Magnesium deficiency and associated pathologies: Part 2
"Magnesium is no trace element: it is an essential giant mineral"
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
TL;DR:
Also available with the translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
- In Part 1 we looked into how severe magnesium depletion (hypomagnesemia) can almost double your risk of dying from coronary heart disease (CHD), stroke, thrombosis, atrial fibrillation (AF), sudden cardiac death (SCD), or a ruptured aneurysm.
I recommend reading that first, if only for an introduction to magnesium. I briefly touched on NHS primary care guidance to practitioners, but generally, the advice is not getting out there.
We need to progress to a more holistic, preventative approach instead of only treating disease when you are symptomatic, and the damage is already done, perhaps over many years.
Heart Issues Linked to Low Magnesium, but Science Takes a 'Wrong Turn'
In 2013, the upshot of a "groundbreaking" review covering what was known about cardiovascular disease from as early as 1937 found that low magnesium levels — not high cholesterol or consumption of too much saturated fat — are the leading cause of many aspects of heart disease.
Review: “The Magnesium Hypothesis of Cardiovascular Disease, The Missing Mineral—Magnesium, (The Strong Link of Low Nutritional Magnesium and High Calcium-to-Magnesium Ratio in the Genesis of Cardiovascular Disease), A Review of the Peer-Reviewed Science, by A. Rosanoff, PhD” (2013)

Due to the size of this Substack and the lack of bookmarking, I have decided to make it into a mini-series of` research reviews investigating some of the many other conditions associated with magnesium depletion - either caused by it or made worse.
These findings are particularly relevant to sufferers of “Long COVID” (PASC) or “Long Vax”, as many of the symptoms are due to related pathologies that may also benefit greatly from extra Mg.
To conclude this series, part 4 will consider the effects on the endocrine system and bioavailability studies into magnesium supplements, including dietary sources.
Addressing Mg deficiencies gets complicated by our genetic differences, supplement intolerance, age, microbiota, diseases, drugs, alcohol, and other factors that can all impede absorption.
And complexity can reach another level where, measured as a whole you wouldn’t be classed as deficient but you may be highly deficient in different tissues, such as bone cells or red blood erythrocytes. All this can make it very challenging to diagnose and treat, especially as there is minimal research into these areas.
Off-the-shelf effective treatments do not have much value for shareholders. Money directs research, so no one is rushing to spread the knowledge or investigate further.
Discussion
Although Mg deficiency contributes to the severity and poor prognosis of many conditions, disease is rarely the product of just one factor. It’s almost a game of whack-a-mole to address as many contributory factors as possible to give yourself the best odds of a positive outcome.
Unless contraindicated (which is why you may need professional advice) addressing as many possible deficiencies in a tick-box fashion leans us towards a more holistic approach. Diet and lifestyle should come first though, supplements second.
Don’t overdose, but don’t assume you aren’t deficient either. And review your lifestyle choices - everything that may contribute to the pathology. Synergistic benefits may also help you turn the corner, where everything else failed up to that point.
On the other hand, Mg is not a miracle cure, and it can take many months to correct long term deficiencies. If the damage is too far gone it can help, but don’t expect the impossible.
However, pathologies may have many contributory factors, and addressing deficiencies can certainly go a long way to helping your body heal itself and mitigate the risk of future problems.
- Crohn’s disease
It costs a lot to treat Crohn’s with allopathic meds.
Cost and lack of awareness (or alternatives, maybe?) is hindering the flow of $$$ to pharma:
The global Crohn's disease treatment market is slated to reach a valuation of US$ 11.68 billion by the end of 2023. According to Future Market Insights, the market is projected to rise at a steady 4.3% CAGR until 2033, being valued at US$ 17.80 billion.
…What Could Possibly Hinder Crohn's Disease Treatment Market Demand?
Lack of reimbursement policies and high cost are expected to hinder the growth
There is lack of awareness among the various patients suffering from Crohn’s disease in various countries across the globe. Additionally, the higher cost for this kind of treatment, including the drug as well as other cost is expected to restrain the growth of the Crohn’s disease treatment market.
From: “Global Crohn's Disease (CD) Treatment Market Snapshot (2023 To 2033)“ (2023)
https://www.futuremarketinsights.com/reports/crohns-disease-treatment-market
Instead of expensive meds, there is another way. This was one of the first magnesium specific papers I read in the last two years, and its a stonker.
The subheading "Magnesium is no trace element: it is an essential giant mineral" is a quote from a section title in a review about Crohn’s written by Naser et al in 2014:
“Domino effect of hypomagnesemia on the innate immunity of Crohn’s disease patients”1 is a great example of how conditions can quickly escalate as symptoms create other pathologies, - until you manage to break the doom-loop.
It’s worth going into detail here as gut health is important for Mg absorption and the pathophys pathways apply to other conditions too. Please refer to the Substack on the microbiota and probiotics, for example.
Key takes (emphasis mine throughout):
Digestive diseases play major role in development and complications of other disorders including diabetes.
Crohn’s disease (CD) is an inflammatory bowel disease associated with Mycobacterium avium subspecies paratuberculosis. The inflammation is a complex process that involves the activity of both innate and adaptive immune responses. CD lesions are primarily due to T cell response, however; innate immune response has a significant role in initiating its pathogenesis.
Inflammatory bowel disease (IBD) generally describes a group of conditions sharing the characteristic of chronic inflammation of the gastrointestinal tract. The two most common conditions in this category are ulcerative colitis (UC) and Crohn’s disease (CD)[1]. In both conditions, the immune system is mistaken food particles and normal flora for foreign materials[2,3]. This will induce an immune response attracting the leukocytes to infiltrate the intestine. The result is destruction of intestinal mucosal cells leading to a state of chronic inflammation.
Although the etiology for IBD has not been well established, genetic components[4,8], diet, and environmental factors such as smoking[3] are associated with an increased risk of pathogenesis. Nevertheless, the impact of IBD in the United States creates a huge burden in the health care system, especially for CD with an estimated cost of $2.29 billion annually[10].
A detailed review of factors that can influence the persistence of CD might lead to establishing therapeutic strategies that can maintain remission for relatively longer periods. One of these factors includes nutritional deficiency due to malabsorption of vitamins and minerals such as magnesium, a frequent finding in CD patients particularly during high activity of the disease[11-15]. Magnesium deficiency or hypomagnesemia is very understudied and underestimated especially when it comes to its relation to CD.
Most Mg ion absorption is passive and between cells:
MAGNESIUM HOMEOSTASIS
This is maintained by the cooperation between three organs: intestine, kidneys and bones[32]. In the intestine, distal parts of jejunum and ileum are the most common sites for magnesium uptake[12,32]. Approximately 80%-90% of dietary magnesium absorption is achieved via paracellular transport, which depends on the permeability of tight junctions[32].

In addition, low expression of claudin 1, 3, 4, 5 and 8 proteins in the jejenum and ileum enables the passage of magnesium ions[33]. This mechanism is passive, allowing a majority of magnesium absorption without energy cost[12,32].
Active transport through the cells:
The rest of dietary magnesium is absorbed by the active transcellular transport via TRPM6 and TRPM7[12,32]. The latter mechanism allows magnesium to be transported into the blood from intestine through cell membrane[12,32].
Once absorbed, magnesium is stored mainly in bone tissue but traces can be found in muscles, where it acts as a natural calcium antagonist to control muscle contraction[12,32]. Lastly, most of the magnesium excreted from the body is processed by the kidneys, where 90%-95% of filtered magnesium daily gets retrieved via passive and active transport mechanisms[12,32].
One of the reasons that magnesium can help with many conditions is due to its systemic anti-inflammatory effects. This helps to rank it up with vitamins such as D and minerals such as zinc in dietary importance.
It’s also anathema to the pharma (and “charity research”) model. Much as with ivermectin they would rather you remain as ill as possible and keep paying out for symptom-reducing allopathic meds and lining up for jabs than take anything safe, effective, and off the shelf. That would be bad for shareholder value and stock options!
Several studies have shown the importance of magnesium in inflammation that linked its low levels to many medical conditions such as diabetes type 2[37] (Barbagallo, 2007 #168), obesity, metabolic syndrome, osteoporosis, and cardiovascular diseases (Table (Table22)[12,38].
These are also linked positively to pleiotropic effects on immune cells, angiogenesis, cancer cell proliferation, migration, and metastasis:
Levels of many pro-inflammatory cytokines varies depending on magnesium balance in the body, and among these cytokines, TNF-α IL-1 and IL-6 have the strongest relation[12,29,38].
C-reative protein (CRP) = a marker for systemic inflammation, produced by the liver in response to increases in plasma IL-1β, IL-6 and TNF-α.
Elevated CRP levels (> 10 μg/ml) are also associated with active, advanced cancer disease.2
Inflammation often correlates with cancer progression. Inflammatory healing responses in response to damage or infection trigger epigenetic changes including proliferation, migration, and phenotypic changes (i.e. cell type and function).3
If you have acquired enough somatic mutations, lack effective DNA damage response (DDR) mechanisms, and have impaired cancer surveillance immunity, dividing cells may undergo unregulated progression, leading to tumor formation. Both extrinsic and tumor-intrinsic inflammations can result in immunosuppression, which further facilitates tumor development.4

Also, levels of CRP, a well-studied inflammatory indicator of low-grade and chronic inflammation synthesized by the liver, vary with magnesium status changes as well[12,38].
The authors discuss four principal effects of Mg on inflammatory responses and mediators:
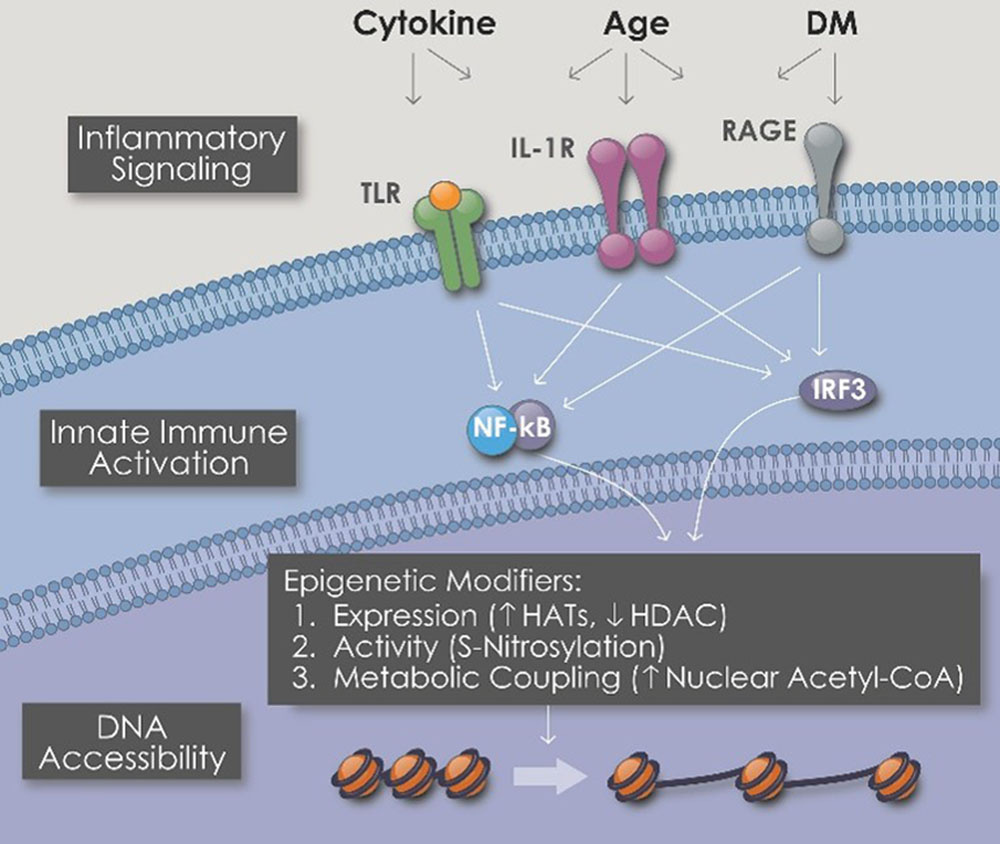
1/ Magnesium as an anti-proinflammatory cytokine. Inhibition of NF-κB activity and increasing levels of IκBα are the backbone for this function (Table (Table22)[29]. NF-κB pathway is stimulated widely in the human body to regulate inflammation, cancer fighting, and cell survival[29].
