Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background:
Cachexia (pronounced kuh-KEK-see-uh) is a “wasting” disorder that causes extreme weight loss and muscle wasting, and can include loss of body fat. This syndrome affects people who are in the late stages of serious diseases like cancer, HIV or AIDS, COPD, kidney disease, and congestive heart failure (CHF).
https://www.healthline.com/health/cachexia
Of particular interest is how Covid-19 vaccines also greatly elevate TNFα and other inflammatory cytokine levels.
For context TNFα was initially named cachectin!
(From https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-e59/comments)
Abstract
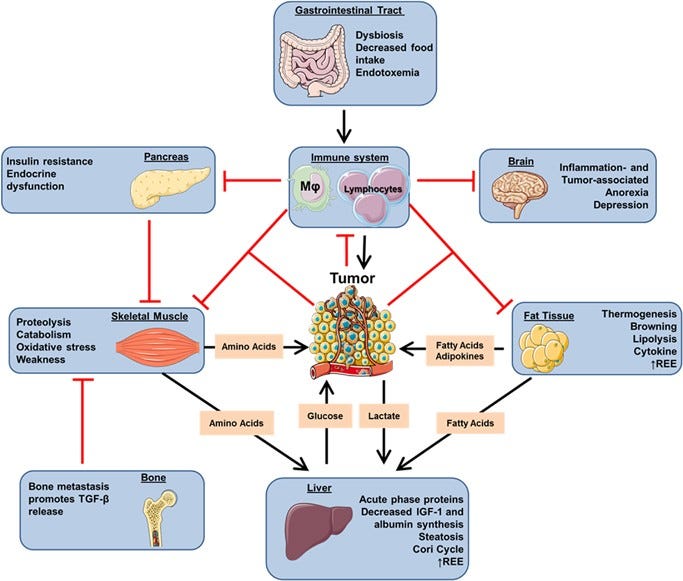
Metabolic reprogramming occurs in tumors to foster cancer cell proliferation, survival and metastasis, but as well at a systemic level affecting the whole organism, eventually leading to cancer cachexia. Indeed, as cancer cells rely on external sources of nitrogen and carbon skeleton to grow, systemic metabolic deregulation promoting tissue wasting and metabolites mobilization ultimately supports tumor growth. Cachectic patients experience a wide range of symptoms affecting several organ functions such as muscle, liver, brain, immune system and heart, collectively decreasing patients’ quality of life and worsening their prognosis. Moreover, cachexia is estimated to be the direct cause of at least 20% of cancer deaths. The main aspect of cachexia syndrome is the unstoppable skeletal muscle and fat storage wasting, even with an adequate caloric intake, resulting in nutrient mobilization – both directly as lipid and amino acids and indirectly as glucose derived from the exploitation of liver gluconeogenesis – that reaches the tumor through the bloodstream. From a metabolic standpoint, cachectic host develops a wide range of dysfunctions, from increased insulin and IGF-1 resistance to induction of mitochondrial uncoupling proteins and fat tissue browning resulting in an increased energy expenditure and heat generation, even at rest. For a long time, cachexia has been merely considered an epiphenomenon of end-stage tumors. However, in specific tumor types, such as pancreatic cancers, it is now clear that patients present markers of tissue wasting at a stage in which tumor is not yet clinically detectable, and that host amino acid supply is required for tumor growth. Indeed, tumor cells actively promote tissue wasting by secreting specific factors such as parathyroid hormone-related protein and micro RNAs. Understanding the molecular and metabolic mediators of cachexia will not only advance therapeutic approaches against cancer, but also improve patients’ quality of life.
Immune system
Inflammation is a double-edged sword in cancer. Aside from the natural role of immune system in controlling tumor growth, ultimate cancer cells hijack the immune system to produce specific cytokines promoting tumor growth, survival and progression.13 Chronic inflammation is also a major driver of cachexia (Table 1), as it affects the function of several tissues such as skeletal muscle, fat, brain and liver.14 Indeed, several pro-inflammatory cytokines promote cachexia: tumor necrosis factor alpha (TNFα), interleukin-6 and -1 (IL-6/IL-1) and interferon gamma.
TNFα, initially named cachectin, is probably the most characterized cytokine in cachexia as it promotes anorexia17 and skeletal muscle wasting mainly through the NF-kB pathway.18 TNFα blockade (etanercept) provided promising results in improving cachexia-associated fatigue in a small cohort of cancer patients.19 However, recent trials using neutralizing antibodies against TNFα showed no benefit, suggesting that targeting TNFα alone is not sufficient to prevent cachexia.20
TNFα can also synergize with interferon gamma21 and IL-122 in promoting muscle wasting. Despite that IL-1 itself promotes anorexia23 using IL-1 receptor antagonist was not sufficient to impair cachexia progression in a rat model.24 Nevertheless, human polymorphisms in IL-1B gene, resulting in augmented levels of IL-1β, were associated with a negative prognostic value,25 indicating the involvement of IL-1 pro-inflammatory cytokines in the pathogenesis of cachexia. A study in cancer patients identified increased circulating levels of cytokines (IL-1α, IL-6 and TNFα), suggesting the presence of a robust network of cytokines collectively promoting cachexia.15 IL-6 can directly drive cachexia in specific murine models26 and acute phase protein in liver and skeletal muscle by STAT3 activation.27 Moreover, IL-6 circulating levels correlate with cachexia development and poor prognosis in prostate cancer patients.28 IL-6 can be produced not only by the immune system but also directly by the tumor,29 further highlighting the direct involvement of tumor cells in driving cachexia. Other members of the IL-6 family such as ciliary neurotrophic factor and leukemia inhibitory factor have also been associated with cachexia development.30, 31 The upregulation of pro-inflammatory cytokines co-occurs with decreased expression of the cytokines hampering inflammation, such as IL-4 -10 and -12.32 Coherently, several treatments controlling excessive inflammation provided beneficial effects on cachexia progression.
Cardiac muscle
The heart is an important target of cachexia. Cardiac alterations are typical in cancer patients and ultimately results in heart failure and arrhythmia, which are two of the concurring causes of death during cachexia. Similarly to skeletal muscle, cardiac wasting involves the activation of protein turnover mediated by the UPR system. Indeed, the heart weight and functionality has been reported to decrease in a murine model of colon cancer developing chronic heart failure. As in skeletal muscle, NF-κB inhibition has been shown to ameliorate cardiac atrophy and functionality in a mouse model of Colon-26-driven cancer cachexia, suggesting novel therapeutic approaches for this severe cause of cancer-cachexia death.
Chronic heart failure has been previously associated with the increase of REE, providing another reason for the increase of energy expenditure in cachectic patients. This increase might be at least in part directly related to an increased metabolism of cardiac tissue, as ex vivo hearts from tumor-bearing rats present an increased oxidative rate.
More: