Magnesium deficiency and associated pathologies: Part 4
"...medicine sent from Heaven"
Reading time:
short story - novelette - novella 1- novel - PhD thesis - War and Peace - U.S. Tax Code
TL;DR:
Also available with the translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
In Part 3 we looked into the role of magnesium and deficiency in arthritis, recovery from renal failure, thyroid gland function, peripheral neuropathy, learning and memory, insomnia, depression, fibromyalgia, and migraine.
To conclude this mini-series the concluding pathophysiological review relates to endocrine function, and how to address magnesium deficiency through both diet and supplements, including bioavailability studies. I also include contributions from Jennifer Depew RD and Dr. Carolyn Dean.
Discussion
- Endocrine effects
The endocrine system is a messenger system consisting of hormonal feedback loops. Regulatory hormones are released by internal glands into the circulation and target distant organs. The seven main endocrine glands are the hypothalamus, the pituitary, the thyroid, the parathyroid, the adrenals, the pineal body, the ovaries, and the testes.
Over or under-production of hormones is associated with a wide range of disorders and syndromes, the commonest being diabetes. We have already discussed some of these, including insulin resistance and diabetes.
I have shortlisted three papers. The first is a study of the positive effects of magnesium on hormones in elderly Italian men, by Maggio et al.
TL;DR: Not only does it make you feel younger, but you may well live longer too!
“Magnesium and anabolic hormones in older men”1 (2011):
Optimal nutritional and hormonal statuses are determinants of successful ageing. The age associated decline in anabolic hormones such as testosterone and insulin-like growth factor 1 (IGF-1) is a strong predictor of metabolic syndrome, diabetes and mortality in older men.
Studies have shown that magnesium intake affects the secretion of total IGF-1 and increase testosterone bioactivity. This observation suggests that magnesium can be a modulator of the anabolic/catabolic equilibrium disrupted in the elderly people. However, the relationship between magnesium and anabolic hormones in men has not been investigated.
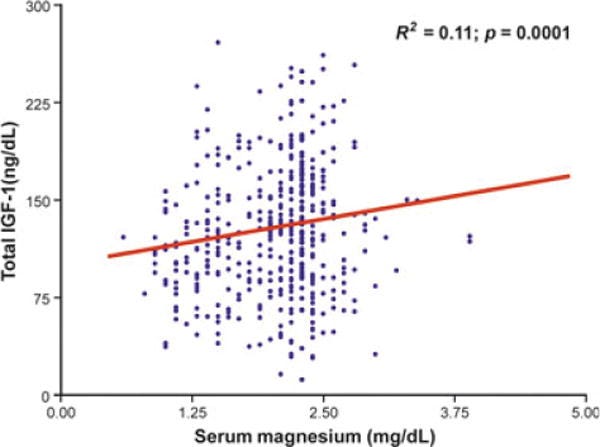
We evaluated 399 ≥65-year-old men of CHIANTI in a study population representative of two municipalities of Tuscany (Italy) with complete data on testosterone, total IGF-1, sex hormone binding globulin (SHBG), dehydroepiandrosterone sulphate (DHEAS) and serum magnesium levels.
β (SE): beta coefficient with standard error, the estimated change in the dependent variable (y) for a one-unit change in a predictor variable (x), while holding all other predictors constant. Larger figures with less variance tend to have higher significance:
Linear regression models were used to test the relationship between magnesium and testosterone and IGF-1. Mean age of the population was 74.18 ± 6.43 (years ± SD, age range 65.2-92.4). After adjusting for age, magnesium was positively associated with total testosterone (β ± SE, 34.9 ± 10.3; p = 0.001) and with total IGF-1 (β ± SE, 15.9 ± 4.8; p = 0.001).
After further adjustment for body mass index (BMI), log (IL-6), log (DHEAS), log (SHBG), log (insulin), total IGF-1, grip strength, Parkinson's disease and chronic heart failure, the relationship between magnesium and total testosterone remained strong and highly significant (β ± SE, 48.72 ± 12.61; p = 0.001).
In the multivariate analysis adjusted for age, BMI, log (IL-6), liver function, energy intake, log (insulin), log (DHEAS), selenium, magnesium levels were also still significantly associated with IGF-1 (β ± SE, 16.43 ± 4.90; p = 0.001) and remained significant after adjusting for total testosterone (β ± SE, 14.4 ± 4.9; p = 0.01). In a cohort of older men, magnesium levels are strongly and independently associated with the anabolic hormones testosterone and IGF-1.
I would prefer to see more clustered results here, with a stronger correlation and steeper regression line, but the association is apparent:

Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4623306/
The second, by Vázquez-Lorente et al, concerns the vitamin D response in post-menopausal women. This is important as vitamin D itself acts like a hormone, and is critical to immune function and calcium balance, see Part 1.
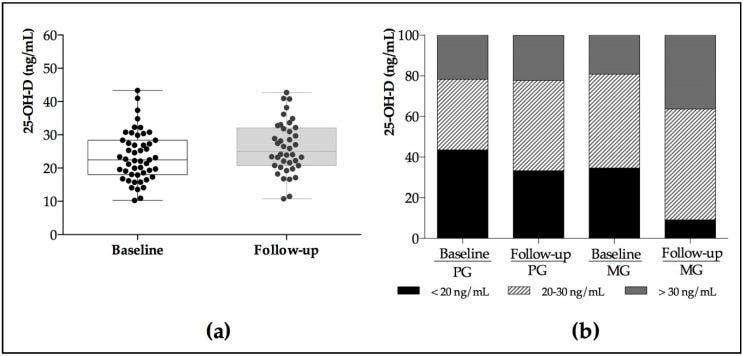
Their study included an eight-week, double-blinded, placebo-controlled, randomized intervention trial. There were 25 women in the placebo group and 27 in the magnesium group (500 mg/day of Mg).
The study doesn’t state the magnesium salt they used, but by looking up the brand I can confirm that, despite it being oxide, it significantly increased vitamin D levels in the intervention group.
“Response of Vitamin D after Magnesium Intervention in a Postmenopausal Population from the Province of Granada, Spain”2 (2020):
Menopause is a stage of hormonal imbalance in women which, in addition to other physiopathological consequences, poses a risk of deficiency of key micronutrients such as magnesium and vitamin D.
A study was made of the influence of a magnesium intervention upon vitamin D status in a postmenopausal population from the province of Granada (Spain).
Fifty-two healthy postmenopausal women between 44–76 years of age were included. Two randomized groups—placebo and magnesium (500 mg/day)—were treated during eight weeks.
Nutrient intake was assessed using questionnaires based on 72-h recall. Vitamin D was analyzed by liquid chromatography—tandem mass spectrometry.
Baseline vitamin D proved deficient in over 80% of the subjects. The administration of magnesium resulted in significantly increased vitamin D levels in the intervention group versus the controls (p < 0.05).
Magnesium supplementation improved vitamin D status in the studied postmenopausal women.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7468838/
The third paper, by Kolanu et al, was similar to the second and investigated the effects of Mg on thyroid hormones of 165 women around menopausal age:
“Activities of Serum Magnesium and Thyroid Hormones in Pre-, Peri-, and Post-menopausal Women”3 (2020):
Abstract
Introduction: Females go through a complex hormonal variation once they reach menarche. The menstrual cycle repeats every month regularly and is dependent on the normal functioning of the hypothalamus, pituitary, and ovarian hormones. The overall wellness of the females during the menstrual cycle depends greatly on nutritional status. It is common that women develop menstrual cycle-related symptoms and are routinely prone to thyroid dysfunction. The present study is carried out to assess the activities of Mg and thyroid hormones in pre-, peri-, and post-menopausal women.
Methods: A total of 165 women were recruited in the study after satisfying the inclusion criteria. An equal number of age-matched subjects were included as controls. All the subjects included in the study were selected from the patients attending various out-patient departments of the Prathima Institute of Medical Sciences, Karimnagar, Telangana, India. Blood samples from each subject were collected and analyzed by a semi-automated analyzer for the activities of Mg, and thyroid hormones tetra-iodothyronine (T4), tri-iodothyronine (T3), and thyroid-stimulating hormone (TSH).
Results: There was a statistically significant relationship between the serum Mg activities and the thyroid hormones between the study subjects and the control group. The activities of the serum Mg (1.72±0.33) in relation to the TSH (5.09±7.54) in the cases were found statistically significant (p <0.001) when compared to the serum Mg (1.8±0.20) in relation to the TSH in the control group (2.41±2.05). The activities of Mg were noted to fall in women through the peri (1.70±0.43), and postmenopausal age (1.60±0.34). There was a significant increase in the activities of TSH in women of premenopause (4.27±5.76), perimenopause (5.65±8.53), and postmenopausal age (7.19±11.07).
Conclusion: From the results of the present study, it can be concluded that the women reaching menopause could suffer from hypomagnesemia and inturn may develop thyroid and other hormonal disorders.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6996468/
The virtual health spar
“Your path to recovery begins the moment you cross the threshold.”
Grab a robe and chill…
Thanks must go to Jennifer for her diagnosis of chronic deficiency of magnesium advice at the US nonprofit Arthritis Foundation, together with some other great suggestions relating to magnesium, health, and wellbeing.
Nature is healing even in images in an office waiting room. Abstract art with jagged or straight geometry was not soothing. Nature or more serene abstract art was soothing. Driving to work had more health benefits when there was water/nature along the route instead of all urban.
Jennifer Depew, RD
From “_agnesium _atters - Part 3 by Doorless Carp. Where is that missing Mg in medical treatment?”
https://denutrients.substack.com/p/_agnesium-_atters-part-3-by-doorless
I agree with Jennifer. Our virtual health spar needs some nature scenes hanging, and perhaps an aquarium in its waiting room.

Let us begin with a soothing bath, in Epsom salts, naturally.
WebMD has some useful advice:
Why Take an Epsom Salts Bath?
You pulled a muscle lifting weights at the gym. Your skin won't stop itching. Or maybe your arthritis is acting up. Is there anything you can do, besides wait it out?
Your grandma could have the answer. Epsom salts have been used for hundreds of years to ease all kinds of aches, pains, and skin troubles. A simple soak in the tub may help you feel better.
What Are They?
Despite the name, Epsom salts aren't like the stuff you put on your fries. They're called salts because of their chemical structure. The "Epsom" part is a place in England where they're found in natural springs.
You can find them in most drugstores, usually around the aspirin and laxatives. Many grocery and natural food stores also carry them. A large box costs just a few dollars.
They're not the same as Dead Sea salts, a blend of minerals found only in the Dead Sea in the Middle East. The water and light there supposedly help with skin diseases, arthritis, and other health problems.
Epsom salts are also different from fancy bath crystals. They may not be made from the same chemicals. Plus they often have oils, colors, and perfumes to relax you and soften your skin.
How Do They Work?
In water, they break down into magnesium and sulfate. The theory is that when you soak in an Epsom salts bath, these get into your body through your skin. That hasn't been proven, but just soaking in warm water can help relax muscles and loosen stiff joints.
People use Epsom salts baths as a home treatment for:
Arthritis pain and swelling
Bruises and sprains
Fibromyalgia, a condition that makes your muscles, ligaments, and tendons hurt, and causes tender points throughout your body
Ingrown toenails
Psoriasis, a disease that causes red, itchy, scaly skin
Sore muscles after working out
Soreness from diarrhea during chemotherapy
Sunburn pain and redness
Tired, swollen feet
While there are plenty of folk remedy claims, there aren't a lot of studies to back them up. Taking this type of bath probably won't hurt you, but if you have health concerns, check with your doctor first.
How to Take an Epsom Salts Bath
The water should be very warm -- not hot, but comfortable to the touch. Add the Epsom salts while the water is running to help them dissolve.
For a standard-sized tub, use the amount suggested on the package, usually 1 to 2 cups, or the amount recommended by your doctor. Don't use Epsom salts in a hot tub, whirlpool, or other tub with jets unless the manufacturer says it's OK.
Keep the part of your body that hurts in the water for at least 12 minutes. Just relax.
Check with your doctor about how long and how often you should soak. You may need to do it just once for an ingrown toenail, or every day if you have arthritis pain.
The following essay was published in 1917 by Colonel RD Rudolf. It would appear that magnesium and its external application goes through cycles of rediscovery.
Prof. Rudolf refers to topical anaesthetic effects, the therapeutic use of magnesium back in the 17th century (Samuel Pepys era), and how Epsom salts acquired their name:4

