High viral loads: what drives fatal cases of COVID-19 in vaccinees? – an autopsy study (2022)
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
In a nutshell:
Once again, many of those most in need of enhanced protective immunity are finding the exact opposite is happening post transfection, the law of unintended consequences.
Incredibly (or perhaps not so given the institutional corruption of our age) this is the first study of its kind, certainly that I can recall:
High viral loads: what drives fatal cases of COVID-19 in vaccinees? – an autopsy study (2022)
Abstract
The rate of SARS-CoV-2 infections in vaccinees has become a relevant serious issue. This study aimed to determine the causes of death, histological organ alteration, and viral spread in relation to demographic, clinical-pathological, viral variants, and vaccine types for deceased individuals with proven SARS-CoV-2 infection after vaccination who died between January and November 2021. Twenty-nine consecutively collected cases were analyzed and compared to 141 nonvaccinated control cases. Autopsies were performed on 16 partially and 13 fully vaccinated individuals. Most patients were elderly and suffered from several relevant comorbidities. Real-time RT-PCR (RT-qPCR) identified a significantly increased rate of generalized viral dissemination within organ systems in vaccinated cases versus nonvaccinated cases (45% vs. 16%, respectively; P = 0.008) mainly with Ct-values of higher than 25 in non-respiratory samples. However, vaccinated cases also showed high viral loads, reaching Ct-values below 10, especially in the upper airways and lungs. This was accompanied by high rates of pulmonal bacterial or mycotic superinfections and the occurrence of immunocompromising factors, such as malignancies, immunosuppressive drug intake, or decreased immunoglobulin levels. All these findings were particularly accentuated in partially vaccinated patients compared to fully vaccinated individuals. The virus dissemination observed in our case study may indicate that patients with an impaired immune system have a decreased ability to eliminate the virus. However, the potential role of antibody-dependent enhancement must also be ruled out in future studies. Fatal cases of COVID-19 in vaccinees were rare and often associated with severe comorbidities or other immunosuppressive conditions.
Results
Representativity of the cohort
Given the 303 cases of deceased individuals with COVID-19 during the time of vaccine availability in the Augsburg Center, the 42 (14%) deceased individuals with COVID-19 after vaccination, 23 of which (autopsy rate 55%) are included in this study, indicates that the study cohort is representative.
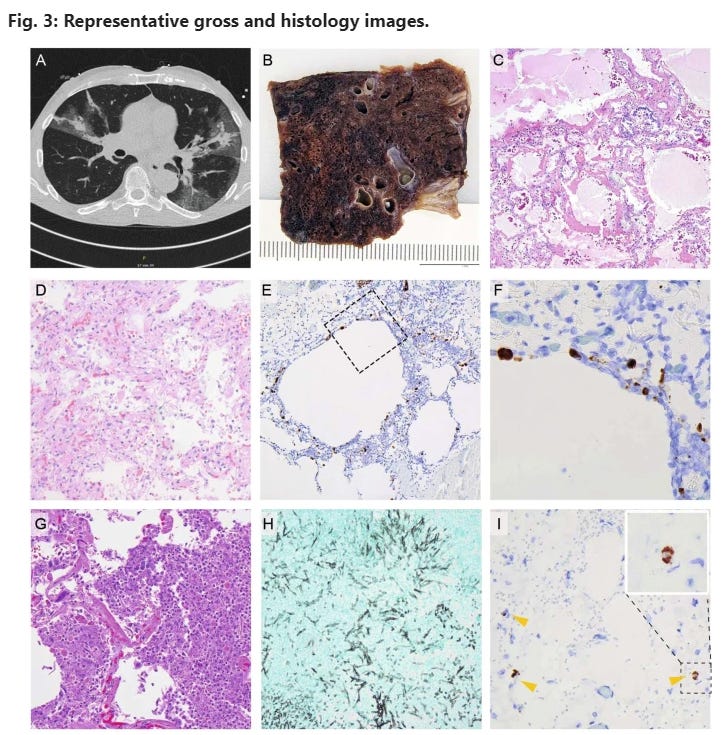
A CT-scan of a COVID-19 pneumonia after single vaccination. B Macroscopic image; formalin-fixed; lung parenchyma is widely destroyed with dark areas of hemorrhage and loss of spongious morphology. C H&E 40x magnification; acute DAD with prominent hyaline membranes. D H&E 200x magnification; organizing DAD with fibroblastic proliferation and loss of alveolar spaces. E RNA-ISH 100x magnification; high viral infection of pneumocytes and probably macrophages around emphysematic alveolar structures (F) higher magnification of the area in E marked by a square. G H&E 400x magnification; acute bacterial pneumonia with dense aggregates of granulocytes within the alveolar spaces. H Grocott 200x magnification; Invasive aspergillosis of the lung. I) RNA-ISH 300x magnification; infection of three histiocytic cells (arrow heads) within the adventitia of the aorta, insert: higher magnification of the positive cell within the dashed square.
