Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #28: Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
- A walkthrough of the paper with definitions
Stephanie Seneff, Greg Nigh, Anthony, M.Kyriakopoulos & Peter A.McCullough
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Definitions
Interferon: a protein released by animal cells, usually in response to the entry of a virus, which has the property of inhibiting virus replication.
G-quadruplex
In molecular biology, G-quadruplex secondary structures (G4) are formed in nucleic acids by sequences that are rich in guanine.[2] They are helical in shape and contain guanine tetrads that can form from one,[3] two[4] or four strands.[5] The unimolecular forms often occur naturally near the ends of the chromosomes, better known as the telomeric regions, and in transcriptional regulatory regions of multiple genes, both in microbes[6][7] and across vertebrates [8][7] including oncogenes in humans.[9] Four guanine bases can associate through Hoogsteen hydrogen bonding to form a square planar structure called a guanine tetrad (G-tetrad or G-quartet), and two or more guanine tetrads (from G-tracts, continuous runs of guanine) can stack on top of each other to form a G-quadruplex.
DNA Oxidation Contribution to Diseases
Furthermore, the concentration of 8-oxo-dG is a known biomarker of oxidative stress within a cell, and excessive amount of oxidative stress has been linked to carcinogenesis and other diseases.[56] When produced, 8-oxo-dG, has the ability to inactivate OGG1, thus preventing the repair of DNA damage caused by the oxidation of guanine.[50] The possible inactivation allows for un-repaired DNA damages to gather in non-replicating cells, like muscle, and can cause aging as well.[55] Moreover, oxidative DNA damage like 8-oxo-dG contributes to carcinogenesis through the modulation of gene expression, or the induction of mutations.[55] On the condition that 8-oxo-dG is repaired by BER, parts of the repair protein is left behind which can lead to epigenetic alterations, or the modulation of gene expression.[57][58] Upon insertion of 8-oxo-dG into thymidine kinase gene of humans, it was determine that if 8-oxo-dG was left unchecked and not repaired by BER, it can lead to frequent mutations and eventually carcinogenesis.
Cancer
Telomeres
G-quadruplex forming sequences are prevalent in eukaryotic cells, especially in telomeres, 5` untranslated strands, and translocation hot spots. G-quadruplexes can inhibit normal cell function, and in healthy cells, are easily and readily unwound by helicase. However, in cancer cells that have mutated helicase these complexes cannot be unwound and leads to potential damage of the cell. This causes replication of damaged and cancerous cells. For therapeutic advances, stabilizing the G-quadruplexes of cancerous cells can inhibit cell growth and replication leading to the cell's death.
More:
https://en.wikipedia.org/wiki/G-quadruplex
Exosome (vesicle)
Exosomes are membrane-bound extracellular vesicles (EVs) that are produced in the endosomal compartment of most eukaryotic cells.[1][2][3] The multivesicular body (MVB) is an endosome with intraluminal vesicles (ILVs) that bud inward into the endosomal lumen. If the MVB fuses with the cell surface (the plasma membrane), these ILVs are released as exosomes.
Background
Exosomes contain various molecular constituents of their cell of origin, including proteins and RNA. Although the exosomal protein composition varies with the cell and tissue of origin, most exosomes contain an evolutionarily-conserved common set of protein molecules. The protein content of a single exosome, given certain assumptions of protein size and configuration, and packing parameters, can be about 20,000 molecules.[16] The cargo of mRNA and miRNA in exosomes was first discovered at the University of Gothenburg in Sweden.[17]
The content of exosomes changes depending on the cells of origin, and they thereby reflect their originating cells. Analysis of the dynamic variation of exosomes may provide a valuable means of monitoring diseases.[18] In that study, the differences in cellular and exosomal mRNA and miRNA content was described, as well as the functionality of the exosomal mRNA cargo. Exosomes have also been shown to carry double-stranded DNA.[19]
Exosomes can transfer molecules from one cell to another via membrane vesicle trafficking, thereby influencing the immune system, such as dendritic cells and B cells, and may play a functional role in mediating adaptive immune responses to pathogens and tumors.[20][21] Therefore, scientists who are actively researching the role that exosomes may play in cell-to-cell signaling, often hypothesize that delivery of their cargo RNA molecules can explain biological effects. For example, mRNA in exosomes has been suggested to affect protein production in the recipient cell.[17][22][23] However, another study has suggested that miRNAs in exosomes secreted by mesenchymal stem cells (MSC) are predominantly pre- and not mature miRNAs.[24] Because the authors of this study did not find RNA-induced silencing complex-associated proteins in these exosomes, they suggested that only the pre-miRNAs, but not the mature miRNAs in MSC exosomes, have the potential to be biologically active in the recipient cells. Multiple mechanisms have been reported to be involved in loading miRNAs into exosomes, including specific motifs in the miRNA sequences, interactions with lncRNAs localized to the exosomes, interactions with RBPs, and post-translational modifications of Ago.[25]
Conversely, exosome production and content may be influenced by molecular signals received by the cell of origin. As evidence for this hypothesis, tumor cells exposed to hypoxia secrete exosomes with enhanced angiogenic and metastatic potential, suggesting that tumor cells adapt to a hypoxic microenvironment by secreting exosomes to stimulate angiogenesis or facilitate metastasis to more favorable environment.
More:
https://en.wikipedia.org/wiki/Exosome_(vesicle)
microRNA
A microRNA (abbreviated miRNA) is a small single-stranded non-coding RNA molecule (containing about 22 nucleotides) found in plants, animals and some viruses, that functions in RNA silencing and post-transcriptional regulation of gene expression.[1][2] miRNAs function via base-pairing with complementary sequences within mRNA molecules.[3] As a result, these mRNA molecules are silenced, by one or more of the following processes: (1) cleavage of the mRNA strand into two pieces, (2) destabilization of the mRNA through shortening of its poly(A) tail, and (3) less efficient translation of the mRNA into proteins by ribosomes.[3][4]
miRNAs resemble the small interfering RNAs (siRNAs) of the RNA interference (RNAi) pathway, except miRNAs derive from regions of RNA transcripts that fold back on themselves to form short hairpins, whereas siRNAs derive from longer regions of double-stranded RNA.[5] The human genome may encode over 1900 miRNAs,[6] although more recent analysis suggests that the number is closer to 2,300.[7] However, only about 500 human microRNAs represent bona fide miRNA in the manually curated miRNA gene database MirGeneDB.[8]
miRNAs are abundant in many mammalian cell types[9][10] and as extracellular circulating miRNAs.[11] Circulating miRNAs are released into body fluids including blood and cerebrospinal fluid and have the potential to be available as biomarkers in a number of diseases.[11][12] MiRNAs appear to target about 60% of the genes of humans and other mammals.[13][14] Many miRNAs are evolutionarily conserved, which implies that they have important biological functions.[15][1] For example, 90 families of miRNAs have been conserved since at least the common ancestor of mammals and fish, and most of these conserved miRNAs have important functions, as shown by studies in which genes for one or more members of a family have been knocked out in mice.[1]
More:
https://en.wikipedia.org/wiki/MicroRNA
Stimulator of interferon genes
Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173) and MPYS/MITA/ERIS is a protein that in humans is encoded by the STING1 gene.[5]
STING plays an important role in innate immunity. STING induces type I interferon production when cells are infected with intracellular pathogens, such as viruses, mycobacteria and intracellular parasites.[6] Type I interferon, mediated by STING, protects infected cells and nearby cells from local infection by binding to the same cell that secretes it (autocrine signaling) and nearby cells (paracrine signaling.) It thus plays an important role, for instance, in controlling norovirus infection.[7]
STING works as both a direct cytosolic DNA sensor (CDS) and an adaptor protein in Type I interferon signaling through different molecular mechanisms. It has been shown to activate downstream transcription factors STAT6 and IRF3 through TBK1, which are responsible for antiviral response and innate immune response against intracellular pathogen.[8]
More:
https://en.wikipedia.org/wiki/Stimulator_of_interferon_genes
Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs (pre-proof, 2022)
Abstract
The mRNA SARS-CoV-2 vaccines were brought to market in response to the public health crises of Covid-19. The utilization of mRNA vaccines in the context of infectious disease has no precedent. The many alterations in the vaccine mRNA hide the mRNA from cellular defenses and promote a longer biological half-life and high production of spike protein. However, the immune response to the vaccine is very different from that to a SARS-CoV-2 infection. In this paper, we present evidence that vaccination induces a profound impairment in type I interferon signaling, which has diverse adverse consequences to human health. Immune cells that have taken up the vaccine nanoparticles release into circulation large numbers of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites. We also identify potential profound disturbances in regulatory control of protein synthesis and cancer surveillance. These disturbances potentially have a causal link to neurodegenerative disease, myocarditis, immune thrombocytopenia, Bell's palsy, liver disease, impaired adaptive immunity, impaired DNA damage response and tumorigenesis. We show evidence from the VAERS database supporting our hypothesis. We believe a comprehensive risk/benefit assessment of the mRNA vaccines questions them as positive contributors to public health.
Plasmablast A short-lived, proliferating antibody-secreting cell arising from B cell differentiation.
The type-I interferons (IFN) are cytokines which play essential roles in inflammation, immunoregulation, tumor cells recognition, and T-cell responses.
