Epidemiological update: SARS-CoV-2 Omicron sub-lineages BA.4 and BA.5
13 May 2022
Last update: 16th May ‘22.
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Just in:
BA.5 is fast becoming the dominant variant. The transfected are by all accounts virtually defenceless.
John Paul has just posted a great update on how the transfected lack mucosal antibodies or ability to recognise the N protein:
Reasons for Vaccine failure vs Omicron
Antigens go brrrrr
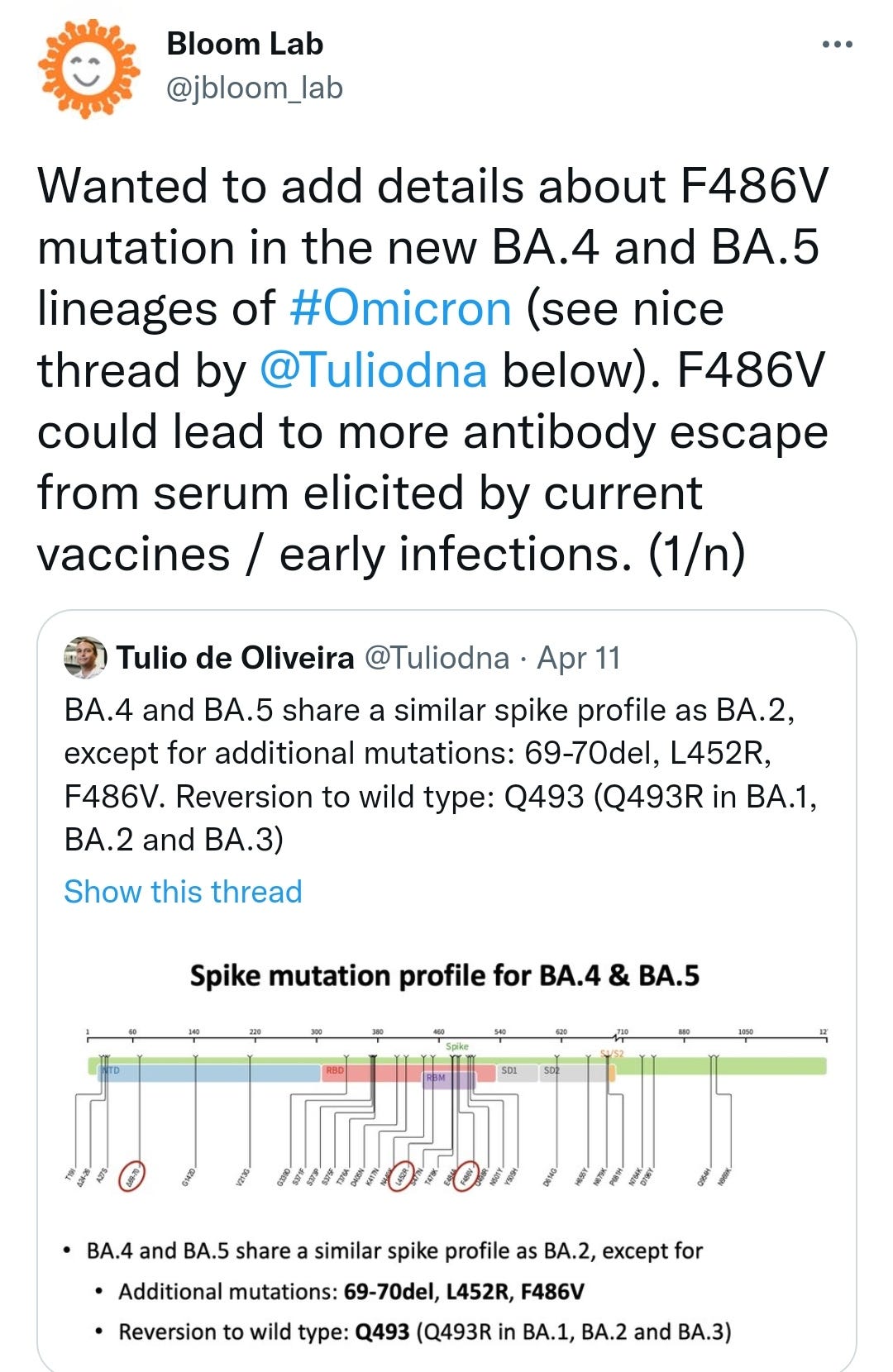
Mutations
L452R in BA.5 is particularly concerning as it confers the additional glycosylation & fusogenicity of the Delta variant, ie to replicate rapidly in the lungs and what Geert refers to as trans-infection. Glycoproteins help make the virus look like self to evade immune surveillance and, taking a feature of HIV, allows it to use dendritic cells to ferry it around the body without being neutralised. This is why BA.5 is considered a variant of concern, ie a public health risk.