“Toll-like receptors (TLRs) are an important family of receptors that constitute the first line of defense system against microbes. They can recognize both invading pathogens and endogenous danger molecules released from dying cells and damaged tissues and play a key role in linking innate and adaptive immunity.”5
“Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are a specialized group of intracellular proteins that play a critical role in the regulation of the host innate immune response. NLRs act as scaffolding proteins that assemble signaling platforms that trigger nuclear factor-κB and mitogen-activated protein kinase signaling pathways and control the activation of inflammatory caspases.”6
Expression of cytokines IL-6 and TNF-α is induced during inflammatory responses triggered by TLR and NLR, which stimulates a downstream pathway to translocate NF-κB into nucleus for pro-inflammatory cytokines production[29,39].
2/ Oxidative stress and magnesium. In an experiment conducted on magnesium deficient rats, there was a 40% increase in the level of superoxide anions and nitric oxide levels (Table (Table22)[12].
Superoxide (ROS) is genotoxic and linked to DNA damage and carcinomas such as gastric MALT lymphomas:7
Also, there were increased levels of neutrophilic basal superoxide anions, as well as prostacyclin, prostaglandin E2, and thromboxane A2[12]. Red blood cells glutathione levels were decreased in the same experiment showing declining body antioxidant potentials in increased oxidative stress as a result of low cellular magnesium levels[12];
NMDA = The N-methyl-D-aspartate receptor is a glutamate receptor and predominantly Ca2+ ion channel found in neurons. The NMDA receptor is ionotropic, meaning it is a protein which allows the passage of ions through the cell membrane.

3/ Magnesium effect on NMDA receptors. Magnesium is a natural calcium antagonist and this was discussed in role of magnesium in muscle contraction[32]. From another perspective, NMDA receptors have a threshold of activation and it is lowered in states of decreased extracellular magnesium levels[38]. This will lead to an increase in calcium influx into the cell through NMDA receptors, resulting in increased production of pro-inflammatory prostaglandin E2, which was decreased upon blocking NMDA receptors[12,38].
Also, as calcium levels increases intracellular, the level of SP increases as a result stimulating NK-1 receptors leading to production of inflammatory mediators from macrophages, monocytes and neutrophils[38]. It is noteworthy to mention that the increase in NK-1 and substance P are well-known findings in IBD[12].
In addition to that, magnesium binds to the regulatory gates of calcium channels limiting calcium influx into the cell, and low extracellular magnesium levels will enhance the calcium influx triggering a greater inflammatory response[38];
4/ Magnesium, gut microbiota and intestinal permeability. It has been established before that gut microbiota [mainly bifidobacteria; a gram positive, non-motile anaerobic bacteria (Table (Table22)[42]] are decreased in endotoxemia, high fat mass index and glucose utilization disturbances[43,44].
Similarly, in another experiment, cecal content of bifidobacteria and lactobacilli were decreased in short-term (four days) magnesium deficient rats[43].
On the other hand, prolonged magnesium deficiency (21 d) has actually increased the cecal content of the mentioned bacteria, suggesting an adaptive response by the bacteria and an established demand for magnesium[43]. Bifidobacteria are microorganisms known for their ability to lower intestinal LPS content and thus enhance the mucosal barrier performance[43,45].
Another link between magnesium and the microbiota. Increased systemic exposure to LPS via the gut is not good:
As the drop of magnesium levels decreases the cecal bacterial content, it also causes change in intestinal mucosal barrier, where mRNA of two of the junction proteins (ZO-1 and Occ) were noticed to decrease in ileum and proximal colon resulting in increased intestinal permeability for bacterial products and especially LPS to be increased systemically[43].
This has implications for exposure to endotoxin (LPS) adulterated experimental synthetic mRNA agents. Mg doesn’t just help protect the gut by suppressing pro-inflammatory monocytes, but by many other mechanisms too.
TNF-a and Il-6 are also linked to neoplastic transformation and pathogenesis of colitis-associated colorectal cancer (CAC):8
Accordingly, it was noticed that expression of CD14 receptors that bind LPS was elevated in gut in magnesium deficient mice, as well as increased expression of CD68 supporting the infiltration of monocytes in proximal colon[43]. The overall content of mRNA of TNF-α and IL-6 in proximal colon was increased in magnesium deficient mice[43].
Implications for colon cancer:
These findings showed the effect of low magnesium on cellular inflammatory stress, which seemed to be limited to proximal colon rather than ileum[43]. Prolonged magnesium deficiency has an impact on the composition of gut microbiota as more bifidobacteria and lactobacilli will be present, and less bacteroids in the intestine[43]; and (5) Magnesium and C-reactive protein.
High levels of CRP were linked to obesity, metabolic syndrome, cardiovascular diseases and IBD[47]. They all share having an inflammatory component in their etiology and CRP was the tested variable in many studies[12].
Back to our mineral, magnesium is a significant immunomodulator that affects many inflammatory responses, and therefore its homeostasis is crucial for the overall body homeostasis. Low magnesium levels (< 1.2 mg/dL) were correlated to elevated levels of TNF-α, IL-1β, IL-6 and hs-CRP in plasma[12,38,46].
A study conducted on 5007 children (1999-2002) showed a significant increase in risk of having high CRP (1.94 times more) in children taking less than 50% of magnesium RDI[38].
This should be shouted from the rooftops:
One of the most interesting findings about magnesium and hs-CRP is that it was developed at University of South Carolina, showing that magnesium is the highest dietary factor in a 42-item dietary anti-inflammatory index they made for the study[46,48].
Adapted from “Table 2. Foods and constituents included in the Inflammatory Index”:
Note that dietary and endogenous lipids possess pro- and anti-inflammatory properties,9 and iron can be both anti-inflammatory (when addressing deficiency) or pro-inflammatory (a consequence of loss of intestinal barrier integrity).10
Deficiency as both cause and effect, the slippery slope:
IBD adds a major cause for developing hypomagnesemia at different rates ranging from 13% to 88% of patients[14]. This deficiency is caused by many factors in CD including anorexia, food avoidance, intestinal surface loss due to diarrhea, fistulae or surgery as well as malabsorption[14].
Intestinal uptake of magnesium is defected dramatically as inflammatory processes of CD result in villus atrophy and fistulae formation, on top of increased bowel movement not allowing the time for magnesium absorption[32].
As the majority of magnesium absorption occurs passively, there will be no sufficient concentration gradient for magnesium uptake in intestine, as well as destructed enterocytes, losing the active transport component of magnesium absorption[32].
Usually during remission of the disease, the body demand for macronutrient is covered by diet. However, micronutrient loss is frequent and supplementation is usually required even during remission of the disease[15].
“Parenterals are sterile preparations that are injected intravascularly, administered into body tissues or into visceral cavities.”11
Due to the chronic and extensive damage of intestinal mucosal cells, oral magnesium supplement is not recommended and parenteral forms are encouraged since the bioavailability will not be a concern in this case[13].
As CD progresses the feedback mechanisms multiply. Impaired absorption of vitamin D further impairs Mg absorption, and so on:
Claudin proteins involved in paracellular mechanism of magnesium absorption (the major mechanism) are regulated by active vitamin D, thus in CD, loss of fat soluble vitamins including vitamin D will lead to decreased magnesium absorption and hypomagnesemia[32].
Surgery may be necessary, but is ultimately counter-productive:
As 75% of CD patients will require surgery at some point due to intestinal disease complications, short bowel syndrome will be a major cause of malabsorption affecting the levels of many nutrients including magnesium as well[14].
Circulating cytokines act like the Ozempic diet drug (a subject for a later Substack), further impairing absorption. The dominoes fall:
Also, magnesium absorption in the intestine is subjected to the amount of protein in diet and this is decreased in CD due to anorexia produced by circulating cytokines and food avoidance by the patients because of abdominal pain[32]. For all of those factors, magnesium will be in negative balance in CD patients (Figure 1).
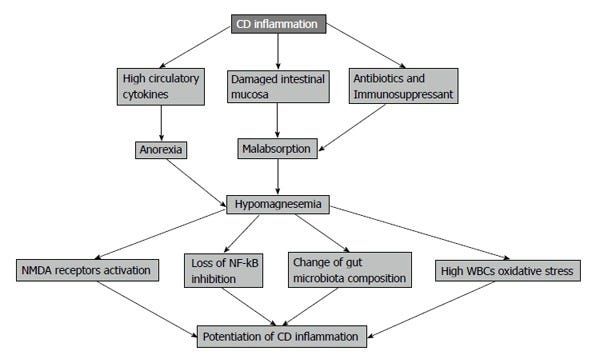
Less magnesium leads to calcium-induced abnormal smooth muscle function, i.e. increased bowel movements, further worsening the...you know:
Role of magnesium in muscle contraction as a calcium antagonist is essential for intestinal smooth muscle function in creating efficient peristalsis. It allows for periods of relaxation following contraction cycles caused by calcium.
SP = Substance P, an 11-amino acid-long neuropeptide expressed by the central nervous system (CNS), the peripheral nervous system, and immune cells: “Current strategies to target SP in human diseases such as gastrointestinal, respiratory inflammation, and other conditions are heavily concentrated upon blocking its high-affinity receptor NK1R via small peptide or non-peptide NK1R antagonists. However, none of the SP against have reached clinical use.“12
Also, SP is a regulator of smooth muscle contractility and hypomagnesemia elevates its levels leading to abnormal intestinal smooth muscle function. This function is essential to control bowel movement frequency in CD which is increased as a result of magnesium deficiency[32].
