Smallpox & monkeypox vaccine mythology, aegrescit medendo
The cure is worse than the disease
Updates:
4th June ‘22: Vaccinia and latent HIV reactivation.
5th June ‘22: Monkeypox lab origins, preliminary analysis.
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Introduction
This Substack reviews the history of smallpox vaccination in part 1.
The safety & efficacy of a vaccine candidate against monkeypox and mechanisms of vaccine induced cardiomyopathy are reviewed in part 2.
It concludes with “Whither monkeypox vaccination” which discusses different approaches to the currently adopted one.
Part 1: Smallpox
Whilst Edward Jenner’s famous discovery undoubtedly saved many lives, it also contributed to many deaths for various reasons.
The net effect was that at the population level net mortality didn't fall over time, if anything it went up (where have we seen that before?)
The main causes were:
Immunosuppressed, malnourished children were unable to mount an effective immune response. It was like pushing on a string.
The live cowpox virus could be as lethal to these individuals as smallpox itself.
Good Manufacturing Practice (GMP) was a thing of the future. Vaccine preparations varied in quality and variant used, and could even be contaminated with anthrax spores. It was also often traceable back to smallpox itself, not cowpox…
For those who haven't read it I can recommend the excellent “Dissolving Illusions: Disease, Vaccines, and The Forgotten History” (2013) by Suzanne Humphries MD, and it is appropriate to include several quotes in this Substack.
In many ways we haven't really progressed from the Victorian era:
Fresh vesicles subsequently formed around the vaccination pocks coalescing with them and causing them to spread. They developed also on the face, head, body, and in the mouth, the later prevented the child from suckling, and it died exhausted on the 45th day after vaccination.
– Case of a healthy child after vaccination, March 13, 1891
Try revaccination—It never will hurt you,
For revaccination has this one great virtue:
Should it injure or kill you whenever you receive it,
We all stand prepared to refuse to believe it.
– From a circular signed “The Doctors,” 1876
And any acquired immunity, if the vax didn't kill you first time round, wasn't lifelong:
…it is observed that all un-revaccinated children over one and a-half years of age, or thereabouts, and all revaccinated persons whose revaccinations are more than three years old, i.e., the vast majority of the entire population—are unprotected.{106}
Another practitioner named Dr. Olesen claimed that revaccination should be done annually.
Recent successful vaccination is an absolute protection against smallpox. Protection lasts from six months to twelve months and often much longer. Revaccination is advisable once a year.
Since the late 1700s, the medical profession has supported vaccination, even though there was never a trial where one group was vaccinated and compared to another group of the same size that was not vaccinated.{109}
The CDC admits that, even now, the level of antibody that protects against smallpox infection is unknown.{110} When the authors of Dissolving Illusions were growing up in the United States, children were considered vaccinated and immune simply by revealing the scar of vaccination years after the procedure.
The standardization and purity of smallpox vaccines was lackluster even after the eradication of smallpox from the United States. Dr. Beddow Bayly’s 1952 statements should leave everyone wondering how such a vaccine could have possibly been responsible for eradication of any disease:
When we recall that vaccine lymph is derived, in the first place, either from a smallpox corpse, the ulcerated udder of a cow, or the running sores of a sick horse’s heels, the choice depending upon the country of its origin and the firm which manufactures it, it is hardly to be wondered at that it has far-reaching ill effects on the human constitution. Years ago, the Lancet declared that “no practitioner knows whether the lymph he employs is derived from smallpox, rabbit-pox, ass-pox, or mule-pox.”{117} Our own Ministry of Health has long confessed to complete ignorance of the ultimate source of its own supply of lymph; but last year Dr. A. Downie stated in the British Medical Journal that “the strain of vaccinia virus used for the routine preparation of lymph in this country [England] is believed to have been derived from a case of smallpox in Cologne during the last century.” That, of course, disposes of the whole theory of cow-pox vaccination.{118}
When Jenner published his paper in 1798 claiming lifelong immunity to smallpox and promoting his technique, many doctors who had seen smallpox follow cowpox challenged his doctrine at a meeting of the Medico-Convivial Society.
But he [Jenner] no sooner mentioned it than they laughed at it. The cow doctors could have told him of hundreds of cases where smallpox had followed cow-pox…{122}
In 1799 Dr. Drake, a surgeon from Stroud, England, conducted an experiment to test Jenner’s new preventive using vaccine obtained directly from Edward Jenner. The children were then challenged with a smallpox inoculation to see if the cowpox procedure had been effective.
In three of them, a lad aged seventeen and two of the Colborne children (one four years, the other fifteen months), the cowpox vesicles came to early maturity and were scabbed under the usual time. The lad was inoculated with smallpox on the 20th December, being the eight day from his vaccination, and the two children on the 21st, being again the eight day. They all developed smallpox, both the local pustules and the general eruption with fever.{123}
Dr. Hughes, another doctor from Stroud, reported that the children subsequently developed smallpox and suggested that the vaccination technique failed. Jenner received the report but decided to ignore the results.
Human behaviour never changes:
Surgeons and doctors were paid well to perform vaccination and embraced it as a new form of income. It is therefore quite significant that so many doctors wrote to medical journals about their experiences. However, just like today, the believers ignored the voices of the medical dissenters, which led to ordinary people speaking out in the lay media.
A case fatality rate of 33% indicates zero efficacy. Perhaps not surprising if smallpox was the source of the lymph:
In the 1844 smallpox epidemic, about one-third of the vaccinated contracted a mild form of smallpox, but roughly 8 percent of those vaccinated still died, and nearly two-thirds had severe disease.{131}
A letter to a newspaper in 1850 claimed there were more admissions to the London SmallPox Hospital in 1844 than during the smallpox epidemic of 1781 before vaccination began. The author also noted that one-third of the deaths from smallpox were in people who had previously been vaccinated.
Daily experience now unhappily shows an altered state of things: small pox, in spite of vaccination, is rapidly on the increase… There were more admissions to the London SmallPox Hospital in 1844 than in the celebrated smallpox epidemic of 1781 before vaccination was introduced. I shall also select the Registrar’s returns of one of the country districts (Bradford) to show how little protection vaccination afforded in the last quarter of that year, 1844: 118 [181?] deaths from smallpox were recorded, 60, or nearly one-third, of which had been vaccinated.{132}
The more things change, the more they stay the same:
In 1898 Dr. Wilder also noted that during the 1871–1872 pandemic, the vaccinated often contracted severe smallpox more rapidly than the nonvaccinated.
Never, however, did the faith in vaccination receive so rude a shock as in the Great SmallPox Epidemic of 1871 and 1872. Every country in Europe was invaded with a severity greater than had ever been witnessed during the three preceding centuries. In England, the number of deaths from the disease was increased from 2,620 in 1870 to 23,126 in 1871 and 19,064 in 1872, falling again to 2,634 in 1873. Upon the Continent, particularly in France and Germany, the visitation was even more severe. In Bavaria, for example, with a population vaccinated more than any other country of Northern Europe, except Sweden, which experienced the greatest that had ever been known.
What was even more significant, many vaccinated persons in almost every place were attacked by smallpox before any unvaccinated persons took the disease.{139}
As resistance to vaccination understandably grew, mandates were enacted:
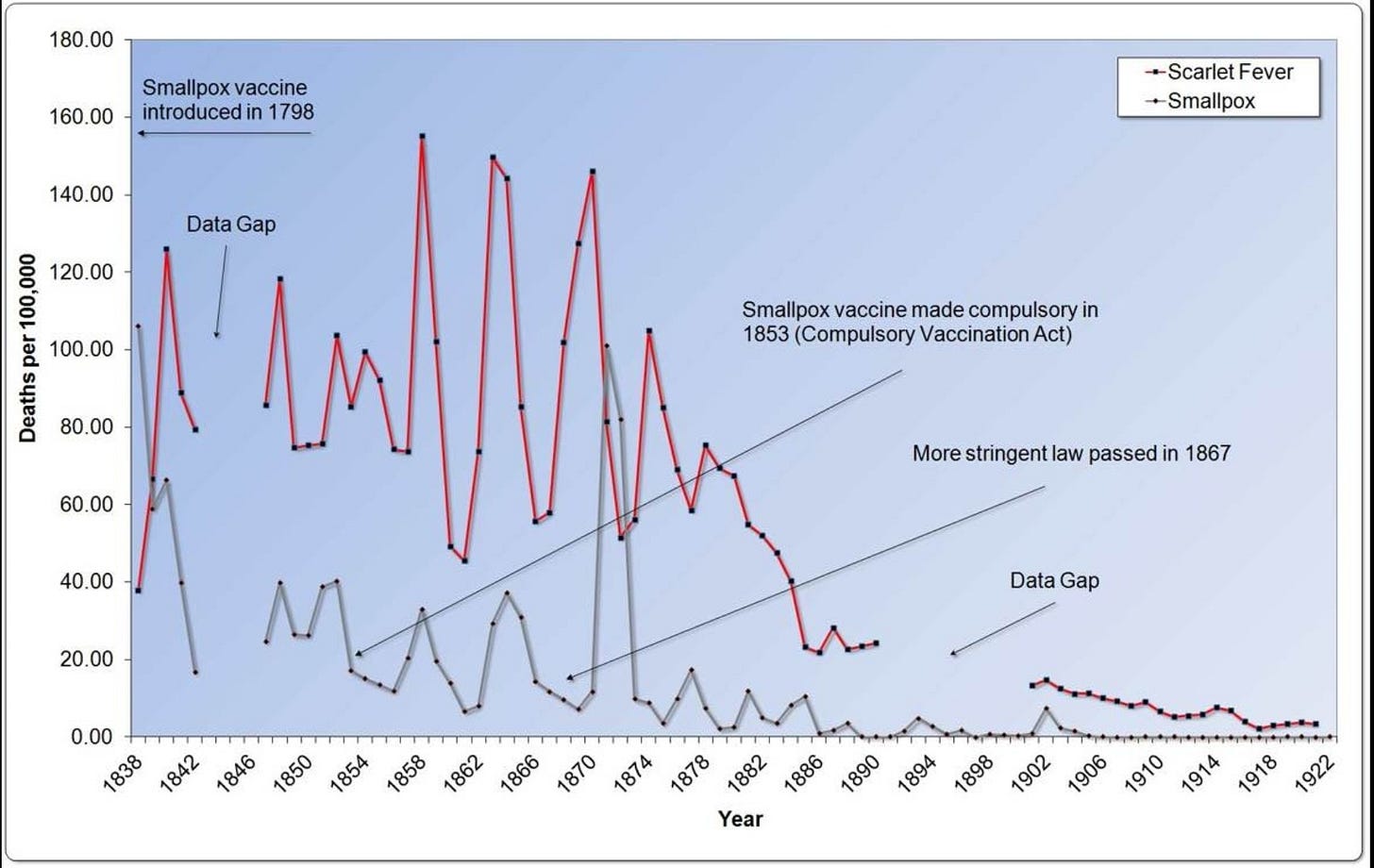
In 1840, as doctors and citizens realized that vaccination was not what it was promised to be, vaccine refusals increased. Governments passed various laws to force people to be vaccinated. Vaccination was made compulsory in England in 1853, with stricter laws passed in 1867. In the United States, Massachusetts created a set of comprehensive vaccination laws in 1855.
