Safe and effective? What you are not being told about influenza vaccination
Alternative therapeutics are available
Reading time: about 70 minutes.
Updates:
29th October ‘23: IgG4 class switching in children.
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Pathologies associated with influenza vaccination
Introduction
Sapporo is the fifth most populous city in Japan and the largest city north of Tokyo on the northernmost island of Japan, Hokkaido.
Around 2019 a 14 year old girl from Sapporo received an injection of inactivated quadrivalent influenza vaccine, containing thimerosal. This is a preservative that contains mercury.
Five days after vaccination she developed fever, chest pain, nausea and sore throat. Two days later she felt faint and her mother took her to an emergency clinic, where she collapsed and was duly referred to the Sapporo Medical University Hospital with cardiopulmonary arrest.
Her cardiac rhythm was a pulseless wide QRS tachycardia, where the ventricles are not effectively moving blood out of the heart and there is no cardiac output. Mechanical circulatory support was started immediately but as CPR was not immediately successful an ECMO machine was then used to oxygenate her blood, together with intra-aortic balloon pumping (IABP).
The IABP and ECMO were removed on day 11 and on day 69 she was transferred to another hospital for rehab.
Results of endomyocardial biopsy showed that there was massive infiltration of CD3- and CD68-positive cells and various degrees of cardiomyocyte necrosis in all of 3 endomyocardial specimens, whereas infiltration of eosinophils or giant cells was not observed. A histologic diagnosis of lymphocytic myocarditis was made. Acute myocarditis is a rare but potentially fatal complication of the influenza vaccination.

From: “Hemodynamic Collapse After Influenza Vaccination: A Vaccine-Induced Fulminant Myocarditis?” (2020)
https://www.sciencedirect.com/science/article/pii/S0828282X20304499
We associate post experimental mRNA COVID-19 gene agents with T-cell infiltration and myo and pericarditis, especially of young people since 2021 but not the flu vax. If she had collapsed anywhere other than where she did with excellent care at the Medical University Hospital then this case report would have had a much more tragic outcome. As it is the cardiomyocyte necrosis will have led to lifechanging consequences with a poor prognosis, given the severity of the injuries1.
Perhaps we need to give it more attention?
To that end, this Substack will start with a look at some longer term public health related data.
I will then review papers investigating some of the pathologies associated with influenza vaccination, what “effectiveness” means in reality and conclude by looking at research into some of the many antiviral therapeutics. Often these may be considered as safer and more effective than vaccination - especially for those with impaired immunity that impairs vaccinal immune response in the first place.

Background
Since 2022 various governments have been limiting their gathering and reporting of both vaccine adverse event data and associated mortality.
As with the ZOE Health Study app2, search engines can be used to indicate health related trends data, albeit not with high statistical power due to confounding factors.
You feel something is wrong with you or someone else or you’ve seen a news report. You may start Googling in response to find out more?
I started using Google Trends data for Canada to look into cancer and comorbidity trends over the last five years.
@EthicalSkeptic posts excellent analysis which provides a useful benchmark:

With lymphoma, apart from an unexplained spike around November 2020 there is little discernible trend when viewed over 5 years:
The data is somewhat noisy and the note line is where “an improvement to our data collection system was applied from 1/1/22”. One persons “improvement” is another persons evidence of baseline or data manipulation. Either way the next step was to zoom out to see data from the last 20 years.
“Improvements” were also made on 1/1/11 and 1/1/16, but this is a lot more interesting:
There is now a distinct uptick visible in the last 3 years, as with the @EthicalSkeptic analysis:
With glioblastoma, a type of brain cancer, we can also see a rising trend from around 2004. On 1/1/11 they improved their geographical assignment:
Now you may say an upward trend is to be expected as more of us have been using search engines over the last two decades. You cannot escape the browsers, they are on our mobiles, tablets, some smart TV’s and the odd fridge too no doubt. Its certainly a factor and its not clear if Google Trends corrects for this? But if that is the case then why are lung cancer cases trending downwards?
As @EthicalSkeptic said about cancer, “This is a big ship. It takes a while to turn it around” so the rate of decline will lag the decline in smoking by many years, but it lends some confidence to our trends data. Which is not good.
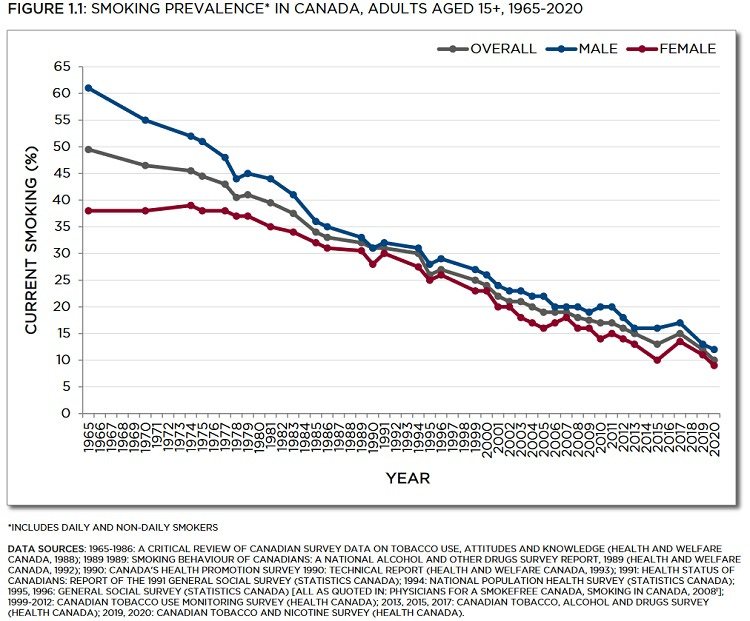
I then searched for “chest pain” and the shocking search engine trends data, predating 2020 even is the reason for this Substack and the reason for all these graphs:
There is a huge, growing problem with declining public health and its not all down to COVID-19 and experimental jabs. Its been building for years and we know many are behavioural or dietary in origin: drinking, obesity, drug abuse, poverty. These are the things the media focus on and the problem is becoming too big to hide.

You will not see reference to child and adult vaccine schedules, corticosteroid misuse & over-prescription3, glyphosate4 and pesticide contamination or allopathic drugs, to name but a few.
Hat tip to Dr John B for posting these:
30 common cleaning products: "... 193 unique hazardous chemicals were identified with potential impacts on the respiratory system, development, reproduction and cancer risk."
https://www.newsweek.com/popular-cleaning-products-increase-cancer-risk-1826778
In our blood: how the US allowed toxic chemicals to seep into our lives
"... the majority of the 86,000 consumer chemicals registered with the Environmental Protection Agency have never received vigorous toxicity testing."
Human exposure to per- and polyfluoroalkyl substances & other emerging contaminants in drinking water:
https://www.nature.com/articles/s41545-023-00236-y#Sec2
Detected: carbamazepine (a recalcitrant pharmaceutical compound used as anticonvulsant and mood stabilizer),... terbuthylazine (herbicide)."

Lifestyle choices cannot explain the temporal association to autistic spectrum disorders too. (A retracted paper, naturally):

I’ve worked with severely autistic children and adults and in my opinion “better diagnosis and more advanced diagnostic criteria” cannot explain all of this increase or sharp trend changes seen with unsmoothed data:


A significant decline in public health is now being seen in the workforce of major economies like the US and UK:
Lengthening waiting lists for medical attention don’t help, but they don’t make you sick in the first place either:
The level of illness among the UK population is costing lives and harming the economy, a report has warned - after the number of people off work due to long-term sickness hit another record high.
More than 2.6 million people now do not have jobs because of their health, according to latest employment data from the Office for National Statistics (ONS).
The all-time high comes after an additional 491,433 adults were added to the official total in the three months from May to July, figures released on Tuesday revealed.
The Institute for Public Policy Research (IPPR) said in a report on Wednesday that the issue had become a "serious fiscal threat" to the UK - and to individuals' health.
The thinktank blamed long NHS waiting lists and other problems faced by the public in accessing treatment, and said reform was urgently needed to avert "killer" costs while also ending second-rate care.
From: https://news.sky.com/story/number-of-long-term-sick-hits-record-high-of-2-6-million-12959783
The hypothesis behind this Substack is that influenza vaccination over the last twenty years is a significant co-factor in this, and I will present supporting evidence.
Actually I don’t need too. The UK’s “National Health Service” (NHS), or should that be “National Help-Pharma Service” has firmly nailed its colours to the mast and tells us why vaccination is “safe and important”.
Which ones? Well all of them, naturally:

Flu vaccine is especially safe and effective.
Its for kids, adults, anyone that can fog a mirror basically:
Look you can even pick one up with your weekly shopping, what are you waiting for?
Even better, have a COVID booster in one arm and the flu shot in the other.
Smile! You’ve had your annual jab. Life’s great!
Well I hate to rain on their parade, but relying on NHS level information would not have allowed the 14 year old girl in Japan or her parents to have informed consent. We saw much the same wording for the mRNA gene shots:

Pathologies associated with influenza vaccination
Flu vax rates are generally high and staying high, eg 60-70% of Canadians aged 65 and over5:
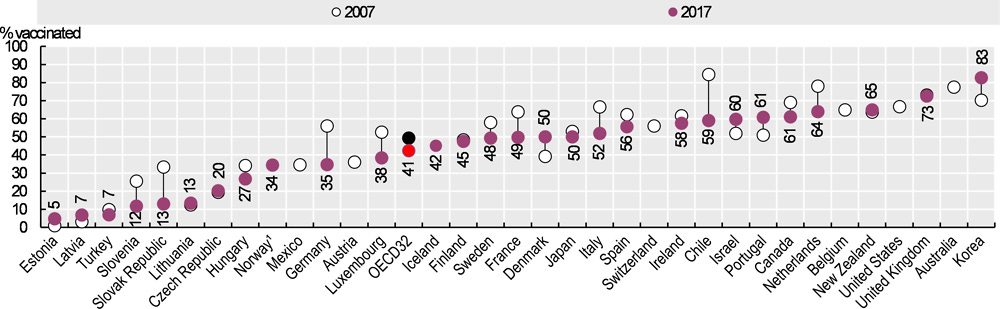
From a survey of Italians 42% of those who took COVID-19 transfection agents also took the flu jab in the 2020/21 season. And about a third of young people were willing to be vaccinated, with refusal rates reported at only 12.6%6.
We have sufficient numbers vaccinated to explain the clinical signals7:
An analysis of billed office visits vs vaccine status found that unvaccinated children were far healthier and made less visits. Published by Lyons-Weiler & Thomas in 2020, you won’t be surprised that the paper has since been retracted8:

NB Since writing this a petition has been started to get it unretracted:
Chest pain
Unfortunately the full paper is by request from the authors only, but we have an abstract from 2005 by Kaplan et al, and they suggested additional studies be conducted.
An incidence rate of 1% should at least warrant a warning on the vaccine information websites, let alone further studies?
You may also ask why this was funded by the US Army? As with their smallpox vax program, presumably their recruits were reporting to the sick bay or unable to perform intensive exercise?
“Chest Pain: An Unrecognized Side Effect Temporally Associated with Influenza Vaccine”9
RATIONALE: Rare cases of pericarditis have been reported in the literature following influenza vaccination. Since the incidence of post vaccine chest pain has not been defined as a reported side effect, this study was designed to determine the frequency and severity of chest pain complaints temporally associated with influenza vaccine.
METHODS: Healthy subjects between 18 to 64 years of age who received a dose of influenza vaccine in the late fall of 2004 were monitored for side effects using a web-based 4 day symptom diary (day 0-3 post vaccination). Severity of pain symptoms was quantified using a 0 to 5 visual analogue scale.
RESULTS: 629 subjects (mean age 48.7 years, 44.4% female) completed the post vaccination diary. Chest pain was reported by 20 subjects. Seven (1%) subjects reported pain > 2 on at least one day with a mean peak pain score of 3/5. One subject reported peak chest pain > 4. Chest pain lasting at least 2 days with peak severity > 2/5 was reported by 5 subjects (0.7%). The greatest frequency of chest pain was on the 2nd day after immunization. There were no hospitalizations or emergency room visits.
CONCLUSIONS: Chest pain occurs as a distinct side effect temporally associated with influenza vaccination. Rarely, the severity meets pain guideline criteria for treatment (VAS > 2/5). Additional studies in larger populations are needed to better define this side effect and its etiology.
Footnotes
Funding: Dept of US Army
A more recent case report, this time from 2022 by Nakamura et al reports new-onset cardiac symptoms occurring in 2.6% of cases after influenza vaccination, or 1 in 38.
As you should always reference primary research from reviews [ref #12] please see: “A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination” by Engler et al (2015)10:
The chest pain data and increases in sudden adult death are by no means extremely rare, they are actually quite common. I suspect mRNA gene agent induced pathologies are more aligned to those from smallpox vaccination, as per the jump in VAERS reports.
And as few clinical trials have been conducted using negative controls or against other vaccines or mRNA gene agents, especially for SARS-CoV-2, then further increased synergistic and cumulative cardiovascular injury becomes all the more likely.
Key extracts from “Acute Lymphocyte Myocarditis Associated with Influenza Vaccination”11 (emphasis mine):
Abstract
An elderly patient was admitted to our hospital for acute heart failure soon after receiving influenza vaccination. On admission, chest radiography revealed pulmonary edema. An electrocardiogram showed poor R progression, and echocardiography showed diffuse hypokinesis and myocardial edema. The serum troponin level was elevated. A histopathological evaluation indicated active myocarditis with lymphocyte-predominant infiltrates. A drug-induced lymphocyte stimulation test (DLST) was positive. The patient rapidly recovered from heart failure after treatment with conventional heart failure drugs, such as intravenous diuretics and vasodilators. These experimental data and the clinical course suggest that influenza vaccination was responsible for heart failure due to acute lymphocyte myocarditis.
Echocardiography showed not only diffuse hypokinesis of the left ventricle wall but also myocardial tissue edema and slight pericardial effusion (Fig. 3A). The cardiac troponin T level was elevated, and the blood level of N-terminal pro-B-type natriuretic peptide was over 2,000 pg/mL (reference range, 0-450 pg/mL) (Table 1). A drug-induced lymphocyte stimulation test (DLST) for the vaccine was performed, with a stimulation index (SI) of 180% considered to indicate a positive response. The influenza vaccine administered to this patient gave a positive response with an SI of 199%.
Immunohistochemical staining showed that inflammatory infiltrates were predominantly leukocyte common antigen (LCA/CD45)-positive lymphocytes and CD68-positive macrophages (Fig. 5). Few eosinophils were noted in this case. We performed stress perfusion imaging using adenosine Thallium-201(Tl) myocardial perfusion single photon emission computed tomography (SPECT) on the sixth hospital day. It did not indicate a myocardial perfusion defect, so we were able to rule out the possibility of ischemic heart disease, such as acute coronary syndrome. Taken together, the laboratory findings and the patient's clinical course suggested that influenza vaccination had induced acute myocarditis, resulting in acute heart failure and pulmonary edema.
We carefully monitored his respiratory and hemodynamic states after the acute clinical phase. He had no recurrence of heart failure and was discharged on the 40th hospital day. At the four-month follow-up visit, the echocardiographic findings of diffuse wall motion abnormality, myocardial tissue edema, and pericardial effusion and electrocardiogram findings of ST elevation and T-wave inversion had completely resolved (Figs. 2C and and3B,3B, Table 2). No clinical signs of heart failure indicating recurrence of myocarditis were observed.
A question mark remains regarding permanent damage or cardiac remodelling after myocarditis12, which may be ongoing for 24 months13 and is associated with negative outcomes14. His serum troponin T level was still at 1260 pg/mL or 0.126 ng/ml by day 40, against a reference range of <450 pg/mL.
And as with experimental mRNA gene agents, rates of subclinical myocardial cell damage after influenza vaccination are highly likely to be significantly higher than 2.6%.
When you look for it, you may find - in this study of 301 Thai adolescents who had received 2 doses of the BNT162b2 COVID-19 transfection agent 18% presented with ECG findings:

Objectives. The present study investigated whether myocyte injury can be assessed sensitively by measurement of serum levels of cardiac troponin T (cTnT) in patients with clinically suspected myocarditis and whether cTnT levels may predict the results of histologic and immunohistologic analysis of endomyocardial biopsy specimens.
Background. Conventionally used laboratory variables often fail to show myocyte injury in patients with clinically suspected myocarditis, possibly because of a low extent of myocardial injury in these patients. Sensitive variables for myocyte injury have not yet been investigated.
Conclusions. Measurement of serum levels of cTnT provides evidence of myocyte injury in patients with clinically suspected myocarditis more sensitively than does conventional determination of cardiac enzyme levels. Myocardial cell damage may be present even in the absence of histologic signs of myocarditis. Additional immunohistologic analysis often shows lymphocytic infiltrates in these patients. Elevated levels of cTnT are highly predictive for myocarditis in this group.
From: “Cardiac Troponin T in Patients With Clinically Suspected Myocarditis” (1997)
https://www.sciencedirect.com/science/article/pii/S0735109797003173
Cancer
Interleukin-6 (IL-6) is a pro and anti-inflammatory cytokine associated with oncogenesis and autoimmunity, via the STAT3 axis15. TNFa has a similar association via STAT316.
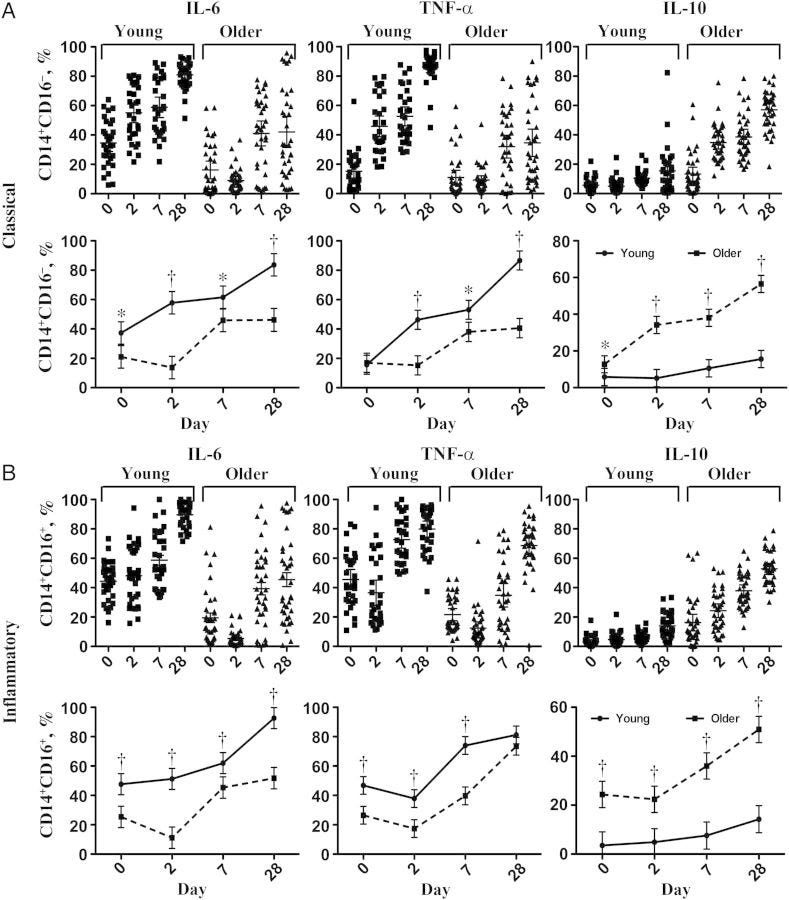
Mohanty et al (2015) conducted a study of in vivo innate immune responses in monocyte populations from 67 young (aged 21-30 years) and older (aged ≥65 years) adults before and after influenza vaccination. They found that CD14+ and CD16+- monocytes were induced by vaccination.
Key takes from “Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults”17:
In classical CD14(+)CD16(-) and inflammatory monocytes, production of tumor necrosis factor α and interleukin 6, as measured by intracellular staining, was strongly induced after vaccination. Cytokine production was strongly associated with influenza vaccine antibody response; the highest levels were found as late as day 28 after vaccination in young subjects and were substantially diminished in older subjects.
IL-6, TNFa and CD14+CD16- monocytes remained elevated for up to 28 days, a long time if you have a tumour:

Snap quiz
Q: What type of monocyte has also been associated with myocarditis?
A: If you said “CD14+CD16-” then you’ve earned yourself a free trip to the ER:
Flow cytometric analysis from all three patients indicated that CD14+CD68+, but not CD16+CD68+, macrophages, are present in the heart (Figure S3C). This further supports the hypothesis that CD14+CD16− monocytes are the main source of cardiac macrophages in human myocarditis. In conclusion, human CD14+ and mouse Ly6Chi monocyte subsets are the major contributors to the cardiac macrophage population during myocarditis development.
From: “The Cardiac Microenvironment Instructs Divergent Monocyte Fates and Functions in Myocarditis” (2019)
So we have found some of the weapons at the crime scene, are there any cancer victims?
Yes there are, but you need to do some digging around. This is unsurprising as it goes against the “safe and effective” mantra and funding tends to govern what is researched18.
The abstract to this Danish paper from 2021 by Gögenur et al starts with presumably the expected outcome but their conclusion is for further clinical studies to investigate some of their “contradictory results”.
Key takes from “Association of influenza vaccine and risk of recurrence in patients undergoing curative surgery for colorectal cancer”19, (emphasis mine):
Background
There is increasing evidence that the inactivated influenza vaccine contains immunostimulatory properties that favor cytotoxicity and benefit survival in large population-based studies. This study aimed to determine whether an influenza vaccine was associated with risk of recurrence, overall mortality, and disease-free survival in patients undergoing curative surgery for colorectal cancer.
Results
A total of 9869 patients were included, with 5146 patients receiving an influenza vaccine between one year before and six months after surgery. In a multivariate Cox regression model, there was no association with risk of recurrence (HR 0.94, 95% CI 0.85–1.05), overall mortality (HR 0.95, 95% CI 0.87–1.03), and disease-free survival (HR 1.01, 95% CI 0.94–1.09). In patients receiving the vaccine between six and twelve months before surgery, we found an association to decreased risk of recurrence (HR 0.78, 95% CI 0.67–0.91) but no association with overall mortality (HR 1.04, 95% CI 0.93–1.17) or disease-free survival (HR 0.97, 95% CI 0.88–1.07). Subgroup analysis of patients revealed contradictory results.
Conclusion
We believe that this study's findings support the need for further clinical studies to investigate the causal effects of the influenza vaccine on oncological outcomes.
A statistically significant signal was found in under 65’s who were vaccinated within one to six months of undergoing curative surgery for colorectal cancer in Denmark in 2009–2015, but not in the group who received it between one year before and six months after surgery:
Risk of recurrence
Receiving an influenza vaccine at any time point between one year before surgery and six months after surgery did not associate with the risk of recurrence (HRadjusted1 0.93, 95% CI 0.83–1.03) (Table 3). When stratifying for the period of vaccine, a statistically significant association between receiving an influenza vaccine between six and twelve months before surgery and reduced risk of recurrence was found, both in unadjusted analysis and when controlling for age, sex, CCI, UICC stage, colon or rectal cancer, elective or acute surgery, year of surgery, education level and income level (HRadjusted1 0.78, 95% CI 0.67–0.90). This association was not found in other periods, but a trend in the opposite direction was noted in patients receiving the vaccine between one to six months after surgery (HRadjusted1 1.12 95% CI 0.97–1.28). The competing risk analysis confirmed the above results.
Recurrence that may have been initiated by vaccination was also associated with reduced disease-free survival for the under 65’s:
In general, the preplanned and exploratory subgroup analyses did not differ from the main results. However, in the subgroup of patients below 65, a statistically significant association between receiving an influenza vaccine and an increased risk of recurrence, overall mortality, and worse disease-free survival was seen (eTables 1–3). The subgroup consisted of 2716 patients, of which only 19% (525 patients) received an influenza vaccine.
When excluding patients who received more than one vaccine, a decreased overall mortality was noted in patients receiving the vaccine between zero to thirty days after surgery (HRadjusted1 0.68 95% CI 0.49–0.95) (eTable 3) compared with unvaccinated patients.
Recurrence was greatest in stage I-II cancer patients. By stage 2 a tumour is larger and more difficult to treat, even without spread.
By stage III, or “locally extensive disease” a tumour has spread locally and may have reached the lymph nodes20:
When stratifying patients according to the UICC stage (eTables 1–3), the association to reduced risk of recurrence in patients receiving the vaccine between six to twelve months before surgery persisted in patients with UICC III cancers, while there only was a trend in patients with UICC I-II cancers. The same was evident about overall mortality, while for disease-free survival when comparing vaccine vs. no vaccine an association to lower disease-free survival was noted.
Natural killer (NK) cells are associated with clearance of cancer cells, but tumour cells can quickly become resistant to these. Recurrences initiated by repeat boosting beyond 12 months after surgery were not assessed by this study.
…there are robust evidences about the effect of the immunoediting process on cancer cells, these cells also affect NK cells inducing a progressive loss of their cytotoxic capability and IFN-γ secretion [142,143]. In particular, cancer stem cells (CSCs) may play a significant role in this process. CSCs represent a subpopulation of tumor cells highly resistant to chemotherapy and radiotherapy that have been classically thought to be susceptible to NK-cell mediated killing due to their low MHC class I expression [144,145]. Accordingly, the efficacy of the NK cell targeting of CSCs has been demonstrated in several solid tumors in experimental models, including of colorectal cancer and melanoma [146,147,148]. However, secretion of suppressor cytokines or shedding of soluble NKG2D ligands by CSCs may play a key role in decreasing NK cell-mediated cytotoxicity and IFN-γ secretion [149,150].
From: “Mechanisms of Resistance to NK Cell Immunotherapy” (2020)
In recent years, the effects of unaltered influenza vaccines on the immune system have been elucidated. The early studies showed an increase in and prolonged NK cell activity after influenza vaccination that also abrogated the detrimental effects of surgery on NK cell activity [Citation6,Citation7]. NK cells are an essential factor in clearing cancer cells in circulation, as cancer cells lack MHC type 1 expression, the main feature that determines whether NK cells will eliminate a cell or not [Citation17,Citation18].
This is nice to know but not how the flu vax is being administered. And Ivermectin can have the same effect21:
The recent preclinical studies have shown that intratumoral application of the influenza vaccine in itself can modulate the local tumor microenvironment in favor of cytotoxic immunity and its capability to ‘convert’ tumors to increase susceptibility to immune checkpoint inhibitors [Citation5]. These results are encouraging and should lead to clinical studies investigating whether the influenza vaccine could be repurposed, especially when considering the safety profile of the influenza vaccine and its regular use in comorbid and immunocompromised patients [Citation19].
Quick take: “Don’t give the flu vax to cancer patients”:
Our results show that receiving an influenza vaccine well before the surgery, when cancer has not been diagnosed yet, is associated with a reduced risk of recurrence. This is, however, not accompanied by a likewise reduced overall mortality or disease-free survival. Although recurrence is a primary factor in death after colorectal cancer surgery, other events can cause death, and it would require a more extensive study population to determine this effect.
The authors note that reduced recurrence with stage 3 patients may be due to the chemotherapy they were receiving as a standard treatment:
We also note with interest that the subgroup of patients with UICC III cancers seemingly is the driver of the association to a reduced risk of recurrence, as only a trend toward a reduced risk of recurrence was seen in patients with UICC I–II cancers. Stage III cancers are more susceptible to recurrence, which could be an explanation for this. Patients with stage III cancers also as a standard receive adjuvant chemotherapy, which presumably also would affect our outcomes.
They go on to discuss possible confounding factors, but conclude their impacts to be negligible:
However, the possible impact of these factors can be considered when considering the unadjusted and adjusted analysis of overall mortality, where the covariates included have a significant effect on the estimates. In the analysis of the risk of recurrence, however, we find that the included covariates have a negligible impact on estimates. Preliminary studies investigating the influenza vaccine in an oncological setting, also found that other vaccines had an impact on the immune system, although not as potent [Citation6]. Information on other vaccines was not available in this study.
In 2018, Paessler asked: “Should safety of the flu vaccine for cancer patients be reexamined?”22
Key takes, (emphasis mine):
Seasonal flu vaccine is recommended as the best protection for cancer patients against influenza infection. Recent in silico and experimental data suggest that antibodies elicited with influenza vaccine could activate bradykinin receptor B2-associated signaling pathway, which is also involved in cell proliferation and migration of tumor cells. These results point to an urgent need for the reexamination of safety of influenza vaccine(s) in cancer patients.
It may induce myocarditis in some, but reduce the risk of major cardio-vascular events in others:
Several epidemiological studies have suggested that vaccination against seasonal influenza virus(es) significantly reduces the risk of major cardio-vascular events in patients with acute coronary syndrome or coronary artery disease2,3.
The molecular mechanism underlying protection of flu vaccine against cardiovascular diseases is unknown; however, this phenomenon is independent from vaccine efficacy against influenza A infections. Recently, it was suggested that the vaccine against influenza A viruses could elicit agonistic antibodies for bradykinin receptor B2 (BKB2R), which activates a BRB2R-associated signaling pathway that may contribute to the protection against cardiovascular diseases4.
Moreover, it has been established that antibody activation of BKBR2 is possible, with all biological activities associated with it5. Recently, a monoclonal antibody with agonistic BKBR2 activity as well as anti-influenza A activity has been patented for multiple purposes6. Taken together, these data support the hypothesis of “molecular mimicry” between BKBR2 and hemagglutinin (HA) of influenza A viruses that may allow for generation of cross reactive antibodies.
In addition, it was found that levels of kinins in biological fluids of cancer patients are increased and that activation of kinin receptors expressed on cancer cells produces an increase in cell proliferation and migration of tumor cells [reviewed in Ref. 7].
Additionally, it has been demonstrated that tumor growth is increased by stimulation of kinin receptors expressed on other cells within the tumor microenvironment7, and that bradykinin and its receptors are involved in pathogenesis of numerous common cancers (gastric8, hepatocellular9, brain10, bladder11, renal12, prostate13 and breast14). These data point out that, because of possible activation of BKB2R with antibodies elicited by influenza vaccine, safety of this vaccine in cancer patients is an important issue.
No long term studies:
Screening of the clinical trials database (clinicaltrial.gov) for trials that investigated safety of the influenza vaccine in cancer patients with solid tumors (literature data connect pathogenesis of this type of tumors with BKB2R-pathway) revealed only five completed studies (Table 1). Patients were monitored in the period between 21 days and 6 months following vaccination and results were released only for one study (NCT01666782 in Table 1) for the monitoring time frame of 21 days.
These short-term studies suggest that influenza vaccination is effective and safe in cancer patients in general1,15–17. However, long-term studies might be needed to test the hypothesis that if antibodies elicited by the seasonal flu vaccine may contribute to activation of BKB2R in cancer patients with potentially far-reaching consequences.
In conclusion, previously published results suggest that influenza vaccines could produce antibodies with BKB2R-agonistic activity. On the other hand, experimental and clinical data showed that activation of the bradykinin pathways plays an important role in pathogenesis of several common solid tumors. All these data suggest that until the role of the influenza vaccine in activation of BKB2R is clarified, vaccination of cancer patients against flu should be taken with some caution, and vaccines need to be monitored beyond the flu season.
This especially concerns children with cancer, who represent the most vulnerable population of oncology patients. In addition, previous in silico analysis of informational properties of BKB2R and HAs from different influenza A viruses suggested that flu vaccines are not equally efficient in production of agonistic antibodies for BKB2R4. This opens the possibility for selection of antigens with low crossreactivity with BKB2R and design influenza vaccines incapable of inducing production of cross-reactive antibodies for safer use in cancer patients.
Autoimmunity
Epitope: “a group of amino acids or other chemical groups exposed on the surface of a molecule, frequently a protein, which can generate an antigenic response and bind antibody”23.
Although lacking the number of homologous epitopes seen with mRNA gene agents24, you escalate the risk of inducing autoimmune disorders the more strains you add or especially by going “universal”. They need to do this as the virus constantly mutates, to get ahead of the likely dominant strains that year:
What is quadrivalent flu vaccine?
A quadrivalent influenza (flu) vaccine is designed to protect against four different flu viruses, including two influenza A viruses and two influenza B viruses.
Learn more about the vaccine composition for the current flu season vaccines.
Why was quadrivalent flu vaccine developed?
For many years, flu vaccines were designed to protect against three different flu viruses: an influenza A(H1N1) virus, an influenza A(H3N2) virus and one influenza B virus. Adding a B virus from the second lineage was done to give broader protection against circulating flu viruses.
Who can get quadrivalent flu vaccine?
All flu vaccines in the United States are quadrivalent. Different vaccines are approved for different age groups. Information on approved flu vaccines for the current flu season, and age indications for each vaccine are available in CDC’s Table: U.S. Influenza Vaccine Products for the 2023-2024 Season.
From: “Quadrivalent Influenza Vaccine” (2023)
Labombarde et al used a murine model (ie lab mice🐭) to investigate the pathogenic risks of adding more and more epitopes.
First, a look at the variable head and stalk regions of the viral surface protein, hemagglutinin (HA):


Key takes and an excellent graphical abstract from “Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity”, (2022)25.
Pharma are constantly pushing vaccinology to the right as their experiments tend to indicate more neutralising antibodies that way, which is pretty much all they seem to be focused on:
In brief
Labombarde et al. investigate whether broadly reactive influenza antibodies, which may also bind self-antigens, contribute to autoimmunity. They demonstrate that induction of these antibodies also supports development of autoreactive antibodies in both humans and mice. Transferring broadly reactive monoclonal antibodies exacerbates autoimmunity in the context of inflammation or genetic susceptibility.
Variable by name, mutations in the head region can quickly lead to viral escape:
Protective antibodies induced by influenza A virus vaccination or infection are primarily directed at epitopes in the variable head region of the viral surface protein, hemagglutinin (HA), and are typically specific for one influenza subtype (Angeletti and Yewdell, 2018a; Krammer, 2019).
Targeting these conserved epitopes is the basis for developing a universal influenza vaccine that provides immunity against distinct influenza strains (Ekiert and Wilson, 2012; Krammer, 2016; Angeletti and Yewdell, 2018a; Corti and Lanzavecchia, 2013; Corti et al., 2011; Ellebedy et al., 2014; Krammer, 2019; Sui et al., 2011; Wrammert et al., 2008). However, it remains unclear why these antibodies are so uncommon, despite repeated exposure to conserved influenza epitopes through vaccination or infection.
Anti-dsDNA antibodies are seen with lupus26; anti-insulin antibodies with insulin autoimmune syndrome (IAS) and hypoglycemia27:
Recent data demonstrate that antibodies targeting conserved HA epitopes are more likely to be polyreactive than antibodies specific for the variable region of HA (Andrews et al., 2015; Bajic et al., 2019; Guthmiller et al., 2020; Khurana et al., 2020). Polyreactivity refers to the ability of an antibody to bind multiple, molecularly distinct antigens and was found to be an inherent property of antibodies binding the conserved HA stalk region (Guthmiller et al., 2020). Broadly reactive influenza antibodies also bound other proteins including insulin, lipopolysaccharide (LPS), and double-stranded DNA (dsDNA), demonstrating a propensity for autoreactivity.
These data raise the possibility that self-tolerance mechanisms may limit the antibody response to the conserved regions of influenza virus. In support of this, vaccination or infection with the 2009 pandemic H1N1 strain, which generated more broadly neutralizing antibodies than previous seasonal strains (Andrews et al., 2015; Li et al., 2012; Pica et al., 2012; Thomson et al., 2012; Wrammert et al., 2011), increased the risk of autoimmune disorders, including narcolepsy and Guillain-Barré syndrome (GBS) (Barker and Snape, 2014; Hao et al., 2019; Kwong et al., 2013; Nohynek et al., 2012; Vellozzi et al., 2014; Vogel, 2015).
To test whether enhancing broadly reactive influenza antibodies also increased autoreactive antibodies, we utilized a mouse model of vaccination in which C57BL/6 mice were given HKx31, an H3N2 strain of influenza, intraperitoneally (i.p.).
To test whether autoreactive antibodies in the serum of HKx31 + RAP mice also bound influenza, or if these antibodies developed in parallel, we measured reactivity to histones by ELISA before and after serum was adsorbed against influenza proteins.
For a positive control, we adsorbed serum against histones, which removed approximately 70% of histone-specific antibodies (Figure 2A). Remarkably, nearly 60% of the histone-specific antibodies were also removed when adsorbed against HKx31, while less than 10% were adsorbed with insulin (Figure 2A), indicating that the antibodies that bind self-proteins also bind influenza.
You really don’t want to lose your self tolerance here:
Broadly reactive influenza antibodies spontaneously develop in mice with defects in B cell tolerance
The fact that autoreactive antibodies and broadly reactive influenza antibodies increase concurrently, and that broadly reactive influenza monoclonal antibodies also bind self-antigens, suggests that self-tolerance mechanisms may limit the generation and/or maintenance of broadly reactive influenza antibodies.
These data demonstrate that mice with higher levels of broadly reactive influenza antibodies developed more severe disease in a model caused by a bacterium associated with GBS in humans.
Think MS-type degenerative neurological disorders: “Antibodies reactive against myelin-associated glycoprotein (MAG) and gangliosides are associated with various neurological pathologies (Cutillo et al., 2020; Lardone et al., 2010).”:
Together, these data demonstrate that humans infected with a novel strain of influenza, which induced higher levels of broadly reactive influenza antibodies compared to previous years, also exhibited an increase in antibodies reactive to MAG and gangliosides. This finding is consistent with our data from the murine models and is particularly important given that influenza infection during the 2009–2010 season was associated with increased cases of GBS (Ghaderi et al., 2016; Kwong et al., 2013; Vellozzi et al., 2014).
As the goal of a universal influenza vaccine is to generate durable, broadly reactive influenza antibodies, a critical question is whether elevated levels of broadly reactive influenza antibodies could exacerbate autoimmune diseases. We demonstrate that the enhanced generation of broadly reactive influenza antibodies also increased autoreactive antibodies and exacerbated disease in four distinct models of autoimmunity.
Importantly, our data demonstrate that the transfer of relatively small amounts of broadly reactive influenza monoclonal antibodies exacerbated autoimmunity in the presence of inflammation or underlying defects in tolerance. Thus, an increase in broadly reactive influenza antibodies alone is not sufficient to induce autoimmune disease. However, in the context of a secondary infection, additional inflammation, or a genetic susceptibility, broadly reactive influenza antibodies can exacerbate autoimmunity. It is important to note that the broadly reactive influenza monoclonal antibodies that enhanced autoimmunity in mice mirrored the binding patterns of broadly reactive influenza monoclonal antibodies isolated from humans.
While broadly reactive influenza antibodies are rare relative to strain-specific antibodies, they tend to occur more frequently after exposure to novel influenza virus strains (Andrews et al., 2015; Ellebedy et al., 2014; Joyce et al., 2016; Li et al., 2012; Pica et al., 2012; Thomson et al., 2012; Wheatley et al., 2015; Whittle et al., 2011; Wrammert et al., 2011).
However, as the immune response continues, self-tolerance mechanisms operating within clonal selection typically reduce levels of autoreactive antibodies over time (Gitlin et al., 2016; Zhang et al., 2016). Thus, broadly reactive influenza antibodies may not be durable because they are more likely to be polyreactive and therefore subject to self-tolerance mechanisms. In support of this, there is evidence of a significant decline in the levels of broadly reactive influenza antibodies following different vaccine strategies to novel influenza strains (Andrews et al., 2017; Feldman et al., 2019; Fukuyama et al., 2020; Wheatley et al., 2015).
Our data reveal that a critical consequence of promoting the maintenance of broadly reactive influenza antibodies is increased susceptibility to autoimmune disease in the event of additional challenges to self-tolerance checkpoints.
James Lyons-Weiler warned of this in the context of mRNA gene agents28. No-one listened:
Finally, our data highlight how self-tolerance mechanisms contribute to the evolutionary fitness of viruses. Epitopes that are avoided due to self-tolerance will not be subjected to immune pressure, and as a result, these epitopes will be more stable or conserved as viruses evolve. Consequently, the conserved epitopes have a higher probability to induce cross-reactive antibodies against self-proteins. Other viruses likely benefit from self-tolerance mechanisms to avoid immune pressure. For example, while several broadly neutralizing HIV antibodies have been identified, these antibodies are rare and have been shown to be autoreactive (Haynes et al., 2005; Liu et al., 2015). Thus, it is imperative to consider the autoreactive potential of antibodies when designing vaccines aimed at generating durable antibodies to epitopes that are naturally avoided by the immune response and particularly the conserved influenza epitopes that are evaded by immune systems of multiple species as evolutionarily diverged from humans as lampreys (Altman et al., 2015).

