Last update: 15th May ‘22.
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
Global mystery hepatitis outbreak spreads to Asia and Canada
Japan reports child with acute liver disease of unknown origin, and Canada investigating similar cases, with nearly 200 now recorded worldwide
Wed 27 Apr 2022
A mysterious liver disease that has infected children in a dozen countries around the world has reached Asia, with a case reported in Japan.
The case in Japan of acute hepatitis – or inflammation of the liver – of unknown origin was flagged by local authorities on 21 April in a child who had tested negative for adenovirus – a possible cause being investigated worldwide – and Covid-19.
The patient had not had a liver transplant, the health ministry said on Monday, without giving further details.
At least one child has died from mystery strain of severe hepatitis, WHO confirms
Canada’s Public Health Agency said on Tuesday it was investigating reports of severe acute hepatitis of unknown origin in young children. It did not reveal the number of cases or their location.
So far across the world, 190 mystery cases of acute hepatitis in children have been reported, with 140 of them in Europe, mostly in the UK (110 cases). Further cases have been found in Israel and in the United States. Seventeen children became so sick they needed liver transplants.
The US Centers for Disease Control and Prevention said in a nationwide health alert last week that the first US cases were identified in October in Alabama. The first UK cases were recorded in January.
On Saturday, the World Health Organization (WHO) said at least one death had been reported in connection the outbreak. The UN health agency said the cases were reported in children aged between one month and 16 years. The WHO did not say in which country the death occurred.
Hepatitis is usually caused by one of several contagious hepatitis viruses, but these have not been found in the affected children. Jaundice, diarrhoea and abdominal pain are among reported symptoms.
One theory being investigated by the UK Health Security Agency is that a lack of exposure to the common adenovirus – which usually causes stomach upsets and colds – during the coronavirus pandemic has led to more severe illness among children. Of 53 cases tested in the UK, 40 (75%) showed signs of adenovirus infection.
Public Health Scotland’s director, Jim McMenamin, told Reuters that work was under way to understand if an adenovirus has mutated to cause more severe disease, or if it could be causing the problems “in tandem” with another virus, including possibly Sars-CoV-2, the virus that causes Covid-19.
UK officials said there was “no link” between the cases and the Covid-19 vaccine, because none of the children affected by hepatitis had received a jab.
Andrea Ammon, director at the European Centre for Disease Prevention and Control in Stockholm, said: “So far there is no connection between the cases and no association to travel.”
The disease appeared in previously healthy children, Ammon said.
Number of UK children suffering from hepatitis rises to 145
Concerns rise about surge as scientists say lack of exposure to viruses during Covid restrictions could be factor
Hannah Devlin Science correspondent
Fri 29 Apr 2022
The number of children in the UK suffering from severe hepatitis has risen to 145 as concerns mount about the mysterious surge in cases.
The UK Health Security Agency (UKHSA) announced an increase of 34 cases but said most children have recovered and no children have died. There has been no increase from the 10 children who have required a liver transplant, reported on Monday.
Scientists believe there could be a link to adenovirus infection, which has been detected in the majority of cases, but are continuing to investigate the cause. Hepatitis is not a common side-effect from adenovirus, which typically causes cold-like symptoms and nausea, so scientists are investigating whether a co-infection, including with Covid-19, or non-infectious causes such as food poisoning, drug or metal exposure, could be playing a role.
Global mystery hepatitis outbreak spreads to Asia and Canada
“We are also exploring whether increased susceptibility due to reduced exposure during the Covid pandemic could be playing a role, or if there has been a change in the genome of the adenovirus,” UKHSA said in a statement.
Dr Meera Chand, the agency’s director of clinical and emerging infections, said: “We know that this may be a concerning time for parents of young children. The likelihood of your child developing hepatitis is extremely low. However, we continue to remind parents to be alert to the signs of hepatitis – particularly jaundice, which is easiest to spot as a yellow tinge in the whites of the eyes – and contact your doctor if you are concerned.”
Prof Deirdre Kelly, a paediatric hepatologist at Birmingham Women’s and Children’s NHS trust and part of a group working with UKHSA to investigate the cases, said that the surge seemed likely to be related to the pandemic and that the group had found a medical report from 1924 describing an increase in child hepatitis after the 1918 influenza pandemic. “They also had a surge in hepatitis, which is fascinating,” Kelly said.
She added that the far higher numbers of cases seen in the UK compared with Europe was likely to be due to a better reporting system. The UK has centralised liver treatment centres, meaning that milder cases could be picked up quickly.
Chand advised parents to ensure that children follow normal hygiene measures including thorough handwashing. “As always, children experiencing symptoms such as vomiting and diarrhoea should stay at home and not return to school or nursery until 48 hours after the symptoms have stopped,” she said.
Hepatitis is the term used to describe inflammation of the liver. It's usually the result of a viral infection or liver damage caused by drinking alcohol.
There are several different types of hepatitis, most of which are outlined below.
Some types will pass without any serious problems, while others can be long-lasting (chronic) and cause scarring of the liver (cirrhosis), loss of liver function and, in some cases, liver cancer.
https://www.nhs.uk/conditions/hepatitis/
Ok, so what the heck is going on?
The first thing to point out is that there is no single cause of hepatitis and some children will unfortunately contract the disease at the best of times, just not in these numbers.
VAERS cases exist linked to breast feeding, but as most of these cases are over 6 months old that isn’t the focus of this Substack.
Next we have the well known transfection induced attack on the liver: cytotoxic LNPs, the sulfatide inflammatory pathway1, pathogenic priming against mitochondrial M2 protein and autoimmune T lymphocytic mediated damage.
But again although not a primary concern because most of these cases were reportedly in the unvaccinated there may be a linguistic slight of hand here where they were called unvaccinated just because they weren't current with boosters or within the 14 day cut-off, which cannot be excluded:
SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis
(Tobias Boettler et al. J Hepatol. 2022.)
Abstract
Background & aims:
Autoimmune hepatitis episodes have been described following SARS-CoV-2 infection and vaccination but their pathophysiology remains unclear. Here, we report the case of a 52-year-old male, presenting with bimodal episodes of acute hepatitis, each occurring 2-3 weeks after BNT162b2 mRNA vaccination and sought to identify the underlying immune correlates. The patient received first oral budesonide, relapsed, but achieved remission under systemic steroids.
Methods:
Imaging mass cytometry for spatial immune profiling was performed on liver biopsy tissue. Flow cytometry was performed to dissect CD8 T cell phenotypes and identify SARS-CoV-2-specific and EBV-specific T cells longitudinally. Vaccine-induced antibodies were determined by ELISA. Data was correlated with clinical labs.
Results:
Analysis of the hepatic tissue revealed an immune infiltrate quantitatively dominated by activated cytotoxic CD8 T cells with panlobular distribution. An enrichment of CD4 T cells, B cells, plasma cells and myeloid cells was also observed compared to controls. The intrahepatic infiltrate showed enrichment for CD8 T cells with SARS-CoV-2-specificity compared to the peripheral blood. Notably, hepatitis severity correlated longitudinally with an activated cytotoxic phenotype of peripheral SARS-CoV-2-specific, but not EBV-specific CD8+ T cells or vaccine-induced immunoglobulins.
Conclusions:
COVID19 vaccination can elicit a distinct T cell-dominant immune-mediated hepatitis with a unique pathomechanism associated with vaccination induced antigen-specific tissue-resident immunity requiring systemic immunosuppression.
Lay summary:
Liver inflammation is observed during SARS-CoV-2 infection but can also occur in some individuals after vaccination and shares some typical features with autoimmune liver disease. In this report, we show that highly activated T cells accumulate and are evenly distributed in the different areas of the liver in a patient with liver inflammation following SARS-CoV-2 vaccination. Moreover, within these liver infiltrating T cells, we observed an enrichment of T cells that are reactive to SARS-CoV-2, suggesting that these vaccine-induced cells can contribute to the liver inflammation in this context.