THE USE OF EPSOM SALTS, HISTORICALLY CONSIDERED
BY COLONEL R. D. RUDOLF,
Professor of Therapeutics in the University of Toronto
THAT there is nothing new under the sun is a common saying, and never was this truer than in regard to the use of that old medicinal friend, magnesium sulphate. This drug has, of course, been used internally as a mild hydragogue for many generations, but until recently, it would appear from modern literature, its external employment had not been thought of. Thus Dr. N. H. Choksy wrote in The Lancet of February 4th, 1911, "that the common and homely drug known as 'Epsom Salts' possessed any other property save the one usually associated with it was scarcely known up to within three years ago. The anaesthetic effects resulting from its subcutaneous application, however, induced Dr. Henry Tucker, of Philadelphia General Hospital, to apply it for the relief of pain in local inflammatory conditions, with rather surprising results. For apart from the relief of pain and discomfort, it was found that it controlled and eventually led to the cure of the inflammatory process. Numerous observations in gonorrheal epididymitis and orchitis, gonorrhoeal rheumatism, acute articular rheumatism, neuritis, etc., gave equally satisfactory results."
And Dr. Tucker himself, in the Therapeutic Gazette of April, 1907, and again in that of June, 1908, elaborates this external use of the drug and gives details as to its employment. Thus, a saturated solution should be applied to the inflamed part, 15 to 20 layers of ordinary gauze being constantly kept wet with it. The gauze should not be removed for twenty-four hours and the parts then washed and the dressing reapplied. There is found to be marked bleaching of the surface, which is not followed by any deleterious effects. It causes numbness and tingling in the hands of the attendants, which may last for twenty-four hours.
The external use of magnesium sulphate thus became established and since then has been much used and to my mind beneficially used in various superficial inflammatory conditions, especially, perhaps, erysipelas. But it can now be shown that this external use of the remedy is only an echo of the very ancient, in fact the most ancient, use of the salt. During the first years after the discovery of these waters they were only used externally, and it remained for the third Lord Dudley North to suggest that this all-healing drug might also be used internally with benefit. As is commonly known, the spring was discovered at Epsom, by a farmer called Henry Wickes, or Wicker, in the dry summer of 1618. This man happened to find on the Epsom Common a small hole containing water. He dug it larger and then brought his thirsty cattle to drink there, but the beasts would have none of it. This led to much talking and further examination of the well, and then someone suggested that it might be a medicinal water, and soon the local people began to use it as such in the bathing of various open sores and painful affections. This was the only use that was made of the well for years, until, indeed, Lord Dudley North, who lived somewhere near, began to take the waters as a medicine. In 1645 he published a book, called "The Forest of Varieties", and in it he claims to have been the first to have made known "the virtues of both the Epsom and the Tonbridge waters to the King's sick subjects, the journey to the German spas being too expensive and inconvenient to sick persons, and great sums of money being thereby carried out of the Kingdom." According to Nehemiah Crew, also, physician and secretary to the Royal Society, who wrote a treatise in 1695 on "The bitter cathartic salt in the Epsom waters", which book is in the library of the Royal Society of Medicine, where I had the chance of seeing it, Lord North was the first to take the waters as a medicine. He had been in the habit of visiting the German spas, as he "laboured under a melancholy disposition." He used it with success and regarded it "as medicine sent from Heaven." By 1688, according to Gordon Home in his "Epsom, its History and Surroundings", "it was a common occurrence for doctors to order a visit to Epsom, for in the ‘Domestic State Papers’ of June 29th, in that year, we read: ‘Chatham Dockyard. John Owen to Pepys. I beg leave of absence for twelve days, being afflicted with _______ and advised to drink Epsom waters.’" Soon all the fashionable world was flocking to Epsom, and they continued to do so until in 1753 that popular physician, Dr. Richard Russel, introduced sea-bathing. The diversion in this direction was fatal to Epsom. Yet the Epsom well is still there, now surrounded by fruit trees, and the water still retains its original qualities. But where are the hosts of fashionable and more humble people who used to throng the London road, riding, driving, walking or being carried to the famous well?
A great Canadian military convalescent hospital, with its four thousand patients, is now nearby, and no doubt much "mag. sulph." is used in assisting these poor fellows back to health, but it is a salt made from dolomite or kieserite that is given, rather than the original waters from the adjacent well. And this is only used internally, unless, indeed, the medical officers have been reading rather recent medical literature and are applying what they, from this, believe to be the new external method, or unless, indeed, they happen to be versed in medical history and hence know that they are only then again employing what was the original method of using this healing solution in the very place where they are now practising their art.
Received for publication October 2nd, 1917.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1584988/
The original well at Epsom is capped now, but it was exposed in the 1990s while undergoing repair work. If this is representative of the original source then I understand why the cattle refused to take a sip:

Modern techniques are contradictory and tend to show that it works through topical exposure, even though it shouldn’t!
…To get through the skin, a substance must penetrate the epidermis or has to be absorbed by sweat glands or hair follicles. The stratum corneum is the outermost layer of the epidermis consisting of dead cells (corneocytes). This layer is composed of about 15 to 20 layers of flattened cells with no nuclei and cell organelles. Their cytoplasm shows filamentous keratin. These corneocytes are embedded in a lipid matrix composed of ceramides, cholesterol, and fatty acids. The stratum corneum functions to form a water-repellent barrier to protect underlying tissue from infection, dehydration, chemicals and mechanical stress [9]. Overcoming this layer in significant quantities is only possible for lipophilic substances. In magnesium chloride solution, magnesium is present in ionized form and therefore not able to penetrate a lipophilic layer. In addition, the radius of the hydrated magnesium ion (86 pm) has been reported to be 400 times higher than its dehydrated form, leading to the assertion that it is almost impossible for magnesium ions to pass through biological membranes [2].
From: “Myth or Reality—Transdermal Magnesium?“ (2017)
Upon topical application of magnesium solution, we found that magnesium penetrates through human stratum corneum and it depends on concentration and time of exposure. We also found that hair follicles make a significant contribution to magnesium penetration.
From: “Permeation of topically applied Magnesium ions through human skin is facilitated by hair follicles“ (2016)

