A single-cell atlas reveals shared and distinct immune responses and metabolism during SARS-CoV-2 and HIV-1 infections
(preprint, 2022)
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
https://threadreaderapp.com/thread/1481683717785624577.html
https://twitter.com/OntarioDoctor2/status/1510258964289753091?s=19
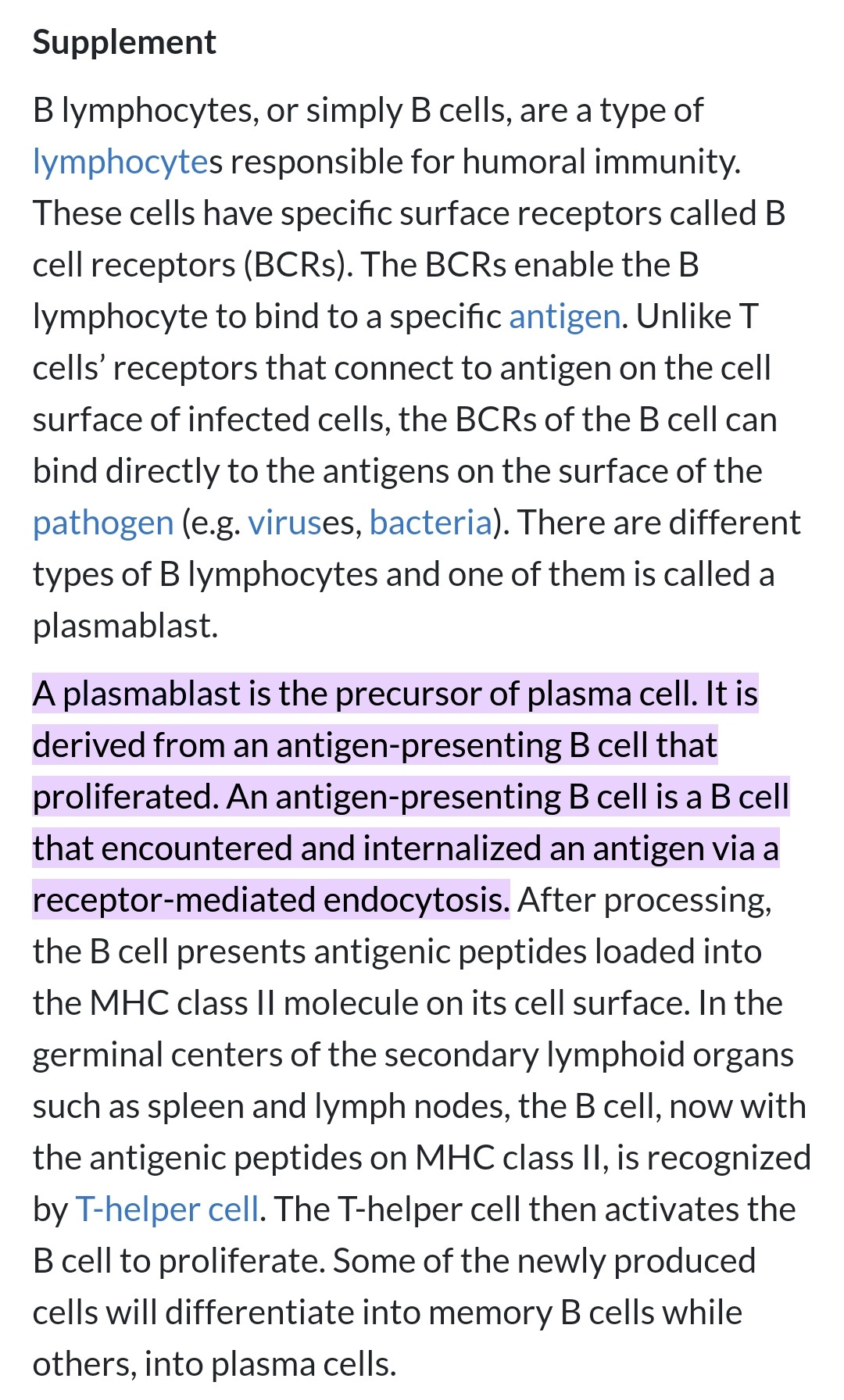
Plasmablast:
https://www.biologyonline.com/dictionary/plasmablast
What is the Difference Between CD4 and CD8 T Cells
“The main difference between CD4 and CD8 T cells is that the CD4 T cells are the helper T cells, which assist other blood cells to produce an immune response, whereas the CD8 T cells are the cytotoxic T cells that induce cell death either by lysis or apoptosis.”