Traditional Chinese Medicine (TCM) has been using Danshen, the dried root of a common medicinal herb Salvia miltiorrhiza Bge for at least 2000 years.
Even today it is used in many prescriptions as a treatment for gastrointestinal diseases (including Crohn’s13), cardiovascular diseases, and certain gynaecological diseases .14
Studies have shown that Danshen extracts contain ∼9 mM magnesium as free Mg2+ ions, which account for its efficacy as a neuroprotective NMDA antagonist.
In contrast, Western medicine still plays with poisons. I’m not exaggerating:
Most of drugs used to control CD have a long list of side effects and a possible toxicity with chronic use. Magnesium has shown great potentials on affecting the same pathways involved in CD inflammation as many therapeutic agents.
On the other hand, magnesium is not expected to be cytotoxic and this hypothesis is very promising if magnesium is tested on specific regimens to block NF-κB signaling pathway in CD patients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4127587/
- Neurological disorders, migraine & pain
From “The Effect of Magnesium Deficiency on Neurological Disorders: A Narrative Review Article“ (2019):
Recent studies have associated Mg-deficiency with many neurological disorders, such as cerebral vasospasm, Alzheimer's disease, stroke, and migraine.
Results: Magnesium is related to neurological disorders on the basis of the study of animals and humans experiments. Furthermore, these nervous systems related diseases include cerebral vasospasm, Alzheimer's disease, Parkinson's disease, stroke and migraine.
Conclusion: Magnesium has effects on neurological disorders, such as its utility in cerebral vasospasm, Alzheimer's disease, Parkinson's disease, stroke and migraine.
Cerebral vasospasm:
Hypomagnesemia is associated with acute focal vasospasm in the coronary arteries (17). Mg plays a key role in the regulation of the excitability of cell membranes. This element antagonizes the NMDA receptor (2, 13) on the cell surface and intracellular voltage-gated calcium channels (13, 18). Thus, calcium entry to ischemic neurons, which is crucial for the activation of cellular apoptotic pathways, is impeded. Mg is a neuroprotective agent in different models of cerebral ischemia (19). Hence, Mg deficiency should be considered as a cause of various neurological symptoms.
Stroke:
Mg treatments exhibited neuroprotection in some disorders, such as global cerebral ischemia, neonatal hypoxia, and coronary artery bypass grafting (13, 26). And contribute to stroke patient recovery from neurologic deficits (27). The basis of neuroprotection may be due to that magnesium deficiency could be associated with the onset of an inflammatory response leading to increasing circulating levels of cytokines, which triggers oxidative responses in endothelial cells (28, 29). There is a statistically significant inverse association between magnesium intake and stroke risk (30, 31).
Alzeihmers disease (AD):
Increasing the concentration of Mg2+ in the extracellular fluid ([Mg2+]) results in a permanent increase in synaptic plasticity in the hippocampal neuronal network cultured in vitro to enhance learning and memory in experimental rats (4). Mg deficiency has been emerging as a risk factor for AD. The level of Mg diet is critical to maintain synaptic plasticity, and the decline in hippocampal synaptic connections has been associated with impaired memory (42). Cognitive decline is associated with the prevention of AD.
Parkinson’s disease (PD):
α-synuclein promoter is a susceptibility factor for idiopathic PD and it plays a key role in the pathophysiology of PD. Early-onset PD has been linked to two point mutations in the gene that encodes α-synuclein, suggesting that disease may arise from accelerated fibrillization (48). An experiment showed the concentration of magnesium in the cortex, white matter, basal ganglia and brainstem of PD brain is low (50). The exact cause of its pathological changes is still not very clear, genetic, aging, oxidative stress, etc. Oxidative damage caused by magnesium deficiency is also reflected in PD (51). In Parkinson’s disease, magnesium levels are reduced (49).
Migraine:
Wherever there is pain and suffering there is someone waiting to cash in:
The overall global migraine market in 2022 was valued at US$9.6 billion and is projected to be worth $17.5 billion by 2027, with a compound annual growth rate (CAGR) of 9.4%. The increased usage of anti-CGRP drugs will drive the growth of the migraine market.
From: “The pipeline and market for migraine drugs” (2023)
Let us consider an alternative approach, at lower cost and with less side effects:
When trigeminal ganglion and its fibers are stimulated, the release of substance P, calcitonin gene-related peptide, and other neuropeptides can be increased. These active substances in adjacent brain blood vessels can cause vascular expansion and throbbing headache (54). Three approaches can lead to migraine caused by Mg deficiency. First, Mg deficiency can alter the release of neurotransmitter. Second, the platelets can become hyper aggregated. Third, cortical spreading depression is promoted (22). Therefore, magnesium can improve mitochondrial oxidative phosphorylation, 5-HT neurotransmission and the NO system during migraine (55). Serum Mg levels are significantly lower in migraine patients, and that the normal population is associated with the frequency of migraine attacks. These phenomena support the use of Mg in migraine prevention and treatment (56).
https://pubmed.ncbi.nlm.nih.gov/31223564/
From: “Magnesium in headache“ (2011):
A study measuring ionized magnesium levels in 40 patients during an acute migraine attack found that 50% had levels below 0.54 mmol/l (normal adult range 0.54-0.65 mmol/l) (Mauskop et al., 1995), with all subjects having total serum magnesium levels within normal limits. Intravenous administration of 1g of magnesium sulfate was most effective in those with low ionized magnesium, with 86% of patients reporting sustained pain relief over 24 hours in those found to have low serum ionized magnesium, while this was the case in only 16% of patients with normal levels. This finding was extended to patients with various headache types, including migraine without aura, cluster head-ache, chronic migraine and chronic tension type headache (Mauskop et al., 1996), with most patients demonstrating low ionized magnesium levels. In addition, high serum ionized calcium to magnesium ratios were found in all headache types except for in those patients with chronic tension-type headaches. Based on these findings, it has been suggested that tension type headache may possibly be discriminated from chronic migraine based on serum ionized magnesium levels (Mauskop et al., 1994).
Recent research has elucidated the aura phase of migraine, which affects up to 5% of the adult population (Agostini and Aliprandi, 2006). Migraine aura is the presentation of characteristic neurological symptoms usually developing prior to the onset of the painful phase of a migraine headache, believed to be due to a phenomenon known as cortical spreading depression (CSD). CSD was originally described by Leao (1944) and is an intense depolarization of neuronal and glial membranes, with alterations in membrane resistance and ion flow. There is subsequent massive release of glutamate and potassium as well as an increase in intracellular sodium and calcium. This results in a strong wave of depolarization that spreads across contiguous neuronal tissue. It can be triggered by depolarization of a small region of brain tissue or by direct application of excitatory amino acids, and activation of the N-methyl-D aspartate (NMDA) receptor can evoke CSD (Gorji et al., 2001). There are characteristic alterations in cerebral blood flow, with an initial brief oligemia followed by a profound hyperemia, and a mild, long-lasting oligemia (Otori et al., 2003). Precisely how the aura phase of a migraine evolves into the painful phase remains unknown, and it has been theorized that it is due to the action of a number of inflammatory proteins including calcitonin gene-related peptide (CGRP), nitric oxide and vasoactive peptide (Goadsby et al., 1990), which feed into the trigeminal nerve and generate pial artery dilation and CSD, and, ultimately, headache.
https://www.ncbi.nlm.nih.gov/books/NBK507271/
- Osteoporosis
These figures don’t include treatment costs, lost productivity etc:
The global market for osteoporosis medications was estimated to be about USD 15.70 billion in sales in 2022 and is expected to grow to USD 23.78 billion by 2030. The global market for osteoporosis medications is expanding due to the rising incidence of osteoporosis.
From: “Osteoporosis Drugs Market [Latest Reports] Business Description and Corporate Strategies in Globe till 2030” (2024)
From “Skeletal and hormonal effects of magnesium deficiency” (2009):
Mg deficiency has been associated with a number of clinical disorders including osteoporosis. Osteoporosis is common problem accounting for 2 million fractures per year in the United States at a cost of over $17 billion dollars. The average dietary Mg intake in women is 68% of the RDA, indicating that a large proportion of our population has substantial dietary Mg deficits.
“Osteoclasts are the cells that degrade bone to initiate normal bone remodeling and mediate bone loss in pathologic conditions by increasing their resorptive activity.”15
Substance P makes another appearance. It has been induced therapeutically in TCM for at least 2,500 years by stimulating neurogenic inflammatory spots, more commonly known as acupoints.16
“In recent years, many studies have investigated the efficacy of acupuncture for the treatment of primary osteoporosis in clinical practice, and the findings have shown that acupuncture is effective in improving osteoporosis”.
This is paradoxical, but it may work by mediating analgesia too.17
We have therefore induced dietary Mg deprivation in the rat at 10%, 25% and 50% of recommended nutrient requirement. We observed bone loss, decrease in osteoblasts, and an increase in osteoclasts by histomorphometry. Such reduced Mg intake levels are present in our population. We also investigated potential mechanisms for bone loss in Mg deficiency. Studies in humans and and our rat model demonstrated low serum parathyroid hormone (PTH) and 1,25(OH)(2)-vitamin D levels, which may contribute to reduced bone formation. It is known that cytokines can increase osteoclastic bone resorption. Mg deficiency in the rat and/or mouse results in increased skeletal substance P, which in turn stimulates production of cytokines. With the use of immunohistocytochemistry, we found that Mg deficiency resulted in an increase in substance P, TNFalpha and IL1beta. Additional studies assessing the relative presence of receptor activator of nuclear factor kB ligand (RANKL) and its decoy receptor, osteoprotegerin (OPG), found a decrease in OPG and an increase in RANKL favoring an increase in bone resorption. These data support the notion at dietary Mg intake at levels not uncommon in humans may perturb bone and mineral metabolism and be a risk factor for osteoporosis.

We have demonstrated that a dietary Mg depletion as high as 50% NR in the rat results in reduction of bone mass. This level is similar to the Mg intake of a substantial proportion of our population and therefore may be a risk factor for osteoporosis. Dietary deficiency in other nutrients, such as calcium, may worsen this effect.
https://pubmed.ncbi.nlm.nih.gov/19828898/
- Accelerated aging
Pro-inflammatory cytokines18, fibrosis and ROS from neutrophils are all associated with accelerated aging. And then you have endocrine effects to consider:
From: “A connection between magnesium deficiency and aging: new insights from cellular studies” (2008):
Abstract
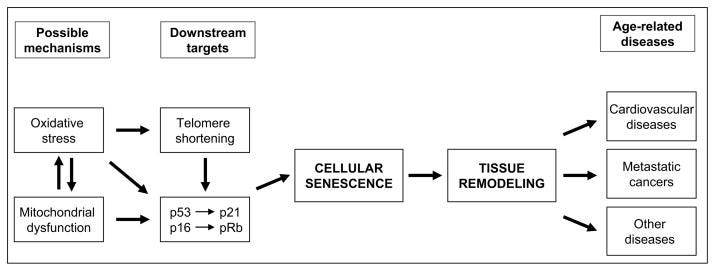
Most human cells can only replicate a limited number of times in cultures before they lose the ability to divide, a phenomenon known as replicative senescence, which seems to play a role in aging at the organismal level. Recent studies have shown that culture in low magnesium (Mg) accelerates the senescence of human endothelial cells and fibroblasts. Given the numerous critical roles of Mg, it seems likely that Mg inadequacy would interfere with cellular metabolism, which could affect the senescence process. Since i) several pieces of evidence link low Mg to aging and age-related diseases and ii) the Occidental diet is relatively deficient in Mg, we propose that broadly correcting nutritional intakes of Mg might contribute to healthier aging and the prevention of age-related diseases.