This work had further support from a mouse study by Bernard-Valnet et al in 2022.
Key takes from “Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy”29 (emphasis mine):
Abstract
Narcolepsy with cataplexy or narcolepsy type 1 is a disabling chronic sleep disorder resulting from the destruction of orexinergic neurons in the hypothalamus. The tight association of narcolepsy with HLA-DQB1*06:02 strongly suggest an autoimmune origin to this disease. Furthermore, converging epidemiological studies have identified an increased incidence for narcolepsy in Europe following Pandemrix® vaccination against the 2009–2010 pandemic ‘influenza’ virus strain.
Here, we developed a mouse model allowing to track and study the T-cell response against ‘influenza’ virus haemagglutinin, which was selectively expressed in the orexinergic neurons as a new self-antigen. Pandemrix® vaccination in this mouse model resulted in hypothalamic inflammation and selective destruction of orexin-producing neurons.
Additional studies revealed pathways potentially involved in the disease process. Notably, the interferon-γ pathway was proven essential, as interferon-γ-deficient CD8 T cells were unable to elicit the loss of orexinergic neurons.
Our work demonstrates that an immunopathological process mimicking narcolepsy can be elicited by immune cross-reactivity between a vaccine antigen and a neuronal self-antigen. This process relies on a synergy between autoreactive CD4 and CD8 T cells for disease development.
A reassessment of the risk of narcolepsy in children in England after receipt of the AS03-adjuvanted H1N1 pandemic vaccine concluded to a 7-fold elevated risk of onset of narcolepsy after Pandemrix®, which was confined to the first year after vaccination.14 Interestingly, a recent report suggests that treatment with immune checkpoint inhibitors could cause narcolepsy in genetically predisposed persons.15 Thus, antigen-specific as well as more general immune stimulation have been associated with development of narcolepsy.
Effects on immunity in children
The problem of original antigenic sin (OAS) prompted one of my earliest Substacks and it is particularly associated with influenza. Whatever strains were used in your first flu jab are what your main memory B recalled antibodies are likely to be primed for, allowing for immune escape to occur.
If the new strain is markedly different you may break OAS, but then risk generating autoreactive antibodies that may lead to disorders such as MS, GBS or narcolepsy, as discussed previously.
Original antigenic sin, also known as antigenic imprinting, the Hoskins effect,[1] or immunological imprinting,[2] is the propensity of the immune system to preferentially use immunological memory based on a previous infection when a second slightly different version of that foreign pathogen (e.g. a virus or bacterium) is encountered. This leaves the immune system "trapped" by the first response it has made to each antigen, and unable to mount potentially more effective responses during subsequent infections. Antibodies or T-cells induced during infections with the first variant of the pathogen are subject to repertoire freeze, a form of original antigenic sin.
Also see:
“Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #8: Original Antigenic Sin: How First Exposure Shapes Lifelong Anti–Influenza Virus Immune Responses” (Dec. ‘21)
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-f8d/comments
In 2011, Bodewes et al compared CD8+ T cell development in vaccinated vs unvaccinated healthy control children. They found that annual vaccination impairs the development of anti-viral CD8+ T cells compared to the control, but not CD4+ T helper cells.
CD8+ T cells (often called cytotoxic T lymphocytes, or CTLs) are very important for immune defence against intracellular pathogens, including viruses and bacteria, and for tumour surveillance. When a CD8+ T cell recognises its antigen and becomes activated, it has three major mechanisms to kill infected or malignant cells. The first is secretion of cytokines, primarily TNF-α and IFN-γ, which have anti-tumour and anti-viral microbial effects.
From: “Cells T CD8+”, https://www.immunology.org/public-information/bitesized-immunology/cells/cells-t-cd8
Key takes from “Annual Vaccination against Influenza Virus Hampers Development of Virus-Specific CD8+ T Cell Immunity in Children”:30
Infection with seasonal influenza A viruses induces immunity to potentially pandemic influenza A viruses of other subtypes (heterosubtypic immunity). We recently demonstrated that vaccination against seasonal influenza prevented the induction of heterosubtypic immunity against influenza A/H5N1 virus induced by infection with seasonal influenza in animal models, which correlated with the absence of virus-specific CD8+ T cell responses.
Its great to see a study using unvaccinated controls instead of the pseudo controls as discussed in “Turtles all the way down: Vaccine Science and Myth.”31:
Annual vaccination of all healthy children against influenza has been recommended, but the impact of vaccination on the development of the virus-specific CD8+ T cell immunity in children is currently unknown. Here we compared the virus-specific CD8+ T cell immunity in children vaccinated annually with that in unvaccinated children. In the present study, we compared influenza A virus-specific cellular and humoral responses of unvaccinated healthy control children with those of children with cystic fibrosis (CF) who were vaccinated annually.
Similar virus-specific CD4+ T cell and antibody responses were observed, while an age-dependent increase of the virus-specific CD8+ T cell response that was absent in vaccinated CF children was observed in unvaccinated healthy control children.
Our results indicate that annual influenza vaccination is effective against seasonal influenza but hampers the development of virus-specific CD8+ T cell responses. The consequences of these findings are discussed in the light of the development of protective immunity to seasonal and future pandemic influenza viruses.
Furthermore, it can be hypothesized that the use of these vaccines interferes with the induction of heterosubtypic immunity and virus-specific CD8+ T cell responses otherwise induced by natural infections, especially in children who are immunologically naïve to influenza viruses (7). We tested this hypothesis in mice and ferrets and confirmed that the use of inactivated A/H3N2 vaccines prevented the induction of heterosubtypic immunity to a lethal infection with influenza A/Indonesia/5/05 (H5N1) virus otherwise induced by infection with A/H3N2 influenza virus (4–6). The prevention of heterosubtypic immunity by H3N2 vaccination correlated with reduced virus-specific CD8+ T cell responses.
Furthermore, epidemiological data obtained during the 2009 pandemic suggest that previous vaccination against seasonal influenza increased the risk of infection with the antigenically distinct influenza A/H1N1 pandemic virus in children and the risk of medically attended illness caused by this virus in adults (23, 25, 37).
To this end, we collected peripheral blood mononuclear cells (PBMCs) and plasma samples from cystic fibrosis (CF) patients and otherwise healthy children undergoing correctional surgery. Since CF patients are at risk for complications caused by influenza virus infections, annual influenza vaccination is recommended from the time of CF diagnosis onwards. PBMCs of the study subjects were tested for the presence of virus-specific T cells by intracellular gamma interferon (IFN-γ) staining, and plasma samples were tested for the presence of virus-specific antibodies against various influenza A virus strains and a number of control antigens used in the national immunization program.
Study subjects. Children with CF who received inactivated influenza vaccine annually and unvaccinated children who visited the hospital to undergo correctional surgery were enrolled in this study. Inclusion criteria for CF children were age between 2 and 9 years with a first recorded vaccination against seasonal influenza viruses before or at 4 years of age and annual vaccination subsequently, no clinical signs of acute disease at the time of blood collection, no chronic treatment with immunosuppressive medications, and no laboratory-confirmed infection with influenza A/H1N1(2009) before or at the time of blood collection.
Inclusion criteria for healthy control children were age between 2 and 9 years, no vaccination against seasonal influenza, no chronic treatment with immunosuppressive medications, and no clinical signs of disease at the time of blood collection. Blood samples were collected during autumn of 2009 and winter of 2009-2010.
IgG antibody responses were similar between the two study groups.
A and B compare age and influenza virus specific CD8+ IFN-γ+ T-cell responses, C and D compare CD4+ IFN-γ+ responses. Each dot represents an individual:
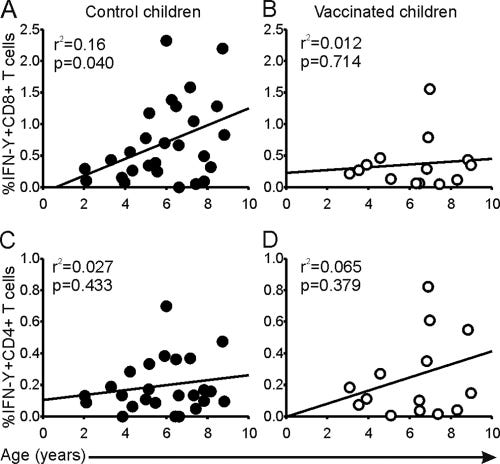
In children younger than 5 impaired CD8+ IFN-γ+ T cell responses were found in the vaccinated children when the cells were exposed to the positive control superantigen Staphylococcus enterotoxin B (SEB).
Hence its not just influenza immune response that is impaired, but bacterial too, potentially leading to more billable visits.
A and B are CD4+ responses; C and D for CD8+; E and F plot CD8+ IFN-γ+ T cell responses to SEB:
In conclusion, this runs opposite to the reasons given to justify vaccination:
A number of studies have demonstrated that annual vaccination reduces the morbidity and mortality caused by seasonal influenza in children and is (cost-)effective (23, 34–36). However, long-term annual vaccination using inactivated vaccines may hamper the induction of cross-reactive CD8+ T cell responses by natural infections and thus may affect the induction of heterosubtypic immunity. This may render young children who have not previously been infected with an influenza virus more susceptible to infection with a pandemic influenza virus of a novel subtype.
Beware the risk of inducing autoimmune disorders, but live-attenuated vaccines are probably better. Unfortunately the mRNA gold rush is moving further away from these, if anything:
Therefore, we argue for the development and use of vaccines that could induce broadly protective immune responses in children. For example, it has been demonstrated that live attenuated influenza vaccines induce virus-specific CD8+ T cell responses (21, 23a). In addition, it has been demonstrated that live attenuated influenza vaccines are also effective against drift variants in children (1, 2, 19).
There were three deaths out of only 501 participants in this stage 2 mRNA flu jab clinical trial of over 18’s32, and all these serious AE’s were judged unrelated - I think we’ve heard that one before?
One of these had even filed a report of grade 1 injection site pain, as this was sustained for 2-6 days after transfection, poor guy:
Three unsolicited SAEs were reported within 28 days of vaccination in the mRNA-1010 groups; all events were assessed as not related by the Investigator. Two SAEs were reported by participants with a significant medical history of cardiovascular disease (n = 1, mRNA-1010 100 µg (cardiac failure congestive in a participant with a history of chronic systolic heart failure and aortic insufficiency/stenosis; event was considered related to medication non-compliance); n = 1, mRNA-1010 50 µg (angina pectoris with negative troponins in a participant with a history of coronary artery disease)). The third SAE was a fatal event of unwitnessed cardiac arrest occurring 15 days after vaccination in a 67-year-old male with a medical history of diabetes mellitus, hypertension, and obesity. The participant reported grade 1 injection site pain on Days 2 through 6 after vaccination but did not report any AEs or safety complaints during the scheduled routine safety call at Day 12 after vaccination (3 days prior to his death). This event and the other 2 above-mentioned SAEs were reviewed by the Data Safety Monitoring Board and were considered unrelated to study vaccination. No events of myocarditis or pericarditis were reported.
From: “Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis”, (2023)
Related to this, in 2012 Cowling et al randomized 115 children to receive either a placebo or trivalent inactivated influenza vaccine (TIV). They found that the TIV recipients had a 4.4 fold increased risk of contracting virologically-confirmed non-influenza infections over the controls (95% confidence interval: 1.31-14.8)33.
We can therefore add impaired generalised antiviral responses to impaired bacterial superantigenic CD8+ responses, and it made no difference to their risk of flu infection either, despite serological evidence of efficacy.
Although it should have worked, it didn’t.
Antibodies are no correlate of immunity, the jabbing was completely pointless. (Emphasis mine):
Being protected against influenza, TIV recipients may lack temporary non-specific immunity that protected against other respiratory viruses.
…Among the 115 participants who were followed up, the median duration of follow-up was 272 days (interquartile range, 264–285 days), with no statistically significant differences in age, sex, household size, or duration of follow-up between TIV and placebo recipients.
…There was no statistically significant difference in the risk of confirmed seasonal influenza infection between recipients of TIV or placebo.
…TIV recipients had significantly lower risk of seasonal influenza infection based on serologic evidence.
From: “Increased Risk of Noninfluenza Respiratory Virus Infections Associated With Receipt of Inactivated Influenza Vaccine”, (2012)
And this study from 2006 by Carrat et al analyzed yearly influenza infection rates by age. They concluded that repeated vaccination at a young age substantially increased the risk of influenza in older age.
Key takes from “Repeated influenza vaccination of healthy children and adults: borrow now, pay later?”34:
SUMMARY
A growing number of publications are recommending annual influenza vaccination of healthy children and adults. However, the long-term consequences of repeated influenza vaccination are unknown. We used a simple model of recurrent influenza infection to assess the likely impact of various repeated influenza vaccination scenarios. The model was based on a Markovian framework and was fitted on annual incidence rates of influenza infection by age. We found that natural influenza infection reduced the risk of being re-infected by 15·4% (95% confidence interval 7·1–23·0). Various scenarios of repeated influenza vaccination were then simulated and compared with a reference scenario where vaccination is given from age 65 years onwards. We show that repeated vaccination at a young age substantially increases the risk of influenza in older age, by a factor ranging between 1·2 (vaccination after 50 years) to 2·4 (vaccination from birth). These findings have important implications for influenza vaccination policies.