The immune response to vaccination as compared to natural infection is lacking in plasmablasts and type 1 interferons, which are actively suppressed:
1. Introduction
In this paper, we explore the scientific literature suggesting that vaccination with an mRNA vaccine initiates a set of biological events that are not only different from that induced by infection but are in several ways demonstrably counterproductive to both short- and long-term immune competence and normal cellular function. These vaccinations have now been shown to downregulate critical pathways related to cancer surveillance, infection control, and cellular homeostasis. They introduce into the body highly modified genetic material. A preprint has revealed a remarkable difference between the characteristics of the immune response to an infection with SARS-CoV-2 as compared with the immune response to an mRNA vaccine against COVID-19 (Ivanova et al., 2021). Differential gene expression analysis of peripheral dendritic cells revealed a dramatic upregulation of both type I and type II interferons (IFNs) in COVID-19 patients, but not in vaccinees. One remarkable observation they made was that there was an expansion of circulating hematopoietic stem and progenitor cells (HSPCs) in COVID-19 patients, but this expansion was notably absent following vaccination. A striking expansion in circulating plasmablasts observed in COVID-19 patients was also not seen in the vaccinees. All of these observations are consistent with the idea that the anti-COVID-19 vaccines actively suppress type I IFN signaling, as we will discuss below. In this paper we will be focusing extensively, though not exclusively, on vaccination-induced type I IFN suppression and the myriad downstream effects this has on the related signaling cascade.
Little real world evidence they do anything to stop transmission or severity of infection. Antibodies do not equal immunity and quickly fade. New variants quickly show vaccine escape:
It is now clear that the antibodies induced by the vaccines fade in as little as 3–10 weeks after the second dose (Shrotri et al., 2021), such that people are being advised to seek booster shots at regular intervals (Centers for Disease Control and Prevention, 2021b). It has also become apparent that rapidly emerging variants such as the Delta and now the Omicron strain are showing resistance to the antibodies induced by the vaccines, through mutations in the spike protein (Yahi et al., 2021). Furthermore, it has become clear that the vaccines do not prevent transmission of the disease, but can only be claimed to reduce symptom severity (Kampf, 2021a). A study comparing vaccination rates with COVID-19 infection rates across 68 countries and 2947 counties in the United States in early September 2021, found no correlation between the two, suggesting that these vaccines do not protect from spread of the disease (Subramanian and Kumar, 2947). Regarding symptom severity, even this aspect is beginning to be in doubt, as demonstrated by an outbreak in an Israeli hospital that led to the death of five fully vaccinated hospital patients (Shitrit et al., 2021). Similarly, Brosh-Nissimov et al. (2021) reported that 34/152 (22%) of fully vaccinated patients among 17 Israeli hospitals died of COVID-19.
The increasing evidence that the vaccines do little to control disease spread and that their effectiveness wanes over time make it even more imperative to assess the degree to which the vaccines might cause harm. That SARS-CoV-2 modified spike protein mRNA vaccinations have biological impacts is without question. Here we attempt to distinguish those impacts from natural infection, and establish a mechanistic framework linking those unique biological impacts to pathologies now associated with vaccination. We recognize that the causal links between biological effects initiated by mRNA vaccination and adverse outcomes have not been established in the large majority of cases.
The JAK-STAT signaling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death, and tumour formation. The pathway communicates information from chemical signals outside of a cell to the cell nucleus, resulting in the activation of genes through a process called transcription. There are three key parts of JAK-STAT signalling: Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs), and receptors (which bind the chemical signals).[1] Disrupted JAK-STAT signalling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.[1]
Interferon regulatory factor 9 is a protein that in humans is encoded by the IRF9 gene, previously known as ISGF3G.IRF9 has been shown to interact with STAT2[8][9] and STAT1.[8]
Death receptor 4 (DR4), also known as TRAIL receptor 1 (TRAILR1) and tumor necrosis factor receptor superfamily member 10A (TNFRSF10A), is a cell surface receptor of the TNF-receptor superfamily that binds TRAIL and mediates apoptosis.[5][6]
Due to disrupting the JAK_STAT signalling and apoptosis (cell death) pathways of cancer cells due to downregulating interferons you are at greater risk of getting a range of cancers and/or neurodegenerative disorders:
2. Interferons: an overview with attention to cancer surveillance
Once TRAIL is bound by IRF9, it is then able to act as a ligand for Death Receptor 4 (DR4) or DR5, initiating a cascade of events involving production of caspase 8 and caspase 3, and ultimately triggering apoptosis (Sayers, 2011). Dysregulation of this pathway, through suppression of either IFN-α or IRF9 and the resulting failure to bind TRAIL-R, has been associated with several hematologic malignancies (Testa, 2010) and has been shown to increase the metastatic potential in animal models of melanoma, colorectal cancer, and lymphoma (Finnberg and El-Deiry, 2008).
IFN-α both initiates and orchestrates a wide range of cancer suppressing roles. Dunn et al. (2005) showed that IFN-α plays an active role in cancer immunoediting, its locus of action being hematopoietic cells that are “programmed” via IFN-α binding for tumor surveillance. It is via the exceedingly complex interactions between type I IFNs and IRF7 and IRF9 in particular that a great deal of antiproliferative effects are carried out. This is evidenced by the large number of studies showing increased tumor growth and/or metastases associated with a wide number of cancer types.
For example, Bidwell et al. (2012) found that, among over 800 breast cancer patients, those with high expression of IRF7-regulated genes had significantly fewer bone metastases, and they propose assessment of these IRF7-related gene signatures as a way to predict those at greatest risk. Use of microRNA to target IRF7 expression has also been shown to enhance breast cancer cell proliferation and invasion in vitro (Li et al., 2015). Zhao et al. (2017) found a similar role for IRF7 in relation to bone metastases in a mouse model of prostate cancer. Regarding the anti-cancer mechanism behind IRF7 expression, Solis et al. (2006) found that IRF7 induces transcription of multiple genes and translation of their downstream protein products including TRAIL, IL-15, ISG-56 and CD80, with the noted therapeutic implications.
IRF9, too, has a central role to play in cancer surveillance and prevention. Erb et al. (2013) demonstrated that IRF9 is the mediator through which IL-6 augments the anti-proliferation effects of IFN-α against prostate cancer cells. Tian et al. (2018) found IRF9 to be a key negative regulator of acute myeloid leukaemia cell proliferation and evasion of apoptosis. It does so, at least in part, through acetylation of the master regulatory protein p53.
Both IFN-α and IRF9 are also apparently necessary for the cancer-preventative properties of a fully functional BRCA2 gene. In a study presented as an abstract at the First AACR International Conference on Frontiers in Basic Cancer Research, Mittal and Chaudhuri (2009) describe a set of experiments which show for the first time that BRCA2 expression leads to increased IFN-α production and augments the signal transduction pathway resulting in the complexing of IRF9, STAT1 and STAT2 described previously. Two years prior, Buckley et al. (2007) had established that BRCA1 in combination with IFN-γ promotes type I IFNs and subsequent production of IRF7, STAT1, and STAT2. Thus, the exceedingly important cancer regulatory genes BRCA1 and BRCA2 rely on IRF7 and IRF9, respectively, to carry out their protective effects. Rasmussen et al. (2021) reviewed compelling evidence that deficiencies of either IRF7 or IRF9 lead to significantly greater risk of severe COVID-19 illness. Importantly, they also note that evidence suggests type I IFNs play a singularly important role in protective immunity against COVID-19 illness, a role that is shared by multiple cytokines in most other viral illnesses including influenza.
As will be discussed in more detail below, the SARS-CoV-2 spike glycoprotein modifies host cell exosome production. Transfection of cells with the spike protein's gene and subsequent SARS-CoV-2 spike protein production results in those cells generating exosomes containing microRNAs that suppress IRF9 production while activating a range of pro-inflammatory gene transcripts (Mishra and Banerjea, 2021). Since these vaccines are specifically designed to induce high and ongoing production of SARS-CoV-2 spike glycoproteins, the implications are ominous. As described above, inhibition of IRF9 will suppress TRAIL and all its regulatory and downstream apoptosis-inducing effects. IRF9 suppression via exosomal microRNA should also be expected to impair the cancer-protective effects of BRCA2 gene activity, which depends on that molecule for its activity as described above. BRCA2-associated cancers include breast, fallopian tube, and ovarian cancer for women, prostate and breast cancer for men, acute myeloid leukaemia in children, and others (National Cancer Institute, 2021).
Vaccination has also been demonstrated to suppress both IRF7 and STAT2 (Liu et al., 2021). This can be expected to interfere with the cancer-protective effects of BRCA1 as described above. Cancers associated with impaired BRCA1 activity include breast, uterine, and ovarian cancer in women; prostate and breast cancer in men; and a modest increase in pancreatic cancer for both men and women (Cancer risk and BRCA1 gene, 2021).
Reduced BRCA1 expression is linked to both cancer and neurodegeneration. BRCA1 is a well-known breast cancer susceptibility gene. BRCA1 inhibits breast cancer cell proliferation through activation of SIRT1 and subsequent suppression of the androgen receptor (Zhang et al., 2016). In a study conducted by Suberbielle et al. (2015), reduced levels of BRCA1 were found in the brains of Alzheimer's patients. Furthermore, experiments with knocking down neuronal BRCA1 in the dentate gyrus of mice showed that DNA double-strand breaks were increased, along with neuronal shrinkage and impairments in synaptic plasticity, learning and memory.
Analysis detailed in a recent case study on a patient diagnosed with a rare form of lymphoma called angioimmunoblastic T cell lymphoma provided strong evidence for unexpected rapid progression of lymphomatous lesions after administration of the BNT162b2 mRNA booster shot (Goldman et al., 2021). Comparisons of detailed metrics for hypermetabolic lesions conducted immediately before and 21 days after the vaccine booster revealed a five-fold increase after the vaccine, with the post-booster test revealing a 2-fold higher activity level in the right armpit compared to the left one. The vaccine had been injected on the right side. It is worth pointing out in this regard that lymphoid malignancies have been associated with suppression of TRAIL-R1 (MacFarlane et al., 2005).
