Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #30: Immunoglobulin class switching to IgG4
Updates:
30th December ‘22:
Contents page - Hat tip to Christopher; LNPs; contribution by Dr Christina Parks.
3rd January ‘23:
Synopsis added. Soluble FAS ligand and oncology considered.
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
If received via email I recommend clicking on the hyperlinked title to read the latest correctly formatted version in full, in a browser. Unfortunately it is not possible to email out revised versions.
Contents:
Lipid Nanoparticles and Cytokines
Double Strand Breaks, P53 & BRCA1
Induction of DSBs by mRNA vaccines
Synopsis
Repeated doses of experimental mRNA gene transfection agents, especially in those reinfected, induce antibody class switching to relatively high levels of a non-neutralizing, anti-inflammatory immunosuppressive type, IgG4.
Inflammatory signalling pathways, cytokines, microRNAs and other factors such as sFAS are involved in this process and are discussed.
As class switching involves induction of DNA double strand breaks during V(D)J recombination in an environment of agent induced depletion of tumor suppressors P53 & BRCA, DSB repair may fail.
This leads to genomic instability and potentially cancers including lymphoma, typically with a lead time of 2-12 years.
And some cancer cell types will be more prone to undergo tumorigenesis and metastasis if relatively high levels of IgG4 antibodies persist, ie higher grade and more aggressive.
Introduction
As, quite rightly, almost every biomed author on Substack is reviewing this paper I will not be re-inventing the wheel but instead focusing on areas that have so far received less attention and are in keeping with the series of Substacks started a year ago: signalling pathways involved and aspects of immunology relating to consequential carcinogenesis.
For anyone not familiar with the back story here yet, Jessica Rose does a great job reviewing this recently published peer reviewed paper:
A paper was published in Science Immunology on December 22, 2022 entitled: “Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination”1 and it explains in wonderful detail how a class of antibody that commands a non-inflammatory response (more like tolerizing) is prominent in people who have been repeatedly injected with the modified mRNA COVID-19 injectable products.
Translation: Instead of the intended pool of spike-specific neutralizing IgG antibodies being dominant in multiply-injected people, a pool of antibodies associated with spike-specific tolerance are dominant in multiply-injected people.
Besides the tolerizing capacity, they also showed that the phagocytic enabling capacities were much reduced overall. These activities lead to clearance of viral pathogens. Reduce them → reduction in viral clearance capacity.
More:
In a follow-up Substack about IgG4 related disease and fibrosis, Jessica discusses consequential autoimmune disorders including forms of vascular disease and how these are already being recorded on VAERS:
I reviewed vasculitis research previously:
Baicalin is of therapeutic value here by selectively regulating follicular regulatory T cell differentiation, thus suppressing autoimmune reactivity and helping to reduce the symptoms of systemic lupus erythematosus (SLE).
Yang et al (2020) investigated1:
Abstract
Baicalin is a natural compound isolated from Chinese herb, which has been reported as an anti-inflammatory drug. Here, we demonstrated that Baicalin treatment could reduce urine protein, inhibit anti-ds-DNA antibody titers, and ameliorate lupus nephritis in MRL/lpr lupus-prone mice. Baicalin inhibited Tfh cell differentiation and IL-21 production, but promoted Foxp3+ regulatory T cell differentiation including part of follicular regulatory T (Tfr) cells. Intravenous injection of Baicalin-induced Foxp3+ regulatory T cells could relieve nephritis, inhibit Tfh cell differentiation and IL-21 production. Baicalin inhibited mTOR activation, reduced mTOR agonist-mediated Tfh cell expansion and increased Tfr cells. These data suggest that Baicalin attenuates lupus autoimmunity by up- and downregulating the differentiation of Tfr cells and Tfh cells, respectively. Baicalin and ex vivo expanded Foxp3+ regulatory T cells are promising therapeutics for the treatment of lupus.
It is also of value in preventing and treating vascular disease such as atherosclerosis2.
Further reading:
MicroRNA’s and Cytokines
In pathophysiology, signalling pathways can affect several different cellular processes downstream, leading to different pathological outcomes from a single causative agent. Our job gets even more complicated as this often involves feedback mechanisms and crosstalk.
In this case various inflammatory cytokines get upregulated by mRNA transfection and one microRNA (miRNA) can affect several genes or a single gene can have its expression promoted or inhibited by more than one miRNA.
Of relevance here, various interleukins and the “nasty cytokine”, tumor growth factor beta (TGF-β) are involved or a consequence of class switching to IgG4. Different research papers may focus on a different one, but IL-4, IL-10 and IL-13 are cited most often.
Garavelli et al (2018)3 reviewed the subject: The Multifaceted Interface Between Cytokines and microRNAs: An Ancient Mechanism to Regulate the Good and the Bad of Inflammation:
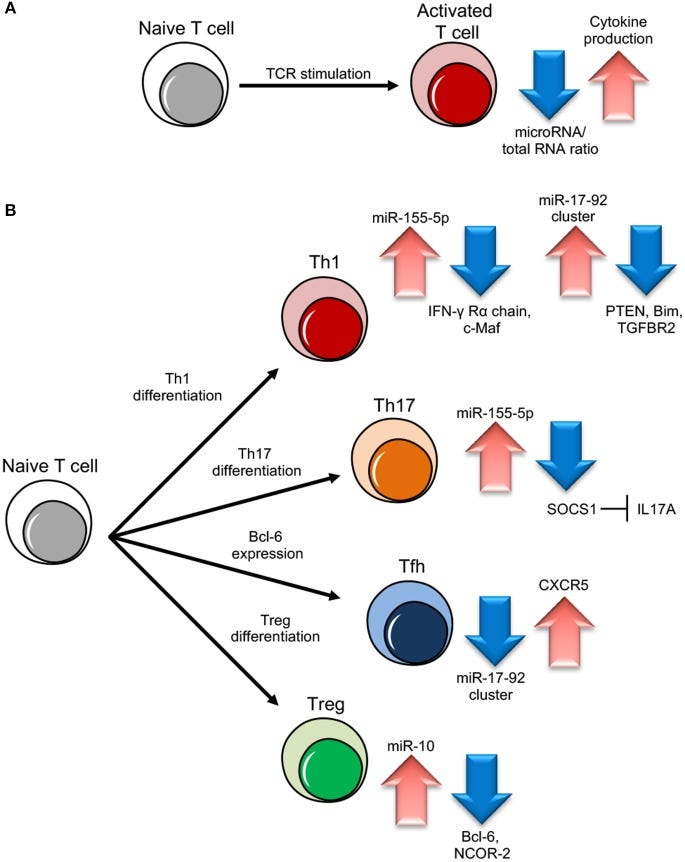
MicroRNAs (miRNAs) are evolutionary conserved small non-coding RNA molecules that affect gene expression by binding to target messenger RNAs and play a role in biological processes like cell growth, differentiation, and death. Different CD4+ T cell subsets such as Th1, Th2, Th17, and T regulatory cells, exert a distinct role in effector and regulatory-type immune responses. miRNAs have been shown to respond to dynamic micro-environmental cues and regulate multiple functions of T cell subsets including their development, survival and activation. Thus, miRNA functions contribute to immune homeostasis, on the one side, and to the control of immune tolerance, on the other. Among the most important proteins whose expression is targeted by miRNAs, there are the cytokines, that act as both key upstream signals and major functional outputs, and that, in turn, can affect miRNA level. Here, we analyze what is known about the regulatory circuit of miRNAs and cytokines in CD4+ T lymphocytes, and how this bidirectional system is dysregulated in conditions of pathological inflammation and autoimmunity.
miRNA role in different T cell subset differentiation. (A) Upon naive T cell activation, increase in cytokine production is dependent on miRNA/total RNA ratio decrease. (B) During Th1 cell differentiation, up-regulation of miR-155-5p and miR-17-92 results in suppression of IFN-γ Rγ chain and c-Maf and PTEN on one side and Bim and TGFBR2 on the other, important to block Th2 differentiation and unlock cell proliferation respectively. During Th17 differentiation, miR-155-5p induction leads to SOCS1 inhibition, which in turn unleashes IL17A production. During follicular helper T cells (Tfh) cell differentiation, CXCR5 up-regulation, important for migration into follicles, is dependent on miR-17-92 cluster downregulation. During Treg cell differentiation, the increase of miR-10 expression blocks the expression of Bcl-6 (Thf differentiation) and NCOR-2 (Th17 maturation).
Th17 is discussed here:
miRNAs are “rheostats,” capable to fine-tune mammalian gene expression. Single miRNAs may only marginally regulate target genes but, when the cell responds to environmental changes, the coordinated modulation of tens of miRNAs altogether is a powerful strategy to efficiently affect many components of a genetic network.
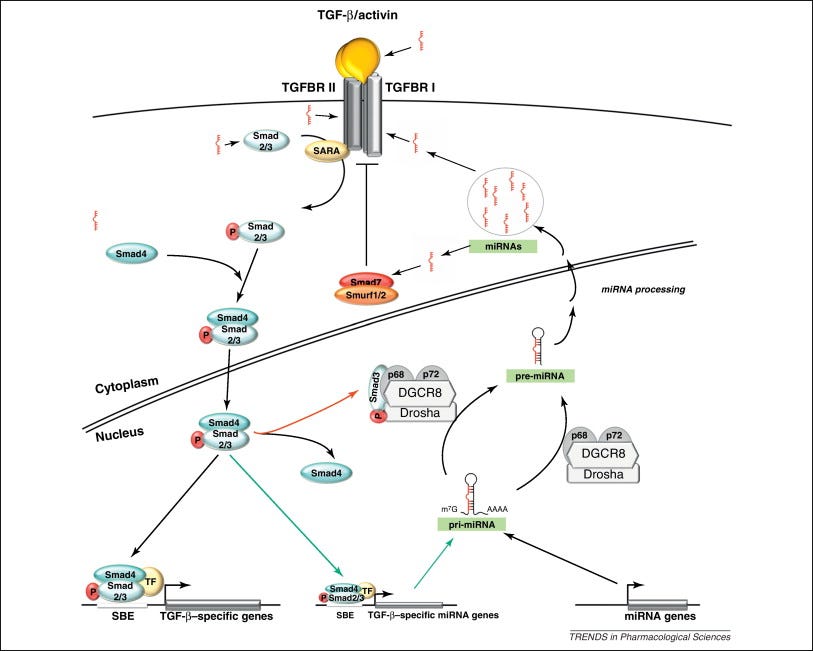
This is an excellent reference from 2021 by RF Fujii:
Quantum microRNA Assessment of COVID-19 RNA Vaccine: Hidden Potency of BNT162b2 SASR-CoV-2 Spike RNA as MicroRNA Vaccine4.
They analysed which miRNAs are being upregulated:
Also see:
And:
TGF-β is elevated in part due to spike protein binding to receptors of and suppression of ACE2 (Carvacho and Piesche, 2021)5. Regardless of source, the effect is the same:
As previously mentioned, infection with SARS‐CoV‐2 reduces the expression of ACE2. 23 That seems counterproductive since ACE2 appears to be the main receptor that the virus uses to infect cells. Nevertheless, a reduced expression of ACE2 in COVID‐19 patients could be the potential source for TGF‐β. ACE2 usually catalysed Angiotensin II (AngII) to Ang1‐7. Ang1‐7 is a biological active peptide that binds and activates the Mas receptor. This ACE2/Ang1‐7/Mas pathway counterbalances RAAS (renin, angiotensin, aldosterone system), promoting the activation of anti‐inflammatory pathways. 30 Reduced ACE2 leads to an overactive RAAS, which provoked a local vascular inflammation and, through aldosterone, activates TGF‐β production.