Dietary sources and sample menu
PubMed is an excellent source of research papers, but less so for healthy living advice. Hence as part of my research for Part 4, I contacted Dr. Carolyn Dean, author of the definitive reference “The Magnesium Miracle” (2017).
Not only did Carolyn respond with some very useful information, but she kindly sent me the latest revision of this classic book, together with her most recent publication: “Magnesium: The Missing Link to Total Health” (Dec 2023).
Carolyn gave some useful feedback about the above studies:
In the testosterone Mg study, should you make a remark that it’s unfortunate that even current research uses the serum Mg test whereas it’s been proven over and over again to be highly inaccurate and less useful than the ionized Mg test. A more accurate test would likely provide much cleaner data.
In the second paper, Vit D and Mg, would it be helpful to say that because Mg is responsible for 80% of known metabolic functions (Workinger 2018), and amount of any Mg will have a beneficial effect. However, it’s the fully absorbed liquid picometer Mg that will support all 80% of known metabolic functions.
I love this study because so few people know about the activation of Vit D by Mg.
Source: Dr. Carolyn Dean.
Jennifer has penned a few posts this week on thyroid function. Its complex and autoimmune antibodies to thyroid peroxidase (TPO) are also associated with fibromyalgia:
For the third paper, just an FYI. The thyroid requires 9 minerals to make thyroid hormones, one of them is Mg.The rest are
iodine, selenium, zinc, molybdenum, boron, copper, chromium, manganese. And countless customers (including myself) have gotten off their thyroid meds by taking my multiple mineral, ReMyte.
Source: Dr. Carolyn Dean.
Additional comment:
1. The Missing Link lists over 65 medical conditions that may actually be the result of Mg deficiency.
2. Workinger 2018 stated that Mg is responsible for 80% of known metabolic functions.
3. The intake of sufficient Mg to overcome Mg deficiency is often hampered by the laxative effect because most Mg compounds are poorly absorbed and act as laxatives.
4. The serum Mg test is useless in predicting Mg deficiency. I have those references in the books I’ve attached and you can search for them.
5. My company, New Capstone (dba RnAReSet - www.rnareset.com and www.ddrcarolyndean.com) is studying absorption using the iMg test. Ionized Magnesium, which Drs Burton and Bella Altura championed. I’ll attach the first paper published we published with highlights.
6. Because of my own severe Mg deficiency, for 10 years after publishing The Mg Miracle 2003, I searched for a company that would research and formulate a non-laxative Mg. Nobody was interested. I found a chemist and worked with him to create ReMag - a picometer-sized, stabilized ion of Mg. Whereas before ReMag, 50mg of a Mg compound would give me the laxative effect, I was able to take 1,200 mg of ReMag to remove my Mg deficiency symptoms including moderately severe heart palpitations. After a year and a half my dozen Mg deficiency symptoms were gone and now I only require 300-450mg of ReMag a day.
Having a non laxative Mg is The Key to solving Mg deficiency symptoms. Otherwise people just give up when they develop diarrhea and turn to drugs or dozens of supplements to treat their symptoms.
7. Supplement companies, including mine are hampered in promoting Mg because the FDA tells us that if we make a claim, or have a testimonial on our websites that sayw or implies that Mg can “cure” disease, then we are making a health claim and could be shut down. We would have to do the billion dollar drug trial to “prove” our claim. They don’t accept the thousands of studies that show positive health results.
8. I’m afraid that we cannot obtain enough Mg from our diet. 100 years ago the USDA said we were getting 500mg of Mg daily, now we’re lucky to get 200mg. Then there is the extreme ultraprocessed food industry - 70% of the American diet is ultraprocessed. it has no Mg and no nutrients of any kind. Then there is the extreme stress that we are living under - i say most people have PTSD today! Then there are all the drugs giving - for Mg deficiency conditions - that deplete more Mg, especially the fluoride drugs like Prozac, Cipro, Lipitor, Diflucan, and even inhaled surgical anesthetics. Fluoride is broken down from these drugs by intestinal microbes then it binds to Mg making a brittle compound that deposits in tendons joints and ligaments. Cipro has an FDA black box warning about tendon rupture.
So, we can not depend on food to save us and we have to have a non laxative magnesium - ReMag.
9. Mg compounds are fighting for supremacy and market share presently but it’s all a moot point. If you are Mg deficient you want Mg, not taurine, glycine, malic acid, etc. etc. Most people are shocked at how little elemental Mg is in Mg compounds. Mg L’threonate is especially disturbing considering that the excessive marketing of the product makes claims that it’s the best absorbed into the brain. I’ll also attach my Mg L’threonate rant.
Source: Dr. Carolyn Dean.
Further to point 7, in the UK we have the “1939 Cancer Act”. This is better termed the “1939 Cancer Treatment Pharma Monopoly Act”. By law, it is illegal to advertise any cancer treatment which is not approved by the state. It is also illegal to offer any claimed cancer treatment, prescribe any claimed cancer treatment, or offer any non-state-sanctioned cancer treatment advice.
The justification for this is to stop snake oil salesmen from cashing in by selling dangerous or ineffective “cures” to desperate people. Irony and hypocrisy stare you in the face. It has the effect of stopping doctors from being doctors, prescribing to their best judgment.
The paradox is that if you don’t want to follow the conventional cut, burn, or poison therapies of Western allopathic cancer medicine then you are forced into either the veterinarian or unregulated grey markets to source your therapeutics - away from qualified medics, prescription grade drugs, and straight to dubious sources the law was supposedly trying to restrict.
Emphasizing that these are NOT claimed cancer cures, I go into more depth about supplements later in the review.
Elemental magnesium content of various salts:
Magnesium Salt Amount of Elemental Magnesium per 500 mg Salt
Magnesium oxide 300 mg
Magnesium carbonate 150 mg
Magnesium hydroxide 144 mg
Dimagnesium malate 95 mg
Dolomite 75 mg
Magnesium citrate 75 mg
Magnesium malate 75 mg
Magnesium chloride 60 mg
Magnesium lactate 60 mg
Magnesium glycinate 50 mg
Magnesium sulfate 50 mg
Magnesium taurate 50 mg
Magnesium l’threonate 34 mg
Magnesium orotate 30 mg
Magnesium gluconate 25 mg
Source: Dr. Carolyn Dean.
Thoughts On Magnesium L’threonate (Magtein)
Every week I’m asked about this new Magnesium L’threonate product, Magtein. Threonate is a sugar acid bound to magnesium. The January 28, 2010 issue of Neuron published a paper titled, “Enhancement of Learning and Memory by Elevating Brain Magnesium.” The study was done on rats and researchers said that “Our findings suggest that an increase in brain magnesium enhances both short-term synaptic facilitation and long-term potentiation and improves learning and memory functions.” The study compares several different forms of magnesium for absorption into the cerebrospinal fluid and found that Magtein measured only 7 percent higher at day 24 of intake. They admit that threonate by itself does not have “any positive effect on memory.”
With this 7 percent increase in the CSF, promoters of Magtein say theirs is the only magnesium that crosses the blood brain barrier. That is an inaccurate statement. Giving superpowers to their product obscures the reality that any magnesium could produce some or all of these same effects. The treatment of migraines, seizures, stroke, head injuries and other nervous system problems with even the highly unabsorbed magnesium oxide (at 4%) shows that all magnesium works at the neuron level, which means all or part of it gets into the brain. See the 24 chapters in Magnesium in the Central Nervous System for more evidence.
I had the notion that Magnesium L’threonate was a L’threanine compound. L’threnine is an amino acid found in green tea and mushrooms said to improve anxiety, stress, and insomnia. But L’threonate has nothing to do with L’threanine. L-Threonate, or Threonic acid, is a metabolite of ascorbic acid, nothing to do with L’threanine.
Personally, when I take Magtein, I get the laxative effect, which means to me that it’s not fully absorbed at the cellular level like some other forms of magnesium. In one formulation, three capsules of Magtein gives you 144mg of magnesium. I would have to take about 20 capsules a day to meet my magnesium requirements. And I wouldn’t get the therapeutic effect before developing diarrhea. From a quick google search, Magtein is a patented product, so this animal study is being used to promote the product. Therefore every opportunity is used to say it’s better than the competition making people feel that if they don’t take this one, they are not getting any benefit from the others. In August 2013, I heard from a supplier of magtein that “There has been a vendor price increase, up to 400% for magnesium threonate.” The research must be paid for, so create the demand and then raise the price is a standard marketing practice.
When you read the free eBook, Magnesium in the CNS it will convince you that all magnesiums (to some extent, cross the BBB and can have a positive effect on the brain. https://www.adelaide.edu.au/press/titles/magnesium/ However, I do find my ReMag is the best absorbed allowing a therapeutic effect without any laxative effect.
Source: Dr. Carolyn Dean.
See Forbes Health report that ReMag is the best Liquid Mg on their Mg list:
Best Magnesium Supplements Of 2024, According To Experts
Source: Dr. Carolyn Dean.
By addressing magnesium deficiency you are targeting the root causes of many diseases. In effect, magnesium is a broad-spectrum therapeutic that rivals baicalin, vitamin D, or ivermectin for efficacy, and the dilemma is what to leave out from any review. Carolyn’s 2017 book has room to go into much more detail, with more conditions described over 600 pages than these four Substacks can, with her wealth of experience.
George Eby, whose foundation provided funds to educate members of Congress about magnesium and presented them with copies of The Magnesium Miracle, has a personal interest in magnesium, having suffered from magnesium deficiency for many years. He is convinced that magnesium deficiency causes 50 to 70 percent of all our chronic illnesses and accounts for a huge share of medical and hospital expenses. He’s also concerned that because most of us have grown up in a society with widespread magnesium deficiency, which began about 100 years ago with the large-scale refining of grain, we are unable to recognize that we are deficient.
Source: “The Magnesium Miracle” (2017).
- Nutrient deficiency of refined foods
Even with the most magnesium-dense foods you need to consume a lot of them in combination to get an RDA of 400 mg, which is why most of us need to supplement. It is possible from diet alone, but it’s a challenge and you risk monotonous mealtimes.
We have the same requirements and dietary shortfalls with many vitamins and minerals, especially vitamin D in the more northerly or southerly latitudes where we don’t get 12 months of adequate UV exposure. To this end, I can recommend the “dminder” app for planning your exposure time. It is satisfying to watch the calculated vitamin D3 iu’s ticking-up in real-time.
https://dminder.ontometrics.com/
To obtain enough magnesium from the diet takes special care, knowledge of magnesium-rich foods, and a knowledge of whether or not the soil in which the plants are grown is fertilized with minerals. Even so, we still need to supplement with magnesium. In 2005, Eby produced a DVD showing a long list of foods with the amounts necessary to deliver 400 mg of magnesium. Imagine 1.8 ounces of rice bran, which provides 400 mg of magnesium, beside 54.25 ounces of doughnuts, which is the quantity you have to eat to obtain the same amount of magnesium. Or visualize a few ounces of nuts compared to 25 ounces of white bread. Such a picture, showing the nutrient deficiency of refined foods, is worth a thousand words.
Source: “The Magnesium Miracle” (2017)
Food: Portion (in ounces) containing 400 mg
Rice bran, crude: 1.8
Wheat bran, crude: 2.3
Cocoa, dry powder: 2.65
Pumpkin seeds: 2.65
Brazil nuts: 3.9
Peanut butter: 3.9
Kellogg’s All-Bran: 3.9
Cashew nuts: 4.80
Almonds: 5.1
Rolled oats: 5.1
Unsweetened chocolate: 5.2
Molasses: 5.3
Buckwheat flour: 5.65
Oat bran: 5.9
Wheat germ: 5.9
Peanuts: 8.0
Tortilla chips: 14.5
Dinner rolls: 16.6
Whole-wheat bread: 17.3
English muffins: 20
Potatoes: 21.7
Spaghetti: 22.0
Macaroni: 22.0
Tortillas: 22.0
Potato flour: 22
Crackers: 22.75
Melba toast: 25
White bread: 25
Potato chips: 26
Brown sugar: 48
Doughnuts: 54.25
* Statistics from USDA data
Source: “The Magnesium Miracle” (2017)
I once worked on trials at an organic research organization. We regularly remineralized the soil with crushed Magnesian limestone, sourced from a nearby quarry.
Calcium is rarely a problem in our diet, and if you have Mg covered, you are probably getting enough Ca too.
Organic Food
Many people would say that organic food should be higher in magnesium than the offerings from Big Agriculture, but that may not be the case. If your organic farmer does not remineralize the land with minerals in the form of rock dust, the plants will be as magnesium-deficient as plants from any other farmland.
Seek out organic farmers who are educated about crop rotation, who test regularly for mineral deficiencies in the soil, and who use mineral-rich fertilizers.
Source: “The Magnesium Miracle” (2017)
- Magnesium content of selected foods
These are only for guidance as the nutritional value will vary according to the quality of the soil they were grown in:
Table 2: Magnesium Content of Selected Foods
Food - Milligrams (mg) per serving - Percent DV*
Pumpkin seeds, roasted, 1 ounce - 156 - 37
Chia seeds, 1 ounce - 111 - 26
Almonds, dry roasted, 1 ounce - 80 - 19
Spinach, boiled, ½ cup - 78 - 19
Cashews, dry roasted, 1 ounce - 74 - 18
Peanuts, oil roasted, ¼ cup - 63 - 15
Cereal, shredded wheat, 2 large biscuits - 61 - 15
Soymilk, plain or vanilla, 1 cup - 61 - 15
Black beans, cooked, ½ cup - 60 - 14
Edamame, shelled, cooked, ½ cup - 50 - 12
Peanut butter, smooth, 2 tablespoons - 49 - 12
Potato, baked with skin, 3.5 ounces - 43 - 10
Rice, brown, cooked, ½ cup - 42 - 10
Yogurt, plain, low fat, 8 ounces - 42 - 10
Breakfast cereals, fortified with 10% of the DV for magnesium, 1 serving - 42 - 10
Oatmeal, instant, 1 packet - 36 - 9
Kidney beans, canned, ½ cup - 35 - 8
Banana, 1 medium - 32 - 8
Salmon, Atlantic, farmed, cooked, 3 ounces - 26 - 6
Milk, 1 cup - 24–27 - 6
Halibut, cooked, 3 ounces - 24 - 6
Raisins, ½ cup - 23 - 5
Bread, whole wheat, 1 slice - 23 - 5
Avocado, cubed, ½ cup - 22 - 5
Chicken breast, roasted, 3 ounces - 22 - 5
Beef, ground, 90% lean, pan broiled, 3 ounces - 20 - 5
Broccoli, chopped and cooked, ½ cup - 12 - 3
Rice, white, cooked, ½ cup - 10 - 2
Apple, 1 medium - 9 - 2
Carrot, raw, 1 medium - 7 - 2
*DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for magnesium is 420 mg for adults and children age 4 years and older [11]. FDA does not require food labels to list magnesium content unless magnesium has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
