Any extracts used in the following article are for non commercial research and educational purposes only and may be subject to copyright from their respective owners.
Abstract
This Substack is a scientific literature review of research into the therapeutic benefits of three Echinacea species. A PubMed 10 year search for “Echinacea” returned 467 results. Some of the more cited research is presented here for review:
Echinacea purpurea was shown to broadly inhibit coronaviruses and SARS-CoV-2 in vitro. In 2021 Nicolussi et al used PCR testing to compare the rates of infection and viral loads in adults and children administered 2,400mg and 1,200mg respectively of Echinaforce extract tincture over 4 months vs the control with excellent results: viral loads in nasal secretions were significantly reduced by 98.5%.
For preventing and treating the common cold a controversial meta-analysis from 2014 by Karsch-Volk et al concluded there were either statistically insignificant or minor benefits.
In 2011, Hudson & Vimalanathan posted their meta analysis of reviews Echinacea—A Source of Potent Antivirals for Respiratory Virus Infections. They found that all strains of human and avian influenza viruses tested, including a Tamiflu resistant strain as well as herpes simplex, RSV and rhinoviruses (a common cold virus) were very sensitive to E purpurea preparations. Their results suggested intracellular inhibition and significant viricidal activity, including by inhibition of replication, although effects were sometimes weak at non-cytotoxic concentrations.
Fusco et al (2010) found that Echinacea-treated mice had lower systemic and pulmonary KC (human IL-8) and IL-10 levels and lower systemic IFN-γ levels following influenza infection. They suggested that E. purpurea alters the clinical course of influenza infection in mice through modulation of cytokines and not direct antiviral activity. Although aerial parts of the plant did not show antiviral activity, they found that root extracts did in fact demonstrate this.
Another finding was that “Echinaforce” inhibits mucin secretion, one of the most discomforting symptoms and the cause of coughs, sore throat or even bronchitis.
A further study by Vimananathan et al in 2017 indicated that virus-induced bacterial adhesion and cytokine storms associated with respiratory infections could be inhibited by E purpurea.
A 2009 murine study into the reduction of latent herpes simplex virus type-1 (HSV-1) reinfections demonstrated efficacy when taken as a prophylactic.
In 2007, a paper by Pillai et al further supports polysaccharides as being potent immunostimulants. Water soluble, but not fat-like extracts from all parts of the plant all produced substantial immunostimulatory activity.
Upregulation of NF-kB is a marker for infection. In 2008, Matthias et al identified 2 different alkyamides in Echinacea root extract, one of which stimulated the immune response, the other modulated it.
Prophylaxis appears important for reducing viral levels. Sharma, Schoop & Hudson (2009) used a standardised Echinacea preparation (Echinaforce) and concluded that symptoms may be reduced at any stage of infection: “under real life conditions of Echinacea consumption, the virus-induced stimulation of pro-inflammatory cytokines can be effectively reversed or alleviated.”
A study from 2007 by Zhai et al concluded that all three species promoted T-cell proliferation, anti-viral interferons and innate & adaptive immune responses, but E. angustifolia or E. pallida may have more anti-inflammatory potential.
A 2017 study by Chiou et al. (only abstract available) researched antioxidant, antidiabetic, and antihypertensive properties. They found that both chlorogenic acid and caffeic acid demonstrated high ACE-inhibitory activity. Their in vitro results suggested that E. purpurea extract and CAD have good potential for managing hyperglycemia and hypertension.
An interesting study by Tsai et al (2012) found that “cichoric acid has a strong growth-inhibitory effect against colon cancer cells, presumably resulting from the reduced telomerase activity and the induction of apoptosis.”
A human clinical trial by Yotsawimonwat et al (2010) showed an increase in overall skin hydration and a reduction in skin wrinkles by 10%-14% when using Echinacea as either a face cream or gel.
Contraindications, interactions with chemotherapeutic drugs, bioavailability and dosage recommendations are then considered.
Discussion
Of the nine species in the group, 3 are typically used in herbal supplements - E purpurea, E angustifolia and E Pallida.
The plants roots and upper parts are used medicinally, and taken as tinctures, extracts, teas & tablets.
Biologically active compounds include caffeic acid, alkamides, phenolic acids, rosmarinic acid, polyacetylenes and cichoric acids. These have high antioxidant properties and can protect against cancer, ageing and cardiovascular diseases. In addition, it has been demonstrated that alkamides have key roles in inhibiting prostaglandin E2 production. It was reported that Echinacea prevents caspase-3 accumulation and protects cell against apoptosis.
In summary, the Echinacea group of flowering plants are linked to many health benefits, such as reduced inflammation, improved immunity and lower blood sugar levels.
This substack will review some of the more significant research into this and provide guidance on safety and dosage.
A member of the daisy family Asteraceae, Echinacea are native to North America.
Also known as the purple coneflower, they frequent open wooded areas & prairies.
Antiviral activity
Echinacea purpurea was shown to broadly inhibit coronaviruses and SARS-CoV-2 in vitro. In 2021 Nicolussi et al used PCR testing to compare the rates of infection and viral loads in adults and children administered 2,400mg and 1,200mg respectively of Echinaforce extract tincture over 4 months vs the control with excellent results: viral loads in nasal secretions were significantly reduced by 98.5%, with Ct-values 31.1 [95% CI 26.3; 35.9] versus 25.0 [95% CI 20.5; 29.5] (p = 0.0479).1
Abstract
Echinacea purpurea was shown to broadly inhibit coronaviruses and SARS-CoV-2 in vitro. This review discusses the available clinical evidence from randomized, blinded and controlled human studies. Two RCTs with results on enveloped viruses, respectively coronavirus infections during prevention treatment were detected. Incidence and/or viral loads were measured by RT-PCR and symptom severity was recorded. Jawad et al. (2012) collected nasopharyngeal swabs from adults (N=755) over 4 months of continuous prevention. Overall, 24 and 47 enveloped virus infections occurred, including 21 and 33 coronavirus detections [229E; HKU1; OC43] with Echinaforce® extract [2’400mg daily] and placebo, respectively (p=0.0114). Ogal et al. (2021) administered the same extract [1’200mg] or control for 4 months to children (4 – 12 years) (N=203). Echinacea reduced the incidence of enveloped virus infections from 47 to 29 (p=0.0038) whereas 11 and 13 coronavirus detections [229E, OC43, NL63] were counted (p>0.05). Respiratory symptoms during coronavirus infections were significantly lower with area-under-curve AUC=75.8 (+/-50.24) versus 27.1 (+/-21.27) score points (p=0.0036). Importantly, viral loads in nasal secretions were significantly reduced by 98.5%, with Ct-values 31.1 [95% CI 26.3; 35.9] versus 25.0 [95% CI 20.5; 29.5] (p = 0.0479). Results from clinical studies confirm the antiviral activity found for Echinacea in vitro, embracing enveloped respiratory pathogens and therefore coronaviruses as well. Substantiating results from a new completed study seems to extrapolate these effects to the prevention of SARS-CoV-2 infection. As hypothesized, the testified broad antiviral activity of Echinacea extract appears to be inclusive for SARS-CoV-2.
4. Discussion
Echinacea owns a long tradition for the prevention and the acute treatment of respiratory tract infections and recent investigations attributed antiviral, immune-modulatory and anti-inflammatory pharmacological actions to the medicinal plant [20,26,36–38]. A wide series of respiratory viruses were shown to be sensitive to lipophilic extracts of freshly harvested Echinacea purpurea and an obvious specificity towards enveloped pathogens could be verified [39]. The extract appears to block interaction of viral docking receptors (e.g. hemagglutinin on influenza) with structures on the target cell and is thus expected to prevent infection, although the detailed mechanism of action still has to be elucidated [37].