In 2010, Nakashima et al published a paper making the connection between these cytokines and IgG4-related tubulointerstitial nephritis6, a frequent cause of acute kidney injury (AKI)7.
It is paywalled so I will post the short abstract instead:
Abstract
Background: IgG4-related tubulointerstitial nephritis (TIN) shows characteristic serum IgG4 elevation and increased IgG4-positive plasma cells in the renal interstitium, and inclusion of TIN as an IgG4-related systemic disease has been suggested. IgG4 is the rarest IgG subclass and is a Th2-dependent isotype with low affinity for target antigen. Although the pathogenesis of this disease has not been elucidated, positive serum immune complex and hypocomplementemia in some patients with this disease suggest that immune complex mechanisms are involved in the causation of this disease.
Method: We selected 20 cases of histological diagnosed TIN. These cases were etiologically different and included 4 cases of IgG4-related TIN. We extracted RNA from paraffin embedded biopsied kidney and evaluated expression levels of various cytokines for each case by real time PCR.
Results: Comparison of cytokine production patterns among different disease-associated TINs revealed that IgG4-related TIN exhibited a quite distinct pattern. On the one hand, there was no expression of IL-2, IFN-gamma IL-17 and IL-6, whereas production of IL-4, IL-10 and TGF-beta was, on the other hand, remarkably increased in IgG4-related TIN.
Conclusion: Based on these cytokine production results, Th2 and Treg appear to play a central role in IgG4-related TIN.
Next I performed a literature search to cross check the 3 main PULS blood markers discussed in the last Substack for any correlations with IgG4.
With these experimental gene agents, for every pathology encountered you will sometimes get another related one "for free" due to overlapping signalling pathways, so its always worth cross referencing.
As you can imagine this will also complicate diagnosis and treatment, unless you are addressing the root cause.
As a reminder, HGF increased 205% over the pre-vac baseline, IL-16 by 234% and sFas by 209%.
HGF was not associated in any research papers (though this doesn’t exclude the possibility.)
IL-16 appears to downregulate IL-13, which should have a negative effect on IgG4 levels but research by Bassam et al (2006) didn’t discuss direct cause and effect8.
No effect of IL-16 was observed on IL-4 mRNA expression. Treatment with IL-16 resulted in increased levels of IL-10 and IL-18 in allergen-stimulated cell culture. Neutralization of IFN-γ, IL-12, IL-10 or IL-18 did not alter the inhibitory effects of IL-16 on IL-13 and IL-5 secretion by allergen-stimulated PBMC. IL-16 did not modify IL-13 synthesis by anti-CD3-stimulated CD4+ T cells, but it significantly reduced the production of IL-5. These data suggest that IL-16 may play an important immunoregulatory role in allergic states in response to allergen.
Punnonen et al (1993) conducted an in vitro study: Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells9:
The present study demonstrates that IL-13 induces IgG4 and IgE synthesis by human B cells. IL-13-induced IgG4 and IgE synthesis by unfractionated peripheral blood mononuclear cells and highly purified B cells cultured in the presence of activated CD4+ T cells or their membranes. IL-13-induced IgG4 and IgE synthesis is IL-4-independent, since it was not affected by neutralizing anti-IL-4 monoclonal antibody. Highly purified, surface IgD+ B cells could also be induced to produce IgG4 and IgE by IL-13, indicating that the production of these isotypes reflected IgG4 and IgE switching and not a selective outgrowth of committed B cells. IL-4 and IL-13 added together at optimal concentrations had no additive or synergistic effect, suggesting that common signaling pathways may be involved.
Soluble FAS and elevated IgG4
Soluble FAS ligand was found to be associated with elevated IgG410 by van Asten et al (2021), yet another factor to consider as this will also be elevating the risk of developing IgG4 related autoimmune disorders:
We performed a screen using a secreted protein library to identify novel factors that promote this process and may be used to combat undesired Ab formation. We tested the differentiating capacity of 756 secreted proteins on human naive or memory B cell differentiation in a setting with suboptimal T cell help in vitro (suboptimal CD40L and IL-21). High-throughput flow cytometry screening and validation revealed that type I IFNs and soluble FAS ligand (sFASL) induce plasmablast differentiation in memory B cells. Furthermore, sFASL induces robust secretion of IgG1 and IgG4 Abs, indicative of functional plasma cell differentiation. Our data suggest a mechanistic connection between elevated sFASL levels and the induction of autoreactive Abs, providing a potential therapeutic target in autoimmunity. Indeed, the modulators identified in this secretome screen are associated with systemic lupus erythematosus and may also be relevant in other autoimmune diseases and allergy.
From 2001, Song et al published the paper: Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer11.
They took biopsies from 12 patients with colon adenocarcinoma and assessed for serum sFasL, apoptosis of peripheral mononuclear cells, immunohistochemical detection of FasL protein, apoptosis assessment of tumour-infiltrating lymphocytes, primary cell culture of colon cancer, western blot analysis for sFasL in supernatants, sFasL-induced apoptosis in Jurkat cell line and serum FasL and apoptosis of peripheral blood lymphocytes.
They concluded that soluble FAS ligand assists immune evasion of colon cancer cells. This together with sFasL mediated elevation of immunosuppressive IgG4 would lead to a poor prognosis:
Serum levels of sFasL were significantly elevated in all colon cancer patients with mFasL expression in tumour tissues (n = 8). In these patients, the number of apoptotic lymphocytes was significantly increased within tumour and peripheral blood. Furthermore, sFasL was present in the corresponding supernatants and induced apoptosis of Jurkat cells in a dose-dependent manner. These findings suggest that mFasL-positive colon cancer cells release sFasL, and thus may induce apoptosis of host lymphocytes as a potential mechanism for immune evasion.
From 2004 by Kim et al, its not just colon cancer: The role of Fas ligand and transforming growth factor β in tumor progression12:
Despite the fact that expression of Fas ligand (FasL) in cytotoxic T lymphocytes (CTLs) and in natural killer (NK) cells plays an important role in Fas-mediated tumor killing, During tumor progression FasL-expressing tumor cells are involved in counterattacking to kill tumor-infiltrating lymphocytes (TILs). Soluble FasL levels also increase with tumor progression in solid tumors, and this increase inhibits Fas-mediated tumor killing by CTLs and NK cells.
Unfortunately researchers haven’t yet resolved all the signalling pathways involved with sFasL, which is unfortunate considering the mass scale of the rollout of experimental gene transfection agents.
From 2020:
Furthermore, it is clear that soluble factors like Tfh cytokines IL-21 and IL-4 are key Tfh cytokines for effective B cell differentiation and that IFN-γ promotes, among other things, migration to inflamed tissue 11,14,15. Yet, several other secreted proteins such as IL-10 and type I IFNs affect B cell differentiation in humans, indicating that the role of soluble factors to modulate this process is underexplored16–18.13
Lipid Nanoparticles and Cytokines
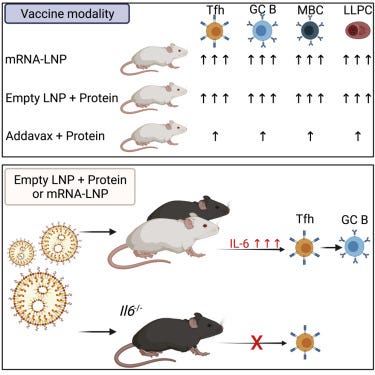
LNPs are known to act as adjuvants to mRNA transfection agents. A paper from 2021 by Alameh et al cites interleukin-6 induction as being upregulated significantly14. Could IL-6 be inducing class switching to IgG4?
Summary
Adjuvants are critical for improving the quality and magnitude of adaptive immune responses to vaccination. Lipid nanoparticle (LNP)-encapsulated nucleoside-modified mRNA vaccines have shown great efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but the mechanism of action of this vaccine platform is not well-characterized. Using influenza virus and SARS-CoV-2 mRNA and protein subunit vaccines, we demonstrated that our LNP formulation has intrinsic adjuvant activity that promotes induction of strong T follicular helper cell, germinal center B cell, long-lived plasma cell, and memory B cell responses that are associated with durable and protective antibodies in mice. Comparative experiments demonstrated that this LNP formulation outperformed a widely used MF59-like adjuvant, AddaVax. The adjuvant activity of the LNP relies on the ionizable lipid component and on IL-6 cytokine induction but not on MyD88- or MAVS-dependent sensing of LNPs. Our study identified LNPs as a versatile adjuvant that enhances the efficacy of traditional and next-generation vaccine platforms.
Next to cross-reference the literature for IL-6 to IgG4 interactions.
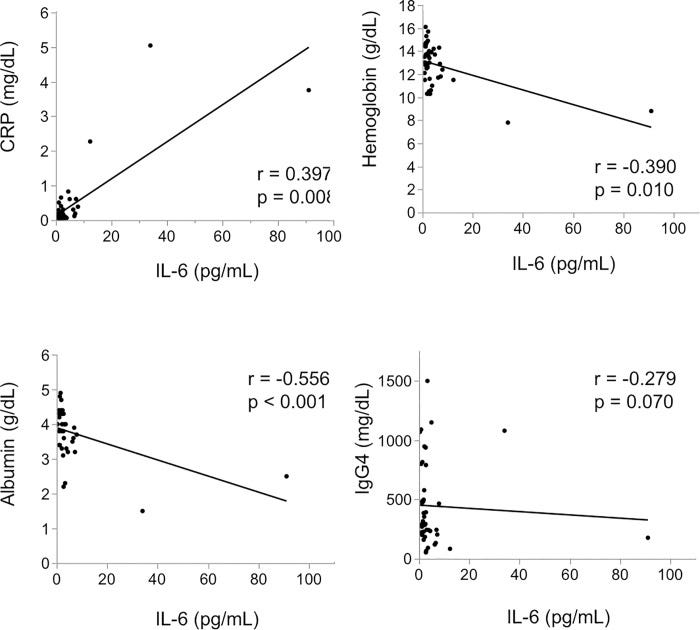
In 2020, Tsukuda et al examined the clinical picture at the onset of 43 patients who were diagnosed with IgG4-RD in their hospital and were measured for serum IL-6 prior to steroid treatment: Clinical implications of elevated serum interleukin-6 in IgG4-related disease.15.
If there is a correlation its appears to be secondary to inflammation rather than direct cause-effect.
Results
The median level of serum IL-6 was 2.2 pg/mL. There was a significant correlation between IL-6 and C-reactive protein (CRP) level (r = 0.397, p = 0.008), hemoglobin level (r = -0.390, p = 0.010) and albumin level (r = -0.556, p < 0.001). When 43 patients were divided into two groups by using a cut-off IL-6 of 4 pg/mL, the high IL-6 group showed higher age, lower albumin, higher CRP and higher aspartate aminotransferase (AST) (age p = 0.014, albumin p = 0.006, CRP p <0.001, AST p = 0.009). Hepatic swelling and splenomegaly were significantly more prevalent in the high IL-6 group than it was in the low IL-6 group (liver p < 0.001, spleen p = 0.020). Biliary tract involvement tended to admit more in the high IL-6 group (p = 0.060).
Conclusion
Serum IL-6 level at the onset of IgG4-RD may be significantly correlated with clinical inflammatory parameters and it may also be associated with involvement of the bile duct, liver, and spleen.
There was no significant correlation between IL-6 level and IgG4 level (r = -0.279, p = 0.070).
Regarding possible indirect association and crosstalk, Gupta et al (2018) did find a synergistic association between IL-6 and IL4 that can promote tumor progression and metastasis 16:
IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors
Here we explored how cytokines of the tumor milieu, interleukin (IL)-6 and IL-4, interact to influence target gene expression in primary human monocyte-derived macrophages (hMDMs). We show that dual stimulation with IL-4 and IL-6 synergistically modified gene expression.
