New variant human coronaviruses and antivirals: how Ralph Baric of UNC was ahead of his time
Back to the Future? A lab mouse investigates🐭
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Official Narratives
It goes something like this:
In December 2019 an infected bat and a pangolin in China decided to get jiggy, then invited in a passing Raccoon Dog, and all three infections inadvertently created a novel coronavirus.
The raccoon dog was subsequently offered for sale at a Wuhan fish market, coughed on the stall holder, surfaces & customers, thus adding humans to the list of infected.
Apart from being spread locally at Wuhan it was also shipped around the world on contaminated boxes of frozen squid tentacles or something like that.
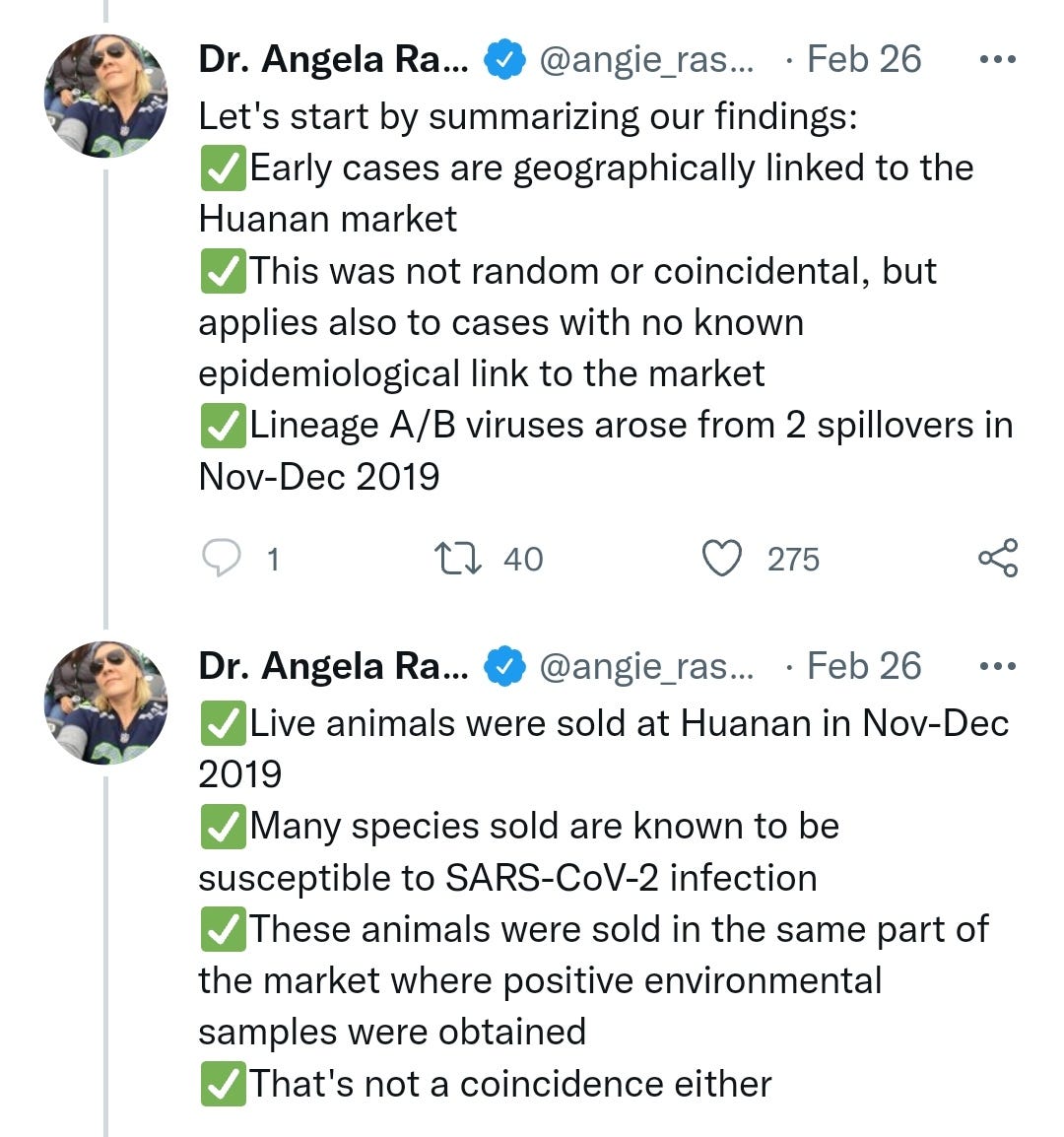
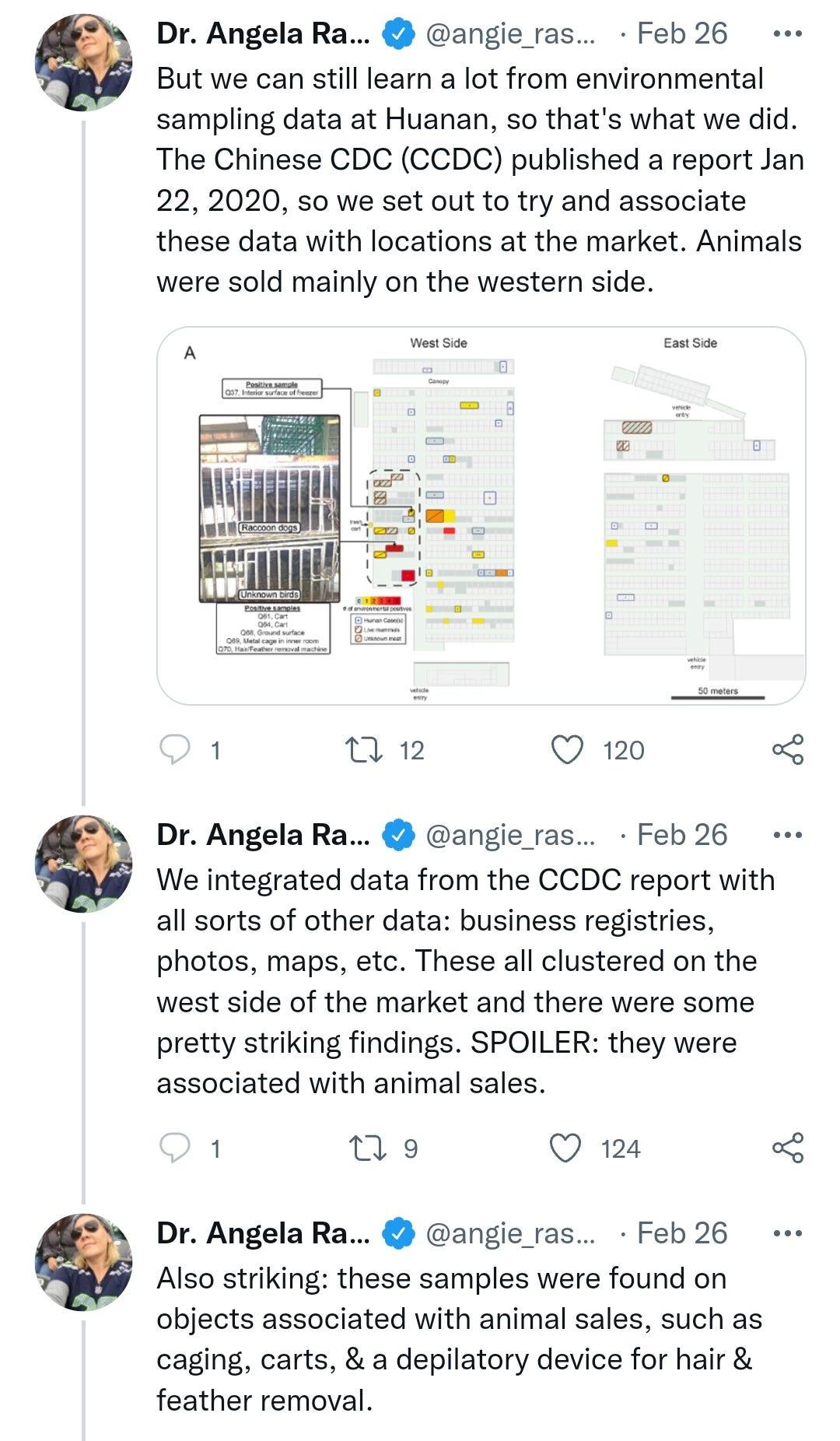
Dr Angie Ra-Ra-Rasmussen finally settled the science on viral origins by performing retrospective data sampling and statistical analysis on the fish market.
