Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) is an autoimmune disorder.
Kamesh et al (2002) describe the pathology and immunosuppressive treatments. Its quite an old study but describes the clinical challenges well, and the poor prognosis if left untreated:1
Inflammation and necrosis of blood vessel wall occurs in a dozen or so primary vasculitic disorders. An attempt to classify these diverse forms of vasculitis resulted in the Chapel Hill international consensus definitions, which used the vessel size as the determinant of classification (1). Wegener granulomatosis, microscopic polyangiitis, and Churg Strauss syndrome are described as small-vessel vasculitides and are acknowledged to be commonly associated with antineutrophil cytoplasm antibodies (ANCA). These diseases share a common pathology with focal necrotizing lesions, which affect many different vessels and organs; in the lungs, a capillaritis may cause alveolar hemorrhage; within the glomerulus of the kidney, a crescentic glomerulonephritis may cause acute renal failure; in the dermis, a purpuric rash or vasculitic ulceration may occur. Wegener granulomatosis and Churg Strauss syndrome have additional granulomatous lesions (for further review, see reference 2). The incidence of these diseases is increasing, with more than 20 per million affected and occurring more often in an elderly population (peak age, 55 to 70 yr) (3).
Pathogenesis
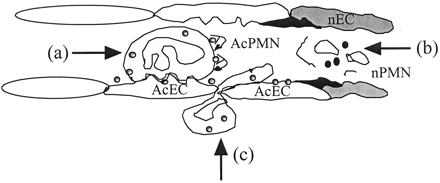
Understanding the pathogenesis of ANCA-associated vasculitis is important for the development of novel therapeutic agents, and important advances have been made in recent years (4,5). ANCA are thought to contribute to the pathogenesis of these small-vessel vasculitides, activating cytokine-primed neutrophils and monocytes, which express the ANCA antigens, proteinase 3 and myeloperoxidase, on their surface. Neutrophils respond by developing the capability of adhering to cytokine-activated endothelial cells, generating a respiratory burst, releasing proteolytic granule contents, and secreting proinflammatory cytokines. ANCA also interfere with the normal processes of resolution of inflammation. Neutrophil apoptosis is dysregulated by ANCA activation of neutrophils, preventing apoptotic cell removal, which in turn allows progression to secondary necrosis, a highly phlogistic event. Electron microscopy studies show the presence of necrotic leukocytes within the intracapillary space (as schematically shown in Figure 1).
Figure 1. Initiation of vasculitic lesions in small vessels by ANCA-activating cytokine-primed neutrophils in the wrong place and at the wrong time. Local release of inflammatory cytokines such as tumor necrosis factor (TNF), both prime neutrophils so that target antigens for ANCA are expressed on the cell surface and locally activate endothelial cells to upregulate their expression of adhesion molecules. ANCA can then bind and activate neutrophils (AcPMN) leading to (a) enhanced adhesion of activated neutrophils to activated endothelial cells (AcEC), (b) dysregulated apoptosis, secondary necrosis (nPMN), and (c) enhanced neutrophil migration across the endothelial barrier. Endothelial cells may be damaged directly by inflammatory mediators released from activated neutrophils, or they may be damaged as neutrophils undergo secondary necrosis in the vascular lumina, amplifying inflammation. After initiation of the vasculitic lesion by the interactions of neutrophils, ANCA, and endothelial cells, further mononuclear leukocytes are recruited, further enhancing vascular inflammation and injury.
The endothelial cell is also important in localizing inflammation. Endothelial cells develop an activated phenotype in ANCA-associated vasculitis with enhanced expression of adhesion molecules that promotes interaction with circulating inflammatory cells. ANCA activation can convert rolling neutrophils to stationary adherent cells that are well placed to mediate endothelial injury. Release of proinflammatory mediators, including nitric oxide, reactive oxygen species, and proteolytic enzymes, all might contribute to directly damaging the endothelial cell. Proteinase 3 and myeloperoxidase can bind to endothelial cells; indeed endothelial cells express receptors for proteinase 3. Myeloperoxidase can induce endothelial cell detachment, whereas proteinase 3 can cause direct apoptosis of these cells. Furthermore, ANCA can bind to the endothelial-bound antigens, inducing endothelial cell cytotoxicity.
Activation of endothelial cells and neutrophils is important for the early development of vasculitic lesions, and progression of these lesions is accompanied by T cell and monocyte recruitment. T cell-mediated immunity is thought to contribute to the pathogenesis of ANCA-associated vasculitis. Several studies have documented the ability of peripheral blood T cells from patients with either active or quiescent disease to proliferate in response to proteinase 3 or myeloperoxidase. T cell activation has also been shown to persist after disease remission with reduced CD28, a costimulatory molecule for T cell activation, and increased CD69, an early marker of T cell activation. These and other studies (see reference 6 for review) suggest that T cells may contribute to the remitting/relapsing nature of ANCA-associated vasculitis and show the failure of current therapies to suppress immune disease processes and induce tolerance.
Clinical Features
Constitutional symptoms, such as fever, myalgia, anorexia, weight loss, malaise, and night sweats, are common in vasculitis. In Wegener granulomatosis, there is a predilection for the upper and lower respiratory tracts and the kidneys to be involved. Upper respiratory tract symptoms include rhinorrhea, epistaxis, sinusitis, otitis media, collapse of the nasal bridge, and tracheal stenosis. Lung disease presents with cough, hemoptysis, and dyspnea and can progress to life-threatening pulmonary hemorrhage. Renal manifestations are common in the form of hematuria, proteinuria, and red cell casts, and there can be a rapid deterioration in renal function. Severe ocular abnormalities, such as episcleritis, uveitis, proptosis, and optic nerve ischemia, can also occur. The disease can involve the gut, causing ischemia and hemorrhage, the heart, causing myocardial ischemia, and the peripheral nervous system, causing mononeuritis multiplex. A detailed review of the clinical findings and presentations may be found in references 7–9.
