Updates:
4th December ‘22:
(Effects of melatonin & N1-methylpseudouridine substitution in miR-21).
17th August ‘23:
(Contents page added).
Contents
Further discussion about ARNTL (BMAL1)
BNT162b2 and adenovirus mRNA vectors
Circadian rhythm disruption: clinical observations
Haematopoietic stem cell transplantation
Atherosclerotic Plaque Rupture
Background
Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation (2018)
miRNAs are small non-coding RNAs, with an average 22 nucleotides in length. Most miRNAs are transcribed from DNA sequences into primary miRNAs (pri-miRNAs) and processed into precursor miRNAs (pre-miRNAs) and mature miRNAs. In most cases, miRNAs interact with the 3′ UTR of target mRNAs to suppress expression (14). However, interaction of miRNAs with other regions, including the 5′ UTR, coding sequence, and gene promoters, have also been reported (15).
Furthermore, miRNAs have been shown to activate gene expression under certain conditions (16). Recent studies have suggested that miRNAs are shuttled between different subcellular compartments to control the rate of translation, and even transcription (17).
miRNAs are critical for normal animal development and are involved in a variety of biological processes (18). Aberrant expression of miRNAs is associated with many human diseases (19, 20). In addition, miRNAs are secreted into extracellular fluids. Extracellular miRNAs have been widely reported as potential biomarkers for a variety of diseases and they also serve as signaling molecules to mediate cell-cell communications
miR-21
Hat tip to @arkmedic.
miR-21 is an oncogenic MicroRNA that inhibits apoptosis, ie it may facilitate cancer growth.
In this paper the researchers found that miR-21, along with other named microRNAs, can contribute to the proliferation of cancerous CTCL T cells and lead to the exhaustion of healthy CD8+ T-cells which would otherwise be regulating these.
The full paper is paywalled:
MicroRNA Regulation of T-Cell Exhaustion in Cutaneous T Cell Lymphoma (2021).
Cutaneous T cell lymphoma (CTCL) is characterized by a background of chronic inflammation, where malignant CTCL cells escape immune surveillance. To study how microRNAs (miRs) regulate T-cell exhaustion, we performed miR sequencing analysis, qRT-PCR, and in situ hybridization on 45 primary CTCL samples, three healthy skin samples, and CTCL cell lines, identifying miR-155-5p, miR-130b-3p, and miR-21-3p. Moreover, miR-155-5p, miR-130b-3p, and miR-21-3p positively correlated with immune checkpoint gene expression in lesional skin samples and were enriched in the IL-6/Jak/signal transducer and activator of transcription signaling pathway by gene set enrichment analysis. Further gene sequencing analysis showed decreased mRNA expression of the major negative regulators of Jak/signal transducer and activator of transcription signaling: SOCS, PIAS, and PTPN. Transfection of MyLa and HuT78 cells with anti–miR-155-5p, anti‒miR-21-3p, and anti‒miR-130b revealed a considerable increase in SOCS proteins along with a significant decrease in the levels of activated signal transducer and activator of transcription 3 and immune checkpoint surface protein expression as well as decreased cell proliferation.
Downregulation of miR-155, miR-130, and miR-21 in CTCL cell lines decreased CTCL cell growth and facilitated CD8+ T-cell–mediated cytotoxic activity, with concordant production of IFN-γ and CD107a expression. Our results describe the mechanisms of miR-induced T-cell exhaustion, which provide a foundation for developing synthetic anti-miRs to therapeutically target the tumor microenvironment in CTCL.
https://www.jidonline.org/article/S0022-202X(21)02479-9/fulltext
T cell exhaustion
CD8+ T cell exhaustion (2019)
CD8+ T cells are important for the protective immunity against intracellular pathogens and tumor. In the case of chronic infection or cancer, CD8+ T cells are exposed to persistent antigen and/or inflammatory signals. This excessive amount of signals often leads CD8+ T cells to gradual deterioration of T cell function, a state called "exhaustion."
Circadian rhythms
Various pathologies are associated with a disrupted circadian rhythm. This paper explores the signalling and hormonal pathways. Note the importance of BMAL1 expression for regulating circadian rhythms:
Circadian rhythms in immunity (2020)
Abstract
Purpose of review
This review is focused on the existing evidence for circadian control of innate and adaptive immune responses to provide a framework for evaluating the contributions of diurnal rhythms to control of infections and pathogenesis of disease.
Recent findings
Circadian rhythms driven by cell-autonomous biological clocks are central to innate and adaptive immune responses against microbial pathogens. Research during the past few years has uncovered circadian circuits governing leukocyte migration between tissues, the magnitude of mucosal inflammation, the types of cytokines produced, and the severity of immune diseases. Other studies revealed how disruption of the circadian clock impairs immune function or how microbial products alter clock machinery.
Summary
Revelations concerning the widespread impact of the circadian clock on immunity and homeostasis highlight how the timing of inflammatory challenges can dictate pathological outcomes and how the timing of therapeutic interventions likely determines clinical efficacy. An improved understanding of circadian circuits controlling immune function will facilitate advances in circadian immunotherapy
Keywords: clock, migration, allergy, asthma, microbiota, infection
(There is a typo in the figure, BMAL1, not BMAL11)
Organization of central and cell-intrinsic peripheral oscillators.
The central oscillator is comprised of the SCN in the hypothalamus, which receives light signals and transmits rhythmic cues to the peripheral oscillators via hormone and neural pathways that are further modulated by timing of food intake and other environmental cues. The peripheral clock machinery is comprised of three interlocking feedback loops. BMAL1 and CLOCK binding to E-box elements drives transcription of multiple clock-controlled genes. As PER and CRY accumulate, they translocate back into the nucleus to repress BMAL1:CLOCK driven gene transcription, including that of PER and CRY. REV-ERBα and RORα alternatively regulate expression of RORE element associated genes, including BMAL1 and NFIL3. NFIL3 and DBP in turn alternatively regulate expression of genes like PER via D-box elements. Each element modulates expression of clock-controlled genes that may either contribute to circadian expression of other genes or have a circadian expression profile without feeding back into the molecular clock machinery.
