Cancer Megathread #34: SARS-CoV-2, synthetic spike protein and heat shock protein mimicry
Another day, another cancer mechanism
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Also available with the translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc:
TL;DR: Vector vaccines and Pfizer’s BNT162b2 have been demonstrated to clinically significantly increase levels of autoimmune antibodies to heat shock proteins including HSP60 and HSP70. Elevated levels persisted for at least 2 months. This was due to molecular mimicry of spike protein antigens. Imbalances in HSP levels are associated with a range of diseases and accelerated progression.
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
A section on heat shock proteins (HSPs) was originally going to be included in IgG4 class switching and Immunoglobulin G4-related aortitis - Part V: Plasma cells and Th1>Th2 cytokines. The relevance being that higher expression of stress-related genes was associated with protein expression in the endoplasmic-reticulum. This is a common factor to both IgG4-RD and Pfizer-induced spike protein expression. It deserved its own Substack, if not a literature review paper, due to the pathologies associated with this. These include autoimmune disorders and tumour progression.
The literature describes the presence of HSP autoimmune antibodies (autoAbs) as being a marker for the progression of many cancers, as do the HSPs themselves. I would go further than this and wish to highlight that this may be a problem because there is evidence that at low levels HSPs may act as tumour suppressors, hence autoAbs to them may be favourable for cancer progression.
Discussion
What are heat shock proteins?
As part of the evolutionary process, protein stability is crucial so that the organism carrying a beneficial mutation is able to survive long enough to reproduce, due to having a selective advantage. Heat stress is a problem at the cellular level as high temperature tends to cause proteins to become unstable. Temperature changes shift the equilibrium between folded and unfolded states1.
Heat shock proteins are a family of cytoprotective molecular chaperones. Their role in the health of the organism is to ensure that proteins that become misfolded due to stress are either refolded correctly or degraded. If the rate of accumulation of misfolded proteins exceeds the rate at which HSPs and other processes can deal with them then misfolded proteins may accumulate.
These may even form amyloid fibrils with ordered cross-β-sheet structures. These are associated with many neurodegenerative disorders, such as Parkinson’s Disease, Alzheimer’s disease and amyotrophic lateral sclerosis2. ALS is a fatal disorder, that is also known as Lou Gehrig's disease or motor neuron disease.
The first hypothesis we can make is that if autoAbs to HSPs cause their concentration to fall, this may be associated with the accelerated progression of many diseases that involve misfolded proteins.
In short, the levels of HSPs need to be kept in a tight range until we need them to respond to the presence of misfolded proteins or to protect a cell from ER stress-induced apoptosis. Any condition, drug or agent that disturbs this delicate balance may, in the longer term, help contribute to the progression of some cancers or neurodegenerative disorders.
This is a Richardson, or ribbon diagram depiction of HSP70, with its flexible “lid”:

In terms of action, HSP70 processes misfolded proteins in a cycle. With the lid open, an aggregation of misfolded proteins is presented by HSP40. These may then be further processed in two ways with the lid closed - either their destruction via ubiquitination, or spontaneous refolding followed by release as a protein in its native state.
Behold the HSP sausage factory at work:

Apart from acting as a chaperone for misfolded proteins, HSP70 has dual roles, depending on whether it is expressed in the intracellular or extracellular space.
Within the cell, it can act as an anti-inflammatory cytokine, whereas outside the cell HSP70 may trigger pro-inflammatory pathways. Cancer progression is more associated with intracellular HSP70.
An adopted term for proteins with this property is “chaperokine”:
Chaperokine is a term recently coined to better describe the dual role of extracellular heat shock protein as both chaperone and cytokine. The augmentation of intracellular Hsp72 expression has clearly been demonstrated to be cytoprotective; with the ability to activate anti-apoptotic and anti-inflammatory pathways, exerting inhibitory effects on cell cycle progression and suppressing genes important in proliferation and differentiation.
On the other hand, the role of extracellular Hsp72 is only now being elucidated and has been demonstrated to play a cytostimulatory role by enhancing proinflammatory cytokine and chemokine synthesis, up-regulate co-stimulatory molecule expression, enhance the maturation of dendritic cells and promote antitumour surveillance. This paper covers the most recent advances in elucidation of the mechanism by which the chaperokine activity of Hsp72 is transduced, addresses its biological significance and, finally, covers how it is being harnessed to produce novel therapeutic agents.
From: “Hsp70: a chaperokine” (2008)
Intracellular vs extracellular roles. As the caption reads, in pathologies “… imbalance leads to disease progression”:

Engineered mRNA agents and HSP autoAbs
The first paper, “Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies” (2023) by Böröcz et al. discusses how the Pfizer product mimics SARS-CoV-2 viral pathology in such a way that it markedly increases autoAbs to heat shock proteins 60 and 70. Nice work!
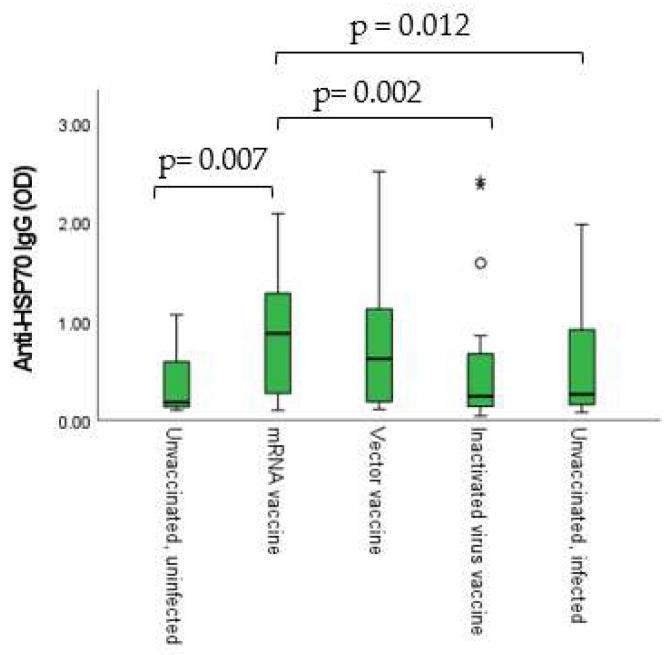
As shown in Figure 7, statistically significant positive connections were found between the immunization-induced anti-SARS-CoV-2 humoral and cellular immune responses and the anti-HSP70 IgG levels.