Adapted from: “Magnesium. Fact Sheet for Health Professionals”
https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
USDA FINDINGS
United States Department of Agriculture (USDA) surveys of women’s diet find that:
Approximately 25 percent of their magnesium is supplied by grain products. This means you should try to include a variety of whole grains and avoid white bread and pasta, which are devoid of minerals. If you crave bread (and sugar), you may be feeding yeast in your intestines and should try to limit your intake to achieve weight loss and blood sugar balance. Or you may be craving nutrients that your body knows are absent from refined grains—you eat more carbs, but the empty calories don’t seem to satisfy a deeper need. You have to eat a pound and a half of white bread to get the RDA of magnesium.
Another 25 percent of women’s magnesium intake comes from fruits and vegetables.
The high-protein foods—meat, poultry, and fish—provide only about 18 percent of total magnesium intake. Since high-protein diets are now becoming more popular, you must make sure to increase your magnesium when on such a diet.
Fats, sweets, and beverages supply only 14 percent of the total magnesium intake. These foods, especially refined fats and sweets, should be limited because they contain empty calories devoid of nutrients. Also, sugar causes a big drain on magnesium, which is required in high amounts to metabolize it.
Nuts and seeds and their butters are the richest sources of magnesium, but they are not even mentioned in most food surveys.
Source: “The Magnesium Miracle” (2017)
A MAGNESIUM EATING PLAN
Upon rising: Juice of ½–1 fresh lemon in warm water. Sweeten with stevia.
BREAKFAST
CROCKPOT CEREAL
•Choose two grains, one nut, and one seed from the following: organic buckwheat, millet, rye, oats, amaranth, quinoa, sunflower seeds, pumpkin seeds, almonds, cashews, filberts, walnuts, pecans.
•Dress with flaxseed oil and organic fresh or frozen blueberries, cherries, strawberries, raspberries, banana, peach, or pear.
DIRECTIONS:
For one person, put 2 oz of dry mixture, including nuts and seeds, and 5 oz of water in a 1-quart crockpot. Cook overnight. In the morning place in a bowl, add 2–4 oz of fruit, mix, and add 2 tbsp flaxseed oil or 1 tbsp butter (organic) or 2 tbsp ground flaxseeds (ground fresh in a coffee grinder).
You may add rice milk, almond milk, or coconut milk as desired. For extra magnesium, sprinkle on wheat germ (kept in the freezer).
SUBSTITUTIONS:
1.Instead of using a crockpot, you can soak 2 oz of grain mixture overnight in 5 oz of water, with or without 2 tbsp of plain yogurt or kefir. In the morning bring to a boil and let simmer on the lowest setting for 20 minutes. You may have to add more water to avoid dryness. Serve with 2 tbsp ground flaxseeds (ground fresh in a coffee grinder).
2.Instead of soaking overnight, cook 2 oz of grain mixture in 6 oz of water. Bring to a boil and let simmer for 30 minutes.
LUNCH (Choose one)
1.Brown rice and vegetables, cooked with 2 oz dulse or other sea vegetables
2.Leafy green salad, soup with added sea vegetables, and Essene sprouted bread or Ezekiel bread
3.Fish, greens (collards, spinach, Swiss chard), and salad
4.Egg omelet with sautéed vegetables
5.Chicken with vegetables
DINNER (Choose one)
1.Soup (with sea vegetables) and salad
2.Stir-fried grains (leftover grains from breakfast) and vegetables
3.Roasted vegetables with wild rice
4.Salad with cooked organic legumes (kidney beans, lentils, black beans, pinto beans, chickpeas)
5.Wheat-free pasta (rice, spelt, kamut) with pesto, tomato sauce, and green vegetables
6.Mixed salad with avocado
SNACKS
1.Raw vegetables
2.Dried fruit (prunes and figs)
3.Shelled nuts and seeds (raw, not processed or salted)
4.Baked blue corn chips
5.Popcorn
6.Chocolate Frozana (see recipe in Chapter 9)
DRINKS
1.Pure, clean water
2.Green tea
3.Freshly squeezed lemon juice and water
4.Cranberry juice sweetened with stevia
5.Kukicha (roasted twig tea)
6.Bone broth (see recipe in Appendix C)
Kukicha twig tea, also known as bancha twig tea, is a Japanese blend of green tea made of roasted stems, stalks, and twigs of the tea plant, Camellia sinensis. It has a mildly nutty and slightly creamy sweet flavor. There are many variations of how to brew kukicha tea, depending on the taste you want. Stir 1 to 3 tsp in 6–8 oz of boiling water and steep for 1–6 minutes. This tea is said to be high in calcium, but I could not find any laboratory data to confirm.
Source: “The Magnesium Miracle” (2017)
- Supplements
To optimize bioavailability and to minimize laxative-like effects the objective is to mimic how we receive it through diet: generally in low doses and not all at once.
Carolyn recommended taking it as a low-dose solution of picometre ionic magnesium chloride throughout the day, (ReMag). They also used magnesium chloride (Chronomag) in the fibromyalgia study (Part 3)
I knew years ago, not from my doctor, but from the work of “disinformation spreader” Dr. Mercola that I needed more Mg in my diet to balance the D, K2, K, and zinc I was taking for cardio benefits.
Before I could find a better source, my magnesium supplementing “career” started with a daily tablet containing magnesium oxide with a B-complex. Last year I graduated up to 600 mg l-threonate capsules.
I took one per day, which gave me an enormous 40 mg of elemental magnesium. I knew I needed to supplement but mistakenly thought that this would be enough to complement my diet.
When I started researching the subject I realized I would need to at least quadruple this dose to have much effect. But this was going to become very expensive, very quickly.
I’ve tried magnesium glycinate powder, at less than 1/5th the price of l-threonate, but have yet to try ReMag. From what Carolyn says, you can probably take quite a large dose without laxative effects, even if you are sensitive to the oxide form.
As for magnesium glycinate, you also need to take a lot of it to get enough elemental magnesium, and the bittersweet taste is not for everyone.
1/2 teaspoon = 2.5 ml = 1500 mg = 150 mg elemental Mg.
This is why dietary or low-lax sources of magnesium are preferred, and you should make sure that you balance your supplementation too:
Magnesium cannot be taken out of context, either in a research setting or in your body. For example, you should increase magnesium intake when you consume more vitamin D. Magnesium is necessary to convert dietary vitamin D into one of the hormones that makes efficient use of calcium in bone formation.11, 12 Vitamin B6 increases the amount of magnesium that can enter cells; as a result, these two nutrients are often taken together. In one experiment, serum vitamin E levels improved after magnesium supplementation.13 We also know that magnesium and the essential fatty acids (EFAs, found in fish, nuts, seeds, and flaxseed oil) are interdependent; each works much more efficiently when the other is present in sufficient amounts.
Source: “The Magnesium Miracle” (2017)
- Top 10 supplements
From “10 Types of Magnesium (and What to Use Each For)”5 (2023):
1. Magnesium citrate
Magnesium citrate is a form of magnesium that’s bound with citric acid.
This acid is found naturally in citrus fruits, giving them their tart, sour flavor (3).
Magnesium citrate is one of the more common magnesium supplement formulations and can be purchased in stores worldwide.
A small study of 14 male participants suggests that this type is among the most bioavailable forms of magnesium, meaning it’s more easily absorbed in your digestive tract than other forms (4Trusted Source).
It’s typically taken orally to replenish low magnesium levels. Due to its natural laxative effect, it’s also sometimes used at higher doses to treat constipation.
It’s occasionally marketed as a calming agent to help relieve symptoms associated with depression and anxiety, but more research is needed on these uses (5Trusted Source).
2. Magnesium oxide
Magnesium oxide is a salt that combines magnesium and oxygen.
It naturally forms a white, powdery substance and may be sold in powder or capsule form (6).
This type isn’t typically used to prevent or treat magnesium deficiencies, as some studies report that it’s poorly absorbed by your digestive tract (7Trusted Source).
Instead, people use it more frequently to relieve uncomfortable digestive symptoms, such as heartburn, indigestion, and constipation. Some may also use it to treat and prevent migraine episodes, but more research is needed to confirm that magnesium deficiency can contribute to migraine attacks (8Trusted Source, 9Trusted Source).
ReMag and other studies used formulations based on this:
3. Magnesium chloride
Magnesium chloride is a magnesium salt that includes chlorine — an unstable element that binds well with other elements, including sodium and magnesium, to form salts.
It’s well absorbed in your digestive tract, making it a great multi-purpose supplement. You can use it to treat low magnesium levels (2Trusted Source, 7Trusted Source, 10).
People take magnesium chloride most frequently in capsule or tablet form, but it may also be an ingredient in topical products like lotions and ointments.
Although people use these skin creams to soothe and relax sore muscles, little scientific evidence links them to improved magnesium levels (11).
4. Magnesium lactate
Magnesium lactate is the salt formed when magnesium binds with lactic acid.
This acid is produced by your muscle and blood cells and is manufactured as a preservative and flavoring agent (12).
Indeed, magnesium lactate is utilized as a food additive to regulate acidity and fortify foods and beverages. It’s less popular as an over-the-counter dietary supplement.
Your digestive tract easily absorbs magnesium lactate, which may also be gentler on your digestive system than other types. This may benefit people who need to take large doses of magnesium regularly or don’t easily tolerate other forms.
In a study of 28 people with a rare condition that required high doses of magnesium daily, those who took a slow-release tablet of magnesium lactate reported fewer digestive side effects than the control group (13Trusted Source).
Other studies likewise reveal that this form may help treat stress and anxiety, but more research is needed (14Trusted Source).
5. Magnesium malate
Magnesium malate includes malic acid, which occurs naturally in foods like fruit and wine. This acid has a sour taste and is often added to food to add flavor or acidity.
Research suggests that magnesium malate is very well absorbed in your digestive tract, making it a great option for replenishing your magnesium levels (15Trusted Source).
Some people report that it’s gentler on your system and may have a less laxative effect than other types. This may be beneficial, depending on your specific needs.
Magnesium malate is occasionally recommended to treat fibromyalgia and chronic fatigue syndrome symptoms. But while some studies have found there may be benefits, more high quality studies are needed (16Trusted Source).
Dr. Carolyn Dean was skeptical of the need for the taurate form, as almost all sources of magnesium benefit the heart:
6. Magnesium taurate
Magnesium taurate contains the amino acid taurine.
Research suggests that adequate intakes of taurine and magnesium play a role in regulating blood sugar. Thus, this form may promote healthy blood sugar levels (17Trusted Source).
Magnesium and taurine also support healthy blood pressure (1Trusted Source8, 19Trusted Source).
A 2018 animal study revealed that magnesium taurate significantly reduced blood pressure in rats with high levels, indicating that this form may bolster heart health (20Trusted Source).
Keep in mind that more human research is needed.
Threonate. If you go this route, budget for up to 6 capsules or 3000 mg / day to make much of a difference.
7. Magnesium L-threonate
Magnesium L-threonate is the salt formed from mixing magnesium and threonic acid, a water-soluble substance derived from the metabolic breakdown of vitamin C (21).
This form is easily absorbed. Animal research notes it may be the most effective type for increasing magnesium concentrations in brain cells (22Trusted Source).
Magnesium L-threonate is often used for its potential brain benefits and may help manage certain brain disorders, such as depression, Alzheimer’s disease, and age-related memory loss. Nonetheless, more research is needed (23Trusted Source).
As discussed earlier:
8. Magnesium sulfate
Magnesium sulfate is formed by combining magnesium, sulfur, and oxygen. It’s commonly known as Epsom salt. It’s white with a texture similar to that of table salt.
While you can consume it as a treatment for constipation in capsule form or dissolve the powder in water, it has an unpleasant taste. Using too much or using it too often can be dangerous (24Trusted Source).
You can dissolve magnesium sulfate in bathwater to soothe sore, achy muscles and relieve stress. It’s also sometimes included in skin care products like lotion or body oil.
Although adequate magnesium levels can play a role in muscle relaxation and stress relief, little evidence suggests that this form is well absorbed through your skin (11Trusted Source).
Magnesium glycinate
Consider using a different form to glycinate if you find the smell of fish repulsive - it has those overtones. This is no coincidence. Glycine is a non-essential amino acid used in fish nutrition; it’s an important antioxidant that is involved in glutathione synthesis.
You have to look after your Cyprinus carpio:6
…Glycine is a non-essential amino acid that is involved in glutathione structure, and is also involved in the antioxidant system in fish. Moreover, glycine stimulates the immune system. This study shows that a period of 8-week feeding with a diets supplemented with 5 g/kg glycine can improve growth performance, erythrocyte stability, and humoral and mucosal immunity in common carp. So, the present results can be used in carp diet production to support higher growth and health.
From “Effects of Dietary Glycine Supplementation on Growth Performance, Immunological, and Erythrocyte Antioxidant Parameters in Common Carp, Cyprinus carpio” (2023)
It is also a useful amino acid for humans, too.
NB Glycine is NOT, repeat NOT, in any way an alternative cancer treatment or adjunct.
#1939CancerAct
…We conducted a nested case-control study involved 129 incident pancreatic cancer cases and 258 individually matched controls within a prospective cohort study of 18,244 male residents in Shanghai, China.
…Results: Odds ratios (95% confidence intervals) of pancreatic cancer for the highest quartile of serine and glycine were 0.33 (0.14−0.75) and 0.25 (0.11−0.58), respectively, compared with their respective lowest quartiles (both p’s < 0.01). No significant association with risk of pancreatic cancer was observed for other serine- or glycine related metabolites including cystathionine, cysteine, and sarcosine.
Conclusion. The risk of pancreatic cancer was reduced by more than 70% in individuals with elevated levels of glycine and serine in serum collected, on average, more than 10 years prior to cancer diagnosis in a prospectively designed case-control study. These novel findings support a protective role of serine and glycine against the development of pancreatic cancer in humans that might have an implication for cancer prevention.