In addition, a meanwhile completed additional clinical study was identified on clinicaltrials.gov (ID: NCT05002179), investigating an Echinacea purpurea preparation from fresh herbs in dosages of 2,400 mg – 4,000 mg/d extract per day over 5 months in comparison with non-treatment. The same preparation as in Ogal et al. was used but at the recommended posology for adults. The study was carried out in N=120 adults from November 2020 until May 2021 and routinely collected naso- / oropharyngeal / blood samples for detection of respiratory virus infections, including SARS-CoV-2. Results suggest a signifi-cantly reduced risk for SARS-CoV-2 infections, measured either as symptomatic Covid-19 illness, RT-PCR positive sample or by seroconversion. Summarized over all phases of prevention, 21 and 29 samples tested positive for any virus in the Echinacea and control group, of which 5 and 14 samples tested SARS-CoV-2 positive (RR=0.37, Chi square test, p=0.03). Overall, 10 and 14 symptomatic episodes occurred, of which 5 and 8 were COVID- 19 (RR=0.70, Chi-square test, p>0.05). EF treatment when applied during acute episodes significantly reduced the overall virus load by at least 2.12 log10 or approx. 99% (t-test, p<0.05), the time to virus clearance by 8.0 days for all viruses (Wilcoxon test, p=0.02) and by 4.8 days for SARS-CoV-2 (p>0.05) in comparison to control. Finally, Echinacea treatment significantly reduced fever days (1 day vs. 11 days, Chi square test, p=0.003) [40]. Findings still await publication in peer-reviewed journal and need to be treated with appropriate caution. They were nevertheless mentioned in the current review for the sake of completeness and because they seem to confirm applicability of antiviral benefits of Echinacea to a broad range of coronaviruses, including SARS-CoV-2. Despite limitations associated with this review (e.g. low number of studies) we believe that our findings are highly relevant as they provide a rather consistent picture on antiviral and preventive benefits of Echinacea in coronavirus infections overall. Also, they provide important implications for the preventive use of Echinacea during Covid-19 epidemic. The reduction of coronavirus loads is medicinally highly relevant in whereas virus concentrations appear to correlate with community transmission, influence illness severity and progression in adults and children [41–44]. Two clinical studies have shown over 98.5% reduction of coronavirus concentration in nasal secretions obtained from children and adults treated with Echinacea. Evidence is still missing that vaccines significantly reduce viral loads, especially of SARS-CoV-2 delta variant and this asset would be a decisive argument for use of Echinacea during Covid-19 pandemic. Notably, all cited studies applied the same Echinacea purpurea extract (Echinaforce®) either as diluted tincture or as tablets, both of which were kept in mouth for a while prior to swallowing. As already hypothesized by Signer et al., pharyngeal administration of the extract may be key to inactivate respiratory viruses at the main entry site prior to infection [19]. Further could any viral attenuation in this region prevent further dissemination of nasal infections to the lungs, as seen with severe progressions of Covid-19. Indeed, a recent meta-analysis found a significant prevention of pneumonia secondary to viral respiratory tract infections upon 2 to 4 months Echinacea prevention [45]
5. Conclusions
Echinacea purpurea (L.) Moench exhibits direct antiviral activity against a broad range of respiratory pathogens, including coronaviruses. Extracts support the tonic production of interferon and modulate inflammatory cytokines (TNF-a). Nowadays, clinical evidence exists to show how enveloped viruses are effectively prevented in adults and children and this review extrapolates efficacy to coronaviruses as well. Yet unpublished clinical results on SARS-CoV-2 and in vitro experiments provide a good rational for general preventive effects against coronavirus infections.
Funding: This research received no external funding.
Conflicts of Interest: MO, AW, GG and PK declare no conflict of interest. SN and RS are consultants to A.Vogel AG, Switzerland
For preventing and treating the common cold this meta-analysis from 2014 by Karsch-Volk et al2 concluded there were either statistically insignificant or minor benefits:
Abstract
Background
Echinacea plant preparations (family Asteraceae) are widely used in Europe and North America for common colds. Most consumers and physicians are not aware that products available under the term Echinacea differ appreciably in their composition, mainly due to the use of variable plant material, extraction methods and the addition of other components.
Objectives
To assess whether there is evidence that Echinacea preparations are effective and safe compared to placebo in the prevention and treatment of the common cold.
Search methods
We searched CENTRAL 2013, Issue 5, MEDLINE (1946 to May week 5, 2013), EMBASE (1991 to June 2013), CINAHL (1981 to June 2013), AMED (1985 to February 2012), LILACS (1981 to June 2013), Web of Science (1955 to June 2013), CAMBASE (no time limits), the Centre for Complementary Medicine Research (1988 to September 2007), WHO ICTRP and clinicaltrials.gov (last searched 5 June 2013), screened references and asked experts in the field about published and unpublished studies.
Selection criteria
Randomized controlled trials (RCTs) comparing mono-preparations of Echinacea with placebo.
Data collection and analysis
At least two review authors independently assessed eligibility and trial quality and extracted data. The primary efficacy outcome was the number of individuals with at least one cold in prevention trials and the duration of colds in treatment trials. For all included trials the primary safety and acceptability outcome was the number of participants dropping out due to adverse events. We assessed trial quality using the Cochrane ’Risk of bias’ tool.
Main results
Twenty-four double-blind trials with 4631 participants including a total of 33 comparisons of Echinacea preparations and placebo met the inclusion criteria. A variety of different Echinacea preparations based on different species and parts of plant were used. Evidence from seven trials was available for preparations based on the aerial parts of Echinacea purpurea.
Ten trials were considered to have a low risk of bias, six to have an unclear risk of bias and eight to have a high risk of bias. Ten trials with 13 comparisons investigated prevention and 15 trials with 20 comparisons investigated treatment of colds (one trial addressed both prevention and treatment).
Due to the strong clinical heterogeneity of the studies we refrained from pooling for the main analysis. None of the 12 prevention comparisons reporting the number of patients with at least one cold episode found a statistically significant difference. However a post hoc pooling of their results, suggests a relative risk reduction of 10% to 20%. Of the seven treatment trials reporting data on the duration of colds, only one showed a significant effect of Echinacea over placebo. The number of patients dropping out or reporting adverse effects did not differ significantly between treatment and control groups in prevention and treatment trials. However, in prevention trials there was a trend towards a larger number of patients dropping out due to adverse events in the treatment groups.
Authors’ conclusions
Echinacea products have not here been shown to provide benefits for treating colds, although, it is possible there is a weak benefit from some Echinacea products: the results of individual prophylaxis trials consistently show positive (if non-significant) trends, although potential effects are of questionable clinical relevance.
AUTHORS’ CONCLUSIONS
Implications for practice
The most important recommendation for consumers and clinicians is to be aware that the available Echinacea products differ greatly. The overwhelming majority of these products have not been tested in clinical trials. It has been shown that labeling of products marketed in health food stores can be incorrect (Gilroy 2003). Our exploratory meta-analyses suggest that at least some Echinacea preparations may reduce the relative risk of catching a cold by 10% to 20%. A risk reduction of 15% would mean that if 500 out of 1000 persons receiving a placebo would catch a cold this figure would be 425 of 1000 persons with an Echinacea product. This is clearly a small effect of unclear clinical relevance. Furthermore, we cannot say which Echinacea products have an effect of this size, or a greater or lesser effect. While there are some hints that both alcoholic extracts and pressed juices that are based primarily on the aerial parts of E. purpurea have beneficial effects on cold symptoms in adults, the evidence for clinically relevant treatment effects is weak. There are still many remaining doubts due to the fact that not all trials using such preparations show even a trend towards an effect.
If those with comorbidities are excluded this could negatively skew the results as these are the very individuals that tend to have suppressed immunity and would likely have demonstrated the most benefits - if you are already healthy its hard to boost your immunity any further to demonstrate efficacy.
Exclusion of what the authors consider “biased studies” could also, ironically, bias the results of their own study. Their conclusions appear to be out of step with the bulk of research in this review.
Another aspect is that taking when you are symptomatic of a cold, unless taken at the first signs eg sore throat, the viral load may be too high by then to treat adequately, just as we see with coronavirus and ivermectin or hydroxychloroquine.
Interesting comment about allergic rash reactions in children:
As randomized controlled trials include limited numbers of participants and often exclude persons with relevant comorbidity, a review of such trials can only contribute limited knowledge on safety issues. The number of patients dropping out or reporting adverse effects did not differ significantly between treatment and control groups in prevention and treatment trials. However, in prevention trials there was a trend towards a larger number of patients dropping out due to side effects or reporting side effects in the treatment groups. The most relevant potential adverse effects of Echinacea preparations are probably allergic reactions (Huntley 2005; Mullins 2002). One trial suggested an absolute 5% increase in rash in children (Taylor 2003). Parenteral application of Echinacea preparation should be discouraged, as there is no evidence of either safety or effectiveness.
Implications for research
In principle, further research is clearly desirable given the widespread use of Echinacea products. However, given the multiplicity and diversity of products on the market applying the knowledge gained from such studies will remain a challenge to persons without in-depth knowledge of herbal preparations. The use of chemically well-defined preparations is recommended to improve comparability of results from different studies. It would be desirable if experts in research on common colds could develop recommendations for a core set of outcome measures to be used and reported in randomized clinical trials. Trials investigating the prevention of colds need large sample sizes as the potential effects of Echinacea products are likely to be small.
There was some negative feedback about the review. And I would add that a relative risk reduction of up to 20%, an NNT of 5 would be viewed as an extremely favourable result with most allopathic medicinals, and this is without considering synergism with other therapeutics or effects of taking it as a prophylactic:
FEEDBACK
Duration of Echinacea dosage
Summary
I wish to comment on the Cochrane review ’Echinacea for preventing and treating the common cold’. It is claimed in the Hot Topic of the Month (Relief from coughs and colds), August 2001, p. 5, para. 6.1, that “the German drug regulatory authority recommends that it be used for no longer than eight weeks at a time”. I have asked the Consumer Network about the evidence for this and been told that it is not available. Nevertheless, I think that if it is indeed a recommendation of the German drug regulatory authority, it should be mentioned in both the review and the abstract.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
We appreciate this comment. It is correct that the German drug regulatory authority recommends that Echinacea preparations should not be taken for longer than eight weeks at a time. While there is no evidence that longer intake can be harmful, such a precaution seems justified in the absence of data on long-term use. We included a statement on this issue in the review conclusion section ’Implications for practice’.
