist) before addition of BA or GW-9662 (10 nM) alone for 5 days, followed by MBP immunofluorescence staining. Nuclei were stained with DAPI (blue). Scale bar, 50 μm. A quantitative analysis was performed for numbers of MBP+ mature oligodendrocytes. Data are shown as mean values ± SEM and analyzed by two-tailed Student t-test (n = 5 each group). *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the control group. BA = baicalin; MBP = myelin basic protein; OPC = oligodendrocyte precursor cell; PPARγ = peroxisome proliferator-activated receptor γ; PPRE = peroxisome proliferator element.
Discussion
…Scutellaria baicalensis, of which BA is the major active compound, has been used for the treatment of a variety of acute and chronic hepatitis and inflammatory disease. BA has been shown to improve memory impairment in Alzheimer disease models and promote the proliferation and differentiation of rat neural stem cells. Our previous study demonstrated that BA effectively ameliorates the severity of clinical disease of EAE, an animal model of MS. In this study, we demonstrated that BA-induced OPC differentiation in vitro and promoted CNS remyelination, alleviated astrocyte activation, reduced inflammatory cytokine production, and improved motor dysfunction at effective doses in a noninflammatory demyelination model through the PPARγ signaling pathway. Given that chronic inflammation and white matter damage in the CNS are 2 hallmarks of demyelination diseases, an ideal therapeutic drug has both anti-inflammatory and myelin repair capacities and would be highly beneficial. As a bioactive flavone that is isolated from S. baicalensis and officially listed in the Chinese Pharmacopoeia, BA has low toxicity and is well tolerated when administered orally in both humans and rodents. In this study, BA dose (100 mg/kg/d) that proved effective for demyelinating disease treatment is approx. 59 times lower than that of its reported acute toxicity (LD50 = 6 g/kg/d). Of note, pharmacokinetic study had clearly demonstrated that the administration of BA could cross the blood–brain barrier and is detectable in the cortex, hippocampus, and striatum, indicating its function in the CNS disorders.
In general, our findings give an important insight into the myelin repair capacities of BA through promoting OLG maturation and remyelination in different models via the PPARγ signal pathway. These studies, together with well-known anti-inflammation activity of BA, its safety record in clinical, its low cost and easy availability traits, pave the way for clinical applications in the demyelination diseases, for which there is currently no effective therapy.
Study Funding
This study was supported by the Chinese National Natural Science Foundation (Grant No. 81771345, 82071396, 31970771), the Natural Science Foundation of Shaanxi Province, China (Grant No. 2019KJXX-022, 2021ZDLSF03-09), the Fundamental Research Funds for the Central Universities (Grant No. GK202105002, GK202007022, 2019TS080, 2020CSLZ010).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
In our final paper on autoimmunity, also from 2022, Wang et al conducted a literature review of Scutellaria baicalensis georgi as a promising candidate for the treatment of autoimmune diseases.98
Abstract
Autoimmune diseases a group of disorders elicited by unexpected outcome of lymphocytes self-tolerance failure, and the common members of which include multiple sclerosis, systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, and type 1 diabetes mellitus, etc. The pathogenesis of autoimmune diseases is not fully understood and the current therapeutic regimen’s inefficacy in certain cases coupled with low rates of success, exorbitant financial burden, as well as numerous side effects, which do open new avenues for the role of natural products as novel therapeutic agents for auto-inflammatory disorders. Scutellaria baicalensis Georgi is a well-known and widely-recognized herbal medicine with certain ameliorative effect on diverse inflammation-involved dysfunction. Though recent advances do highlight its potential to be applied in the fight against autoimmune diseases, the specific mechanism and the related opinion on the exploring possibility are still limited which hampered the further progress. Here in this timeline review, we traced and collected the evidence of how Scutellaria baicalensis Georgi and its bioactive contents, namely baicalin, baicalein, wogonoside and wogonin affect autoimmune diseases. Moreover, we also discussed the clinical implications and therapeutic potential of Scutellaria baicalensis Georgi and its bioactive contents in autoimmune diseases treatment.
Keywords: scutellaria baicalensis, autoimmune diseases, inflammatory response, inflammasome, inflammatory injury
WGI: Wogonin
BAI: Baicalin
BAE: Its aglycone, baicalein
STR: Scutellarin
SBG: Scutellaria baicalensis georgi.
One of the reasons there has been so much research into S. baicalensis and its therapeutic compounds is due to the great number of signalling pathways that they interact with, marked by the red stars:

Their review then discusses the signalling pathways involved with autoimmune disorders of the brain and spinal cord, skin and hair, endocrine glands, joints, autoimmune uveitus and the digestive system.
Key excerpts:
Brain and spinal cord
The best-known ADS which occur at brain and spinal cord include multiple sclerosis, narcolepsy and optical neuromyelitis. Multiple sclerosis is characterized as localized demyelination and constant overactivated neuroinflammation can be observed during the progress. Likewise, abnormally activated astrocytes mediate transduction of eIF-2α/ATF4/CHOP signaling and downstream elevated caspase-11 noncanonical inflammasome. LW-213, a derivative of WGI, was reported to modulate eIF-2α/ATF4/CHOP axis in human cutaneous T-cell (Yu et al., 2021), and given the fact that WGI could pass the blood brain barrier to regulate eIF-2α/ATF4/CHOP in neurons (Chen et al., 2015), it is likely to ameliorate multiple sclerosis via inhibiting caspase-11.
Similarly, cuprizone model is also an established murine model of multiple sclerosis. BAE reversed cuprizone-induced increase in Iba1-positive microglia, GFAP-positive astrocytes, as well as the gene expressions of CD11b, GFAP, TNFα, IL-1β, and iNOS to suppress neuroinflammation (Hashimoto et al., 2017; Sanadgol et al., 2017). It is also worth mentioning that scutellarin (STR) inhibited cuprizone-induced inflammatory response in neural stem cells, thereby rescuing behavioral deficits in this mouse model of multiple sclerosis, even STR is not one of the highest-content compounds from SBG (Wang et al., 2016).
Skin and hair
Skin autoimmune diseases, such as psoriasis vulgaris, alopecia areata and systemic lupus erythematosus (SLE) affects approximately 200 million people globally. BAI and ex vivo expanded Foxp3+ regulatory T cells were recently reported to be promising therapeutics strategy for SLE treatment since it was able to reduce Tfh cell differentiation, IL-21 production and mTOR activation while promote the differentiation of Foxp3+ regulatory T cell (Yang et al., 2019). On the other hand, BAE demonstrated reno-protective effect in a mouse model of lupus nephritis, which is regarded as a representative manifestation in SLE. Authors also shed the light of the underlying mechanisms, indicating the ROS production, Nrf2/heme-oxygenase (HO)-1, NLRP3 inflammasome, as well as NF-κB phosphorylation are the dominant pathways regulated by BAE (Li D. et al., 2019).
Endocrine glands
Distinct from type 2 diabetes that caused by unhealthy lifestyles, T1DM is an undesirable consequence of autoimmune destruction (Syed, 2022). WGI demonstrated certain protective effect on diabetic renal injury, the mechanism involves its modulation on BCL-2-mediated autophagy and apoptosis and the inhibition of downstream pro-inflammatory cytokine production, which is likely to be associated with its inhibitory effect on osteopontin expression through enhancing PPARα activity (Zhang Y. M. et al., 2015; Liu et al., 2022).
Also in this model, BAI repaired T1DM-induced renal injury partly through modulating Klotho promoter methylation (a well-recognized endogenous inhibitor against renal fibrosis), as well as suppressing epithelial-to-mesenchymal transition (EMT) and microRNA-124/TLR4/NF-κB axis to repair renal fibrosis (Zhang S. et al., 2020; Zhang X. T. et al., 2020).
Joints
Rheumatoid arthritis (RA) is an autoimmune disease that brings about constant chronic joint inflammation with no cure yet. In mice model of collagen-induced arthritis (CIA) which is widely used to mimic joint inflammatory symptoms in human RA (Wu et al., 2022), BAI treatment alleviated radiographic and histologic abnormalities in the hind paw joints of CIA rats, and the underlying mechanism was associated with its modulation on TLR2/MYD88/NF-κB p65 signaling as well as the blockage of IL-1β, TNF-α and IL-6 production (Wang H. Z. et al., 2014; Xu et al., 2018; Bai et al., 2020). BAI also lowered relevant proinflammatory cytokines including TNF-α, IL-1β, IL-6, MMP-2, MMP-9, NO and COX-2 secretions in e synovial fluids and tissues of CIA rats to interfere JAK/STAT signaling transduction, which attributed to its restoring of pressure pain thresholds and clinical arthritis scores (Wang G. et al., 2018). In addition to collagen II, RA animal model established by complete Freund’s adjuvant (CFA) is also well-recognized because it shares similar experimental designs, immunological and pathological characteristics with RA in human beings (Huang et al., 2020). In this model, WGI reduced arthritic score and paw thickness-mediated by CFA and decreased IL-1β, IL-6, and TNF-α production via blocking MAPK and NF-κB signaling (Huang et al., 2020). Of notes, ICAM-1 was reported to occur in RA and meanwhile IL-17 are abundantly expressed in synovial fibroblasts. Since IL-17 not only mediates the synthesis of ICAM-1 and diverse cytokines (IL-6 and TNF-α, etc), but also accelerates collagen degradation via diminishing collagen and proteo glycan synthesis while enhancing bone erosion, its inhibition was believed to be an effective way to ameliorate RA. BAI rescued ankle swelling in Rats model of RA through negatively-regulating Th17 cell population expansion in spleen, and this effect was exerted through reducing the gene expression of RORgt, a dominant transcription factor in Th17 cell differentiation (Dinda et al., 2017; Sun and Gu, 2019).