Anti-HSP70 IgG levels showed statistically significant differences between the different vaccination groups. The difference was the most prominent between the inactivated virus vaccine and the mRNA vaccine groups (p = 0.002) (Figure 8).
Both vector vaccines and mRNA vaccines both mimic the effects of natural infection on anti-HSP70 autoAbs.
Examples of vector vaccines include Janssen/Johnson & Johnson, AstraZeneca and the University of Oxford COVID-19 vaccines:
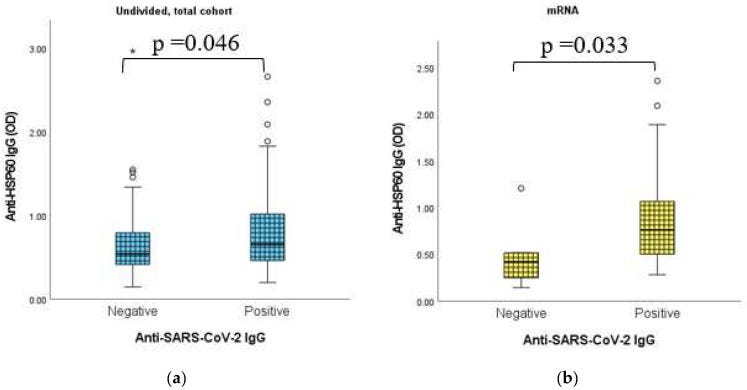
Anti-HSP60 autoAbs were also upregulated by mRNA vaccines, and there was a direct correlation with anti-SARS-CoV-2 IgG antibodies.
IgG sub-classes were not stated:
… HSP70 has already been described to work as a “double agent”, acting inside and outside the cell [56]. It has been found that Hsp70-derived epitopes interact with the immune cell components, consequently stimulating the humoral autoimmune response and production of the anti-HSP70 autoantibodies [56].
Bearing in mind the de facto bioweapons lab origins of the virus, this may not be a coincidence:
Considering the phenomenon of molecular mimicry, it has already been described that 17 human HSP proteins belonging to inter alia Hsp60, Hsp70 and Hsp90 chaperones share immunogenic epitopes (at least six amino acids) with SARS-CoV-2 proteins, as analyzed by the free Immune Epitope Database and Analysis Resource [57].
Plasticity of immune response is important:
Evidently, the plasticity, being one of the most important features of the immune response, is also true in the case of HSPs [58]. Through molecular mimicry, the infection may cause the exposure of nonself-antigens to the immune system. The evolutionary conservation of heat shock proteins induces cross-reactivity with self-HSP antigens [58].
The greater the levels of IgG antibodies and anti-viral type-2 interferon-gamma (IFN-γ), the more HSP70 autoAbs you tend to find. As we saw in Figure 7, this also applies to engineered mRNA agents:
Considering all of these, it was expected that we would find connections between anti-HSP70 results and the immunization-induced immune response. In fact, a statistically significant positive connection was found between IgG isotype humoral antibody levels and anti-HSP70 results, as well as between IFN-γ release assays (IGRA) and anti-HSP70 results. Additionally, connections were observable between the different vaccination groups (vaccine types) and the anti-HSP70 IgG levels.
But what does this mean, with regard to disease or vaccine side effects? Our next papers suggest that these autoAbs may, for starters, be associated with hearing loss. In this case, due to molecular mimicry, HSP70 autoAbs also target cells expressing an inner-ear endothelial protein. It’s called the Cogan peptide, and it shares sequence homology with several proteins. These include CD148, gap-junction protein connexin 26 and ANCA autoAbs, amongst others.
Although infection increases levels of HSP autoAbs its less of a problem for you if unvaccinated as you typically quickly become immune to the virus, viral loads are low and the number of repeat infections is low.
Whereas if you are boosted the immune stimulatory effects are, by design, similar to having a severe COVID infection for the first time, every time. And the resultant impaired immune responses and IgG4-associated breakthrough infections further increase the risk of triggering autoimmune disorders.
Previously, I’ve written about engineered mRNA agents and elevated vasculitis risk.
Key takes from “Cogan's syndrome: anti-Hsp70 antibodies are a serological marker in the typical form“3 (2014) by Bonaguri et al.:
Abstract
Background: Cogan's syndrome (CS) is a rare autoimmune vasculitis characterized by ocular inflammation and sensorineural hearing loss. CS is divided into a "typical" form with non-syphilitic interstitial keratitis and audiovestibular symptoms, and an "atypical" form with ocular involvement affecting structures other than the cornea. Anti-Hsp70 antibodies were found at variable levels in patients presenting with various forms of autoimmune sensorineural hearing loss (ASNHL).
Objectives: To assess the correlation between anti-Hsp70 antibodies and specific ASNHL subgroups.
Methods: We divided 112 subjects into four groups: 14 subjects with typical CS, 24 with atypical CS, 55 with ASNHL, and 19 control subjects (healthy subjects and patients with systemic autoimmune diseases but no sensorineural hearing or audiovestibular alterations). Patients were tested for serological autoimmunity markers including anti-Hsp70.
Results: Positivity of the anti-Hsp70 antibody test was highest in the typical CS group (92.9%) and lowest in the control group (5.2%). The test was positive in 52.7% of patients in the ASNHL group and 16.6% in the atypical CS group. The paired comparison analysis between groups showed that sensitivity of anti-Hsp70 in the typical CS group was significantly higher, as compared to the other three study groups.
Conclusions: Anti-Hsp70 antibodies can be considered a serological marker of "typical" CS. "Atypical" CS is conceivably a sort of "melting pot" of different forms of autoimmune diseases still characterized by ocular inflammation and sensorineural hearing loss but whose antigenic characteristics need to be further defined.
Correlation isn’t causation, but I have written at length about autoimmune pathologies and aneurysms:
… The disease was probably present long before this formal identification, and a prominent example is the celebrated composer Ludwig von Beethoven [2]. CS is a rare autoimmune vasculitis characterized by ocular inflammation and sensorineural hearing loss; 10% of patients have abdominal aneurysms. General symptoms of the acute inflammatory phase are often non-specific and may include headache,fever, dizziness, arthralgia and abdominal pain. As a result,patients may first be referred to a neurologist for vertigo, or to a surgeon for abdominal symptoms [3].
A lab test may come back negative, but this doesn’t mean you don’t have Cogan's syndrome:
… Laboratory tests commonly used to detect autoimmune diseases are rarely positive for CS. A negative result does not exclude the possibility of the disease. Therefore, diagnosis remains essentially clinical and chiefly involves ophthalmolo-gists and otolaryngologists. If not promptly identified, CS may progress to deafness, severe visual impairment, and disability.
Around half the patients with Cogan’s syndrome have HSP70 autoAbs:
… Multiple studies support an autoimmune pathogenesis for CS. They indicated various antibodies directed against inner ear, corneal and endothelial antigens as possible etiology and serological markers of the disease [15,16]: in particular, anti-bodies against a peptide antigen (Cogan peptide) which shares sequence homology with CD148 and connexin 26, ANCA,and anti-Hsp70 antibodies. In the present study, the rate of CS patients (typical and atypical forms) who tested positive for anti-Hsp70 antibodies at least once during the follow-up was similar to that found in a prior pilot study (45% vs. 50%)[13].
From a paper dating back to 2003, we learn that some autoAbs can indeed attack your auditory cilia (inner-ear hair cells), resulting in deafness:

… The homologies we recorded included laminin and ladinin (another cell-adhesion molecule) and kinesin and calcineurin. Autoantibodies against these molecules are seen in autoimmune diseases of the skin or collagen. Microtubule-associated kinesins are contained in the cilia and flagella of eukaryotic and prokaryotic cells. Kinesin-2 is needed for transport pathways in motile and non-motile cilia and flagella; in these organisms it is essential for sensory functions in ciliated neurons such as the otic neuroepithelium. An immune response against ciliated or flagellated microorganisms can thus become an autoimmune response against ciliated human cells—eg, inner-ear hair cells— because of molecular mimicry between exogenous and endogenous kinesins.
From: “Cogan's syndrome as an autoimmune disease“ (2003)
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(03)12478-6/fulltext
As with abdominal aneurysms, there is also a link between IgG4-RD and autoimmune sensorineural hearing loss (SNHL)4 5. Not unrelated to this, a study from 1995 by Rauch et al. found that 47% of patients with Menière's syndrome had HSP70 autoAbs. Symptoms of Menière's include fluctuating sensorineural hearing loss, episodic vertigo, and tinnitus.
Of those with bilateral Menière's (i.e. both ears affected), 59% had HSP70 autoAbs6.
Key takes from the 2006 paper, “Antibody reactivity to heat shock protein 70 and inner ear-specific proteins in patients with idiopathic sensorineural hearing loss“ by Tebo et al.7:
Aetiology: The cause, or causes of disease.
Idiopathic: Any disease or condition that arises spontaneously, without a known cause.
… Deafness is attributable to autoimmunity in a subset of adult patients with sensorineural hearing loss (SNHL) of unknown aetiology.
… HSP70 antibodies have been reported in many inflammatory diseases [18] and may mark the early onset of hearing loss.
From:
https://pmc.ncbi.nlm.nih.gov/articles/PMC1810414/

As a reminder of the vax-apartheid segregation we were subjected to, this was from August 2021 and Paul was in attendance:

Duration of autoimmunity to HSP60 & HSP70
From “Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies” we learned that the autoAbs were measured in blood drawn after 2 months, which further confirms the correlation with mRNA-induced IgG levels, which may take 2-3 months to normalise.
The concern here is that just as anti-Spike antibodies may persist for years (as in IgG4-RD, Long-COVID or Long-Vax), anti-HSP60 & anti-HSP70 autoAbs may also persist indefinitely. This becomes all the more likely if the Spike epitopes have homology to many human HSP epitopes. Alarmingly, there is potentially no practical limit to repeat exposure and duration.
And regardless of that, each booster will also likely boost your anti-HSP autoAbs too. Many people that I know just had yet another booster, making it something like their 12th? Naturally, by the old definition, this is no “vaccine” as it doesn’t induce long-term immunity. It’s a drug, a gene therapy agent, and an extremely poor, contaminated and dangerous one at that.
From 2024, a paper by Sitko et al. found low levels of anti-HSP autoAbs in asymptomatic individuals, - it’s normal to have some. But they also report that, as with Lupus (SLE), the presence of autoimmune abs may pre-date symptomatic disease by as long as ten years, and levels of anti-HSP autoAbs often correlate not just with IgGs, but with other autoantibodies too.
Key takes from “Detection of autoantibodies to heat shock protein 70 in the saliva and urine of normal individuals”8:
It is believed that antibodies reacting with self-molecules present in the serum of healthy individuals are part of natural autoantibody pool with multiple regulatory functions. On the other hand, some autoantibodies (e.g., typical of autoimmune bullous skin diseases or systemic lupus erythematosus) may be present before the onset of the disease and serve as specific predictive biomarkers.
We have previously found that antibodies directed to human Hsp60, Hsp70, and Hsp90 were significantly higher in the serum of patients with dermatitis herpetiformis (Duhring disease) during the active phase of the disease and their levels were significantly lower in remitting patients.
Interestingly, levels of anti-Hsp autoantibodies positively correlated with the levels of disease-specific autoantibodies directed against epidermal or tissue transglutaminase (18).
Similarly, the titer of circulating autoantibodies against Hsp40, Hsp60, and Hsp90 were significantly higher in patients with coeliac diseases and positively correlated with autoantibodies directed to tissue transglutaminase (19).
Another intriguing study showed that patients with systemic lupus erythematosus had at least one disease-specific autoantibody present before the disease onset (3) and a subset of patients with autoimmune blistering skin diseases had specific autoantibodies directed to various structural skin molecules present from several months up to 10 years before becoming symptomatic (4).