https://pediaa.com/what-is-the-difference-between-cd4-and-cd8-t-cells/
The paper:
A single-cell atlas reveals shared and distinct immune responses and metabolism during SARS-CoV-2 and HIV-1 infections
Summary
SARS-CoV-2 and HIV-1 are RNA viruses that have killed millions of people worldwide. Understanding the similarities and differences between these two infections is critical for understanding disease progression and for developing effective vaccines and therapies, particularly for 38 million HIV-1+ individuals who are vulnerable to SARS-CoV-2 co-infection. Here, we utilized single-cell transcriptomics to perform a systematic comparison of 94,442 PBMCs from 7 COVID-19 and 9 HIV-1+ patients in an integrated immune atlas, in which 27 different cell types were identified using an accurate consensus single-cell annotation method. While immune cells in both cohorts show shared inflammation and disrupted mitochondrial function, COVID-19 patients exhibit stronger humoral immunity, broader IFN-I signaling, elevated Rho GTPase and mTOR pathway activities, and downregulated mitophagy. Our results elucidate transcriptional signatures associated with COVID-19 and HIV-1 that may reveal insights into fundamental disease biology and potential therapeutic targets to treat these viral infections.
Highlights
COVID-19 and HIV-1+ patients show disease-specific inflammatory immune signatures
COVID-19 patients show more productive humoral responses than HIV-1+ patients
SARS-CoV-2 elicits more enriched IFN-I signaling relative to HIV-I
Divergent, impaired metabolic programs distinguish SARS-CoV-2 and HIV-1 infections
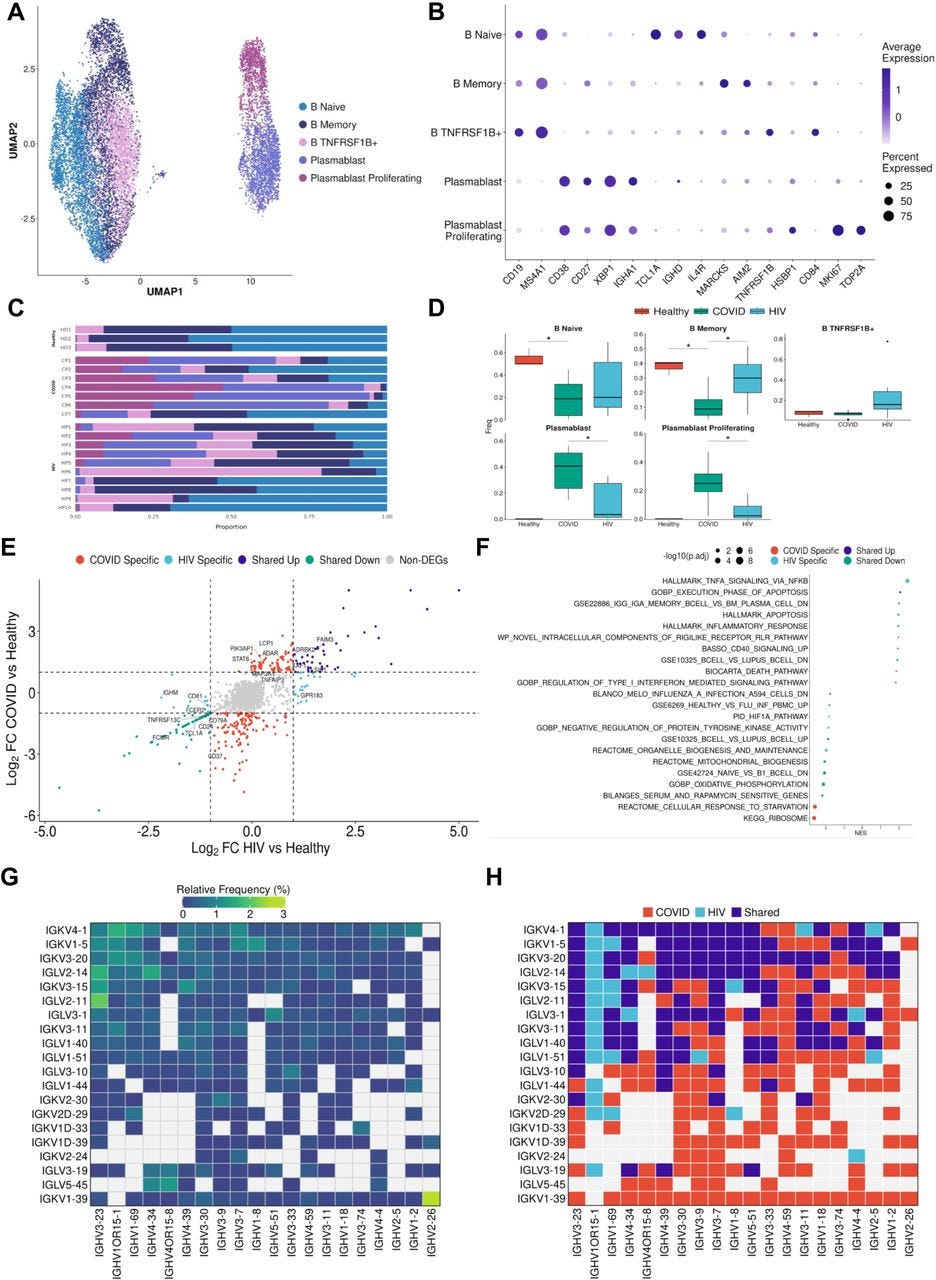
…Consistent with prior COVID-19 studies that have shown extensive plasmablast expansion in patients (Bernardes et al., 2020; De Biasi et al., 2020; Kuri-Cervantes et al., 2020), we found that COVID-19 patients have significantly higher proportions of plasmablasts and proliferating plasmablasts compared to healthy controls (Figures 4C and 4D). Consistent with this result, we found that COVID-19 patients have significantly lower proportions of naïve and memory B cells compared to healthy controls (Figures 4C