Study of clinical data finds that experimental mRNA shots increase your 5yr risk of acute coronary syndrome (ACS) from 11% to 25%
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Substack limitations
Once Substacks’ support the use of internal hyperlinks I will add them to the abstract. Otherwise please use a “Find In Page” keyword search to navigate or click on the footnote text numbers.
If received via email I recommend clicking on the hyperlinked title to read the latest correctly formatted version in full, in a browser. Unfortunately it is not possible to email out revised versions.
Introduction
Just over a year after publication the paper prompting the title of this piece deserves hilighting again with interpretation as unfortunately it proved to be quite prescient, its clear warnings going unheeded.
This substack will also consider potential therapeutics, dietary adjustments and the potential reversibility of such myocardial damage.
Synopsis
Our friends the “fact checkers” are a great resource here and attempted to bury the original story. Whilst being unable to disprove the findings they actually appear to add credence to the conclusions in the process1. Nice work.
A study published in the Lancet, for example, which included nearly 90,000 COVID-19 patients in Sweden and compared them with similar patients who did not have the disease, found that having COVID-19 was associated with more than triple the risk of having a first-time heart attack in the first two weeks after falling ill. The study also identified COVID-19 as a possible risk factor for ischemic stroke.
The abstract, which by nature is a brief summary and in this case represented preliminary research that is not peer-reviewed, concludes that “mRNA vacs dramatically increase inflammation on the endothelium and T cell infiltration of cardiac muscle and may account for the observations of increased thrombosis, cardiomyopathy, and other vascular events following vaccination.” The abstract is not part of a full scientific paper, and was presented as a poster at AHA’s Scientific Sessions online program on Nov. 13. None of the mRNA COVID-19 vaccines have been linked to thrombosis.
On the last point case reports and analysis beg to differ, for example: Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine (2021)2 or Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study (2021)3:
(CVST = cerebral venous sinus thrombosis)
Main outcome measures The primary outcomes were hospital admission or death associated with thrombocytopenia, venous thromboembolism, and arterial thromboembolism within 28 days of three exposures: first dose of the ChAdOx1 nCoV-19 vaccine; first dose of the BNT162b2 mRNA vaccine; and a SARS-CoV-2 positive test. Secondary outcomes were subsets of the primary outcomes: cerebral venous sinus thrombosis (CVST), ischaemic stroke, myocardial infarction, and other rare arterial thrombotic events.
…increased risk of arterial thromboembolism after BNT162b2 mRNA vaccination (1.06, 1.01 to 1.10 at 15-21 days) and after SARS-CoV-2 infection (2.02, 1.82 to 2.24 at 15-21 days). Secondary analyses found increased risk of CVST after ChAdOx1 nCoV-19 vaccination (4.01, 2.08 to 7.71 at 8-14 days), after BNT162b2 mRNA vaccination (3.58, 1.39 to 9.27 at 15-21 days), and after a positive SARS-CoV-2 test; increased risk of ischaemic stroke after BNT162b2 mRNA vaccination (1.12, 1.04 to 1.20 at 15-21 days)
“Shows its working”. Seriously:
Dr. Douglas S. Harrington, a pathologist, is the chairman of Predictive Health Diagnostic Company, which owns the cardiac test used by Gundry, called the PULS Cardiac Test. He told us the numbers in the abstract are being misused by vaccine opponents.
“It is not proof that people should not get vaccines. What it should be interpreted as is proof that the vaccine works,” he told us in an interview.
I will leave it there and let you draw your own conclusions.
The PULS Cardiac Test
PULS stands for Protein Unstable Lesion Signature. It measures key clinical risk factors including age, sex, diabetic status, family history and protein biomarkers to give you a 5 year risk score of suffering ACS, heart attack or stroke 4.
As atherosclerotic disease progression may be asymptomatic until quite advanced this can be a useful wakeup call to change your lifestyle and diet before too much irreversible damage has been done.
The PULS Cardiac Test is a blood test designed to help identify people who appear healthy but may have active heart disease which could result in a heart attack. This test detects the early stages of heart disease by recognizing blood vessel injury and unstable plaque formation, even in patients who otherwise have no signs or symptoms. The PULS Cardiac Test measures protein markers in the blood that are linked to unstable plaque to see if a heart attack may be likely in the next five years.5
In the abstract by R Gundry (8 Nov. 2021)6 he describes how a PULS score was taken from 566 patients aged 28-97, equal numbers M-F, both 3 to 5 months before the first shot and from 2 to 10 weeks following the 2nd mRNA COVID shot.
Markers for the cardiac damaging pro-inflammatory cytokine, interleukin-16 (IL-16) increased from 35+/-20 above the norm to 82 +/- 75 above the norm post vax, an average increase of 234%.
Soluble Fas (sFas) is an inhibitor of apoptosis, and markers for that increased from 22+/- 15 above the norm to 46+/-24 above the norm post vax, an average increase of 209%.
