Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
If received via email I recommend clicking on the hyperlinked title to read the latest correctly formatted version in full, in a browser. Unfortunately it is not possible to email out revised versions.
Contents:
The VAX003 (AIDSVAX B/E) vaccine trial
Failure of the RV144 and VAX003 vaccine trials
Background
All attempts to create safe and effective vaccines against HIV appear to have been met with failure to date: ie in confirmatory clinical trials they failed to meet acceptable endpoints in the short and/or long term for multiple reasons, despite looking encouraging in earlier phases.
An article in Nature: Another HIV vaccine failure: where to next? (2013)1 asked 4 research scientists to comment on the high-profile failure of an HIV vaccine trial HVTN505.
A question of ethics and the potential involvement of regulatory committees was also raised due to signs of vaccine-mediated enhancement in some of the trials:
The recent high-profile failure of an HIV vaccine trial (HVTN 505) raises some key questions for researchers in this field 1 . The authors tested a vaccine regimen consisting of a DNA vector encoding HIV-1 Gag, Pol, Nef and Env proteins followed by a recombinant adenovirus type 5 (rAd5) boost in an at-risk population in the US, with ∼1,250 individuals given the vaccine and a similar number given a placebo. The vaccine did not reduce the rate of HIV-1 acquisition or the viral-load set point in the population studied. We asked four experts for their opinions on this trial and how they think the HIV field should move on from this disappointing result.
In HIV/AIDS vaccine development, concepts rapidly evolve, but facts, such as those obtained from randomized, double-blind, placebo-controlled clinical efficacy trials, come extremely slowly. Although the outcome of one such trial, HVTN 505, was disappointing, it establishes an all-too-infrequent factual benchmark by which evolving vaccine concepts can be judged1.
The HVTN 505 HIV-1 vaccine trial reached a definitive conclusion: the vaccine had no protective efficacy against acquisition of infection or subsequent viral load1. This failure mirrors the outcome of two earlier efficacy trials (STEP and Phambili) of another rAd5 vector vaccine expressing Gag, Pol and Nef2. There is consensus, then—the rAd5 vectors tested to date have been ineffective. But there are indications of deeper problems: a presentation at the recent Barcelona AIDS Vaccine meeting described how the rAd5 vector was directly responsible for a higher HIV-1 infection rate compared to placebo in the South African Phambili trial—and by an unknown mechanism (G. Gray, University of the Witwatersrand, personal communication). This disturbing conclusion may have serious implications for rAd5-based vaccines.
There was also a greater HIV-1 infection rate in the STEP vaccine arm compared to placebo, but various confounding variables preclude conclusions about vaccine-mediated enhancement2. A key question is whether it can be proven that any adenoviral vector vaccines for HIV-1 and other pathogens, including ones based on alternative serotypes, are safe enough for large-scale testing in HIV-endemic areas. If the Phambili enhancement mechanism is unknown, how can we be sure that the problem is absent from similar vectors? Several microbicide or passive antibody trials in Africa have also enhanced HIV-1 infection4. How will ethics and regulatory committees now react to the Phambili trial? Is it time to be prudent and switch to more potent next-generation vectors? If so, more attention should be given to what HIV proteins the vectors actually express. Preventing HIV infection consistently may require the additional induction of virus-neutralizing antibodies, something the tested rAd5 vectors were never designed to do. Does that omission account for their failure or, worse, the enhancement seen with the Phambili vaccine?
“Modest efficacy” of the RV144 clinical trial?
The HVTN 505 report emphasizes that no vaccine-mediated enhancement of HIV-1 infection occurred1. A late upwards separation of the vaccine and placebo infection-rate curves is not significant (although the number of 'infection events' in the vaccine group at that time seems greater than when the infection rate for the placebo group darted upwards in the RV144 trial, thereby creating modest efficacy3).
The VAX003 (AIDSVAX B/E) vaccine trial
The AIDSVAX B/E double blinded phase 3 clinical trial of 2500 participants took place in Bangkok, Thailand from March 1999 to August 2000. It used a genetically engineered version of HIV envelope protein gp120. HIV positive volunteers were excluded.
Official Title:
A Phase III Trial to Determine the Efficacy of AIDSVAX B/E Vaccine in Intravenous Drug Users in Bangkok, Thailand
Brief Summary:
The purpose of this study is to determine if the vaccine, AIDSVAX B/E, will protect intravenous drug users from becoming infected with HIV.
Detailed Description:
Volunteers are immunized and followed for a minimum of 2 years. Any volunteer that becomes infected with HIV-1 is followed every 4 months post infection for up to 36 months. Behavior effects associated with study participation are assessed.