Autoimmune uveitis is a potentially blinding disease in humans. Use of animal models to study basic mechanisms of disease revealed that thymic expression of retinal antigens eliminates most autoreactive lymphocytes through central tolerance and positively selects natural regulatory T cells.99
Autoimmune uveitis
Targeting immunological inflammation has been regarded as a promising way to prevent pathogenesis of experimental autoimmune uveitis (EAU), a sight-threatening ocular inflammatory disorder. Zhu and colleagues declared BAI demonstrated protective effect on EAU due to its inhibition on intraocular inflammatory process, as well as infiltrated inflammatory cells and transcriptions of proinflammatory cytokines like IL-17A, IFN-γ and TNF-α. Moreover, it is observed that BAI promoted regulatory T cells’ amounts and frequency while restrained those of Th1 and Th17. Similar results were obtained in in-vitro study which further drew a conclusion that BAI was a promising candidate for EAU treatment since it modulated reg/Teff balance as a inducer of aryl hydrocarbon receptor (Zhu et al., 2018). Besides, the pathogenesis of EAU could also be rescued by BAE administration with an underlying mechanism of IL-17 inhibition (Li et al., 2016). Research concerning the interaction between WGI, WGS, or SBG and EAU is yet limited.
Digestive system
Comprised with ulcerative colitis and Crohn’s disease, inflammatory bowel disease (IBD) is characterized as intestinal microbiota alterations which are linked with a wide range of autoimmune conditions (Spalinger et al., 2022). A recent released review concentrated on the up-to-date studies investigating the affection of baicalin on IBD, indicating BAI can be a promising therapeutic prospect as a potential supplementary agent due to it reversed IBD by virtue of anti-inflammation and antioxidant properties, immune regulation, as well as maintenance of intestinal barrier and intestinal flora balance (Wang X. et al., 2022). Liang and colleagues found that BAI, BAE, and the combination of the two flavonoids (those extracted either young or withered SBG) blocked NF-κB and MAPK signaling to alleviate clinical symptoms and signs of IBD with different potency, they also repaired the abnormal organ indices and normalized the blood biochemistry (Liang et al., 2019). Besides, a novel agent named PF2405 which enriched with BAE, oroxylin A and WGI had outstanding preventive and therapeutic activities in trinitrobenzene sulfonic acid or dextran sulfate sodium-induce colitis mice.
Note that using a mouse model SBG extract appeared to induce rather than suppress autoimmune hepatitis, but more research is needed:
Autoimmune hepatitis (AIH) is another autoimmune disease occurring in digestive system. Abnormal apoptosis in activated lymphocytes accelerates the development of AIH. In AIH animal model established by concanavalin A, BAE was observed to reduce Bcl-2/Bax ration and meanwhile restraining caspase-9/-3 activation to further induce apoptosis in activated lymphocytes and eventually ameliorate AIH (Zhang et al., 2013). Of interest, SBG extract itself was reported to be a AIH model inducer when intraperitoneally-administrated to mice (Wang J. Y. et al., 2014). The reason for these controversial results may be related to the potential opposite effect of SBG constituents on AIH. Given the fact that reports on how AIH and constituents affect AIH remain limited, further studies concerning the effect of BAI, WGI and WGS on AIH are desperate to figure out the underlying mechanisms.
Discussion
…SBG is one of the most important and high-frequently used traditional Chinese medicine where dried roots preparation of ‘Huang-qin’, as well as bioactive constituents, particularly of those flavonoids, are applied for liver and pulmonary complaints and as alternative and complementary treatment for various of carcinoma, such as prostate, bladder, breast, and liver cancer (Bonham et al., 2005; Zhao Q. et al., 2019; Cao et al, 2020; Kong et al., 2021; Yao et al., 2021). However, no particular attention concerning how SBG affect ADS in a systematic way has been paid by far. By combing through the current reported evidence, even the impact of SBG on ADS symptoms is also broad-active like chemical-modified drugs, it has characteristic of multi-component and multi-targeting which potentiates mutual-reinforcement at both pharmacokinetic and pharmacodynamics level as displayed in Figure 2 and Table 2, and this goal is achieved through 1) superimposing potency on one certain target, 2) expanding the scope of efficacy or 3) promoting the intracellular transport of SBG flavonoids via affecting membrane transport proteins like P-gp and organic cation transporters (Wu et al., 2016). Compared with the chemical-modified agents currently used in clinic to reverse ADS, SBG oral preparation and its bioactive ingredients have advantage of relatively low toxicity of only causing symptoms such as stomach discomfort and muscle soreness, which suggest that it a promising candidate to be widely applied in clinical practices (Zhao T. et al., 2019). Indistinct diagnosis of different types of ADS has been being an enormous challenge for young clinicians. To cope with the diverse symptoms, patients may receive drug combination which is likely to lead to the accumulation of adverse reactions. As other traditional Chinese medicine, the characteristic of SBG is multi-component and multi-targeting which makes it possible to avoid toxicity superposition since no obvious adverse reaction is observed in oral preparation of SBG (Zhao Q. et al., 2019).
Although the issue of bioavailability is always being questioned for SBG’s flavonoids or even the majorities of small-molecular compounds with natural resource, SBG and its flavonoids were reported to have ameliorative effect on disorders occurred in multiple systems, thereby suggesting those flavonoids were able to reach targeted tissues via bloodstream and crossing the blood-brain barrier to perform therapeutic effect on ADS took place in diverse systems illustrated in Figure 3. The commercial interest and the increasing demand of SBG contributed to the appearance of huge gap of its development. Given the evidence provided by available scientific validation, SBG and its principal flavonoids, namely BAI, BAE, WGI and WGS, are potential candidates since they block abnormal auto-inflammatory response which occurred in a wide range of ADS including multiple sclerosis, EAU, SLE, T1DM, RA, IBD, as well as AIH. As discussed in previous chapters in this review, SBG and its principal flavonoids modulates diverse mechanistic routes to maintain their inflammatory-preventing and immune-regulatory actions. Notably, pathways of JAK/STAT/mTOR, BAX/Bcl2/caspase-3/9, TLR4/NF-κB/PPAR, IL-4/PI3K/Akt/GSK3β, TGF-β/Smad2/3, NLRP3/caspase-1/GSDMD and ROS/Nrf2/HO-1 are the key ones modulated by SBG. Nevertheless, there is still a long way before fully uncover the underlying molecular of SBG ameliorates ADS. In recent years, an increasing body of evidence ascertained canonical and noncanonical inflammasome activation pave the way for the development and exacerbation of ADS.

Slight contradiction in the text here in that they say in their discussion that in practice bioavailability issues did not stop SBG’s flavonoids from ameliorating disorders in multiple systems, yet in their conclusion they say this “needs to be addressed”:
Conclusion
To sum up, to introduce a new generation of treating regimen for ADs with fewer life-threatening side effect, it is desperate to explore novel small-molecule alternative medicine from natural resources. With advantages of multi-targeting and broadly-active with low adverse reactions and less financial burden, SBG and its flavonoids are promising candidates for the treatment of diverse ADS including multiple sclerosis, systemic lupus erythematosus, type 2 diabetes and the complications, rheumatoid arthritis, autoimmune uveitis, inflammatory bowel disease, and autoimmune hepatitis. Nevertheless, bioavailability issue need resolving and detailed information on the underlying molecular mechanism and gender difference in this process need to be addressed and supplemented before SBG and its flavonoids can be translated from bench to bedside.
Funding
This work is supported by the National Natural Science Foundation of China (82104491), the “Xinglin Scholar” Scientific Research Promotion Plan of Chengdu University of Traditional Chinese Medicine (BSH2020024), and the Postdoctoral Science Foundation of China (2021M693789).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Dosing, contraindications and administration in a clinical setting
Taken from the excellent “HERBAL ANTIVIRALS Natural Remedies for Emerging & Resistant Viral Infections” by Stephen Harrod Buhner (2013):
He rates Chinese skullcap as one of the top seven available antiviral herbs:
Exploring Scutellaria Species
The Chinese do use another skullcap species, Scutellaria barbata, which is
considered much weaker but still specific for certain conditions. There is,
however, an important distinction about the medicines made from these
various skullcap species. The most studied species, and the one consideredthe strongest, is S. baicalensis and with that species the Chinese use
the root only. (See “Part Used” and “Western Botanic Practice” for more
on all this.) With S. barbata and the various American species the aerial
parts are used. This difference, aerial part versus root, makes all the
difference in the medicinal impacts of the plants. The leaves just aren’t
as strong, especially for viral infections. After several millennia of use,
the Chinese consider all other skullcaps to be inferior to S. baicalensis in
their medicinal effects. And I assume (makes an ass of u and me) that
they must have tried the roots of the other species and found them not
as strong; nevertheless, what if they didn’t . . . ? Perhaps we in the West
should do a little experimenting with some roots of our own.Now, that being said, there are 200, or 300, or 350 species in the
genus Scutellaria . . . taxonomists are absolutely positive about those
figures. Many of the various skullcaps are used similarly as medicines
in the regions in which they grow; most of them contain the same con
stituents. However, there has been very little study on species other
than S. baicalensis. As illustration, there are over 600 journal articles on
PubMed on S. baicalensis (and its major constituents), but only 88 on
Scutellaria barbata, just 24 on Scutellaria lateriflora (the main American
species), a mere eight on S. viscidula, six on S. indica, four on S. racemosa,
three on S. galericulata, and one each on S. regeliana, S. incana, S. taiwan
ensis, and S. austrotaiwanensis. There’s even less on the others.Some studies have shown that S. baicalensis, S. barbata, S. lateriflora,
and S. racemosa have similar constituents in their roots — but then the
roots of those other species are almost never used in medicine making so there is no way to know if in practice they are as potent as the Chinese
skullcap root. (If you try roots other than those of S. baicalensis, let me
know how they work. Let’s start something here.)S. lateriflora, a.k.a blue skullcap, mad dog skullcap, Virginia skullcap,
hoodwort (along with S. galericulata, i.e., marsh or common skullcap), is
the species most commonly used in the United States. S. racemosa is
more common in South America and S. barbata is the other main species
used in traditional Chinese medicine. Constituent studies have found that
those three varieties all contain baicalin, baicalein, scutellarin, wogonin,
melatonin, and serotonin — the constituents considered to be the most
active in Chinese skullcap. Other studies, on the roots of S. viscidula and
S. amoena, found very similar constituents in them. It does seem that
most of the species contain baicalein, baicalin, and wogonin at the very
least — though the amounts in differing species have not been comparatively studied to any degree.However, one study that did examine the constituent levels in the roots
of Scutellaria planipes and compared them with S. baicalensis found that
the root constituents were very similar in nature, degree, and number,
and were equally active as antibacterials and antiallergics. Another study
(Chinese) showed that the root of Scutellaria rivularis stimulated the production of monoclonal antibodies to encephalitis virus E protein, just as
the root of S. baicalensis does.So . . . it may turn out that many of the skullcaps can be used interchangeably with S. baicalensis as medicines — but only if the roots are
used, which they generally aren’t. Again, I think the roots of various skullcap
species should be explored to find out if they can be used interchangeably
with the Chinese variety. So, if you are using the American skullcaps, try
harvesting some of the root of whatever species you are growing, and make
it into medicine and find out how it works. (Then e-mail me.)
