Updates:
17th June ‘22: (Tincture Taste Test)
7th January ‘23: (Hyperlinked contents page. Browser support varies)
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents:
Artemisinin compounds and anti-cancer activity
Efficacy of artemisinin and its derivatives in the treatment of type 2 diabetes melitus
Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis
Artemisia annua and pulmonary hypertension (PH)
Dosing guidance and contraindications
Tincture of Artemisia annua: The all important taste test
Abstract
This Substack is a scientific literature review of much of the research relevant to treating COVID-19 and transfection sequelae using the therapeutic herb Artemisia annua, including citations from other topic themed bibliography reviews.
Malarial treatments are discussed because the dosing techniques, toxicity and efficacy are relevant and well researched.
It explores the rational for finding effective antivirals against COVID-19 as a matter of some urgency, including a presentation of correlative anecdotal and official data on the harm being caused by currently adopted public health policy.
The botanical history of the herb Artemisia annua and its principle bioactive components and minerals are detailed.
A passage submitted by contributing author Charles Wright is featured: “THE WORLD HEALTH ORGANIZATION'S POSITION ON THE USE OF WHOLE LEAF AND CHEMICAL DERIVATIVES OF A. ANNUA”. This reports on how the stance of the WHO has changed in the last 20 or so years from supporting scientifically-proven traditional medicine to one of collaborating with social media platforms to suppress the discussion of the potential of whole leaf A. annua to treat SARS2.
A paper is reviewed that found strong anti-HIV activity with low toxicity when taken as a tea. As with malaria patients, this a very popular low cost and accessible treatment in Africa, with research studies findings of efficacy comparable to allopathic medications, although results varied according to sample sets used. No particular compound could be isolated that was responsible for this.
Research papers published into antiviral activity against COVID-19 are reviewed. One study concluded: “Results suggest that oral consumption of A. annua hot-water extracts (tea infusions), could provide a cost-effective therapy to help stave off the rapid global spread of these variants...” Another paper found via in vitro studies that later variants required greater artemisinin concentrations to achieve the same degree of viral inhibition.
Of particular note, greater efficacy than from hydroxychloroquine was indicated against mild to moderate COVID in one study. Another study concludes: “Artemisinin showed significant inhibition of 3CL protease activity but not Spike/ACE-2 binding.”
The water soluble artemisinin derivative artesunate shows particular promise as a compound which demonstrates anticancer, antimalarial and anti-amyloid properties, amongst others.
The next section further explores Artemisinin compounds and their anti-cancer properties. Research found multiple modes of action and synergistic effects for many of the therapeutic actions of A. annua.
Positive efficacy of artemisinin and its derivatives for the treatment of type 2 diabetes mellitus are reviewed. Once again, multiple modes of action are responsible.
A study by Kiss et al (2021) demonstrated that the artemisinin derivative artensuate attenuates levels of an amyloid precursor protein in an Alzheimer's disease (AD) mouse model.
A paper exploring therapeutic efficacy in the experimental model of multiple sclerosis using mice found that “The brain histology shows the absence of plaque formation in the artemisinin treated group.”
Another study found that dihydroartemisinin (DHA),an active form of artemisinin, alleviates pulmonary hypertension (PH) through the ELAVL2/miR-503/PI3K/AKT pathway (ie an autoimmune signalling pathway involving a microRNA), which might provide a basis for new therapeutic strategies.
The review concludes with dosing guidance from multiple sources, any contraindications, advice on growing, harvesting, drying and making tea infusions from A. annua and a further contribution by Charles on his recommendations for future research strategies.
Background
This review should be read alongside ones for Berberine1, NAC2 , a collation of therapeutics3 and MS4 with a view towards using in combination where applicable to gain synergistic benefits. Due to time pressures this cannot be a fully comprehensive, paraphrased literature review as Artemisia is one of the most researched herbs and, unlike berberine, it has hundreds of active ingredients that many of which could probably support a review on their own.
A PubMed search using “Artemisia annua” as keywords returned 836 research paper results over the last 10 years and 132 in the last year alone.
Several books have also been written just for this genera, taking months or years of work for each or/and taking contributions from a long list of researchers.
For this reason other relevant scientific literature reviews are considered, with the focus being on those concerning antiviral efficacy and safe effective dosing given the current population level transfection induced immunosuppression and increased virulence of escaped COVID-19 variants, for which many of the current first-line allopathic medications are now less than effective and in many cases harmful too.
Indeed it is a terrible indictment of modern allopathic medicine and public health policy that it has become so corrupted that we need to dust off our old herbal and Chinese medicine reference books to look for salvation in a safe, effective and affordable manner. As you will see there is a lot to learn from them.
Medicine really has regressed that far and it's incredible that some doctors must sue just for the right to be doctors who can prescribe as they see fit, eg with “horse wormer”, because it’s one of the few off-the-shelf therapeutics that actually works pretty well.5
There is a strong temporal relationship between each nations mass transfection rollout from December 2020 and 20-80% surges in COVID-19 cases, excess deaths, and a 200-1000% surge in myocarditis, sudden adult death syndrome and cancer diagnoses. Recurrence even in those free of the disease for 10 years are now common, and relapses are often very aggressive.
Reported this week alone, the first one about “stage IV lymphomas coming out of nowhere”:
https://twitter.com/VaccinatedFox/status/1534206501916745728?s=19
“Died in their sleep”. This sort of tragic incident was extremely rare with otherwise healthy young people until the last few months. I explored the pathophysiology of cardiac remodelling and fibrosis in detail in the Substack on monkeypox (ie rebadged smallpox) vaccines.6
And it explains why SADS (corrected=“post COVID vaccine deaths”) cases are significantly increasing of late despite the victims being boosted many months ago now. It may not become symptomatic for years when you suddenly suffer a cardiac arrest with little to no warning, cardiac remodeling takes time and can be a ticking time bomb. Arrhythmia can also lead to clot formation which may be fatal when the clot moves and blocks an artery to your brain or a vital organ.7
“The estimated mortality from SADS was 0.16/100 000 per annum (95% CI 0.12 to 0.21)”8, or about 1 in 625,000.
https://euroweeklynews.com/2022/06/08/sudden-adult-death-syndrome-sads/
https://twitter.com/GBNEWS/status/1534620276335443969?s=19
https://twitter.com/rosellacottage/status/1535129796082298880?s=19
@Storiesofinjury
This Twitter feed was just taken down by the censors.
I am pleased to have the following screenshots on record and that they have a website that is still accessible, though with a lower public profile:
https://community.covidvaccineinjuries.com/
This is why we need to make multi-role therapeutics and dosage advice available for all ASAP.
Healing the degree of endothelial damage mediated diseases & tumor growths being reported here though would be challenging, to put it mildly, - prevention is a much more effective strategy.
There may be some reporting bias but the pattern is consistent with other clinical data sources.
“In a time of universal deceit, telling the truth is a revolutionary act.”
I am indebted to Mr Charles Wright for sharing his “Review of Literature on the anti-SARS2 Activity of Whole Leaf Artemisia annua”9 and research references:
THE WORLD HEALTH ORGANIZATION'S POSITION ON THE USE OF WHOLE LEAF AND CHEMICAL DERIVATIVES OF A. ANNUA
In November 2001, The World Health Organization met in Shanghai, China to discuss treatments of malaria using chemicals derived from A. annua.
The WHO report recommended that several chemicals derived from A. annua, such as artemether, artesunate, dihydroartemisinin, should be developed for with other drugs in combination to treat malaria. These combination drugs have come to be referred to as Artemisinin-based combination therapy (ACT). The WHO did not recommend that two chemicals derived from the same plant should be used in combination or that the antiviral effects of the whole plant should be researched.
Report: Meeting on Antimalarial Drug Development, Shanghai, China, November 16-17 2001.
Chinese scientists are now working on the development of several combinations of artemisinin drugs with longer-acting synthetic drugs. WHO considers such combinations very promising as they may provide affordable, short-course treatments that could prove useful for large-scale use, even in areas of intense malaria transmission. ... There is a need for more antimalarial combination treatments containing artemisinin derivatives.
Later on May 4, 2020, as SARS2 was reported to be spreading, the WHO Regional office of Africa released a statement with an extremely misleading headline:
WHO supports scientifically-proven traditional medicine.
"WHO recognizes that traditional, complementary and alternative medicine has many benefits and Africa has a long history of traditional medicine and practitioners that play an important role in providing care to populations. Medicinal plants such as Artemisia annua are being considered as possible treatments for COVID-19 and should be tested for efficacy and adverse side effects."
"Africans deserve to use medicines tested to the same standards as people in the rest of the world. Even if therapies are derived from traditional practice and natural, establishing their efficacy and safety through rigorous clinical trials is critical."
"As efforts are under way to find treatment for COVID-19, caution must be taken against misinformation, especially on social media, about the effectiveness of certain remedies." The World Health Organization later announced that they had collaborated with Twitter, Facebook, Instagram, LinkedIn, Snapchat, Tiktok, Pinterest, and Youtube to "fight COVID19 misinformation." As one of the individuals who tried to spread the word of the anti-SARS2 potential of whole leaf A. annua on social media, I can attest that social media platforms did collaborate with the WHO to suppress the discussion of the potential of whole leaf A. annua to treat SARS2.
The herb Artemisia annua
The specific name annua is Latin and means year or annual and refers to the annual biological cycle of this plant.
Common names include Sweet Annie, Sweet sagewort, Sweet woodworm, and Chinese woodworm and is called Qing Hao in China.
Artemisia annua is an annual plant in the Asteraceae (i.e. sunflower) family. It was originally native to China, but is now localized in many countries, including scattered areas of North America.
The whole plant has medicinal properties, but dried leaves are most commonly used.
Unlike the previously reviewed berberine, the therapeutic properties of annua come from a range of bioactive components and minerals10: Of around 46 phenolics that have been isolated at least 3 of these are important anti-viral/anti-cancer compounds: quercetin, myricetin and luteolin.

Please note that this is different to the common wormwood, Artemisia absinthium which is moderately poisonous, gives the alcoholic liqueur absinthe its flavour, can damage the nervous system and cause mental deterioration. This toxicity is attributed to thujones (alpha-thujone and beta-thujone), which constitute 0.25–1.32% in the whole herb and 3–12% of the oil.
Antiviral activity - HIV
A 2012 paper by Lubbe et al conducted quantitative in vitro measurements of the anti-HIV activity of Artemisia annua, administered as a tea11. They found strong anti-HIV activity with low cytotoxicity. They didn’t attempt to identify all the specific active ingredients but noted the effect was not due to artemisinin content but from another chemical compound and recommended further research:
Ethnopharmacological relevance: Artemisia annua contains the well-known antimalarial compound artemisinin, which forms the backbone of the global malaria treatment regime. In African countries a tea infusion prepared from Artemisia annua has been used for the treatment of malaria only for the past 10-20 years. Several informal claims in Africa exist that the Artemisia annua tea infusions are also able to inhibit HIV. Since HIV is a relatively newly emerged disease, the claims, if substantiated, could provide a very good example of "ethnopharmacology in overdrive". The objective of this study was to provide quantitative scientific evidence that the Artemisia annua tea infusion exhibits anti-HIV activity through in vitro studies. A second objective was to determine if artemisinin plays a direct or indirect (synergistic) role in any observed activity. This was done by the inclusion of a chemically closely related species, Artemisia afra, known not to contain any artemisinin in our studies.
Materials and methods: Validated cellular systems were used to test Artemisia annua tea samples for anti-HIV activity. Two independent tests with different formats (an infection format and a co-cultivation format) were used. Samples were also tested for cellular toxicity against the human cells used in the assays.
Results: The Artemisia annua tea infusion was found to be highly active with IC(50) values as low as 2.0 μg/mL. Moreover we found that artemisinin was inactive at 25 μg/mL and that a chemically related species Artemisia afra (not containing artemisinin) showed a similar level of activity. This indicates that the role of artemisinin, directly or indirectly (synergism), in the observed activity is rather limited. Additionally, no cellular toxicity was seen for the tea infusion at the highest concentrations tested.
Conclusion: This study provides the first in vitro evidence of anti-HIV activity of the Artemisia annua tea infusion. We also report for the first time on the anti-HIV activity of Artemisia afra although this was not an objective of this study. These results open the way to identify new active pharmaceutical ingredients in Artemisia annua and thereby potentially reduce the cost for the production of the important antimalarial compound artemisinin.
In this paper we report on the remarkable in vitro anti-HIV activity of the Chinese medicinal herb Artemisia annua L. (Asteraceae). This plant is mainly used to treat malarial infections as it contains artemisinin (ART) an important antimalarial ingredient. This compound (and its derivatives) is now being used in combination with other antimalarials that possess a different mechanism of action in a drug regime called Artemisinin Combination Therapies. Although the traditional use of the plant in the form of an uncontrollable tea infusion to treat malaria is still widespread, it is strongly discouraged due to fears that a low content of ART may lead to the emergence of resistance (De Ridder et al., 2008). In order to address this aspect we recently demonstrated (Van der Kooy and Verpoorte, 2011) that by using the correct preparation methods the tea infusion can contain up to 95% of the ART present in the plant material.
A survey about such use of the plant in Kenya and Uganda conducted from 2009 to 2011 revealed some interesting additional observations: more than half of the respondents had started using Artemisia annua for ailments other than malaria. Of these, about half had started to use Artemisia annua to treat HIV/AIDS (Willcox et al., 2011). In another survey of treatments prescribed by herbalists for people living with HIV/AIDS in Cameroon, Artemisia annua was one of the most frequently mentioned plants (Noumi, 2011). In both studies a tea was prepared from the plant, either alone or with other plant species.
Something is biologically active or working in combination, but they were unable to identify what?
A literature survey revealed that various pharmacological activities of Artemisia species have recently been described by Bora (2011). Reported antiviral activities include the inhibition of HSV-1 and -2 by the essential oil of Artemisia arborescens, the inhibition of hepatitis B by a tablet containing Artemisia capillaris, and in vitro inhibition of HIV replication in H9 lymphatic cells by isolated compounds of Artemisia capillaris. The compounds responsible for inhibiting HIV replication were two flavonoids, arcapillin and isorhamnetin, and a coumarin aesculetin (Wu et al., 2001). A methanol extract of Artemisia annua was tested in a syncytium inhibition assay, which is based on the interaction between the HIV-1 envelope and the cellular membrane protein CD4 on T-lymphocytes (Chang, 2003). Some inhibition was seen at the concentration tested (15%), even though it was relatively low compared to some of the other 80 plants in the test series (Jung and Schinazi, 1994). Reports of other Artemisia species showing anti-HIV activity are limited to Artemisia caruifolia (Ma et al., 2001). Four compounds were isolated from a methanol extract of which N1,N5,N10-tri-p-coumaroylspermidine showed around 70% inhibition of HIV-1 protease at a concentration of 100 μg/mL. The three dicaffeoylquinic acid derivatives also isolated during this study did not show any appreciable activity against HIV-1 protease. Cos et al. (2008) did, however, report that 3,5-dicaffeoylquinic acid does indeed show good activity against HIV integrase although controversy remains around its potency and activity. In a metabolomic investigation of Artemisia annua and Artemisia afra coumaroylspermidine was not detected in either species tested (Liu et al., 2010a). No other reports could be found that this compound has been identified in Artemisia annua keeping in mind that Artemisia annua is probably one of the best studied Artemisia species. Moreover, the level of activity reported for this compound (around 100 μg/mL) is also far higher than the activity we report in this study for the tea infusion. No other reports could be found that any other extract of Artemisia annua significantly exhibits HIV expression.
In this study we prepared tea infusions of nine Artemisia annua, one Artemisia afra, and one Rooibos tea sample and tested their anti-HIV activity in vitro. We also determined the solid content of the different tea infusions in order to quantitatively determine the activity. The objective in this study was to provide quantitative scientific evidence that the Artemisia annua tea infusion exhibits anti-HIV activity.
ART = Artemisinin
2.1. Plant material
Artemisia annua samples were obtained from the breeding programme of Anamed (Germany), collected in different years from different countries. Dr. Martin Hirt identified the plants as being Artemisia annua. Due to the fact that ART is known only to occur in appreciable quantities in this species, our quantitative analysis of ART in all the Artemisia annua samples acts as an additional positive identification of the plant material.
Typical concentration of ART in the leaves was between 0.50-1.00%:
They used boiling water for infusion, whereas later we will see that cold extraction, tinctures or dried leaves provide more of the active ingredients.
Tea infusions using A. annua have a bitter but cooling taste according to reports, and the longer it brews the more bitter it becomes, but you can flavour it with dried peppermint or fennel to make it more palatable.
2.3. Tea preparation
Tea infusions were prepared as described earlier (Van der Kooy and Verpoorte, 2011). Ninety mg of plant material were carefully weighed off, and 10 mL of boiling distilled water was added to each sample. The samples were allowed to simmer for 3 min, after which 1.5 mL was filtered into HPLC vials (0.2 μm syringe filter) whilst it was still hot. The samples were sealed and sent for anti-HIV analysis.
The half maximal inhibitory concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function. IC50 is a quantitative measure that indicates how much of a particular inhibitory substance (e.g. drug) is needed to inhibit, in vitro, a given biological process or biological component by 50%.[1] The biological component could be an enzyme, cell, cell receptor or microorganism. IC50 values are typically expressed as molar concentration.12
The positive control Efavirenz (EFV) is a synthetic non-nucleoside reverse transcriptase (RT) inhibitor with antiviral activity. Efavirenz binds directly to the human immunodeficiency virus type 1 (HIV-1) RT, an RNA-dependent DNA polymerase, blocking its function in viral DNA replication. 13
HeLa cells are a line of immortal human cervical cancer cells frequently used for assay testing. Of course this is not the same way that in vivo exposure would occur, but it is essential to do it this way first. It’s a means to control the number of variables in order to demonstrate efficacy using a methodology that is repeatable and at concentrations that are comparable with a known good positive control.
2.5. HIV bioassay
Various sample sets were prepared. The first set contained tea prepared from 1 sample in triplicate, the second set of samples was prepared from all plant samples in order to confirm the original result. Due to two samples being damaged on transport a third sample set was analysed consisting of the duplicate samples of the damaged samples.
The anti-HIV analysis was conducted using a validated cellular system by testing each sample at various dilutions in triplicates. Experimental results were used to determine the IC50 values for each test sample. Two independent examinations were conducted and, importantly, no cytotoxicity was observed. The utilised format “iFIGS” (Infection format of “Fusion-induced gene stimulation”) (Klimkait et al., 1998) represents an in vitro infection system: defined full length HIV-1 plasmids produce infectious virus after a transfection of DNA into human HeLa cells. During 48–60 h post transfection the cells release infectious HIV particles into the culture supernatant. This cell-free supernatant containing viral particles is then quantified and used as inoculum to infect reporter cells that contain a lacZ gene under the control of the HIV control region. Thereby, upon infection with HIV, the reporter gene will be induced in a quantifiable fashion, and the product, beta galactosidase, allows quantification of inhibitory effects of new chemical compounds or extracts. This system was infected in the presence of the sample (Artemisia annua tea) or Efavirenz (EFV) as a standard control drug. Efavirenz is a potent non-nucleosidic RT inhibitor currently in clinical use.
For a second analysis the “deCIPhR” system (“dual-enhancement of Cell Infection to Phenotype Resistance”) which is a co-cultivation procedure, in which the transfected cells are directly co-cultivated in the presence of drug with reporter cells. Both the infection (iFIGS) and co-cultivation (deCIPhr) format produced similar results, but the deCIPhR format permits direct and more rapid cell-to-cell spread of the virus and is therefore more stringent (more demanding on any inhibitor). As a consequence slightly higher drug concentrations are typically required for full inhibition.
Results. Artemisinin content excluded as being directly responsible for the anti-HIV effects:
From Table 1 it can be calculated that ART degrades by 10–30% over a 2 year period if the material is stored dry and at room temperature (exposed to light and air). The results furthermore suggest that the ART content does not correlate well with the activity data presented in Table 2. No correlation can be drawn between the content of ART and the observed activity indicating that ART is probably not the main active compound in the tea infusion. Moreover, the pure ART standard was found to be inactive at 25 μg/mL. The maximum content of ART in the tea samples can be calculated as follow (for sample 1): 90 mg leaf material X 0.0036 (% ART)/1909 (dilution factor). The maximum content of ART for sample 1 is around 170 ng/mL (we assume a 100% ART extraction efficiency and no degradation). Furthermore, the most active sample (sample 1) had one of the lowest concentrations of ART whilst the sample with the highest content of ART had one of the lowest activities (sample 9). This indicates that ART does not appear to play any direct significant role in the observed activity.
Activity of Artemisia afra and synergism:
Another possible explanation for the antiviral activity is synergism between ART and other compounds in the extract. With the inclusion of Artemisia afra (not containing ART) and the observed activity of this sample we can state that the possibility that synergism involving ART is rather limited, although it cannot be completely excluded. We have shown (Van der Kooy et al., 2008, Liu et al., 2010a) that these two species are chemotaxanomically closely related with the major exception that ART has not yet been detected in any Artemisia afra specimen. This does, however, not mean that the compound(s) responsible for the activity are the same in both species. With the inclusion of Aspalathus linearis we wanted to show that a well known tea (Rooibos) will not give a positive result and thereby act as a negative control. The tea prepared from this plant species did not show any activity, as was expected. Our intention was to have two non-ART-producing species as negative controls but to our surprise Artemisia afra showed a similar level of anti-HIV activity as Artemisia annua.
This is the first report of Artemisia afra possessing significant in vitro anti-HIV activity and adds credence to the reports of Mulholland and Drewes (2004) that patients given Artemisia afra in combination with standard HIV treatment reported improvement of symptoms compared to patients taking only standard HIV treatments. Keeping in mind the massive burden of HIV in southern Africa, can Artemisia afra become a flagship for Traditional African Medicines as asked by Liu et al. (2009). Here we provide scientific evidence that indeed this plant may have the potential to become a flagship for traditional African medicines although much more research will be needed.
Activity of Artemisia annua:
The activity of the Artemisia annua tea infusions were found to be between 2.0 and 58.0 μg/mL. According to Cos et al. (2008) any pure natural product with an activity of below 25 μg/mL should be considered to have significant antiviral activity. If we look at the results of sample set 2, the IC50 values for the tea infusions ranged between 2.0 and 14.8 μg/mL for the iFIGS bioassay format and 7.0–22.7 μg/mL for the more stringent deCIPhR bioassay format. The tea infusion therefore show potent activity in both bioassays used in this study. Based on this we can describe the tea infusion, consisting of many compounds, to be highly active. To place this into a better perspective we adjusted the IC50 values in order to take into account the variability of the bioassay. This calculation is based on the reported IC50 values for the positive control EFV (Table 2).
Table 2. The results are presented as the solid content for each sample and the dilution factor needed for each sample to reach the IC50. Based on these two results the IC50 was calculated as μg/mL. Not all samples were tested in both bioassays:
First IC50 μg/mL column is for the iFIGS bioassay format, second IC50 μg/mL column is for the more stringent deCIPhR bioassay format. Look for values below 25:
To have a better comparison between all the samples and to minimise the effect of variation in the bioassay, we adjusted all the IC50 values to the lowest (1 nM) and highest (48 nM) reported value for the positive control. These corrected IC50 values are presented in Table 3. This gives us a more accurate picture of the range of activity and the comparison between all the samples. The activity range for Artemisia annua can now be given as between 0.6 and 216.0 μg/mL and for Artemisia afra between 1.0 and 48.0 μg/mL. Sample 9 which was the first sample tested now also gives a far better comparison to the result obtained during its second analysis. Without the correction the IC50 was found to be 115.6 μg/mL (EFV = 25 nM) and in the second test 14.8 μg/mL (EFV = 4 nM). With the IC50 correction the lowest activity is now 4.6 and 3.7 μg/mL respectively (EFV = 1 nM).
Table 3. Corrected IC50 values for the iFIGS analysis. These values were obtained by adjusting all IC50 values to the lowest (1 nM) and highest value (48 nM) obtained for the positive control EFV. This gives a better comparative analysis by minimising the effect of bioassay variability.
Standardised lowest and highest IC50’s. Lower is better:
The activities of all the samples appear to be relatively closely related indicating that the concentration of the active compound(s) in the samples is probably very similar keeping in mind the inherent variability of the sample preparation and the bioassay. This also indicates that the storage period (oldest sample ∼10 years) and cultivation site does not seem to play a significant role in the presence/absence or quantity of these active compounds. No correlation can therefore be drawn between activity and site of cultivation or the age of the samples. In our current study our objective was not to identify the active components but to provide a quantitative measurement of the in vitro anti-HIV activity of Artemisia annua.
In their conclusion there is concern by the WHO that continued use of tea infusions with low doses of ART for treating HIV may lead to resistance in the malarial parasite. I would consider this unlikely as Artemisia has been used by Chinese herbalists as a remedy for many illnesses and fevers, presumably including malaria for thousands of years without resistance by Plasmodium falciparum14. And natural therapies use whole plant extracts, not extracted single compounds.
Despite this voice, however, the uncontrolled use of the plant material in its traditional way will continue. This does create a potential problem. We aim at addressing this issue in two different ways. (1) We decided to publish our in vitro findings in order to create awareness of the full potential of Artemisia annua. At the moment there are shortages of ART on the world market leading to enormous price fluctuations. If a distinct anti-HIV compound(s) can be identified in this plant we may be able to produce a second lead compound or even a separate “active pharmaceutical ingredient” (API) from this plant. These API's may contribute to reducing the production cost of ART, if it can be co-produced with the use of existing facilities. Currently ART is extracted with apolar solvents (e.g. hexane) from which it is then purified. We have recently developed a polar (ethanol) extraction and purification protocol for ART and it is foreseen that these polar anti-HIV compound(s) will be co-extracted to some extent with the use of this protocol (Liu et al., 2011). If possible, this may lead to a stabilising economic effect on the ART world market. (2) It does not seem to be wise to principally ignore the long-standing traditional use of Artemisia annua. We rather suggest undertaking a thorough scientific investigation in order to fully understand what compounds are responsible for which activity. This could be followed by cultivating specific Artemisia annua cultivars containing the respective compounds at the exploitable levels. The identification of the key ingredients responsible for the observed activity will allow possibly managing, and more importantly, controlling their production in the plant. We have now embarked on a full metabolomic analysis with the aim to identify and quantify all key components in the tea infusion. Further careful examination as well as independent confirmation of the results presented in this paper will be essential before the discovery of new antiviral activities can lead to an expanded clinical exploitation from known and already industrially established plant-based preparations.
No conflicts of interest were recorded:
We would like to thank Heino Heyman of the University of Pretoria (South Africa) for performing the first anti-HIV bioassay. This work was conducted without any funding. Tea extracts are available on request.
Antiviral activity - Covid-19
Several papers have been published on this over the last two years. The first from 2021 by Nair et al also used hot water extracts which showed potent activity against various Covid-19 variants including delta in vitro.15
Overoptimistic expectations for future vaccine efficacy here though, which is why we are where we are:
Abstract
Ethnopharmacological relevance For millennia in Southeast Asia, Artemisia annua L. was used to treat “fever”. This medicinal plant is effective against numerous infectious microbial and viral diseases and is used by many global communities as a source of artemisinin derivatives that are first-line drugs to treat malaria.
Aim of the Study The SARS-CoV-2 (Covid-19) global pandemic has killed millions and evolved numerous variants, with delta being the most transmissible to date and causing break-through infections of vaccinated individuals. We further queried the efficacy of A. annua cultivars against new variants.
Materials and Methods Using Vero E6 cells, we measured anti-SARS-CoV-2 activity of dried-leaf hot-water A. annua extracts of four cultivars, A3, BUR, MED, and SAM, to determine their efficacy against five fully infectious variants of the virus: alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and kappa (B.1.617.1).
Results In addition to being effective against the original wild type WA1, A. annua cultivars A3, BUR, MED and SAM were also potent against all five variants. IC50 and IC90 values based on measured artemisinin content ranged from 0.3-8.4 μM and 1.4-25.0 μM, respectively. The IC50 and IC90 values based on dried leaf weight (DW) used to make the tea infusions ranged from 11.0-67.7 μg DW and 59.5-160.6 μg DW, respectively. Cell toxicity was insignificant at a leaf dry weight of ≤50 μg in the extract of any cultivar.
Conclusions Results suggest that oral consumption of A. annua hot-water extracts (tea infusions), could provide a cost-effective therapy to help stave off the rapid global spread of these variants, buying time for broader implementation of vaccines.
Multiple compounds including ART showed antiviral activity in previous studies:
Recently, we showed that hot-water extracts of dried leaves of seven cultivars of the medicinal plant, Artemisia annua L., used for millennia to treat malaria fever (Hsu, 2006) and sourced from four continents, prevented SARS-CoV-2 replication in vitro (Nair et al., 2021). Recently, anti-SARS-CoV-2 efficacy of A. annua extracts was independently confirmed (Zhou et al., 2021).