I've edited the thread for brevity, but feel free to read the whole thing, impressive stuff worthy of a Nobel I think we can all agree:
https://mobile.twitter.com/angie_rasmussen/status/1497692666997329923
COVID-19 wasn't declared a global pandemic until 11th March 2020 and the rest is history.
Remdesivir (AKA RunDeathIsNear!) was the first notable one out of the gate.
Reading this now it's laughably bad and you can see good trial protocols being broken, supposedly on the grounds of ethics but as with the gene therapy trials it was to unblind the controls and (literally?) bury any potentially bad news. It's a familiar pattern from the last two years:
Hopes rise for coronavirus drug remdesivir (4th May 2020)
Despite conflicting studies, results from largest trial yet show the antiviral speeds up recovery, putting it on track to become a standard of care in the United States.
An experimental drug — and one of the world’s best hopes for treating COVID-19 — could shorten the time to recovery from coronavirus infection, according to the largest and most rigorous clinical trial of the compound yet. On 1 May, the US Food and Drug Administration (FDA) granted an ‘emergency use authorization’ for clinicians to use the drug, called remdesivir, which is administered intravenously, in hospitals for people with severe COVID-19.
Remdesivir interferes with the replication of some viruses, including SARS-CoV-2, which is responsible for the current pandemic. On 29 April, Anthony Fauci, director of the US National Institute of Allergy and Infectious Diseases (NIAID), announced that a clinical trial in more than 1,000 people had showed that those taking remdesivir recovered in 11 days on average, compared with 15 days for those on a placebo.
“Although a 31% improvement doesn’t seem like a knockout 100%, it is a very important proof of concept,” Fauci said. “What it has proven is that a drug can block this virus.”
There were also fewer deaths among trial participants who received the drug, he said, but that trend was not statistically significant. The shortened recovery time, however, was significant, and was enough of a benefit that investigators decided to stop the trial early, he said, to ensure that those participants who were receiving placebo could now access the drug. Fauci added that remdesivir would become a standard treatment for COVID-19. The FDA’s authorization is not a final drug approval, and can be revoked when the conditions required for emergency use are no longer in effect. Distribution of the drug in the United States will be under government control.
Rollercoaster ride
The news comes after weeks of data leaks and on a day of mixed results from clinical trials of the drug. The drug’s maker, Gilead Sciences of Foster City, California, announced on the same day that in its own trial, more than half of 400 participants with severe COVID-19 had recovered from their illness within two weeks of receiving treatment. But the study lacked a placebo-controlled arm, making the results difficult to interpret.
With so much uncertainty, the remdesivir-watchers were waiting anxiously for final results from the NIAID trial, which were not expected until the end of May. In lieu of a vaccine, which could still be more than a year away, effective therapies are crucial in reducing deaths and limiting economic damage from the pandemic. Yet, despite the flood of small clinical trials, no therapy has been convincingly shown to boost survival in people with COVID-19.