Microscopic polyangiitis is very similar to Wegener granulomatosis. Renal involvement is very common, and pulmonary hemorrhage can also occur. However, no granuloma formation is seen, and upper airway involvement is rare.
In Churg-Strauss syndrome, the disease is characterized by hypereosinophilia, asthma, and necrotizing vasculitis. Vasculitis can affect the skin, peripheral nerves, muscles, and the intestine. Renal involvement is usually mild, and severe renal failure is uncommon.
The vascular pathology is shared between the disorders. In the kidney, early lesions show focal segmental necrosis of the glomeruli, which later progress to crescent formation. Granulomatous inflammation is predominant in Wegener disease, and these lesions are common in the respiratory tract. In addition, Churg-Strauss syndrome has eosinophilic infiltrates.
Diagnosis
The diagnosis of ANCA-associated vasculitis is made on the basis of the clinical findings, by biopsy of a relevant involved organ (typically kidney, nasal mucosa, or occasionally lung) and the presence of ANCA. Testing for ANCA using both indirect immunofluorescence and antigen-specific enzyme-linked immunosorbent assay is recommended and provides a high sensitivity (approximately 99%) and good specificity (approximately 70%) (10,11).
Treatment in ANCA-Associated Vasculitis
The prognosis of untreated ANCA-associated vasculitis is poor, with up to 90% of patients dying within 2 yr, usually due to respiratory failure (12). The introduction of cyclophosphamide and high-dose corticosteroids by Hoffman et al. (12) and Fauci et al. (13) in the 1970s markedly reduced the mortality. Cyclophosphamide alkylates DNA guanidine nucleotides, induces lymphopenia, particularly of B lymphocytes, and suppresses Ig responses. The combination of prednisolone and cyclophosphamide, now viewed as standard therapy, leads to control of disease in 80 to 90% of patients. However, these regimes are associated with treatment-related morbidity in over 50% of patients, including steroid-induced diabetes, bladder and lymphoproliferative malignancy, and infertility (12,14) There appears to be little role for prednisolone alone. When compared with combined cyclophosphamide and prednisolone, prednisolone alone is associated with a lower remission rate (56 versus 85% in one study), a higher relapse rate, and a higher mortality rate (15).
Treatment has converted this acutely fatal disease into a chronic relapsing disorder with accumulating morbidity. Current treatment is toxic and contributes to morbidity and mortality. Treatment must be tailored to the stage and severity of disease to balance the dangers of disease against those of treatment.
For successful treatment vasculitis must be recognized and treated early before permanent scarring occurs. Disease monitoring tools have been developed to aid distinction of tissue damage by active disease that would be amenable to treatment from that caused by healing scars or treatment. For example, the Birmingham Vasculitis Activity Score (BVAS) and vasculitis damage index (VDI) score active disease and chronic damage respectively (for review, see reference 16). These tools have been particularly useful in clinical studies, contributing to definitions of clinical remission and relapse, which are necessary end points of therapeutic trials.
Reference #13 was co-authored by a doctor that currently holds a very senior position in the US administration, is a strong advocate for mass transfection, and should therefore be fully aware of the pathophysiology and prognosis for those potentially affected by the very same gene experimental gene therapies being promoted:
13 Sneller MC, Hoffman GS, Talar-Williams C, Kerr GS, Hallahan CW, Fauci AS: An analysis of 42 Wegener’s granulomatosis patients treated with methotrexate and prednisolone. Arthritis Rheum 38: 608–613, 1995
https://onlinelibrary.wiley.com/doi/abs/10.1002/art.1780380505?sid=nlm%3Apubmed
Bouiller et al (2014) investigated the contribution of antineutrophil cytoplasmic antibodies to severe cardiomyopathy, and again stress the importance of early diagnosis and treatment:2
Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare form of systemic vasculitis in which cardiac involvement is frequent and severe, and accounts for half of EGPA-related deaths. ANCA-positive EGPA differs from ANCA-negative EGPA in that the former is significantly associated with renal involvement, peripheral neuropathy and biopsy proven vasculitis, whereas the latter is associated with cardiac involvement. Herein, we report a case of EGPA with myocarditis in a woman, who was successfully treated with steroids and cyclophosphamide. This report highlights the importance of diagnosing cardiac involvement in EGPA early, especially in ANCA-negative patients.
By 2016 the 5 year mortality rate was still 28%, even with treatment, and 93% within 2 years if untreated. Various cardiac manifestations are described, including pericarditis and myocarditis. Cardiac involvement of patients with EPGA is usually asymptomatic.
Immunosuppressives are recommended.
Życińska and Borowiec review “Cardiac manifestations in antineutrophil cytoplasmic autoantibody (ANCA) — associated vasculitides”:3
The overall mortality rate of GPA with cardiac involvement has been reported to be between 15-45% [17]. Estimates of the frequency of cardiac involvement in GPA vary widely,with a North American study reporting a rate of 33.3% and no association with a higher rate of relapse or premature death [16], while a European study reported cardiac involvement in 46% of GPA patients assessed with electrocardiography and echocardiography, along with increased all-cause and cardiovascular mortality,
CARDIAC MANIFESTATION IN GRANULOMATOSIS WITH POLYANGIITIS
(GPA)
…Coronary artery involvement is rare and is characterized by coronary arteritis and subsequent coronary artery thromboembolism [21]. Magnetic resonance imaging (MRI) study of coronary arteries in GPA patients revealed that in some patients coronary arteries dilatation can be observed [22]. Morbini et al. report an extreme example of an elderly female with intimal inflammation in multiple sites of a coronary tree with and without atherosclerosis which triggered coronary thrombosis. She died after cardiac arrest from a clinically unrecognized systemic autoimmune-inflammatory disorder with necrotizing arteritis. Autopsy showed findings typical of GPA and systemic arteritis with fibrinoid necrosis. Although there were no clinical signs of cardiac involvement, the coronary arteries showed inflammation associated with multiple thrombi [23]. The ischemia in GPA patients may be due to the involvement of the small vessels secondary to the vasculitic process rather than the atherosclerosis as it responds to immunosuppressive therapy with reversal of ischemic changes [24].There are several cases that described patients with GPA and myocardial infarction [25-28]. In Danish National Hospital register patients had an increased rate of myocardial infarction within 5 years of diagnosis of GPA when compared to the general population.