Given the universally recognized importance of optimally functioning BRCA1/2 for cancer prevention and given the central role of the TRAIL signal transduction pathway for additional cancer surveillance, the suppression of IRF7 and IRF9 through vaccination and subsequent SARS-CoV-2 spike glycoprotein production is extremely concerning for long-term cancer control in SARS-CoV-2 mRNA genetic vaccine injected populations.
Making an effective mRNA vaccine has always been extremely challenging. By changing the amino acids they were able to make them much more resistant to immune attack which would have limited how many copies of spike protein could be created before it stopped working. It increases translation efficiency:
3. Considerations in the design of mRNA vaccines
However, with time it became clear that there were problems with this approach, both because the intense reaction could cause flu-like symptoms and because IFN-α could launch a cascade response that would lead to the breakdown of the mRNA before it could produce adequate amounts of SARS-CoV-2 spike glycoprotein to induce an immune response (de Beuckelaer et al., 2016). A breakthrough came when it was discovered experimentally that the mRNA coding for the spike protein could be modified in specific ways that would essentially fool the human cells into recognizing it as harmless human RNA. A seminal paper by Karikó et al. (2005) demonstrated through a series of in vitro experiments that a simple modification to the mRNA such that all uridines were replaced with pseudouridine could dramatically reduce innate immune activation against exogenous mRNA. Andries et al. (2015) later discovered that 1-methylpseudouridine as a replacement for uridine was even more effective than pseudouridine and could essentially abolish the TLR response to the mRNA, preventing the activation of blood-derived dendritic cells. This modification is applied in both the mRNA vaccines on the market (Park et al., 2021).
It was recommended they assessed their efficacy at triggering innate imunity, but this was not done:
Rather prophetically, the extensive review by Forni and Mantovani (2021) has raised serious questions about the development of innate immunity by the mRNA SARS-CoV-2 genetic vaccinations. As the authors declared: “Due to the short development time and the novelty of the technologies adopted, these vaccines will be deployed with several unresolved issues that only the passage of time will permit to clarify.” Subsequently, the authors recommended including certain molecules such as the long pentraxin PTX3 as representative humoral immunity markers to assess the early activation of innate immune mechanisms and the underlying reactogenicity under the BIOVACSAFE consortium protocols (Forni and Mantovani, 2021; Weiner et al., 2019). However, to the best of our knowledge these safety protocols have not been included in the assessment of induced innate immunity by the SARS-CoV-2 mRNA genetic vaccines (Mulligan et al., 2020).
Because the interferon mediated immune responses have been bypassed the immune response is inferior and less able to cope with new variants:
In this regard, in the case of SARS-CoV-2 BNT162b2 mRNA vaccine, unlike the immune response induced by natural SARS-CoV-2 infection, where a robust interferon response is observed, those vaccinated with BNT162b2 mRNA vaccines developed a robust adaptive immune response which was restricted only to memory cells, i.e., an alternative route of immune response that bypassed the IFN mediated pathways (Mulligan et al., 2020). Furthermore, due to subsequent mutations in the SARS-CoV-2 spike protein, there is a substantial loss of neutralizing antibodies induced by the BNT162b2 mRNA vaccine compared to those conferred by the SARS-CoV-2 mutants alone (Collier et al., 2021). In that respect, as vaccine developers admit: “Vaccine RNA can be modified by incorporating 1-methylpseudouridine, which dampens innate immune sensing and increases mRNA translation in vivo.” (Mulligan et al., 2020; Katalin Karikó et al., 2008). Bearing in mind the multiple mutations that SARS-CoV-2 develops, as for example in the Brazil outbreaks (Timmers et al., 2021), an effective immune response that prevents the spread of SARS-CoV2 mutants necessarily involves the development of a robust IFN-I response as a part of the innate immune system. This response also requires the involvement of a functional NF-κB response. Unfortunately, spike glycoprotein overexpression dismantles the NF-κB pathway responses, and this molecular event can be augmented by spike-protein-coding mRNAs (Kyriakopoulos and McCullough, 2021; Jiang and Mei, 2021).
To further protect against degradation and to act as an adjuvant the mRNA was also encased in a lipid nanoparticle and the end caps of the mRNA changed to look less like a genuine virus attack.
Cationic lipid nanoparticles can also be cytotoxic in their own right and may cause cellular oxidation or dampen nerve signalling through suppressed potassium gating in the case of SM-102.1
For successful mRNA vaccine design, the mRNA needs to be encapsulated in carefully constructed particles that can protect the RNA from degradation by RNA depolymerases. The mRNA vaccines are formulated as lipid nanoparticles containing cholesterol and phospholipids, with the modified mRNA complexed with a highly modified polyethylene glycol (PEG) lipid backbone to promote its early release from the endosome and to further protect it from degradation (Hou et al., 2021). The host cell's existing biological machinery is co-opted to facilitate the natural production of protein from the mRNA through endosomal uptake of a lipid particle (Hou et al., 2021). A synthetic cationic lipid is added as well, since it has been shown experimentally to work as an adjuvant to draw immune cells to the injection site and to facilitate endosomal escape. de Beuckelaer et al. (2016) observed that “condensing mRNA into cationic lipoplexes increases the potency of the mRNA vaccine evoked T cell response by several orders of magnitude.” Another important modification is that they replaced the code for two adjacent amino acids in the genome with codes for proline, which causes the spike glycoprotein to stay in a prefusion stabilized form (Wrapp et al., 2020).
The SARS-CoV-2 spike glycoprotein mRNA is further “humanized” with the addition of a guanine-methylated cap, 3′ and 5′ untranslated regions (UTRs) copied from those of human proteins, and finally a long poly(A) tail to further stabilize the RNA (Kyriakopoulos and McCullough, 2021). In particular, researchers have cleverly selected the 3′UTR taken from globins which are produced in large quantities by erythrocytes, because it is very effective at protecting the mRNA from degradation and maintaining sustained protein production (Orlandini von Niessen et al., 2019). This is to be expected, since erythrocytes have no nucleus, so they are unable to replace the mRNAs once they are destroyed. Both the Moderna and the Pfizer vaccines adopted a 3′UTR from globins, and the Pfizer vaccine also uses a slightly modified globin 5′UTR (Xia, 2021). de Beuckelaer et al. (2016) aptly summed up the consequences of such modifications as follows: “Over the past years, technical improvements in the way IVT [in vitro transcribed] mRNAs are prepared (5′ Cap modifications, optimized GC content, improved polyA tails, stabilizing UTRs) have increased the stability of IVT mRNAs to such extent protein expression can now be achieved for days after direct in vivo administration of the mRNA.”
A study found the mRNA can persist undegraded in lymph nodes, expressing spike protein for at least 60 days after vaccination. Implications for lymphoma mediation and persistently elevated inflammatory cytokine levels, which could also lead to osteoporosis.
A recent early-release study has found that the mRNA in the COVID-19 vaccines is present in germinal centers in secondary lymphoid tissue long after the vaccine is administered, and that it continues to synthesize spike glycoprotein up to at least sixty days post-vaccination (Röltgen et al., 2022). This suggests that immune cells taking up the mRNA in the arm muscle migrate into the lymph system to the lymph nodes, presumably in order to expose B-cells and T-cells to the toxic antigen. The persistence of the mRNA in the lymph nodes and its sustained synthesis of SARS-CoV-2 spike glycoprotein reflect the clever engineering involved in the mRNA technology, as described above.
In the end, it is through utilization of nanolipids and sophisticated mRNA technology that the normal immune response to exogenous RNA is evaded in order to produce a strong antibody response against an exogenous RNA virus.
To get more spike protein out of the mRNA before it degrades they used something called codon optimisation, but without testing for the effects first in vitro or in animals or humans.
This can lead to impaired immune responses, cancers, or protein misfolding, which is implicated in many degenerative diseases:
4. GC enrichment and potential G4 (pG4) structures in vaccine mRNAs
Recently, members of our team investigated possible alterations in secondary structure of mRNAs in SARS-CoV-2 vaccines due to codon optimization of synthetic mRNA transcripts (McKernan et al., 2021). This study has shown that there is a significant enrichment of GC content in mRNAs in vaccines (53% in BNT162b2 and 61% in Moderna mRNA-1273) as compared to the native SARS-CoV-2 mRNA (36%). The enriched GC content of mRNAs is the result of codon optimization performed during the development of the mRNAs used in SARS-CoV-2 vaccines, apparently without determining the effect on secondary structures, particularly the Guanine quadruplex (G quadruplex) formation (McKernan et al., 2021).
Codon optimization describes the production of synthetic, codon-optimized polypeptides and proteins used in biotechnology therapeutics (such as the synthetic mRNAs used for SARS-CoV-2 vaccination). The altered codon assignments within the mRNA template dramatically increase the quantity of polypeptides and/or proteins produced (Mauro and Chappell, 2014). Synonymous codon replacement also results in a change in the multifunctional regulatory and structural roles of resulting proteins (Shabalina et al., 2013). For this reason, codon optimization has been cautioned against due to its consequent changes causing perturbation in the secondary conformation of protein products with potentially devastating effects on their resulting immunogenicity, efficacy and function (Zhou et al., 2013; Agashe et al., 2013). Notably, various human diseases are the result of synonymous nucleotide polymorphisms (McCarthy et al., 2017).