A more intriguing, longer-term correlation is between anti-HSP autoAbs and an IL-4-associated Th2-skewed immune response. Linked to rheumatoid arthritis, this is also related to IgG4-RD and tolerance.
Due to their associations, as HSPs have dual roles as chaperokines we do have grounds to make the hypothesis that both COVID and vaccine-induced anti-HSP autoAbs may be associated not just with IgG4 autoAbs, but with Th2-like cytokine shifts and diseases such as rheumatoid arthritis, neurodegenerative disorders and cancer too.
AutoAbs that only target Th1-associated extracellular HSP60 may skew the immune system to Th2, as it is thrown out of balance. And as per the previous figure, “… imbalance leads to disease progression”.
Anti-inflammatory Th2 cytokines include IL-4, IL-5, IL-10, and IL-13.
From “Autoantibodies to heat shock proteins 60, 70, and 90 in patients with rheumatoid arthritis“ (2019) by Mantej et al.9:
Autoantibodies to HSP are associated with Th1-like and Th2-like cytokine levels in RA. Although the levels of circulating anti-Hsp60, anti-Hsp70, and anti-Hsp90 autoantibodies revealed no significant correlations with disease activity (DAS 28) and joint damage based on the Steinbrocker criteria (Table 1), some significant associations between anti-HSP and serum levels of Th1-like and Th2-like cytokine have been found in RA (Table 1).
Positive correlations between serum levels of anti-Hsp60 IgG and IL-4 (Th2-like cytokine) and between serum levels of anti-Hsp90 IgG and IFN-ɣ(Th1-like cytokine), as well as anti-Hsp90 IgA and rheumatoid factor (RF), were found to be statistically significant in RA (Table 1).
In addition, a significant inverse correlation was found for serum levels of anti-Hsp70 IgM and TNF-α(Th1-like cytokine) in RA (Table 1). No significant associations between serum levels of anti-HSP and the remaining cytokines IL-2, IL-6, and IL-10 were found (data not shown).

