The link between transfection, autoimmune diseases, cardiovascular disease and cancer.
Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Background
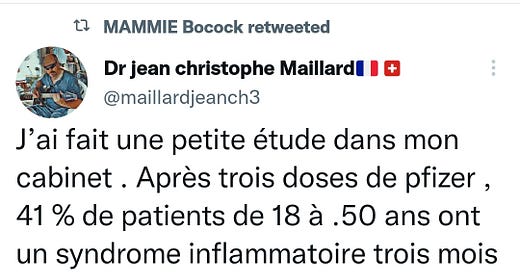
An informative tweet caught my attention today. No way to verify it but the doctor describes himself as a Swiss“médecine interne générale” or the medical specialty dealing with the prevention, diagnosis, and treatment of internal diseases.
I’ve no reason to doubt the authenticity:
https://twitter.com/maillardjeanch3/status/1517983339722027010?s=19
What is it?
“Lupus, technically known as systemic lupus erythematosus ( SLE ), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary between people and may be mild to severe.”
It must be said that the presence of anti-lupus antibodies does NOT mean they will all develop the condition, it is perfectly normal to carry some of these and at lower levels they don’t present a problem. Testing to confirm a diagnosis is complicated and time consuming.1
Either way, autoimmune disorders or a variant called pathogenic priming has long been expected due to the homology between vaccinal spike protein epitopes and human tissues:
Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity (2020)
Abstract
Homology between human and viral proteins is an established factor in viral- or vaccine-induced autoimmunity. Failure of SARS and MERS vaccines in animal trials involved pathogenesis consistent with an immunological priming that could involve autoimmunity in lung tissues due to previous exposure to the SARS and MERS spike protein. Exposure pathogenesis to SARS-CoV-2 in COVID-19 likely will lead to similar outcomes. Immunogenic peptides in viruses or bacteria that match human proteins are good candidates for pathogenic priming peptides (similar to the more diffuse idea of "immune enhancement"). Here I provide an assessment of potential for human pathogenesis via autoimmunity via exposure, via infection or injection. SAR-CoV-2 spike proteins, and all other SARS-CoV-2 proteins, immunogenic epitopes in each SARS-CoV-2 protein were compared to human proteins in search of high local homologous matching. Only one immunogenic epitope in a SARS-CoV-2 had no homology to human proteins. If all of the parts of the epitopes that are homologous to human proteins are excluded from consideration due to risk of pathogenic priming, the remaining immunogenic parts of the epitopes may be still immunogenic and remain as potentially viable candidates for vaccine development. Mapping of the genes encoding human protein matches to pathways point to targets that could explain the observed presentation of symptoms in COVID-19 disease. It also strongly points to a large number of opportunities for expected disturbances in the immune system itself, targeting elements of MHC Class I and Class II antigen presentation, PD-1 signaling, cross-presentation of soluble exogenous antigens and the ER-Phagosome pathway. Translational consequences of these findings are explored.
Keywords: Autoimmunity; COVID-19; Immune Enhancement; Pathogenic priming; SARS-CoV-2.
https://pubmed.ncbi.nlm.nih.gov/32292901/
OK, so we know the theory and we have anecdotes. Over to the Johns Hopkins Lupus Center for potential pathological outcomes. I don’t subscribe to the lipid hypothesis of cardiovascular disease or salt and hypertension, but the main thing here is the message. Apart from hypertension & atherosclerosis, did you know that 25% of lupus sufferers get pericarditis, 10% get myocarditis?
We are potentially storing up an enormous future public health disaster due to autoimmune disorders, in addition to all the other potential pathologies disclosed previously.
These experimental gene therapy transfections must be halted immediately pending further investigations.
How Does Lupus Affect the Cardiovascular System
Lupus can affect the cardiovascular system, which includes your heart and blood vessels. In fact, cardiovascular disease, not lupus itself, is the number one cause of death in people with SLE. Therefore, it is very important that you take steps to maintain optimal cardiovascular health. Do not smoke, since smoking increases the risk of cardiovascular disease. A low-fat, low-cholesterol diet is also essential. Focus on eating whole grains, vegetables, and lean sources of protein. Limit your sodium (i.e., salt) intake, since sodium levels are directly linked to blood pressure. In addition, try to exercise at least 30 minutes per day. This goal can be difficult for people with lupus who experience reoccurring joint and muscle pain, fatigue, and other symptoms. However, engaging in low-impact daily activities such as walking, biking, yoga, Tai chi, and other forms of stretching may help to alleviate some of this pain while also helping you to maintain a healthy weight and strong cardiovascular system.
Hypertension (High Blood Pressure)
Fifty percent of people with lupus experience hypertension (high blood pressure), which is defined as a blood pressure of greater than 140/90 mmHg. In addition, many more lupus patients have blood pressures greater than the normal 120/80 mmHg limit. The most common causes of high blood pressure in people with lupus are obesity, kidney disease, and long-term steroid use. Other medications, such as cyclosporine (Neoral, Sandimmune, Gengraf) can also cause elevations in blood pressure.
High blood pressure can lead to kidney failure, stroke, heart failure, and heart attack. Since cardiovascular disease is the number one cause of death in people with lupus, it is very important that your blood pressure is brought to the healthy 120/80 mmHg level and kept there.
While it is important that you take steps yourself to help achieve and maintain optimum cardiovascular health, diet and exercise alone may be insufficient in controlling your blood pressure. Therefore, your doctor may prescribe a medication that works to lower, control, and/or maintain your blood pressure. Each medication works in a different way, and your doctor will work with you to evaluate and prescribe the blood pressure medication that best suits your personal condition.
Atherosclerosis
Atherosclerosis is the most common manifestation of cardiovascular disease in people with lupus. The condition is caused by the accumulation of cholesterol and other substances (known collectively as plaque) along the inner linings of arteries. Eventually, this plaque can harden and restrict the flow of blood to various parts of the body, including the heart and brain; if it ruptures, it can cause a clot to form, leading to complications like heart attack and stroke. Research has shown that being overweight and having high blood pressure are the two most important predictors of heart disease in SLE. In fact, the risk of heart attack in women with lupus aged 35-44 is 50-times greater than that of women without lupus, and for everyone with lupus the risk is increased 7 to 9-fold. Even though lupus itself contributes to the development of atherosclerosis, traditional factors, such as smoking, obesity, and high blood pressure, can exacerbate the condition and must be controlled.
Pericarditis
Pericarditis is the most common heart problem associated with active lupus and occurs in about 25% of people with SLE. The condition occurs when the pericardium—the thin membrane surrounding your heart—becomes swollen and irritated, causing it to leak fluid around the heart. People with pericarditis may experience pressure pains that are worse lying down and better sitting up. Your doctor will work with you to decide the best course of treatment for pericarditis. Often, NSAIDs or steroids are effective in reducing the inflammation associated with this condition.