Klaus Linde
Contributors
David Potter
Comment posted 02/06/2005
In 2011, Hudson & Vimalanathan posted their meta analysis of reviews Echinacea—A Source of Potent Antivirals for Respiratory Virus Infections.3 They found that all strains of human and avian influenza viruses tested, including a Tamiflu resistant strain as well as herpes simplex, RSV and rhinoviruses (a common cold virus) were very sensitive to E purpurea preparations. Their results suggested intracellular inhibition and significant viricidal activity, including by inhibition of replication, although effects were sometimes weak at non-cytotoxic concentrations when tested as isolates:
Abstract
Extracts of Echinacea species have been used traditionally in North America for the control of symptoms of colds, influenza, and other diseases, and some of them have become very popular as “herbal medicines”. Recent studies have revealed that preparations derived from certain species and plant parts, but not all of them, possess potent antiviral activities, at non-cytotoxic concentrations, particularly against membrane-containing viruses. Thus all strains of human and avian influenza viruses tested (including a Tamiflu-resistant strain), as well as herpes simplex virus, respiratory syncytial virus, and rhinoviruses, were very sensitive to a standardized Echinacea purpurea preparation. In mechanistic studies the influenza virus-specific hemagglutinin and neuraminidase were inhibited. In addition some extracts displayed anti-inflammatory activity in virus-infected cells, and numerous other effects on the expression of cellular genes. Multiple components, either discrete compounds or mixtures, appeared to be responsible for the various antiviral activities.
Keywords: antiviral, Echinacea, phytomedicine, respiratory viruses, respiratory infection, anti-inflammatory
However, a problem with commercial Echinacea extracts in general (and in common with many other herbal products) is their inadequate characterization and standardization. Consequently different commercial sources, derived from different species and plant parts, and with resulting distinctive chemical compositions, may show different combinations of bio-activities, or in some cases relatively little bioactivity [17]. The result of this is that research studies, and especially clinical studies, have yielded inconsistent results. It is also important to ensure that any antiviral activity detected in a herbal preparation is really significant, that is to say, the extract should be able to inactivate a substantial amount of virus at a practical non-cytotoxic dosage, and therefore the assay techniques should reflect this requirement.
Early reports of antiviral activity of Echinacea [18] indicated that several different methanol and aqueous extracts derived from E. purpurea could partially protect cultured cells from infection by influenza A virus, herpes simplex virus type 1, or vesicular stomatitis, viruses. This suggested an intracellular inhibition, although the possibility of a virucidal activity was not reported.
Later studies supported the concept of Echinacea species as a potential source of antiviral activities. Cheminat et al. [19] isolated and characterized a group of caffeoyl derivatives from dried and fresh E. pallida plants, and examined two of them, cichoric acid and echinacoside, as well as caffeic acid, a constituent of E. purpurea, for activity against the replication of vesicular stomatitis virus (a membrane containing RNA virus) in a mouse cell line. Activity was relatively weak however, except at high and cytotoxic concentrations, and the possibility of virucidal activity was not tested.
Binns et al. [20] examined extracts from a variety of different species and plant parts for antiviral activity against herpes simplex virus. Assays were designed to test virucidal activity or viral growth inhibition, and they also incorporated exposure to light in case photosensitizers were involved (these are often found as bioactive constituents of medicinal plants, ref. 21). The results are summarized in Table 3. Many of the extracts showed significant but relatively weak activity, although the hexane root extract of E. purpurea and the ethanol inflorescence extract of E. sanguinea were more substantial. Pure cichoric acid was also moderately active, and could therefore contribute to the activity of certain extracts.
Vimalanathan et al. (Table 3, ref. [22]) evaluated different solvent fractions of E. purpurea aerial parts for activity against several viruses, in the presence and absence of light during the reactions. Aqueous extracts were active against herpes simplex virus and influenza virus, but these activities were not dependent on light exposure. In contrast the ethyl acetate fraction of the ethanol extract contained impressive activity against both viruses and which was due to a photosentizer. No activity against rhinovirus was detected. A polysaccharide-enriched fraction was also tested and found to contain only a relatively weak activity. Data are summarized in the form of MICs (minimum inhibitory concentrations) in Table 4.
Similar studies were carried out to compare root extracts from E. purpurea, E. angustifolia and E. pallida (23). The aqueous fraction of E. purpurea roots, which was almost devoid of caffeic acid derivatives and alkylamides, showed impressive activity against HSV and influenza virus (Table 4). The E. angustifolia root extract contained moderate activity against all three viruses (HSV, influenza, and rhinovirus) in the ethyl acetate fraction, but no activity in the aqueous extracts. In contrast, E. pallida root extracts were devoid of antiviral activity.
Several different compounds appear to contribute to antiviral efficacy, both with aqueous and alcohol based extracts:
However, in a more recent study, a series of aqueous and ethanol extracts of E. pallida aerial parts showed significant virucidal activity against HSV-1 and HSV-2 [24], and some of the extracts also appeared to inhibit virus replication within infected cells. The different extracts had distinct chemical profiles, as expected, but the authors concluded that combinations of components, rather than individual compounds, were responsible for these different activities. In recent tests we found that an ethanol extract of E. angustifolia aerial parts was as active against HSV as a corresponding E. purpurea extract (unpublished observations), thus indicating that there may be similar antiviral compounds in aerial part extracts of all three common Echinacea species (E. purpurea, E. angustifolia, and E. pallida). It is also clear that several different compounds contribute to the overall antiviral activity of a given extract, although many other extracts are devoid of activity.
They then press the need for standard extracts and report on the popular preparation sold as “Echinaforce”. Alcohol based extracts had greater antiviral activity:
4. The Need for Standard Extracts
The presence of multiple antiviral activities among different extracts and fractions suggests that many kinds of Echinacea preparation, such as tinctures, sprays, tablets, teas, etc. could be beneficial in the treatment of colds and flu, although not all preparations are likely to be effective. In fact a recent study on 10 commercial preparations highlighted the variability of antiviral activity between different preparations, although lot-to-lot variation was less evident [17]. In general ethanol based extracts had greater antiviral activity than aqueous extracts; but it was not possible to identify a specific component responsible for the activity. Furthermore there was no correlation between antiviral activity and anti-inflammatory activity.
Recent detailed studies with the standardized preparation Echinaforce® (comprising ethanol extracts of E. purpurea, 95% aerial parts plus 5% roots; abbreviated EF) showed that this preparation was very active as a virucidal agent against viruses with membranes, as indicated in Table 2. In addition to HSV-1 and respiratory syncytial virus, all tested human and avian strains of influenza A virus, as well as influenza B virus, were susceptible [25,26]. In addition rhinovirus was also equally susceptible at the relatively high concentrations of Echinaforce® recommended for oral consumption (1:10 dilution, equivalent to 1.6 mg/mL dry weight/volume). Under these conditions more than 105 infectious viruses could be killed within 5 min.
Again, the earlier you take it the greater the efficacy:
In contrast Echinaforce® was found to be less effective against intracellular virus [25]. Consequently virus already present within a cell could be refractory to the inhibitory effect of Echinaforce®, but virus particles shed into the extracellular fluids would be vulnerable [25,26]. Therefore the actions of the Echinaforce® would be manifest during initial contact with the virus, i.e. at the inception of infection, and also during transmission of virus from infected cells.
Unlike with Tamiflu, repeatedly taking it does not lead to resistance:
Additional experiments showed that continuous passage of influenza A virus in cell cultures in the presence of Echinaforce® did not result in the emergence of resistant strains, whereas passage of the virus through successive cultures in the presence of Tamiflu rapidly generated Tamiflu-resistance. Furthermore Tamiflu-resistant virus remained fully susceptible to Echinaforce® [26]. Therefore continuous usage of Echinaforce® in the population would be less likely to generate resistant strains of virus than Tamiflu or other anti-influenza compounds currently in the market. Recent studies have illustrated the relative ease with which resistant strains of influenza virus can arise [6,27]. Furthermore Echinaforce® could also be useful as an accessory treatment for individuals undergoing anti-influenza therapy with agents such as Tamiflu.
Not just replication, but binding and viral entry can be inhibited:
It was shown by hemagglutination assays that this extract (EF) inhibited the receptor binding activity of influenza A viruses, over a range of EF concentrations including that recommended for oral consumption, suggesting that EF interfered with viral entry into the cells, thus effectively rendering the virus non-infectious [26]. EF also inhibited neuraminidase activity in vitro (unpublished results), suggesting that the active compounds could block influenza virus entry and spread by acting on at least two virion targets. However, the susceptibility of other viruses, which do not rely on HA or NA functions, to Echinaforce® indicates that additional molecular targets must be accessible.
Echinaforce also inhibits mucin secretion, one of the most discomforting symptoms and the cause of coughs, sore throat or even bronchitis:
5. Mucin Secretion
Most sufferers of colds and other respiratory problems would agree that secretion of excessive mucus is one of the more annoying symptoms, and accordingly many pharmaceuticals have been designed to relieve this feature of a cold or flu, usually with the accompaniment of undesirable side effects. Rhinoviruses induced the secretion of excess MUC5A, the dominant respiratory mucin, in bronchial epithelial cells in culture, and in cultured airway tissues, and Echinaforce® reversed this secretion in both systems [28], suggesting that this could be an additional benefit of Echinacea treatment. This result was supported by histochemical examination of cultured airway tissues, which revealed the conspicuous presence of muco-polysaccharide-filled goblet cells resulting from rhinovirus infection, whereas EF treated and infected tissues appeared normal.