In summary, although further research is needed to confirm
the present data, based on our report and previous observa-
tions, humoral immune response against autologous Hsp60
and Hsp70 is not in contradiction with the known immuno-
suppressive activity of these chaperones in RA.
Further support for the hypothesis is from “Pathological Relevance of Anti-Hsp70 IgG Autoantibodies in Epidermolysis Bullosa Acquisita” (2022) by Tukaj et al.10
Epidermolysis bullosa acquisita (EBA) is (was?) a rare autoimmune blistering skin disease:
Living or dead cells can actively or passively release Hsp70 to the extracellular space, respectively, where it influences both innate and adaptive immune cells.
The role of extracellular Hsp70 in autoimmunity, however, is still enigmatic due to conflicting outcomes regarding its contribution to the development or maintenance of pathological conditions, depending on type of diseases, experimental conditions, and the general cellular niche (1, 5, 11).
For instance, Hsp70 treatment suppressed psoriasis and autoimmune arthritis in preclinical models (11, 12). On the other hand, extracellular Hsp70 has an inflammation-promoting role in EBA (5). In more detail, circulating levels of Hsp70 were significantly elevated in mice with experimental EBA as compared to naïve mice, and Hsp70-treated EBA mice had a more intense clinical disease severity compared to untreated EBA mice (5).
… the mechanism of anti-Hsp70 IgG production in EBA patients is still not fully understood but may possibly be linked to skin tissue damage-related release of Hsp70 to the extracellular space with consecutive activation of the humoral immune response.
An indirect pathophysiological significance of anti-Hsp70 IgG in EBA patients may be evidenced by the positive correlation between serum levels of anti-Hsp70 IgG and IFN-γ, the latter known to be implicated in EBA (14–16).
Since a positive correlation between circulating anti-Hsp90 IgG and IFN-ɣ has been previously also found in patients with rheumatoid arthritis (4), it is likely that anti-Hsp autoantibodies are involved in the development of some autoimmune diseases in an IFN-ɣ-associated manner.
The pathophysiological relevance of anti-Hsp70 antibodies was experimentally demonstrated in the murine model of EBA. In this well-established antibody transfer-induced EBA model, anti-COL7 antibodies lead to neutrophil infiltration into the skin which directly causes subepidermal blister formation by ROS- and MMP-mediated disruption of adhesion molecules (6).
Heat shock proteins and cancer
A duality of function, HSP70 may promote cancer, or it may inhibit tumour growth:
HSP70 can not only promote tumor progression, enhance tumor cell resistance and inhibit anticancer effects but also induce an anticancer response by activating immune cells.
HSP70 has a variety of cancer-related functions. HSP70 is abundantly expressed in cancer and has a wide range of activities.
HSP70 is versatile in various ways, including promoting angiogenesis, inhibiting cellular senescence, enhancing tumor cell metastasis and serving as a biomarker for liquid biopsy.
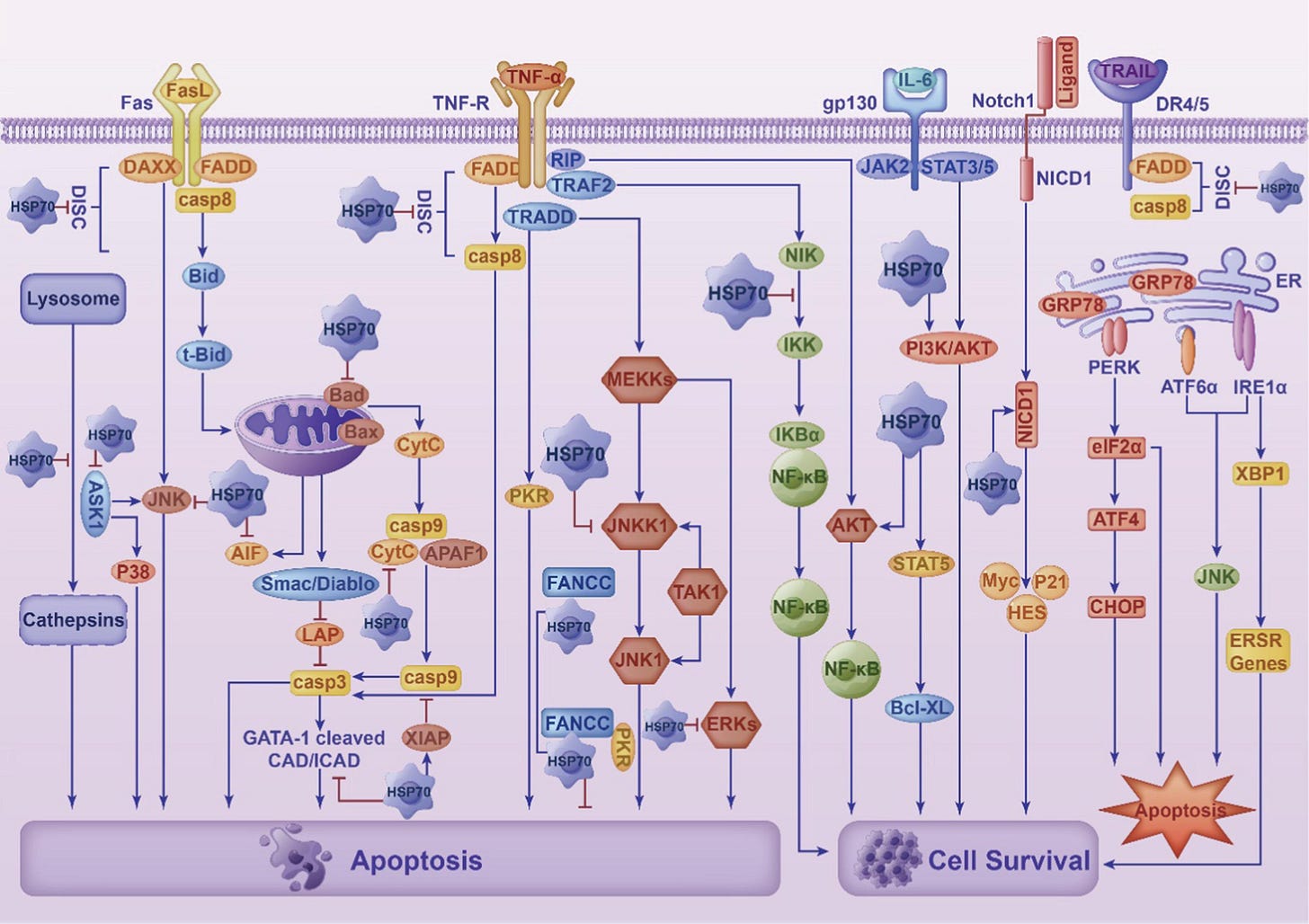
From: “The multifunction of HSP70 in cancer: Guardian or traitor to the survival of tumor cells and the next potential therapeutic target“ (2023)
https://www.sciencedirect.com/science/article/pii/S1567576923008159
In this study “Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation” from May 2024 by Xu et al11. it was found to degrade programmed death ligand 1 (PD-L1), which causes apoptosis of T cells, thereby reducing tumour-associated immunosuppression:
Abstract
Immune checkpoint inhibition targeting the PD-1/PD-L1 pathway has become a powerful clinical strategy for treating cancer, but its efficacy is complicated by various resistance mechanisms.
One of the reasons for the resistance is the internalization and recycling of PD-L1 itself upon antibody binding. The inhibition of lysosome-mediated degradation of PD-L1 is critical for preserving the amount of PD-L1 recycling back to the cell membrane.
In this study, we find that Hsc70 promotes PD-L1 degradation through the endosome-lysosome pathway and reduces PD-L1 recycling to the cell membrane. This effect is dependent on Hsc70-PD-L1 binding which inhibits the CMTM6-PD-L1 interaction.
We further identify an Hsp90α/β inhibitor, AUY-922, which induces Hsc70 expression and PD-L1 lysosomal degradation. Either Hsc70 overexpression or AUY-922 treatment can reduce PD-L1 expression, inhibit tumor growth and promote anti-tumor immunity in female mice; AUY-922 can further enhance the anti-tumor efficacy of anti-PD-L1 and anti-CTLA4 treatment.
Our study elucidates a molecular mechanism of Hsc70-mediated PD-L1 lysosomal degradation and provides a target and therapeutic strategies for tumor immunotherapy.
Overexpression of other heat shock proteins, such as HSP27, is associated with a poor cancer prognosis, including accelerated development, invasiveness and metastasis. Hepatocellular carcinoma (HCC) is one such cancer.
In 2021, Gruden et al. discussed the association between cancer, HSP27 and its autoAbs: “Serum levels of anti-heat shock protein 27 antibodies in patients with chronic liver disease”12:
Heat shock protein 27 (HSP27), an intracellular molecular chaperone, is involved in the pathogenesis of cancer by promoting both tumor cell proliferation and resistance to therapy. HSP27 is also present in the circulation and circulating HSP27 (sHSP27) can elicit an autoimmune response with production of antibodies. Levels of sHSP27 are enhanced in patients with hepatocellular carcinoma (HCC).
What protects our healthy cells from ROS & apoptosis also protects cancer cells:
Besides assisting protein refolding and regulating proteostasis under stressful conditions, HSP27 stabilizes the cytoskeleton through its actin-capping activity, reduces oxidative stress, and prevents apoptosis (Welsh and Gaestel 1998). These cytoprotective functions are deleterious in the context of cancer.
Recently, HSP27 has been implicated in the pathogenesis and progression of HCC. HSP27 promotes proliferation and invasion of HCC cells and HSP27 expression correlates with HCC incidence, tumor differentiation, and α-fetoprotein level (Liang et al. 2018). Moreover, HSP27 overexpression predicts poor overall survival in HCC patients (Choi et al. 2019; Liang et al. 2018; Wang et al. 2016).
Cancer cells may release it to the circulation:
Although HSP27 is primarily known as an intracellular chaperone, in stressed/cancer cells, HSP27 can also be exposed on the plasma membrane and/or released either in soluble form or associated with extracellular vesicles (EVs), predominantly exosomes.
Apart from IgG4-RD, there is also a link between soluble HSP27, lowered cholesterol levels and reduced risk of cardiovascular disease. The cholesterol-lipid hypothesis of CVD has long been debunked, but the evidence shows that the anti-inflammatory effects of statins may be of limited benefit.
We also discussed how M1U-modified mRNA vaccines have immunomodulatory effects by steric inhibition of pattern recognition receptors (PRRs), especially TLR4. See “Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?”13:
Soluble HSP27 lowers cholesterol levels and exerts important both anti-inflammatory and immune-modulatory effects by binding to Toll-like receptors (Batulan et al. 2016).
A link to IgG4-RD, as well as tumour progression:
This is of relevance in tumor progression as soluble HSP27 can also enhance vascular endothelial growth factor (VEGF) transcription (Thuringer et al. 2013) and triggers differentiation of monocytes to macrophages with immunotolerizing phenotypes that lose tumoricidal activity and become proangiogenic (Banerjee et al. 2011).
Immune complexes with HSP27 and improved prognosis with cardiovascular disease:
Extracellular HSP27 can induce an autoimmune response with production of anti-HSP27 antibodies (Conroy et al. 1998). Anti-HSP27 levels are reduced in patients with cardiovascular diseases (CVD) compared with that of healthy controls, and they have been proposed as a biomarker of CVD.
Moreover, recent studies have shown that formation of HSP27-anti-HSP27 immune complexes (HSP27-ICs) potentiates the activity of HSP27 as chaperokine (Shi et al. 2014; Shi et al. 2020), and there is growing interest on the potential therapeutic exploitation of the anti-inflammatory properties of HSP27-ICs in CVD.
Soluble HSP27 promotes cancer progression, whereas, as you would expect, increased levels of autoAbs to it are associated with improved survival prospects with breast cancer:
Data on anti-HSP27 antibodies in cancer are limited; however, anti-HSP27 antibody levels are increased in sera from women with ovarian (Olejek et al. 2009) and breast carcinoma (Conroy et al. 1998; Homaei-Shandiz et al. 2016) compared with that from healthy controls. Furthermore, anti-HSP27 levels are associated with improved survival in patients with breast cancer (Conroy et al. 1998), suggesting the possible use of anti-HSP27 antibody levels as a diagnostic/prognostic biomarker of cancer.
Due to dsDNA contamination14 and activation of the cGas-sting pathway, research shows why Pfizer’s BNT162b2 may also promote cancer progression by increasing levels of a transmembrane receptor for advanced glycation end-products (RAGE).
RAGE (receptor for advanced glycation endproducts), also called AGER, is a 35 kilodalton transmembrane receptor[5] of the immunoglobulin super family which was first characterized in 1992 by Neeper et al.[6]
Its name comes from its ability to bind advanced glycation endproducts (AGE), which include chiefly glycoproteins, the glycans of which have been modified non-enzymatically through the Maillard reaction.
In view of its inflammatory function in innate immunity and its ability to detect a class of ligands through a common structural motif, RAGE is often referred to as a pattern recognition receptor.
RAGE also has at least one other agonistic ligand: high mobility group protein B1 (HMGB1). HMGB1 is an intracellular DNA-binding protein important in chromatin remodeling which can be released by necrotic cells passively, and by active secretion from macrophages, natural killer cells, and dendritic cells.
The interaction between RAGE and its ligands is thought to result in pro-inflammatory gene activation.[7][8] Due to an enhanced level of RAGE ligands in diabetes or other chronic disorders, this receptor is hypothesised to have a causative effect in a range of inflammatory diseases such as diabetic complications, Alzheimer's disease and even some tumors.