The NIAID results put a new sheen on remdesivir. The NIAID did not release detailed safety data. The study in China found no significant difference between remdesivir and placebo in the frequency of adverse events, but 12% of people who received remdesivir dropped out of the study due to side effects including nausea and cardiopulmonary failure, compared to only 5% on placebo.
Fauci said the finding reminded him of the discovery in the 1980s that the drug AZT helped to combat HIV infection. The first randomized, controlled clinical trial showed only a modest improvement, he said, but researchers continued to build on that success, eventually developing highly effective therapies.
Gilead began to ramp up production of remdesivir well before the NIAID results came out. By the end of March, the company had produced enough to treat 30,000 patients. And by streamlining its manufacturing process and finding new sources of raw materials, Gilead announced, it hopes to produce enough remdesivir to treat more than one million people by the end of the year.
More:
https://www.nature.com/articles/d41586-020-01295-8
Molnupiravir (AKA MoreNewThalidomide) is another pandemic wonder drug that seemed to appear out of nowhere, with a horrible safety profile full of gaps and poor efficacy in reality.
The immunocompromised who might have benefited need to take it very early on after becoming symptomatic in order to have much antiviral effect, but the safety profile precludes it being taken as a prophylactic by anyone, making it completely impractical & utterly pointless, you have to know in advance you are going to be sick enough to warrant the risk of taking it, much like with Remdesivir.
On the other hand if they had been promoting hydroxychloroquine or ivermectin as prophylactics or at the first possible symptoms outcomes would have been radically different.
Molnupiravir: long-term safety questions linger as approvals approach (2021)
Some experts have concerns about the safety of Merck’s investigational Covid-19 antiviral molnupiravir.
Molnupiravir safety concerns
Molnupiravir’s mechanism against Covid-19 has some experts concerned about its mutagenic potential in human cells. One study has suggested that the drug, though intended to disrupt only viral RNA, could also incorporate into and cause mutations in human DNA.
The study’s authors say mutations in host DNA could potentially “contribute to the development of cancer, or cause birth defects either in a developing foetus or through incorporation into sperm precursor cells”.
Other experimental nucleoside analogue compounds have been found to cause birth defects in animals; former head of US Biomedical Advanced Research and Development Authority Rick Bright said offspring from animals treated with drugs similar to molnupiravir “had been born without teeth and without parts of their skull”.
Professor Ron Swanstrom of the University of North Carolina’s Department of Biochemistry and Biophysics is also concerned about the safety of the drug. While molnupiravir is “potentially an important antiviral strategy”, he says, it’s simply not possible to know what the long-term effects of the compound could be for humans.
“The question that I don’t have an answer for, and it’s unknown, as far as I’m concerned: is it a totally acceptable risk that means nothing?” Swanstrom says. “Or is it a more significant risk, but we won’t know the outcome for 10 years?”
Accounting for molnupiravir’s mutagenic potential
Merck has said it has “conducted a comprehensive nonclinical programme to characterise the safety profile of molnupiravir”, including assays that are designed to provide a “robust measure” of a compound’s ability to cause mutations.
Animals were given molnupiravir for longer and at higher doses than administered in human studies, Merck has noted, and the data indicates the drug “is not mutagenic or genotoxic in in vivo mammalian systems”. The company did not respond to a request for comment for this story.
Swanstrom is not convinced. In his view, the assays used in Merck’s animal studies are unlikely to be able to determine what may happen to mammalian DNA several years after the medicine is first administered.
Either way, Merck has acknowledged mutagenic drugs’ potential to mutate non-viral cells in its inclusion criteria for the Phase III study. Participants in the trial could not be pregnant or breastfeeding, and those of childbearing potential had to abstain from heterosexual sex or use highly effective contraception for 28 days from the start of study intervention. Individuals taking part in the study were also required to refrain from donating sperm.
“In a foetus every cell, virtually, is dividing,” Swanstrom explains. “So every cell has the potential to incorporate mutations – that’s the worst of all possible times [to take a mutagenic drug].”