From: “The Association between Serum Serine and Glycine and Related-Metabolites with Pancreatic Cancer in a Prospective Cohort Study“ (2022)
9. Magnesium glycinate
Magnesium glycinate is formed from elemental magnesium and the amino acid glycine.
Your body employs this amino acid in protein construction. It also occurs in many protein-rich foods, such as:
fish
meat
dairy
legumes
Animal studies suggest that glycine on its own can help improve sleep and treat some inflammatory conditions, including heart disease and diabetes. But more robust studies are needed to further support this (25Trusted Source, 26Trusted Source).
Magnesium glycinate is easily absorbed and may have calming properties. It may help reduce mental health issues, such as: (1Trusted Source)
anxiety
depression
stress
insomnia
Yet, there is limited scientific evidence on these uses, so more studies are needed.
10. Magnesium orotate
Magnesium orotate includes orotic acid, a natural substance involved in your body’s construction of genetic material, including DNA (27).
It’s easily absorbed and doesn’t have the strong laxative effects characteristic of other forms (28Trusted Source).
Early research suggests it may promote heart health due to orotic acid’s unique role in the energy production pathways in your heart and blood vessel tissue (28Trusted Source, 29).
As such, it’s popular among competitive athletes and fitness enthusiasts, but it may also aid people with heart disease.
One 2009 study of 79 people with severe congestive heart failure found that magnesium orotate supplements were significantly more effective for symptom management and survival than a placebo (28Trusted Source).
Yet, this form is significantly more expensive than other magnesium supplements. Based on the limited evidence, its benefits may not justify the cost for many people.
- Bioavailability
From “Physiology of a Forgotten Electrolyte—Magnesium Disorders“7 (2023) by Ray et al.
This is in order of absorption in the GI tract, once dissolved. Faster is not necessarily better, but generally correlates with reduced laxative effects:
In humans, rank-order absorption of Mg2+ salts were shown to be aspartate >lactate>>citrate>glycinate>oxide>chloride>gluconate.93 Mg2+ acetate was absorbed significantly better than a slow-release Mg2+ chloride formulation.94 Mg2+ aspartate, lactate and slow-release chloride all increased urinary Mg2+ concentrations compared to controls; only Mg2+ oxide did not.95 The discrepancy in chloride findings may reflect improved absorption with slow-release formulations. Thus, organic Mg2+ salts are generally best absorbed, followed perhaps by Mg2+ chloride, then Mg2+ oxide. Tendency to cause loose stools is likely inversely proportional to absorbability of the salts.
If affordability influences how many capsules you take each day then this indirectly influences bioavailability, along with offputting laxative effects such as from citrate or malate:
CONCLUSION
Mg2+ is an important but often overlooked electrolyte, which is frequently not even tested for. Identification of new genes, causing rare forms of inherited hypomagnesemia has improved our understanding of tubular Mg2+ handling. In the clinical setting it is mostly side effects of medications contributing to hypomagnesemia. Therapy of hypomagnesemia remains challenging despite different forms of Mg2+ supplements.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10291516/
I agree with Dr. Carolyn Dean that L-threonate isn’t the only form of Mg that can increase levels in the brain. It may be the best in short-term trials but citrate and glycinate aren’t far behind.
In “Enhancement of Learning and Memory by Elevating Brain Magnesium”8 (2010) Slutsky et al. treated rats with different magnesium compounds to demonstrate enhancement of learning abilities and memory. There are three recognized types of memory, and MgT benefited all three: long-term, short-term, and working.
Although they refer to chloride, citrate, glycinate, and gluconate in the results section they only reported findings for chloride, citrate, threonate, and gluconate + milk, without saying why glycinate was excluded.
Also note that if the Y-axis on “A” started at zero then the results would appear a lot more clustered:
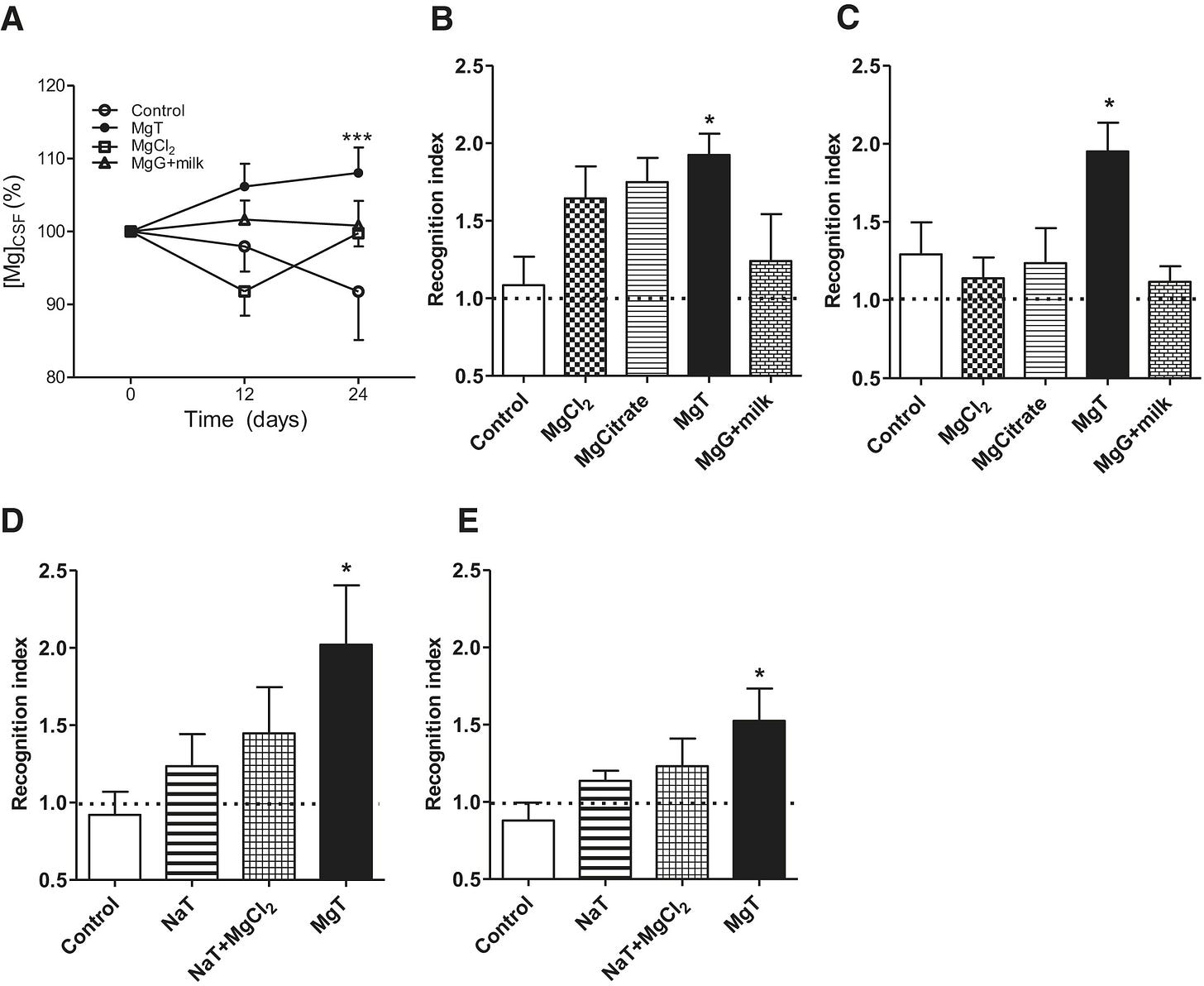
Fold changes are highlighted in bold.
n, numbers treated, are 10 or less:
(A) Elevation of magnesium concentration in the cerebrospinal fluid ([Mg2+]CSF) following treatment with different magnesium compounds. Total Mg2+ in CSF was measured before magnesium treatment (day 0), 12, and 24 days after magnesium treatment. Two-way ANOVA analysis revealed significant effect of treatment (F3,69 = 4.76, p = 0.0045, n = 6–8). Data were calculated and presented as a percentage of baseline level.
(B) Rat performance during a short-term memory test (10 min retention interval) evaluated by novel object recognition test.
(C) Long-term memory test (12 hr) using novel object recognition test. One-way ANOVA analysis revealed significant effect of treatment on short-term memory (F4,34 = 2.89, p = 0.037, n = 7–9) and long-term memory (F4,31 = 4.50, p = 0.005, n = 5–10). Post hoc test revealed significant effect of magnesium-L-threonate (MgT) on short term and long-term memory.
(D and E) Effects of sodium-L-threonate (NaT) alone and when combined with magnesium chloride (MgCl2) on short-term memory (D) and long-term memory (E). ANOVA analysis revealed a significant effect of treatment on short-term memory (F3,33 = 2.90, p = 0.049, n = 8–10) and long-term memory (F3,34 = 3.23, p = 0.034, n = 9–10). Post hoc test revealed significant effect of magnesium-L-threonate (MgT). See also Figures S2 and S3.
Magnesium-L-Threonate Treatment
Magnesium-L-threonate (604 mg/kg/day) was administered via drinking water (50 mg/kg/day elemental Mg2+). The average drinking water per day was determined (∼30 ml/day), and the dose was dissolved in the daily drinking amount. Rat chow contained 0.15% of elemental Mg2+. We also monitored the food intake to determine the amount of Mg2+ intake from food.
To convert a rat dose to human we can divide by 6.29, which gives 8 mg/kg human equivalent dose (HED) or 560 mg elemental Mg2+, which is almost double the RDA - it’s a large dose and a mouthful of capsules!
A conflict of interest was declared by one of the authors, Guosong Liu:
Acknowledgments
…G.L. declares that he is a cofounder of Magceutics, a company whose goal is to develop drugs to treat age-dependent memory decline and Alzheimer's disease.
This paper has been cited 263 times.
Full paper:
https://www.sciencedirect.com/science/article/pii/S0896627309010447
Carolyn’s study highlights from Dr. Taylor Wallace of “Circulating Ionized Magnesium as a Measure of Supplement Bioavailability: Results from a Pilot Study for Randomized Clinical Trial” (2020) by Zhan et al:10
NUTRIENTS PAPER
1. iMg (ionized magnesium testing) is the better marker for magnesium. Serum magnesium is bound up and not usable. Magnesium ions are available to use in the cells.
2. Using a molecular size analyzer (Malvern Zetasizer Nano ZS, Malvern, UK), we confirmed that the majority of the particle size of ReMag® formulation is in the picometer range. The test was repeated three times.
3. ReMag goes to the ionized magnesium status and is not bound up. Its small size allows it to be absorbed higher up in the GI tract and avoid the laxative effect.
4. Mg Citrate and Mg Oxide will eventually be ionized somewhat but they revert back to a compound state immediately and therefore don’t have as high an absorption efficiency.
5. Elevated ionized magnesium was measurable in the blood within 2-4 hours. Our head of research, Dr. Taylor Wallace said that this fluctuation with one single dose is very important. He said he would not have anticipated mineral electrolyte levels changing with 1 single dose.
6. Clinical investigations have demonstrated that ionized magnesium, but not serum magnesium, is depressed in a number of clinical conditions such as migraine, individuals with noninsulin-dependent diabetes, patients with asthma, and women with high-risk pregnancies
7. A subclinical magnesium deficiency can occur when serum magnesium levels are within the lower reference range. Therefore, serum total magnesium levels may not be sufficient for diagnosis of deficiency.
8. Our findings of a more robust ionized response, when compared to serum magnesium, are similar to those reported by Altura et al.
Conflicts of Interest
TCW serves on the Scientific Advisory Board for The Vitamin Shoppe and has received research grants from Pfizer Consumer Healthcare. All his conflicts are listed at www.drtaylorwallace.com. CMW is on the scientific advisory boards of the Yogurt in Nutrition Initiative (YINI) and the U.S. Food and Drug Administration and serves on the Board of Trustees of the International Life Sciences Institute (ILSI). JZ, SJB, SC, VA, LAS, and NG-M have no conflicts of interest to disclose.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7281967/
I searched PubMed using the keywords “bioavailability” and “ReMag”, which returned two results. The first was the previous paper from 2020, and the second was from 2023, a randomized control trial with 23 participants, reported by Baidoo et al:
“Relationship between short-term self-reported dietary magnesium intake and whole blood ionized magnesium (iMg2+) or serum magnesium (s-Mg) concentrations”11 (2023).
Key takes:
The design of this study is a retrospective, secondary data analysis of data obtained from a pilot, randomized, placebo-controlled, crossover trial (NCT04139928) that aimed to assess the bioavailability of a single dose of a novel, picometer-sized, magnesium chloride formulation (ReMag®) [8].
For the main study, during each of the three clinic visits, participants consumed a single dose (300 mg) of magnesium chloride or a lemon juice placebo, and the pharmacokinetics of absorption was assessed in blood samples over a 24-h period.
Although with a small sample size (n number), their trial found that whole blood ionized magnesium (iMg2+) does respond to supplemental magnesium intake.
The authors discussed how results varied between different studies, and serum magnesium does not necessarily correlate to your overall magnesium status or food sources. They did not support the use of iMg2+ alone as a biomarker for making dietary adjustments:
Despite findings from us [8] and others [9,10], suggesting that iMg2+ responds to supplemental magnesium intake, results from this current analysis did not support its use as a dietary biomarker.
One reason for the lack of a correlation between dietary Mg intake and both iMg2+ and s-Mg is that when intake of Mg is low, the body releases Mg reserves from the bone to help maintain Mg homeostasis thus limiting the use of iMg2+ and s-Mg as a dietary biomarker [22].
It may only increase iMg2+ when you supplement effectively or have a large dose in your diet:
Another possible explanation could be that iMg2+ may only be a useful marker for supplemental or acute dietary intake of magnesium.
Disclosure statement
NGM and TCW are co-investigators on an investigator-initiated unrestricted educational grant from New Capstone, Inc., the manufacturer of ReMag®. TCW has received speaker honoraria from Nova Biomedical. V.Y.A.B is sponsored by the National Institutes of Health (T32HL007909) training grant. CDT’s research was in part funded by an award from the National Institute of Diabetes and Digestive and Kidneys Diseases Award #R01DK132385. KT report no conflict of interest.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10088968/
From 2018, Uysal et al used Sprague Dawley rats in the following study. The title describes the work very well:
“Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best?”.12
Magnesium acetyl taurate gave the best results in almost every metric, but malate resulted in the highest area under the curve.