Part Used
The root and the root only — generally only from plants older than 3
years. There is reason to believe that the increase in pharmacological
action of Chinese skullcap over the usual American species used as
medicinals is due to the difference between using the root in Chinese
practice and the leaf in American practice. As far as I know there hasbeen virtually no examination of the root as a medicinal in any of the
Western species.
There are, as well, no substantive studies on the difference between
roots and leaves of any species of skullcap as regards their chemical
compounds. The few I have seen that touch on it do show substantialdifferences between the leaf and the root and this really does need
to be explored, especially if American herbalists are insisting that
the American skullcaps are interchangeable with the Chinese. (And
some of them are.) However, as some researchers have commented,
“The results showed that the components and relative contents of the
essential oils among flowers, stem, leaves, roots, and seeds have significant differences.” This is, of course, true of nearly all plants on Earth
and one of the most basic understandings of herbal medicine.Preparation and Dosage
Medicinals prepared from this plant are a bit hard to find in the United
States, though not impossible. (I know only a few sources for Chinese
skullcap root tinctures, but hundreds for the American leaf tinctures.)TINCTURE
If making it yourself, again, use the root. After harvesting the root,
cut it into easy-to-use pieces, let it dry in a cool, shaded location, then
powder the root pieces and tincture them. The ratio should be 1:5 (one
part herb, five parts liquid), with the liquid being 50 percent alcohol,
50 percent water. Take 1/4–1/2 teaspoon 3x daily. In acute conditions,
double that. Remember: If using for CNS damage or encephalitis,
you want to flood the brain and CNS with the compounds over a long
enough time period to sharply reduce the inflammation and protect
and restore the neural structures of the brain. I think the tincture best
for this purpose.
For sleep: The plant and root are high in melatonin, so they can help
with sleep. If you are using it for that, take just before bedtime, 1/2
teaspoon of the tincture.
Fresh leaf tincture: If you want to use the aerial parts of the plant, get
or make a 1:2 tincture of the fresh leaves and stems. Tonic doses
run from 10–30 drops up to 6x daily but I have taken up to 1/2
ounce of the tincture at a time (of the American skullcaps anyway)
without side effects; there apparently are none even at high doses.POWDER
The Chinese dosages are large, as usual, generally 3–9 grams at a
time. Most of the clinical studies and trials used similar dosing. If you
are using capsules this is the dosage range you should be exploring,
divided into three equal doses every 4 hours or so. Capsules are pretty
much impossible to find but you can get the powdered herb fairly easily. I would use 1 teaspoon of the powdered root 3–6x daily.
Note: The herb reaches peak levels in the plasma and body organs
in about 1 hour and only lasts in the body for about 4 hours, so you
really do need to dose about every 3–4 hours.AS A WASH
The fresh juice of the plant can be used as an eye wash for eye infections, as can the cooled infusion or decoction of the root.Side Effects and Contraindications
Side effects from skullcap are rare, mostly gastric discomfort and
diarrhea. It should not be used during pregnancy. Caution should be
exercised if you are taking pharmaceuticals as it can increase the bioavailability of the drugs, thus increasing their impacts. It may interact additively with blood-pressure-lowering drugs. Type 1 diabetics
should exercise strong caution with the herb as it can affect insulin
and blood sugar levels.Herb/Drug and Herb/Herb interactions
Lots. Chinese skullcap is a synergist, perhaps as efficacious as licorice,
ginger, and piperine, and should probably be added to that category
of herbs. Among other things it inhibits the NorA efflux pump, which
inactivates some forms of antibiotic resistance. Like the other synergists I know of, it is also a strong antiviral, which is beginning to
stimulate speculation. Nevertheless, the herb strongly affects pharmaceuticals and herbs taken along with it.
Baicalein, one of the major compounds in S. baicalensis, is synergistic with ribavirin, albendazole, ciprofloxacin, amphotericin B.S. baicalensis is strongly inhibitive of CYP3A4, a member of the
cytochrome oxidase system. And this inhibition is dose dependent; the
more you take, the more it is inhibited. CYP3A4 is a type of enzyme,
strongly present in the liver, and is responsible for catalyzing reactions
involved in drug metabolism. Many of the pharmaceuticals that are
ingested are metabolized by the CYP3A4 system, meaning that some
portion of the drug is inactivated, usually by being altered to another
molecular form. With pharmaceuticals, the normal dosage range you
are given is adjusted to take this metabolization into account. If you
are using Chinese skullcap, then less of the pharmaceutical is going to
be metabolized. In some cases this will make the impacts of the drug
stronger, with the bioavailable dose higher. With other drugs it is the
metabolites created by CYP3A4 that are active in the body. In this
circumstance, since the herb inhibits CYP3A4, the metabolites of the
pharmaceutical you are taking will be reduced in degree and have less
effect in the body. Acetaminophen, codeine, cyclosporin, diazepam,
erythromycin, and so on are all affected in one way or another. The
herb does affect the amount of antibiotics that enter the system.To make things more complicated, one of the herb’s constituents,
oroxylin A, is a strong P-glycoprotein inhibitor. P-glycoprotein is
strongly present in the blood-brain barrier, the lining of the GI tract,
renal tubular cells, capillary endothelial cells, and the blood-testes
barrier. It reduces the amount of substances that cross over those barriers in order to protect what is on the other side. (This is why berberine is mostly confined to the GI tract.) P-glycoprotein inhibitors allow
more of a substance to cross barriers that are high in P-glycoprotein.
That means that if you are taking skullcap, any substance taken with
it will end up in higher levels in the bloodstream, thus increasing its
impacts in the system.This means that Chinese skullcap will act through two different
mechanisms to increase drug and herb uptake in the body.
Also, because cancer cells use P-glycoprotein as a form of efflux
pump in order to eject drugs designed to kill them from the cancer
cells, Chinese skullcap will increase the effectiveness of anticancer
drugs by inhibiting P-glycoprotein-mediated cellular efflux. Paclitaxel
uptake, for example, was increased over twofold when administered
with oroxylin A.
All this applies equally to herbs and supplements that you take
along with skullcap. If you are using this herb along with licorice,
which is also a potent synergist, through its own mechanisms (as is
ginger), everything else you take will be much more potent and pronounced in the body. (This is one reason why licorice and skullcap are
considered such important medicines in Chinese practice and why
they are commonly added to many herbal combinations.) Keep this
in mind.
Use to Treat
Viral infections, especially pandemic influenzas and encephalitis, respiratory infections, pneumonia, infections that affect the central nervous
system (essentially any infection that has accompanying meningitis or
encephalitis such as viral encephalitis or meningitis, mycoplasma, Lyme,
viral and bacterial CNS infections, and so forth), impaired brain function,
fevers, intermittent fevers, GI tract disorders with accompanying inflammation, diarrhea and dysentery, hepatitis, nephritis, urinary tract infections,
nervous irritability, epileptic seizures, convulsions, sleep disruptions. You
can also use Chinese skullcap as supportive therapy in cancer.Note: The root tincture of this plant is extremely specific for reducing
inflammation in the brain, reducing the cytokine cascades initiated by
viral and other microbial agents in the CNS, and alleviating CNS impacts
of those microbes. It should be used in any treatment of viral or bacterial
CNS infection.Other uses
Some cultures use the leaves as a steamed vegetable; the dried leaves
are common as a tea.
From “Herbal Remedies Handbook” by Andrew Chevallier (2007), who rates tincture above capsules above taking as a decoction from the root. This also correlates with the analysis of extracts taken with water vs ethanol:
Drug Interaction Report
5 potential interactions found for the following 16 drugs:100
bioflavonoids
ivermectin
artesunate
echinacea
NAC (acetylcysteine)
Coenzyme Q10 (ubiquinone)
methylene blue
doxycycline
milk thistle
goldenseal
scullcap
Quercetin (bioflavonoids)
turmeric
chondroitin / glucosamine
melatonin
Vitamin K2 (menaquinone)
A note on capsule or tablets derived from S. baicalensis as sources of baicalin and other flavonoids.
In an analysis of 132 root powder samples using high performance liquid chromatography (HPLC), baicalin concentrations ranged from 11.54-15.40%.101
TCM generally uses 3-9g doses per day of S. baicalensis, or around 400-1200mg baicalin, although cumulative beneficial effects have been seen in the studies at much lower doses.