Role of the clock in immune cell development
The majority of immune cell lineages possess intrinsic clocks that provide temporal control of development, differentiation, migration, and function of these cells [2, ●3]. In some cases, the circadian clock machinery contributes to development and differentiation of immune cell lineages. A primary example is NFIL3, which is required for the development of innate lymphoid cells (ILCs), including natural killer (NK) cells [14–18]. In the case of interferon-gamma (IFN-γ) producing ILC1 and NK cells, NFIL3 is essential both for development of these cells from a common ILC progenitor [15, 16, 19, 20], as well as, for the homeostatic maintenance of these lineages within intestines and lymphoid tissues [17, 18]. In contrast, group 3 ILC only require NFIL3 at the level of the common ILC progenitor [15].
Similar to the role of NFIL3 in ILC, the development of B cells depends on BMAL1. Bmal1−/− mice exhibit marked deficits in peripheral B cell numbers and immunoglobulin (IgG) titers [21]. However, cell transfer and conditional gene deletion experiments revealed a B cell-extrinsic role of BMAL1 in modulating the bone marrow microenvironment in which B cells develop [21, 22].
The circadian clock machinery is also implicated in the development of specific subsets of CD4⁺ T cells. Neither global nor T-cell-specific ablation of Bmal1 appreciably affected overall or subset-specific frequencies of T cells [21, 22]. However, loss of NFIL3 in T cells resulted in increased frequencies of T helper 17 (Th17) cells [23], whereas mice expressing a dominant-negative CLOCK protein or lacking either REV-ERBα or RORα exhibited reduced Th17 frequencies [23, 24]. Given the vital role of Th17 cells in intestinal health and immunity, the role of clock genes in Th17 homeostasis likely serves to balance the activity of the cells for optimal protection against pathogens with minimal tissue damage.
Clocks control leukocyte trafficking
During homeostasis, inflammatory cells undergo daily flux between the bone marrow, blood, and tissues. As a result, the number of circulating leukocytes in both mice and humans changes dynamically over the day [25, 26]. The rhythmic trafficking of immune cells during homeostasis is largely controlled via cell-extrinsic mechanisms [27]. Central clock cues from adrenergic nerves regulate expression of C-X-C motif chemokine ligand 12 (CXCL12) by bone marrow stromal cells. CXCL12 is a vital bone marrow retention signal and this mechanism controls the rhythmic egress of cells into the periphery.
Similar cell-extrinsic mechanisms control temporal variation in leukocyte numbers in tissues [28]. During acute inflammation, the rhythmic release of CXCL5 from bronchiolar epithelial Club cells in the lung promotes increased recruitment of neutrophils during the resting phase (daytime in mice) [29]. Elevated endogenous glucocorticoids dampen CXCL5 and neutrophil recruitment during the active phase (nighttime in mice), and BMAL1 deletion in Club cells abrogates this circadian circuit, resulting in a non-rhythmic pro-inflammatory influx of neutrophils into the lung.
Of note, diurnal expression of glucocorticoids also regulates IL-7 receptor (IL-7R) and CXCR4 in T cells [●30]. Circulating T cell frequencies in mouse blood peak during the resting phase when IL-7R expression is low, whereas elevations in IL-7R expression during the active phase enhanced T cell survival and accumulation in spleen and lymph nodes. As a result of active phase accumulation of T cells in secondary lymphoid tissues, antigen-specific T cell responses and humoral immunity induced by systemic bacterial infections and soluble antigen during this active phase were enhanced [●30].
A recent organism-wide analysis revealed that both the microenvironment and leukocyte-autonomous oscillations in migratory factors control the time-of-day-dependent homing of particular cell subsets to specific organs [●●31]. In multiple lymphoid and nonlymphoid tissues, pro-migratory molecules like intercellular adhesion molecule 1 (ICAM1), ICAM2, and vascular cell adhesion molecule 1 (VCAM1) undergo rhythmic oscillations. In contrast, addressins and selectins display tissue-restricted oscillations in the liver (E-selectin), lymph node (mucosal vascular addressin cell adhesion molecule 1, MAdCAM-1), gut (CD44 and peripheral lymph node addressin, PNAd), and skin (CD44). Correspondingly, some chemokine receptors (CXCR4) and adhesion molecules (P-selectin glycoprotein ligand-1, PSGL-1) exhibit circadian oscillations in nearly every leukocyte subset analyzed, including lymphocytes, myeloid cells, and granulocytes [●●31]. Genetic ablation of the clock in either the endothelium or in leukocyte ablates these time-of-day differences. Similarly, paired phasing of CC-chemokine receptor 7 (CCR7) on T cells and high endothelial venules CCL21 favored accumulation of mouse T cells in lymph nodes during the active phase [●32]. The authors conclude that an extensive circadian trafficking zip code system guides homeostatic leukocyte migration between circulation and organs.
Cell-intrinsic clocks also coordinate immune defense during inflammatory insult. Trafficking of inflammatory monocytes from blood to infected tissues is constrained by BMAL1 regulation of CXCR4 and CCL2 expression [25]. Likewise, BMAL1 regulation of CXCL2 expression modulates the migratory properties of circulating neutrophils, which is antagonized by CXCR4 [●33]. This process favors the egress of neutrophils from blood vessels of mice during the active phase, thereby enhancing anti-microbial activity within tissues. Disruption of this internal timer resulted in intravascular accumulation of neutrophils that predisposed mice to fatal vascular injury [●33]. Collectively, these studies reveal that leukocyte localization within the body is under strict environmental and cell-intrinsic circadian control that is vital to limit harmful pathology and promote optimal immune defense. In addition, these adaptations presumably optimize energy expenditure while pairing appropriate immune responses with the likely timing of pathogen encounter.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7357859/
Further discussion about ARNTL (BMAL1)
Aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL) or brain and muscle ARNT-Like 1 (BMAL1) is a protein that in humans is encoded by the ARNTL gene on chromosome 11, region p15.3. It's also known as BMAL1, MOP3, and, less commonly, bHLHe5, BMAL, BMAL1C, JAP3, PASD3, and TIC.
Research has revealed that ARNTL is the only clock gene without which the circadian clock fails to function in humans. ARNTL has also been identified as a candidate gene for susceptibility to hypertension, diabetes, and obesity, and mutations in ARNTL have been linked to infertility, gluconeogenesis and lipogenesis problems, and altered sleep patterns. ARNTL, according to genome-wide profiling, is estimated to target more than 150 sites in the human genome, including all of the clock genes and genes encoding for proteins that regulate metabolism.[
Circadian properties are due to how long it takes for 2 types of protein, CER and CRY, to accumulate.