Keywords: Autoimmune hepatitis; CD8+ T cell; COVID-19; Immunosuppression; vaccination; virus-specific T cell.
https://pubmed.ncbi.nlm.nih.gov/35461912/
“Rare” = “quite common”
Which COVID-19 Vaccines are Adenovirus-Based? (13th April ‘22)
The AstraZeneca vaccine has been paused for younger demographics in several countries due to blood clotting concerns, and the FDA and CDC just called for the Johnson & Johnson COVID-19 vaccine to be temporarily paused as the same potential rare side effect is researched. Here’s a look at which COVID-19 vaccines are based on adenovirus vectors.
Here Are the COVID-19 Vaccines that Use Adenovirus Vectors
Both Johnson & Johnson’s vaccine and the AstraZeneca/Oxford vaccine use adenovirus vectors. Johnson & Johnson’s vaccine utilizes an Ad26 vector (a human adenovirus) believed to have less natural immunity in the population, Children’s Hospital of Philadelphia reported. The AstraZeneca/Oxford vaccine (called Covishield in India) uses a chimpanzee-based vector called ChAdOx1. This same vector was used in a 2018 trial on 24 people for a MERS vaccine, CEN reported.
Russia’s Sputnik V vaccine uses Ad26 for its first dose, followed by Ad5 for its second dose, Children’s Hospital of Philadelphia reported.
China’s CanSino Biologics also has an adenovirus vector vaccine for COVID-19, CEN reported. This uses the Ad5 vector, which has in the past brought up questions about effectiveness since many humans have some pre-existing immunity to that particular adenovirus strain.
ImmunityBio is developing an adenovirus-vector vaccine based on Ad5. They are conducting a trial using a combination of an oral vaccine and an under-the-tongue vaccine. Some hope that Ad5 delivered this way might have less of an issue with pre-existing immunity in the population.
Altimmune, Stabilitech BioPharma, and Vaxar are testing Ad5 vaccines using nasal sprays or pills, CEN reported. Altimmune’s AdCOVID is adenovirus-based but is delivered intranasally. On March 15, the results from a transgenic mouse model were announced from a preclinical trial.
ReiThera (once called Okairos) is testing a COVID-19 vaccine using a gorilla-derived adenovirus vector, CEN reported. This GRAd-COV2 vaccine candidate just started its Phase 2/3 clinical study on March 18. The study is named COVITAR.
Other Vaccines that Use Adenovirus Vectors
Adenovirus-based vaccines use the adenovirus as a vector to deliver an antigen. In the case of Johnson & Johnson’s vaccine, part of the adenovirus’s genetic instructions are replaced with coronavirus genes for making the spike protein. When injected, the cells make the spike protein on their own surface, teaching your immune system to recognize the spike proteins and create antibodies against them. The spike protein can’t cause COVID-19 by itself, and the adenovirus vector can’t replicate.
This isn’t the first time that adenoviruses have been used as vectors for vaccines and vaccine trials. The adenovirus was first used as a form of genetic therapy in the 1990s, but this was stopped after a teen boy died from the therapy in 1999 due to massive inflammation. Although this inflammation was harmful for gene therapy, scientists determined it could be helpful for vaccine delivery, CEN reported.
However, another concern with adenovirus vectors is that humans may have pre-existing immunity to certain strains or develop it after the first dose. This is less of a concern with chimpanzee-based adenovirus vectors used in some vaccines, Clinical Trials Arena reported.
The concern has played out in the past. Merck & Co. tested an HIV vaccine using an Ad5-based adenovirus vector. Because humans had a pre-existing immunity to this vector, the trials were halted in 2007 when it became known that the vaccines didn’t work or might even increase the risk of infection, CEN reported.
CanSino later developed an Ebola vaccine using an Ad-5 vector, which was approved by China in 2017 for emergency use only. There was still concern that pre-existing immunity to that particular vector might hurt that vaccine’s effectiveness, CEN reported.
Johnson & Johnson uses an adenovirus vector for its Ebola vaccine, Reuters reported. The vaccine was rolled out in the eastern Congo in 2019 for its first real-world setting after passing clinical trials. The vaccine required two injections about eight weeks apart. The first uses Janssen’s AdVac technology (Ad26) for the vector, which is the same that’s used for the COVID-19 vaccine, Johnson & Johnson shared. The second dose utilizes a different vector from MVA-BN technology. J&J has also been working on vaccines for RSV, Zika, and HIV using the Ad26 vector, CEN reported. These also use a booster shot with a different vaccine.
Adenovirus vector vaccines are also being researched for HIV, the flu, and Zika vaccines, the CDC reported.
Okairos has developed a chimpanzee-based adenovirus vector vaccine for malaria, CEN reported. Oxford has done the same with its own chimpanzee vector (ChAdOx1) and is testing it for MERS, AIDS, malaria, and tuberculosis. The MERS vaccine was tested on 24 people in a trial, showing elevated antibodies in people who got the highest dose.
https://heavy.com/news/which-covid-19-vaccines-adenovirus/
Immunopathology explored
In the majority of these case studies the adenovirus itself didn’t cause liver inflammation, but where present it was localised and in response to lymphocytic white cell response:
Adenovirus Hepatitis: Clinicopathologic Analysis of 12 Consecutive Cases From a Single Institution (2017)
Abstract
Adenoviruses are common pathogens that usually cause self-limited infections. However, in the immunocompromised host they can cause severe infections involving multiple organs including the liver. A search of the pathology database at Stanford University Medical Center (1995 to 2016) identified 12 cases of adenovirus hepatitis including biopsy and autopsy specimens. There were 8 pediatric patients, 7 of which had received orthotropic liver transplants and 1 of which was receiving chemotherapy for lymphoblastic leukemia. There were 4 adult patients, of which 1 was actively receiving chemotherapy for chronic lymphocytic leukemia and 2 had undergone hematopoietic stem cell transplantation for hematologic malignancies. One patient had lymphoplasmacytic lymphoma and had received chemotherapy over a year prior but was not receiving therapy at the time he contracted adenovirus hepatitis. In all cases, histologic sections showed nonzonal coagulative hepatocyte necrosis and characteristic intranuclear inclusions. Hepatocyte necrosis ranged from spotty to massive. The majority of cases (7/12; 58%) had no associated inflammation. If present, inflammation was focal and lymphohistiocytic. In 1 case, findings were focal within the liver, requiring an image-guided biopsy. This patient underwent a simultaneous nontargeted liver biopsy that lacked histologic evidence of adenovirus. Among the pediatric patients, 63% (5/8) died secondary to organ failure, while there was 100% (4/4) mortality in the adult population.
Note the date of of the letter, the alarm HAD been raised!
ChAdOx1 = AstraZeneca ChAdOx1-S/nCoV-19 [recombinant], COVID-19 vaccine
Can ChAdOx1, a replication-deficient adenoviral (Ad) vector used to deliver SARS-Cov2 spike protein as a vaccine, recombine with the homologous human Ad to generate a novel Ad+Spike virus? (Created: June 25, 2020)
AUTHORS
Sandeep Chakraborty
Letter
The development of a vaccine for Covid19 is being expedited [1]. The underlying technology for the vaccines are varied: `nucleic acid (DNA and RNA), virus-like particle, peptide, viral vector (replicating and non-replicating), recombinant protein, live attenuated virus and inactivated virus' [2]. Among these, ChAdOx1, a genetically modified, weakened version of a common cold virus (adenovirus) is now in human clinical trials [3].