and 4D). Interestingly, we found a significant enrichment of TNFRSF1B+ B cells in HIV-1+ patients (Figure 4D). These B cells are a subset of effector memory-like B cells given their expression memory B-cell marker genes (intermediate expression of AIM2 and CD27) and upregulation of TNFRSF1B and CD84. CD84 has been shown to be upregulated on a subset of memory B cells that exhibit higher levels of proliferation and has been associated with B cell activation and signal transduction (Tangye et al., 2002), while TNFRSF1B encodes for a TNF-receptor protein that has been known to induce TNF-mediated apoptosis. While HIV-1+ patients also had increased proportions of plasmablast and proliferating plasmablast subsets compared to healthy controls, these responses were more moderate compared to COVID-19 patients. We found COVID-19 plasmablasts to express higher levels of MKI67, IGHM, CCR2, and XBP1 compared to HIV-1 plasmablasts, suggesting their elevated proliferation and maturation (Figure S4D).
Figure 4:
B cells in COVID-19 show more robust plasmablast response and antibody diversity relative to HIV-1
(A) UMAP embeddings of B cells colored by subtype.
(B) Dot plot of canonical B cell marker expression across subtypes.
(C) Stacked bar plots of the relative frequency of subtypes present in each patient.
(D) Box plots of the relative frequency of subtypes present in each condition. Significance testing was doing using student’s t-test. “*”: P < .05.
(E) Double differential gene expression plot of genes that are differentially expressed between COVID-19 patients compared to healthy controls or differentially expressed between HIV-1 patients compared to healthy controls.
(F) Dot plot of enriched biological pathways from differentially expressed genes that were found to be upregulated (right, positive) or downregulated (left, negative) compared to healthy controls.
(G) Heatmap of top 20 light chain (Y axis) and heavy chain (X axis) combinations found in HIV-1+ and COVID-19 patients.
H) Heatmap indicating the light chain/heavy chain combinations that are either unique to HIV-1 (light blue), COVID-19 (red), or shared across the two diseases (dark blue).
