Self-amplifying mRNA (saRNA) Part 1: The Japanese registration documents
Dr Stebel's walkthrough, translated and annotated by DC
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
EU wants to approve saRNA Kostaive Part 1
Japanese registration documents page 1-12 (of 68)
EU wants to approve saRNA Kostaive Part 2
Japanese registration documents page 13-18 (of 68)
EU wants to approve saRNA Kostaive Part 3
Japanese Regulatory Documents page 19-33 (of 68)
EU wants to approve saRNA Kostaive Part 4
Japanese registration documents page 34-57 (of 68)
EU wants to approve saRNA Kostaive Part 5
Japanese Registration Documents Page 58-End
EU wants to approve saRNA Kostaive Part 6
Post-marketing data of the two-month follow-up and Japanese manufacturer information
Introduction
Backstory
“The lady doth protest too much”.
How I was fact-checked by a founder figure from the saRNA cult, and this led me to start asking questions:
The Kostaive approval story
On 27th November 2023, vaccine manufacturer CSL announced that Japan's Ministry of Health, Labour and Welfare had approved the first self-amplifying mRNA COVID vaccine, “ARCT-154”, for adults1. CSL worked on this as a joint venture with Arcturus Therapeutics.
Moving forward to 10th December 2024, Ghostfromthefuture tagged me and asked me about the history of ARCT-154. Further reading revealed that CSL Seqirus also made the AstraZeneca COVID vaccine2 (now withdrawn as unsafe and ineffective) and that in November 2022 they inked a deal with Arcturus worth a cool $4.3 Billion3.
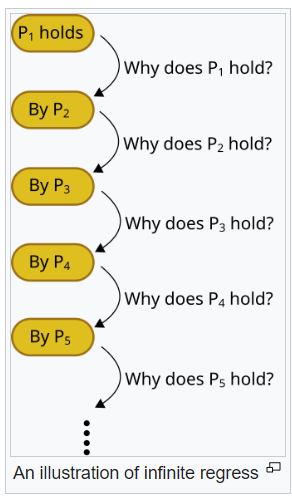
With that much capital invested something as trifling as regulator intervention will have already long been considered and excluded as a significant risk to the project. And the potential future earnings from this and other “turtles all the way down”4 bridged derivative products could potentially be huge.
The tower of turtles is a cross-reference of acceptability (i.e. infinite regress), with no underlying long-term data available of significance to support any such claims for either safety or effectiveness.
It’s already happening.
While researching my literature review for Part 2, I came across this paper, published in September:

This Substack is a translated, consolidated version of the walkthrough of the Japanese registration documents by Dr Sabine Stebel. A Biologist, her expertise is in protein engineering and directed evolution.
In short, it’s a pre-approval by the regulator for CSL-Arcturus to proceed straight to commercial manufacture, to put their product in the arms of most Japanese adults, and all this with no long-term clinical trial data available. Or did they borrow Ralph Baric’s time machine?
The Japanese approval report is an empty vessel of a document that makes a lot of noise but lacks substance. It defers many of the regulatory requirements for information to the future - i.e. via “post-marketing reports”.
The justification for this dereliction of duty is mostly by reference to Moderna and Pfizer’s mRNA COVID products, laughable as this is, given their abysmal track record.
Sabine’s work provides an excellent context for Part 2, which reviews research that helps to answer if saRNA technology is a friend or foe of cancer? I will post this as a follow-up Substack, given the length.
Discussion
Sabine asked if I had any European public assessment reports (EPAR) for saRNA products. I couldn’t find any, because EPARs are a set of documents describing the evaluation of authorised medicines, and these haven’t been approved in Europe yet5.
The next step would be to review the Japanese equivalent. I found this link to a pdf, and fortunately, it didn’t require translation. Their version of the European Medicines Agency (EMA) is the Pharmaceuticals and Medical Devices Agency (PMDA).
PR Newswire’s take on the approval:
The approval is based on clinical evidence supporting the safety and effectiveness of CSL and Arcturus Therapeutics' sa-mRNA COVID-19 vaccine, including published data demonstrating superior immunogenicity to Omicron BA 4/5 compared to a conventional mRNA COVID-19 vaccine booster and follow-up data demonstrating duration of immunity lasting up to one year.
From: “Japan's Ministry of Health, Labor and Welfare Approves CSL and Arcturus Therapeutics' Updated Self-amplifying mRNA COVID-19 Vaccine for Protection against JN.1 Strain, to be Distributed in Japan by Meiji Seika Pharma“ (13th September 2024)
Initial thoughts on the report, after a quick readthrough:
So because nucleic acids are “natural” that poses no concern?
Seriously?
I think their only concern was the dollar signs.
Never tested in humans or primates for teratogenicity.
Kostaive = ARCT-154
Note: ARCT-021 is the LNP carrier.
Do you see the logic here? The approval comes first, the safety data is later via post-marketing reports. Really?
= Presumed safe.
It is not classified as a biological product, presumably to bypass associated restrictions and safeguards.
"Conditional approval", but in reality, they won't backtrack.
Dr Stebel’s 6 Substack walkthrough is well worth reading in full, and we considered co-authoring the work. Instead, to save time, Sabine let me translate and reproduce her work in English, in one Substack, translated from German and Japanese, as needed.
The findings only get worse with a deep dive. One reader commented that they thought the approval had been written with the assistance of AI and that probably no one had read it. I couldn’t disagree.
If you think of the so-called “regulators” as being sales reps for the medical industry things begin to make a lot more sense.

A walkthrough of the “Report on the Deliberation Results”
EU wants to approve saRNA Kostaive Part 1
Japanese registration documents page 1-12 (of 68)
Approval Conditions - First vaccinate, then see how harmful it was and whether it worked at all
Intramuscularly, of course, exactly the application for which the lipid of the pKs is NOT suitable.
Just by the way:
ATX-126 and potassium sorbate have never been used for intramuscular injections before. Where are the toxicity studies? I'm only asking for a friend.
I make homebrew wine, meads & melomels (fermented honey). Potassium sorbate may be used as a fermentation stopper to reduce the risk of creating bottle bombs.
But after reading papers about sorbate I won’t be using it.

It renders yeast inactive by causing severe mitochondrial damage6. It’s also carcinogenic through causing chromosome aberrations, chromatid exchanges7 and mutations, due to its ability to enhance mitochondrial ROS (free radical) production.
The legal limit for potassium sorbate in the US is 300 mg/l when used as a wine stabilizer8 and 1000 mg/l when used as a preservative in fruit butters, jellies, preserves, and related products9.
I subscribe to the multihit model of cancer10, whereby although the contribution of a single factor may not reach statistical significance in a controlled laboratory study, it may become significant in vivo due to synergistic effects. For example:
Sorbate and nitrite form several species of direct-acting mutagens and genotoxic agents when present together at pH's mimicking gastric conditions. Two of the mutagens have been identified as ethylnitrolic acid and 1,4-dinitro-2-methylpyrrole. Mutagen formation is blocked by ascorbate at low pH. Ascorbate at eightfold molar excess leads to inactivation of 1,4-dinitro-2-methylpyrrole near neutral pH but does not destroy the mutagenic nitro compound at low pHs. The combination of sorbate with nitrite represents a potential health risk in the absence of adequate inactivating levels of ascorbate (vitamin C).
From: “Review: putative mutagens and carcinogens in foods. II: sorbate and sorbate-nitrite interactions“ (1983)
I recommend ignoring the MSM, managed AI and government regulatory advice and trying to limit your exposure to all such factors as much as possible.
These also include glyphosate11, BPA12, Teflon (PTFE)13, aluminium14, Flouride15 etc. Sorbate has no place as a novel excipient in a vaccine.
On the pharmacokinetics of Kostaive, they indeed use unscientific “weasel words” that don’t tell you - inform you - of anything useful, but sound impressive:
No independent safety pharmacological studies because rabbits tolerated the product well...
I would like to remind you of the TGN1412 disaster15 16. Animal experiments say NOTHING about safety in humans. TGN1412 was "only" an antibody and not a complex genetic prodrug.
However, the authority saw it differently than I did:
I already mentioned that you have to keep an eye out for weasel words. Here we have two:
"… is expected to show" is not the same as "shows that it is effective".
"… no particular special safety concerns" is not the same as "there are no safety concerns".
Who needs non-clinical pharmacokinetic studies of a complex product?
Even normal modRNA has complex pharmacokinetics without officially replicating itself (which it does).
Pharmacokinetics are completely overrated, who needs that?
And again the game, "because it is a (genetic) vaccine, we can simply omit certain studies":
This product is also distributed throughout the body and was no longer detectable in plasma after 31 days. Well, it was still in the muscle and in the lymph nodes as well. However, the authors of the document do not write when it was out.
After all, it doesn't seem to go into the brain and testicles but otherwise everywhere and there it stays for 2 months (with the mouse).
That was the first 12 pages and it's creepy.
So that it doesn't get too long, I'll divide it into several articles.
Conclusion page 1-12
Spike protein is used again as a false antigen.
