What You Should Know About Growth Plate Injuries (2021)
Risks from experimental gene therapy transfection
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background
This should be read alongside previous Substacks and further details the specific risks to childhood musculoskeletal development due to repeated, sustained exposure of growth plates to transfection induced proinflammatory cytokines. Triggered autoimmune disorders are another huge risk factor.
Such effects may take months or years to become apparent, by which time the damage is done and may not be easily remedied through surgery, drugs or manipulation.
Although myocarditis is the primary concern, with as many as 50% having some degree of asymptomatic damage and as many as 1 in 30 having symptomatic damage requiring a trip to A&E1 my concern is that effects on growth may be insidious & affect many more of the transfected.
Adults have some choice in what harms they expose themselves to but the same cannot be said of our offspring. It's simply too great a risk to take and will have long long consequences in terms of quality of life and ability to earn a living, there are far too many unknowns.
I considered neurological damage for example:
"England to offer Covid jab to five to 11-year-olds"
The pathophysiology of childhood exposure to elevated levels of Reactive Oxygen Species (ROS)
https://doorlesscarp953.substack.com/p/england-to-offer-covid-jab-to-five?s=w
Such experimental gene therapies must be withdrawn immediately pending further investigations and never applied to children where the risks are infinite and the benefits negligible, as they are at zero effective risk from the virus, or if so immunocompromised as to be at risk would not benefit from transfection either.
IU researchers study long-term effects of COVID-19 on bone growth (2022)
New paper is now published in BONE
https://doorlesscarp953.substack.com/p/iu-researchers-study-long-term-effects?s=w
Also:
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #6: consistent pathophysiological alterations after vaccination with COVID-19 vaccines. + New paper warns of osteoporosis.
Physical trauma can damage the circulation, in much the same way that spike protein can damage the microvasculature & endothelia:
What You Should Know About Growth Plate Injuries (2021)
Steps you can take to protect your young athlete
We all know that nutrition is important for your child’s growth and development. But your child’s growth — particularly height — also depends on bone growth plates.
When those growth plates become damaged through a sports-related injury or accident, they not only can be painful but also can affect how well and how long your child’s arms, legs, hands and feet grow.
What is a growth plate?
Growth plates are thin cartilage discs at the end of long bones in children and adolescents.
“As the cartilage cells in the discs multiply, the bones grow longer,” says pediatric orthopedic surgeon R. Tracy Ballock, MD.
Growth plates continue to lengthen bones until about age 14 in girls and age 16 in boys,” Dr. Ballock says. “In both genders, the surge of estrogen at puberty causes growth plate cartilage to change into solid bone.”
Growth plates disappear when the skeleton reaches maturity and the bones stop growing. However, bones can stop growing earlier — stunting physical development and causing functional problems — if the growth plates become severely damaged, Dr. Ballock says.
How growth plates get damaged
Growth plate injuries happen in a similar fashion to broken bones. They can be caused by:
Trauma such as a fall or collision: About 30% of growth plate injuries are from playing contact sports, such as football, soccer and basketball, Dr. Ballock says. Another 20% are due to recreational activities, such as skateboarding and skiing. The remaining 50% are from general accidents.
Overuse: “Little League shoulder” is one example. Chronic stress on the shoulder from too much ball-throwing causes microdamage to the growth plate. Pain and swelling result. Similar damage can occur in gymnasts’ wrists as well as the body of any young athlete involved in repetitive training.
“When a limb becomes stressed in some way, it breaks wherever it’s weakest,” Dr. Ballock says. “Cartilage is weaker than bone, so in children, injuries can be more common in the growth plate.”
How to detect growth plate damage
If your child complains of pain in the shoulder or joints, don’t decide that there is no injury because your child can wiggle their fingers or toes or there is no swelling or bruising. Those signs aren’t reliable indicators.
The most reliable sign of a growth plate injury is tenderness at a single point, Dr. Ballock says.
“It may not hurt anywhere outside that point,” Dr. Ballock says. “And it might not look like anything is wrong.”
Other indicators include:
Inability to continue activity due to pain.
Change in performance.
A deformed limb.
If there’s a growth plate injury, doctors can treat it with casts or splints.
“Casts or splints keeps the limb immobile so it can heal better,” Dr. Ballock says. “If a bone is out of alignment, we may need to perform surgery to reposition it and possibly keep the bone growing.”