Aging is characterized by the progressive deterioration of physiological functions. This complex process has been proposed to be under the influence of both genetic and environmental (nutrition, lifestyle) influence at a ratio of approximately 30:70 [1], not counting stochastic variation. Several pieces of evidence link low magnesium (Mg) to aging and age-related diseases. In turn, it is noteworthy that aging itself constitutes a risk factor for Mg deficit [2, 3], thus creating a vicious circle that drives the clinical patterns seen in aging. Interestingly, a long-term moderate Mg-deficient diet aggravates cardiovascular risk associated with aging in rats by significantly increasing blood pressure and inflammatory markers [4]. In addition, low Mg is associated with age-associated memory decline [5], neurodegenerative disease [6], decreased muscle performance [7], insulin-resistance [8], osteoporosis [9], and development of some cancers [10] in human studies. All these data underscore the contribution of Mg deficiency to aging in vivo. The cellular mechanisms that drive the aging phenotype are not fully characterized, but growing evidence supports the involvement of senescent cells in these processes. Given the numerous critical roles of Mg, it seems likely that Mg inadequacy would interfere with cellular metabolism, which could affect the senescence process.
Only recently, some light has been shed on the cellular and molecular mechanisms involved, as it has been demonstrated that culture in low Mg promotes cellular senescence of human endothelial cells and fibroblasts [11, 12].
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790427/
- Muscle cramps
Magnesium supplementation as an antispasmodic treatment for muscle cramps is fairly well known.
But not well enough:
Marketresearch.biz reports that the The Antispasmodics Drugs Market size is expected to be worth around USD 28.8 Bn by 2032 from USD 12.1 Bn in 2022, growing at a CAGR of 9.3% during the forecast period from 2023 to 2032
From: “Antispasmodics Drugs Market Size and Growth Potential: Unveiling Growth Potential and Forecasted Outlook for 2032“ (2023)
From: “Muscle cramps and magnesium deficiency: case reports.“ (1996):
Case report 1
A 17-year-old military recruit presented on sick parade with aching in his muscles and thighs, and generalized tenderness in all his skeletal muscles. He walked gingerly on tiptoe, as his calf muscles contracted spasmodically when his feet became dorsiflexed. He admitted to having exercised over and above his normal army training and later listed his excesses: repeated runs of between 10 to 15 km, weight training, 16 sprints varying from 100 m to 400 m, followed by half an hour of swimming. Three days before reporting sick, he experienced weakness of his legs, which "began to wobble." The day before becoming sick, his legs "felt like jelly," and on the morning when he reported sick, he could "hardly move" his limbs due to muscle spasm.
The only abnormality indicated by laboratory investigations was a serum magnesium concentration of 0.54mmol/L (reference range 0.7 to 1.5mmol/L).
When results of the serum magnesium levels became available, an immediate intravenous infusion of 3 g of magnesium sulfate 50% weight-to-volume ratio in 500mL N saline was given over 6 hours. On the following day, he received a further intravenous infusion of 5 g of magnesium sulfate in a total of 1500mL N saline. Within 48 hours, he had fewer muscle pains, and the tetany had resolved. Apart from a transient mild generalized headache, he had no adverse symptoms.
After 4 days he was completely pain free, and the tetany was gone. His only complaint was mild tenderness in the left calf. He was then discharged, and after 2 weeks, in which time he refrained from excessive physical exercise, was symptom free. He returned to military training but was warned not to participate in extra-curricular runs and exercise. Three months later he underwent a 19-km run with full pack and equipment and experienced normal muscle aches and pain but no further tetany.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2146789/
- Tinnitus
I suspect these market projection figures are also conservative due to mass administration of experimental gene therapy agents:
“According to the Vaccine Adverse Events Reporting System (VAERS), 12,247 cases of coronavirus post-vaccination tinnitus have been reported till September 14, 2021.”19
Global revenue from the ringing ears treatment market was estimated at US$ 2.5 Bn in 2021, with the market projected to move ahead at a CAGR of 2.8% to reach a valuation of US$ 3.2 Bn by the end of 2031. Subjective tinnitus indication is expected to account for a market value of US$ 3.1 Bn by 2031.
From: “Ringing ears treatment market” (2022)
https://www.persistencemarketresearch.com/market-research/ringing-ears-treatment-market.asp
From: “Relationship between serum magnesium level and subjective tinnitus“ (2016):
Abstract
Objectives: This study aims to assess the relationship between serum magnesium level and bilateral subjective tinnitus.
Patients and methods: The study included a total of 76 patients (36 males, 40 females; mean age 48.5±6.5 years; range 43 to 65 years) suffering from severe bilateral subjective tinnitus (accepted as severe and catastrophic according to tinnitus severity index) as the study group and 86 healthy participants (42 males, 44 females; mean age 43.8±7.3 years; range 40 to 61 years) as the control group. Serum magnesium levels of both groups were measured and compared statistically.
Results: The serum magnesium concentration was significantly lower in the study group compared to the control group (1.8±0.2 vs. 2.3±0.4 mg/dL, p=0.03).
Conclusion: The significant association between serum magnesium level and tinnitus shows the importance of magnesium in the pathophysiology of subjective tinnitus.
Discussion
In the literature, very few studies have investigated the relationship between tinnitus and magnesium. In most of these studies, the effect of magnesium therapy was investigated as a treatment choice for tinnitus and for noise-induced hearing loss, which often causes tinnitus. Cevette et al.[6] examined the potential benefit of magnesium by diminishing the severity of tinnitus and claimed that magnesium supplementation is useful in reducing the perception of tinnitus. Xiong et al.[7] examined the relation between the cochlear magnesium content and noise-induced hearing loss and suggested that the cochlear magnesium content affects the severity of cochlear damage after acoustic trauma. Gordin et al.[8] determined that magnesium treatment improves nerve regeneration, hearing recovery and prognosis in idiopathic sudden hearing loss. Joachims et al.[9] and Attias et al.[10] investigated the effect of magnesium on noise-induced hearing loss. Both studies found that magnesium was significantly protective against noise-induced cochlear damage. The exact mechanism of magnesium supplementation in tinnitus remains unclear. Magnesium deficiency is related to the reduction of microcirculation and is responsible for the formation of free radicals in the inner ear. It is proposed that magnesium supplementation improves the microcirculation, reduces inflammatory cytokines and oxidative stress in the cochlea by maintaining normal membrane characteristics.[4,11-13]
From: “Phase 2 study examining magnesium-dependent tinnitus“ (2011):
Abstract
Background: Recent studies in noise-induced and idiopathic sensorineural hearing loss have suggested that magnesium supplementation may lessen both hearing loss and the severity of tinnitus in patients. Further epidemiological evidence indicates that all age groups of Americans fall short of the recommended daily allowance for magnesium by 100 mg daily.
Purpose: The purpose of this study was to examine any potential benefit in lessening the severity of tinnitus in patients taking supplemental magnesium.
Research design: The study was a single-arm, open-label, before-and-after study of oral magnesium (532 mg per day) in 26 patients for 3 months. Tinnitus severity was evaluated and recorded daily by the patient using the Tinnitus Distress Rating (TDR) scale of 0 (no tinnitus) to 10 (worst possible tinnitus). The Tinnitus Handicap Inventory (THI) was administered before and at the end of the study, and scores were converted to the grades of the 5-item Tinnitus Severity Scale (TSS). The purpose of this phase 2 study was to investigate whether the treatment was effective at all, and, as such, a placebo control was not performed. All data were collected at Mayo Clinic in Scottsdale, Arizona, between March 6 and December 10, 2008.
Study sample: Patients with moderate to very severe tinnitus (TDR score of 3 through 8).
Intervention: Daily magnesium supplementation, 532 mg; patient completion of the THI; and daily self-report of TDR.
Data collection and analysis: The main outcome measures were mean TDR scale scores and THI scores as converted to TSS grades. The primary analysis was done on the basis of intention to treat.
Results: Twenty-six patients were enrolled; 19 completed the study. The extent of handicap, as measured by THI/TSS, for subjects with slight or greater impairment was significantly decreased (P=.03). Patients who ranked slight or greater on the THI/TSS before intervention showed a significant decrease in the severity of their tinnitus at post-testing (P=.008).
Conclusion: The results suggest that magnesium may have a beneficial effect on perception of tinnitus-related handicap when scored with the THI.
SUMMARY
The present study indicated that subjects showed significant improvement in their self-rating of tinnitus with a magnesium supplement of 532 mg daily for 3 months. Further investigations should control for the placebo effect.
https://pubmed.ncbi.nlm.nih.gov/22249877/
- Cancer
The Brainy Insights estimates that the USD 146.72 billion in 2022 global oncology/cancer drugs market will reach USD 311.81 billion by 2032. Continued progress in genomics and biomarker discovery offers opportunities for precision medicine in oncology.
From: “Oncology/Cancer Drugs Market Size Worth $311.81 Billion by 2032 | CAGR: 7.83% by The Brainy Insights” (2024)
https://finance.yahoo.com/news/oncology-cancer-drugs-market-size-010000971.html
Hardly needing any introduction, a PubMed keyword search returns 5,220 results:
https://pubmed.ncbi.nlm.nih.gov/?term=%28cancer%29+AND+%28magnesium%29&sort=date
From: “Magnesium and cancer: more questions than answers“ (2011):
Abstract
The relationship between Mg and cancer is still a puzzle to disentangle. The knowledge derived from preclinical studies reveals a complex scenario in which low magnesium has both anti- and pro-tumour effects, such as inhibition of tumour growth at its primary site and facilitation of tumour implantation at its metastatic sites. In different cell types, neoplastic transformation dramatically disrupts the controlled and coordinated fluctuations of intracellular magnesium, an event that offers selective advantages to the cells. It is difficult to translate the lesson learnt from experimental models to humans. Based on epidemiological studies, Mg deficiency seems to be linked to increased risk of some types of cancers. The demonstration of an impairment of magnesium homeostasis in oncologic patients further complicates the field. We need more translational and clinical data to draw firm conclusions about the contribution of magnesium to tumours.
Inadequate dietary intake of Mg is not the only cause of Mg deficiency. Mg homeostasis is very tightly tuned in the intestine and the renal tubules through a complex network of transporters, some of which have recently been defined at the molecular level (Quamme, 2010). Consequently, Mg deficiency accompanies chronic gastrointestinal and renal diseases, therapies with some classes of diuretics or anticancer drugs, and also complicates diabetes mellitus. In addition, it is common in alcoholics and in the elderly (Ford and Mokdad, 2003).