…Collectively, our findings suggest that IL-4 and IL-6 cooperate to alter the human macrophage transcriptome, endowing hMDMs with pro-tumorigenic properties.
In summary, our study provides evidence for a pro-tumorigenic polarization of hMDMs by IL-4/IL-6. Our data support the central role of STAT3 as a transcription factor driving IL-6-elicited alterations of the macrophage transcriptome and reveal a novel role of BATF transcription factor in shaping the transcriptional response of IL-4/IL-6 stimulated macrophages, suggesting its potential importance as a target to suppress pro-tumorigenic properties of TAMs.
Double Strand Breaks, P53 & BRCA1
Unrepaired DSB’s are a source of genomic instability ie potential tumorigenesis17:
DNA double strand breaks (DSBs) are considered the most cytotoxic type of DNA lesion. They can be introduced by external sources such as ionizing radiation (IR), by chemotherapeutic drugs such as topoisomerase poisons and by normal biological processes such as V(D)J recombination. If left unrepaired, DSBs can cause cell death. If misrepaired, DSBs may lead to chromosomal translocations and genomic instability. One of the major pathways for the repair of IR-induced DSBs in mammalian cells is non-homologous end-joining (NHEJ).
Think of P53 as being like a piercer in a Victorian cotton mill, looking out for broken threads and repairing them as quickly as possible:
Spike protein impairs the repair of DSB’s by suppressing this “guardian of the genome” by penetrating the nucleus and inhibiting P53 and BRCA protein expression.
The paper reviewed here was later retracted, but I have it on good authority that this was not at the request of the authors, and not for fraud or using experimental techniques that haven’t been peer reviewed as appropriate before, but because their choice of words in the conclusions were not approved by Corporate:
Retraction to: Cellular & Molecular Immunology 10.1038/s41423-020-0424-9, published online 7 April 2020
The authors have retracted this article1. After the publication of this article, it came to the authors attention that in order to support the conclusions of the study, the authors should have used primary T cells instead of T-cell lines. In addition, there are concerns that the flow cytometry methodology applied here was flawed. These points resulted in the conclusions being considered invalid.
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #2: SARS–CoV–2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency/comments
Note how they used known DSB initiators such as gamma radiation for comparison and full length transfected spike protein had a greater effect at inhibiting repair than if it has been cleaved to S1 & S2.
Unfortunately, they found that full length SP was in the nucleus in their study:

To determine how the spike protein inhibits both NHEJ and HR repair pathways, we analyzed the recruitment of BRCA1 and 53BP1, which are the key checkpoint proteins for HR and NHEJ repair, respectively. We found that the spike protein markedly inhibited both BRCA1 and 53BP1 foci formation (Figure 3D–G). Together, these data show that the SARS–CoV–2 full–length spike protein inhibits DNA damage repair by hindering DNA repair protein recruitment.
Discussion/conclusions:
…This suggests that the use of antigenic epitopes of the spike as a SARS–CoV–2 vaccine might be safer and more efficacious than the full–length spike. Taken together, we identified one of the potentially important mechanisms of SARS–CoV–2 suppression of the host adaptive immune machinery. Furthermore, our findings also imply a potential side effect of the full–length spike–based vaccine. This work will improve the understanding of COVID–19 pathogenesis and provide new strategies for designing more efficient and safer vaccines.
A physiological assessment from 2015 by Björkman et al: Aberrant recombination and repair during immunoglobulin class switching in BRCA1-deficient human B cells concluded that BRCA1 is also important to avoid the risk of genomic instability resulting from failed NHEJ during CSR18.
If both BRCA and P53 are depleted as per the previous paper then this could further ratchet up the risk of lymphoma:
The tumor-suppressing activity of BRCA1 has mainly been attributed to its roles in HR and checkpoint control (9, 10). In light of our findings here, the involvement of BRCA1 in regulating the NHEJ pathway may also contribute to its cancer-preventing function. The importance of efficient repair through NHEJ, especially in lymphocytes, becomes evident when studying mice double deficient for p53 and any of the c-NHEJ factors, as most of them develop aggressive lymphomas that often harbor translocations involving the Ig loci (51). Moreover, we have previously shown that DNA repair genes are frequently mutated in human B-cell lymphomas, and NHEJ mutations are associated with IgH translocations in these tumors (52). With the contribution of BRCA1 to both HR and NHEJ, two repair pathways that are important for the maintenance of genome stability and resolution of AID-induced DSBs during CSR, BRCA1 could function as a tumor suppressor in mature B cells. Accordingly, one of the most common tumors observed in BRCA1-deficient mouse models is lymphoma (20).
Induction of DSBs by mRNA vaccines
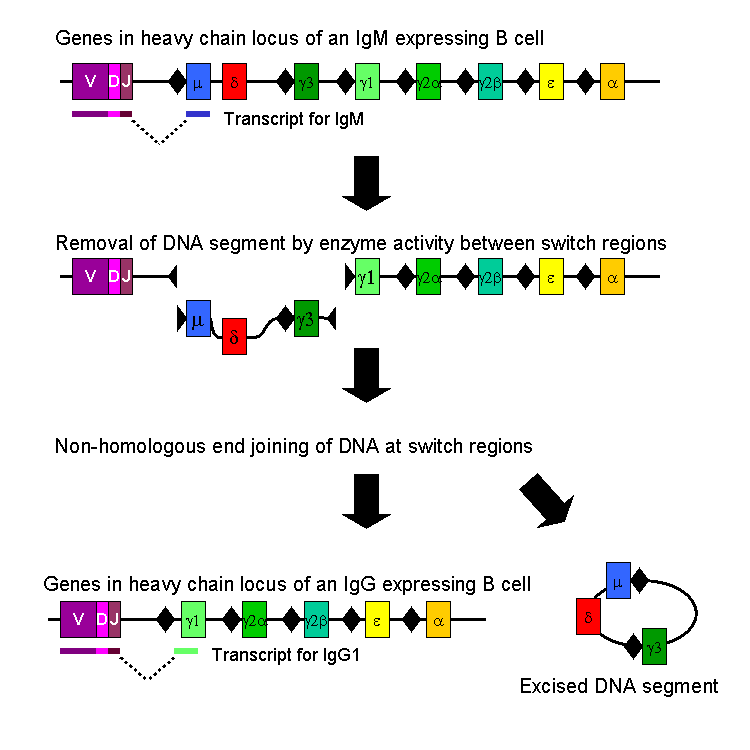
In the paper of interest by Irrgang et al (2022) Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination19 the authors discussed how IL-4 and IL-10 are inducing the class switching via activation of the enzyme AID.
Activation-induced cytidine deaminase (AID) is the enzyme that catalyzes SHM in antibody variable (V) regions. It is expressed in GC B cells and also mediates class switch recombination (CSR) of constant (C) region genes (18, 19). IgG3 encoded by the γ3 C-region is the most upstream (5′) Cγ-region in the immunoglobulin heavy chain gene locus on chromosome 14 (Fig. 1A). Ongoing activity of AID can lead to switching towards more downstream Cγ regions, i.e. γ1, γ2 and γ4, which encode IgG1, IgG2 and IgG4 (20, 21). CSR is highly regulated during an immune response. The C-region to which a B cell switches is modulated by cytokines and B-cell activators at the level of transcription of non-rearranged heavy chain constant genes.
Further research is warranted to find the specific mechanisms, but they suspect persistent expression or presence of antigens in the lymph nodes. Alterations to the T follicular helper cells was another possibility (hence the reference to Baicalin earlier):
Furthermore, we observed significantly higher IgG4 levels after two doses of Comirnaty mRNA vaccine compared to a heterologous immunization regimen with a primary Vaxzevria vaccination followed by one dose of Comirnaty, although the total anti-spike IgG response was comparable. This argues against the hypothesis that repeated exposure to the spike protein itself triggers the unusual IgG4 response. It is currently not clear whether or to what extent the Comirnaty mRNA vaccination or the short interval of immunizations are responsible for the observed long-lasting GC reactions (12–14), but a prolonged presence of vaccine mRNA or antigen in the lymph node might be a potential explanation (12). Furthermore, a robust and persistent T follicular helper cell (TFH) response for up to six months after mRNA vaccination has been described in draining lymph nodes (17), which might be involved in the regulation of CSR by recurrent interactions of GC B cells with TFH cells.
AID at work. Going back to the grim cotton mill analogy, its as if you have taken the thread, pinched a loop, trimmed it off and re-joined the ends20:
Where this becomes problematic is if you get “off-target” DSB formation. Its like someone running amok in the mill with a pair of scissors whilst the piercers (P53) are off duty.
And P53 has already been suppressed by full length spike protein in the nucleus. Read on…
Lymphoma and Sarcoma
Robbiani at al (2009) performed a murine study to investigate AID, DSBs and lymphoma:
AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations21
Summary
Cancer initiating translocations such as those associated with lymphomas require the formation of paired DNA double-strand breaks (DSBs). Activation-induced cytidine deaminase (AID) produces widespread somatic mutation in mature B cells; however the extent of “off-target” DSB formation and its role in translocation-associated malignancy is unknown. Here we show that deregulated expression of AID causes widespread genome instability, which alone is insufficient to induce B cell lymphoma; transformation requires concomitant loss of the tumor suppressor p53. Mature B cell lymphomas arising as a result of deregulated AID expression are phenotypically diverse and harbor clonal reciprocal translocations involving a group of Immunoglobulin (Ig) and non-Ig genes which are direct targets of AID: this group includes miR-142, a previously unknown micro-RNA target which is translocated in human B cell malignancy. We conclude that AID produces DSBs throughout the genome, which can lead to lymphoma associated chromosome translocations in mature B cells.
B cell lymphoma
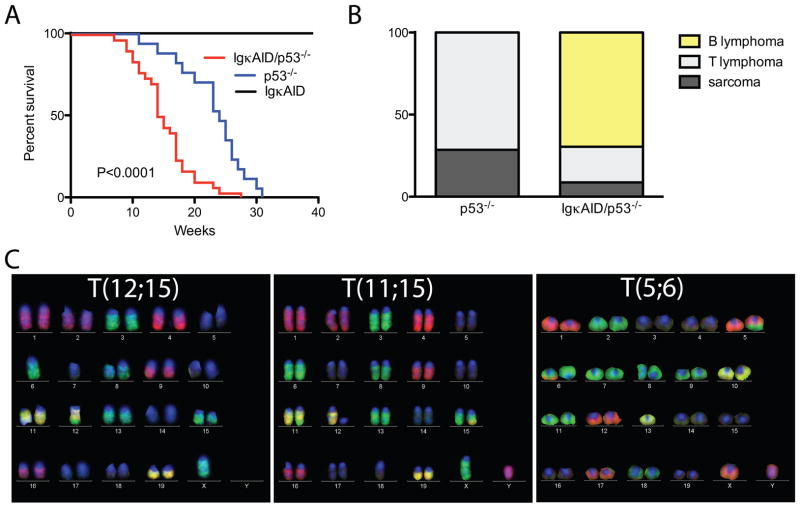
Despite high levels of c-myc/IgH translocations in stimulated B cells expressing deregulated AID, B cell lymphomas did not develop in IgκAID or in three other previously reported strains of AID transgenic mice (Figure 3A and (Muto et al., 2006; Okazaki et al., 2003; Shen et al., 2008)). All eight of the IgκAID mice survived beyond 40 weeks (Figure 3A). One potential explanation for this unexpected result is that B cells are protected from AID mediated DNA damage induced transformation by gatekeepers such as p53, which trigger cell cycle arrest or apoptosis (Bassing et al., 2003; Green and Kroemer, 2009; Kruse and Gu, 2009; Lowe et al., 2004; Ramiro et al., 2006a). Consistent with this idea, gatekeeper genes, or genes that control them such as Bcl6 (Phan and Dalla-Favera, 2004), are frequently deleted or mutated in mature human B cell lymphomas (Kuppers, 2005).