Did this work? Not exactly.
In fact, more people died from smallpox in the 20 years after the strict compulsory laws than in the 20 years prior.
…smallpox, after having almost wholly disappeared from our community during the thirty or forty years which followed the introduction of vaccination in 1800, gradually regained its foothold in Boston, where it continued to prevail almost uninterruptedly, although with varying intensity, from 1839, when the disease for the first time assumed the form of a distinct epidemic, up to 1873. During this period of thirty-five years the course of smallpox has been marked by a succession of epidemic paroxysms, generally by intervals of several years, during which a varying number of sporadic cases has testified to the more or less constant presence of the disease. The latest epidemic that of 1872-1873, having proved fatal to 1040 persons, was the most severe that has been experienced in Boston since the introduction of vaccination.{142}
The same pattern of more severe epidemics was to be repeated throughout highly vaccinated populations in the Western world.
Sanitation and nutrition, every time:
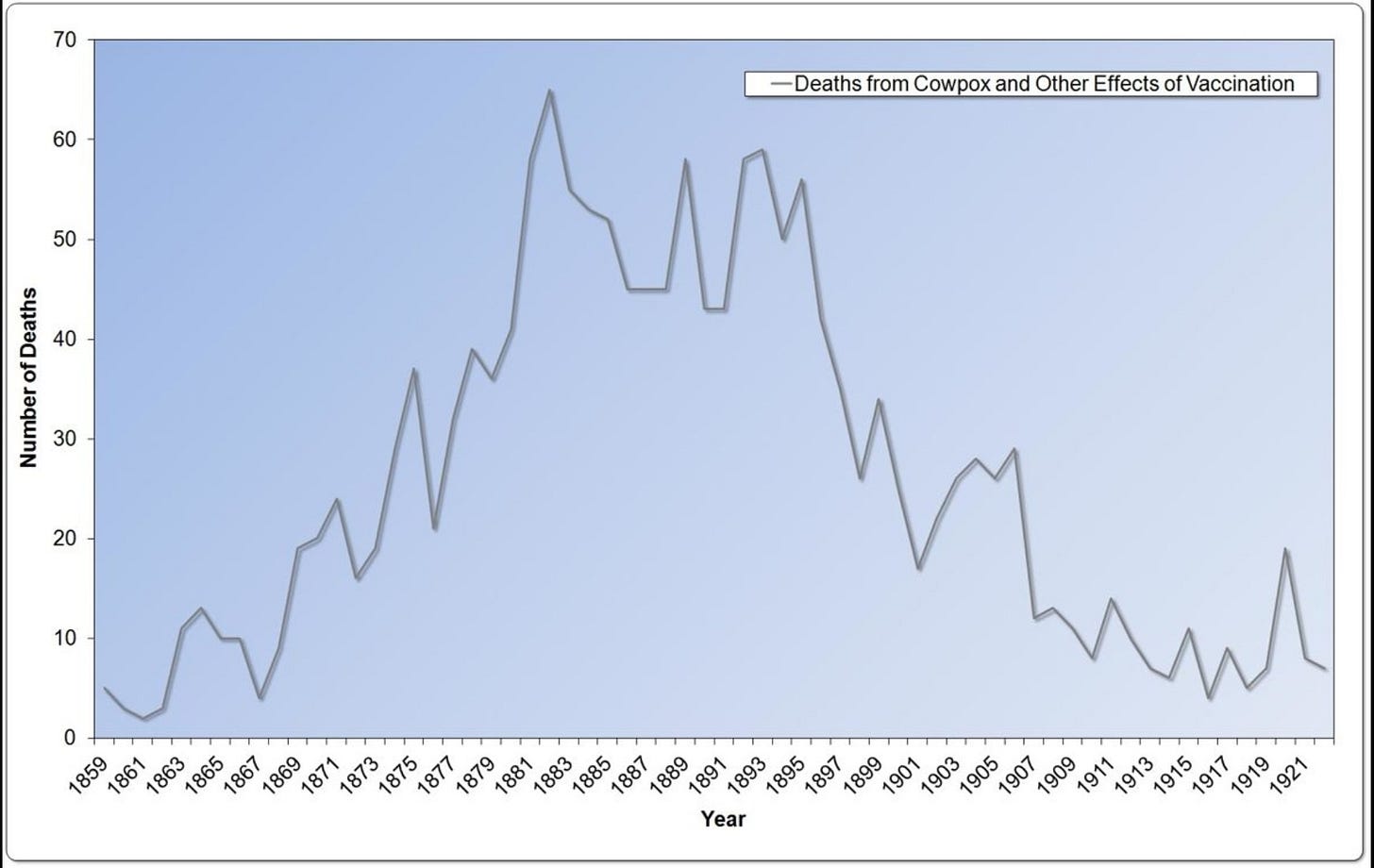
The death rate for smallpox declined after 1872, but there is no evidence that vaccination had anything at all to do with it. In the early 1900s, death from smallpox all but vanished from England (Graph 4.5). Interestingly, the pattern of smallpox deaths mirrored almost perfectly a much bigger killer—scarlet fever, a bacterial toxin-mediated disease. There was a scarlet fever toxin vaccine, which was never widely used because it had severe consequences to many of its recipients. A marked decline in scarlet fever death occurred long before any antibiotic was used.
Some may look at the graphs and think that the vaccine just needed longer to have its effect. But after 1872, vaccination coverage rates slowly declined from a high of nearly 90 percent. Coverage rates plummeted to only 40 percent by 1909 (Graph 4.6). Despite declining vaccination rates, smallpox deaths remained low, vanishing to near zero after 1906. Smallpox vaccination has always correlated positively to epidemics in the countries that collected data in the vain hope of proving the vaccine’s worth.
Just as now, many harrowing cases of needless child deaths. Being compliant didn't work and how compulsory vaccination was halted in England:
After the 1872 pandemic, even more people lost confidence in vaccination. They began asking the question as to whether better sanitation, hygiene, improved housing, nutrition, and isolation of cases were the best ways to deal with smallpox. These ideas, which clashed with the medical profession and governmental laws, culminated in a large demonstration in 1885 against compulsory vaccination in the small manufacturing town of Leicester, England. People had had enough. The tide was about to turn against both the medical profession and the law.
Although vaccination for smallpox had been used since the year 1800, the government did not begin to enforce it until the first acts in 1840 and 1853. The 1853 law set the governmental machinery in place to require every child to be vaccinated within three months of birth.
Through a series of legal acts over the years, the British government had, by the time of the protest in Leicester, made refusing smallpox vaccination a crime punishable by fines or imprisonment.
The penalties fell disproportionately on the poor who, if they could not afford to pay the fine for noncompliance with the vaccination law, would have the settlement forcibly offset by seizure and sale of their furniture. From the Leicester Mercury, January 1884:
A man named Arthur Ward had two children injured through vaccination and refused to submit another one to the operation. A fine was imposed and on 24th November two police officers called for the penalty, or in default to ticket the goods. The husband was out at the market, and the poor woman had no money to pay. The goods downstairs were considered insufficient to cover the amount, and the officers demanded to go upstairs. The woman refused to allow this, and an altercation took place, and harsh language was used by the officers, who threatened to take her husband to prison, terrifying Mrs. Ward. At that time she was pregnant, and the shock to the system, and the fright, were of such a character that symptoms ensued which ultimately led to a premature confinement, and on 26th December she gave birth to a still-born child. She never recovered and last week she expired. The doctor who had attended Mrs. Ward said that although he believed in vaccination he did not think it was the duty of any professional man to carry out the laws in the outrageous and brutal manner in which they were enforced.{193}
Leicester, March 23, 1885:
Because of the serious and sometimes fatal results of the procedure, and the government’s steadfast support of forced vaccination through fines and imprisonment, the people were motivated to revolt. In great numbers, they took to the streets of Leicester to protest. At the time of the demonstration, thousands of prosecutions were being brought against parents who refused vaccination for their children.
The widespread opposition to the enforcement of the compulsory clauses of the Vaccination Acts which exists in Leicester culminated yesterday in a great demonstration, which was carried out very successfully. The position which the inhabitants of the town have assumed with regard to this question is due to a variety of causes. At the present moment there are over 5,000 persons being summoned for refusing to comply with the law.… summonses issued in the year 1884 only reached seven, or a little over one summons in every two months, while at the present moment forty-five summonses are being heard and disposed of every week. But even the disposal of forty-five defendants every week is not sufficient to meet the requirements of the case, and the defaulters and the objectors increase faster than the cases can be dealt with.{194}
The crowd gathered in Leicester from a number of different counties in England and included people of all professions.