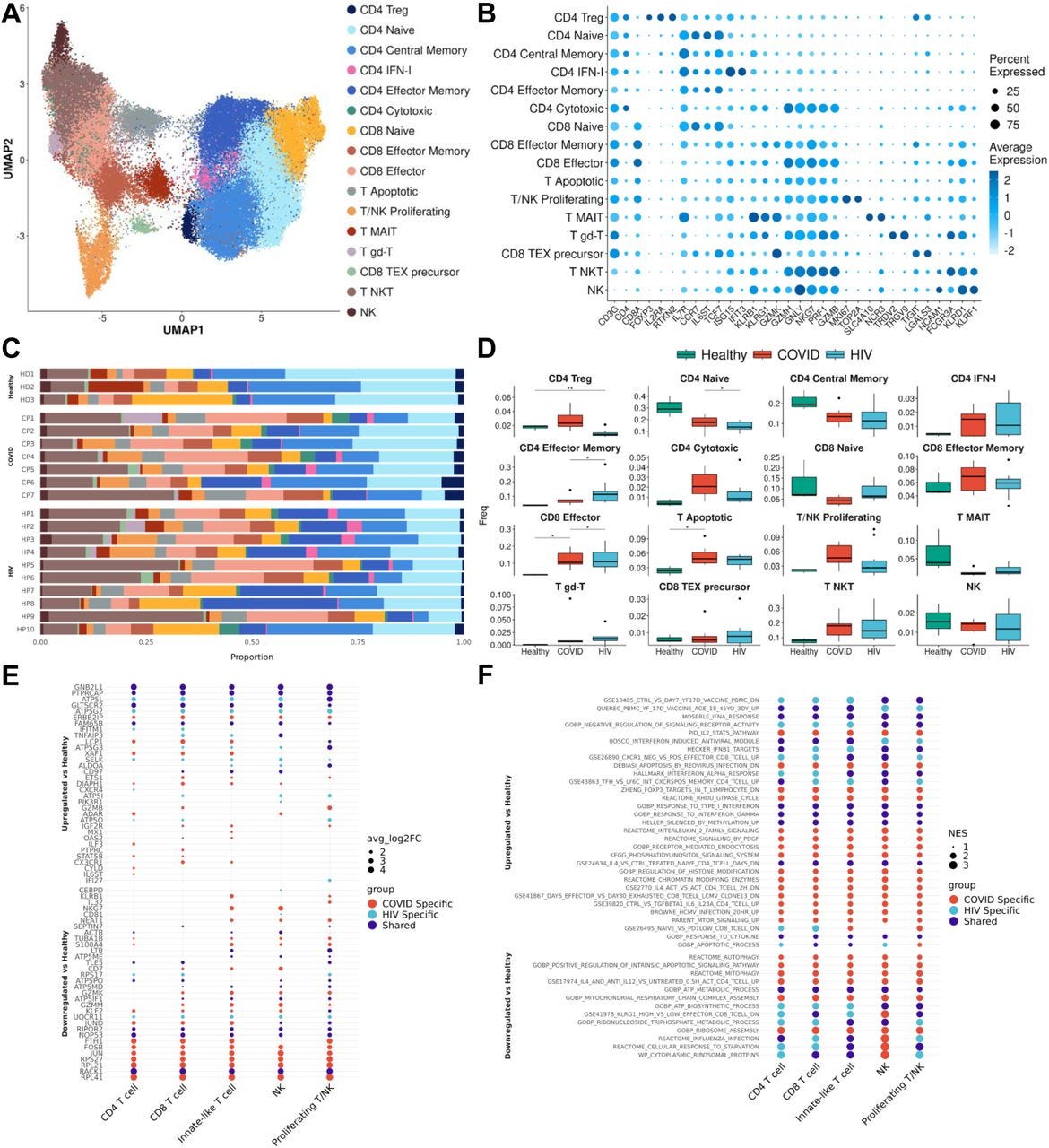
…After integration and clustering of only T cells and NK cells, we found 16 subpopulations in total (Figures 5A and 5B). Among those, three subpopulations were particularly noteworthy. We found an increase in IFN-I+ CD4+ cells in both COVID-19 and HIV-1+ patients, showing high expression of IFN-I-stimulated genes ISG15 and IFIT3, as well as IL7R, which were reported to be upregulated by IFNβ in CD4+ T cells (Hoe et al., 2010). The second is apoptotic T cells (Figures 5A and 5B), which feature a high expression of mitochondrial genes characteristic of dying cells (Zhu et al., 2020). These two clusters are enriched in both COVID-19 and HIV-1+ patients compared to healthy controls, although COVID-19 patients have an even higher proportion of IFN-1+ CD4+ T cells compared to HIV-1+ patients (Figures 5C and 5D). The enrichment of apoptotic T-cell subpopulations in both groups is consistent with the notion that HIV-1 infection leads to low levels of CD4+ T cells through pyroptosis of abortively infected T cells (Doitsh et al., 2014) and apoptosis of uninfected bystander cells (Garg et al., 2012) as well as the observation that severe COVID-19 patients frequently experienced lymphopenia (Chen et al., 2020b). Finally, there exists a third cytotoxic CD4+ T-cell subpopulation, featuring elevated markers of cytotoxicity (GZMH, GNLY, NK7G, PRF1, and GZMB), indicating a more cytotoxic phenotype (Figures 5A and 5B). The frequency of cytotoxic CD4+ T cells is dramatically increased for both cohorts and is higher in COVID-19 patients, suggesting that viral infection can induce CD4+ T cell cytotoxicity and the effect is stronger in SARS-CoV-2 infection (Figures 5C and 5D). The frequency of naïve T cells decreased significantly in COVID-19 and HIV-1+ patients; correspondingly, effector memory T cells and effector T cells are enriched in both patients, with more enrichment in HIV-1+ patients, suggesting these two viral infections can cause different levels of T-cell activation (Figures 5C and 5D).