Endocarditis
Libman-Sacks endocarditis occurs in about 15% of people with lupus. The condition leads to the development of growths called vegetations on the surface of heart valves. Usually this form of endocarditis is associated with antiphospholipid antibodies, which are present in about 50% of people with SLE.
Endocarditis can lead to two major complications: infection and stroke. Vegetations are prime sites for bacterial growth, and an infected valve requires surgical replacement. Visits to the dentist offer opportunities for bacteria to sneak into the blood and land on a heart valve. Therefore, people with severe heart murmurs should speak to their doctor about taking an antibiotic before receiving dental work. In addition, pieces of a vegetation can break off and travel through the blood stream, potentially blocking blood flow to the brain and causing a stroke. If you have a heart vegetation, your doctor will prescribe an anticoagulant to reduce this risk.
Myocarditis
Myocarditis, inflammation of the myocardium or heart muscle, occurs in fewer than 10% of people with lupus. People with this condition often experience a rapid heartbeat and chest pain, and x-rays may show an enlarged heart. Myocarditis may weaken your heart’s ability to pump blood to the rest of your body. Therefore, it is a serious complication that must be closely monitored and treated with high dose steroids for several weeks or months.
https://www.hopkinslupus.org/lupus-info/lupus-affects-body/lupus-cardiovascular-system/
This very recently published and timely paper appears to confirm the pathogenic priming hypothesis, explores why cardiovascular disease is the likely outcome, whilst also moving away from the lipid hypothesis.
Now although epitopes of viral origin are implicated, they are more likely to be vaccinal in origin as this bypasses the defences of the upper respiratory tract, becoming systemic.
Vaccine induced impaired immunity (“VAIDS”), in turn has been demonstrated to increase the rates of infection of the triple dosed up to 5 fold when compared to the unvaccinated, with identical or higher viral loads and reduced IgA mucosal antibodies compared to unvaccinated.2
Selective pressure on the virus due to the use of leaky vaccines is also leading to rapid evolution of escape variants, so the problem is unlikely to go away any time soon.3
For some great micrographs of spike protein expression and T cell proliferation in vascular tissues in action take a look at this Substack on the serious and related pathology of ciliopathy:
Ciliary Dysfunction Secondary to COVID-19. Explanation of the Pathogenesis From Analysis of Human Interactome With Sars-Cov-2 Proteome
https://doorlesscarp953.substack.com/p/ciliary-dysfunction-secondary-to?s=w
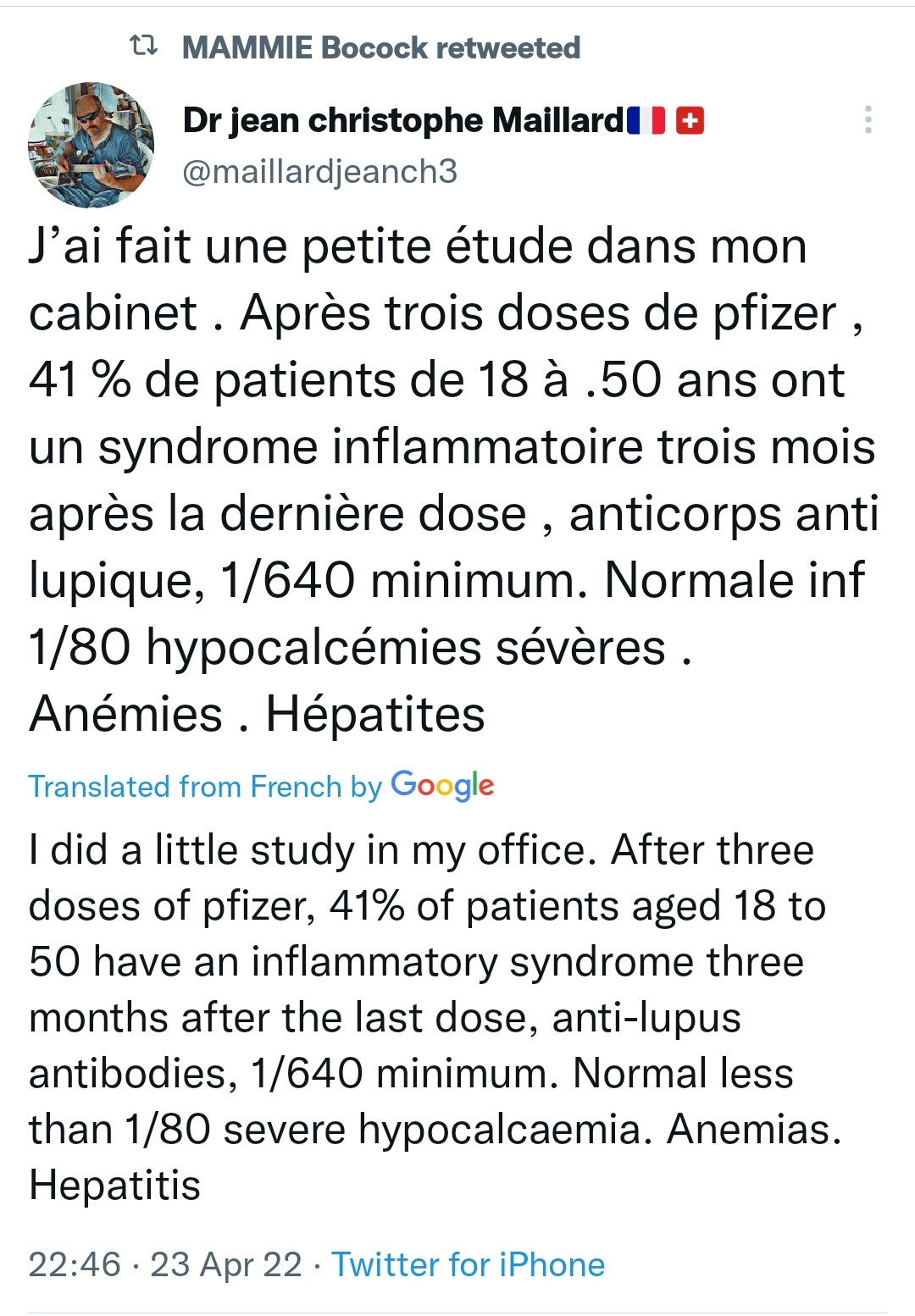
Human Coronary Plaque T Cells Are Clonal and Cross-React to Virus and Self (18th April 22)
Abstract
Background:
Once considered primarily a disorder of lipid deposition, coronary artery disease is an incurable, life-threatening disease that is now also characterized by chronic inflammation notable for the buildup of atherosclerotic plaques containing immune cells in various states of activation and differentiation. Understanding how these immune cells contribute to disease progression may lead to the development of novel therapeutic strategies.