Hepatocyte growth factor (HGF) is a pleiotropic cytokine promoting proliferation, migration and survival in several cell types. This marker increased from 42+/-12 above the norm to 86+/-31 above the norm post vax, an average increase of 205%.
We will explore these biomarkers in more detail later.
The abstract in full:
This clinic has been using the PULS Cardiac Test (Predictive Health Diagnostics Co., Irvine, CA) a clinically utilized measurement of multiple protein biomarkers, which generates a score predicting the 5 yr risk (percentage chance) of a new Acute Coronary Syndrome (ACS) called the PULS Score. The score is based on changes from the norm of multiple protein inflammatory biomarkers including IL-16, a proinflammatory cytokine, soluble Fas, an inducer of apoptosis, and Hepatocyte Growth Factor (HGF) which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue, among other markers. Elevation above the norm increases the PULS score, while decreases below the norm lowers the PULS score. The PULS score has been measured every 3-6 months in our patient population for 8 years. Recently, with the advent of the mRNA COVID 19 vaccines (vac) by Moderna and Pfizer, we tracked the changes of the PULS score and three of the inflammatory markers it measures in all of our patients consecutively receiving these vaccines.
This report summarizes those results. A total of 566 pts, aged 28 to 97, M:F ratio 1:1 seen in a preventive cardiology practice had a previously scheduled PULS test drawn from 2 to 10 weeks following the 2nd mRNA COVID shot and was compared to the pt’s PULS test drawn 3 to 5 months previously pre-shot. Each vac pt’s PULS score and inflammatory marker changes were compared to their pre-vac PULS score, thus serving as their own control. There was no comparison made with unvaccinated patients or pts treated with other vaccines.
Baseline IL-16 increased from 35+/-20 above the norm to 82 +/- 75 above the norm post-vac; sFas increased from 22+/- 15 above the norm to 46+/-24 above the norm post vac; HGF increased from 42+/-12 above the norm to 86+/-31 above the norm post vac. These changes resulted in an increase of the pre vac PULS score of predicted 11% 5 yr ACS risk to a post vac PULS score of a predicted 25% 5 yr ACS risk, based on data which has not been validated in this population. No statistical comparison was done in this observational study.
In conclusion, the mRNA vacs numerically increase (but not statistically tested) the markers IL-16, Fas, and HGF, all markers previously described by others for denoting inflammation on the endothelium and T cell infiltration of cardiac muscle, in a consecutive series of a single clinic patient population receiving mRNA vaccines without a control group.
Apart from the “fact checkers”, the journal editors (or others?) also got to work watering down the wording of the original abstract or adding qualifiers, but most significantly did not disputing the findings7:
The sentence “These changes resulted in an increase of the PULS score from 11% 5 yr ACS risk to 25% 5 yr ACS risk.” has been revised to read “These changes resulted in an increase of the pre vac PULS score of predicted 11% 5 yr ACS risk to a post vac PULS score of a predicted 25% 5 yr ACS risk, based on data which has not been validated in this population.”
The sentence “At the time of this report, these changes persist for at least 2.5 months post second dose of vac.” has been removed from the description of the results.
The sentence “We conclude that the mRNA vacs dramatically increase inflammation on the endothelium and T cell infiltration of cardiac muscle and may account for the observations of increased thrombosis, cardiomyopathy, and other vascular events following vaccination” has been revised to read “In conclusion, the mRNA vacs numerically increase (but not statistically tested) the markers IL-16, Fas, and HGF, all markers previously described by others for denoting inflammation on the endothelium and T cell infiltration of cardiac muscle, in a consecutive series of a single clinic patient population receiving mRNA vaccines without a control group.”
One might say that validation is being seen by way of the 25-sigma post-COVID increase in the number of deaths classified as "circulatory heart and uncertain related (RXX) disorders"8:
When you see people concerned that sudden heart deaths seem to be happening more frequently.
They are. They used to be happening because of Covid.
But now Covid is not the issue. Something else is.
@EthicalSkeptic
It would be useful to have a further striated breakdown of the risk of acute coronary syndrome by age, health profile and for doses greater than 2.
The 1 in 4 risk over 5 years is very unlikely to apply evenly across the range of profiles and longer term clinical data with further research is clearly needed here.
What is Acute Coronary Syndrome?
The mayoclinic provides a useful overview9:
Overview
Acute coronary syndrome is a term used to describe a range of conditions associated with sudden, reduced blood flow to the heart.
One such condition is a heart attack (myocardial infarction) — when cell death results in damaged or destroyed heart tissue. Even when acute coronary syndrome causes no cell death, the reduced blood flow changes how your heart works and is a sign of a high risk of heart attack.
Acute coronary syndrome often causes severe chest pain or discomfort. It is a medical emergency that requires prompt diagnosis and care. The goals of treatment include improving blood flow, treating complications and preventing future problems.