Figure 5:T cells in COVID-19 and HIV-1 show varied IFN-I and activation signatures(A) UMAP embeddings of T cells colored by subtype.(B) Dot plot of canonical T cell marker expression across subtypes.(C) Stacked bar plots of the relative frequency of subtypes present in each patient.(D) Box plots of the relative frequency of subtypes present in each condition. Significance testing was doing using student’s t-test. “*”: P < .05, “**”: P < .01.(E) Dot plots of the key genes differentially upregulated (top) or downregulated (bottom) compared to healthy controls.(F) Dot plot of enriched biological pathways from differentially expressed genes that were found to be upregulated (top) or downregulated (bottom) compared to healthy controls.
Figure 6:
IFN-I signaling is correlated with divergent biological functions in COVID-19 versus HIV-1
(A) Network plot of genes related to IFN-I signaling that are differentially upregulated in COVID-19 (Right), HIV-1 (Left), or jointly upregulated compared to healthy controls. Genes are colored based on Log-2-fold change in expression in HIV-1+ versus COVID-19 patients.
(B) Violin plots of the normalized gene expression of the three IFN-I signatures in A), split across conditions (top) and major cell populations (bottom).
(C) Normalized gene expression plots of IFN-I genes in COVID-19 (top) and HIV-1 (bottom).
(D) Network plot of key pathways correlated with IFN-I signaling in COVID-19 (left) and HIV-1+ patients (right). Size of each center corresponds to the number of genes present in the pathway. Genes are colored based on Log-2-fold change in expression in HIV-1+ versus COVID-19 patients.
(E) Violin plot of the normalized gene expression of the actin polymerization pathway across each condition, split by selected cell subsets exhibiting high expression.
(F) Violin plot of the normalized gene expression of the TLR signaling pathway across each condition, split by selected cell subsets exhibiting high expression.
…Using bicorrelation analysis on disease-specific IFN-I genes, we found a much higher number of top correlated genes and enriched pathways in COVID-19 compared to HIV-1 (Figure 6D). Notably, COVID-19 IFN-I correlated genes were enriched in MAPK signaling and interleukin signaling, which have been implicated in inflammation, thrombosis, and pulmonary injury and cytokine storm, respectively (Figure 6D, left).