Methods:
We used single-cell technology and in vitro assays to interrogate the immune microenvironment of human coronary atherosclerotic plaque at different stages of maturity.
Results:
In addition to macrophages, we found a high proportion of αβ T cells in the coronary plaques. Most of these T cells lack high expression of CCR7 and L-selectin, indicating that they are primarily antigen-experienced, memory cells. Notably, nearly one-third of these cells express the HLA-DRA surface marker, signifying activation through their TCRs (T-cell receptors). Consistent with this, TCR repertoire analysis confirmed the presence of activated αβ T cells (CD4<CD8), exhibiting clonal expansion of specific TCRs. Interestingly, we found that these plaque T cells had TCRs specific for influenza, coronavirus, and other viral epitopes, which share sequence homologies to proteins found on smooth muscle cells and endothelial cells, suggesting potential autoimmune-mediated T-cell activation in the absence of active infection. To better understand the potential function of these activated plaque T cells, we then interrogated their transcriptome at the single-cell level. Of the 3 T-cell phenotypic clusters with the highest expression of the activation marker HLA-DRA identified by the Seurat algorithm, 2 clusters express a proinflammatory and cytolytic signature characteristic of CD8 cells, while the other expresses AREG (amphiregulin), which promotes smooth muscle cell proliferation and fibrosis, and, thus, contributes to plaque progression.
Conclusions:
Taken together, these findings demonstrate that plaque T cells are clonally expanded potentially by antigen engagement, are potentially reactive to self-epitopes, and may interact with smooth muscle cells and macrophages in the plaque microenvironment.
https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.121.320090
Next back to the title of this Substack. There are many pathways for transfection triggered autoimmune disorders, cardiovascular disease and carcinogenesis, explored in detail previously, but is there a common link? Reactive Oxygen Species (ROS) generation is one of the key factors and I broached the subject at the start of the year, before these papers came to print.4
In short, spike protein binds to ACE2 (angiotensin converting enzyme 2), this leads to the upregulation of ANGII (angiotensin II) and then redox-dependent pathways involving the stimulation of NADPH oxidases.5
ROS, in turn then causes dysregulation of regulatory T-cells and several other components of an otherwise healthy immune system, leading to either cancer and/or autoimmune diseases in epigenetically susceptible individuals as detailed below.
“Epigenetically susceptible individuals” are those whose behaviour and environment have changed how their genes work in a negative sense. Examples include through poor diet and lack of exercise, smoking, old age and due to immune weakening infections...or due to transfection.
AIDs in this case refers to autoimmune disorders.
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. Referring specifically to mRNA gene therapy it is used interchangeably with “vaccination”, but it technically shouldn’t be as the latter traditionally will “contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism.”
Cancer and Autoimmune Diseases: A Tale of Two Immunological Opposites?
(25 January 2022)
The present article compares, side-by-side, cancer and autoimmune diseases in terms of innate and adaptive immune cells involvement, MHC Class I and Class II expression, TGFβ effect, immune modulating drugs effect and the effect of reactive oxygen species. The change in the inflammatory immune reaction during the progress of cancer and the effect of this change on the comorbidity of autoimmune diseases and cancer are discussed. The similar inflammatory properties of autoimmune diseases and early cancer, and the contrasting inflammatory properties of autoimmune diseases and advanced cancer elucidate the increased incidence of many types of cancer in patients with pre-existing autoimmune diseases and the decreased cancer-specific mortality of these patients. Stage-dependent effects of reactive oxygen-species on tumor proliferation are an additional probable cause for these epidemiological observations. The relationship: {standardized incidence ratio (SIR)} > {cancer-specific hazard ratio (HR)} for cancer patients with a history of autoimmune diseases is substantiated and rationalized.
“The regulatory T cells (Tregs /ˈtiːrɛɡ/ or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells.[1] Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells.[2] Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.[3]”
https://en.wikipedia.org/wiki/Regulatory_T_cell
T-helper 17 cell induced pathologies have been explored previously.6
Introduction
…The present work expands on the immunological properties of cancer and autoimmune diseases (AIDs) in relation to the binary classification proposed. During its course of growth, cancer progress from a pro-inflammatory, “low Treg” disease, to an anti-inflammatory, “high Treg” disease. This change is observed not only within the tumor microenvironment (TME) but also in the systemic circulation [Ref. 2 and references therein]. On the other hand, many autoimmune diseases are characterized by a decreased Tregs function or frequency (4), and may be regarded as proper pro-inflammatory “low Treg” chronic diseases. In addition, as will be described later, reactive oxygen species (ROS) exert opposite effects on the promotion of AIDs and advanced cancer. For these reasons, AIDs and advanced cancer can be considered immunological opposites. In contrast, AIDs and early cancer are triggered and promoted by a similar anti-inflammatory environment. As will be portrayed later, perspicacity of these time-related relationships helps in explaining the increased risk of cancer development in patients with AID history, a commonly reported observation which was referred to in the literature as “a paradox” (5). Noticing these unique relationships also helps in elucidating the longer survival of cancer patients with pre-existing AIDs compared to cancer patients without documented AIDs.
Cancer
The Involvement of T Cells in Cancer
CD8+ T cells attack cancer cells directly, and are of primary importance among several anti-cancer mechanisms reported in animals and man. By recognizing peptide-MHC-I complexes, CT8+ T cells identify cancer cells and destroy them through the release of perforin and the activation of the FAS apoptosis pathway (6).
Tregs suppress the function of CD8+ T cells (7, 8) and thus promote cancer. In addition, Tregs inhibit the proliferation of memory T cells (9) and of effector T cells such as Th1 (10) and Th2 (11). Th1 cytokine IL-2 demonstrated an anti-tumor activity in experimental models (12). A recombinant form of IL-2 is approved in several countries for the treatment of malignant melanoma and renal cell carcinoma. However another Th1 cytokine, IFNγ, exhibits pro- and anti-tumor effects (13).
In contrast to Th1 mediated immunity which is in general anti-cancer, Th2 mediated immunity present pro- and anti-tumor effects (14).
…Collectively, accumulation of Treg and Th17 cells in the tumor microenvironment along with increased levels of both cells in blood are observed in many cancers.