Symptoms
The signs and symptoms of acute coronary syndrome usually begin abruptly. They include:
Chest pain (angina) or discomfort, often described as aching, pressure, tightness or burning
Pain spreading from the chest to the shoulders, arms, upper abdomen, back, neck or jaw
Nausea or vomiting
Indigestion
Shortness of breath (dyspnea)
Sudden, heavy sweating (diaphoresis)
Lightheadedness, dizziness or fainting
Unusual or unexplained fatigue
Feeling restless or apprehensive
Chest pain or discomfort is the most common symptom. However, signs and symptoms may vary significantly depending on your age, sex and other medical conditions. You're more likely to have signs and symptoms without chest pain or discomfort if you're a woman, older adult or have diabetes.
PULS biomarkers in more detail
The clinic found a temporal increase in these biomarkers in association with the administration of 2 shots of experimental gene “therapies”.
It is important to note that these are by no means the only biomarkers and cardiovascular disease can be mediated by many other factors than those discussed in this substack. I considered some of these in several other substacks, eg:
Interleukin-16
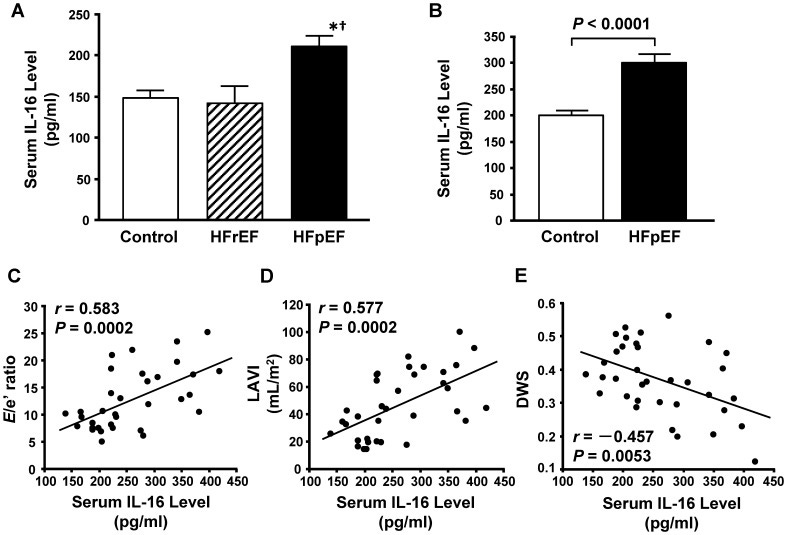
From 2013, Tamaki et al published a paper on how the pro-inflammatory cytokine IL-16 promotes cardiac fibrosis and myocardial stiffening in cases of heart failure with preserved ejection fraction10.
They found that half of all patients with chronic heart failure (CHF) had chronic heart failure with preserved left ventricular (LV) ejection fraction (HFpEF) and that there is no established therapy for this. They investigated the inflammatory mediators and mechanisms and concluded that IL-16 is a key mediator, and preventative blockade may be a therapeutic option to prevent the damage occurring in the first place.
Restated, you might say primum non nocere, or “first, do no harm”, by not being administered any agents that may elevate IL-16 along with making lifestyle and dietary changes.
Recent evidence has shown that activation of the immune system plays an important role in CHF. Immune activation caused by myocardial injury, bacterial translocation and peripheral tissue hypoxia is thought to result in the production of pro-inflammatory mediators including tumor necrosis factor-α, interleukin (IL)-1β and IL-6 from mononuclear cells or the myocardium itself. These mediators have been reported to worsen CHF through their detrimental effect on myocardial contractility, LV remodeling or endothelial function [8], [9]. Increased circulating levels of cytokines or chemokines have been shown to be associated with the severity of clinical symptoms and increased mortality.
Figure 1. Elevated serum interleukin-16 (IL-16) levels in patients with heart failure with preserved ejection fraction. A, Serum IL-16 levels in controls and patients with heart failure with reduced (HFrEF) or preserved ejection fraction (HFpEF) measured by a multiplex-bead array assay. *P<0.05 vs. control group. † P<0.05 vs. HFrEF group. B, Serum IL-16 levels in controls and patients with HFpEF measured by enzyme-linked immunosorbent assay. C through E, Correlations of serum IL-16 level and the ratio of early transmitral flow velocity to septal mitral annular early diastolic velocity (E/e′ ratio) (C), left atrial volume index (LAVI) (D) and diastolic wall strain (DWS) (E) in controls and HFpEF patients combined.

Soluble Fas ligand
Fas ligand (FasL or CD95L or CD178) is a type-II transmembrane protein that belongs to the tumor necrosis factor (TNF) family. Its binding with its receptor induces apoptosis. Fas ligand/receptor interactions play an important role in the regulation of the immune system and the progression of cancer.11
A study by Nishigaki et al (1997) reported that apoptotic cell death occurs in the myocytes of dogs with chronic heart failure, and that hypoxia is frequently seen in advanced CHF and can stimulate Fas/APO-1 receptors to induce apoptosis12.