It takes 18 hours for expression of BMAL1 protein to peak, and 6 hours for the other clock control genes to peak:
After the PER and CRY proteins have accumulated to sufficient levels, they interact by their PAS motifs to form a large repressor complex that travels into the nucleus to inhibit the transcriptional activity of the CLOCK:BMAL1 heterodimer. This inhibits the heterodimer activation of the transcription of Per and Cry genes, and causes protein levels of PER and CRY drop. This transcription-translation negative feedback loop (TTFL) is modulated in the cytoplasm by phosphorylation of PER proteins by casein kinase 1ε or δ (CK1 ε or CK1 δ), targeting these proteins for degradation by the 26S proteasome. The TTFL loop of nocturnal mice transcription levels of the Bmal1 gene peak at CT18, during the mid-subjective night, anti-phase to the transcription levels of Per, Cry, and other clock control genes, which peak at CT6, during the mid-subjective day. This process occurs with a period length of approximately 24 hours and supports the notion that this molecular mechanism is rhythmic.
An interesting study on how mechanical stimulation can time shift peak expression of BMAL- 1.
Note that in this study it initially peaked at around 7.00pm.
Oscillations of the circadian clock protein, BMAL-1, align to daily cycles of mechanical stimuli: a novel means to integrate biological time within predictive in vitro model systems (2022)
Abstract
Purpose
In vivo, the circadian clock drives 24-h rhythms in human physiology. Isolated cells in vitro retain a functional clockwork but lack necessary timing cues resulting in the rapid loss of tissue-level circadian rhythms. This study tests the hypothesis that repeated daily mechanical stimulation acts as a timing cue for the circadian clockwork. The delineation and integration of circadian timing cues into predictive in vitro model systems, including organ-on-a-chip (OOAC) devices, represent a novel concept that introduces a key component of in vivo physiology into predictive in vitro model systems.
Methods
Quiescent bovine chondrocytes were entrained for 3 days by daily 12-h bouts of cyclic biaxial tensile strain (10%, 0.33 Hz, Flexcell) before sampling during free-running conditions. The core clock protein, BMAL-1, was quantified from normalised Western Blot signal intensity and the temporal oscillations characterised by Cosinor linear fit with 24-h period.
Results
Following entrainment, the cell-autonomous oscillations of the molecular clock protein, BMAL-1, exhibited circadian (24 h) periodicity (p < 0.001) which aligned to the diurnal mechanical stimuli. A 6-h phase shift in the mechanical entrainment protocol resulted in an equivalent shift of the circadian clockwork. Thus, repeated daily mechanical stimuli synchronised circadian rhythmicity of chondrocytes in vitro.
Conclusion
This work demonstrates that daily mechanical stimulation can act as a timing cue that is sufficient to entrain the peripheral circadian clock in vitro. This discovery may be exploited to induce and sustain circadian physiology within into predictive in vitro model systems, including OOAC systems. Integration of the circadian clock within these systems will enhance their potential to accurately recapitulate human diurnal physiology and hence augment their predictive value as drug testing platforms and as realistic models of human (patho)physiology.
Circadian oscillations of the clock protein, Bmal-1, align to daily patterns of mechanical stimuli
a Schematic of the entrainment protocol by daily patterns of mechanical stimulation and subsequent sampling time-course under constant conditions. Cyclic biaxial stretch (10%, 0.33 Hz) was applied for 12 h alternating with 12 h rest for 3 days, to entrain the circadian clock, before sampling during constant unloaded conditions. Group 1 (red) and group 2 (blue) cultures where cultured and sampled in parallel, but group 2 had a 6 h phase delay or “jet lag” in the entrainment protocol.
b Quantification of the circadian clock protein, BMAL-1, from normalised Western blot signal intensity, showing that cell autonomous oscillations of BMAL-1 protein align to daily patterns of mechanical stimuli. A linear model was fit to the data points of each group using Cosinor package (R studio) with 24 h periodicity (p < 0.001). There were 11 datapoints per time series illustrated, with replicate timeseries yielding comparable results. The shaded areas on the graphs illustrate times of mechanical stimulation anticipated for each group, according to the prior loading protocol shown in a. The cell autonomous circadian oscillations in BMAL-1 demonstrated a nadir in the early anticipated loaded phase and peak in the early anticipated rest phase.
c Overlay comparison of the cosinor linear models from B shows a 6-h phase delay in clock oscillations in response to a 6-h shift in loading protocol.
d Array data illustrating mechanosensitive Per1 gene expression in response to a single 5 h bout of mechanical stimuli (n = 3).
Together, these data demonstrate that daily patterns of mechanical stimuli acts as a novel timing cue that is sufficient to re-set the circadian clock within in vitro systems
Full paper:
https://link.springer.com/article/10.1007/s44164-022-00032-x
Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart (2014)
Abstract
Circadian clocks are cell autonomous, transcriptionally based, molecular mechanisms that confer the selective advantage of anticipation, enabling cells/organs to respond to environmental factors in a temporally appropriate manner. Critical to circadian clock function are 2 transcription factors, CLOCK and BMAL1. The purpose of the present study was to reveal novel physiologic functions of BMAL1 in the heart, as well as to determine the pathologic consequences of chronic disruption of this circadian clock component. To address this goal, we generated cardiomyocyte-specific Bmal1 knockout (CBK) mice. Following validation of the CBK model, combined microarray and in silico analyses were performed, identifying 19 putative direct BMAL1 target genes, which included a number of metabolic (e.g., β-hydroxybutyrate dehydrogenase 1 [Bdh1]) and signaling (e.g., the p85α regulatory subunit of phosphatidylinositol 3-kinase [Pik3r1]) genes. Results from subsequent validation studies were consistent with regulation of Bdh1 and Pik3r1 by BMAL1, with predicted impairments in ketone body metabolism and signaling observed in CBK hearts. Furthermore, CBK hearts exhibited depressed glucose utilization, as well as a differential response to a physiologic metabolic stress (i.e., fasting). Consistent with BMAL1 influencing critical functions in the heart, echocardiographic, gravimetric, histologic, and molecular analyses revealed age-onset development of dilated cardiomyopathy in CBK mice, which was associated with a severe reduction in life span. Collectively, our studies reveal that BMAL1 influences metabolism, signaling, and contractile function of the heart.
Keywords: chronobiology; circadian; metabolism; signaling; transcriptome.