The effect of Th17 cells on cancer is usually limited. Inhibition of IL-23, a key cytokine for Th17 maintenance and expansion, by IL-23 inhibitor risankizumab, does not increase long-term cancer risk (28). Similarly, long-term treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis patients with IL-17A inhibitor secukinumab, demonstrated a long-term low risk of malignancy (29). A pooled analysis of other long-term studies with secukinumab presented similar results (30). Another IL-17A inhibitor, ixekizumab, also demonstrated a long-term low risk of cancer (31). However other studies with these and other IL-23 inhibitors display conflicting results with pro-tumor or anti-tumor effects (32). It may be concluded that Th17 cells do not present significant anti-cancer or pro-cancer activities.
The Involvement of Neutrophils in Cancer
Tumor associated neutrophils (TANs) display plasticity in the TME. In the absence of TGFβ they attain a pro-inflammatory and anticancer mode of action (N1 phenotype) while in the presence of TGFβ they present an anti-inflammatory and pro-cancer response (N2 phenotype). The N1 phenotype which produces high levels of TNFα, NO and H2O2, prevails in the TME of early tumors when the level of TGFβ is low. The N2 phenotype predominates at later stages when TGFβ accumulates (33). Circulating neutrophils in tumor-bearing mice display two sub-populations classified by their densities in peripheral blood mononuclear cells (PBMC) fraction, after density gradient centrifugation of whole blood. High density neutrophils (HDN) prevail in tumor-free mice while low density neutrophils (LDN) are the dominant neutrophils in tumor-bearing mice. HDN were cytotoxic towards cancer cells in culture, whereas LDN were innocuous. Similar to TANs, high density neutrophils transform into the low density type in a TGFβ-dependent manner (33). It is not surprising therefore that circulating blood of patients with advanced-stage lung cancer was reported to be enriched with LDN, relative to early-stage patients, or healthy controls (34).
The Involvement of Macrophages in Cancer
Macrophages can be classified as pro-inflammatory macrophages (M1) or anti-inflammatory macrophages (M2). M1 macrophages promote inflammation by the secretion of inflammatory cytokines such as TNFα, IL-1α, IL-1β, IL-6, IL-12, IL-18, and IL-23, and by the generation of ROS and reactive nitrogen species. They also express high levels of MHC that allow the activation of the adaptive immune arm. M2 macrophages secrete high levels of IL-10, PGE2 and TGFβ, cytokines that play an anti-inflammatory role. M2 macrophages express high levels of arginase-1, mannose receptor, and low levels of MHC class II complex (35).
Tumor associated macrophages (TAMs) include both phenotypes, M1 and M2. It has been proposed that macrophages display a pro-inflammatory antitumor effect (M1 phenotype) in early cancer and an anti-inflammatory pro-tumor effect (M2 phenotype) in established cancer (36).
The Effects of ROS on Cancer
The tumor microenvironment is rich in reactive oxygen species. As the tumor develops, ROS accumulate in the TME of many solid cancers, as a result of intensive production by tumor cells mitochondria and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. In addition, ROS are released into the TME by cancer-associated fibroblasts (CAFs), TAMs, and myeloid-derived suppressor cells (MDSCs) (37). The effects of ROS on cancer proliferation are complex. They are partly mediated by the interaction of ROS with Tregs, but other pathways are possible. These interactions are presented below.
ROS Are Required for Tregs Function
When mitochondrial oxidative phosphorylation in Tregs is impaired by the ablation of mitochondrial respiratory chain complex III in mice, the suppressive capacity of these Treg cells is lost without altering Treg cell proliferation and survival (38).
ROS and Tregs Mutually Induce Each Other’s Activity
It has been demonstrated in vitro and in vivo that ROS generated by macrophages NADPH oxidase complex induce Treg frequency and function (39). In addition it was shown in psoriasis rat model that ROS prevent imiquimod-induced psoriatic dermatitis by enhancing Tregs function (40). On the other hand, TGFβ excreted by Treg cells induced the generation of ROS by NADPH oxidase (41, 42). In accordance with these observations, Treg-induced immunosuppression in the tumor microenvironment is mediated by Tregs-generated ROS (43).
ROS Induce MHC-I Expression in Cancer Cells (Which Promote the Anti-Cancer Effect of CD8+ T Cells)
MHC-I expression in tumor cells has been shown to increase following oxidative stress (44, 45). This increased expression of MHC-I facilitates the identification of cancer cells by CD8+ T cells and boosts tumor attack by these T cells.
Reduction in ROS Production by Dendritic Cells Hampers Their MHC-I Machinery and Impairs Their Role in Anti-Cancer Immunity
Mitochondrial reactive oxygen species have a role in cross-presentation of MHC-I antigens by plasmacytoid dendritic cells. Reduction in mitochondrial generation of reactive oxygen species by plasmacytoid dendritic cells resulted in a substantial decrease in the ability of these cells to induce a CD8+ T cell reaction following cross-presentation (46). This behavior was also reported in normal dendritic cells, where NADPH oxidase isoform NOX2 (an enzyme with a prominent role in mitochondrial reactive oxygen species generation) was found essential for an efficient antigen cross-presentation to CD8+ T cells. Lack of NOX2 in DCs resulted in impaired cross-presentation (47). Dendritic cells from chronic granulomatous disease patients with an impaired catalytic subunit of NOX2 have shown defective cross-presentation of soluble antigens to CD8+ T cells (48). Since dendritic cells are major players in the control of cancer by adaptive immunity (49), a reduction in ROS production by dendritic cells is expected to promote cancer.
The MAPK pathway has been considered in depth concerning GP120 and microglial cell activation leading to “brain fog”,7 plus P53 related pathology.8
Moderately-High ROS Levels Promote Solid Cancers While Low ROS Levels Are Cytostatic and Excessive ROS Levels Are Cytotoxic
As discussed above, ROS effect on tumor growth may be mediated by Tregs. However other routes for the effect of ROS on malignancy are possible. At the pre-cancerous stage, ROS may drive cancer initiation by inducing oxidative damage and base pair substitution mutations in tumor suppressor genes such as TP53 (50). As the tumor grows, the increased level of ROS in the TME induces tumor proliferation by enhancing Tregs activity. In addition ROS activate several canonical pathways involved in tumor propagation such as the NF-κB pathway, the MAPK pathway (including ERK1/2, JNK, MAPK-11 and MAPK1) and the PI3K/PTEN pathway (37). Reactive oxygen species also support the development of tumor metastases by driving epithelial–mesenchymal transition (EMT) (48, 51, 52) and by anoikis inhibition (anoikis is a type of programmed cell death induced by cell detachment from extracellular matrix) (53).