If we take the lower range of 11% baicalin then the most that root extracts can be concentrated is around 1:10, and even then it is likely that some beneficial flavonoids will be removed in the manufacturing process.
Many tablets appear to contain around 500mg of baicalin. If you see a tablet or capsule with root extracts concentrated at greater than about 1:10 without stating the baicalin content then perhaps consider asking for this information or considering a different manufacturer?
A Certificate of Analysis may also be available.
One of the reasons that tinctures are recommended over tablets & capsules is that the fundamental approach is one of addition rather than subtraction of material in order to reduce the size to something that can be swallowed easily.
In tinctures, ethanol is a better solvent and preservative agent than water, as we have seen earlier. In consequence the extracts should be more broad spectrum and beneficial.
In 2013, Mbagwu et al analysed 5 commercially available tinctures to determine the concentration of drug they contained per ml:102
Tinctures of S. baicalensis
Different sample tinctures of S. baicalensis were provided by Dr Oliva Corcoran of the school of health and Biosciences of the university of east London. The samples were labelled SB1 (70%, 1:5), SB2 (45%, 1:1), SB3 (25%, 1:3) and SB4 (45%, 1:3), noting their concentration of alcohol and drug to extraction ratio.
Considering the hydroalcohol extract yield from 1 ml volume of each of the tinctures (Table 1), it was observed that SB2 sample tinctures gave the highest yield followed by SB4. The low yield of SB1 and SB3 can be attributed to the drug-to-extraction solvent ratio as both have the smallest ratio of 1:5 and 1:3 respectively.
Working with the findings of Geo et al., 2008 that only three major flavonoids (Baicalin, Biacalein and Wogonin) are present in the hydroalcohol extracts, SB1 with an extract yield of 117.9 mg will contain about 58 mg of baicalin, 47mg of baicalein and 13 mg of wogonin. These values are derivations from Gao’s assertion showing baicalin to be 4.5 times the amount of woganin and biacalein 3.7 times wogonin. Applying the same assumption, biomarker content as per the 219.6 mg extract yield for SB2 gives approximately 8 mg of baicalin, 206 mg of baicalein, and 5 mg of woganin. This means that baicalein in SB2 is about 26 times the proportion of baicalin and 41 times that of wogonin. Considering the 98.5 mg extract yield from SB3 sample tincture, the relative amount of the biomarkers will be approximately 91mg foe baicalin, 6 mg for baicalein and 2 mg for wogonin. This is derived from the proportionality ratio of 40.5:2.5:1 for biomarkers baicalin, baicalein and wogonin respectively. The same assumption could not be applied for the SB4 sample tincture as their relatively high extract yield could not be reconciled with their documented low total flavonoids biomarker content, suggesting the contributions of other solutes aside flavonoids in them. It is therefore most likely that baicalin and baicalein contributes equally to the observed effect seen with the SB2 tincture while for SB3 is baicalin. However, no single biomarker can be solely implicated for any effect observed with the SB4 tinctures.
1ml of a 1:1 tincture would contain 1000mg of the herb, 1ml of 1:3 tincture: 330mg.
The recommended daily dose is 7.5-10ml in total or around 750-2200mg daily standardized to flavonoids, baicalin, or baicalein, based on the above analysis.
The Functional Medicine Approach to COVID-19: Additional Research on Nutraceuticals and Botanicals recommends 750 -1500mg daily:103
What does skullcap tea taste like?
Although skullcap is a member of the mint family, it does not have a minty taste. Skullcap has a bitter, earthy taste. Many people use sweeteners with it to make it more palatable.104
To conduct my own taste test I obtained a bottle of 45%, 1:3 tincture and added 4ml to about 100ml of cold water.
In comparison to Artemisia annua the scent is less sweet, more akin to Echinacea but with earthier notes. Even at this dilution some of the bitterness comes out, but no hint of menthol. It’s not unpleasant and doesn’t linger on the palate but you may wish to take with something like lemon balm to counter the bitterness.
Administration in a clinical setting
Dr Johanna Dienert has been administering baicalin in combination as follows for some time (examples only, the following were case specific and are not medical advice):
Azi = Azithromycin (an antibiotic with antiviral properties)
Baic = Baicalin
Querc = Quercetin (a plant flavonol from the flavonoid group of polyphenols)
HCQ = Hydroxychloroquine
NAC = N-acetylcysteine (a powerful antioxidant)
Ambroxol = a drug that breaks up phlegm, used in the treatment of respiratory diseases associated with viscid or excessive mucus.
ASS = Acetylsalicylic acid (“aspirin”)
For your relief. I start with Azi, when I see a pulmonary superinfection combined with prolonged sinusitis symptoms. Mycoplasma is often hiding in chronic sinusitis and when immunosuppressed causing downstairs infection as well. Never started that earlier than 14 days into the infection. Prior to that its Baic/Querc/Zinc or Baic/HCQ/Zinc with NAC & Ambroxol, a bit of ASS, lots of Antioxidants via Tea (cistus, green), in Fevers Kefir for IL-6 inhibition and in neurological progression even nicotine gums.
I've done the first combing throught my data: 49 cases of Baicalin treatment (5×Early, 23×late Therapy) in akute, post-covid (14) and post-vac (9). 2 cases of Hepatitis are most interesting for me... one of them recovered under my treatment, the other was psychiatizized and noone saw the Transaminases rise (ANA positive). 7 Neurology cases with persistent symptoms under late therapy, but most made significant subjective improvement. Thats just the rough count. 4x Coagulopathy.
The regimen is to have it combined at least with zinc and NAC. In a few vulnerable I added HCQ, most got Quercetin as endosomal entry inhibitor. The Post-Vac and Hepatitis cases were supported with specific liver herbals (Silymarin, Dandelion, Artichoke, Superoxide Dismutase, Ornithin). First Hepatitis case was near normal after control, second improved drastically within 14 days of Baicalin. When added the Liver-Remedy the gut disturbance went away and the faeces had colour again. Waiting for hepatologist assessment now…I can't believe that lady was told she's nuts!!!
I'm careful with all CYP relevant meds (mainly psychopharmacologica), combination with herbal antidepressant.
She never drinks, but she even had raised Choline Esterase Values... not a good sign in Hepatitis... she has ANA of 1:800. Autoimmune-Hepatitis post-vac (3x), plus infection. She is improving!
Worst thing to improve is the neurological issues. It's like if you are sticking in the mud and have to watch slow mo... only two dropped out, one post-vac did react with sensitivity and one of my long-covid cases chose to take a different advice, she is now in a severe tumor situation. Exacerbation of a formerly present benign breast cyst, now two G2/G3 tumors (not vaxxed!).
The age and time of therapy is also interesting. The ones with problems are YOUNG!!!
I had only one vulnerable case with early therapy, that still has a few mild issues. Cerebral ischaemia in the history. Post-covid palpitations and exhaustion, but able to work.
Not all can. I have a few post-vac and post-covids that have not been able to work for >1/2 year.
11 started treatment in a later after onset and have not fully recovered. 7 recovered.
5 early treatments of vulnerable without any sequalea... even though with MS or Crohns disease.
Some of my PrEPed vulnerable never got any covid yet... although 3x vaxxed.
The cognitive improvement of my 6 neuro-cases was phenomenal!
The relatives started to notice... most reported a time of about 14 days.
Max dosage in cancer treatment is 1200mg/d and not more than 20mg/kg Bodyweight. You need to do the calc. I usually have 300mg and take 1 to 4 a day, depending on the situation. In acute infection I go with the old wisdom in immunological dysregulations... "HIT HARD AND EARLY"!
Once we have sufficient data I will add Kaplan-Meier curves here to graphically compare outcomes.
Conclusion
A key flavonoid that is readily and legally available in most countries, thousands of research papers and years of use in TCM with demonstrated low toxicity have proven the worth of baicalin, its derivatives and its parent plant, Scutellaria baicalensis for the treatment of many infections, neurological, autoimmune disorders and cancers, to name just a few.
Whether taken on its own or in combination with other therapeutics this deserves a top place in every medicine cupboard and its benefits need to be disseminated further in Western medical practice, with further clinical trials and research into improving its already good bioavailability and range of applications.
And finally...Its over to you!
This is a reader supported publication, please comment below if there are any therapeutics or aspects of pathophysiology you would be particularly interested in seeing reviewed at some point?
Please be as specific as you can as some subjects can be very broad in scope - even a simple compound like baicalein interacts with many, many signalling pathways, as we have seen, but all suggestions are welcome and I will do my best to accommodate them.
Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References
Baicalin - Wikipedia
Baicalein - Wikipedia
Glycoside - Wikipedia
Flavanone - Wikipedia
Glucuronide - Wikipedia
Scutellaria - Wikipedia
Scutellaria baicalensis - Wikipedia
MAPK/ERK pathway - Wikipedia
Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q, Li X. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol. 2019 Sep;71(9):1353-1369. doi: 10.1111/jphp.13129. Epub 2019 Jun 24. PMID: 31236960.
https://academic.oup.com/jpp/article/71/9/1353/6121997?login=false
Gad SC, Spainhour CB, Shoemake C, Pallman DR, Stricker-Krongrad A, Downing PA, Seals RE, Eagle LA, Polhamus K, Daly J. Tolerable Levels of Nonclinical Vehicles and Formulations Used in Studies by Multiple Routes in Multiple Species With Notes on Methods to Improve Utility. Int J Toxicol. 2016 Mar-Apr;35(2):95-178. doi: 10.1177/1091581815622442. Epub 2016 Jan 10. PMID: 26755718.