Lipids diluted with NaCl, an ionic solution, are unacceptable
False pKs for intramuscular injection
Lack of toxicological and pharmacological studies
Was simply introduced in Japan during ongoing studies.
Animal models and their respective use are doubtful.
Again, only IgG measured.
Distributed throughout the body and detectable for 2 months
https://drbine.substack.com/p/eu-will-sarna-kostaive-zulassen-teil
EU wants to approve saRNA Kostaive Part 2
Japanese registration documents page 13-18 (of 68)
Where and for how long were the spike protein and the ionizable lipid found in the test animals?
Nothing new here. Same same but different.
The spike protein was found after only 24-48 hours in
the muscles
of the lungs (spike protein measurable after only 2 hours)
the lymph nodes
the ovaries (spike protein measurable after just 2 hours)
and plasma
After 15 days, it was still found in the lymph nodes after a dose of 25 μg (but only once and because once is none, you can probably ignore it) and after a dose of 25 g the spike protein was still detectable in the lymph nodes. After 8 days, the spike protein could no longer be detected in the lungs and ovaries. The damage caused by the protein over time (it fuses cells together and cells that produce foreign proteins are killed by the immune system) has not been investigated.
Rabbits are killed after 31 and 57 days.
After 31 days, the saRNA was still detectable in the muscle and in the spleen, but no longer in other tissues.
After 57 days, the saRNA was no longer detectable even in rabbits.
However, the ionizable lipid ATX-126 was still detectable in plasma, muscle, lymph nodes and ovaries.
Now the question is how the manufacturers themselves have detected the lipid. That's very interesting, BioNTech/Pfizer and Moderna didn't do that. I don't know if there are detection methods for ALC-0315 and SM-102 to see if they accumulate in the liver.
The lipid goes everywhere, but no toxicologically significant histopathological findings were observed in these tissues 31-57 days after injection. Rabbits therefore seem to tolerate the lipid to such an extent for the time being.
However, the fact that the lipid can be found everywhere also means that the RNA reaches every organ. This document deals with this very openly and pleasingly honestly.
I would add that if mutations have been induced or occurred naturally in these tissues then it is far too soon for malignant transformation to occur. Even abnormal cell nuclei may not be visible in this period.
The same may be said for other long-term conditions such as autoimmune antibody-associated disorders, or fibrosis.
2-year continuous-exposure rodent bioassays (RCBs) are the benchmark method to screen for human carcinogens16, with lifetime-exposure studies being the ideal17.
Is the ionizable lipid and RNA degraded?
We don't have any data, we haven't even bothered to measure it, we just assume that the mRNA and the S protein are already broken down by the body somehow.
That confuses me now. First they say that the metabolites of the ionizable lipid are excreted in urine, and now it says that no excretion studies have been conducted. That contradicts each other.
Non-clinical pharmacokinetics
But I am reassured that there are no particular problems. What about the normal problems that aren't special?
No special problems is not the same as no problems.
There are problems.
Just not special ones, but the usual problems inherent in technology.
This is not particularly reassuring.
"Because of the absence of reverse transcriptase, sequences of mRNA-2105 are not incorporated into host cell DNA."
Pure, easily refutable assertion. In addition to the good old Line-1, there are the following reverse transcriptases in human cells:
Ribozymes are RNA molecules with catalytic activity (https://flexikon.doccheck.com/de/Ribozym). There are probably also some that function as reverse transcriptase. A reverse transcriptase ribozyme - PubMed (nih.gov)
polymerase η (eta): Human DNA polymerase η has reverse transcriptase activity in cellular environments - PubMed (nih.gov)
Polymerase θ (theta): Polθ reverse transcribes RNA and promotes RNA-templated DNA repair - PubMed (nih.gov)
In addition, the RNA does not have to integrate at all to cause damage2, fragments that act as microRNAs and switch genes on and off uncontrollably are sufficient. This was awarded the Nobel Prize in 20243.
In addition, the manufacturer itself has already admitted that the product contains residual plasmid DNA:
That's DNA, that's how it integrates.
This contradiction, this incompleteness, should have been noticed by the reviewers.
"Kostaive is an LNP-formulation of mRNA-2105. After intramuscular administration, it is expected to be incorporated by endocytosis into muscle cells and antigen presenting cells (e.g., dendritic cells) at the administration site (Vaccines. (Basel) 2019;7(4):122, Vaccines. (Basel) 2021;9(2):147, etc.). mRNA-2105 released from LNP into cytoplasm is expected to be amplified by replication mechanisms of VEEV replicase19) and translated into S-protein antigen by ribosome within the cell, thereby exhibiting the expected effect. The amplified mRNA and the translated protein within the cell are expected to be degraded and removed by intracellular nuclease and protease."
Weasel words again.
"… expected to" means nothing other than, "we hope or suspect that", because we have not measured that. It may be that the protein is not produced in the cytoplasm but in the endoplasmic reticulum and then shedded via exosomes. They also expect the replication mechanism (the built-in copying machine) to increase the mRNA (for how long? how often?), thereby enhancing the effect. What the expected effect is, is not specified. Possibly the shortening of the life of the injected? And then you expect that the copy machine and the spike protein will somehow, magically, disappear into thin air.
“Usually, administered mRNA is rapidly metabolized as is the case with nucleic acid in the body, whereas mRNA encapsulated in LNP is incorporated into cells without being metabolized. The biodistribution of LNP-encapsulated mRNA depends mainly on the components and particle size of LNP but not on the incorporated mRNA itself.”
Free mRNA is immediately destroyed by the body, RNA encapsulated in LNP enters the cells of each organ uncontrollably, no matter what sequence is used.
Pfizer’s engineered mRNA also gets distributed everywhere, including across the blood-brain barrier (BBB), via exosomal trafficking18.
“Placental transfer of Kostaive was discussed based on the results of the fertility studies, embryo-fetal studies, and developmental toxicity studies on ARCT-021 .”
Without analyzing the original animal study data, these statements are pretty worthless.
“mRNA-2002 was undetectable in placenta or fetal tissues in any treatment group studied, except in the plasma (8.19 pg/mL) of a fetus in 1 of 20 samples in the 10 µg group. ATX-126 was detectable in placenta in the 10 and 20 µg groups (275.0 ± 33.9 ng/g in the 10 µg group, 387.6 ± 117.8 ng/g in the 20 µg group), but not detected in plasma or tissues of fetuses in either of the groups. The above results suggest that ARCT-021 is unlikely to be transferred from maternal animals to fetuses. The same is considered to apply to Kostaive.”
We have detected mRNA-2002 in a fetus and the lipid in the placenta of some animals. In those where the lipid was detected in the placenta, we did not find the lipid in the fetus. What about the breakdown products of the lipid? Have they been found in the fetus?
And because we found our mRNA in a fetus, we conclude that the product is not transferred to fetuses.
Yes, of course, you can see it that way.
How many animals were there? 20?
If this was already the case with only 20 animals, I would not support the conclusion of low probability.
1 in 20 is a common side effect!
In a case of 10,000, it would still be a rare risk and the authors know that, so they use subjunctives and words like "probably not" at one in 20.
So if the child is transfected in every twentieth pregnancy, this is a "probably not transmitted from mother to fetus" for the authors.
However, the authority concludes:
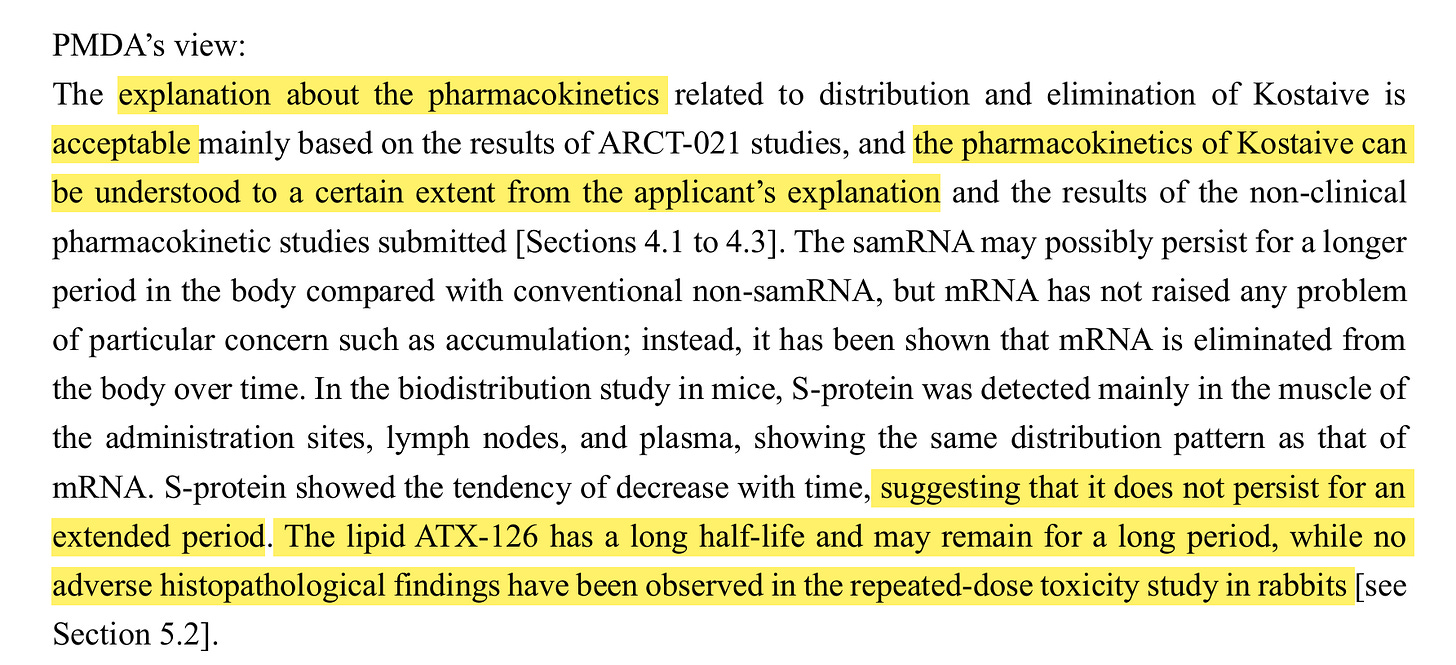
"The explanation about the pharmacokinetics related to distribution and elimination of Kostaive is acceptable mainly based on the results of ARCT-021 studies, and the pharmacokinetics of Kostaive can be understood to a certain extent from the applicant’s explanation and the results of the non-clinical pharmacokinetic studies submitted."