In an experiment where GC-rich and GC-poor versions of mRNA transcripts for heat shock protein 70 were configured in the context of identical promoters and UTR sequences, it was found that GC-rich genes were expressed several-fold to over a hundred-fold more efficiently than their GC-poor counterparts (Kudla et al., 2006). This is partly because all of the preferred mammalian codons have G or C nucleotides in the third position. It is also well documented that AU-rich elements in the 3’ UTRs can destabilize mRNA (Otsuka et al., 2019). What may be of particular concern is the fact that GC enrichment content in vaccine mRNAs results in an enhanced ability for potential G-quadruplex (pG4) formations in these structures, and this could cause onset of neurological disease (Wang et al., 2021). Remarkably, the human prion protein (PrP) genetic sequence contains multiple G4 forming motifs, and their presence may form the missing link in the initial conversion of PrP to the misfolded form, PrPsc (Olsthoorn, 2014). PrP binding to its own mRNA may be the seed that causes the protein to misfold. This observation is particularly concerning in light of the fact that the SARS-CoV-2 spike glycoprotein has prion-like characteristics (Tetz and Tetz, 2022).
On the one hand, the GC content has a key role in the modulation of translation efficiency and control of mRNA expression in mammals (Babendure et al., 2006). Especially during translation initiation, the GC content operating as a cis-acting mRNA element orchestrates the 43S ribosomal pre-initiation complex attachment and thereafter the assembly of the eukaryotic translation initiation factor 4F (eIF4F) complex. One representative example of this system in action is the regulation of α and β globin mRNA expression through their 5′ untranslated regions (5′UTRs) (Babendure et al., 2006).
On the other hand, the presence of pG4s in RNAs is implicated in cancer biology as key determinants of the regulation of G4 RNA binding proteins such as helicase (Herdy et al., 2018). Generally, the G-quadruplexes in RNAs have essential roles in a) the regulation of gene expression, b) the localization of ribonuclear proteins, c) the mRNA localization and d) the regulation of proto-oncogene expression (Fay et al., 2017).
Regarding SARS-CoV-2, relevant studies reveal overwhelming similarities between SARS-CoV-2 pG4s, including in RNA coding for SARS-CoV-2 spike glycoprotein, and those sequenced in the human transcriptome (Zhang et al., 2020). Thus, it can be inferred that synthetic mRNAs in vaccines carrying more pG4 structures in their coding sequence for SARS-CoV-2 spike glycoprotein will amplify and compound the potential post-transcriptional disorganization due to G4-enriched RNA during natural SARS-CoV-2 infection. Moreover, the cellular nucleic acid binding protein (CNBP), which is the main cellular protein that binds to the SARS-CoV-2 RNA genome in human-infected cells (Schmidt et al., 2021), binds to and promotes the unfolding of SARS-CoV-2 G4s formed by both positive and negative sense template strands of the SARS-CoV-2 RNA genome. A similar modulation of CNBP on vaccine mRNA G4s and promotion of G4 equilibrium towards unfolded conformations create favorable conditions for miRNA binding, and this will have a direct impact on miRNA-dependent regulation of gene expression (Rouleau et al., 2017).
Co-infection with other viruses may induce further creation of G-quadruplexes. Cancers could result:
The negative-sense RNAs are intermediate molecules produced by the replicase transcriptase complex (RTC) formed by the nonstructural proteins of coronaviruses (including SARS-CoV-2) to provide efficiency in replication and transcription (Bezzi et al., 2021; Sola et al., 2015). This, however, introduces another potentially serious complication associated with vaccination. Co-infection with other negative sense RNA viruses such as hepatitis C (Jaubert et al., 2018) or infection by other coronaviruses contemporaneous with vaccination periods would provide the necessary machinery of RTC to reproduce negative sense intermediates from synthetic mRNAs and therefore amplify the presence of pG4s by negative sense templates. This would result in further epitranscriptomic dysregulation (Spiegel et al., 2020).
Summarizing the topic to this point, the enrichment of GC content in vaccine mRNA will inevitably lead to an increase in the pG4 content of the vaccines. This, in turn, will lead to dysregulation of the G4-RNA-protein binding system and a wide range of potential disease-associated cellular pathologies including suppression of innate immunity, neurodegeneration, and malignant transformation (Herdy et al., 2018).
The authors go into further detail here of how cellular lifespans can be disrupted, leading to either premature cell death or, more likely, cancers. They give lymphoma as an example.
The vaccine generated G4s compete for RNA binding proteins and MicroRNAs from a limited pool of both. This means that there are not enough to regulate human G4s, upsetting homeostasis and disrupting the cell cycle regulatory mechanism:
As described elsewhere, during the cellular translation of vaccine mRNAs, an increased assembly of a number of RNA binding protein helicases, such as eIF4A bound to eIF4G, will occur (Kyriakopoulos and McCullough, 2021). The presence of increased pG4s in synthetic mRNAs can potentially amplify binding of RNA binding proteins and miRNAs. This form of molecular crowding of protein components (helicases) with great affinity for G4 binding (Rouleau et al., 2017) will decrease the number of RNA binding proteins binding G4s normally available for miRNA regulation. This loss of RNA binding proteins as well as miRNA availability for regulation by binding to G4s can dramatically alter the translational regulation of miRNAs present in cells and thereby disrupt essential regulation of oncogene expression. An example is the p16-dependent regulation of the p53 tumor suppressor protein (Rouleau et al., 2017; Al-Khalaf and Aboussekhra, 2018).
This process is exceedingly complicated yet tantamount to cellular homeostasis. So, again, it merits summarizing. If pG4s accumulate, as would be expected with an increased amount of GC content in the vaccine mRNA, this would have an effect of increasing potential G4 structures available during translation events and this can affect miRNA post-transcriptional regulation. This, in turn, would either favor greater expression of the oncogenes related to a range of cancers, or drive cells towards apoptosis and cell death (Weldon et al., 2018). The case study described earlier in this paper strongly supports the hypothesis that these injections induce accelerated lymphoma progression in follicular B-cells (Goldman et al., 2021).
miRNA binding recognition patterns are imperfectly complementary to their target regions, and for this reason they are referred to as “master regulators,” since one miRNA affects a plethora of different targets (Rouleau et al., 2018). The multitude of pG4s in the mRNA of the vaccine would predictably act as decoys, distracting miRNAs from their normal function in regulating human protein expression. The increase in G4 targets due to the vaccine would decrease the availability of miRNAs to target human-expressed G4s for regulation of gene expression. This can result in downregulation of miRNA expression which is implicated in cardiovascular pathology (Small and Olson, 2011), onset of neurodegeneration (Abe and Bonini, 2013), and/or cancer progression (Farazi et al., 2013).
The G4 quadruplexes thus created by codon optimisation can also lead to a reduction in available microRNA’s to indirectly regulate a major tumor suppressor called P53, again potentially leading to a wide range of cancers. You can see where we are going with this:
In most respects within epitranscriptomic machinery, miRNAs are involved in translation repression. One example, vital for cellular normal housekeeping, is that of Mouse double minute 2 homolog (MDM2), a physical negative regulatory protein of p53. P53 itself is considered the master regulator of the cellular tumor suppression network of genes. P16 controls the expression of many miRNAs, and, via miR-141 and mIR-146b-5p binding to MDM2 mRNA, it induces the negative regulation of MDM2, thus enabling p53 ubiquitination and promotion of cell survival upon DNA damage events (Al-Khalaf and Aboussekhra, 2018). Dysregulation of miRNAs that control MDM2 suppression of p53 would predictably lead to an increased risk to a range of cancers (Ozaki and Nakagawara, 2011).
Lack of type 1 interferons is associated with severity of infection and paradoxically the vaccines can downregulate these, leading to more severe disease and infection in the 2 weeks post-vaccination:
5. Type I IFNs and COVID-19
Type I IFNs play an essential role in fighting viral infections, and deficiencies in type I IFN signaling have been associated with poor outcomes from COVID-19 in multiple studies. These cases are often associated with autoantibodies to type I IFNs. As reviewed below, type I IFNs have been used with some success in treating severe COVID-19, particularly if administered very early in the disease process. If, as argued above, the mRNA vaccines interfere with type I IFN signaling, this could lead to increased susceptibility to COVID-19 in the two weeks following the first vaccine, before an antibody response has been initiated.
A severe infection with a disrupted interferon response is associated with production of the neurotoxic and oncogenic cytokines TNF & Il-6:
A multi-author study by researchers in Paris, France, involving a cohort of 50 COVID-19 patients with varying degrees of disease severity, revealed that patients with severe disease were characterized by a highly impaired type I IFN response (Hadjadj et al., 2020). These patients had essentially no IFN-β and low IFN-α production and activity. This was associated with a persistent blood viral load and an exacerbated inflammatory response, characterized by high levels of tumor necrosis factor α (TNF-α) and Il-6. The authors proposed type I IFN therapy as a potential treatment option. A paper by several researchers in the United States also identified a unique and inappropriate inflammatory response in severe COVID-19 patients, characterized by low levels of both type I and type III IFNs along with elevated chemokines and elevated expression of Il-6 (Blanco-Melo et al., 2020).
15% of severe Covid infections in those who are deficient in type 1 interferons:
A surprising number of people have neutralizing autoantibodies against type I IFNs, although the underlying etiology of this phenomenon is not understood. A study using longitudinal profiling of over 600,000 peripheral blood mononuclear cells and transcriptome sequencing from 54 patients with COVID-19 and 26 controls found a notable lack of type I IFN-stimulated gene responses in myeloid cells from patients with critical disease (van der Wijst et al., 2021). Neutralizing autoantibodies against type I IFNs were found in 19% of patients with critical disease, 6% of patients with severe disease, and 0% of patients with moderate disease. Another study based in Madrid, Spain revealed that 10% of patients with severe COVID-19 disease had autoimmune antibodies to type I IFNs (Troya et al., 2021). A multi-author study based in France found that COVID-19 mortality was significantly more frequent in patients with neutralizing autoantibodies against type I interferon than those without neutralizing antibodies (55% vs. 23%) (Chauvineau ‐ Grenier et al., 2022). Finally, Stertz and Hale (2021) note that, whether due to autoantibodies or perhaps loss-of-function polymorphisms associated with interferon system genes, deficiencies in interferon production are associated with as many as 15% of all life-threatening COVID-19 cases.