Swanstrom is also concerned about molnupiravir’s long-term effect on the sperm cells produced by those who take the pills.
“Merck’s talking specifically about just the generation of sperm during the treatment,” he says. “I worry more about a step back, the germ cells that are going to sit there and make new sperm cells next year or 10 years from now.
“That’s another potential risk, that you’re actually making these really bad things, germline mutations, that affect the genes that are present in the next organism.”
A short course of treatment
In human studies, molnupiravir was administered orally twice daily for five days. It’s a relatively short treatment course, but this offers little reassurance to Swanstrom that the drug’s potentially harmful effects will be minimised.
“It’s long enough to mutagenise the virus, and at a pretty high level – enough to kill the virus by mutations,” he says. “The idea of feeding people a mutagen orally, I think we have to acknowledge that this is new territory that we don’t understand.”
Additionally, the researchers behind the study of molnupiravir’s mutagenic potential in mammalian cells wrote: “It seems unlikely that a short course of therapy would spare the host from this exposure, because both RNA precursors that affect the virus and DNA precursors that would affect the host pass through the common ribonucleoside diphosphate intermediate.”
Post-approval measures
Despite some experts’ concerns about its safety in humans, molnupiravir seems set for approval; governments are desperate for Covid-19 treatments that can be taken at home, reducing the burden on hospitals, and results from studies of molnupiravir show it to be pharma’s most promising candidate.
More:
https://www.pharmaceutical-technology.com/features/molnupiravir-safety-questions-approvals-approach/
How antiviral pill molnupiravir shot ahead in the COVID drug hunt (9th October 2021)
The Merck pill, which could become the first oral antiviral COVID treatment, forces the SARS-CoV-2 coronavirus to mutate itself to death.
The pharmaceutical firm Merck announced last week that an antiviral pill it’s developing can cut hospitalizations and deaths among people with COVID-19 by half. The results haven’t yet been peer reviewed. But if the drug candidate, molnupiravir, is authorized by regulators, it would be the first oral antiviral treatment for COVID-19. By contrast, the other currently authorized drugs must be delivered intravenously or injected.
A pill could make treating patients earlier on in their infection much easier — and more effective. It could also keep hospitals from overflowing, especially in places where vaccination rates are still low, such as many low- and lower-middle-income countries. Molnupiravir was so effective in a phase 3 trial involving COVID-19-positive people at risk of severe illness that clinicians halted enrolment early.
COVID-19 is not the first disease caused by a coronavirus to seriously impact humans. But the 2002–04 severe acute respiratory syndrome (SARS) epidemic fizzled out quickly, and the Middle East respiratory syndrome (MERS) outbreak in 2012 never became widespread — meaning that drugmakers had little incentive to develop antivirals against these diseases.
So when the first cases of COVID-19 emerged in late 2019, “there wasn’t a portfolio of antivirals waiting”, says Saye Khoo, an infectious-disease physician at the University of Liverpool, UK, who has led a clinical trial of molnupiravir.
Hot pursuit
Molnupiravir began as a possible therapy for Venezuelan equine encephalitis virus at Emory University’s non-profit company DRIVE (Drug Innovation Ventures at Emory) in Atlanta. But in 2015, DRIVE’s chief executive George Painter offered it to a collaborator, virologist Mark Denison at Vanderbilt University in Nashville, Tennessee, to test against coronaviruses. “I was pretty blown away by it,” Denison remembers. He found that it worked against multiple coronaviruses: MERS and mouse hepatitis virus.
Painter also recruited his collaborator Plemper to test the drug against influenza and respiratory syncytial virus. After the pandemic hit, however, plans changed. DRIVE licensed the compound to Ridgeback Biotherapeutics in Miami, Florida. Plemper, too, pivoted to coronaviruses, and tested the compound in ferrets. It silenced the virus’s ability to replicate, he says, but it also suppressed the virus’s transmission from infected ferrets to uninfected ones. Merck’s data hint that might also be true in humans: molnupiravir appeared to shorten the duration of SARS-CoV-2’s infectivity in trial participants with the virus.