The demonstration… drew delegates from all parts of the country, while many letters of sympathy were received not only from England, Scotland, and Ireland, but from Jersey, France, Switzerland, Belgium, Germany, and America. Most of the large towns in the kingdom sent special banners, the Yorkshire, Irish, and Scotch being very prominent. The anti-vaccinationists in Jersey sent a very elaborate banner setting forth that the Acts had been four times defeated there, while the Belgium banner had this inscription in French—“Neither fines nor imprisonment will prevent vaccine being a poison nor the vaccination laws an infamy.”{195}
Organizers of the event estimated the number at attendance to have been between 80,000 and 100,000. Mr. Councillor Butcher of Leicester presided and congratulated the crowd on the magnificent and elaborate display. He said:
…the exemplary conduct of the many thousands of people who had attended the demonstration showed that they were determined only to use fair and constitutional means to bring about a repeal of the Acts.{200}
He addressed the audience:
Many present had been sufferers under the Acts, and all they asked was that in the future they and their children might be let alone. They lived for something else in this world than to be experimented upon for the stamping out of a particular disease. A large and increasing portion of the public were of opinion that the best way to get rid of smallpox and similar diseases was to use plenty of water, eat good food, live in light and airy houses, and see that the Corporation kept the streets clean and the drains in order. If such details were attended to, there was no need to fear smallpox, or any of its kindred; and if they were neglected, neither vaccination nor any other prescription by Act of Parliament could save them.{201}
The people of Leicester prevailed and brought this pseudoscientific nightmare to a close, the compulsory vaccination laws were eventually repealed:
Thousands of brave people set off a historical rebellion that successfully countered a prevailing medical belief and heavy-handed government rule. The medical profession proclaimed that the Leicester residents would suffer greatly for their decision to turn their backs on vaccination. They prognosticated that this unvaccinated town with its “highly flammable material”{206} would suffer with the “dread disease”{207} that would spread like “wild-fire on a prairie”{208} and decimate the population.
But the leaders of Leicester held steadfast to what they knew was right and successfully implemented their plan of sanitation, hygiene, and isolation—instead of vaccination. Their grand experiment would test the very notions of freedom of choice, self-determination, and the heart of a flawed medical belief.
For the full story and many other case studies I recommend reading the book.
Part 2: Monkeypox
I won't be going into great virological depth here as currently it's something of a media hyped, vaccine/WHO promoting propaganda exercise, a nothingburger with an R number less than 1 and transmitted in a similar fashion to HIV, effectively a gender specific STD.
Just like with Covid-19 for most people the imported West African clade presents as a comparatively mild, self limiting disease with a case fatality ratio of less than 1, including all those with comorbidities, ie its actually quite difficult to die from, let alone catch in the first place.1
However there is evidence of a lab based origin or potential gain of function work, and there is the possibility it could function like a binary weapon, the other component being immunosuppression mediated by Coronavirus “vaccines”, so it's one to watch for transmission rates. That would require aerosol spread rather than direct person to person to become an issue.
@Kevin_McKernan:
There is something fishy about the monkey pox sequences.
50 mutations is too many for a 2018 DNA virus.
There is also a deletion which hints at serial passaging.
https://virological.org/t/discussion-of-on-going-mpxv-genome-sequencing/802
And this thread added 5th June ‘22:
https://threadreaderapp.com/thread/1532852045614665732.html
From the pharma viewpoint, smallpox vaccines are interchangeable with monkeypox vaccines.
Smallpox vaccines have also been associated with very high rates of myocarditis:
In 2015, US military physicians described a study of 1,081 healthy young soldiers who received a smallpox vaccine as part of their military service. [Smallpox vaccine is not used in the civilian population.] It is known to cause a high rate of side effects, including myo- and peri-carditis, heart attacks and heart failure.
They found:
5 soldiers or 0.046% (about 1 in 216 vaccine recipients) developed a clinical case of myo or pericarditis. This is over 200 times the expected rate!
But an additional 31 vaccine recipients had elevated cardiac enzymes
Adding these 2 groups together (36 out of 1081 soldiers) we find that one in 30 soldiers had lab-diagnosed cardiac inflammation. The 31 didn't complain of symptoms. But in the military, it never pays to complain.
They too were at elevated risk of a cardiac arrhythmia and/or reduced cardiac function, and may have been at higher risk of a myocardial infarction.
We don't know how common mild or subacute myo/pericarditis is in young Americans after Covid vaccines, because the US health agencies have neither performed a similar study, demanded such a study from the vaccine manufacturers (while it is the responsibility of both FDA to request and the manufacturers to perform), and the FDA and CDC have kept the databases hidden that might help at least identify the "clinical" cases, the ones who complained and sought medical care.
The CDC or FDA could also have contracted with this group of military physicians to perform a similar study of Covid vaccine recipients.
Dr. Michael Nelson, this study's second author, was made a member of the FDA vaccine advisory committee for Covid vaccines. But he has been mum about the potential similarities between the covid and smallpox vaccine side effects, and didn't publicly mention this study when he spoke at the VRBPAC meetings.
In 2003, another group of military and civilian physicians (including at least 2 vaccine zealots as coauthors: Greg Poland and John Grabenstein) published a study of US soldiers receiving smallpox vaccine, in which they did not look carefully for cases. How common was myocarditis in their study? One case in 12,818 soldiers. They found 400 times fewer cases than the authors of the 2015 study. The full text can be downloaded here as a pdf.
How hard are CDC and FDA looking for Covid vaccine myo-pericarditis cases? We heard about no prospective studies at the VRBPAC and ACIP (the FDA and CDC vaccine advisory committees') meetings in June.
Below is the abstract, and here is the full text of the 2015 military study:
New onset chest pain, dyspnea, and/or palpitations occurred in 10.6% of SPX-vaccinees and 2.6% of TIV-vaccinees within 30 days of immunization (relative risk (RR) 4.0, 95% CI: 1.7-9.3). Among the 1081 SPX-vaccinees with complete follow-up, 4 Caucasian males were diagnosed with probable myocarditis and 1 female with suspected pericarditis. This indicates a post-SPX incidence rate more than 200-times higher than the pre-SPX background population surveillance rate of myocarditis/pericarditis (RR 214, 95% CI 65-558). Additionally, 31 SPX-vaccinees without specific cardiac symptoms were found to have over 2-fold increases in cTnT (>99th percentile) from baseline (pre-SPX) during the window of risk for clinical myocarditis/pericarditis and meeting a proposed case definition for possible subclinical myocarditis. This rate is 60-times higher than the incidence rate of overt clinical cases. No clinical or possible subclinical myocarditis cases were identified in the TIV-vaccinated group.
If you think one in thirty is impossibly high, a Finnish study of military recruits published in 1978 found the same 3% rate after smallpox and DTP vaccination, based on EKG changes.
Covid vaccines may be causing similar high rates of cardiac inflammation too. But today, who's counting?
And did this cause the military to stop vaccinating for smallpox, a disease wiped out in 1977? No. Military smallpox vaccinations continued.
Nass, Meryl MD, How common is myocarditis? It hugely depends how hard you look. For smallpox vaccine in military recruits, 1 in 30 had clinical or subclinical myo or pericarditis/PLOS One, (Wednesday, July 7, 2021)
https://anthraxvaccine.blogspot.com/2021/07/how-common-is-myocarditis-it-hugely.html
Now we consider “JYNNEOS”, the monkeypox vaccine of the hour. It's a repurposed 2 dose live, non-replicating smallpox vaccine.
To get “immunised” with it is very much a personal decision based on your circumstances and future epidemiological developments. I present the evidence here and personally would advise extreme caution.
The memorandum wreaks of a now all too familiar bias and lacked long term safety or immunological efficacy data.
Extracts from the BLA Clinical Review Memorandum, a Biologics License Application (BLA) dated 10/25/2018 and downloadable from:
https://www.fda.gov/media/131870/download
Jynneos is an attempt at a safer smallpox vaccine (“MVA-BN”). For an eradicated disease.
And who might these “bioterrorists” be they refer to?
Smallpox is a highly contagious infectious disease caused by variola virus with a mortality rate of 30-40%. Smallpox was declared officially eradicated in 1980. Following the official declaration of smallpox eradication, routine vaccination 3 programs against smallpox were discontinued, leading to a growing majority of the world’s population lacking immunity to smallpox. The intentional release of variola virus, a recognized agent of potential bioterrorist intent, could therefore have devastating effects. The only currently licensed smallpox vaccine, ACAM2000, is a live, replicating vaccinia virus based smallpox vaccine. ACAM2000 is contraindicated in severely immunocompromised individuals who are not expected to benefit from the vaccine. ACAM2000 is also limited to use in individuals at high risk of smallpox because of severe side effects, such as progressive vaccinia in less severely immunocompromised individuals for whom the vaccine is not contraindicated, eczema vaccinatum in individuals with atopic dermatitis, myopericarditis in smallpox vaccine naïve individuals, fetal vaccinia in pregnant women, and spread of vaccine virus beyond the vaccination site (generalized vaccinia) or to contacts of vaccinees. Therefore, an unmet medical need exists for a smallpox vaccine with an improved safety profile.
Monkeypox is a rare viral zoonosis with symptoms similar to those seen in smallpox patients. Although it is clinically less severe than smallpox, it can be fatal. Case fatality in monkeypox outbreaks has been between 1% and 10%. With the eradication of smallpox in 1980 and subsequent cessation of smallpox vaccination, monkeypox virus has emerged as the most important orthopoxvirus. Monkeypox occurs sporadically in central and western Africa’s tropical rainforest. A monkeypox outbreak was first confirmed in the U.S. in 2003. There is no specific treatment or approved vaccine for monkeypox although Advisory Committee on Immunization Practices (ACIP) recommends that ACAM2000 be used for prevention of monkeypox in individuals at high risk of exposure (e.g., lab workers who handle monkeypox virus).