Tumor necrosis factor alpha (TNFa) and IL-6 levels were increased significantly only in patients with severe, functional class IV CHF and sFas levels increased with severity of functional classification, independent of the underlying disease.
Hepatocyte growth factor (HGF)
Hepatocyte growth factor (HGF) or scatter factor (SF) is a paracrine cellular growth, motility and morphogenic factor. It is secreted by mesenchymal cells and targets and acts primarily upon epithelial cells and endothelial cells, but also acts on haemopoietic progenitor cells and T cells. It has been shown to have a major role in embryonic organ development, specifically in myogenesis, in adult organ regeneration, and in wound healing.13
Hepatocyte growth factor (HGF) is an angiogenic, cardioprotective factor important for tissue and vascular repair. High levels of HGF are associated with chronic inflammatory diseases, such as coronary artery disease (CAD) and periodontitis, and are suggested as a marker of the ongoing atherosclerotic event in patients with CAD.14
In 2001, Kitta et al published a paper on how HGF can protect cardiac myocytes against oxidative stress-induced apoptosis, in part via activating the MEK-MAPK anti-inflammatory signalling pathway15 . This pathway appears to trigger cell survival signals. HGF is thus cardioprotective and elevated levels are a marker of myocardial damage.
In 2019 Radik et al published the results into the effects of HGF on rats16. They found that pulmonary hypertension development was associated with the progressive elevation of HGF plasma levels, correlating with the relative mass of the right ventricle.
Also from 2019, Zhu et al explored the association between HGF and the prognosis of ischemic stroke17. Their conclusion was that elevated serum HGF was associated with adverse clinical outcomes in ischemic stroke patients with abnormally high levels of lipids in the blood ie "dyslipidemia".
Low HDL-C (high density lipoprotein cholesterol, aka "good cholesterol) exhibited a particularly significant association. Indeed the highest odds ratios of the primary outcome associated with elevated HGF were 2.13 (95% CI, 1.45–3.14; Ptrend<0.001) for patients with dyslipidemia, ie doubling of the risk of an adverse outcome and 0.81 (95% CI, 0.54–1.22; Ptrend = 0.310) for those with normal lipids, ie a reduced risk.
Put another way, these elevated biomarkers may not just be associated with a greater 5 year risk of heart failure but also of other related pathologies including pulmonary hypertension, stroke or thrombosis. Dietary changes may reduce the likelihood of some of these outcomes.
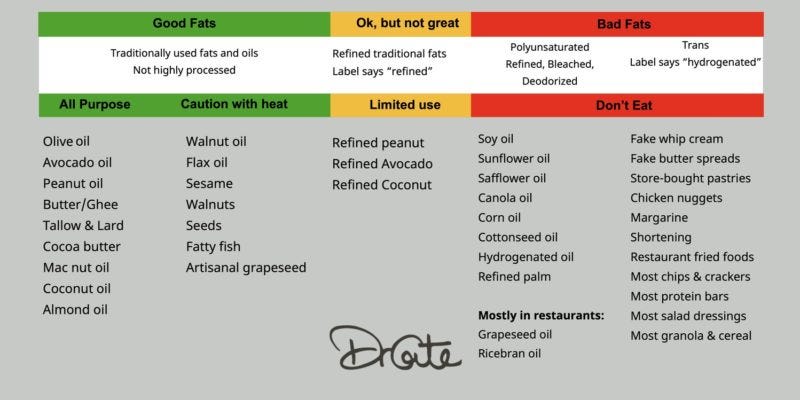
Despite public health advice to the contrary, as elevated serum HDL is associated with either neutral to net positive cardiovascular effects then consider elevating the following in your diet instead of vegetable oils that promote low-density lipoprotein (LDL), which is prone to oxidation and inflammation the consequential promotion of foam cells in arterial endothelia, ie atherosclerosis:
Although HDL cholesterol levels are partly determined by genetics, there are many things a person can do to naturally increase their levels.
This includes eating healthful fats, such as olive oil, coconut oil, and fatty fish, and avoiding harmful trans fats. Getting regular exercise, quitting smoking, and eating antioxidant-rich foods are also effective for increasing HDL cholesterol.
The habits and practices that raise HDL cholesterol often provide other health benefits, and they are key components of a healthful lifestyle.18
A quick note about butter. My choice is to include grass fed butter in my diet. A rich yellow in colour due to the presence of antioxidant carotenoids it's also a good source of vitamin K2 and others.
Regarding lipoprotein types its not as simple as saying it's rich in LDLc therefore to exclude it - there are further subclasses of LDLc grouped by particle size and not all are bad. As butter is the large particle type it does not penetrate vascular endothelia in the same way but is instead protective overall.
For further reading see Froyen’s narrative review of the effects of fat consumption on low-density lipoprotein particle size from 202119.
In 2020, Ambreen et al used rabbits in a study of the effects of a south Asian herb, Murraya koenigii (curry leaves), on atherosclerosis induced by consuming repeatedly heated mixed vegetable oils20.