Furin - Wikipedia
Kibirev VK, Osadchuk TV, Vadziuk OB, Garazd MM. New non-peptide inhibitors of furin. Ukr Biokhim Zh (1999). 2010 Mar-Apr;82(2):15-21. Russian. PMID: 20684240.
https://www.researchgate.net/publication/239527131_New_non-peptide_inhibitors_of_furin
Osadchuk TV, Shybyryn OV, Kibirev VK. Chemical structure and properties of low-molecular furin inhibitors. Ukr Biochem J. 2016 Nov-Dec;88(6):5-25. doi: 10.15407/ubj88.06.005. PMID: 29235831.
PCSK9 - Wikipedia
Nhoek P, Chae HS, Masagalli JN, Mailar K, Pel P, Kim YM, Choi WJ, Chin YW. Discovery of Flavonoids from Scutellaria baicalensis with Inhibitory Activity Against PCSK 9 Expression: Isolation, Synthesis and Their Biological Evaluation. Molecules. 2018 Feb 24;23(2):504. doi: 10.3390/molecules23020504. PMID: 29495284; PMCID: PMC6100156.
SREBF1 Gene - Sterol Regulatory Element Binding Transcription Factor 1
Bao J, Zhu L, Zhu Q, Su J, Liu M, Huang W. SREBP-1 is an independent prognostic marker and promotes invasion and migration in breast cancer. Oncol Lett. 2016 Oct;12(4):2409-2416. doi: 10.3892/ol.2016.4988. Epub 2016 Aug 10. PMID: 27703522; PMCID: PMC5038874.
PCSK9 - Wikipedia
Mahboobnia K, Pirro M, Marini E, Grignani F, Bezsonov EE, Jamialahmadi T, Sahebkar A. PCSK9 and cancer: Rethinking the link. Biomed Pharmacother. 2021 Aug;140:111758. doi: 10.1016/j.biopha.2021.111758. Epub 2021 May 28. PMID: 34058443.
https://www.sciencedirect.com/science/article/pii/S0753332221005400?via%3Dihub
Moore OA, Gao Y, Chen AY, Brittain R, Chen YC. The Extraction, Anticancer Effect, Bioavailability, and Nanotechnology of Baicalin. J Nutr Med Diet Care. 2016;2(1):11. doi: 10.23937/2572-3278.1510011. Epub 2016 Mar 22. PMID: 27790646; PMCID: PMC5079443.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010 Apr 2;141(1):52-67. doi: 10.1016/j.cell.2010.03.015. PMID: 20371345; PMCID: PMC2862057.
Lin SR, Fu YS, Tsai MJ, Cheng H, Weng CF. Natural Compounds from Herbs that can Potentially Execute as Autophagy Inducers for Cancer Therapy. Int J Mol Sci. 2017 Jul 1;18(7):1412. doi: 10.3390/ijms18071412. PMID: 28671583; PMCID: PMC5535904.
Tando T, Ishizaka A, Watanabe H, Ito T, Iida S, Haraguchi T, Mizutani T, Izumi T, Isobe T, Akiyama T, Inoue J, Iba H. Requiem protein links RelB/p52 and the Brm-type SWI/SNF complex in a noncanonical NF-kappaB pathway. J Biol Chem. 2010 Jul 16;285(29):21951-60. doi: 10.1074/jbc.M109.087783. Epub 2010 May 11. PMID: 20460684; PMCID: PMC2903353.
Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015 Oct 22;6(10):e1942. doi: 10.1038/cddis.2015.271. PMID: 26492375; PMCID: PMC4632300.
Tumor-associated macrophage - Wikipedia
Dicer - Wikipedia
Small interfering RNA - Wikipedia
Martin BN, Wang C, Willette-Brown J, Herjan T, Gulen MF, Zhou H, Bulek K, Franchi L, Sato T, Alnemri ES, Narla G, Zhong XP, Thomas J, Klinman D, Fitzgerald KA, Karin M, Nuñez G, Dubyak G, Hu Y, Li X. IKKα negatively regulates ASC-dependent inflammasome activation. Nat Commun. 2014 Sep 30;5:4977. doi: 10.1038/ncomms5977. PMID: 25266676; PMCID: PMC4298287.
TRAF2 - Wikipedia
Shen RR, Zhou AY, Kim E, O'Connell JT, Hagerstrand D, Beroukhim R, Hahn WC. TRAF2 is an NF-κB-activating oncogene in epithelial cancers. Oncogene. 2015 Jan 8;34(2):209-16. doi: 10.1038/onc.2013.543. Epub 2013 Dec 23. PMID: 24362534; PMCID: PMC4067463.
Hua F, Li CH, Gao YC, Li J, Meng F. Molecular mechanism and role of NF-κB in the early diagnosis of cervical cancer. J BUON. 2019 Mar-Apr;24(2):720-728. PMID: 31128029.
MicroRNA 138-1 - Wikipedia
Ge A, Liu L, Deng X, Luo J, Xu Y. Exploring the Mechanism of Baicalin Intervention in Breast Cancer Based on MicroRNA Microarrays and Bioinformatics Strategies. Evid Based Complement Alternat Med. 2021 Dec 20;2021:7624415. doi: 10.1155/2021/7624415. PMID: 34966436; PMCID: PMC8712139.
Shen C, Wang J, Xu Z, Zhang L, Gu W, Zhou X. ONECUT2 which is targeted by hsa-miR-15a-5p enhances stemness maintenance of gastric cancer stem cells. Exp Biol Med (Maywood). 2021 Dec;246(24):2645-2659. doi: 10.1177/15353702211038496. Epub 2021 Aug 9. PMID: 34365839; PMCID: PMC8669165.
Takebayashi K, Nasu K, Okamoto M, Aoyagi Y, Hirakawa T, Narahara H. hsa-miR-100-5p, an overexpressed miRNA in human ovarian endometriotic stromal cells, promotes invasion through attenuation of SMARCD1 expression. Reprod Biol Endocrinol. 2020 Apr 16;18(1):31. doi: 10.1186/s12958-020-00590-3. PMID: 32299427; PMCID: PMC7161200.
Huang E, Liu R, Chu Y. miRNA-15a/16: as tumor suppressors and more. Future Oncol. 2015;11(16):2351-63. doi: 10.2217/fon.15.101. PMID: 26260813.
Liu GX, Ma S, Li Y, Yu Y, Zhou YX, Lu YD, Jin L, Wang ZL, Yu JH. Hsa-let-7c controls the committed differentiation of IGF-1-treated mesenchymal stem cells derived from dental pulps by targeting IGF-1R via the MAPK pathways. Exp Mol Med. 2018 Apr 13;50(4):1-14. doi: 10.1038/s12276-018-0048-7. PMID: 29650947; PMCID: PMC5938007.
Fold change - Wikipedia
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng P, Chen A, Huang H. The Fascinating Effects of Baicalein on Cancer: A Review. Int J Mol Sci. 2016 Oct 9;17(10):1681. doi: 10.3390/ijms17101681. PMID: 27735841; PMCID: PMC5085714.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5085714/?report=classic
Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000 Nov 28;160(2):219-28. doi: 10.1016/s0304-3835(00)00591-7. PMID: 11053652.
Silibinin - Wikipedia
Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006 Nov-Dec;26(6B):4457-98. PMID: 17201169.
Xu P, Zhou H, Li YZ, Yuan ZW, Liu CX, Liu L, Xie Y. Baicalein Enhances the Oral Bioavailability and Hepatoprotective Effects of Silybin Through the Inhibition of Efflux Transporters BCRP and MRP2. Front Pharmacol. 2018 Oct 26;9:1115. doi: 10.3389/fphar.2018.01115. PMID: 30416442; PMCID: PMC6212553.
Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 2000 Sep 24;276(2):534-8. doi: 10.1006/bbrc.2000.3485. PMID: 11027509.
CCR5 - Wikipedia
CXCR4 - Wikipedia
Dr Ah Kahn Syed. How to BLAST your way to the truth about the origins of COVID-19. (2021).
https://arkmedic.substack.com/p/how-to-blast-your-way-to-the-truth
Wu Zhang X, Leng Yap Y. Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. Theochem. 2004 May;677(1):73-76. doi: 10.1016/j.theochem.2004.02.018. Epub 2004 Apr 2. PMID: 32287546; PMCID: PMC7141560.
Prashant Pradhan, Ashutosh Kumar Pandey, Akhilesh Mishra, Parul Gupta, Praveen Kumar Tripathi, Manoj Balakrishnan Menon, James Gomes, Perumal Vivekanandan, Bishwajit Kundu. Uncanny similarity of unique inserts in the 2019-nCoV spike protein to HIV-1 gp120 and Gag. bioRxiv 2020.01.30.927871; doi: https://doi.org/10.1101/2020.01.30.927871
Li ZL, Buck M. Neuropilin-1 Assists SARS-CoV-2 Infection by Stimulating the Separation of Spike Protein Domains S1 and S2. bioRxiv [Preprint]. 2021 Jan 19:2021.01.06.425627. doi: 10.1101/2021.01.06.425627. Update in: Biophys J. 2021 Jul 20;120(14):2828-2837. PMID: 33442700; PMCID: PMC7805474.
Oliveira ASF, Ibarra AA, Bermudez I, Casalino L, Gaieb Z, Shoemark DK, Gallagher T, Sessions RB, Amaro RE, Mulholland AJ. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys J. 2021 Mar 16;120(6):983-993. doi: 10.1016/j.bpj.2021.01.037. Epub 2021 Feb 18. PMID: 33609494; PMCID: PMC7889469.
Riese A, Eilert Y, Meyer Y, Arin M, Baron JM, Eming S, Krieg T, Kurschat P. Epidermal expression of neuropilin 1 protects murine keratinocytes from UVB-induced apoptosis. PLoS One. 2012;7(12):e50944. doi: 10.1371/journal.pone.0050944. Epub 2012 Dec 10. PMID: 23251405; PMCID: PMC3518474.