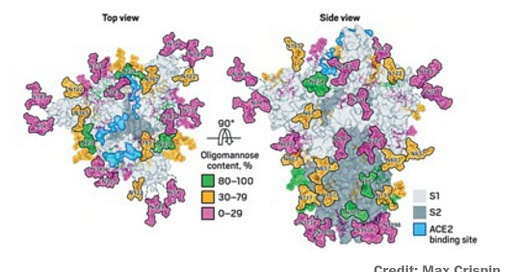
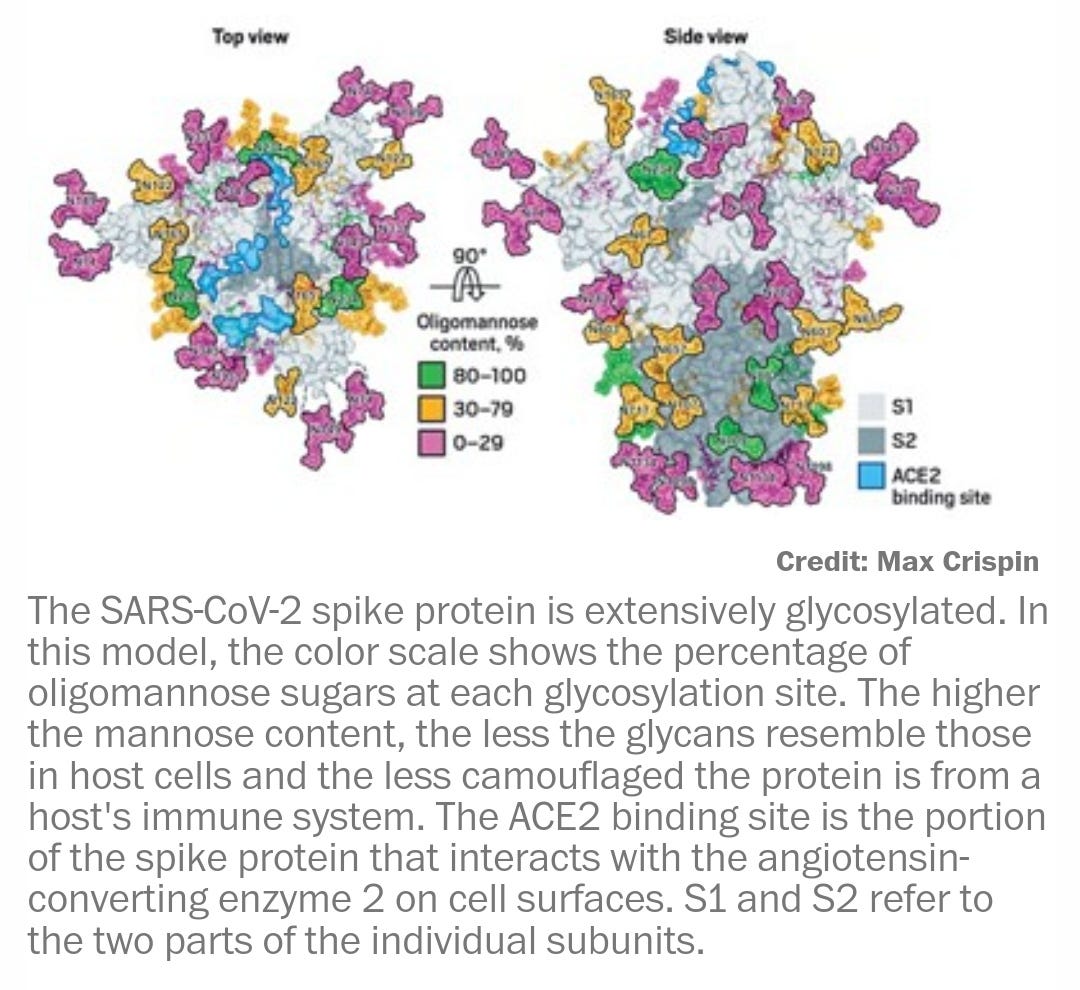
Adding the missing sugars to coronavirus protein structures (2020)
Most of the recently reported viral structures have left out the carbohydrate decorations that help mask the proteins from our immune system.