The ChAd vector (Chimpanzee adenovirus) was introduced in 2012 Chimpanzee adenovirus Y25 [4]. A large proportion of human adults possess significant titres of neutralising antibodies to human Adv, hence the requirement for a different adenovirus. The deletion of a single transcriptional unit, E1, ensures these viruses cant replicate. Other genes like the E3 region may also be deleted. Now, in the Covid19 vaccine ChAdOx1, the spike protein gene from MERS-CoV strain Camel/Qatar/2/2014 `was inserted into the E1 locus of a genomic clone of ChAdOx1 using site-specific recombination' [5].
One of the theories about the genesis of SARS-Cov2 is recombination with coronaviruses from pangolins [6]. Whether or not it happened in SARS-Cov2, there is no denying that such recombinations do happen.
How do we know that the spike protein wont be inserted into a human adenovirus using recombination?
Human adenovirus shares 95% homology to ChAd. The spike protein may be inserted after the E1 protein in a viable human virus. What will happen after that to the virus is anyone's guess. Note, that there is precedence for such recombinant adenoviruses - using `ping-pong" zoonosis and anthroponosis', where the genome of a promiscuous pathogen is `embedded with evidence of unprecedented multiple, multidirectional, stable, and reciprocal cross-species infections of hosts from three species (human, chimpanzee, and bonobo)' [7].
Another critique - co-stimulation in host cells
A spike protein from SARS-Cov2, which is supposed to bind to ACE2 and CD147 [8], has been inserted in an adenovirus. The adenovirus has its own host-cell receptor preferences [9] - what will be the consequences of co-stimulation in those cells in which both these receptors are expressed?
Full letter:


Developments in Viral Vector-Based Vaccines (2014)
Abstract
Viral vectors are promising tools for gene therapy and vaccines. Viral vector-based vaccines can enhance immunogenicity without an adjuvant and induce a robust cytotoxic T lymphocyte (CTL) response to eliminate virus-infected cells. During the last several decades, many types of viruses have been developed as vaccine vectors. Each has unique features and parental virus-related risks. In addition, genetically altered vectors have been developed to improve efficacy and safety, reduce administration dose, and enable large-scale manufacturing. To date, both successful and unsuccessful results have been reported in clinical trials. These trials provide important information on factors such as toxicity, administration dose tolerated, and optimized vaccination strategy. This review highlights major viral vectors that are the best candidates for clinical use.
Keywords: viral vector, vaccine, CTL, MVA, adenovirus
1. Introduction
Viral vectors are regarded as potential tools for gene therapy and vaccines. Their utility is based on the ability of viruses to infect cells. In general, the advantages of viral vectors are as follows: (a) high efficiency gene transduction; (b) highly specific delivery of genes to target cells; and (c) induction of robust immune responses, and increased cellular immunity.
The concept of viral vector vaccines is different from that of subunit vaccines, as the latter help prevent infectious diseases by eliciting a humoral response. Recombinant viral vectors have potential for therapeutic use because they enable intracellular antigen expression and induce a robust cytotoxic T lymphocyte (CTL) response, leading to the elimination of virus-infected cells. Despite their efficacy, viral vectors present unavoidable problems that need to be addressed. In the near future, viral vector-based vaccines may be increasingly used to fight major diseases, such as HIV-1 and malaria. In some vectors, stable expression of the interesting gene is achieved via viral integration mechanisms. Integration into the host genome can lead to cancer. Another obstacle to the clinical use of viral vectors is the presence of pre-existing immunity against the vector. This is caused by previous exposure to the virus and the production of neutralizing antibodies that reduce vaccine efficacy.
The development of viral vectors requires a high biological safety level in order to gain public acceptance. Therefore, non- (or low-) pathogenic viruses are often selected. In most cases, viruses are genetically engineered to reduce or eliminate pathogenicity. Additionally, most viral vectors are replication-defective. For example, in adenovirus-based vectors, the E1A and E1B encoding regions, which are needed for replication in infected cells, are deleted and replaced with the target gene.
When using a viral vector, it is important to assess the potential implications by understanding the epidemiological and virological characteristics. In this review, we describe several representative viral vectors with respect to risk and benefit, optimized vector-design attempts, and clinical results. Moreover, we discuss the combined use of viral vectors in prime-boost vaccination regimens.
3.2. Adenovirus Vectors
Approximately 50 human adenovirus (Ad) serotypes have been identified. Among them, human Ad serotype 5 (Ad5) has been widely investigated as a gene delivery vector because it can be easily produced in high titers. Ad5 contains a 36-kb double-stranded, linear DNA genome; it has a capsid shell with hexon and penton structures and no envelope. Because the Ad fiber protein mediates Ad binding to the coxsackievirus and Ad receptor (CAR), it is a major determinant of viral tropism. Various clinical syndromes are causes by Ad infection, including acute respiratory disease, pharyngoconjunctival fever, and gastroenteritis.
Recombinant Ad vectors are widely used because of their high transduction efficiency, high level of transgene expression, and broad range of viral tropism. They can infect both dividing and non-dividing cells.
Most Ad vectors are replication-defective because of deletion of the E1A and E1B viral gene region. Often, the E3 genes are also deleted to provide space for the transgene. A popular method for Ad vector production involves two steps. First, the Ad vector plasmid is transfected into E1-complementing cell lines (the 293 cell lines are frequently used). The Ad vector then propagates in the infected 293 cells in culture. Second, the vector is collected from infected cells and purified by ultracentrifugation.
Because most people have been exposed to an Ad serotype, the presence of pre-existing anti-Ad immunity is a disadvantage of the Ad vector. Ad contains three main structural proteins, hexon, penton, and fiber. These proteins are the major targets of the humoral and cellular immune responses against Ad5 [38,39]. Antibodies against the hypervariable regions (HVRs) of the hexon protein dominate the neutralizing responses [40]. Modification of these HVRs and the fiber knob domain has been investigated as a way to evade pre-existing immunity [41,42,43].
Although replication-defective Ad has reduced its overall virulence, a further improvement in the clinical safety profile of Ad vectors is required. For example, because CAR is expressed at a high level in hepatocytes, Ad5-based vectors have a strong tropism for liver parenchymal cells, which increases hepatotoxicity [44]. Modification of the fiber protein of Ad can alter its tropism and reduce liver toxicity [45]. Another approach is modulation of the host immune response by reducing viral gene expression. Deletion of the E2 and E4 viral gene regions lowers toxicity by reducing the vector-derived immune response in infected cells. This vector is called a 2nd generation Ad vector [46].
Modification of the Ad fiber protein also improves the efficiency of gene transduction because fiber protein determines the virus’s tropism. The RGD-fiber Ad vector, which incorporates an Arg-Gly-Asp (RGD) peptide into the fiber knob domain, enhances transduction into T cells and dendritic cells [47]. Our group generated a highly immunogenic chimeric Ad5 vector that expresses the Ad35 fiber protein [45].
Recombinant Ad vector-based vaccines have been examined in clinical trials against HIV-1 [48,49], influenza [50], and solid tumors [51]. Ad vectors are well tolerated, and a Phase I study of an HIV-1 vaccine identified 1010 virus particles (VP) as the best-tolerated dose [52]. Despite these efforts, a major Phase IIb trial failed, as the Ad vector further amplified a pre-existing immune response against Ad5, during a STEP Study [10]. This result revealed that vector-based vaccines such as the above could provide conducive environments for HIV-1 replication, thereby contradicting the clinical safety outcomes of a previous HIV-1 study [53]. On the contrary, a Phase I Ad5-based TB vaccine demonstrated immunogenic efficacy, in spite of a pre-existing anti-Ad immunity [54]. In order to evaluate the safe use of Ad-based vectors, a better understanding of the host immune response against different antigens seems necessary.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4494222/#__ffn_sectitle
The conundrum of current anti-SARS-CoV-2 vaccines (2021)
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has given rise to the urgent need for vaccines and therapeutic interventions to address the spread of the SARS-CoV-2 virus. SARS-CoV-2 vaccines in development, and those being distributed currently, have been designed to induce neutralizing antibodies using the spike protein of the virus as an immunogen. However, the immunological correlates of protection against the virus remain unknown. This raises questions about the efficacy of current vaccination strategies. In addition, safety profiles of several vaccines seem inadequate or have not yet been evaluated under controlled experimentation. Here, evidence from the literature regarding the efforts already made to identify the immunological correlates of protection against SARS-CoV-2 infection are summarized. Furthermore, key biological features of most of the advanced vaccines and considerations regarding their safety and expected efficacy are highlighted.