Antiviral efficacy inversely correlated to artemisinin (ART) content (Nair et al., 2021). Others also observed that compared to A. annua, A. afra, a related perennial species lacking ART, was equally effective vs. SARS-CoV-2 with IC50 values of 0.9-3.4 and 0.65 mg/mL extract, respectively (Nie et al., 2021). Although these results indicated that both A. annua and A. afra have potent anti-SARS-CoV-2 activity in vitro and that the effect is not ART dependent, it was unclear whether A. annua is effective against emerging variants.
Here we report in vitro efficacy against new variants of four of the seven originally studied A. annua cultivars.
They used a standardised hot water extraction from 10g dried leaves/L, boiled for 10 minutes and analysed for ART by by gas chromatography-mass spectrometry (GC-MS) before assaying against Vero E6 (originally from African Green Monkey kidney epithelial cells16) at various dilutions to determine the IC50’s for Covid-19 variants USA WA1; alpha, B1.1.7; beta, B1.351; gamma, P.1; delta, B.1.617.2; and kappa, B.1.617.1.
Results showed that ART alone significantly inhibited COVID-19, but low ART cultivars and the non-ART control A. afra were more or similarly effective, so other compounds are biologically active. No cytotoxicity was observed at the concentrations being used.
A. annua hot-water extracts inhibited the recently evolved variants of SARS-Cov-2 (Figure 1) with calculated IC50 values normalized to the ART content of each tea infusion ranging from 1.1 - 7. 9 μM for the gamma, delta, and kappa variants. Although already reported by (Nair et al., 2021), WT(WA1), alpha, and beta variants were included for direct experimental comparison (Figure 1; Table 1). The lowest IC50 values were from the BUR cultivar and the highest were from the SAM cultivar. As previously shown (Nair et al. 2021), there was an inverse correlation between ART in extracts and anti-viral efficacy. The lowest ART content (BUR) yielded the greatest potency (the lower the IC50, the more potent the drug/extract), providing evidence that ART is not the only active antiviral agent in these extracts. The Nie et al. (2021) preprint further validated that ART was not the only anti-SARS-CoV-2 compound in the extracts by showing that aqueous extracts of the PAR cultivar of Artemisia afra, an Artemisia species lacking ART, had an IC50 of 4.1 mg/mL, within the range of 3.1-13.0 mg dried extract/mL of the A. annua cultivars studied therein. As already reported for extracts used in this study, no cytotoxicity was observed at a dry weight of ≤50 μg in the extract of any cultivar (Nair et al. 2021).
Note that later variants required higher IC50 concentrations. And it's a log scale, each point is 10 fold greater.
Increased glycosylation of the spike protein is suspected as a factor, and John Paul has recently observed reduced efficacy of ivermectin in his recipients, finding better results with berberine (previous review).
Kappa is B.1.617.1 and Delta is B.1.617.2:
IC50’s from aqueous extracts were difficult to compare with previous research, so they also used dried leaves to try to get a better comparison. Ethanolic extractions had IC50’s about 50% lower so were more potent than aqueous extracts. Low temperature extraction and poor water solubility may be the key here, tinctures vs tea.
Although Zhou et al. (2021) also showed that A. annua hot-water extracts had anti-SARS-CoV-2 efficacy, it is difficult to compare their IC50 values because they did not test the same viral strain, use the same plant cultivars, or make their extracts and apply them to viral infected cells using the same procedures. Using A. annua aqueous extracts against the BavPat 1/2020 strain of SARS-CoV-2 in Vero E6 cells, the IC50 values were 390 and 260 μg dried extract/mL for pretreated and treated cells, respectively. For pretreatment, extract was added 1.5 h before virus infection and for treatment, drug was added 1 h after virus infection. Ethanolic extracts yielded IC50 values about 50% lower, and thus were more potent than the aqueous extracts. To compare results of both studies, we calculated the dry mass of leaves equivalent to their reported IC50 to be 941.2 mg. The IC50 mass reported in (Nair et al., 2021) ranged from 13.5-57.4 μg, varying by cultivar. The leaf dry mass IC50s in this study for the gamma, delta, and kappa variants ranged from 38.2-50.7, 42.4-67.7, and 37.0-45.0 μg leaf DW, respectively. The three orders of magnitude difference between this study and Zhou et al. (2021) likely result from the above noted differences in methodology.
ART and its derivatives have some anti-SARS-CoV-2 activity (Cao et al., 2020; Gendrot et al., 2020a; Gendrot et al., 2020b; Nair et al., 2021; Zhou et al., 2021). However, in those reports where there are direct comparisons with Artemisia extracts, ART is not the only active phytochemical, suggesting there are other antiviral compounds in the plant. A. annua contains a rich assortment of identified phytochemicals (Ferreira et al., 2010), some of which have activity against human coronavirus proteins. For example, quercetin and myricetin have inhibitory activities against SARS-CoV NTPase/helicase with IC50s of 0.1 and 2.7 μM, respectively, and luteolin has an IC50 of 10.6 μM against SARS-CoV in Vero E6 cells (Russo et al., 2020). Investigating other potential anti-SARS-CoV-2 phytochemicals found in A. annua and A. afra is warranted.
In conclusion the authors refer to sustained efficacy against different variants (albeit with dose variations) and urge the WHO to update their trials. The team themselves propose testing in rodents, but at the time of writing I have been unable to find anything published:
Hot-water (tea infusion) extracts of A. annua are active against SARS-CoV-2 and its variants alpha, beta, gamma, delta, and kappa. In our original report, anti-SARS-CoV-2 activity inversely correlated with ART content. Herein, similar responses are noted for gamma, delta, and kappa wherein the A. annua cultivar with the lowest ART content, BUR, generally had the lowest (most effective) IC50. These results demonstrate the potential of the extracts as treatments in the global fight against this constantly evolving virus. We urge WHO to consider including extracts and encapsulated dried leaves in their announced clinical trials that already include artesunate (Kupferschmidt, 2021). We aim to test preclinical models of SARS-CoV-2 in rodent models (Dinnon et al., 2020; Gu et al., 2020) that could help advance A. annua as an inexpensive therapeutic in parts of the world where logistic issues such as delivery require longer time to achieve vaccination levels that would ultimately quell this pandemic.
7.0 Conflict of Interest Statement
Authors declare they have no competing conflicts of interest in the study.
Next paper from 2021 and Orege et al published a literature review including an analysis of minerals & artemisinin’s:
Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective17
Essential oils in Artemisia
Essential oils are compounds usually networked or multiplexed with volatile molecules such as terpenes and aromatic components that are phenol derivatives. They have a broad spectrum of bioactivity due to the presence of several active ingredients or secondary metabolites with varying modes of action, which make them play vital roles in nature, ranging from antibacterial, antiviral, antifungal, etc. (Dhifi et al. 2016). Artemisia species are an excellent source of essential oils such as pinene, thujyl alcohol, cadinene, phellandrene, thujone, etc. and have been reported to achieve remarkable success for several biological activities including, analgesic, anti-coccidial, anti-diabetic, antifungal, antiviral, anti-herpes virus, and lots more (Kumar and Kumari 2018; Martínez et al. 2012).
Mode of action and toxicity studies. Recent in silico studies predict it should have stronger antiviral properties than hydroxychloroquine:
Anti-viral and immune-stimulatory potentials of Artemisia and Artemisia-based Products against SARS-CoV-2
Artemisia spp. had earlier been reported to consist of essential phytochemicals that contribute to its inhibitory role against viruses (Bora and Sharma 2010). Before the outbreak of COVID-19, some ethnopharmacological studies on Artemisia derivatives revolved around their retroviral properties (Efferth 2018; Jana et al. 2017; Laila et al. 2019; Lubbe et al. 2012), capacity to minimize the replication of herpes viruses (Efferth et al. 2008; Milbradt et al. 2009; Naesens et al. 2006; Nagamune et al. 2007) and inhibition of hepatitis B and C viruses (Dai et al. 2016; Paeshuyse et al. 2006; Qi et al. 2013; Romero et al. 2005), etc. Noteworthily, the bioactive constituents present in A. annua have demonstrated activity against several viruses such as bovine viral diarrhoea (Romero et al. 2006), Epstein-Barr Virus, and Hepatitis B Virus (Haq et al. 2020). Earlier, some authors reported the use of A. annua against SARS coronavirus which appeared in 2002 Lin et al. (2003). The presence of flavonoids, quercetin, and di-caffeoylquinic acid in the plant inhibits the activity of MERS-CoV-3 CLPro, an enzyme that is similarly produced by SARS-CoV-2 (Jo et al. 2019, 2020). Interestingly, in a Vero cell-based, 3-(4,5-dimethylthiazol-2-yl-)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay for virus-induced cytopathic effect (CPE) screening analysis of medicinal plant extracts with antiviral potentials against SAR-CoV viral strain (α-coronavirus), A. annua alongside three other plants demonstrated a substantial inhibitory effect (Li et al. 2005). The results showed that A. annua, a highly efficacious species demonstrated a CC50 of 1053.0(± 92.8) µg/ml and EC50 of 34.5 ± 2.6 µg/mL with a selective index > 31 as compared to interferon-α that was > 100,000(± 710.1) and 660.3(± 119.1) respectively, indicating its ability to inhibit SARS-CoV-2 penetration and replication.
Since its discovery as an antiviral agent by a Chinese scientist (Qian et al. 1982), several studies have revealed the promising role of Artemisinin and its derivatives in the inhibition of viruses (Efferth et al. 2008). Artemisinin has been revealed to inhibit replication and penetration of viruses both in vivo and in vitro as well as generating enhanced host type I interferon response (Wang et al. 2020a). The replication of Hepatitis C replicon, a single-stranded RNAvirus just like SARS-CoV-2 was reported to be inhibited by artemisinin (Obeid et al. 2013). Very recently, a study on molecular dynamic using computer-aided drug discovery (CADD) revealed that artemisinin and its derivatives could be more potent than hydroxychloroquine (HCQ) in silico. In addition to that, artemisinin and its derived molecules showed an extra mode of interaction by binding to Lys353 and Lys31-binding hotspots of SARS-CoV-2 spike protein and producing a better Vina docking score of − 7.1 kcal/mol for artenilic acid than − 5.5 kcal/mol for hydroxychloroquine (Sehailia and Chemat 2020). The study further revealed that the formed complexes interfered and remained stable on the SARS-CoV-2 Spike protein receptor site. Besides the antiviral activity, Artemisia contains a high concentration of zinc, which is reported to be effective for the immunomodulation effect of host response and increase in CD4 level (Honscheid et al. 2009). It should be noted that the antioxidant ability of Artemisia enhances immune defense.
Clinical interventional studies of Artemisia and Artemisia–based products as mono- or combined therapy in the face of COVID-19
From a safety point of view, hundreds of phytochemicals present in A. annua have been revealed to be below recommended toxicity limits (Duke 1992; Lutgen 2019; Yang et al. 2010). Some antiviral agents including repurposed off-label drugs such as CQ, HCQ, Redmesivir, etc. have been in the spotlight as frontline therapies for COVID-19 (Bolarin et al. 2020). However, some of them have demonstrated cardiotoxicity concerns among many other after-administration side-effects (Yang et al. 2010). Notably, Artemisinin has been reported to possess a better and lower toxicity profile compared with CQ and HCQ (Cheong et al. 2020). As such, clinicians can have minimal worries should higher dosage application become necessary. Also, its flexibility as a combination therapy with other drugs suggests its potential usage for the treatment of patients with cases of co-infections.
The mechanism of viral inhibition is not clearly understood, but thought to be via inhibiting 3CLpro, stimulation of T-cell generation and by suppressing inflammatory cytokines.
“The viral 3-chymotrypsin-like cysteine protease (3CL pro) enzyme controls coronavirus replication and is essential for its life cycle”.18
Generally, the mode of action of active ingredients from natural products against coronaviruses is through suppressing virus infection which in turn reduces the viral load (Jassim and Naji 2003). Specifically, the mode of action of A. annua on Spike protein of the SARS-CoV-2 is not clearly understood. Nevertheless, it has been reported to be by inhibiting the enzymatic activity of chymotrypsin-like protease (3CLpro) (Law et al. 2020). A. annua stimulates adaptive immunity by generating CD8 and CD4 lymphocytes responsible for the production of antibodies targeting SARS-CoV-2 and down-regulating the production of pro-inflammatory cytokines prostaglandin E2 (PGE2), TNF-α, interleukin-6 (IL-6), interleukin-10 (IL-10), thus increasing CD4 count and CD4/CD8 ratio (Poisson-Benatouil 2020). Cytokine storms decrease the number of Treg cell in COVID-19 infected patients, and leads to functionally exhausted CD8 and CD4 lymphocytes which ultimately affects human immune systems and cause severe respiratory failure (De Biasi et al. 2020).
Another antiviral literature review from 2021, this time by Andréa D Fuzimoto.19
Nineteen studies were retrieved. From these, fourteen in silico molecular docking studies demonstrated potential inhibitory properties of artemisinins against coronavirus-host proteins including 3CLPRO, S protein, N protein, E protein, cathepsin-L, helicase protein, nonstructural protein 3 (nsp3), nsp10, nsp14, nsp15, and glucose-regulated protein 78 receptor. Collectively, A. annua constituents may impede the SARS-CoV-2 attachment, membrane fusion, internalization into the host cells, and hinder the viral replication and transcription process. This is the first comprehensive overview of the application of compounds from A. annua against SARS-CoV-2/coronavirus disease 2019 (COVID-19) describing all target proteins. A. annua's biological properties, the signaling pathways implicated in the COVID-19, and the advantages and disadvantages for repurposing A. annua compounds are discussed. The combination of A. annua's biological properties, action on different signaling pathways and target proteins, and a multi-drug combined-therapy approach may synergistically inhibit SARS-CoV-2 and assist in the COVID-19 treatment. Also, A. annua may modulate the host immune response to better fight the infection.
Keywords: Artemisia annua; Artemisinin; COVID-19; Main protease; SARS-CoV-2; Spike protein.
Substitute RNA viruses are often used due to safety concerns, but the knowledge should be transferrable:
Coronaviruses are very contagious pathogens that often require a biosafety level 3 (BSL-3) laboratory to handle them [20]. Due to the high risk of viral manipulation, the inability to access a BSL-3 laboratory or adapt a BSL-2 laboratory, and other factors, researchers must use other viral models for coronavirus research. Some examples are pseudotyped viruses that do not replicate, viruses that can be researched in a BSL-2 laboratory, and RNA viruses that may provide insights and results relevant to the RNA-based coronaviruses. For instance, HCV, HIV, human coronavirus-OC43, mouse hepatitis virus, and HIV-luc/SARS pseudotyped virus are some of the commonly used viruses [21]. RNA viruses include SARS, MERS, HIV, HCV, Ebola virus, influenza, polio measles, dengue virus, and adult human T-cell lymphotropic virus type 1 [22]. Thus, the previous research on viral infections provided a strong background to further the SARS-CoV-2 research.
Greater efficacy than HCQ indicated against mild to moderate COVID:
…a recent controlled clinical trial investigated the anti-SARS-CoV-2 effects of artemisinin-piperaquine (AP) [47]. Patients diagnosed with mild to moderate COVID-19 were divided into two groups and the majority of the patients had a moderate form of the disease (82.6% in the AP group and 88.9% in the control group). One group of 23 patients received AP while the control group with 18 patients received a combination of HCQ-arbidol [47]. Both drug combinations were used as antiviral and symptomatic treatments. The AP group took significantly less time to reach undetectable levels of SARS-CoV-2 than the controls, requiring (10.6 ± 1.1) d and (19.3 ± 2.1) d, respectively (P = 0.001, 0.005) [47]. Considerable reduction was also found in the percentage of undetectable RNA on days 7, 10, 14, 21, and 28 and in the length of hospital stay in the AP group. No patients progressed to a severe or critical disease stage, and adverse reactions in both groups were mild. The authors pointed to research limitations, such as sample size and trial design and advised the monitoring of electrocardiograph and liver enzymes. Nevertheless, they recommended the use of AP for COVID-19 prevention and treatment of mild to moderate cases (8 tablets—artemisinin 500 mg/piperaquine 3000 mg during 7 d) [47]. Hence, ACTs could be a viable antiviral resource to assist in the treatment of SARS-CoV-2 infection, but more research is needed.
In silico docking studies reviewed.
“Any substance that binds specifically and reversibly to a biomacromolecule to form a larger complex and alters its activity or function is called a ligand. In the PDB, drugs, metals, and small molecules are also called ligands.”20
Molecular docking simulations were used to predict the interrelation between a small molecule ligand (e.g., drugs, herbs, or phytochemicals) and viral/host proteins [56]. The docking process examines the ligand conformation, position, and orientation in relation to the docking site, and assesses the binding affinity [56]. It is important to note that, conventional drugs, herbs and natural compounds go through the same or similar testing methods to identify possible antiviral agents against the SARS-CoV-2 [21]. In silico studies often screen and test hundreds or even thousands of pharmaceutical drugs, herbs, and phytocompounds to identify drug candidates.
This should pinpoint the phytocompounds in A. annua most likely responsible for binding to viral receptors:
Below, the computational simulations that investigated the A. annua phytocompounds and their effects on the coronavirus and host proteins are reviewed. Also, due to different methodologies and data interpretation of the studies, for the present review, the binding score of ≤ –7.0 kcal/mol will be considered appreciable to provide a baseline for reporting and comparing. However, additionally examined compounds-drugs and their scores can be visualized in Table 1. A summary of the A. annua compounds that reached the score of ≤ –7.0 kcal/mol is shown in Fig. 1 .
Summary of the Artemisia annua phytocompounds as potential SARS-CoV-2/host protein inhibitors. These compounds attained appreciable antiviral activity by inhibiting SARS-CoV-2/host proteins with binding scores of ≤ –7.0 kcal/mol. The stronger binding interaction among the artemisinins was reached by artesunate-N protein (–8.8 kcal/mol). The results were attained by in silico studies except those that are highlighted as in vitro studies (e.g. arteannuin B and dihydroartemisinin inhibited the N protein in vitro; artesunate inhibited N protein in vitro and in silico; and the rest of the compounds were in silico). For the A. annua phytocompounds and a full list of scores and other results, see Table 1. SARS-CoV-2: severe acute respiratory syndrome coronavirus-2.
IV administered artesunate is also the first-line drug for the treatment of severe chemo-resistant malaria in the United States.21
Artemisinin was one of the compounds that interacted with the 3CLPRO active binding sites [39]. SARS-CoV 3CLPRO, or the main protease (MPRO), is a viral enzyme responsible for the cleavage of polyproteins (pp1a and pp1ab) into functional proteins important for the coronavirus replication. 3CLPRO is considered to be a promising drug target and its inhibition would hinder viral replication. Although artemisinin was not the best possible 3CLPRO inhibitor among the compounds, it had a promising estimated free binding energy (ΔG) of –7.15 kcal/mol [39]. The free binding energy represents how strongly the binding was modeled to occur, as opposed to how accurately the fit occurs. Other investigated drugs and compounds were better binders, such as rutin, curcumin, emetine, HCQ, ritonavir, and lopinavir. Rutin was the most potent inhibitor, with ΔG of –9.55 kcal/mol [39]. HCQ had similar docking energy to artemisinin, with a ΔG of –7.75 kcal/mol. In the simulation, artemisinin bound to the SARS-CoV-2 3CLPRO through an alkyl hydrophobic interaction with MET49 and CYS145 residues and a π-alkyl interface with HIS163 [39]. This result was somewhat corroborated by another in silico study that examined 36 compounds for their SARS-CoV-2 3CLPRO inhibition potential [40]. Artesunate had a docking score of –6.46 kcal/mol, while artemisinin had a stronger docking score of –7.78 kcal/mol [40]. Both CQ and HCQ were used as positive controls, with binding scores of –7.12 kcal/mol and –7.35 kcal/mol, respectively. Although other phytocompounds were better binders such as betulinic acid (–10.0 kcal/mol), artemisinin had a better binding score than the controls.
Findings from another study:
Overall, the simulations revealed that artesunate had significant binding energy, a stable and tight docking to residues, and exhibited five active site interactions. The authors concluded that artesunate is one of the four agents, together with epigallocatechin gallate, withaferin, and dolutegravir, that may act as anti-SARS-CoV-2 3CLPRO.
Inhibition of SARS-CoV-2 by binding to ACE2. They found that it had more binding potential than HCQ, but less than other compounds:
The coronavirus S protein binds to the hACE-2, and substances that prevent the coronavirus S protein from docking to the hACE-2 receptor may inhibit the infection [7]. After further analysis, the authors recommended the prioritization of artenimol as a candidate for future clinical trials, as most artemisinin derivatives end up being converted to artenimol in the body. However, the binding scores of most artemisinins were below what many researchers would consider appreciable (from –6.0 to –6.8 kcal/mol) and artelinic acid showed the best score among the compounds (–7.1 kcal/mol). Still, artemisinin and its derivatives were less toxic and more efficient at docking at the SARS-CoV-2 S protein than HCQ (–5.5 kcal/mol)
Moreover, another computational study examined if antimalarial, HIV-protease inhibitor, anti-inflammatory, and antibiotic drugs had a good affinity to the S protein [49]. They verified that the antimalarial agents, artemisinin, CQ, HCQ, mefloquine, and pyrimethamine, had poor anti-SARS-CoV-2 S glycoprotein activity when compared with other drugs [49]. Artemisinin had a binding score of –6.8 kcal/mol and an estimated inhibitor constant (KI) of 15.37 μmol/L, and formed two bonds with the amino-acid residues Asn460 and Lys462 [49]. For this study, other drugs had more potential to hinder the S protein from binding to the ACE-2 receptor, such as lopinavir (–9.1 kcal/mol), ritonavir (–8.0 kcal/mol), cobicistat (–8.3 kcal/mol), erythromycin (–9.0 kcal/mol), and spiramycin (–8.5 kcal/mol) [49]. Also, the HIV protease inhibitors, anti-inflammatory, and antimicrobial drugs formed more bonds with a series of other S protein amino acid residues (e.g., from 3 to 6 hydrogen)
Other compounds probably have greater binding affinity for the spike glycoprotein:
Further structural analysis showed that, although artemisinin had a low binding capacity, it had the second-best interaction and a good fit between the interface of ACE-2/S protein. Andrographolide had the best binding score, with six residue interactions (Asp-30, Asn-33, His-34, Pro-389, Arg-393, and Tyr-505), while artemisinin was bound to four residues (His-34, Ala-387, Pro-389, and Tyr-505) [51]. Also, upon the toxicity testing, andrographolide and artemisinin exerted the lowest toxicity levels of the compounds. However, additional molecular dynamics simulation to predict the stability of the bounds showed that artemisinin had the least preferable docking score. Overall, the authors concluded that the more stable compounds andrographolide and pterostilbene would be the best options for further study.
Against CTSL.
“CTSL is a member of the lysosomal cysteine protease family, whose major function is proteolysis of antigens produced by pathogens. It is mainly detected in Golgi apparatus and traffic-related organelles like endosomes and lysosome 16”.22
3.2.3. Study that investigated CTSL
In another molecular docking study from 2007, 26 compounds (10,458 natural product molecules) from the Traditional Chinese Medicine Database (TCMD) were screened using MDL28170 as a template [38]. MDL28170 was recognized as an effective CTSL inhibitor. CTSL is an endosomal protease that plays an important role in membrane fusion and the internalization of the coronavirus. Thus, CTSL is an important target protein for therapeutic interventions in SARS-CoV infections. The compound MOL736 (aurantiamide acetate) extracted from A. annua was detected as an efficient inhibitor of the host CTSL protein and it was more “matchable” than MDL28170 [38]. Thus, this study showed this A. annua’s isolate as a potential therapeutic resource in the fight against SARS-CoVs.
Next, studies into target protein interactions were discussed. An “adding up of benefits” is an apt description, it doesn’t seem to excel in any particular area but collectively is very effective, so many modes of action & synergism. This should also make escape all the more challenging for the virus:
3.2.4. Studies that investigated multiple target proteins
Another research group examined the in silico ability of 25 natural compounds and their potential as inhibitors of SARS-CoV-2 3CLPRO and host glucose-regulated protein 78 (GRP78) [46]. The GRP78 is a master chaperone protein that responds to accumulated unfolded or misfolded proteins in the cells [58]. When translocated to the cell membrane, it can bind to region IV of the SARS-CoV S protein (the highest binding affinity of –9.8 kcal/mol), thus mediating viral entry into the host cells [58]. Agents that inhibit the GRP78 cell receptor could offer another antiviral therapeutic option. Four compounds displayed promising activity as antiviral agents: withaferin, artemisinin, curcumin, and andrographolide. All four phytochemicals showed the ability to bind to both SARS-CoV-2 3CLPRO and GRP78 substrate-binding domain, with withaferin displaying the strongest binding energy (–9.83 kcal/mol). Also, all four compounds satisfied the “rule of five” in drug-likeness, according to SwissADME, and the selected proteins demonstrated a high probability of druggability [46]. Yet, for our analysis here, artemisinin exhibited a favorable binding score of –8.06 and –7.89 kcal/mol for 3CLPRO and GRP78, respectively. In another investigation, the researchers performed a molecular docking analysis of 171 essential oil components against SARS-CoV-2 3CLPRO, RdRp, the S protein binding domain, and hACE-2. Additionally, they tested the SARS-CoV-2 endoribonuclease (SARS-CoV-2 Nsp15/NendoU) and the SARS-CoV-2 ADP-ribose-1’-phosphatase (SARS-CoV-2 ADRP) [45]. The non-structural protein, nsp15, is an endoribonuclease that cleaves RNA at uridylate and is required for viral infection. The inhibition of the coronavirus ADRP may reduce the multiplication of the virus, providing another target for therapeutic intervention. The study included most of the A. annua essential oil constituents and showed the binding scores for each target protein. The best docking scores were reached by (E,E)-α-farnesene, (E)-β-farnesene, and (E,E)-farnesol [45]. However, the docking energies of these components were somewhat weak, compared with other proteins. The authors concluded that the essential oil components would not make good coronavirus/host protein inhibitors, but they could work synergistically with other antiviral agents to provide relief for the COVID-19 symptoms [45]. As mentioned by the authors, linalool, β-caryophyllene, and 1,8-cineole provide anti-inflammatory and antinociceptive properties, and camphor has an antitussive action that could add up to the benefits of A. annua for the COVID-19. Additionally, the authors listed the in vitro inhibitory effects of several Artemisia species and their essential oil constituents against different viruses. A. vulgaris L. essential oils produced 100% reduction of yellow fever virus at 100 μg/mL; A. princeps var. orientalis reduced 64% of the murine norovirus-1 (MNV-1) at 0.01% of the essential oil; and A. arborescens L. inhibited HSV-1 and HSV-2 infected Vero-6 cells with IC50 of 2.4 and 4.1 μg/mL, respectively.
In another study, the binding capacity of FDA-approved drugs and chemicals against a series of SARS-CoV-2/host proteins such as RdRp, helicase protein, nucleocapsid (NC or N) protein, S protein RBD, E protein, nsp10, nsp14, and nsp15 were examined [50]. The screened drugs included artesunate, artemether, antimalarial pharmaceutical drugs (CQ, quinine, primaquine, amodiaquine, mefloquine, and proguanil), the antiviral drugs galidesivir, remdesivir, pirodavir, and others. Artesunate exhibited binding activity with E protein, helicase protein, nucleocapsid (NC) protein, and with the non-structural proteins nsp10, nsp14, and nsp15 [50]. Artesunate did not interact with S protein RBD or RdRp.
I would certainly shortlist the derivative artesunate as it is quite broad spectrum in its binding affinity:
…artesunate was classified as “may be chosen” and artemether was “not chosen” as SARS-CoV-2/host inhibitors even though all binding scores were ≤ –7.0 kcal/mol [50]. From these two, the best binding score was attained by the artesunate-N protein interaction (–8.8 kcal/mol). Regardless, the reported results on artesunate and artemether showed potential antiviral activity from proteins that not previously been reported. Also, the authors proposed four drug combinations for “proper and effective management of COVID-19;” two of them included artesunate. The rationale for their proposed combination of artesunate-drug or drug-drug regimens that would include “immune boosters” also requires more investigation and careful analysis. On the other hand, another research study screened and tested 123 antiviral drugs as nsp15 inhibitors [52]. The top three candidates that reached significant binding scores of ΔG ≤ –7.0 kcal/mol were simeprevir (–8.4 kcal/mol), paritaprevir (–7.5 kcal/mol), and artesunate (–7.2 kcal/mol) [56] The molecular dynamics simulations revealed that the nsp15 drug complexes were stable and had structural integrity. Nonetheless, in some evaluations, simeprevir and paritaprevir were more stable than artesunate, and the binding free energy utilizing the MM/PBSA approach showed that the interaction of artesunate with nsp15 was non-spontaneous and infeasible [52]. Thus, in this instance artesunate was not considered a good candidate as a nsp15 inhibitor.