Myocarditis is a rare condition due to GPA. Weidhase at el presented a case of a 28 year-old male suffering from Wegener's granulomatosis, who died suddenly with signs of cardiac failure. Autopsy revealed diffuse granulomatous and necrotizing giant cell myocarditis [39]. There are few cases of patients with GPA and constictive [sic] pericarditis. It was described in patient with GPA who presented with progressive exertional dyspnoea and orthopnoea. The diagnosis was confirmed with cardiac catheterisation and patient was referred for pericardial stripping.
CARDIAC MANIFESTATION IN MICROSCOPIC POLYANGIITIS (MPA)
Microscopic polyangiitis shows lower prevalence than GPA, it affects more men than women and starts at the age of 50 years. Renal involvement is the most common manifestation (79% patients) in MPA patients. However, MPA may also involve the nervous system, the skin, the musculoskeletal system and gastrointestinal system [46, 47]. Cardiac involvement is uncommon and usually occurs in the context of multi-system involvement. In the series of 85 MPA patients only 10% had pericarditis, cardiac failure occurred in 18% and myocardial infarction in 2% [47]. In another cohort of Chinese patients cardiac involvement was present in one-fifth of patients, with pericardial involvement (pericarditis and pericardial effusion), cardiomyopathy, aortic incompetence and rhythm disturbances being described
CARDIAC MANIFESTATION IN EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS (EGPA)
Eosinophilic granulomatosis with polyangiitis is a rare form of systemic, necrotizing small-vessel vasculitis. EGPA is characterized by bronchial asthma, eosinophilia, and eosinophilic tissue infiltration of various organs with granuloma formation [5,49]. It is a rare disease with the annual incidence of 2.4-6.8 cases/million inhabitants, and prevalence of 11-14 cases/million in the general population [49]. Its frequency is highest at the age of 40-60 years with equal gender and ethnic distribution. The pathogenesis of the disease is still unclear, however, it is often assumed to be an autoimmune disease due to altered immune response and presence of antineutrophil autoantibodies (ANCA) in about 40% of patients [50]. In Sinico et al. study, ANCA-positive patients were more likely to have disease manifestation due to small-vessel vasculitis, including necrotising glomerulonephritis, mononeuritis and purpura, whereas ANCA-negative cases were more likely to have cardiac and lung involvement [51]. EGPA is a systemic disease and may affect almost any organ. Most of patients complain of general symptoms, such as malaise, fatigue, fever, arthralgia and weight loss. The respiratory tract involvement consists of asthma and is present in almost all patients. Typical for EGPA is also neurological involvement, that can be found in up to 76% of patients[52]. Of the three types of AAV, cardiac involvement is the most common in EGPA, seen in approximately 16-92% of patient. Cardiac involvement, whether subclinical or clinically manifest, is a poor prognostic factor. Two main mechanism are postulated in the development of cardiac involvement in EGPA patients: vasculitis-related ischemia and eosinophilic infiltration of the myocardium. Any cardiac structure can be involved, and patients present with myocarditis with cardiomyopathy and heart failure, pericarditis with pericardiac effusion(up to 25% of patient), ventricular and supraventricular arrhythmias, coronary arteritis, valvulopathy, intracavitary cardiac thrombosis and sudden cardiac death [52]. There are some cases in literature describing dilated cardiomyopathy in patients with EGPA [53,54]. Jeong et al. presented a case of reversible dilated cardiomyopathy and intracardiac thrombi which was diagnosed by MRI in EGPA patient presented with multifocal cerebral infarction [55]. Ischemia and myocardial infarction in EGPA patients are combined with the inflammatory process and eosinophil accumulation, with epicardial coronary arteries rarely demonstrating any changes during the coronary angiography [56]. Cardiovascular involvement is usually an early manifestation, but it can also occur later in the course of the disease. Majority of patients with heart involvement are asymptomatic. Subclinical cardiac involvement has been described using multimodality screening with electrocardiography, transthoracic echocardiography, and MRI in up to 90 % of patients with EGPA in clinical remission [52]. Moreover, Szczeklik et al. showed in their study that greater degree of peripheral blood eosinophilia at baseline was correlated with higher prevalence of rhythm disturbances and lower left ventricle ejection fraction [52]. Therefore, all patients with EGPA should be studied not only with a detailed history of cardiac symptoms and ECG, but also with echocardiography. In patients with any abnormalities in echocardiography cardiac magnetic resonance should be performed. Cardiac involvement carries a poor prognosis. It causes 50% of the deaths of EGPA patients and is the first cause of death of these patients. Therapy with high-dose corticosteroids plus immunosuppressive agents, particularly cyclophosphamide in case of myocardial inflammation is an life-saving procedure [57]. The use of immunosuppressives to control the activity of EGPA in addition to conventional heart failure medical therapy should be considered in patients with depressed left ventricle systolic function due to myocarditis.