DISCUSSION
The purpose of the present study was to investigate the importance of BMAL1 in the heart, through the generation of cardiomyocyte-specific BMAL1 knockout (CBK) mice. Following validation of the cardiac-restricted nature of this model (Figure 1 and Supplemental Figure 1), we identified 19 direct putative BMAL1 target genes, with known functions in metabolism and signaling (Table 1). Subsequent validation studies revealed that aberrant regulation of Bdh1 and Pik3r1 in CBK hearts was associated with impaired ketone body metabolism (Figure 3) and signaling (Figure 4) respectively. We next tested the hypothesis that a physiologic role of BMAL1 is for metabolic adaptation of the heart to fasting; we observed that CBK hearts exhibit a fasting-like metabolic profile (depressed glucose utilization and increased fatty acid oxidation), even in the fed state (Figure 5). Finally, we report that chronic BMAL1 ablation in the heart results in an age-onset cardiomyopathy (Figure 6) and reduced lifespan (Figure 7 and Table 2). Collectively, these studies highlight novel functions for the circadian clock component BMAL1 in the heart.
Full paper:
BNT162b2 and adenovirus mRNA vectors
MicroRNA transfection agent BNT162b2 synthesises homologous miR-21 amongst many other miRNA’s due to viral mimicry.
This is a problem because the spike protein expressing mRNA can persist in the lymph node germinal centres for several months, constantly expressing miRNAs including miR-21 which may be distributed systemically via exosomes:
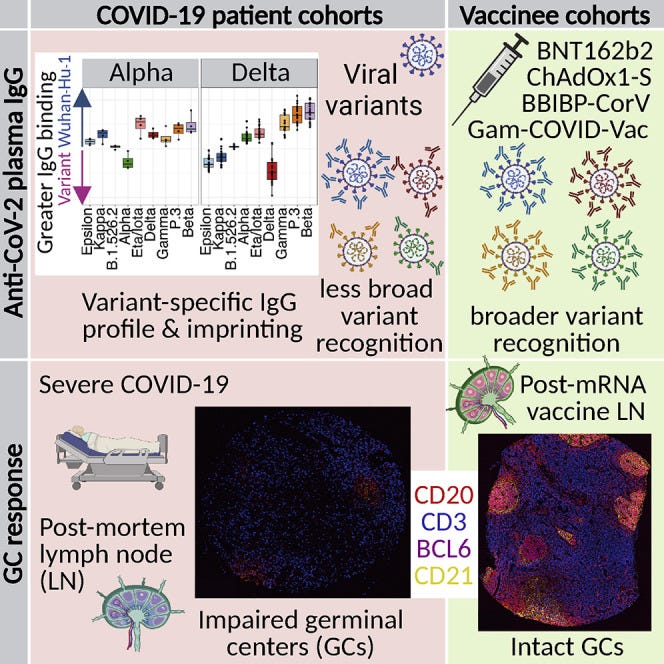
Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination (2022)
https://www.cell.com/cell/fulltext/S0092-8674(22)00076-9
Quantum microRNA Assessment of COVID-19 RNA Vaccine: Hidden Potency of BNT162b2 SASR-CoV-2 Spike RNA as MicroRNA Vaccine (2021)
Also see:
Abstract
Objective: The pandemic of coronavirus disease 2019 (COVID-19) is caused by infection with severe respiratory syndrome human coronavirus 2 (SARS-CoV-2). The spike (S) RNA of SARS-CoV-2 is used to build the COVID-19 vaccine. Although the level of immune response elicited by the full-length S RNA vaccine and the Receptor Binding Domain (RBD) vaccine that is a part of S is similar, the full-length S RNA vaccine is safer and lower reactogenicity than the RBD vaccine. However, the reason has not yet been clarified. On the other hand, the COVID-19 RNA vaccine with the S sequences may produce viral microRNAs (miRNAs). But there are no miRNA assessments for the safety of the COVID-19 vaccines. Therefore, evaluation as miRNA vaccines is necessary for risk management of vaccination.
Materials and Methods: miRNA Fold was used for pre-miRNA prediction in SARS-CoV-2 S RNA. MRmicro- T was used for protein target search. The integrated network algorithm as the miRNA entangling sorter (METS) analysis by using quantum miRNA language plus Artificial Intelligence (AI) were used as previously described.
Results: In computation, sixteen SARS-CoV-2-S-derived miRNAs bound to the negative strand S RNA with quite strong avidities. Further, CovS-miR-21 downregulated Rho associated coiled-coil containing protein kinase (ROCK2) and aryl hydrocarbon receptor nuclear translocator like (ARNTL), and CovSmiR- 3 decreased lysine methyltransferase 2C (KMT2C). Therefore, in the METS analysis, CovS-miR-21 suppressed the function of Ras homolog family member A (RhoA)/Rock2 signaling and circadian rhythm, and CovS-miR-3 inhibited histone H3-K4 methylation.
Conclusion: We found that BNT162b2 inhibits SARS-CoV-2 replication through degradation of negative strand viral RNAs that are completely paired with SARS-CoV-2 S-derived miRNAs. Further, CovS-miR-21 derived from BNT162b2 restores circadian rhythm and attenuate immunogenicity. Quantum miRNA assessments showed that the BNT162b2 RNA vaccine has a character of miRNA vaccine and is an excellent vaccine with high efficacy and low side-effects.
Keywords: COVID-19; SARS-CoV-2; BNT162b2; RNA vaccine; microRNA; Computer simulation; In silico; Circadian rhythm; Rho pathway
Circadian rhythm controller of CovS-miR-21
The robustness of circadian rhythm is implicated in the oscillating expression of clock gene family, such as clock circadian regulator (CLOCK), Aryl Hydrocarbon Receptor Nuclear Translocator Like (ARNTL, BMAL1), Period Circadian Regulators (PERs), Cryptochrome Circadian Regulators (CRYs). Disturbance of these clock genes lead to pathophysiology for cardiovascular system, whereas the stabilization is protective. Although ARNTL was reduced by CovS-miR-21 with miR-206 (Figure 4), deletion of circadian rhythm-related gene BMAL1 has exacerbated acute bronchiolitis caused Sendai and influenza A virus in mice. Therefore, CovS-miR-21 is an aggravating factor for SARS-CoV-2 pathogenicity because low levels of BMAL1 expression promoted RNA viral replication. As shown in Figure 5, ARNTL expression is various among seasons in human blood sample and the lowest levels of ARNTL has been observed during the winter months coinciding with the peak season for respiratory viral epidemics (Figure 5, a right panel). In the case of COVID-19 pandemic, since ARNTL was suppressed by Covs-miR-21 from SARS-CoV-2 S, infectivity and mortality of SARS-CoV-2 infection are acceleratively both higher in colder climates.
