Tumor Suppressor Protein p53 & BRCA related cancers
Experimental gene therapy transfections and public health implications
Updated 11th May ‘22
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Dr. James E. Olsson,
Genetic Engineering, Johns Hopkins 2014, Biomedical and Cancer Research:
"Make sure to tell your primary that that was not supposed to happen according to what you heard on the television when they are writing you a referral to an oncologist. Tell your primary "fact check" said it wasn't so.”
Background:
Transfection is the process of introducing nucleic acids into eukaryotic cells by nonviral methods. Using various chemical or physical methods, this gene transfer technology enables the study of gene function and protein expression in a cellular environment.
BRCA:
BRCA1 and BRCA2 are the first two genes found to be associated with inherited forms of breast cancer. Both genes normally act as tumor suppressors, meaning that they help regulate cell division. When these genes are rendered inactive due to mutation, uncontrolled cell growth results, leading to breast cancer. Women with mutations in either gene have a much higher risk for developing breast cancer than women without mutations in the genes.
BRCA1 and BRCA2 are the names for two different genes that are associated with inherited or familial breast cancer. Everyone has two copies of these genes in all of their cells, and when they're unmutated, they serve to protect the cell against turning into a cancer cell. Some people are born with a specific mutation or different mutations in these genes, and these individuals are more susceptible to cancer. If they're a woman, they're at a greatly increased risk of having breast cancer in their lifetime. If they're a man, they may be at an increased risk of prostate cancer. People who inherit mutations have one copy of this gene that is not working. If, by chance, in some of their cells, either in their breast tissue or maybe their prostate tissue, the particular cell loses the other copy, that cell that has now lost both copies of BRCA1 or both copies of BRCA2 is unable to repair its DNA. And when it replicates its DNA during cell division, many, many more mistakes enter into that replicated DNA. Those mistakes make it more likely that cell will go on and start down the path to become a tumor.
Lawrence C. Brody, Ph.D.
https://www.genome.gov/genetics-glossary/BRCA1-BRCA2
S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein p53 and BRCA: an In Silico Study
Abstract
Novel coronavirus disease 2019 (COVID-19) is the biggest threat to human being globally. The first case was identified in a patient with flu symptoms along with severe acute respiratory syndrome in Wuhan, China in December 2019 and now it has spread in more than 200 countries. COVID-19 is more lethal in the elderly and people with an underlying condition such as asthma, cancer, diabetes. Here we performed bioinformatic analysis to investigate the interaction of S2 subunit protein of SARS-nCoV-2 of novel coronavirus with tumor suppressor proteins p53 and BRCA-1/2. In this short communication we report the interaction between S2 subunit proteins with tumor suppressor proteins for the first time. This preliminary result will open up a new direction to investigate the effect of a novel coronavirus in cancer patients.
In December 2019, an outbreak of pneumonia was reported in Wuhan, China which was caused by a new strain of coronavirus called novel coronavirus (nCoV). The novel coronavirus disease-2019 (COVID-19), which started spreading globally, was latter announced as a pandemic by WHO. Till April 30, 2020, there are 3,090,445 infections and 217,769 deaths worldwide [1]. Previously known SARS-CoV which caused severe acute respiratory syndrome (SARS) has 79.5% sequence similarity with SARS-nCoV-2 (nCoV). The pernicious nCoV has been causing severe flu-like symptoms along with pneumonia specially in the elderly and people with ailments like hypertension, asthma, cancer, diabetes, etc. [2,3] but detail understanding of infection is still lacking.