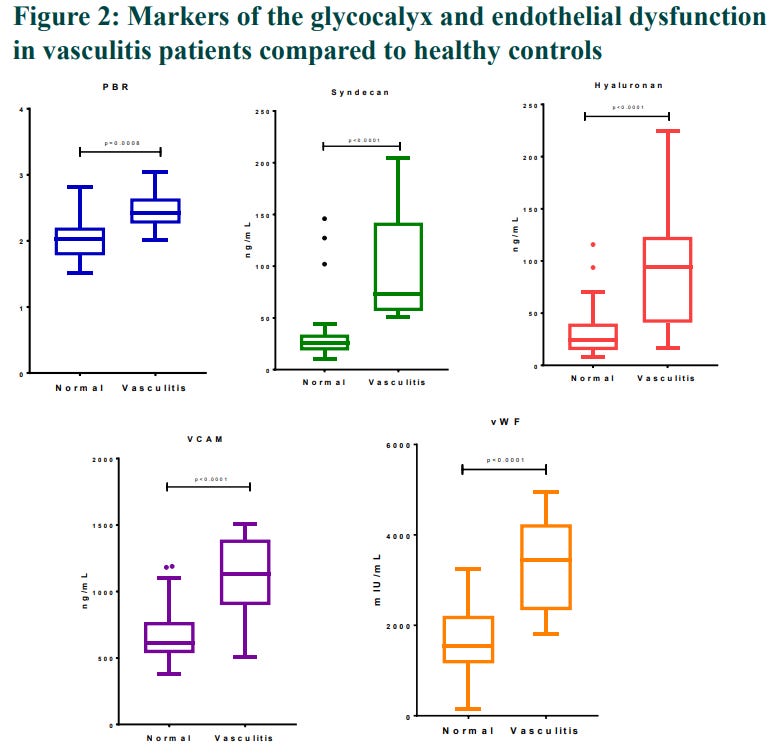
In 2019, Liew et al published a paper: Endothelial Glycocalyx is Damaged in Acute ANCA-Associated Vasculitis and is Improved After Treatment4
This may be read in conjunction with:
They investigated the effects of plasma exchange (PEX) therapy and of 3 months of immunosuppressive therapy:
Figure 2 as follows isn’t very clear but significantly elevated levels were observed, short answer is that they found autoimmune mediated markers of glycocalyx damage, indicative of cardiovascular disease:
PBR (um): Perfused boundary region. “PBR characterizes penetration of red blood cells inside glycocalyx and its thickness can have profound impact on microcirculation and other vascular parameters.”5
Syndecan-1 (ng/mL): “…is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane (type I) heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.
Altered syndecan-1 expression has been detected in several different tumor types. Syndecan 1 can be a marker for plasma cells.”6
Hyaluronan (ng/mL): (KD: Kawasaki disease, CAL: coronary artery lesions). Increased serum hyaluronan may indicate glycocalyx shedding. “Serum syndecan-1 and hyaluronan levels at the acute phase were significantly elevated in KD patients with the CALs than in those without CALs. Serum hyaluronan, not syndecan-1, was determined as the most contributory parameter to predict CALs by a multiple logistic analysis.”7
VACM-1 (ng/mL): Vascular cell adhesion molecule.
“The VCAM-1 protein mediates the adhesion of lymphocytes, monocytes, eosinophils, and basophils to vascular endothelium. It also functions in leukocyte-endothelial cell signal transduction, and it may play a role in the development of atherosclerosis and rheumatoid arthritis.
Upregulation of VCAM-1 in endothelial cells by cytokines occurs as a result of increased gene transcription (e.g., in response to Tumor necrosis factor-alpha (TNF-α) and Interleukin-1 (IL-1)) and through stabilization of Messenger RNA (mRNA) (e.g., Interleukin-4 (IL-4)). The promoter region of the VCAM-1 gene contains functional tandem NF-κB (nuclear factor-kappa B) sites. The sustained expression of VCAM-1 lasts over 24 hours.
…Certain melanoma cells can use VCAM-1 to adhere to the endothelium, VCAM-1 may participate in monocyte recruitment to atherosclerotic sites, and it is highly overexpressed in the inflamed brain. As a result, VCAM-1 is a potential drug target.”8
vWF (mlU/mL): von Willebrand factor “…is a blood glycoprotein involved in hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis.”9
Markers improved after plasma exchange therapy, but apart from for PBR this wasn’t sustained out to 3 months. One can surmise that there was a recurrence of glycocalyx damage mediated by returning antineutrophil cytoplasmic antibodies.
Candlesticks: Baseline - PostPex - 3 months
A search of OpenVAERS reports 2895 cases including the word “vasculitis”. The real figure will be many times this due to the underreporting factor and errors in diagnosis/classification.10
Subclinical cases which manifest in later months and years are unlikely to be filed either.
“As of 6 a.m. EDT Aug. 31, a total of 224,113,439 Americans had been fully vaccinated, or 67.5 percent of the country's population, according to the CDC's data.”11
Mass vaccination, the clues in the name. A doubling of pre-2021 case numbers to say 2% of the “fully vaccinated” may not sound much, but that's potentially another 2 million patients requiring urgent diagnosis and treatment, or may, worst case, be at 80% risk of death within 3 years. And that's just in the USA.
Also to consider, risk may be cumulative with additional boosters and/or reinfection.
This site has some excellent photos of typical clinical presentations for different types of vasculitis:
https://www.vasculitis.org.uk/about-vasculitis/vasculitis-images
(Formerly Wegener’s Granulomatosis)
This morning I felt ok but after fixing breakfast the symptoms came back and they feel like the same symptoms I had when I had the vasculitis in 2009-2010 and that concerned me so I discussed it with a Neurologist and Immunologist who I will reach out to after the holidays to schedule an appointment
I received my shot at appx 1300 hours. By 1700 hours I had a flare up of Vasculitis symptoms. I began to get little red dots on both of my lower legs a total of about 10. They began really light and over the night got brighter and bigger. I went to the doctor the next morning and was given a prescription for a steroid and antibiotic which I first took at 2000 hours on 12/31/2020. By the next evening my dots began to get lighter. As of today all but one have faded away. The one I have is really light. I did have some of the usually listed symptoms, slight headache, runny nose, hard to breathe.
12 days after receiving the vaccine the patient developed red painful bruises to both feet that start mildly, but continued to worsen over the next several days. The patient went to see his dermatologist and was diagnosed with Leukocytodastic Vasculitis.