In partially vaccinated patients, the lungs were the most affected organs. High viral loads could be detected by RT-qPCR, with a median Ct-value of 21 (range: 14–31), confirmed by RNA-ISH (Fig. 3), which showed a strong correlation with the Ct-values (R = 0.819, P < 0.0001) in a semiquantitative analysis (Fig. 4). Another remarkable observation in this collection was the high rate of malignancies in their medical history of 56%. Again, this is considerably higher compared to the completely vaccinated cases (3 vs. 10, 23%; P = 0.130) and the naïve control cohort (30 vs. 112, 21%; P = 0.005).
In contrast to the partially vaccinated cases, the viral spread in fully vaccinated cases was restricted to the upper airways and lungs in eight of the 13 cases, whereas viral dissemination throughout the organ system was seen in five cases. Again, histological changes in the organs were similar to nonvaccinated cases, with relevant impairment of the lungs. The median RT-qPCR Ct-value of the lungs was 23 (range: 17–27), similar to the partially vaccinated cases (median 21, range 14–31).
Discussion
To the best of our knowledge, this is the first series of autopsies of fatal cases of COVID-19 in SARS-CoV-2-vaccinated individuals. The lack of reliable studies and data make it difficult to assess the situation of vaccinated individuals. Therefore, we started to assess viral dissemination in the context of demographic and clinical data to identify potential factors that foster a fatal course of COVID-19 in vaccinees. The aim of this study was to investigate a cohort of 29 fatal COVID-19 cases in vaccinees by collecting all available metadata including SARS-CoV-2 antibody testing and by using necropsy, in situ hybridization, RT-qPCR analysis, and whole-genome sequencing to analyze the course of infection, allowing a substantiated disease and strain characterization.
The focus was on the comparison between partially vaccinated (vaccination interval not completed) and fully vaccinated cases (vaccination interval completed). Moreover, a collection of 141 consecutive cases from nonvaccinated individuals from the Augsburg autopsy series served as controls. Overall, the cases in vaccinees represent about one-third of all deceased in the Augsburg medical center, showing a similar but not identical demographic feature, with a slightly lower proportion of women and a slightly higher age compared to the total collective. All fully vaccinated cases came from the University Medical Center Augsburg, while six of the 16 partially vaccinated cases were contributed by other academic centers.
This study includes two fundamentally different post-vaccination situations, i.e., with partial and full vaccinations. In fully vaccinated cases, the type of infection was classified according to Schieffelin et al.7, taking so-called “vaccine nonresponders” into account. However, in our study group of fully vaccinated cases, real “breakthrough infections” occurred in the majority of individuals, and only two of ten cases were defined as likely “vaccination failure,” which therefore might play a limited role in lethal infections. For vaccination failure, it has to be clarified whether it was a primary failure (e.g., nonresponders, application errors, etc.) or loss of vaccination response over time, as recently described in Israel42. In our study group, based on serological data, a primary failure due to nonresponse, e.g., during steroid treatment, is the most likely cause in both described cases (C17) (C29)43.
The macroscopic and histomorphological findings in the partially vaccinated deceased individuals were similar to the findings in the nonvaccinated cases. Most patients died due to COVID-19 pneumonia with typical DAD. Superinfections (Supplementary Tables 1 and 2) occurred at a relatively high frequency (11 of 29), including aspergillosis (four cases). This is considerably more frequent compared to our previous results17 but rarer than reported in deceased patients after long-term treatment44. Other organs showed no histological alterations that could be associated with SARS-CoV-2 infection. However, a high rate of viral dissemination detected by RT-qPCR within the organ system was an unanticipated result in this study, which was especially accentuated in the partially vaccinated compared to fully vaccinated cases (11 of 16 vs. five of 13, respectively; P = 0.144). In several cases, RT-qPCR identified the RNA of SARS-CoV-2 in all investigated samples, including cerebrospinal fluid, CNS, and soft tissues. This is in strong contrast to a previously published collection of the Augsburg series of nonvaccinated lethal SARS-CoV-2 infections, in which the frequency of viral dissemination was rare, with a rate of only 16% (three of 19)17 instead of 69%. In this context, it seems especially important to compare the results of different cohorts within the same analytic system. Other authors have reported results we classify in this study as “disseminated” at high frequencies45,46, but they used different settings and methods.