Glycosylation can act as camouflage because the sugars on viral proteins come from the animal or person that has been infected. Viruses commandeer the enzymatic machinery that host cells use to add sugars to their own proteins and get those enzymes to attach glycans to viral proteins.
All the mass spec data point to the SARS-CoV-2 spike protein’s being heavily glycosylated, Crispin says. “It’s covered in carbohydrates, but it’s slightly lower than in HIV,” he says. HIV is so densely glycosylated that the enzymes that process the sugars on its surface can’t easily reach them. SARS-CoV-2’s sparser glycosylation means that the sugars are more naturally processed than the ones in HIV. But it also suggests that the coronavirus’s glycan shield may not be as effective as that of HIV.
Full article:
https://cen.acs.org/biological-chemistry/proteomics/Adding-missing-sugars-coronavirus-protein/98/i16
SARS-CoV-2 spike L452R mutation increases Omicron variant fusogenicity and infectivity as well as host glycolysis (March ‘22)
Dear Editor,
The SARS-CoV-2 Omicron variant has rapidly displaced the Delta variant and spread across the world. The Omicron variant harbors over 60 mutations, and 15 of the mutations are located in the receptor-binding domain (RBD).1 Compared with parental virus and previous variants, the Omicron variant is characterized by decreased hospitalization rates and less severe disease in patients.2 However, how the Omicron variant reduces pathogenicity remains unclear. The Omicron variant has diminished fusogenicity, although the underlying mechanism is unknown. Fusogenicity was shown to be associated with pathogenicity in SARS-CoV-2 patients.3 The L452R mutation, one of the most frequent mutations (Fig. 1a, b), is the only RBD domain mutation that emerges in the Delta variant but is absent in the Omicron variant (Fig. 1c). It has been reported that L452R mutation increases SARS-CoV-2 fusogenicity and infectivity.4 Here, we developed an L452R mutated Omicron variant (Omicron-L452R) and found that the Omicron-L452R variant rescued fusogenicity and strengthened the high infectivity by enhancing the cleavage of the spike protein. Notably, Omicron-L452R greatly enhanced the ability of Omicron to infect lung tissues of humanized ACE2 mic Furthermore, the Omicron-L452R variant dramatically enhanced glycolysis in host cells. Our data suggest that the decreased fusogenicity of the Omicron variant is due to a lack of the L452R mutation present in the Delta variant.
SARS-CoV-2-infected cells fuse with neighboring cells to form multinucleated cells named syncytia. Syncytia may contribute to virus transmission, immune evasion, and pathogenicity. It has been reported that infected syncytial pneumocytes are present in 87% of deceased SARS-CoV-2 patients, and syncytia formation correlates with disease severity. The Omicron variant has exhibited diminished fusogenicity and pathogenicity compared with the Delta variant and parental virus. Because of the essential role of the L452R mutation in SARS-CoV-2 fusogenicity and infectivity, we investigated the effect of L452R on the fusogenicity of the Omicron variant. Consistent with a previous report, compared to the parental virus (B.1), the Omicron variant almost lost fusogenicity, and the Delta variant demonstrated greatly enhanced fusogenicity. Importantly, the Omicron-L452R variant exerted markedly enhanced fusogenicity (Fig. 1d). Efficient syncytia formation depends on the cleavage of the spike (S) protein at the S1/S2 site.5 Next, we investigated the effect of variants and parental virus on spike protein cleavage. In agreement with the syncytia formation assay, the Omicron variant reduced the cleavage of the SARS-CoV-2 S protein compared with the Delta variant and parental virus, whereas the Omicron-L452R variant greatly enhanced the cleavage of the S protein (Fig. 1e). It has been reported that mutations in the Omicron spike protein introduce electrostatic contacts and enhance the interaction between the S1 and S2 subunits, which results in reduced S protein cleavage. Whether the L452R-Omicron variant promotes cleavage of the S protein by decreasing the interaction of the S1 and S2 subunits remains to be investigated.