Figure 5: Summary of the quantum miRNA assessment for BNT162b2. BNT162b2-derived miRNAs inhibit SARS-CoV-2 replication via degradation of the negative strand viral RNA by completely paired RNA-dependent gene silencing (blue arrows), which would induce the high efficacy (85-91%) on 15-28 days after the first dose of BNT162b2 vaccination. BNT162b2-derived CovS-miR-21 would restore circadian rhythm and attenuate immunogenicity while CovS-miR-3 would induce Th1 promotion and antibody production. Therefore, CovSmiR- 21 and CovS-miR-3 would play an important role for lower incidence and high neutral Ab production plus Th1 promotion, respectively. The circadian oscillation is seasonally synchronized with immune cells’ gene expression in healthy people (the right panel) and SAR-CoV-2 S-derived CovS-miR-21 would make a narrow peak in the summer season (red arrows). On the other hand, BNT162b2-derived CovS-miR-21 could restore the circadian rhythm by direct beating the viral genome. Substantially as described as similia similibus curantur, BNT162b2-CovS-miR-21 would attenuate immunogenicity via suppression of clock gene. The pandemic of COVID-19 would be successfully decreased by BNT162b2.
…On the contrary, CovS-miR-21 from BNT162b2 would limit innate immunity via decreasing of ARNTL in circadian-rhythm. ARNTL knockout mice has rendered macrophages unable to sustain inflammatory reaction and M1 macrophages enhanced ARNTL expression. Thus, these data strongly suggest that inflammation by vaccine injection could be attenuated by CovSmiR- 21 as described above. Subsequently, since CoVS-miR-21 was a viral pathogenic factor, adenovirus- or vaccinia virus-based DNA vaccines should consider additional side-effects of SARS-CoV-2 S-derived miRNAs as circadian rhythm disturber because SARSCoV- 2 S DNA in adenovirus vector may be integrated into the host chromosome as a character of viral vectors. For example, as the relationship of subarachnoid hemorrhage to circadian oscillation via CRYs and PER2 has been widely studied spontaneous brain injury may be induced by SARS-CoV-2 S-derived miRNA.
https://crimsonpublishers.com/aics/fulltext/AICS.000552.php
I really should have looked into the circadian aspect more before now...look what the authors are telling us!
Over suppressing ARNTL (BMAL-1) for months, even if it may restore circadian rhythm, is not good if it leads to immunosuppression, a pro tumor environment or cardiac damage as discussed earlier.
Their text even contradicts itself with respect to restoration of circadian rhythm:
…since CoVS-miR-21 was a viral pathogenic factor, adenovirus- or vaccinia virus-based DNA vaccines should consider additional side-effects of SARS-CoV-2 S-derived miRNAs as circadian rhythm disturber.
And apart from increased risk of subarachnoid haemorrhage, the risk of other cardiovascular accidents is also increased by elevated levels of miR-21 itself, eg due to pre-existing atherosclerosis.
A synopsis from this paper is that as miR21 suppresses immunity and inhibits apoptosis. Atherosclerotic plaques may be allowed to build up until they break away. And maybe kills you in the process if it goes on to block a key artery in, for example, your lung, heart or brain.
Think of it as a series of controlled brushwood fires being replaced instead by the whole forest going up in flames, taking you with it (California anyone?)
The clearance of apoptotic cells by professional and non-professional phagocytes - a process termed 'efferocytosis' - is essential for the maintenance of tissue homeostasis. Accordingly, defective efferocytosis underlies a growing list of chronic inflammatory diseases.
-Efferocytosis in health and disease (2020)
MicroRNA-21 Controls Circadian Regulation of Apoptosis in Atherosclerotic Lesions (2021)
Clinical Perspective
What Is New?
In atherosclerotic lesions, diurnal regulation of apoptosis with a peak at the beginning of the active phase results in synchronized changes in the necrotic core size because of a temporal mismatch between apoptosis and efferocytosis.
The diurnal oscillation of miR-21-5p and miR-21-3p controls the molecular clock output in atherosclerotic lesions by triggering the daily rhythm of apoptosis via targeting proapoptotic Xaf1 (XIAP-associated factor 1).
Molecular clock genes also oscillate in human atherosclerotic plaques and regulate circadian expression of the miR-21 strands antiphase to that of lesional apoptosis, thereby promoting the morning peak of apoptosis.
What Are the Clinical Implications?
A macrophage death clock controlled by Mir21 may enhance lesion growth and susceptibility to rupture, indicating that the molecular clock can have detrimental effects under pathological conditions.
The molecular clock in lesional macrophages may contribute to the circadian pattern of myocardial infarction, which could be a target for preventive measures to limit the mismatch between apoptosis and efferocytosis, and thus reduce plaque vulnerability in the morning.
The necrotic core partly formed by ineffective efferocytosis increases the risk of an atherosclerotic plaque rupture. Microribonucleic acids contribute to necrotic core formation by regulating efferocytosis and macrophage apoptosis. Atherosclerotic plaque rupture occurs at increased frequency in the early morning, indicating diurnal changes in plaque vulnerability.
In cancer cells, miR-21-5p has been shown to inhibit apoptosis by targeting programmed cell death 4.39miR-21-5p and miR-21-3p reduced macrophage and EC apoptosis by suppressing Xaf1, which promotes TNFα (tumor necrosis factor α)–induced apoptosis by antagonizing the caspase-inhibitory activity of XIAP and by XIAP-independent release of mitochondrial cytochrome c.
Increased apoptosis at ZT1 was associated with enhanced efferocytosis in Mir21–/–- mice. However, the diurnal increase in apoptosis in Mir21+/+ mice at the transition to the active phase exceeded the lesional capacity of macrophages to remove dead cells, suggesting that efferocytosis is not diurnally regulated. The temporal mismatch between apoptosis and efferocytosis resulted in accumulation of apoptotic cells in the necrotic core at the transition to activity. Thus, Mir21 expression in macrophages promotes atherosclerosis because the diurnal changes of the necrotic core do not come full circle, like those of the lesional apoptosis, but start again each day at an increased level.
Conclusions:
Our findings suggest that the molecular clock in atherosclerotic lesions induces a diurnal rhythm of apoptosis regulated by circadian Mir21 expression in macrophages that is not matched by efferocytosis, thus increasing the size of the necrotic core.