The RV144 vaccine trial
The Thai army-led trial RV144 used the ALVAC® HIV vaccine as the prime and the AIDSVAX® B/E vaccine for two booster shots.
Results from the failed 2003 combined study were published in the NEJM in 20092. Boosting with AIDSVAX B/E occurred at weeks 12 and 24:
Abstract
Background: The development of a safe and effective vaccine against the human immunodeficiency virus type 1 (HIV-1) is critical to pandemic control.
Methods: In a community-based, randomized, multicenter, double-blind, placebo-controlled efficacy trial, we evaluated four priming injections of a recombinant canarypox vector vaccine (ALVAC-HIV [vCP1521]) plus two booster injections of a recombinant glycoprotein 120 subunit vaccine (AIDSVAX B/E). The vaccine and placebo injections were administered to 16,402 healthy men and women between the ages of 18 and 30 years in Rayong and Chon Buri provinces in Thailand. The volunteers, primarily at heterosexual risk for HIV infection, were monitored for the coprimary end points: HIV-1 infection and early HIV-1 viremia, at the end of the 6-month vaccination series and every 6 months thereafter for 3 years.
Results: In the intention-to-treat analysis involving 16,402 subjects, there was a trend toward the prevention of HIV-1 infection among the vaccine recipients, with a vaccine efficacy of 26.4% (95% confidence interval [CI], -4.0 to 47.9; P=0.08). In the per-protocol analysis involving 12,542 subjects, the vaccine efficacy was 26.2% (95% CI, -13.3 to 51.9; P=0.16). In the modified intention-to-treat analysis involving 16,395 subjects (with the exclusion of 7 subjects who were found to have had HIV-1 infection at baseline), the vaccine efficacy was 31.2% (95% CI, 1.1 to 52.1; P=0.04). Vaccination did not affect the degree of viremia or the CD4+ T-cell count in subjects in whom HIV-1 infection was subsequently diagnosed.
Conclusions: This ALVAC-HIV and AIDSVAX B/E vaccine regimen may reduce the risk of HIV infection in a community-based population with largely heterosexual risk. Vaccination did not affect the viral load or CD4+ count in subjects with HIV infection. Although the results show only a modest benefit, they offer insight for future research. (ClinicalTrials.gov number, NCT00223080.)
Anything less than 50% would normally be considered a fail.
Although a phase 3 trial of VaxGen bivalent gp120 AIDSVAX B/E vaccine alone involving injection-drug users showed no effect on HIV-1 acquisition,21 a phase 2 trial of an ALVAC-HIV (vCP1521) prime with an AIDSVAX B/E boost showed induction of prespecified cellular and humoral immune responses and was consistent with criteria for advancement to a large test-of-concept study.17 In October 2003, our study was initiated in a population at community risk for HIV infection.8
Bivalent subtype B AIDSVAX B/B gp120 did not protect high-risk men who have sex with men,34-36 and AIDSVAX B/E did not protect Thai injection-drug users21 from infection with HIV-1. The Step trial of Merck recombinant adenovirus type 5 (rAd5) HIV-1 vaccine containing subtype B gag, pol, and nef in high-risk men who have sex with men was stopped because of futility and possibly higher rates of infection in vaccine recipients.37
Though early studies of canarypox–gp120 subunit prime–boost regimens were promising,10-13 advanced-phase testing of subtype B ALVAC-HIV (vCP1452) and AIDSVAX B/B was canceled because CD8+ reactivity on ELISPOT was too low.12
Failure of the RV144 and VAX003 vaccine trials
Related studies:
Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination (2022)
https://www.science.org/doi/10.1126/sciimmunol.ade2798
mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine (2023)
https://www.frontiersin.org/articles/10.3389/fimmu.2022.1020844/full
The implications for increased incidence of many cancers such as lymphoma are discussed:
Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines
In 2014, Chung et al posted an analysis of the Fc-effector profiles and subclass selection mediated by the RV144 and VAX003 clinical trials3.
They concluded that partial efficacy of the RV144 trial was due to Fc-mediated effector responses through the selective induction of functional immunoglobulins IgG1 and IgG3.
You need antibodies with all three properties for therapeutic efficacy:
Antibody-dependent cellular cytotoxicity (ADCC).
Antibody-dependent cellular phagocytosis (ADCP).
Complement-dependent cytotoxicity (CDC).
The Fc-mediated effector functions of antibodies include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), and have been shown to be crucial for the therapeutic efficacy of most clinically approved antibodies.