The modified cap structure of the vaccine mRNA helps to deplete eukaryotic translation initiation factor 4E protein (eIF4E), which can lead to cancers and reduced resistance to viral infections due to dysregulating M6A. 2
6. Are the methylation strategies for cellular housekeeping generally omitted by vaccine mRNAs?
Methylation of mRNAs has been evolutionarily devised to control translation of transcripts and therefore expression of genes by a complex cascade of methylator (writers), de-methylator (eraser) and reader proteins. Adenosine methylation is the most abundant epitranscriptomic mRNA modification, and it occurs at multiple sites across the mRNA molecule (Zaccara et al., 2019). A key methylation of adenosine “N6-methyladenosine (m6A)” specifically in the 5′ UTR of mRNAs regulates normal cell physiology, the inflammatory response and cancer progression. The role and mechanisms of m6A in human disease is extensive, and it is excellently covered in other comprehensive reviews (Yang et al., 2020; Knuckles and Bühler, 2018). Foremost among these, the SARS-CoV-2 molecular vaccination induces cell stress conditions, as is described by the elevated NF-κB signaling after vaccination (Liu et al., 2021; Koo et al., 2010).
Under conditions of cellular stress, which can be induced by a viral infection or disease states such as cancer, m6A mediates mRNAs to undergo translation preferentially in a cap-independent way (Meyer et al., 2015). As discussed previously, this is opposite to the impact of mRNA SARS-CoV-2 vaccination, which drives cells toward a cap-dependent translation. Furthermore, under diversified conditions of cellular stress, there is an overwhelming induction of transcriptome-wide addition of m6A that causes an increased number of mRNAs to possess 5′UTRs enriched with m6A (Meyer et al., 2015).
Eukaryotic translation initiation factor 4E (eIF4E) is the initial mRNA cap-binding protein that directs ribosomes to the cap structure of mRNAs, in order to initiate translation into protein. The dependence on cap-dependent translation of vaccine mRNAs will consume a surplus of eIF4E availability needed to translate an unnaturally high number of synthetic mRNAs. However, cap-independent translation takes place without requiring eIF4E to be bound to eIF4F. The competition for ribosomes will shift towards the cap-independent translation of transcripts, since the mRNAs undergoing cap-independent translation are equipped, apart from internal ribosome entry sites (IRES), with special binding motifs that bind to factors that actively recruit mRNAs to the ribosome cap-independent translational enhancers (CITEs) (Shatsky et al., 2018).
Furthermore, this also means that eIF4E, which is a powerful oncogene regulator and cell proliferation modulator, will sustain its activities by this competition for an unnaturally prolonged period of time, trying to counterbalance the competition between robustly-capped mRNAs in vaccines and IRES-containing mRNAs (Kyriakopoulos and McCullough, 2021; Svitkin et al., 2005). This type of condition results in dysregulation of co-transcriptional m6A mRNA modifications and seriously links to molecular progressions of various cancers (Han and Choe, 2020), as well as creating predisposing conditions for subsequent viral infections (Svitkin et al., 2005).
Spike protein from vaccination can travel all over the body in exosomes. In the brain or in contact with other nerve cells this can lead to nerve cell damage. Inflammatory microRNA ‘s miR148a and miR-590, unique to vaccination vs Covid, can also be carried in exosomes to cause pathological effects.3
As an aside, I would also be very grateful if someone would explain why a paper found a sequence in BNT162b2 that translates to a potent downregulator of tumor suppressors and contributor to cardiovascular disease in the form of microRNA-21? Where are the regulators?4
7. Exosomes and MicroRNAs
In a collaborative effort by a team of researchers from Arizona and Connecticut, it was found that people who were vaccinated with the mRNA vaccines acquired circulating exosomes containing the SARS-CoV-2 spike glycoprotein by day 14 following vaccination (Bansal et al., 2021). They also found that there were no circulating antibodies to the spike glycoprotein fourteen days after the first vaccine. After the second vaccine, however, the number of circulating spike-glycoprotein-containing exosomes increased by up to a factor of 12. Furthermore, antibodies first appeared on day 14. The exosomes presented spike glycoprotein on their surface, which, the authors argued, facilitated antibody production. When mice were exposed to exosomes derived from vaccinated people, they developed antibodies to the spike glycoprotein. Interestingly, following peak expression, the number of circulating spike-glycoprotein-containing exosomes decreased over time, in step with the decrease in the level of antibodies to the spike glycoprotein.
In humans, there is an abundance of mostly asymptomatic picornavirus infections like the Safford Virus with an over 90% seroprevalence in young children and adults (Zoll et al., 2009). In either case, whether an apoptotic event due to a stress-like condition (Rusk, 2008) or an mRNA-cap-driven-like carcinomatous effect (De Paolis et al., 2021), the miRNA levels will be increased due to the increased epitranscriptomic functioning and enhanced mRNA decay. Because of the high demand for gene expression, high levels of certain miRNAs will be expected to be contained in exosomes via P bodies (Yu et al., 2016).
Also, under conditions of overwhelming production of SARS-CoV-2 spike glycoprotein due to SARS-CoV-2 molecular vaccination, it would of course be expected that a significant proportion of over-abundant intracellular spike glycoproteins would also be exported via exosome cargoes (Wei et al., 2021).
Mishra and Banerjea (2021) investigated the role of exosomes in the cellular response of SARS-CoV-2 spike-transfected cells. They wrote in the abstract:
“We propose that SARS-CoV-2 gene product, Spike, is able to modify the host exosomal cargo, which gets transported to distant uninfected tissues and organs and can initiate a catastrophic immune cascade within Central Nervous System (CNS).”
Their experiments involved growing human HEK293T cells in culture and exposing them to SARS-CoV-2 spike gene plasmids, which induced synthesis of spike glycoprotein within the cells. They found experimentally that these cells released abundant exosomes housing spike glycoprotein along with specific microRNAs. They then harvested the exosomes and transferred them to a cell culture of human microglia (the immune cells that are resident in the brain). They showed that the microglia readily took up the exosomes and responded to the microRNAs by initiating an acute inflammatory response. The role of microglia in causing neuroinflammation in various viral diseases, such as Human Immunodeficiency Virus (HIV), Japanese Encephalitis Virus (JEV), and Dengue, is well established. They proposed that long-distance cell-cell communication via exosomes could be the mechanism by which neurological symptoms become manifest in severe cases of COVID-19.
In further exploration, the authors identified two microRNAs that were present in high concentrations in the exosomes: miR-148a and miR-590. They proposed a specific mechanism by which these two microRNAs would specifically disrupt type I interferon signaling, through suppression of two critical proteins that control the pathway: ubiquitin specific peptidase 33 (USP33) and IRF9. Phosphorylated STAT1 and STAT2 heterodimers require IRF9 in order to bind IFN-stimulated response elements, and therefore IRF9 plays an essential role in the signaling response. The authors showed experimentally that microglia exposed to the exosomes extracted from the HEK293 culture had a 50% decrease in cellular expression of USP33 and a 60% decrease in IRF9. They further found that miR-148a specifically blocks USP33 and miR-590 specifically blocks IRF9. USP33 removes ubiquitin from IRF9, and in so doing it protects it from degradation. Thus, the two microRNAs together conspire to interfere with IRF9, thus blocking receptor response to type I interferons.
A study by de Gonzalo-Calvo et al. (2021) looked at the microRNA profile in the blood of COVID-19 patients and their quantitative variance based upon disease severity. Multiple miRNAs were found to be up- and down-regulated. Among these was miR-148a-3p, the guide strand precursor to miR-148a. However, miR-148a itself was not among the microRNAs catalogued as excessive or deficient in their study, nor was miR-590. It appears from these findings that miR148a and miR-590 and their inflammatory effects are unique to vaccination-induced SARS-CoV-2 spike glycoprotein production.
Vaccinal exosomes can travel to the brainstem along the vagus nerve from the spleen, leading to all sorts of deleterious effects, as you can imagine. I will add here that CJD uses the same pathway…
Tracer studies have shown that, following injection into the arm muscle, the mRNA in mRNA vaccines is carried into the lymph system by immune cells and ultimately accumulates in the spleen in high concentrations (Bahl et al., 2017). Other studies have shown that stressed immune cells in germinal centers in the spleen release large quantities of exosomes that travel to the brain stem nuclei along the vagus nerve (as reviewed in Seneff and Nigh (2021)). The vagus nerve is the 10th cranial nerve and it enters the brainstem near the larynx. The superior and recurrent laryngeal nerves are branches of the vagus that innervate structures involved in swallowing and speaking. Lesions in these nerves cause vocal cord paralysis associated with difficulty swallowing (dysphagia) difficulty speaking (dysphonia) and/or shortness of breath (dyspnea) (Gould et al., 2019; Erman et al., 2009). We will return to these specific pathologies in our review of VAERS data below.