3 ways to prevent growth plate injuries
You may not be able to prevent growth plate injuries, but you and your child can take precautions. Dr. Ballock recommends these three:
Don’t play one sport year-round. “Kids need at least three months off from their chosen sport to allow growth plate microdamage to heal,” Dr. Ballock says. “It’s also wise to change physical activities for a few months each year to work other muscle groups.”
Use protective equipment. Recommended gear will vary by sport, but it’s also important for recreational activities. For example, wrist guards may help prevent injuries in skateboarders.
Avoid risky recreational activities. Jumping on a trampoline is a common risky activity, especially if more than one child is on the trampoline, and accounts for many bone fractures in children.
No matter how it happens, if you suspect your child has injured a growth plate, it’s time to make a doctor’s appointment for an evaluation.
“Sometimes injuries can heal on their own,” Dr. Ballock says. “But without treatment, kids will have more pain and a higher risk of more severe, possibly growth-stunting damage.”
https://health.clevelandclinic.org/what-you-should-know-about-growth-plate-injuries/
Imaging of Pediatric Growth Plate Disturbances (2017)
Abstract
The growth plates, or physes, are visible on virtually all images obtained in skeletally immature children. The proper function of these growth plates depends on an intricate balance between chondrocyte proliferation, which requires nourishment from the epiphyseal vessels, and chondrocyte death, which requires the integrity of the metaphyseal vessels. Therefore, injury to the growth plate (ie, direct insult) or vascular compromise on either side of the growth plate (ie, indirect insult) can cause growth plate dysfunction. Direct growth plate insults occur most commonly with Salter-Harris fractures, and injuries that allow the transphyseal communication of vessels are at a higher risk for subsequent transphyseal bone bridge formation. Indirect insults lead to different sequelae that are based on whether the epiphyseal blood supply or metaphyseal blood supply is compromised. Epiphyseal osteonecrosis can result in slowed longitudinal bone growth, with possible growth plate closure, and is often accompanied by an abnormal secondary ossification center. In contrast, the disruption of metaphyseal blood supply alters endochondral ossification and allows the persistence of chondrocytes within the metaphysis, which appear as focal or diffuse growth plate widening. Imaging remains critical for detecting acute injuries and identifying subsequent growth disturbances. Depending on the imaging findings and patient factors, these growth disturbances may be amenable to conservative or surgical treatment. Therefore, an understanding of the anatomy and physiologic features of the normal growth plate and the associated pathophysiologic conditions can increase diagnostic accuracy, enable radiologists to anticipate future growth disturbances, and ensure optimal imaging, with the ultimate goal of timely and appropriate intervention.Introduction
Bone formation occurs by way of intramembranous and endochondral ossification. In general, flat bones develop by means of intramembranous growth and long bones develop by means of endochondral growth. With intramembranous growth, bone formation occurs directly from the mesenchyme. With endochondral growth, bone formation occurs on a cartilaginous framework at the growth plate (1,2).
Primary “discoid” and secondary “spherical” growth plates are found at the ends of long bones. The primary growth plate is responsible for the longitudinal growth of the bone. This growth plate is bound peripherally by the fibrous perichondrium. The secondary growth plate, or acrophysis, is responsible for the enlargement of the secondary ossification center (SOC) within the cartilaginous epiphysis and is the only growth center in smaller bones, such as the apophyses, carpal and tarsal bones, and sesamoid bones (1) (Fig 1).
The primary growth plate is a critical component of the immature skeleton. Impairment and dysfunction of this structure can result in lifelong morbidity, including limb length discrepancy, angular deformity, and altered joint biomechanics. However, the exact clinical implications of the growth disturbance depend on the anatomic location, the severity of the deformity, and the remaining growth potential. For example, disturbances that involve the weight-bearing lower extremities in younger children are less well tolerated, are more likely to require intervention, and more often result in early osteoarthritis and joint replacement. For proper function of the primary growth plate, the zonal arrangement and cellular function must be orderly and the vascular supply from the adjacent epiphysis and metaphysis must be intact. Injury to either the growth plate (direct injury) or the adjacent epiphysis and metaphysis (indirect injury) can lead to growth plate dysfunction and future growth disturbance.The most common insult mechanism is trauma, which can directly or indirectly injure the growth plate. Less common mechanisms include vascular compromise, infection, inflammation, radiation, and tumor (3); these insults are more likely to primarily affect the epiphysis and metaphysis and only indirectly injure or secondarily involve the growth plate. Indirect insults may cause very different sequelae based on whether the epiphyseal blood supply or metaphyseal blood supply is compromised.