…Notably, our analysis also revealed disease-specific altered metabolism profiles. We characterize a decrease in OXPHOS and ribosome biogenesis in response to both SARS-CoV-2 and HIV-1 infection. Virus-induced reduction of OXPHOS has been previously characterized in other diseases and could be a result of oxidative stress triggered by mitochondrial clustering (Khan et al., 2015). Viral hijacking of ribosomal function is also crucial to viral replication and survival in the host (Li, 2019). Disruption of these viral interactions could be advantageous for COVID-19 and HIV-1 treatment. We also unveiled molecular metabolic pathways which could also be targeted for therapy. For example, we found enriched proteasomal genes in COVID-19 patients. It has been proposed that proteasome inhibitors may be a possible therapy for COVID-19, since proteasome inhibitor may interfere with the viral replication processes and reduce the cytokine storm associated with various inflammatory conditions (Longhitano et al., 2020). Consistent with our observation of the upregulation of Rho GTPase in COVID-19 patients; the plausibility of using Rho kinase inhibitors to treat COVID-19 has been discussed, as they can restore the activity and level of ACE2 which is inhibited by SARS-CoV-2 without increasing the risk of infection (Abedi et al., 2020b). Although remained to be investigated in immune cells, a recent study demonstrated that small GTPase RhoA activation drives increased cellular glycolytic capacity (Wu et al., 2021a) which is typically associated with reduced mitochondrial metabolism, in agreement with the upregulation of Rho GTPase and disrupted mitochondrial function in COVID-19 patients. Moreover, Rho GTPases have been linked to additional key metabolic controls such as mTOR signaling pathways. (Mutvei et al., 2020; Senoo et al., 2019). Finally, we found COVID-19 specific upregulation of the mTOR pathway, and thus its inhibitors may also be used for treatment, since mTOR inhibitors can adjust T cells by induction of autophagy without apoptosis, reduce viral replication, restore T-cell function, and decrease cytokine storm (Mashayekhi-Sardoo and Hosseinjani, 2021).
In conclusion, our study provides a comprehensive comparison of the immunological landscape of SARS-CoV-2 and HIV-1 infections in humans. The high resolution of single-cell RNA sequencing, diversity of patient samples, and large dataset allowed us to unveil important shared and disease-specific features that offer insight into the next generation of antiviral treatments. Through cell type-specific analysis, we found a common enrichment of activated B cells and plasmablasts, inflammatory monocyte and effector T cell subsets, and cytokine signaling that appear to drive the antiviral response to SARS-CoV-2 and HIV-1. We also found dendritic cells and monocytes to be highly interactive with adaptive immune cells in both diseases, but found that innate cells in COVID-19 appear to be more capable of immunosuppressive function through CTLA-4 and TIM-3-mediated interactions. We also found that the cytokine response was more diverse in COVID-19 patients, which is highlighted by IL-2, IL-4, and IL-20 signaling, while HIV-1+ individuals primarily exhibited high levels of NF-kB signaling. Our analysis corroborated pathways in COVID-19 patients that have already shown therapeutic benefits, but further in vitro and in vivo experiments are necessary to measure the contribution of other molecular pathways (such as Rho GTPase and IL-2 signaling) that also appear to be distinctively enriched. Overall, our study provides a roadmap to help develop novel drugs to treat COVID-19 and HIV-1 infections.
Full paper:
https://www.biorxiv.org/content/10.1101/2022.01.10.475725v1.full
If it's ROS then transfection mediated spike protein can do the same.
Published: 11 March 2022
ACE2-independent infection of T lymphocytes by SARS-CoV-2
Abstract
SARS-CoV-2 induced marked lymphopenia in severe patients with COVID-19. However, whether lymphocytes are targets of viral infection is yet to be determined, although SARS-CoV-2 RNA or antigen has been identified in T cells from patients. Here, we confirmed that SARS-CoV-2 viral antigen could be detected in patient peripheral blood cells (PBCs) or postmortem lung T cells, and the infectious virus could also be detected from viral antigen-positive PBCs. We next prove that SARS-CoV-2 infects T lymphocytes, preferably activated CD4 + T cells in vitro. Upon infection, viral RNA, subgenomic RNA, viral protein or viral particle can be detected in the T cells. Furthermore, we show that the infection is spike-ACE2/TMPRSS2-independent through using ACE2 knockdown or receptor blocking experiments. Next, we demonstrate that viral antigen-positive T cells from patient undergone pronounced apoptosis. In vitro infection of T cells induced cell death that is likely in mitochondria ROS-HIF-1a-dependent pathways. Finally, we demonstrated that LFA-1, the protein exclusively expresses in multiple leukocytes, is more likely the entry molecule that mediated SARS-CoV-2 infection in T cells, compared to a list of other known receptors. Collectively, this work confirmed a SARS-CoV-2 infection of T cells, in a spike-ACE2-independent manner, which shed novel insights into the underlying mechanisms of SARS-CoV-2-induced lymphopenia in COVID-19 patients.
https://www.nature.com/articles/s41392-022-00919-x
Natural immunity? Yes please. Since 2020 we have learned that the transfection agents are also cytotoxic as well as immunosuppressive. Which means you are then being exposed to both a number of boosters for negative benefit and enhanced viral infection as a bonus, double jeopardy.