HEK293 cells were originally derived from cultures taken from the kidney of a human fetus several decades ago and immortalized through infection with adenovirus DNA. While they were extracted from the kidney, the cells show through their protein expression profile that they are likely to be of neuronal origin (Shaw et al., 2002). This suggests that neurons in the vagus nerve would respond similarly to the SARS-CoV-2 spike glycoprotein. Thus, the available evidence strongly suggests that endogenously produced SARS-CoV-2 spike glycoprotein creates a different microRNA profile than does natural infection with SARS-CoV-2, and those differences entail a potentially wide range of deleterious effects.
The authors restate the consequences of suppressing type I interferon responses.
Re-activation of latent viruses can be associated with brain cancer or more severe disease.5
A central point of our analysis below is the important distinction between the impact of vaccination versus natural infection on type I IFN. While vaccination actively suppresses its production, natural infection promotes type I IFN production very early in the disease cycle. Those with preexisting conditions often exhibit impaired type I IFN signaling, which leads to more severe, critical, and even fatal COVID-19. If the impairment induced by the vaccine is maintained as antibody levels wane over time, this could lead to a situation where the vaccine causes a more severe disease expression than would have been the case in the absence of the vaccine.
Another expected consequence of suppressing type I IFN would be reactivation of preexisting, chronic viral infections, as described in Section 9.
pluripotent: (of an immature cell or stem cell) capable of giving rise to several different cell types.
Impaired DNA repair can inhibit the production and maintenance of of functioning B and T cells and trigger a pathway to carcinogenesis. A study using an attenuated virus vaccine found that “STAT2 and IRF7 were significantly downregulated 28 days after vaccination, indicative of impaired type I IFN responses.“6
8. Impaired DNA repair and adaptive immunity
The immune system and the DNA repair system are the two primary systems that higher organisms rely on for defense against diverse threats, and they share common elements. Loss of function of key DNA repair proteins leads to defects in repair that inhibit the production of functional B- and T-cells, resulting in immunodeficiency. Non-homologous end joining (NHEJ) repair plays a critical role in lymphocyte-specific V(D)J recombination, which is essential for producing the highly diverse repertoire of B-cell antibodies in response to antigen exposure (Jiang and Mei, 2021). Impaired DNA repair is also a direct pathway towards cancer.
A paper published by Liu et al., in 2021 monitored several parameters associated with immune function in a cohort of patients by conducting single-cell mRNA sequencing of peripheral blood mononuclear cells (PBMCs) harvested from the patients before and 28 days after the first injection of a COVID-19 vaccine based on a weakened version of the virus (Liu et al., 2021). While these vaccines are different from the mRNA vaccines, they also work by injecting the contents of the vaccine into the deltoid muscle, bypassing the mucosal and vascular barriers. The authors found consistent alteration of gene expression following vaccination in many different immune cell types. Observed increases in NF-κB signaling and reduced type I IFN responses were further confirmed by biological assays. Consistent with other studies, they found that STAT2 and IRF7 were significantly downregulated 28 days after vaccination, indicative of impaired type I IFN responses. They wrote: “Together, these data suggested that after vaccination, at least by day 28, other than generation of neutralizing antibodies, people's immune systems, including those of lymphocytes and monocytes, were perhaps in a more vulnerable state.” (Liu et al., 2021).
These authors also identified disturbing changes in gene expression that would imply impaired ability to repair DNA. Up to 60% of the total transcriptional activity in growing cells involves the transcription of ribosomal DNA (rDNA) to produce ribosomal RNA (rRNA). The enzyme that transcribes ribosomal DNA into RNA is RNA polymerase I (Pol I). Pol I also monitors rDNA integrity and influences cell survival (Kakarougkas et al., 2013). During transcription, RNA polymerases (RNAPs) actively scan DNA to find bulky lesions (double-strand breaks) and trigger their repair. In growing eukaryotic cells, most transcription involves synthesis of ribosomal RNA by Pol I. Thus, Pol I promotes survival following DNA damage (Kakarougkas et al., 2013). Many of the downregulated genes identified by Liu et al. (2021) were linked to the cell cycle, telomere maintenance, and both promoter opening and transcription of POL I, indicative of impaired DNA repair processes.
One of the gene sets that were suppressed was due to “deposition of new CENPA [centromere protein A] containing nucleosomes at the centromere.” Newly synthesized CENPA is deposited in nucleosomes at the centromere during late telophase/early G1 phase of the cell cycle. This points to arrest of the cell cycle in G1 phase as a characteristic feature of the response to the inactivated SARS-CoV-2 vaccine. Arrest of pluripotent embryonic stem cells in the G1 phase (prior to replication initiation) would result in impaired self-renewal and maintenance of pluripotency (Choi et al., 2013).
Key P53 & BRCA tumor suppressor impairment, ie cancer cells have the opportunity to grow unchecked:7
Two checkpoint proteins crucially involved in DNA repair and adaptive immunity are BRCA1 and 53BP1, which facilitate both homologous recombination (HR) and NHEJ, the two primary repair processes (Zhang and Powell, 2005; Panier and Boulton, 2014). In an in vitro experiment on human cells, the SARS-CoV-2 full-length spike glycoprotein was specifically shown to enter the nucleus and hinder the recruitment of these two repair proteins to the site of a double-strand break (Jiang and Mei, 2021). The authors summarized their findings by saying, “Mechanistically, we found that the spike protein localizes in the nucleus and inhibits DNA damage repair by impeding key DNA repair protein BRCA1 and 53BP1 recruitment to the damage site.”
Another mechanism by which the mRNA vaccines could interfere with DNA repair is through miR-148. This microRNA has been shown to downregulate HR in the G1 phase of the cell cycle (Choi et al., 2014). As was mentioned earlier in this paper, this was one of the two microRNAs found in exosomes released by human cells following SARS-CoV-2 spike glycoprotein synthesis in the experiments by Mishra and Banerjea (2021).
Impaired type 1 interferon signaling means that latent viruses like herpes can be reactivated, leading to shingles. If it is an oncogenic virus like HPV this can also lead to increased risk of carcinogenesis, as per the Substack on GP-120 and HIV (see footnote for link).
9. Reactivation of varicella-zoster
Type I IFN receptor signaling in CD8+ T cells is critical for the generation of effector and memory cells in response to a viral infection (Kolumam et al., 2005). CD8+ T cells can block reactivation of latent herpes infection in sensory neurons (Liu et al., 2000). If type I IFN signaling is impaired, as happens following vaccination but not following natural infection with SARS-CoV-2, CD8+ T cells’ ability to keep herpes in check would also be impaired. Might this be the mechanism at work in response to the vaccines?
Shingles is an increasingly common condition caused by reactivation of latent herpes zoster viruses (HZV), which also causes chicken pox in childhood. In a systematic review, Katsikas Triantafyllidis et al. (2021) identified 91 cases of herpes zoster occurring an average of 5.8 days following mRNA vaccination. While acknowledging that causality is not yet confirmed, “Herpes zoster is possibly a condition physicians and other healthcare professionals may expect to see in patients receiving COVID-19 vaccines” (Katsikas Triantafyllidis et al., 2021). In a letter to the editor published in September 2, 2021, Fathy et al. (2022) reported on 672 cases of skin reactions that were presumably vaccine-related, including 40 cases of herpes zoster and/or herpes simplex reactivation. These cases had been reported to the American Academy of Dermatology and the International League of Dermatologic Societies’ COVID-19 Dermatology Registry, established specifically to track dermatological sequalae from the vaccines. There are multiple additional case reports of herpes zoster reactivation following COVID-19 vaccination in the literature (Psichogiou et al., 2021b; Iwanaga et al., 2021). Lladó et al. (2021) noted that 51 of 52 reports of reactivated herpes zoster infections happened following mRNA vaccination. Herpes zoster itself also interferes with IFN-α signaling in infected cells both through interfering with STAT2 phosphorylation and through facilitating IRF9 degradation (Verweij et al., 2015).
An additional case of viral reactivation is noteworthy as well. It involved an 82-year-old woman who had acquired a hepatitis C viral (HCV) infection in 2007. A strong increase in HCV load occurred a few days after vaccination with an mRNA Pfizer/BioNTech vaccine, along with an appearance of jaundice. She died three weeks after vaccination from liver failure (Lensen et al., 2021).
10. Immune thrombocytopenia
Immune thrombocytopenia is an autoimmune disorder, where the immune system attacks circulating platelets. Immune thrombocytopenic purpura (ITP) has been associated with several vaccinations, including measles, mumps, rubella (MMR), hepatitis A, varicella, diphtheria, tetanus, pertussis (DPT), oral polio and influenza (Perricone et al., 2014). While there is broad awareness that the adenovirus DNA-based vaccines can cause vaccine-induced immune thrombotic thrombocytopenia (VITT) (Kelton et al., 2021), the mRNA vaccines are not without risk to VITT, as case studies have been published documenting such occurrences, including life threatening and fatal cerebral venous sinus thrombosis (Lee et al., 2021; Akiyama et al., 2021; Atoui et al., 2022; Zakaria et al., 2021). The mechanism is believed to involve VITT antibodies binding to platelet factor 4 (PF4) and forming immune complexes that induce platelet activation. Subsequent clotting cascades cause the formation of diffuse microclots in the brain, lungs, liver, legs and elsewhere, associated with a dramatic drop in platelet count (Kelton et al., 2021). The reaction to the vaccine has been described as being very similar to heparin-induced thrombocytopenia (HIT), except that heparin administration is notably not involved (Cines and Bussel, 2021).
Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation.[1] IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes.