Inhibition of the viral stimulated secretion of pro-inflammatory cytokines. Of note here is that Echinaforce tincture was beneficial even with late stage infections - viral load may or may not be reduced but symptoms can still be suppressed:
6. Effects on Virus-Infected Cells
A series of studies by Sharma et al. focused on the application of E. purpurea extracts (including Echinaforce®) to epithelial cells and tissues infected by viruses [25,29-31]. In rhinovirus infected human bronchial and lung epithelial cell lines the virus could stimulate the secretion of more than 30 different cytokines, including the pro-inflammatory IL-1, IL-6, IL-8, and TNFα, which are known to be collectively involved in many of the symptoms common to colds and flu, such as sneezing, fever, sore throat, nasal discharges and inflammation in various respiratory tissues. Certain Echinacea preparations were able to completely or partly reverse this stimulation [25,29-31]. In studies with Echinaforce®, it was shown that EF could be added before or after virus infection, with similar success, and also the results were not affected by virus dose or the time of exposure to EF [29].
Other viruses, including HSV-1, influenza A virus, adenovirus type 3 and 11, and respiratory syncytial virus, stimulated the secretion of pro-inflammatory cytokines, and in each case the stimulation was reversed by EF (Table 5, and ref. [25]). However only live infectious viruses were able to do this, for infection by equivalent doses of ultraviolet-inactivated viruses failed to elicit the responses. This suggests that the virus may have to enter the cells and undergo some degree of gene expression in order to stimulate the cytokine expression or secretion. It is also interesting that viruses such as adenoviruses, which are not vulnerable to direct attack by Echinacea, but could nevertheless stimulate cytokine secretion, were still susceptible to cytokine reversal.
All extracts, whether aqueous or ethanol, from roots, leaves or stems, were responsible for the observed antiviral and anti-inflammatory effects:
In an attempt to correlate immune modulatory effects with specific classes of Echinacea components, various chemically characterized extracts and fractions, derived from three common species of Echinacea, were evaluated for their possible inhibitory effects on the secretion of pro-inflammatory cytokines IL-6 and IL-8 (CXCL-8) by human bronchial epithelial cells infected with rhinovirus type 14. All of the E. purpurea fractions, comprising aqueous or ethanol extracts of roots, leaves and stems, but to a lesser degree flowers, strongly inhibited the secretion of both cytokines [32]. These results suggest that different compounds, or combinations, were responsible for antiviral and anti-inflammatory effects.
Echinacea appears to turn down and help normalise genes related to cytokine expression that the virus has upregulated:
Randolph et al. [33] described changes in levels of expression (in terms of mRNAs and proteins) of several cytokine genes in human blood samples taken at different times after treatment with a commercial blended Echinacea product, and Brovelli et al. [34] found that the expression of several cytokine genes in cultured human monocytes was influenced by the nature of the Echinacea preparation (stage of development and plant part used), presumably a reflection of their different chemical compositions. Altamirano-Dimas et al. [35] analyzed gene expression in human bronchial cells by means of DNA microarrays, following treatment by one of two E. purpurea preparations, a polysaccharide rich aqueous extract and an alkylamide-rich ethanol extract, with or without infection by rhinovirus type 14. Both extracts influenced the expression of many genes, including cytokine genes, although the pattern of expression was different for the two extracts. In addition the virus induced numerous changes, mostly increases in expression, and the extracts tended to decrease (i.e. restore to normal levels) these expression levels.
Effects were multifold and complex, including with dendritic cells. These are antigen seeking cells that are phagocytes and activate T lymphocytes. They have a role as anti-cancer agents too.
Wang et al. [37] described the effects of a butanol fraction, derived from aerial parts of E. purpurea, on gene expression of immune-related molecules in human dendritic cells, which are part of the adaptive immune response, in contrast to the studies described above, which focused on the innate immune response. Many dendritic cell genes were affected, either up regulated or down-regulated. These studies did not include infected cells, but clearly showed the multiple gene effects of this Echinacea preparation.
Benson et al. [38] studied the effects of extracts of E. purpurea, an aqueous extract of roots and an ethanol extract of aerial parts, on selected immune-related proteins in murine dendritic cells, and found a variety of significant responses, reinforcing the concept of multiple consequences of Echinacea exposure of immunologically important cells.
The authors concluded that the antiviral effects of E purpurea aerial parts could be due to compounds including polyphenols, and anti-inflammatory effects due partly to alkylamides or other constituents.
Positive conclusions regarding both viral loads and amelioration of symptoms:
9. Conclusions
Studies on Echinacea extracts have shown that some of them, but not all, possess multiple beneficial actions in the treatment of viral respiratory infections: (1) a direct virucidal activity against several respiratory viruses; (2) reversal of the pro-inflammatory response of epithelial cells and tissues to different viruses; (3) reduction in the excessive secretion of mucin by airway cells and tissues; (4) lack of cytotoxic effects or disruption of tissue integrity by Echinacea in airway cell cultures or tissues, at practical antiviral concentrations; (5) additional potentially positive effects on cellular gene expression. A combination of these beneficial activities could reduce the amount of prevailing viable virus, and their transmission, and also lead to amelioration of the virus-induced symptoms.
Conflict of Interest
The authors declare no conflict of interest.
Fusco et al (2010) found that Echinacea-treated mice had lower systemic and pulmonary KC (human IL-8) and IL-10 levels and lower systemic IFN-γ levels following influenza infection. They suggested that E. purpurea alters the clinical course of influenza infection in mice through modulation of cytokines and not necessarily due to direct antiviral activity:4
Abstract
Influenza infection is a major clinical problem and Echinacea purpurea, a widely consumed botanical product, is purported to alter the course of respiratory infections including influenza. Mice infected with WSN influenza A and treated with E. purpurea polysaccharide extract had less weight loss than untreated mice but similar pulmonary viral titers. Echinacea-treated mice had lower systemic and pulmonary KC and IL-10 levels and lower systemic IFN-γ levels following influenza infection. These suggest that E. purpurea alters the clinical course of influenza infection in mice through modulation of cytokines and not direct antiviral activity.
Keywords: Influenza, Echinacea, Cytokines, Mice
3.2. Echinacea treatment did not alter lung viral titers
To assess the impact of echinacea treatment on viral replication, we measured viral titers in lung homogenates of treated and untreated mice on days 2, 3, 4, 5, 6 and 7 post-infection. Fig. 2 shows mean viral titers for the different treatment groups at each time-point examined, from a representative experiment. Compared to untreated mice, echinacea-treated mice had similar viral titers on all days (p > 0.3 for all days).
Although aerial parts of the plant did not show antiviral activity, they found that root extracts did in fact demonstrate this:
In summary, our results indicate that E. purpurea aerial (non-root) polysaccharide extract does not have evident antiviral effect but modifies influenza-related (1) clinical course and (2) immune response, as measured by cytokines, with decreased pulmonary and systemic KC (human IL-8) early in influenza infection then decreased pulmonary and systemic IL-10 and systemic IFN-γ later in the course of infection. It is interesting to note that, in vitro, root-containing extract from the same echinacea species and a similar preparation method did have antiviral activity, reinforcing the likely unique characteristics of distinct plant parts [9]. It is possible that some form of the extract described in this study could be clinically useful for (1) influenza treatment or (2) prophylaxis against post-viral bacterial pneumonia, which is possibly mediated by elevated IL-10 following influenza [23]. However, the observed KC inhibitory effects could worsen post-influenza pneumonia, so further studies will be required to evaluate the effect of echinacea in this setting. A limitation of our study is that we evaluated a single dosing regimen of echinacea. Further studies are needed to establish the clinically relevant dose in humans. As clinical challenges related to influenza continue to escalate, further, systematic evaluation of this compound may be timely.
No conflict of interest statement was made.
A further study by Vimananathan et al in 2017 indicated that virus-induced bacterial adhesion and cytokine storms associated with respiratory infections could be inhibited by E purpurea5:
Abstract
Viral infections may predispose the airways to secondary bacterial infections that can lead to unfavorable progression of principally self-limiting illnesses. Such complicated respiratory infections include pneumonia, bronchitis, sinusitis, acute otitis media, and sepsis, which cause high morbidity and lethality. Some of the pathogenic consequences of viral infections, like the expression of bacterial adhesion receptors and the disturbance of physical barrier integrity due to inflammation, may create permissive conditions for co-infections. Influenza virus A (H3N2) is a major pathogen that causes secondary bacterial infections and inflammation that lead to pneumonia. The herbal medicine Echinacea purpurea, on the other hand, has been widely used to prevent and treat viral respiratory infections, and recent clinical data suggest that it may prevent secondary infection complications as well. We investigated the role of standardized E. purpurea (Echinaforce® extract or EF) on H3N2-induced adhesion of live nontypeable Haemophilus influenzae (NTHi) and Staphylococcus aureus, along with the expression of bacterial receptors, intracellular adhesion molecule-1 (ICAM-1), fibronectin, and platelet activating factor receptor (PAFr), by BEAS-2B cells. Inflammatory processes were investigated by determining the cellular expression of IL-6 and IL-8 and the involvement of Toll-like receptor (TLR-4) and NFκB p65. We found that influenza virus A infection increased the adhesion of H. influenzae and S. aureus to bronchial epithelial cells via upregulated expression of the ICAM-1 receptor and, to some extent, of fibronectin and PAFr. Echinaforce (EF) significantly reduced the expression of ICAM-1, fibronectin, and PAFr and consequently the adhesion of both bacterial strains. EF also effectively prevented the super-expression of inflammatory cytokines by suppressing the expression of NFκB and possibly TLR-4. These results indicate that E. purpurea has the potential to reduce the risk of respiratory complications by preventing virus-induced bacterial adhesion and through the inhibition of inflammation super-stimulation (cytokine storms).