BN (also referred to as the applicant throughout the document) proposed a 2- dose primary series for use in smallpox vaccine naïve individuals and a single booster dose for use in individuals previously vaccinated with a smallpox vaccine (replicating smallpox vaccine or MVA-BN primary series). They submitted 22 clinical trials to support the effectiveness and safety of MVA-BN for licensure. Among these 22 clinical trials, 7 clinical trials are considered essential by the review team to support the proposed indication and usage. Full clinical study reports were submitted to the BLA for these 7 studies:
• POX-MVA-006: A pivotal Phase 3 non-inferiority trial comparing MVA-BN with ACAM2000 to support safety and effectiveness of MVA-BN in vaccinia naïve healthy subjects
• POX-MVA-013: A placebo-controlled Phase 3 lot consistency trial to establish manufacturing consistency of MVA-BN as well as to support safety of MVA-BN
• POX-MVA-008: A Phase 2 trial to support use of MVA-BN in individuals with atopic dermatitis
• POX-MVA-011: A Phase 2 trial to support use of MVA-BN in HIV-infected individuals
• POX-MVA-005 and -23: A Phase 2 trial and its extension trial, respectively, to support use of MVA-BN in vaccinia experienced individuals
• POX-MVA-024: A Phase 2 trial to support use of MVA-BN in individuals 65 years of age and older
So it starts on a high note, but things go downhill fast from there. Starting efficacy target is only 50%. Safety and efficacy isn’t assessed primarily against an unvaccinated control but against ACAM2000, is this to make it look safer?
Antibodies do not equal immunity. They do pick up that there is no known quantified antibody response to demonstrate immunity:
During the discussions of licensure pathway for MVA-BN, we agreed that the most appropriate approach to licensure for MVA-BN would be to demonstrate vaccine effectiveness compared to ACAM2000 using a primary endpoint of noninferior vaccinia specific neutralizing antibody titers. The non-inferiority margin was pre-specified at 0.5. Given that vaccine antigens and replication competence are different for MVA-BN vs. ACAM2000, and that a vaccinia neutralizing antibody response that predicts protection against smallpox has not been established, we considered that demonstrating vaccine efficacy in animal models showing protection against relevant orthopoxvirus challenge (e.g., monkeypox in NHPs) would be critical to support the immunologic non-inferiority comparison.
And no need to actually trial it against a different species to smallpox as monkeypox is close enough and it worked on monkeys. I’m not sure that’s how it works, its conjecture.
The applicant’s original proposed indication did not include monkeypox. During the review of this submission, we received inquiries from external stakeholders in the US government asking whether the available data for MVA-BN would support an indication for prevention of monkeypox. We determined that immunogenicity data for MVA-BN obtained in humans together with the non-human primate (NHP) data already submitted to BLA 125678/0 support the indication for prevention of monkeypox, since the clinical and non-clinical studies provided multiple lines of evidence that the immune response to MVA-BN provided protection against different orthopoxviruses, and specifically monkeypox in the NHP challenge model. Therefore, we recommended including the monkeypox indication in the product labeling.
They followed up some trial participants for 6 months, but myocardial fibrosis can take years to become symptomatic and still shortens your natural lifespan. These papers are worth reading in full in the context of Covid-19 “vaccine” mediated myo and pericarditis. Small lumen capillaries are particularly prone to obstruction and T-cell infiltration mediates further myocardial injury.
Abstract
Heart injury from many causes can end up in a common final pathway of pathologic remodeling and fibrosis, promoting heart failure development. Dilated cardiomyopathy is an important cause of heart failure and often results from virus-triggered myocarditis. Monocytes and monocyte-like cells represent a major subset of heart-infiltrating cells at the injury site. These bone marrow-derived cells promote not only tissue injury in the short term but also angiogenesis and collagen deposition in the long term. Thus, they are critically involved in the typical tissue fibrosis, which evolves in the dilating ventricle during the process of pathologic remodeling. Recent findings suggest that heart-infiltrating monocyte-like cells indeed contain a pool of progenitors, which represent the cellular source both for accumulation of differentiated monocytes during the acute inflammatory phase and for transforming growth factor-beta-mediated myocardial fibrosis during the later chronic stages of disease. Obviously, a delicate balance of proinflammatory and profibrotic cytokines dictates the fate of bone marrow-derived heart-infiltrating progenitors and directly influences the morphologic phenotype of the affected heart. In this minireview, we provide an update on these mechanisms and discuss their significance in pathologic remodeling and heart failure progression after myocarditis.
Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009 Nov;19(8):247-52. doi: 10.1016/j.tcm.2010.02.005. PMID: 20447565.
https://pubmed.ncbi.nlm.nih.gov/20447565/
Cardiac remodelling in detail. This is post “heart attack” (myocardial infarction or MI) but is applicable in the context of the shared mechanisms of myocardial fibrosis:
Abstract
Ischemic cell death during a myocardial infarction leads to a multiphase reparative response in which the damaged tissue is replaced with a fibrotic scar produced by fibroblasts and myofibroblasts. This also induces geometrical, biomechanical, and biochemical changes in the uninjured ventricular wall eliciting a reactive remodeling process that includes interstitial and perivascular fibrosis. Although the initial reparative fibrosis is crucial for preventing rupture of the ventricular wall, an exaggerated fibrotic response and reactive fibrosis outside the injured area are detrimental as they lead to progressive impairment of cardiac function and eventually to heart failure. In this review, we summarize current knowledge of the mechanisms of both reparative and reactive cardiac fibrosis in response to myocardial infarction, discuss the potential of inducing cardiac regeneration through direct reprogramming of fibroblasts and myofibroblasts into cardiomyocytes, and review the currently available and potential future therapeutic strategies to inhibit cardiac fibrosis.
The fibrotic response after an MI can be classified into two types of fibrosis, namely replacement and reactive fibrosis, both of which are mediated by fibroblasts and myofibroblasts. Replacement fibrosis, i.e. scar formation, is a pivotal process to prevent the rupturing of the ventricular wall after an ischemic insult (van den Borne et al. 2010; Shinde and Frangogiannis 2014). However, the increased mechanical stress post-MI, together with hormonal and paracrine mediators, also induces the expansion of connective tissue in areas remote to the infarction. This reactive fibrosis in the infarct border zone and in the remote uninjured myocardium leads to altered chamber compliance and increased ventricular stiffness thereby compromising cardiac output.
In addition to its effect on cardiac contractility, both the fibrous scar and interstitial fibrosis have been shown to interfere with the normal electrical function of the heart thus predisposing to arrhythmia (for a review, see Francis Stuart et al. 2015). The compact scar may serve as an insulated non-excitable area that anchors re-entrant arrhythmia leading to sustained ventricular tachycardia (Ripplinger et al. 2009). In interstitial fibrosis, the non-conducting fibrillar collagen network between cardiomyocyte sheets might promote re-entrant tachycardia through inducing focal ectopic activity and through slowing or blocking of conduction (Francis Stuart et al. 2015). Additionally, the electronic coupling of myofibroblasts and cardiomyocytes might play a role in fibrosis-induced arrhythmogenesis (Kohl and Gourdie 2014). Not surprisingly, cardiac fibrosis has been identified as an autonomous risk factor in HF: it predisposes HF patients to sudden cardiac death and increases overall mortality independently of the ejection fraction (Gulati et al. 2013).
Following the establishment of a collagen-based matrix at the infarct site, the growth factors and matricellular proteins promoting the survival and activity of myofibroblasts are depleted (van den Borne et al. 2010; Shinde and Frangogiannis 2014). In response, the majority of myofibroblasts are removed from the scarred area, possibly through apoptosis. Moreover, the vascular cells die, and the temporary microvasculature is disintegrated. Whether active inhibitory signaling is involved in suppressing the fibrotic response is unclear. During the maturation phase of MI, collagen turnover by the remaining myofibroblasts continues, and type III collagen is replaced with type I collagen. Type I collagen is further modified by LOX-catalyzed cross-linking. The expression of all four LOX isoforms is increased in the infarct area and in the border zone at 3–7 days post-MI (Gonzalez-Santamaria et al. 2016). This correlates with significant accumulation of mature collagen fibers and extensive remodeling, and LOX inhibition with a pharmacological inhibitor or a neutralizing antibody reduces infarct expansion resulting in improved cardiac function at 28 days post-MI (Gonzalez-Santamaria et al. 2016). Cross-linking of the collagen fibers leads to increased tensile strength and contraction of the scar, which alters the geometry of the chamber and contributes to remodeling in the remote areas of the ventricular wall (van den Borne et al. 2010). In a normal wound healing response, all myofibroblasts are cleared from the scarred area, but in the heart, they have been found to persist in the infarct scar even decades after the insult (Willems et al. 1994). The reason for the continuous myofibroblast presence in the infarct scar is not known but is possibly necessary for the continuous maintenance of the ECM in the continuously contracting environment (van den Borne et al. 2010).
Reactive fibrosis: remodeling of remote myocardium
Most often it is not the necrotic cardiomyocyte loss during MI that causes heart failure but the subsequent remodeling of the non-infarcted left ventricular wall. In pathological remodeling, the fibroblast-mediated expansion of the ECM is accompanied by the hypertrophic growth of cardiomyocytes as the cells try to compensate for the increased workload by growing in size in order to increase cardiac function and decrease ventricular wall tension (Heineke and Molkentin 2006). The increased thickness caused by cardiomyocyte hypertrophy and stiffness attributable to excessive cross-linked collagen and the tonic contraction of fibrous tissue mediated by myofibroblasts compromise the diastolic function of the heart (Weber et al. 2013). This remodeling process is progressive and eventually leads to the development of heart failure.
The exact mechanisms and regulation of reactive fibrosis are unclear, and systematic studies examining the characteristics of fibroblasts in the non-infarcted myocardium are lacking (Shinde and Frangogiannis 2014). One promoting factor is the increased mechanical stress in the non-infarcted left ventricular wall; this stress also induces the activation of latent TGF-β in the non-infarcted myocardium. In addition, the persisting activated myofibroblasts in the infarct scar continue to secrete pro-fibrotic factors that might traverse to the remote areas of the myocardium inducing activation and proliferation of local fibroblasts and increased collagen deposition in the interstitial compartment (interstitial fibrosis) and in the adventitia of coronary vessels (perivascular fibrosis; Weber et al. 2013). Pro-fibrotic factors initiating and sustaining the reactive fibrotic response are described in the next section.