You get a rough impression of what might be happening.
"… acceptable" and "can be understood to a certain extent" do not exactly sparkle with conviction on the part of the authority.
“S-protein showed the tendency of decrease with time, suggesting that it does not persist for an extended period. The lipid ATX-126 has a long half-life and may remain for a long period, while no adverse histopathological findings have been observed in the repeated-dose toxicity study in rabbits.”
The spike protein tends to degrade at some point. Sometime... or not over a longer period of time. Can it be even more vague?
After all, the lipid probably stays in the liver and spleen for a long time, but what the hell doesn't seem to hurt at least 60 days and after that, time will tell.
Drug safety at its best.
Studies into genotoxicity were not required by the “regulator” because “… Kostaive consists of natural nucleic acid”.
Kevin was not impressed:
Genotoxicity and carcinogenicity
Because the mRNA does not contain N1-methyl pseudouridine, the modRNA and the new substances have not yet been tested by anyone on it, we have not done any studies.
Note: “Plörre” in German doesn’t have a direct English equivalent, but it means words like “dishwater”, “stale beer”, “slop”, and “burned coffee”.
You get the picture!
Since the Plörre is not observed experimentally for more than 6 months, we have not (yet) taken the trouble to look for cancer. It can still come, but then it will be too late anyway.
Reproductive toxicity
Reproductive toxicity has been done in rabbits.
Why not in the huACE2 mice?
It has been proven that huACE2 mice were used.
Why were rabbits taken that have a rabbit ACE2 receptor in the placenta and no huACE2 receptor?
How can one conclude reproductive toxicity in this way?
The animal model is completely unsuitable for this.
Due to the wrong animal models, no toxicity could be seen, so everything is OK.
Clinical Pharmacology
What is clinical pharmacology?
According to DocCheck: "Clinical pharmacology is a subfield of human medicine with numerous overlaps with pharmacy. Clinical pharmacology focuses on the research, documentation and evaluation of medicinal products of all kinds in their application to patients. The focus of this department is on drugs both before and after their approval. Clinical pharmacology can be seen as a link between the clinic and pharmacology. The aim is to ensure the gentlest and most effective pharmacological treatment possible for patients."5
Wikipedia defines clinical pharmacology as follows:
"Clinical pharmacology is a field of human medicine. It deals with all aspects of the use of drugs in humans, both before (phases I–III) and after approval of a drug (phase IV). Clinical pharmacology thus closes the gap between basic pharmacology and medical practice. Their main goal is to use medicines as effectively and safely as possible. In clinical practice, close cooperation with clinical pharmacy is important."6
"The most effective and safe use of medicines" does not seem to me to be acutely important. That's why they saved themselves directly.
Result
A lipid that is difficult to break down, whose unspecified degradation products are excreted in the urine.
Spike protein in all organs for days. This does not happen with a normal infection.
Although it has admitted that it is contaminated by plasmid residues, it is claimed that the mRNA does not integrate and ignores at least 4 reverse transcriptases in the human cell.
In one in 20 pregnancies, the RNA was detected in the fetus, which corresponds to a definition of "common", but is interpreted by the manufacturers as "does not occur". The ionizable lipid goes into the placenta. What about the breakdown products of the lipid?
Reproductive toxicity has not been studied in animals with humanized ACE2.
Genotoxicity and carcinogenicity have not been investigated.
Clinical pharmacology has been completely dispensed with.
6 pages full of catastrophic data.
That was "only" 6 (SIX) pages.
I would have slapped the applicant around the ears and sent him home humiliated.
https://drbine.substack.com/p/eu-will-sarna-kostaive-zulassen-teil-e46
EU wants to approve saRNA Kostaive Part 3
Japanese Regulatory Documents page 19-33 (of 68)
This part of the review focused on clinical trials. By now you won’t be surprised that they haven’t moved on from 2020. Their obsession is still with antibodies/schmantibodies, which aren’t the correlate of long-term immunity. And IgG4 is the disgraced uncle that no one wants to talk about.
Before the rollout of the first COVID vaccines, a panel of experts was interviewed for their comments about the following statement about antibodies and immunity:
In the WHO’s (World Health Organisation’s) daily press conference on 17th April, Dr Maria van Kerkhove the WHO’s technical lead for COVID-19, said that there was no evidence yet that antibody tests can show that an individual is immune to or protected from the disease.
Prof Adam Finn, Professor of Paediatrics, University of Bristol, said:
“In people who have had the virus, there is specific immunity – immunity to a particular thing – which is made up of two parts: B cell responses (antibodies) and T cell responses (which don’t involve antibodies). Both are involved in the immune response to the virus and for this virus we don’t know how important each type is, but most likely both matter to some extent. These parts of the immune system respond to different components of the virus called antigens and different parts of each antigen, called epitopes or, in the case of T cells, peptides. When infected, you make responses to multiple epitopes from multiple antigens – some are useful in protecting against future infection and some are not.
“Most of the time we do tests for antibody responses. It’s possible to measure T cell responses too but more difficult to do and more difficult to interpret. But it’s important to realise that someone might fail to make protective antibody responses but still be protected by their T cells.
… Prof Andrew Easton, Professor of Virology, University of Warwick, said:
“An infection leads to the production of a range of antibodies directed against the infectious agent, in this case the coronavirus. It is well understood that while some of these, called neutralising antibodies, have the ability to inactivate the invading virus, others do not, these are non-neutralising antibodies. The neutralising activity of different antibodies can use different mechanisms to prevent the virus from causing an infection.
… A serology test does not discriminate between neutralising and non-neutralising antibodies; a discriminatory test is much more complex and slow.”
… Prof Paul Hunter, Professor in Medicine, UEA, said:
… “It is possible that IgG antibodies (one class of antibodies), which an antibody test would probably measure, will indicate a past infection but may not protect against future COVID-19 infections as another class antibody (IgA) are more important in viral respiratory infections. However, immune responses to viral infections can be complex involving both the production of different antibody classes and cellular immunity.
… Prof Gordon Dougan, Department of Medicine, University of Cambridge, said:
“People are being tested in some countries e.g. China for antibodies to SARS2-Cov19 in their blood or other clinical samples using antibody tests. Provided the test is specific and sensitive (unfortunately many being sold are not!), it tells us if our immune system has seen (detected) the virus or that we have been exposed to it or infected. However, the presence of antibody in the blood to the virus itself does not mean people are protected in the case of COVID-19. Antibody for some diseases does not correlate with protection after infection or even after vaccination.
… “In addition to simply testing for antibody, we can test for antibodies that kill or neutralise the virus but those tests are not so easy to run and will not be offered widely. Even a neutralising antibody does not absolutely mean you are protected. There is still much to learn in this regard with COVID-19.
“For vaccine development, developers will try to use an immunological correlate of protection to license a COVID-19 vaccine. This could be a neutralising (blocking) antibody titre that prevents viral growth or even kills the virus. The problem is that for many vaccines neutralising titres do not completely correlate with protection e.g. rotavirus vaccines. Some viruses need intracellular as well as extracellular neutralisation complicating the situation. Not so simple I am afraid. We will likely know in the coming months where all of this ends up.
From: “expert reaction to comments made by Dr Maria van Kerkhove at the WHO that there is no evidence antibody tests can show that an individual is immune or is protected from the infection“ (April 18, 2020)
Now we know “where all of this ends up” - an ongoing public health disaster with many millions of iatrogenic deaths.
Sabine walks us through each of the four studies cited in the report. The TL;DR is that the side effects they looked for lasted for up to 28 days in one study, whereas antibody titers were highly variable and quickly waned in most:
ARCT-021-04
Samplesize: 600 subjects [300 aged 18 to 55 years, 300 aged ≥56 years; 150 each in groups A, B, C or D] at 15 study sites in the USA and Singapore.