The relative risks of influenza episodes, based on simulated cohorts of vaccinees and non-vaccinees, are depicted in Figure 2 a. Influenza vaccination from birth increases the risk of episodes at all ages after 65 years by a factor of 2·4 (95% CI 1·9–2·5), by comparison with annual influenza vaccination starting after age 65 years. Vaccination of individuals after age 50 years increases the risk of influenza infection after 65 years by a factor of 1·16 (95% CI 1·13–1·20). Other simulated scenarios gave intermediate values.
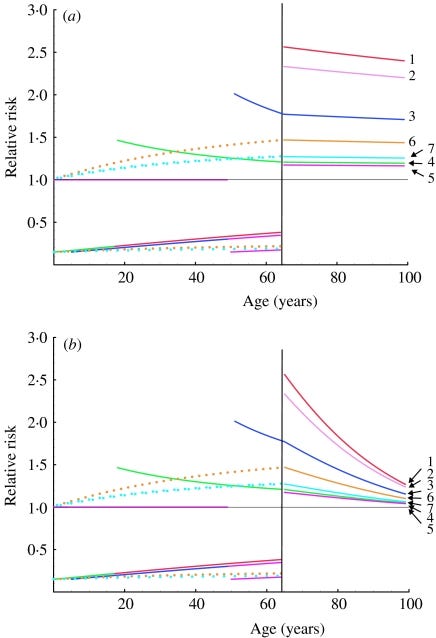
From: “Fig.2. Relative risks of influenza infection in to different scenarios of repeated influenza vaccination. Relative risks are calculated as the ratio of influenza infection rates expected in individuals in the scenarios listed in the Table, to influenza infection rates in individuals vaccinated yearly from 65 years. (a) The calculations assume constant β and VEP values. (b) Values for β and VEP decrease linearly with age between 65 years and 100 years (from 0·17 to 0·05 and from 85% to 25% respectively).”, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2870374/ We also conducted a simulation in which vaccine effectiveness declines in old age: as expected, the absolute difference in the number of influenza episodes after 65 years of age between the reference scenario and other simulations increased, but the corresponding relative risk of infection was only slightly modified.
Under the plausible assumption that protection against influenza infection lasts longer after naturally acquired infection than after vaccination, we show that repeated vaccination at a young age substantially increases the risk of influenza in older age. We integrated numerous hypotheses into the model. We assumed that influenza vaccination did not stimulate long-term cross-protective immunity, even though the live attenuated vaccine is accompanied by viral replication in the nose and thus mimics mild natural influenza infection [48].
Our model could be modified to examine how yearly infection with a live vaccine strain might compete with natural influenza infection. However, we are unaware of examples where vaccination confers stronger and more sustained protection than natural infection against subsequent infection, and do not, therefore, believe that our results would be markedly modified.
It would also have been easy to integrate different types or subtypes of influenza virus, or to compute different degrees of vaccine effectiveness or immune responses to natural infection with age, but none of these adjustments would have affected the overall results.
Our model does not deal with herd immunity. There are several reports suggesting that mass vaccination of children can result in a reduction in morbidity or mortality among non-vaccinated adults and elderly subjects [49, 50].
However, herd immunity would not affect individual relative risks, and long-term repeatedly vaccinated individuals would remain at a higher risk than newly vaccinated individuals when entering old age.
ACKNOWLEDGEMENTS
We acknowledge the financial support of the French National Institute of Health and Medical Research (INSERM).
DECLARATION OF INTEREST
None.
Leading us into the next section, in 2020 Wehenkel analysed data sets from countries with more than 0.5 million inhabitants and found a positive association between COVID-19 deaths and influenza vaccination rates35.
The author includes links to the source data so that you can conduct your own analysis or “fact checking”.
The publisher reminds us that “correlation (which this article reports upon for a specific age group) does not necessarily equal causation.” (Apart from when it fits the narrative of the day).
NB This was from before the mRNA gene jab rollouts. A key factor may well be the impaired CD8+ antiviral T cell responses:
The results showed a positive association between COVID-19 deaths and IVR of people ≥65 years-old. There is a significant increase in COVID-19 deaths from eastern to western regions in the world. Further exploration is needed to explain these findings, and additional work on this line of research may lead to prevention of deaths associated with COVID-19.
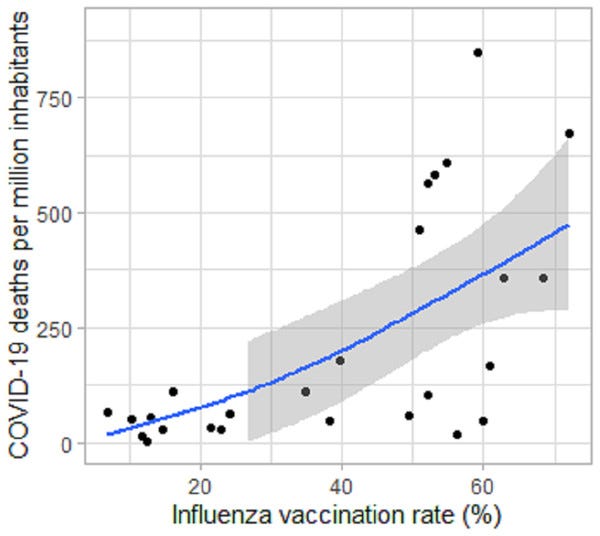
From: “Positive association between COVID-19 deaths and influenza vaccination rates in elderly people worldwide”, (2020)
Added 29th October ‘23:
IgG4 class switching in children
Hat tip to my colleague Alberto who hypothesised that this may be the case.
There aren’t many studies in this area, which is understandable, but in 2012 Nakyama et al investigated the IgG1 (which you want) and IgG4 (which you don’t) antibody responses to an alum-adjuvanted H5N1 whole virion inactivated vaccine or WIV.
Its not clear how relevant the alum is to class switching, and a 2017 study found it was more associated with IgE rather than allergen-specific IgE or IgG436.
Key takes from “Alum-adjuvanted H5N1 whole virion inactivated vaccine (WIV) induced IgG1 and IgG4 antibody responses in young children”37.
In this report, IgG subclass antibody responses were investigated, and most serum samples were positive for IgG1 antibody before immunization. A significant response (more than 4-fold increase) of IgG1 antibody was observed in 67/193 (34.7%) and that of gG4 antibodies in 42/193(21.8%).
Children <4 years of age showed a significant increase in IgG subclass antibodies but those ≥4 years showed lower responses. Alum- adjuvanted H5N1WIV induced an efficient immune response in young children especially <4 years.
…However, when it was administered to young infants and children at a reduced dose, 7.5 or 3 μg, a high body temperature (≥38.0 °C) was observed in >60% of recipients <7 years of age, but, unexpectedly, NT antibody titers were higher than those observed in the clinical trial in adults.
IgG4 class switching appears to be an immune damping mechanism in response to upregulation of anti-inflammatory cytokines. If class switched Bmem cells are recalled on the next exposure to the virus then a higher viral load is likely due to imprinted immunosuppression. The effect was greatest the younger the vaccinee:
Human IgG1 reflected not only Th1 cytokine response but also Th2 cytokine activation. IgG4 subclass switch depends on IL-4 and IL-13, which are considered part of a Th2 response.
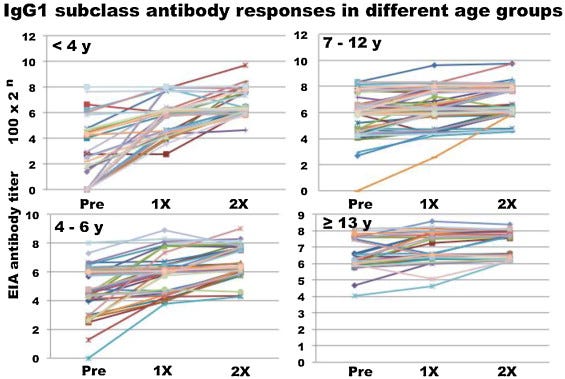
The IgG4 response is shown in Fig. 3A. In 134 recipients, IgG4 antibody was negative before vaccination without a significant response after two dose vaccinations and 42 recipients (21.8%) showed positive responses.
This is a huge response, almost equal to igG1:
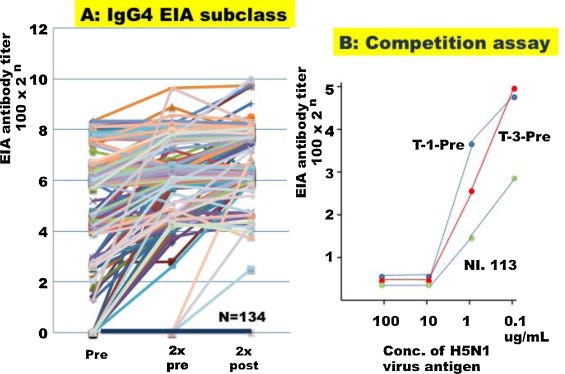
The authors conclude by warning against using alum adjuvanted flu vaccines in the very young and suggest reformulation.
I would suggest not using it at all and instead make sure they are well nourished.
In this report, a significant IgG1 and IgG4 antibody responses were observed after immunization with alum-adjuvanted H5 WIV especially in young infants <4 years. It provided efficient immune response in young naïve infants. Considering the previous report that alum-adjuvanted vaccine induced inflammatory cytokines, including IFN-α, IL-1ß, IL-6, and TNF-α, they would modulate the expression of co-stimulatory molecules recognized by naïve CD4 helper T cells. Therefore, the IgG4 antibody response seems to be T cell-dependent, induced by innate immune impacts of WIV with alum adjuvant. But, it caused high incidence of febrile reactions, and efficient influenza vaccine formulation for priming in young infants is expected with low incidence of febrile reactions.
…In a recent study the combination of influenza antigen with MF59 or Alum gave a strong IgG1 antibody responses associated with IL-5 producing T cells indicating Th2 skewing, whilst combination of haemagglutinin with the cationic liposomal adjuvant CAF01 led to a more Th1 and Th17 biased cellular response.
…Alum is the most commonly included adjuvant in influenza vaccines, but even then is only included in five vaccines. The other adjuvants used are virosomes (Inflexal V), MF59 (FluAd), AS03 (Pandemrix). AF03 was licensed for as part of Humenza, but this product was never marketed. Heat labile enterotoxin (LT) was licensed as part of Nasalflu, but this has been withdrawn.
…Strikingly the immunological mechanism of action of Alum is still not entirely understood.
From: “Adjuvanted influenza vaccines” (2018)
Influenza vaccine efficacy
Guessing the dominant strain(s) in the coming year is a challenge, and quite often they get it wrong as the virus mutates away.
2020 data is absent as there is “Not enough data to compute”. Which is curious as there has never been as much population level PCR testing as there was that year. I would like to see the data they did have?
As judged by standards set by the European Medicines Agency (EMA), almost all of these boosters would have been judged as failures and unfit for approval:
EMA has not set a minimum level of efficacy for approval. This is because it looks at the overall balance of safety and efficacy for each vaccine individually before concluding on whether or not it will approve the vaccine.
For example, a vaccine could have other advantages, such as:
very few side effects;
easy storage and delivery;
good results in a specific age group or type of population that may respond less well to other types of vaccines.
Nevertheless, medicine developers have been asked to design studies to demonstrate a rate of efficacy of at least 50%.
From: “COVID-19 vaccines: studies for approval”
To measure efficacy, the CDC use a combination of randomized controlled trials (RCTs) and observational studies.
There are many confounding factors that need to be allowed for. For example using a test-negative design where the person with a respiratory infection has sought medical attention, is enrolled in a care setting and influenza is confirmed by PCR vs data gleaned from randomised testing of the wider population.
And if the outcome you use to assess effectiveness is hospitalisation or worse then it means you are being misled if you think the jab will stop you getting flu or being very ill for days, laid up in bed. That would still count as “effectiveness”:
…The ratio of vaccinated to unvaccinated persons (i.e., the odds of flu vaccination) is compared among patients with and without laboratory-confirmed flu. In this way, a test-negative design study estimates VE by comparing vaccination rates among persons with confirmed flu illness (also called “cases”) versus persons with similar illness who do not have flu (also called “controls”) based on laboratory tests. The test-negative design reduces selection bias due to health care seeking behaviors. Other observational study designs have also been used to estimate flu vaccine effectiveness.
What are vaccine effectiveness point estimates and confidence intervals?
CDC typically presents flu vaccine effectiveness (VE) as a single point estimate: for example, 60%. This point estimate represents the reduction in risk provided by a flu vaccine. CDC vaccine effectiveness studies measure different outcomes. For example, outcomes measured can include laboratory-confirmed flu illness (that results in a doctor’s visit), hospitalizations or intensive care unit (ICU) admissions. For these outcomes, a VE point estimate of 60% means that on average the flu vaccine reduces a person’s risk of that flu outcome by 60%.
From: “How Flu Vaccine Effectiveness and Efficacy are Measured”
As this is an average from all their data we really need to know efficacy grouped by age? As they don’t provide an easily accessible breakdown we need to look for other studies for clues as to real world efficacy in the elderly.
In 2022, Burton et al published a study where they took blood samples from 10 healthy vaccinated volunteers aged 22-36 and 10 healthy vaccinated volunteers aged 67-86. They had all received the 2016–2017 trivalent seasonal influenza vaccine (TIV).
Sera and peripheral blood mononuclear cells (PBMCs) were then analysed for anti-influenza antibody production and B cell subsets.
Their study shows that the aged human germinal centre GC reaction and memory B cell response following vaccination is defective.
Key takes from “The memory B cell response to influenza vaccination is impaired in older persons”38:
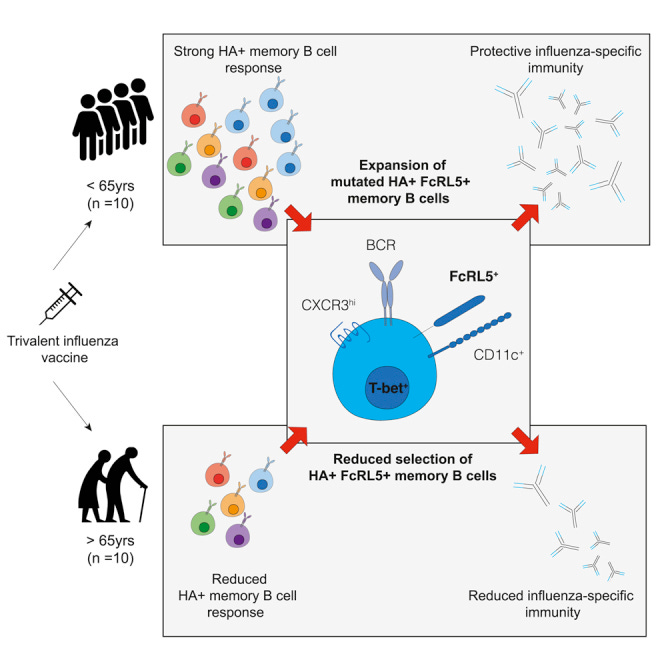
Aging is associated with a decline in the efficacy and quality of the humoral immune response to infection and vaccination, resulting in incomplete protection from infection. In this study we use the trivalent influenza vaccine and HA labeling to track vaccine-specific B cells before and after immunization of younger and older persons. We show that the formation of Bmem cells is attenuated in individuals aged 67–86 years old, including the impaired formation of FCRL5+ B cells that have the transcriptional signature characteristic of T-bet+ B cells (Li et al., 2016), collectively referred to here as “atypical memory B cells.”
Together, our data show that atypical memory B cells expand to a lesser degree in older persons and show reduced evidence of positive selection, indicative of impaired GC function following vaccination in older people.
While HA-specific FcRL5+ B cells identified here display many overlapping features with dysfunctional B cells, our study and others (Andrews et al., 2015; Sutton et al., 2021) suggest that FcRL5+ B cells form part of a “typical” immune response following vaccination associated with robust recall responses to infection (Kim et al., 2019) and with intact capacity to undergo class-switch recombination (Weiss et al., 2009; Muellenbeck et al., 2013; Portugal et al., 2015; Li et al., 2016) and SHM (Muellenbeck et al., 2013; Portugal et al., 2015).
These results contrast with accumulations of dysfunctional CD21low B cells associated with aging. We propose that this discrepancy is likely due to the investigation of vaccine-specific responses here, as opposed to the polyclonal B cell response.
FCRL5+ B cells from older individuals showed evidence of reduced positive selection, despite comparable levels of class-switch recombination and somatic hypermutation between older and younger individuals. These data support several reports suggesting that while the mechanism of somatic hypermutation is intact in aging, selection after mutation is impaired (Banerjee et al., 2002; Dunn-Walters, 2016; Lazuardi et al., 2005).
You start at less than 50% efficacy, factor in impaired memory B response and then you must expect a further significant decline in efficacy in the very population subset that the vaccine is targeting.
In this study there is virtually no response at day 7 or by day 42 in older vaccinees:

In summary, we report a defect in the differentiation and expansion of vaccine-specific Bmem following immunization in older persons. Importantly, this work identifies a defect in the expansion of FcRL5+ Bmem in older people following seasonal influenza vaccination. Understanding how Bmem cell formation and recall is altered with age is central to understanding how we can harness vaccinations to provide effective and durable protection against infection in older people, a population at high risk of mortality and morbidity from infections.
A further study by the CDC using flu VE data from 2018-19, which started out at only 29%, further declined by 7.5% per 30 days post vaccination against H3N2-associated hospitalisation. Zero VE would have been reached in months.
Key takes from “Waning Vaccine Effectiveness Against Influenza-Associated Hospitalizations Among Adults, 2015–2016 to 2018–2019, United States Hospitalized Adult Influenza Vaccine Effectiveness Network”39 (2020):
Thank you NHS for bringing it forward to early September in the UK.
January-February is going to be interesting for respiratory infection rates.
Mounting evidence that vaccine-induced immunity wanes over the course of an influenza season [1, 2] suggests that early vaccination (ie, in July and August) may result in suboptimal immunity before the end of the influenza season, particularly among older adults.
To balance the need to immunize before influenza circulation with concern about waning immunity, US guidelines recommend that vaccination be offered by the end of October.
Here, we report estimates of intraseason waning of vaccine protection against influenza-associated hospitalizations observed among adults enrolled in the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN).
Datasets included 3016 participants for analysis of VE against influenza A(H3N2), 1492 participants for analysis of VE against influenza A(H1N1)pdm09, and 1060 participants for analysis of VE against influenza B/Yamagata–associated hospitalizations. The mean participant age was 61, 59, and 60 years in the influenza A(H3N2), A(H1N1)pdm09, and B/Yamagata analyses, respectively.
These are wide error bars too, which is indicative of inconsistencies in the source data and phenotypic variations in response to vaccination:
Maximum VE was observed shortly after vaccination, followed by an absolute decline in VE of about 8%–9% per month. Rate of decline was similar across influenza subtypes. Among older adults, we observed a slightly faster rate of decline of 10%–11% per month.
Our results contribute to evidence suggesting that influenza vaccine protection may wane significantly in a matter of months. The public health implications of these findings warrant closer examination because even a 1- to 2-month delay in annual vaccination could improve VE by 10%–20%. If such an approach does not encroach on the annual influenza season, delay vaccine delivery, or reduce vaccine uptake, it could lead to appreciable gains in public health benefits given the large burden of influenza morbidity and mortality annually in the US.
Alternative therapeutics
By now, on the balance of information available from these studies, you have probably concluded that vaccination is at best of limited short term benefit.
And at worst we see data indicating increased rates of bacterial and viral infections, together with cumulative heart damage of varying degrees and a significant tailwind for the growth or recurrence of some cancers, albeit not on the scale of the experimental mRNA gene agents.
But doing nothing isn’t a sensible option either for most of us.
The short lived reduced mortality from vaccinations may be reason enough in itself for some - that is a decision to be made between you and your physician. The purpose of this Substack is to help you make a more informed choice either way.
Before I knew more about nutrition and health I had two bouts of influenza in the 1990’s. The first time I was bedridden, unable to eat and feverish with hallucinations for 3 days. It was the worst viral infection I have ever had. And I was much younger then so can well imagine the effects on those with even more impaired immunity than I had through lifestyle. Think “student diet” and you get the picture.