Their conclusion was that M koenigii provided an accessible, cheap alternative to statins, one that it is free of associated side effects including renal hypertrophy, haemorrhagic stroke, hepatomegaly, and myopathy.
Cholesterol is transported to the liver for metabolism, by HDL, whereas endothelial injuries and formation of atheroma are triggered by oxidized LDL molecule. Therefore, reduction of LDL and TC, along with HDL level enhancement is targeted to prevent atherosclerosis.
Physico-chemical characteristics of the cooking oils change when they are heated beyond a certain limit as several chemical reactions take place in the presence of moisture and air. Oils degenerate and produce volatile substances, unwanted monomers, polymers, isomers, and free radicals [13]. In cooking oil fatty acids (FAs) naturally exist in the cis-isomer form, but during thermal oxidation they convert into trans-isomers, possessing physical properties similar to saturated FAs [14]. Several animal studies demonstrate that consumption of thermally oxidized vegetable oils increased the risk of CVDs, like hypertension with reduced vasorelaxation responses [15], endothelial malfunction [16], lipid peroxidation [17], atherosclerosis [18], and oxidative stress [19]. However, regardless of all these studies, the practice of reusing and overheating the cooking oils while processing the food seems to continue.
In Pakistan, commercially available oils are mostly a blend of two or more edible oils, and the most common available blend of an equal ratio of olive, canola, and sunflower oils was focused in this study, to have the model that mimics the human situation. Vegetable oils are repeatedly heated when used for food processing (frying) at household and commercial levels. We used repeatedly heated mixed vegetable oils (RHMVO) to induce hyperlipidemia and atherosclerosis. We designed this study to investigate the medicinal effects of curry leaves in different doses, in RHMVO-induced atherosclerotic models.
Vegetable oils that have not been reheated aren’t off the hook either due to inhibition of the cardioprotective vitamin K2, as investigated by Okuyama et al (2016)21:
Summary: Statin-induced suppression of prenyl intermediates in the cholesterol biosynthetic pathway has been linked to stimulated atherosclerosis and heart failure. On the other hand, certain types of vegetable oil and hydrogenated oil shortened the survival of stroke-prone spontaneously hypertensive rats by decreasing platelet number, increasing hemorrhagic tendency and damaging kidney functions, which could not be accounted for by their fatty acid and phytosterol compositions. These vegetable oils and medicines such as statin and warfarin share, in part, a common mechanism to inhibit vitamin K2-dependent processes, which was interpreted to lead to increased onset of CVD, DM, chronic kidney disease, bone fracture and even mental disorder. Impaired vitamin K2-dependent processes by some types of vegetable oils and medicines, but not plasma high low density lipoprotein cholesterol, were proposed as the cause of CVD, DM and other lifestyle-related diseases. High n-6/n-3 fatty acid ratio of ingested foods, but not animal fats, was emphasized to be another risk factor for many of the diseases described above.
Key messages: To date, no randomized controlled trials (RCTs) have been performed to prove the above interpretation. However, the opposite types of RCT trials have been performed by increasing the intake of high-linoleic vegetable oils and reducing that of animal fats, which resulted in increased CVD and all-cause mortality. The amounts of these vegetable oils to exhibit adverse effects in animal studies are not huge (<6 energy %), which should not be overlooked nor disregarded.
K2 depletion is one factor, and in 2018, DiNicolantonio and O’Keefe published a paper discussing how the omega-6 polyunsaturated fat linoleic acid contributes significantly to the oxidative potential of vegetable oils22:
The low-density lipoprotein (LDL) oxidation hypothesis gained traction during the 1980s because it was noted that in general, native unoxidised LDL does not cause foam cell formation. In other words, LDL had to become oxidised first in order for atherosclerosis to develop. Indeed, it was later discovered that oxidised LDL (oxLDL) caused direct toxic effects to the cell, recruitment and entry of monocytes into the subendothelial layer and increased foam cell formation5 leading to increased atherosclerosis and inflammation.6 Moreover, oxLDL was found to be higher in patients with CAD compared with normal patients and oxLDL was able to better identify patients at an elevated risk of heart disease.7–9 Moreover, OxLDL and autoantibodies to oxLDL are found in atherosclerotic lesions.10–12 Furthermore, patients with progressive carotid atherosclerosis have more antibodies to oxLDL versus those without progression.9 Thus, the evidence is resounding that oxLDL is important in the formation of atherosclerosis.
…dietary linoleic acid, especially when consumed from refined omega-6 vegetable oils, gets incorporated into all blood lipoproteins (such as LDL, VLDL and HDL) increasing the susceptibility of all lipoproteins to oxidise and hence increases cardiovascular risk.