Immunoglobulin A (IgA, also referred to as sIgA in its secretory form) is an antibody that plays a role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined.[3] In absolute terms, between three and five grams are secreted into the intestinal lumen each day.[4] This represents up to 15% of total immunoglobulins produced throughout the body.[5]
Immunoglobulin M (IgM) is one of several isotypes of antibody (also known as immunoglobulin) that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antigen.[1][2] In the case of humans and other mammals that have been studied, the spleen, where Plasmablasts that are responsible for antibody production reside, is the major site of specific IgM production.[3][4]
Platelet factor 4 (PF4) is a small cytokine belonging to the CXC chemokine family that is also known as chemokine (C-X-C motif) ligand 4 (CXCL4) . This chemokine is released from alpha-granules of activated platelets during platelet aggregation, and promotes blood coagulation by moderating the effects of heparin-like molecules. Due to these roles, it is predicted to play a role in wound repair and inflammation.[5] It is usually found in a complex with proteoglycan.
It has been shown that the mRNA vaccines elicit primarily an immunoglobulin G (IgG) immune response, with lesser amounts of IgA induced (Wisnewski et al., 2021), and even less IgM production (Danese et al., 2021). The amount of IgG antibodies produced is comparable to the response seen in severe cases of COVID-19. It is IgG antibodies in complex with heparin that induce HIT. One can hypothesize that IgG complexed with the SARS-CoV-2 spike glycoprotein and PF4 is the complex that induces VITT in response to mRNA vaccines. It has in fact been shown experimentally that the receptor binding domain (RBD) of the spike protein binds to PF4 (Passariello et al., 2021).
A spike protein complex and/or vaccine mediated microRNA-148a can elevate the risk of thrombocytopenia:
Platelets normally circulate with an average lifespan of only five to nine days, so they are constantly synthesized in the bone marrow and cleared in the spleen. Antibody-bound platelets, subsequent to platelet activation via Fcγ receptors, migrate to the spleen where they are trapped and removed through phagocytosis by macrophages (Crow and Lazarus, 2003). Fully one third of the body's total platelets are found in the spleen. Since the mRNA vaccines are carried into the spleen by immune cells initially attracted to the injection site in the arm muscle, there is tremendous opportunity for the release of spike-glycoprotein-containing exosomes by dendritic cells in the spleen synthesizing spike protein. One can speculate that platelet activation following the formation of a P4F/IgG/spike protein complex in the spleen is part of the mechanism that attempts to clear the toxic spike glycoprotein.
We mentioned earlier that one of the two microRNAs highly expressed in exosomes released by human cells exposed to the SARS-CoV-2 spike glycoprotein was miR-148a. miR-148a has been shown experimentally to suppress expression of a protein that plays a central role in regulating FcγRIIA expression on platelets. This protein, called T-cell ubiquitin ligand-2 (TULA-2), specifically inhibits activity of the platelet Fcγ receptor. miR-148a targets TULA-2 mRNA and downregulates its expression. Thus, miR-148a, present in exosomes released by macrophages that are compelled by the vaccine to synthesize SARS-CoV-2 spike glycoprotein, acts to increase the risk of thrombocytopenia in response to immune complexes formed by spike glycoprotein antigen and IgG antibodies produced against the spike glycoprotein.
MicroRNA’s from the vaccine can suppress interferon regulatory factor 9 (IRF9) synthesis via PPAR-α. This leads in turn to lower levels of a sulfatide that helps stop blood clotting, is anti-inflammatory and anti-fibrotic. Lower levels of this combined with a high LDL cholesterol diet can lead to increased oxidative stress in the liver and a range of diseases resembling those caused by alcohol abuse:
11. PPAR-α, sulfatide and liver disease
As we have already stated, an experiment by Mishra and Banerjea (2021) demonstrated that the SARS-CoV-2 spike glycoprotein induces the release of exosomes containing microRNAs that specifically interfere with IRF9 synthesis. In this section we will show that one of the consequences of suppression of IRF9 would be reduced synthesis of sulfatide in the liver, mediated by the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α).
Sulfatides are major mammalian serum sphingoglycolipids which are synthesized and secreted mainly from the liver (Lu et al., 2019). They are the only sulfonated sphingolipids in the body. Sulfatides are formed by a two-step process involving the conversion of ceramide to galactocerebroside and its subsequent sulfation. Sulfatide is expressed on the surface of platelets, erythrocytes and lymphocytes. Serum sulfatides exert both anti-coagulative and anti-platelet-activation functions. The enzyme in the liver that synthesizes sulfatide, cerebroside sulfotransferase, has specifically been found to be induced by activation of PPAR-α in mice (Kimura et al., 2012). Therefore, reduced expression of PPAR-α leads to sulfatide deficiency.
PPAR-α ligands exhibit anti-inflammatory and anti-fibrotic effects, whereas PPAR-α deficiency leads to hepatic steatosis, steatohepatitis, steatofibrosis, and liver cancer (Wang et al., 2020b). In 2019, an experiment was conducted by a team of researchers in Japan on mice with a defective gene for PPAR-α (Lu et al., 2019). These mice, when fed a high cholesterol diet, were susceptible to excess triglyceride accumulation and exacerbated inflammation and oxidative stress in the liver, along with increased levels of coagulation factors. The mice also manifested with decreased levels of sulfatides in both the liver and the serum. The authors hypothesized that cholesterol overload exerts its toxic effects in part by enhancing thrombosis, following abnormal hepatic lipid metabolism and oxidative stress. They showed that PPAR-α can attenuate these toxic effects through transcriptional regulation of coagulation factors and upregulation of sulfatide synthesis, in addition to its effects in ameliorating liver disease. They proposed that therapies such as fibrates aimed at activating PPAR-α might prevent high-cholesterol-diet-induced cardiovascular disease.
Tracer studies have shown that the mRNA from mRNA vaccines migrates preferentially to the liver and spleen, reaching higher concentration there than in any other organs (Bahl et al., 2017). Thus, there is potential for suppression of IRF9 in the liver by the vaccine. IRF9 is highly expressed in hepatocytes, where it interacts with PPAR-α, activating PPAR-α target genes. A study on IRF9 knockout mice showed that these mice developed steatosis and hepatic insulin resistance when exposed to a high-fat diet. In contrast, adenoviral-mediated hepatic IRF9 overexpression in obese mice improved insulin sensitivity and ameliorated steatosis and inflammation (Wang et al., 2013).
Multiple case reports in the research literature describe liver damage following mRNA vaccines (Zin Tun et al., 2021; Dumortiera, 2022; Mann et al., 2021). A plausible factor leading to these outcomes is the suppression of PPAR-α through downregulation of IRF9, and subsequently decreased sulfatide synthesis in the liver.
Vaccine mediated sulfatide deficiency can trigger autoimmune attacks on nerve cells, leading to GBS according to this theory:
12. Guillain Barré syndrome and neurologic injury syndromes
GBS is an acute inflammatory demyelinating neuropathy associated with long-lasting morbidity and a significant risk of mortality (Cr é ange, 2000). The disease involves an autoimmune attack on the nerves associated with the release of pro-inflammatory cytokines.
GBS is often associated with autoantibodies to sulfatide and other sphingolipids (Ilyas et al., 1991). Activated T-cells produce cytokines in response to antigen presentation by macrophages, and these cytokines can induce autoantibody production through epitope spreading (Vanderlugt and Miller, 2002). The antibodies, in turn, induce complement activation, which causes demyelination and axonal damage, leading to severe injury to peripheral neurons (Kuwahara and Kusunoki, 2018). The SARS-CoV-2 spike glycoprotein has been shown to bind to heparan sulfate, which is a sulfated amino-sugar complex resembling the sulfated galactose in sulfatide (Kalra and Kandimalla, 2021). Thus, it is conceivable that the spike glycoprotein also binds to sulfatide, and this might trigger an immune reaction to the spike-glycoprotein-sulfatide complex.
As described in the previous section, impaired sulfatide synthesis in the liver due to suppression of IRF9 will lead to systemic sulfatide deficiency over time. Sulfatide deficiency can have major impact in the brain and nervous system. Twenty percent of the galactolipids found in the myelin sheath are sulfatides. Sulfatide is a major component of the nervous system, found in especially high concentrations in the myelin sheath in both the peripheral and the central nervous system. Deficiencies in sulfatide can lead to muscle weakness, tremors, and ataxia (Honke, 2013), which are common symptoms of GBS. Chronic neuroinflammation mediated by microglia and astrocytes in the brain leads to dramatic losses of brain sulfatide, and brain deficiencies in sulfatide are a major feature of Alzheimer's disease (Qiu et al., 2021). Mice with a defect in the ability to synthesize sulfatide from ceramide show an impaired ability to maintain the health of axons as they age. Over time, they develop redundant, uncompacted and degenerating myelin sheaths as well as deteriorating structure at the nodes of Ranvier in the axons, causing the loss of a functionally competent axoglial junction (Marcus et al., 2006).
Spike protein binding to ACE2 upregulates angiotensin II (ANGII) levels, which causes oxidative damage and inflammation to neurons if it happens in the brain. Cleaved S1 spike segments can easily cross the blood brain barrier, and exosomes are suspected too. Neurodegenerative disease or rapid deterioration of existing conditions like Alzhemers’s Disease can result.
To the optic nerve this can lead to irreversible blindness.
Tinitus is positively associated to ANGII levels and the resulting hypertension.