Whereas interstitial fibrosis stiffens the myocardium and thereby leads to diastolic and systolic dysfunction, reactive fibrosis in the adventitia of the coronary arteries and arterioles (perivascular fibrosis) can cause narrowing of the vessel lumen and has been associated with impaired coronary blood flow (Dai et al. 2012). This might decrease the oxygen supply to the myocardium thereby compromising the survival of cardiomyocytes and predisposing them to ischemic cell death.
More:
Talman, V., & Ruskoaho, H. (2016). Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell and tissue research, 365(3), 563–581. https://doi.org/10.1007/s00441-016-2431-9
On cardiac amyloidosis. This is more relevant to spike protein infiltration but certainly worth covering within the context of Covid-19 transfection2. Useful diagnostic information too:
Cardiac AL amyloidosis
Light chain amyloidosis is consequent on a clonal plasma cell proliferative disorder in which misfolded immunoglobulin light chains are deposited as amyloid fibrils in multiple organs, including the heart in about half of cases. Cardiac dysfunction in AL amyloidosis results from extracellular infiltration of the myocardium, but there is often also evidence for a cardiotoxic effect exerted by pre-fibrillar light chain aggregates. The severity of cardiac dysfunction is the major determinant of morbidity and mortality.3
Light chain amyloidosis is the most commonly diagnosed type of cardiac amyloidosis. One or many vital organ systems may be involved, commonly the kidneys, liver, peripheral and autonomic nervous systems and soft tissues. The heart is frequently affected and is the only clinically involved organ in some patients.
Clinical presentation reflects the varying multisystem deposition of amyloid. Examination findings may reflect soft tissue and small vessel amyloid infiltration and include macroglossia, periorbital pupura, submandibular gland enlargement and nail dystrophy. Fatigue and weight loss are common. Hepatic or splenic infiltration may cause palpable organomegaly. Renal dysfunction is common, usually presenting as nephrotic range proteinuria.
Early cardiac amyloidosis is a major diagnostic challenge. The classical features of ‘right-sided’ congestive heart failure may not be evident until cardiac disease is very advanced. Elevated jugular venous pressure, a third heart sound, hepatomegaly and peripheral oedema may be very subtle or absent in patients who have already started diuretics.
Peripheral neuropathy is relatively common, presenting with paraesthesia or dysaesthesia typically in a ‘glove and stocking’ distribution. Autonomic neuropathy is an important diagnostic clue, manifesting as orthostatic hypotension, alternating diarrhoea and constipation and erectile dysfunction.
Monoclonal immunoglobulin or free light chains can be identified in the serum and/or urine of at least 95% of patients using sensitive assays, but are often missed in routine serum electrophoresis. The absence of a detectable clone is problematic for diagnosis and monitoring response to treatment.
Martinez-Naharro, A., Hawkins, P. N., & Fontana, M. (2018). Cardiac amyloidosis. Clinical medicine (London, England), 18(Suppl 2), s30–s35, https://pubmed.ncbi.nlm.nih.gov/29700090/.18-2-s30
Back to the Jynneos application. Antibody titres were common endpoints in the trials, even though there is no known threshold of efficacy or assessment of whether they could actually work against monkeypox.
They know this and state this but proceeded nonetheless as per, for example, trial POX-MVA-005:
POX-MVA-005 was a Phase 2 trial to compare immunogenicity of two doses of MVA-BN in vaccinia-naïve healthy subjects and a single dose of MVA-BN in vaccinia-experienced healthy subjects who were vaccinated with the first generation of smallpox vaccines over 25 years ago. The primary endpoint was vaccinia-specific SCR derived from the ELISA specific antibody titers two weeks after the last vaccination. The study enrolled 549 vaccinia-naïve subjects and 204 subjects who were previously vaccinated with the first generation of smallpox vaccines.
Studies POX-MVA-005/023 and POX-MVA-011 used of PRNT, and Studies POX-MVA-008 and POX-MVA-024 used of PRNT. of PRNT used in these studies were insufficiently validated and were not accepted by CBER assay reviewers. The PRNT assay issue precluded us from making any conclusion regarding vaccine effectiveness among the study populations, including for use of a single booster dose in smallpox vaccine experienced individuals. In addition, the primary endpoints for these studies were SCR determined by MVA-based ELISA, which is not considered clinically meaningful for inferring vaccine effectiveness. Therefore, the data obtained from these studies were not sufficient to support vaccine effectiveness of two doses of MVA-BN specifically in HIV-infected individuals or individuals with AD subjects, nor to support licensure of a single dose (or to inform timing of a single booster dose) in individuals previously vaccinated with a smallpox vaccine. However, it was reasonable to conclude that the 2-dose regimen of MVA-BN would be as effective in smallpox vaccine experienced individuals as compared to smallpox vaccine naïve individuals, so smallpox vaccine experienced individuals were included in the approved indication for the 2-dose regimen. Similarly, there is no physiologic reason to suspect decreased effectiveness of MVA-BN in individuals with AD, and benefit-risk of MVA-BN may still be favorable in HIV-infected individuals. Therefore, there is no reason to specifically exclude individuals with AD or infected with HIV from the general indication for use of this vaccine.
The following is totally unacceptable, given that elevated troponin is an indicator of myocardial injury, which may be asymptomatic for months or years until it presents as cardiac arrest or heart disease.
“Post-marketing pharmacovigilance” means that you become the unwitting trial participant, with little chance of the product being pulled regardless of how dreadful the realised safety profile is:
4.6 Pharmacovigilance
Although no safety signal regarding cardiac events has been identified from the studies, up to 18.4% of subjects in two of the 22 clinical trials were reported to have abnormal, asymptomatic troponin-I elevations following vaccination. These troponin-I elevations were not accompanied by clinically significant ECG changes or other findings on cardiology evaluation and were of uncertain clinical significance. We find the applicant’s proposed plan to assess spontaneously reported cardiac data as part of the routine post-marketing pharmacovigilance plan acceptable.
Lymphadenopathy = swollen lymph nodes, a marker of suppressed immunity and/or autoimmune disorders3.
Arthralgia = joint pain, another condition associated with autoimmune disorders, just as elevated troponin and peri & myocarditis is.
Note the grade 3 percentages.
Group 1 = Jynneos
Group 2 = ACAM2000
10.9% of the Jynneos candidates analysed had grade 3 induration, presumably at the injection site but they don't break it down further. Think “bee sting” sort of swelling and pain:
Induration: “Localized hardening of soft tissue of the body. The area becomes firm, but not as hard as bone.”
“Grade 3 events are serious and interfere with a person’s ability to do basic things like eat or get dressed. Grade 3 events may also require medical intervention.4”
Aegrescit medendo.
6.1.10.1 Populations Enrolled/Analyzed
Full Analysis Set (FAS)
FAS was defined as all subjects who had received at least one dose of trial vaccine and for whom any post vaccination safety or immunogenicity data were available.
The safety analysis and secondary supportive immunogenicity analyses were performed on the FAS.
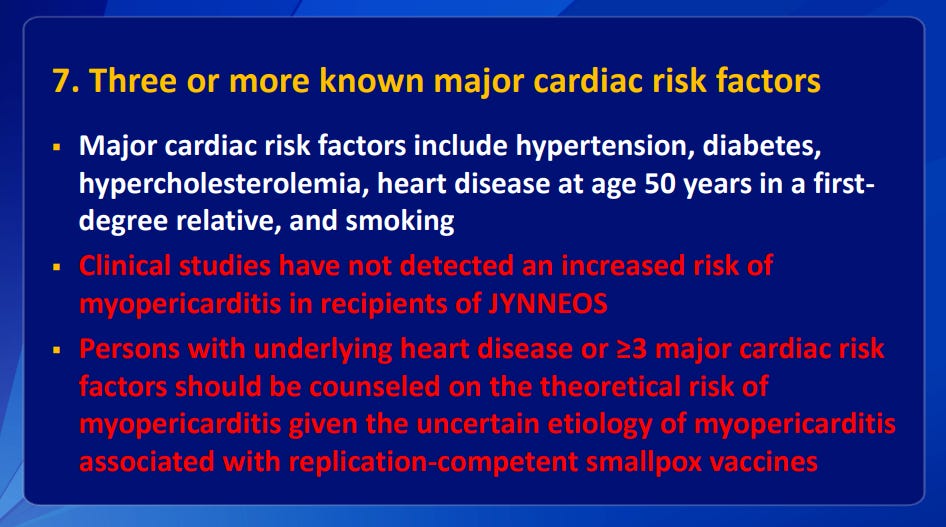
Slides from a presentation by the Advisory Committee on Immunization Practices (ACIP). Despite the trials data and their own assessment they managed to get any risk of peri or myocarditis removed from the package insert:
Clinical guidance for the use of JYNNEOS. ACIP Meeting November 3 , 2021
Once on the program, expect to be chased for boosters every 2 years with risk of cumulative cardiac damage:
Those who need it the most benefit the least. Welcome back to Victorian England:
That’s a NO then:
Just like spike protein expressing transfection is replication deficient. That’s also a NO:
From the application up to 2.1%, that’s 1 in 50, had a cardiac adverse event of special interest (AESI). That’s over 10 times the placebo rate. And they had another 127 cases that didn’t meet the threshold of 2 times the upper limit of normal (ULN). HIV, Alzheimer's Disease and autoimmune associated symptoms were strong correlates:
So the troponin studies were more sensitive, is that the explanation?
Who made the assessment about the suspected pericarditis, why was it “unlikely related to MVA-BN”?