Antibodies do not equal immunity, especially spike protein specific Ab’s:
1.1. What is the immunological correlate of protection against SARS-CoV-2 infection?
Most commonly, the term “correlate of protection” refers to a laboratory parameter associated with protection from a clinical disease [2]. When this concept is applied to the immune response against SARS-CoV-2, there are several uncertainties. For instance, data from a detailed immunological study on hundreds of infected patients, extended for up to 8 months after symptoms onset, did not provide definitive conclusions about the mechanisms of protective immunity [3]. On this basis, it has been proposed that a coordinated action of CD4+ T cells, CD8+ T cells, and neutralizing Abs is necessary to control SARS-CoV-2 infection [3,4]. Results from a study on rhesus macaques indicated that adequate levels of anti-SARS-CoV-2 immunoglobulin G (IgG) can protect against infection and that cellular immunity contributes to protection in the case of subprotective Ab titers [5].
With regard to the virus-induced humoral response in humans, specific Ab responses were found to be elevated in severely ill patients, but remained moderate-to-low or even undetectable in asymptomatic subjects and patients with mild disease [[6], [7], [8], [9], [10], [11]]. This applied to anti-S protein and anti-receptor-binding domain (RBD) Abs, that is, Abs recognizing the S domain binding to the angiotensin-converting enzyme 2 (ACE-2) cell receptor, and to all Ab classes [[12], [13], [14], [15]]. The highest levels of neutralizing Abs, that is, the anti-S Abs identified for their specific ability to block virus entry in in vitro assays, were found in patients with more severe illness [15,16]. Serological time course analysis carried out on hospitalized patients failed to demonstrate a correlation between anti-S Ab levels and patient outcomes [15]. However, many deceased patients developed very high levels of anti-S, anti-RBD, and neutralizing Abs [15,[17], [18], [19]]. In addition, the kinetics of the decay of anti-S, anti-RBD, and neutralizing Abs was strongly correlated [15]. This evidence goes against the hypothesis that the quality, rather than quantity of Ab response, may predict the patient’s outcome.
Taken together, these clinical observations raise serious questions about the correlates of protection against a SARS-CoV-2 infection.
Antibodies do not equal immunity! And in this case that which doesn’t kill us doesn’t make us stronger, we become dependent with the law of diminishing returns putting the vaccinee in a dangerous spot:
Ab waning may not necessarily imply unresponsiveness in a subsequent antigen recognition, considering that humoral immunity can be promptly reactivated in the presence of a pool of memory B cells. In the case of SARS-CoV-2 infection, different studies have demonstrated the persistence of circulating virus-specific memory B cells for up to 8 months [3,[22], [23], [24]]. However, the formation of a pool of memory B cells in airway tissues remains uncertain. Lymphocytes residing in the lungs are essentially a self-renewing cell population, and this population is only minimally replenished by circulating cells [25]. Thus, the presence of virus-specific circulating memory B cells would not necessarily mirror adequate levels of immunity in lung tissues, which are the region most involved in SARS-CoV-2 pathogenesis. This may be a critical issue in the case of anti-SARS-CoV-2 vaccines, since the rapid decrease in Ab response in the absence of an effective pool of memory B cells in the lungs may imply the requirement of frequent booster doses.
A wealth of data supports the conclusion that this strategy leads to a robust humoral response [[30], [31], [32]] associated with apparent protection from severe symptoms [[33], [34], [35]]. To the best of our knowledge, mRNA-based vaccines do not present major safety concerns, besides short-term adverse reactions of limited severity. However, at present, nothing is known about unpredictable, long-term adverse reactions that could have been monitored only by extended phase III clinical trials. In addition, the limited observation times preclude a reliable evaluation regarding the duration of humoral response and need for Ab response reactivation.
Once the replicative recombinant adenovirus has been generated, why could it not be transmitted readily to the unvaccinated, especially to immunonaive children??
This is the crux of the public health nightmare being faced. And just as with coronavirus quasispecies storms, there is no reason to think these are rare, unique events, they can be spontaneously generated anywhere viral vector vaccines are being used, regardless of political borders.
“Catastrophic” is a word the authors do not use lightly:
1.5. Vaccines based on non-human adenoviral vectors
In an effort to elude the neutralization effects of pre-existing immunity, vaccines based on vectors from non-human primate adenoviruses have been produced and are currently being administered [[46], [47], [48], [49]]. The underlying technology is essentially the same as that used to produce vaccines based on vectors from human adenoviruses. After the first inoculation, anti-vector immunity would strongly decrease the immunogenicity of these vaccine preparations. Most importantly, the delivery of non-human adenovirus-based vectors puts vaccine recipients who were previously infected with human adenoviruses at a risk of generating new and unpredictable chimeric virus species. In fact, severely pathogenic virus species may arise as a result of recombination events. Recombination is quite frequent within adenovirus genomes, as largely documented by data obtained by sequencing genomes from people co-infected with different human adenovirus types [[50], [51], [52]]. In vaccinated individuals, a non-human viral vector may enter a single cell that is already infected with a human adenovirus. Considering the very high sequence homology between human and non-human primate adenoviruses (> 95 %), intracellular recombination events are likely to occur. Even if at present only theoretical, the likelihood that a similar, potentially catastrophic event may occur is expected to increase with the increase in the number of vaccinations.
Full paper:
https://www.sciencedirect.com/science/article/pii/S1359610121000265
In depth study on the E1B protein and how it contributes to adenovirus replication:
The biology of the adenovirus E1B 55K protein (2019)
Abstract
The adenovirus E1B 55K (E1B) protein plays major roles in productive adenoviral infection and cellular transformation. Interest in E1B increased because of the potential of adenoviruses as therapeutic vectors, and the E1B gene is commonly deleted from adenovirus vectors for anticancer therapy. E1B activities are spatiotemporally regulated through SUMOylation and phosphorylation, and through interactions with multiple partners that occur presumably at different intracellular sites and times postinfection. E1B is implicated in the formation of viral replication compartments and regulates viral genome replication and transcription, transcriptional repression, degradation of cellular proteins, and several intranuclear steps of viral late mRNA biogenesis. Here, we review advances in our understanding of E1B during productive adenovirus replication and discuss fundamental aspects that remain unresolved.
Keywords: DNA replication; E1B 55K; E1B 55kDa; adenovirus; cellular transformation; mRNA export; post-transcriptional processing; protein degradation; replication compartments; viral oncogene.
There is still an awful lot we don’t know. Maybe would have been best to have some more answers before injecting millions of experimental subjects - if you don’t fully understand the mechanism of inhibition (or not) then by definition you cannot have the full safety data:
As detailed before, for many years the formation of the E1B-E4Orf6 E3 ubiquitin-ligase has been regarded as the central role for E1B activities during viral replication (reviewed in [3]), including efficient viral late mRNA export. In the light of the new evidence on the role of E1B in crucial steps of viral replication, from formation of RCs to production of viral mRNA, it seems overly simplistic to consider that multifunctionality may solely stem from protein degradation. Rather, the outcomes of the interactions that E1B can establish during different times postinfection and in different cellular locations (Table 1) should be determined.
Ultimately, each of the viral replication steps that may be regulated by E1B will have to be scrutinized to reveal the specific molecular functions of the protein during viral replication and cellular transformation. This in turn will contribute to understanding the basis for selective adenoviral replication in tumor versus normal cells and to improving the design and development of oncolytic adenoviruses.