Coronaviruses (CoVs) belong to coronaviridae family and are the largest RNA viruses identified till date. SARS-nCoV-2 contains a spike (S) protein which consists of two subunits S1 and S2. S1 aids the virus to infect human cells by binding to human angiotensin-converting enzyme 2 (hACE2) and S2 mediates the membrane fusion process. S2 subunit is further divided (N-terminal to C-terminal) into fusion peptide (FP), hepted repeat 1 (HR-1), hepted repeat 2 (HR-2), transmembrane domain (TM), and cytoplasmic domain (CP). After infection to host receptor, the HR-1 and HR-2 domain of S2 subunit interact with each other to form six-helix bundle (6-HB) fusion core, bringing viral and cellular membrane into close proximity for fusion and infection [4]. That is why it is very important to study the interaction of S2 subunit with other proteins, to gain insight in to its function and interaction with other potential proteins which have a central role in human diseases. This would unravel the possible mechanism of COVID-19 infection and severity in humans who are already suffering from an ailment.
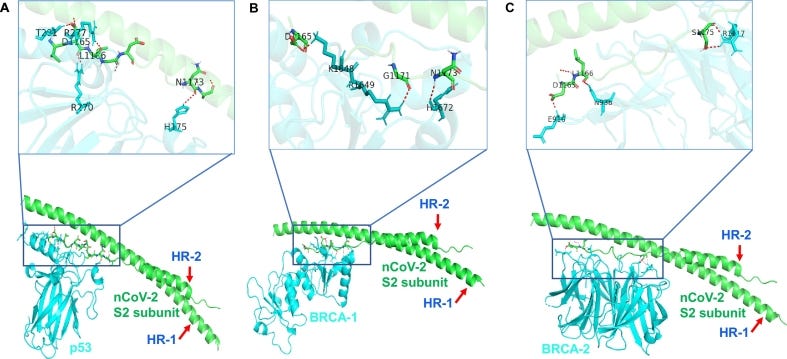
Here, we have investigated the interaction of S2 subunit to tumor suppressor and cell cycle-related proteins. HADDOCK 2.2 software [5,6] was used to analyze the interaction and found that S2 subunit of SARS-nCov-2 strongly interacts with p53 and BRCA-1/2 proteins (Figure 1). p53 and BRCA are the well-known tumor suppressor proteins, that regulate downstream genes in response to numerous cellular stress and are frequently mutated in human cancer [5,6]. Interestingly we found p53, BRCA-1 and BRCA-2 interact with heptic repeat-2 region of S2 subunit through C- terminal domain. PDB ID of these proteins was extracted from RCSB Protein Data Base (PDB) and details of crystal structure IDs and interacted amino acid residues are mentioned in the figure legend. This short bioinformatic analysis is a first time report and significant since COVID-19 is more fatal in people with underlying conditions specially lung diseases, diabetes and cancer. Therefore, further research is needed to understand COVID-19 effect in cancer patients and the detailed role of these interactions.
Analysis of S2 subunit of SARS-nCoV-2 interaction with tumor suppressor proteins. (A) Interaction between S2 Subunit of nCoV (Green color PDB ID: 6LXT [4] asp 1165, leu 1166, asn 1173) with p53 protein (Cyan color PDB ID: 3EXJ [7] thr 281, arg 270, arg 277, his 175) (B) Interaction between S2 Subunit of nCoV (Green color PDB ID: 6LXT [4] asp 1165, gly 1171, asn 1173) with BRCA-1 protein (Cyan color PDB ID: 4Y18 [8] lys 1648, arg 1649, his 1672) (C) Interaction between S2 Subunit of nCoV (Green color PDB ID: 6LXT [4] asp 1165, leu 1166, ser 1175) with BRCA-2 protein (Cyan color PDB ID: 3EU7 [9] glu 916, leu 938, arg 1117). HR: hepted repeat. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
This paper was published the following year, and confirmed that both viral and transected spike protein interact with and impaired 2 important tumour suppressors:
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #2: SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro (2021)
“To determine how the spike protein inhibits both NHEJ and HR repair pathways, we analyzed the recruitment of BRCA1 and 53BP1, which are the key checkpoint proteins for HR and NHEJ repair, respectively. We found that the spike protein markedly inhibited both BRCA1 and 53BP1 foci formation (Figure 3D–G). Together, these data show that the SARS–CoV–2 full–length spike protein inhibits DNA damage repair by hindering DNA repair protein recruitment.”