Keywords: Echinacea, Hemophilus influenzae; Influenza virus; Pneumonia, ICAM-1; Respiratory complications; Staphylococcus aureus; Superinfection.
A 2009 murine study into the reduction of latent herpes simplex virus type-1 (HSV-1) reinfections6. This is of particular interest due to transfection induced suppression of antiviral immunity. Herpes reactivation is a frequently reported adverse event, sometimes involving facial paralysis if the virus is reactivated adjacent to a nerve axon or leading to recurrence of ocular herpetic infection (eye infection):
“Ocular herpetic infection may be activated by COVID-19 (BNT162b2) mRNA vaccine. Treating physician should be alert to such associations, and patients should be followed closely.”7
Abstract
Objective: During the latency period of herpes simplex virus type-1 (HSV-1), the virus can occasionally reactivate, travel back to the eye and cause recurrent ocular disease. As this condition arises from the ability of HSV-1 to produce a dormant infection, effective medication to prevent the virus enter a latent state should prevent it. In this study, we applied Echinacea polysaccharide (EP) fraction as prophylactic mediator for latency prevention. Methods: In order to investigate the protective properties of EP, we evaluated its immunostimulatory functions on different immune aspects that play important roles in latency prevention (particularly IFN-γ as one of the main indicators of cellular immunity and latency). Finally, we assessed establishment of latency by detection of thymidine kinase gene in trigeminal ganglia of BALB/c mice. Results: We demonstrated that EP promotes immune response, leading to a reduced latency rate, and it has a promising effect on latency prevention. Conclusion: EP was able to exert an antiviral action on the development of recurrent HSV-1 disease when supplied prior to infection.
Echinacea purpurea extract has been used in prophylaxis and therapy of various viral infections, mainly respiratory tract infections in animals and humans [7] . Echinacea is perhaps best known for its reputed immunostimulating properties [8] . In vitro studies have shown that Echinacea acts directly on a number of cell types, including natural killer cells [9] , polymorph nuclear leukocytes [10] and macrophages [11] . Pharmacological investigations have shown immunomodulatory activities of cichoric acid, alkamides and polysaccharides from E. purpurea [12] . It appears that the immunostimulating effects of Echinacea result from polysaccharides surrounding tissue cells which provide protection from pathogenic invasions [12] . E. purpurea polysaccharides (EPs) possess strong macrophage-activating properties. Although they also have some effect on B cell proliferation, their main targets seem to be macrophages [13]
“Delayed-type hypersensitivity (DTH) is a unique type of cell-mediated immunity. The name originated from the skin test used in the diagnosis of tuberculosis and denotes cellular infiltrates causing induration and erythema at the skin test site within 24 to 72 hours. DTH was coined to describe the reaction to the tuberculosis skin test and to differentiate between delayed cellular skin test results and antibody-mediated immediate skin test results.”8
KOS (a nonvirulent HSV-1 strain) is the positive control, and PBS the negative control for comparison. Higher is better. This footpad test demonstrates a level of immunity to the latent virus:
Stimulation of antiviral gamma interferon by E purpurea. Higher is better:
Discussion
HSV infection is widespread. It is estimated that 70– 90% of people over the age of 18 have antibodies to HSV-1 and/or HSV-2 and carry the latent virus, with approximately 25% showing clinical symptoms [3] . During the life of a latently infected individual, the virus can occasionally reactivate, travel back to the eye and cause recurrent disease. A major cause of corneal scarring is that induced by HSV-1 after reactivation from latency [3, 4] . In order to break the vicious circle of the latency, recurrence and renewed latency of the virus, it is essential to induce a protective immune response for clearance of the virus itself, since little or even no latency can then reoccur in the case of primary or recurrent infection. The most important solution is therefore an immunostimulatory substance, whereby the virus is killed.
Mouse survival:
Our findings implied that the treatment with EP as an immunostimulator, before lethal challenge, enhances the efficient immune responses leading to reduced viral multiplication in the eye. Subsequently, EP significantly affected and diminished the establishment of a latent infection. As described before, we also confirmed in vivo the anti-herpetic effect of EP and showed that it significantly reduced the incidence and the severity of ocular disease with respect to untreated infected mice [17] . To our knowledge, in this study for the first time we report that EP does induce T cell proliferation. This has been attributed to macrophage activation to stimulate IFN-y production in association with the secondary activation of T lymphocytes. The great production of IFN-y by spleen cells from EP-treated mice suggested that injection of EP would induce an appropriate Th1 immune response [22] . DTH and splenocyte proliferation results also confirmed immunity. On the other hand, it has been shown that IFN-y is one of the major factors in latency prevention [20, 25, 26] . Although there is ongoing controversy in the literature as to whether the immune-stimulating properties of Echinacea are attributable to its polysaccharide component, or whether it is the alkylamides that are responsible, this study once again verified the immunostimulatory effects of the polysaccharide component.
A synergism between polysaccharides and efficacy demonstrated with no significant difference to the positive control (=good), but it should be taken as a prophylactic if you are at risk:
Numbers of active polysaccharides have been isolated from E. purpurea. Frequently, these include a 45-kDa polysaccharide fraction isolated from cultivated plants as well as 2 neutral fucogalactoxyloglucans (10 and 25 kDa) and a 75-kDa acid arabinogalactan from large-scale plant cell culture. It has been suggested that they synergize to mediate a protective effect [27] . When injected into mice, arabinogalactan was found to activate macrophages and was associated with an increased production of TNF-a , IL-1 and interferons [24] . Therefore, macrophage responses and consequent IFN-y induction to these polysaccharides can reduce latency rates. In comparison to the KOS group (as a positive control group), EP induced IFN-y with insignificant difference. Because of complete latency protection of KOS mice, it seems that EP has high prospective ability for improvement of its efficiency against latency in further studies. In conclusion, EP proved to exert an antiviral action on the development of recurrent HSV-1 disease when supplied prior to infection.
No conflict of interest statement was provided.
Suppression of proinflammatory cytokines and enhancement of innate and adaptive immune functions
In 2007, the paper by Pillai et al further supports polysaccharides as being potent immunostimulants.9 Water soluble, but not fat-like extracts from all parts of the plant all produced substantial immunostimulatory activity.
Flow cytometric analysis enables the researcher and clinician to enumerate lymphocyte subsets in peripheral blood mononuclear cells (PBMC).10
Abstract
Introduction: When directly exposed to various echinacea fractions, human leukocytes ex vivo are strongly stimulated to proliferate and to produce immunostimulation and inflammatory cytokines. A comparison of fractions containing lipoidal small molecules and high-molecular-weight water-soluble polysaccharides indicates that the latter are substantially more potent as immunostimulants. Echinacea purpurea (L.) Moench, E. angustifolia DC, and E. pallida (Nutt.), Nutt. extracts, and each plant part contain significantly potent constituents. Flow cytometric techniques were utilized.
Objectives: This study was undertaken to determine whether flow cytometry could measure immunostimulant activity present in echinacea and, if so, which species produced more activity, which plant part was the most active, and whether the organic soluble or the aqueous extractables were more active. Ex vivo human clinical material was employed.
Design: Echinacea extracts were analyzed using flow cytometric techniques. The immunostimulation assays were measured in triplicate.
Methods: Samples dissolved in dimethyl sulfoxide (DMSO) were added to 200 microL of heparinized blood mixed with 50 muL of phosphate buffer, vortexed, and incubated to allow adequate time for immune-cell stimulation. Fifty (50) microL of the stimulated blood samples were added to each of a reagent cocktail consisting of 20 microL of CD4FITC/CD69PE/CD3PerCP expressed on the helper/inducer T-lymphocyte subset; CD8FITC/CD69/PE/ CD3PerCP expressed on the human suppresser/cytotoxic T-lymphocytes and on a subset of natural killer lymphocytes; CD19FITC/CD69PE/CD45PerCP expressed on B-lymphocytes; or CD56FITC/CD69PE/CD45PerCP expressed on NK lymphocytes. Four hundred and fifty (450) microL of 1 X FACS lysing solution was added and incubated in the dark (rt, 30 minutes) and then subjected to flow cytometric analysis. All reported readings are the average of several determinations. Positive controls consisted of phorbol myristyl acetate (PMA) (50 ng/mL), phytohemagglutinin (10 microg/mL), CD2/CD2R (positive activation control)(5 microL/250 muL of reaction), and negative controls consisted of dimethyl sulfoxide (2% in RPMI-1640), RPMI-1640 medium, and cyclosporin A (10 microg/mL).
Results: The main immunostimulatory activity of echinacea resides in the water-soluble materials rather than the lipoidal small molecules. E. purpurea, E. Pallida, and E. angustifolia leaves, stems, flowering tops, and roots all produce substantial immunostimulatory activity.
Conclusions: The use of flow cytometry demonstrates a link between the polysaccharides in echinacea and the biologic immunostimulatory effect that has therapeutic relevance, and strong evidence for this immunostimulant property is presented.