(A) Survival curve for n=8 IgκAID, n=17 p53−/− and n=30 IgκAID/p53−/− mice. The P value refers to the difference between IgκAID/p53−/− and p53−/− (log-Rank test). The median survival for IgκAID/p53−/− is 14.5 weeks, for p53−/− it is 24 weeks. (B) Phenotype of tumors at necropsy (n=7 p53−/− and n=23 IgκAID/p53−/−). (C) Representative M-FISH images of reciprocal translocations found in B cell lymphomas of IgκAID/p53−/− mice.
To determine whether p53 is protective against AID-induced B cell lymphomas in vivo we produced IgκAID/p53−/− mice. In contrast to IgκAID mice, all 30 of the IgκAID/p53−/− mice died within 28 weeks and they succumbed more rapidly to malignancy than p53−/− controls (P<0.0001, Figure 3A and 3B). In addition, tumors that developed in IgκAID/p53−/− mice differed from those in the p53−/− controls: the former were predominantly mature B cell lymphomas, whereas the p53−/− controls developed thymic lymphomas and sarcomas (Figure 3B and (Jacks et al., 1994)).
In summary we have at least 2 mechanisms that may lead to increased risk of developing lymphomas and sarcomas, with decreased survival time:
Upregulation of AID expression and consequential DSBs via cytokine signalling.
Downregulation of the tumor suppressors P53 & BRCA1, concurrent with this.
There are 2 potentially pro-tumorigenic aspects to mRNA vac induced class switching. The latter could be classed as “initiation” via induction of DSBs & oncogenic mutations.
The second could be classed as “immunosuppression” and will be discussed next.
The first was published by Kinker et al in 2021 and correlates high levels of IgG3 with prolonged survival in patients with somatic hypermutation (SHM)22
Plasma cells generated within tumor-associated TLSs may reside locally and produce significant amounts of tumor-specific antibodies, as shown for breast cancer (Coronella et al., 2002; Pavoni et al., 2007), melanoma (Erdag et al., 2012), non-small cell lung cancer (Lohr et al., 2013; Germain et al., 2014), and high-grade serous ovarian cancer (Montfort et al., 2017). Importantly, the isotype and specificity of such antibodies can drive distinct immune responses (Sharonov et al., 2020). The IgG1 antibody class is of primary importance for anti-tumor cytotoxic responses, as these antibodies can bind to Fcγ receptors and trigger antibody-dependent cellular cytotoxicity and phagocytosis, complement activation, and enhance antigen presentation by dendritic cells (Gilbert et al., 2011; Boyerinas et al., 2015; Carmi et al., 2015). Accordingly, high intratumoral IgG1 has been associated with longer patient survival, while IgA may drive an opposite pattern (Welinder et al., 2016; Bolotin et al., 2017; Isaeva et al., 2019). Additionally, analysis of more than 5,000 TCGA RNA-seq samples revealed that high levels of IgG3–1 switch are associated with prolonged survival in patients with high SHM rates, whereas IgG3–1 levels are not prognostic in low SHM samples, underscoring the role of SHM in generating BCR sequences with high binding affinity to the exposed tumor antigens (Hu et al., 2019).
Apart from the previously referred to IgG4, IL-10 and IL-21, they also discussed how TGF-β-producing Bregs are also involved in the suppression of CD8 T cell anti-tumor activities.
Taking the opposite approach, in 2020 Yu et al published a paper on which IgG subclasses to use in developing anti-tumor therapeutic antibodies23 .
Put another way, which antibodies you would definitely not choose to elevate via vaccination over the others without having long term in vivo and clinical data first:
IgG has four subclasses, named IgG1, IgG2, IgG3, and IgG4 [25]. Although all the subclasses have more than 90% identity on the amino acid level, each subclass however has a unique profile with respect to the length of hinge region, the number of inter-chain disulfide bonds, and Fc-effector functions. Differences in the profile of IgG subclasses are summarized in Table 2 [26]. Among the four IgG subclasses, IgG3 demonstrates the highest affinity binding to most FcγRs, but is not selected routinely as a therapeutic format due to its long hinge region and polymorphic nature, both of which increase the risk of stability and immunogenicity [28], thus IgG3 will not be discussed further in this review. Of the remaining subclasses, IgG1 demonstrates the highest affinity for all FcγRs and is a potent activator of ADCC and ADCP. IgG4 only has high affinity for FcγRI but weak affinities for all other receptors, and is a poor inducer of Fc-mediated effector functions. IgG2 has high affinity for the H131 form of FcγRIIA, but no measurable or weak affinity for FcγRI and all other FcγRs (Table 2).
Depiction of tumor microenvironment. Tumor microenvironment (TME) is specially built up by tumor cells creating an ideal environment for tumor cell growth. TME is full of immunosuppressive cells (such as Tregs, M2 macrophages) and soluble factors (such as VEGF, TGF-β, FGL-1) secreted by tumor cells that play roles in promoting angiogenesis and tumor growth, in induction of Tregs and M2 macrophages. Tumor cells can also initiate epigenetic silencing pathways preventing immune cells from infiltration into TME. The best rationale for developing BsAb would be to simultaneously inhibit tumor growth and activate long-lasting immunity against tumors.
Working model of CD47xCD20 Bispecific Molecule (IMM0306). IMM0306 targeting CD47xCD20 (Left) is composed of CD20 antibody and the first domain of SIRPα which is connected to the N-terminal of the heavy chain of the antibody. The Fc domain was engineered to have enhanced ADCC/ADCP activity. When administrated, IMM0306 will block the “don't eat me” signal by binding to CD47 and blocking CD47-SIRPα interaction between cancer cells and macrophages, in parallel, it will also strongly activate innate immune cells via Fc-FcγRIIA/FcγRIIIA interaction leading to fully killing of the cancer cells
Melanoma
In this author’s view by Karagiannis et al from 2013 the inhibitory but non-neutralising effects of IgG4 antibodies on melanoma are discussed24.
The role of B cells and antibodies in cancer is insufficiently understood but is receiving increasing attention. We have recently identified IgG4 as an antibody subclass elicited by melanoma-associated interleukin-10-driven inflammation. In this setting, IgG4 exhibit inefficient immunostimulatory capacity and block the cytotoxic activities of other antibodies. These previously unappreciated mechanisms of immune escape may constitute promising targets for the development of novel anticancer immunotherapies.
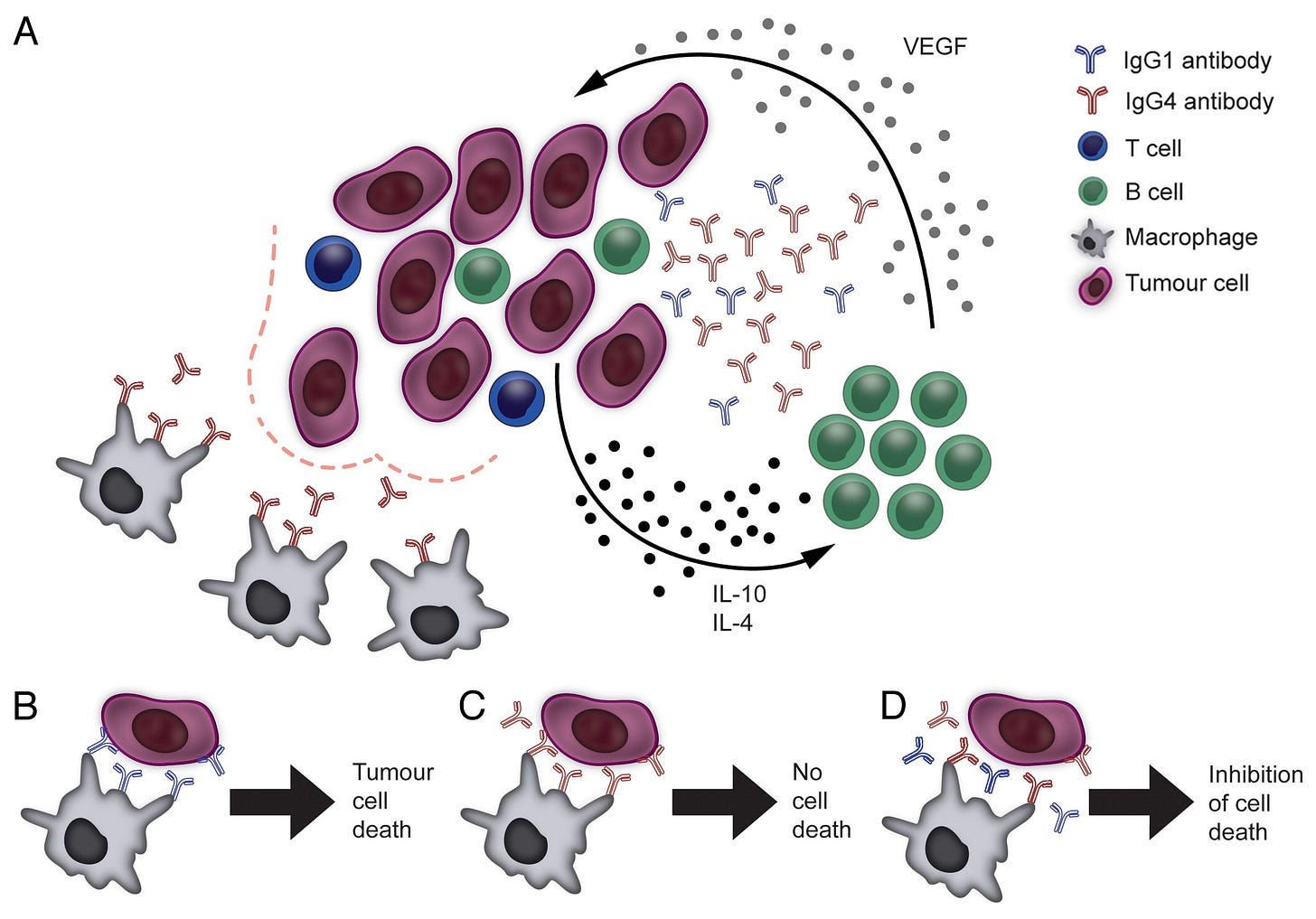
The tumor itself can polarize B cells to secrete IgG4 antibodies by releasing TH2 cytokines such as IL-10 and IL-4 as well as by stimulating B cells to produce vascular endothelial growth factor (VEGF).