Key takes:
The aim of this study is to investigate the bioavailability of five different magnesium compounds (magnesium sulfate, magnesium oxide, magnesium acetyl taurate, magnesium citrate, and magnesium malate) in different tissues.
Following a single dose 400 mg/70 kg magnesium administration to Sprague Dawley rats, bioavailability was evaluated by examining time-dependent absorption, tissue penetration, and the effects on the behavior of the animals.
Pharmacokinetically, the area under the curve calculation is highest in the magnesium malate.
The magnesium acetyl taurate was found to have the second highest area under the curve calculation. Magnesium acetyl taurate was rapidly absorbed, able to pass through to the brain easily, had the highest tissue concentration level in the brain, and was found to be associated with decreased anxiety indicators.
Magnesium malate levels remained high for an extended period of time in the serum. The commonly prescribed dietary supplements magnesium oxide and magnesium citrate had the lowest bioavailability when compared to our control group.
More research is needed to investigate the bioavailability of magnesium malate and acetyl taurate compounds and their effects in specific tissues and on behavior.
Higher is better:

Even in rats, it took up to 8 hours for malate and sulphate levels to peak in plasma, and 4 hours for malate to peak in red blood cells.
We know from previous studies that a lot of passive and some active absorption occurs in the jejunum and ileum, which takes time to transit.
Malate is the top curve:
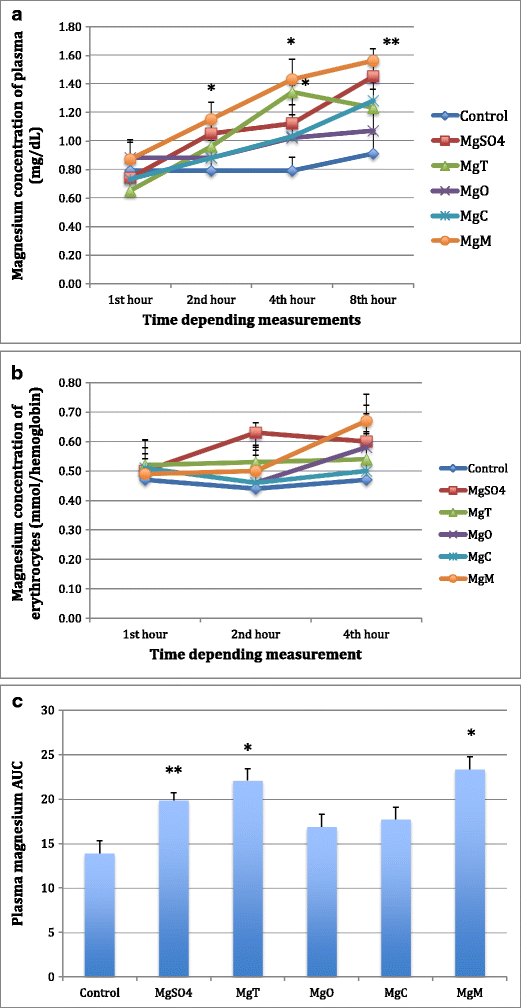
By contrast, AT levels in the muscles were much lower than the other salts.
Figure “a” is for brain tissue levels, and “b” for muscle.
It would have been interesting to compare acetyl taurate’s peak in the brain with chloride, the ReMag version, and L-threonate.

The full paper is paywalled:
https://link.springer.com/article/10.1007/s12011-018-1351-9
The next study, from 2019 and by Ates et al, took similar metrics but used 103 mice and 3 different doses in five groups. This time they used magnesium acetyl taurate and malate again, as well as glycinate and citrate.
Key takes from “Dose-Dependent Absorption Profile of Different Magnesium Compounds”13 (2019):
It has been demonstrated that absorption of organic magnesium compounds is better than absorption of inorganic compounds.
The aim of this study is to investigate transitions to tissues of different organic magnesium compounds in different doses and whether there is a difference in the organic acid–bounded compounds (magnesium citrate and magnesium malate) and the amino acid–bounded compounds (magnesium acetyl taurate and magnesium glycinate), associated with transition and bioavailability.
They even investigated the effects of splitting the dose throughout the day, which should in theory improve net bioavailability (area under the curve):
In addition, the effects of split dosages of high doses in a high volume of solvent on tissue magnesium levels are being investigated, because galenic formulation problems are regarded to prepare convenient dosage that can be taken once a day.
All magnesium compounds were administered as three different doses, 45, 135, and 405 mg/70 kg elemental magnesium, were given per orally to Balbc mice.
Half doses every 12 hours:
In a second set of experiments, 405 mg/70 kg high dose was divided into two doses of 202.5 mg/70 kg each and administered every 12 h.
Acetyl taurate for the brain, again:
Brain, muscle tissues, and serum magnesium levels measured in all experimental groups and control 24 h later. Brain magnesium levels were found increased in all magnesium acetyl taurate administered subjects.
Citrate for muscle and brain:
Magnesium citrate increased muscle and brain magnesium levels in a dose-independent manner.
In mice, splitting the dose did not make much difference to tissue levels:
We showed that dividing high doses of daily administered magnesium compounds did not sufficiently increase tissue magnesium levels.
Mice were divided into five groups: (1) control group (n = 7), (2) magnesium acetyl taurate (n = 21), (3) magnesium glycinate (n = 21), (4) magnesium citrate (n = 21), and (5) magnesium malate (n = 21).
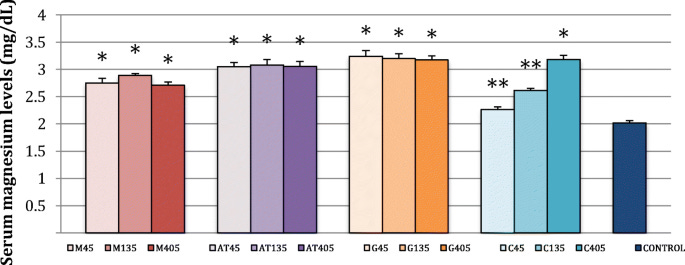
In brain tissue, high-dose glycinate rivals acetyl taurate, but only at the highest dose. Low-dose citrate and malate were little better than the control.
A study period longer than 24 hours would have been useful to see if the gaps closed at lower doses. These are grouped by dose, in mg/70 kg:

With muscles, results were generally tightly clustered and mediocre, the clear outlier being high-dose citrate:

Apart from low-dose citrate, all compounds quickly elevated serum Mg:
Split dosing didn’t improve brain Mg levels, and malate was similar to the control in both regimes:
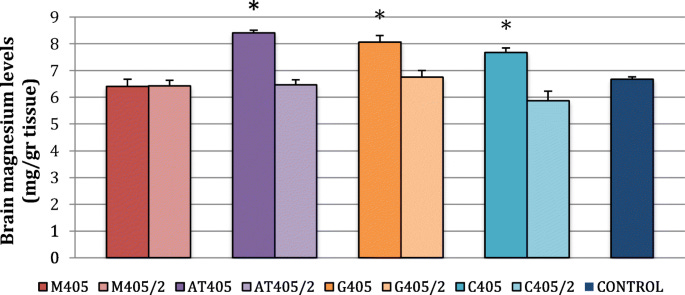
This wasn't an investigation into the long-term treatment of hypomagnesemia, but even so, it is interesting to see the effects after 24 hours. Again, high-dose citrate was best for muscles, which may be useful if you are spasmodic (cramps):

Even serum doses benefited most from a single vs a split dose:

Conclusions from the study:
(1) magnesium acetyl taurate increases brain magnesium levels independent of dose; both low and high doses are equally effective;
(2) magnesium citrate compounds increase muscle magnesium levels in only after high-dose administration; and
(3) administration of divided high-dose daily magnesium compounds does not sufficiently increase tissue magnesium levels. There is a need for further research on long-term administration of different magnesium compounds and their effect on other tissues.
Full paper (paywalled):
https://link.springer.com/article/10.1007/s12011-019-01663-0