Atherosclerotic plaque rupture and myocardial infarction occur more often in the morning than in the rest of the day.
Another hat tip to @arkmedic :
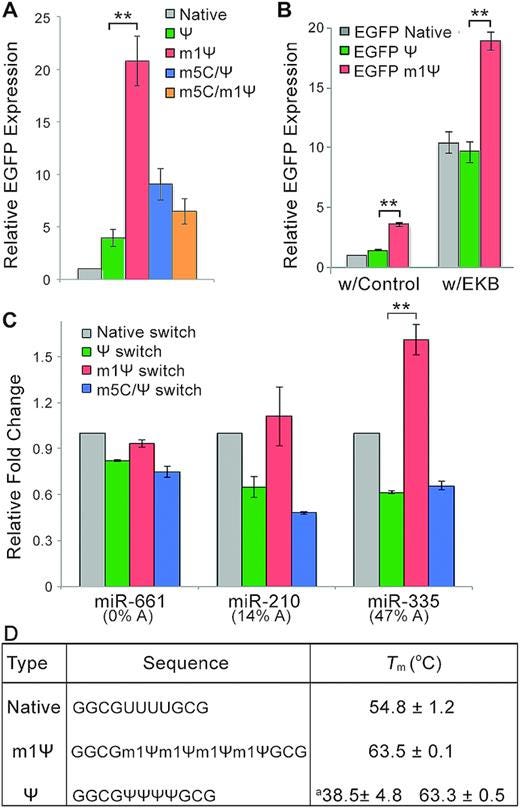
Not only is homologous mirR-21 being expressed for months, but it is a 20 fold “turbocharged” version due to the substitution of uridine with N1-methylpseudouridine:
N1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells (2020)
Key excerpts.
As shown in Figure 1C, the m1Ψ-containing switch exhibited the highest fold-change and significantly outperformed the conventional m5C/Ψ switch, suggesting it has the highest sensitivity for miR-21-5p mimic.
(EGFP is a fluorescent marker protein)
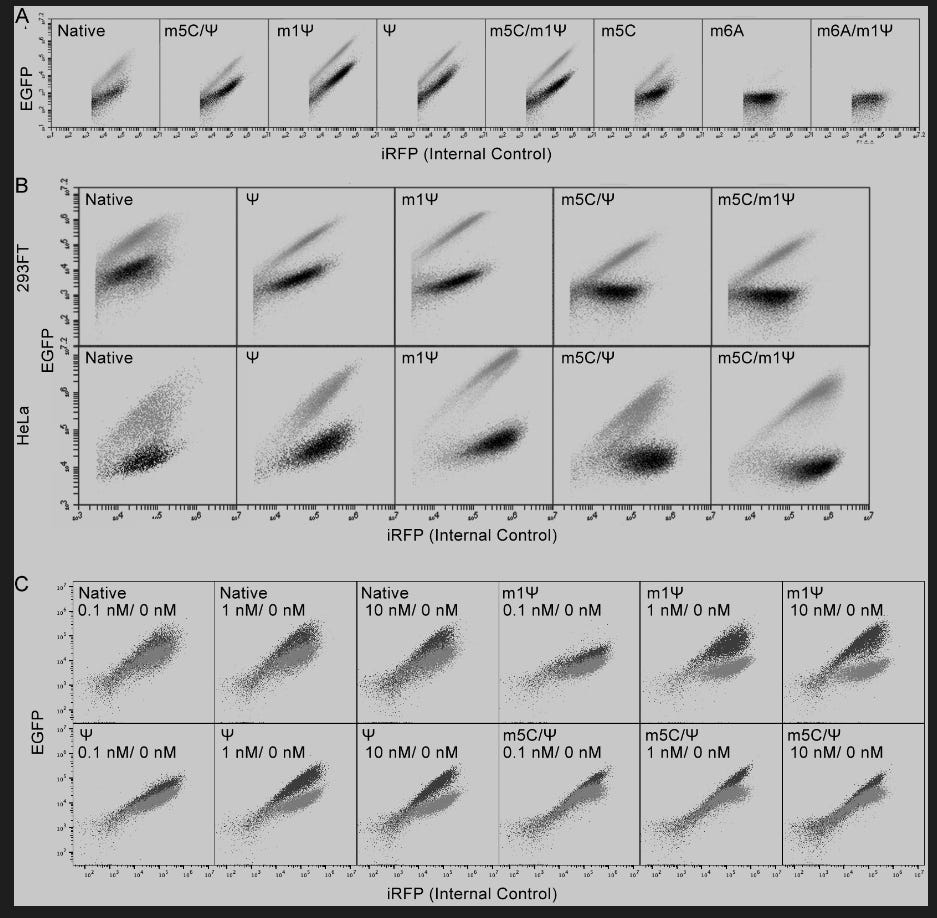
EGFP expression from m1Ψ-containing miR-21- or MS2CP-sensing switch was higher than that with other modified bases in both 293FT and HeLa cells (Supplementary Figure S2A, B).
Figure S2. (A) Representative dot plots showing the expression of EGFP from miR-21-5p-responsive EGFP mRNAs with different base modifications and the expression of iRFP from internal control iRFP mRNAs in 293FT cells. Gray dots show the expression from cells without co-transfection of miR-21-5p mimic; black dots show the expression from cells with co-transfection of miR-21-5p mimic. (B) Representative dot plots showing the expression of EGFP from MS2CP-responsive EGFP mRNAs with different base modifications and the expression of iRFP from internal control iRFP mRNAs in 293FT and HeLa cells. Gray dots show the expression from cells without co-transfection of MS2CP-coding mRNA; black dots show the expression from cells with co-transfection of MS2CP-coding mRNA. (C) Representative dot plots showing the expression of EGFP from miR-21-5p-responsive mRNA circuits (Fig. 4C) with different base modifications and the expression of iRFP from internal control iRFP mRNAs in 293FT cells. Gray dots show the expression from cells without co-transfection of miR-21-5p mimic; black dots show the expression from cells with co-transfection of miR-21-5p mimic.
A log scale on the right and ~20 fold increase in gene switching efficacy over conventional miR-21:
Figure S1. Characterization of mRNA switches. (A) Dose-response fold-change curve of conventional m5C/Ψ miR-21-5p-responsive switch in 293FT cells co-transfected with 1 pmol of miR-21-5p mimic or negative control mimic. Click Beetle Luciferase mRNA is co-transfected at reverse gradient concentrations to maintain a same total amount of transfected mRNAs among all samples. (B) Dose-response curve of MS2CP-responsive switch with MS2CP mRNA in 293FT cells. Relative switch expression is normalized to the condition without MS2CP mRNA. Results show the mean ± SD (n=3).