Full paper:
https://febs.onlinelibrary.wiley.com/doi/10.1002/1873-3468.13694
This is a study in to how E1B deleted adenovirus can be de-attenuated by recombination with cytomegalovirus promotors. They attempted to make this specific to the tumor cell lines so as not to, er, accidentally kill the host:
E1B-55K-Deleted Adenovirus Expressing E1A-13S by AFP-Enhancer/Promoter Is Capable of Highly Specific Replication in AFP-Producing Hepatocellular Carcinoma and Eradication of Established Tumor (2002)
Here, we constructed a recombinant replication-competent adenovirus (rRCAd; AdAFPep/Rep) that expresses both E1A-13S driven by the α-fetoprotein (AFP) enhancer/promoter (AFPep) lacking any silencers in the 5′-flanking region of the AFP gene, and 55K-deleted E1B driven by the cytomegalovirus (CMV) promoter. We then examined the feasibility of gene therapy utilizing this virus for AFP-producing hepatocellular carcinoma (HCC). AdAFPep/Rep lysed all the AFP-producing HCC cell lines (HuH7, HepG2, PLC/PRF/5 (P5)) examined at a multiplicity of infection (MOI) as low as 0.1 and did not lyse primary human hepatocytes (Hc) at a MOI as high as 100, indicating that the rRCAd virus can lyse AFP-producing HCC cells with a higher specificity and potency than previously reported. Furthermore, this virus was capable of complete eradication of a preestablished HuH7-cell tumor by a single intratumoral injection of 108 plaque-forming units (pfu) of AdAFPep/Rep. Thus, AdAFPep/Rep may be applicable for clinical use.
Key Words
E1B is implicated in the formation of viral replication compartments and regulates viral genome replication and transcription, transcriptional repression, degradation of cellular proteins, and several intranuclear steps of viral late mRNA biogenesis.
Nevertheless, these tumor-specific promoters are generally not as powerful as nonspecific viral promoters currently used for clinical gene therapy; thus, when used for in vivo suicide gene therapy, they failed to induce complete regression of tumors even in animal models [18, 19].
Therefore, to substantiate antitumor activity, Hallenbeck et al. [20] recently generated a rRCAd (named AvE1a04i), which was constructed to encode the E1A gene, one of the genes essential for viral replication, under the control of an artificial AFP promoter. The modified Ad was intended to replicate in and lyse only AFP-producing HCC cell lines. However, as hepatocytes in hepatitis are known often to express AFP (though not at levels as high as hepatocytes in hepatoma), to prevent viral replication in AFP-expressing hepatocytes, these authors raised the tumor selectivity of rRCAd by intentionally inserting six copies of distal silencers in an artificial AFP promoter.
In other words the coronavirus vaccine ChAdOx1 adenovirus had the gene for E1B protein silenced so that once it has created the spike protein in your cell it is inert or attenuated.
...Except that the viral RNA is quite able to get the machinery back through recombination with other viruses, the part of the first paper as in "and 55K-deleted E1B driven by the cytomegalovirus (CMV) promoter.".
Another name for a CMV? Herpes. And it's everywhere. And the researchers were fully aware of the risks of off-target collateral damage:
One of the most important issues of using a replication-competent virus is without doubt the specificity. Our virus, having the tumor-specific expression of E1A-13S by AFPep (Fig. 1), together with E1B-55K deletion, was designed to have double assurance of specificity, and it was indeed found to be highly specific for AFP-producing tumors, exerting no cytotoxicity to Hc, which express no detectable AFP (FIG. 3, FIG. 7). The tumor specificity was also confirmed by the experiment that showed no bystander effects [33] of AdAFPep/Rep in a mixed culture of infected tumor cells and noninfected normal hepatocytes (Fig. 6). Incidentally, the fact that the ONYX-015 proved cytotoxic to normal diploid cells (Hc) indicates that the type of cell in which the virus can replicate is not restricted to the cells that have deficient or mutated p53, as had been initially speculated. Although application of rRCAd in practical use for HCC may be through the hepatic artery [34, 35] to avoid the dissemination of the virus throughout the whole body, there is still a risk of replication in distant, dividing normal diploid cells such as gastrointestinal epithelial cells. AdAFPep/Rep, in this regard, is endowed with assured safety not to replicate in these dividing normal diploid cells because its replication is under the control of AFPep.
Although the AFP promoter is indeed specific and potent, AFP-producing hepatocytes are quite often present in inflamed liver associated with hepatitis; therefore, if rRCAd equipped with an AFP promoter that has an intact E1B region infects these hepatocytes, they may readily undergo cytolysis because very low levels of E1A expression may be sufficient for Ad production [36]. In this regard, another important feature of our virus, deletion of E1B-55K, will nullify this possible risk.
To make the adenovirus shuttle vector, the following five fragments were cloned into pAdBglII (provided by M. Imperiale, University of Michigan, MI) as described [39]: AFP enhancer, AFP promoter, E1A-13S [50] terminated with simian virus-40 (SV40) polyadenylation signal (pA), E1B-19K [50] (provided by E. White, Cold Spring Harbor Laboratory, NY) under control of a CMV promoter (CMVp), and bovine growth hormone (BGH) pA of pRc/CMV (Invitrogen). The resulting shuttle vector was designated pAdBglII-AFPep/E1A-13S/CMVp/E1B-19K.
Full paper:
https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(02)90589-7
In this paper the tail is wagging the dog, adenovirus is re-activating latent cytomegalovirus in the lungs. This was published back in 1994!
Adenovirus E1A 13S gene product up-regulates the cytomegalovirus major immediate early promoter (1994)
Abstract
Latent cytomegalovirus (CMV) infection is often reactivated in the lung. We postulated that this reactivation could occur by stimulation of the CMV major immediate early (IE) promoter by other viruses that infect the lung. The specific aim of this study was to investigate whether adenovirus early proteins could stimulate the CMV IE promoter in inflammatory cells. We transfected the monocyte/macrophage THP-1 cell line and the T-lymphocyte Jurkat cell line with plasmids coding for adenovirus E1A 12S or 13S proteins, along with a plasmid containing the CMV IE promoter region linked to the chloramphenicol acetyltransferase (CAT) reporter gene. In unstimulated THP-1 cells, the E1A 13S gene product increased CMV IE CAT activity by 18-fold compared with cells containing the control E1A plasmid. This effect was not seen in cells transfected with the E1A 12S plasmid. There was a similar effect of the E1A 13S gene product in LPS-stimulated THP-1 cells. In unstimulated Jurkat cells, the E1A 13S gene product stimulated CMV IE CAT activity by 19-fold compared with cells containing the E1A control plasmid; the E1A 12S gene product had no effect. There was a similar effect of the 13S E1A gene product in phorbol myristate acetate-stimulated Jurkat cells. These findings demonstrate that the CMV IE promoter can be stimulated by early viral proteins of adenovirus in inflammatory cells. These observations could be important for understanding the reactivation of latent CMV infection.
Full paper:
and:
Cytomegalovirus and adenovirus infections and diseases among 75 paediatric unrelated allogeneic bone marrow transplant recipients (2004)
Abstract
Viral infections remain a major complication of allogeneic bone marrow transplantation. A population of children who underwent unrelated allogeneic bone marrow transplantation in a single centre has been followed-up for viral infections and diseases. We describe the detection of cytomegalovirus (CMV) and adenovirus among 75 children transplanted between 1989 and 2000. CMV was detected among 22 patients (29%) and adenovirus among 19 patients (25%); they were associated with clinical diseases in 10 and 8 patients, respectively. Four patients had adenovirus and CMV coinfection. The obvious risk factor for CMV infection is seropositivity of the recipient prior to transplantation. Adenovirus is detected significantly more frequently when conditioning regimen includes anti-thymocyte or anti-lymphocyte globulin. Diseases associated with adenovirus have been correlated with a significantly higher mortality rate, stressing the need for the implementation of a systematic virological survey for this virus and for the evaluation of therapeutic protocols including new molecules.