In contrast, repeated AIDSVAX B/E protein boosts elicited non-neutralising monofunctional antibody responses influenced by IgG4 selection:
These data suggest that subclass selection differences associated with coordinated humoral functional responses targeting strain-specific protective V2 loop epitopes may underlie differences in vaccine efficacy observed between these two vaccine trials.
To expand on this:
We hypothesized that the RV144 and VAX003 trials elicited different humoral functional profiles that could provide key insights into the mechanism by which immunization strategies skew humoral immunity. Here, we show that RV144 elicited a polyfunctional Ab response associated with the selective induction of IgG3 Abs. By contrast, VAX003 elicited a monofunctional Ab response strongly influenced by the selection of the more functionally inert IgG4 Ab subclass. This skewed IgG4 subclass profile was promoted by repeated administration of gp120 protein vaccination, marked by a concomitant decrease in IgG3 subclass levels and alterations in Ab functionality with repeated protein exposure. Moreover, whereas VAX003 induced a highly focused IgG4 Ab response against the crown of the V2 loop, the RV144 vaccine induced both IgG1 and IgG3 Abs targeting this region, preferentially targeting strains blocked by the vaccine but with limited coverage of breakthrough sequences. Thus, both trials induced suboptimal humoral immune profiles, but qualitative analyses probing beyond Ab titer and neutralization indicate that selection of functional Ab subclasses that target vulnerable envelope regions may be potent contributors to vaccine efficacy.
VAX003 induced higher gp120 binding titres and higher Fc-effector profiles compared to RV144, so antibody titre and function alone could not explain the lack of efficacy. They investigated further:
RV144 induces qualitatively different polyfunctional Ab responses. VAX003 induced higher gp120 binding titers (1, 2) (fig. S1, A to C) and consequently linked higher Fc-effector profiles compared to RV144 (fig. S1, D to I), suggesting that neither Ab titer nor Ab function alone could account for the reduced risk of infection observed in the RV144 trial. Therefore, we sought to determine whether there were qualitative differences in nonneutralizing Fc-effector profiles between the two vaccine trials.
Although both RV144 and VAX003 induced IgG1, VAX003 alone induced markedly higher titres of IgG4:
Fig. 2. gp120-specific IgG subclass selection in the RV144 and VAX003 trials. A customized Luminex subclass assay was conducted using purified bulk IgG isolated from RV144 and VAX003 samples to determine the levels of gp120-specific IgG1, IgG2, IgG3, and IgG4 Abs. A positive threshold was determined for each subclass by assaying 20 RV144 and 10 VAX003 placebo samples and determining median fluorescence intensity (MFI) + 2 SDs for each subclass to ensure a 95% confidence interval. (A) The frequency of subjects positive for each of the IgG subclasses is depicted in the bar graph for RV144 (red) and VAX003 (blue). P values were calculated by Fisher’s exact test. (B to E) Differences in the overall quantity of each subclass are depicted in the dot plots for (B) IgG1, (C) IgG2, (D) IgG3, and (E) IgG4 for each vaccine trial, based on the respective % IgG subclass MFI from total HIV-specific IgG MFI. Differences in isotype frequencies between the two trials were calculated by Mann-Whitney test.
After conducting further experiments, they concluded that the high titres of IgG4 were one of the key factors contributing to the lack of Fc-mediated effector functions, despite the presence of IgG3. This is also a feature of melanoma tumors:
Subclass selection represents one of the key Ab features exploited during the design of monoclonal therapeutics (21, 22) to potentiate/ impair Ab function. Of the four IgG subclasses, IgG1 and IgG3 are associated with higher affinities for Fcg receptors (12) and, consequently, stronger activation of Fc-mediated effector functions. By contrast, IgG2 and IgG4 have weaker affinities for Fcg receptors, with IgG4 having 10-fold weaker affinity than IgG1 (23). Malaria-specific immunity has been associated with skewed subclass selection, with IgG1/IgG3 selection associated with immunity and IgG2-biased responses associated with the lack of protection (24–26). Likewise, Ab functionality was closely linked to distinct subclass profiles in RV144 and VAX003 vaccinees. When compared with VAX003, RV144 induced higher levels of IgG3 Abs compared to VAX004, whereas VAX003 promoted higher levels of IgG1, IgG2, and IgG4 Ab levels. Moreover, longitudinal analysis of alum-adjuvanted VAX003 demonstrated an expected IgG1-biased response with increased titers and functionality after repeated boosting. However, boosting simultaneously resulted in reduced polyfunctionality linked to the concomitant induction of IgG4 responses. In melanoma, both antigen-specific and bulk IgG4 Abs have been shown to inhibit Ab-guided tumoricidal activity by reducing Fc receptor activation (22). Previous studies in the VAX004 trial also showed an antagonistic effect of gp120- specific IgG2 Abs (27). Likewise, depletion of IgG4 Abs from VAX003, where some individuals induced HIV-specific IgG responses consisting of up to 30% IgG4, resulted in significant increases in Fc-mediated effector functions, supporting an antagonistic role for IgG4 Abs in VAX003.