A final review of artensuate also showed high binding affinities and “significant inhibition” in one study. Please note this is just one compound from A.annua and artemisinin itself didn’t have the required bonding affinity against a nonstructural RNA-binding protein or PLRO:
“The SARS-CoV-2 coronavirus encodes an essential papain-like protease domain as part of its non-structural protein (nsp)-3, namely SARS2 PLpro, that cleaves the viral polyprotein.”23
One more study screened 108 FDA-approved anti-inflammatory and antiparasitic drugs against SARS-CoV-2/host target proteins 3CLPRO, PLPRO, RdRp, S protein, helicase protein, nsp1, nsp3, nsp4, nsp9, and nsp16–nsp10 [53]. Although some researchers do not make a clear distinction between PLPRO and nsp3, the latter is a multi-domain protein and PLPRO is one of the catalytic domains of nsp3 [53]. Artesunate inhibited the nsp3 and formed H-bonds with Ala154, Phe156, Asp157, and Leu126, and interacted with residues Val49, IIe23, Ala52, and Phe156 in other interface types [53]. The antiparasitic drugs showed higher inhibitory actions. Although artesunate was not the most potent (–8.1 kcal/mol), it did exert significant inhibition [53]. Also, another in silico study tested Ayurvedic herbs and spices, and pharmaceutical drugs for their potential uses against SARS-CoV-2/host proteins, including 3CLPRO (nsp5), PLPRO (nsp3), RdRp, helicase protein (Hel), S protein, M protein, NC protein, E protein, hACE-2 receptor, nsp1, nsp2, nsp4, nsp6–nsp16, ExoN, and NendoU [54]. Artemisinin which was included in this study only inhibited nsp2 and PLPRO with binding energies of –5.174 and –6.134 kcal/mol, respectively [54]. However, other compounds, such as epicatechin and hesperidin reached a multi-protein higher inhibition.
Potential mechanisms of Artemisia annua and its phytocompounds against SARS-CoV-2/COVID-19. The combination of A. annua’s biological properties, action on different signaling pathways, and target proteins may synergistically inhibit the SARS-CoV-2, decrease inflammation, modulate the host immune response, and alleviate the COVID-19 symptomatology. SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; COVID-19: coronavirus disease 2019.
Artemisinins moderate immune responses, as discussed previously. Rheumatoid arthritis and SLE (lupus) are interesting to see in the review as autoimmune disorders can be particularly challenging to treat long term safely eg corticosteroids can lead to osteoporosis, cardiovascular damage, immune suppression and even tumorigenesis.
As an aside, these are often prescribed to cancer patients, which is ironic:
Corticosteroids are prescribed frequently in oncology practice to reduce swelling and pain caused by cancer and may also be used to control and prevent nausea and vomiting caused by chemotherapy.24
Corticosteroids include glucocorticoids and corticosterones. Elevated glucocorticoid levels increase the activity of the negative regulator murine double minute 2 (MDM2) through induction of the serum-and-glucocorticoid-regulated kinase (SGK1) and mediate the inhibition of p53 (27). P53 can initiate DNA repair, cell cycle arrest, aging, and apoptosis, which are related to the body's ability to inhibit tumor formation and respond to various types of cancer treatment (28). Therefore, the loss or impairment of the p53 function mediated by corticosteroids can considerably promote tumorigenesis. Obradović et al. (29) found that the increase in glucocorticoids during breast cancer progression was related to a lower survival rate. Increased hormone levels could lead to the activation of glucocorticoid receptors that were involved in the activation of multiple processes in metastasis and the up-regulation of kinase orphan receptor 1 (ROR1) at distant metastatic sites. Inhibition of ROR1 expression can reduce metastasis and prolong the survival rates of breast cancer patients.25
A. annua’s biological properties and signalling pathways discussed.
MMP’s (matrix metallopeptidases ie peptide & protein destroyers: matrix degradation) & VEGF (vascular endothelial growth factor) can promote cancer metastasis & abnormal angiogenesis.
Angiogenesis is how a tumor grows by the growth of blood vessels from surrounding tissues into it, often stimulated by VEGF so that it is supplied with oxygen, nutrients and waste drainage. Inhibition of VEGF is just one way to inhibit tumor growth.
The effects of artemisinins have been investigated in models of asthma, chronic obstructive pulmonary diseases, lung cancer, nasopharyngeal carcinoma, and acute lung injury [2]. A. annua derivatives have shown antioxidant, anti-inflammatory, anti-pulmonary fibrosis, antimetastatic, anti-angiogenic, antimucus, and anti-tumor proliferation activities that act through several signaling pathways benefitting these respiratory diseases [2]. Notably, artemisinins can regulate the expression of pro-inflammatory cytokines, NF-κB, MMPs, and VEGFs [2]. In general, the anti-inflammatory properties of the artemisinins are due to inhibition of toll-like receptors, Syk tyrosine kinase, phospholipase C-γ, phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), mitogen-activated protein kinase, signal transducer and activator of transcription-1/3/5, NF-κB, specificity protein 1, and nuclear erythroid 2-related factor 2/antioxidant response element signaling pathways [63]. In an RA study, artesunate downregulated the secretion of IL-1β, IL-6, and IL-8 through the inhibition of NF-κB and the regulation of the PI3K signaling pathway [64]. Also, the flavonoids, casticin and chrysosphenol-D, the monoterpene, 1,8-cineol, rosmarinic acid, and chlorogenic acid of A. annua have shown anti-inflammatory properties [65]. On another note, in a murine model of ulcerative colitis, the artemisinin analogue SM934 suppressed neutrophils and macrophages in the colon tissues, inhibited NF-κB, and decreased pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) [66]. Artesunate affected innate immunity in vivo and in vitro by suppressing macrophages, dendritic cells, IL-12, and TNF-α in another study of colitis [67]. Also, dihydroarteannuin improved the symptoms of SLE by inhibiting TNF-α in the macrophages of mice in vitro and in vivo and by blocking the NF-κB nuclear translocation in vivo [68]. Thus, these studies exemplify the potentially beneficial influence of A. annua and its phytochemicals on the innate immune response of the host.
A kinase is an enzyme that catalyses the transfer of a phosphate group from ATP to a specified molecule.
“p21-activated kinase 1 (Pak1) is a member of the highly conserved family of serine/threonine protein kinases regulated by Ras-related small G-proteins, Cdc42/Rac1. It has been previously demonstrated to be involved in cardiac protection.”26
“Ras is a family of related proteins which is expressed in all animal cell lineages and organs. All Ras protein family members belong to a class of protein called small GTPase, and are involved in transmitting signals within cells (cellular signal transduction).”27
One review article examined the role of the p21-activated kinase-1 (PAK-1) in the coronavirus pathogenesis and proposed artemisinin as a PAK-1 blocker to act against coronaviruses, suppress lung fibrosis and inflammation, and boost immune function [71]. The authors also looked at the effects of CQ as a PAK-1 inhibitor and suggested that PAK-1-blockers could assist in the current pandemic [71]. PAK-1 is an enzyme encoded by the PAK-1 gene, which belongs to the serine/threonine PAK family and is activated by RAC/CDC42, the RAS-related GTPases (p21) [72], [73]. PAKs are distributed throughout body tissues and are essential for different cellular functions, such as cytoskeletal modeling, focal adhesion assembly, cell migration, survival, mitosis, and transcriptional modulation and protein synthesis involving ERK and NF-κB [72]. The PAK-1 activity has been linked to a variety of diseases, such as cancer and tumors, viral and bacterial infections, inflammatory diseases, like asthma and arthritis, acquired immune deficiency, type 2 diabetes, hypertension, neuronal diseases, like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, depression, and epilepsy, and others [73]. PAK-1 gets activated during the infection in patients with malaria, influenza A, HIV, and HPV and contributes to the replication of these pathogens [73]. Thus, PAK-1 could also offer another route for therapeutic intervention in coronavirus infection. According to the authors, one of the advantages of using PAK1-blockers is that they are not needed for normal cell growth, thus they would cause no side effects to people or animals when treating PAK-1-dependent diseases [73]. Interestingly, another PAK-1 blocker, curcumin, has also shown anti-COVID-19 activity [74]. Therefore, the hypothesis that artemisinin acts as a PAK-1 blocker offers another mode of action against COVID-19.
4.3. Advantages of repurposing of A. annua and its phytocompounds for the SARS-CoV-2
A series of docking simulations reviewed herein demonstrated the ability of A. annua phytocompounds to bind SARS-CoV-2/host proteins to hinder the viral replication process. The modeled binding strength between the drug or natural compound and proteins, their interaction, fit, and stability are some of the factors that suggest a molecule’s potential as an efficient inhibitor. However, other factors may influence which molecules are good antiviral candidates. The advantages of repurposing artemisinins include low toxicity, safer higher dosages, few side effects, low cost, and easy production [2], [12]. Many artemisinins have gone through pharmacokinetic and pharmacodynamic studies demonstrating good profiles [2]. These phytocompounds have been tested for malaria and are being used through different modes of administration, including oral, intravenous, intramuscular, and rectal [2]. Artemisinins can also sensitize other drugs, providing an attractive motive to combine them with other drugs such as the current ACT protocols [2]. Drug-combination therapy targeting different mechanisms may delay drug resistance and increase treatment effectiveness [12]. For instance, the concentration of artesunate that showed effectiveness against HBV, HCMV, and HepG2 2.2.15 cells is similar to the concentration used for malaria.
4.4. The use of the A. annua whole plant vs. its isolates
Since A. annua has a variety of phytochemicals that may target different proteins and signaling pathways, the question of whether using the whole plant instead of its separate isolates would attain better results and if it would reach the desired bioavailability to address the infection is an important one. In a very informative study, researchers examined the absorption, distribution, metabolism, and excretion of the dried leaves of A. annua (DLA) vs. pure artemisinin in vitro and in vivo [65]. They also used a rat model to investigate the effects of these herbal agents on systemic inflammation. The results showed that the whole plant taken orally in the form of extracts and teas made from DLA was more bioavailable than pure artemisinin [65]. These preparations inhibited artemisinin-metabolizing enzymes (CYP2B6 and CYP3A4) which made the DLA-delivered artemisinin pass through the liver unmetabolized, causing it to be more available in the tissues [65]. Also, the artemisinin contained in the DLA had better absorption in the tissues of the female rats. Furthermore, after administration to the lipopolysaccharide-challenged rats, which exhibited systemic inflammation, both DLA and pure artemisinin significantly reduced the serum TNF-α in females, while DLA alone reduced TNF-α and IL-6 in the males. No effects were noticed on the anti-inflammatory cytokine IL-10, suggesting that other signaling pathways may be involved [65]. The authors hypothesized that DLA provided better absorption and bioavailability of its artemisinin due to reduced liver enzyme activity, or it worked through the additive effect of several compounds providing a better outcome. Regardless, it was evident that the use of the whole plant was more effective than the isolated compound [65]. In a Caco-2 permeability assay, the same group of researchers indicated that the intestinal permeability of the DLA-delivered artemisinin was significantly greater by 37% compared with pure artemisinin [75]. This suggested that one or more phytocompounds in A. annua may increase intestinal absorption of artemisinin [75]. In a previous study on the use of DLA for malaria, the authors also showed that artemisinin delivered through DLA was about four times more soluble than the pure artemisinin, and that the essential oil fraction of A. annua contributed to the increase in artemisinin solubility in the DLA [76]. Remarkably, 18 severe malaria patients who were unresponsive to the WHO-approved ACT protocol (Coartem®) and IV artesunate, after six months, were given DLA tablets (0.5 g DLA per os, twice daily for 5 d), as last resort compassionate care [77]. For those in a coma or too young to swallow, the tablets were crushed, mixed with water, and delivered by nasogastric tube. Of the 18 ACT-resistant patients, all recovered completely with the use of DLA and experienced no side effects. Thus, due to the known low bioavailability and possibility of resistance of artemisinin, which poses some disadvantages to its use, whole-plant A. annua could be a valuable and inexpensive resource to assist with the coronavirus pandemic. Not surprisingly, traditional medicines have been using whole plant extracts for thousands of years, and science has been re-discovering its benefits. The compounds in the DLA tablet analyzed in this study consisted of artemisinin (10.97 mg), deoxyartemisinin (2.54 mg), arteannuin B (0.89 mg), artemisinin acid (1.30 mg), and dihydroartemisinic acid (3.91 mg). It also contained chlorogenic acid 10.94 mg, coumarins (scopoletin 2.47 mg), flavonoids (18.32 mg), and non-artemisinic terpenes (camphor 0.27 mg, phytol 0.56 mg) [77]. Some of these compounds may work synergistically with artemisinin, the target proteins of the SARS-CoV-2/host and signaling pathways of COVID-19.
The combination of A. annua’s biological properties, action on different signaling pathways and target proteins, and a combined therapy approach may synergistically inhibit the SARS-CoV-2 and assist making therapies to attenuate the COVID-19 symptoms. The addition of other herbs and phytochemicals that have also shown antiviral activity, anti-coronavirus and host protein inhibition, and other properties may contribute to designing therapies that modulate the host immune system to better fight the infection [21]. A big limitation of the present review is the small number of included peer-reviewed studies. The different methodologies, algorithms, and software used by the docking studies also limited the comparison of results.
In conclusion, binding affinity and in vitro & in silico studies alone are not enough to predict (or explain) observed antiviral activity due to other interactions, more research and trials are warranted.
The author declared he received no funding and there was no conflict of interest:
A. annua and its phytocompounds may be able to inhibit the SARS-CoV-2/host proteins 3CLPRO, S protein, CTSL, N protein, E protein, helicase protein, nsp3, nsp10, nsp14, nsp15, and GRP78 receptor. Thus, collectively, the A. annua constituents may impede the SARS-CoV-2 attachment, membrane fusion, and internalization into the host cell, and hinder the viral replication and transcription process. The best inhibitions were attained by the interactions of artesunate-3CLPRO (–8.0 kcal/mol), artemisinin-3CLPRO (–8.06 kcal/mol), artemether-N protein (–8.0 kcal/mol), artesunate-N protein (–8.8 kcal/mol), artesunate-nsp3 (–8.1 kcal/mol), artesunate-nsp14 (–8.4 kcal/mol), and artesunate-nsp15 (–8.2 kcal/mol). Other appreciable scores of ≤ –7.0 kcal/mol were also reported. To date, no peer-reviewed articles confirmed a significant binding of A. annua and its compounds directly with RdRp, PLPRO, hACE-2 receptor, S/ACE-2 complex, 3a protein, or additional non-structural proteins that are important for the coronavirus replication.
Not only the binding strength of an herb-compound to the viral proteins is important, but also the type of bond, fit, and stability should be considered when electing anti-SARS-CoV-2 options. The in vitro and in silico studies alone are not enough to determine the best antiviral candidates, and other factors need to be considered. Although in some studies the artemisinins did not show the highest binding capacity, the combination of their antiviral action, target-protein inhibition, and biological properties may synergistically contribute to the treatment of SARS-CoV-2 and intervene in additional signaling pathways to favorably influence the COVID-19 pathogenesis. Importantly, A. annua may re-modulate the host’s innate and adaptive immune system and assist in reducing the cytokine storm, ARDS, and COVID-19 symptoms. Advantages for repurposing artemisinins include low toxicity, safer higher dosages, few side-effects, cost-effectiveness, easy production, pre-existing pharmacokinetic and pharmacodynamic studies showing good profiles, well-understood modes of administration, drug sensitization to design drug and/or herbal-combination therapies, reduction of drug resistance (when drugs are associated), and dosage predictability based on other disease protocols. The evidence we reviewed here supports future research and clinical trials to understand the use of A. annua for the SARS-CoV-2 infection, COVID19 prevention, reduction of severity, treatment of different phases of the disease, and management of symptoms.
The following study by Dogan et al in 2021 further supports evidence for artemisinin causing significant inhibition of 3CL protease activity but not Spike/ACE-2 binding.28
Importance of proteases to the virus:
“The genomes of some viruses encode one massive polyprotein, which needs a protease to cleave this into functional units (e.g. the hepatitis C virus virus and the picornaviruses ). These proteases (e.g. TEV protease) have high specificity and only cleave a very restricted set of substrate sequences.”29
“Many viruses encode one or more proteases as a common strategy to sustain replication with a compacted genome. The viral genome encodes a polyprotein with an embedded viral protease that cleaves the polyprotein at several specific sites to generate mature viral proteins. Viral proteases are therefore essential for replication, which makes them ideal therapeutic targets.”30
Abstract
Introduction
Numerous efforts in natural product drug development are reported for the treatment of Coronavirus. Based on the literature, among these natural plants Artemisia annua L. shows some promise for the treatment of SARS-CoV-2.
Objective
The main objective of our study was to determine artemisinin content by liquid chromatography electrospray ionisation tandem mass spectrometry (LC-ESI-MS/MS), to investigate the in vitro biological activity of artemisinin from the A. annua plants grown in Turkey with various extracted methods, to elaborate in silico activity against SARS-CoV-2 using molecular modelling.
Methodology
Twenty-one different extractions were applied. Direct and sequential extractions studies were compared with ultrasonic assisted maceration, Soxhlet, and ultra-rapid determined artemisinin active molecules by LC-ESI-MS/MS methods. The inhibition of spike protein and main protease (3CL) enzyme activity of SARS-CoV-2 virus was assessed by time resolved fluorescence energy transfer (TR-FRET) assay.
Results
Artemisinin content in the range 0.062–0.066%. Artemisinin showed significant inhibition of 3CL protease activity but not Spike/ACE-2 binding. The 50% effective concentration (EC50) of artemisinin against SARS-CoV-2 Spike pseudovirus was found greater than 50 μM (EC45) in HEK293T cell line whereas the cell viability was 94% of the control (P < 0.01). The immunosuppressive effects of artemisinin on TNF-α production on both pseudovirus and lipopolysaccharide (LPS)-induced THP-1 cells were found significant in a dose dependent manner.
Conclusion
Further studies of these extracts for COVID-19 treatment will shed light to seek alternative treatment options. Moreover, these natural extracts can be used as an additional treatment option with medicines, as well as prophylactic use can be very beneficial for patients.
This paper published in February this year by Gurung at al investigated artemisinin and its derivatives as possible inhibitors of SARS-CoV-2 Nsp1.31 As we saw earlier, binding energies aren’t the whole story:
Abstract
The need for novel antiviral treatments for coronavirus disease 2019 (COVID-19) continues with the widespread infections and fatalities throughout the world. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the deadly disease, relies on the non-structural protein Nsp1 for multiplication within the host cells and disarms the host immune defences by various mechanisms. Herein, we investigated the potential of artemisinin and its derivatives as possible inhibitors of SARS-CoV-2 Nsp1 through various computational approaches. Molecular docking results show that artemisinin (CID68827) binds to Nsp1 with a binding energy of −6.53 kcal/mol and an inhibition constant of 16.43 µM. The top 3 derivatives Artesunate (CID6917864), Artemiside (CID53323323) and Artemisone (CID11531457) show binding energies of −7.92 kcal/mol, −7.46 kcal/mol and −7.36 kcal/mol respectively. Hydrophobic interactions and hydrogen bonding with Val10, Arg11, and Gln50 helped to stabilize the protein–ligand complexes. The pharmacokinetic properties of these molecules show acceptable properties. The geometric parameters derived from large-scale MD simulation studies provided insights into the changes in the structural topology of Nsp1 upon binding of Artesunate. Thus, the findings of our research highlight the importance of artemisinin and its derivatives in the development of drugs to inhibit SARS-CoV-2 Nsp1 protein.
In conclusion:
Molecular modelling approaches were used to investigate artemisinin and its derivatives as inhibitors of SARS-CoV-2 Nsp1, a key virulence factor suppressing the host’s immunological responses. The study shows that Artemisinin and its derivatives including Artesunate, Artemiside, and Artemisone bind to Nsp1 with high binding affinity and establishes hydrogen bonds and hydrophobic interactions with key residues. These molecules possess favourable drug-like properties and pharmacokinetic characteristics. The binding of the best-ranked molecule, Artesunate modulates the activity of Nsp1 by lowering the flexibility of the backbone atoms, reducing the structural compactness, increasing the solvent accessible surface area and boosting the intramolecular hydrogen bond interactions. Thus, artemisinin and its derivatives hold a great promise in developing into new antiviral treatments against SARS-CoV-2.
This literature review from 2022 by Ahmad et al focuses on the antiviral effects of A annua on COVID-19 and 4 key possible mechanisms:32
Abstract
COVID-19 is a worldwide pandemic. Currently, there are a few approved effective antiviral drugs against COVID-19. Therefore, an effective way to treat an emerging disease is to use existing medicines, which usually have a safety profile. A large number of compounds are produced from traditional medicinal plants and some of them that have antiviral activity could be used as therapeutics, such as Artemisia annua L. Here, we update the information on the therapeutic effects and possible antiviral mechanisms of A. annua and their derivatives against severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection will be updated. The A. annua derivatives might be effective alternatives for COVID-19 treatment. A. annua might act against the SARS-CoV-2 infection by inhibiting its invasion, ACE2, CD147, and TMPRSS2 expression, virus replication, reducing oxidative stress and inflammation by attenuating Nrf2 and NF-kB signaling, and mitigating lung damage in patients with COVID-19. However, clinical effectiveness needs to be demonstrated.
Chemically, A. annua L contains sterols, terpenes, flavonoids, phenolic, and polysaccharides.8,11 Artemisinin is a bioactive component that is extracted from A. annua L., has high efficiency and low toxicity, and is approved by the Food and Drug Administration9,12 and the World Health Organization (WHO) for the control of malaria.13,14 Artemisinin and other compounds from A. annua L. have been used in the management of several types of diseases, including autoimmune diseases, diabetes, cancer, parasitosis, viral infections, and atherosclerosis.4,8,9,12 The methanolic extracts from A. annua L. might be valuable for antiviral therapy, because they have higher activity against the Herpes Simplex virus type-1 than acyclovir.12 The ethanolic extracts of A. annua L. have significant antiviral activity against SARS-coronavirus with a 50% cytotoxic concentration (CC50) of 1,053 ± 92.8 µg/mL and 50% effective concentration (EC50) of 34.5 ± 2.6 µg/mL. These observations suggest that A. annua L. might be valuable for the treatment of COVID-19.15 In addition, A. annua L. might have potent inhibitory activities against the Epstein-Barr Virus, Cytomegalovirus, Herpes Simplex Virus 1, Human Herpes Virus 6A, and Human Papillomavirus.8
Molecular modeling was used to show that phytocompounds of artemisinin could bind to COVID-19.16 Furthermore, A. annua L. has potent anti-inflammatory activity that might inhibit or reduce inflammation and lung injury in COVID-19 patients.17 In the current article, the possible mechanisms underlying the actions of A. annua L. against SARS-CoV-2 will be highlighted.
It is well known that A. annua L. contains active compounds that have antiviral activities, such as artemisinin, arteannuin B, artemisinic acid, quinic acid, caffeic acid, quercetin, rutin, and crysosplenetin.8 Artemisinin and artesunate can inhibit the replication of the Hepatitis C Virus, which, similar to the SARS-CoV-2 virus, is a positive-sense single-stranded RNA virus.13 In addition, Artesunate can inhibit cytomegalovirus20 and JC polyomavirus replication.13
A. annua L. can inhibit SARS-CoV-2 viral invasion and replication, and reduce oxidative stress, consequently reducing inflammation during COVID-19 (Fig. 1). The following route might provide a critical target for the development of effective antiviral agents. The pharmacological mechanisms of A. annua L. and their compounds might lead to the development of potential antiviral agents against COVID-19.
NF-κB signaling, as well as assisting viral replication, has a key role in inflammation, innate immunity, cancer initiation and progression.33
Of interest, a previous study reported that androgens can upregulate the expression of TMPRSS2 protein and ACE2.25 Artemisinin can induce androgen receptor degradation via the 26S proteasome and disrupt the androgen response.26 Therefore, Artemisinin might inhibit SARS-CoV-2 infection by limiting the expression of ACE-2 and TMPRSS2 in sensitive cells (Fig. 1).18
CD147 is a transmembrane glycoprotein encoded by the Basigin gene in humans. CD147 can increase the synthesis of matrix metalloproteinases (MMPs) and pro-inflammatory cytokines.14 CD147 and MMPs expression can be enhanced by protein kinase (PK) and mitogen-activated protein kinase (MAPK) signaling.14 Artemisinin at 20–80 µg/mL inhibited the expression of CD147 in human cells. In addition, artemisinin strongly blocked PMA-induced CD147 expression by attenuating PKCδ and MAPK phosphorylation in human monocytes.14 Artesunate inhibited human cytomegalovirus replication by reducing PK activity.27 Therefore, artemisinin might be effective in the control of SARS-CoV-2 infections.
A. annua L. against SARS-CoV-2 replication: 3CLpro
The conserved 3-chymotrypsin-like protease (3CLpro) or main protease (Mpro), controls coronavirus transcription and virus replication. Their inhibition can reduce virus replication. A recent study showed that A. annua L. could inhibit the enzymatic activity of 3CLpro that is produced by SARS-CoV-2 during COVID-19 infection, which inhibits COVID-19 replication.17
A. annua L. against SARS-CoV-2 infection: Nrf2 and NF-kB
Nrf2 is a transcription factor and can regulate the cellular antioxidant response. Nrf2 signaling can reduce oxidative stress, which contributes to disease progression.28 In addition, Nrf2 can attenuate pulmonary fibrosis by upregulating antioxidant expression and defense enzymes.29 Enhanced oxidative stress is associated with pulmonary fibrosis and acute respiratory distress syndrome. Of note, pulmonary fibrosis contributes to the progression of COVID-19, which leads to high mortality in COVID-19 patients.30 Hence, modulation of Nrf2 activity might be valuable for the control of COVID-19 related pulmonary fibrosis.
A. annua L. can activate Nrf2 signaling that suppresses oxidative stress and inflammation.28,31A. annua L. has potent antioxidant activity and high phenolic content.32 Artesunate, an A. annua L. derivative, is a promising agent that could improve lung fibrosis by inhibiting the activity of profibrotic molecules.33 Artemisinin, which is a derivative of A. annua L., is an Nrf2 activator and has antioxidant and anti-inflammatory effects. Treatment with artemisinin inhibits bleomycin-induced lung damage in wild-type mice.28 Mechanistically, artemisinin can activate and stabilize Nrf2 by reducing its ubiquitination and degradation. An A. annua L. medicinal approach that targets Nrf-2 might offer antioxidant activity for humans against tissue damage and antifibrotic activity against SARS-CoV-2 infection and confer protection against tissue damage in other organs.
NF-κB is a protein complex that regulates gene transcription, cell survival, and stimulates pro-inflammatory cytokine productions.34 NF-κB signaling contributes to the pathogenesis of lung disease, including Acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome, and respiratory viral infections.13,34 The increase in cytokine production results in a cytokine storm and leads to the accumulation of fluid in the air sacs of alveoli, which causes suffocation.22 During COVID-19, severe disease can induce a cytokine storm, and cause fatal inflammation, leading to multiple organ dysfunction syndromes.13,22 Therefore, the inhibition of NF-κB signaling might be valuable in the control of COVID-19.13,22
Artesunate is an A. annua L. derivate and can inhibit NF-κB signaling, limiting virus replication. Artesunate has been demonstrated to inhibit chloroquine-like endocytosis, which might be effective for the treatment of SARS-CoV-2 infection.13 Therefore, artesunate might inhibit NF-κB signaling during SARS-CoV-2 infection to attenuate the cytokine storm. Artesunate might have the anti-inflammatory activity to reduce the inflammatory response, pro-inflammatory cytokine production, and lung inflammation that is caused by SARS-CoV-2 infection.
Future directions
The methanolic extracts of A. annua L. might be an appropriate candidate for antiviral therapy, because they have the highest antiviral potential against other viral replication. Furthermore, A. annua L. has shown preclinically that it has potent activities against SARS-CoV-2 infection and might be important for the control of COVID-19. However, the therapeutic efficacy and safety of these potential medicines for the treatment of COVID-19 need to be tested in clinical trials.
Conclusions
Repurposing drugs is an effective strategy to discover a therapeutic agent for the treatment of COVID-19. The A. annua L. derivatives might be potential candidates in COVID-19 treatment. A. annua L. can fight against SARS-CoV-2 infection by inhibiting its entry, ACE2, CD147, TMPRSS2, and S expression in host cells and reducing its replication by inhibiting 3CLpro. Furthermore, A. annua L. derivatives can reduce oxidative stress by increasing Nrf2 activity, inhibiting NF-κB signaling, reducing pro-inflammatory cytokine production, cytokine storm, inflammation, lung damage, and fatal inflammation that is caused by SARS-CoV-2. Their therapeutic efficacy and safety in the treatment of COVID-19 patients in clinical trials are urgently required.