“This suggests that the use of antigenic epitopes of the spike as a SARS–CoV–2 vaccine might be safer and more efficacious than the full–length spike. Taken together, we identified one of the potentially important mechanisms of SARS–CoV–2 suppression of the host adaptive immune machinery. Furthermore, our findings also imply a potential side effect of the full–length spike–based vaccine. This work will improve the understanding of COVID–19 pathogenesis and provide new strategies for designing more efficient and safer vaccines.”1
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency/comments?s=w
If we equate the effects of down-regulation of P53 expression by spike protein with the effects due to mutations of the TP53 gene, both of which lead to reduced amounts of tumor suppressor protein, then we can look at the findings of prior research into this to give us some clinical indications of what cancers are likely to become more prevalent.
One such study shows that breast cancer is particularly affected, with a prognosis at least twice as poor as for other p53 correlated cancers:
Clinical Outcomes and Correlates of TP53 Mutations and Cancer (2010)
Abstract
The initial observation that p53 accumulation might serve as a surrogate biomarker for TP53 mutation has been the cornerstone for vast translational efforts aimed at validating its clinical use for the diagnosis, prognosis, and treatment of cancer. Early on, it was realized that accurate evaluation of p53 status and function could not be achieved through protein-expression analysis only. As our understanding of the p53 pathway has evolved and more sophisticated methods for assessment of p53 functional integrity have become available, the clinical and molecular epidemiological implications of p53 abnormalities in cancers are being revealed. They include diagnostic testing for germline p53 mutations, and the assessment of selected p53 mutations as biomarkers of carcinogen exposure and cancer risk and prognosis. Here, we describe the strengths and limitations of the most frequently used techniques for determination of p53 status in tumors, as well as the most remarkable latest findings relating to its clinical and epidemiological value.
…
SOMATIC TP53 MUTATIONS AS BIOMARKERS OF CANCER RISK IN PREDISPOSING CONDITIONS
The overrepresentation of C to T transitions in the tumor spectra of TP53 mutations has prompted the proposal that endogenous reactive molecules, such as nitric oxide, may contribute to cancer etiology (Ambs et al. 1997). Nitric oxide is overproduced in chronic inflammatory diseases, such as ulcerative colitis, that are cancer prone and have high TP53 mutational load in nontumor tissues (Hussain et al. 2000a). The interplay of nitric oxide production and TP53 mutation may lead to clonal selection and tumor growth in sporadic colon cancer as well (Ambs et al. 1998; Goodman et al. 2004). Other chronic inflammatory diseases that predispose to cancer, such as hemochromatosis and Wilson disease (Ambs et al. 1999), are also associated with detectable TP53 mutations in nontumor tissue (Hussain et al. 2000b). There would be clear clinical benefit from the availability of sensitive assays that would identify mutations in individuals at risk for cancer before overt tumor development (Olivier et al. 2004). Chronic inflammation and cancer risk is an exiting field for research that may yield early diagnostic and therapeutic opportunities (Hussain and Harris 2007).
Thus, the presence of TP53 mutations with a specific pattern or representing a plausible biological mechanism can be used clinically to study the etiology of cancers as well as screening tools for cancer predisposition and risk.
TP53 Abnormalities as Biomarkers of Cancer Prognosis
A multitude of retrospective studies have associated abnormal p53 protein expression as well as somatic mutation with poor survival or lack of response to therapy. Nonetheless, the clinical significance of p53 status for patient outcome has been (Roth 1999) and continues to be one of the most controversial areas of p53 research (Hoff 2005; Munro et al. 2005). The problem largely stems from the inherent complexity of the p53 pathway, the abundance of mutations in different tumor types and clinical stages, and their disparate effects, as well as lack of randomized prospective studies compounded by heterogeneity in experimental designs, sample types, and techniques. In addition, it would perhaps be naïve to expect that a ubiquitously mutated gene would have the same clinical value regardless of context. That being said, there have been encouraging findings regarding survival prediction by TP53 mutations. This is illustrated in Figure 2 using data extracted from the IARC TP53 database and updated with the addition of 20 sequencing-based studies published between 2007 and 2009. For cancers of the breast, head and neck, liver, hematopoietic, and lymphoid systems, a majority of studies show an association of TP53 mutation with worsened survival (Fig. 2). For cancers of the bladder, brain, lung, colon, esophagus, and ovary, about as many studies found an association as did not. For cancers of the pancreas, prostate gland, rectum, and stomach, the number as well as the cumulative number of patients in the respective cohorts reported by each publication were small. The largest patient cohorts have been reported for breast and colorectal carcinomas.