They hypothesise that repeat boosting without using strong adjuvants contributed to the class switching to IgG4 observed in these trials:
The uncharacteristic induction of high levels of IgG4 in the alum based VAX003 regimen may be related to the repeated administration of seven large doses of vaccine antigens in the absence of sufficiently potent adjuvant signals that may have driven excessive B cell receptor triggering. Allergy studies have similarly observed that continuous exposure to high doses of antigen can result in suppressed inflammatory responses through elevated levels of antigen-specific IgG4 (28). However, little is known about the mechanism by which vaccines tune Ab subclass selection. Upon activation, a naïve B cell can functionally tune its Ab response from IgM to the production of IgG, IgE, or IgA through class switch recombination (29), a highly regulated process largely controlled by cytokines and T helper–provided signals (30). IgG3 Ab responses are induced robustly during acute HIV infection but decline rapidly with progressive disease (31). Because IgG3 responses were induced by protein immunization alone (VAX003 at visit 5), it is unlikely that the viral vector prime alone was responsible for the selection of functional Ab responses. However, it is possible that the viral vector prime may have provided key adjuvanting signals preventing further class switch recombination to less functional subclasses during RV144 boosting and that protein immunization in alum alone, in the absence of additional strong inflammatory priming signals in VAX003, may have progressively driven class switch recombination from IgG3 to the less functional IgG1 and finally to IgG4.
Others factors they consider are that IgG2 Abs induced by VAX003 at early time points may have competed and blocked the recruitment of ADCC activity or that RV144 may have been more effective at inducing IgG3, with superior functionality:
Alternatively, IgG3 responses may be intrinsically transient because of their short serum half-life and may inevitably be lost in all vaccinees over time, as was observed in most RV144 vaccinees at week 52 (fig. S5). However, tetanus boosting specifically promotes IgG3 recall responses (32, 33), suggesting that IgG3 responses are not inherently transient, are retained in memory, and can be efficiently recalled. Thus, future vaccine design efforts aimed at defining the signals that may program and boost IgG3 responses specifically in the absence of class switch recombination may promote greater polyfunctional protection from infection.
Even at as low as 10% of total circulating antibodies, IgG3 can form complexes with IgG1, whereas low levels of IgG4 can have an equivalent outsize effect by competitively inhibiting rather than promoting Fc-mediated effector activities.
Formation of IgG1 complexes with subclasses in the absence of IgG4 appears to be necessary in order to drive Ab functionality:
ADCP: Antibody-dependent cellular phagocytosis.
ADCC: Antibody-dependent cellular cytotoxicity.
The depletion of IgG3 Abs from RV144 samples highlights the functional importance of HIV-specific IgG3 responses. Specifically, IgG3 depletion resulted in decreases in Fc-mediated effector activities and the loss of polyfunctionality, suggesting that despite being low in abundance, IgG3 Abs are potent effectors of the vaccine-induced humoral immune response. Despite the negative correlation between IgG3 responses and functionality in VAX003 (Fig. 3), depletion of IgG3 also resulted in a loss of ADCP, but not ADCC, further emphasizing the functional importance of this low-abundance Ab subclass in driving Ab functionality. The unexpected lack of a correlation between total IgG3 levels and ADCP activity in VAX003 (Fig. 3) may be related to the strong association between IgG3 levels and elevated levels of less functional Ab subclasses (IgG2 and IgG4; fig. S4) that may compete within the bulk polyclonal pool to diminish functionality, rather than to a direct antagonistic effect of IgG3 Abs in VAX003. Because the induction of ADCC and ADCP requires the engagement of low-affinity Fc receptors, Abs must form immune complexes to recruit the effector function of the innate immune cells. Therefore, IgG3 Abs, which represent less than 10% of total circulating Abs, likely cooperate with IgG1 to drive Ab functionality. Along these lines, although IgG3 depletion eliminated the polyfunctional profile of RV144 Abs, it did not completely abrogate all Ab functionalities, suggesting that additional Ab subclasses, especially IgG1, may coordinately drive Ab functionality. Thus, future efforts aimed at defining additional functional IgG1 Ab subpopulations that may function independently or in synchrony with IgG3 may provide critical insights in the design of a vaccine aimed at eliciting effective control of HIV.