Unlike with berberine, I am yet to see the results of any such trials and I’m not sure they ever took place, or ever will, unless we organise them ourselves.
No conflicts of interest were registered by the authors.
Artemisinin compounds and anti-cancer activity
There is an interesting paper on this, although the subject came up repeatedly in the antiviral studies. From 2012 by Lai, Singh & Sasaki.
Multiple modes of action include by reacting with iron to form cytotoxic free radicals that can selectively kill cancer cells, aided by various anti-angiogenic, anti-inflammatory, anti-metastasis, and growth inhibition effects:34
Abstract and Figures
Artemisinin contains an endoperoxide moiety that can react with iron to form cytotoxic free radicals. Cancer cells contain significantly more intracellular free iron than normal cells and it has been shown that artemisinin and its analogs selectively cause apoptosis in many cancer cell lines. In addition, artemisinin compounds have been shown to have anti-angiogenic, anti-inflammatory, anti-metastasis, and growth inhibition effects. These properties make artemisinin compounds attractive cancer chemotherapeutic drug candidates. However, simple artemisinin analogs are less potent than traditional cancer chemotherapeutic agents and have short plasma half-lives, and would require high dosage and frequent administration to be effective for cancer treatment. More potent and target-selective artemisinin-compounds are being developed. These include artemisinin dimers and trimers, artemisinin hybrid compounds, and tagging of artemisinin compounds to molecules that are involved in the intracellular iron-delivery mechanism. These compounds are promising potent anticancer compounds that produce significantly less side effect than traditional chemotherapeutic agents.
They do caution about lack of potency and high doses of up to 100 mg/kg/day are required, but no significant side effects were reported:
Artemisinin and its monomer analogs are generally not potent enough to assure cancer cure due to their relatively low toxicity toward cancer cells and short half lives. More potent compounds are needed. Many attempts have been made to increase the cancer cytotoxicity potency. These include artemisinin dimers, tetraoxanes and hybrids. Another approach is to deliver artemisinin compounds to cancer cells by tagging them to cancer cell targeting molecules.
Artemisinin monomers
The anticancer effects of artemisinin have been reported in the early 1990s by Woerdenbag et al.[3]. Most of the earlier research was mainly on the monomer analogs. The most prolific researcher in this area has been Thomas Efferth of the Johannes Gutenberg University.
The common artemisinin monomers (artemisinin, dihydroartemisinin, artesuante and artemether (Fig. 1)) have been tested on many different types of cancer cells (Table 1).Results indicate they are toxic to cancer cells with IC50s in the 10–20 μM range. Only few studies had simultaneously tested the compounds on normal cells [1,2,4–11]. In general, artemisinin compounds have been shown to be more toxic toward cancer cells than their corresponding normal cells. Many molecular mechanisms have been investigated. Artemisinins affect many different cellular pathways that are involved in cellular development, proliferation, and apoptosis. Apoptosis is a commonly reported effect [6,7,12–31], as well as arrest in cell cycle [9,11,32–37], particularly at the G0/G1phases. Thus, both cell death and growth inhibition occur. However, the site of action is not clear. There are reports of involvement of mitochondria and the apoptotic pathway [5,7,9,20–22,38–41]. There are also reports suggesting extra-mitochondrial mode of action [7,30,44]. Involvements of iron/heme [1,2,9,14,23,38,40–45] and reactive oxidative species [5,7,12,20,30,46–51] have also been implicated as we had previously hypothesized [1].
Many cellular molecular pathways involved in cell growth and processes of cancer development have been studied. Two processes that have repeatedly been reported to be affected by artemisinins are inhibition of nuclear factor kappaB (NF-κB) [27,32,52–56] and decrease in vascular endothelial growth factor (VEGF) [16,57–63] activities. Effects on other cellular pathways have also been reported including NOXA [5], mitogen-activated protein kinase(MAPK) [53,64], hypoxia-inducible factor 1α(HIFα)[59,65], Wnt/β−catenin [66,67], survivin [24], COX[68], c-MYC oncoprotein [18,23,69], epidermal growth factor (EGF) [70], and tumor necrosis factor α(TNFα)[56]. These molecular effects could explain the apoptotic, anti-angiogenic [15,16,54,58–63,71–86]; anti-inflammatory[55,56,68,87–90]; anti-metastasis [61,91–95], and cell cycle inhibition effects of artemisinin compounds. Of course, most of these changes in cellular molecular activities could result from an increase in free radical activity in cancer cells due to the reaction of artemisinins with iron.
Artemisinin monomers have been tested on many animal models of cancer, including sarcoma, leukemia, fibrosarcoma, glioma, oesteosarcoma, and cancers of the breast, pancreas, ovary, liver, and colon. Studies are summarized in Table 2. A general conclusion is that these monomers can retard cancer growth. However, high doses up to 100 mg/kg/day are required to achieve significant effect. No significant side effects have been reported. Several studies [6,8,96,97]have shown that dihydroartemisinin is synergistic with various traditional chemotherapeutic anticancer drugs. These findings warrant the use of artemisinin compounds as primary or adjuvant agents for cancer treatment. However, only five human case reports have so far been published: laryngeal squamous cellcarcinoma/artesunate [98]; metastatic uveal melanoma/artesunate [99]; pituitary macroadenoma/artemether [100]; non-small cell lung cancer/artesunate [101]; and cervical cancer/dihydroartemisinin [102]
Synthetic hybrid derivatives have been developed and some showed great potential:
Ricci et al.[81] studied artemisinin-glycolipid hybrids and reported high antiangiogenic activity of these compounds comparable to fumagillin and thalidomide. Glycolipids have been shown to have anti-angiogenic effects. Recently, artemisinin-glycolipid hybrids from 12β(c-c)-type deoxoartemisinin and glycolipids have also been tested on cancer cells [110]. They have potent anticancer activity above that of artemisinin or glycolipid alone on several cancer cell lines. Notably, they are five times more potent than cisplatin and paclitaxel on oral cancer cells.
Ductal carcinoma in mice = mammary glands, representative of early stage breast cancer in humans.35
Noori et al.[116]reported that artemisinin was an immunosuppressive agent. It was shown to suppress delayed hypersensitivity to sheep blood cells in mice. More recently, Noori et al.[117] reported that dihydroartemisinin stimulated the delayed hypersensitivity against sheep blood cells and reduced growth of ductal carcinoma in mice. Noori and Hassan [118] further reported that dihydroartemisinin decreased IL-4 and the level of CD4(+)CD25(+)Foxp3(+) T-lymphocytes in mice. Laugroudi et al.[119]reported that artemisinin reduced Treg cells in tumor and increased IFN gamma/IL-4 ratio in splenoctye cultures. Increase in Treg cells in tumors is correlated with tumor progression. More recently, Noori and Hassan [120] reported that Tehranolide inhibited proliferation of MCF-7 breast cancer cells by induction of G0/G1cell cycle arrest and apoptosis.
The authors do register conflicts of interest with regard to the synthetic derivative compounds discussed in the rest of the paper:
Conflict of interest statement
The authors are co-inventors of technologies, of which the patents are owned by the University of Washington, related to artemisinin-tagged transferrin, artemisinin-tagged transferrin receptor binding peptides, and artemisinin-dimer hydrazone. These technologies are licensed to Holley Pharmaceuticals (China) and Artemisia Biomedical (USA) for commercial development.
A literature search of nearly 200 papers on PubMed from 2015 by AK Das:36
Abstract
The anti-malarial drug artemisinin has shown anticancer activity in vitro and animal experiments, but experience in human cancer is scarce. However, the ability of artemisinins to kill cancer cells through a variety of molecular mechanisms has been explored. A PubMed search of about 127 papers on anti-cancer effects of antimalarials has revealed that this class of drug, including other antimalarials, have several biological characteristics that include anticancer properties. Experimental evidences suggest that artemisinin compounds may be a therapeutic alternative in highly aggressive cancers with rapid dissemination, without developing drug resistance. They also exhibit synergism with other anticancer drugs with no increased toxicity toward normal cells. It has been found that semisynthetic artemisinin derivatives have much higher antitumor activity than their monomeric counterparts via mechanisms like apoptosis, arrest of cell cycle at G0/G1, and oxidative stress. The exact mechanism of activation and molecular basis of these anticancer effects are not fully elucidated. Artemisinins seem to regulate key factors such as nuclear factor-kappa B, survivin, NOXA, hypoxia-inducible factor-1α, and BMI-1, involving multiple pathways that may affect drug response, drug interactions, drug resistance, and associated parameters upon normal cells. Newer synthetic artemisinins have been developed showing substantial antineoplastic activity, but there is still limited information regarding the mode of action of these synthetic compounds. In view of the emerging data, specific interactions with established chemotherapy need to be further investigated in different cancer cells and their phenotypes and validated further using different semisynthetic and synthetic artemisinin derivatives.
I will highlight a few interesting entries, but the whole review is worth reading for a background on all the pathways.
“In pharmacology, pleiotropy includes all of a drug's actions other than those for which the agent was specifically developed”.37
Artemisinins also have pleiotropic effects. The sensitivity and resistance of tumor cells depend on the mRNA expression of angiogenesis-related genes suggesting artemisinins exert their antitumor effects at least partly by inhibiting tumor angiogenesis. This was validated in 6 out of 30 angiogenesis related genes in one study by microarray data.[39] Artemisinins may also be chemopreventive in addition to being antiproliferative since many chemopreventive drugs have antiangiogenic features.
Anticancer activity of artemisinin has been demonstrated primarily in vitro and in animal models. In a study, testing 55 cell lines showed that artesunate showed inhibitory effects against leukemia, colon, melanoma, breast, ovarian, prostate, central nervous system, and renal cancer cells.[39] The semisynthetic derivative DHA showed remarkable antineoplastic activity against pancreatic, leukemic, osteosarcoma, and lung cancer cells.[40] Moreover, artemisone was superior to artemisinin and showed better synergism with other anti-cancer agents.[41]
Artemisinin has also been found to act either directly by causing DNA damage or indirectly by interfering with several signaling pathways involved in carcinogenesis. The indirect DNA damage seems to be commoner than direct damage. In pancreatic cells, artesunate caused DNA fragmentation and membrane damage. Low doses of artesunate were associated with oncosis-like cell death, whereas higher concentrations caused apoptosis. But, extent and type of such damage can depend on the phenotype and the origin of cell line, varying in time- and dose-dependent manner. Notably, higher sensitivity to artesunate was observed in rapidly growing cell lines compared to slow growing cancer cells.[40]
Alternatively, DHA, artesunate, and artemether may possibly modulate genes and proteins coordinating growth signals, apoptosis, proliferative capacity, angiogenesis, tissue invasion, and metastasis. Complex interactions through different pathways may enhance the anticancer effect of these endoperoxide drugs leading to growth control and cell death.
Artemisinins usually promote apoptosis rather than necrosis in most cases although both have been reported. Induction of apoptosis is a major advantage of artemisinins’ anti-malignancy action since there is no associated inflammation or cell damage due to necrosis. Artemisinin-induced necrosis is associated with low levels of ATP and defective apoptotic mechanisms.
Silymarin is also excellent at suppressing Pgp protein pumps which increases cellular uptake of chemotherapeutic agents.38
Many tumors develop drug resistance over time. A leading cause is a drug efflux generated by overexpression of membrane protein pumps resulting in ineffective/low drug concentrations.[67] Cytotoxicity of artemisinins has shown to be unaffected in otherwise resistant/multiresistant cancer cells. One study revealed that genes related with resistance to the established anticancer drugs such as MDR1 P-glycoprotein (Pgp), MRP1, and BCRP had no impact on artemisinins effect, and confirmed later on that the antitumor activity of artemisinin remains intact when resistance to other agents is present.[40] Artemisinins are effective in doxorubicin, metrotexate, and hydroxyurea-resistant cancer lines without cross-resistance. Furthermore, artesunate proapoptotic effect is not affected in a doxorubicin-resistant leukemia cell line; rather it potentiates doxorubicin's apoptotic effects.[8] In another study, intact anticancer potency of artesunate was found equally in chemoresistant and chemosensitive neuroblastoma cell lines and primary neuroblastoma cultures.[68] Here, sensitivity to artesunate was intact in vincristine, doxorubicin, cisplatin, topotecan, mephalan, and etoposide-resistant cells. Only one cell line showed low sensitivity to artesunate which was related to low ROS formation and high expression of glutathione cysteine ligase (GCL), where depletion of glutathione mediated by a GCL inhibitor improved artesunate sensitivity. Pgp or p53 attenuation did not affect the sensitivity to artesunate. DHA has shown better efficacy in cell lines such as cholangiocarcinoma and hepatocarcinoma compared to other drugs; moreover, upregulation of MDR1, MRP1-2, or MRP3 had no effect on its potency.
Artemisinins Toxicity
Dose-dependent toxicity is a major problem of anticancer therapy that can be overcome by increasing its efficacy with lower toxic drug concentrations. In spite of a wide use of artemisinin derivatives, toxicity in humans is negligible. The toxicity of artemisinin-like compounds can occur with long-term use, but treatments up to one year have shown no adverse effects.[17] DHA is the most neurotoxic artemisinin derivative. This has been reported in animal studies in a dose- and time-dependent manner (≥7 days).[81] Hence, rapid elimination of artemisinin in oral form is safer than slow-release/oil-based intramuscular formulations.[5] Clinical doses for malaria is 3 times higher than its anticancer activity.[40] Thus, artemisinin may have benefits as an anticancer agent, as it can be used in combination without increased side effects while efficacy and dose-reduction of more toxic anticancer agents can be possible. Brainstem neurotoxic encephalopathy has been reported in animal studies usually associated with long-term high-dose treatments.[82] Fatal overdose, especially in children may occur.
Artemether and artesunate have been used with good tolerability and lack of significant side effects in a variety of cancers such as laryngeal squamous cell carcinoma, malignant skin cancer, pituitary macroadenoma and advanced non-small cell lung.[17,83,84] They showed a substantial reduction of tumor size, increased survival with a significant improvement in disease control and metastasis reduction, especially with combination chemotherapy.[17] No new artesunate-related side effects were reported.[85]
Conclusion
Widely used as antimalarials for long, this drug class has diverse biological properties including strong anticancer activity; but how the antitumor activity is exerted following artemisinin activation is still not well-understood but is associated with multiple mechanisms, including reactive oxygen species (ROS), oxidative DNA damage, sustained DNA double-strand breaks, and apoptosis. A better understanding of common mechanisms under similar conditions in different cell systems will greatly help developing targeted artemisinin derivatives. Their ability to kill cancer cells through multiple and heterogeneous molecular events is documented, although the exact molecular basis of artemisinin-induced cell damage is not fully known. Apart from NF-κB, survivin, NOXA, HIF-1α, and BMI-1, other molecules need investigation that may influence drug response, drug interactions, mechanisms of resistance, and associated effects in normal cells. Experimental evidences, mostly animal studies indicate that artemisinins and its derivatives may be a therapeutic alternative in the future, particularly in highly metastatic and aggressive cancers without developing drug resistance. Synthetic endoperoxides may act synergistically with other anticancer drugs without additional side effects. However, the benefits of artemisinins and DHA with specific and established chemotherapy in the clinical setting need to be further explored in different cancers by co-targeting multiple pathways to minimize shifting of cancer biomarkers and drug toxicity. Simultaneously, long-term therapy with artemisinins will require close monitoring. Artemisinin antagonistic reactions and resistance must be cautiously validated using different semisynthetic derivatives. DHA, artesunate, and artemether are the endoperoxides currently licensed for therapeutic use. Overall, discovery of artemisinin compounds’ antitumor effect has opened new vistas and the need for further large scale studies in this regard including candidate genes and cancer biomarkers.
No conflicts of interest were declared.
From Artemisia annua, a Traditional Plant Brought to Light (2020) by Septembre-Malaterre et al. Multiple therapeutic effects are discussed including antioxidant activities, antidiabetic activities, cytotoxic and antitumor effects, immunomodulatory effects (eg autoimmune diseases like SLE/Lupus), on amyloidogenesis, antibacterial and antifungal activities, antiviral activities and several antiparasitic activities:39
5.3. Cytotoxic and Antitumor Effects
Several studies suggest that artemisinin may not be the most active antitumor compound in Artemisia annua [94,101]. Artemisia annua contains a variety of other biologically active substances [30,101,177], suggesting that this plant could be a source of new herbal anticancer therapies.
Lang et al. [30] demonstrated that Artemisia annua extract free of artemisinin has antitumor activity in vitro and in vivo and identified active compounds. In vitro data were validated in two in vivo cancer models, the chick chorioallantoic membrane (CAM) assay and the orthotopic breast cancer xenografts in nude mice. The Artemisia annua extract, inhibited the viability of breast (MDA-MB-231 and MCF-7), pancreas (MIA PaCa-2), prostate (PC-3), non-small lung cell (A459) cancer cells. Likewise, the extract’s most abundant ingredients, chrysosplenol D, arteannuin B, and casticin, inhibited the viability of MDA-MB-231 breast cancer cells. The extract induced the accumulation of multinucleated cancer cells within 24 h of treatment and increased the number of cells in the S and G2/M phases of the cell cycle, followed by loss of mitochondrial membrane potential, caspase-3 activation, and the formation of an apoptotic hypodiploid cell population. Further, the extract inhibited cancer cell proliferation, decreased tumor growth, and induced apoptosis in vivo in triple negative breast cancer (TNBC) and MDA-MB-231 xenografts grown on CAM as well as in nude mice.
Essential oil isolated from Artemisia annua (100 µg/mL) induced apoptosis in SMMC-7721 hepatocarcinoma cells by nuclear chromatin fragmentation and cytoplasmic condensation [178].
Another study showed that a water-soluble polysaccharide with a molecular weight of 6.3 × 104 Da isolated from Artemisia annua inhibited the growth of HepG2 cells in a dose-dependent manner. phenylindole dihydrochloride (DAPI) staining and flow cytometric analysis revealed that the soluble polysaccharide suppressed cell proliferation via induction of the p65-dependent mitochondrial signaling pathway, as evidenced by the increased activation of caspase-3 and -9, negative regulation of Bcl-2 protein, increased regulation of Bax protein and release of cytochrome c from mitochondria into the cytosol, and suppression of the nuclear factor κB (NF-κB) p65 [179].
Several mechanisms of action regarding the antitumor activities of artemisinin and its derivatives have been identified [99]. The oxidative stress response has a major role, as it has been demonstrated that the endoperoxide moiety is crucial for the bioactivity of artemisinin-type drugs. Its cleavage leads to Reactive Oxygen Species (ROS) formation and presumably oxidative stress. The authors found numerous statistically significant associations between cellular response to artemisinin and mRNA expression of genes involved in oxidative stress response [180,181,182,183]. Artemisinin induces oxidative DNA-damage in dose-dependent manner [99,148]. ROS and oxidative DNA lesions tremendously affect cellular integrity, leading to perturbations in cellular replication and division mechanisms, which ultimately cause cell cycle arrest and cell death. This mechanism is also true for artemisinin-type drugs. Cell cycle arrest has been reported to occur at G1 or G2 checkpoints, presumably depending on individual defects of tumor cell lines in the cell cycle machinery. All these cascades of events lead to cell apoptosis [99]. Depending on the cell model, both mitochondrial (intrinsic) and the extrinsic FAS-receptor-driven pathways of apoptosis can be induced by artemisinin with upregulated Fas/CD95 expression, breakdown of the mitochondrial membrane potential, cytochrome C release, PARP (poly (ADP-ribose) polymerase) cleavage and caspase 3/9activation [184,185]. Other cell death mechanisms induced by artemisinin-type drugs in tumor cells include non-apoptotic cell death mechanisms such as autophagy, necrosis, necroptosis, oncosis (ischemic cell death), anoikis (anchorage-dependent cell death) and ferroptosis [99]. It has been described that ferrous iron enhances the cytotoxicity of artemisinin-type drugs against tumor cells and that the form of iron-dependent cell death termed ferroptosis is tightly linked to artemisinin and its derivatives [186,187].
5.4. Immunomodulatory Effects
Artemisinin and its derivatives have been the subject of several studies on their immunoregulatory properties [188]. They modulate key effectors of the immune system, including toll-like receptors (TLRs) [189,190].
Wojtkowiak-Giera et al. [190] presented two studies demonstrating the immunomodulatory effect of Artemisia annua water extracts on TLR2 and TLR4 immune system components. The first evaluated the effects of Artemisia annua extracts on the expression of TLR2 and TLR4 in the brains of mice with Acanthamoeba infection. The Artemisia annua extract significantly reduced the level of TLR2 expression and altered the level of TLR4 expression.
TLRs are a family of transmembrane proteins belonging to several innate immune receptors located primarily on cells of the immune system and others such as lung cells. These receptors play a key role in the recognition of pathogens, including parasites (by recognizing molecular patterns associated with pathogens (PAMPs) or molecular patterns associated with host-derived damage (DAMPs)) and induce inflammatory mediators production [191]. TLR 2 and 4 are the best known and most studied members of this family [192].
The second study evaluated the effects of Artemisia annua extracts on TLR2 and TLR4 expression in the lungs of mice with acanthamoebiasis [188]. Extracts from Artemisia annua can modulate the expression of both TLRs. The effect of artemisinin and derivatives was suggested to be associated with a decrease in TLR2 expression, TLR4 mRNA expression was found to be increased. Artemisia annua extracts were hence suggested to have anti-inflammatory properties by reducing TLR2 mRNA expression.
Similar effects were reported by Li et al. [193] in in vitro experiments where artesunate, a widely used artemisinin derivative, inhibited the secretion of TNF-α from murine peritoneal macrophages induced by heat-killed Staphylococcus aureus via decreased TLR2 mRNA expression.
Artesunate also decreased the expression of TLR4 and TLR9 mRNA. TLR4 is a receptor that induces inflammatory response activation by the recruitment of adaptor proteins such as MyD88 that leads to the activation of the nuclear factor NF-κB and the production of pro-inflammatory cytokines. It should be noted that artesunate can inhibit the LPS-induced expression of TLR4, MyD88 and NF-κB by blocking the degradation of the inhibitor of NF-κB (IκB) [194].
Artemisinin and its derivatives have been tested for their anti-inflammatory activities in numerous models of auto-immune and allergic conditions.
Artesunate, dihydroartemisinin, artemether and the water-soluble derivative SM905 have been reported to possess protective effects against experimental models of rheumatoid arthritis (RA) [195,196,197,198].
In an experimental RA model, the attenuation of inflammatory symptoms and prevention of tissue damage were obtained with artesunate. Artesunate was found to induce the suppression of proinflammatory cytokines including TNF-α, GM-CSF, IL-1β, IL-6, IL-8 and IL-17α via inhibition of the mitogen activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt and NF-κB signaling pathways [196,198,199]. Artemisinin and derivatives have also been shown to exert anti-angiogenic activities in RA, acting as inhibitors of angiogenesis-related factors such as matrix metalloproteinase-2 (MMP-2) and MMP-9, Vascular Endothelial Growth Factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) [196,200]. Artemisinin and its derivatives have demonstrated effective antiarthritic properties in RA, with comparable efficacy but a significantly reduced side effect profile as compared to methotrexate [17].
In an experimental murine model of Systemic Lupus Erythematosus (SLE), oral artesunate at 125 mg/kg/d over 16 weeks exhibited comparable immunosuppressive effects to cyclophosphamide, by repressing monocyte chemoattractant protein 1 (MCP-1) and B-cell-activating factor (BAFF) levels, leading to a significant reduction in anti-nuclear antibody and anti-double-strand DNA (dsDNA) antibody production, proteinuria, serum creatinine as well as related renal pathology [201].
Studies have also revealed that a 3–8 week regime of oral water-soluble artemisinin analog SM934 (2.5 and 10 mg/kg/d) exhibited pronounced suppression of proteinuria, glomerulonephritis, development of Th-1 and Th-17 cytokine profiles, and increases in anti-dsDNA, IgG2a and IgG3 antibodies, while promoting increases in Th-2 responses, and serum IL-10 and IL-4 levels in experimental murine models of SLE [202,203].
SM934 demonstrated mixed actions on different subsets of T cells, suppressing the memory/effector T cells, while promoting regulatory T cell development. Notably, these studies have revealed that SM934 can exhibit extensive protective effects in chronic systemic inflammatory condition, comparable to a clinically effective corticosteroid drug like prednisolone [202] or immunosuppressant like rapamycin [203].
In lupus nephritis, a severe and frequently-occurring secondary kidney-specific inflammation following SLE, oral dihydroartemisinin (5–125 mg/kg/d) was found to suppress serum levels of anti-dsDNA antibody and TNF-α and abrogate renal pathology in mice via blockade of NF-κB p65 subunit nuclear translocation [204]. Besides, oral artesunate (150 mg/kg/d) has demonstrated stronger protective effects than prednisone in experimental lupus nephritis, by lowering serum levels of TNF-α and IL-6, and NF-κB p65 subunit and transforming growth factor beta 1 (TGF-1β) expressions in renal tissues [205]. Furthermore, artesunate combined with prednisone was found to induce higher expression of glucocorticoid receptor α (GRα) in peripheral blood mononuclear cells (PBMCs) and to enhance transcriptional coactivator P300/CBP protein expression in renal tissues when compared to prednisone alone in lupus nephritis mice [206].
Artesunate has been shown to possess therapeutic actions against inflammatory bowel disease (IBD) [207]. Artesunate (150 mg/kg/d) dramatically mitigated colon pathology and inflammatory damage in experimental colitis induced by dextran sulfate sodium salt (DSS) or trinitrobenzene sulfonic acid (TNBS). These anti-inflammatory effects of artesunate corroborated well with the suppression of Th-1 and Th-17 cytokines, IFN-γ and TNF-α via the inhibition of NF-κB activities [17].
Artemisinin and its derivatives have been also shown to have anti-allergic activities, which are linked to their immunosuppressive effects mediated by the downregulation of NF-κ p65 subunit, T-bet and IFN-γ expressions [208]. A Chinese trial on 90 subjects with allergic skin disorders has demonstrated that topical artesunate exerts potent efficacy against eczema, erythema multiforme, polymorphous sunlight eruption and hydroa aestivale, and moderate effectiveness against atopic dermatitis, psoriasis vulgaris and dermatomyositis [17].
Cheng et al. [209] demonstrated that artesunate (3–30 mg/kg) prevented IgE-mediated vascular permeability in a passive cutaneous anaphylaxis mouse model, and blocked IgE-induced mast cell degranulation in the lungs, increase in plasma histamine level, and subsequent hypothermia. In RBL-2H3 cells and mature human mast cells, artesunate was found to directly inhibit IgE-induced mast cell degranulation, by blocking Syk tyrosine kinase phosphorylation, the downstream phospholipase Cγ (PLCγ) activation, and elevation in inositol trisphosphate (IP3) and intracellular Ca2+ levels. These findings strongly support a therapeutic role for artemisinin and its derivatives in the treatment of mast-cell-mediated allergic responses.
Artesunate has been shown to protect against experimental allergic asthma. At 3–30 mg/kg/d, artesunate given intraperitoneally markedly inhibited both ovalbumin- and house dust-mite-induced total and eosinophil counts in bronchoalveolar lavage fluid, anti-inflammatory effects comparable to dexamethasone [210,211]. Furthermore, artesunate drastically suppressed aeroallergen induced increases in Th-2 cytokines and chemokines, IL-17, IL-33, MUC5AC, and adhesion molecules in the airways [210]. These protective effects by artesunate in allergic asthma have been associated with its pronounced inhibition of the PI3K/Akt signaling cascade and NF-κB activation. In contrast to the formation of free ROS via cleavage of the endoperoxide bond by heme iron in its structure as a mechanism to kill Plasmodium spp. parasites and to induce cytotoxic effects in cancer cells, in allergic asthma, artesunate was found to decrease the levels of oxidative and nitrosative damage markers including 8-hydroxy-2-deoxyguanosine, 8-isoprostane and 3-nitrotyrosine, in inflamed airways. These antioxidative effects of artesunate were correlated with the inhibition of expression of NADPH oxidases and iNOS, and elevation of superoxide dismutases and catalase, probably via the induction of nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) by artesunate in allergic airways [210]. Four cases of IgE-mediated anaphylactic reactions to oral and intravenous artesunate have been described. These allergic reactions to artemisinin and its derivatives are considerably rare [212].