Our final study by Blancquaert et al used the futuristic-sounding Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) to select two of 15 magnesium formulations with contrasting bioavailability predictions - the best and the worst.
These two were then selected for in vivo testing in 30 subjects, and all 15 formulations were tested in vitro to study absorption and dissolution properties. Short-term absorption profiles and bioavailability were assessed, for a maximum of 6 hours following intake.
Key takes from “Predicting and Testing Bioavailability of Magnesium Supplements”14 (2019):
In vivo bioavailability was compared following one acute ingestion by monitoring blood magnesium concentrations up to 6 h following intake.
The in vitro tests showed a very wide variation in absorption and dissolution of the 15 magnesium products.
In the in vivo testing, a significant different serum magnesium absorption profile was found up to 4 h following supplement ingestion for the two supplements with opposing in vitro test results.
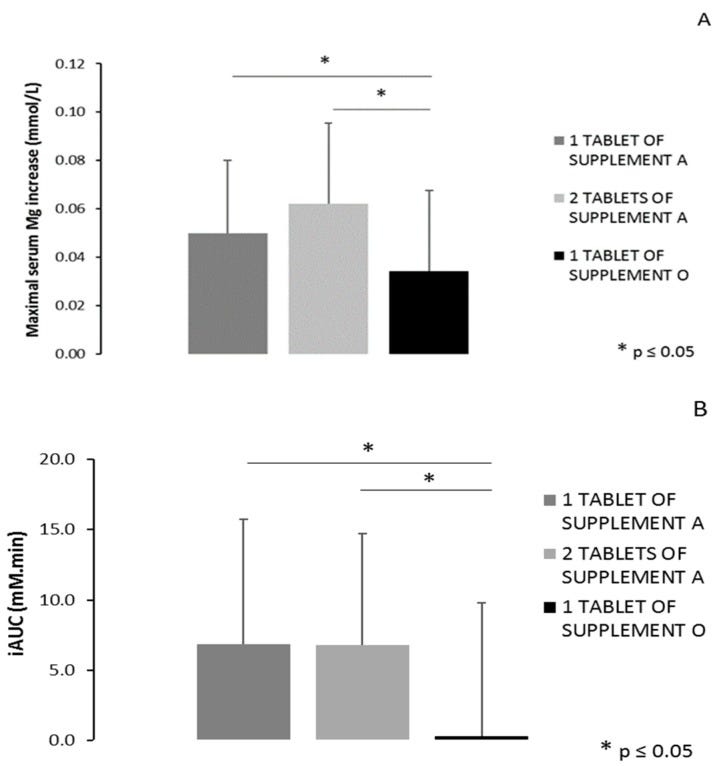
Moreover, maximal serum magnesium increase and total area under the curve were significantly different for both supplements (+6.2% vs. +4.6% and 6.87 vs. 0.31 mM.min, respectively).
The SHIME simulator model appeared to be validated:
Collectively, poor bioaccessibility and bioavailability in the SHIME model clearly translated into poor dissolution and poor bioavailability in vivo. This provides a valid methodology for the prediction of in vivo bioavailability and effectiveness of micronutrients by specific in vitro approaches.
So which two supplements were selected as best and worst for solubility and bioavailability? I think we can guess the MgO will be the worst. They tested 15 branded product formulations, but not 15 different magnesium salts:
Results
3.1.1. In Vitro SHIME Simulation
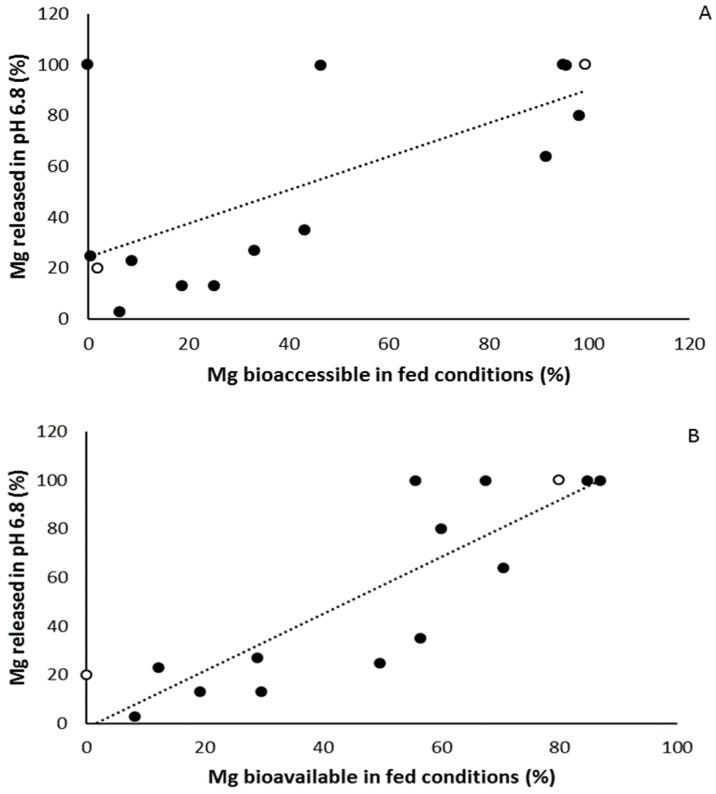
The SHIME® technology was applied to simulate the behavior of 15 different Mg2+ formulations in the stomach and small intestine under fasted and fed conditions (Table 1). Total Mg2+ content (mg) was measured upon dosing the content of the stomach, and small intestinal compartment in either simulated fasted or fed conditions.
By taking into account the total amount of Mg2+, present in each formulation, the proportional Mg2+ release was calculated. Since Mg has its highest solubility in an acidic environment such as the stomach, this is described as bioaccessible Mg. However, Mg is mainly absorbed in the distal part of the small intestine, and this is referred to as the bioavailable Mg.
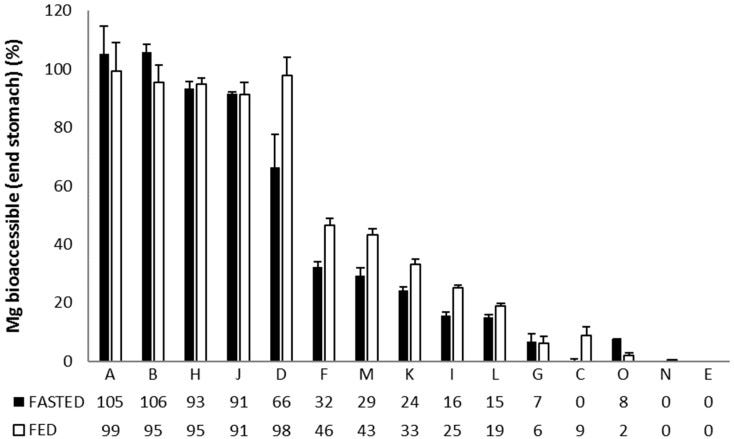
The Mg2+ release from the different formulations in the stomach (Figure 1), reveals three main response scenarios: (1) Complete or very high release of Mg2+ under both fasted and fed conditions: Ultractive Magnesium (A), Magné Vie B6 (B), Polase (H) and High Absorption Magnesium (J), (2) complete release of Mg2+ under fed conditions with a lower release under fasted conditions: Mag 2 (D), (3) no or limited release of Mg2+: Magnerot (F), Magnesium Citrate 200 mg (M), MagOx 400 (K), Biolectra (I), Magnesium 500 mg (L), Mag 2 24 (G), Promagnor (C), B-Magnum (O), Slow-Mag (N), Magnesium Verla (E) (in order from intermediate to no release).
This is an assessment of relative solubility in simulated stomach acid, not bioavailability.
The top 5:
“A” is oxide and glycerophosphate (196 mg).
“B” is citrate (100 mg).
“H” is also citrate (19 mg).
“J” is a chelate, glycinate lysinate (100 mg).
“D” is carbonate (100 mg).
The worst is “E”, Mg citrate + Mg bis (hydrogen-l-glutamate) (40 mg).
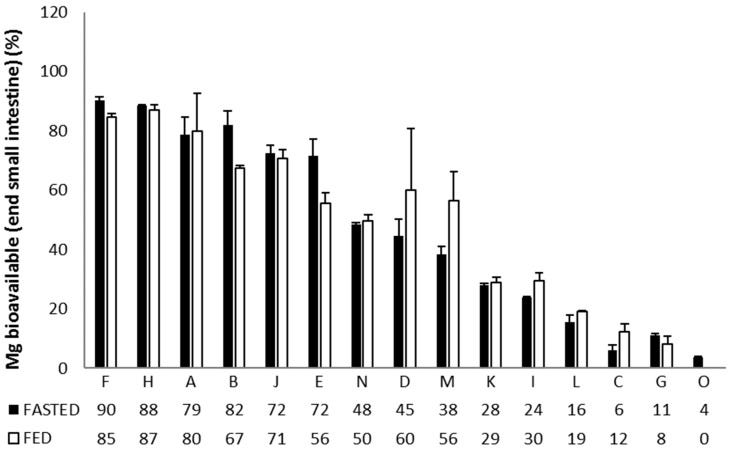
Next, they considered absorption from the small intestine. This changes the order somewhat, but there is a correlation.
The top 5:
“F” is orotate dihydrate (32. 8 mg).
“H” is citrate (19 mg).
“A” is oxide and glycerophosphate (196 mg).
“B” is citrate (100 mg).
“J” is a chelate, glycinate lysinate (100 mg).
The worst is “O”, Mg oxide (450 mg), as expected.
Go organic
In vitro testing was next.
The best:
We don’t know the proportions but I would caution against “A” due to the oxide content and risk of laxative effects etc.
19 mg of citrate in “H”? Really? That’s almost a placebo. But then it is made by the Pfizer clowns.
“A” is oxide and glycerophosphate (196 mg); “B” is citrate (100 mg); “F” is orotate dihydrate (32. 8 mg); “H” is citrate (19 mg); “J” is a chelate, glycinate lysinate (100 mg);
Not so hot:
This is curious as citrate is both one of the best and one of the worst. It may be down to the formulation.
“E” is citrate + Mg bis (hydrogen-l-glutamate) (40 mg) and “M” is citrate (200 mg):
Taken together the results on the in vitro experiments, magnesium supplements partially or completely consisting of organic magnesium formulation such as Supplement A, B, F, H, and J show the highest rate of both bioaccessibility, bioavailability and dissolution, although this was not found for the organic magnesium Supplements E and M, indicating that the magnesium formulation is not the only determining factor.
Of interest here is the importance revealed of taking your supplements with food, and not when fasted. Eating strongly correlates with increased bioaccessibility and bioavailability.
What’s the difference?
“Bioaccessibility measures the proportion of a compound consumed in a meal that is released from the food matrix during digestion in the luminal content and is accessible for absorption in the small intestine or biotransformed by the gut microbiota.”
“…bioavailability refers to the amount of compounds that completes the route passing through the digestive tract, is absorbed, and reaches the target tissues in the intact or metabolized form to perform its bioactivity or to be stored.”15
The white dots denote shortlisted formulations. The best was “A”, oxide and glycerophosphate (196 mg); the worst was “O”, oxide only (450 mg):
Glycerophosphate itself has been reported, not by coincidence, to be one of the least laxative, fat-soluble salts:
In vivo bioavailability testing of “A”, oxide and glycerophosphate (196 mg) and “O”, oxide (450 mg) concluded the shortlisting.
Serum levels trended up for about 2 hours and then declined slightly but remained elevated for 6 hours when compared to the control:

Doubling the 196 mg dose of “A”, oxide and glycerophosphate, increased the maximum serum level reached, but didn’t increase total bioavailability (iAUC, area under the curve).
Why didn’t they test a double dose of “O”, oxide only (496 mg)? They don’t say, but probably due to the risk of oxide-induced osmotic diarrhea.
Save that dose for the pharma SHIMEballs.
It is known that serum magnesium values reflect only 1% of the body magnesium content [14] since the absorbed magnesium disappears quickly from the circulation and is distributed into body stores (bone, muscle, and soft tissues) and excreted renally.
It is known that serum magnesium concentrations are therefore not necessarily a good reflection of total body magnesium status.
The magnesium tolerance test, in which the percentage of magnesium retained after parenteral administration is determined, is often offered as an alternative [4].
You can test for 24 hours, but you don’t need to:
Interestingly, many studies in the literature use 24 h urinary magnesium excretion as an estimate of bioavailability following supplement ingestion. This parameter was also tested in the current study, but no significant differences were found in urinary magnesium excretion in both phase one and two.
The present results further support the general pattern that inorganic magnesium compounds are not as easily absorbable as organic magnesium compounds by both in vitro and in vivo testing.
Did the oxide work synergistically with the organic glycerophosphate?
The selected supplement which had one of the highest predicted bioavailability differs from other formulations as it contains both inorganic (Mg oxide) and organic (Mg glycerophosphate) magnesium salts, while the supplement with the worst predicted bioavailability consists of only inorganic magnesium (Mg Oxide).
Ingredient ratios of “A”. That’s a lot of oxide:
Supplements: Ultractive magnesium contains both organic and inorganic Mg2+ salts. One tablet consists of 248.72 mg Mg oxide (containing 60% Mg, corresponding to 149 mg Mg) and 380.72 mg Mg glycerophosphate (containing 12.37% Mg, corresponding to 47 mg Mg). In total, one tablet thus contains 196 mg elementary Mg.
As Carolyn noted, the drawback of organic sources is that you need to take a high dose:
The disadvantage of organic sources of magnesium holds that, although they have a higher solubility, they provide only limited levels of elementary magnesium in contrast to inorganic salt which offers a high loading of elementary magnesium.
But this has an upper limit that cannot necessarily be solved by doubling up. We saw in an earlier study that splitting the doses didn’t help either:
Yet, in phase B of the current study, no additive effect of ingestion of two tablets of Supplement A (396 mg elementary magnesium) compared to one tablet (196 mg elementary magnesium) on serum magnesium levels was found.
Moreover, ingestion of one tablet of Supplement A led to higher serum magnesium levels compared to one tablet of Supplement O, although the latter contains more than twice as much elementary magnesium (196 vs. 450 mg elementary magnesium, respectively).
Solubility is more important for bioavailability than the amount of elemental Mg:
These findings suggest that the solubility of a magnesium supplement is of greater relevance for in vivo bioavailability than the loading (amount elemental magnesium).
Although upper intake levels have been set in the US and EU, this is not informed guidance as it takes no account of bioaccessibility, bioavailability, or the risk of osmotic diarrhea:
One important notice is that the tolerable upper intake levels (UL) for supplemental magnesium are set at 350 mg per day in the US and 250 mg per day in Europe.
As in vivo bioavailability of one tablet of Supplement A (196 mg magnesium) was not higher compared to ingestion of two tablets (392 mg magnesium), and the ingestion of two tablets exceeds the UL, ingestion of only one tablet should be recommended.
Although not putting you at risk of overdosing on Mg, we have seen the UL of magnesium oxide for some people is very much lower than 250 mg:
For Supplement O, containing 450 mg magnesium per tablet, the UL is thus exceeded, but it is highly unlikely that this effectively led to an overdose, as no bioavailable magnesium could be measured in serum following one acute ingestion.
The authors acknowledge that there might be some absorption after 6 hours:
One limit of the current study is that serum magnesium levels were collected up to 6 h following supplement ingestion. Magnesium is primarily absorbed in the distal parts of the small intestine and in the colon. Although magnesium absorption is known to decrease from 4–5 h post-ingestion, it cannot fully be excluded that a minor part of the ingested magnesium might not yet be absorbed within the 6 h timeframe during which serum was collected.
In conclusion:
The in vivo Mg bioavailability was not related to the Mg content of the supplements, but rather to the in vitro solubility and bioaccessibility.
Moreover, monitoring of serum magnesium levels was found to be a valuable measure of in vivo bioavailability. The in vitro methodology is useful for the prediction of bioavailability of micronutrients in general and can be a helpful approach to assure proper supplement use in future intervention studies and to avoid non-efficient micronutrient supplements on the market.
Full paper:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6683096/
- Magnesium and probiotics
These studies were originally cited in the Gut microbiota and colorectal cancer Substack, and inspired this mini-series to investigate further.
You can take your magnesium supplements with probiotics. In fact, it’s better if you do. The two work synergistically as probiotic bacteria promote magnesium uptake and magnesium promotes probiotic bacteria:
Effects of probiotic and magnesium co-supplementation on mood, cognition, intestinal barrier function and inflammation in individuals with obesity and depressed mood: A randomized, double-blind placebo-controlled clinical trial
Probiotic-driven advancement: Exploring the intricacies of mineral absorption in the human body
Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota
Conclusion
Dietary sources should come first, but regarding supplements, there are no hard and fast rules. Each compound has its advantages and disadvantages, depending on your tolerance, absorption profile, and where your deficiencies may be. Chelated organic salts tend to have higher bioavailability, up to a point, and reformulated chloride may be a means to increase your intake further, as splitting doses of organic salts didn’t increase net uptake in the studies cited.
For muscles and skeletal tissues, citrate performed well in the studies. Whereas for the brain the results weren’t as clear-cut between threonate, malate, acetyl taurate, and glycinate. If studies were followed up for longer than 24 hours I suspect the gaps would narrow further still.
And then we have the conundrum of topical magnesium, whether applied by soaked gauze pads, sprays, or bathing. Skin is largely impermeable to magnesium ions, yet it works, measurably, and predates “taking the waters” internally. Absorption via hair follicles and sweat glands may suffice.
I hope you found this mini-series useful. It’s certainly opened my eyes, and the research findings confirm that it is, indeed, an “essential giant mineral”.
Just keep that to yourself though.
Disclaimer
This site is strictly an information website reviewing research into potential therapeutic agents. It does not advertise anything, or provide medical advice, diagnosis, or treatment. This site does not promote any of these as potential treatments or offer any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses, or pharmacists. This content is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Any extracts quoted in the previous article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
References
Parazzini F, Di Martino M, Pellegrino P. Magnesium in the gynecological practice: a literature review. Magnes Res. 2017;30(1):1-7. doi:10.1684/mrh.2017.0419
Vázquez-Lorente H, Herrera-Quintana L, Molina-López J, et al. Response of Vitamin D after Magnesium Intervention in a Postmenopausal Population from the Province of Granada, Spain. Nutrients. 2020;12(8):2283. doi:10.3390/nu12082283
Kolanu BR, Vadakedath S, Boddula V, Kandi V. Activities of Serum Magnesium and Thyroid Hormones in Pre-, Peri-, and Post-menopausal Women. Cureus. 12(1):e6554. doi:10.7759/cureus.6554
Rudolf RD. The use of Epsom salts, historically considered. Can Med Assoc J. 1917;7(12):1069-1071. Accessed April 17, 2024. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1584988/
Types of Magnesium and Their Benefits. Healthline. Published November 21, 2019. Accessed March 28, 2024. https://www.healthline.com/nutrition/magnesium-types
Abbasi M, Taheri Mirghaed A, Hoseini SM, Rajabiesterabadi H, Hoseinifar SH, Van Doan H. Effects of Dietary Glycine Supplementation on Growth Performance, Immunological, and Erythrocyte Antioxidant Parameters in Common Carp, Cyprinus carpio. Animals (Basel). 2023;13(3):412. doi:10.3390/ani13030412
Ray EC, Mohan K, Ahmad S, Wolf MTF. Physiology of a Forgotten Electrolyte—Magnesium Disorders. Adv Kidney Dis Health. 2023;30(2):148-163. doi:10.1053/j.akdh.2022.12.001
Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of Learning and Memory by Elevating Brain Magnesium. Neuron. 2010;65(2):165-177. doi:10.1016/j.neuron.2009.12.026
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27-31. doi:10.4103/0976-0105.177703
Zhan J, Wallace TC, Butts SJ, et al. Circulating Ionized Magnesium as a Measure of Supplement Bioavailability: Results from a Pilot Study for Randomized Clinical Trial. Nutrients. 2020;12(5):1245. doi:10.3390/nu12051245
Ansu Baidoo VY, Thiagarajah K, Tekwe CD, Wallace TC, Gletsu-Miller N. Relationship between short-term self-reported dietary magnesium intake and whole blood ionized magnesium (iMg2+) or serum magnesium (s-Mg) concentrations. Ann Med. 55(1):2195702. doi:10.1080/07853890.2023.2195702
Uysal N, Kizildag S, Yuce Z, et al. Timeline (Bioavailability) of Magnesium Compounds in Hours: Which Magnesium Compound Works Best? Biol Trace Elem Res. 2019;187(1):128-136. doi:10.1007/s12011-018-1351-9
Ates M, Kizildag S, Yuksel O, et al. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol Trace Elem Res. 2019;192(2):244-251. doi:10.1007/s12011-019-01663-0
Blancquaert L, Vervaet C, Derave W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients. 2019;11(7). doi:10.3390/nu11071663
Rodrigues DB, Marques MC, Hacke A, Loubet Filho PS, Cazarin CBB, Mariutti LRB. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Current Research in Food Science. 2022;5:228-233. doi:10.1016/j.crfs.2022.01.002












![Soothing mountain river in Japan [Gif] | Nature gif, Rocky river, Amazing nature Soothing mountain river in Japan [Gif] | Nature gif, Rocky river, Amazing nature](https://substackcdn.com/image/fetch/$s_!odLK!,w_1456,c_limit,f_auto,q_auto:good,fl_lossy/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F66be08bf-1ee3-4d6b-830d-8b519060eae3_800x450.gif)



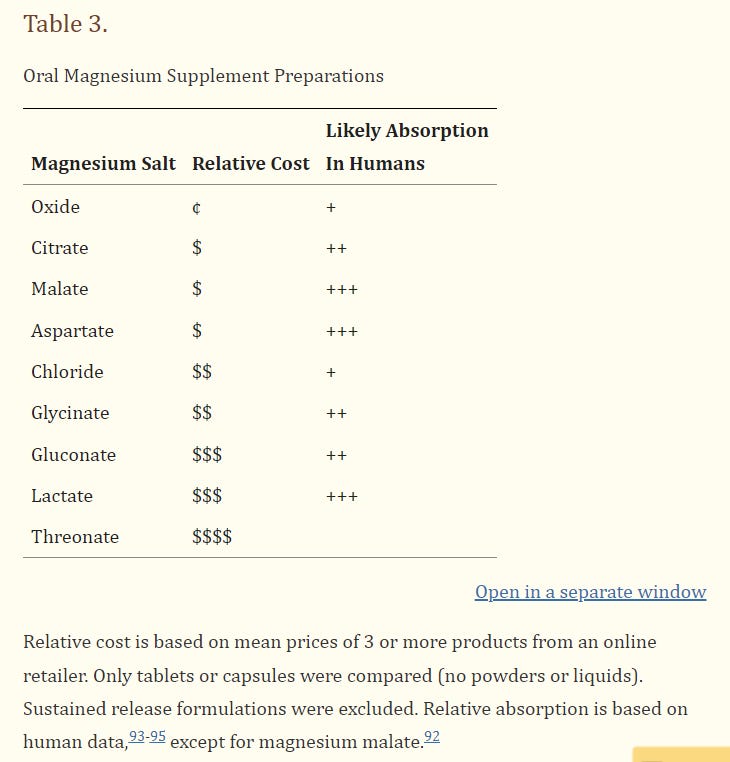




I was asked about "magnesium oil", is it any good ? I wasn't aware of solubility in oil, and it's not as such, it's actually a saturated solution of the chloride:
"... Magnesium oil is made from a mixture of magnesium chloride flakes and water. When these two substances are combined, the resulting liquid has an oily feel, but isn’t technically an oil. Magnesium chloride is an easy-to-absorb form of magnesium that may be able to raise levels of this nutrient within the body when applied topically to the skin."
https://www.healthline.com/health/magnesium-oil-benefits
Thanx as always, DC. Debonking the threonate scam was big for me.
Also, you have the best gifs on Substack
Also also, TYVM for boosting my friend Jen @denutrients