In conclusion, antibody titre alone can not be used to predict efficacy, IgG subclasses and functional activity also need to be considered when designing a vaccine:
Thus, these data argue that vaccine strategies aimed at eliciting favorable subclass selection with broader coverage of the V2 loop may provide enhanced protection from infection. Moreover, measures of vaccine protective efficacy that move beyond titer alone and include variables such as Ab subclass and functional activity may provide critical new insights into the potential antiviral activity of Abs that extends beyond virus neutralization.
Comparison of Antibody Responses Induced by RV144, VAX003, and VAX004 Vaccination Regimens
And in 2017, Karnasuta et al compared antibody responses induced by the RV144, VAX003 and VAX004 HIV vaccine clinical trials4.
After 8 years, boosting with either the HIV/AIDSVAX B/E combination or AIDSVAX B/E alone (RV305) still increased IgG4 antibody titres, implying that long term class switching has occurred due to imprinting and this may not be reversed by further boosting.
…polyfunctionality in RV144 was tethered to IgG1 and IgG3 responses to Env with IgG3 acting as a surrogate to a coordinated IgG1 and IgG3 response.18 Evidence suggests that polyfunctional CD4+ T cells provided help for antibody generation as a correlate in primary analysis.7
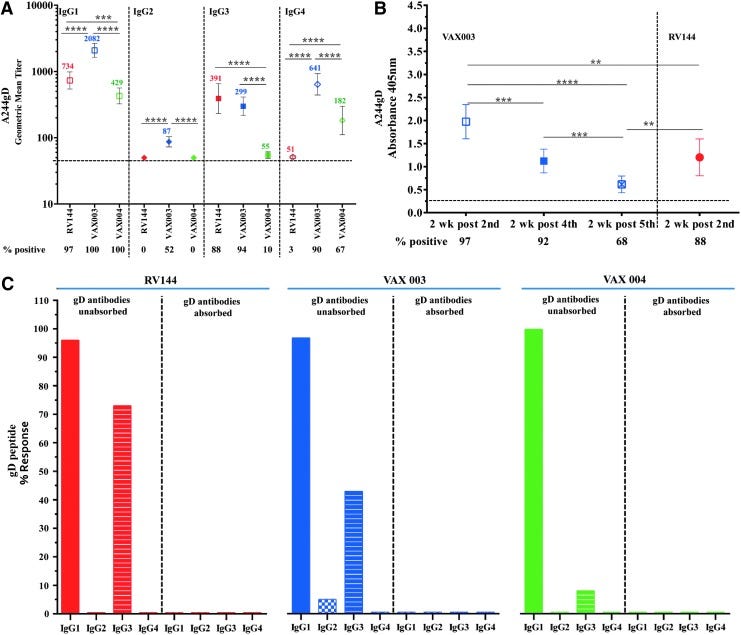
IgG antibody subclasses (IgG1-IgG4) to A244gD gp120 envelope
To characterize IgG subclass responses, we used gp120 A244gD and samples 2 weeks post last injection in RV144 (visit 8, week 26) and 2 weeks post fourth injection in VAX003 and VAX004 (week 54, A09) (Fig. 6A). All three vaccine regimens induced gp120 IgG1 antibodies with significantly higher responses in VAX003 (2,082, 100%) followed by RV144 (734, 97%) and VAX004 (429, 100%) (Fig. 6A). Low IgG2 responses (87, 52%) were detected only in VAX003. IgG3 antibodies were detected in VAX003 (299, 94%) and RV144 (391, 88%) (Not significant), while IgG3 responses in VAX004 were significantly lower in magnitude and frequency (55, 10%) than in RV144 and VAX003. IgG4 responses were significantly higher in VAX003 (641, 90%) than in VAX004 (182, 67%) and RV144 (51, 3%) (Fig. 6A).