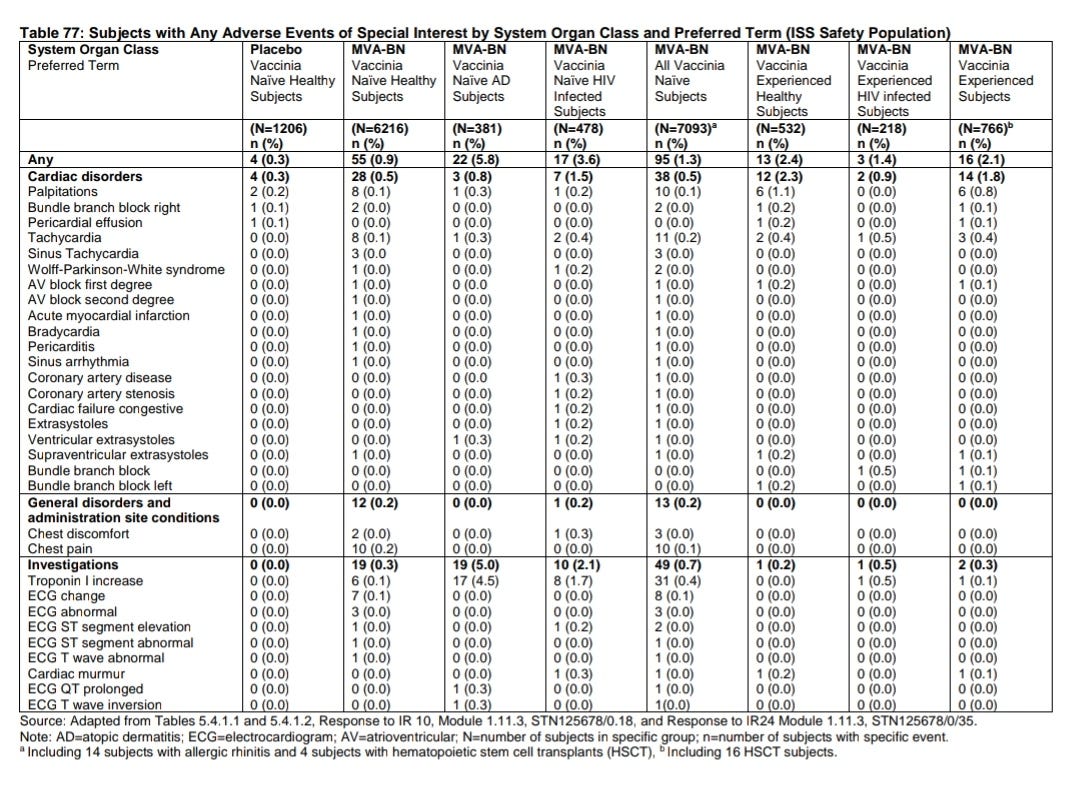
Cardiac AESIs were reported to occur in 1.3% (95/7,093) of MVA-BN recipients and 0.2% (3/1,206) of placebo recipients who were smallpox vaccine-naïve. Cardiac AESIs were reported to occur in 2.1% (16/766) of MVA-BN recipients who were smallpox vaccine-experienced. The higher proportion of MVA-BN recipients who experienced cardiac AESIs was driven by 28 cases of asymptomatic post-vaccination elevation of troponin-I in two studies: POX-MVA011, which enrolled 482 HIV-infected subjects and 97 healthy subjects, and POX-MVA-008, which enrolled 350 subjects with atopic dermatitis and 282 healthy subjects. An additional 127 of asymptomatic post-vaccination elevation of troponin-I above the ULN but not above 2 times the ULN were documented in MVA-BN recipients throughout the clinical development program, 124 of which occurred in studies POX-MVA-011 and POX-MVA-008. Proportions of subjects with troponin-I elevations (> ULN) were similar between healthy (13.7%) and HIV-infected (11.5%) subjects in POX-MVA-011 and between healthy (18.9%) and atopic dermatitis (18.0%) subjects in POX-MVA-008.
Overall, the number of subjects with AESIs in this clinical development program was relatively low. Except for one case of suspected pericarditis that was assessed as unlikely related to MVA-BN and isolated mild to moderate increases of troponin levels with unknown clinical significance, there were no other reported cardiac AESIs. Among the 22 studies, all the studies except for studies POXMVA-008 and POX-MVA-011 had few subjects with post-vaccination elevation of troponin-I. The applicant postulates that the increased proportion of subjects with post-vaccination elevation of troponin-I is related to the use of a more sensitive troponin assay. Among these two studies, 188 subjects were assessed for troponin-I with a ”conventional” troponin assay, and 934 subjects were assessed for troponin-I with a “high sensitivity” troponin assay. The “high sensitivity” troponin assay used in these two studies was not cleared by FDA. Among the 188 subjects whose troponin-I was assessed with the “conventional” troponin assay, no subject reported post-vaccination elevation of troponin-I, while 144 out of 934 subjects whose troponin-I was assessed by the “high sensitivity” troponin assay reported post-vaccination troponin-I elevation. All subjects with elevated troponin-I levels underwent a cardiologist workup and no clinically meaningful cardiac abnormality was identified among these subjects. Since there was no placebo control in these two studies, the clinical relevance of the increased proportion of subjects with subclinical, yet abnormal troponin-I is unknown.
“Not detected an increased risk of myopericarditis”, was it the same, a bit less maybe? This is misleading wordage, up to 18% of the HIV, AD & atopic dermatitis (eczema) groups having elevated troponin levels is strong evidence of elevated risk of subclinical peri or myocarditis or future fibrosis induced cardiomyopathy, in addition to the AESI’s in other groups:
Here they are warning of vaccine-associated enhanced disease (VAED). Thanks.
There is no strong evidence that Jynneos is actually doing anything (apart from killing cardiomyocytes).
Trust us, OK, we’re experts:
“False sense of security”:
Something missing? Cardiac risk events which you unconvincingly “believe to be lower” and not at placebo levels?
So with unconfirmed efficacy against actual monkeypox in humans, antibody titres at unknown levels long term, with no T or memory B-cell studies and a skewed risk profile with no long term safety data leaning towards administering to only the most vulnerable (apart from if they immunocompromised) then you would expect Jynneos to be withdrawn or strictly restricted.
That’s not quite how it worked out:
Following confirmation that monkeypox has made its way to the U.S., the government ordered millions of doses of a vaccine that protects against the virus.
Bavarian Nordic, the biotech company that makes the vaccine, has announced a $119 million order placed by the U.S., with the option to buy $180 million more if it wants. Should that second option be exercised, it would work out to approximately 13 million doses.
The order will convert existing smallpox vaccines, which are also effective against monkeypox, into freeze-dried versions, which have a longer shelf life. The converted vaccines will be manufactured in 2023 and 2024, the company says.
Bavarian Nordic has worked with the U.S. government since 2003 to develop, manufacture and supply smallpox vaccines. To date, it says, it has supplied nearly 30 million doses to the Department of Health and Human Services.
The U.S. isn’t the only country stocking up on the vaccine. On Thursday, Bavarian Nordic said an unidentified European country had secured a contract to obtain the vaccine.
“While the full circumstances around the current monkeypox cases in Europe remain to be elucidated, the speed of which these have evolved, combined with the potential for infections beyond the initial case going undetected, calls for a rapid and coordinated approach by the health authorities, and we are pleased to assist in this emergency situation,” said Paul Chaplin, president and CEO of Bavarian Nordic in a statement.
U.S. government places $119 million order for 13 million freeze-dried Monkeypox vaccines, (May 19, 2022)
https://fortune.com/2022/05/19/monkeypox-vaccine-purchase-2022-us-government/
BAVARIAN NORDIC TO MANUFACTURE FIRST FREEZE-DRIED DOSES OF SMALLPOX VACCINE UPON EXERCISE OF CONTRACT OPTION BY THE U.S. GOVERNMENT
USD 119 million option exercised for the manufacturing of freeze-dried JYNNEOS® in 2023 and 2024
This represents the first set of options with a total value of USD 299 million to convert the existing bulk vaccine, previously purchased by BARDA, to approximately 13 million freeze-dried JYNNEOS doses
https://www.bavarian-nordic.com/investor/news/news.aspx?news=6569
4th June ‘22:
Vaccinia and latent HIV reactivation
Smallpox vaccination history has another surprise in store.
This timely post piqued my interest:
Wait, what? I was able to find a source for this and related content:
https://www.hivireland.ie/wp-content/uploads/1987_Print_Media_005_May_11th_to_15th-WebSize.pdf
Some big names quoted in these articles. Whether by contaminated needle reuse or viral reactivation (or both?), the conspiracy theory becomes plausible, indeed plausible enough to sponsor research into the latter.
We see why later.
The key mechanism behind the hypothesis is hi-lighted:
The main focus of the project consists in the studies of interactions of vacccinia virus and HIV-1 with the host; specifically responses of the atopic organism towards infection with vaccinia virus and the effect of redox potential on reactivation of the latent HIV-1 will be studied. The research should provide a basis for new therapeutic and vaccination approaches usable in infections with these viruses.
Research Objectives:
To develop a mouse model of eczema vaccinatum in Nc/Nga mice or in a different mouse model of atopic dermatitis, and to study immune responses of the atopic organism to vaccinia virus.
To test the antiviral effect of selected derivatives of the ethacrynic acid and other agents with the anti-poxviral effect in vivo; in tissue culture, to study the exact mechanism of action of the agents, the type of cell death of the infected cells of different embryonic origin and its significance for induction of the immune responses in infection with vaccinia virus
To characterize the molecular mechanism of action of redox-modulating agents on reactivation of the latent HIV-1 and to verify the results obtained in vitro in tissue cultures also in primary peripheral lymphocytes of healthy donors and HIV+ patients ex vivo.
Further information can be found at www.phenogenomics.cz or at the link
https://biocev.lf1.cuni.cz/
Content of the research:
Our primary research focus is to study virus-host interactions, namely interactions between vaccinia virus and HIV-1. The research should provide the basis for new therapeutic and vaccination approaches usable in infections with these and other viruses.