Graphical representation of the number of studies that have shown an association or lack of association of TP53 mutation with poor survival. The cumulative number of patients in all cohorts reported in those studies is indicated as n for each cancer type. Data were extracted from the IARC p53 database R13 release http://www-p53.iarc.fr and were updated with 20 new studies appearing in 2006–2009.
Colorectal Cancer
Two large meta-analysis reports on the predictive value of p53 abnormalities on outcome of colon cancer show contradictory results regarding response to chemotherapy, but an essentially similar lack of significant effect on survival prediction (Munro et al. 2005; Russo et al. 2005). In contrast, a sequencing-based, prospective study on primary operable colorectal cancer patients undergoing resective surgery showed that specific TP53 mutations were associated with worse prognosis (Bazan et al. 2005). Similarly, a subset of functionally inactive mutations predicted poor survival in late stage colorectal cancers (Iacopetta et al. 2006).
Breast Cancer
Breast cancer seems to show the clearest association of TP53 mutation with poor survival in large, prospective, sequence-based studies. The presence of TP53 mutation was an independent predictor of cancer-specific death in a large cohort of women with primary breast cancer and associated with poorest prognosis when combined with the absence of progesterone receptor (Olivier et al. 2006). The overall picture that is emerging from breast cancer indicates that missense mutations that affect DNA binding are particularly deleterious and are associated with the worse survival, whereas nonmissense mutations in the DNA-bindings motifs or null mutations are associated with intermediate reduction of survival compared with no mutations (Olivier et al. 2006; Alsner et al. 2008). Kaplan-Meier survival curves from the paper by Olivier et al. (2006) illustrating this finding are shown in Figure 3. In another large prospective study, TP53 mutation was found to be associated with poor survival as well as “basal-like” and “ERBB2+” gene expression subgroups, which themselves had very high mortality (Langerod et al. 2007). Missense TP53 mutations also conferred higher risk of recurrence and death compared with wild-type TP53 in women with node-negative breast cancer (Ozcelik et al. 2007). In contrast to the analysis of TP53 mutations, the overall clinical value of p53 immunostaining in breast cancer is poor with respect to other clinical parameters, including Her-2 positivity (Soerjomataram et al. 2008). Thus, the routine use of p53 immunostaining is not recommend in clinical practice (Harris et al. 2007).
Kaplan-Meier survival curves of patients with breast cancer stratified by the type of TP53 gene mutation found in their tumor. Survival of patients without mutation or with a silent mutation within exons 5 to 8 (blue line), with a missense mutation within exons 5 to 8 but outside the DNA binding domains (red line), with a missense mutation in the DNA binding domains (green line), and with a mutation other than missense within exons 5 to 8 (black line). (Reprinted, with permission, from Olivier et al. 2006 [© AACR].)
Head and Neck Cancers
A prospective, multicenter trial analyzed TP53 mutation status in squamous-cell carcinoma of the head and neck through p53 GeneChip and found that presence of any TP53 mutation had an adverse effect on survival, but those mutations that resulted in disrupted DNA binding led to an even worse prognosis (Poeta et al. 2007). In agreement, TP53 mutations in direct DNA contact areas resulted in accelerated tumor progression and reduced therapeutic responsiveness in head and neck squamous cell carcinoma (Erber et al. 1998; Temam et al. 2000).