From: “RAGE (receptor)”
The vaccine mimics pathologies of the disease, which isn’t perhaps the best design of therapeutic to inject repeatedly into billions. This paper from 2024 discusses the pathways in depth. Key takes from “Self-DNA driven inflammation in COVID-19 and after mRNA-based vaccination: lessons for non-COVID-19 pathologies“ by Martin Heil15:
So, if no viral cDNA is synthesized, which DNA activates innate immunity in COVID-19? It turns out that fragments of the host’s ‘self-DNA’ activate the before mentioned dsDNA sensors to trigger - eventually detrimental - inflammation and cell death (Figure 2).
Several groups reported that SARS-CoV-2 infection generates oxidative stress, damages the mitochondrial genome, destabilizes the mitochondrial membrane and subsequently, triggers a release of mitochondrial (mt)DNA to the cytosol (9, 13, 18, 41, 42).
From Wiki: “AIM2 is a cytoplasmic sensor found in hematopoietic cells that recognizes the presence of double-stranded DNA (dsDNA) of microbial or host cellular origin.”
“NLRP3 is expressed predominantly in macrophages and as a component of the inflammasome,[7][8]: 436 detects products of damaged cells such as extracellular ATP and crystalline uric acid. Activated NLRP3 in turn triggers an immune response… These signals release HSP90 and SGT1 from and recruit ASC protein and caspase-1 to the inflammasome complex. Caspase-1 within the activated NLRP3 inflammasome complex in turn activates the inflammatory cytokine, IL-1β.”
Thereby, mtDNA becomes accessible to cGAS, AIM2 or NLRP3 (9, 18, 22). Second, SARS-CoV-2-infected cells can undergo syncytia formation, a cell-to-cell fusion that generates multi-nucleated cells and therefore, is associated with DNA damage, nuclear membrane blebbing and a release of chromatin – including nuclear (genomic) DNA (nDNA) - to the cytosol, where it is sensed by cGAS (9, 13, 17, 23, 36).
Moreover, the DNA of dying infected cells can trigger inflammation and pyroptotic cell death in immune bystanders, either because the DNA of engulfed cells becomes exposed to TLR9, or because DNA that these cells release to the extracellular space serves as an inflammasome-activating signal (9, 13).
Multiple reports on elevated levels of anti-dsDNA antibodies in the plasma of vaccinees (126–132) indicate that increased cfDNA levels might be common in this group, although direct evidence for elevated levels of cfDNA in plasma of vaccinated individuals is scarce (but see (127)).
DNA damage is associated with both the virus and the Pfizer agent:
Post-mortem transcriptomic analyses of cardiac tissues of COVID-19 patients revealed an enrichment of DNA damage and repair, heat shock, and cell cycle control among the predominant upregulated genes (288).
An increase in oxidative stress and in the number of DSBs has been observed in PBMCs from older individuals at 24 h after vaccination with BNT162B2 (280), and several p53-controlled genes, including those related to apoptosis and DNA-repair, were overexpressed in PBMCs from a patient who developed myocarditis after BNT162b2 vaccination (hence, likely as a consequence of CoV-2 S expression in the absence of infection) (289).
Fibrosis is linked to the galectin-3 fold of Spike, bringing us full circle to one of the IgG4-associated initiators. The Spike RBD and HIV-sourced gp120 local loops are also implicated:
The consequences of these alterations comprise cell cycle arrest in the S1-phase, the activation of a pro-inflammatory senescence-associated secretory phenotype that exhibits elevated resistance to programmed cell death (190, 246), and enhanced expression of NF-κB and of the ACE2 promoter (290, 291).
A distinct cancer-promotion pathway16:
In fact – as described earlier for HIV-1 (15, 292–294) - SARS-CoV-2 induces NF-κB, which inhibits the DNA damage-activated transcription factor p53. P53 is involved in cell-cycle control as part of the DDR, including the decision ‘DNA repair versus elimination of cells’ (295, 296).
Perhaps Ralph Baric could tell us more about this, as he worked for years on HIV and using serial passage for SARS weaponization via gain-of-function17:

And Daszak let the mask slip back in 2016:

Returning to our paper:
Since the early nineties of the last century, HIV-1 is known to damage the DNA of its host and activate genes involved in the DNA damage response (DDR) (294, 311–314), and HIV-1 is also well known for its ability to fuse its host cells and thereby reach 4-5 times higher reproduction rates (304, 315).
In the case of HIV-I, the bipartite envelope glycoprotein (Env) performs the two essential functions of binding to receptors on the surface of target cells and fusion among host-cell and viral membranes, including the formation of a fusion pore to deliver the viral core into the cell cytoplasm (306).
Apparently, the virus employs the same protein to destabilize the nuclear envelope. In this context, it seems important to recall that SARS-CoV and SARS-CoV-2 are closely related and that consequently, the S2 domain of the SARS-CoV spike protein and of CoV-2 S are highly similar (2, 34, 270).
Importantly, SARS-CoV spike, in turn, shares multiple structural similarities with the gp41 unit of the envelope glycoprotein of HIV-1 (135, 316). Based on these similarities, which are underlined by the cross-reaction of CoV-2 S-directed non-neutralizing polyclonal antibodies with gp41 (317), these proteins can be expected to interact with cell membranes via the same mechanism.
Furthermore, while there should be no need to repeat that HIV-1 infects mobile immune cells (in particular CD4+ T helper cells), it seems worth to mention that evidence for SARS-CoV-2 doing the same is accumulating.
SARS-CoV-2 has been shown to infect monocytes, macrophages and B-cells (22, 28, 318), and at least Pontelli et al (318) present evidence supporting a successful reproduction of the virus in these cells, although at low rates.
Since excessive infiltration of mobile pro-inflammatory cells such as macrophages and T-helper 17 cells has been found in lung tissues of patients with COVID-19 (11), it appears at least possible that SARS-CoV-2 can apply a strategy that was considered unique to HIV-1: triggering local inflammation to attract mobile immune cells and infect these cells to achieve systemic distribution throughout the host.
If you were infected prior to vaccination you were protected from IgG4 class switching, but the author discusses how prior infection leads you to being hypersensitive to dsDNA.
Macrophage priming is key to this, as we discussed in a previous Substack. He doesn’t mention this in the context of contamination, but the implication is of an enhanced cGas-STING pathway, RAGE and RAGE-associated HSP expression, with all its pathologies.
Their figure includes a feature-length caption, but it shows how cyclic GMP-AMP Synthase (cGAS) signals various inflammatory or pathogenic responses after sensing dsDNA. RAGE is also a dsDNA sensor, and the only one in the cell membrane. It also signals these pathways, which include HSPs (not shown):