Fig 6. Comparison of IgG subtypes binding to rgp120 HIV-1 CRF01_AE A244gD protein and gD peptide. (A) IgG subtype (1–4) binding to rgp120 A244gD; RV144 (red symbol) samples at 2-weeks post second (week 26, visit 8), VAX003 (blue symbol) and VAX004 (green symbol) samples at 2-weeks post fourth (week 50, visit A09) vaccination. Color coded numbers in the plot area show GMTs. Numbers under x-axis show percentage of positive samples (A405 ≥ 0.25) and responses above dotted line are considered positive. Symbols are GMTs with 95% confidence intervals. ***p < .001; ****p < .0001. (B) IgG3 antibodies binding to rgp120 A244gD; VAX003 (blue symbols) samples at 2-weeks post second (week 6, visit A05), fourth (week 50, visit A09) and fifth (week 74, visit A11), and RV144 at 2-weeks post second (week 26, visit 8) AIDSVAX B/E vaccination. Numbers under x-axis show percentage of positive samples (A405 ≥ 0.25) and responses above dotted line are considered positive. Symbols are means and bars indicate standard deviations. **p < .01; ***p < .001; ****p < .0001. (C) Samples were preincubated with HSV-1 gD peptide before adding to ELISA plates with gD peptide as the capturing antigen and compared to gD unabsorbed plasma. Antibody responses with A405 ≥ 0.25 (2.5 times the background, wells without capturing antigen) were considered positive. Solid colors: IgG1, checkered columns: IgG2, and stripped columns: IgG3. RV144 samples in red at 2-weeks post second injection (visit 8), VAX003 and VAX004 samples, are in blue and green respectively, at 2-weeks post fourth (visit A09) AIDSVAX vaccinations. HSV-1, herpes simplex virus 1.
IgG4 antibodies to A244gD protein were detected in VAX trials after four protein immunizations (A09) but were not detected in RV144 at peak immunogenicity (visit 8). The lack of IgG4 responses in RV144 might be attributed to the ALVAC vaccinations. We therefore, investigated the presence of IgG4 in VAX003 after two protein immunizations (week 6, A05), the same proteins boosts as in RV144. We showed that only 1/50 vaccinees had IgG4 to A244gD in VAX003 at visit A05, comparable to 1/32 in RV144 at visit 8 (data not shown) suggesting that IgG4 were induced after multiple protein immunizations.
IgG2 responses were only detected in 5% of samples in VAX003 and none of the vaccines induced IgG4 antibodies to the gD peptide at the visits tested.
The gD peptide induced IgG1, IgG2, and IgG3, but not IgG4. Increasing the number of immunizations in the VAX trials decreased the gD peptide-specific responses because IgG4 (not binding to gD) was induced while IgG3 (binding to gD) levels declined. The reasons for IgG4 subclass discrimination to gD peptide are not clear. IgG4 generation was associated with increased number of protein boosts.
Since IgG3 antibodies are polyfunctional, this may have affected downstream events with FC receptors. IgG3 antibodies can fix complement, have high affinity for FcγR:IgG3>IgG1>IgG2>IgG4 (reviewed in Forthal et al.33), and have been associated with protection in several other infections.34,35 Spontaneous control of HIV infection in the absence of antiretroviral therapy is associated with the generation of high levels of p24 and gp120-specific IgG1 and the maintenance of gp120-specific IgG3 antibodies.36
We showed that IgG4 antibodies to A244gD were induced in both VAX003 and VAX004 after four protein boosts but not in RV144 after two protein immunizations, agreeing with similar studies.15 However, when we investigated the generation of IgG4 in VAX003 after two protein vaccinations we did not detect significant differences between RV144 and VAX003 indicating that more than two protein immunizations are needed to induce IgG4 antibodies. In other studies, multiple HIV-1 Env protein immunizations induced IgG4.37 While IgG4 was induced with repeated vaccinations in VAX003, IgG3 decreased gradually and after five protein immunizations responses were significantly lower than earlier boosts and RV144 and just above the background. These observations show a dynamic change of IgG subclass contribution, IgG3 decreasing and IgG4 increasing, to the total IgG with multiple protein immunizations and consequently an alteration of downstream cellular events that depend on the interaction of IgG subclasses with the Fc receptors on effector cells. Therefore, multiple protein boosts can alter the concentration and subclasses of IgG to V1 V2 scaffolds and other epitopes.