The Omicron variant spike protein binds human ACE2 and facilitates its entrance and confers Omicron increased infectivity in human primary nasal epithelial cultures and other ACE2-positive cells. Consistent with previous data, the pseudovirus infection assay indicated that the Omicron variant demonstrated enhanced entry into Huh-7, 293T-ACE2, and H1299-ACE2 cells compared to the Delta variant and parental virus, and the Omicron-L452R variant further increased viral entry into these cells (Fig. 1f). In summary, the Omicron-L452R variant not only rescued fusogenicity but also strengthened infectivity, suggesting that the Omicron-L452R variant is a very risky variant.
To obtain available energy and nucleotides for viral replication, SARS-CoV-2 induces metabolic reprogramming in host cells in a manner similar to the Warburg effect in cancer cells. However, the effect of the Delta and Omicron variants on glycolysis has not been investigated. We found that the Delta and Omicron-L452R variants increased lactate production more than the Omicron variant in Huh-7, 293T-ACE2, and H1299-ACE2 cells, although the Omicron variant enhanced lactate production in H1299-ACE2 cells (Fig. 1g). In agreement with lactate production, the extracellular acidification rate (ECAR) assay indicated that the Delta and Omicron-L452R variants highly stimulated glycolysis in Huh7, H1299-ACE2, and 293T-ACE2 cells, while the Omicron and parental viruses slightly increased glycolysis flux (Fig. 1h). Therefore, the Delta and Omicron-L452R variants may use enhanced glycolysis to support their high fusogenicity and high infectivity. It has been shown that SARS-CoV-2 infection increases the expression of hexokinase (HK2) and pyruvate kinase isozyme (PKM), key rate-limiting enzymes in glycolysis, and promotes lactate production, resulting in enhanced host glycolysis. Whether the Omicron-L452R variant stimulates host glycolysis by regulating glycolysis-related enzymes remains to be elucidated.
Next, the infectivity of the Omicron-L452R variant was investigated in K18-hACE2 transgenic mice in vivo. The bioluminescence of infected mice was measured to indicate the pseudovirus infection severity. Consistent with live SARS-CoV-2 virus, the Delta variant has the highest infectivity. The Omicron variant modestly inhibited infectivity, whereas Omicron-L452R significantly enhanced infectivity (Fig. 1i). qRT-PCR was performed to detect the infected pseudovirus copies. Compared to mice infected with the parental virus, the virus copies in the Omicron-infected mouse lung tissues were reduced, while the virus copies in the Omicron-L452R variant-infected mouse lung tissues were markedly enhanced (Fig. 1j). Therefore, the Omicron-L452R variant enhanced the ability of Omicron to infect lung tissues in vivo.
The Omicron variant is characterized as less pathogenic and more transmissible than the parental and previous variants, which may be due to crystal structure changes caused by over 30 mutations in the Spike protein. In this investigation, we found that the further mutation of L452R in the Omicron variant not only rescued fusogenicity but also strengthened the high infectivity. Importantly, the Omicron-L452R variant enhanced the ability of Omicron to infect lung tissues of humanized mice. By dramatically enhancing the anaerobic glycolysis of host cells, the Omicron-L452R variant obtains enough energy and nucleotide material to replicate in human cells. Taken together, the L452R mutation dramatically increases the risk of the Omicron variant. Our data explain why the Omicron variant has decreased fusogenicity and suggest that whether the L452R mutation is present in the Omicron variant should be closely monitored, and specific therapeutic antibodies and vaccines that target the L452R mutation should be developed.
Full paper:
https://www.nature.com/articles/s41392-022-00941-z
https://twitter.com/jbloom_lab/status/1513537998314356736?s=20
Geert Vanden Bossche Predictions on evolution Covid 19 pandemic [UPDATE May 2022]
"I SERIOUSLY expect that a series of new highly virulent and highly infectious SARS-CoV-2 (SC-2) variants will now rapidly and independently emerge in highly vaccinated countries all over the world and that they will soon spread at high pace. I expect the current pattern of repetitive infections and relatively mild disease in vaccinees to soon aggravate and be replaced by severe disease and death. Unfortunately, there is no way vaccinees can rely on assistance from their innate immune system to protect against coronaviruses as their relevant innate IgM antibodies are increasingly being outcompeted by infection-enhancing vaccinal Abs, which are continuously recalled due to the circulation of highly infectious Omicron variants. In contrast, Omicron’s high infectiousness would enable the non-vaccinated to train their innate immune defense against SC-2 while the infectious and pathogenic capacity of the new SC-2 variants would be debilitated in the non-vaccinated for lack of infection-enhancing Abs in their blood. Unless..."