Another study showed that the serum level of Mg is frequently decreased in patients with solid tumours, independent of therapies, and that this decrease correlated to the stage of malignancy (Sartori et al., 1992). This finding seems to be due to the avidity of the tumours for Mg, which behave as Mg traps.
Several epidemiological studies have provided evidence that a correlation exists between dietary Mg and various types of cancer. A high content of Mg in drinking water seems to protect from liver and oesophageal cancer (Tukiendorf and Rybak, 2004; Yang et al., 2002). In addition, Mg content in drinking water was inversely correlated with death by breast, prostate, and ovarian cancers, whereas no correlation existed for other tumours (Yang et al., 2000a; 2000b; 2002; Chiu et al., 2004). In particular, a high serum calcium Ca:Mg ratio has been suggested as a novel risk factor which increases the development of postmenopausal breast cancer (Sahmoun and Singh, 2010), the most commonly diagnosed cancer among women in North America and Europe, with a mortality that ranks second only to lung cancer (Jemal et al., 2010).
It should also be pointed that Mg might have a role in colon cancer prevention. Indeed, large epidemiological studies in Sweden, in the Netherlands, in the USA and in Taiwan have demonstrated an association between low intake of Mg and the risk of colon cancer (Larsson et al., 2005; Folsom et al., 2006; van den Brandt et al., 2007; Chiu et al., 2010) and a large population- based prospective study in Japan showed a significant inverse correlation between dietary intake of Mg and colon cancer in men, but not in women (Ma et al., 2010). Intriguingly, the association between low intake of Mg and colon cancer seems to be due, at least in part, to the increased formation of N-nitroso compounds, most of which are potent carcinogens (Chiu et al., 2010).
https://www.ncbi.nlm.nih.gov/books/NBK507261/
From: “Magnesium: The overlooked electrolyte in blood cancers?“ (2020):
In solid malignancies, hypomagnesemia at diagnosis portends a worse prognosis. However, little is known about prognosis in patients with hypomagnesemia and blood cancers in general; lymphoma more specifically. Hypomagnesemia has been associated with a higher viral load of the Epstein Barr virus, a virus associated with a multitude of hematologic malignancies.
3.2. Magnesium deficiency leads to lymphoid hyperplasia
Magnesium deficiency produces exaggerated growth of lymphoid tissues including the thymus [68]. The deficiency might produce the effects of making cells more malignant or become malignant by selecting for naturally occurring mutants, increasing mutation frequency thereby making more likely the emergence of resistance, or by altering the virus-cell relationships so that gene products conferring increased adaptability are produced [69].
Animal studies have shown that when young rats are fed a magnesium deficient diet, about 20% of the animals develop thymic tumors after several months and a small subset of rats develop thymic lymphomas [70]. Of note, the degeneration of the thymus is not specific to magnesium deficiency; it is also seen in zinc deficiency [71].
3.4. Magnesium deficiency and leukocyte dysfunction
There is increasing interest in the link between monocytes/macrophages and the development of malignancy and specifically lymphoproliferative disorders. Monocytes play a multitude of roles in malignancy, both pro and anti-tumoral. For example, they may secrete tumoricidal mediators, promote angiogenesis, recruit lymphocytes and eventually differentiate into tumor-associated macrophages [86].
Neutrophils can be bad news:
…What is emerging is a complex, rather heterogeneous picture with both pro- and anti-tumorigenic roles, which apparently differs with cancer type and disease stage. Furthermore, we will discuss the well-known role of neutrophils as myeloid-derived suppressor cells (MDSC), and also on the role of neutrophils as important effector cells during antibody therapy in cancer. It is clear that neutrophils contribute substantially to cancer progression in multiple ways, and this includes both direct effects on the cancer cells and indirect effect on the tumor microenvironment.
From: “Neutrophils in cancer“ (2016)
How might monocyte responses change in response to magnesium deficiency? Older studies have demonstrated that white blood cell subpopulations change; leading to an increased number of neutrophils and decreased B cells [90,91]. Studies in rats have demonstrated peripheral vasodilation of the ears in response to magnesium deficiency which is associated with increased histamine levels from mast cell degranulation [92]. In fact, macrophages from magnesium deficient rats have enhanced responses to stimulation which may be secondary to increased production of inflammatory cytokines [70]. Magnesium deficiency is associated with greater adhesion of monocytes to endothelial cells as a result of increased pro-inflammatory factors. Interestingly, in human umbilical vein endothelial cells cultured in magnesium rich concentrations, there is attenuation of the endothelial cell responses to lipopolysaccharide (LPS) [93].
Multiple studies have demonstrated an association between hypomagnesemia and type 2 diabetes mellitus (T2DM). Patients with T2DM and hypomagnesemia tend to have rapid disease progression and increased complications related to their diabetes [101]. Oral supplementation with magnesium increases insulin sensitivity and metabolic control in patients with T2DM [102]. We hypothesize that impaired T cell activation in insulin resistant patients may be mediated by magnesium deficiency in a subgroup of patients with T2DM. Insulin plays a pivotal role in the activation of T cells and this link with magnesium should be explored further in mechanistic studies.
It is possible that magnesium deficiency may lead to hyper-responsive monocytes that are immunosuppressive. When this is coupled with malignancy, it may attenuate optimal responses to cancer immunotherapy. Finding a target for an immunosuppressive monocyte in patients with lymphoma while optimizing magnesium levels may make the tumor microenvironment less favorable and increase responses to immunotherapy.
Higher dietary intake of magnesium decreases the risk of colorectal cancer, pancreatic cancer and lung cancer [[121], [122], [123], [124], [125], [126], [127]]. In the EPIC study, there was a reduced incidence of pancreatic cancer in overweight men supplemented with 100 mg per day of magnesium [128]. In breast cancer patients, it has been observed that increased dietary magnesium intake decreases all-cause mortality [129]. Molecular studies have demonstrated that in breast cancer, neoplastic cells have increased expression of magnesium transport channels which increases intracellular concentrations leading to enhanced tumor growth [130]. In patients with advanced ovarian cancer, frequent occurrence of hypomagnesemia during chemotherapy is predictive of shorter survival [131]. Taken together, these studies highlight the importance of a diet enriched with magnesium to decrease the incidence of solid cancers and the possibility that hypomagnesemia is associated with poor outcomes in cancer patients undergoing treatment.
Few studies have specifically investigated magnesium levels in patients with lymphoma. One such study performed in a small cohort of patients found a statistically significant reduction in serum magnesium levels in patients with either leukemia or lymphoma compared to control patients. Interestingly, the magnesium levels in these patients were considered in the lower limit of normal. These results do not rule out the possibility that patients with lymphoma and leukemia could have intracellular magnesium depletion [134]. As discussed previously, serum magnesium levels do not always reflect total body magnesium status, and thus these studies should be interpreted with caution [25,26].
As discussed in Part 1:
Other mineral deficiencies have been implicated in poor prognosis in patients with lymphoma. Previous work has demonstrated that vitamin D insufficiency is linked to poor prognosis in patients with malignancy including lymphoma [137]. In fact, vitamin D insufficiency is an independent and adverse prognostic risk factor for overall survival in patients with leukemia, DLBCL and peripheral T cell lymphoma. Vitamin D3 replacement is associated with improved outcomes in patients with DLBCL with vitamin D deficiency [138]. 1,25(OH)2D stimulates magnesium absorption in the intestinal tract. Vitamin D supplementation can increase circulating levels of magnesium. Conversely, magnesium is a necessary cofactor for vitamin-D binding proteins.
Little and often may be better, and it may be a moving goalpost as conditions may increase your need for magnesium, leading to new-onset deficiencies:
IV use is necessary in patients with severe hypomagnesemia and/or those in whom the enteral route cannot be used. Of note, IV magnesium replacement is inefficient. An abrupt increase in serum magnesium levels will lead to temporary suppression of magnesium reabsorption in the loop of Henle and up to 50% of the IV magnesium will be excreted [23]. Additionally, when replacing magnesium in a non-emergent setting, administration over 12 to 24 h may be more effective as abrupt increases in serum magnesium could lead to magnesium wasting from the kidneys. Hence, replacing magnesium over a longer period will minimize this wasting and would facilitate proper distribution of magnesium in to respective body compartments [24].
Oral magnesium is more commonly prescribed as it leads to more consistent increases in mean serum magnesium concentration. Oral magnesium given in high amounts that is higher than the upper limit set by the Food and Nutrition Board for daily consumption, causes diarrhea which can decrease patient compliance [23,143]. Organic magnesium salts including magnesium citrate are considered superior to inorganic magnesium salts such as magnesium oxide, as magnesium oxide has a fractional absorption is less than 5% [144] In studies involving rodents and humans, organic magnesium salts have been demonstrated to have higher bioavailability and do not interfere with gastric acid secretion [12,[145], [146], [147]].A recent case report successfully administered magnesium over 24 h via a syringe pump subcutaneously, however there remains little published information about the efficacy and feasibility of this route [148].

Topical magnesium delivery is one of the oldest forms of therapy for skin diseases, the most well-known being Dead Sea therapy and Epsom bath salts. Grober et al. summarizes the data regarding transdermal magnesium [149]. In order to further investigate the possible role of transdermal magnesium applications, Chandrakanth et al. found that hair follicles are responsible for magnesium penetration through the skin. It appears that magnesium ions are indeed able to cross the stratum corneum in a time dependent process [150]. Another study measuring the passive permeation of topical magnesium across cadaver skin found that topical magnesium was absorbed successfully. [151].
To further characterize if transdermal magnesium therapy is effective, one pilot study consisting of 25 participants received transdermal delivery of 56 mg magnesium/day (a low dose compared to commercial transdermal Mg2+ products available) showed a larger percentage rise in both serum and urinary markers from pre to post intervention compared to subjects using the placebo cream. Statistical significance was achieved only for serum Mg2+ in a subgroup of non-athletes [152].
Grober et al. summarizes the data regarding transdermal magnesium and conclude that there is not enough evidence to recommend the topical application of magnesium [149].
Practice points
Clinicians should be encouraged to screen for magnesium deficiency in ill patients and those with malignancy.
Counsel patients about maintaining a varied diet that includes foods rich in magnesium
Serum magnesium levels are commonly measured in ill patients. Those with low levels are truly deficient but even those with normal blood levels can have total body deficiency
Decreased magnesium intake may be associated with an increased risk of developing both solid and hematologic malignancies.
Cheap and effective, with fewer courses of chemo needed as a consequence.
This does not support the pharma/charity research business model:
…The proper detection and management of pancreatic cancer-related malnutrition syndromes are of primary importance and deserve a specific and multidisciplinary (clinical nutrition, oncology, etc.) approach to improve survival, but also the quality of life. In this context, the introduction of a "Nutritional Oncology Board" in routine daily practice, aimed at assessing an early systematic screening of patients and at implementing nutritional support from the time of disease diagnosis onward seems to be the right path to take.