Lung Cancer
Among patients with stage I NSCLC, survival was higher in those with wild-type p53 than in those with mutant p53 (Ahrendt et al. 2003), but TP53 mutations were not associated with the very limited survival of cases with advanced-stage NSCLC (Lim et al. 2009). TP53 mutations were, however, found to be significantly associated with recurrence of NSCLC (Ludovini et al. 2008).
Prostate Cancer
Abnormal p53 expression in prostate cancer was related to increased risk of disease-specific death as well as the development of distant metastasis at 5 years (Che et al. 2007). Most importantly, this study provided support for using p53 as a marker to stratify patients undergoing androgen deprivation and radiotherapy, by showing that long-term androgen deprivation may significantly improve the cause-specific survival for those with abnormal p53 (Che et al. 2007).
Ovarian Cancer
A meta-analysis of 62 studies addressing the prognostic significance of p53 abnormalities using IHC or mutational analysis found that overall p53 aberrant status confers poor survival, but the effect was modest (de Graeff et al. 2009).
Hematopoietic Cancers
Although the prevalence of TP53 mutations in chronic lymphocytic leukemia (CLL) is low (<10%), TP53 disruption is frequently observed in CLL through del17p13, and both these abnormalities are predictive of poor survival (Zenz et al. 2008; Rossi et al. 2009). Also, most of TP53 mutations found in CLL cluster within the DNA binding domain of p53.
Lymphoid Cancers
An international collaborative study on 477 patients with diffuse large B-cell lymphoma (DLBCL) showed that p53 DNA-binding mutants have a worse effect on overall survival in DLBCL than WT p53 or mutants in other domains (Young et al. 2008).
Sequencing-based randomized prospective studies have started to uncover the prognostic value of TP53 mutational analysis. Overall, the finding that null mutations have a somewhat intermediate effect on survival gives support to “gain-of-function” properties that may translate into newly acquired transcriptional functions (Weisz et al. 2007).
…
Two recent discoveries related to p53 may have significant clinical implications. MicroRNAs are small, evolutionarily conserved noncoding RNAs that exert diverse regulatory activities on mRNA stability and translation (Ambros 2001). They are frequently deleted and/or disregulated in cancer (Calin and Croce 2006) and can be biomarkers of cancer diagnosis, prognosis, and therapeutic outcome (Bartels and Tsongalis 2009). Certain microRNAs, e.g., mir-34a, b, and c, are transcriptionally transactivated by p53 (Chang et al. 2007; He et al. 2007; Raver-Shapira et al. 2007; Tarasov et al. 2007), whereas others, e.g., mir-125b, negatively regulate its expression (Le et al. 2009). So, it is becoming apparent that not only do TP53 mutations affect its downstream functions, such as apoptosis and senescence, but also epigenetic or mutational changes in related microRNAs need to be considered, because abnormalities in microRNAs, such as methylation of mir-34 (Lujambio et al. 2008) and/or amplification of mir-125b (Bousquet et al. 2008) may affect downstream p53 functions (Fig. 5). In addition, the discovery of p53 isoform variants arising from alternative splicing and promoter usage may also have clinical implications (Bourdon et al. 2005). These variants can interact with wild-type p53 through either dominant–negative or cotransactivating functional modulation. Most importantly, p53 isoforms are expressed in human cancers (Boldrup et al. 2007; Fujita et al. 2009) so that certain isoforms may alter the function of wild-type p53 and complicate the interpretation of nonmutant TP53 and immunohistochemical results in prediction of cancer risk, prognosis, and therapeutic response.
MicroRNAs are integral to the p53 network and their abnormalities may have clinical implications. MicroRNAs mir-34a, b, and c, are transcriptionally transactivated by p53 and are downstream effectors of p53 function. MicroRNA mir-125b negatively regulates p53 expression. In addition to TP53 mutations and deletions, abnormalities in microRNAs, such as methylation of mir-34 (Lujambio et al. 2008) and/or amplification of mir-125b (Bousquet et al. 2008) may affect downstream p53 functions.