Link to full article:
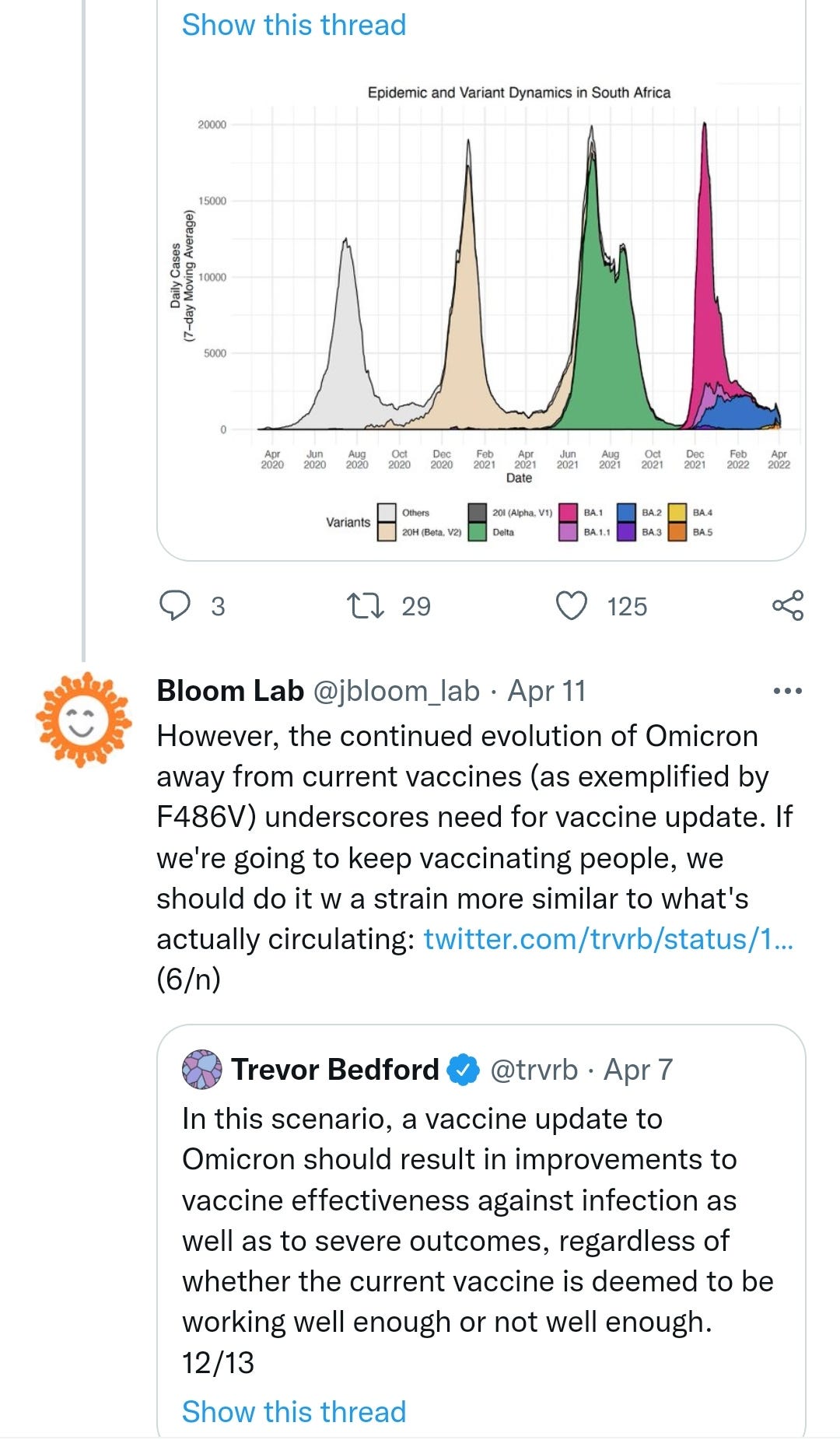
As is the norm these days, no article is allowed to conclude without a pro-vaccination spin. Even though the body of the preceding text was all about why that is a particularly bad idea that fails repeatedly, this leading, along with other factors, to an increase in hospitalisations & excess deaths of the transfected.