Comparison of antibody functionality showed that RV144 induced highly functional IgG3, whereas VAX003 after seven protein immunizations elicited mono-functional antibodies with high levels of IgG4.38 IgG subclass switching from highly functional antibodies to IgG4 may pose a new challenge for HIV vaccines as multiple immunizations and vaccine regimens may generate durable antibody responses but antibodies may not be of the highly functional IgG1 and IgG3 subclasses. Factors that determine subclass contribution to total IgG are not fully understood but adjuvants, antigens, vaccine dose, route of administration, age, and vaccine production methodologies could play a role.39 Decreased IgG3 and increased of IgG4 and IgG1 subclasses were observed in RV305, a follow-up study in which RV144 uninfected volunteers who received all vaccinations 6–8 years earlier were boosted with ALVAC-HIV/AIDSVAX B/E or AIDSVAX B/E alone or ALVAC-HIV alone.40,41
In all three HIV-1 trials, the antibody responses observed a typical saw-tooth pattern and were poorly sustained over time. Conflicting results have been observed42,43 but none match the long duration of antibody responses observed with other common viral and vaccine antigens.44 It is hypothesized that plasma cells are imprinted with a predetermined lifespan based on the magnitude of B cell signaling that occurs during the induction of an antigen-specific humoral immune response.45 Moreover, additional AIDSVAX B/E, two late boosts given 8 years post last RV144 vaccination of the ALVAC-HIV/AIDSVAX B/E combination or AIDSVAX B/E alone (RV305) did increase IgG1 and IgG4 but not IgG3 subclass antibodies.40,41
Author Disclosure Statement
Co-author James Tartaglia is an employee of Sanofi Pasteur. Co-authors Faruk Sinangil and Donald P. Francis are employees of GSID. Other co-authors declare no competing interests.
Boosting with AIDSVAX B/E Enhances Env Constant Region 1 and 2 Antibody-Dependent Cellular Cytotoxicity Breadth and Potency
Then in 2020, Easterhoff et al also studied the effect of late boosting of the previous discussed vaccinees in follow up RV305 HIV-1 vaccine trial (NCT01435135)5.
They found that although late boosting of the previous vaccinees increased ADCC breadth and potency, boosting in this case could not totally reverse the effects of class switching to IgG4 of VAX003 and VAX004 vaccinees, and repeated boosting led to a further decline in ADCC.
4 clonal lineage members were isolated from PBMCs collected from the same vaccine recipient 2 weeks after the second boost with AIDSVAX B/E alone given in the RV305 clinical trial (RV305 group II). Thus, this B cell clonal lineage belongs to a long-lived memory B cell pool started by the RV144 vaccine regimen and boosted many years later with the RV305 vaccine regimen.
They caution that even with the RV144 trial repeat boosting may not be effective:
Thus, clonal lineages with fine epitope specificities capable of being matured to increased ADCC breadth and potency greatly benefited from the two AIDSVAX B/E boosts given in the RV305 clinical trial. It should be noted that in VAX003 and VAX004 clinical trials ADCC responses peaked at 3 to 4 immunizations and declined after 5 to 7 immunizations (20). Collectively these data indicate that while the RV144 clinical trial was underboosted, repetitive subsequent boosting beyond RV305 does not necessarily lead to continuously better functional antibody outcomes.
They suggest that boosting with long rest periods of greater than or equal to 1-2 years may be necessary to avoid skewing antibody subclasses.
Improving vaccine-induced NNAb effector function will also require more detailed immunological studies on the timing and frequency of boosting. In the VAX003 (ClinicalTrials registration no. NCT00002441) and VAX004 (ClinicalTrials registration no. NCT00002441) trials, frequent protein immunizations skewed Env-specific antibody subclass usage from the highly functional IgG3 to IgG4 (21,–23). The RV305 boosts that were studied here occurred several years (6 to 8 years) after the final RV144 boost, unlike previous HIV-1 vaccine trials. Whether the boosting interval can be shortened without skewing antibody subclass usage is not known, but it is possible that boosting with long rest intervals (≥1 to 2 years) will be necessary.
Above is the only direct reference to IgG4 in the entire paper.
Conflicts of interest may be a factor:
B.F.H., G.F., and D.E. have patents submitted on antibodies listed in this paper.
Conclusions
Antibodies are not a correlate of immunity, but studies have shown that the presence of relatively low levels of IgG4 can cause impaired Fc-mediated effector functionality. B-cell imprinting may be implicated and the effects appeared to persist for at least 6 - 8 years. Use of strong adjuvants may partially reverse this, but this also carries its own risk profile.
According to these studies, boosting more than 3-4 times is not recommended and the gap between boosters should be 1-2 years minimum to minimise the risk of class switching and imprinting - provided there isn’t persistent exposure to antigens during this period.
However, research has found evidence for this with some experimental mRNA gene therapies6, and re-infections can increase exposure further, mediating yet more class switching according to the previously referenced works.
These experimental transfection agents and subsequent boosters should be withdrawn immediately as experimental data indicates that antibody titres and not subclasses such as IgG4 or other contributors to an effective immune response were adequately considered in their design or assessed for in clinical trials until now.