Full paper:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC2829964
p53 and the pathogenesis of skin cancer (2007)
Abstract
The p53 tumor suppressor gene and gene product are among the most diverse and complex molecules involved in cellular functions. Genetic alterations within the p53 gene have been shown to have a direct correlation with cancer development and have been shown to occur in nearly 50% of all cancers. p53 mutations are particularly common in skin cancers and UV irradiation has been shown to be a primary cause of specific 'signature' mutations that can result in oncogenic transformation. There are certain 'hot-spots' in the p53 gene where mutations are commonly found that result in a mutated dipyrimidine site. This review discusses the role of p53 from normal function and its dysfunction in pre-cancerous lesions and non-melanoma skin cancers. Additionally, special situations are explored, such as Li-Fraumeni syndrome in which there is an inherited p53 mutation, and the consequences of immune suppression on p53 mutations and the resulting increase in non-melanoma skin cancer in these patients.
Editorial on role of p53 in esophageal cancer from a meta-analysis of 16 studies by Fisher et al. (2017)
"Considering that the presence of low-moderate heterogeneity across studies is not statistically significant, this systematic review shows that TP53 mutation is associated with a statistically significant negative effect on patient OS with an HR 1.48 (95% CI: 1.16 to 1.90, P=0.002). Fourteen of the total 16 studies reports that the median survival time of patients assessed as having mutated TP53 was 18.9 months compared with 26.2 months for patients with non-mutated TP53."
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5506115/
https://twitter.com/kimdechurch/status/1524026216881266691?s=20
Autoimmune mediated depletion of many tumor suppressors was researched in this excellent paper. This potential pathology is additional to that discussed above and perhaps one of the most alarming papers I have seen in its implications, and received almost no publicity. Once autoimmune responses have been primed it is currently not possible to stop these, only to try to reduce the sequale.
“This article described a vast peptide overlap between SARS-CoV-2 spike gp and tumor-suppressor proteins, and supports autoimmune cross-reactivity as a potential mechanism underlying prospective cancer insurgence following exposure to SARS-CoV-2. Clinically, the findings call for close surveillance of tumor sequelae that possibly could result from the current coronavirus pandemic.”
“…might equate to induce or enhance carcinogenesis in almost all of the human organs, from brain and liver to lung and bones. Examples of the cancers that might be evoked/potentiated by exposure to SARS-CoV-2 in the next future are T cell acute lymphoblastic leukemia, oligodendrogliomas, breast/ovarian cancers, sarcoma, malig-nant mesothelioma, B cell chronic lymphocytic leukemia, and non-small cell lung carcinoma, among the others.”
“SARS-CoV-2 by infection or vaccination ends to divert onto available immune determinants that, in the present case, are the common determinants present in the tumor-suppressor human proteins. Pathologically, one has to consider that usually an anamnestic secondary immune response is char-acterized by high avidity and high affinity, besides being quantitatively relevant. Therefore, as a final result, exposure to SARS-CoV-2 by infection and/or vaccination can trigger immediate and violent cross-reactive attacks against the proteins that protect the human being from carcinogenesis.”