As per the above letter and work by Geert Vanden Bossche, I respectfully disagree that “There is currently no indication of any change in severity for BA.4/BA.5 compared to previous Omicron lineages.”:
Epidemiological update: SARS-CoV-2 Omicron sub-lineages BA.4 and BA.5
As of 12 May 2022, ECDC has reclassified Omicron sub-lineages BA.4 and BA.5 from variants of interest to variants of concern.
BA.4 and BA.5 were first detected in South Africa in January and February 2022, respectively, and since then they have become the dominant variants there. Both lineages contain the amino-acid substitutions L452R, F486V, and R493Q in the spike receptor binding domain compared to BA.2. Preliminary studies suggest a significant change in antigenic properties of BA.4 and BA.5 compared to BA.1 and BA.2, especially compared to BA.1. Additionally, there is an increasing trend in the variant proportions for BA.5 observed in Portugal the recent weeks, accompanied by an increase in COVID-19 case numbers and test positivity rate.
The Portuguese National Institute of Health estimated that BA.5 already accounted for ~37% of the positive cases as of 8 May 2022. The estimated daily growth advantage for BA.5 over BA.2 is 13%, which is similar to the 12% daily growth advantage previously reported by South Africa. Assuming such growth rate, BA.5 will become the dominant variant in Portugal by 22 May 2022.
The currently observed growth advantage for BA.4 and BA.5 is likely due to their ability to evade immune protection induced by prior infection and/or vaccination, particularly if this has waned over time. Limited available data from in vitro studies evaluating sera from unvaccinated individuals who have experienced a prior BA.1 infection indicate that both BA.4 and BA.5 are capable of escaping immune protection induced by infection with BA.1. Such unvaccinated individuals are unlikely to be protected against symptomatic infection with BA.4 or BA.5. Whilst sera from vaccinated individuals performed better in invitro studies done thus far, protection derived from currently available vaccines does wane over time against the Omicron variant.
There is currently no indication of any change in severity for BA.4/BA.5 compared to previous Omicron lineages.
Taken together, this indicates that the presence of these variants could cause a significant overall increase in COVID-19 cases in the EU/EEA in the coming weeks and months. The overall proportion of BA.4 and BA.5 in the EU/EEA is currently low but the high growth advantages reported suggest that these variants will become dominant in the EU/EEA in the coming months. Based on the limited data currently available, no significant increase in infection severity compared to the circulating lineages BA.1 and BA.2 is expected. However, as in previous waves, if COVID-19 case numbers increase substantially, some level of increased hospital and ICU admissions is likely to follow.
ECDC encourages countries to remain vigilant for signals of BA.4 and BA.5 emergence. Early variant detection critically relies on sensitive and representative testing and genomic surveillance, with timely sequence reporting. Representative testing policies are required to reliably estimate the contribution of these variants to ongoing viral circulation, as well as to accurately determine the extent these variants may contribute to any observed increases in severe outcomes in the population, such as increases in hospital or ICU admissions.
The public health benefit of administering a second mRNA COVID-19 booster dose was recently assessed by ECDC to be clearest in those aged 80 years and above and immediate administration of a second booster dose in this population was found to be optimal in situations of continued high or increasing viral circulation.
Continued close epidemiological and vaccine effectiveness monitoring is essential in order to rapidly detect signals of increased SARS-CoV-2 circulation or risk of severe disease among vaccinated individuals. If such signals emerge, a second booster may be considered for some or all adults 60 years and older and for other vulnerable groups. Countries should have plans in place for the rapid deployment of booster doses in these population groups.
For all age groups, it remains a priority to improve COVID-19 vaccine uptake of the primary course and first booster dose in populations who have yet to receive them.
Updated: 16th May ‘22
It is no coincidence that one of the most heavily vaccinated countries in the world, Portugal, is also expected to be one of the first to have “rag doll” BA.5 as the dominant variant.
This is entirely as predicted due to original antigenic sin from the long extinct Wuhan variant they were immunised (for life?) against, each new variant mediating a renewed back boost of non-neutralising antibodies, and with memory B & T cells and other active responses suppressed as explored in the other Substacks.







"As is the norm these days, no article is allowed to conclude without a pro-vaccination spin. Even though the body of the preceding text was all about why that is a particularly bad idea that fails repeatedly..."
I've noticed this too; apparently it's the only way to get published.
BA 2.1. point...something and BA. 4 and 5 are going to make really hard to mess with the data.