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #6: consistent pathophysiological alterations after vaccination with COVID-19 vaccines
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #10: SARS-CoV-2 spike protein induces monocyte apoptosis and interleukin-8 production
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #2: SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency/comments?s=w
If the cells are persistently expressing a cytotoxic & de facto carcinogenic agent you cannot switch off then this is an extremely bad idea. Think lymphadenopathy or lymphoma sort of bad. Think glioblastoma & breast cancer relapse sort of bad.
Gone in 2 days it is not:
SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses (2021)
Abstract
SARS-CoV-2 mRNA-based vaccines are about 95% effective in preventing COVID-191,2,3,4,5. The dynamics of antibody-secreting plasmablasts and germinal centre B cells induced by these vaccines in humans remain unclear. Here we examined antigen-specific B cell responses in peripheral blood (n = 41) and draining lymph nodes in 14 individuals who had received 2 doses of BNT162b2, an mRNA-based vaccine that encodes the full-length SARS-CoV-2 spike (S) gene1. Circulating IgG- and IgA-secreting plasmablasts that target the S protein peaked one week after the second immunization and then declined, becoming undetectable three weeks later. These plasmablast responses preceded maximal levels of serum anti-S binding and neutralizing antibodies to an early circulating SARS-CoV-2 strain as well as emerging variants, especially in individuals who had previously been infected with SARS-CoV-2 (who produced the most robust serological responses). By examining fine needle aspirates of draining axillary lymph nodes, we identified germinal centre B cells that bound S protein in all participants who were sampled after primary immunization. High frequencies of S-binding germinal centre B cells and plasmablasts were sustained in these draining lymph nodes for at least 12 weeks after the booster immunization. S-binding monoclonal antibodies derived from germinal centre B cells predominantly targeted the receptor-binding domain of the S protein, and fewer clones bound to the N-terminal domain or to epitopes shared with the S proteins of the human betacoronaviruses OC43 and HKU1. These latter cross-reactive B cell clones had higher levels of somatic hypermutation as compared to those that recognized only the SARS-CoV-2 S protein, which suggests a memory B cell origin. Our studies demonstrate that SARS-CoV-2 mRNA-based vaccination of humans induces a persistent germinal centre B cell response, which enables the generation of robust humoral immunity.
Fig. 1: Plasmablast and antibody response to SARS-CoV-2 immunization.
a, Study design. Forty-one healthy adult volunteers (ages 28–73, 8 with a history of SARS-CoV-2 infection) were enrolled and received the BNT162b2 mRNA SARS-CoV-2 vaccine. Blood was collected before immunization, and at 3, 4, 5, 7 and 15 weeks after immunization. For 14 participants (ages 28–52, none with a history of SARS-CoV-2 infection), FNAs of ipsilateral axillary lymph nodes (LNs) were collected at 3, 4, 5, 7 and 15 weeks after immunization. b, c, ELISpot quantification of S-binding IgG- (b) and IgA- (c) secreting plasmablasts (PBs) in blood at baseline, and at 3, 4, 5 and 7 weeks after immunization in participants without (red) and with (black) a history of SARS-CoV-2 infection. d, Plasma IgG titres against SARS-CoV-2 S (left) and the RBD of S (right) measured by ELISA in participants without (red) and with (black) a history of SARS-CoV-2 infection at baseline, and at 3, 4, 5, 7 and 15 weeks after immunization. Dotted lines indicate limits of detection. Symbols at each time point in b–d represent one sample (n = 41). Results are from one experiment performed in duplicate.
Full paper:










Another great post, thank you. I added some of this info to my post and mentioned yours:
https://medquotes.substack.com/p/not-new-arginine-hiv-tat-lab-tool
This post looks at HIV-1 Tat arginine sequences that seem to appear in Spike, and pertains both to destruction of immune cells and to mitochondrial dysfunction.