More:
https://pubs.rsna.org/doi/full/10.1148/rg.2017170029
The pathophysiology of the growth plate in juvenile idiopathic arthritis (2006)
Abstract
Children with chronic inflammatory diseases, such as juvenile idiopathic arthritis (JIA), suffer from a variety of growth disorders. These range from general growth retardation to local acceleration of growth in the affected limb. These disorders are associated with the increased production of proinflammatory cytokines, which may influence growth through a local effect in the growth plates of long bones and/or systemic effects throughout the whole body. In this article we review these aspects and also discuss the evidence for interaction between the inflammatory cytokine and growth-signalling pathways.
Topic: cytokine signal transduction arthritis, juvenile rheumatoid child epiphysial cartilage insulin-like growth factor i inflammatory disorders systemic drug effect
Child growth is multifactorial, and requires the tightly controlled mobilization and utilization of energy, coordinated by several biochemical and physiological regulatory mechanisms. Impaired linear growth is commonly encountered in children suffering from chronic inflammatory diseases such as juvenile idiopathic arthritis (JIA), both at disease presentation and following treatment with glucocorticoids. In these children, maintenance of growth is a complex process that is influenced by a number of different mechanisms, including not only the steroid therapy, but also other factors such as the disease process, nutritional status, endocrine status and the response of the body to inflammatory mediators. The growth abnormalities observed in children with inflammatory diseases are often associated with delayed onset of sexual maturation and altered bone development. Evidence of an imbalance of proinflammatory cytokines in patients with inflammatory diseases includes the positive correlation of serum and synovial cytokine concentrations with JIA disease activity [1, 2], an increase in antagonists or soluble receptors with a flare of arthritis [3] and the effectiveness of JIA therapies that involve cytokine modulation [4, 5]. The proinflammatory cytokines that have been reported to play a major role in JIA include interleukin 1β (IL-1β), tumour necrosis factor α (TNF-α) and interleukin 6 (IL-6) [6–8]. These proinflammatory cytokines may influence child growth through systemic effects and/or a local effect at the level of the growth plate of long bones.
…
TNF-α and apoptosis
TNF-α has two cell surface receptors, TNFR1 and TNFR2. Binding of TNF-α to TNFR1 initiates an apoptotic response, which is simultaneously activated by TNF-α through the Fas-associated death domain (FADD), a protein that triggers the pro-apoptotic caspases and cell death. TNF-α has been reported to induce apoptosis in chondrogenic cells [75] and fetal rat metatarsal cultures [77]. TNF-α-induced apoptosis may be a major contributor to the growth abnormalities observed in children suffering from inflammatory diseases.
…
A number of clinical studies suggest a direct link between factors produced during chronic inflammation and growth failure [45, 48, 49]. In systemic JIA, impairment of linear growth is seen during periods of disease activity, with subsequent normalization of growth rate during remission [48, 49]. In patients with systemic JIA and growth defect treated with GH, growth velocity during treatment appears inversely correlated with the intensity of inflammation [45].
It is likely that cytokines may influence the development of these abnormal growth patterns in children with inflammatory diseases, through both systemic effects and local effects at the level of the growth plate.