The low Ct-values of nasopharyngeal swabs and lung samples, the latter with abundant viral detection by RNA-ISH, underline high viral loads in vaccinated deceased individuals, again with accentuation in partially vaccinated individuals. However, the previous series17 did not include VOCs. Therefore, it cannot be ruled out that the reported increased viral loads are, in part, also a consequence of the respective circulating viral variants. However, because we also found this effect in non-VOC vaccinees and observed anecdotical restricted dissemination of VOCs, including the delta and gamma variants in non-vaccinees (data not shown), it is probable that the dissemination phenotype observed here is not related to the given variant. A recently published study showed that a single shot of AZD1222 or BNT162b2 exhibited a relevant protective effect against infection with SARS-CoV-247. However, this does not equate to complete protection, and individual fatal courses (e.g., also related to preexisting disease conditions) are supported by our data. The PCR-based viral detection in samples from other locations than lung could be confirmed only in part by RNA-ISH in a few cases. This is most likely due to its reduced sensitivity compared to the PCR. We and others have shown this previously17,18,48. The fact that we could demonstrate a correlation between the Ct-values from the lung and the non-respiratory samples with the corresponding RNA-ISH results support this interpretation. In these cases, viral ISH signals were not found in parenchymal or soft tissue cells but in histiocytic cells. This is in concordance with the lack of a relevant parenchymal impairment based on the histomorphological findings. Viremia investigated by RT-PCR analysis of blood serum was detected only in one out of seven cases. This single case showed an exceedingly low Ct-value of the nasopharyngeal swab and was classified as non-disseminated. The six samples with negative serum-PCR-results were obtained from cases classified as disseminated. Serum testing by PCR has recently been shown being efficient to detect SARS-CoC-2 viremia providing a high prognostic relevance49,50. Even if we cannot rule out viremia completely it seems unlikely to be the underlying mechanism.
Despite the lack of COVID-19-specific histopathological alterations of non-respiratory organs the distribution of the virus in concert with low Ct-values in the nasopharyngeal swabs and lung samples remains a relevant result in the majority of the cases in this series.
Two major contrary theses could explain this viral spread: 1) the vaccination itself and 2) the constitution of the individual. The first is mediated by antibody-dependent enhancement (ADE)51,52,53,54,55, which is known from other viral infections, such as dengue56, Ebola57, and HIV58. In ADE, antibodies do not eliminate the virus or do so only to a reduced extent; instead, they promote viral uptake into the host’s cells. Virus-bound IgG is carried into immune cells by Fc-receptor-mediated internalization. The extent to which ADE plays a role in coronavirus infections is unclear. Reports advocating the existence of ADE in coronavirus infections are based on experiments using cell cultures59,60 or animal models61. However, there is currently no evidence indicating that ADE is a relevant mechanism counteracting the protective role of anti-spike protein antibodies generated by vaccines in humans. A large study of 20,000 patients receiving COVID-19 convalescent plasma reported no safety concerns62, which can also be considered a powerful argument against the relevant role of ADE in humans. Currently, no assays or biomarkers have been established to prove ADE in vivo.1 Immune cell infiltration, including eosinophils indicating an adverse immune reaction, is restricted to T-helper cell-mediated responses and is not related to ADE 63.
Focusing on potential patient-related factors, the immune system is of major interest in the context of failing viral elimination. Both collections in this study are characterized by a high median age and a high rate of potentially immune compromising conditions, such as cancer history (12 individuals), intake of immunosuppressive drugs (three individuals), asplenia (one individual), or decreased immunoglobulin levels (three individuals). One or more of these conditions were found in 69% and 40% of partially and fully vaccinated patients, respectively. A very recent clinical study underlines the role of immune compromission64. The finding that negative nucleocapsid antibody testing was associated with strongly increased or generalized viral dissemination in fully vaccinated cases (Table 1 and Supplementary Table 2) further supports the hypothesis that the immune system of these patients was no longer able to elicit a primary response versus the SARS-CoV-2 nucleocapsid protein, while spike-specific antibodies were often present or even boosted to high titers (Table 1 and Supplementary Table 2). In terms of cancer, a recently published study showed that malignancies are important risk factors for COVID-19, hospitalization, and death65. One explanation for this finding is the lower rate of seroconversion after vaccination of cancer patients in general as a result of immunosuppression (disease and therapy)66,67. The same is true for immunosuppressive antirheumatic drugs 43.
A general limitation of autopsy studies such as ours is the rather small case number. In an ongoing pandemic, inhomogeneities regarding the included variants might further weaken the study. Nevertheless, the consecutively collected cases with an appropriate rate can be assumed to be representative enough to draw relevant conclusions.
Overall, this is the first series of fatal courses of COVID-19 after vaccination that was analyzed in detail using a broad range of diagnostic techniques. As a major outcome, it can be concluded that most of the deceased were elderly patients with a high number of comorbidities. Lethal SARS-CoV-2 infection in vaccinated individuals therefore seems to be a very rare event and is mainly connected with high age and additional underlying factors, such as chronic diseases. A high viral infection, both in terms of the spread within the organ system and viral load in the respiratory system (detected by RT-qPCR), together with high rates of immunocompromising conditions, are the most striking findings of this study, which were accentuated in cases with an incomplete vaccination status.