Summary
The consumption of the omega-6 polyunsaturated fat linoleic acid has dramatically increased in the western world primarily in the form of vegetable oils. OxLDL is thought to play an important role in atherosclerosis formation; however, it is the oxidised linoleic acid contained in LDL that leads to harmful OXLAMs, which induces atherosclerosis and CHD. Thus, reducing the amount of dietary linoleic acid, mainly from industrial vegetable/seed oils, will reduce the amount of linoleic acid in LDL and likely reduce oxLDL as well as the risk for CHDcoronary heart disease.
In summary, numerous lines of evidence show that the omega-6 polyunsaturated fat linoleic acid promotes oxidative stress, oxidised LDL, chronic low-grade inflammation and atherosclerosis, and is likely a major dietary culprit for causing CHD, especially when consumed in the form of industrial seed oils commonly referred to as ‘vegetable oils’.
Can the damage be reversed?
An informative editorial by Nikolaos G Frangogiannis (2019), asked the question:
Can Myocardial Fibrosis Be Reversed?23
In principle yes, as cardiac fibroplasts exhibit phenotypic plasticity. In other words revert back to a quiescent or apoptotic state as part of a healing process.
In practice its not as simple as that, and in this case prevention is many times better than cure.
Cardiac extracellular matrix (ECM) is a complex meshwork of fibers comprised of matrix proteins in which cardiac myocytes, fibroblasts, leukocytes, and cardiac vascular cells reside.24
Fibrosis accompanies most cardiac pathophysiological conditions. Because the adult mammalian heart has negligible regenerative capacity, sudden massive loss of cardiomyocytes following myocardial infarction heals through formation of a collagen-based scar, leading to development of “replacement fibrosis.”
In a wide range of human populations, the presence and extent of cardiac fibrosis predicts adverse outcome. In both heart failure with preserved ejection fraction and in heart failure with reduced ejection fraction, the extent of cardiac fibrosis is associated with increased mortality and a higher incidence of hospitalizations 1, 2, 3. Adverse outcome in subjects with prominent fibrosis may reflect perturbations in systolic or diastolic ventricular function, or the occurrence of arrhythmias and electrical conduction defects.
Although in repair of the infarcted heart, activation of fibroblasts is transient, serving to form a scar that preserves the structural integrity of the ventricle (8), persistent or overactive fibroblast responses may lead to excessive deposition and cross-linking of ECM proteins in the cardiac interstitium, reducing cardiac compliance and perturbing propagation of the electrical impulse. Inhibition of fibrosis and restoration of normal myocardial structure and function remain a major visionary goal in the treatment of heart failure. However, to what extent established cardiac fibrosis can be reversed remains unknown.
The ability of activated fibroblasts to revert into a quiescent phenotype, as noted in the current study, is not surprising. Fibroblasts exhibit remarkable phenotypic plasticity.
The process of reversing myocardial fibrosis may prove fatal in itself due to being pro-inflammatory.
Chemokines can recruit leukocytes to the area and cytokines can ironically mediate further myocardial pathology:
However, clearance of fibrotic changes and restoration of normal cardiac architecture and function in the failing heart require a lot more than deactivation of cardiac fibroblasts.
The Challenges of Reversing CARDIAC Fibrosis
In order to improve cardiac function, reversal of cardiac fibrosis requires both localized resorption of excessive ECM and restoration of normal myocardial structure. The negligible endogenous regenerative potential of the adult mammalian heart, the biological properties of the cross-linked ECM, and the inherent proinflammatory actions of matrix fragments (which may be recognized as danger signals by resident immune cells) pose several major challenges in designing strategies aimed at reversing cardiac fibrosis (Figure 1). First, degradation of large areas of replacement fibrosis could be catastrophic unless accompanied by robust cardiac regeneration. Despite extensive research in the field, there are currently no effective approaches to regenerate the adult human heart. Second, cross-linked interstitial and perivascular matrix may be highly resistant to proteases. Matrix cross-linking enzymes, such as lysyl oxidases (13) and transglutaminase-2 (14), are markedly induced in remodeling and failing hearts, and may cause formation of irreversible fibrotic foci (Figure 1B). Third, even when locally confined to the interstitium, ECM degradation could exert potent proinflammatory actions. Matrix fragments can act as damage-associated molecular patterns, activating cytokine and chemokine-mediated inflammation. Unless rapidly cleared, these fragments may have adverse consequences on myocardial structure and function.
If high levels of crosslinked ECM have already formed then it may not be reversible in any case:
Co-Operation of Several Distinct Cell Types May be Needed to Remodel the Fibrotic Interstitium
In the absence of an effective regenerative approach, fibrosis may only be reversible if it is confined to the interstitium, and it is not associated with high levels of crosslinked ECM. Although deactivation of fibroblasts would be required to reverse fibrosis, TGF-β inhibition alone may not be sufficient. In experimental models of cardiac fibrosis, induction and activation of TGF-β is followed by endogenous repression of Smad-dependent signaling. Although this endogenous process may prevent expansion of fibrosis, it is clearly not sufficient for reversal. Reversing fibrosis is an active process that may require co-operation of interstitial cell subsets, immune cells, vascular cells, and cardiomyocytes (Figure 1A). In a study analyzing samples biopsied from patients with ischemic cardiomyopathy undergoing aortocoronary bypass surgery, we found that myocardial segments that recovered function following revascularization exhibited higher interstitial cellularity, increased levels of the matricellular protein tenascin-C (15), and accentuated inflammatory activity.