Subjects were stratified by age (18-55, ≥56) and randomized.
The dosing regimen in each group was as follows:
Group A: A single dose of ARCT-021 7.5 μg followed by the administration of a placebo (physiological saline) after 28 days.
Group B: 2 doses of ARCT-021 5 μg 28 days apart.
Group C: 2 doses of ARCT-021 7.5 μg 28 days apart.
Group D: 2 doses of placebo 28 days apart.
Positive: The placebo was saline
Of 143 subjects who received ONE dose of 7.5μg, approximately 7% developed a fourfold increased antibody titer at baseline
Of 145 subjects who received TWO doses of 5μg, about 34% developed a fourfold increased antibody titer at baseline
Of 144 subjects who received TWO doses of 7.5μg, approximately 35% developed a fourfold increased antibody titer at baseline
Whether you get two cans of 5μg or 7.5μg hardly makes a difference, it depends on the two doses.
BUT
even after two doses, "only" 1/3 form a fourfold increased antibody titer at baseline.
You can also see that in group A, which received table salt as a second shot, the titer had already dropped massively again in 6 out of 10.
The effect is anything but long-lasting. What can be seen positively indicates that Spike will not be produced for too long.
BUT, if the "protection" is already dwindling so quickly, what is the point of the effort?
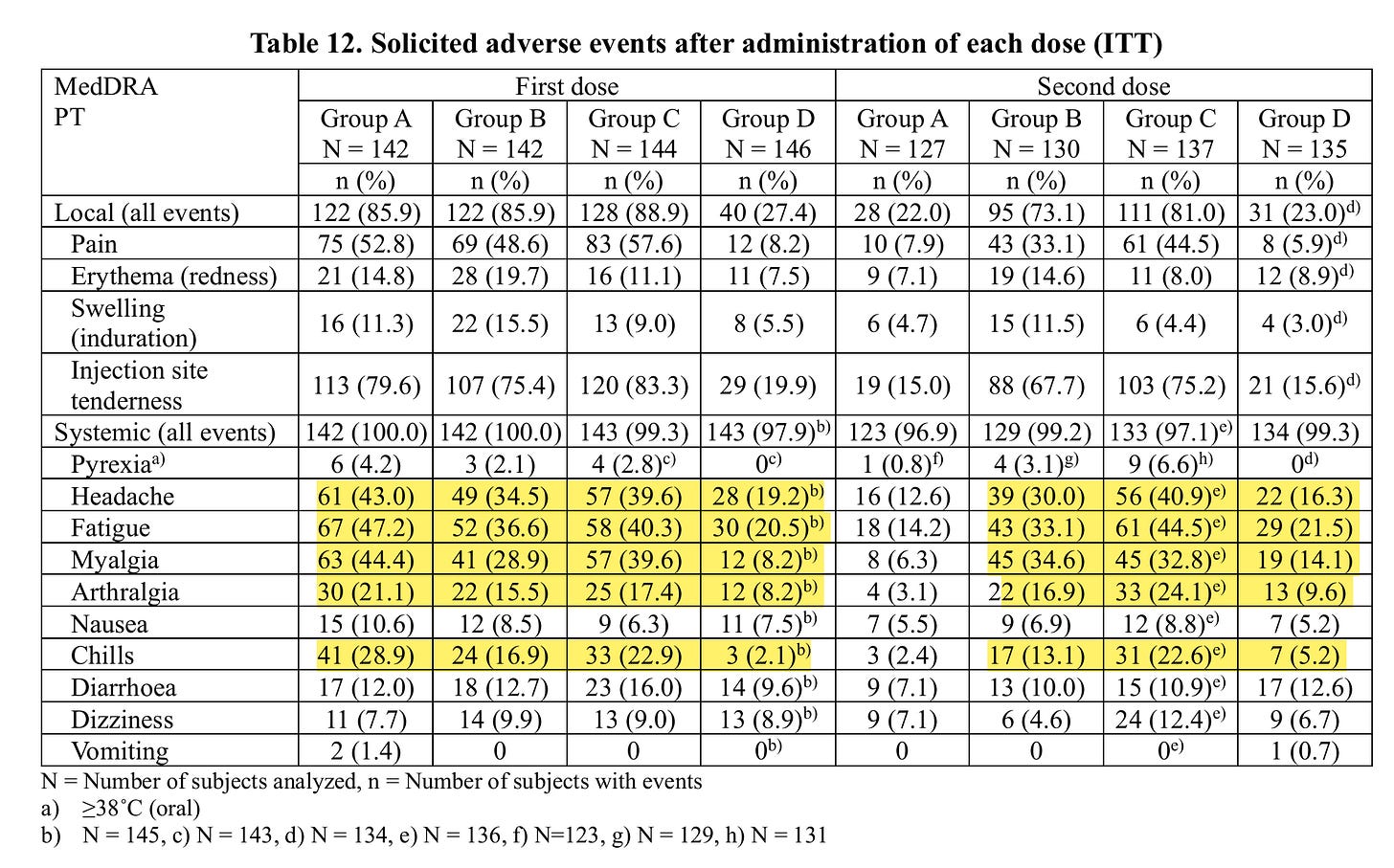
The side effects up to 7 days after the injection for this minimal, short-term effect can be found in this table:
D is placebo!
Pyrexia = fever
Myaligia = muscle pain
Athralgia = joint pain
Verum: Drugs that contain active pharmaceutical substances and are used in clinical trials.
All systemic side effects were significantly increased in A-C that got Verum the first time.
At the second dose, A and D received placebo and B and C verum
It is astonishing that the side effects are lower with A than in the pure placebo group, perhaps because after the side effects the first time you were so relieved that this time there was nothing to notice in comparison that you dealt with small aches and pains in a more relaxed way.
The side effects up to 28 days after the injection:
Looks inconspicuous overall.
There was also no death.
Result:
No information on how long the saRNA is supposed to "protect" at all.
There is no evidence that memory cells may or may not form in the bone marrow.
Partly quite strange study design, which I don't really understand from the document.
Antibody titers seem to drop again quickly, which I see as a good sign that spike production does not last long in most people.
In the short term, there are more side effects than the modRNA "classics", in the long term, the products don't seem to take anything away from each other, even in terms of their uselessness.
https://drbine.substack.com/p/eu-will-sarna-kostaive-zulassen-teil-3d9
EU wants to approve saRNA Kostaive Part 4
Japanese registration documents page 34-57 (of 68)
From this part, the summary of the PMDA (Pharmaceuticals and Medical Devices Agency) begins1 at.
One could say that the PMDA once again formulates in its own words how it understood the data of the applicant/sponsor. A lot of data is therefore repeated. So I'll leave them out of this article if I've already covered them in previous parts.
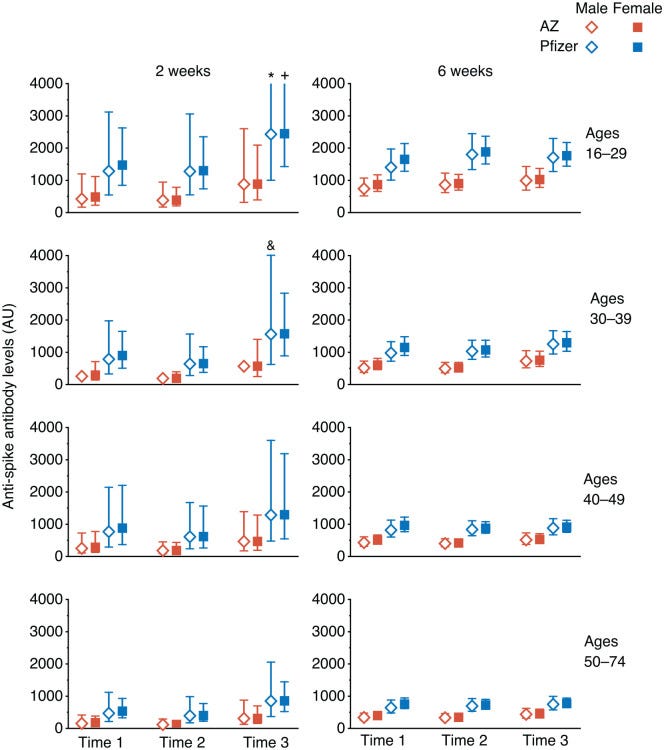
The PMDA decided that there was no need to study different ethnic groups because other vaccines didn’t demonstrate significant differences, therefore no ethnic groups need consideration. Riiight. This is absurd, especially when you consider that even the time of day of vaccination can have a significant effect on short-term immune response.
Time 3 on the right of the charts is from vaccinations given between 3 and 9 pm:
Or how entire tribes were wiped out by common diseases after contact with the first European settlers because their immune systems and T cell repertoires were entirely different:
It is estimated that 95 percent of the indigenous populations in the Americas were killed by infectious diseases during the years following European colonization, amounting to an estimated 20 million people.14 While Europeans coming to the New World had herd immunity or were asymptomatic carriers of many of these diseases, Native American populations, who were being exposed to pathogens for the first time, experienced deadly epidemics. This has been termed the “Virgin Soil Effect”.15
From: “A Historical Perspective of Healthcare Disparity and Infectious Disease in the Native American Population“ (2022)
What?! Do I understand that correctly? Because this stuff didn't kill Vietnamese, one concludes that there are no differences between Europeans or blacks?! For me, Vietnamese are still Asians and not long-noses or do so many different ethnicities live in Vietnam?