More:
https://www.nature.com/articles/d41586-021-02783-1
So before the pandemic hit Molnupiravir hadn't been tested on coronaviruses since 2015 for MERS & mouse hepatitis virus.
And why would it have been? No-one could have known in advance or predicted the future inter-generic recombinant events at the Wuhan Fish Market and got funding to develop it for some random combination that could be anything, finding the “needle in a haystack” from an infinite world of possibilities.
Other viruses were far more prevalent and worthy of antiviral development funding at the time, like influenza and RSV. Apart from for our friend Ralph who seems to have an uncanny knack of being right about the need to save mankind from new variant coronaviruses, months & years ahead of the herd.
He seemed to arrive as a visitor from the future, working with or on a couple of antivirals that later were to be pushed extensively, contributing huge profits to the manufacturers.
GS-5734 is a synonym for Remdesivir.
EIDD-2801 for Molnupiravir.
And EIDD-2801 acts as a prodrug in the body, ie it changes into EIDD-1931 / N4-Hydroxycytidine, which is the active ingredient that inhibits viral replication.
So what was Ralph Baric working on?
In 2017 he co-published this:
Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5567817/#__ffn_sectitle
And in 2019, months before the animal antics at the fish market he was working on EIDD-1931/N4-Hydroxycytidine, the active ingredient of Molnupiravir:
Small-Molecule Antiviral β-d-N4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance.
Journal of virology (2019-10-04)
https://www.sigmaaldrich.com/GB/en/tech-docs/paper/1382290
It takes genius to have the foresight for events ahead of their occurrence, and he was happy to warn us all and reported his work on Remdesivir:
GS-5734 inhibits a range of emerging coronaviruses (8th March 2018)
Researchers report on GS-5734, a promising experimental broad-spectrum antiviral drug, previous studies on GS-5734 have shown that the drug inhibits strains of SARS and MERS coronaviruses that infect human airways and the lower respiratory tract, as well as infection by the Ebola virus.
The researchers now report that the drug also inhibits murine hepatitis virus, or MHV, which is closely related to several human coronaviruses that can cause respiratory tract infections, sometimes as severe as SARS.
“These viruses are poised to cause outbreaks,” says molecular virologist Ralph C. Baric at the University of North Carolina at Chapel Hill, “and most emerging viruses are cyclic in nature.” In the case of SARS, the coronavirus caused an outbreak that began in 2003 and was eventually controlled through public health efforts. However, many pre-epidemic forms of the virus are still around. “We need to have broad-based and potent drugs on a shelf to control future epidemics.”
The researchers tested the drug on mini models of human lungs consisting of airway epithelial cells, collected from lung transplants. Because those cells express the genes and proteins of the airways that are targeted by coronavirus infections, researchers can use them as a facsimile for host tissue, says Dr Baric.
GS-5734 may be useful in treating a wide range of infections caused by coronaviruses, including contemporary and epidemic strains, as well as those that are poised to jump from an animal host to a human in the future, says Dr Baric who co-led the study with Mark Denison, a paediatrician and infectious disease expert whose lab at Vanderbilt University in Nashville, Tennessee, focuses on coronaviruses. The researchers also collaborated with Gilead Sciences, a biopharmaceutical company that is developing a phase I clinical trial of the drug for people with MERS in Saudi Arabia.
In another new development, the researchers identified how the virus fights back against the drug, which is crucial information for predicting whether an antiviral might be effective in human hosts.
Like many antivirals, GS-5734 is a nucleoside analogue, a class of drugs that work by inhibiting the replication of the virus. However, because viruses evolve so rapidly, they quickly develop mutations that confer resistance to these drugs. Researchers at Vanderbilt identified the genetic mutations in the coronaviruses triggered by exposure to GS-5723. Those mutations, however, significantly weakened the virus, which suggests that the drug may be effective enough to outpace viral evolution.