From: “Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board“ (2021)
Research agenda
Develop an accurate test with high sensitivity for magnesium deficiency.
Provide evidence that a magnesium deficient diet is associated with increased risk of developing cancer and the importance of nutritional oncology.
Identify whether magnesium deficiency portends a worse outcome in patients with hematologic malignancies and whether magnesium replacement alters outcomes
Further characterize the association between magnesium deficiency and EBV viral loads
https://www.sciencedirect.com/science/article/abs/pii/S0268960X20300266?via%3Dihub
A showcase for its broad-spectrum efficacy, not only can Mg help prevent cancers but it can also significantly reduce (ie by about half!) the required opioid dose of cancer patients and their associated side effects.
Magnesium L-threonate (threonic acid) is made by bonding Mg to a breakdown product of vitamin C to increase its bioavailability:
“Oral application of magnesium‐L‐threonate enhances analgesia and reduces the dosage of opioids needed in advanced cancer patients—A randomized, double‐blind, placebo‐controlled trial”20 (2023)
L-TAMS: magnesium‐L‐threonate.
Abstract
Purpose
To investigate the effects of oral administration of magnesium‐L‐threonate, a novel magnesium compound, on the analgesic effect of opioids in patients with advanced cancer.
Methods
We performed a prospective, randomized, double‐blind trial at a tertiary hospital in Shanghai, China. Eligible cancer patients who took opioids orally were assigned randomly to receive L‐TAMS capsules (1.5 g or 2.0 g according to weight) or a placebo (starch capsules). The primary outcome was the increase in the daily oral dose of morphine in each of the two groups, measured at 7, 14, 21, 30, 60, and 90 days during this trial.
Results
A total of 116 patients from the oncology and pain departments, including inpatients and outpatients, were screened; 83 were enrolled. The increases in daily morphine doses began to differ from day 30 (L‐TAMS group 9.85 mg/d vs. Placebo group 20.49 mg/d, p < 0.05); the differences persisted on day 60 (L‐TAMS group 15.96 mg/d vs. Placebo group 29.06 mg/d, p < 0.05) and on day 90 (L‐TAMS group 21.20 mg/d vs. Placebo group 40.44 mg/d, p < 0.01).
Conclusions
L‐TAMS outperforms a placebo in enhancing the analgesic effect of opioids and reducing the necessary opioid dosage. Moreover, L‐TAMS can significantly relieve opioid‐induced constipation. These advantages may be beneficial to patients with advanced cancer.
OMEDDs: oral morphine equivalent daily doses.

Pain intensity scores were similar, despite the lower OMEDDs:
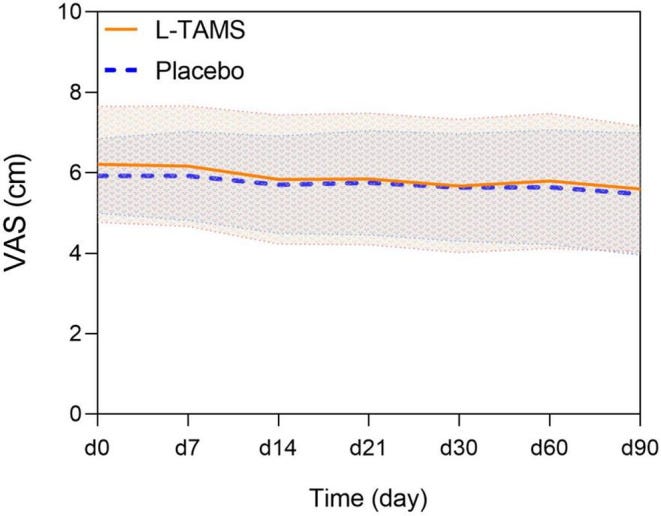
Constipation scores:

3.3. Adverse events
During our trial, no serious side effects, such as respiratory depression, sensorimotor disorder or heart block, occurred in the two groups. Aside from OIC, the most common side effect was nausea (L‐TAMS Group 8 vs. Placebo Group 10, p = 0.56, chi‐square test). Other common side effects included vomiting, pruritus, dizziness, and urine retention. There was no significant difference between the two groups in side effects (Table S9).
Our results revealed that although the opioid dosage of the two groups continued to increase, a safe daily oral dose of L‐TAMS (1.5 g or 2.0 g/day) significantly reduced the increase in opioids in patients with advanced cancer compared with the placebo group starting on day 30. On day 90, this difference was even more pronounced.
The mode of action is through multiple pathways, and once more is related to the NMDA receptors.
Cyclic guanosine monophosphate (cGMP) is a cyclic nucleotide derived from guanosine triphosphate (GTP) and acts as a second messenger of intracellular signals:
The mechanism of the effect of Mg2+ on opioid tolerance is not very clear. Conventional wisdom holds that Mg2+ is a natural antagonist of the N‐methyl‐D‐aspartic acid receptor (NMDAR), can physically block the Ca2+ channels coupled with NMDARs, and inhibits Ca2+/CaMKII‐dependent nitric oxide synthase (NOS) activity. 23 , 24 , 25 Decreased NO production ultimately affects intracellular cGMP production, which, in turn, is manifested as enhanced morphine analgesia through changes in cGMP‐dependent protein kinase activity. 7 , 24 Moreover, the activation of NMDAR can also cause the activation of protein kinase C (PKC), which plays a vital role in the development of morphine tolerance. The activity of cPKC is also affected by intracellular Ca2+ and DG concentrations, and there are positive regulatory sites for cPKC on NMDARs, resulting in positive feedback. 26 In addition to the mechanism based on NMDARs, a recent molecular simulation experiment confirmed that in the presence of a physiological concentration of Na+, a slightly increased concentration of extracellular Mg2+ could preferentially bind to the extracellular part of the μ‐opioid receptor and regulate the G‐protein‐binding region of the receptor to promote the maintenance of receptor activity. Thus, the dissociation effect of agonists was weakened, which enhanced the binding affinity of agonists and the analgesic effect of opioids. 27
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9972038/
- Magnesium and diabetes
The authors in the review about Mg and blood cancers hypothesized that magnesium can decrease insulin resistance (IR) and that untreated IR can lead to impaired T-cell responses due to suppressed T-cell metabolism. This means that magnesium-depleted obese individuals, in particular, would be at increased risk of infections and cancer.
Two studies help to confirm the hypothesis. A 2018 in vitro study by Tsai et al:
“Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection”21:
Highlights
Insulin receptor signaling controls T cell proliferation and cytokine production
T cell-intrinsic insulin resistance dampens T cell pro-inflammatory function
T cell insulin receptor stimulation drives protective immunity against influenza
Insulin receptor modulates T cell function through controlling cell metabolism
Summary
T cells represent a critical effector of cell-mediated immunity. Activated T cells engage in metabolic reprogramming during effector differentiation to accommodate dynamic changes in energy demands. Here, we show that the hormone, insulin, and downstream signaling through its insulin receptor shape adaptive immune function through modulating T cell metabolism. T cells lacking insulin receptor expression (LckCre+ Insrfl/fl) show reduced antigen-specific proliferation and compromised production of pro-inflammatory cytokines. In vivo, T cell-specific insulin receptor deficiency reduces T cell-driven colonic inflammation. In a model of severe influenza infection with A/PR8 (H1N1), lack of insulin receptor on T cells curtails antigen-specific immunity to influenza viral antigens. Mechanistically, insulin receptor signaling reinforces a metabolic program that supports T cell nutrient uptake and associated glycolytic and respiratory capacities. These data highlight insulin receptor signaling as an important node integrating immunometabolic pathways to drive optimal T cell effector function in health and disease.
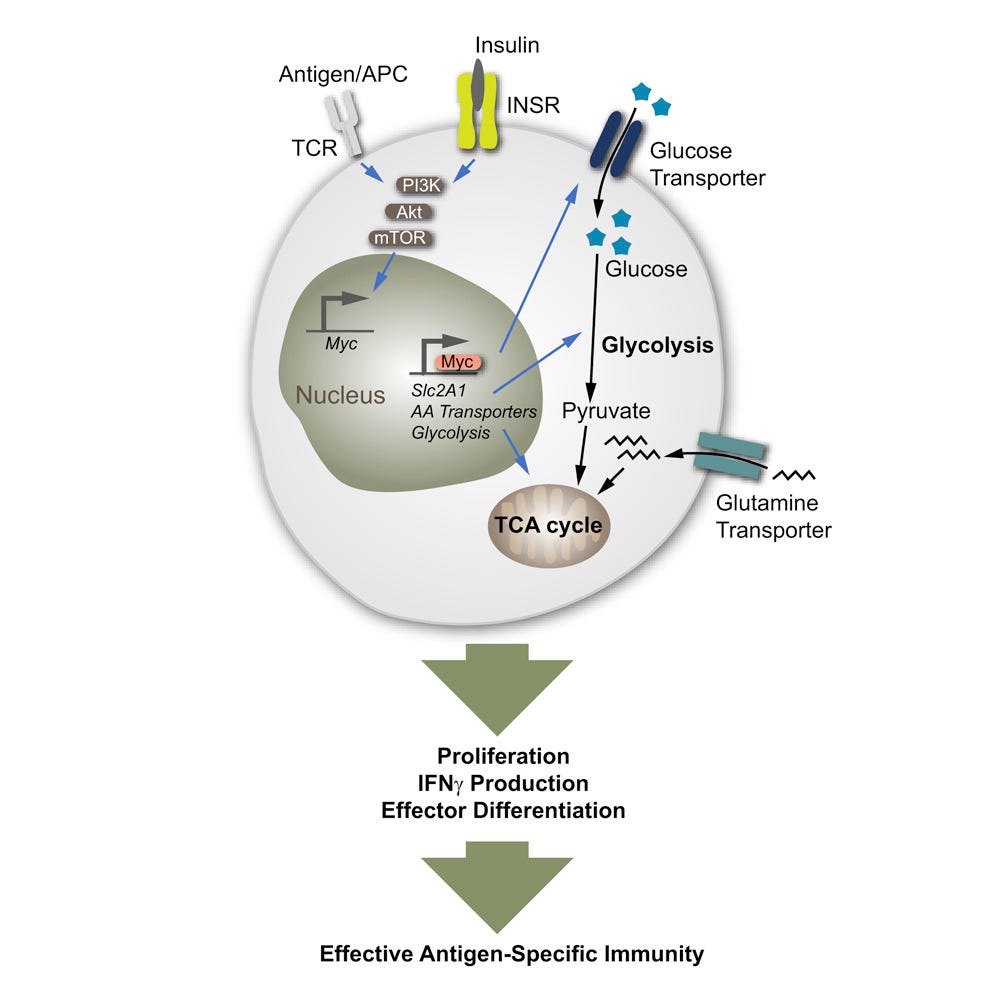
One of my favorite words to see in a paper:
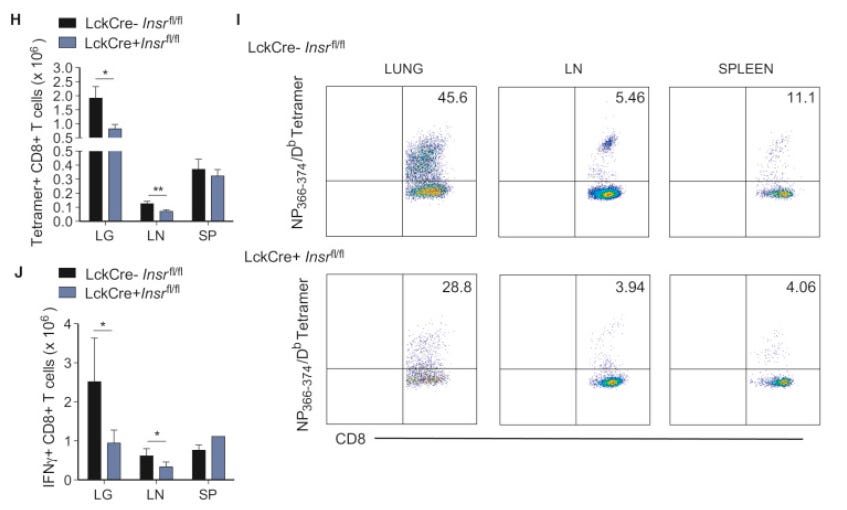
Striking differences were also seen in the CD8+ T cell compartment during influenza infection. Infection of the PR8 strain in mice generated a robust CD8+ T cell-mediated response, with nucleoprotein (NP366–374) and polymerase acidic protein (PA224–233)-specific responses dominating in the primary acute (day 10) phase (Cukalac et al., 2014). Accordingly, NP366–374/H2-Db tetramers stained a significant proportion of the CD8+ T cells in the lungs and draining lymph nodes of INSR-competent mice (Figures 4I and S4C).