These and other pro-cancerous effects of increased ROS levels drive tumor progression. However, very high levels of oxidative agents may result in tumor tissue damage. Excessive ROS levels induce cell death by apoptosis, necrosis and ferroptosis (54). Hence, a moderately-high level of ROS within the TME would be optimal for tumor growth and sustainability (55–57). A tight control on ROS balance is therefore required for tumor progression. It may be stated that moderately-high intra- and extra-cellular ROS levels promotes solid cancer propagation, metastases and angiogenesis, whereas very high levels induce cancer cell death and low levels are cytostatic.
The Involvement of MHC-I in Cancer
MHC-I Induces the Suppressive Function of Tregs Which Promotes Cancer
Using a mouse model, Mu et al. have shown that MHC-I transcription is enhanced by the transcription factor FoxP3 in T cells. This resulted in a higher expression of MHC-I in CD4(+)CD25(+) regulatory T cells than in conventional CD4(+)CD25(-) T cells. The authors also found that MHC-I expression by Tregs contributes to their regulatory function (58). High suppressive Treg activity drives advanced cancer and metastases (2). Mu et al. mention a study by Joetham et al. reporting that CD8+ T cells interaction with MHC-I on Tregs is required for Tregs activation (59). In relation to this observation, TCRs expressed in peripheral Treg cells can recognize foreign antigens with high affinity. Tregs induce antigen-specific suppression mainly by Treg-DCs interaction. A mechanism by which DCs present antigens to Treg cells as part of MHC-II, by means of Treg TCR, has been proposed. This process eventually results in the generation of antigen-specific tolerogenic DCs (60). One may speculate that antigens presented by DCs to Tregs may further bind to MHC-I to form protein-MHC-I complex. This complex may help CD8+ T cells in recognizing Tregs (the way CD8+ T cells recognize cancer cells), before they activate them by a direct contact.
TGFβ Suppresses MHC-I Expression
The suppression of MHC-I expression by TGF-β1 has been demonstrated in the TGFβ1 null mouse where elevated mRNA levels of MHC-I (and MHC-II) were detected compared to normal or TGFβ1 heterozygous littermates (61). Incubation of two human uveal melanoma cell lines in the presence of TGF-β generated more than 50% decrease in MHC-I antigen expression (62).
Tumors Escape Immune Control Through the Loss of MHC-I Antigen Presentation Machinery
As explained above, peptide-MHC-I complexes guide tumor attacks by CD8+ T cells. Many solid tumors demonstrate decreased expression of MHC-I antigen presentation (mediated through different pathways), and thus evade cancer immune control by CT8+ T cells (63).
The Involvement of MHC-II in Cancer
MHC-II-mediated antigen presentation to CD4+ T cells supports immune response by both T helper cells and CD8+ T cells [CD4+ T cells are required for CD8+ priming and function (64)]. Even though MHC-II is expressed mainly by professional antigen presenting cells (macrophages, dendritic cells, and B cells) and by thymic epithelial cells, other cells may express MHC-II, including cancer cells. Indeed, in addition to changes in MHC-I expression by cancer cells, changes in the expression of MHC-II by cancer cells are also observed (65). The frequencies of MHC-II alleles in cancer patients are different from these observed in healthy subjects. These differences, which depend on the type of cancer, were found to correlate with the risk of developing the malignancy and with the response to treatment (66). It is tempting to believe that CD+4 T cells (and MHC-II expression) are important for anti-tumor immunity. In line with this, the response to PD-1 blockage in a synergistic murine model of melanoma required CD4+ T cells in addition to CD8+ T cells, and a costimulation by dendritic cells and macrophages (67).
Tregs Deplete MHC-II From Dendritic Cells
Akkaya et al. have shown in a mouse model that one of the mechanisms by which regulatory T cells induce immune suppression is by the depletion of peptide-MHC-II complex from DCs (68). Since advanced cancer is a “high Treg” disease, this regulatory mode of action is expected to promote immune escape at advanced cancer stages.
TGFβ Reduces MHC-II Expression in Macrophages
Delvig et al. demonstrated that by reducing MHC-II expression on macrophages, TGFβ blocked antigen presentation of two T cell epitopes. In addition, TGFβ reduced the constitutive expression of MHC-II transactivator (CIITA), invariant chain, and HLA-DO mRNA (69). These findings are supported by another study, reporting of CIITA gene-attenuation mediated by Tregs (70). Since Treg cells are a major source of TGFβ, these results are consistence with the earlier observation of MHC-II depletion mediated by Tregs.
MHC-II Expression Declines During the Course of Cancer Progression
Treg cells activity (number and function) as well as TGFβ levels increase (in the TME and in the circulation) during the course of cancer development. Based on the findings aforementioned, MHC-II expression is expected to decline during the course of cancer progression. In line with this, MHC-II allele HLA-DRB1*07 expression was higher in lymph nodes of patients with early stage non-small cell lung cancer, compared to patients with an advanced stage of the disease (71).
Taken together, MHC-II expression is important for an effective anti-tumor immunity. Treg-mediated downregulation of MHC-II expression promotes tumor immune escape.
Immune suppression can mediate carcinogenesis:
The Tumorigenic Effect of AIDs Drug-Treatment
Non-Specific-Action Immunosuppressants Induce Cancer
Considering the antitumor effects of CD8+ T cells and Th1 cells, it is not surprising that non-specific-action (but not target specific) immunosuppressive drugs increase the risk of cancer. For example, malignancy risk in transplant patients using immunosuppressive drugs such as cyclosporine or azathioprine increases 4 - 500 fold compared to age-matched controls in the general population
Autoimmune Diseases
The Involvement of T Cells in Autoimmune Diseases
Autoimmune diseases are a typical example of pro-inflammatory chronic disease (1). Many AIDs display impaired Tregs function (4). Moreover, the lack of Tregs induces AIDs…Since Treg function is impaired in autoimmune diseases, CD4+ T cells activity intensifies. In particular, Th1 and Th17 cells are involved in the pathogenesis of many AIDs (74). Th17 cells frequency or function increases in multiple sclerosis (MS), psoriasis, RA, inflammatory bowel disease, and SLE (75).
Th2 cells mediate allergic immune responses (76) and also play a pathogenic role in systemic sclerosis and ulcerative colitis (74). Systemic frequency of Th2 in RA patients is similar to that in healthy controls (77). The data regarding Th2 cytokines concentration in SLE is conflicting (78, 79).
CD8+ T cells frequency (in affected organs or in the circulation) increases or their function is boosted in MS, systemic sclerosis, T1D, SLE, and severe aplastic anemia (80).
The Involvement of Neutrophils in Autoimmune Diseases
Neutrophils exhibit a pro-inflammatory function in AIDs. However their modus operandi may vary from one disease to another. The involvement of neutrophils in RA and SLE are described below as representative examples.