Acknowledgement
A shout out must go to Charles Rixey for making the connection, finding this paper and prompting further investigations:
…I came across this "Nevertheless, the recent discovery of DC-SIGN expression on activated B cells and of its role in relevant pathogenic processes in HIV infection, such as polyclonal Ig class switching" and I'm pondering... what if the IgG4 problem is ALSO tied back to the gp120 inserts, in a way that is exacerbated by the mRNA method of jabbing?
HIV-1 Envelope Triggers Polyclonal Ig Class Switch Recombination through a CD40-Independent Mechanism Involving BAFF and C-Type Lectin Receptors (2006)
References:
Another HIV vaccine failure: where to next?. Nat Med 19, 1576–1577 (2013). https://doi.org/10.1038/nm.3413
Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH; MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209-20. doi: 10.1056/NEJMoa0908492. Epub 2009 Oct 20. PMID: 19843557.
Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014 Mar 19;6(228):228ra38. doi: 10.1126/scitranslmed.3007736. PMID: 24648341.
Karnasuta C, Akapirat S, Madnote S, Savadsuk H, Puangkaew J, Rittiroongrad S, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Tartaglia J, Sinangil F, Francis DP, Robb ML, de Souza MS, Michael NL, Excler JL, Kim JH, O'Connell RJ, Karasavvas N. Comparison of Antibody Responses Induced by RV144, VAX003, and VAX004 Vaccination Regimens. AIDS Res Hum Retroviruses. 2017 May;33(5):410-423. doi: 10.1089/AID.2016.0204. Epub 2017 Jan 30. PMID: 28006952; PMCID: PMC5439458.
Easterhoff D, Pollara J, Luo K, Tolbert WD, Young B, Mielke D, Jha S, O'Connell RJ, Vasan S, Kim J, Michael NL, Excler JL, Robb ML, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Nitayaphan S, Sinangil F, Tartaglia J, Phogat S, Kepler TB, Alam SM, Wiehe K, Saunders KO, Montefiori DC, Tomaras GD, Moody MA, Pazgier M, Haynes BF, Ferrari G. Boosting with AIDSVAX B/E Enhances Env Constant Region 1 and 2 Antibody-Dependent Cellular Cytotoxicity Breadth and Potency. J Virol. 2020 Jan 31;94(4):e01120-19. doi: 10.1128/JVI.01120-19. PMID: 31776278; PMCID: PMC6997759.
Turner JS, O'Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, Lei T, Thapa M, Chen RE, Case JB, Amanat F, Rauseo AM, Haile A, Xie X, Klebert MK, Suessen T, Middleton WD, Shi PY, Krammer F, Teefey SA, Diamond MS, Presti RM, Ellebedy AH. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021 Aug;596(7870):109-113. doi: 10.1038/s41586-021-03738-2. Epub 2021 Jun 28. PMID: 34182569; PMCID: PMC8935394.






I would recommend looking into this:
"An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy"
https://pubmed.ncbi.nlm.nih.gov/32819973/
The pursuit of HIV vaccines was a con game from the start. Before I proceed, let me be clear. My decade-plus examination of HIV and AIDS in dozens of federal court cases proved that:
1. HIV IS REAL: I bought, examined, and photographed copies of Montagnier’s original LAV.
2. AIDS IS REAL: The immune systems of all living organisms are eventually compromised by entropy, age, and various toxins, environments, and behaviors.
3. No one has ever proven that HIV causes AIDS.
So-called HIV “co-discoverer” Robert Gallo never proved that HIV attacks cells or causes AIDS. SCIENCE published his four papers without peer review. They were never peer reviewed.
* https://omsj.org/reports/Gallo1984a.pdf
* https://omsj.org/reports/Gallo1984b.pdf
* https://omsj.org/reports/Gallo1984c.pdf
* https://omsj.org/reports/Gallo1984d.pdf
Hundreds of other "researchers" have since uncritically referenced his unproven hypothesis.
Gallo’s “co-discoverer”, Luc Montagnier, admitted that HIV can be cured within weeks with clean water and good nutrition WITHOUT the need for drugs or vaccines. https://youtu.be/PyPq-waF-h4?t=1040
According to Peter Duesberg (who mapped the genome of the retrovirus), all retroviruses are non-pathogenic. Thus, HIV is a) non-pathogenic, b) doesn’t naturally exist in the human body, and c) no one ever proved otherwise. https://omsj.org/reports/Duesberg1988.pdf
Real scientists would establish a cause for a disease before developing interventions to fight fake diseases. Arguing cures for fake diseases generates billions, but taxpayer shakedowns have nothing to do with infectious disease mortality that became statistically irrelevant by 1955. https://www.omsj.org/reports/Armstrong%201999.pdf
Respectfully...