Very little is known regarding the molecular signals that may be implicated in reverse remodeling of the fibrotic heart. The absence of robust animal models to study reversal of fibrosis and the difficulties in documenting the serial changes associated with reverse remodeling pose major challenges in understanding the cell biological mechanisms.
Treatments are being developed, but they appear to be mostly preventative in nature and results are generally mixed or contradictory.
With diabetes one "benefit" is that you or your doctor already knows that you are at risk of cardiovascular disease and may well be on medications before too much irreversible damage has ocurred.
Research into the efficacy of ACE inhibitors and glucose lowering drugs were reviewed by Sharma et al (2022)25:
Historically, treatments specifically targeting diabetic cardiomyopathy, and the associated cardiac fibrosis, have been sorely lacking. However, with the introduction and more widespread use of newer classes of glucose-lowering treatments such as SGLT2 inhibitors and GLP-1 agonists, alternate and more efficacious approaches of targeting diabetes-induced HF have emerged. SGLT2 inhibitors in particular, robustly attenuate cardiac remodelling and dysfunction commonly associated with diabetes, and of the available anti-diabetic therapies, have been the most successful at reducing this pathology and HF in a number of different patient populations. However, there is still much that still needs to be clarified. The mechanism by which SGLT2 inhibitors and GLP-1 agonists exert reductions in MACE and/or hospitalisations for HF still remains a mystery, especially given that SGLT2 is not expressed in the heart (Wright et al., 2011). Whether these benefits are secondary to, or independent of, any improvements in cardiac fibrosis are yet to be fully resolved.
Conclusion
Experimental mRNA gene transfection agents should be withdrawn from use ASAP.
The risk-benefit ratio appears to be negative across all age groups and health profiles.
Multiple research papers, clinical case reports and analysis support this conclusion, especially as any cardiovascular damage may well be permanent in nature and irreversible.
The precautionary principle applies here. First, do no harm, - get off the booster train.
If you are in a high risk group or are showing any related symptoms then get yourself checked out at the earliest opportunity.
There is a case to be made for large scale cardiovascular health assessments of those at risk, but this may not be feasible as it could well comprise up to around 80% of the population of many countries.
Another paper calls for similar actions too, but for cancer.
Targeting and/or rationing of health care is inevitable.
These data are disturbing and invite to immediately intensify clinical surveillance in oncology and to undertake rigid cancer prevention actions, including healthy lifestyle and continuous controls.
No Credible Evidence COVID-19 mRNA Vaccines ‘Dramatically Increase’ Heart Attack Risk, Contrary to Flawed Abstract
Dias L, Soares-Dos-Reis R, Meira J, Ferrão D, Soares PR, Pastor A, Gama G, Fonseca L, Fagundes V, Carvalho M. Cerebral Venous Thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021 Aug;30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. Epub 2021 May 25. PMID: 34111775; PMCID: PMC8148614.
Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study (2021). BMJ 2021;374:n1931.
PULS (PROTEIN UNSTABLE LESION SIGNATURE) CARDIAC TEST – WHAT IS YOUR 5 YEAR RISK OF A HEART ATTACK OR STROKE?
Abstract 10712: Observational Findings of PULS Cardiac Test Findings for Inflammatory Markers in Patients Receiving mRNA Vaccines (8 Nov 2021)
https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712
Correction to: Abstract 10712: Mrna COVID Vaccines Dramatically Increase Endothelial Inflammatory Markers and ACS Risk as Measured by the PULS Cardiac Test: a Warning
(21 Dec 2021)
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001053
Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, Omori Y, Tsukamoto Y, Ikeya Y, Kawai M, Kumanogoh A, Hagihara K, Ishii R, Higashimori M, Kaneko M, Hasuwa H, Miwa T, Yamamoto K, Komuro I. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One. 2013 Jul 19;8(7):e68893. doi: 10.1371/journal.pone.0068893. PMID: 23894370; PMCID: PMC3716890.
Fas ligand - Wikipedia
Nishigaki K, Minatoguchi S, Seishima M, Asano K, Noda T, Yasuda N, Sano H, Kumada H, Takemura M, Noma A, Tanaka T, Watanabe S, Fujiwara H. Plasma Fas ligand, an inducer of apoptosis, and plasma soluble Fas, an inhibitor of apoptosis, in patients with chronic congestive heart failure. J Am Coll Cardiol. 1997 May;29(6):1214-20. doi: 10.1016/s0735-1097(97)00055-7. PMID: 9137215.