Artemisinin was demonstrated to be capable of extenuating amyloidogenesis and neuroinflammation in a model of Alzheimer’s disease (AD) in APPswe/PS1dE9 double transgenic mice [213]. Artemisinin (40 mg/kg) given intraperitoneally daily for 30 days abrogated β-secretase activity and decreased neurotic plaque burden in AD mouse model. These anti-inflammatory effects of artesunate have been ascribed to the inhibition of NF-κB activity and the activation of NALP3 inflammasome. Another therapeutic prospect for artemisinin and derivatives has been investigated in an experimental rat model of endometriosis. Artesunate at 150 and 300 mg/kg/d, given intragastrically for 4 weeks, increased apoptosis index and significantly reduced Bcl-2 and microvascular density of the implanted ectopic endometrium, with protective effects comparable to a modified progestogen danazol [214].
Artemisinin derivatives can inhibit P-glycoprotein mediated return of chemotherapeutics to the gut lumen, helping to counter multidrug resistance (MDR). No requirement to take with milk thistle (silymarin) to increase uptake. A study by Wang et al (2019):40
Abstract
Background: Artemisinin was isolated and identified in 1972, which was the starting point for a new era in antimalarial drug therapy. Furthermore, numerous studies have demonstrated that artemisinin and its derivatives exhibit considerable anticancer activity both in vitro, in vivo, and even in clinical Phase I/II trials. P-glycoprotein (P-gp) mediated multi-drug resistance (MDR) is one of the most serious causes of chemotherapy failure in cancer treatment. Interestingly, many artemisinin derivatives exhibit excellent ability to overcome P-gp mediated MDR and even show collateral sensitivity against MDR cancer cells. Furthermore, some artemisinin derivatives show P-gp-mediated MDR reversal activity. Therefore, the interaction between P-gp and artemisinin derivatives is important to develop novel combination treatment protocols with artemisinin derivatives and established anticancer drugs that are P-gp substrates.
Purpose: This systematic review provides an updated overview on the interaction between artemisinin derivatives and P-gp and the effect of artemisinin derivatives on the P-gp expression level.
Results: Artemisinin derivatives exhibit multi-specific interactions with P-gp. The currently used artemisinin derivatives are not transported by P-gp. However, some of novel synthetized artemisinin derivatives exhibit P-gp substrate properties. Furthermore, many artemisinin derivatives act as P-gp inhibitors, which exhibit the potential to reverse MDR towards clinically used anticancer drugs.
Conclusion: Therefore, studies on the interaction between artemisinin derivatives and P-gp provide important information for the development of novel anti-cancer artemisinin derivatives to reverse P-gp mediated MDR and for the design of rational artemisinin-based combination therapies against cancer.
Keywords: Artemisinin; Cancer; Chemotherapy; Multidrug resistance; Natural products; P-glycoprotein.
Efficacy of artemisinin and its derivatives in the treatment of type 2 diabetes melitus
A paper by Jiang et al from 2020. As per a now familiar theme, artemisinins have multiple modes of action in treating T2DM:41
Abstract
Type 2 diabetes mellitus (T2DM) is a chronic disease that has become a global public health problem. Studies on T2DM prevention and treatment mostly focus on discovering therapeutic drugs. Artemisinin and its derivatives were originally used as antimalarial treatments. In recent years, the roles of artemisinins in T2DM have attracted much attention. Artemisinin treatments not only attenuate insulin resistance and restore islet ß-cell function in T2DM but also have potential therapeutic effects on diabetic complications, including diabetic kidney disease, cognitive impairment, diabetic retinopathy, and diabetic cardiovascular disease. Many in vitro and in vivo experiments have confirmed the therapeutic utility of artemisinin and its derivatives on T2DM, but no article has systematically demonstrated the specific role artemisinin plays in the treatment of T2DM. This review summarizes the potential therapeutic effects and mechanism of artemisinin and its derivatives in T2DM and associated complications, providing a reference for subsequent related research.
Overview of Artemisinin and Its Derivatives
The properties of artemisinins determine their roles. Artemisinin is insoluble in oil and water and is the starting material of semisynthetic derivatives such as artemether, dihydroartemisinin, artesunate, and arteether (O’Neill and Posner, 2004; Gautam et al., 2009). These compounds are characterized by a short half-life, fast onset of effects, and low oral bioavailability (19%–35%) (Thomas et al., 1992; Navaratnam et al., 2000). The artemisinin compounds mentioned above are lipophilic, with the exception of artesunate, which is the only artemisinin derivative for which an intravenous formulation is available; dihydroartemisinin, artemether and arteether are currently administered intramuscularly in various oil formulations (Navaratnam et al., 2000; Krishna et al., 2001). Compared with other artemisinin derivatives, intramuscular artemether and arteether have a longer half-life, which may be attributed to the “depot” effect and/or the local blood supply and the slow and prolonged absorption of the sesame oil preparations at the site of injection (Ashton et al., 1998; Li et al., 1999; Visser et al., 2014).
The transformation of artemisinin and its derivatives into the primary metabolite dihydroartemisinin mostly depends on the action of the liver cytochrome P450 isozyme family, except for artesunate, which depends on the action of common esterases.
IR = insulin resistance.
DKD = diabetic kidney disease.
DR = diabetic retinopathy.
Aβ = amyloid beta.
Discussion
As a range of drugs with huge potential for the treatment of metabolic diseases, artemisinin and its derivatives play critical roles in the therapy of T2DM as well as its related complications. Firstly, through horizontally comparisons of the properties and tissue distribution of artemisinins, it is helpful for us to understand the therapeutic effects and mechanism of artemisinins on diabetes and its complications and provide directions and ideas for future research. For example, artemisinins, except arteether, can pass through the blood-brain barrier; thus, we can infer from the experimental results of Albasher and Zeng that artemisinin may attenuate diabetic cognitive impairment (Zeng et al., 2017; Albasher et al., 2020). Because of these characteristics, artemether, dihydroartemisinin, and artesunate deserve further study to determine their effects on diabetic cognitive impairment. Moreover, artesunate and artemether are distributed in skeletal muscles and liver, which may align with their function of ameliorating IR. Similarly, artesunate distributed in the eyeball has also been confirmed to alleviate eye diseases (Cheng et al., 2013; Ge et al., 2019). Furthermore, artemisinin can pass through blood-placenta barriers (Niu et al., 1985), and artesunate can be distributed in testicular tissue; therefore, we need to pay attention to the reproductive toxicity of these two drugs. The pharmacological understanding of artemisinin and its derivatives is incomplete, and there are still many gaps that need to be filled (Karbwang et al., 1997; Navaratnam et al., 2000; Gautam et al., 2009).
Attenuating IR and restoring islet cell function are two pathways through which artemisinins can alleviate T2DM. Because of their close relationship, obesity, IR, and inflammation often exert cross-influences on each other and continuously promote the development of diseases. Accumulating evidence confirms that artemisinin and its derivatives can break any step of a vicious cycle by modulating adipose production, differentiation, and consumption and inhibiting the inflammatory response to reverse metabolic dysfunction. Artemisinin and its derivatives also act on islet cells by promoting insulin secretion, protecting pancreatic islet ß cells, and achieving islet α-cell to ß-cell transdifferentiation by reversing a number of abnormal proteins and RNAs, thereby inhibiting the development and progression of T2DM.
In addition to playing a crucial role in treating T2DM, artemisinins can also participate in the therapy of diabetic complications through a series of molecular pathways. Notably, in numerous studies, inhibiting the expression of key inflammatory pathways or inflammatory factors and attenuating the chronic inflammation state will significantly improve the functions of related organs. Attenuating inflammation seems to be an important therapeutic mechanism, even for diabetes and all the complications that have been studied. In addition, artemisinin and its derivatives have demonstrated great promise as regulators of oxidative stress, especially in diabetic nephropathy, diabetic retinopathy, and diabetic cognitive impairment. Specifically, artemisinins ameliorate DKD by regulating metabolism, restoring mitochondrial function, modulating RAS, and altering a range of related abnormal molecules. The technology of next-generation sequencing can be applied to identify related genes and pathways influenced by artemisinin. For DR, according to one study, artesunate is more effective than the anti-VEGF drug Avastin and can also effectively prevent the occurrence of retinal detachment (Zong et al., 2016). On the one hand, artesunate can induce apoptosis of epithelial cells and reduce neovascularization by inducing oxidative stress; on the other hand, artemisinin can protect retinal epithelial cells from damage caused by the oxidative stress state of diabetes (Zong et al., 2016; Yan et al., 2017). Therefore, we speculate that the effects of artemisinin are different based on the different pathological conditions of epithelial cells. Generally, artemisinins act as balancing agents and thus can reverse the pathological state and stabilize the intracellular environment. The protective effects of artemisinins on nerve cells are also significant. In addition to anti-inflammatory and antioxidative effects, artemisinins also decrease the level of Aβ, upregulate neurotrophic factors, modulate the apoptosis of neurons, and change the composition of the gut microbiota to reverse cognitive impairment. Cardiovascular benefits are among the criteria for evaluating diabetes drugs. In addition to reducing early inflammation, reducing fiber formation can also help prevent the occurrence and development of diabetic cardiomyopathy. It is also worth noting artemisinin’s cardiovascular protective function. Metformin also has a positive role in protecting the cardiovascular system, and artemisinin shows certain advantages that are similar to metformin to some degree. Artemisinins play protective roles in atherosclerosis by inhibiting inflammation, promoting macrophage autophagy, and improving the expression of endothelial vascular protection genes. However, the toxicity of artemisinin and its derivatives has gradually been recognized. The side effects of artemisinin and its derivatives have become an obstacle to the treatment of diabetes. In addition to inducing possible damage to islet ß cells (Chen et al., 2020), artemisinins also have adverse effects upon its inappropriate use in the course of treatment and doses, which limit clinical application and need to be addressed seriously (Farombi et al., 2015; Singh et al., 2015; Cao et al., 2020). Although artemisinins have not shown obvious toxicity in various experiments, ways to reduce side effects to the greatest extent possible while retaining the maximum therapeutic effect may be the next issues to be addressed.
The hypoglycemic effects of artemisinin have been verified in most experiments, but the differences in the experimental results and some questions are still worth further study. 1) Different animal models should be considered. Sex has been considered to play an important role in the metabolism of artemisinin and its semisynthetic derivatives. The free fraction of artemisinin in the plasma of male rats was significantly lower than that of female rats (Ashton et al., 1999). Most studies use a single-sex model, and the effects of sex on the results were not studied, which is a limitation to clinical application. Therefore, it is crucial to perform further studies to assess the general applicability of artemisinin and its derivatives for the treatment of diverse patients with diabetes. The use of different mouse strains may be one of the most likely reasons for the discrepant results. In Ins1-H2B-mCherry × Gcg-Cre × Rosa26-stop-YFP triple transgenic mice and Glucagon-CreERT2; Rosa-LSL-eYFP male mice, α-cell to ß-cell transdifferentiation was not observed; however, they were observed in the αTC1 and Min6 cell lines and transgenic (Gcga: GFP)ia1 − (ins: NTR-mcherry)ml10 zebrafish. Designing a model that better fits the simulated situation may lead to a conclusion more in line with the true situation. 2) Different mediators should be considered. Different media can cause the same result from different processes; for example, palmitate, high glucose levels, and proinflammatory cytokines can cause ß-cell failure through different pathways. Artemisinin and its derivatives have been shown to respond to reverse inflammatory factor-induced ß cell damage but not to palmitate-induced ß cell damage. It has been demonstrated that artemisinins exert effects in an inflammatory environment rather than in a state where free fatty acids are abundant. Therefore, assessing the disease states of the body in which artemisinin functions may expand the scope for the application of artemisinins. 3) Dose and duration should be considered. Side effects are different based on the dose and duration of artemisinin treatments in different diseases. By reviewing the adverse effects, we found that much attention should be paid to suitable artemisinins, reasonable doses and courses of treatment. Hence, artemisinin analogs can be better exploited for therapeutic interventions. 4) Relatively unknown areas should be explored. Traditional Chinese medicine, such as berberine, is potent in modulating gut microbiota (Zhang et al., 2012; Zheng et al., 2018). Different artemisinin derivatives might have differential roles in changing the gut microbiota. In addition, different dietary and living conditions of the mice may lead to different outcomes through changes in the gut microbiota. 5) Without improved technology, efficacy may be affected by contaminating proteins and RNAs; the original droplet-based single-cell transcriptome contains up to 20% contaminating transcripts, indicating that there are still some defects in the detection and extraction methods at this stage. The development of a greater number of efficient separation and purification methods and techniques will greatly improve the accuracy and the precise understanding of the drug effects. 6) Species-dependent effects need to be considered. Marquina-Sanchez’s report demonstrated that the efficacy of artemisinin is species-dependent. Although abundant evidence shows the great potential of artemisinins in the treatment of T2DM and related complications in diabetic models, there is still reasonable doubt about its efficacy in humans. 7) Multitargeted effects need to be explored. From reviewing various experiments, we suggest that artemisinins are multitargeted in the treatment of diabetes and its related complications. However, there are many unresearched but very valuable targets for the reversion of diabetic pathological changes. Further research will contribute to a comprehensive understanding of the roles of artemisinins in T2DM.
In summary, artemisinin and its derivatives play vital roles in the treatment of T2DM, while the clinical application of artemisinin is still challenging. It is essential to further study the interaction between artemisinins and T2DM and then provide clear reasons to use artemisinin as a potential treatment for T2DM and its complications, paving the way for the future cure of diabetes in patients.
No conflicts of interest were registered.
Of particular note, Kiss et al (2021) demonstrated that artensuate attenuates levels of a an amyloid precursor protein in an Alzheimer's disease (AD) mouse model:42
Abstract
Alzheimer's disease (AD) is the most frequent form of dementia, characterized histopathologically by the formation of amyloid plaques and neurofibrillary tangles in the brain. Amyloid β-peptide (Aβ) is a major component of amyloid plaques and is released together with carboxy-terminal fragments (CTFs) from the amyloid precursor protein (APP) through proteolytic cleavage, thought to contribute to synapse dysfunction and loss along the progression of AD. Artemisinins, primarily antimalarial drugs, reduce neuroinflammation and improve cognitive capabilities in mouse models of AD. Furthermore, artemisinins were demonstrated to target gephyrin, the main scaffold protein of inhibitory synapses and modulate GABAergic neurotransmission in vitro. Previously, we reported a robust decrease of inhibitory synapse proteins in the hippocampus of 12-month-old double transgenic APP-PS1 mice which overexpress in addition to the Swedish mutated form of the human APP a mutated presenilin 1 (PS1) gene and are characterized by a high plaque load at this age. Here, we provide in vivo evidence that treating these mice with artemisinin or its semisynthetic derivative artesunate in two different doses (10 mg/kg and 100 mg/kg), these compounds affect differently inhibitory synapse components, amyloid plaque load and APP-processing. Immunofluorescence microscopy demonstrated the rescue of gephyrin and γ2-GABAA-receptor protein levels in the brain of treated mice with both, artemisinin and artesunate, most efficiently with a low dose of artesunate. Remarkably, artemisinin reduced only in low dose the amyloid plaque load correlating with lower levels of mutated human APP (hAPPswe) whereas artesunate treatment in both doses resulted in significantly lower plaque numbers. Correspondingly, the level of APP-cleavage products, specifically the amount of CTFs in hippocampus homogenates was reduced significantly only by artesunate, in line with the findings in hAPPswe expressing cultured hippocampal neurons evidencing a concentration-dependent inhibition of CTF-release by artesunate already in the nanomolar range. Thus, our data support artemisinins as neuroprotective multi-target drugs, exhibiting a potent anti-amyloidogenic activity and reinforcing key proteins of inhibitory synapses.
“Gephyrin is a protein that in humans is encoded by the GPHN gene. This gene encodes a neuronal assembly protein that anchors inhibitory neurotransmitter receptors to the postsynaptic cytoskeleton via high affinity binding to a receptor subunit domain and tubulin dimers.”43
A positive impact on type 2 diabetes and obesity is implied:
Interestingly, artemisinins, a group of plant derived sesquiterpene lactone components and antimalarial drugs in clinical use were proposed recently as potential therapeutic agents for AD due to the fact that their use in mouse models of AD resulted in decreased inflammation and plaque load and improved cognitive functions (Shi et al., 2013; Ho et al., 2014; Qiang et al., 2018; Zhao et al., 2020). Moreover, these substances were found to bind to the receptor binding pocket of gephyrin in vitro (Kasaragod et al., 2019). In pancreatic islet cells, this binding was reported to increase GABA-ergic signaling in a gephyrin-dependent manner and resulting in elevated insulin secretion (10 μM arthemeter) (Li et al., 2017), whereas in studies with cultured spinal cord and hippocampal neurons a decrease of glycinergic signaling and a reduction of gephyrin and GABAAR co-clustering, was detected using high concentrations of artemisinins (50 μM artesunate) (Kasaragod et al., 2019). Most recently, Kasaragod and coworkers have also shown that artemisinins bind to and inhibit the enzyme pyridoxal kinase, which synthesizes pyridoxal 5-phosphate, an essential cofactor of glutamic acid decarboxylase (GAD) involved in the synthesis of GABA, thus resulting in a reduced inhibitory neurotransmission in vitro (Kasaragod et al., 2020).
The identification of gephyrin as a primary mammalian target of artemisinins (Li et al., 2017) prompted us to investigate a possible rescue of gephyrin expression in 12-month-old APP-PS1 mice using two different doses of artemisinin or its semi-synthetic derivative artesunate, the latter being most effective for the treatment of cerebral malaria (WHO, 2015), but to our knowledge was not yet tested in AD-models. We demonstrate, that, upon administration for several weeks both artemisinin compounds restored gephyrin and γ2-GABAAR levels in the brain of 12-month-old animals. These results are in agreement with our earlier observations in young asymptomatic APP-PS1 mice which were treated for 6 weeks with artemisinin. Those findings provided evidence for an increase of gephyrin protein level and elevated gephyrin phosphorylation in the hippocampus of 3-month-old APP-PS1 after treatment with artemisinin (Kiss et al., 2021). In addition, in our present study with 12-month-old animals a striking reduction of amyloid plaque load was detected by low doses of artemisinin and artesunate. Interestingly, artesunate treatment resulted first of all in a dose-dependent decrease of CTFs-levels and soluble Aβ concentrations as measured in the hippocampus homogenates. These results reinforced by the findings in hAPPswe expressing cultured hippocampal neurons treated with different concentrations of artesunate provide important new insight into the neuronal effects of artemisinins in vivo.
The authors declared no conflict of interest.
Also from Artemisia annua, a Traditional Plant Brought to Light (2020):
5.2. Antidiabetic Activities
Aqueous extracts of Artemisia annua show significant anti-hyperglycemic and anti-hypoinsulinemia activities in diabetic animals. In fact, significant decrease in blood glucose level occurred in animals receiving 28.5 mg/kg twice a day of the aqueous extract [26]. This may be due to stimulation of the secretion of insulin by β cells, inhibition of α cells of the pancreatic islets, or by enhancing insulin activity [174].
In addition, an important link between oxidative stress, inflammatory response and insulin activity is now well established. This can be explained by the ability of antioxidants to protect against the deleterious effects of hyperglycemia and also to improve glucose metabolism. Generally, these antioxidants are flavonoids which were demonstrated to act on biological targets involved in type 2 diabetes mellitus such as α-glycosidase, glucose cotransporter or aldose reductase [175].
The anti-hypoinsulinemic effect of essential oil components of Artemisia annua extract (camphor, germacreneD, artemisia ketone,1,8-cineole) may be attributed to its protective effect against hepatocyte damage through inhibition of the lipopolysaccharide (LPS)-elicited expression of the proinflammatory mediators IL-1β (Interleukin 1 beta), TNF-α (tumor necrosis factor alpha), COX-2 (cyclooxygenase 2) and iNOS (Inducible nitric oxide synthase)
Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis
A PubMed search only returned one result for a different species, A. dracunculus44, but the National Library of Medicine hosts a paper relevant to A. annua published in 2017.45
As per the neuroprotective properties referred to earlier, the results in a murine model were encouraging. They found that artemisinin elevated Th2 cell counts antagonised autoimmune Th1 macrophages:46
Why this is important. In short it helps stop the body attacking itself.
“Type 1 T helper (Th1) cells produce interferon-gamma, interleukin (IL)-2, and tumour necrosis factor (TNF)-beta, which activate macrophages and are responsible for cell-mediated immunity and phagocyte-dependent protective responses. By contrast, type 2 Th (Th2) cells produce IL-4, IL-5, IL-10, and IL-13, which are responsible for strong antibody production, eosinophil activation, and inhibition of several macrophage functions, thus providing phagocyte-independent protective responses.”47
Abstract
Context: The immune system through T-helper 1 (Th1) and Th17 cells play a critical role in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), whereas the Th2 responses inhibit myelin degeneration. Artemisinin, as an anti-malaria as its agent, has been used widely in the treatment of malaria, shifts the lymphocyte responses from Th1 to Th2.
Objective: In this study, we have investigated the therapeutic effects of artemisinin on the EAE treatment.
Materials and methods: EAE was induced in the inbred C57BL6 mice. High and low doses of prednisolone and artemisinin were injected daily with the control and test groups, respectively. The spleen and the brain of the mice were removed and used for ELISA and histological studies.
Results: The mean weight of mice was significantly (p value < .05) higher in artemisinin-treated group compared with the untreated group, whereas, the mean EAE score of mice was significantly (p value < .05) lower in the artemisinin-treated group compared with the untreated group. The brain histology shows the absence of plaque formation in the artemisinin treated group. The concentration of IFN-γ in the low dose of artemisinin treated group showed significantly (p value < .05) lower in comparison to the untreated group. IL-4 concentration was significantly (p value < .05) higher in the treated groups than the control group.
Conclusions: Since, artemisinin can shift the immune responses from Th1 to Th2, therefore, it can be helpful in the treatment of MS after more investigation.
Keywords: Artemisinin; autoimmune diseases; cytokines; experimental autoimmune encephalomyelitis; multiple sclerosis.
Artemisia annua and pulmonary hypertension (PH)
PH mediated congestive heart failure can be a fatal complication caused by COVID-19 infections, which may also be a consequence of both transfection induced immunosuppression and/or mass vaccination pressured virulent escape variants like BA.5:48
Abstract
Coronavirus disease 2019 (COVID-19) is a primary respiratory infectious disease, which can result in pulmonary and cardiovascular complications. From its first appearance in the city of Wuhan (China), the infection spread worldwide, leading to its declaration as a pandemic on March 11, 2020. Clinical research on SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) suggests that the virus may determine changes in the pulmonary hemodynamics through mechanisms of endothelial dysfunction, vascular leak, thrombotic microangiopathy, and venous thromboembolism that are similar to those leading to pulmonary hypertension (PH). Current available studies report echocardiographic signs of PH in approximately 12 to 13% of hospitalized patients with COVID-19. Those with chronic pulmonary obstructive disease, congestive heart failure, pulmonary embolism, and prior PH are at increased risk to develop or worsen PH. Evidence of PH seems to be associated with increased disease severity and poor outcome. Because of the importance of the pulmonary hemodynamics in the pathophysiology of COVID-19, there is growing interest in exploring the potential therapeutical benefits of inhaled vasodilators in patients with COVID-19. Treatment with inhaled nitric oxide and prostacyclin has shown encouraging results through improvement of systemic oxygenation, reduction of systolic pulmonary arterial pressure, and prevention of right ventricular failure; however, data from randomized control trials are still required.
ELAVL2 = In humans, the ELAV like RNA binding protein gene family has four members (ELAVL1-4). ELAVL RNA binding proteins recognize AU-rich elements in the 3' UTRs of gene transcripts and thereby regulate gene expression post-transcriptionally. The protein encoded by this gene binds to several 3' UTRs, including its own and also that of FOS, ID, and POU5F1. This gene encodes ELAVL2 and, like ELAVL3 and ELAVL4, is expressed specifically in neurons and primarily localizes to the cytoplasm. This protein also forms a cytosolic complex with the normally nuclear-localized ELAVL1 protein. Alternative splicing of this gene results in multiple transcript variants encoding distinct protein isoforms.49
Dihydroartemisinin (DHA) can act as an effective therapeutic by promoting the expression of ELAVL2 according to this murine model:50
Abstract
Dihydroartemisinin (DHA) is an active form of artemisinin extracted from the traditional Chinese medicine Artemisia annua, which is used to treat malaria. Previous studies have shown that DHA has a therapeutic effect on pulmonary hypertension (PH), but its specific mechanism has not been fully elucidated. In this study, a hypoxia-induced PH mouse model was established and DHA was administered as a therapeutic intervention. We measured hemodynamics and right ventricular (RV) hypertrophy and observed hematoxylin and eosin (HE) staining of lung tissue sections, which proving the therapeutic effect of DHA on PH. Furthermore, cell counting kit-8 and 5-ethynyl-2'-deoxyuridine (EdU) cell proliferation assay kit were performed to examine cell proliferation of pulmonary artery smooth muscle cells (PASMCs) cultured in hypoxia or in normoxia. Transwell migration chamber assay was performed to examine cell migration of the same cell model. Consistent with the therapeutic effect in vivo, DHA inhibited hypoxia-induced cell proliferation and migration. Through high-throughput sequencing of mouse lung tissue, we screened ELAVL2 as a key RNA binding protein (RBP) in PH. Mechanistically, DHA inhibited the proliferation and migration of PASMCs by promoting the expression of ELAVL2 and regulating the miR-503/PI3K/AKT pathway. The binding relationship between ELAVL2 and pre-miR-503 was verified by RNA binding protein immunoprecipitation assay. In conclusion, we first propose that DHA alleviates PH through the ELAVL2/miR-503/PI3K/AKT pathway, which might provide a basis for new therapeutic strategies of PH.
As an aside, regulation of microRNA-503 should moderate cardiovascular disease and carcinogenic activity too:51
Abstract
microRNAs (miRs) are short, non-coding RNAs that regulate gene expression by mRNA degradation or translational repression. Accumulated studies have demonstrated that miRs participate in various biological processes including cell differentiation, proliferation, apoptosis, metabolism and development, and the dysregulation of miRs expression are involved in different human diseases, such as neurological, cardiovascular disease and cancer. microRNA-503 (miR-503), one member of miR-16 family, has been studied widely in cardiovascular disease and cancer. In this review, we summarize and discuss the studies of miR-503 in vitro and in vivo, and how miR-503 regulates gene expression from different aspects of pathological processes of diseases, including carcinogenesis, angiogenesis, tissue fibrosis and oxidative stress; We will also discuss the mechanisms of dysregulation of miR-503, and whether miR-503 could be applied as a diagnostic marker or therapeutic target in cardiovascular disease or cancer.
Keywords: angiogenesis; cancer; cardiovascular disease; microRNA-503; microRNAs; oxidative stress.
Dosing guidance and contraindications
Charles shared some excellent research findings on this, which I will expand on later:
SUPERIORITY OF DRIED LEAF EXTRACT OVER CHEMICAL DERIVATIVES
This section is a brief review of a few studies that compared blood samples after ingestion of whole leaf A. annua and pure artemisinin.
WEATHERS ET AL 2011: Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases.
Weathers et al (2011) measured the amount of artemisinin in the blood of mice following the administration of pure artemisinin and the dried leaves of A. annua. "There was 45 times more pure artemisinin fed to the mice than the amount fed via A. annua leaves, yet almost the same amount of (artemisinin) appeared in the bloodstream."
ELFAWAL: Dried Whole Plant Artemisia annua as an Antimalarial Therapy
Elfewal et al (2012) tested the effectiveness of dried leaf A. annua against pure artemisinin. "We found conclusive evidence that orally ingested, powdered dried leaves of whole plant A. annua kills malaria parasites (in mice) more effectively than a comparable dose of pure drug."
https://charleswright1.substack.com/p/review-of-literature-on-the-anti?sd=pf&s=r
“The Lost Book of herbal Remedies” by Apelian & Davis (2020) gives advice for the more toxic variety of Wormwood, Artemisia absinthium, but the warning of possible miscarriages is theoretically valid for all varieties:
Warning: Take wormwood under the supervision of a medical professional. Use small doses and take it for no longer than 4 weeks at a time, then give the body a rest before repeating treatment if needed. Do not take wormwood if you are pregnant or breastfeeding. It can cause miscarriages. Do not use wormwood if you are allergic to it or other members of the daisy family.
An excellent guide to growing and harvesting A. annua.