More:
https://academic.oup.com/rheumatology/article/45/1/11/2899323
Added 5th April:
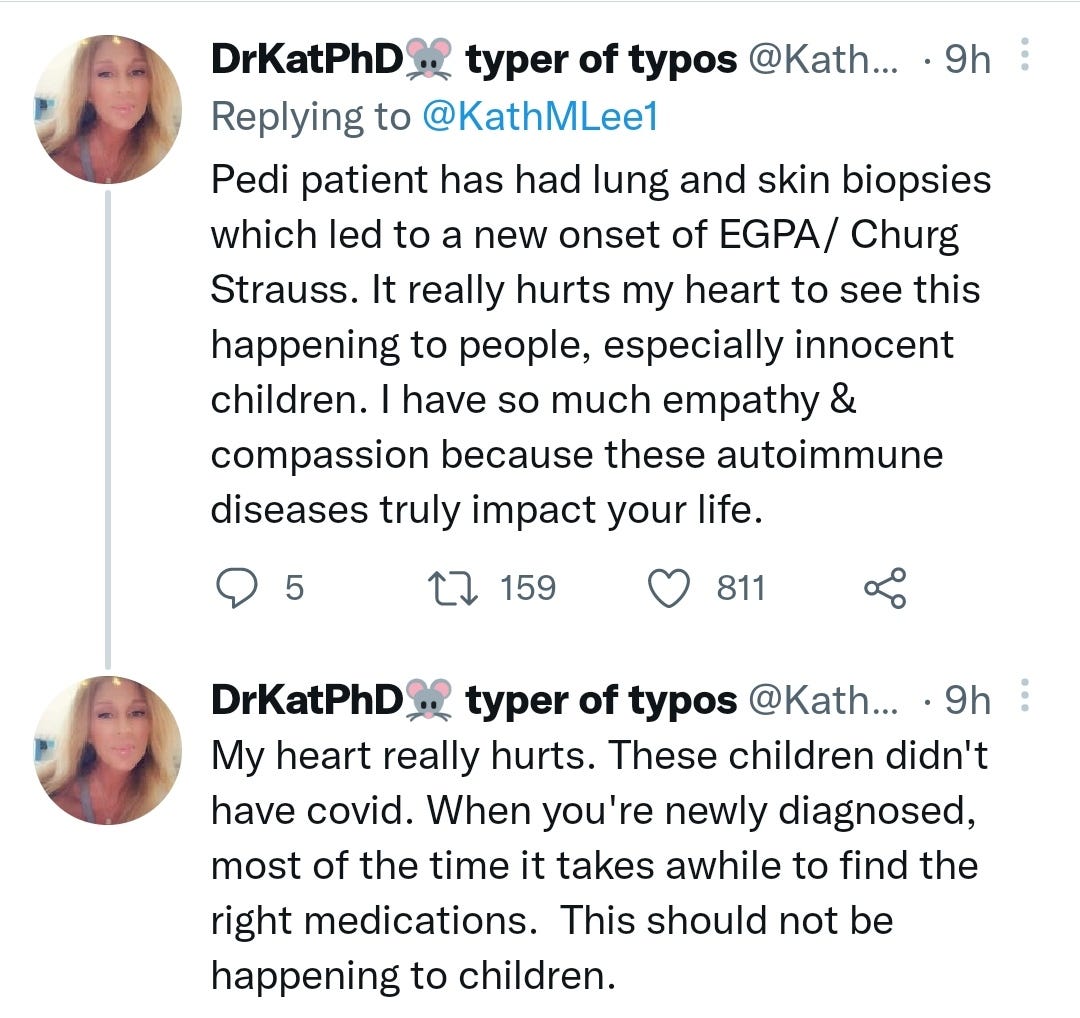
https://twitter.com/KathMLee1/status/1511094985562677258?s=19
Flare of rheumatoid arthritis after COVID-19 vaccination (2021)
With the COVID-19 pandemic, there has been great uncertainty about whether the virus could exacerbate autoimmune diseases such as rheumatoid arthritis given that infection can lead to an overactivation of the immune system, which is thought to play a part in severe cases in the general population.1, 2 A review of the literature shows there has been one case report so far of a flare of rheumatoid arthritis after infection with SARS-CoV-2.3
Three COVID-19 vaccines have been approved for emergency use in the USA so far.4 Two of the vaccines, BNT162b2 (BioNTech-Pfizer) and mRNA-1273 (Moderna), are novel mRNA-based vaccines delivered via lipid nanoparticles. The clinical trials of these vaccines allowed for patients with rheumatic disease to participate in the later stages of the trials, but excluded patients on immunosuppressive agents.5, 6 Therefore, it is not fully known whether these vaccines might provoke flares of underlying rheumatic conditions as a result of immune activation or non-specific adjuvant effects. There are reports of other vaccines, such as those against tetanus, rubella, hepatitis B, and influenza, triggering rheumatoid arthritis, but causality has never been proven and an association has never been reproduced in large, controlled studies.7 Molecular mimicry is thought to be one mechanism by which autoimmunity can occur, in which similarities between viral peptides and self-peptides can stimulate immune activation, but this has not been proven in rheumatoid arthritis.8 The American College of Rheumatology issued guidance regarding COVID-19 vaccination on Feb 8, 2021, and acknowledged a theoretical risk of flare of autoimmune disease after vaccination with moderate consensus.9
Here, we present a case of a White male, aged 55 years, with non-erosive, seropositive rheumatoid arthritis (positive for rheumatoid factor, anticyclic citrullinated peptide antibodies, antinuclear antibodies, and anti-Ro antibodies) who had been in sustained clinical remission for more than 2 years and developed an acute flare of his rheumatoid arthritis 12 h after the second BNT162b2 vaccination.