On the back a zone of redness / urticarial up to the spine; Shivering; Malaise; Painful feeling at the spine / back, band-shaped to the front of the thorax; Extensive zone of vasculitis from armpit to right hip crest (A4 size vertical); Nausea; Burning feeling; Painful feeling at the spine / back, band-shaped to the front of the thorax; alk phosphatases very slightly increased (108 U / l); Blood clotting problem; Fever; Sudden death; Skin problem; Highly elevated D dimers 3227; This is a spontaneous report from a contactable physician downloaded from a regulatory authority-WEB, regulatory authority number BE-FAMHP-DHH-N2021-88081. A 87-year-old female patient received the first dose of bnt162b2 (COMIRNATY, Solution for injection, lot number was not reported), via an unspecified route of administration on 26Mar2021 at a single dose for covid-19 immunisation…death 06Apr2021 sudden and unexpected in the morning On 06Apr post mortem PCR covid negative one RXTX and RX abdomen showed no major signs. clinical examination was not abnormal except for vasculitis.
Ischemic stroke; This is a spontaneous report from a contactable physician downloaded from the Regulatory Authority (RA)-WEB, regulatory authority number FR-AFSSAPS-SE20211242. A 52-year-old male patient received his first dose of bnt162b2 (COMIRNATY), as an intramuscular single dose, administered in left arm, on 23Apr2021 (Lot Number: EX0893), for covid-19 immunisation. Medical history included infarct myocardial in 2015, ST elevation acute coronary syndrome treated with thrombolysis and drug-eluting stents insertion in left anterior descending coronary artery in 2015, sensory polyneuropathy of lower limbs under follow-up from 2006, dyslipidemia, active smoking/ ongoing tabaquism, alcohol consumption. Concerning COVID-19: the patient was not a risk to develop a severe COVID-19 (history of infection and testing, if any, was unknown). Chronic treatment/ concomitant medications included DL-lysine acetylsalicylate (KARDEGIC, strength 75), oxazepam (SERESTA, strength 50 mg) in the evening, nebivolol (strength 5), perindopril (strength 4), eplerenone (INSPRA, strength 25) once daily. Course of the event: On 23Apr2021, the patient was vaccinated for the first time with COVID-19 mRNA Vaccine (nucleoside modified) (COMIRNATY), by intramuscular route in the left arm. On 12May2021 in the evening, the patient developed right upper arm paresis, considered as not significant by the patient. On 13May2021, aphasia prompted visit to the emergency room. Brain magnetic resonance imaging revealed ischemic stroke in the left middle cerebral artery territory. Transthoracic echocardiography returned normal. Electrocardiogram showed sinus rhythm. Neck Doppler ultrasound was unremarkable. DL-lysine acetylsalicylate was continued. The initial course was favorable. On 21May2021, the patient experienced consciousness impairment with meningeal irritation. Computed tomography disclosed hemorrhagic transformation of previous stroke with severe hematoma, intraventricular bleeding and dilation of ventricular system. Subfalcine herniation was also observed. On 22May2021, neurological condition rapidly worsened, required surgical management with hematoma drainage, placement of external ventricular drain in the left frontal horn and intracranial pressure sensor. During and after procedure, the patient presented with bilateral mydriasis. Following discharge from the intensive care unit, intracranial pressure was uncontrollable. No more surgery was indicated given the poor clinical condition and the severity of cerebral lesions. Intracranial hypertension was refractory, uncontrollable. On 23May2021, the patient died. The final diagnosis and cause of death was Ischemic stroke.
Symptoms
Anti-cyclic citrullinated peptide antibody, Anti-neutrophil cytoplasmic antibody positive vasculitis, Antinuclear antibody, Antiphospholipid antibodies, Beta-2 glycoprotein antibody, Blood bilirubin, Blood cholesterol, Blood fibrinogen, Blood triglycerides, Borrelia test, CSF pressure, Cardiolipin antibody, Coagulation factor V level, Coagulation factor VIII level, Computerised tomogram, Computerised tomogram head, Cytomegalovirus test, Echocardiogram, Electrocardiogram, Epstein-Barr virus test, Fundoscopy, Glomerular filtration rate, HIV test, HTLV-1 test, HTLV-2 test, Haemoglobin, Hepatitis B virus test, Hepatitis C virus test, High density lipoprotein, Investigation, Ischaemic stroke, Low density lipoprotein, Magnetic resonance imaging head, Platelet count, Protein C, Protein S, Prothrombin time, Rheumatoid factor, SARS-CoV-2 test, Toxoplasma serology, Treponema test, Ultrasound Doppler, Vascular imaging, White blood cell count, Whole body scan
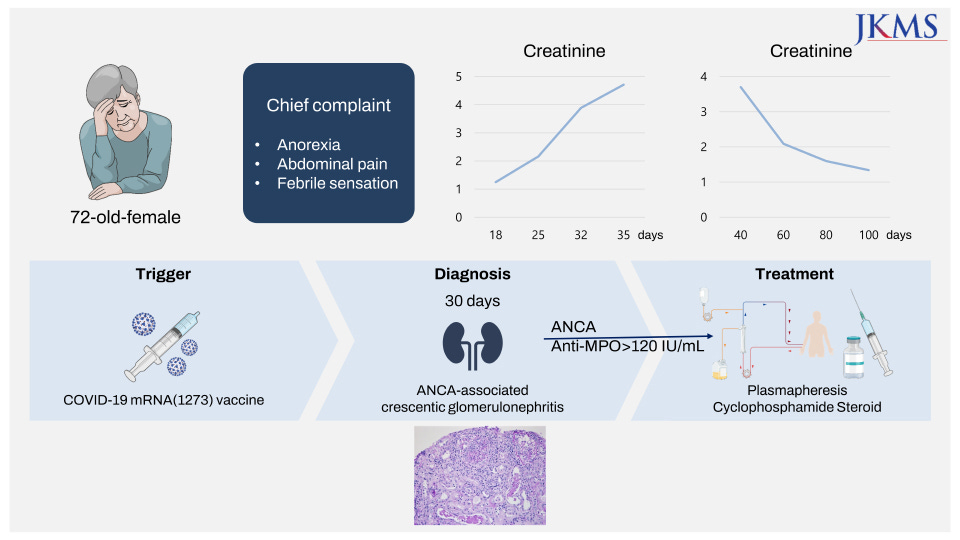
On 4th July ‘22, Kim et al published A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination12
Abstract
Despite that clinical trials have been examining the safety profile of coronavirus disease 2019 (COVID-19) vaccines, there are concerns about long-term side effects as the number of vaccinations increases. Herein, we report a case of new-onset renal-limited anti-myeloperoxidase (MPO) antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis after booster vaccination with the mRNA 1273 (Moderna) vaccine. A 72-year-old woman with no specific past history, and who had a normal renal function, developed ANCA-associated vasculitis following heterologous booster with mRNA1273 (Moderna) vaccine. After a kidney biopsy, she was diagnosed with ANCA-associated pauci-immune crescentic glomerulonephritis. Her renal function and constitutional symptoms have been improved with treatment with plasmapheresis, intravenous cyclophosphamide and steroid pulse therapy (intravenous 500 mg of methylprednisolone sodium succinate for 3 days) followed by a reduced steroid regimen.