In effect, this is a self sustaining positive feedback mechanism as the resultant tumor cells can induce further secretion of IgG4 antibodies, thus sustaining the cycle and the class switching:
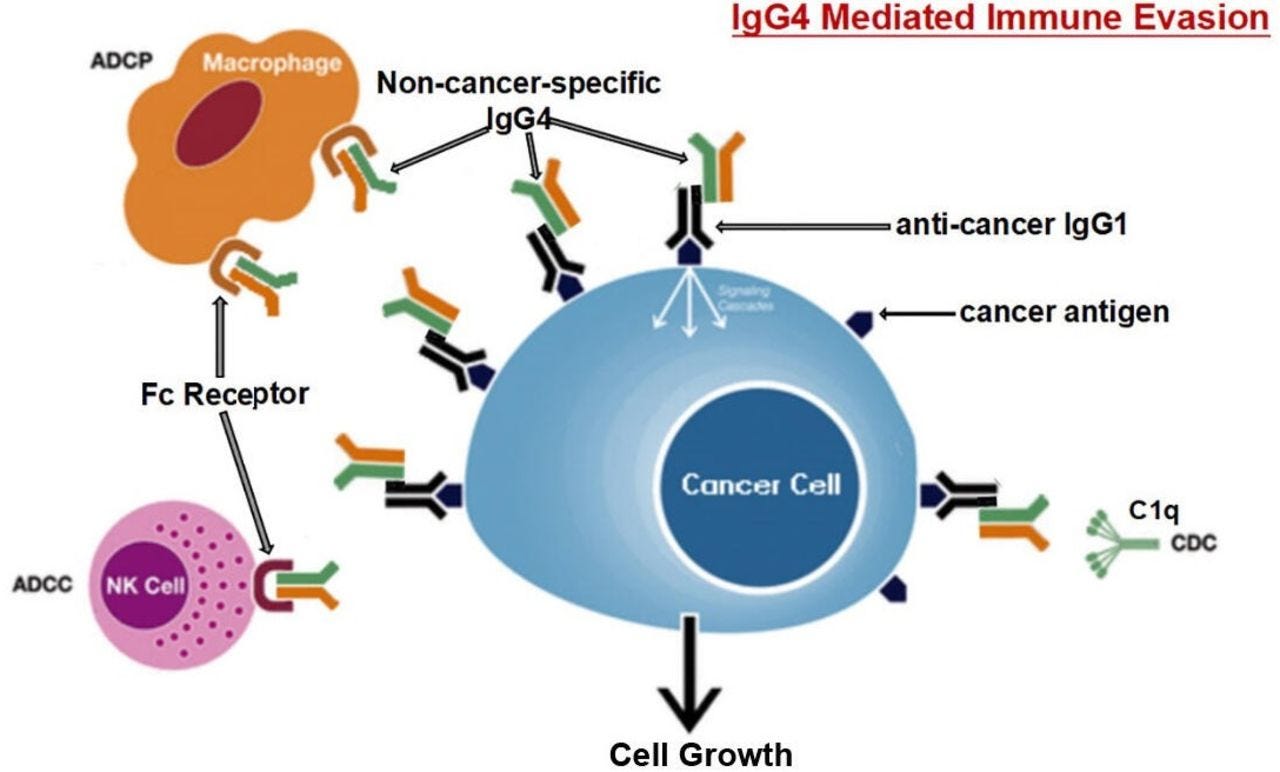
Mechanisms underpinning the IgG4 bias of the tumor microenvironment and the suppression of immune effector cells by IgG4s. (A) Malignant cells, aided by immune and stromal cells of the tumor microenvironment, can polarize B cells to secrete IgG4 antibodies by releasing TH2 cytokines such as interleukin (IL)-10 and IL-4 as well as by stimulating B cells to produce vascular endothelial growth factor (VEGF). This may be part of a feedback circuitry delivering constant class-switching and activation signals to tumor-infiltrating B cells. IgG4 antibodies are poor activators of antitumor effector cell functions (bottom left). (B) Tumor-specific IgG1 antibodies can mediate effective antibody-dependent cellular cytotoxicity by immune effector cells (e.g., monocytes/macrophages) through the activation of FcγRI (CD64) signaling. (C) IgG4 antibodies do not activate effector cells to kill malignant cells as they are inefficient at inducing FcγRI signaling upon binding to the receptor. (D) IgG4 antibodies can impair IgG1-dependent tumor cell killing by competing for the binding of FcγRI receptors on immune effector cells.
In summary, we demonstrated that IgG4 antibodies, promoted by tumor-induced TH2-biased inflammatory conditions, impair antitumor immunity by inhibiting immune effector cell activation. Our findings are supported by a recent report demonstrating the existence of IgG4-positive infiltrates in extrahepatic cholangiocarcinoma,10 and not only reveal a previously unappreciated aspect of B cell-cancer cell interactions but also provide novel insights into the mechanisms contributing to tumor immune escape.
Then in 2015, Karagiannis et al added to the body of research by conducting a clinical trial involving 167 melanoma patients and 104 healthy volunteers to assess IgG4 as a predictor of disease progression, plus conducted a peripheral blood B cell analysis of 47 melanoma patients and 24 healthy volunteers25.
They found that high IgG4 levels correlated negatively with survival rates:
Abstract
Emerging evidence suggests pathological and immunoregulatory functions for IgG4 antibodies and IgG4+ B cells in inflammatory diseases and malignancies. We previously reported that IgG4 antibodies restrict activation of immune effector cell functions and impair humoral responses in melanoma. Here, we investigate IgG4 as a predictor of risk for disease progression in a study of human sera (n = 271: 167 melanoma patients; 104 healthy volunteers) and peripheral blood B cells (n = 71: 47 melanoma patients; 24 healthy volunteers). IgG4 (IgG4/IgGtotal) serum levels were elevated in melanoma. High relative IgG4 levels negatively correlated with progression-free survival (PFS) and overall survival. In early stage (I-II) disease, serum IgG4 was independently negatively prognostic for progression-free survival, as was elevation of IgG4+ circulating B cells (CD45+CD22+CD19+CD3-CD14-). In human tissues (n = 256; 108 cutaneous melanomas; 56 involved lymph nodes; 60 distant metastases; 32 normal skin samples) IgG4+ cell infiltrates were found in 42.6% of melanomas, 21.4% of involved lymph nodes and 30% of metastases, suggesting inflammatory conditions that favor IgG4 at the peripheral and local levels. Consistent with emerging evidence for an immunosuppressive role for IgG4, these findings indicate association of elevated IgG4 with disease progression and less favorable clinical outcomes. Characterizing immunoglobulin and other humoral immune profiles in melanoma might identify valuable prognostic tools for patient stratification and in the future lead to more effective treatments less prone to tumor-induced blockade mechanisms.
Keywords: B cells; IgG4; biomarker; cancer inflammation; humoral response; immunomodulation; immunomonitoring; immunosuppression; melanoma; prognosis.
And in 2022, Yu et al conducted a systematic literature review into the risk of malignancy in patients with IgG4-related disease26:
IgG4-related disease (IgG4-RD), featuring dense lymphoplasmacytic IgG4+ plasma cells, storiform fibrosis and obliterative phlebitis in histopathology, is a chronic fibroinflammatory autoimmune disease with multisystemic involvement. It is crucial to differentiate malignant disease from IgG4-RD for the application of glucocorticoids, the first-line treatment for IgG4-RD.
Their results for the standardized incidence ratios (SIRs) of cancers if you have IgG4-RD’s were as follows:
Overall risk of cancer: 257% (SIR 2.57 95% CI 1.72–3.84)
Pancreatic: 407% (SIR 4.07 95% CI 1.04–15.92)
Lymphoma: 6917% (SIR 69.17 95% CI 3.91–1223.04)
Respiratory and gastric cancers: Not significant (SIR 2.14 95% CI 0.97–4.75, SIR 0.95 95% CI 0.24–3.95, respectively)
Compared with the general population, patients with IgG4-RD appear to have a higher risk of overall cancer, especially pancreatic and lymphoma. The risk of lung and gastric cancer was not different between IgG4-RD patients and the general population.
In 2020, Wang et al found a correlation between IgG4, esophageal cancer, metastasis and poor prognosis27. Of note here, they also found that the anti-cancer immunotherapy drug nivolumab could, paradoxically induce a "hyperprogressive disease" state, AKA "turbo cancer".
Antigen specificity was not a requirement either:
Background: Recent impressive advances in cancer immunotherapy have been largely derived from cellular immunity. The role of humoral immunity in carcinogenesis has been less understood. Based on our previous observations we hypothesize that an immunoglobulin subtype IgG4 plays an essential role in cancer immune evasion.
Methods: The distribution, abundance, actions, properties and possible mechanisms of IgG4 were investigated with human cancer samples and animal tumor models with an extensive array of techniques both in vitro and in vivo.
Results: In a cohort of patients with esophageal cancer we found that IgG4-containing B lymphocytes and IgG4 concentration were significantly increased in cancer tissue and IgG4 concentrations increased in serum of patients with cancer. Both were positively related to increased cancer malignancy and poor prognoses, that is, more IgG4 appeared to associate with more aggressive cancer growth. We further found that IgG4, regardless of its antigen specificity, inhibited the classic immune reactions of antibody-dependent cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis and complement-dependent cytotoxicity against cancer cells in vitro, and these effects were obtained through its Fc fragment reacting to the Fc fragments of cancer-specific IgG1 that has been bound to cancer antigens. We also found that IgG4 competed with IgG1 in reacting to Fc receptors of immune effector cells. Therefore, locally increased IgG4 in cancer microenvironment should inhibit antibody-mediated anticancer responses and help cancer to evade local immune attack and indirectly promote cancer growth. This hypothesis was verified in three different immune potent mouse models. We found that local application of IgG4 significantly accelerated growth of inoculated breast and colorectal cancers and carcinogen-induced skin papilloma. We also tested the antibody drug for cancer immunotherapy nivolumab, which was IgG4 in nature with a stabilizing S228P mutation, and found that it significantly promoted cancer growth in mice. This may provide an explanation to the newly appeared hyperprogressive disease sometimes associated with cancer immunotherapy.
Conclusion: There appears to be a previously unrecognized immune evasion mechanism with IgG4 playing an essential role in cancer microenvironment with implications in cancer diagnosis and immunotherapy.
Keywords: antibody specificity; immune evasion; immunotherapy; tumor microenvironment.
IgG4 is a unique antibody that has the lowest concentration among IgG subtypes in healthy individuals, and its function has not been well understood.3–5 IgG4 was regarded as a ‘blocking antibody’ because of its reduced ability to trigger effector immune reactions.6 7 Therefore whatever molecules IgG4 reacts to, the subsequent immune reaction was subdued.