More:
https://academic.oup.com/nar/article/48/6/e35/5742781
Circadian rhythm disruption: clinical observations
This was a reverse study, but it does contain a passage of interest:
Association of sleep duration and quality with immunological response after vaccination against severe acute respiratory syndrome coronavirus‐2 infection (2022)
Although there are few studies concerning the effect of vaccination on sleep, their results seem noteworthy. Vaccination against Salmonella typhi in healthy volunteers raised interleukin‐6 levels 2 h after administration and led to disruptions during night‐time sleep and frequent awakenings, compared to those who were administered placebo (Sharpley et al., 2016). Other studies with vaccines against HAV or influenza virus Η1Ν1 in healthy adults proved that a night of sleep deprivation after vaccination is enough to observe a reduced immunity response against HAV at 4, 8, and 16 weeks later, as well as reduced H1N1‐specific antibodies 5 days after vaccination in males (Benedict & Cedernaes, 2021; Lange et al., 2011). The immunological difference between the two groups was observed even a year after vaccination (Lange et al., 2011).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9348328/
Long COVID: Disrupted sleep, fatigue common months after infection (2022)
Researchers at the Cleveland Clinic found that nearly two-thirds of people are fatigued, and about half experience sleep disruption months after an acute COVID-19 infection.
Moderate-to-severe sleep disruption is three times more common among Black people after recovering from COVID-19.
Anxiety is also linked to increased long COVID sleep disruption.
The study emphasizes the need to characterize race-specific determinants and disparities in COVID-19 survivors.
Trouble sleeping and fatigue are among the often-reported symptoms of the condition known as “long COVID.” New research from the Cleveland Clinic in Ohio presents the findings of researchers investigating sleep issues in people who have recovered from COVID-19.
According to the research, nearly half of those who recovered from COVID-19 experience at least moderate sleep issues.
The research was presented in June at Sleep 2022, a meeting of the Associated Professional Sleep Societies, a joint venture of the American Academy of Sleep Medicine (AASM) and the Sleep Research Society (SRS).
There is a risk of developing long COVID even for the vaccinated, and researchers suggest the condition can persist for years for some people.
The researchers analyzed the experiences of 962 Cleveland Clinic ReCOVer Clinic patients between February 2021 and April 2022. The individuals filled out the sleep disturbance and fatigue questionnaire sections of the National Institutes of Health’s Patient-Reported Outcomes Measurement Information System (PROMIS).
The clinic found that its Black patients were more than three times more likely to have moderate-to-severe sleep disturbances after recovering from COVID-19.
Another factor that was associated with a higher than average incidence of sleep disturbance was anxiety.
After factoring for age, race, sex, and body mass index, the analysis concluded:
After recovery from COVID-19, 41.3% of patients reported at least moderate sleep disturbances, and 8% described severe sleep issues.
More than two-thirds of patients (67.2%) reported moderate fatigue.
Lead study author Dr. Cinthya Pena Orbea tells Sleep 2022:
“Our study suggests that the prevalence of moderate to severe sleep disturbances is high and that [the] Black race confers increased odds to suffer from moderate to severe sleep disturbances, highlighting the importance to further understand race-specific determinants of sleep disturbances in order to develop race-specific interventions.”
Haematopoietic stem cell transplantation
A murine study investigating the contribution of sleep to the success of bone marrow stem cell transplantation.
A lack of sleep impairs HSC migration capacity:
Sleep disruption impairs haematopoietic stem cell transplantation in mice (2015)
Abstract
Many of the factors affecting the success of haematopoietic cell transplantation are still unknown. Here we show in mice that donor sleep deprivation reduces the ability of its haematopoietic stem cells (HSCs) to engraft and reconstitute the blood and bone marrow of an irradiated recipient by more than 50%. We demonstrate that sleep deprivation downregulates the expression of microRNA (miR)-19b, a negative regulator of the suppressor of cytokine signalling (SOCS) genes, which inhibit HSC migration and homing. Accordingly, HSCs from sleep-deprived mice have higher levels of SOCS genes expression, lower migration capacity in vitro and reduced homing to the bone marrow in vivo. Recovery of sleep after sleep deprivation restored the reconstitution potential of the HSCs. Taken together, this study provides insights into cellular and molecular mechanisms underlying the effects of sleep deprivation on HSCs, emphasizing the potentially critical role of donor sleep in the success of bone marrow transplantation.
https://www.nature.com/articles/ncomms9516
Melatonin
Melatonin may restore BMAL-1 levels to counter some of the pathologies, as well as being therapeutic itself via other signalling pathways:
Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival
(2019)
Abstract
The circadian rhythm is driven by a master clock within the suprachiasmatic nucleus which regulates the rhythmic secretion of melatonin. Bmal1 coordinates the rhythmic expression of transcriptome and regulates biological activities, involved in cell metabolism and aging. However, the role of Bmal1 in cellular- survival, signaling, its interaction with intracellular proteins, and how melatonin regulates its expression is largely unclear. Here we observed that melatonin increases the expression of Bmal1 and both melatonin and Bmal1 increase cellular survival after oxygen glucose deprivation (OGD) while the inhibition of Bmal1 resulted in the decreased cellular survival without affecting neuroprotective effects of melatonin. By using a planar surface immunoassay for PI3K/AKT signaling pathway components, we revealed that both melatonin and Bmal1 increased phosphorylation of AKT, ERK-1/2, PDK1, mTOR, PTEN, GSK-3αβ, and p70S6K. In contrast, inhibition of Bmal1 resulted in decreased phosphorylation of these proteins, which the effect of melatonin on these signaling molecules was not affected by the absence of Bmal1. Besides, the inhibition of PI3K/AKT decreased Bmal1 expression and the effect of melatonin on Bmal1 after both OGD in vitro and focal cerebral ischemia in vivo. Our data demonstrate that melatonin controls the expression of Bmal1 via PI3K/AKT signaling, and Bmal1 plays critical roles in cellular survival via activation of survival kinases.