Neutrophils in RA patient’s joints are cytotoxic and neutrophils in the circulation of RA patients are primed for the production of ROS, in contrast to those in healthy subjects. Proteases released from lysosomal granules of neutrophils promote RA inflammation (81). Elevated levels of myeloperoxidase (MPO), a cytotoxic enzyme released from neutrophils granules, are found in blood, synovial fluid and tissues of RA patients. MPO increases vascular permeability and allows the penetration of pro-inflammatory immune cells. In addition, MPO attracts more neutrophils to the site of inflammation. Neutrophils in RA synovial fluid secrete inflammatory cytokines such as TNF, B cell-activating factor (BAFF) and receptor activator of nuclear factor kappa B ligand (RANKL). Neutrophil extracellular traps (NETs) are webs of histones and DNA fibers that participate in pathogens eradication. Citrullinated histones comprise around 70% of all NETs proteins. NETs can promote the inflammatory effects of fibroblast-like synoviocytes. NETs formation which is enhanced in RA, is believed to trigger autoimmunity to citrullinated proteins (81).
Similar to their role in RA, neutrophils in SLE display a pro-inflammatory function. They are mainly of the LDN phenotype and secrete type I IFN, IFN-γ, IL-6, IL-8 and TNFα. Like RA, SLE is characterized by enhanced NETs formation. NETs promote immune response in SLE by exposing antigens that are normally shielded by plasma membrane, by reducing T cells activation threshold, and by activation of autoreactive B-cells (82).
The Involvement of Macrophages in Autoimmune Diseases
In many AIDs macrophages display the M1 pro-inflammatory phenotype (RA, SLE, primary biliary cholangitis, Sjögren’s syndrome, T1D). However, M2 phenotype has been detected in fibrotic AIDs such as systemic sclerosis and inflammatory bowel disease (83). In multiple sclerosis (MS), even though most macrophages in disease lesions display M1 characteristic, a large fraction of macrophages displays both M1 and M2 markers (84).
The Effect of ROS on Autoimmune Diseases
Low ROS Levels Promote Autoimmune Disorders
Chronic granulomatous disease (CGD) is a rare genetic disorder caused by defects in any of the five subunits of the NADPH oxidase complex. An increased frequency of several autoimmune diseases has been reported in CGD patients (85). Experimental evidence (observed in human and animals) supports a link between ROS deficiency and autoimmune diseases such as systemic lupus erythematosus (SLE) (86–88), rheumatoid arthritis (RA) (89–92), psoriasis (93) and Guillain–Barré syndrome (94)
Low ROS Levels Impair Treg Function Which in Turn Drive Autoimmunity, While Elevated Levels of ROS Attenuate Autoimmunity
Using a mouse model, Kim et al. have shown that imiquimod-induced psoriatic dermatitis was attenuated by elevated ROS levels, whereas lower ROS levels which induced Tregs dysfunction aggravated the disease (40). It is plausible that impaired Treg function under low ROS levels promoted this autoimmune disease.
Very High ROS Levels (Oxidative Stress) Underlie Tregs Damage That Drive SLE and Experimental Autoimmune Encephalitis (EAE)
Strickland et al. demonstrated that oxidant-treated T cells induced lupus-like disease in mice. In addition, genes known to be associated with lupus in SLE patients and animal models of SLE were upregulated by ROS. This effect was most pronounced with peroxynitrite (ONOO-) as the oxidant (95). Peroynitrite is at equilibrium with peroxynitrous acid which rapidly decays in non-alkaline media to •NO2 and •OH free radicals (~ 30% yield) and the rest of peroxynitrous acid isomerizes quickly [k = 1.2 sec-1 (96)] to the nitrate ion (NO−3)(NO3−) (97). For the treatment of T cells, Strickland et al. have used 20 µM peroynitrite solution (95). This highly oxidative medium triggered the disease.
T cell mitochondrial dysfunction has been proposed as the generator of oxidative stress in SLE (98). Mitochondrial oxidative stress and DNA damage was reported in Treg cells extracted from patients with different AIDs. This mitochondrial oxidative stress and DNA damage which resulted in Treg cell death, was also observed in the EAE mouse model (99). Oxidative stress has been shown to affect the Treg/Th17 balance in SLE (100).
It is evident that oxidative stress (which is synonymous with very high ROS levels) promotes autoimmunity.
Immunosuppressive Agents Induce ROS Generation in Renal Transplant Patients
Administration of several immunosuppressive agents to renal transplant recipients (with an uneventful postoperative course and stable renal function) before transplantation, indicated a statistically significant increase in ROS levels (101).
The Effect of TGFβ on Autoimmune Diseases
TGFβ Deficiency Induces Autoimmunity
Blocking TGFβ signaling in T cells induces autoimmunity in mice (102, 103).
The Involvement of MHC-I in Autoimmune Diseases
MHC-I Effect on AIDs Is Ambiguous and Restricted to Few AIDs Only
…It can be concluded that MHC-I is not involved with the pathogenesis of all AIDs and its effect is controversial.
The Involvement of MHC-II in Autoimmune Diseases
Genetic studies have confirmed that associations between MHC and autoimmune diseases are mostly related to MHC-II alleles. Certain MHC-II alleles increase the probability of developing specific AIDs (a positive association) while other alleles reduce this probability (a negative association). Grave’s disease, narcolepsy, autoimmune thyroiditis, RA, MS, T1D, SLE, ulcerative colitis and Crohn’s disease are all associated with MHC-II polymorphism (109, 110). Ulcerative colitis and Crohn’s disease are associated (but less consistently) with Class I alleles as well (110).
Strong induction of MHC -II expression in mice retina was observed in an experimental mouse-model of autoimmune uveitis (111). Upregulated endothelial MHC-II expression has been reported in dilated cardiomyopathy, RA, SLE, MS and Crohn’s disease patients (112).
Pathogen-stimulated PBMC from subjects homozygous for autoimmune vitiligo high-risk SNP haplotype, demonstrated increased production of IFN-γ and IL-1β than cells from subjects homozygous for a low-risk haplotype (113).
Collectively, AIDs are consistently associated with MHC-II polymorphism. MHC-II expression is upregulated in AIDs and induces a pro-inflammatory effect.
Cancer Drug-Treatment Drives Autoimmunity
Checkpoint Inhibitors Induce Autoimmunity in Patients With a Pre-Existing Autoimmune Disease
Autoimmune disease patients treated with checkpoint inhibitors for cancer are at increased risk of AID relapses. More than 30% of these patients develop flares of their pre-existing AIDs and some develop new autoimmune manifestations.