NFkappaB, also known as NF-kB or Nuclear factor kappa-light-chain-enhancer of activated B cells is a protein complex that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.11
Upregulation of NF-kB is therefore a marker for infection. In 2008, Matthias et al identified 2 different alkyamides in Echinacea root extract, one of which stimulated the immune response, the other modulated it:12 This is important to note when you are making up herbal preparations:
Abstract
The effects of Echinacea and several of its phytochemical components on NFkappaB expression by Jurkat cells (a human T-cell line) were investigated in vitro. In the absence of stimulation, Echinacea and its components exerted no significant effect on basal NFkappaB expression levels. In the presence of endotoxin (LPS), NFkappaB expression was decreased. However, this decrease was significantly reversed by treatment with cichoric acid, an Echinacea root extract (prepared from both Echinacea angustifolia and Echinacea purpurea) and the alkylamide fraction derived from this combination. For the phorbol myristate acetate stimulation of Jurkat cells, effects on NFkappaB expression were mixed. Depending on the concentration, cichoric acid and a 2,4-diene alkylamide significantly induced NFkappaB levels, whereas a 2-ene alkylamide caused a significant inhibition. In contrast, both the Echinacea and the mixed alkylamide fraction exerted no effect. The alkylamide results indicate that the two basic forms of these compounds present in Echinacea may have opposing effects. These opposing effects demonstrate the importance of a knowledge, not only of the phytochemical make-up of a herbal preparation, but also of the actions of each component and the consequences of differing relative amounts in the preparation being investigated.
Prophylaxis appears important for reducing viral levels, but here we have another study concluding that symptoms may be reduced at any stage of infection: “under real life conditions of Echinacea consumption, the virus-induced stimulation of pro-inflammatory cytokines can be effectively reversed or alleviated.”
Sharma, Schoop & Hudson (2009) also used standardised Echinacea preparation (Echinaforce)13. Suppression of interleukin-6 is valuable as elevated levels are correlated with tumorigenesis14 and interleukin-8 is correlated with atherosclerosis and cardiovascular disease.15 This is particularly relevant to sufferers of post vaccination sequalae (PASC) due to the persistence of S1 spike protein in monocytes.16
Abstract
Numerous Echinacea preparations are available on the market for the prevention and treatment of cold and 'flu symptoms and inflammatory conditions associated with infections. Most of these preparations are consumed orally in the form of aqueous or ethanol extracts and tinctures. Since the recommended consumption normally involves a brief local exposure to the diluted preparation at an unspecified time in relation to the actual infection, then it is important that experimental models for the evaluation of Echinacea reflect these limitations. A line of human bronchial epithelial cells, in which rhinoviruses stimulate the production of pro-inflammatory cytokines, was used to evaluate several relevant parameters. The chemically characterized Echinacea preparation (Echinaforce) was capable of inhibiting completely the rhinovirus induced secretion of IL-6 (interleukin-6) and IL-8 (chemokine CXCL-8) in these cells, regardless of whether the Echinacea was added before or after virus infection, and in response to a range of virus doses. This inhibitory effect was also manifest under conditions resembling normal consumption with respect to the duration of exposure to Echinacea and the Echinacea dilution. It is concluded that under real life conditions of Echinacea consumption, the virus-induced stimulation of pro-inflammatory cytokines can be effectively reversed or alleviated.
Echinacea source.
The test material was Echinaforce®(A. Vogel Bioforce AG, Switzerland), a 65% ethanol extract of freshly harvested aerial parts of Echinacea purpurea supplemented with 5% E. purpurea roots. This preparation was essentially free of polysaccharides, and contained the following caffeic acids and alkylamides (caffeic acid 0, caftaric acid 264.4μg/mL, chlorogenic acid 40.2μg/mL, cichoric acid 313.8μg/mL, cynarin 0,echinacoside 6.9μg/mL, PID 8/9 36.3μg/mL). The composition was determined (courtesy J. T. Arnason, University of Ottawa) by HPLC as described in Binns et al. (2002)
RV + E is of note here, IL-6 and IL-8 were both inhibited to control levels by Echinacea:
Figure 1 also shows the dramatic inhibitory effect of Echinacea on induced IL-6 and -8, which were often reduced to control levels. Similar results were obtained for any of the time points chosen, 24 –96 h (not shown).However, there was no significant effect of Echinacea on the levels of these cytokines in control uninfected cells (Fig. 1).These results were unaffected by the passage number of the BEAS-2B cells; passage 8 and passage 54 cells showed similar responses to RV infection and to Echinacea inhibition (not shown). In addition rhinovirus type 1A(RV 1A), which uses a different cellular receptor from RV 14 (LDL instead of ICAM-1), showed results similar to RV 14.Furthermore similar results were obtained when the BEAS-2B cells were replaced by A549 human lung epithelial cells, or by human skin fibroblast cells (data not shown).
As per other studies, beneficial suppression of both cytokines were observed regardless of what stage of infection it was first administered at. This could be particularly useful for COVID-19 sufferers who missed the early symptoms at low viral load before getting treatment (berberine has similar properties17)
A dose effect can be observed, but preparations can still be efficacious even when diluted considerably:
Echinacea dose effect
A common question about Echinacea consumption is: how much is appropriate for its success in counteracting cold symptoms? To determine if the inhibitory capacity of Echinacea was dose dependent, as might be expected, the anticytokine effect of different doses was examined, using the same experimental conditions described above. The previous tests (Fig. 1) utilized a 1:100 dilution of Echinacea, equivalent to a final concentration of 160μg/mL. However inhibition was still observed with dilutions up to1:400, and occasionally at 1:800, but there was clearly a dose response effect, as shown in Fig. 3. This suggests that the preparation could be diluted considerably and should still be active. None of the Echinacea doses used in these studies appeared to have adverse effects on the cells.
High viral load didn’t stop Echinacea bringing cytokine levels down to those of the control:
Effect of virus concentration (multiplicity of infection, MOI)
Another variable that could affect the outcome of a cold is the amount of virus acquired in the infection.
One would reasonably expect greater amounts of virus to produce more intense symptoms. To test this increasing concentrations of virus (RV14) were used, from 0.01 to 1.0 pfu/cell, which resulted in successively greater amounts of cytokine induction, for both IL-6 and IL-8 (Fig. 4), although even at a multiplicity of infection of 1.0 infectious virus per 100 cells there was still a substantial induction in cytokine secretion after 48 h. Nevertheless, in all cases Echinacea was able to reverse these responses and bring the cytokine levels down to the control levels previously indicated in Fig. 2. These data are shown for A-549 cells: but similar results were obtained for BEAS-2B cells.
As per the abstract, this study aimed to consider the effects of relatively brief exposure to therapeutic doses.
Just a 5 minute exposure to 1:10 Echinacea significantly reduced IL-6 levels:
Duration of exposure to Echinacea
In order to mimic the natural consumption of Echinacea, experiments were carried out in which Echinacea (at the usual 1:100 dilution) was added to the RV-infected cells for various times, and then the Echinacea washed off the cells. Cytokine secretion was then measured 24 h later. A short exposure of only 5 min did not significantly affect the RV-induction of cytokines; however, with increasing exposure to Echinacea, the more effective was the cytokine inhibition (Fig. 5). This suggests that under normal conditions of oral Echinacea consumption, inhibition of RV-induced cytokine secretion could be substantial, even with a high virus input. Although the results presented in Fig. 5 indicate that a brief 5 min exposure to 1:100 Echinacea was not very effective, nevertheless the results in Table 1 show that a 5 min exposure to 1:10 Echinacea (a more realistic dose in practice) gave a significant reduction in IL-6.
No conflict of interest statement was given.
This study from 2007 by Zhai et al concluded that all three species promoted T-cell proliferation, anti-viral interferons and innate & adaptive immune responses, but E. angustifolia or E. pallida may have more anti-inflammatory potential:18
Gavaged mice: fed directly into their stomachs.
A vehicle control is used in studies in which a substance (e.g., saline or mineral oil) is used as a vehicle for a solution of the experimental compound. In a vehicle control, the supposedly innocuous substance is used alone, administered in the same manner in which it will be used with the experimental compound.19
Abstract
Echinacea preparations are commonly used as nonspecific immunomodulatory agents. Alcohol extracts from three widely used Echinacea species, Echinacea angustifolia, Echinacea pallida, and Echinacea purpurea, were investigated for immunomodulating properties. The three Echinacea species demonstrated a broad difference in concentrations of individual lipophilic amides and hydrophilic caffeic acid derivatives. Mice were gavaged once a day (for 7 days) with one of the Echinacea extracts (130 mg/kg) or vehicle and immunized with sheep red blood cells (sRBC) 4 days prior to collection of immune cells for multiple immunological assays. The three herb extracts induced similar, but differential, changes in the percentage of immune cell populations and their biological functions, including increased percentages of CD49+ and CD19+ lymphocytes in spleen and natural killer cell cytotoxicity. Antibody response to sRBC was significantly increased equally by extracts of all three Echinacea species. Concanavalin A-stimulated splenocytes from E. angustifolia- and E. pallida-treated mice demonstrated significantly higher T cell proliferation. In addition, the Echinacea treatment significantly altered the cytokine production by mitogen-stimulated splenic cells. The three herbal extracts significantly increased interferon-alpha production, but inhibited the release of tumor necrosis factor-gamma and interleukin (IL)-1beta. Only E. angustifolia- and E. pallida-treated mice demonstrated significantly higher production of IL-4 and increased IL-10 production. Taken together, these findings demonstrated that Echinacea is a wide-spectrum immunomodulator that modulates both innate and adaptive immune responses. In particular, E. angustifolia or E. pallida may have more anti-inflammatory potential.