The good news is that influenza viruses are relatively easy to impair by almost every therapeutic in our arsenal. And no, that doesn’t include “Pot Noodles™”.
If it was genuinely a “health service” the NHS and other agencies would be making this information freely available and sponsoring further clinical research, instead of having only a rusty hammer in the toolbox.
I must also caution that viral infections in the elderly should be taken seriously as they can lead to the threat of secondary infections such as bacterial pneumonia40 that may prove fatal to the infirm (hat tip to Jikky’s #3tablets campaign and Jessica Rose for raising awareness).
I won’t go into great depth here with dosing etc.
For further reading see my other Substacks or this post:
Not medical advice as we all have different tolerances, ages and med histories, but I would start with making sure you have sufficient C, D and zinc from your diet or by supplementation and keep NAC etc back for the moment you first become symptomatic.
Daily aspirin ticks many boxes and you can incorporate some of the others into your diet or via infusions.
This is by no means the definitive list, these are only the ones I could think of but you get the idea!
“The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections”41, (1999):
Overall, reported flu and cold symptoms in the test group decreased 85% compared with the control group after the administration of megadose Vitamin C. Conclusion: Vitamin C in megadoses administered before or after the appearance of cold and flu symptoms relieved and prevented the symptoms in the test population compared with the control group.
“Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials”42, (2022):
The study finding indicates that substitution with vitamin D significantly reduces the risk of influenza infections (RR = 0.78, 95% CI:0.64-0.95). No evidence of publication bias was observed.
“ZINC INTAKE AND RESISTANCE TO H1N1 INFLUENZA”43, (2010):
Zinc deficiency is relevant to H1N1 influenza because it decreases cell-mediated immunity.4 At the practical level, zinc treatment has been found to be efficacious for a variety of infections4; in low-income Mexican Americans aged 6 to 7 years who are not visibly ill but have mild zinc deficiency (normal plasma zinc), a randomized trial showed that the administration of zinc and other micronutrients together were significantly more efficacious for cell-mediated immunity than was administration of other micronutrients alone.5
“The Role of Zinc in Antiviral Immunity”44, (2019):
In vitro replication of influenza (PR/8/34) is significantly inhibited by the addition of the zinc ionophore pyrrolidine dithiocarbamate (110), perhaps through inhibition of the RNA-dependent RNA polymerase (RdRp), as had been suggested 30 y earlier (111). In similar fashion, severe acute respiratory syndrome (SARS) coronavirus RdRp template binding and elongation was inhibited by zinc in Vero-E6 cells (60). Moreover, zinc salts were shown to inhibit respiratory syncytial virus, even while zinc was incubated with HEp-2 cells only before infection, and then removed (72).
“N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus”45, (2009):
The antioxidant N-acetyl-L-cysteine (NAC) had been shown to inhibit replication of seasonal human influenza A viruses. Here, the effects of NAC on virus replication, virus-induced pro-inflammatory responses and virus-induced apoptosis were investigated in H5N1-infected lung epithelial (A549) cells. NAC at concentrations ranging from 5 to 15 mM reduced H5N1-induced cytopathogenic effects (CPEs), virus-induced apoptosis and infectious viral yields 24 h post-infection. NAC also decreased the production of pro-inflammatory molecules (CXCL8, CXCL10, CCL5 and interleukin-6 (IL-6)) in H5N1-infected A549 cells and reduced monocyte migration towards supernatants of H5N1-infected A549 cells. The antiviral and anti-inflammatory mechanisms of NAC included inhibition of activation of oxidant sensitive pathways including transcription factor NF-kappaB and mitogen activated protein kinase p38…AC inhibits H5N1 replication and H5N1-induced production of pro-inflammatory molecules. Therefore, antioxidants like NAC represent a potential additional treatment option that could be considered in the case of an influenza A virus pandemic.
“Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study”46, (2017):
Aspirin was found to be highly effective against influenza A H1N1 virus. The antiviral activity against further respiratory RNA viruses was less distinct. Respiratory syncytial virus was minimally inhibited. However, the activity of aspirin against rhinoviruses was more pronounced. Aspirin demonstrated antiviral activity against all human rhinoviruses (HRV), but the effect on members of the "major group" viruses, namely HRV14 and HRV39, was greater than on those of the "minor group," HRV1A and HRV2.
“Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry”47, (2015):
Here we found that quercetin inhibited influenza infection with a wide spectrum of strains, including A/Puerto Rico/8/34 (H1N1), A/FM-1/47/1 (H1N1), and A/Aichi/2/68 (H3N2) with half maximal inhibitory concentration (IC50) of 7.756 ± 1.097, 6.225 ± 0.467, and 2.738 ± 1.931 μg/mL, respectively. Mechanism studies identified that quercetin showed interaction with the HA2 subunit. Moreover, quercetin could inhibit the entry of the H5N1 virus using the pseudovirus-based drug screening system. This study indicates that quercetin showing inhibitory activity in the early stage of influenza infection provides a future therapeutic option to develop effective, safe and affordable natural products for the treatment and prophylaxis of IAV infections.
“Inhibition of influenza A virus replication by resveratrol”48, (2005):
We found that RV strongly inhibited the replication of influenza virus in MDCK cells but that this activity was not directly related to glutathione-mediated antioxidant activity. Rather, it involved the blockade of the nuclear-cytoplasmic translocation of viral ribonucleoproteins and reduced expression of late viral proteins seemingly related to the inhibition of protein kinase C activity and its dependent pathways. RV also significantly improved survival and decreased pulmonary viral titers in influenza virus-infected mice. No toxic effects were observed in vitro or in vivo. That RV acts by inhibiting a cellular, rather than a viral, function suggests that it could be a particularly valuable anti-influenza drug.
“Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions”49, (2019):
The crude pomegranate peel extract and its n-butanol and ethyl acetate fractions had the highest inhibitory effect against influenza A virus with IC50 value of 6.45, 6.07 and 5.6 μg/ml in MDCK cells, respectively. Our results also showed that, the production of virus was significantly reduced upon treatment with crude extract, n-butanol and ethyl acetate fractions in a dose-dependent manner (p<0.05).
“Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal?”50, (2020):
From 2012 onwards, there have multiple reports that ivermectin has antiviral properties [4,5,7,8,9,10,11,12,13,14,15,16,17] towards a growing number of RNA viruses, including human immunodeficiency virus (HIV)-1, influenza, flaviruses such as dengue virus (DENV) and Zika virus (ZIKV) and, most notably, SARS-CoV-2 (COVID-19)
“Baicalin inhibits influenza virus A replication via activation of type I IFN signaling by reducing miR‑146a”51, (2019):
Here, it was demonstrated that baicalin inhibits the H1N1 and H3N2 viruses in A549 cells. Subsequently, it was found that miR‑146a was markedly downregulated by treatment of baicalin. Additionally, further experiments revealed that miR‑146a was able to promote the replication of H1N1 and H3N2 by targeting TNF receptor‑associated factor 6 (TRAF6), a pivotal adaptor in the interferon (IFN) production signaling pathway, to downregulate type I IFN production, and enrichment of miR‑146a eliminated the anti‑IVA effects of baicalin on the H1N1 and H3N2 viruses. Additionally, in vivo experiments demonstrated that baicalin could protect mice during H1N1 infection. Taken together, our findings firstly illustrated the anti‑IVA molecular mechanism of baicalin and provide new evidence for targeting miRNAs to prevent and treat viral infection, such as the H1N1 and H3N2 viruses.
“Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice”52, (2018):
The results showed that berberine strongly suppressed viral replication in A549 cells and in mouse lungs. Meanwhile, berberine relieved pulmonary inflammation and reduced necrosis, inflammatory cell infiltration, and pulmonary edema induced by viral infection in mice when compared with vehicle-treated mice. Berberine suppressed the viral infection-induced up-regulation of TLR7 signaling pathway, such as TLR7, MyD88, and NF-κB (p65), at both the mRNA and protein levels. Furthermore, berberine significantly inhibited the viral infection-induced increase in Th1/Th2 and Th17/Treg ratios as well as the production of inflammatory cytokines.
“Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways”53, (2018):
The results showed that curcumin could directly inactivate IAV, blocked IAV adsorption and inhibited IAV proliferation. As for the underlying mechanisms, we found that curcumin could significantly inhibit IAV-induced oxidative stress, increased Nrf2, HO-1, NQO1, GSTA3 and IFN-β production, and suppressed IAV-induced activation of TLR2/4/7, Akt, p38/JNK MAPK and NF-κB pathways.
“Anti-influenza Viral Effects of Honey In Vitro: Potent High Activity of Manuka Honey”54, (2014):
Manuka honey efficiently inhibited influenza virus replication (IC50 = 3.6 ± 1.2 mg/mL; CC50 = 82.3 ± 2.2 mg/mL; selective index = 22.9), which is related to its virucidal effects. In the presence of 3.13 mg/mL manuka honey, the IC50 of zanamivir or oseltamivir was reduced to nearly 1/1000th of their single use.
“Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial”55, (2015):
Recovery from illness was comparable in the 2 treatment groups at 1.5% versus 4.1% after 1 day, 50.2% versus 48.8% after 5 days, and 90.1% versus 84.8% after 10 days of treatment with Echinaforce Hotdrink and oseltamivir, respectively. Noninferiority was demonstrated for each day and overall (95% CI, 0.487–0.5265 by generalized Wilcoxon test). Very similar results were obtained in the group with virologically confirmed influenza virus infections and in a retrospective analysis during the peak influenza period. The incidence of complications was lower with Echinaforce Hotdrink than with oseltamivir (2.46% vs 6.45%; P = 0.076) and fewer adverse events (particularly nausea and vomiting) were observed with Echinaforce Hotdrink.
“Echinacea purpurea aerial extract alters course of influenza infection in mice”56, (2010):
Influenza infection is a major clinical problem and Echinacea purpurea, a widely consumed botanical product, is purported to alter the course of respiratory infections including influenza. Mice infected with WSN influenza A and treated with E. purpurea polysaccharide extract had less weight loss than untreated mice but similar pulmonary viral titers. Echinacea-treated mice had lower systemic and pulmonary KC and IL-10 levels and lower systemic IFN-gamma levels following influenza infection. These suggest that E. purpurea alters the clinical course of influenza infection in mice through modulation of cytokines and not direct antiviral activity.
“Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE₂ from RAW 264.7 macrophage-like cells”57, (2010):
Seventeen extracts and 4 alkylamides were tested for the ability to inhibit production of cytokines, chemokines, and PGE₂ from RAW 264.7 macrophage-like cells infected with the H1N1 influenza A strain PR/8/34. The alkylamides undeca-2Z,4E-diene-8,10-diynic acid isobutylamide, dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamide, dodeca-2E,4E-dienoic acid isobutylamide, and undeca-2E-ene-8,10-diynoic acid isobutylamide suppressed production of TNF-α and PGE₂ from infected cells. Dodeca-2E,4E-dienoic acid isobutylamide was especially effective at inhibiting production of these mediators and also strongly inhibited production of G-CSF, CCL2/MCP-1, CCL3/MIP-1α and CCL5/RANTES…Our in vitro experiments suggest that E. purpurea extracts have the potential for use in alleviating the symptoms and pathology associated with infections with influenza A; however, further study will be necessary to define procedures necessary to unmask the alkylamide activity in crude extracts.
Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References
Xu T, Ding W, Tariq MA, et al. Molecular mechanism and therapy application of necrosis during myocardial injury. J Cell Mol Med. 2018;22(5):2547-2557. doi:10.1111/jcmm.13575
COVID — ZOE. Accessed September 13, 2023. https://zoe.com/learn/category/covid
Corticosteroids: Mechanisms of action, contraindications, inhibition of p53-induced apoptosis and alternative medications. Accessed September 13, 2023. https://doorlesscarp953.substack.com/p/corticosteroids-mechanisms-of-action
Zhang L, Rana I, Shaffer RM, Taioli E, Sheppard L. Exposure to Glyphosate-Based Herbicides and Risk for Non-Hodgkin Lymphoma: A Meta-Analysis and Supporting Evidence. Mutat Res. 2019;781:186-206. doi:10.1016/j.mrrev.2019.02.001
OECD. Health at a Glance 2019: OECD Indicators. OECD; 2019. doi:10.1787/4dd50c09-en
Domnich A, Grassi R, Fallani E, et al. Changes in Attitudes and Beliefs Concerning Vaccination and Influenza Vaccines between the First and Second COVID-19 Pandemic Waves: A Longitudinal Study. Vaccines. 2021;9(9):1016. doi:10.3390/vaccines9091016
Domnich A, Grassi R, Fallani E, et al. Changes in Attitudes and Beliefs Concerning Vaccination and Influenza Vaccines between the First and Second COVID-19 Pandemic Waves: A Longitudinal Study. Vaccines. 2021;9(9):1016. doi:10.3390/vaccines9091016
Lyons-Weiler J, Thomas P. Relative Incidence of Office Visits and Cumulative Rates of Billed Diagnoses Along the Axis of Vaccination. International Journal of Environmental Research and Public Health. 2020;17(22):8674. doi:10.3390/ijerph17228674
Kaplan MR, Nelson M, Klote M, Engler R. Chest Pain: An Unrecognized Side Effect Temporally Associated with Influenza Vaccine. Journal of Allergy and Clinical Immunology. 2006;117(2):S207. doi:10.1016/j.jaci.2005.12.819
Engler RJM, Nelson MR, Collins LC, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
Nakamura R, Ando S ichi, Kato S, Kadokami T. Acute Lymphocyte Myocarditis Associated with Influenza Vaccination. Intern Med. 2022;61(15):2307-2313. doi:10.2169/internalmedicine.8855-21
Tschöpe C, Cooper LT, Torre-Amione G, Van Linthout S. Management of Myocarditis-Related Cardiomyopathy in Adults. Circulation Research. 2019;124(11):1568-1583. doi:10.1161/CIRCRESAHA.118.313578
Hassell ME, Vlastra W, Robbers L, et al. Long-term left ventricular remodelling after revascularisation for ST-segment elevation myocardial infarction as assessed by cardiac magnetic resonance imaging. Open Heart. 2017;4(1):e000569. doi:10.1136/openhrt-2016-000569
Azevedo PS, Polegato BF, Minicucci MF, Paiva SAR, Zornoff LAM. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq Bras Cardiol. 2016;106(1):62-69. doi:10.5935/abc.20160005
Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2020;33(3):127-148. doi:10.1093/intimm/dxaa078
de Jong PR, Schadenberg AWL, van den Broek T, et al. STAT3 regulates monocyte TNF-alpha production in systemic inflammation caused by cardiac surgery with cardiopulmonary bypass. PLoS One. 2012;7(4):e35070. doi:10.1371/journal.pone.0035070
Mohanty S, Joshi SR, Ueda I, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;211(7):1174-1184. doi:10.1093/infdis/jiu573
Research Funding - an overview | ScienceDirect Topics. Accessed September 15, 2023. https://www.sciencedirect.com/topics/nursing-and-health-professions/research-funding
Gögenur M, Fransgård T, Krause TG, Thygesen LC, Gögenur I. Association of influenza vaccine and risk of recurrence in patients undergoing curative surgery for colorectal cancer. Acta Oncologica. 2021;60(11):1507-1512. doi:10.1080/0284186X.2021.1967444
A global language: cancer stages and TNM Classification | UICC. Accessed September 15, 2023. https://www.uicc.org/global-language-cancer-stages-and-tnm-classification
Draganov D, Han Z, Rana A, Bennett N, Irvine DJ, Lee PP. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. NPJ Breast Cancer. 2021;7(1):22. doi:10.1038/s41523-021-00229-5
Paessler S, Veljkovic V. Should safety of the flu vaccine for cancer patients be reexamined? Published online January 2, 2018. doi:10.12688/f1000research.13428.1
Epitope - an overview | ScienceDirect Topics. Accessed September 16, 2023. https://www.sciencedirect.com/topics/chemistry/epitope
Autoimmune disorders: COVID-19, spike protein & homologous epitopes. Published July 2, 2022. Accessed September 15, 2023. https://doorlesscarp953.substack.com/p/autoimmune-disorders-covid-19-spike
Labombarde JG, Pillai MR, Wehenkel M, et al. Induction of broadly reactive influenza antibodies increases susceptibility to autoimmunity. Cell Rep. 2022;38(10):110482. doi:10.1016/j.celrep.2022.110482
Wang X, Xia Y. Anti-double Stranded DNA Antibodies: Origin, Pathogenicity, and Targeted Therapies. Frontiers in Immunology. 2019;10. Accessed September 16, 2023. https://www.frontiersin.org/articles/10.3389/fimmu.2019.01667
Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6(17):335. doi:10.21037/atm.2018.07.32
Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun. 2020;3:100051. doi:10.1016/j.jtauto.2020.100051
Bernard-Valnet R, Frieser D, Nguyen XH, et al. Influenza vaccination induces autoimmunity against orexinergic neurons in a mouse model for narcolepsy. Brain. 2022;145(6):2018-2030. doi:10.1093/brain/awab455
Bodewes R, Fraaij PLA, Geelhoed-Mieras MM, et al. Annual Vaccination against Influenza Virus Hampers Development of Virus-Specific CD8+ T Cell Immunity in Children▿. J Virol. 2011;85(22):11995-12000. doi:10.1128/JVI.05213-11
Anonymous. Turtles All The Way Down: Vaccine Science and Myth. (O’Toole Z, J.D MH, eds.). The Turtles Team; 2022.
https://www.amazon.co.uk/Turtles-All-Way-Down-Vaccine/dp/9655981045
Lee IT, Nachbagauer R, Ensz D, et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis. Nat Commun. 2023;14(1):3631. doi:10.1038/s41467-023-39376-7
Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54(12):1778-1783. doi:10.1093/cid/cis307
CARRAT F, LAVENU A, CAUCHEMEZ S, DELEGER S. Repeated influenza vaccination of healthy children and adults: borrow now, pay later? Epidemiol Infect. 2006;134(1):63-70. doi:10.1017/S0950268805005479
Wehenkel C. Positive association between COVID-19 deaths and influenza vaccination rates in elderly people worldwide. PeerJ. 2020;8:e10112. doi:10.7717/peerj.10112
Hoyt AEW, Schuyler AJ, Workman LJ, Conaway M, Heymann PW, Steinke JW, et al. Changes in production of total immunoglobulin E but not food allergen-specific IgE or IgG4 in at-risk children after exposure to aluminum adjuvant. Journal of Allergy and Clinical Immunology. 2017 Feb 1;139(2):AB142.
Nakayama T, Kumagai T, Ishii KJ, Ihara T. Alum-adjuvanted H5N1 whole virion inactivated vaccine (WIV) induced IgG1 and IgG4 antibody responses in young children. Vaccine. 2012 Dec 14;30(52):7662–6.
Burton AR, Guillaume SM, Foster WS, et al. The memory B cell response to influenza vaccination is impaired in older persons. Cell Rep. 2022;41(6):111613. doi:10.1016/j.celrep.2022.111613
Ferdinands JM, Gaglani M, Martin ET, et al. Waning Vaccine Effectiveness Against Influenza-Associated Hospitalizations Among Adults, 2015–2016 to 2018–2019, United States Hospitalized Adult Influenza Vaccine Effectiveness Network. Clin Infect Dis. 2021;73(4):726-729. doi:10.1093/cid/ciab045
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8499703/pdf/ciab045.pdf
Jung HS, Kang BJ, Ra SW, et al. Elucidation of Bacterial Pneumonia-Causing Pathogens in Patients with Respiratory Viral Infection. Tuberc Respir Dis (Seoul). 2017;80(4):358-367. doi:10.4046/trd.2017.0044
Zhu Z, Zhu X, Gu L, Zhan Y, Chen L, Li X. Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials. Front Nutr. 2021;8:799709. doi:10.3389/fnut.2021.799709
Gorton HC, Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J Manipulative Physiol Ther. 1999;22(8):530-533. doi:10.1016/s0161-4754(99)70005-9
Palamara AT, Nencioni L, Aquilano K, et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191(10):1719-1729. doi:10.1086/429694
Moradi MT, Karimi A, Shahrani M, Hashemi L, Ghaffari-Goosheh MS. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J Med Biotechnol. 2019;11(4):285-291.
Jans DA, Wagstaff KM. Ivermectin as a Broad-Spectrum Host-Directed Antiviral: The Real Deal? Cells. 2020;9(9):2100. doi:10.3390/cells9092100
Li R, Wang L. Baicalin inhibits influenza virus A replication via activation of type I IFN signaling by reducing miR‑146a. Mol Med Rep. 2019;20(6):5041-5049. doi:10.3892/mmr.2019.10743
Wu W, Li R, Li X, et al. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses. 2015;8(1):6. doi:10.3390/v8010006
Yan YQ, Fu YJ, Wu S, et al. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother Res. 2018;32(12):2560-2567. doi:10.1002/ptr.6196
Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir Viruses. 2017;11(1):85-92. doi:10.1111/irv.12421
Dai J, Gu L, Su Y, et al. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int Immunopharmacol. 2018;54:177-187. doi:10.1016/j.intimp.2017.11.009
Sandstead HH, Prasad AS. ZINC INTAKE AND RESISTANCE TO H1N1 INFLUENZA. Am J Public Health. 2010;100(6):970-971. doi:10.2105/AJPH.2009.187773
Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The Role of Zinc in Antiviral Immunity. Adv Nutr. 2019;10(4):696-710. doi:10.1093/advances/nmz013
Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N. Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch Med Res. 2014;45(5):359-365. doi:10.1016/j.arcmed.2014.05.006
Rauš K, Pleschka S, Klein P, Schoop R, Fisher P. Effect of an Echinacea-Based Hot Drink Versus Oseltamivir in Influenza Treatment: A Randomized, Double-Blind, Double-Dummy, Multicenter, Noninferiority Clinical Trial. Curr Ther Res Clin Exp. 2015;77:66-72. doi:10.1016/j.curtheres.2015.04.001
Fusco D, Liu X, Savage C, et al. Echinacea purpurea aerial extract alters course of influenza infection in mice. Vaccine. 2010;28(23):3956-3962. doi:10.1016/j.vaccine.2010.03.047
Cech NB, Kandhi V, Davis JM, Hamilton A, Eads D, Laster SM. Echinacea and its alkylamides: effects on the influenza A-induced secretion of cytokines, chemokines, and PGE₂ from RAW 264.7 macrophage-like cells. Int Immunopharmacol. 2010;10(10):1268-1278. doi:10.1016/j.intimp.2010.07.009
Geiler J, Michaelis M, Naczk P, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79(3):413-420. doi:10.1016/j.bcp.2009.08.025