Lee YJ, Yeo IJ, Choi DY, Yun J, Son DJ, Han SB, Hong JT. Amyloidogenic, neuroinflammatory and memory dysfunction effects of HIV-1 gp120. Arch Pharm Res. 2021 Jul;44(7):689-701. doi: 10.1007/s12272-021-01340-8. Epub 2021 Jul 23. PMID: 34302237; PMCID: PMC8300079.
Inducible Nitric Oxide Synthase
https://www.sciencedirect.com/science/article/pii/B9780080552323605094
Prostaglandin-endoperoxide synthase 2 - Wikipedia
https://en.wikipedia.org/wiki/Prostaglandin-endoperoxide_synthase_2
Woo KJ, Lim JH, Suh SI, Kwon YK, Shin SW, Kim SC, Choi YH, Park JW, Kwon TK. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology. 2006;211(5):359-68. doi: 10.1016/j.imbio.2006.02.002. Epub 2006 Mar 29. PMID: 16716805.
Alvarez S, Serramía MJ, Fresno M, Muñoz-Fernández MA. HIV-1 envelope glycoprotein 120 induces cyclooxygenase-2 expression in astrocytoma cells through a nuclear factor-kappaB-dependent mechanism. Neuromolecular Med. 2007;9(2):179-93. doi: 10.1007/BF02685891. PMID: 17627037.
Noh K, Nepal MR, Jeong KS, Kim SA, Um YJ, Seo CS, Kang MJ, Park PH, Kang W, Jeong HG, Jeong TC. Effects of baicalin on oral pharmacokinetics of caffeine in rats. Biomol Ther (Seoul). 2015 Mar;23(2):201-6. doi: 10.4062/biomolther.2014.134. Epub 2015 Mar 1. PMID: 25767690; PMCID: PMC4354323.
Yu HY, Zhu Y, Zhang XL, Wang L, Zhou YM, Zhang FF, Zhang HT, Zhao XM. Baicalin attenuates amyloid β oligomers induced memory deficits and mitochondria fragmentation through regulation of PDE-PKA-Drp1 signalling. Psychopharmacology (Berl). 2022 Mar;239(3):851-865. doi: 10.1007/s00213-022-06076-x. Epub 2022 Feb 1. PMID: 35103832.
What is mitochondrial fragmentation?
https://www.aatbio.com/resources/faq-frequently-asked-questions/What-is-mitochondrial-fragmentation
Mitochondrial fragmentation
https://www.neurotar.com/contract-research/mitochondrial-fragmentation/
Phosphodiesterase-4 inhibitor - Wikipedia
Synaptic plasticity - Wikipedia
Coley AA, Gao WJ. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol Biol Psychiatry. 2018 Mar 2;82:187-194. doi: 10.1016/j.pnpbp.2017.11.016. Epub 2017 Nov 21. PMID: 29169997; PMCID: PMC5801047.
H-89 - Wikipedia
Beta-Actin's Role in Neuronal Plasticity
https://www.novusbio.com/antibody-news/antibodies/beta-actins-role-in-neuronal-plasticity
Tu L, Wu ZY, Yang XL, Zhang Q, Gu R, Wang Q, Tian T, Yao H, Qu X, Tian JY. Neuroprotective effect and mechanism of baicalin on Parkinson's disease model induced by 6-OHDA. Neuropsychiatr Dis Treat. 2019 Dec 31;15:3615-3625. doi: 10.2147/NDT.S165931. PMID: 32099367; PMCID: PMC6997193.
Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011 Apr 1;508(1):1-12. doi: 10.1016/j.abb.2010.12.017. Epub 2010 Dec 19. PMID: 21176768; PMCID: PMC3065393.
Lu QY, Zhang L, Moro A, Chen MC, Harris DM, Eibl G, Go VL. Detection of baicalin metabolites baicalein and oroxylin-a in mouse pancreas and pancreatic xenografts. Pancreas. 2012 May;41(4):571-6. doi: 10.1097/MPA.0b013e318232e130. PMID: 22158070; PMCID: PMC3310302.
Ha J, Zhao L, Zhao Q, Yao J, Zhu BB, Lu N, Ke X, Yang HY, Li Z, You QD, Guo QL. Oroxylin A improves the sensitivity of HT-29 human colon cancer cells to 5-FU through modulation of the COX-2 signaling pathway. Biochem Cell Biol. 2012 Aug;90(4):521-31. doi: 10.1139/o2012-005. Epub 2012 May 18. PMID: 22607196.
Chen Y, Yang L, Lee TJ. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochem Pharmacol. 2000 Jun 1;59(11):1445-57. doi: 10.1016/s0006-2952(00)00255-0. PMID: 10751555.
RELA - Wikipedia
Baicalin as a treatment for SARS infection - Patent US-7605135-B2 - PubChem (nih.gov)
Haixia Su, Sheng Yao, Wenfeng Zhao, Minjun Li, Jia Liu, WeiJuan Shang, Hang Xie, Changqiang Ke, Meina Gao, Kunqian Yu, Hong Liu, Jingshan Shen, Wei Tang, Leike Zhang, Jianping Zuo, Hualiang Jiang, Fang Bai, Yan Wu, Yang Ye, Yechun Xu. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro.
bioRxiv 2020.04.13.038687; doi: https://doi.org/10.1101/2020.04.13.038687
https://www.biorxiv.org/content/10.1101/2020.04.13.038687v1.full
Shuanghuanglian - Wikipedia
Zhang TB, Yue RQ, Xu J, Ho HM, Ma DL, Leung CH, Chau SL, Zhao ZZ, Chen HB, Han QB. Comprehensive quantitative analysis of Shuang-Huang-Lian oral liquid using UHPLC-Q-TOF-MS and HPLC-ELSD. J Pharm Biomed Anal. 2015 Jan;102:1-8. doi: 10.1016/j.jpba.2014.08.025. Epub 2014 Aug 30. PMID: 25222137; PMCID: PMC7126236.
Ni L, Wen Z, Hu X, Tang W, Wang H, Zhou L, Wu L, Wang H, Xu C, Xu X, Xiao Z, Li Z, Li C, Liu Y, Duan J, Chen C, Li D, Zhang R, Li J, Yi Y, Huang W, Chen Y, Zhao J, Zuo J, Weng J, Jiang H, Wang DW. Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front Med. 2021 Oct;15(5):704-717. doi: 10.1007/s11684-021-0853-6. Epub 2021 Apr 28. PMID: 33909260; PMCID: PMC8079840.
Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin Drug Investig. 2020 Aug;40(8):683-686. doi: 10.1007/s40261-020-00933-3. PMID: 32533455; PMCID: PMC7290142.
Bhattacharjee A, Kumar B. Phytomelatonin: a comprehensive literature review and recent advance on medicinal meadow. Int J Hydro. 2018;2(3):396-403. DOI: 10.15406/ijh.2018.02.00102
Zaidan R, Geoffriau M, Brun J, Taillard J, Bureau C, Chazot G, Claustrat B. Melatonin is able to influence its secretion in humans: description of a phase-response curve. Neuroendocrinology. 1994 Jul;60(1):105-12. doi: 10.1159/000126726. PMID: 8090277.
Jin Z, Huang J, Zhu Z. Baicalein reduces endometriosis by suppressing the viability of human endometrial stromal cells through the nuclear factor-κB pathway in vitro. Exp Ther Med. 2017 Oct;14(4):2992-2998. doi: 10.3892/etm.2017.4860. Epub 2017 Aug 1. PMID: 28912852; PMCID: PMC5585734.
Bcl-2 - Wikipedia
Liang S, Deng X, Lei L, Zheng Y, Ai J, Chen L, Xiong H, Mei Z, Cheng YC, Ren Y. The Comparative Study of the Therapeutic Effects and Mechanism of Baicalin, Baicalein, and Their Combination on Ulcerative Colitis Rat. Front Pharmacol. 2019 Dec 13;10:1466. doi: 10.3389/fphar.2019.01466. PMID: 31920656; PMCID: PMC6923254.
Chinese skullcap seems to correct PEM/the energy problem in ME (2020)
Ishfaq M, Zhang W, Hu W, Waqas Ali Shah S, Liu Y, Wang J, Wu Z, Ahmad I, Li J. Antagonistic Effects Of Baicalin On Mycoplasma gallisepticum-Induced Inflammation And Apoptosis By Restoring Energy Metabolism In The Chicken Lungs. Infect Drug Resist. 2019 Oct 1;12:3075-3089. doi: 10.2147/IDR.S223085. PMID: 31632098; PMCID: PMC6781171.
Mycoplasma gallisepticum - Wikipedia
Zhang Y, Li X, Ciric B, Ma CG, Gran B, Rostami A, Zhang GX. Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci Rep. 2015 Nov 30;5:17407. doi: 10.1038/srep17407. PMID: 26616302; PMCID: PMC4663791.
Xu J, Liu J, Yue G, Sun M, Li J, Xiu X, Gao Z. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol Med Rep. 2018 Jul;18(1):1149-1154. doi: 10.3892/mmr.2018.9054. Epub 2018 May 23. PMID: 29845272.
JAK-STAT signaling pathway - Wikipedia
The Central Role of Enzymes as Biological Catalysts
Wang X, Yu JY, Sun Y, Wang H, Shan H, Wang S. Baicalin protects LPS-induced blood-brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int Immunopharmacol. 2021 Jul;96:107725. doi: 10.1016/j.intimp.2021.107725. Epub 2021 May 28. PMID: 34162131.
https://www.sciencedirect.com/science/article/pii/S1567576921003611
NFE2L2 - Wikipedia
Superoxide dismutase - Wikipedia
Ai RS, Xing K, Deng X, Han JJ, Hao DX, Qi WH, Han B, Yang YN, Li X, Zhang Y. Baicalin Promotes CNS Remyelination via PPARγ Signal Pathway. Neurol Neuroimmunol Neuroinflamm. 2022 Feb 1;9(2):e1142. doi: 10.1212/NXI.0000000000001142. PMID: 35105686; PMCID: PMC8808354.