No single compound is responsible for the immunological activities:
Isolation and characterization of bioactive phytochemicals in Echinacea extracts used in studies are essential. A few widely accepted active compounds of Echinacea include lipophilic alkamides and polar caffeic acid derivatives (cichoric acid and echinacoside).23,24 The in-depth investigation of these active compounds seems to be helpful for the better understanding of the biological nature of Echinacea. However, a purified phytochemical does not mimic the immunological effects of whole plant extracts. It appears that the immunopharmacological activities of Echinacea depend on a combination of several active compounds,25 but not any single individual constituent. In addition, extracts of Echinacea are commercially available and economical, but commercially available purified compounds are expensive and not approved for human consumption. Thus, studies using Echinacea extracts are still of great value. Animal experiments have shown that a combination of Echinacea extracts demonstrated greater effects than extracts of single plants (reviewed by Bodinet et al.17), but using a mixture of plants makes it difficult to distinguish the biological significance of each plant or identify redundancy in effects.
Our preliminary studies using oral administration of Echinacea found that the dry powder or alcohol extract of E. purpurea root could stimulate splenic T-cell proliferation and NK cell cytotoxicity.
Echinacea administration
This study consisted of two independent experiments with an identical study design. After acclimation, the mice were randomly assigned to five groups. Groups 1–3 were gavaged with one of the three Echinacea preparations. Group 4 was gavaged with an equal volume of 5% ethanol as vehicle control. Group 5 served as a no gavage control (no treatment group). The vehicle control and the no gavage control were established to control for the effects of vehicle as well as handling stress. The Echinacea preparations were orally administered to the animals at 130 mg/kg of body weight once daily for 7 consecutive days using an animal feeding needle. This dosage and regimen were chosen based on an extrapolation of the dose recommended for humans (4 g of powder/day for an average 65-kg human × 1 week).
“A large body of evidence indicates the existence of functionally polarized CD4+ T-cell responses based on their profile of cytokine secretion. Type 1 T helper (Th1) cells produce interferon-gamma, interleukin (IL)-2, and tumour necrosis factor (TNF)-beta, which activate macrophages and are responsible for cell-mediated immunity and phagocyte-dependent protective responses. By contrast, type 2 Th (Th2) cells produce IL-4, IL-5, IL-10, and IL-13, which are responsible for strong antibody production, eosinophil activation, and inhibition of several macrophage functions, thus providing phagocyte-independent protective responses.”20
IFN-a inhibits viral replication, TNF-a and IL-1B are pro-inflammatory and pro-tumor:
TH1-cell activation will in turn activate macrophages that protect against intracellular pathogens. The effects of Echinacea on phagocytosis and cytokine production by macrophages have been extensively investigated in vitro and in vivo, but the results were rather inconsistent (see reviews by Barrett6 and Percival7). Macrophages are important as a first line of defense against infections. Upon activation, they may secrete many pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-12, and IL-6. However, inflammatory processes subsequently need to be down-regulated to allow healing. These divergent and, at times, seemingly contradictory effects reflect the dichotomy of macrophages as both pro- and anti-inflammatory effectors in response to host environmental changes. Recently one study25 reported opposing effects of Echinacea on cytokine gene expression when used in vitro versus oral administration in vivo. The in vitro data found a short-term (6-h) exposure of human monocytic cell line THP-1 cells to E. purpurea stimulates the expression of inflammation-related genes, such as IL-1β, TNF-α, IL-8, intracellular adhesion molecule-1, and cyclooxygenase-2. However, oral administration of Echinacea in vivo induced a reduction in the expression of inflammation-related genes, but increased the IFN-α expression in healthy individuals.25 The effects of Echinacea on cytokine production vary considerably, depending on the experimental conditions used (e.g., animal model, cell culture model, and the treatment scheme). Our animal model in this study demonstrated Echinacea exerted a strong inhibition on the TNF-α and IL-1β production by macrophages in the presence of LPS, suggesting that Echinacea has anti-inflammatory activity, as demonstrated previously.3,4 The down-regulation of these two important inflammatory mediators might be associated with increased production of IL-4. IL-4 supports the differentiation of CD4+ into TH2-type cells and simultaneously suppresses the development of TH1-type cells. On macrophages, IL-4 acts in an anti-inflammatory manner to inhibit the production of pro-inflammatory cytokines, i.e., IL-1β and TNF-α.
The researchers were unable to identify which other phytochemicals gave E. angustifolia and E. pallida their strong immunomodulatory effects compared to E. purpurea:
Undoubtedly, the observed differential effects of these three Echinacea species on certain immune parameters are associated with their variation in phytochemical composition. Among the phytochemicals, amides, echinacoside, and cichoric acid are thought of as the main active compounds responsible for the immunomodulatory action of alcohol extracts of Echinacea.24,37,38 E. purpurea has been reported to have a mix of constituents different from the other two species.24,38 Chromatographic analysis of our preparations showed that E. purpurea lacks echinacoside, but contains cichoric acid. Most, though not all, amides were present in the three Echinacea preparations and made up a major part of all identifiable phytochemicals, especially for E. angustifolia and E. purpurea preparations. Among the three Echinacea species, E. purpurea is believed to have the strongest potency on the immune system.39 It is unexpected that E. purpurea displayed a weaker potential to stimulate TH2- and TH1-type cytokine production than E. angustifolia and E. pallida, especially since the HPLC results demonstrated that the E. purpurea extract contained high levels of amides and cichoric acid, with the latter proven to have stronger immunostimulatory effects than echinacoside.38 So in this study, other phytochemicals, but not amides and cichoric acid, in the E. angustifolia and E. pallida preparations may be responsible for the strong immunomodulatory effects on T lymphocytes.
In conclusion, the present orogastric administration studies with three different Echinacea species have proven them to be effective immunomodulators. We found that three different species of Echinacea exhibit multiple modulating effects on immune function. They stimulate not only nonspecific, innate immune response, but also specific, adaptive immune function, suggesting that Echinacea possesses an immunomodulating potential for the overall immune system. The effects of Echinacea were more robust in immune responses that were suppressed by the daily handling in the vehicle control group as compared to the no gavage group. To our knowledge, this is the first study that demonstrates the relevance of Echinacea’s immune-enhancing effects in conjunction with a mild stress.
Research was publicly funded:
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences (grant P01ESO12020) and the Office of Dietary Supplements, National Institutes of Health. This work was performed as part of the Center for Research on Dietary Botanical Supplements at Iowa State University and the University of Iowa. The authors are grateful to Dr. Chalermpol J. Lekcharoensuk for his technical assistance and statistical analyses and Dr. Diane Birt for good suggestions during review of the manuscript.
Antioxidant, Antidiabetic, and Antihypertensive Properties
A 2017 study by Chiou et al21. The full paper is paywalled which prevents a full review, but the anti-oxidant, glucose regulating and non-allopathic ACE-inhibitory effects are encouraging for those with type 2 diabetes or hypertension, ie for cardiovascular health in general.
They do recommend further research to develop the active ingredients as drugs. This might be challenging due to losing synergistic benefits:
Abstract
The extraction yield, total phenols, caffeic acid derivatives (CAD), and antioxidant properties of 50% ethanolic Echinacea purpurea flower extract were determined. The in vitro inhibitory effects of 50% ethanolic extract and CAD on α-amylase, α-glucosidase, and angiotensin-converting enzyme (ACE) linked with type 2 diabetes were also investigated. The extraction yield, total phenols, and total CAD of the extract were 27.04%, 195.69 mg CAE/g and 78.42 mg/g, respectively. Cichoric acid (56.03 mg/g) was the predominant CAD compound in the extract. The extract exhibited good antioxidant properties. The extract and CAD inhibited α-amylase, α-glucosidase, and ACE activities in a concentration-dependent manner. Among the tested samples, chlorogenic acid, and caffeic acid (IC50 of 1.71-1.81 mg/mL) had the highest α-amylase inhibitory activity, cichoric acid (IC50 of 0.28 mg/mL) showed higher α-glucosidase inhibitory activity. Both chlorogenic acid and caffeic acid (IC50 of 0.11-0.14 mg/mL) demonstrated higher ACE-inhibitory activity. The in vitro results suggest that E. purpurea extract and CAD have good potential for managing hyperglycemia and hypertension. Overall, the data suggest it is a choice for developing antihyperglycemia and antihypertension compounds from field-grown E. purpurea.
Keywords: Echinacea purpurea; angiotensin-converting enzyme; antioxidant activity; caffeic acid derivatives; α-Amylase; α-glucosidase.
Anticancer properties
An interesting study by Tsai et al (2012)22 found that “cichoric acid has a strong growth-inhibitory effect against colon cancer cells, presumably resulting from the reduced telomerase activity and the induction of apoptosis.”
The researchers suggested further research be conducted into this:
Abstract
Ethnopharmacological relevance: Echinacea is a top-selling herbal supplement that acts as immunostimulant. It has been used to treat common cold, coughs, bronchitis and upper respiratory infections. It is also a popular product used in anticancer therapy. The cytotoxic effects of Echinacea on cancer cells are still not clear. The aims of this study were to provide a preliminary validation of the effects of 50% aqueous ethanol extract of Echinacea purpurea flowers and its major compound, cichoric acid, on human colon cancer cells Caco-2 and HCT-116.