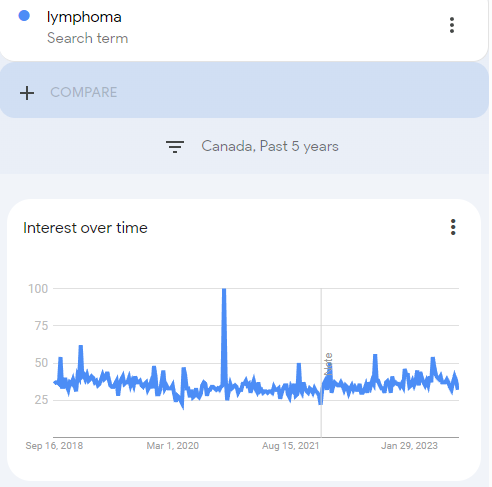


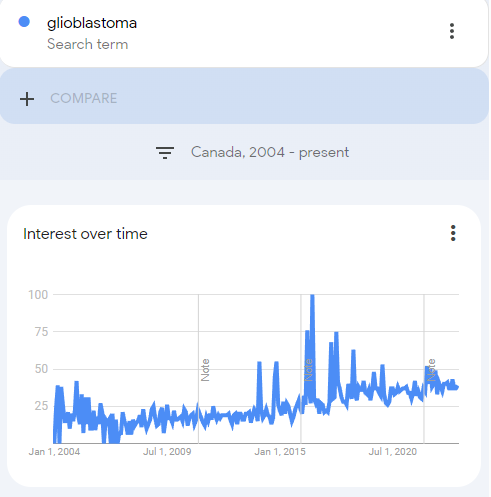







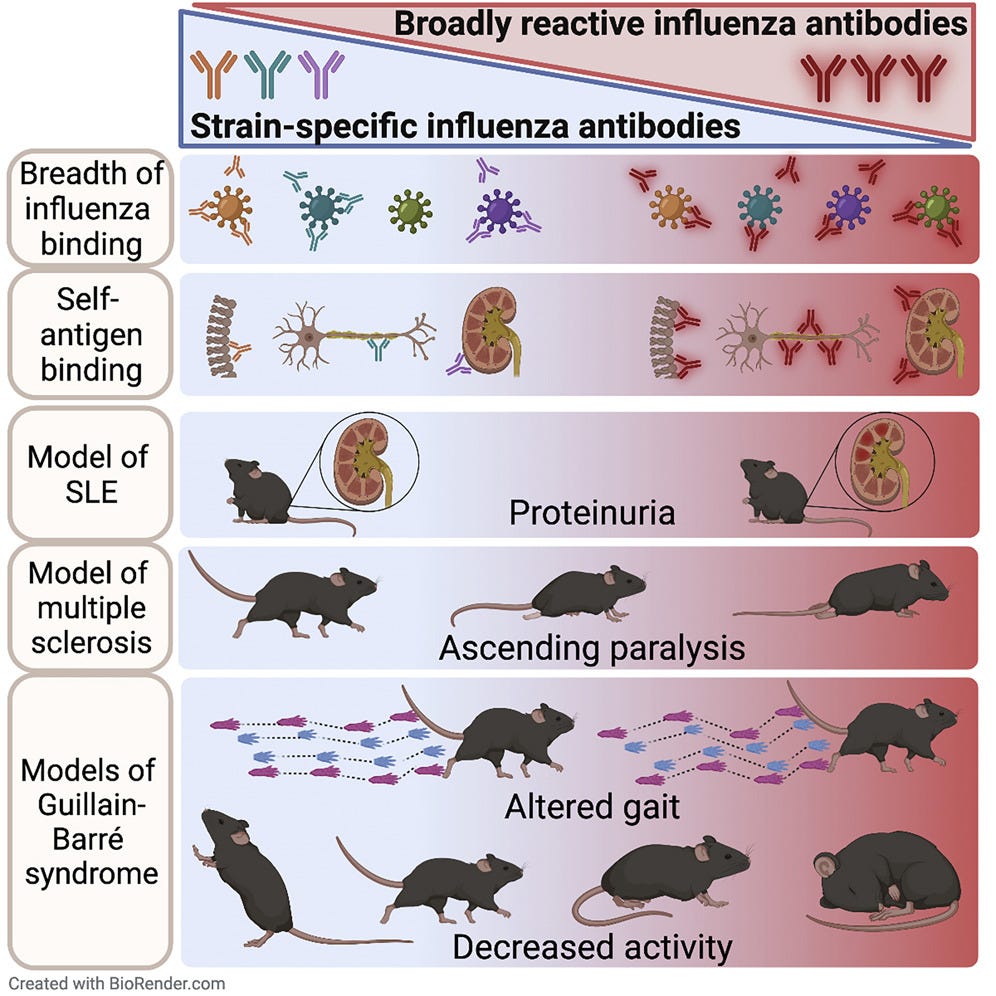







Quite an old study but, as per the Cochrane reviews, things haven't changed much since:
Study: Flu shots in elderly don't cut mortality rate
Robert Roos February 16, 2005
https://www.cidrap.umn.edu/influenza-vaccines/study-flu-shots-elderly-dont-cut-mortality-rate
Hat tip @jikkyleaks
Why have three long-running Cochrane Reviews on influenza vaccines been stabilised?
https://community.cochrane.org/news/why-have-three-long-running-cochrane-reviews-influenza-vaccines-been-stabilised