“The location being targeted doesn’t seem to have much capacity to evolve and escape the effects of the drug, says Dr Baric. “Drug resistance was very difficult to achieve.”
Epidemics caused by emerging coronaviruses can be devastating. During a SARS epidemic that began in Asia in 2003, for example, more than 8,000 people in 29 countries became infected, and 774 died, according to data from the World Health Organization. The WHO also reports that since September 2012, more than 2,000 people in 27 countries have become infected with MERS, and 750 have died from the infection.
https://www.europeanpharmaceuticalreview.com/news/73459/gs-5734-inhibits-coronaviruses/
And then on November 13–15, 2018:
Advances in respiratory virus therapeutics – A meeting report from the 6th isirv Antiviral Group conference
14. Hot topics and late-breakers
14.1. Antiviral design against emerging and pre-epidemic coronaviruses
Ralph Baric, University of North Carolina, Chapel Hill, NC, USA.
Viruses that emerge from zoonotic reservoirs present unique challenges for the development of treatment and/or vaccination strategies, as preparation requires being ready to combat viruses that have yet to be identified. The highly pathogenic human coronaviruses SARS-CoV and MERS-CoV originated in bat zoonotic reservoirs and then passed through different intermediate hosts prior to infecting humans. Bat coronavirus strains that share varying homology to MERS- and SARS-CoV are still circulating and represent the pre-epidemic strains most likely to make the jump into humans (Anthony et al., 2017; Becker et al., 2008; Menachery et al., 2015, 2016; Sheahan et al., 2008a, 2008b). As many novel bat coronavirus strains were identified solely as genomic sequence isolated from bat guano, characterization of these strains beyond sequence analysis was not initially possible.
To determine if SARS-CoV-like bat coronaviruses could replicate using non-human primate or human cellular attachment proteins, the Baric laboratory cloned individual bat coronavirus spike genes into the SARS-CoV infectious clone backbone and isolated replication competent viruses. The SARS-CoV/bat-CoV spike viruses were evaluated in cell lines expressing either the human, bat, or civet angiotensin converting enzyme 2 (ACE2, cellular receptor) to determine host species specificity, and to identify candidate bat-CoV strains to generate infectious clones based on the entire bat coronavirus genome. Four of the six SARS-CoV/bat-CoV spike viruses tested could use all three ACE2 molecules for entry and replicated to high titers (>106 plaque forming units/mL) (Becker et al., 2008; Menachery et al., 2015; Rockx et al., 2007). These four bat coronavirus strains were then isolated from infectious clones derived from their respective genomes and the resulting virus progeny used to characterize replication kinetics in immortalized cell lines and primary human lung cell cultures and to perform in vivo vaccination or treatment experiments in comparison to wild type SARS-CoV. SHC014 and WIV1 replicated to high titers, the same titers as wild type SARS-CoV, in primary lung cell cultures suggesting that these strains were fully competent to infect humans. In murine disease models of highly pathogenic human coronavirus infection the bat coronavirus strains were attenuated in comparison to wild type mouse adapted SARS-CoV. Vaccination studies demonstrated that vaccines specific for SARS-CoV were not effective against the bat coronavirus strains and antibody treatments that were protective for SARS-CoV failed against the bat strains (Becker et al., 2008; Menachery et al., 2015; Rockx et al., 2007, 2008). These data highlight how crucial it is to evaluate all treatment options on more than just the current and past human strain(s) and SARS- and MERS-CoV so that appropriate treatment and vaccination strategies1 are stock piled to be prepared for future epidemics.