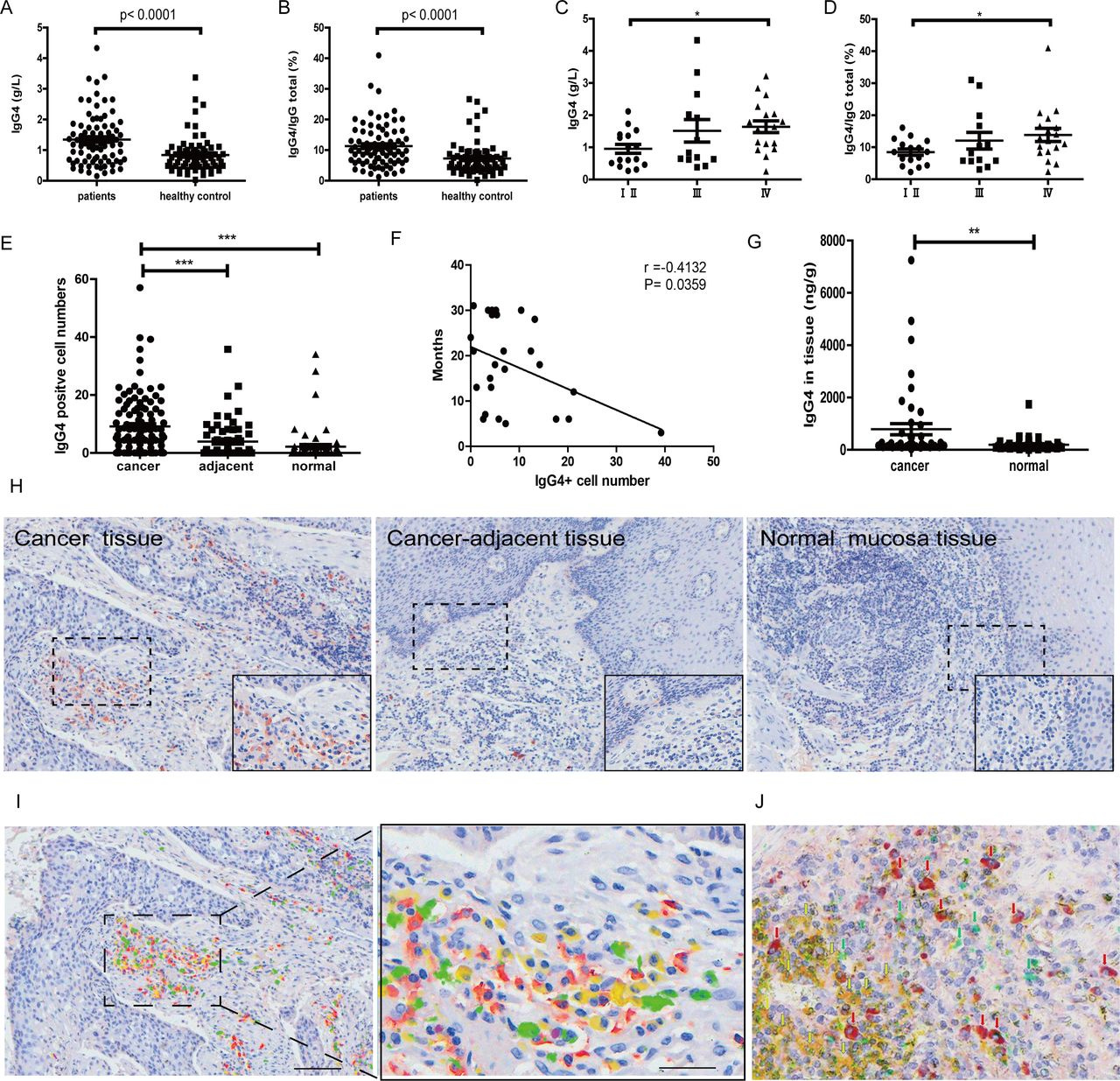
Significant increase of IgG4 and IgG4:IgG total in serum and IgG4-positive B lymphocytes in esophageal cancer. (A) IgG4 in serum of esophageal squamous cell cancer (ESCC) (n=82) was significantly higher when compared with healthy controls (n=70) (p<0.0001). (B) IgG4:IgGtotal ratio in ESCC (n=82) was significantly higher than that in matching healthy adults (n=70) (p<0.0001). (C) IgG4 in stage Ⅳ (n=18) was significantly higher than those in stages Ⅰ and Ⅱ (n=16) (p<0.01). (D) IgG4:IgGtotal in serum of stage IV ESCC (n=18) was significantly higher than those in stages Ⅰ and Ⅱ (n=16) (p<0.05). (E) Scatter diagram of IgG4-positive cell numbers in cancer (cancer), adjacent normal tissue (adjacent) and normal tissues. IgG4-positive lymphocytes in and around the esophageal cancer mass (n=110) are significantly more abundant than those in the adjacent normal tissue (n=60) and normal lymphoid tissues (n=63) (***p<0.001 for both). Increases of IgG4-positive lymphocytes were most abundant in areas of cancer cell proliferation. (F) The increase of IgG4-positive cell numbers was related to the prognoses of the patients. More IgG4-positive cells were associated with worse outcome (p<0.05). (G) IgG4 concentration in cancer tissue (n=46) was significantly higher than that in adjacent normal tissue (n=46) (p<0.01). (H) Immunohistochemistry of IgG4 in esophageal cancer tissues. From left to right are IgG4 in cancer tissues, cancer-adjacent tissue and normal lymphoid tissue (tonsil). It clearly demonstrates that IgG4-positive lymphocytes (red) were markedly increased (left) in comparison with normal lymphoid tissue (right) and with tumor-adjacent normal tissue (middle). Scale bar: 100 µm. (I) Demonstration of four subpopulations of IgG-containing plasma cells with multiple immunostaining (SDS method) in cancer. Each subclass has its own distribution pattern and one plasma cell only produces one subclass of IgG. IgG1: yellow; IgG2: green; IgG3: purple; IgG4: red. (J) On the same tissue section, a triple immunostaining was performed with the SDS method to demonstrate the distribution and relationship among CD3-positive T cells (yellow), IgG1-positive B cells (greens) and IgG4-positive B cells (red). Each cell type has its own distribution, and no overlap between different cell types is observed. SDS, stain-decolorize-stain.
Positive staining is good, in this case:
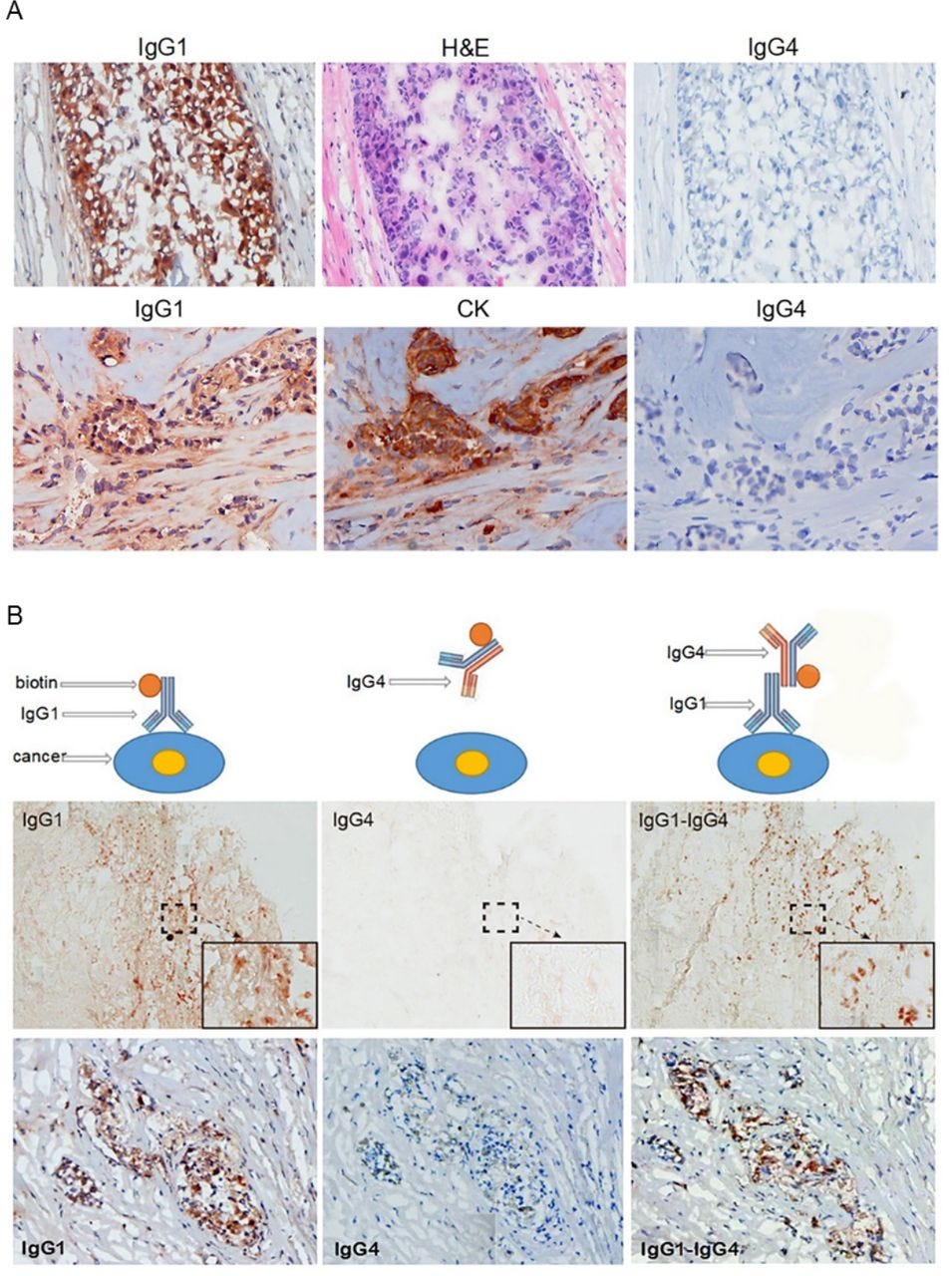
IgG4 extracted from a patient with cancer reacted to cancer-bound IgG1 and blocked antibody-mediated cancer immunity. (A) Upper panel: These photos serve as an example of the reactivity of IgG1 and IgG4 extracted from patients with cancer. IgG1 from the serum of a patient with breast cancer was labeled with biotin and stained a frozen cancer tissue section of the same patient. Cancer cells were positively stained by IgG1 (left). The cancer cells were confirmed by their characteristic histopathology of H&E staining (middle). IgG4 from serum of the same patient labeled with biotin and applied on the same cancer on a consecutive section was completely negative (right). Lower panel: Another breast cancer positively stained by IgG1 from the patient’s serum (left). The cancer cells were identified by positive immunostaining of cytokeratin (CK) on a consecutive section (middle). IgG4 from the same patient was not reactive to the same cancer on a consecutive section (right). (B) The upper panel illustrates the principle of the experimental reactions, and the middle and lower panels show staining results from two patients with breast cancer. Left: IgG1 from a patient with cancer positively reacted to frozen cancer tissue of the same patient (brown cells). Middle: IgG4 from the same patient with cancer applied to consecutive sections, but did not react to the same cancer. Right: However, when unlabeled IgG1 was applied to the same cancer tissue section followed by biotin-labeled IgG4, the cancer cells were positively stained (brown cells).
They then discussed how IgG4 antibodies were found to react to other IgGs (IgG1, IgG2, IgG3 and IgG4) via an Fc-Fc mechanism, and that this reaction was across species, but IgG4 did not react to other Ig subtypes (IgM, IgE, IgA or IgD).