Although there is no immune correlate, the antibody titer is equated with immunity. They don't even distinguish between IgG and IgG4 and even ignore IgG4 completely. Work with professionals for once.
Turtles-on-turtles again. “Approved vaccines” are not valid controls:
“The clinical data package is designed based on a series of “Principles for the Evaluation of Vaccines Against the Novel Coronavirus SARS-CoV-2” issued by PMDA and is acceptable.”
“… the applicant’s policy to evaluate the immunogenicity using approved vaccines as the controls is acceptable.”
Acceptable.
Soso.
Compare his Plörre with another Plörre, which is useless and has no protective effect, in the same distorted way as the first useless Plörre obtained approval.
This is acceptable?
Yes, they used the early unblinded placebo trick again, lame as the placebo was due to them comparing vaccinated against vaccinated.
When you see columns labelled “placebo” in the tables think “ComiRNAty” instead.
What’s the excuse this time? There is none, other than it shows that they expected there to be a significant risk of severe AE reports and deaths if they let the efficacy surveillance period run for much longer than 2 months:
They even pull off the stunt with the dissolution of the placebo group for ethical reasons:
“In the circumstance where COVID-19 vaccine became available by the mass vaccination campaign, a long-term follow-up survey of a placebo-controlled study would be an ethical problem because subjects include elderly and those with co-morbid conditions associated with high risk of severe COVID-19. It was decided to limit the efficacy surveillance period to 2 months and to administer the study vaccine (Kostaive or placebo) alternately to all of the participating subjects.”
So did I understand the study correctly after all. There was no real placebo at all, at least not in the long term.
Again, the authorities contradict themselves.
If it’s only effective enough for 2 months it’s a drug, not an immunising vaccine.
In this table, the antibodies remain high longer with Kostaive than with Comirnaty.
THIS is NOT a good sign, unless saRNA would really form memory cells in the bone marrow, which we don't know (and memory cells wouldn't be good in that case either).
If the titer remains high for longer, spike protein must also be produced for longer and ensure the generation of antibodies in the organism. The antibody titers are a surrogate parameter for detecting spike load. Long, high titer thus corresponds to longer high spike loading.8
But, the titer also drops at some point with saRNA and not much slower than with modRNA, which doesn't take much.
I wonder if this is measured in BAU, as in our study. Unfortunately, the units are missing here.
The Japanese authority is of the erroneous opinion:
“GMC and SRR of the neutralizing antibody against the Omicron variant after the primary series were low.”
Low?!
Nowadays, you are already considered very well protected below 384 BAU, above which there is no longer an official measurement. What is low here?!
They just want to be able to continue boosters with this "reasoning":
“Since the neutralizing antibody titer against XBB.1.5.6 lineage of the Omicron variant is lower than the titer against BA.4-5 lineage, development of a vaccine against XBB.1 lineages will be necessary.”
Always keep the titer in a range that indicates that spike protein is still circulating and harming people in the long term. That's what I read out here.
In plain language: No idea whether the measurement contains any meaningful indications about the effect and even predicts that the saRNA is efficient. We don't know if a high titer offers more protection, so always keep the titer up with boosters.
How can you directly contradict yourself so often in consecutive sections in a document?
Has anyone ever proofread that?
Th1 dominant immune response, as in the adenovirus products.
BUT
What has not been measured?
IL-6, IL-10, autoantibodies, heart markers? The stuff does not cause heart damage, spike protein is also produced and a lipid that has not been tested for heart compatibility is used.
In other words, the substances that ultimately indicate the problems.
If you don't measure these substances, you won't find any problems.
So when lawyers tweet new markers9 or there are publications about new inflammatory markers, just collect the information. That would be really nice. And maybe you could tell the doctors (after someone forced out the PSURS via FOIA/IFG) at some point what kind of shit they injected their patients).
But we don't say that to the young people who don't need this plörre at all. Young people in particular should still get injected to protect the elderly. That's how it was, wasn't it?
Grade refers to the severity of the AE.
The CTCAE displays Grades 1 through 5 with unique clinical descriptions of severity for each AE based on this general guideline:
Grade 1
Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
Grade 2
Moderate; minimal, local or noninvasive intervention indicated; limiting age appropriate instrumental ADL*.
Grade 3
Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self care ADL**.
Grade 4
Life-threatening consequences; urgent intervention indicated.
Grade 5
Death related to AE19.
I wouldn't say it's just tendency.
I was always of the opinion that if you have to do statistics, you don't have any results. Real results can be seen in the raw data. Like here. You can see that very well in the raw data.
There was no clear difference in the incidence of adverse events between age groups, although a comparison is difficult due to the extremely limited number of older subjects studied.
I still see an age difference in the raw data.
Myocarditis/pericarditis, GBS and VAED
“Adverse events related to myocarditis/pericarditis, Guillain-Barre syndrome, or VAED/VAERD were not observed in any of the study data submitted.”
The weasel word in this sentence is "study data submitted". That doesn't mean that these cases didn't exist, they weren't submitted, just like Pfizer, where they were also embezzled.10
“Most of the adverse events observed were mild or moderate and transient.”
Just like the mild or moderate and transient myocarditis in modRNA products.
“Since Kostaive is considered to share a similar modality with RNA vaccines, vaccine in the same class, cautions against the risk of these adverse events are still required as are the cases with vaccines in the same class.”
“No significant difference was observed in the types or incidence of adverse events between the Kostaive group and the control vaccine Comirnaty group in the clinical study on booster dose.”
No differences between saRNA and the modRNA plörre is not good news when you know how bad the modRNA plörre is.
People with pre-existing conditions
Study ARCT-154-01 (summarized data from parts 1, 2, 3a and 3b before the crossover): In subjects with underlying diseases, adverse events occurred in 729 of 2,103 subjects (37.7%) in the Kostaive group and in 783 of 1,953 subjects (40.1%) in the placebo group. Adverse events occurred in 109 of 2,103 subjects (5.2%) in the Kostaive group and in 85 of 1,953 subjects (4.4%) in the placebo group. Adverse events leading to discontinuation of the study occurred in 3 of 2,103 subjects (0.1%) in the Kostaive group and in 18 of 1,953 subjects (0.9%) in the placebo group.
We also know something like this from the BioNTech/Pfizer studies, where there were "placebos" that were worse than Verum. How this could have happened is still unclear today. For a while you suspected that something was swapped. But it is strange to see this effect again. Here it often happens that the remedy has probably made people healthier in general.
Study ARCT-154-J01: In subjects with pre-existing conditions, adverse events occurred in 11 of 63 subjects (17.5%) in the Kostaive group and in 13 of 48 subjects (27.1%) in the Comirnaty group. Adverse reactions occurred in 9 of 63 subjects (14.3%) in the Kostaive group and in 8 of 48 subjects (16.7%) in the Comirnaty group, and adverse reactions occurred in 9 of 63 subjects (14.3%) in the Kostaive group and in 8 of 48 subjects (16.7%) in the Comirnaty group. There were no serious adverse events or adverse events that resulted in death.
Badmouthing the competition is part of good manners. But now even T-online says that the side effects are stronger with saRNA than with modRNA.11
Pregnant and breastfeeding women
Pregnant Women In Study ARCT-154-01
“In the animal study conducted using ARCT-021, a vaccine manufactured based on the same platform as Kostaive, no direct or indirect adverse effect was observed on the fertility, embryofetal development, delivery, or postnatal development.”
Different vaccine, no spike protein and probably no huACE2 mice so completely useless.
“In non-clinical studies, vaccination with Kostaive did not pose any safety concerns for the parental animals or for the off-spring [see Section 5.5], but the applicant’s explanation is understandable because there is no experience of vaccination with Kostaive in pregnant or lactating women and excretion of Kostaive in milk has yet to be investigated and unknown. The same precautions as those for approved RNA vaccines should be raised for pregnant and lactating women.”
So no precautions at all. Cleverly formulated.
But still:
“Still, given the limited data available on the safety of using Kostaive during pregnancy, safety for pregnant women has not been established. The use of Kostaive during pregnancy should be considered only when potential benefits outweigh the potential risks for the mother and the fetus.”
And that's where the dog is buried:
The alleged benefit in relation to the ignored risk.
Breastfeeding women
“Lactating women were not included in clinical studies of Kostaive. Whether Kostaive is excreted into breast milk is unknown. The clinical necessity of Kostaive and its potential influences on breastfeeding should be considered when vaccinating lactating women with Kostaive.”
No data, but if the doctor considers the benefit to be great...
The usual game that we already know from modRNA.
Since it is LNP, the results will be very similar to the "classics".
Cross-vaccination yes/no?