Full paper:
https://pubmed.ncbi.nlm.nih.gov/14695667/
Full thread:
https://threadreaderapp.com/thread/1521579651734200320.html
We could have answers, but no-one wants to bring the house of revenue crashing down and get sued. They must know, but no-one is talking:
https://twitter.com/sanchak74/status/1522043992552083456?s=19
Its not just a reckless endeavour, it’s malicious as it was virtually inevitable with this scale of use and the viral vectors they selected. At best you could say they took a calculated gamble, but it didn’t pay off. Instead it is going catastrophically wrong by all accounts.2
To constitute a crime, there must be an actus reus (guilty act) accompanied by the mens rea (guilty mind). Negligence shows the least level of culpability, intention being the most serious, and recklessness being of intermediate seriousness, overlapping with gross negligence. The distinction between recklessness and criminal negligence lies in the presence or absence of foresight as to the prohibited consequences. Recklessness is usually described as a "malfeasance" where the defendant knowingly exposes another to the risk of injury. The fault lies in being willing to run the risk.
https://en.wikipedia.org/wiki/Criminal_negligence
I will keep this Substack updated.
Dr. James Olsson is on to this and doing great work:
Added 8th May ‘22:
Unfortunately I got banned for life in April ‘21 for mentioning Ivermectin so cannot thank James directly or request a threadreader, but I can read posts and consider these worthy of reproducing here in full.
More on the pathology of adenovirus mediated autoimmune hepatitis:
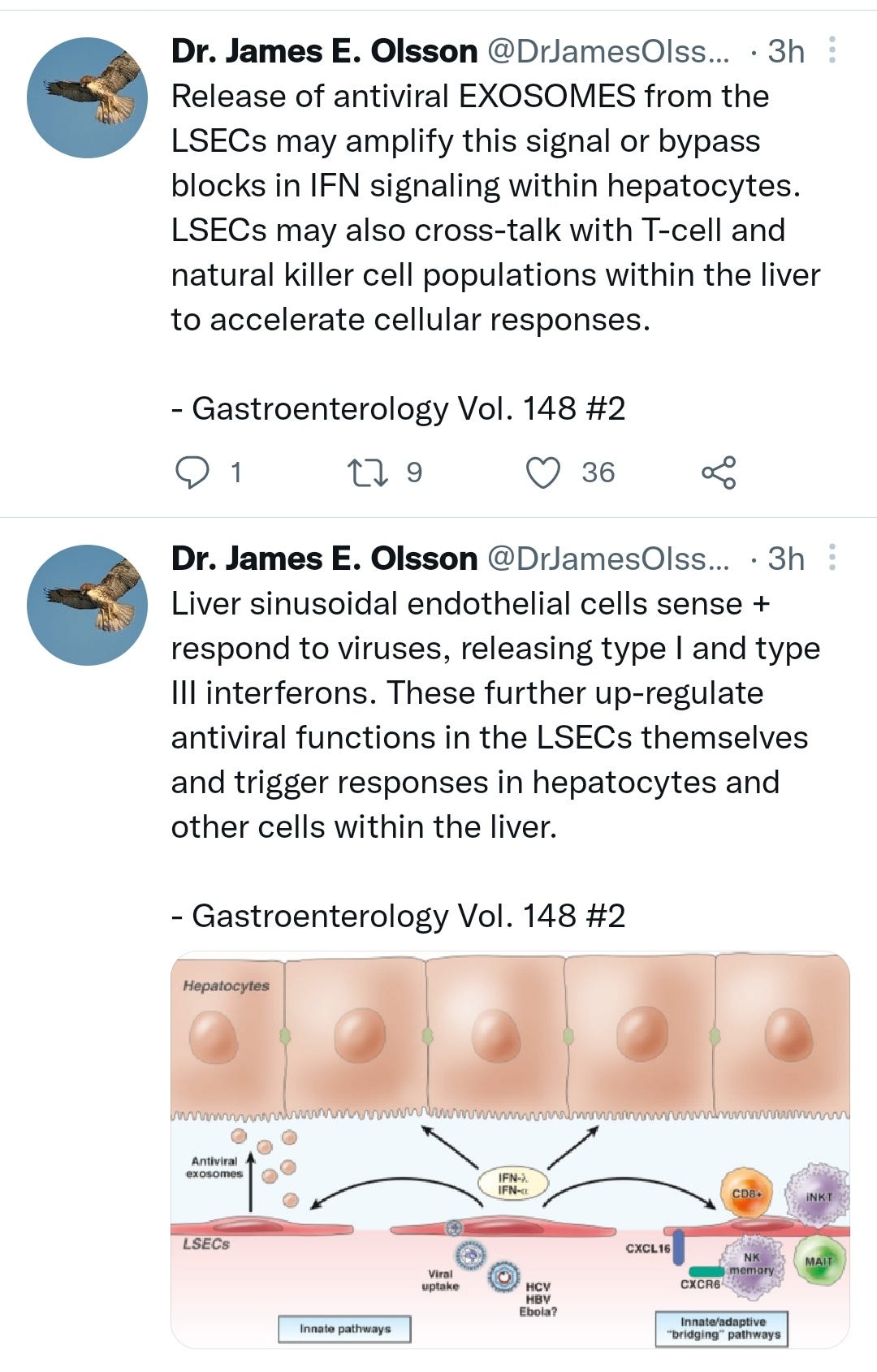
https://twitter.com/DrJamesOlsson/status/1523145349367738368?s=19
Brazil Rejects the Gamaleya Vaccine
(28 Apr 2021)
We have two pieces of news about the Gamaleya Institute's "Sputnik-V" vaccine today. Neither of them are going to be enjoyable to go into.
First off, many may have heard that the Brazilian regulatory authorities had a hearing yesterday to see if this vaccine would be approved for use there. They have turned it down, for several reasons. (Update: here are their slides, in Portuguese). Among these are questions about the manufacturing and scale-up processes, which I have to say have not been very well documented for this vaccine. Readers may recall the reports from Slovakia about the authorities there getting what appeared to be completely different formulations of the vaccine all shipped together, so there is some room for clarity about how these processes are controlled. But the bigger news was that Anvisa, the Brazilian drug agency, said that every single lot of the Ad5 Gamaleya shot that they have data on appears to still have replication-competent adenovirus in it. Let's back up for a second to appreciate what the means, for readers who don't do this stuff for a living. The next few paragraphs are background; skip if you like.
…Which is why the news that the Sputnik vaccine contains replicating adenovirus was surprising and unwelcome. As mentioned, this is probably not going to cause big problems in its vaccinated population, but it's a completely unnecessary risk. And if such a vaccine is going into tens of millions of people (or more), it seems certain that there will be some people harmed by this avoidable problem. If you're going to make a replication-competent vaccine, make one and run the clinical trials with it, and if you're asking for regulatory approval for a replication-incompetent one you shouldn't show up with an undefined mixture of replicating and non-replicating viral particles instead. This sort of thing calls into question the entire manufacturing and quality control process, and I can see why the Brazilian regulators are concerned.