As the anticancer drug Cetuximab uses IgG1 as the mediating antibody, binding or competition with IgG4 stopped it working, with dramatic results:
IgG4 (including nivolumab) significantly accelerated breast cancer cell and colon cancer cell growth in two immune potent mouse models in vivo
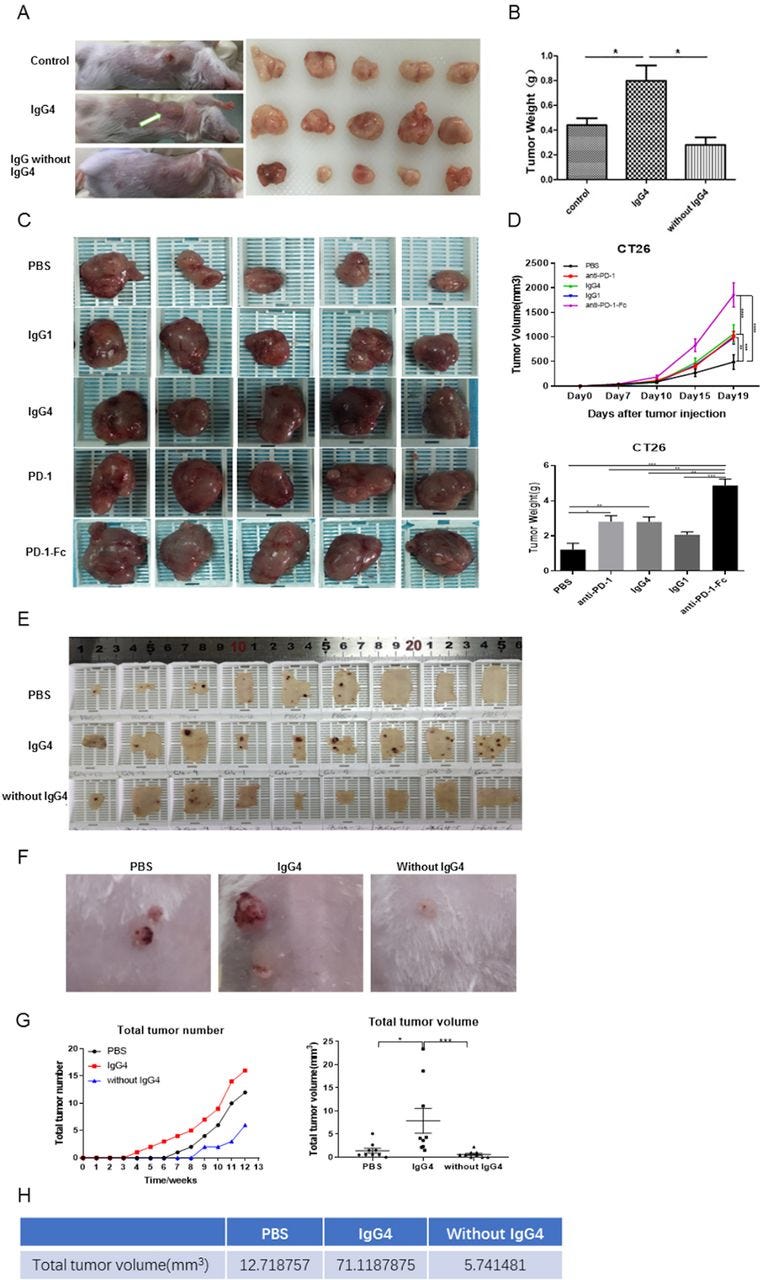
The above results point to a mechanism that IgG4 plays an important role in local immune evasion by blocking immune responses mediated by cancer-specific IgG antibodies. To further examine this mechanism mediated by such antibodies, we performed in vivo studies to verify this hypothesis with immune competent mouse models. In one model, we injected non-cancer-specific IgG4 into a location where breast cancer cells were inoculated subcutaneously. In this group of mice, cancer cell growth was significantly increased, resulting in a much larger cancer mass by 21 days in comparison with other groups (injections of PBS or IgG without IgG4) (figure 5A,B). As there is no direct effect of IgG4 on cancer cell growth (figure 4I), these results unequivocally confirmed that IgG4 can inhibit local immune reaction and thereby promote cancer growth in vivo through immune evasion.
IgG4 accelerated cancer cell growth in three immune potent mouse models.
(A,B) Non-cancer-specific IgG4 accelerated breast cancer cell growth. Local inoculation of mouse breast cancer cells (4T1, 1×105 cells per mouse) into immune competent mice (BALB/c, body weight 20 g, n=5) resulted in sizeable tumor masses in 21 days. Local injections of non-cancer-specific IgG4 resulted in tumor masses that doubled the size of those similarly injected with ‘IgG without IgG4’ or with PBS. As there is no direct effect of IgG4 on cancer cell growth (figure 4I), these results clearly demonstrate that IgG4 can effectively promote tumor growth by inhibiting local immunity. (C,D) IgG4, anti-PD-1 (nivolumab) and anti-PD-1-Fc induced significant tumor (CT26 mouse colon cancer) progression in mice. Five groups of mice were tested by injecting IgG4, nivolumab (IgG4 subtype), nivolumab-Fc, IgG1 and PBS. By 19 days, IgG4, nivolumab and nivolumab-Fc induced significant progression of tumor size in comparison with the other groups. (E–G) IgG4 significantly accelerated skin papilloma formation in a carcinogen-induced skin tumor model in immune potent mice. IgG4 significantly accelerated tumor development and growth in comparison with control (PBS), which in turn had more tumor formation than the group treated with IVIG without IgG4. By 12 weeks, the total tumor volume in IgG4-treated group was more than 12 times larger than the group that was treated with IVIG without IgG4, and was six times larger than the group treated with PBS. (E) The removed back skin of mice from the three groups (at 15 weeks). The tumors are shown in dark-brown color. (F) Tumors at higher magnification. (G) Changes of tumor numbers and volume over time of the three groups. (H) Total tumor volumes of the three groups at 12 weeks after treatment. *p<0.05, **p<0.01, ***p<0.001. IVIG, intravenous IgG; PBS, phosphate buffered saline; PD-1, programmed cell death-1.
Diagrammatic illustration of proposed mechanism of cancer-initiated B lymphocyte-derived IgG4-mediated immune evasion. Chronic stimulation by cancer antigens induces class switch of B lymphocytes to produce IgG4. Such increased IgG4 can react to cancer-bound IgG with its Fc-Fc binding property and also to Fc receptors of immune effector cells. With its unique structural and biological property, increased IgG4 in cancer microenvironment mediates an effective immune escape for cancer.
ADCC, antibody-dependent cell-mediated cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, complement-dependent cytotoxicity; NK, natural killer cells.
These actions of IgG4 found in this study represent a previously unrecognized dimension of cancer immunology. In addition to going through specific antigens and receptors, this response uses the Fc fragment of IgG4 to react with the Fc of other antibodies and to Fc receptors of immune effector cells. This mechanism may provide new insights into the immunopathology of cancer, IgG4-related diseases and immune tolerance in general.
Future Perspectives
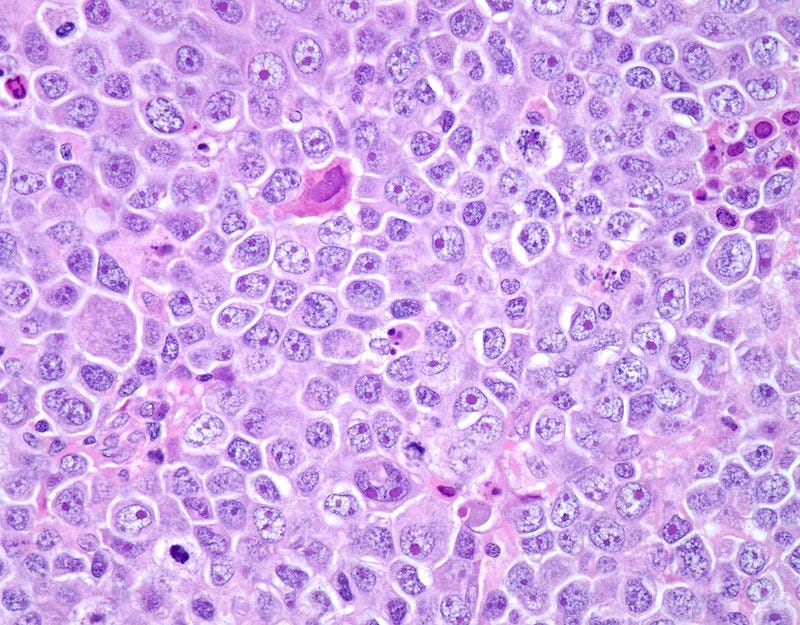
Diffuse large B-cell lymphoma is the most common type of lymphoma and accounts for about 35% of all cases of non-Hodgkin lymphoma. It is an aggressive malignancy and presents with rapidly enlarging lymph nodes or tumor masses at extranodal sites. There is diffuse proliferation of large to medium-sized lymphoid cells which can be classified into centroblasts and immunoblasts. Some cases have markedly pleomorphic anaplastic cells. This high magnification photomicrograph of diffuse large B-cell lymphoma involving a lymph node shows cellular detail. The immunoblasts have round vesicular nucleus and a single prominent nucleolus with abundant basophilic cytoplasm.
Although many pro-cancer mechanisms have been discussed in this and other Substacks it does not mean that a large percentage of the population is about to develop lymphoma, sarcoma, melanoma etc.
Each tumor starts out as a single cell, a process known as tumor clonality.
Fortunately the mutated cells are usually detected and destroyed by apoptosis or the DNA is repaired long before before this process can get underway. And even if transfected, after a few months spike protein and inflammatory cytokine expression should abate just as tumor suppressor levels are being restored and hematopoetic stem and derivative cell counts recover, so you should be able to clear such microscopic tumors whilst still at an early stage.
Age, lifestyle, inherited genes, repeat infections and epigenetics all play a part. Some of these we can change for the better, some not.
But if you continue to expose yourself to sources of spike protein then your risk profile will increase proportionately. And multiple clinical reports of “turbo cancer” cases mean that the book is being re-written. This is particularly a risk for those with a weak (or weakened) immune system and for those whose cancers are in remission, possibly from decades before.
Question: Clonality of tumour cells28
Does all cancer cells come from a single clone of cells or are there evidences of polyclonal cancer cells too?
Answer
Most tumors are probably not strictly clonal; all cancer cells in a tumor seldom have the exact same set of mutations. Tumors tend to be subclonal, with all cancer cells having a single common ancestor but with later evolution resulting in some mutations shared among all cancer cells but others restricted to sub-populations of cancer cells. This fact and its implications for therapy were noted 40 years ago by Nowell (Science 194:23-28, 1976), and verified by much recent work as reviewed for example by McGranahan and Swanton (Cancer Cell 27:15-26, 2015).
A tumor might need to have a billion (109109) or so cancer cells to be clinically detectable. Thus many years and many cell divisions have passed since the time that a single cell developed the set of mutations originally needed to produce the invasive tumor. With a probability of some uncorrected mutation at each cell division, particularly in cancer cells that often have defects in DNA replication and repair and in chromosome separation, it is essentially impossible for all of the billion cancer cells in the tumor to have identical sets of mutations. See, for example, the work of Bozic et al (eLife 2: e000747,2013). So although there is a clonal origin of the tumor, the tumor is typically a collection of related but genetically distinct populations best considered to be subclones.
Differences among subclones present a major barrier to traditional cytotoxic therapies, to more modern targeted therapies, and to the immunotherapies that presently receive so much attention. Even if most of the tumor is sensitive and responds, a subclone whose mutations somehow provide resistance to the therapy will be able to repopulate the tumor thereafter. Bozic and Nowak (PNAS 111:15964-8, 2014) estimated that a clinically detectable tumor may have 10 or more subclones resistant to any targeted therapy, due to mutations accumulated during the subclinical growth of the tumor.
As examples from immunotherapy for cancer, McGranahan et al recently showed (Science 351:1463-1469, 2016) that responses to immune checkpoint inhibition in melanoma and non-small cell lung cancer were best in patients with clonal rather than subclonal tumor neoantigens for the immune system to attack. Zaretzky et al (New Engl J Med 2016, epub ahead of print) identified subclonal mutations in interferon-receptor signaling and antigen presentation in some patients, mutations enriched in the recurrent tumors following immune checkpoint inhibition and thus likely accounting for relapse following that therapy.
It is possible in principle for a single physical tumor to have multiple cells of origin. Tumors often develop from a region of tissue that has been subject to field cancerization, where carcinogenic challenges have produced many cells with mutations that lead to dysregulated but not invasive behavior. This can lead to multiple independent cancers arising from the same general location, as individual cells later develop the full complement of mutations needed for an invasive tumor. In principle, such multiple cancers might coalesce into a single tumor mass so that the tumor is polyclonal, but I'm not aware of any well documented cases. So it seems that tumors are seldom truly polyclonal with multiple independent cells of origin.
Recently, it has been appreciated that the initiating chromosomal events in other B-cell malignancies may precede the development of overt disease by many years.29
I reached out to cellular and molecular biologist Dr Christina Parks and asked her thoughts on whether the class switching was possibly reversible?