These studies confirm that infection with SARS-CoV-2 causes a long-term reprogramming of the immune system, particularly in macrophages. Thus, it seems plausible that in vaccinees who had an infection before being vaccinated, the effects of SARS-CoV-2 expression (including DNA damage) occurred in the context of a primed DNA-sensing machinery: a situation that can strongly enhance its immunogenic potential.
Others discovered that S1 and S2 proteins administered intraperitoneally triggered enhanced concentrations of IL-6, IL-1β, and TNFα (16 hr post treatment) in WT mice but not in mice lacking TLR2, which indicates a role of TLR2, rather than TLR4 (279).
In addition, CoV-2 S induced an enhanced release of ATP and IL-1β from human lung epithelial cells (249) and cultured microglial cells (BV2 line) and in the latter, it also induced the expression of the purinergic eATP receptor P2X7 (343).
I conclude that SARS-CoV-2-mediated immune priming can enhance the DNA-damaging and pro-inflammatory effects of CoV-2 S and cause certain responses to pass a threshold or point of no return, reaching those dimensions that we see in the severe adverse effects of the Spike-based mRNA vaccines.
If you don’t look, you won’t find:
Even phase III vaccine trials usually exclude individuals who show preexisting immunity due to previous infection, but the vaccination campaigns included significant proportions of the entire population at a time point at which many people had already passed through an infection with SARS-CoV-2, and pre-existing immunity was seldom checked in these mass vaccination events.
In summary, immune priming represents an example of a mechanism that could generate different outcomes of vaccination depending on an earlier – perhaps non-symptomatic and not detected – infection with SARS-CoV-2.
With a creepy similarity to “Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies”, a study from 2002 “Elevated levels of antibodies against 70 kDa heat shock proteins in the sera of patients with HIV infection” by Kocsis et al.18 discusses a pathology that could almost be describing the Pfizer product too:
Abstract
Heat shock proteins (Hsp), especially 70 kDa heat shock protein (Hsp70) play an important role in the life cycle of HIV-1 virus. Hsp70 is overexpressed in HIV-infected cells and this is the most abundant Hsp associated with HIV virions.
The aim of our study was to investigate whether HIV infection increases the extent of specific humoral immune response against Hsp70. The serum concentration of anti-Hsp70 IgG antibodies was measured in 47 HIV-infected patients, and 62 healthy, HIV-seronegative persons.
Nineteen patients on highly active anti-retroviral therapy (HAART) were followed for 24 months in a longitudinal study. Anti-Hsp70 antibodies were measured by ELISA, using recombinant human Hsp70.
SARS-CoV-2 or the vaccine are NOT HIV, but there are commonalities to both types of pathology, and potentially in prognosis:
Levels of anti-Hsp70 antibodies were significantly (P < 0.0001) higher in the HIV-infected patients (median: 1409 (25th–75th percentile: 1031–2214) AU/ml) than in healthy control subjects (626 (429–970) AU/ml).
In 19 HIV patients, serum levels of anti-Hsp70 antibodies significantly (P < 0.001) decreased during 24 (11–41) months HAART (1309 (887–2213) AU/ml before and 640 (386–959) AU/ml during HAART), accompanied by viral load reduction and CD4+ count elevation.
With the Pfizer product it is due to HSP-like antigens on Spike and RAGE pathways:
It is concluded that HIV-infection induces a marked increase in the anti-Hsp70 antibody levels, which is consistent with the enhanced expression of Hsp70 on the surface of HIV-infected cells and/or incorporation of the protein into the membrane of HIV virions. J. Med. Virol. 71:480–482, 2003. © 2003 Wiley-Liss, Inc.
HIV elevates HSP27 and HSP70. In turn, HSP70 promotes the replication of HIV:
According to Wainberg et al. [1997], acute HIV infection results in 8- to 20-fold increase in the mRNA and protein level of 27 and 70kDa Hsp. Induction was viral dose-dependent and abrogated by HIV-1 neutralizing antibodies and antibodies to CD4 [Furlini et al., 1994; Wainberg et al., 1997].
If you are a HIV patient the implication is that Pfizer mRNA and RAGE signalling may lead to increased viral loads and relapse:
It was suggested [Agostini et al., 2000] that Hsp70 plays a role in the nuclear import of HIV-1 preintegration complexes. HIV infection-induced increased expression and redistribution of Hsp70 on the cell surface may influence late stage of infection and may also be involved in the prevention of apoptosis [Mosser et al., 1997].
Hsp70 interact with HIV-1 gag proteins during formation and release of HIV-1 virion from the infected cells, and as demonstrated recently by Gurer et al.[2002], Hsp70 and certain other heat shock proteins (Hsp27, Hsp40, Hsp60, and Hsc 70) incorporate into the membrane of HIV virions. According to their observations, Hsp70 was the most abundant heat shock protein associated with HIV virions.
Increased expression of Hsp70 on the surface of HIV infected cells and its incorporation into the membrane of HIV virions may lead to increased immune response against this protein.
Although it may act as an adjuvant, the authors caution that HSP70 autoAbs may also affect HSP-HIV protein complexes:
It is also possible that high anti-Hsp70 titer is the consequence of overall stress response and cellular activation. Based upon the adjuvant properties of Hsp70 and promising results obtained with Hsp-based cancer vaccine, use of Hsp–HIV protein complexes as HIV-vaccines was proposed recently [Brenner and Wainberg, 2001].
Pfizer’s product also acts as HSP-based vaccine, and of course, such investigations never took place. Even now, awareness of this must be minimal, which is why I’m discussing it.
Since according to our present findings, anti-Hsp70 antibodies are present in high titers of in almost 50%of the HIV-infected patients, the effect of these antibodies on the Hsp–HIV protein complexes should be investigated before the design of heat shock protein (Hsp) based HIV vaccines.
This study “Extracellular vesicle-mediated amyloid transfer to neural progenitor cells: implications for RAGE and HIV infection“ from 2020 by András et al.19 is quite concerning:
Abstract
Amyloid beta (Aβ) deposition was demonstrated to be elevated in the brains of HIV-infected patients and associated with neurocognitive decline; however, the mechanisms of these processes are poorly understood.
The goal of the current study was to address the hypothesis that Aβ can be transferred via extracellular vesicles (ECVs) from brain endothelial cells to neural progenitor cells (NPCs) and that this process can contribute to abnormal NPC differentiation.
Mechanistically, we focused on the role of the receptor for advanced glycation end products (RAGE) and activation of the inflammasome in these events.
ECVs loaded with Aβ (Aβ-ECVs) were readily taken up by NPCs and Aβ partly colocalized with the inflammasome markers ASC and NLRP3 in the nuclei of the recipient NPCs. This colocalization was affected by HIV and RAGE inhibition by a high-affinity specific inhibitor FPS-ZM1.
If we reverse this null hypothesis, RAGE pathways lead to increased amyloid deposition in the brains of HIV-infected patients and contribute to neurocognitive decline:
Blocking RAGE resulted also in an increase in ECV number produced by brain endothelial cells, decreased Aβ content in ECVs, and diminished Aβ-ECVs transfer to NPC nuclei. Interestingly, both Aβ-ECVs and RAGE inhibition altered NPC differentiation.
Overall, these data indicate that RAGE inhibition affects brain endothelial ECV release and Aβ-ECVs transfer to NPCs. These events may modulate ECV-mediated amyloid pathology in the HIV-infected brain and contribute to the development of HIV-associated neurocognitive disorders.
I discussed this in the context of brain fog, Spike and its 3 neurotoxic gp120 local loops. Gp120 is also associated with HIV-associated neurocognitive disorder (HAND):
HIV-infected brains were shown to have increased amyloid beta (Aβ) deposition [1,2,3,4,5,6]. This phenomenon has been linked to the development of cognitive dysfunction based on the observation that early beta-amyloidosis in HIV-infected patients was associated with HIV-associated neurocognitive disorders (HAND) [3, 7].
Aβ deposition occurs mostly in the perivascular space [3, 7,8,9], which points to the brain microvessels having a role in amyloid pathology.
In support of this notion, the blood-brain barrier (BBB), a critical player in the brain infection by HIV and the development of HIV-associated cerebrovascular comorbidities [10, 11], was postulated to regulate Aβ homeostasis as an interface contributing to Aβ accumulation in the brain [12].
Indeed, it was demonstrated that the receptor for advanced glycation end products (RAGE) can mediate Aβ transport across the BBB and accumulation in the brain [13]. Similarly, RAGE was shown to be involved in HIV-induced accumulation of Aβ in brain endothelial cells, a structural component of the BBB [14].
Based on the observations that a) HIV can increase RAGE expression in brain endothelial cells [14], b) HIV-induces Aβ accumulation in brain endothelial cells via a RAGE-dependent mechanism [14], and c) RAGE may be involved in microvesicle secretion [27], we hypothesize in the current study that RAGE may be a key player in the HIV-induced brain endothelial ECV release and Aβ-ECVs transfer to NPCs.
In addition, because both HIV infection [28] and Aβ pathology [29, 30] were linked to the inflammasome pathway, and RAGE was shown to signal through the NLR family pyrin domain containing 3 (NLRP3) inflammasome [31], we further aimed to examine the impact of Aβ-ECV transfer on the NLRP3 inflammasome in NPCs.