More:
https://www.nature.com/articles/s41598-019-55663-0/#
Atherosclerotic Plaque Rupture
Multiple factors lead to plaque build-up and eventual breakaway for high risk individuals. “The straw that breaks the camel’s back” indeed:
Atherosclerotic Plaque Rupture
Identifying the Straw That Breaks the Camel’s Back (2016)
Introduction
The idiom “the straw that breaks the camel’s back” describes scenarios where seemingly minor incidents eventuate in a sudden, unexpected, and often detrimental downfall. In the case of atherosclerosis, over time multiple subclinical cellular events result in the development of unstable, vulnerable atherosclerotic lesions, which leads to the rupture of atherosclerotic plaques, culminating in the often catastrophic clinical manifestation of myocardial infarction or ischemic stroke. In this review, we first summarize lessons learned from autopsy studies, clinical investigations, and animal models mainly published in ATVB. We then present recent experimental studies published in ATVB that shed light on the underlying pathophysiological development of plaque instability and disruption, and how differential and sometimes maladaptive responses at cellular levels contribute to this complex process. These many publications in ATVB are a testament to the journal’s leading role in research on atherosclerotic plaque instability, an area of research with utmost translational relevance.
Although postmortem examinations provide invaluable information on histopathologic characteristics of vulnerable plaques, they are inherently limited by selection bias, and only lesions at the most advanced stage have been studied. Furthermore, they are snapshot in nature and cannot provide detailed information on the natural history of plaque progression. This deficiency is, in part, bridged by clinical studies that use intracoronary imaging in patients. In the PROSPECT study (The Providing Regional Observations to Study Predictors of Events in the Coronary Tree), 697 patients were followed up for 3.4 years, and multimodality intracoronary imaging was used to interrogate and characterize lesions in all 3 coronary vessels. Investigators have found that thin-cap fibroatheroma indeed confers higher risk of adverse outcomes (4.9% versus 1.3%; P<0.001). However, the fact that only 4.9% of these lesions eventually give rise to clinical events limits the clinical utility of the criterion of plaque vulnerability, as used in PROSPECT, as a risk-stratifying tool. In addition, this further highlights the knowledge gap and the need for a more mechanistic understanding and better identification of characteristics reflecting plaque vulnerability.
Cholesterol and Other Lipids Boosting Plaque Instability
Clinical studies have established a close link between low-density lipoprotein cholesterol and plaque instability.
Intraplaque Hemorrhage as a Consequence and Amplifier of Plaque Instability
Intraplaque hemorrhage is one of the cardinal histological features of vulnerable plaques and is thought to result either from microruptures with intraplaque bleedings or from leaky neovascularization within the plaque.
Platelets as Therapeutic Targets in Plaque Instability?
Platelets play a pivotal role in atherogenesis. Their binding to the endothelium precedes the appearance of leukocytes in plaques and, as such, platelets are early players in atherogenesis. Also infusion of activated platelets accelerates atherosclerosis. Furthermore, platelets are clearly involved in the often fatal event of thrombotic vessel occlusion occurring with plaque rupture.
Monocytes/Macrophages as Central Players in Plaque Instability
The atherosclerotic lesion burden has been demonstrated to be positively correlated with the number of circulating monocytes in mice and the presence of coronary artery disease in patients. Vulnerable, rupture-prone plaques have abundant monocytes/macrophages, particularly in the shoulder region of thin caps, indicating their causative role in plaque rupture and also rendering macrophages good drug targets.
Emerging Role of Lymphocytes in Plaque Instability
Multiple lymphocyte populations are found in vulnerable plaques. Natural killer T (NKT) cells, which bridge the gap between innate and adaptive immunity, accumulate in human rupture-prone shoulder regions of vulnerable plaques, together with dendritic cells.
Artery Tertiary Lymphoid Organs: A Future Focus of Plaque Instability
It has been known for >30 years that adventitial immune cell infiltrates are associated with advanced human atherosclerotic plaques, with structural organizations that suggest generation of local humoral responses. In ruptured plaques, the infiltrates seem to be greater in number than in nonruptured human atherosclerotic plaques, suggesting involvement in the pathology of plaque instability.
Summary
It is easy to see the breaking of the camel’s back, as it is typically an obvious, final, and dramatic scene that catches everyone by surprise. Yet it is difficult to see the straw itself and to understand all the strain that silently builds up and contributes to the final event. Similarly, the rupture of unstable atherosclerotic plaques is a complex process ultimately representing a maladaptive response to hemodynamic conditions, lipid accumulation, arterial injury, necrosis, and inflammation. Growing understanding of the many facets of this pathological process offers multiple opportunities for early detection of unstable plaques with biomarkers and novel molecular imaging technologies, and hopefully will ultimately lead to the development of utterly needed plaque-stabilizing drugs.
https://www.ahajournals.org/doi/10.1161/ATVBAHA.116.307993
Conclusion
There is much more in the full review, but in summary it would be advisable to avoid any agents that may elevate homologous oncogenic miR-21 and other miRNAs for a sustained period of time, repeatedly, especially if you have co-morbidities like cancer or atherosclerosis.
For balance, the COVID-19 viral infection may also present similar pathologies to these groups. However, post transfection the risk of infection is enhanced after around 3 months according to multiple studies, hence there is a double benefit to restricting your exposure to specific transfection agents.












As always, Brainy Doorless Carp,THANK YOU!
I know about miRNA and breast cancer as cited below.
https://pubmed.ncbi.nlm.nih.gov/18812439/MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis
Uh, oh. the CLOCK. I am up all hours struggling with all of this hard, hard science.
I also wanted you to know that I know some one who definitely had a number of small strokes - lacunas- and not one of them was in the morning. Anomalies?
Advice for cancer patients, please. Avoid the jabs. Watch the CLOCK. And what else? Thank you, Jennifer, for the study about polyphenols, and the importance of pomegranate!
Thank you.!!!!!
P.S. In reference to the cartoon, Walter Chesnut was just discussing SADS, and how young people can have the hearts of an old person.
https://rumble.com/v1y5rak-streaming-with-walter-chesnut-sudden-adult-death-syndrome.html
START AT TWENTY-NINE MINUTES
I'm on a small, international (non-science) team collecting obit data. Dropping like flies age 0-75 (we don't collect 75+). Astonishing. Can't keep up with the obits. The obit writers can't keep up (we can tell by the quality of the obits). Sudden deaths; sudden and lethal cancers; sudden everything. And shocked/grieving families are gently coerced into asking for donations to pharma-funded "foundations"...