“Taking account of the observations that the immunogenicity and safety in these clinical studies were similar regardless of the number of times of SARS-CoV-2 vaccination before the study, together with the findings on approved RNA vaccines in the same class (JAMA. 2023;6:e232598, MMWR. 2022;71:971-76), it is acceptable to provide booster doses of Kostaive without any specific limitations to the number of prior SARS-CoV-2 vaccinations.”
What now? Two completely different statements in one document. Has no one proofread it?
“These data on approved vaccines suggest the efficacy and safety of the booster dose with Kostaive regardless of the type of the vaccine administered in the past. Nevertheless, the fact that Kostaive has never been administered to individuals with the history of receiving SARS-CoV-2 vaccines other than RNA vaccine will be included in the package insert to raise caution.”
So somehow there is (no) data or something, so we write a precaution in the package insert that we don't have to include at all.
Also a solution.
How often can you take the shot?
“PMDA’s view:
SARS-CoV-2 infection status in Japan 50 ) does not necessitate vaccination every 3 months, and individuals vaccinated ≥3 months before were enrolled in Study ARCT-154-J01, and results did not show any clear difference in the immunogenicity or safety between groups with different vaccination intervals. Also, given that approved RNA vaccines can be administered at ≥3 month intervals, it is acceptable to establish the interval of vaccination with Kostaive at ≥3 months, as proposed by the applicant.”
So, put it in every 4 months?!
It doesn't get any more vague than that.
Post-marketing study
There should be a post-marketing study, where is it accessible?
GLP
We are still looking at GLP (good laboratory practice) and GCP (good clinical practice). We don't have any final results yet.
Overall assessment
No particular problems.
The weasel-word is "special". Since they have already compared themselves with Comirnaty, no special problems are to be expected in a direct comparison.
https://drbine.substack.com/p/eu-will-sarna-kostaive-zulassen-teil-02a
EU wants to approve saRNA Kostaive Part 5
Japanese Registration Documents Page 58-End
I’ve reproduced Part 5 in full here.
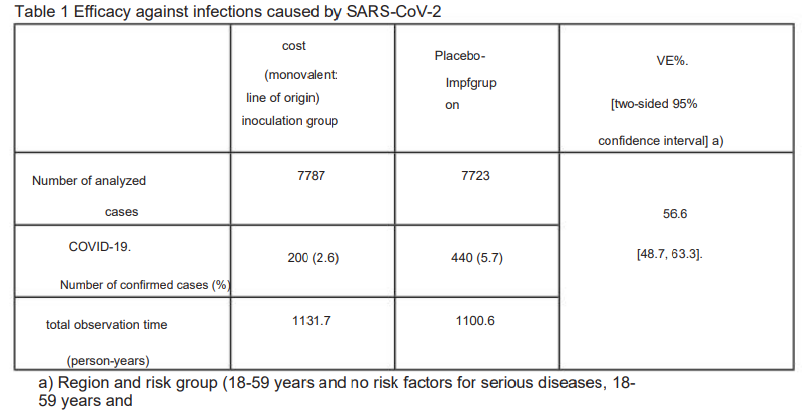
I would be interested in the mechanisms of action too. It might be important, you never know, especially due to the “turtles” tower of approvals that will follow this train wreck of uncertain pathologies, unknown mechanisms and a vaccine efficacy rate that barely made it to 56.6%20. In other words, it’s a flop.
Even with cherry-picked cohorts it’s barely above the WHOs threshold of 50%21, let alone the 60-70% VE required for herd immunity, according to one model22.
Regardless of this, an approval anywhere is now effectively an approval everywhere:
New regulatory recognition routes for medicines will be established using approvals from Australia, Canada, the European Union, Japan, Switzerland, Singapore and the United States, the Medicines and Healthcare products Regulatory Agency has announced today.
This means that patients will have access to safe and effective medicines that have been approved by trusted regulatory partners in other countries.
… These recognition routes, which have been facilitated by existing international partnerships such as those developed through the Access Consortium and Project Orbis, mark the start of a new international recognition framework for medicines that will be in place by the first quarter of 2024.
The new framework will allow the MHRA to make the most of the expertise and decision-making of trusted regulatory partners to streamline assessments of specific products. As a result, cutting-edge medicines that have been approved in other countries will get to UK patients more quickly, with cost reductions and streamlined regulatory processes for industry.
From: “MHRA announces new recognition routes to facilitate safe access to new medicines with seven international partners“ (2023)
The final part of the analysis includes the comments made during the expert discussion and subsequent review by the Pharmaceuticals and Medical Devices Agency (PMDA).
Now come the comments of the Japanese "experts(TM)" at the very end.
I would like to have the data, it must have been submitted somewhere after a year?
And in detail, also with regard to the kinetics of the LNP. I would like to have this + genetics + the immunological part.
The mechanism of action of Comirnaty is currently still unknown, just by the way. Note the 5 question marks in this chart from November 2022.
This was then submitted:
I would say: They missed the point here!
Extremely low is a completely useless term. Extremely low compared to what? Are there approximate values? If it is still measurable, is it already extremely low?
And the RNA with a copy machine is unlikely to persist for extremely long. How likely? How long is not extremely longer?
Such terms in scientific texts do NOT work at all. So-called deictic terms are used here, such as extremely longer or extremely low. But these terms need a reference for the extremum.
"The speaker can use them to refer to people, time and places. The reference objects change depending on the utterance situation. The interpretations of these expressions depend on the context of the utterance."3
Not even a reference is named here that would make the term "extreme" interpretable.
“The active-control vaccine (Comirnaty) used in the Japanese phase III study (Study ARCT-154-J01) is a replicon-non-containing RNA vaccine.”
“No significant difference was observed in the duration of solicited adverse events between the Kostaive group and the Comirnaty group in the clinical study. On the basis of the above results, PMDA considers that the replicon in Kostaive is unlikely to cause extreme persistence or prolongation of the symptoms of adverse reactions compared with approved RNA vaccines.”
Unfortunately, THAT is not correct. Officially, modRNA may not replicate, but the DNA remnants from production integrate into the cells. Spike production thus lasts for years. If you don't notice any difference to Kostaive, it doesn't look like the product will deactivate anytime soon.
Their words, not mine. But that's true, I'd say.
In 2023, the titer for immunity was still unclear? Seriously? What are the values actually for?
I would therefore assume that this data should be available to the EU by now, as it had to be prepared for the Japanese authorities.
Because it is a completely new active ingredient, is the stuff first unleashed on people for 8 (EIGHT) years to cause potential damage?
What do they mean by non-organic product?!
The experts(TM) didn't ask any of the questions I asked myself. The questions give the impression of alibi questions, so that you have asked something.
The answers are subterranean and partly missed the point (in my opinion).
EU wants to approve saRNA Kostaive Part 6
Post-marketing data of the two-month follow-up and Japanese manufacturer information
Part 6 is a work in progress due to translation challenges. For German or Japanese speakers please follow the links in the Substack:
Roger Bittel sent me the link through one of his contacts, where you can find the Japanese information about what happened to the saRNA injected.
Translated with deepl.com because I don't know Japanese. So I can't control the translation either. I'm completely dependent on AI. The (translated) documents are available in this article for download or as a link.
Specialist portal
Interim reports are transparently and directly accessible on the specialist portal, so you don't have to complain first, as in the wild west of the EU.
But I can't translate most of the documents because you can't copy the text into Deepl and Deepl can't translate the pdf either. The rights of the pdfs are limited and I don't know how to remove that.
Two people developed heart failure, which is described as hyperpulsatile or pulsating. I don't know if that's because of the translation. I guess it's heart failure with an increased stroke rate. The English document says "pulsatile heart failure" and "hyperpulsatile heart failure".
Heart failure is ALWAYS fatal.
NYHA is a measure of damage to the heart. There are four stages:
Stage I: No symptoms despite demonstrable heart disease; Unrestricted physical performance in everyday life
Stage II: Reduced performance and discomfort during heavy physical exertion (climbing stairs over two floors, hiking on uneven terrain); however, the patients feel comfortable at rest or during light activity
Stage III: Symptoms even during everyday light physical exertion such as walking on a flat track; Freedom from complaints in peace
Stage IV: Symptoms already at rest; Physical activities are not possible without causing symptoms
If shortness of breath already occurs during everyday light exertion (NYHA III), 25% of those affected die within one year, and in the case of shortness of breath already at rest (NYHA IV), the figure is over 50%.1
Two cases of “high-output heart failure” were recorded, and I suspect it’s been lost in the translation.
What is high-output heart failure?
High-output heart failure is a condition in which your heart is initially working normally (either with reduced or preserved ejection fraction) but can’t keep up with your body’s increasing need for more blood. Your heart ultimately becomes weak and can no longer pump blood effectively throughout your body.
https://my.clevelandclinic.org/health/diseases/24660-high-output-heart-failure
From the package insert, using a Google-translated version:
Who may not be vaccinated
Self-explanatory. I have no idea how long it lasts, at least that's honest. Will this also be written directly in the German package inserts?
What is my CAPRA response to LNPs?