“Crescentic glomerulonephritis (GN) is a chronic immune-mediated disease which causes severe glomerular inflammation and injury, and often leads to irreversible kidney failure. It is a common cause of morbidity and mortality worldwide. GN is a major contributor to the escalating health burden associated with CKD.”13
DISCUSSION
After the rapid authorization of COVID-19 vaccines worldwide, there have been concerns about de novo ANCA-associated glomerulonephritis following COVID-19 vaccination. There has been debate about the causality and relationship between vaccination and new-onset vasculitis. Among the patients who developed ANCA-associated vasculitis, symptoms appeared not only after the first dose but also after the second dose, and in some patients, symptoms worsened with serial doses. Given that ANCA vasculitis is a rare disease, with an incidence of 1.5–16 per 1 million person-year and a prevalence of 9–95 per 1 million person-year, it is noteworthy that the reported cases of ANCA-vasculitis after COVID vaccination increase. Notably, this patient’s symptoms began after she received the second dose of ChAdOx1 nCoV-19 (Oxford AstraZeneca) COVID-19 vaccine. Although there are certain possibilities that the immune cascades may have started on her initial doses of Oxford AstraZeneca vaccines, we believe her third dose with mRNA1273 (Moderna) played a critical role in developing ANCA-associated vasculitis. The evidence that there have been more reported cases of ANCA vasculitis following mRNA vaccines than following mRNA vector vaccines supports the idea. Furthermore, the lipid-nanoparticle-formulated mRNA vaccines coding for the SARS-CoV-2 full-length spike protein can induce immunogenic pathways. It has been previously demonstrated that mRNA vaccines can promote more robust adaptive and native immune reactions via prolonged S-specific germinal center B-cell responses and antigen-specific Th1-skewed immunity. It is hypothesized that these promoted immune responses can provide an effective defense mechanism against SARS-CoV-2; however, they might also provoke glomerular diseases in predisposed individuals. Moreover, a recent comparative study on using the same vaccine as the primary series (homologous boosters) and using a different vaccine (heterologous boosters) showed that the immunogenicity of heterologous boosting was generally similar to or greater than those of homogenous boosting. Further studies are required to confirm whether the increased immune response of heterologous injection of COVID-19 vaccinations contributes to a higher incidence of ANCA-associated vasculitis. Therefore, clinicians should be aware of de novo ANCA vasculitis following COVID-19 vaccination in this pandemic situation.
On 19th August ‘22, Irure-Ventura et al published their findings:
Increased induction of de novo serum ANCA and ANCA-associated vasculitis after mass vaccination against SARS-CoV-214
Abstract
Different immune-mediated diseases have been described after SARS-CoV-2 vaccination, with antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) being one of the possible side effects. In this study, a total of 35 patients presented ANCA for the first time during 2021, with the number during 2019 being 15. Twenty-seven out of thirty-five patients developed ANCA after vaccination. Two of them developed these antibodies after receiving the first dose (7.4%), and 25 patients developed ANCA after the second dose of the vaccine (92.6%), with BNT162b2 being the main vaccine received by these patients. In 97.1% of the patients who developed ANCA during 2021, the positivity of ANCA was accompanied by systemic involvement, with renal and respiratory tracts being the main organs affected. Therefore, an increase in the development of AAV has been observed during 2021 in comparison with 2019, which could be due to the administration of SARS-CoV-2 vaccine.
Keywords: Clinical finding; Disease; Health sciences.
ANCA-associated vasculitis (AAV) is characterized by the development of autoantibodies against antigens in cytoplasmic granules of neutrophils affecting predominantly to small vessels, with myeloperoxidase (MPO) or proteinase 3 (PR3) being the main antigens toward which these autoantibodies are directed. Although the development of ANCA after influenza vaccination has been previously reported, most of the studies that analyze the possible relationship between SARS-CoV-2 vaccination and the development of ANCA and AAV correspond to case reports.
Results
A total of 35 patients presented ANCA and/or anti-GBM antibodies for the first time during 2021, with the number during 2019 being 15. Throughout 2019 and 2021, a similar number of ANCA and/or anti-GBM antibodies determinations were carried out. Specifically, this study was performed in 1,287 patients in 2019 and 1,434 patients during 2021. Monitorization of the mentioned autoantibodies was not taken into account. Consequently, the percentage of patients who debuted with these autoantibodies in 2019 was 1.17%, whereas in 2021 this percentage increased to 2.44%, the differences observed being statistically significant (p = 0.020) (Figure 1). Mean age of the patients included was 65.8 and 63.1 years in 2019 and 2021, respectively. No statistically significant difference on gender was observed. In 2019, the most frequent ANCA was anti-MPO, being present in 80.0% of the patients. However, in 2021, an increase in the positivity for anti-PR3 antibody was observed, which was presented in 45.8% of the patients. Despite nonsignificant differences in the frequency of anti-PR3 antibodies between 2019 and 2021, an important increase of the detection of this autoantibody was observed in 2021
Only 8 out of 35 patients detected in 2021 developed ANCA before receiving SARS-CoV-2 vaccine. Of the remaining 27 patients, 2 patients developed ANCA after receiving the first dose (7.4%), being BNT162b2 the vaccine administrated in all cases, and 25 patients developed ANCA after the second dose of the vaccine (92.6%), being the vaccination schedule received at that moment: two doses of BNT162b2, two doses of mRNA-1273, two doses of AZD1222, and one dose of Ad26.CoV2.S plus one dose of mRNA-1273, in 87.5%, 4.2%, 4.2%, and 4.2%, respectively. The median time for ANCA detection was 24 days after the first dose (IQR 6–42 days) and 102 days (IQR 46.5–130.5 days) after the second dose of the vaccine.