Peroxisome proliferator-activated receptor gamma - Wikipedia
https://en.m.wikipedia.org/wiki/Peroxisome_proliferator-activated_receptor_gamma
Wang J, Chen S, Zhang J, Wu J. Scutellaria baicalensis georgi is a promising candidate for the treatment of autoimmune diseases. Front Pharmacol. 2022 Sep 16;13:946030. doi: 10.3389/fphar.2022.946030. PMID: 36188625; PMCID: PMC9524225.
Immunopathogenesis of Experimental Uveitic Diseases
https://www.sciencedirect.com/science/article/pii/B9780128093245013900
Quantification of Baicalin Content in Scutuellaria Baicalensis Powder (Herbal Supplements) by Vis-NIRS
Mbagwu, Sonne & Ilodigwe, E. & Ajaghaku, Daniel & Samuel, Uzodinma & Osonwa, Uduma. (2013). Cytotoxic and Apoptotic Effect of Commercial Tinctures of Scutellaria baicalensis on Lung Cancer Cell Lines ARTICLE INFO ABSTRACT. Journal of Applied Pharmaceutical Science. 3. 52-59. 10.7324/JAPS.2013.30209.
The Functional Medicine Approach to COVID-19: Additional Research on Nutraceuticals and Botanicals. (linked pdf).
What Is Chinese Skullcap?
https://www.verywellhealth.com/the-health-benefits-of-skullcap-89584
