Full paper:
https://www.nature.com/articles/s41379-022-01069-9
And a newly published related paper on the inferior n-protein seroconversion of the transfected as above, resulting in late and impaired adaptive immune response, vaccine enhanced infection and immune escape:
Anti-nucleocapsid antibodies following SARS-CoV-2 infection in the blinded phase of the mRNA-1273 Covid-19 vaccine efficacy clinical trial (Preprint. April 19, 2022.)
Abstract
Importance The performance of immunoassays for determining past SARS-CoV-2 infection, which were developed in unvaccinated individuals, has not been assessed in vaccinated individuals.
Objective To evaluate anti-nucleocapsid antibody (anti-N Ab) seropositivity in mRNA-1273 vaccine efficacy trial participants after SARS-CoV-2 infection during the trial’s blinded phase.
Design Nested analysis in a Phase 3 randomized, placebo-controlled vaccine efficacy trial. Nasopharyngeal swabs for SARS-CoV-2 PCR testing were taken from all participants on Day 1 and Day 29 (vaccination days), and during symptom-prompted illness visits. Serum samples from Days 1, 29, 57, and the Participant Decision Visit (PDV, when participants were informed of treatment assignment, median day 149) were tested for anti-N Abs.
Setting Multicenter, randomized, double-blind, placebo-controlled trial at 99 sites in the US.
Participants Trial participants were ≥ 18 years old with no known history of SARS-CoV-2 infection and at appreciable risk of SARS-CoV-2 infection and/or high risk of severe Covid-19. Nested sub-study consists of participants with SARS-CoV-2 infection during the blinded phase of the trial.
Intervention Two mRNA-1273 (Moderna) or Placebo injections, 28 days apart.
Main Outcome and Measure Detection of serum anti-N Abs by the Elecsys (Roche) immunoassay in samples taken at the PDV from participants with SARS-CoV-2 infection during the blinded phase. The hypothesis tested was that mRNA-1273 recipients have different anti-N Ab seroconversion and/or seroreversion profiles after SARS-CoV-2 infection, compared to placebo recipients. The hypothesis was formed during data collection; all main analyses were pre-specified before being conducted.
Results We analyzed data from 1,789 participants (1,298 placebo recipients and 491 vaccine recipients) with SARS-CoV-2 infection during the blinded phase (through March 2021). Among participants with PCR-confirmed Covid-19 illness, seroconversion to anti-N Abs at a median follow up of 53 days post diagnosis occurred in 21/52 (40%) of the mRNA-1273 vaccine recipients vs. 605/648 (93%) of the placebo recipients (p < 0.001). Higher SARS-CoV-2 viral copies at diagnosis was associated with a higher likelihood of anti-N Ab seropositivity (odds ratio 1.90 per 1-log increase; 95% confidence interval 1.59, 2.28).
Conclusions and Relevance As a marker of recent infection, anti-N Abs may have lower sensitivity in mRNA-1273-vaccinated persons who become infected. Vaccination status should be considered when interpreting seroprevalence and seropositivity data based solely on anti-N Ab testing
Trial Registration ClinicalTrials.gov NCT04470427
Question Does prior mRNA-1273 vaccination influence anti-nucleocapsid antibody seroconversion and/or seroreversion after SARS-CoV-2 infection?
Findings Among participants in the mRNA-1273 vaccine efficacy trial with PCR-confirmed Covid-19, anti-nucleocapsid antibody seroconversion at the time of study unblinding (median 53 days post diagnosis and 149 days post enrollment) occurred in 40% of the mRNA-1273 vaccine recipients vs. 93% of the placebo recipients, a significant difference. Higher SARS-CoV-2 viral copy number upon diagnosis was associated with a greater chance of anti-nucleocapsid antibody seropositivity (odds ratio 1.90 per 1-log increase; 95% confidence interval 1.59, 2.28). All infections analyzed occurred prior to the circulation of delta and omicron viral variants.
Meaning Conclusions about the prevalence and incidence of SARS-CoV-2 infection in vaccinated persons based on anti-nucleocapsid antibody assays need to be weighed in the context of these results.
Full paper:
https://www.medrxiv.org/content/10.1101/2022.04.18.22271936v1.full
“That is, there’s no clinical way to distinguish antibody-dependent enhancement from just a severe case of an infectious disease. And that can complicate analysis of a candidate vaccine. “Vaccine enhancement of disease” would show up in a clinical trial as more people receiving a vaccine getting sick than the participants getting placebo.”
Can Some Antibodies Worsen COVID-19? The Odd Situation of Enhancement - DNA Science