Migraines. One theory is that spike protein containing exosomes can travel along the vagus nerve from the spleen to the brain stem, deplete IRF9, elevate ANGII & oxidation8, reduce sulfatide levels and the resulting autoimmune attack on the nerves causes chronic pain:
Angiotensin II (Ang II), in addition to its profound effects on cardiovascular disease, also plays a role in inflammation in the brain leading to neurodegenerative disease (Lanz. et al., 2010). The SARS-CoV-2 spike glycoprotein contains a unique furin cleavage site not found in SARS-CoV, which allows the extracellular enzyme furin to detach the S1 segment of the spike glycoprotein and release it into circulation (Letarov et al., 2021). S1 has been shown to cross the blood-brain barrier in mice (Rhea et al., 2021). S1 contains the receptor binding domain that binds to ACE2 receptors, disabling them. When ACE2 receptor signaling is reduced, Ang II synthesis is increased. Neurons in the brain possess ACE2 receptors that would be susceptible to disruption by S1 released from spike-glycoprotein-containing exosomes or spike-glycoprotein-producing cells that had taken up the nanoparticles in the vaccines. Ang II enhances TLR4-mediated signaling in microglia, inducing microglial activation and increasing the production of reactive oxygen species leading to tissue damage, within the paraventricular nucleus in the brain (Rodriguez-Perez et al., 2015).
Elevated levels of Ang II is a causal factor in neurodegeneration of the optic nerve, causing optic neuritis, which can result in severe irreversible visual loss (Guo et al., 2017). Multiple case reports have described cases of optic neuropathy appearing shortly after mRNA vaccination for COVID-19 (Maleki, 2021; Barone et al., 2021). Other debilitating neurological conditions are also appearing shortly after vaccination, where a causal relationship is suspected. A case study based in Europe tracking neurological symptoms following COVID-19 vaccination identified 21 cases developing within a median of 11 days post-vaccination. The cases had diverse diagnoses including cerebral venous sinus thrombosis, nervous system demyelinating diseases, inflammatory peripheral neuropathies, myositis, myasthenia, limbic encephalitis, and giant cell arteritis (Kaulen et al., 2021). Khayat-Khoei et al. (2021) describe a case series of 7 patients, ages ranging from 24 to 64, presenting with demyelinating disease within 21 days of a first or second mRNA vaccination. Four had a prior history of (controlled) MS, while three were previously healthy.
Hearing loss and tinnitus are also well-known side effects of COVID-19. A case study involved a series of ten COVID-19 patients who suffered from audiovestibular symptoms such as hearing loss, vestibular dysfunction and tinnitus (Jeong et al., 2021). The authors demonstrated that human inner ear tissue expresses ACE2, furin and the transmembrane protease serine 2 (TMPRSS2), which facilitates viral entry. They also showed that SARS-CoV-2 can infect specific human inner ear cell types.
Another study evaluating the potential for the SARS-CoV-2 virus to infect the ear specifically examined expression of the receptor ACE2 and the enzymes furin and TM-PRSS2 various types of cells in the middle and inner ears of mice. They found that ACE2 and furin were “diffusely present in the eustachian tube, middle ear spaces, and cochlea, suggesting that these tissues are susceptible to SARS-CoV-2 infection.” (Uranaka et al., 2021). Tinnitus is positively associated with hypertension, which is induced by elevated levels of Ang II (Rodrigues Figueiredo et al., 2016).
Headache is a very common adverse reaction to the COVID-19 mRNA vaccines, particularly for people who are already susceptible to headaches. In a study based on a questionnaire involving 171 participants, the incidence of headaches was found to be 20.5% after the first vaccine, rising to 45.6% after the second shot (Sekiguchi et al., 2021). A case study described a 37-year-old woman suffering from a debilitating migraine attack lasting for 11 days following the second Pfizer/BioNtech mRNA vaccine (Consoli et al., 2021).
Steroids are often used as adjunct therapy to treat migraine (Huang et al., 2013). Dexamethasone and other steroids stimulate PPAR-α receptors in the liver through the steroid receptor, thus offsetting the effects of IRF9 suppression (Lemberger et al., 1994). A theory for the origins of migraine involves altered processing of sensory input in the brainstem, primarily trigeminal neurons (Dodick and Silberstein, 2006). The trigeminal nerve is in close proximity to the vagus nerve in the brainstem, so spike-glycoprotein-carrying exosomes could easily reach it along the vagal route. Magnetic resonance imaging has revealed that structural changes in the trigeminal nerve reflecting aberrant microstructure and demyelination are a characteristic feature of people who suffer from frequent migraine headaches (Mungoven et al., 2020). A potential factor linked to either SARS-CoV-2 infection or mRNA vaccination is an excessive level of Ang II in the brainstem due to SARS-CoV-2 spike glycoprotein inhibition of ACE2 receptors. ACE inhibitors and Ang II receptor antagonists have become popular drugs to treat migraine headaches off-label (Tronvik et al., 2003; Nandha and Singh, 2012). Migraine headache could thus arise from both the spike glycoprotein's disruption of ACE2 receptors and the destruction of the myelin sheath covering critical facial nerves through a microglial inflammatory response and loss of sulfatide. The source of that spike glycoprotein could be either exogenous or endogenous.
Peripheral neuropathy, often shortened to neuropathy, is a general term describing disease affecting the peripheral nerves, meaning nerves beyond the brain and spinal cord.[1] Damage to peripheral nerves may impair sensation, movement, gland, or organ function depending on which nerves are affected; in other words, neuropathy affecting motor, sensory, or autonomic nerves result in different symptoms. More than one type of nerve may be affected simultaneously. Peripheral neuropathy may be acute (with sudden onset, rapid progress) or chronic (symptoms begin subtly and progress slowly), and may be reversible or permanent.
A ganglion is a group of neuronal bodies.
13. Bell's palsy
Bell's palsy is a common cranial neuropathy causing unilateral facial paralysis. Even in the Phase III clinical trials, Bell's palsy stood out, with seven cases appearing in the treatment arm as compared to only one in the placebo group (FDA, 2021a; FDA, 2021b). A case study reported in the literature involved a 36-year-old man who developed weakness in the left arm one day after vaccination, progressing to numbness and tingling in the arm and subsequent symptoms of Bell's palsy over the next few days. A common cause of Bell's palsy is reactivation of herpes simplex virus infection centered around the geniculate ganglion (Eviston et al., 2015). This, in turn, can be caused by disruption of type I IFN signaling.
Full paper:
https://www.sciencedirect.com/science/article/pii/S027869152200206X
Thank you for reading, please subscribe and share to raise awareness.
Moderna & SM-102: Fact checking the fact checkers
A sting in the tail
https://doorlesscarp953.substack.com/p/moderna-and-sm-102-fact-checking?s=w
More on M6A and cancer:
Regulation of Gene Expression Associated With the N6-Methyladenosine (m6A) Enzyme System and Its Significance in Cancer (2021)
https://www.frontiersin.org/articles/10.3389/fonc.2020.623634/full
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #20: MiR-200, a new star miRNA in human cancer
Effect of exosomal miRNA on cancer biology and clinical applications (2018)
https://doorlesscarp953.substack.com/p/effect-of-exosomal-mirna-on-cancer?s=w
The type I interferon-alpha mediates a more severe neurological disease in the absence of the canonical signaling molecule interferon regulatory factor 9. (2010)
https://doorlesscarp953.substack.com/p/the-type-i-interferon-alpha-mediates?s=wSARS-
CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia
(Front. Immunol., 14 April 2021)
https://doorlesscarp953.substack.com/p/sars-cov-2-spike-targets-usp33-irf9?s=w
Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination (2021)
https://doorlesscarp953.substack.com/p/cutting-edge-circulating-exosomes?s=w
Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination (2021)
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #21: BNT162b2 derived miR-21
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-880?s=w
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #27: Further investigations into GP120, IL-6 & multidrug resistance
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-298?s=w
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #2: SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency/comments?s=w
S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein p53 and BRCA: an In Silico Study (2020)
Experimental gene therapy transfections and public health implications
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency/comments?s=w






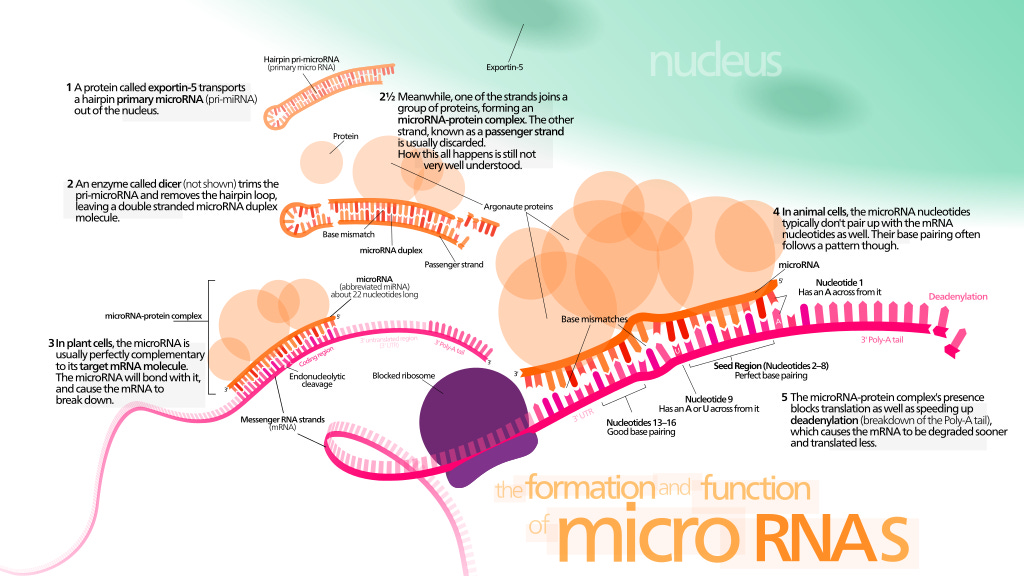


I can't wait to read through this, I have been looking for someone to break down this paper. Appreciate the hand-holding for us lay people!
Thank you so much for this breakdown for the layperson!