Note that SARS-CoV-2 has prion-like domains (PrDs)20 and also elevates Aβ levels, leading to the accelerated decline of dementia patients.
I saw Facebook reports from carers and relatives of the rapid cognitive decline of those in Care homes after vaccination. Within days they often lost self-care abilities and mobility, and sometimes just stared into space.
These groups were quickly censored and taken down.
… We inspected the transcriptomic and interactomic profiles by comparing the COVID-19 cohort against the control cohort and the AD cohort against the AD+COVID-19 cohort. SARS-CoV-2 in patients without AD mainly activated processes related to immune response and cell cycle. Conversely, 21 key nodes in the interactome are deregulated in AD. Interestingly, some of them are linked to beta-amyloid production and clearance. Thus, we inspected their role, along with their interactors, using the gene ontologies of the biological process that reveals their contribution in brain organization, immune response, oxidative stress and viral replication. We conclude that SARS-CoV-2 worsens the AD condition by increasing neurotoxicity, due to higher levels of beta-amyloid, inflammation and oxidative stress.
From: “SARS-CoV-2 Exacerbates Beta-Amyloid Neurotoxicity, Inflammation and Oxidative Stress in Alzheimer’s Disease Patients“ (2021)
RAGE is linked to upregulation of HSP90β21, HSP7022, and HSP2723. In this study, the researchers demonstrated that diabetes can promote pancreatic cancer via related glyceraldehyde (GA)-derived advanced glycation-end products (GA-AGEs).
Key takes from “Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion“ (2017) by Takata et al.24:
The dose-dependent production of some high-molecular-weight (HMW) complexes of HSP90β, HSP70, and HSP27 was observed following administration of GA. We considered HMW complexes to be dimers and trimers with GA-AGEs-mediated aggregation. Cleaved caspase-3 could not be detected with WB. Furthermore, 10 and 20 μg/mL GA-AGEs-BSA was 27% and 34% greater than that of control cells, respectively (P < 0.05 and P < 0.01).
CONCLUSION
Although intracellular GA-AGEs induce pancreatic cancer cell death, their secretion and release may promote the proliferation of other pancreatic cancer cells.
… Type 2 DM (T2DM), the most common type of DM, has been shown to increase the risk of pancreatic cancer by more than 50%; furthermore, T2DM patients with pancreatic cancer have worse prognosis and shorter survival time than those without the disease[5,6].
PANC-1 and MIA-PaCa-2 are pancreatic cancer cell lines.
WB: Western blot protein analysis:
AGE has been detected in human pancreatic cancer cell lines[29,30]. WB revealed high expression levels of RAGE in PANC-1 and MIA-PaCa-2 cells, and low levels in BxPC-3 cells[30]. GA-AGEs-BSA may promote the proliferation of PANC-1 as well as IL90 and HuH7 cells through the GA-AGEs-RAGE system.
As we see in the next paragraph, the kicker here is that both HSP70 and HSP90 are hypothesised by the authors to be GA-AGEs, and they even highlight differences between intracellular and extracellular effects on pancreatic cancer cells:
In conclusion, intracellular and extracellular GA-AGEs induced PANC-1 cell death and proliferation, respectively. This suggests that, although intracellular GA-AGEs induce pancreatic cancer cell death, their secretion and release may induce the proliferation of other pancreatic cancer cells.
In this investigation, we did not examine GA-AGEs; however, we consider the monomer HSP90α, monomer HSP90β, and HMW complexes of HSPs to be GA-AGEs. However, two recent studies identified GA-AGEs generated by Hsc70 and heterogeneous nuclear ribonucleoprotein M in Hep3B cells treated with GA or fructose[16,31].
If the monomers and aggregates of HSPs are identified as GA-AGEs in the future, this indicates that GA-AGEs were first identified in human pancreatic ductal carcinoma cells. Identifying intracellular GA-AGEs will reveal the mechanism of cell death.
In some cancers the reverse is true, and it’s the intracellular HSPs that are promoting cancer:
In addition, GA-AGEs secreted or released into the conditioned medium of cultured PANC-1 cells, which generate intracellular GA-AGEs, may demonstrate that extracellular GA-AGEs promote cancer.
If confirmed (and only if they want to, as profit comes first) they may need to ditch the old chemo drugs. As it is, pancreatic cancer is one of the cancers associated with a very poor prognosis, due to late-stage diagnoses and metastasis. The five-year survival rate averages only 12%:
If the mechanism through which T2DM promotes PDAC relies on GA-AGEs as key factors, the current research on drug therapy for PDAC may change[1,32].
More on how proteins in the HSP70 family are associated with cancer:
The 70 kDa heat shock proteins (HSP70s) are a group of highly conserved and inducible heat shock proteins. One of the main functions of HSP70s is to act as molecular chaperones that are involved in a large variety of cellular protein folding and remodeling processes. HSP70s are found to be over-expressed and may serve as prognostic markers in many types of cancers. HSP70s are also involved in most of the molecular processes of cancer hallmarks as well as the growth and survival of cancer cells. In fact, many effects of HSP70s on cancer cells are not only related to their chaperone activities but rather to their roles in regulating cancer cell signaling. Therefore, a number of drugs directly or indirectly targeting HSP70s, and their co-chaperones have been developed aiming to treat cancer.
… Though lots of evidence showed that the HSP70 family, especially HSPA5, indirectly promoted the expression of ERK, whether HSP70 has a direct effect on ERK still remains unclear and needs to be further explored [45,46,47,48,49,50,51,52]. Nevertheless, the majority of studies suggest that HSP70 plays an activating role in regulating the RTKs-RAS-RAF-MEK-ERK signaling pathway.