Four patients included in the present study presented SARS-CoV-2 infection during the follow-up. However, in all four cases the developed of ANCA occurred after vaccination and before the viral infection.
In 97.1% of the patients who developed ANCA during 2021, the positivity of ANCA was accompanied by systemic involvement (Table 2). The activity index following BVAS score of the patients who debuted with AAV during 2021 was 18.8 (SD 6.5) in comparison with those patients who debuted in 2019 in whom a mean of 11.4 (SD 5.2) was observed, with no statistically significant differences (p = 0.741). Renal and respiratory tracts were the main organs affected. Kidney biopsy was carried out in 8 of the patients who presented renal manifestations during 2019 (80%) and 2021 (47%). In the rest of the cases, the kidney biopsy could not be carried out due to the clinical status of the patients. In all of the biopsied patients who debuted with ANCA during 2019 and 2021, the biopsy showed data of pauci-immune extracapillary glomerulonephritis.
Most patients in our series developed ANCA after the second dose of the vaccine (68.6%), but the detection of these autoantibodies varied from 6 days after the first dose to more than 5 months after the second dose.
Unlike expected, in Southern European countries including Spain, the incidence of anti-MPO antibodies usually is higher than anti-PR3 antibodies. However, in the present study a relevant increase in the frequency of anti-PR3 antibodies in 2021 (45.8%) compared with 2019 (20.0%) was observed. There is no clear explanation for such a finding. A possible limitation of the present study could be that ANCA were only tested using chemiluminescent methods for anti-MPO and anti-PR3 antibodies. ANCA were not tested using IIF, so other ANCA specificities different from those mentioned could not be detected.
New-onset autoimmune manifestations following SARS-CoV-2 vaccination are being reported extensively, molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants being the main mechanisms through which these vaccines trigger autoimmune phenomena. Previous studies indicate that the use of human papillomavirus, hepatitis B, and influenza vaccines may trigger the onset or exacerbations of autoimmune diseases by molecular mimicry inducing autoimmunity (Segal and Shoenfeld, 2018). However, whether the association between SARS-CoV-2 vaccine and autoimmune manifestations is coincidental or causal remains to be elucidated. Further studies and longer-term follow-up studies are necessary to elucidate the underlying mechanisms and identify the exact cause of these autoimmune manifestations.
On 26th July ‘22, Bolkhovitina et al published their findings on Short-Term Effect of SARS-CoV-2 Spike Protein Receptor-Binding Domain-Specific Antibody Induction on Neutrophil-Mediated Immune Response in Mice.15
Their hypothesis is as follows. Unfortunately the clinical case reports indicate a net increase in cases of autoimmune mediated vascular damage vs those being protected by transfection:
In accordance with the trained immunity concept, vaccination can induce the alteration of innate immune cell functions [8]. In the present study, we demonstrated the alteration of neutrophil functional activity, i.e., NET formation ability, during the specific antibody induction. Although our data suggest the antibody-mediated effects on neutrophil maturation and the suppression of neutrophil reactivity as a result of vaccination, it is worth mentioning that not all the antibodies have a protective effect in the inflammation. Autoantibodies, such as antinuclear antibodies (ANA) and antiplatelet autoantibodies (APA), are thought to be the causes of COVID-19 complications [37,38]. While APA are associated with thrombocytopenia, ANA can be responsible for the vasculitis in COVID-19 complications [39,40]. Interestingly, anti-neutrophil cytoplasmic antibodies (ANCA), directed against proteins of neutrophil cytoplasmic granules, were detected in COVID-19 patients [41,42]. ANCA can be induced by the NET remnants. Moreover, ANCA can facilitate netosis [43]. Several cases reports support the evidence of post-COVID-19 ANCA-associated vasculitis [44]. The data provided in the present study demonstrate that RBD-specific antibody induction suppresses NET formation, supporting the preventive effects of vaccination in the case of vascular complications. Unfortunately, a number of case reports demonstrate that neutrophil-associated complications and vasculitis can be triggered by vaccination [45,46]. Enhanced pathogen-induced neutrophil recruitment in the case of RBD-specific antibody induction observed in the present study can in part explain the adverse reactions to vaccination.
Thus, the effects of SARS-CoV-2 spike protein RBD-specific antibody induction are complex and not restricted to adaptive immunity activation. Among the short-term effects of specific antibody induction on innate immune response are the enhancement of neutrophil recruitment, circulating neutrophil maturation, and reactivity suppression.
This is where our story gets interesting, “ANCA-associated granulomatous vasculitis” was renamed from “Wegener’s granulomatosis” due to its association with a Nazi doctor who may have experimented on prisoners:
What’s in a name? How Wegener’s granulomatosis became GPA16
Physician and history buff Eric L. Matteson, MD, professor of medicine in the division of rheumatology at the Mayo Clinic College of Medicine and Science, explained the complicated scenario on Healio’s Rheuminations podcast. “Friedrich Wegener was a physician pathologist who trained pre-World War II, but during World War II he was a member of the brown shirts, or SA,” he said. “He was assigned as chief pathologist in occupied Poland.”