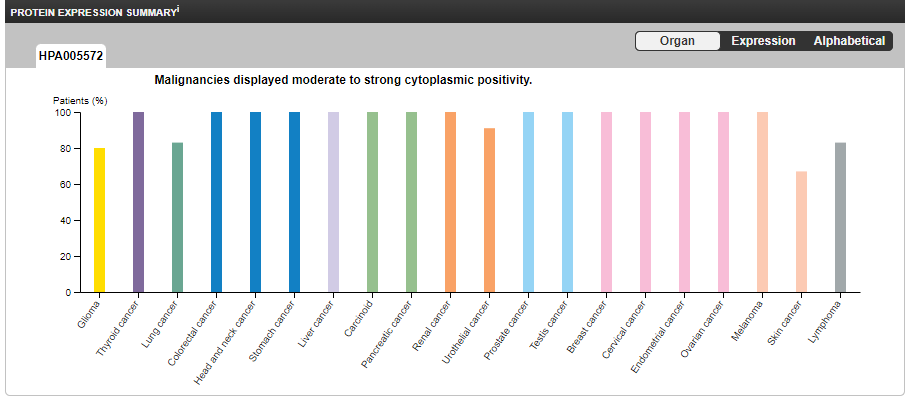


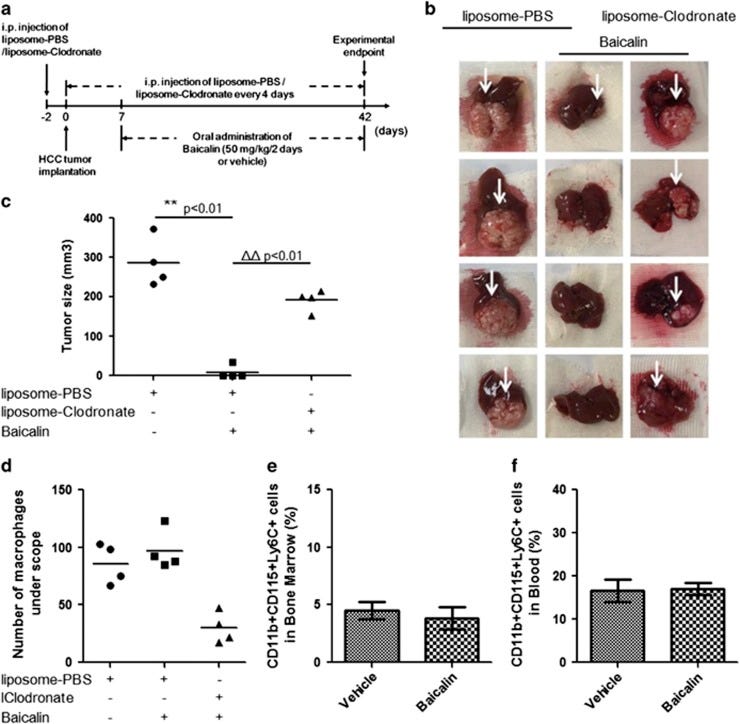

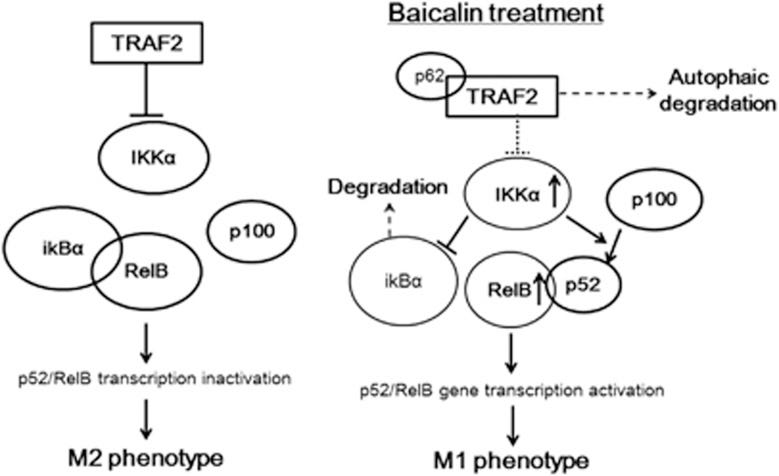
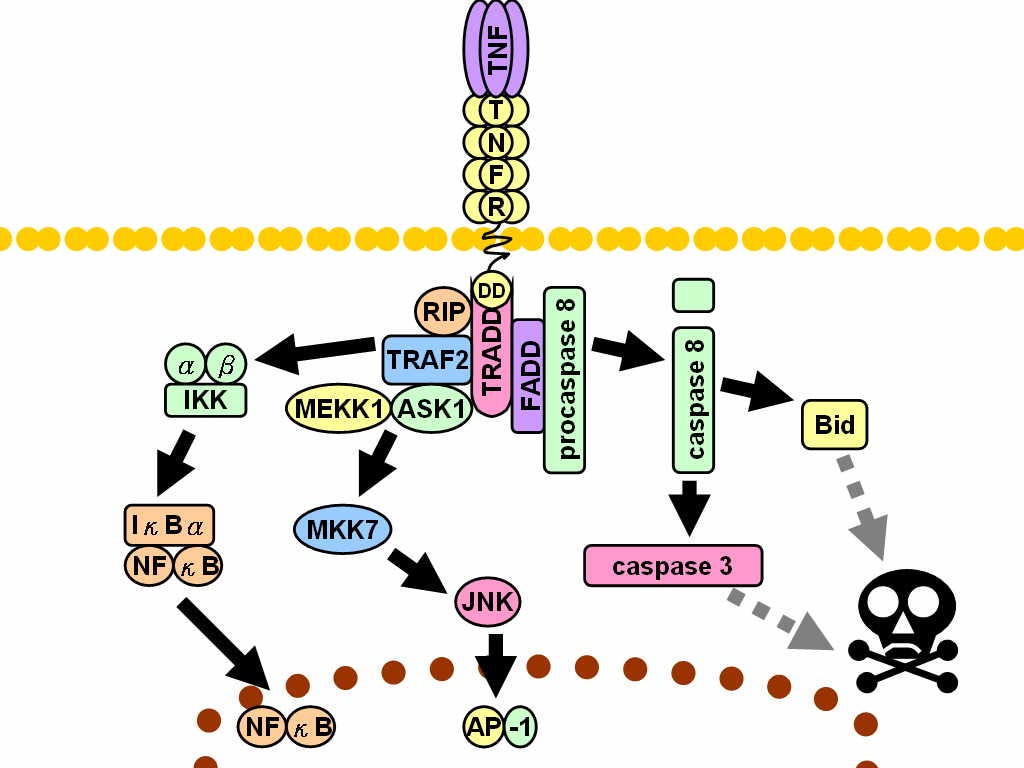

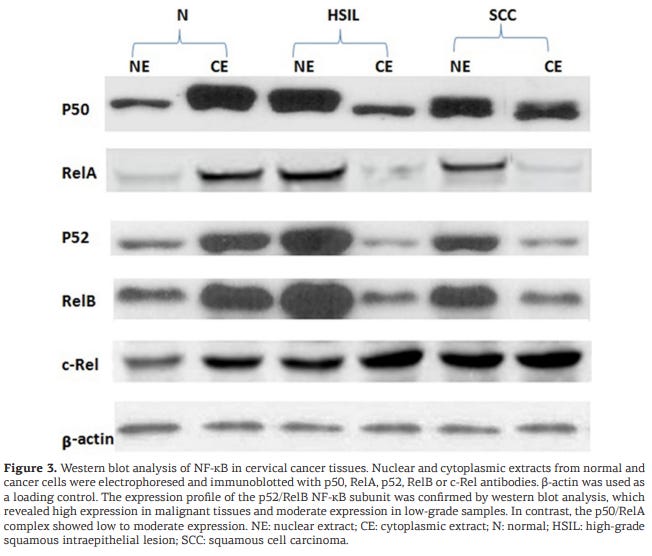
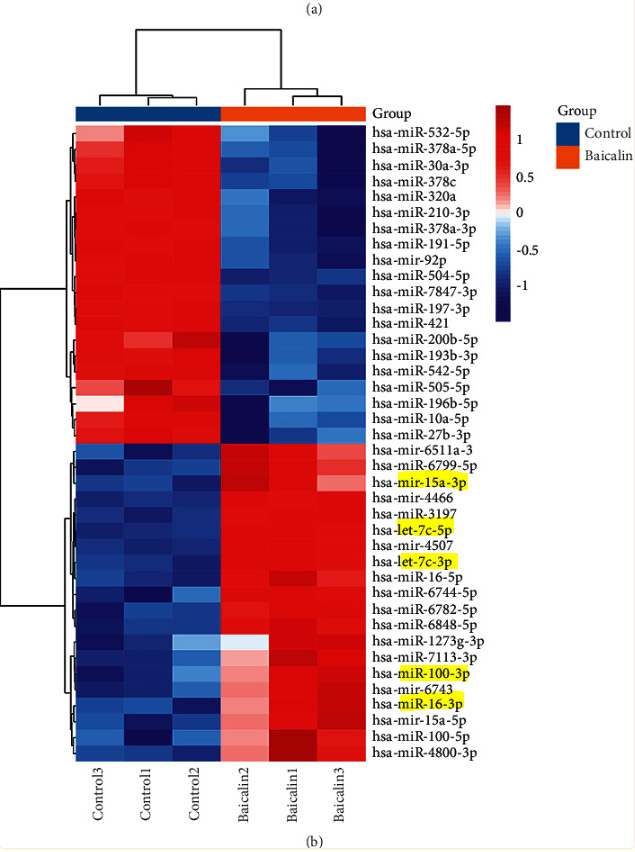





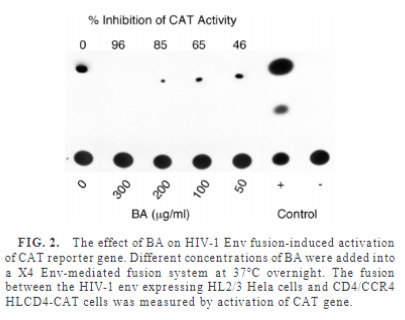
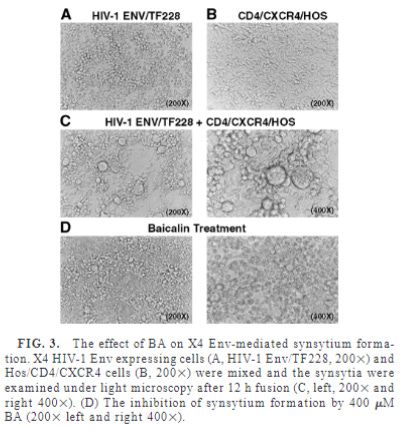





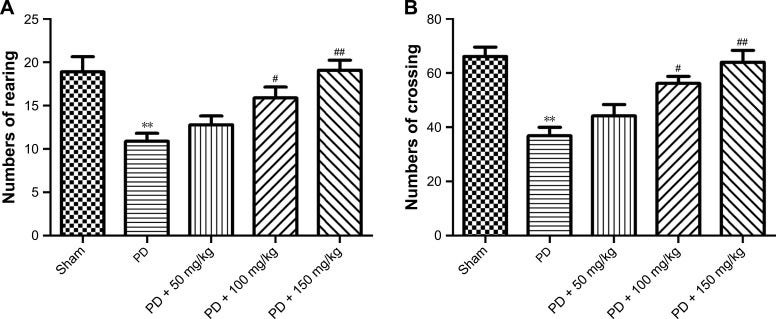

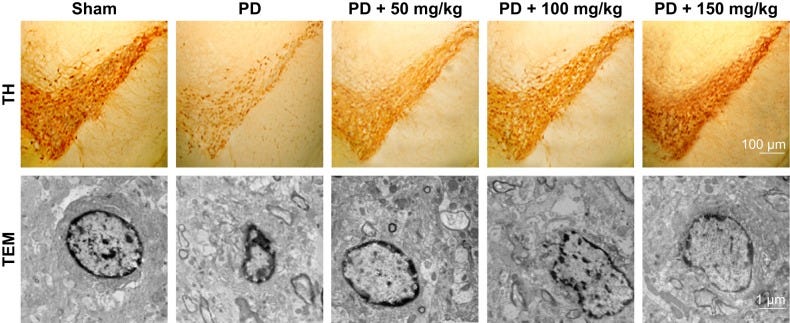
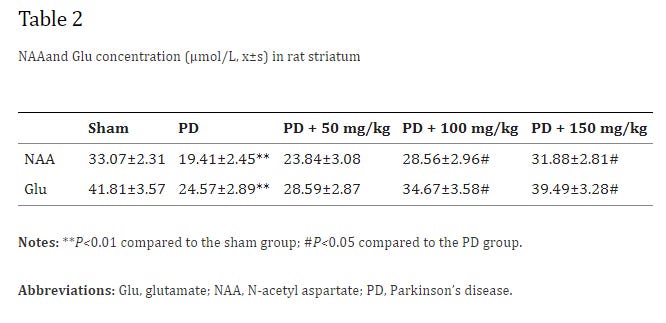



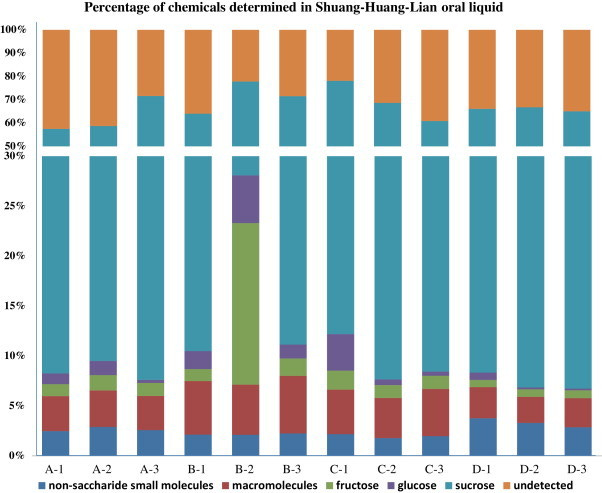
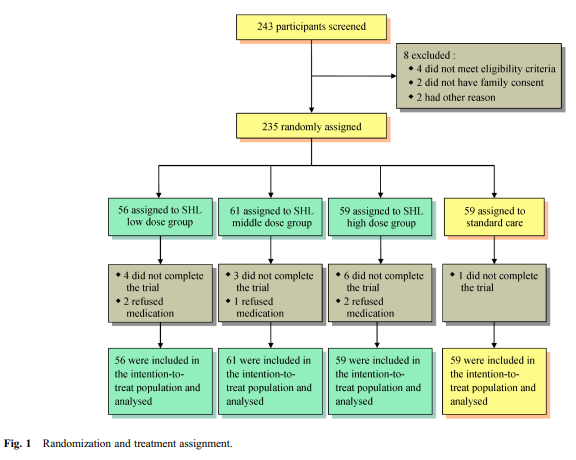
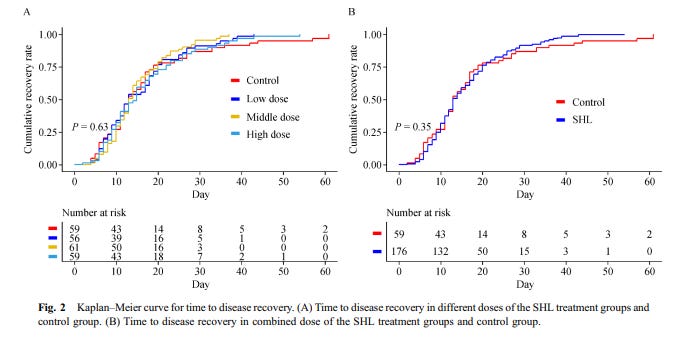
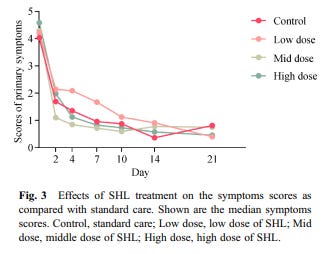



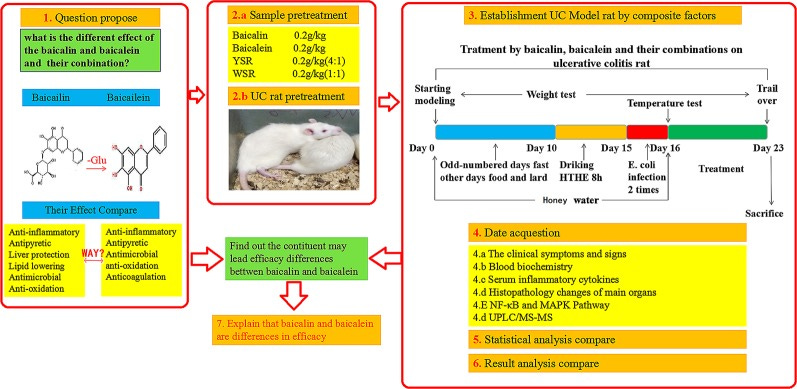

























So many "Old fashioned remedies" have been suppressed by the EVIL MegaPharmas seeking to inflate their already grossly obscene & evil profiteering.
The wisdom of the ancients kept them from a lot of pain, suffering & disease without doctors (aka White coated legal highly addictive & mainly toxic, Drug Peddlers.)
Excellent herb. I’ve been taking it daily for years. Carp, you should find this database interesting: herbsum.onuniverse.com