So this appears to be as the result of clonal expansion of the aberrant B-cells after aberrant somatic hypermutation (antibody formation). If so, then the question is just how do we get rid of these clones. My hunch is that the numbers of these IgG4 B cells would die out over time IF the people were not constantly exposed to the spike protein by circulating virus and those continually getting boosted and/or shedding the spike protein. I thought it was interesting that the effect was not seen when AstraZeneca was used for the first vaccine. I think its likely that the aberrant antibody formation is happening because of spike deposition in the germinal centers causing prolonged inflammation/activation. I wonder if the amount of spike protein generated by the AZ vax is comparable or if it dies of more quickly due to it being a DNA vaccine. In my mind the cell is going to continuously make protein from mRNA, but may not transcribe non-genomic DNA, and even if it does, that RNA is not stabilized and will have a short half life, so will not produce as much protein. IN the end, this is the result of using gene therapy for a vaccine.
I just can't....
Thank you for reading or subscribing and supporting these reviews.
Selected therapeutics have been discussed here, and this list is being updated regularly:
These data are disturbing and invite to immediately intensify clinical surveillance in oncology and to undertake rigid cancer prevention actions, including healthy lifestyle and continuous controls.
From Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Immune Response to Cancer Onset via Molecular Mimicry and Cross-Reactivity (2021)
References
Yang J, Yang X, Yang J, Li M. Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 2019 Feb 13;10(2):140. doi: 10.1038/s41419-019-1315-9. PMID: 30760702; PMCID: PMC6374440.
Zheng X, Wang P, Jia M, Li Q, Zhang A, Zhou Q. Baicalin Alleviates Thrombin-Induced Inflammation in Vascular Smooth Muscle Cells. Biomed Res Int. 2022 Jan 21;2022:5799308. doi: 10.1155/2022/5799308. PMID: 35097121; PMCID: PMC8799346.
Garavelli S, De Rosa V, de Candia P. The Multifaceted Interface Between Cytokines and microRNAs: An Ancient Mechanism to Regulate the Good and the Bad of Inflammation. Front Immunol. 2018 Dec 21;9:3012. doi: 10.3389/fimmu.2018.03012. PMID: 30622533; PMCID: PMC6308157.
Fujii RF. Quantum microRNA Assessment of COVID-19 RNA Vaccine: Hidden Potency of BNT162b2 SASR-CoV-2 Spike RNA as MicroRNA Vaccine (2021)
Carvacho I, Piesche M. RGD-binding integrins and TGF-β in SARS-CoV-2 infections - novel targets to treat COVID-19 patients? Clin Transl Immunology. 2021 Mar 18;10(3):e1240. doi: 10.1002/cti2.1240. PMID: 33747508; PMCID: PMC7971943.
Nakashima H, Miyake K, Moriyama M, Tanaka A, Watanabe M, Abe Y, Sato H, Nakamura S, Saito T. An amplification of IL-10 and TGF-beta in patients with IgG4-related tubulointerstitial nephritis. Clin Nephrol. 2010 May;73(5):385-91. doi: 10.5414/cnp73385. PMID: 20420800.
Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol. 2017 Apr;32(4):577-587. doi: 10.1007/s00467-016-3394-5. Epub 2016 May 7. PMID: 27155873; PMCID: PMC5099107.
El Bassam S, Pinsonneault S, Kornfeld H, Ren F, Menezes J, Laberge S. Interleukin-16 inhibits interleukin-13 production by allergen-stimulated blood mononuclear cells. Immunology. 2006 Jan;117(1):89-96. doi: 10.1111/j.1365-2567.2005.02269.x. PMID: 16423044; PMCID: PMC1782191.
Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal Malefyt R, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3730-4. doi: 10.1073/pnas.90.8.3730. PMID: 8097323; PMCID: PMC46375.
van Asten SD, Unger PP, Marsman C, Bliss S, Jorritsma T, Thielens NM, van Ham SM, Spaapen RM. Soluble FAS Ligand Enhances Suboptimal CD40L/IL-21-Mediated Human Memory B Cell Differentiation into Antibody-Secreting Cells. J Immunol. 2021 Jul 15;207(2):449-458. doi: 10.4049/jimmunol.2001390. Epub 2021 Jul 2. PMID: 34215657.
Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer. 2001 Sep 28;85(7):1047-54. doi: 10.1054/bjoc.2001.2042. PMID: 11592778; PMCID: PMC2375090.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2375090/pdf/85-6692042a.pdf
Kim R, Emi M, Tanabe K, Uchida Y, Toge T. The role of Fas ligand and transforming growth factor beta in tumor progression: molecular mechanisms of immune privilege via Fas-mediated apoptosis and potential targets for cancer therapy. Cancer. 2004 Jun 1;100(11):2281-91. doi: 10.1002/cncr.20270. PMID: 15160330.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.20270
Saskia D. van Asten, Peter-Paul Unger, Casper Marsman, Sophie Bliss, Tineke Jorritsma, Nicole M. Thielens, S. Marieke van Ham, Robbert M. Spaapen. Soluble FAS Ligand directs human memory B cells towards antibody-secreting cells. bioRxiv 2020.12.04.411959; doi: https://doi.org/10.1101/2020.12.04.411959Now published in The Journal of Immunology doi: 10.4049/jimmunol.2001390
https://www.biorxiv.org/content/10.1101/2020.12.04.411959v1.full
Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, Gaudette BT, Soliman OY, Pine M, Hicks P, Manzoni TB, Knox JJ, Johnson JL, Laczkó D, Muramatsu H, Davis B, Meng W, Rosenfeld AM, Strohmeier S, Lin PJC, Mui BL, Tam YK, Karikó K, Jacquet A, Krammer F, Bates P, Cancro MP, Weissman D, Luning Prak ET, Allman D, Locci M, Pardi N. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2022 Jun 14;55(6):1136-1138. doi: 10.1016/j.immuni.2022.05.007. Erratum for: Immunity. 2021 Dec 14;54(12):2877-2892.e7. PMID: 35704995; PMCID: PMC9195404.
Tsukuda S, Ikeura T, Ito T, Nakamaru K, Masuda M, Hori Y, Ikemune M, Yanagawa M, Tanaka T, Tomiyama T, Yamaguchi T, Ando Y, Uchida K, Fukui T, Nishio A, Terasawa R, Tanigawa N, Okazaki K. Clinical implications of elevated serum interleukin-6 in IgG4-related disease. PLoS One. 2020 Jan 17;15(1):e0227479. doi: 10.1371/journal.pone.0227479. PMID: 31951598; PMCID: PMC6968836.
Gupta S, Jain A, Syed SN, Snodgrass RG, Pflüger-Müller B, Leisegang MS, Weigert A, Brandes RP, Ebersberger I, Brüne B, Namgaladze D. IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors. Oncoimmunology. 2018 Jul 30;7(10):e1494110. doi: 10.1080/2162402X.2018.1494110. PMID: 30288360; PMCID: PMC6169572.
Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009 Feb 1;417(3):639-50. doi: 10.1042/BJ20080413. PMID: 19133841; PMCID: PMC2975036.
Björkman A, Qvist P, Du L, Bartish M, Zaravinos A, Georgiou K, Børglum AD, Gatti RA, Törngren T, Pan-Hammarström Q. Aberrant recombination and repair during immunoglobulin class switching in BRCA1-deficient human B cells. Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):2157-62. doi: 10.1073/pnas.1418947112. Epub 2015 Feb 2. PMID: 25646469; PMCID: PMC4343177.
Irrgang P, Gerling J, Kocher K, Lapuente D, Steininger P, Habenicht K, Wytopil M, Beileke S, Schäfer S, Zhong J, Ssebyatika G, Krey T, Falcone V, Schülein C, Peter AS, Nganou-Makamdop K, Hengel H, Held J, Bogdan C, Überla K, Schober K, Winkler TH, Tenbusch M. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol. 2022 Dec 22:eade2798. doi: 10.1126/sciimmunol.ade2798. Epub ahead of print. PMID: 36548397.
Immunoglobulin class switching - Wikipedia
https://en.wikipedia.org/wiki/Immunoglobulin_class_switching#
Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, Klein IA, Stone G, Eisenreich TR, Ried T, Nussenzweig A, Nussenzweig MC. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell. 2009 Nov 25;36(4):631-41. doi: 10.1016/j.molcel.2009.11.007. PMID: 19941823; PMCID: PMC2805907.
Kinker GS, Vitiello GAF, Ferreira WAS, Chaves AS, Cordeiro de Lima VC, Medina TDS. B Cell Orchestration of Anti-tumor Immune Responses: A Matter of Cell Localization and Communication. Front Cell Dev Biol. 2021 Jun 7;9:678127. doi: 10.3389/fcell.2021.678127. PMID: 34164398; PMCID: PMC8215448.
https://www.frontiersin.org/articles/10.3389/fcell.2021.678127/full
Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol. 2020 May 5;13(1):45. doi: 10.1186/s13045-020-00876-4. PMID: 32370812; PMCID: PMC7201658.
https://jhoonline.biomedcentral.com/articles/10.1186/s13045-020-00876-4
Karagiannis P, Gilbert AE, Nestle FO, Karagiannis SN. IgG4 antibodies and cancer-associated inflammation: Insights into a novel mechanism of immune escape. Oncoimmunology. 2013 Jul 1;2(7):e24889. doi: 10.4161/onci.24889. Epub 2013 May 7. PMID: 24073371; PMCID: PMC3782134.
Karagiannis P, Villanova F, Josephs DH, Correa I, Van Hemelrijck M, Hobbs C, Saul L, Egbuniwe IU, Tosi I, Ilieva KM, Kent E, Calonje E, Harries M, Fentiman I, Taylor-Papadimitriou J, Burchell J, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN. Elevated IgG4 in patient circulation is associated with the risk of disease progression in melanoma. Oncoimmunology. 2015 Jun 3;4(11):e1032492. doi: 10.1080/2162402X.2015.1032492. PMID: 26451312; PMCID: PMC4590000.
https://www.tandfonline.com/doi/full/10.1080/2162402X.2015.1032492
Yu T, Wu Y, Liu J, Zhuang Y, Jin X, Wang L. The risk of malignancy in patients with IgG4-related disease: a systematic review and meta-analysis. Arthritis Res Ther. 2022 Jan 5;24(1):14. doi: 10.1186/s13075-021-02652-2. PMID: 34986892; PMCID: PMC8728936.
https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-021-02652-2
Wang H, Xu Q, Zhao C, Zhu Z, Zhu X, Zhou J, Zhang S, Yang T, Zhang B, Li J, Yan M, Liu R, Ma C, Quan Y, Zhang Y, Zhang W, Geng Y, Chen C, Chen S, Liu D, Chen Y, Tian D, Su M, Chen X, Gu J. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. J Immunother Cancer. 2020 Aug;8(2):e000661. doi: 10.1136/jitc-2020-000661. PMID: 32819973; PMCID: PMC7443307.
Clonality of tumour cells
https://biology.stackexchange.com/questions/50987/clonality-of-tumour-cells#
Frederick Racke, Sabrina Simpson, Beth Christian, Kristie A. Blum, Robert Hasserjian, Weiqiang Zhao; Evidence of Long Latency Periods Prior to Development of Mantle Cell Lymphoma. Blood 2010; 116 (21): 323. doi: https://doi.org/10.1182/blood.V116.21.323.323
https://ashpublications.org/blood/article/116/21/323/65895/Evidence-of-Long-Latency-Periods-Prior-to












I have updated this to include IgG4-related autoimmune diseases and added a hyperlinked contents page to make it more accessible:
Autoimmune disorders: COVID-19, spike protein & homologous epitopes
A literature review
https://doorlesscarp953.substack.com/p/autoimmune-disorders-covid-19-spike
This collection of articles suggests the possibility that it won't only be blood banks that are in need of quality products in the future. Imagine the implications for bone marrow donation.