Insulin-resistant mice had weaker T-cell responses to influenza.
It would have been instructive to compare the immune response of magnesium-supplemented mice too:
On the other hand, LckCre+ Insrfl/fl mice mounted a weaker antigen-specific CD8+ T cell response against influenza nucleoprotein, both in percentages and absolute numbers (Figures 4H, 4I, and S4C). IFNγ, but not TNF-α, responses in the CD8+ T cell compartment were also significantly lower in this strain (Figures 4J and S4G–S4I). Taken together, INSR deficiency compromises both the CD4+ and CD8+ T cell compartments during influenza infection, marking it as a critical stimulatory molecule that promotes optimal antigen-specific CD4+ and CD8+ T cell responses to infection.
Tetramer assays are used for single-cell phenotyping and enumeration, in this case for anti-influenza T-cells.
Grey bars for T-cell counts in H and J, and the bottom row in I are insulin resistant (+Insr):
LG: lungs; LN: lymph nodes; SP: spleens
https://www.cell.com/cell-metabolism/fulltext/S1550-4131(18)30504-7
The second paper of interest is a study by Takaya et al (2004) that discusses the pathways involved.
You need an account to access the full paper:
“Intracellular magnesium and insulin resistance”22
Abstract
Magnesium, the second most abundant intracellular divalent cation, is a cofactor of many enzymes involved in glucose metabolism. Magnesium has an important role in insulin action, and insulin stimulates magnesium uptake in insulin-sensitive tissues. Impaired biological responses to insulin is referred to as insulin resistance. This review was designed to reach a better understanding of the mechanism involved in the correlation between magnesium and insulin resistance. Intracellular magnesium concentration is low in type 2 diabetes mellitus and in hypertensive patients. In patients with type 2 diabetes an inverse association exists between the plasma magnesium and insulin resistance due to intracellular changes. The suppressed intracellular magnesium concentration may result in defective tyrosine kinase activity and modify insulin sensitivity by influencing receptor activity after binding or by influencing intracellular signaling and processing. Intracellular magnesium deficiency may affect the development of insulin resistance and alter the glucose entry into the cell.
Conclusions: Magnesium is required for both proper glucose utilization and insulin signaling. Metabolic alterations in cellular magnesium, which may play the role of a second messenger for insulin action, contribute to insulin resistance.
https://pubmed.ncbi.nlm.nih.gov/15319146/
A study of patients with a genetic defect leading to deficiencies of magnesium transporter 1 (MAGT1) also discussed the role of Mg in natural killer (NK) and CD8+ T-cell immune responses.
Epstein–Barr virus (EBV) is associated with Hodgkin’s lymphoma (HL). The implication here is that if you are Mg deficient then you increase your risk of developing HL.
“Mg2+ Regulates Cytotoxic Functions of NK and CD8 T Cells in Chronic EBV Infection Through NKG2D”23 (2013):
Magnesium to the Rescue
Individuals with X-linked immunodeficiency with Mg2+ defect, Epstein-Barr virus (EBV) infection, and neoplasia (XMEN) disease are genetically deficient for expression of MAGT1, a magnesium transporter. Chaigne-Delalande et al. (p. 186) sought to better understand why these individuals are chronically infected with EBV at high viral loads and are susceptible to the development of lymphomas. CD8+ T cells and natural killer cells, which help to keep EBV infection in check, exhibited reduced cytotoxicity owing to their lower expression of the cell surface receptor NKG2D, which triggers cytolysis upon ligation. Magnesium supplementation in vitro and also in two XMEN patients restored levels of free Mg2+, increased NKG2D expression, and resulted in reduced amounts of EBV+ cells, suggesting that this may be an effective therapeutic approach for XMEN patients.
Mutation of the magnesium transporter 1 (*MAGT1*) gene results in a primary immunodeficiency known as XMEN (X-linked immunodeficiency with Mg2+ defect, EBV infection and neoplasia). MAGT1 selectively transports Mg2+ ions across the plasma membrane into cells, but it has been unclear how the decreased intracellular levels of free (unbound) Mg2+ in patients with XMEN account for the high levels of Epstein–Barr virus (EBV) and hence the predisposition to lymphoma. This study in *Science* shows that intracellular free Mg2+ is required for the cytotoxic activity of T cells and natural killer (NK) cells as it controls the expression of the activating receptor natural killer group 2 member D (NKG2D).
https://www.science.org/doi/10.1126/science.1240094
A later review by Gile et al discussed how hypomagnesemia is linked to EBV and blood cancers. This isn’t just a risk for those with a mutation of the MAGT1 gene.
“Magnesium: The overlooked electrolyte in blood cancers?” (2020):
Mounting evidence in the literature suggests an association between hypomagnesemia and all-cause mortality. In addition, epidemiologic studies have demonstrated that a diet poor in magnesium increases the risk of developing cancer, highlighting its importance in the field of hematology and oncology. In solid malignancies, hypomagnesemia at diagnosis portends a worse prognosis. However, little is known about prognosis in patients with hypomagnesemia and blood cancers in general; lymphoma more specifically. Hypomagnesemia has been associated with a higher viral load of the Epstein Barr virus, a virus associated with a multitude of hematologic malignancies.
Good luck with this. The pharma clowns just aren’t interested:
The goal is to use these data to stimulate additional high-quality and well powered studies to further investigate the role of magnesium in preventing cancer and improving outcomes of patients with malignancy and concomitant magnesium deficiency.
MAGT1 mutations have been associated with EBV infection in patients with Kaposi Sarcoma as well [79]. As a result of these studies, Juan et al. investigated the relationship between magnesium levels and EBV levels measured via quantitative polymerase chain reaction (qPCR) with endemic Burkitt Lymphoma (eBL). They found that Ugandan women with decreased levels of plasma magnesium had elevated EBV viral loads [76]. This finding strengthens the hypothesis that reduced magnesium uptake by NK cells may lead to impairment in EBV control [76]. EBV has been associated with post-transplant lymphoproliferative disorder (PTLD), Burkitt lymphoma; Hodgkin Lymphoma (HL), Diffuse Large B Cell Lymphoma (DLBCL), and T cell lymphomas among other types of malignancy [80]. eBL is EBV positive in nearly 100% of cases [81] while DLBCL is rarely EBV related [82,83]. It is possible that chronic magnesium deficiency may predispose to the development of lymphoma through increased EBV viral load. How the EBV virus leads to lymphoma remains poorly understood, although it is thought to be secondary to a complex interplay between viral and cellular gene expression [84].
https://www.sciencedirect.com/science/article/pii/S0268960X20300266
Meta-analysis of clinical outcomes from several randomized, double-blind controlled trials also helped to confirm the diabetes hypothesis:
From “Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials“24 (2021):
…Compared with placebo, Mg supplementation reduced fasting plasma glucose in people with diabetes. In people at high risk of diabetes, Mg supplementation significantly improved plasma glucose per se, and after a 2 h oral glucose tolerance test. Furthermore, Mg supplementation demonstrated an improvement in insulin sensitivity markers. In conclusion, Mg supplementation appears to have a beneficial role and improves glucose parameters in people with diabetes. Moreover, our work indicates that Mg supplementation may improve insulin-sensitivity parameters in those at high risk of diabetes.
FPG = fasting plasma glucose.
SMD = standardized mean differences, with 95% confidence intervals (CIs).
As shown in Table 1, treatment with Mg significantly reduced FPG at follow-up in 325 participants with diabetes compared to 331 taking placebo (n = 11 studies; SMD = −0.426; 95%CI: −0.782 to −0.07; p = 0.02), this finding was characterized by a high heterogeneity (I2 = 79.0%) (Supplementary Figure S1).
Results were somewhat heterogeneous, perhaps due to confounding factors.
But overall, statistically significant reductions of various markers by 23-65% were found.
HbA1c = glycated haemoglobin A1c, a measure of blood sugar and glucose bound to hemoglobin molecules.
HOMA-IR = homeostatic model assessment-insulin resistance.
Regarding HbA1c levels, Mg supplementation did not improve this parameter in 301 participants compared to 307 participants taking placebo (n = 10 studies; SMD = −0.134; 95%CI: −0.409 to 0.141; p = 0.34; I2 = 63.7%). On the contrary, there was no evidence that Mg supplementation was able to improve fasting insulin or HOMA-IR compared to placebo in four studies including 153 people taking Mg and 149 taking placebo.
Table 1 reports the data regarding the effect of Mg, compared to placebo on glucose and insulin-sensitivity parameters. Overall, Mg supplementation significantly improved FPG in 482 subjects at high risk of diabetes compared to 485 randomized to placebo (11 RCTs; SMD = −0.344; 95%CI: −0.655 to −0.03; p < 0.0001; I2 = 81.2%) (Supplementary Figure S2). After trimming 4 studies at the left of the mean, these results remained statistically significant (SMD = −0.565; 95%CI: −0.860 to −0.271).
2hOGTT = oral glucose tolerance test.