Their website also includes a great 50 minute guide to growing and processing A-3 tea from seed to harvest by pharmacist Dr. Hans Martin who worked with the herb in the Congo to treat malaria. Background to the A-3 program:
Artemisia annua anamed ("A-3") Program
Pharmaceutical companies worldwide sell drugs, that are isolated from the plant Artemisia annua. Wild plants of this species have, however, three disadvantages: they can't grow in the tropical lowlands, they have few leaves, and sometimes they have a too low potency to fight strongly against malaria. The special breed "Artemisia annua anamed" is not genetically manipulated. It is rich in potency, has many leaves, and grows up to 3 m on our fields in more than 100 countries, e.g. Germany, South Africa, Congo, Uganda, Kenya, Brazil etc.! And: everywhere where malaria is prevalent, there is water and therefore the possibility to plant Artemisia annua anamed.
https://www.anamed-edition.com/en/artemisia-annua-anamed.html
How to grow Artemisia Annua
You will need:
Empty cardboard egg boxes or halved toilet roll tubes
Good quality soil
Knife
Seedling tray or extra egg boxes / toilet roll tubes
Garden space or a barrel/large sack
1. Fill the egg box tray or halved toilet roll tubes with soil.
2. Place them into a watertight tray.
3. Sow around 20 seeds into each compartment or roll. Water the roots/sides daily (do not water the fragile seeds/seedlings directly).
4. After around two weeks, very carefully separate and re-pot each seedling, Allocate one container per plant (you can once again use egg boxes or halved toiled roll tubes if you do not have a seedling tray).
5. When the plants are around 10cm in height, they can be replanted in the ground. Make a hole in the earth 70x70x70cm.
6. Half fill the hole with natural garden waste, followed by natural kitchen waste (compost bin contents), and cover with soil. You can skip this step and make a shallower hole if the soil is of excellent fertile quality.
7. Plant the Artemisia plant in the centre.
8. Water all around (but not directly on the leaves of) the Artemisia plant.
9. If the climate is extremely hot, it is a good idea to surround the small plant with loose leaves so that the sun's rays do not destroy the plant.
10. Consider making a mixed bed: Plant Artemisia alongside other crops such as cucumber, maize, potatoes.
11. If you have limited space or need to transport your mature plant, you may use a barrel or large strong sack filled with fertile soil to grow Artemisia in until it is ready to be harvested.
TIPS:
The roots need air. Never confine A-3 plants to plastic cups.
Keep soil loose. An underlayer of loose garden waste and compost bin contents helps with this.
Water the plants generously, once per day, in the morning.
Water the soil/sides - not the delicate seeds or seedlings.
Give the plants plenty of sunshine.
How to make A-3 cuttings
You will need:
Tray or dish, such as a lasagne dish
Net/mesh bag or material
Band to secure net/mesh bag/material
1. Take a container such as a lasagne dish and surround snugly with a net bag (such as the type onions come in). The net should not sag.
2. Fill the dish entirely with water.
3. Cut a small branch from the top of the Artemisia plant and remove the lower leaves on the stem.
4. Place the stem through one of the net holes. The plant should sit comfortably there without slipping through. Repeat until the tray is full, with space between each cutting.
5. Roots should begin to form in 2-3 weeks.
How to harvest Artemisia Annua
You will need:
Ventilated basket
Knife for cutting
Metal tray
Sieve (not extremely fine, ideally flat)
Collection bowl/tray
Plastic airtight storage bag
Hygrometer
1. In months 4, 5, 6 and 7 of the plant's life (providing the plant is healthy and strong), harvest the interior leaves, leaving the outermost leaves intact. The plant will continue to grow as long as conditions are favourable.
2. When harvesting, collect in a ventilated basket.
3. Cut the leaves as you would spinach, and place on a metal tray. You can line this with paper or cloth if desired.
4. Place in full sun. If in an extremely hot climate, place in partial sun. After three days, the herb should be dry.
5. Take a flat sieve and place the dry material inside. Apply a little pressure and move it around inside to encourage the dried leaf to fall through the holes. Make sure there is a solid tray or bowl below to collect the dried leaf.
6. The remaining matter can be used as medicine for animals.
7. Place the dried leaf into a plastic airtight bag, ideally along with a hygrometer. The following day, the meter should indicate not more than 40% relative humidity. Bags of this quality can keep for three years.
8. If the hygrometer indicates too high humidity, dry the herb further and repeat until a good quality is achieved.
https://www.anahitamama.com/growing-artemisia
From “Encyclopedia of Herbal Medicine” by Andrew Chevallier (2016):
ARTEMISIA Medicinal and Aromatic Plants - Industrial Profiles, edited by Colin W. Wright (2002) provides guidance on the most widely available formulations of artemisinin and its derivatives for use against fevers and malaria.
As per previous studies we know these doses may be up to 3 times the required IC50 to treat some cancers and full spectrum extracts are recommended instead for non-malarial diseases to benefit from any synergistic effects.
Recrudescence = “the recurrence of an undesirable condition”, normally with respect to malarial symptoms in this case.
CLINICAL STUDIES
The currently available preparations of artemisinin and its derivatives are artesunate for intravenous injection, oral tablets, and suppositories, artemether in oil for intramuscular injection and as oral capsules, arteether for intramuscular injection, and dihydroartemisinin in tablets (Looareesuwan and Wilairatana, 1997). China and Vietnam are the main producer countries. Artemisinin and its derivatives have been imported into many countries in Southeast-Asia and to a few African countries. The most widely available oral preparations are artemisinin tablets, artemether capsules, artesunate (artesunic acid) tablets, and dihydroartemisinin tablets. Intramuscular preparations are artemether in oil, artesunate as anhydrous powder, and recently a preparation of arteether. The intravenous preparation of artesunate is identical to the preparation for intramuscular injection. Suppository preparations are available for artemisinin, artesunate, and dihydroartemisinin. Table 1 shows the most widely available formulations (WHO, 1998).
Artemisinin and its derivatives have been studied in many countries, e.g., China and Vietnam for many years, some Southeast Asian countries such as Thailand and Myanmar for a few years, and Africa. Artemisinin and its derivatives, artesunate, and artemether give more rapid clearance of fever and malaria parasites than other drugs and survival rates are comparable to those with standard treatment regimens.
The use of artemisinin and its derivatives with other antimalarials seems to be obligatory if a high cure rate is to be obtained. Because of their short half-lives, artemisinin and its derivatives have to be given once or twice daily for 5-7 days. The shorter the duration of drug administration, the higher the rate of recrudescence. A three-day course of these compounds has been associated with about 50% recrudescence. A longer treatment duration, for example of 5-7 days, improves the cure rate to 90-98%, however, patient compliance is poor. The use of antimalarial combinations (i.e. an artemisinin derivative plus mefloquine, tetracycline or doxycycline), may be used for treatment on an out-patient basis because it reduces the duration of drug administration (i.e. 2-3 days us. 5-7 days) and increases patient compliance. Many studies have shown that combinations of these drugs with other antimalarials having a longer half-life gives more advantage. Mefloquine combinations (Karbwang et al., 1992a; Looareesuwan et al., 1992b,c, 1993, 1994a,b, 1995, 1996b,d; Luxemburger et al., 1994; Nosten et al., 1994) with either artemisinin rectally, artesunate orally or parenterally or rectally, artemether orally or parenterally have been studied extensively in uncomplicated and severe malaria. Mefloquine allows a short treatment course due to its long-half life. Combination treatment gives a rapid initial therapeutic response and protects the artemisinin compounds from resistance since the other antimalarial drug should eliminate residual parasites.
Children
Most of the reported information on artemisinin and its derivatives concerns adults, but there are sufficient data to conclude that children also tolerate the drugs very well, that no serious adverse effects have been observed, and that the therapeutic response resembles that of adults with similar levels of immunity (Hien et al., 1991; Taylor et al., 1993; White et al., 1992).
Pregnancy
Non-clinical studies showed no mutagenicity or teratogenicity but the drug caused foetal resorption in rodents even at relatively low doses of 11200-111400 of the LDSo (i.e. above 10 mg) when administered after the sixth day of gestation (Qinghaosu Antimalarial Coordinating Research Group, 1979). There are few reports of the use of artemisinin and its derivatives in pregnancy, and they show no abnormalities in children whose mothers were treated with artemisinin or artemether during the second and third trimesters (Li et al., 1990; Shen 1989; Wang 1989). For the management of uncomplicated malaria in pregnancy, artemisinin and its derivatives can be used in the second and third trimester, but their use in the first trimester is not recommended. In severe malaria, artemisinin derivatives are the drug of choice in the second and third trimester. For the treatment of severe malaria in the first trimester, the advantages of artemisisinin-like drugs over quinine, especially the lower risk of hypoglycaemia, must be weighed against the fact that there is still limited documentation on pregnancy outcomes following their use (WHO, 1998).
Overdosage
No reports on overdosage in human have become available.
Adverse Effects
Over two million patients are estimated to have been treated with artemisinin and its derivatives, and no adverse effects have been noted (Hien et al., 1993; WHO 1994). Cardiac and gut toxicity has also occurred in animals, usually with higher doses (White 1996b). Although transient dose-related reductions in reticulocyte counts were noted in some early preclinical toxicology studies, they have not been observed in human clinical trials. Foetal resorption was observed in some animal toxicology studies (China Cooperative Research Group on Qinghaosu and its Derivatives as Antimalarials, 1982). The principal toxicity in animals is a dose-related selective pattern of neurotoxicity affecting brain stem nuclei involved in auditory relays (Brewer et al., 1994). Although there has been one report (Miller et al., 1997), of a patient with ataxia and slurred speech after artesunate treatment, there is no definite proof of a causal relationship in man; ataxia and slurred speech may be due to other causes such as malaria itself. In some countries, artemisinin and its derivatives can be obtained easily because of wide-spread availability of over-the-counter antimalarial chemotherapy in shops and the market places. Self-treatment, presumptive treatment, and repeated treatment of uncomplicated infections are most frequently performed with oral-dosage forms because these are most convenient for outpatients. Once a drug is released and available, drug toxicity particularly with chronic or repeated administration may become a very important issue (Meshnick et al., 1996).
Treatment of Malaria in Pregnancy
Although, information concerning the risk of artemisinin and its derivatives in pregnancy is incomplete, these drugs should not be withheld from pregnant women in areas where these drugs are indicated (White 1996a; WHO 1994). For pregnant women with uncomplicated malaria, it is preferable to avoid the use of these drugs in the first trimester but for those suffering from severe malaria in areas with low quinine efficacy the risk-benefit ratio may favour their use even in the first trimester.
Prophylaxis
At the present time, there is a strong consensus that artemisinin and its derivatives should not be used as prophylaxis (WHO 1994). This is based on concerns of current uncertainties on the effect of higher doses and frequently repeated doses, and about continuous drug pressure leading to the emergence of resistance.
Drug Interactions
In the rodent malaria P. berghei, artemisinin potentiated the antimalarial effects of mefloquine, primaquine and tetracycline, is additive with chloroquine, and antagonises pyrimethamine, cycloguanil and sulphonamides (Chawira et al., 1987). When mefloquine was given at 6 h after oral artesunate, the mefloquine concentrations were below those observed with mefloquine alone. Later mefloquine administrations had no such effect. Co-administration of artemisinin derivatives and desferrioxamine B to cerebral malaria patients was thought to be useful, combining the rapid parasite clearance of the artemisinin and the potential central-nervous system protection of the iron chelator. Although data from in vitro studies indicate that artesunate and desferrioxamine B are antagonistic in terms of antiparasitic efficacy (Meshnick et al., 1993), subsequent studies in mice have found no evidence of such antagonism (Meshnick, unpubl. obs). In humans there was no evidence of adverse effects or toxicity resulting from this combination (Looareesuwan et at., 1996~).
From “Artemisia annua Prospects, Applications and Therapeutic Uses” by Aftab et al (2018).
The antimalarial compound dehydrosilibin is a derivative of silibin, an active ingredient of milk thistle.
4.4 COMMON PHARMACOLOGICAL AND AGRICULTURAL USES OF A. ANNUA AND SOME OF ITS SECONDARY METABOLITES
Having highlighted some of the different systems that can be used for the enhanced production of artemisinin and the therapeutic uses of this sesquiterpenoid compound, it is equally important to state that the artemisinin used presently in the formulation of ACTs for treatment of malaria is often not cost-effective due to the very expensive process used to extract and purify it from A. annua. Presently, more than 95% of artemisinin is produced in China and Vietnam while the reminder comes from a few other sources in India and Africa. However, as reliable as these drugs (especially the ACTs for malaria treatment) could be, they are not readily available to all patients in malaria-endemic regions due either to the low yield of artemisinin from the native plant as outlined previously or the high cost of the treatments, making them inaccessible to most patients afflicted with malaria. Consequently, in many regions of sub-Saharan Africa with a high incidence rate of malaria, local populations, against the advice of WHO, continue to drink extracts of A. annua leaves as a tea (or infusion) or consume the dried leaves of the plant directly in a porridge mixture not only in the treatment of malaria fever but also for the treatment of anorexia, insomnia, anemia, a lack of appetite, flatulence, stomach ache, jaundice, indigestion, diabetes, sickle-cell anemia, testosterone-induced benign prostatic hyperplasia (Brisibe, unpublished), obesity (Baek et al. 2015), and HIV (Lubbe et al. 2012). Another important factor responsible for this development is that a large part of the population in many developing countries, especially in rural areas of Asia, Africa, and Latin America, do not have confidence in orthodox or Western-based drugs, as all their healthcare needs have always been met by herbal remedies (Brisibe et al. 2009b). Understandably, therefore, both hot water extracts and the dried leaves of the plant consumed in the porridge mixture would contain not only artemisinin but other bioactive compounds, including polymethoxylated flavonoids such as artemetin, casticin, chrysosplenetin, chrysosplenol-D, cirsilineol, and eupatorine, and more than a dozen other sesquiterpenes that abound in the leaves, which have been indicated as important compounds with antimalarial (Elfawal et al. 2014, Willcox 2009) and potentially anticancer activities. Synergistic benefits may also be derived from the presence of other antimalarial compounds such as dehydrosilibin and dimethylallyl campferide. Aside from this, it has been reported that the traditional A. annua tea therapy contained artemisinin as well as some antioxidant compounds mostly flavonoids (Chukwurah et al. 2014, Willcox et al. 2007, Rath et al. 2004). In addition to their bioavailability, these compounds, such as phenols, saponins, flavonoids, alkaloids, and tannins, act to reduce parasitemia independent of artemisinin (Liu et al. 1992), perhaps by inducing an oxidative stress. The presence of other compounds in A. annua leaves has thus raised suspicion as to the possibility of their synergistic role with artemisinin in malaria and cancer treatment (Ferreira et al. 2010). These in planta constituents potentiate and enhance the overall activity of artemisinin (Elford et al. 1987), the reason given for the long-term use of the plant as a tea in China even before the discovery of artemisinin (Ferreira et al. 2010). Consequently, given the complex nature of A. annua and the many bioactive components and nutrients present in its tissues (Bhakuni et al. 2001, Brisibe et al. 2009), it would be simplistic to consider the consumption of either the traditional tea or whole plant material essentially as a monotherapy; a fear expressed by many people, which is understandable. However, this worry appears to be misplaced. Some recent studies have demonstrated that there may be less chance of resistance occurring from the combined use of numerous plant constituents, which enhances the overall activity of artemisinin and can prevent Plasmodium or any other microbial parasite from developing resistance to the compound (Elfawal et al. 2014). In fact, some of Current and future uses of Artemisia annua 67 these recent studies indicate that treatment with the whole A. annua plant provides a multicomponent defense system that is a more efficient means of slowing resistance development than a comparable dose of the purified monotherapeutic artemisinin, which is not only expensive (Elfawal et al. 2015) but also inefficient (Kangethe 2016). On account of the fact that A. annua has such broad potential therapeutic power against Plasmodium parasites, it could effectively be used as a cost-effective means for malaria treatment in many developing countries (Kangethe 2016).
Taken together, these observations have such strong support that apart from the use of WHO-recommended ACTs, some researchers have vigorously campaigned in favor of either re-establishing the use of traditional A. annua tea (Magahalaes et al. 2016, Van der Kooy and Verpoorte 2011) or using dried whole leaves as compressed tablets (Figure 4.2) or pACTs (Elfawal et al. 2012, 2014, 2015; Weather et al. 2011; Brisibe and Chukwurah 2014); with the caveat that the plant material used has high or clinical levels of artemisinin in remote areas where malaria is endemic. Considering that the onset of cerebral malaria and malaria induced coma is fast and the nearest medical facility or hospital could be as far as a journey of 2–3 days away, the use of the plant material (in whole or as A. annua tea therapy) should be investigated scientifically and, hopefully, permitted to sustain a malaria patient to reach a health center stocked with antimalarial drugs (Ferreira et al. 2010).
Weathers and others (2011) also showed that mice fed with dried whole plant material of A. annua had about 40 times more artemisinin in their bloodstream than those fed with a corresponding amount of the pure drug. This amount exceeded eight-fold the minimum concentration of serum artemisinin (10 µg/l) required against P. falciparum, which suggests that the active ingredients contained in the whole plant material were delivered faster and in greater quantity than those from pure drug treatments. In fact, in a human trial in Kenya (Table 4.1, cited from Weathers et al. 2014b), dried A. annua leaf tablets (delivered as pACT), fed to 48 malaria patients, yielded results similar to trials with pure artemisinin, but much less artemisinin was required when the drug was delivered as dried leaves (ICIPE, 2005).
In an exciting recent study, dried leaves of A. annua were included in the diet preparations of streptozocin-induced diabetic rats, resulting not only in a gradual but significant reduction in their blood glucose levels, but also a significant effect in controlling the loss of body weight (Brisibe et al. submitted). Though it may be quite difficult to attribute this observation to the function(s) of any of the chemical constituents of the plant, it is interesting that an earlier pharmacological study demonstrated that Tarralin™, an ethanolic extract of Artemisia dracunculus L., decreases hyperglycemia in rodents with chemically induced diabetes, as well as those genetically prone to diabetic conditions showing insulin resistance (Ribnicky et al. 2006).
Over and above all of the diseases and ailments that can be treated with A. annua, and especially as the incidence of HIV/AIDS becomes more prevalent in different parts of the world with varying consequences, a lot of new drugs (both natural and through organic synthesis) are being evaluated in the fight against the scourge. So far, A. annua has been identified as one of the few medicinal plants to show great promise in this regard (Lubbe et al. 2012), perhaps because its tea infusion stimulates white blood cell activities and has normalizing effects on immune functions of AIDS patients with highly compromised immune systems. It will be a major pharmacological novelty once the anti-HIV effects of A. annua have been unequivocally confirmed in humans.
In addition to what has been previously outlined, A. annua is also a highly nutritive plant as it has been identified as a storehouse of several important nutrients and antinutrients. The leaves are rich in many nutritional components, and a full complement of minerals such as zinc, copper, calcium, magnesium, iron, and so on. Some of these, especially iron, flavonoids, and the antioxidant vitamins that are commonly deficient in many plant-based diets, are abundant in A. annua leaves (Brisibe et al., 2009).
Perhaps the most detailed guidance from all the literature reviewed so far, from the excellent “Artemisia annua - Pharmacology and Biotechnology” by Aftab et al (2014).
4.2 A. annua Tea Infusion Therapy 4.2.1
Chemistry of Tea and its Preparation
To our knowledge, there have been few well-controlled studies examining the extraction recovery and stability of the many compounds in A. annua tea infusion. Recently, however, van der Kooy and Verpoorte (2011) performed a systematic study of different preparations of A. annua therapeutic tea infusion and showed that nearly 93 % of available artemisinin was extracted from dried A. annua leaves, but only under certain conditions. Ideal conditions were 9 g DW leaves L-1 , for 5 min at 100°C. More importantly, they also showed that when stored at room temperature, the tea artemisinin concentration did not significantly decrease, which is important for people in developing countries where malaria is endemic and there is little or no access to refrigeration. Ideally, a liter of tea infusion would be prepared daily and consumed in equal aliquots of about 250 mL over 24 h (van der Kooy and Verpoorte 2011).
Carbonara et al. (2011) detected a wide variety of phenolics including 0.06 mg g-1 DW of the flavonoid, circilineol, in an A. annua tea infusion prepared as follows: 0.5 g in 13 mL boiling water, then leaving the infusion to cool for up to 48 h prior to extraction and measurement. The original starting artemisinin concentration in the leaves was not reported, thus preventing quantification of the relative amount of constituents released into the tea. However, the artemisinin tea concentration remained constant during the 48-h room temperature infusion. Most of the measured phenolics also remained constant for 48 h. It is likely, however, that there was poor extraction of artemisinin mainly because the proportion of dried leaves to boiling water (38 g DW L-1 ) was fourfold greater than that determined to be optimal (9 g DW L-1 ) by van der Kooy and Verpoorte (2011). These authors showed data suggesting that increasing the ratio of dried leaves to water proportionately decreased the amount of extracted artemisinin; thus, at 40 g DW L-1 , only 43 % of the extractable artemisinin (93 %) appeared in the tea, a result also substantiated by Räth et al. (2004). Weathers and Towler (2012) later confirmed a high efficiency of extraction and 24-h stability of artemisinin retrieving about the same amount of artemisinin while using the same optimized tea protocol. However, several measured flavonoids, casticin and artemetin, were neither well extracted nor stable. Artemisinin solubility in water is about 50 mg L-1 (van der Kooy and Verpoorte 2011), so the amount of artemisinin retrieved via hot water tea infusions is reasonable. Clearly, if a tea infusion is to be a therapeutic option, however, it must be consistently and reliably prepared.
4.2.2 Tea Studies in Animals
To our knowledge, there is only one published study in animals using a tea infusion. Atemnkeng et al. (2009) compared parasite clearance in Plasmodium chabaudi chabaudi-infected mice (n = 6) treated twice daily for 6 days with either pure artemisinin or an A. annua tea infusion. Both treatments used an equal dose of artemisinin of 0.011 mg (0.275 mg kg-1 ). This dosage was far lower than used in a third group of infected mice treated with WHO recommended doses of artemisinin of 0.4 mg (10 mg kg-1 ) on day 1, followed by 0.2 mg (5 mg kg-1 ) day-1 for days 2–7 (Table 4.1). After 6 days, only the WHO-dosed mice showed significant reduction in parasitemia; tea-treated mice had at best 50 % parasite clearance (Table 4.1).
4.2.3 Human Trials
Mueller et al. (2000) tested treatment of A. annua (cv. Artemis) tea on adults infected with uncomplicated P. falciparum malaria. The amount of artemisinin measured in the tea, although much lower than the usual dose of pure artemisinin and artemisinin derivatives, showed some success in treatment (Table 4.1). Later, Mueller et al. (2004) used doses of 5 and 9 g for A. annua tea preparations to treat P. falciparum-malaria-infected adults (Table 4.1). The A. annua (cv. Artemis), grown and dried in Germany, was delivered to the eastern Democratic Republic of Congo in prepackaged doses of dried leaves. Although the dried plant material was reported to have 1.4 % artemisinin, only 47 and 94 mg of artemisinin were extracted in a liter of tea prepared from 5 to 9 g of A. annua, respectively, which was <75 % of the original artemisinin in the dried leaves. Unfortunately, the study showed considerable recrudescence in the tea-treated group (Table 4.1). Because of the consistently lower rates of recrudescence in the quinine-treated control group (Table 4.1), it was inferred that most parasite reappearance in the tea-treated patients was the result of recrudescence, not reinfection (Mueller et al. 2004). Using 5 g dried leaves in 1 L, De Donno et al. (2012) showed that A. annua tea infusion was effective against both chloroquine (CQ)-sensitive (D10) and resistant (W2) strains of P. falciparum with IC50s of 7.08 and 5.60 nM, respectively.
In a more recent human tea trial (Blanke et al. 2008), a placebo tea with an alternate antimalarial drug was included on the first day of treatment in parallel with the test A. annua tea. Artemisinin concentration was at the same level as in Mueller et al. (2004), but tea concentration was at best 19 % (94 mg) of standard pure artemisinin treatments (500 mg) per person (Blanke et al. 2008). The A. annua used in this trial was also grown in Germany and sent to the test site in western Tanzania in dried, pre-dosed bags. At days 14 and 28, recrudescence of tea-treated patients was consistently greater than alternate drug treatments (Table 4.1).
Data from therapeutic tea trials in humans and in animals correlate well and unfortunately do not support the use of A. annua tea for treating malaria for the following reasons: animal and human data are comparably negative and compelling, artemisinin dose is not easily controlled, and other potentially useful components in the tea are not readily controlled or extractable. Nevertheless, use of the tea could play a role in malaria prophylaxis (Sect. 4.2.5) or in temporary control of malaria, mainly prevention of coma, until the infected person reaches a hospital or clinic stocked with ACT.
4.2.5 Prophylactic Human Trials
In a randomized clinical trial in Uganda (Ogwang et al. 2011, 2012), artemisia tea was tested as a prophylaxis against malaria in 132 farm workers for 9 months, and any adverse clinical effects were tracked for 12 months. Tea consumed once a week at a 2.5 g adult infusion dose had an unadjusted protective efficacy of 37.5 % (Ogwang et al. 2012), which is better than that reported for vaccines RTS, S/ AS01B and RTS, S/AS02A with protection efficacy of about 30 % in adults (Bojang et al. 2001; Polhemus et al. 2009). It was also superior to FMP1/AS02 vaccine that was reported to confer no protection and also to vaccines LSA-NRC/ AS01 and LSA-NRC/AS02 that elicited antigen-specific antibody and CD4+ T cell responses, but with no protective immunity (Ogutu et al. 2009; Cummings et al. 2010). Tea protective effects also increased with duration of use. Unlike vaccines such as RTS, S/AS02 whose protection wanes within a few weeks (Bojang et al. 2001), the increasing protection trend by artemisia tea suggested that curbing of malaria in a given population is a possibility. Persons who used artemisia tea also had 80 % fewer hospital visits due to fevers, with some individuals in the study community reporting use of the tea for >7 years with no incidence of malaria. More randomized clinical trials of Artemisia tea malaria prophylaxis need to be conducted in different populations and age groups.
A study carried out in Uganda among adults aged 18–60 years found that their immunity to malaria is greater than that in children or the very elderly. Although one might argue that weekly consumption of A. annua tea might lead to emergence of resistance, data soon to be submitted for publication from the Rich and Weathers laboratories in Massachusetts (USA) suggest otherwise.
4.3 A. annua Dried Leaf Consumption as Therapy
4.3.1 Animal Studies
Recently, Elfawal et al. (2012) measured parasitemia in mice infected with P. chabaudi that were fed two different doses (0.6 or 3.0 mg; 24 and 120 mg kg-1 , respectively) of pure artemisinin either in mouse chow or in dried leaves of A. annua. The dried leaves were at least five times more effective, and with a longer lasting response, than the pure drug in reducing parasitemia (Table 4.1). Interestingly, mice needed >45-fold more artemisinin (mixed with mouse chow) than artemisinin consumed via dried leaf in order for artemisinin to be detected in the serum (Weathers et al. 2011).
4.3.2 Human Trials
Except for the early tea trials of Mueller et al. (2000, 2004), in the Democratic Republic of Congo, clinical trials using dried leaf A. annua are scarce in the scientific literature. Although WHO does not encourage either whole plant or tea infusion clinical trials (WHO 2012), some African universities carried out their own trials (personal comm from C Kasongo to P Lutgen). Those involving a very limited number of patients were generally not published, and results were not assessed by polymerase chain reaction (PCR) as later done for clinical trials with ACTs. Their results are briefly described here, with best studies summarized in Table 4.1.
Compared with controls or even other antimalarial drugs, e.g., artesunate– amodiaquine, early unpublished trials mainly used A. annua decoctions and showed significantly greater sensitivity of the decoction with lower late therapeutic failures. In the Democratic Republic of Congo, 54 volunteers suffering from malaria were treated for 10 days with capsules containing powdered leaves of A. annua at decreasing doses. The total amount of dried herb administered per patient was 15 g dried leaves containing 15 mg of artemisinin [0.1 % artemisinin leaf content; Tiruneh et al. (2010)]. All were free of fever after 2 days, and 51 were free of parasites after 10 days.
In an unusual study aimed at preventing severe postoperative malaria at Bangui, Central Africa, capsules containing powdered leaves of A. annua leaves were administered to 25 patients, 22 of them children aged 1–16 years, during surgical interventions for orthopedic disorders (Onimus et al. 2013). The duration of the treatment ranged from 3–4 days with a daily dose of 0.4–0.5 mg artemisinin delivered in 0.4–0.5 g of A. annua dried leaves, 0.1 % artemisinin leaf content. Despite the very low administered dose of artemisinin, average parasitemia in the patients dropped by 62 % with an added benefit of a strong antinociceptive response.