His rheumatoid arthritis was well controlled before the vaccination, and there were no other inciting events, so we believe that this flare might have been triggered by his immune response to a component of the BNT162b2 vaccine. BNT162b2 contains mRNA encoding for the SARS-CoV-2 spike protein encapsulated in lipid nanoparticles, in addition to other components that stabilise the vaccine in the circulation and promote its uptake into cells by endocytosis. Although the mechanism of flare is not known, one could speculate that one of these components might have had non-specific adjuvant effect, or there could have been molecular mimicry between the viral spike peptides and the patient's self-peptides, activating a flare. However, we cannot exclude the possibility that the timing of the flare with regard to vaccination was coincidental. The patient was treated with intra-articular steroids with rapid improvement, and he is once again in clinical remission.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8009616/#__ffn_sectitle
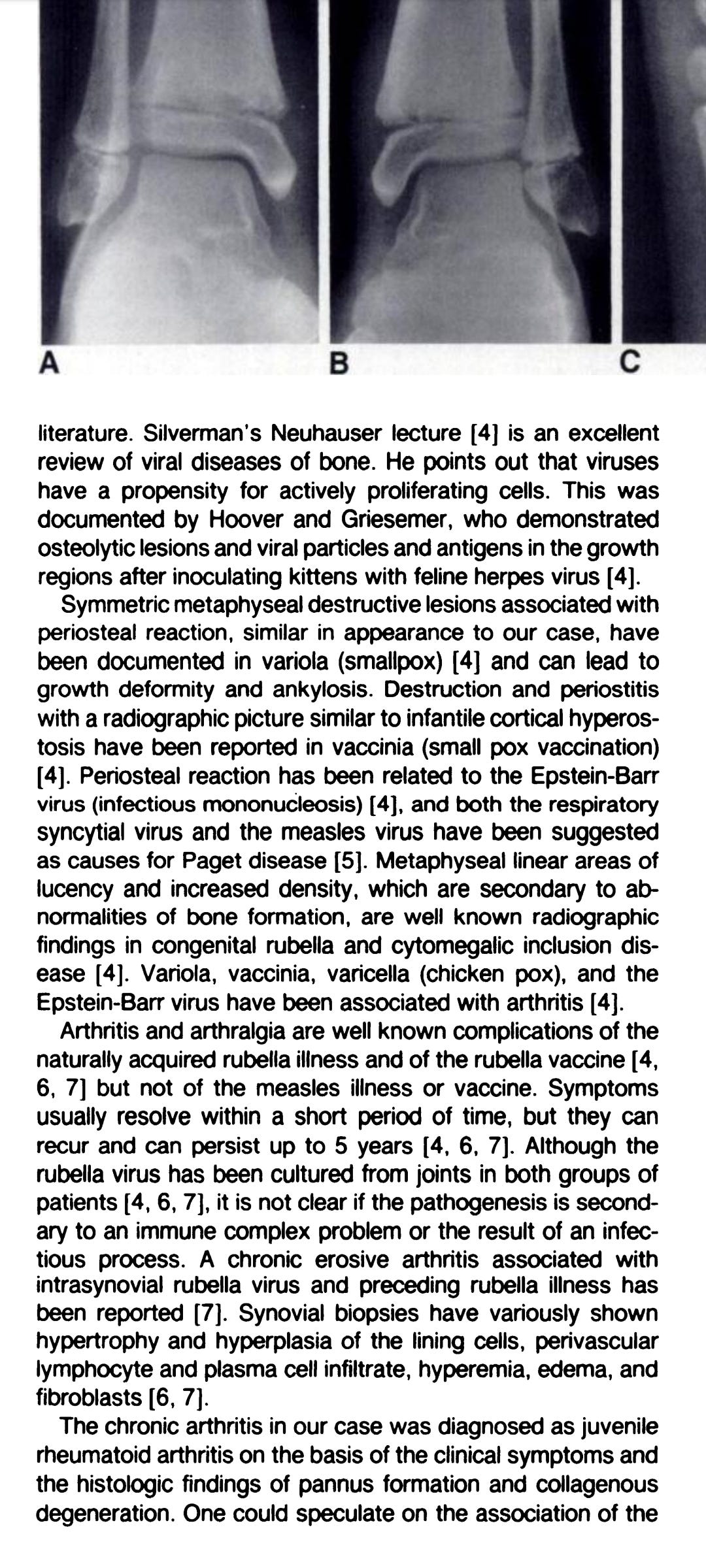
Bone changes after rubella vaccination (1984)
"Arthritis and arthralgia are well known complications of the naturally acquired rubella illness and of the rubella vaccine but not of the measles illness or vaccine. Symptoms usually resolve within a short period of time, but they can recur and can persist up to 5 years"
Key:
Erythema is a type of skin rash caused by injured or inflamed blood capillaries. It usually occurs in response to a drug, disease or infection. Rash severity ranges from mild to life threatening.