Specifically, his post was in the city of Lodz, which Matteson pointed out is the site of the first enclosed ghetto the Nazis created for Jewish citizens. “It is only about 30 miles from the first extermination camp,” he added. “It emerged that, as health officer for that ghetto . . . he oversaw a couple of other physicians who were known to be doing medical experimentation. But, of course, it was not really experimentation, because it was done on victims of oppression, the ghetto inhabitants.”
After more thorough investigation, Woywodt and Matteson published a paper in Rheumatology in 2006 that more fully described the life, both inside and outside of the clinic, of Wegener. Matteson allows that it was not clear that Wegener himself was involved in the experiments, but he was certainly there at the time.
The disease was named for Wegener in 1954. However, Matteson pointed out that this is problematic for one important reason. “[Wegener] wasn’t the first person to describe the disease anyway,” he said. “It turns out a guy named Fritz Klinger, who, of all things, was a roommate of his at medical school, actually described it first in 1931, which is 5 years before Wegener.”
“We recognize the difficulty inherent in seeking a replacement term for a long-established disease name for this complex multisystem illness with highly variable clinical presentations,” Falk and colleagues wrote. They suggested that although the “replacement term is neither perfect nor encompasses all aspects of the pathophysiology and clinical spectrum of the disease,” the new term has utility for a number of reasons. Including “granulomatosis” gives a nod to both the history and the pathology of the syndrome, while including “polyangiitis” acknowledges its multi-vascular nature.
In conclusion, experimental gene therapy treatments should immediately be suspended pending further clinical investigations into transfection mediated autoimmune disorders, amongst other adverse events.
Mass screenings of transfected older and at-risk cohorts for ANCA antineutrophil cytoplasmic antibodies should also be conducted so that treatments can be commenced at the subclinical stage before vascular lesions have had time to develop, with potentially fatal outcomes.
Further reading:
Lavanya Kamesh, Lorraine Harper and Caroline O. S. Savage. ANCA-Positive Vasculitis. (2002).
JASN July 2002, 13 (7) 1953-1960; DOI: https://doi.org/10.1097/01.ASN.0000016442.33680.3E
Bouiller K, Samson M, Eicher JC, Audia S, Berthier S, Leguy V, Humbert O, Martin L, Lorgis L, Cottin Y, Bonnotte B, Lorcerie B. Severe cardiomyopathy revealing antineutrophil cytoplasmic antibodies-negative eosinophilic granulomatosis with polyangiitis. Intern Med J. 2014 Sep;44(9):928-31. doi: 10.1111/imj.12525. PMID: 25201426.
Życińska K, Borowiec A. Cardiac manifestations in antineutrophil cytoplasmic autoantibody (ANCA) - associated vasculitides. Kardiol Pol. 2016;74(12):1470-1476. doi: 10.5603/KP.a2016.0138. Epub 2016 Oct 7. PMID: 27714710.
https://journals.viamedica.pl/kardiologia_polska/article/view/KP.a2016.0138/58089
Liew, Hui & Roberts, Matthew & Mcmahon, Lawrence. The Endothelial Glycocalyx is Damaged in Acute ANCA-Associated Vasculitis and is Improved After Treatment. (2019).
Gorshkov AY, Klimushina MV, Boytsov SA, Kots AY, Gumanova NG. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation. 2018 May;25(4):e12454. doi: 10.1111/micc.12454. PMID: 29608790.
Syndecan 1 - Wikipedia
Gorshkov, AY, Klimushina, MV, Boytsov, SA, Kots, AY, Gumanova, NG. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation. 2018; 25:e12454. https://doi.org/10.1111/micc.12454
VCAM-1 - Wikipedia
von Willebrand factor - Wikipedia
States ranked by percentage of population fully vaccinated
Kim BC, Kim HS, Han KH, Han SY, Jo HA. A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination. J Korean Med Sci. 2022 Jul;37(26):e204. https://doi.org/10.3346/jkms.2022.37.e204
Al Mushafi A, Ooi JD, Odobasic D. Crescentic Glomerulonephritis: Pathogenesis and Therapeutic Potential of Human Amniotic Stem Cells. Front Physiol. 2021 Oct 15;12:724186. doi: 10.3389/fphys.2021.724186. PMID: 34721059; PMCID: PMC8554237.
Irure-Ventura J, Belmar-Vega L, Fernández-Fresnedo G, González-López E, Castro-Hernández C, Rodrigo-Calabia E, Heras-Vicario M, Ruiz San Millán JC, López-Hoyos M. Increased induction of de novo serum ANCA and ANCA-associated vasculitis after mass vaccination against SARS-CoV-2. iScience. 2022 Aug 19;25(8):104847. doi: 10.1016/j.isci.2022.104847. Epub 2022 Aug 2. PMID: 35937087; PMCID: PMC9344695.
Bolkhovitina EL, Vavilova JD, Bogorodskiy AO, Zagryadskaya YA, Okhrimenko IS, Sapozhnikov AM, Borshchevskiy VI, Shevchenko MA. Short-Term Effect of SARS-CoV-2 Spike Protein Receptor-Binding Domain-Specific Antibody Induction on Neutrophil-Mediated Immune Response in Mice. Int J Mol Sci. 2022 Jul 26;23(15):8234. doi: 10.3390/ijms23158234. PMID: 35897803; PMCID: PMC9331224.
What’s in a name? How Wegener’s granulomatosis became GPA















Great research and deep dive. Dr. Bruce Patterson has research along the same lines, but using the monocyte model of pathology. He recommends statins to reduce the glycocalyx damage and reduce adhesion. Early diagnosis and treatment is vital to any resolution, and perhaps proteolytic enzymes like Nattokinase and steroids to reduce inflammation. It is notable that people with PASC have low cortisol levels that control the release of ACTH and CRH. You shine a light into dark corners and will save lives.
I don't speak the language, but I believe I have an idea of what's going on here. Thanks!