Contrary to a common belief, the need to understand pathogenesis of poxvirus infection and post-vaccination complications as well as to develop safe vaccination vectors and/or drugs effective against poxviruses still remains. The main focus of our research is the response of an atopic organism towards vaccinia and other viruses. We have developed our own model of eczema vaccinatum in atopic Nc/Nga mice, used it to characterize immune responses and to compare the risks and efficiencies of vaccinations with vaccinia virus strain WR, Dryvax and non-replicating MVA. In this atopic model, we continue to study the disregulated immune responses towards vaccinia and other viruses.
HIV latency in reservoir cells is the main obstacle to cure HIV/AIDS. Our goal is to identify, characterize and develop new agents to reactivate latent HIV-1. We have described a reactivation potential of heme arginate and we identified several other HIV-1-reactivating agents in vitro in tissue cultures. We are currently focusing on confirmation of the results in primary peripheral lymphocytes of HIV + patients and in primary latency models. We intend to patient selected drug combinations and to seek for a strategic partner to further develop the new HIV treatment.
Potential for Cooperation
We are open to new PhD students and to any type of collaboration/exchange with researchers interested in studying the immune responses of an atopic organism towards vaccinia and other viruses. To further develop new agents effective a reactivation of latent HIV, we are seeking for a strategic investor.
MUDr. Zora Mělková, Ph.D., Study of Vaccinia Virus Interactions with the Host and Reactivation of the Latent HIV-1, (Year ?).
On smallbox based HIV vaccines, as per the last newspaper cutout, co-authored by no less than Luc Montagnier:
Abstract
The sequences encoding the core proteins p55, p25, and p18 of the human immunodeficiency virus (HIV-1) have been inserted into the vaccinia virus genome. Infection of cultured cells with the live recombinant viruses led to the expression of proteins that were recognized by sera from HIV-seropositive individuals. Immunization of mice with the recombinant virus expressing the HIV p25 protein and the p55 precursor yielded high levels of antibodies directed against the corresponding HIV antigens. The data obtained are discussed in terms of the possible use of these live recombinant viruses in the development of a strategy toward an AIDS vaccine.
Rautmann G, Kieny MP, Brandely R, Dott K, Girard M, Montagnier L, Lecocq JP. HIV-1 core proteins expressed from recombinant vaccinia viruses. AIDS Res Hum Retroviruses. 1989 Apr;5(2):147-57. doi: 10.1089/aid.1989.5.147. PMID: 2713165.
https://pubmed.ncbi.nlm.nih.gov/2713165/
Glutathione peroxidase, HERV reactivation and Vaccinia, this time in a protective role:
...The reduced risk of melanoma due to BCG and vaccinia, as well as certain common causes of infectious disease, is shown to be associated with antigenic determinants exhibiting sequence homologies with the HERV-K-MEL-antigen. The latter is a product of a pseudo-gene that is closely associated with the env-gene of the endogenous human retrovirus K (HERV-K). A suppressive immune reaction appears to inhibit the expression of endogenous retroviral genes, such as the HERV-K env-gene, that could otherwise result in malignant transformation years or even decades later. The HERV-K env-protein has homologous amino acid sequences with the human nuclear factor Oxygen Responsive Element Binding Protein (OREBP) that controls the expression of glutathione peroxidase. The formation of this and other redox-enzymes, needed to maintain appropriate levels of the normal intracellular redox potential, seems to be suppressed by the OREBP-homologous protein. The present hypothesis is in accordance with the concept that immune dysregulation due to adverse environmental impacts is a risk factor not only for some autoimmune disorders, as previously described, but also for certain malignancies such as melanoma.
Bernd Krone et al, Protection against melanoma by vaccination with Bacille Calmette-Guerin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control, Eur J Cancer, (2005 Jan).
https://pubmed.ncbi.nlm.nih.gov/15617995/
HIV is very sensitive to the REDOX potential of the cell5. The key here is that Vaccinia encodes a functional glutaredoxin.
What are these? Short answer:
"Glutaredoxins (also known as Thioltransferase) are small redox enzymes of approximately one hundred amino-acid residues that use glutathione as a cofactor. In humans this oxidation repair enzyme is also known to participate in many cellular functions, including redox signaling and regulation of glucose metabolism.[4][5] Glutaredoxins are oxidized by substrates, and reduced non-enzymatically by glutathione.6"
Abstract
Vaccinia virus (VV) was previously shown to encode a functional glutaredoxin, the product of the o2l gene, which is synthesized late in infection, after the onset of DNA replication. Here we report that an open reading frame in the VV genome designated as g4l encodes a protein that has sequence similarity to glutaredoxins and possesses thioltransferase and dehydroascorbate reductase activities. G4L protein in infected cells can be detected as early as 4 hr after infection and is constitutively expressed up to 24 hr postinfection. A protein homologous to G4L and retaining the predicted glutaredoxin active center is encoded by the recently sequenced Molluscum Contagiosum virus (MCV), whereas O2L protein is not conserved, suggesting that the glutaredoxin activity of G4L may be involved in replication of all poxviruses.
Gvakharia BO, Koonin EK, Mathews CK. Vaccinia virus G4L gene encodes a second glutaredoxin. Virology. 1996 Dec 15;226(2):408-11.
doi: 10.1006/viro.1996.0669. PMID: 8955061.
https://pubmed.ncbi.nlm.nih.gov/8955061/
How might glutaredoxin reactivate latent viruses including HIV?
First point of note is the cancer signalling pathways being activated too:
Abstract
Although the use of antioxidants for the treatment of cancer and HIV/AIDS has been proposed for decades, new insights gained from redox research have suggested a very different scenario. These new data show that the major cellular antioxidant systems, the thioredoxin (Trx) and glutathione (GSH) systems, actually promote cancer growth and HIV infection, while suppressing an effective immune response. Mechanistically, these systems control both the redox- and NO-based pathways (nitroso-redox homeostasis), which subserve innate and cellular immune defenses. Dual inhibition of the Trx and GSH systems synergistically kills neoplastic cells in vitro and in mice and decreases resistance to anticancer therapy. Similarly, the population of HIV reservoir cells that constitutes the major barrier to a cure for AIDS is exquisitely redox sensitive and could be selectively targeted by Trx and GSH inhibitors. Trx and GSH inhibition may lead to a reprogramming of the immune response, tilting the balance between the immune system and cancer or HIV in favor of the former, allowing elimination of diseased cells. Thus, therapies based on silencing of the Trx and GSH pathways represent a promising approach for the cure of both cancer and AIDS and warrant further investigation.
Benhar, M., Shytaj, I. L., Stamler, J. S., & Savarino, A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. The Journal of clinical investigation, 126(5), 1630–1639. (2016)
https://doi.org/10.1172/JCI85339
Which brings us back to monkeypox vaccination.
Jynneos is an MVA. Is it expressing glutaredoxin too in quantity?
Please correct me if I'm reading the genome paper incorrectly, but the answer appears to be yes.
It makes sense as in order to generate immunity then you need a high degree of homology, ie protein expression.
Vaccinia viruses re-engineered to express foreign genes are vectors for production of recombinant proteins, the most common being a vaccine delivery system for antigens.[3] Concerns about the safety of the vaccinia virus have been addressed by the development of vectors based on attenuated vaccinia viruses. One of them, the Modified vaccinia Ankara (MVA) virus, is a highly attenuated strain of vaccinia virus that was developed towards the end of the campaign for the eradication of smallpox by Anton Mayr in Munich, Germany. Produced between 1953 and 1968 by more than 500 serial passages of vaccinia virus (from a wild strain discovered by the Turkish vaccine institute of Ankara) in chicken cells[4] (chicken embryo fibroblast), MVA has lost about 10% of the vaccinia genome and with it the ability to replicate efficiently in primate cells. A recombinant MVA-based vector for vaccination with different fluorescent reporter genes was developed by Antonio Siccardi, which indicate the progress of genetic recombination with the transgene of an antigen (green, colorless, red).[5][6]
...Compared to replicating vaccinia viruses, MVA provides similar or higher levels of recombinant gene expression even in non-permissive cells.
...MVA is an attenuated vaccinia virus and does not replicate in the human body as efficiently as vaccinia. However, whether or not MVA can induce the same side effects as vaccinia is not known at this time.
Modified vaccinia Ankara
G4L is the gene that expresses glutaredoxin, and it's conserved in MVA and therefore in Jynneos, with potential public health implications.
Note the reference to cowpox:
Abstract
The complete genomic DNA sequence of the highly attenuated vaccinia strain modified vaccinia Ankara (MVA) was determined. The genome of MVA is 178 kb in length, significantly smaller than that of the vaccinia Copenhagen genome, which is 192 kb. The 193 open reading frames (ORFs) mapped in the MVA genome probably correspond to 177 genes, 25 of which are split and/or have suffered mutations resulting in truncated proteins. The left terminal genomic region of MVA contains four large deletions and one large insertion relative to the Copenhagen strain. In addition, many ORFs in this region are fragmented, leaving only eight genes structurally intact and therefore presumably functional. The inserted DNA codes for a cluster of genes that is also found in the vaccinia WR strain and in cowpox virus and includes a highly fragmented gene homologous to the cowpox virus host range gene, providing further evidence that a cowpox-like virus was the ancestor of vaccinia. Surprisingly, the central conserved region of the genome also contains some fragmented genes, including ORF F5L, encoding a major membrane protein, and ORFs F11L and O1L, encoding proteins of 39.7 and 77.6 kDa, respectively. The right terminal genomic region carries three large deletions: all classical poxviral immune evasion genes and all ankyrin-like genes located in this region are fragmented except for those encoding the interleukin-1β receptor and the 68-kDa ankyrin-like protein B18R. Thus, the attenuated phenotype of MVA is the result of numerous mutations, particularly affecting the host interactive proteins, including the ankyrin-like genes, but also involving some structural proteins.