Previous studies have shown that remdesivir (formerly GS-5734) has broad spectrum antiviral activity and in vivo efficacy against filoviruses, paramyxoviruses, and coronaviruses including MERS-CoV (Lo et al., 2017; Siegel et al., 2017; Warren et al., 2016). Remdesivir pharmacokinetic studies have been completed and this compound is currently being evaluated in clinical trials for Ebola virus efficacy (NCT03719586). Nucleotide/side analogues including remdesivir were tested to determine if they were effective against highly pathogenic human coronaviruses despite previous studies demonstrating that nucleotide analogues generally have poor activity against coronaviruses due to the proofreading enzyme that is part of the viral replication machinery. Studies in human lung cell lines and primary human lung epithelial cell cultures demonstrated that remdesivir is effective against both SARS- and MERS-CoV as well as the pre-epidemic bat-CoV strains described earlier (Sheahan et al., 2017). In parallel, in vitro passage studies were also performed to determine if coronavirus genomes could evolve to replicate in the presence of remdesivir. After 23 passages in the presence of drug, two mutations were identified in the viral RNA dependent RNA polymerase gene (F276L and V553L) that allowed for increased replication in the presence of remdesivir (Agostini et al., 2018). These two amino acid changes decrease viral fitness overall and attenuate SARS-CoV pathogenesis in a mouse model of SARS-CoV induced disease (Agostini et al., 2018). In both prophylactic and therapeutic (24 h post infection) dosing regimens of SARS-CoV infected animals, remdesivir treated animals had a >2 log reduction in viral titers by Day 4 or 5 post infection (Sheahan et al., 2017). Remdesivir treated animals also maintained their starting weight throughout the infection time course while vehicle treated animals lost 20% of their starting weight by Day 4 or 5 post infection (Sheahan et al., 2017). Finally, improved lung function (less airway constriction and reduced times between breaths) was seen when treated animals were compared to SARS-CoV infected untreated control animals (Sheahan et al., 2017). Similar results were seen with MERS-CoV in prophylactic dosing studies performed in the Baric laboratory's MERS-CoV mouse model (Cockrell et al., 2016). In contrast, therapeutic treatment with human monoclonal antibodies did not protect against MERS-CoV induced severe disease or protect from loss of lung function in animal models of highly pathogenic human coronavirus disease (Cockrell et al., 2016). Remdesivir is a promising broad-spectrum antiviral that may prove useful in the treatment of highly pathogenic human coronavirus.
https://www.sciencedirect.com/science/article/pii/S0166354219301585#!
Way to go Ralph.
Thanks for reading.
...”Ah just one more thing sir” as the great detective Columbo would say, just before he nails the suspect who thought they’d got away with the perfect crime.
An excellent Substack by Igor Chudov:
Did Ralph Baric of UNC Design Omicron?
"COVID Bioweapon Against Mice" Patent 11225508
Ralph Baric, whose name is familiar to our readers, is a brilliant scientist at UNC who is the top name in research related to coronavirus modifications.
He is the researcher who
Experimented with adding HIV genes to SARS-1 spikes
Found ways to mutate nsp-14 protein to make SARS-1 to spawn 21 times more variants. Sars-Cov-2 inherited a 5% mutated nsp-14 from SARS-1, and is now producting endless variants, oddly enough
Found ways to enhance function of SARS-1 in 2007, to cause much more severe disease in mice, killing most older mice
Created chimeric coronaviruses engineered to effectively infect humans
Was given a “Moderna Vaccine Candidate” on Dec 12, before Sars-Cov-2 was officially known.
More:
https://igorchudov.substack.com/p/did-ralph-baric-of-unc-design-omicron
He refers to future vaccines but he is not working on these pre-pandemic. It's pure gain of function research. In his own words from the first paragraph:
“To determine if SARS-CoV-like bat coronaviruses could replicate using non-human primate or human cellular attachment proteins, the Baric laboratory cloned individual bat coronavirus spike genes into the SARS-CoV infectious clone backbone and isolated replication competent viruses.”
Gain-of-function research