Breast cancer often has peak incidence with "carcinogen associated incidents" and the predictive power of lymphocytes:
Cancer Morbidity among Methyl Isocyanate Exposed Long-Term Survivors and their Offspring: a Hospital-Based Five Year Descriptive Study (2006 - 2011) and Future Directions to Predict Cancer Risk in the Affected Population
Abstract
The purpose of this study was to update both researchers and clinicians about the cancer incidence in methyl isocyanate (MIC) exposed long-term survivors and in their offspring, focusing on the etiological plausibility. In the time period 2006-2011, cancer morbidity was evaluated in the population surviving after exposure to (MIC) on December 3rd, 1984, in Bhopal. This descriptive study is based on hospital registration of 1261 cancer patients those are MIC gas victims and their subsequently born offspring. Morbidity status was studied on the basis of gender, age, organ and site with relative percentages. Cancers on specific sites, with special reference to breast (n=231) (18.31%), lung (n=103) (8.16%), tongue (n=103) (8.16%), buccal mucosa (n=94) (7.45%), cervix (n=72) (5.70%), and esophagus (n=68) (5.39%) were found in high proportions. Ovary (n=43) (3.40%), brain (n=42) (3.33%), larynx (n=40) (3.17%), non-Hodgkin’s (n=31) (2.45%), gallbladder (n=29) (2.29%), stomach (n=28) (2.22%), head and neck (n=28) (2.22%), liver (n=27) (2.14%), acute lymphoid leukemia (n=24) (1.90%), rectum (n=20) (1.58%), colon (n=20) (1.58%), chronic myeloid leukemia (n=17) (1.34%), alveolus (n=17) (1.34%), Hodgkin’s (n=14) (1.11%), uterus (n=14) (1.11%), multiple myeloma (n=14) (1.11%), and prostate (n=11) (0.87%) lesions were observed less frequently. Remarkably, gradual increase of cancers on different organs and sites were observed in the long-term survivors and their offspring. The present study observed some cancers which were not previously reported in this population.
Full paper:
https://pubmed.ncbi.nlm.nih.gov/22471495/
Update 30th March.
Big hat tip to Walter P Chesnut. Is priongenic spike protein inducing misfolding of P53, leading to amyloidosis as well as carcinogenesis?
https://threadreaderapp.com/thread/1508976433418362880.html
Nosocomial Pneumonia Elicits an Endothelial Proteinopathy: Evidence for a Source of Neurotoxic Amyloids in Critically Ill Patients
https://www.atsjournals.org/doi/10.1164/rccm.201801-0060LE
https://twitter.com/Parsifaler/status/1508939763394523143?s=19
Amyloidogenicity of p53: a hidden link between protein misfolding and cancer
Hao Gong et al. Curr Protein Pept Sci. 2015.
Abstract
Pathogenic aggregation is closely associated with various protein misfolding diseases such as type 2 diabetes mellitus and Alzheimer's disease. Amyloidogenic proteins that have a propensity to assemble into amyloid oligomers and fibrils form the aggregates. The tumor suppressor p53, a transcription factor that regulates the cell cycle and apoptosis, is also amyloidogenic. In tumor models, both wild type and mutant p53 proteins show aggregation kinetics and morphology similar to those of classical amyloidogenic proteins, such as β-amyloid peptide and α- synuclein. Wild type p53 loses its anticancer activity when it aggregates, while p53 mutants with enhanced amyloidogenicity show accelerated aggregation. So far, amyloidogenic p53 mutations have been implicated in more than ten different types of cancer, suggesting a connection between p53 aggregation and cancer. Therefore, inhibition of both inherent and mutation induced p53 aggregation may stabilize p53 in a functional conformation and provide a novel approach to cancer prevention and treatment. Here, we summarize recent findings on carcinogenic aggregation of wild type p53 and its clinical mutants, structure-dependent amyloidogenesis of p53, and several promising strategies based on inhibition of p53 aggregation are also discussed.
https://pubmed.ncbi.nlm.nih.gov/25692950/
This is alarming, and goes some way to answer a question. But how does it relate to transfection? I believe we are seeing the answer to that.
p53 amyloid formation leading to its loss of function: implications in cancer pathogenesis (2015)
"Amyloid forms of proteins have also shown the ability to ‘seed’ or initiate the aggregation of corresponding native protein molecules in the cellular milieu. More importantly, several amyloids possess prion-like ‘infectious’ properties wherein they can amplify themselves and transmit between cells, thus resulting in an extensive dissemination of the disease. In this context, it has been suggested that p53 aggregates possess prion-like properties in cancer"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5596421/#__ffn_sectitle
We DO have a connection…
https://twitter.com/danelledubs/status/1509133838462631951?s=19
To conclude this Substack, are any clinical outcome signals being seen due to this pathophysiology?