Link to pdf:
G.Antoine, F.Scheiflinger, F.Dorner, F.G.Falkner, The Complete Genomic Sequence of the Modified Vaccinia Ankara Strain: Comparison with Other Orthopoxviruses, (1998),
https://www.sciencedirect.com/science/article/pii/S0042682298991231
Can COVID-19 infection be enhanced/reactivated in the same way? It appears not as, unlike HIV it thrives in an oxidative environment.7
Vaccinia immunisation may still promote it indirectly via other pathways such as T cell exhaustion or reactivation of other latent viruses, chipping away at your immune system. Repeated antigenic challenge is not to be encouraged.
Related to Vaccinia vaccination, an excellent paper on vaccine mediated antibody dependent enhancement of viral infections, especially why repeated attempts at creating effective HIV & coronavirus vaccines keep failing:
Abstract
Examples of vaccine-induced enhancement of susceptibility to virus infection or of aberrant viral pathogenesis have been documented for infections by members of different virus families. Several mechanisms, many of which still are poorly understood, are at the basis of this phenomenon. Vaccine development for lentivirus infections in general, and for HIV/AIDS in particular, has been little successful. Certain experimental lentiviral vaccines even proved to be counterproductive: they rendered vaccinated subjects more susceptible to infection rather than protecting them. For vaccine-induced enhanced susceptibility to infection with certain viruses like feline coronavirus, Dengue virus, and feline immunodeficiency virus, it has been shown that antibody-dependent enhancement (ADE) plays an important role. Other mechanisms may, either in the absence of or in combination with ADE, be involved. Consequently, vaccine-induced enhancement has been a major stumble block in the development of certain flavi-, corona-, paramyxo-, and lentivirus vaccines. Also recent failures in the development of a vaccine against HIV may at least in part be attributed to induction of enhanced susceptibility to infection. There may well be a delicate balance between the induction of protective immunity on the one hand and the induction of enhanced susceptibility on the other. The present paper reviews the currently known mechanisms of vaccine-induced enhancement of susceptibility to virus infection or of aberrant viral pathogenesis.
Keywords: Vaccine, Enhancement, ADE, HIV, Lentivirus
W. Huisman,1 B.E.E. Martina, G.F. Rimmelzwaan, R.A. Gruters, and A.D.M.E. Osterhaus,Vaccine-induced enhancement of viral infections, Vaccine. 2009 Jan 22; 27(4): 505–512., Published online 2008 Nov 18. doi: 10.1016/j.vaccine.2008.10.087
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7131326/#__ffn_sectitle
Whither monkeypox vaccination
This study from 2011 also calls out the major flaws in the Jynneos approach and recommends efficacy trials which may also benefit a population at genuine risk, imagine that!
New candidate vaccines have been evaluated in humans for immunogenicity, but since smallpox is eradicated, all efficacy testing has been conducted in animal models. Therefore, none of the products recently developed for the prevention and treatment of variola virus infection have been field-tested in humans, and have been manufactured and deposited into the biodefense stockpile based on animal studies and the presumption they will work in humans in the event of a crisis.
Although T cell-mediated immunity is known to be important for clearing poxvirus infections, protection is strongly associated with antibody responses. This is noted because the complexity and cost of a Phase I vaccine trial is significantly higher if cryopreservation of peripheral blood mononuclear cells (PBMCs) is required. For Phase I testing of candidate poxvirus vaccines, serum collection for vaccine-induced antibody would be sufficient.
Its difficult, time consuming and expensive to do properly, and their target efficacy would be 80% to make it worthwhile, not 50%.
Its a lot quicker, easier and cheaper to just skip all this Phase II & III safety and efficacy nonsense and just sign it off for mass distribution:
Advancing from Phase I safety and immunogenicity testing to a Phase IIb or Phase III efficacy trial would add a new level of complexity not only because of the size, but because the study location would shift from Kinshasa to the Sankuru District in the central part of DRC where there are few health care facilities and few roads making access difficult and creating logistical challenges for managing the delivery of biological products and clinical samples. Performance of epidemiological studies required an elaborate system of vehicles, motorcycles, bicycles, and foot travel to access populations and transport diagnostic samples. Even with an annual incidence approaching 0.2% (in 5–19 year old children), a placebo-controlled efficacy trial with one year of follow-up would require about 20,000 subjects to detect a vaccine efficacy of 80% with 80% power. Fewer subjects would be needed if the follow-up period could be extended, provided immunity was expected to be maintained.
Benefits of vaccine clinical trials in areas endemic for MPX
Performing clinical trials in the DRC would have a number of benefits for distinct constituencies. First, the people of the Congo River Basin who are primarily affected by the disease would benefit. Clinical studies would call attention to the problem of MPX, improve general knowledge about MPX prevention, and potentially improve knowledge about other health conditions by strengthening the public health infrastructure. There would also be intangible benefits that accompany clinical trial activities including stimulation of local economies. Secondly, Congolese people in general and the Congolese health system would benefit because an interventional vaccine trial would require investment in the regulatory infrastructure. In addition, it would expand research capacity by increasing the number of active investigators and staff. With an efficacy of 85% at the current incidence rate, approximately one MPX infection could be prevented for every 600 persons vaccinated.
Vaccine developers of alternative smallpox vaccines and stakeholders in the area of biodefense and emerging infectious diseases would derive benefit from the opportunity to evaluate candidate smallpox vaccines in the field against a virulent orthopoxvirus. This benefit should not be underestimated because live vaccinia inoculation is the only smallpox vaccination that has proven efficacy in humans. All other candidate vaccines will be provisionally approved by applying The Animal Rule 20. Having human efficacy data would provide a significant selection advantage for one candidate vaccine over another.
In the end the authors ask themselves if its all worthwhile? Why not just administer antivirals to the rare cases that crop up and educate at risk groups about safe practices? Why not indeed.
Its the Leicester Method of incidence response & focused quarantine updated for the 21st Century:
Alternatives to smallpox vaccination
Given the risks of adverse events, cost and logistical considerations associated with smallpox vaccination, alternate strategies should also be considered for control of human MPX. An alternative to vaccination could be treatment of incident cases with antiviral therapy to reduce the morbidity and transmission, and by providing access to antibiotics for treatment of secondary bacterial infections. Clinical diagnosis of MPX is relatively easy, thus effective antivirals and supportive clinical care may be more practical options than vaccination at this time.
Reducing the frequency of human MPX infection could be also be accomplished through health education on handling potential animal reservoir species to prevent animal-to-human transmission and by quarantine or contact isolation to prevent human-to-human spread. Defining the factors underlying increased incidence, and their impact on primary versus secondary transmission, is thus a crucial direction for on-going research. Additionally, a better understanding of the mortality and complications associated with monkeypox infection should be assessed. Continued active disease surveillance in endemic regions coupled with household and contact studies with long term follow up would address these important questions.
Further studies are also needed to identify intermediate hosts and animal reservoirs. Smallpox vaccination will not modify the reservoir nor the amount of MPX virus found in amplification species. Introduction of MPX into human populations is dependent upon contact with infected species, thus vaccination alone will not be effective in controlling the geographic spread of MPX as it is determined by the movement of animals and driven largely by the loss of natural habitat.
Rimoin, A. W., & Graham, B. S. (2011). Whither monkeypox vaccination. Vaccine, 29 Suppl 4(Suppl 4), D60–D64.
https://doi.org/10.1016/j.vaccine.2011.09.004
And thank you for reading.
WHO, Monkeypox - United Kingdom of Great Britain and Northern Ireland, (May 18th 2022),
https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON383
Norris, J, Misfolded spike protein could explain complicated COVID-19 symptoms, (May 26, 2022),
Lymphadenopathy at the crossroad between immunodeficiency and autoinflammation: An intriguing challenge, (2021),
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8374228/#__ffn_sectitle
Side Effects in Clinical Trials
https://dipg.org/dipg-research/clinical-trials-for-dipg/side-effects/
Bhaskar A, Munshi M, Khan SZ, Fatima S, Arya R, Jameel S, Singh A. Measuring glutathione redox potential of HIV-1-infected macrophages. J Biol Chem. 2015 Jan 9;290(2):1020-38. doi: 10.1074/jbc.M114.588913. Epub 2014 Nov 18. PMID: 25406321; PMCID: PMC4294471.
Jaswinder Singha, Rajinder S.Dhindsab, Vikram Misrac, Baljit Singhd, SARS-CoV2 infectivity is potentially modulated by host redox status, (2020),
https://www.sciencedirect.com/science/article/pii/S2001037020304839










































thanks for the ammunition!
The WHO has been funding researchers monkeying around with monkeypox since the late 1960s. In Leonard Horowitz's 1996 book _Emerging Viruses: AIDS & Ebola_, he explores the history and lab-creation of the HIV bioweapon, and in chapter 2, he indicates how monkeypox (referred to as one of the "slow" viruses, causing chronic degenerative disease) was of great interest to WHO, CDC, NIB, and NCI scientists between 1968 and 1974. In Chapter 1, we read how the US's Deputy Director of the Department of Defence, per the Congressional Appropriations Hearings in 1969 on the development of immune-system destroying agents for biological warfare, opined that at a total cost of $10 million, within 10 years they could likely produce what was desired -- "a new infective microorganism" that "might be refractory to the immunological and therapeutic processes upon which we depend to maintain our relative freedom from infectious disease." That transcript was classified but later obtained via FOI request.
According to the 1988 Strecker Memorandum, "[t]he United States Defence Department requested and got $10 million to make the AIDS virus in labs as a political/ethnic weapon to be used mainly against Blacks. The feasibility program and labs were to have been completed by 1974-1975; the virus between 1974-1979. The World Health Organization started to inject AIDS-laced smallpox vaccine into over 100 million Africans (population reduction) in 1977. And over 2000 young white male homosexuals (Trojan horse) in 1978 with the hepatitis B vaccine through the Centers for Disease Control/New York Blood Center."
So, while HIV was being created as an immunosuppressive bioweapon, monkeypox was also being researched.
Read the book here:
https://www.bibliotecapleyades.net/ciencia/ciencia_viruses.htm