The medial malleolus is the bony bump on the inner side of the ankle. This is the end of the shin bone (tibia).
Metaphyseal lesions. It refers to an injury to the metaphysis which is the growing plate at each end of a long bone (such as tibia, femur, etc).
https://www.ajronline.org › pdfplus
SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells (2020)
Abstract
Cytokine storm is suggested as one of the major pathological characteristics of SARS-CoV-2 infection, although the mechanism for initiation of a hyper-inflammatory response, and multi-organ damage from viral infection is poorly understood. In this virus-cell interaction study, we observed that SARS-CoV-2 infection or viral spike protein expression alone inhibited angiotensin converting enzyme-2 (ACE2) receptor protein expression. The spike protein promoted an angiotensin II type 1 receptor (AT1) mediated signaling cascade, induced the transcriptional regulatory molecules NF-κB and AP-1/c-Fos via MAPK activation, and increased IL-6 release. SARS-CoV-2 infected patient sera contained elevated levels of IL-6 and soluble IL-6R. Up-regulated AT1 receptor signaling also influenced the release of extracellular soluble IL-6R by the induction of the ADAM-17 protease. Use of the AT1 receptor antagonist, Candesartan cilexetil, resulted in down-regulation of IL-6/soluble IL-6R release in spike expressing cells. Phosphorylation of STAT3 at the Tyr705 residue plays an important role as a transcriptional inducer for SOCS3 and MCP-1 expression. Further study indicated that inhibition of STAT3 Tyr705 phosphorylation in SARS-CoV-2 infected and viral spike protein expressing epithelial cells did not induce SOCS3 and MCP-1 expression. Introduction of culture supernatant from SARS-CoV-2 spike expressing cells on a model human liver endothelial Cell line (TMNK-1), where transmembrane IL-6R is poorly expressed, resulted in the induction of STAT3 Tyr705 phosphorylation as well as MCP-1 expression. In conclusion, our results indicated that the presence of SARS-CoV-2 spike protein in epithelial cells promotes IL-6 trans-signaling by activation of the AT1 axis to initiate coordination of a hyper-inflammatory response.
@arkmedic
When elite athletes had cardiac MRI scans 50% of them had asymptomatic myocarditis, putting them at risk of sudden death. I cannot reference the source but that figure is not implausible.
I have no idea where we will be in a year or so: myocarditis can lead to fibrosis, but the process takes between several months and 10 years. Fibrous scar tissue may not conduct electrical signals properly, you are at risk of arrhythmia and sudden death, out of the blue.
https://gab.com/Doorlesscarp953/posts/107571468253387875
Myocarditis in a Belgian running club.
3 in 170 (about 1 in 50, or even 1 in 25 if they were all in the beginner group).
Bad batch, or this typical? "At least" implies there may have been more, and as they were all hospitalised the asymptomatic cases would not have been picked up, only the more severe cases. This is not looking good.
Translated:
After several incidents, doctors advise against practicing intensive sport after vaccination
At least three young cyclists have been admitted to hospital with heart problems after a race or training in recent weeks.
"How common is myocarditis? It hugely depends how hard you look. For smallpox vaccine in military recruits, 1 in 30 had clinical or subclinical myo or pericarditis"
https://anthraxvaccine.blogspot.com/2021/07/how-common-is-myocarditis-it-hugely.html
https://twitter.com/P_McCulloughMD/status/1506285051469832198?s=19