The response from the Sputnik V camp has not been good. The official Twitter account has accused Anvisa of having "invented fake news" about the vaccine, when what you'd hope to see is more of the good ol' "We stand by our manufacturing process, but take these concerns seriously and are working with the authorities to resolve this question" sort of thing. But no, it's all for "political reasons". Their official statement is no more conciliatory. A tip for the vaccine's manufacturers: don't immediately start accusing your critics of bad faith, especially when they are the regulatory authorities. Step up and act like responsible drug developers: address the issues directly, with transparency, and work to find a solution. Throwing fits on Twitter is not the answer. But that brings us to the next post, coming up shortly. . .
https://www.science.org/content/blog-post/brazil-rejects-gamaleya-vaccine
Added 9th May ‘22:
Thanks @Joyce_Bowen
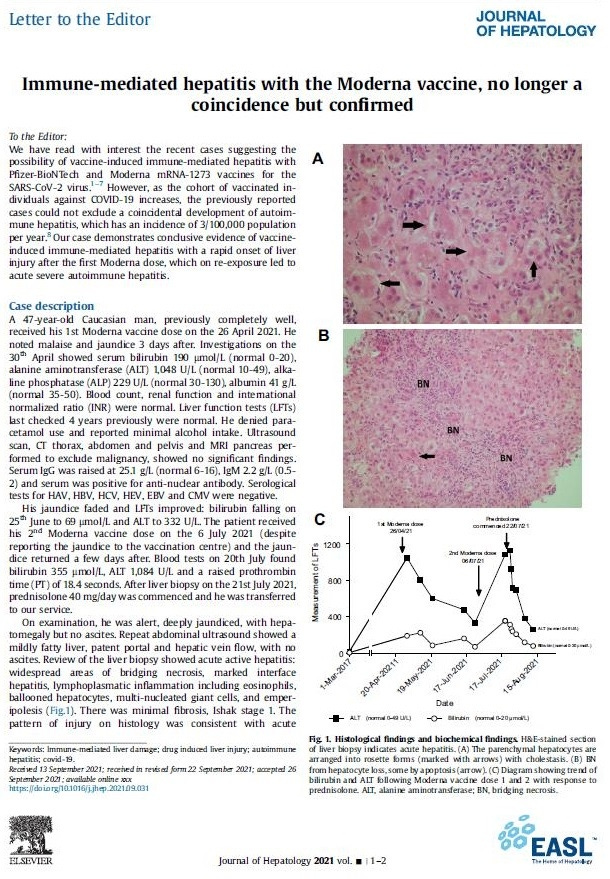
They knew last September [at least the] Moderna caused liver problems. I wouldn’t doubt the rest do, too.
Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed Journal of Hepatology 2021 vol. - j 1–2 accepted 26 September 2021;
https://www.journal-of-hepatology.eu/article/S0168-8278(21)02093-6/fulltext
Translation of a translation. As I read this, there are many unreported cases of child hepatitis in China, at epidemic levels of incidence. BGI has performed genomic sequencing and they know it's a match to some of their viral vector adenovirus vaccines.
If anyone can add to this please comment below?
https://pubmed.ncbi.nlm.nih.gov/27682509/
Added 15th May ‘22:
Maybe these are normal case counts as some are saying - media hype, nudge & a distraction? But a transplant surgeon says this is very unusual for them:
This is not my kid': Mysterious hepatitis wreaked havoc in healthy child with shocking speed
Chinnakotla, a world-renowned surgeon and one of only a few dozen specialists who perform pediatric liver transplants in the United States, put Baelyn on a transplant waiting list.
Children automatically get highest priority, a status called 1A, reserved for those who have hours or days to live.
In an average year, he might do this surgery on 10 children. Most of them need new livers because they were born with autoimmune diseases or birth defects. Maybe one might need a new liver because of sudden liver failure.
"And this year," he said, "we've already seen two children with liver failure and transplanted two children with liver failure. This is very unusual for us."
A medical mystery
Disease detectives aren't sure what's causing these hepatitis cases.