Hepatocyte growth factor - Wikipedia
Lönn J, Starkhammar Johansson C, Kälvegren H, Brudin L, Skoglund C, Garvin P, Särndahl E, Ravald N, Richter A, Bengtsson T, Nayeri F. Hepatocyte growth factor in patients with coronary artery disease and its relation to periodontal condition. Results Immunol. 2011 Dec 30;2:7-12. doi: 10.1016/j.rinim.2011.12.002. PMID: 24371561; PMCID: PMC3862344.
Kitta K, Day RM, Ikeda T, Suzuki YJ. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic Biol Med. 2001 Oct 1;31(7):902-10. doi: 10.1016/s0891-5849(01)00663-3. PMID: 11585709.
Radik M, Kmecova Z, Veteskova J, Malikova E, Doka G, Krenek P, Klimas J. Hepatocyte growth factor plays a particular role in progression of overall cardiac damage in experimental pulmonary hypertension. Int J Med Sci. 2019 Jun 2;16(6):854-863. doi: 10.7150/ijms.31690. PMID: 31337959; PMCID: PMC6643116.
Zhu Z, Wang A, Guo D, Bu X, Xu T, Zhong C, Peng Y, Xu T, Peng H, Chen J, Ju Z, Geng D, He J, Zhang Y. Association between serum hepatocyte growth factor and prognosis of ischemic stroke: The role of blood lipid status. Nutr Metab Cardiovasc Dis. 2020 Mar 9;30(3):492-499. doi: 10.1016/j.numecd.2019.11.005. Epub 2019 Nov 14. PMID: 31831364.
9 ways to increase your HDL cholesterol levels
Froyen, E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: a narrative review. Lipids Health Dis 20, 86 (2021). https://doi.org/10.1186/s12944-021-01501-0
https://lipidworld.biomedcentral.com/articles/10.1186/s12944-021-01501-0#citeas
Ambreen G, Siddiq A, Hussain K, Hussain AS, Naz Z. Repeatedly heated mix vegetable oils-induced atherosclerosis and effects of Murraya koenigii. BMC Complement Med Ther. 2020 Jul 14;20(1):222. doi: 10.1186/s12906-020-03012-4. PMID: 32664977; PMCID: PMC7362559.
Okuyama H, Langsjoen PH, Ohara N, Hashimoto Y, Hamazaki T, Yoshida S, Kobayashi T, Langsjoen AM. Medicines and Vegetable Oils as Hidden Causes of Cardiovascular Disease and Diabetes. Pharmacology. 2016;98(3-4):134-70. doi: 10.1159/000446704. Epub 2016 Jun 2. PMID: 27251151.
DiNicolantonio JJ, O'Keefe JH. Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis. Open Heart. 2018 Sep 26;5(2):e000898. doi: 10.1136/openhrt-2018-000898. PMID: 30364556; PMCID: PMC6196963.
Nikolaos G. Frangogiannis, Can Myocardial Fibrosis Be Reversed?∗, Journal of the American College of Cardiology, Volume 73, Issue 18, 2019, Pages 2283-2285, ISSN 0735-1097, https://doi.org/10.1016/j.jacc.2018.10.094.
https://www.sciencedirect.com/science/article/pii/S0735109719344316?via%3Dihub
Rienks M, Papageorgiou AP, Frangogiannis NG, Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ Res. 2014 Feb 28;114(5):872-88. doi: 10.1161/CIRCRESAHA.114.302533. PMID: 24577967.
Sharma A, De Blasio M, Ritchie R. Current challenges in the treatment of cardiac fibrosis: Recent insights into the sex-specific differences of glucose-lowering therapies on the diabetic heart: IUPHAR Review 33. Br J Pharmacol. 2022 Feb 16. doi: 10.1111/bph.15820. Epub ahead of print. PMID: 35174479.
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15820










I'm helping a team collect data from public obituaries. Mindblowing. Babies, children, teens, young adults, adults (forget age 70+, even). Sudden passings, unexpected passings, in-the-night passings, sudden/aggressive cancers...
Interesting post as always, thank you.
One of the articles above mentions: "cross-linked interstitial and perivascular matrix may be highly resistant to proteases. Matrix cross-linking enzymes, such as lysyl oxidases (13) and transglutaminase-2 (14), are markedly induced in remodeling and failing hearts, and may cause formation of irreversible fibrotic foci (Figure 1B)."
This is interesting in relation to Vojdani and Kharrazian (2020): "Looking at the reaction between SARS-CoV-2 spike protein antibody and tissue proteins (Fig. 1A), we found that the strongest reactions were with transglutaminase 3 (tTG3), transglutaminase 2 (tTG2), ENA, myelin basic protein (MBP), mitochondria, nuclear antigen (NA), α-myosin, thyroid peroxidase (TPO), collagen, claudin 5+6, and S100B."
Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020 Aug;217:108480. doi: 10.1016/j.clim.2020.108480.
So could that mean that there is a transglutaminase-2-similar motif in Spike, similar enough to trigger autoantibodies against the real transglutaminase 2, and similar enough to form irreversible cross-linking in the giant fibrous clots that are being found?