The most thorough study designed to assess clinical efficacy of whole-leaf A. annua was undertaken in a collaborative project between the International Centre of Insect Physiology and Ecology (ICIPE) and Kenya Medical Research Institute (KEMRI) (ICIPE 2005; Table 4.1). The study, conducted at ICIPE Mbita Field campus, Suba District, Western Kenya, was an open-label, non-randomized clinical trial primarily targeted to assess the efficacy, safety, and tolerance of increasing doses of whole-leaf A. annua in the form of tablets (Sawa et al., in preparation). The tablets were made from a hybrid of A. annua grown in the Tanzania highland (2,000–2,200 m altitude) by a Tanzania-based NGO, Natural Uwemba System for Health (NUSAG). Harvested leaves from 8-month-old plants (just before flowering) were dried for *3 weeks under shade, then crushed, finely powdered, homogenized, and pressed into 500 mg tablets under ambient temperature. Randomly selected batches of 100 tablets extracted with hexane, concentrated and analyzed by HPLC with diode array detector showed the artemisinin content of the tablets was highly consistent at 0.74 ± 0.06 % (i.e., *3.7 mg per tablet).
Forty-eight consenting patients aged 15–56 years (average 23.42), with P. falciparum malaria (parasitemia was 0.02–4 %, based on Giemsa-stained blood smears counted against 200 WBC) and hemoglobin levels C8 mg dL-1 , were recruited for the project. Patients were divided into four cohorts and treated with increasing levels of A. annua tablets, ranging from 2 to 5 tablets twice on day 1, followed by 1–4 tablets twice daily for the next 5 days. Although there were three cases of reappearance of parasites in blood smears scattered throughout different cohorts a week following the treatments, all doses were effective in clinical and parasitological regression of malaria with 9–20 % recrudescence at day 28. Patients also suffered no toxic affect; there was no significant change in the serum levels of urea, serum proteins, creatinine, c-glutaryl transferase, serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase, alkaline phosphatase levels, or hemoglobin; pre- and post-electrocardiograms were unchanged (ICIPE 2005).
Thus, despite the relatively low levels of artemisinin in the administered doses (14.8–37 mg on day 1 followed by between 7.4–29.6 mg on days 2–6) and compared to daily doses of 60–90 mg day-1 of leaf artemisinin in a hot water extract for 5 days (Mueller et al. 2000; Räth et al. 2004), by day 28, 75–91 % cure was achieved (Table 4.1). This cure rate was also comparable to or exceeded other results: 500 mg day-1 of pure artemisinin in the form of tablets or capsules (Hien 1994; McIntosh and Olliaro 2010), and similar levels of dihydroartemisinin derivatives (artesunate, artemether, etc.) in the form of tablets or capsules (de Vries and Dien 1996). Furthermore, the positive therapeutic response seemed somewhat independent of the range of dose tested (Table 4.1; ICIPE 2005). Although the oral doses used in the ICIPE (2005) trials were far less than any tea studies, levels of recrudescence were much lower than tea and equivalent to studies using pure artemisinin (Giao et al. 2001; Table 4.1).
These results suggested that there is an important role for the phytochemical blend associated with powdered foliage of whole-leaf A. annua when orally administered as tablets. The results are also consistent with the study by Elfawal et al. (2012) and a study in China on mice infected with P. berghei, which compared the effects of pure artemisinin with crude A. annua extracts (Yao-De et al. 1992). The two products had comparable levels of artemisinin; however, crude preparations were at least 3.5 times more effective in reducing parasitemia than pure artemisinin, indicating a synergistic role played by non-artemisinin constituents in the extracts.
And from the Botanical Institute:52
Sweet Wormwood Safety:
Safety Class: 2b (not for use during pregnancy)
Interaction Class: A (no clinically relevant interactions are expected)
The Botanical Safety Handbook puts Artemsia annua in the safety class of 2b, which means that it should not be used during pregnancy (Gardner & McGuffin, 2013).
Animal studies demonstrate that ingesting A. annua may have the ability to cause embryotoxicity, especially in the 1st trimester.
Sweet wormwood has an interaction class of “A” which suggests that adverse interactions are not expected to occur (Gardner & McGuffin, 2013).
A clinical study showed that taking appropriate doses of artimesian for malaria has a high rate of efficacy and is well tolerated and safe to take for most individuals.
SAFETY SUMMARY:
Artemisia annua is generally well tolerated and safe for most individuals to consume when taken in appropriate dosages. Women who are pregnant should avoid A. annua, especially during the 1st trimester.
Sweet Wormwood Dosing:
Standard dosing for sweet wormwood is as follows:
Tincture:
Take 0.7mL two to four times per day of a 1:4 tincture (in 50% alcohol).
Infusion:
To make an infusion, pour 1 cup boiling water over 1 teaspoon of dried leaf and infuse for 10-15 minutes. For therapeutic use it is recommended to drink 2 to 4 cups a day.
Updated 17th June ‘22:
Tincture of Artemisia annua: The all important taste test
So what’s it like?
I've gone to the top of the 0.7-2.0ml dose range in cold water to bring out any bitterness, but if unsure start at the lowest.
As the name implies it is pleasantly sweet, aromatic. Strong scent but not sickly.
Taste is also sweet & caramel. Bitterness is there but mild to me at this dilution in a 300ml glass (<1%), much less than a gin & tonic. No burning sensation or warming in the mouth, throat or stomach. If anything its slightly cooling from its slight menthol content.
It's not herbally like several infusions I take, you could almost flavour a cocktail with it. And the aroma lingers on the palate long after, quite pleasant, but your experience may be different.
Conclusion
The chemotherapeutic properties of Artemisia annua almost defy modern analysis techniques due to synergistic effects and interactions, but the case has to be made for inclusion in the list of first-line herbals against Covid-19 variants.
As per the last review, very small amounts of dried leaves have demonstrated high cumulative efficacy and with an excellent cytotoxicity profile.
It can also be taken as a tincture or infused in tea, warm or cold, but greater amounts of ingredient are recommended and it may not suit everyone’s palate. And the tincture has 50% greater antiviral efficacy than acqueous extracts as measured by the IC50, ie greater potency.
Further research and clinical trials involving either A. annua extracts alone or in combination therapies (eg with berberine, ivermectin, quercetin, resveratrol, NAC, CBD, echinacea etc) should be commenced forthwith as for many people the range of safe and effective allopathic treatment options to treat Vaccine Acquired Immunodeficiency Syndrome (VAIDS)53 and other sequelae are shrinking fast.
And in a case of “if you can't beat them, join them” some very big names have been sponsoring further research into ART, whilst being careful not to undermine their other pro-vaccine sponsored research:54
The challenge our researchers faced was firstly to increase the amount of artemisinin produced by Artemisia annua plants. Secondly, the challenge was producing enough low cost seed from these improved plants to meet the ongoing demand for ACTs.
The research
This project, funded by the Bill and Melinda Gates Foundation, started by understanding and then improving the biochemistry and genetics of Artemisia annua.
To do this, we analysed the best Artemisia annua plant varieties available, including the commercially available F1 hybrid Artemis and its parents as well as commercial varieties from growers in Africa and Asia.
The insights from this analysis were used in a fast-track plant breeding programme to develop robust F1 hybrid Artemisia varieties that generate excellent yields of the anti-malarial drug artemisinin.
We then worked with experienced Artemisia growers to test new hybrids in field trials across Africa and Asia to make sure that selected varieties were competitive and reliable in the areas where they would be grown commercially.
In addition, we partnered with international seed company East-West Seed, a leading tropical seed producer, to optimise methods for commercial-scale field-based Artemisia hybrid seed production for the first time.
The outcome
We have made a significant impact on the availability of natural resources to tackle malaria.
High-quality, low-cost Artemisia F1 hybrid seed has benefited farmers and extractors in developing countries, with seed sales to African growers and extractors sufficient to produce over 62m ACT treatments from 2014 to date.
In addition to providing a reliable agricultural product, our Artemisia F1 hybrid varieties are sought after by other researchers. We interact with international researchers and Artemisia growers to share new scientific knowledge and freely provide access to elite Artemisia F1 hybrids to scientists researching next generation solutions to ensure future artemisinin availability.
“This project demonstrates how world-class, strategic research can be delivered with impact for the benefit of developing countries.”
Professor Ian Graham
One last note, if conflicts of interest can be resolved there may be many more lives it can save in addition to those successfully treated for malaria:55
Summary
The 2015 Nobel Prize in Physiology or Medicine was awarded to Professor Youyou Tu for her key contributions to the discovery of artemisinin. Artemisinin has saved millions of lives and represents one of the significant contributions of China to global health. Many scientists were involved in the previously unknown 523 Project, and the Nobel Prize given to a single person has not been without controversy. Here we summarized some key events in the 523 Project and present our views on the Award to help the public better understand the rationale of the Nobel committee’s decision, the significance of the discovery, and current issues related to artimisinin in treating malaria.
Conclusions
There is no doubt that Professor Tu (her group) is the first person who brought the A. annua into the 523 Project, the first person who obtained the active ingredient (extract and crystal), and the first person who demonstrated antimalarial activity in human. The impact of artemisinin on public health is immediate and tremendous. Millions of lives have been saved by the use of artemisinin. Additionally, the discovery of artemisinin has led to paradigm shifts in antimalarial research and therapy. There are few scientific discoveries that have the same scale and instant impacts on public health, human productivity, and scientific research as artemisinin has!
It is only fitting to hand over to Charles to conclude this review with his recommendation for future research:
IN CONCLUSION
The language in countless studies of A. annua is universally positive and hopeful for the development of drugs from A. annua to treat Malaria, SARS, SARS2 and other viruses. Available information strongly suggests that the whole leaf of the plant should be tested more scientifically.
In order to perform a statistically correct and medically correct study of A. annua extracts in vitro, I suggest the following. The essential oils of multiple and varying samples of A. annua should be created by a cold press method to preserve the delicate compounds in the plant.
A complete chemical analysis should be done of every sample of the dried leaf matter. A complete chemical analysis should be done of every sample of cold press extracts of the dried leaf matter. These results should be compared to determine what compounds were removed or altered in the cold press extraction process.
After the compounds in the multiple samples of cold press A. annua extracts have been quantified, they may be tested in Vero E6 and Huh7.5 cells infected with all available strains of SARS-CoV-2 to determine the micrograms per milliliter (µg/mL) necessary to achieve EC50 (half maximal effective concentration) responses.
The EC50 values can be described as a "function" of the quantity of the individual compounds in the extracts. The effectiveness of the individual compounds can be sorted using ordinary least squares (OLS) multiple linear regression (MLR).
This test method should greatly increase the understanding of the effectiveness of the individual compounds of A. annua in their natural state in fighting SARS2. It should give a coefficient of effectiveness of every individual compound in A. annua against all SARS2 strains that are tested in vitro.
This method is clearly superior as it captures any synergistic effects, while at the same time testing every individual compound. The test methods used in the reviewed study treat A. annua as a whole substance, and test individual compounds, one at a time. This method also preserves the natural structure of the compounds in the plant.
A similar method could be used for testing infected mice. This method should should also capture how the individual compounds can activate various immune functions in mammals. The dry leaf can also be tested in this manner, as it is not necessary to use extracts.
As for human testing, determining the EC50 values SARS2 in humans will prove more difficult because it has proven elusive for scientists to isolate SARS2 virus in the blood of humans. Nevertheless, various testing methods can still be used, such as measuring a reduction in the symptoms of volunteers who wish to try the most promising samples. I am open for suggestions on how to measure SARS2 in humans.
The image I used for this Substack came from Manfred Taege’s: Artemisia annua (Sweet wormwood) – from sowing to harvest a life-saving plant. The scientific study of compounds in their natural state will provide growers with the information that they need to selectively breed the plant for desired effect, thus bypassing the pharmaceutical industry.
Charles Wright
Review of Literature on the anti-SARS2 Activity of Whole Leaf Artemisia annua, (2022),
https://charleswright1.substack.com/p/review-of-literature-on-the-anti?sd=pf&s=r
Thank you for reading, please share this review widely if you think it will help someone.
Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References:
DoorlessCarp, Therapeutic properties of Berberine, A literature review, (May, 2022),
https://doorlesscarp953.substack.com/p/therapeutic-properties-of-berberine?s=w
DoorlessCarp, N-Acetylcysteine as Adjuvant Therapy for COVID-19 – A Perspective on the Current State of the Evidence (2021), (March, 2022),
https://doorlesscarp953.substack.com/p/n-acetylcysteine-as-adjuvant-therapy?s=w
DoorlessCarp, Therapeutics for Long Covid & Transfection Sequalae, (February, 2022),
https://doorlesscarp953.substack.com/p/therapeutics-for-long-covid?s=w
DoorlessCarp, Therapeutics for Multiple Sclerosis, (February, 2022),
https://doorlesscarp953.substack.com/p/therapeutics-for-multiple-sclerosis?s=w
Doctors Suing Food And Drug Administration Over Ivermectin
https://www.theepochtimes.com/doctors-suing-food-and-drug-administration-over-ivermectin_4515103.html?utm_medium=social&utm_source=twitter&utm_campaign=digitalsub
DoorlessCarp, Smallpox & monkeypox vaccine mythology, aegrescit medendo, The cure is worse than the disease, (June, 2022),
https://doorlesscarp953.substack.com/p/smallpox-and-monkeypox-vaccine-mythology?s=w
Behr, E. R., Casey, A., Sheppard, M., Wright, M., Bowker, T. J., Davies, M. J., McKenna, W. J., & Wood, D. A. (2007). Sudden arrhythmic death syndrome: a national survey of sudden unexplained cardiac death. Heart (British Cardiac Society), 93(5), 601–605. https://doi.org/10.1136/hrt.2006.099598
Wright, C, Review of Literature on the anti-SARS2 Activity of Whole Leaf Artemisia annua,
This review suggests the need for a new proposed testing method, (2022),
Orege, J. I., Adeyemi, S. B., Tiamiyu, B. B., Akinyemi, T. O., Ibrahim, Y. A., & Orege, O. B. . Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective. Advances in Traditional Medicine, 1–12. Advance online publication, (2021). https://doi.org/10.1007/s13596-021-00576-5
Andrea Lubbe, Isabell Seibert, Thomas Klimkait, Frank van der Kooy, Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua, Journal of Ethnopharmacology, Volume 141, Issue 3, 2012, Pages 854-859, ISSN 0378-8741, https://doi.org/10.1016/j.jep.2012.03.024. Https://www.sciencedirect.com/science/article/pii/S0378874112001845
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 64139, Efavirenz; [cited 2022 June 8]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Efavirenz
Cui, L., & Su, X. Z. (2009). Discovery, mechanisms of action and combination therapy of artemisinin. Expert review of anti-infective therapy, 7(8), 999–1013. https://doi.org/10.1586/eri.09.68
M.S. Nair, Y. Huang, D.A. Fidock, M.J. Towler, P.J. Weathers, Artemisia annua hot-water extracts show potent activity in vitro against Covid-19 variants including delta, bioRxiv 2021.09.08.459260; doi: https://doi.org/10.1101/2021.09.08.459260
Orege JI, Adeyemi SB, Tiamiyu BB, Akinyemi TO, Ibrahim YA, Orege OB. Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective. ADV TRADIT MED (ADTM). 2021 May 5:1–12. doi: 10.1007/s13596-021-00576-5. Epub ahead of print. PMCID: PMC8098784.
Tahir Ul Qamar M, Alqahtani SM, Alamri MA, Chen LL. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 Aug;10(4):313-319. doi: 10.1016/j.jpha.2020.03.009. Epub 2020 Mar 26. PMID: 32296570; PMCID: PMC7156227.
Fuzimoto AD. An overview of the anti-SARS-CoV-2 properties of Artemisia annua, its antiviral action, protein-associated mechanisms, and repurposing for COVID-19 treatment. J Integr Med. 2021 Sep;19(5):375-388. doi: 10.1016/j.joim.2021.07.003. Epub 2021 Jul 22. PMID: 34479848; PMCID: PMC8378675.
Intravenous Artesunate for Treatment of Severe Malaria in the United States,
https://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html
Zhao, MM., Zhu, Y., Zhang, L. et al. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Discov 8, 53 (2022). https://doi.org/10.1038/s41421-022-00419-w
Klemm T, Ebert G, Calleja DJ, Allison CC, Richardson LW, Bernardini JP, Lu BG, Kuchel NW, Grohmann C, Shibata Y, Gan ZY, Cooney JP, Doerflinger M, Au AE, Blackmore TR, van der Heden van Noort GJ, Geurink PP, Ovaa H, Newman J, Riboldi-Tunnicliffe A, Czabotar PE, Mitchell JP, Feltham R, Lechtenberg BC, Lowes KN, Dewson G, Pellegrini M, Lessene G, Komander D. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020 Sep 15;39(18):e106275. doi: 10.15252/embj.2020106275. Epub 2020 Aug 26. PMID: 32845033; PMCID: PMC7461020.
Haywood, A., Good, P., Khan, S., Leupp, A., Jenkins-Marsh, S., Rickett, K., & Hardy, J. R. (2015). Corticosteroids for the management of cancer-related pain in adults. The Cochrane database of systematic reviews, 2015(4), CD010756. https://doi.org/10.1002/14651858.CD010756.pub2
Dai, S., Mo, Y., Wang, Y., Xiang, B., Liao, Q., Zhou, M., Li, X., Li, Y., Xiong, W., Li, G., Guo, C., & Zeng, Z. (2020). Chronic Stress Promotes Cancer Development. Frontiers in oncology, 10, 1492. https://doi.org/10.3389/fonc.2020.01492
Wang, Y., Wang, S., Lei, M., Boyett, M., Tsui, H., Liu, W., and Wang, X. (2018) The p21-activated kinase 1 (Pak1) signalling pathway in cardiac disease: from mechanistic study to therapeutic exploration. British Journal of Pharmacology, 175: 1362– 1374. doi: 10.1111/bph.13872.
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.13872
Dogan, K, Erol, E, Didem Orhan, M, et al. Instant determination of the artemisinin from various Artemisia annua L. extracts by LC-ESI-MS/MS and their in-silico modelling and in vitro antiviral activity studies against SARS-CoV-2. Phytochemical Analysis. 2022; 33( 2): 303- 319. doi:10.1002/pca.3088
https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/pca.3088
Zephyr, J., Kurt Yilmaz, N., & Schiffer, C. A. (2021). Viral proteases: Structure, mechanism and inhibition. The Enzymes, 50, 301–333.
Arun Bahadur Gurung, Mohammad Ajmal Ali, Joongku Lee, Mohammad Abul Farah, Khalid Mashay Al-Anazi, Fahad Al-Hemaid, Artesunate induces substantial topological alterations in the SARS-CoV-2 Nsp1 protein structure, Journal of King Saud University - Science, Volume 34, Issue 2, 2022, 101810, ISSN 1018-3647, https://doi.org/10.1016/j.jksus.2021.101810.
https://www.sciencedirect.com/science/article/pii/S1018364721004729
Iftekhar Ahmad, Rahman Ali, Monyck Jeane dos Santos Lopes, Carl Hermann Dino Steinmetz, Faiz Ul Haq, Artemisia annua L. and Its Derivatives: Their Antiviral Effects on COVID-19 and Possible Mechanisms, (2022),
Hoesel, B., & Schmid, J. A. (2013). The complexity of NF-κB signaling in inflammation and cancer. Molecular cancer, 12, 86.
Lai, Henry & Singh, Narendra & Sasaki, Tomikazu. (2012). Development of artemisinin compounds for cancer treatment. Investigational new drugs. 31. 10.1007/s10637-012-9873-z.
Behbod, F., Gomes, A.M. & Machado, H.L. Modeling Human Ductal Carcinoma In Situ in the Mouse. J Mammary Gland Biol Neoplasia 23, 269–278 (2018).
Das A. K. (2015). Anticancer Effect of AntiMalarial Artemisinin Compounds. Annals of medical and health sciences research, 5(2), 93–102.
Pleiotropy (drugs)
Dobiasová S, Řehořová K, Kučerová D, Biedermann D, Káňová K, Petrásková L, Koucká K, Václavíková R, Valentová K, Ruml T, Macek T, Křen V, Viktorová J. Multidrug Resistance Modulation Activity of Silybin Derivatives and Their Anti-inflammatory Potential. Antioxidants (Basel). 2020 May 25;9(5):455. doi: 10.3390/antiox9050455. PMID: 32466263; PMCID: PMC7278776.
Septembre-Malaterre, A., Lalarizo Rakoto, M., Marodon, C., Bedoui, Y., Nakab, J., Simon, E., Hoarau, L., Savriama, S., Strasberg, D., Guiraud, P., Selambarom, J., & Gasque, P. (2020). Artemisia annua, a Traditional Plant Brought to Light. International journal of molecular sciences, 21(14), 4986.
Wang Y, Li Y, Shang D, Efferth T. Interactions between artemisinin derivatives and P-glycoprotein. Phytomedicine. 2019 Jul;60:152998. doi: 10.1016/j.phymed.2019.152998. Epub 2019 Jun 27. PMID: 31301971.
Jiang, Y. Y., Shui, J. C., Zhang, B. X., Chin, J. W., & Yue, R. S. (2020). The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus. Frontiers in pharmacology, 11, 585487.
Kiss E, Kins S, Zöller Y, Schilling S, Gorgas K, Groß D, Schlicksupp A, Rosner R, Kirsch J, Kuhse J. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and C-terminal fragments (CTFs) of the amyloid precursor protein in an AD-mouse model. Mol Cell Neurosci. 2021 Jun;113:103624. doi: 10.1016/j.mcn.2021.103624. Epub 2021 Apr 30. PMID: 33933588.
https://www.sciencedirect.com/science/article/abs/pii/S1044743121000373?via%3Dihub
Safari H, Anani Sarab G, Naseri M. Artemisia dracunculus L. modulates the immune system in a multiple sclerosis mouse model. Nutr Neurosci. 2021 Nov;24(11):843-849. doi: 10.1080/1028415X.2019.1681742. Epub 2019 Oct 31. PMID: 31665978.
Khakzad MR, Ganji A, Ariabod V, Farahani I. Artemisinin therapeutic efficacy in the experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol. 2017 Dec;39(6):348-353. doi: 10.1080/08923973.2017.1379087. Epub 2017 Sep 27. PMID: 28952817.
Dardalhon, V., Korn, T., Kuchroo, V. K., & Anderson, A. C. (2008). Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of autoimmunity, 31(3), 252–256. https://doi.org/10.1016/j.jaut.2008.04.017
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999 Nov;5(4):285-94. doi: 10.1097/00054725-199911000-00009. PMID: 10579123.
Castiglione L, Droppa M. Pulmonary Hypertension and COVID-19. Hamostaseologie. 2021 Dec 21. doi: 10.1055/a-1661-0240. Epub ahead of print. PMID: 34933375.
Cai H, Fan S, Cai L, Zhu L, Zhao Z, Li Y, Yao Y, Huang X, Wang L. Dihydroartemisinin attenuates hypoxia-induced pulmonary hypertension through the ELAVL2/miR-503/PI3K/AKT axis. J Cardiovasc Pharmacol. 2022 May 5. doi: 10.1097/FJC.0000000000001271. Epub ahead of print. PMID: 35512032.
ELAVL2 ELAV like RNA binding protein 2 [ Homo sapiens (human) ]
He Y, Cai Y, Pai PM, Ren X, Xia Z. The Causes and Consequences of miR-503 Dysregulation and Its Impact on Cardiovascular Disease and Cancer. Front Pharmacol. 2021 Mar 8;12:629611. doi: 10.3389/fphar.2021.629611. PMID: 33762949; PMCID: PMC7982518.