We are more concerned with how the post transfection pro-inflammatory environment is conducive to both initiation of autoimmune disorders and carcinogenesis:
Table 1 A comparison of cancer and autoimmune diseases in terms of immunological properties, ROS effect, TGFβ effect and drug effect:
Table 1 reveals that several immune reactions, triggers, cytokines and drug effects associated with advanced cancer are reciprocal to those associated with autoimmune diseases. The immune reaction to advanced cancer may be regarded as a “high Treg” and anti-inflammatory response while immune reaction to autoimmune diseases is a “low Treg” and pro-inflammatory response. On the other hand, the immune reaction to early cancer (prior to immune evasion) is pro-inflammatory, just like the immune reaction to AIDs. The pro-inflammatory environment that underlies AIDs supports the initiation and growth of early malignancy and increases the risk of cancer in AID patients. Indeed, higher incidence of cancer development is reported in many autoimmune diseases (125). In agreement, tumor infiltrating regulatory T cells (which release high amounts of TGFβ in the TME) improve survival in cancers with a long pre-metastatic periods like lymphoma, but have a negative effect on survival in cancers with an early immune evasion like breast cancer, lung cancer, or aggressive squamous cell carcinoma (2).
The standout correlation between autoimmune disease and cancer type is with primary liver cancer, and then hepatobiliary cancer (“of, relating to, situated in or near, produced in, or affecting the liver and bile, bile ducts, and gallbladder”):
Table 2 Standardized incidence ratios (SIR) and cancer-specific hazard ratios (HR) (averaged over a wide-range of autoimmune diseases) for different types of cancer:
Table 2 presents published values of mean standardized incidence ratios (SIR) for different types of cancer, averaged over a large number of AIDs (including 95% confidence intervals). An increased SIR value in cancer patients with a history of AIDs was reported in lung cancer (126), kidney cancer, bladder cancer, prostate cancer (127), hepatobiliary cancer, primary liver cancer, gallbladder cancer, extrahepatic bile duct cancer (128) and multiple myeloma (129). Similarly, SIR for the development of lymphoma, increased in patients with SLE or RA (133). Cancer SIR (averaged over several types of cancer) increased in SLE, Sjogren’s syndrome, systemic sclerosis, and marginally in rheumatoid arthritis (134).
Increased likelihood of cancer may not mean increased likelihood of death from that cancer, as the inflammatory nature of AIDs may reduce the aggressiveness. BUT this doesn’t mean overall mortality is necessarily lower because of conditions such as the aforementioned cardiovascular disease. Choose your poison:
Turning to the survival of cancer patients with AIDs, the pro-inflammatory nature of AIDs in advanced cancer is expected to slow down cancer propagation and to correct the negative effect of AIDs in early cancer. Since cancer-related mortality occurs almost always at advanced stages of the disease, the hazard ratio for cancer-specific mortality is expected to be lower than the cancer standardized incidence ratio. Inspection of Table 2 reveal that in 11 out of 14 types of cancer the relation {SIR > HR} (where HR refers to cancer-specific mortality) holds true, and in 9 types of cancer, cancer-specific mortality in patients with AIDs is even lower compared to patients without an autoimmune background (i.e. HR < 1), even though the effect may be statistically non-significant in several cancers. In addition, a decrease in mortality was reported in breast cancer patients where a statistically significant 54% reduced risk of breast cancer mortality was observed in women with Th1 dominant autoimmune diseases (135).
In this respect, the distinction between cancer-specific mortality and overall mortality is important. Overall mortality is affected by comorbidities other than cancer (mainly by comorbidities related to AIDs) and is higher than cancer-specific mortality. This was recorded, for example, in kidney cancer, bladder cancer and prostate cancer (127). Therefore, the relationship {SIR > HR} may not be valid for the hazard ratio of overall mortality.
It must be delineated that the results presented in Table 2, are averages over a wide range of autoimmune diseases. The effect of a specific autoimmune disease may strongly deviate from the average.
It should also be realized that factors other than immune reaction, may affect the survival of cancer patients with AIDs differently from those cancer patients without AIDs.
For instance, cancer patients with formerly diagnosed AIDs are often undertreated with respect to their malignancy. The major reason for this insufficient treatment is “poor initial performance status or frailty” (136). An increased frequency of leukopenia due to the simultaneous use of immunosuppressive and chemotherapeutic drugs in these patients is a possible reason for shorter anti-cancer treatment periods or lower doses of anti-cancer drugs. In line with this, higher cancer-specific hazard ratios were reported in breast, cervical, and endometrial cancer in a subgroup of patients with severe AIDs (patients with at least 3 hospitalizations) compared to the mean values over the entire group (that included patients with less severe autoimmune disease) (130). The subgroup with a more severe disease was possibly undertreated.
If insufficient treatment of cancer patients with AIDs is considered as a confounding factor in mortality hazard assessment, the beneficial effect of AIDs on cancer-specific mortality may be even larger, and the “true” HR values should be lower than the values presented in Table 2.
To summarize, the incidence of cancer increases in many autoimmune diseases (digestive tract malignancies are exceptions) while cancer-specific mortality of most solid cancers either decreases or is unaffected by pre-existing autoimmune disease. The inequality {SIR > HR} (where HR refers to cancer-specific mortality) is valid in the overwhelming majority of cancer types in patients with formerly diagnosed AID (including digestive tract cancers).