… Anti-cancer therapy research targeting HSP70 has been carried out for more than 20 years. Numerous studies focused on HSP70 inhibitors reported great efficacies; however, there are still many obstacles in the transformation applications. One of the difficulties for developing HSP70 inhibitors is that HSP70 is ubiquitously expressed in the human body and has different isoforms. Further understanding the structure of HSP70, especially the SBD, and designing inhibitors based on this may be an effective way to solve the poor specificity of HSP70 inhibitors.
… HSP70 has a dual role in tumor progression. These two opposing roles of HSP70 may co-exist in the same tumor; therefore, research on the efficacy of targeting HSP70 is best evaluated in the immunocompetent animal models. Moreover, the expression level of HSP70 in tumor cells and the immunogenicity of this tumor may help to choose whether to preferentially verify the efficacy of HSP70 inhibitors or HSP70-based vaccines.
From: “HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances” (2023)
Does this mean that Pfizer’s engineered mRNA is protective against neurodegenerative disorders and cancer? Unfortunately not. AutoAb responses tend to target the more accessible protective extracellular HSP70 proteins, rather than the pro-tumour intracellular proteins initiating all these pathways.
It is also complicated by all the different forms of HSP70 that you would need to target, and the dual roles it has, and how these can vary by cancer type. There are still many gaps in our understanding of the mechanisms involved. Skewing the balance has consequences not limited to deafness.
HSP70 as a tumour suppressor and HSPs and innate immunity
From 2002, Gaston published the review “Heat shock proteins and innate immunity”25.
In the context of anti-tumour immunity, the first role is the recognition of HSPs by T cells. This in itself may lead to more anti-inflammatory effects, which in itself may attenuate levels of pro-oncogenic, pro-inflammatory cytokines such as IL-6:
Initially hsp were investigated primarily as antigens, particularly when it was found that they were rather common targets of both the humoral and T cell-mediated responses to intracellular pathogens like mycobacteria.
Their recognition by T cells in models of autoimmune disease, particularly arthritis and diabetes, gave rise to much speculation that immune responses initially directed against hsp from pathogens might cross-react with self antigens including hsp themselves [1,2].
Since hsp are often up-regulated at sites of inflammation this would provide opportunities for persistent stimulation of cross-reactive hsp-specific T cells [3]. Such speculation continues, although more recent evidence points to anti-inflammatory properties of T cells which recognize self hsp rather than their ability to induce autoimmune disease [4].
HSPs and presentation of tumour antigens to T cells:
A second involvement of hsp in T cell-mediated immunity was demonstrated by the pioneering studies of Srivastava and colleagues, who showed that the chaperone function of many hsp (their ability to bind to and protect other polypeptides) allows them to deliver tumour antigens very effectively to antigen presenting cells [5,6].
Cluster of differentiation 91 (CD91), which also known as low density lipoprotein receptor-related protein 1 (LRP1), alpha-2-macroglobulin receptor (A2MR), or apolipoprotein E receptor (APOER) is a receptor for HSP70 and HSP90 family proteins:
This delivery appears to be receptor mediated, and hsp receptors are now being characterized, such as CD91 which binds several different hsp including two members of the hsp90 family and hsp70 [7].
Linked to this, Annelise highlighted to me the importance of HSPs in augmenting acquired and innate immune responses:
In addition, when fusion proteins are created which contain antigen and part of the sequence of hsp70, the gain in immunogenicity is very marked, especially for the induction of responses by CD8+ T cells [8,9].
Vaccines which mimick HSP antigens or lead to autoAbs may disrupt this delicate balance and essential role, leading to disease progression:
This function is analogous to that reported for antigens coupled to fragments of C3 which are potent inducers of antibody responses [10]. Thus both C3 and hsp might be regarded as physiological adjuvants, and serve as links between the innate and the acquired immune systems.
This brings us to the third immunological role of hsp, namely their ability to stimulate cells of the innate immune system, particularly antigen presenting cells, though interactions with other myeloid cells and endothelial cells have also been described.
The initial observations were that hsp, usually tested as recombinant proteins, could elicit production of cytokines such as IL-1 or TNFα from monocyte/macrophage cells and cell lines [11–16].
Gum disease, a bacterium called Actinobacillus actinomycetemcomitans, HSP60 and bone loss in your jaw:
Another such paper by Ueki et al. [17] appears in this issue of CEI and documents cytokine production by macrophages in response to human hsp60; interestingly the investigators’ initial interest seems to have been in hsp60 from Actinobacillus actinomycetemcomitans, a bacterium implicated in periodontal disease, but this did not induce cytokine production.
My early substacks discussed how vaccine-induced systemic proinflammatory cytokines could shift the balance of cells from bone-producing osteoblasts to bone-destroying osteoclasts. This could be an additional mechanism:
Their initial idea that the bacterial hsp might mediate periodontal disease was modified therefore to suggest that human hsp60 released in response to infection would contribute to bone loss. In view of the accumulating literature on intrinsic stimulatory properties of hsp, it is timely to review current evidence on this novel feature of hsp.
Concluding remarks
HSP autoAbs don’t appear to be directly oncogenic, but indirectly they may promote cancer by skewing HPS levels, inhibiting tumour antigen presentation by APCs, promoting PD-L1 recycling and inducing a shift towards Th2 immunity, which is more tolerogenic.
And dsDNA contaminated engineered mRNA may promote cancer and disease by increasing HSP levels via RAGE pathways.
As ever, more studies are needed to confirm the findings and extent of their impact on disease and progression. Disease mechanisms do not happen in isolation, you also need to consider correlations with other pathologies, such as other autoimmune antibodies or even just Gal-3 (See “Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z”).
A PubMed search for “heat shock protein” returned nearly 9,000 results, therefore there is far more to the story than “just” autoAbs in response to bio-engineered transfection agents, this Substack can only scratch the surface.
There was one aspect that needs further discussion though, and this must wait for Part II. The question is “what involvement do HSPs have in neurological disorders such as myalgic encephalomyelitis/chronic fatigue syndrome (ME), multiple sclerosis (MS) and fibromyalgia syndrome (FMS)?”
Any links to HSPs and their autoAbs are all the more important to research and discuss, along with therapeutics, given that all these conditions are known to be triggered by or exacerbated by experimental SARS-CoV-2 mRNA gene therapies.
References
Zheng J, Guo N, Huang Y, Guo X, Wagner A. High temperature delays and low temperature accelerates evolution of a new protein phenotype. Nat Commun. 2024;15(1):2495. doi:10.1038/s41467-024-46332-6
Rutledge BS, Choy WY, Duennwald ML. Folding or holding?—Hsp70 and Hsp90 chaperoning of misfolded proteins in neurodegenerative disease. The Journal of Biological Chemistry. 2022;298(5):101905. doi:10.1016/j.jbc.2022.101905
Bonaguri C, Orsoni J, Russo A, et al. Cogan’s Syndrome: Anti-Hsp70 Antibodies are a Serological Marker in the Typical Form. The Israel Medical Association journal : IMAJ. 2014;16:285-288.
Gallo JR, Ortiz AC, Paira SO. IgG4-Related Disease and Sensorineural Hearing Loss. Clinical and Experimental Otorhinolaryngology. 2014;7(3):236. doi:10.3342/ceo.2014.7.3.236
Ren Q, Su J, Zhang D, Ding X. Otological IgG4-Related Disease With Inner Ear Involvement: A Case Report and Review of Literature. Ear Nose Throat J. 2022;101(10):NP441-NP444. doi:10.1177/0145561320976411
Rauch SD, San Martin JE, Moscicki RA, Bloch KJ. Serum antibodies against heat shock protein 70 in Menière’s disease. Am J Otol. 1995;16(5):648-652.
Tebo AE, Szankasi P, Hillman TA, Litwin CM, Hill HR. Antibody reactivity to heat shock protein 70 and inner ear-specific proteins in patients with idiopathic sensorineural hearing loss. Clinical and Experimental Immunology. 2006;146(3):427. doi:10.1111/j.1365-2249.2006.03227.x
Sitko K, Mantej J, Bednarek M, Tukaj S. Detection of autoantibodies to heat shock protein 70 in the saliva and urine of normal individuals. Front Immunol. 2024;15:1454018. doi:10.3389/fimmu.2024.1454018
Mantej J, Polasik K, Piotrowska E, Tukaj S. Autoantibodies to heat shock protein 60, 70, and 90 in patients with rheumatoid arthritis. Cell Stress and Chaperones. 2018;24. doi:10.1007/s12192-018-0951-9
Tukaj S, Mantej J, Sitko K, et al. Pathological Relevance of Anti-Hsp70 IgG Autoantibodies in Epidermolysis Bullosa Acquisita. Front Immunol. 2022;13:877958. doi:10.3389/fimmu.2022.877958
Xu X, Xie T, Zhou M, et al. Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation. Nat Commun. 2024;15(1):4237. doi:10.1038/s41467-024-48597-3
Gruden G, Carucci P, Barutta F, et al. Serum levels of anti-heat shock protein 27 antibodies in patients with chronic liver disease. Cell Stress and Chaperones. 2021;26(1):151-157. doi:10.1007/s12192-020-01164-3
Rubio-Casillas A, Cowley D, Raszek M, Uversky VN, Redwan EM. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? Int J Biol Macromol. 2024;267(Pt 1):131427. doi:10.1016/j.ijbiomac.2024.131427
McKernan, Kevin, Yvonne Helbert, Liam T. Kane, and Stephen McLaughlin. Sequencing of Bivalent Moderna and Pfizer MRNA Vaccines Reveals Nanogram to Microgram Quantities of Expression Vector DsDNA per Dose. OSF, April 10, 2023. https://doi.org/10.31219/osf.io/b9t7m.
Heil M. Self-DNA driven inflammation in COVID-19 and after mRNA-based vaccination: lessons for non-COVID-19 pathologies. Front Immunol. 2024;14. doi:10.3389/fimmu.2023.1259879
Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2020;8(3):287-297. doi:10.1016/j.gendis.2020.06.005
Roberts A, Deming D, Paddock CD, et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1):e5. doi:10.1371/journal.ppat.0030005
Kocsis J, Prohászka Z, Bíró A, Füst G, Bánhegyi D. Elevated levels of antibodies against 70 kDa heat shock proteins in the sera of patients with HIV infection. J Med Virol. 2003;71(4):480-482. doi:10.1002/jmv.10507
András IE, Garcia-Contreras M, Yanick C, et al. Extracellular vesicle-mediated amyloid transfer to neural progenitor cells: implications for RAGE and HIV infection. Molecular Brain. 2020;13(1):21. doi:10.1186/s13041-020-0562-0
Tetz G, Tetz V. Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms. 2022;10(2):280. doi:10.3390/microorganisms10020280
Miyata Y, Nakamoto H, Neckers L. The Therapeutic Target Hsp90 and Cancer Hallmarks. Curr Pharm Des. 2013;19(3):347-365. doi:10.2174/138161213804143725
Grunwald MS, Ligabue-Braun R, Souza CS, et al. Putative model for heat shock protein 70 complexation with receptor of advanced glycation end products through fluorescence proximity assays and normal mode analyses. Cell Stress and Chaperones. 2017;22(1):99-111. doi:10.1007/s12192-016-0746-9
Chiappalupi S, Salvadori L, Vukasinovic A, Donato R, Sorci G, Riuzzi F. Targeting RAGE to prevent SARS-CoV-2-mediated multiple organ failure: Hypotheses and perspectives. Life Sci. 2021;272:119251. doi:10.1016/j.lfs.2021.119251
Takata T, Ueda T, Sakasai-Sakai A, Takeuchi M. Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion. World J Gastroenterol. 2017;23(27):4910-4919. doi:10.3748/wjg.v23.i27.4910
Gaston, J. S. H. ‘Heat Shock Proteins and Innate Immunity’. Clinical and Experimental Immunology 127, no. 1 (January 2002): 1. https://doi.org/10.1046/j.1365-2249.2002.01759.x.
















The thought of putting any mRNA or any other covid injection into my body fills me with horror yet people I know have repeatedly been injected, with my neighbour I have lost count how many he has had. My friend developed MND symptoms within two weeks of her 3rd Moderna injection and died within a year, we often talked about how quickly her symptoms developed following her 3rd injection. Incredibly complicated article, I am full of admiration that you can wrap your brain around all of it, I still cannot believe that billions of people were injected with so little thought to the possible detrimental outcomes on their health.
This is a terrific article. I’m not done yet.