Similarly, Mg significantly improved 2hOGTT in 3 studies involving 210 participants (SMD = −0.35; 95%CI: −0.62 to −0.07; I2 = 0%). Finally, compared to placebo, Mg significantly decreases HOMA-IR in 9 studies (340 Mg vs. 344 placebo) (SMD = −0.234; 95%CI: −0.443 to −0.025; p = 0.028; I2 = 43.2%) (Supplementary Figure S3), whilst no effect was observed on HbA1c or serum insulin levels (Table 1).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8619199/
Conclusion
Magnesium deficiency can contribute to the aetiology of many diseases and conditions, including Crohn’s, neurological disorders, migraine, osteoporosis, aging, muscle cramps, tinnitus, cancer and diabetes. And in turn, these conditions can either increase your need for further magnesium or make the prior deficiency worse.
Although correcting the deficiency may not cure you, multiple studies have shown that it can reduce the symptoms, improve the prognosis and decrease the need for allopathic medications that are typically more expensive, less efficacious and with worse side effect profiles.
In Part 3 I will review some of the papers investigating how magnesium deficiency is associated with arthritis, renal failure complications, hypothyroidism, peripheral neuropathy, learning and memory, insomnia, major depression and fibromyalgia.
Disclaimer
This site is strictly an information website reviewing research into potential therapeutic agents. It does not advertise anything, or provide medical advice, diagnosis, or treatment. This site does not promote any of these as potential treatments or offer any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses, or pharmacists. This content is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Any extracts quoted in the previous article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
References
Naser SA, Abdelsalam A, Thanigachalam S, Naser AS, Alcedo K. Domino effect of hypomagnesemia on the innate immunity of Crohn’s disease patients. World J Diabetes. 2014;5(4):527-535. doi:10.4239/wjd.v5.i4.527
Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-Reactive Protein and Cancer—Diagnostic and Therapeutic Insights. Front Immunol. 2020;11:595835. doi:10.3389/fimmu.2020.595835
Cooke JP. Inflammation and Its Role in Regeneration and Repair. Circulation Research. 2019;124(8):1166-1168. doi:10.1161/CIRCRESAHA.118.314669
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and Cancer. Ann Afr Med. 2019;18(3):121-126. doi:10.4103/aam.aam_56_18
El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: an overview. Bulletin of the National Research Centre. 2019;43(1):187. doi:10.1186/s42269-019-0227-2
Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like Receptors in Microbial Recognition and Host Defense. Immunol Rev. 2009;227(1):106-128. doi:10.1111/j.1600-065X.2008.00734.x
Ye H, Liu H, Attygalle A, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102(3):1012-1018. doi:10.1182/blood-2002-11-3502
Yang H, Qi H, Ren J, et al. Involvement of NF-κB/IL-6 Pathway in the Processing of Colorectal Carcinogenesis in Colitis Mice. International Journal of Inflammation. 2014;2014:e130981. doi:10.1155/2014/130981
Andersen CJ. Lipid Metabolism in Inflammation and Immune Function. Nutrients. 2022;14(7):1414. doi:10.3390/nu14071414
Malesza IJ, Bartkowiak-Wieczorek J, Winkler-Galicki J, et al. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia—A Narrative Review. Nutrients. 2022;14(17):3478. doi:10.3390/nu14173478
Tubic-Grozdanis M, Krämer I. Parenteral. In: Le Brun P, Crauste-Manciet S, Krämer I, Smith J, Woerdenbag H, eds. Practical Pharmaceutics: An International Guideline for the Preparation, Care and Use of Medicinal Products. Springer International Publishing; 2023:473-519. doi:10.1007/978-3-031-20298-8_21
Graefe SB, Rahimi N, Mohiuddin SS. Biochemistry, Substance P. In: StatPearls. StatPearls Publishing; 2024. Accessed March 18, 2024. http://www.ncbi.nlm.nih.gov/books/NBK554583/
Zhang S, Luo H, Sun S, et al. Salvia miltiorrhiza Bge. (Danshen) for Inflammatory Bowel Disease: Clinical Evidence and Network Pharmacology-Based Strategy for Developing Supplementary Medical Application. Front Pharmacol. 2021;12:741871. doi:10.3389/fphar.2021.741871
Salvia Miltiorrhiza - an overview | ScienceDirect Topics. Accessed March 18, 2024. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/salvia-miltiorrhiza
Boyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit Rev Eukaryot Gene Expr. 2009;19(3):171-180. doi:10.1615/critreveukargeneexpr.v19.i3.10
Fan Y, Kim DH, Seob Gwak Y, et al. The role of substance P in acupuncture signal transduction and effects. Brain Behav Immun. 2021;91:683-694. doi:10.1016/j.bbi.2020.08.016
Han JS, Xie GX, Zhou ZF, Folkesson R, Terenius L. Acupuncture mechanisms in rabbits studied with microinjection of antibodies against beta-endorphin, enkephalin and substance P. Neuropharmacology. 1984;23(1):1-5. doi:10.1016/0028-3908(84)90208-9
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol. 2018;9:586. doi:10.3389/fimmu.2018.00586
Ahmed SH, Waseem S, Shaikh TG, et al. SARS-CoV-2 vaccine-associated-tinnitus: A review. Ann Med Surg (Lond). 2022;75:103293. doi:10.1016/j.amsu.2022.103293
Wu S, Jin T, Ma B, et al. Oral application of magnesium‐L‐threonate enhances analgesia and reduces the dosage of opioids needed in advanced cancer patients—A randomized, double‐blind, placebo‐controlled trial. Cancer Med. 2023;12(4):4343-4351. doi:10.1002/cam4.4922
Tsai S, Clemente-Casares X, Zhou AC, et al. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 2018;28(6):922-934.e4. doi:10.1016/j.cmet.2018.08.003
JLE - Magnesium Research - Intracellular magnesium and insulin resistance. Accessed March 22, 2024. https://www.jle.com/fr/revues/mrh/e-docs/intracellular_magnesium_and_insulin_resistance__263229/article.phtml
Chaigne-Delalande B, Li FY, O’Connor GM, et al. Mg2+ Regulates Cytotoxic Functions of NK and CD8 T Cells in Chronic EBV Infection Through NKG2D. Science. 2013;341(6142):186-191. doi:10.1126/science.1240094
Veronese N, Dominguez LJ, Pizzol D, Demurtas J, Smith L, Barbagallo M. Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients. 2021;13(11):4074. doi:10.3390/nu13114074






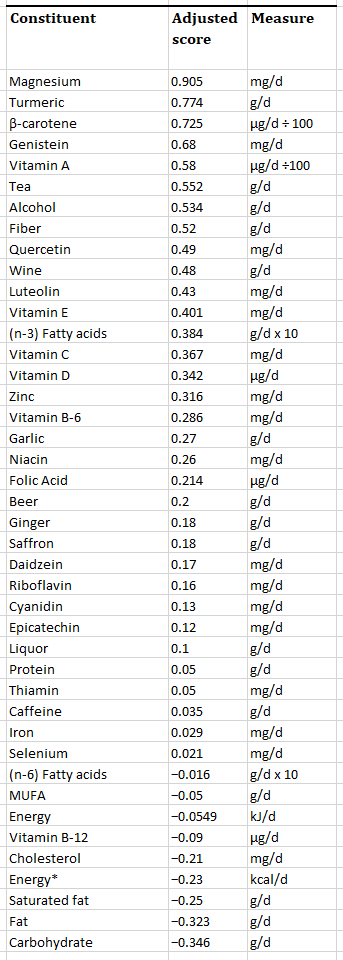
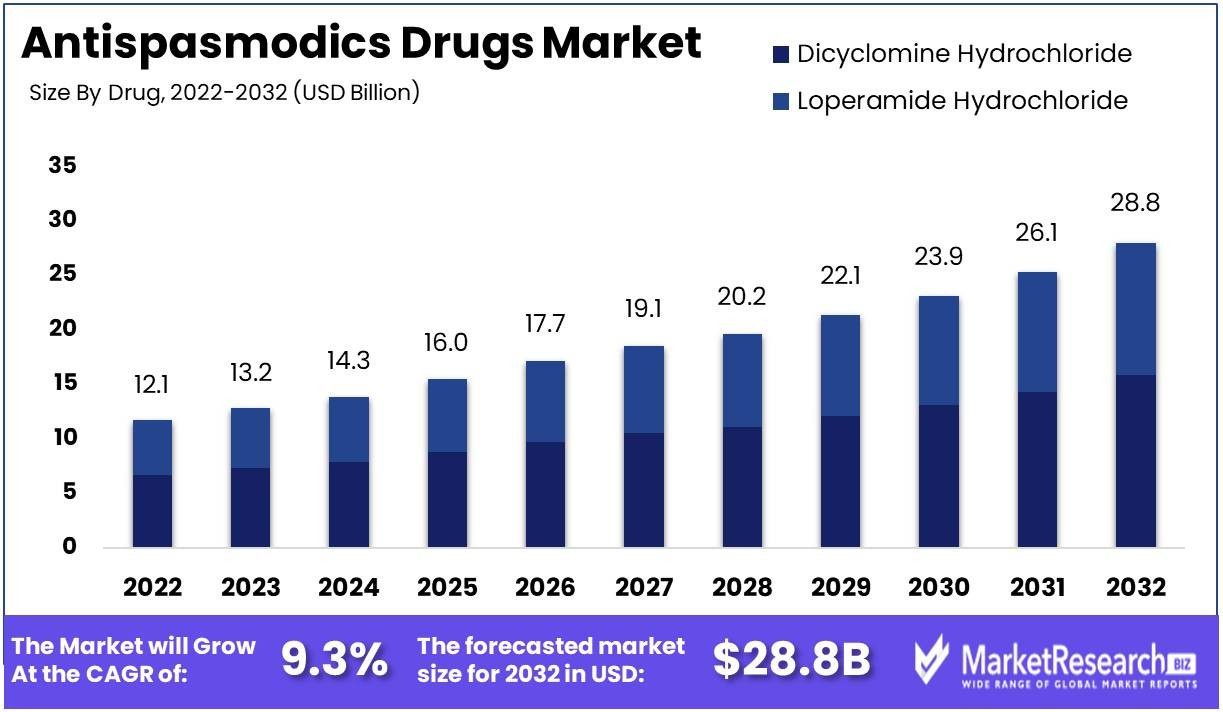




I have had a troubled history with magnesium supplements. I knew I needed magnesium due to my prolonged use of Omeprazole and once I stopped Omeprazole I tried magnesium supplements but because of chronic irritable bowel I had a very low tolerance. By chance I started probiotics to see if it would help and I have never had such a positive result from a supplement before. My chronic irritable bowel disappeared completely and my general anxiety reduced. I then tried Magnesium (Dr's Best magnesium powder) and lo and behold I could tolerate the magnesium with no problem, I am currently managing 300 mgs daily. Thank you for the very interesting article I will now read it again more slowly.
Holy crapola, what a great review. I need to read a couple more times to absorb. Stunningly useful information, since I’m also working on Klotho hormone that controls calcium, and is an important factor in aging. You article complements this research, and makes sense. Also great to know about substance P and is vital role in CD. Thanks for your research.