Very high ROS-inducing repeat boosting with its mitochondrial & DNA damage mediation, followed by months of elevated but lower levels of pro-inflammatory cytokines could put us in the sweet spot for both conditions:
The Effect of Reactive Oxygen Species on Cancer and Autoimmune Diseases
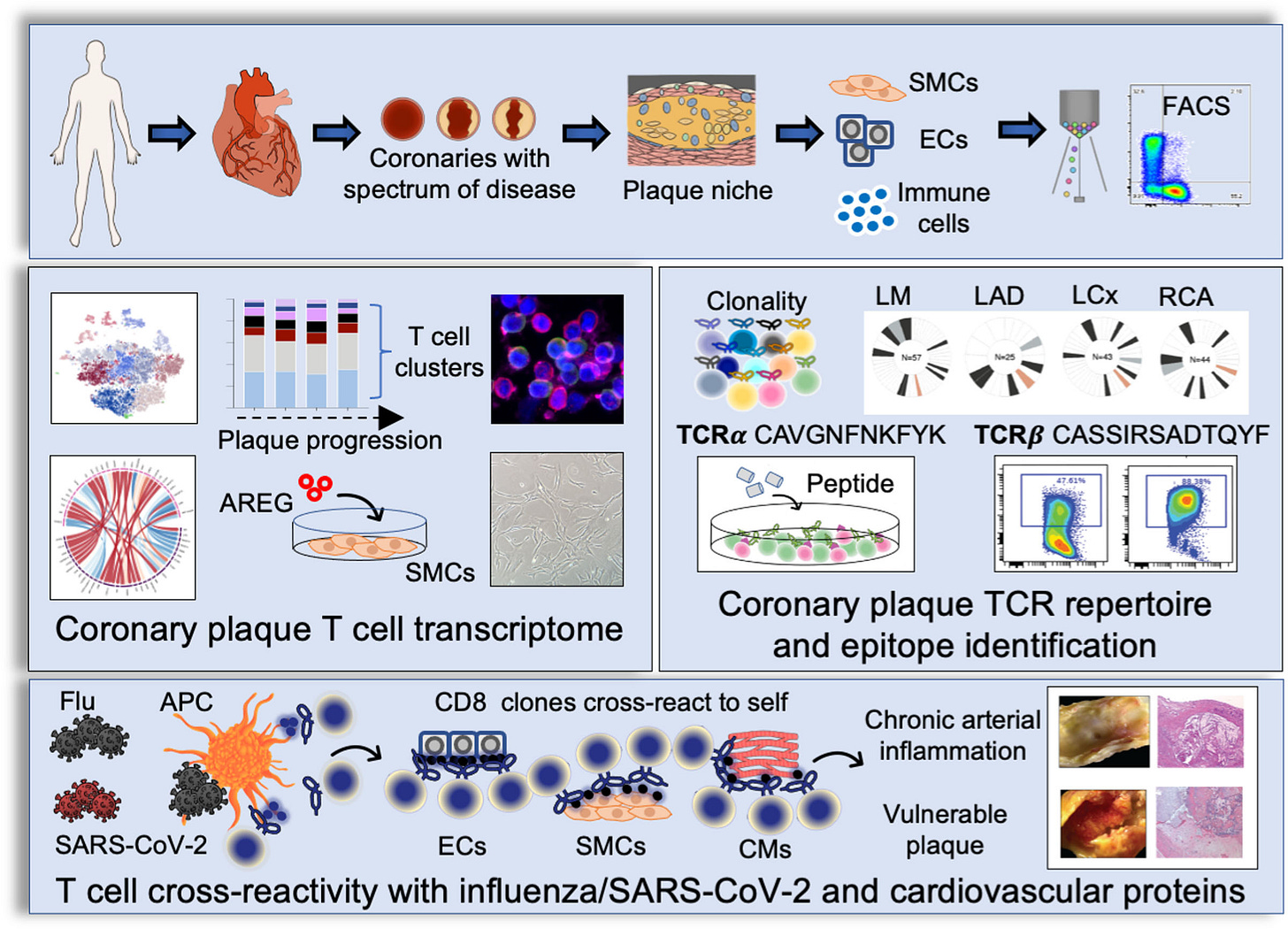
It is noted that ROS levels that promote or suppress cancer are a mirror image of the levels that promote or suppress AIDs (see Figure 1). Whereas cancer proliferate and metastasize best under moderately-high ROS levels, AIDs develop and thrive under either low or very high ROS levels. Even though the high-low borders are not well defined, it is clear that very high ROS levels promote AIDs (98). Reactive oxygen species that accumulate in the circulation of AID patients act as pro-cancer agents at early cancer stages but as anti-cancer agents at advanced stages (50). This stage-related effect is another factor that contributes to the pathogenic effect of AIDs in early cancer and to their beneficial effect in advanced cancer, leading to the relationship {SIR > HR} discussed before.
Summary
Inflammation drives both autoimmune diseases and early stage cancer while it slows down advanced cancer. Low or very high levels of reactive oxygen species induce autoimmunity while cancer propagates optimally at moderately-high levels of reactive oxygen species.
Due to both effects, initiation of cancer and their early growth are promoted by pre-existing autoimmune diseases leading to an increased risk of cancer, while advanced cancer growth and spread are hindered in patients with pre-existing autoimmune diseases, resulting in an improved survival
https://www.frontiersin.org/articles/10.3389/fimmu.2022.821598/full
I like to finish on a positive note where possible, so apart from avoiding experimental transfection with dreadful safety records and negative efficacy, what therapies are available to treat autoimmune disorders? I maintain a generalised list to treat sequalae here with help from several contributors:
Specific to autoimmune disorders, the news isn’t great as there are hundreds of them - almost every cell type can come under attack, one size doesn’t fit all, with conditions ranging from type 1 diabetes to MS to rheumatoid arthritis to Guillain-Barre syndrome. Addressing the root cause of oxidation and immune dysregulation is the nearest to pan-disease targeting you are likely to get.
Allopathic medicine is more focused on symptomatic control, immune suppression via corticosteroids or addressing any related hormonal imbalances:
There are many autoimmune diseases. Some cause distressing symptoms that affect a person’s quality of life but otherwise are not life threatening. Other autoimmune conditions are more serious and can cause lasting tissue damage.
In many cases, management strategies such as taking medication, modifying the diet, and making lifestyle changes can help reduce the symptoms.
A doctor can help diagnose and recommend treatments for specific autoimmune conditions.9
Prevention is the best cure. Once started you can’t just take a reverse vaccine pill and reset your immune responses as they were and undo the ROS mediated damage, you are stuck with the consequences.
Thank you for reading, and I hope this helps raise awareness of some of the conditions that have been unleashed on a subset of the population.
UKHSA Publishes Final Vaccine Data Report – as Vaccine Effectiveness Against Hospitalisation in Over-80s Heads to Zero
https://dailysceptic.org/2022/04/03/ukhsa-publishes-final-vaccine-data-report-as-vaccine-effectiveness-against-hospitalisation-in-over-80s-heads-to-zero/
SARS-CoV-2 variants of concern and variants under investigation in England
Technical briefing 40
8 April 2022
SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling (2021)
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #25: T- helper 17 cells & interleukin 17
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-9f1?s=w
Spike protein (inc vax) induced immunodeficiency & carcinogenesis megathread #27: Further investigations into GP120, IL-6 & multidrug resistance
https://doorlesscarp953.substack.com/p/spike-protein-inc-vax-induced-immunodeficiency-298?s=w
S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein p53 and BRCA: an In Silico Study (2020)
Experimental gene therapy transfections and public health implications
https://doorlesscarp953.substack.com/p/s2-subunit-of-sars-ncov-2-interacts?s=w
List of autoimmune diseases, with symptoms and treatments
https://www.medicalnewstoday.com/articles/list-of-autoimmune-diseases







Thank you, Carp. Your writings are dense and complex, but well worth plodding through again and again. May good health be yours. Cheers.