CARPA reactions are complement-activated pseudoallergies that cannot be predicted, cannot be tested, can occur quickly or slowly and can sometimes be fatal. 5% to 45% of people respond with a CARPA response to nanolipids as opposed to less than 2% to penicillin.2
Seems to go directly to heart failure with saRNA, if you look at the post-marketing document.
This reminds me of my initial research into valproate and the sea of red flags it raised:
The funny thing about section 9 of the package insert is that the swearers said exactly that in 2021: First do an allergy test with the lipid (which an allergist doesn't get so easily). And as long as that is not ruled out, no vaccination. For this, we were defamed and laughed at. Here it is in the package insert.
They mainly injected people with pre-existing conditions, who are exempt here. Who takes blood thinners? Mostly heart patients. These were particularly preferred with modRNA. Seen in this way, a pretty honest package insert. Only suitable for very healthy people (who do not need the product).
Since two completely new substances are used (ATX-126 and potassium sorbate)3), which have not been tested in humans before and CARPA reactions can also occur, the allergist also has a problem with the certificate.
But in the plandemic, everyone who is explicitly excluded here was eligible for vaccination.
Otherwise, it also contains what you already know from the registration document.
Woe betide the EU allows it for under 18.
In the case of myocarditis, one is direct and has even mentioned the dermal fillers:
As far as uselessness is concerned, they are also very transparent: 56.6%, which is actually below the hurdle that the EU had set for approval.
modRNA with foreign bodies and discoloration was still routinely used in the plandemic, was brought to me from vaccination centers. Simply pull it up around the turbidity, they said.
FAQ Page:
Here, the package insert is divided into questions, which ultimately only reflect the content of the package insert by section.
I will post the guidance, translated to English and linked from “Intramuscular Injection of Kostaive”:
As this is a scan, it will not translate without sophisticated OCR:

Which links to this site:
Product information portal link:
Which links to this site:
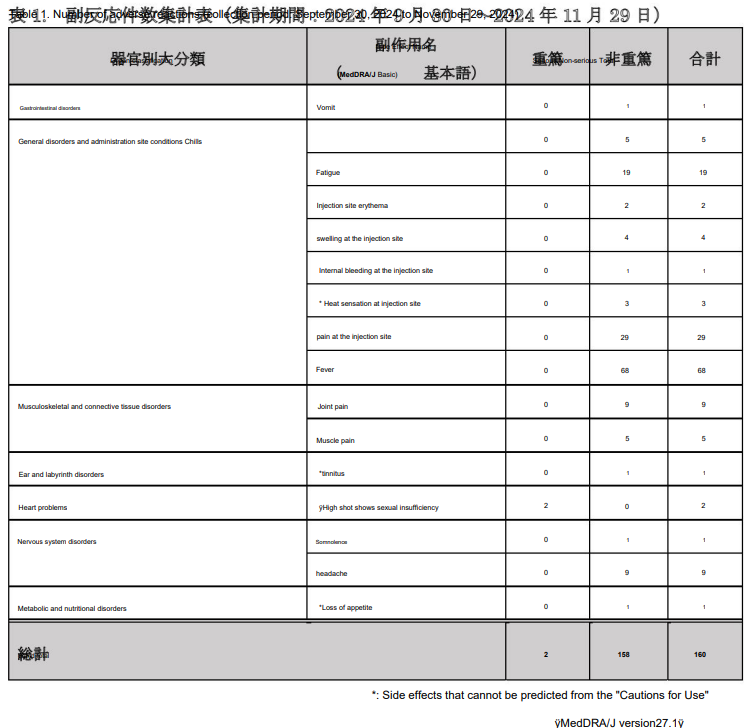
I won’t follow all of these up, as much of the guidance is just a repeat. However, the post-marketing survey results for health professionals have red flags that should cause Kostaive to be recalled under the precautionary principle, given what we know about other engineered RNA products (also used as placebo).
Much like Karikó’s finest work, this Plörre should have never been allowed out of a BSL-3 lab.
“Post-marketing surveillance”.
Note the “unknown side effects” that they couldn’t, and probably didn’t wish to predict.
Google-translated from a pdf:
Result
The manufacturers deal with the problems in the documents, which google or deepl can translate, very openly and directly. They don't try to pretend that this product is particularly efficient with 56.6%. You are transparent that the product is not suitable for those who would need it if it worked and that you actually have hardly any data and do not know how long the product will work at all.
If you get the shit injected anyway, it's your own fault.
Once again, it is politics that markets a useless, harmful product, not the manufacturer.
Breaking news
The bioweapon/shedding hypothesis is that saRNA may be exhaled as exosomes that get aerosolised when we exhale, cough or sneeze and proceed to transfect unwitting recipients, - effectively with an artificial virus.
I don’t buy into this though, because although we know that shedding is a real phenomenon, saRNAs are designed to transfect by their immune-privileged LNP carriers. For the recipient, foreign exosomes like these would quickly be detected and neutralised by mucosal IgA, macrophages, dendritic cells etc.
Even if it did transfect you, although a handful of viruses may be sufficient to infect you, you can’t say the same about aerosolised exosomes containing saRNA. They may be able to amplify RNA for a while, but these aren’t replication-competent.
And we aren’t talking 10µg doses going into your deltoid muscle and lymphatic system, more like the odd particle hitting a mucous membrane.
There is far more to making a completely synthetic virus that could go on to “wipe out humanity” than this.
That said, I am concerned that it could eventually integrate into the RNA of a wild-type alphavirus, leading to gain of function or/and zoonotic transmission.

Drug company sues lawmaker over ‘bioweapon’ claim on vaccine
By KENTA NOGUCHI/ Staff Writer
December 26, 2024 at 18:18 JST
Meiji Seika Pharma Co. in Tokyo’s Chuo Ward (The Asahi Shimbun)
A pharmaceutical company sued a lawmaker, saying he spread unfounded claims that its COVID-19 vaccine was a “bioweapon” and compared the firm to the notorious Unit 731 from World War II.
Meiji Seika Pharma Co. said on Dec. 25 it is seeking 10 million yen ($63,500) in damages from Kazuhiro Haraguchi, a Lower House member of the main opposition Constitutional Democratic Party of Japan.
According to the lawsuit filed at the Tokyo District Court, Haraguchi’s repeated defamatory remarks about the Replicon vaccine have significantly damaged the company’s reputation and finances.
Haraguchi said in a statement through his office, “I have not received the complaint and cannot comment at this time.”
According to Meiji Seika Pharma, Haraguchi has referred to the vaccine as a “bioweapon” on social media, such as X (formerly Twitter) and YouTube, as well as in his own books since around June.
The company said the lawmaker also likened Meiji Seika Pharma to the Imperial Japanese Army’s Unit 731, which conducted experiments on live Chinese and Russian prisoners to develop biological weapons during World War II.
Meiji Seika Pharma said it received a flood of protest calls, forcing it to spend 1.2 million yen to deal with the complaints.
The company also said it lost more than 5.5 billion yen in potential profits from vaccine sales and suffered intangible damage from the harm to its reputation.
The lawsuit seeks 10 million yen to recover part of these losses.
At a news conference, Daikichiro Kobayashi, president of Meiji Seika Pharma, noted that the government has approved the efficacy and safety of the vaccine. But he said the lawsuit is not necessarily about challenging the scientific basis of Haraguchi’s claims.
“It is natural for people to feel uncertainty or concerns about new vaccines,” Kobayashi said.
However, he said Haraguchi’s series of remarks and actions as a lawmaker have gone beyond the bounds of acceptable opinion or commentary.
Meiji Seika Pharma said it had sent a warning letter to Haraguchi in October, urging him to stop making posts that damage the company’s credibility.
After seeing no improvement in the situation, the company decided to proceed with the lawsuit.
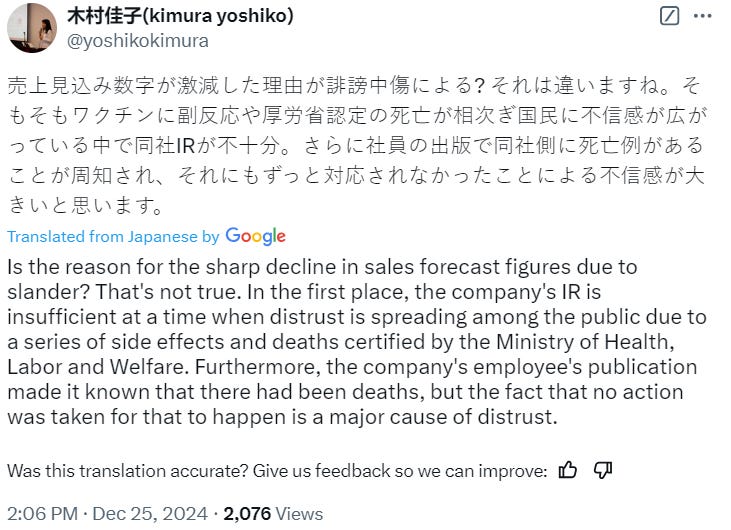
Some of the replies, for context. I have nothing to add here as I don’t have the facts or speak Japanese.
The share price tanked:
This ticker confirms that shareholders share our scepticism, and it predates the headline story.