Dr. Jay Butler, the CDC's deputy director of infectious diseases, said at a briefing last week that the agency was "casting a wide net" to look at all possible exposures and associations.
Even before this outbreak, sudden liver failure cases like this often puzzled doctors.
"I've taken care of a half-dozen or a dozen kids where we did our best look, and we never found a cause for why their livers just failed," said Dr. Beth Thielen, a pediatric infectious disease specialist at the University of Minnesota who has been treating Baelyn. "And some of them got better, or some of them went to transplant, so this happens at some base frequency.
"And I think what has drawn people's attention is that this seems to be happening more frequently, and there does seem to be this association with adenovirus -- not every child, but there does seem to be a larger percentage of these cases that do seem to be associated with adenovirus," Thielen said.
More than half the children in the CDC's investigation -- including Baelyn -- have tested positive for adenovirus 41, a type of virus that ordinarily causes stomach upset and cold-like symptoms. It has never before been linked to liver failure in otherwise healthy children.
Doctors aren't sure how this virus might be involved. It's not clear whether it could be directly damaging the liver or setting off an unusual immune response that's causing the body to attack its own tissues. Another possibility is that adenovirus has an accomplice, a co-factor that could be genetic, environmental or even infectious, that in tandem is leading to these extreme outcomes.
https://edition.cnn.com/2022/05/13/health/hepatitis-child-liver-transplant-baelyn/index.html
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #28: Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
https://doorlesscarp953.substack.com/p/innate-immune-suppression-by-sars-315?s=w
"Product administered to patient of inappropriate age"
This shouldn't be a thing, in my opinion.






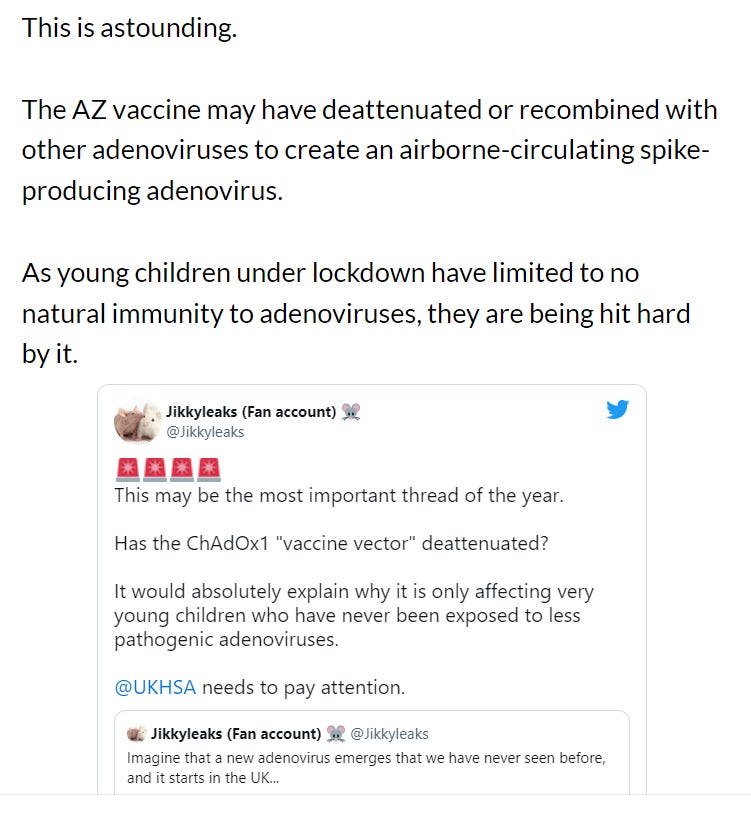

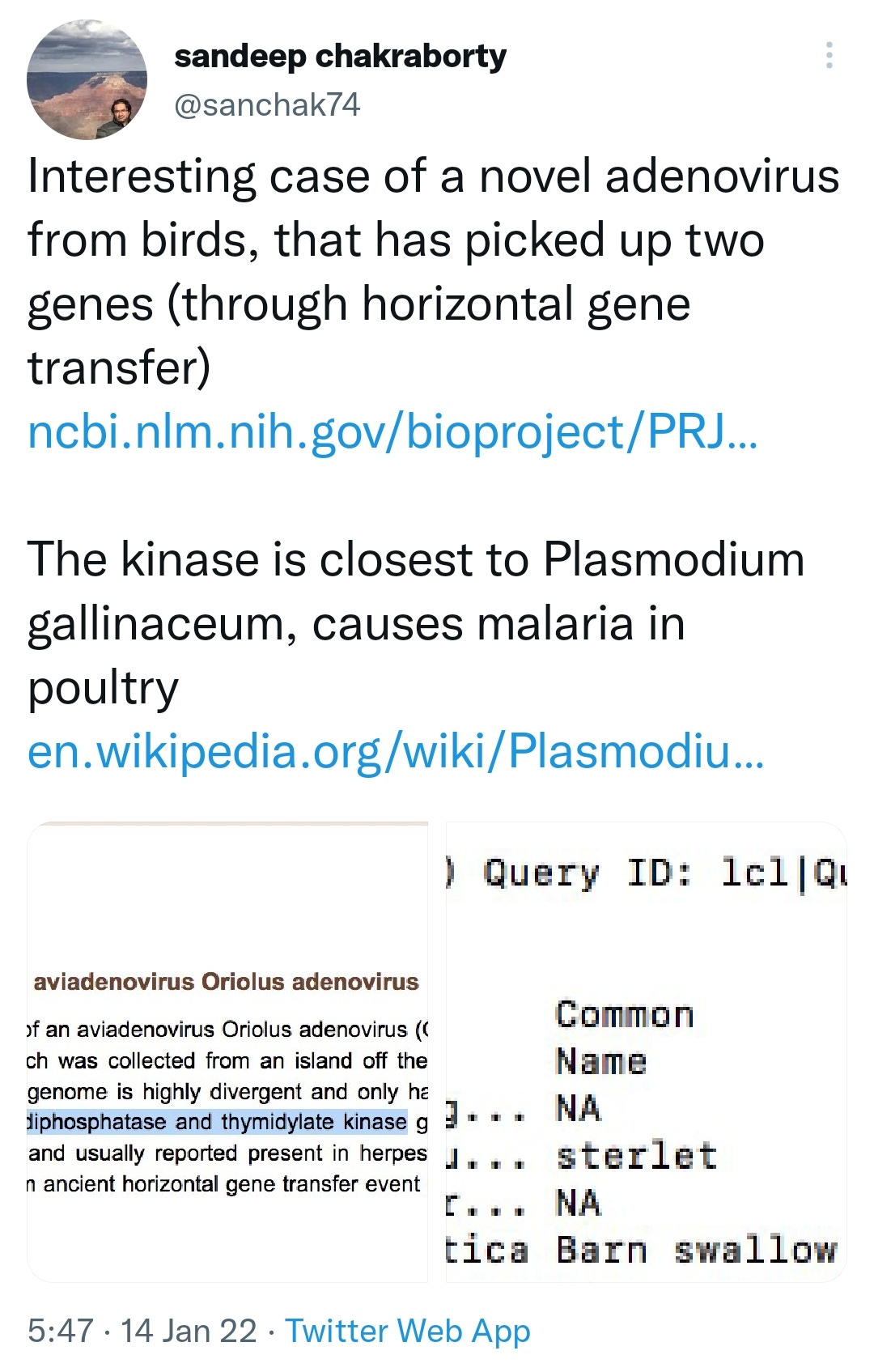





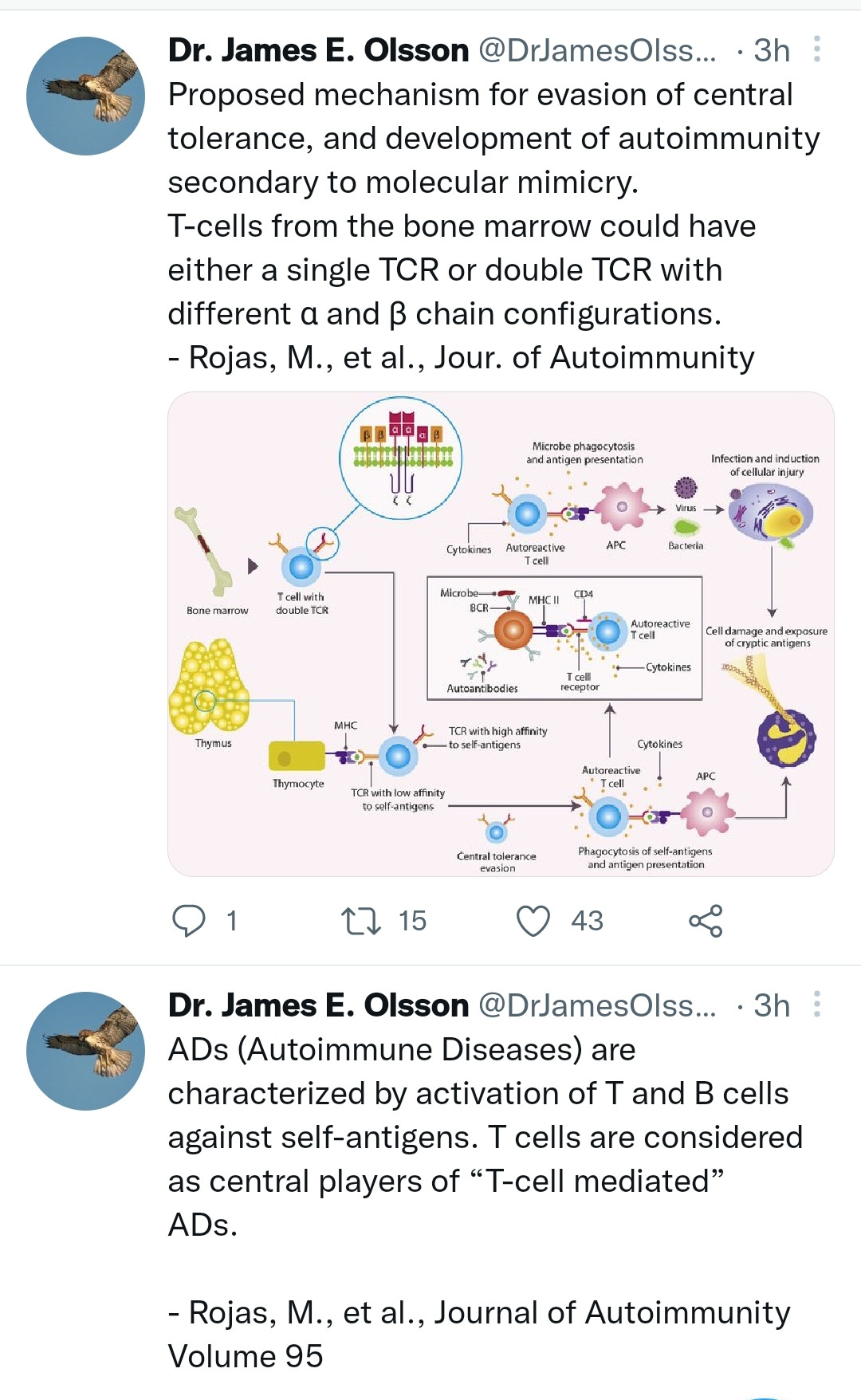






And then there’s this compelling (and terrifying) theory from Walter M Chestnut that has much larger implications:
https://wmcresearch.substack.com/p/are-the-cases-of-hepatitis-in-children?r=ydcjg&utm_campaign=post&utm_source=ARE%20THE%20CASES%20OF%20%22HEPATITIS%22%20IN%20CHILDREN%20ACTUALLY%20SPIKE%20PROTEIN%20AMYLOIDOSIS%20OF%20THE%20LIVER?&utm_medium=ios
Here's some articles on the history of Adeno vectors, including ones where the authors point out that shedding occurs (for instance, from parent to child). The media has been arguing that this mysterious hepatitis in children must come out of the blue, since not all kids have had shots.
https://medquotes.substack.com/p/adenovirus-vectors