Powers D, Artemisia annua: 6 Key Benefits, Dosage, & Safety, (2022),
Vaccine Acquired Immune Deficiency Syndrome (VAIDS): ‘We should anticipate seeing this immune erosion more widely, (Dec 9, 2021),
Tackling malaria with fast-track plant breeding,
https://www.york.ac.uk/research/impact/tackling-malaria-with-plants/
Su, X. Z., & Miller, L. H. (2015). The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Science China. Life sciences, 58(11), 1175–1179. https://doi.org/10.1007/s11427-015-4948-7




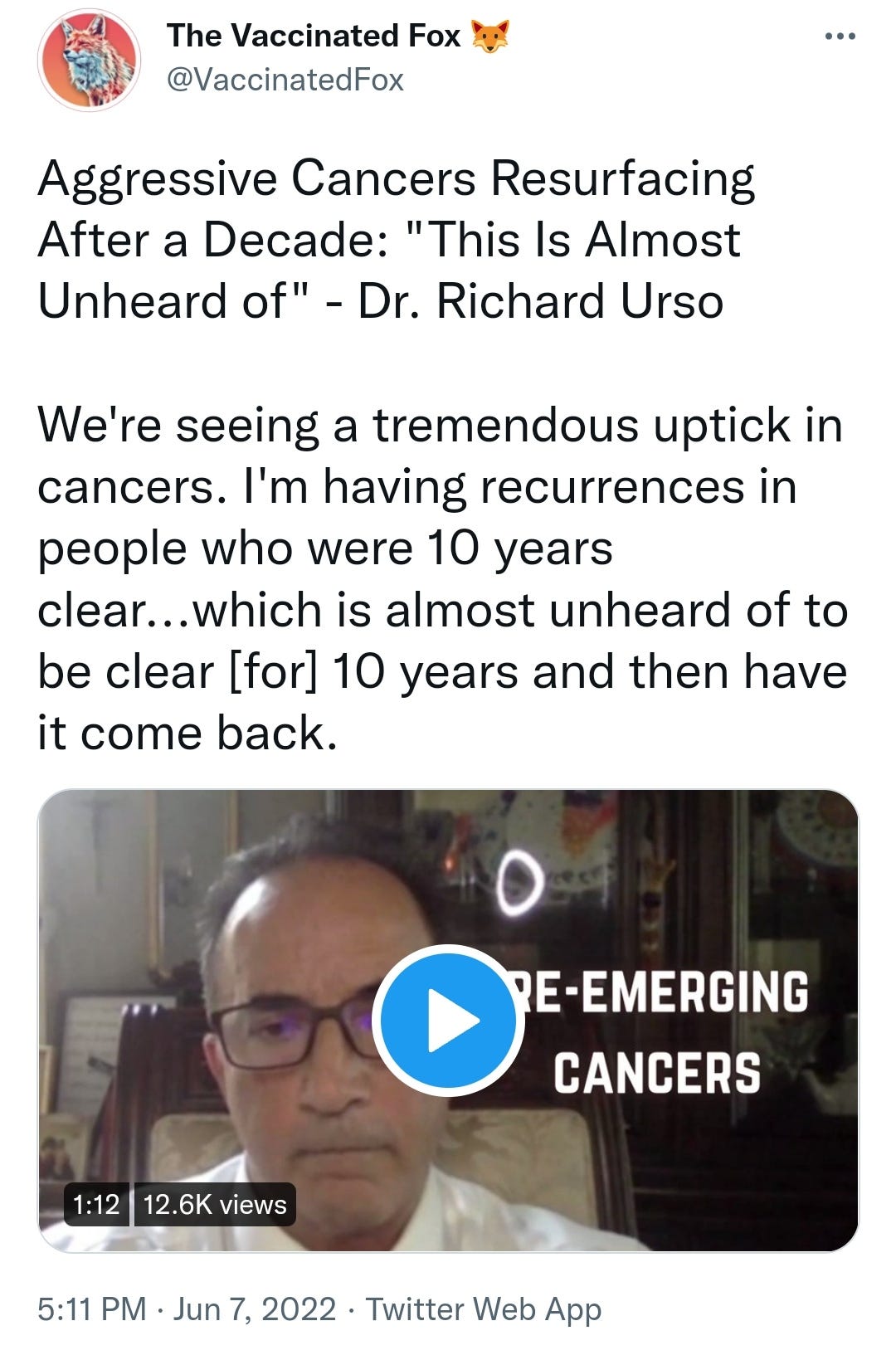
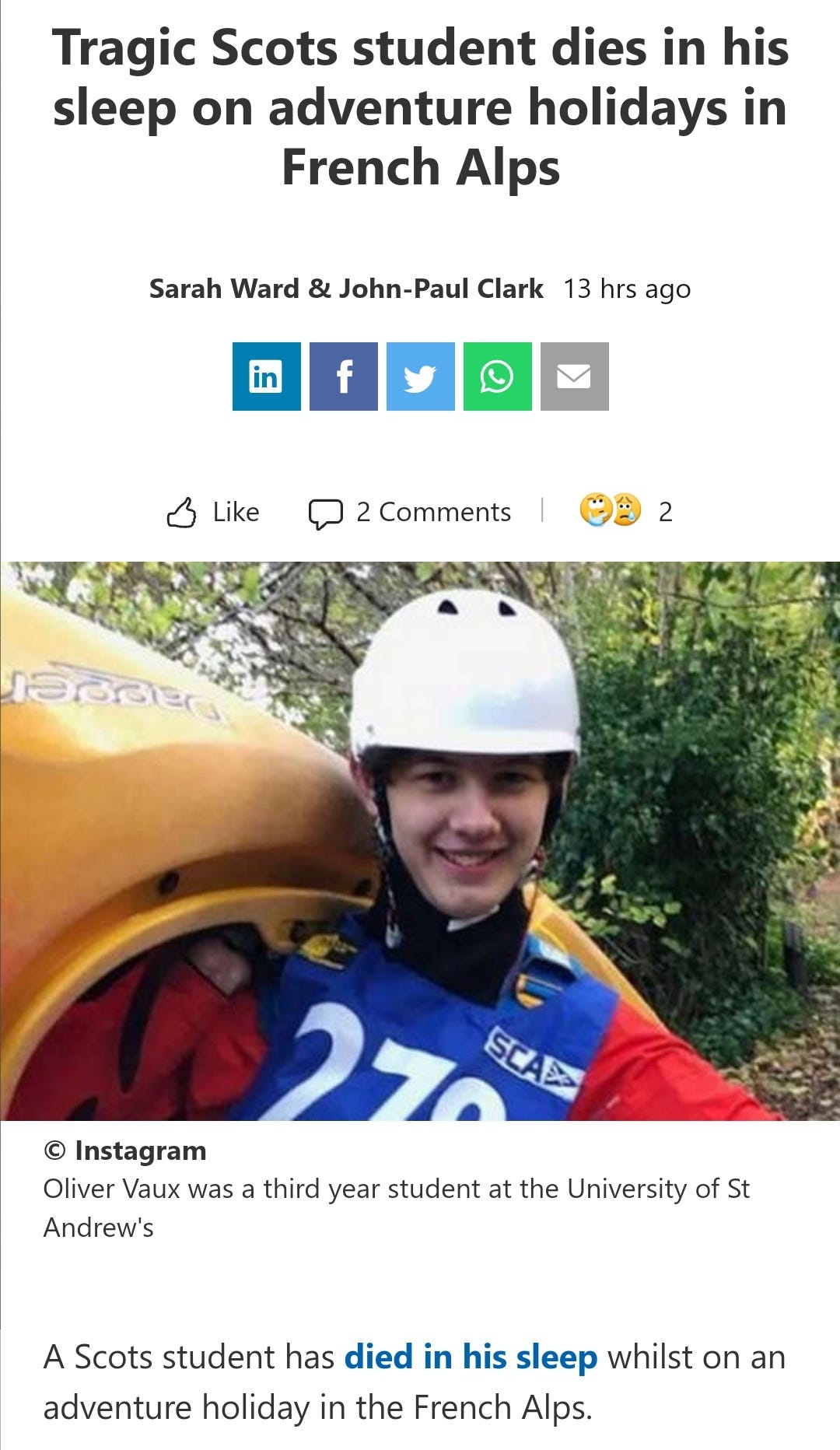

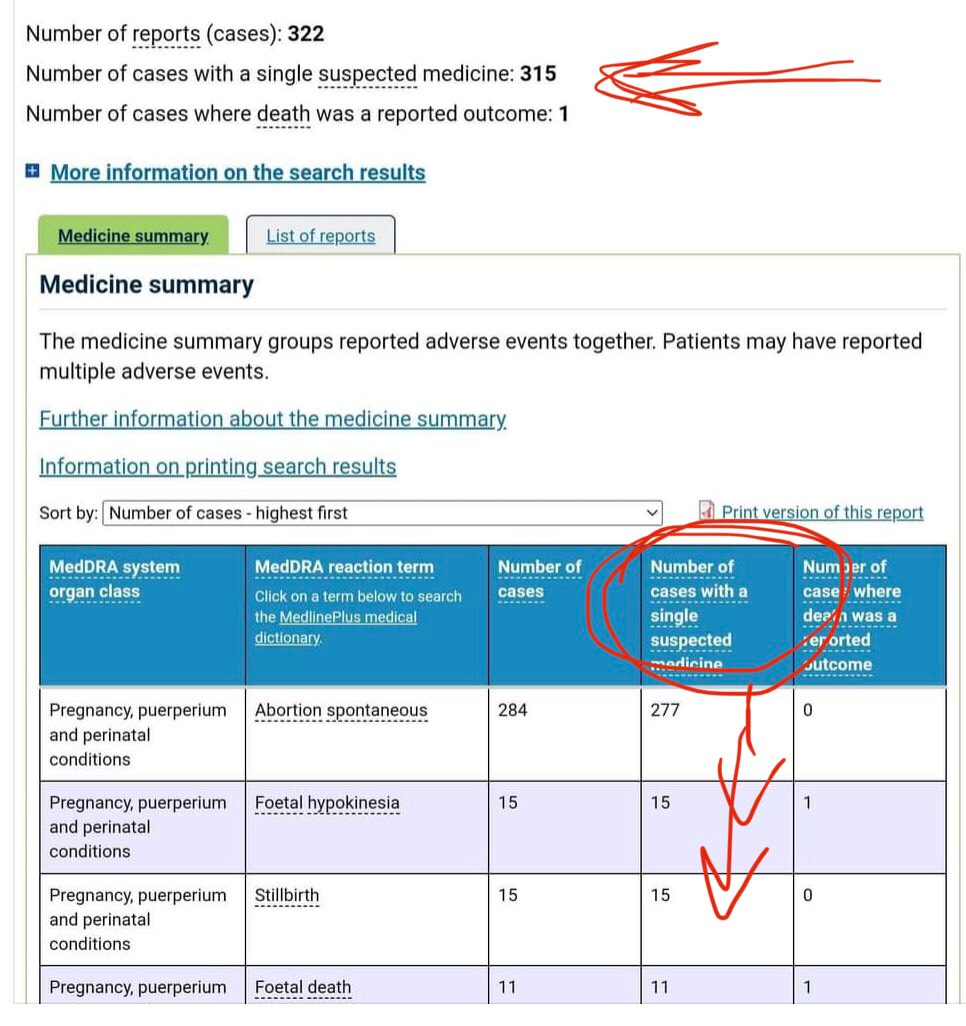
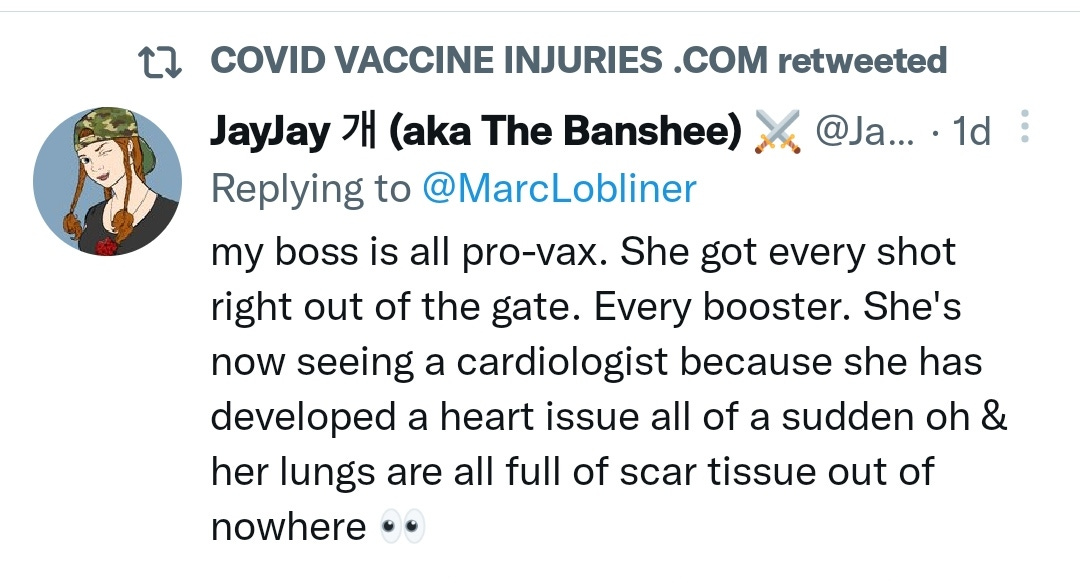
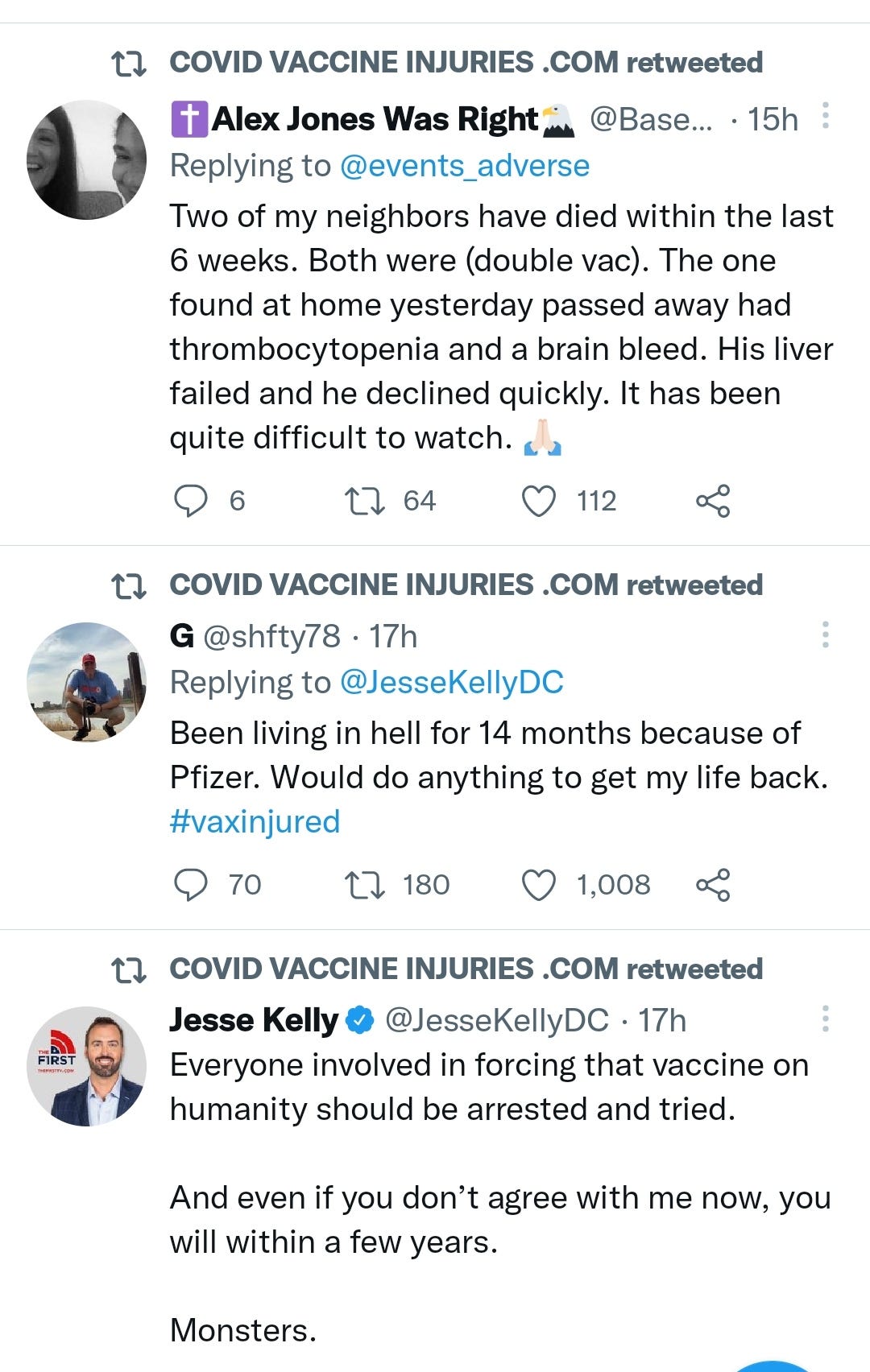
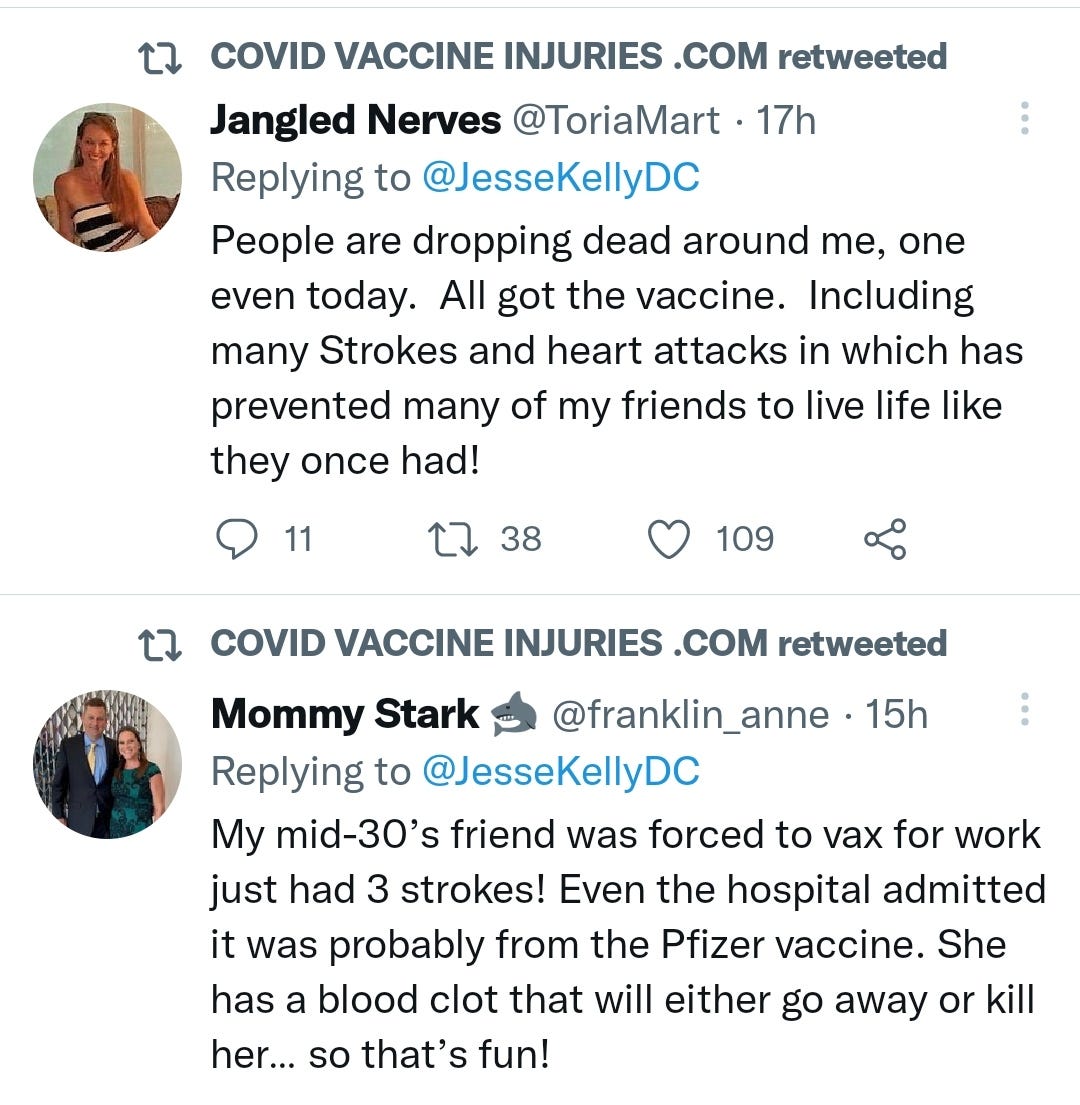
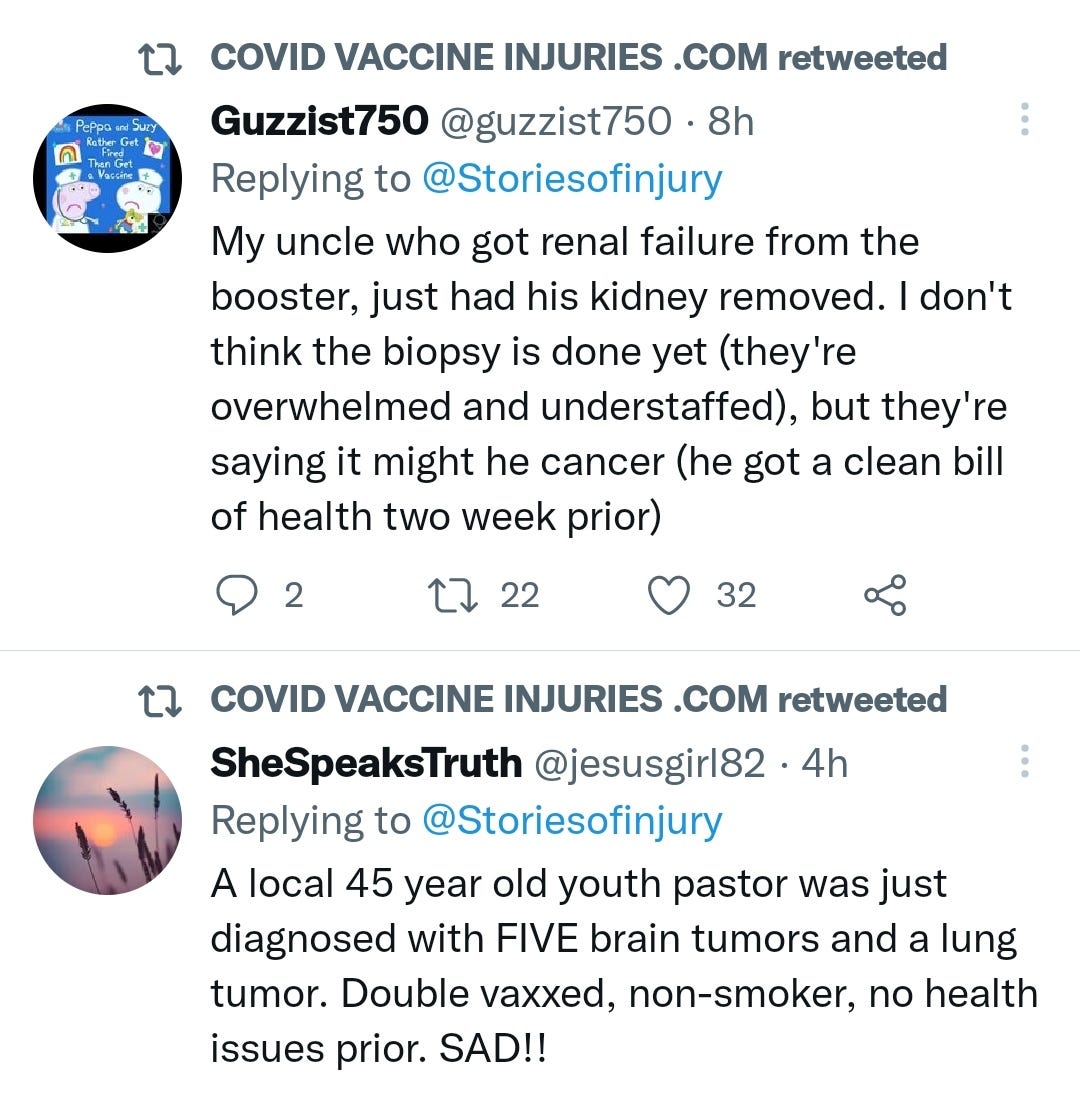









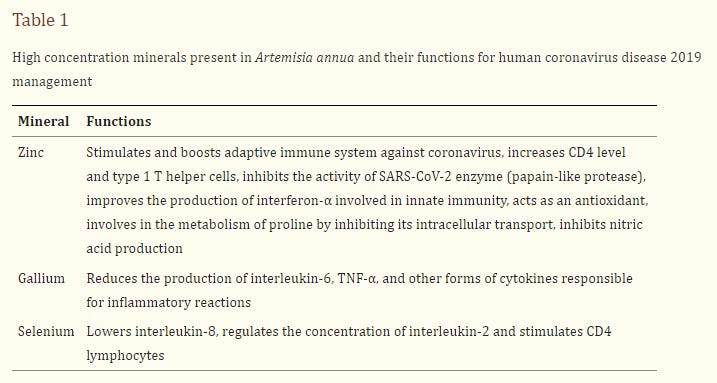
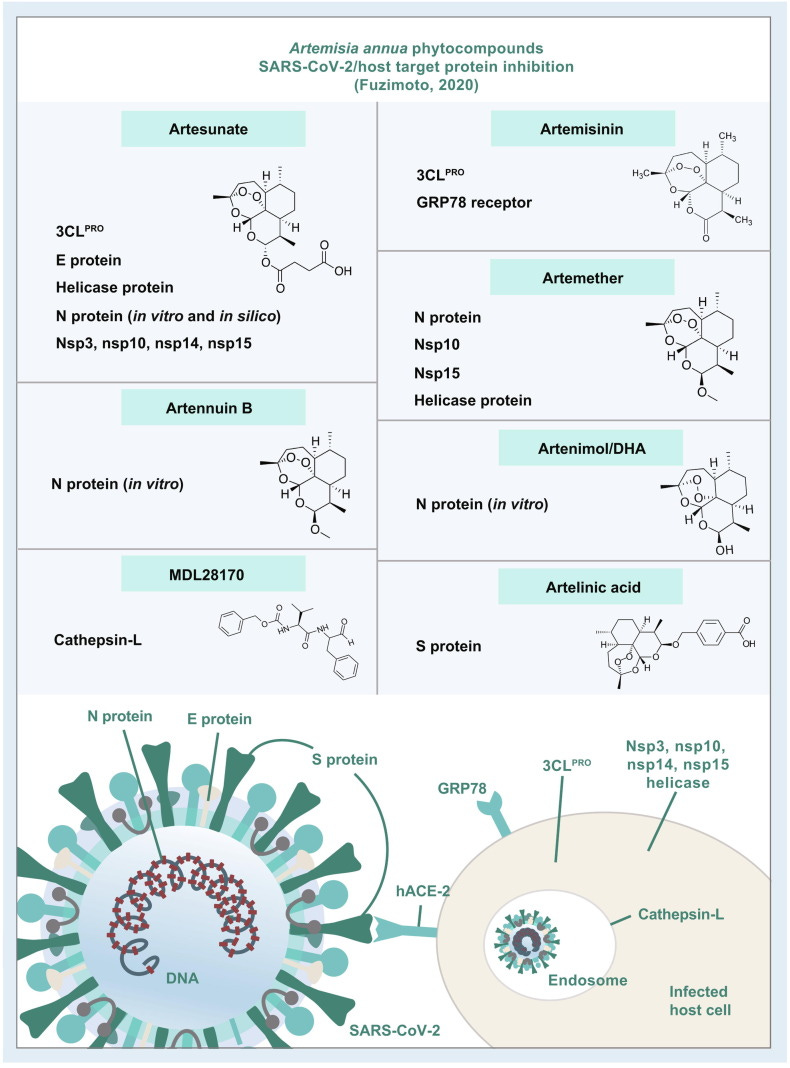
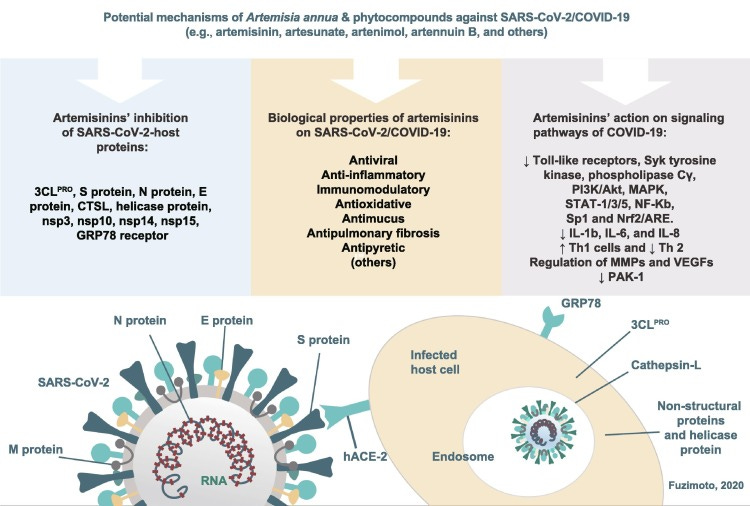

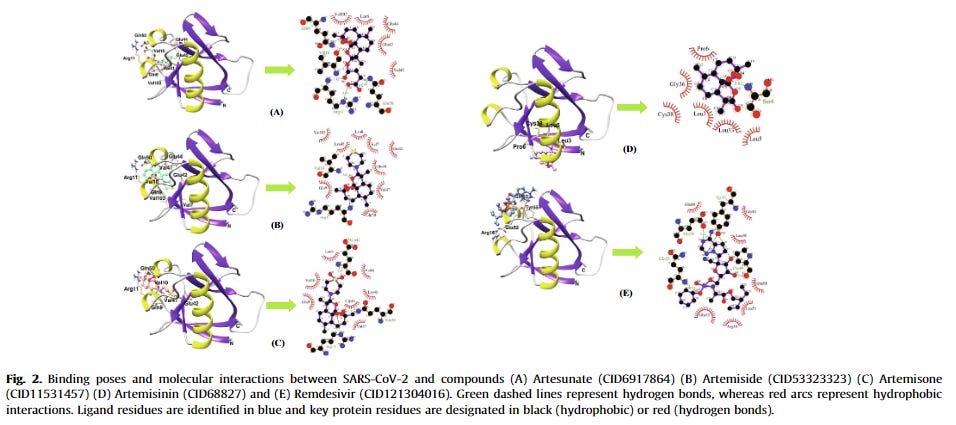
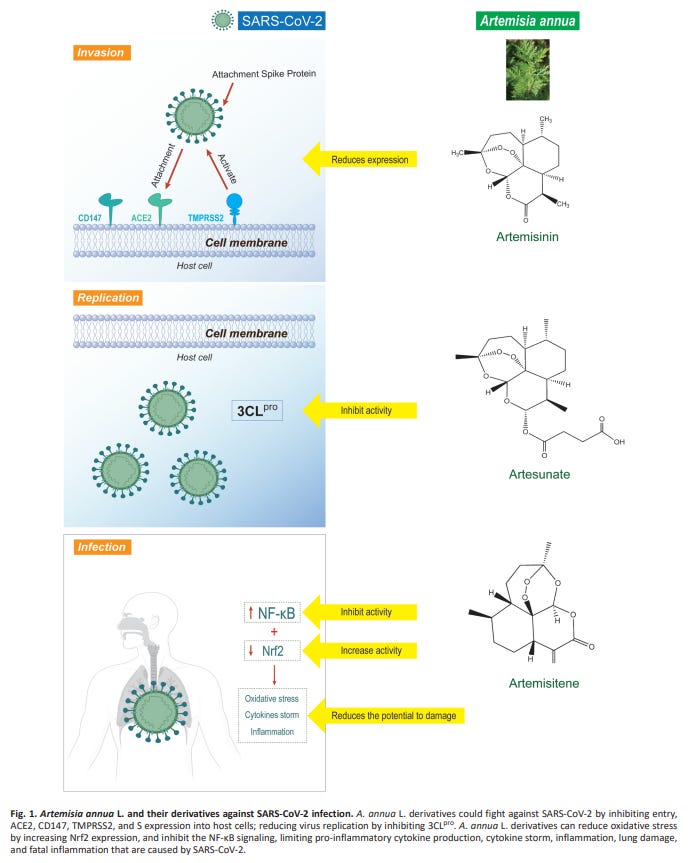








Just discovered your substack. I am a biochemist.. I have yet to read thru your substack so sorry if you have already reported on the commentsI am sharing. I am glad to see the truth for health showcasing chlorine dioxide. We may be needing universal treatments if our support systems collapse. Also ray peats theory of prion amyloidosis may be of interest (available on the internet) Ray speculates that out breaks of bse correlated with radiation releases by fires at Hanford site and Chernobyl. I think we need to rethink many of the paradigms like prions theory in science that we have been fed. Also skullcap is a cheap antivirals I use. I use the whole herb due principal of what we know about vitamin c being not one compound but a mix of cofactors. Try one herb at a time to see how it effects you at first start trial gradually to feel herbs effects. Thoughts for today. I look forward to discovering new ideas and collaborating thru your substack and with your commenters.
Excellently compiled research on Artemisia annua! I was aware of the traditional african medicine use (its one of their powerhouse medicines), the SarsCov2 antiviral inhibition, and Dr Lu's Nobel prize, but the details re diabetes, specifics on the dosages etc, was very interesting.
I particularly found the tidbit on the Bill &Melinda Gates Foundation sponsoring the research on Artemisia annua (particularly as an antiviral), by creating F1 hybrid seeds for production and sale to research companies....🤔😐😑🤦♀️
My, my. Pretty sure theres a term in business used to explain tactics lije this- Owning shares in vaccine companies, that make a particular shot, when your also funding research and own seeds patents on an antiviral that can cure the very thing the vaccine was invented for? I know in life it's called playing both sides of the fence! Im sure the business jargon is much cooler.😉
Nice work DC!