I discussed these previously and if the data from the younger cohorts in military service and reports from clinicians and pathologists are representative then it is looking like its potentially a public health disaster, especially as such signals are being seen at such an early stage as most tumors take years to develop, but aggressive relapses are also being reported.
Kathryn asked: “How quickly do tumours develop?”
The short answer is it varies from tumour to tumour. But overall, it’s slower than you might expect.
According to Professor Trevor Graham, a Cancer Research UK-funded cancer evolution expert, the best evidence for the fact that most cancers grow slowly comes from screening. The screening programmes we have in the UK work “because there’s a long period of time when a tumour may have started to develop but it’s not become dangerous yet”, he says.
Take bowel screening for example. For every cancer that’s picked up during bowel cancer screening, 4 early tumours called adenomas are detected. Although most will never go on to become cancer, some will.
“It seems that tumour growth is started in lots of people, but it never quite makes it to the cancer stage,” says Graham. “That really points to growth often being slow.”
And this doesn’t just apply to bowel cancer. Similar trends are seen in breast cancer, where 1 in 4 breast cancers picked up during screening would never have caused any problems, which points to them being slow growing.
And even with cancers that often seem to be fast growing and aggressive, such as pancreatic cancer, Graham says this could just be down to the point at which they’re detected.
“We think that pancreatic cancers are often detected quite late, so they’ve been growing and evolving for a while. There might be a long and fairly slow development phase, but the first part is just invisible to us,” he says.
But like all good rules of thumb, there are exceptions. Graham works with people with inflammatory bowel disease, who have a higher risk of bowel cancer and are offered more regular bowel screening. This gives researchers a better idea of when tumours have begun to grow.
“Around 1 in 6 cancers develop in the 3 years between screens,” he says. “They won’t be there the first time we test, but they’ll develop before the second time. So they’ve developed relatively quickly.”
https://news.cancerresearchuk.org/2018/10/18/science-surgery-how-quickly-do-tumours-develop/
“Based on data from the Defense Medical Epidemiology Database (DMED), Renz reported that these whistleblowers found a significant increase in registered diagnoses on DMED for miscarriages, cancer, and many other medical conditions in 2021 compared to a five-year average from 2016-2020. For example, at the roundtable Renz stated that registered diagnoses for neurological issues increased 10 times from a five-year average of 82,000 to 863,000 in 2021. There were also increases in registered diagnoses in 2021 for the following medical conditions:
Hypertension – 2,181% increase
Diseases of the nervous system – 1,048% increase
Malignant neoplasms of esophagus – 894% increase
Multiple sclerosis – 680% increase
Malignant neoplasms of digestive organs – 624% increase
Guillain-Barre syndrome – 551% increase
Breast cancer – 487% increase
Demyelinating – 487% increase
Malignant neoplasms of thyroid and other endocrine glands – 474% increase
Female infertility – 472% increase
Pulmonary embolism – 468% increase
Migraines – 452% increase
Ovarian dysfunction – 437% increase
Testicular cancer – 369% increase
Tachycardia – 302% increase2
As advised above, mass cancer screenings should be commenced forthwith and all experimental gene therapies withdrawn immediately pending further investigations by non-conflicted parties.
Thread is far too long to screenshot. But loads of PE's, MI's, Afibs, miscarriages, rapid onset cancers & relapses to stage 4, esp female as P53 path predicted, autoimmune lupus like disorders....
https://twitter.com/AmericaLovingRN/status/1506085454180986886?s=19






















This needs to be WAY more widely discussed…
Why has this stack not garnered more eyeballs on this piece!!!! 🤔🤔🤦♀️🤦♀️🤦♀️
Hang in the Doorless, you do phenomenal work, which SHALL be widely seen in coming months.🙏🙏🙏📣📣📣☎️☎️☎️☎️🎩🎩🎩😊