The approval was at the end of November ‘23:
Concluding remarks
Big Pharma’s Wild West gold rush continues. In the next few months and years we can expect to see many other engineered RNA products fast-tracked into commercial production and distributed everywhere, for all ages.
There could be anything from saRNA influenza vaccines to saRNA RSV vaccines, saRNA avian flu vaccines, and even replacements for antibiotics.
They are going to throw their pasta at the wall and see what sticks. It may be Kostaive this time, it may not.
Much like the infinite regress of the “climate change” scam, at no point are you likely to be offered reliable, useful data or mechanisms to support such claims. Just lots of circular arguments, arm-waving, fraud, and expensive “cures” that are worse than the disease. Regulatory capture has seen to that.
Part 2 will fill in some of the many knowledge gaps from the approval documents.
From their perspective, your job is not to ask too many questions, “to reject the evidence of your eyes and ears” and to just keep rolling your sleeves up.
The ultimate decision on whether to take such products is between you and your doctor, not this Substack, but our work should bring you closer to informed consent. Closer that is, but not fully informed, because we don’t have sufficient data yet. Nobody does. And that in itself should be sufficient reason for an indefinite pause.
References
Global Newsroom | CSL. ‘Japan’s Ministry of Health, Labour and Welfare Approves CSL and Arcturus Therapeutics’ ARCT-154, the First Self-Amplifying mRNA Vaccine Approved for COVID in Adults’. Accessed 13 December 2024. https://newsroom.csl.com/2023-11-28-Japans-Ministry-of-Health,-Labour-and-Welfare-Approves-CSL-and-Arcturus-Therapeutics-ARCT-154,-the-first-Self-Amplifying-mRNA-vaccine-approved-for-COVID-in-adults.
Administration (TGA), Therapeutic Goods. ‘TGA Approves CSL - Seqirus to Manufacture AstraZeneca COVID-19 Vaccine in Australia | Therapeutic Goods Administration (TGA)’. Text. Therapeutic Goods Administration (TGA), 21 June 2022. https://www.tga.gov.au/news/media-releases/tga-approves-csl-seqirus-manufacture-astrazeneca-covid-19-vaccine-australia.
BioSpace. ‘CSL Seqirus and Arcturus Ink $4.3 Billion mRNA Vaccine Deal’, 2 November 2022. https://www.biospace.com/csl-seqirus-and-arcturus-ink-4-3-billion-licensing-deal-to-develop-vaccines.
Anonymous. Turtles All The Way Down: Vaccine Science and Myth. Edited by Zoey O’Toole and Mary Holland J.D. The Turtles Team, 2022. https://www.amazon.com/Turtles-All-Way-Down-Vaccine/dp/9655981045/ref=sr_1_1
European public assessment report | European Medicines Agency (EMA). Accessed December 26, 2024. https://www.ema.europa.eu/en/glossary-terms/european-public-assessment-report
Piper JD, Piper PW. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr Rev Food Sci Food Saf. 2017;16(5):868-880. doi:10.1111/1541-4337.12284
Hasegawa MM, Nishi Y, Ohkawa Y, Inui N. Effects of sorbic acid and its salts on chromosome aberrations, sister chromatid exchanges and gene mutations in cultured Chinese hamster cells. Food Chem Toxicol. 1984;22(7):501-507. doi:10.1016/0278-6915(84)90219-9
27 CFR § 24.246 - Materials authorized for the treatment of wine and juice. LII / Legal Information Institute. Accessed December 26, 2024. https://www.law.cornell.edu/cfr/text/27/24.246
"21 CFR 150 - Fruit butters, jellies, preserves, and related products" (PDF). Government Printing Office. April 1, 2011. Retrieved May 25, 2018.
Valdes Angues R, Perea Bustos Y. SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis. Cureus. 2023;15(12):e50703. doi:10.7759/cureus.50703
Davoren MJ, Schiestl RH. Glyphosate-based herbicides and cancer risk: a post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis. 2018;39(10):1207-1215. doi:10.1093/carcin/bgy105
Khan NG, Correia J, Adiga D, et al. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci Pollut Res Int. 2021;28(16):19643-19663. doi:10.1007/s11356-021-13071-w
Program NT. Tetrafluoroethylene. In: 15th Report on Carcinogens [Internet]. National Toxicology Program; 2021. Accessed December 26, 2024. https://www.ncbi.nlm.nih.gov/books/NBK590807/
Darbre PD. Aluminium, antiperspirants and breast cancer. J Inorg Biochem. 2005;99(9):1912-1919. doi:10.1016/j.jinorgbio.2005.06.001
Zeiger E, Shelby MD, Witt KL. Genetic toxicity of fluoride. Environ Mol Mutagen. 1993;21(4):309-318. doi:10.1002/em.2850210402
Suarez-Torres JD, Orozco CA, Ciangherotti CE. The 2-year rodent bioassay in drug and chemical carcinogenicity testing: Performance, utility, and configuration for cancer hazard identification. Journal of Pharmacological and Toxicological Methods. 2021;110:107070. doi:10.1016/j.vascn.2021.107070
Halmes NC, Roberts SM, Tolson JK, Portier CJ. Reevaluating Cancer Risk Estimates for Short-Term Exposure Scenarios. Toxicological Sciences. 2000;58(1):32-42. doi:10.1093/toxsci/58.1.32
Bansal S, Perincheri S, Fleming T, et al. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J Immunol. 2021;207(10):2405-2410. doi:10.4049/jimmunol.2100637
Common Terminology Criteria for Adverse Events (CTCAE). Published online 2017.
Nature Communications Publishes Pivotal Data Demonstrating Efficacy and Tolerability of CSL and Arcturus Therapeutics’ COVID-19 Vaccine. Global Newsroom | CSL. Accessed December 26, 2024. https://newsroom.csl.com/2024-05-20-Nature-Communications-Publishes-Pivotal-Data-Demonstrating-Efficacy-and-Tolerability-of-CSL-and-Arcturus-Therapeutics-COVID-19-Vaccine
Vaccine efficacy, effectiveness and protection. Accessed December 26, 2024. https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection
Bartsch SM, O’Shea KJ, Ferguson MC, et al. Vaccine Efficacy Needed for a COVID-19 Coronavirus Vaccine to Prevent or Stop an Epidemic as the Sole Intervention. Am J Prev Med. 2020;59(4):493-503. doi:10.1016/j.amepre.2020.06.011













































































































They are coming for your pets. But they don't tell you it's saRNA tech in their bullshit marketing speel, and they certainly don't want you to see the raw data:
USDA Approves MSD Animal Health’s NOBIVAC® NXT Canine Flu H3N2 – The First and Only RNA-Particle Technology Vaccine for Canine Influenza
"... NOBIVAC NXT is a revolutionary, first-of-its-kind vaccine technology for companion animals that leverages RNA-particle technology, allowing for a precise immune response to protect against a wide range of viral and bacterial pathogens."
"... NOBIVAC NXT Canine Flu H3N2 is a nonadjuvanted, low volume 0.5 mL dose vaccine that harnesses the natural ability of the immune system to generate a robust response without compromising comfort or safety."
https://www.msd-animal-health.com/2024/06/25/usda-approval-nobivac-nxt-civ/
***
Efficacy and Safety in Dogs Following Administration of an Alphavirus RNA Particle Canine Influenza H3N2 Vaccine (2024)
"... Alphavirus-derived RNA particles (RP), particularly those based on Venezuelan equine encephalitis virus (VEEV), are a compelling platform for the development of vaccines [10,11]. The RP utilizes alphavirus coat proteins to deliver a propagation-defective, self-amplifying RNA to cells in vivo, where the viral replicase drives RNA amplification and efficient protein expression of inserted antigen-coding sequences [12]. "
"... Data Availability Statement
The datasets presented in this article are unavailable because the data are proprietary information of Merck Animal Health."
https://pmc.ncbi.nlm.nih.gov/articles/PMC11511248/
arcturus patents contain literally HUNDREDS of different nucleotides with which the natural messenger RNA can be substituted with. From all the above it is still not clear what it really is in the injection materials. Kevin is right, what's patented can't be natural. In particular when looking at the lifetime of the measured xyzRNA)?) amounts in BLOOD... And placebo is not defined either... Is it saline, if so why does it cause almost IDENTICAL side effects as the real stuff does. That alone is always indication that the 'safety studies' by pharma cartels, stink, a lot.
That on top of everything makes this entire endeavor the next level crime, since one thing was omitted here. What about people carrying other viruses, genetic material from previous injections? Can that stuff all get translated by the replicons and kill even sooner? The sequences listed in their countless patents call for frequent -PP- mutations, exactly like the one added into Spike... WHy? Because it is NOT digestible.
The EU political arena is disgusting, carrying bought out by NOBODY elected individuals, like von der Leyen, who should have been in prison LONG TIME AGO!