Materials and methods: The cytotoxic effects of Echinacea flower extracts and cichoric acid on cell viability, telomerase activity, DNA fragmentation, β-catenin, caspase-9, and cleavage of poly-ADP-ribose polymerase (PARP) of human colon cancer cell were examined.
Results: The results showed a significant inhibition of proliferation in E. purpurea flower extract and cichoric acid in a dose- and time-dependent manner. Cichoric acid treatment decreased telomerase activity in HCT-116 cells. Moreover, cichoric acid effectively induced apoptosis in colon cancer cells, which were characterized by DNA fragmentation, activation of caspase-9, cleavage of PARP and downregulation of β-catenin.
Conclusions: Our data indicate that cichoric acid has a strong growth-inhibitory effect against colon cancer cells, presumably resulting from the reduced telomerase activity and the induction of apoptosis. The exact mechanism of action should still be determined in future studies. Overall, the effects of 50% aqueous ethanol extract of E. purpurea flowers and cichoric acid may have provided in vitro evidence for the use as chemotherapeutic agents.
Note the reference to inhibiting HIV and pancreatic cell cancer lines, which are notoriously difficult to treat. We will consider contraindications next.
The immuno-stimulating properties of Echinacea products are attributed to the bioactive phytochemicals including caffeic acid derivatives, alkylamides, and polysaccharides. Among these phytochemicals, caffeic acid derivatives, especially cichoric acid, possess many bioactive functions including anti-hyaluronidase activity, protection of collagen from free radical induced degradation, antiviral activity, inhibition of human immunodeficiency virus type 1 integrase and replication, promoting phagocyte activity in vitro and in vivo and a high free radical scavenging property (Bauer and Wagner, 1991, Pellati et al., 2004, Barnes et al., 2005). Thus, cichoric acid is commonly used as marker to determine the medicinal quality of Echinacea products (Thygesen et al., 2007).
Despite its well-known immune enhancing role, the effects of Echinacea on cancer cells are still elusive and paradoxical. It has been shown that Echinacea root extracts have cytotoxic effects on pancreatic cancer cell line in a concentration- and time-dependent manner (Chicca et al., 2007). In contrast, other previous studies showed that Echinacea root extracts may compromise doxorubicin chemotherapy by promoting cell proliferation in breast and cervical cancer cells (Huntimer et al., 2006). Interestingly, there is a report indicating that daily consumption of Echinacea is prophylactic and significantly alleviates leukemia in mouse model (Miller, 2005).
There was no significant difference in cell viability when Caco-2 and HCT-116 cells were treated with various concentrations of 50% aqueous ethanol extract (0–2000 μg/ml) for 24 h (Fig. 1A). However, when treated with 50% aqueous ethanol extract for 48 h, the viability of both cells was significantly reduced in a dose-dependent manner revealed by MTT assay (Fig. 1A). It has been shown that -hexane root extracts from three Echinacea species have potential anticancer activity (Chicca et al., 2007). Thus, our findings further demonstrate that the 50% aqueous ethanol extracts of E. purpurea flower has an anti-proliferative effect on human colon cancer cell lines.
We previously showed that cichoric acid was the most abundant compound in the 50% aqueous ethanol extract of freeze-dried E. purpurea flowers (Tsai et al., 2012). Therefore, the effects of cichoric acid on human colon cancer cell growth were also examined. As illustrated in Fig. 1(B), after incubation with cichoric acid for 24 h, cell viability was significantly decreased when the concentration of cichoric acid was increased to 150 or 200 μg/ml level. However, after incubation for 48 h, all treatments of cichoric acid (50–200 μg/ml) showed significant reduction in the viability of Caco-2 and HCT-116 cells.
Decrease the activity of telomerase and the cancer cell loses its immortality.
Short term treatment with natural products can suffice:
3.2. Cichoric acid decreases the activity of telomerase
Telomerase was reported to be strongly up-regulated in most human cancers, implying its critical function for cell immortalization and turmorigenesis (Kim et al., 1994). To support the anti-proliferation effect of cichoric acid, we examined whether telomerase activity was reduced upon cichoric acid treatment. Our data showed decreased telomerase activity in cichoric acid-treated HCT-116 cells (50–150 μg/ml) revealed by telomeric repeat amplification assay (Fig. 2). Several studies have shown that short-term treatment with natural products is sufficient to repress telomerase activity, leading to induction of apoptosis in cancer cells (Hsu and Lin, 2005, Wang et al., 2009). Additionally, depletion of hTERT, the rate-limiting component of human telomerase, promotes the induction of apoptotic cell death through the mitochondrial pathway (Massard et al., 2006). Together, the molecular mechanism for cichoric acid-induced cytotoxicity and apoptosis in colon cancer cells may involve repression of telomerase activity.
3.3. Cichoric acid induces DNA fragmentation and expression of caspase-9, β-catenin and PARP
To test whether the cytotoxic effect caused by cichoric acid was due to induction of apoptosis, we first checked DNA fragmentation with cichoric acid treatment. As showed in Fig. 3(A) and (B), DNA fragmentation was induced by cichoric acid in a concentration- and time-dependent manner.

Caspase activation is generally considered to be a key process during apoptosis and the PARP is also an important biomarker of apoptosis in many cell types. Caspase-9 has been linked to mitochondrial-dependent apoptosis (Budihardjo et al., 1999). Indeed, cichoric acid was found to activate caspase-9 in a dose-dependent fashion, as evidenced by the increased cleaved caspase-9. On the other hand, cichoric acid at higher doses (100–150 μg/ml) induced PARP cleavage, suggesting the activation of caspase pathway and the ongoing apoptosis. Beta-catenin plays an essential role in the Wnt signalling pathway and has been shown to influence growth and apoptosis in several cancer cell types (Huang et al., 2007, Wang et al., 2010, Liyan et al., 2011). Consistent with these results, we found that the expression of β-catenin was significantly decreased when treated with the doses of cichoric acid that inhibited cell growth and induced apoptosis (100–150 μg/ml) (Fig. 3C).
4. Conclusion
The 50% aqueous ethanol flower extract and cichoric acid could induce significant cytotoxicity in human colon cancer cells. The inhibitory effect of cichoric acid on HCT-116 cell growth could be mediated through the repression of telomerase activity and β-catenin expression, thus leading to induction of apoptosis featuring activation of caspase-9 and cleavage of PARP (Fig. 4). These results have provided a possible mechanism for E. purpurea flower extract-induced in vitro cytotoxicity. However, it requires further investigations to understand the detailed mechanism behind cichoric acid–induced apoptosis in HCT-116 cells. Still, these results have provided mechanism-based strategies for the development of anticancer natural products.
This research was publicly funded.
Skin Health & Anti-Aging Properties
A human clinical trial by Yotsawimonwat et al (2010) with 10 patients showed an increase in overall skin hydration and a reduction in skin wrinkles by 10%-14% when using Echinacea as either a face cream or gel.23
Their study also showed that Echinacea is well tolerated, with none of the patients complaining of skin irritation. Therapeutic benefits were thought to be due to the antioxidant effects of phenolic compounds in the gels and creams they prepared.
They found that low storage stability was a problem due to oxidation of the creams and recommended using fresh product. Tinctures used as base ingredients do not suffer from oxidative degradation to the same extent.
Synopsis
Echinacea purpurea contains many beneficial constituents for protection of skin from oxidative stress and for improving hydration of skin. This study aimed to investigate the stability and dermatological efficacy of E. purpurea cream and gel. Echinacea purpurea extract was incorporated into suitable cream and gel bases. Stability of the extract in the formulations was investigated by determining its residual total phenolic content and antioxidant activity after storage at 4°C, 30°C and 40°C for 6 months. The effect of those formulations on skin irritation, hydration level and wrinkle reduction was evaluated in 10 healthy volunteers, aged 25–40 years. The shelf lives of E. purpurea cream and gel in terms of total phenolic content and antioxidant activity were only 2 and 4 months respectively at 4°C and could be extended up to 7 months by incorporation of α-tocopherol or disodium editate. The corneometer hydration indices increased up to 10.6 AU and 11.4 AU, and the wrinkles decreased 9.47% and 14.92% because of the application of E. purpurea cream and gel for 1 month. Both formulations showed no irritation to skin. Echinacea purpurea cream and gel developed in this study were effective in improving skin hydration and reducing wrinkle, but showed low storage stability.
Conclusions
The E. purpurea formulations developed in this study could effectively improve the hydration of the skin and decrease skin wrinkle without inducing any short-term and long-term irritation. However, the stability of the extract in the formulations was relatively low and needs further study to improve it by using other appropriate techniques such as encapsulation.
Acknowledgement
This study was financially supported by the Graduate School and Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
Contraindications
The possibility of being allergic to Echinacea is one of the most frequent reasons for caution.24 Three types of skin test can be conducted: the scratch test, the intradermal test or the patch test.25
The researchers in an Australian study from 2002 found that of 100 patients referred to them with asthma or allergic rhinitis, 20% of them also tested positive when exposed to Echinacea allergen. This sample was naturally skewed by the referral criteria and the severity of response varied:26
Echinacea, the popular herbal remedy used to strengthen the immune system and fight infection, is reportedly being used by some consumers to treat allergies, asthma and skin disorders. The herb, however, is not recommended for those uses and can have negative effects, a new study shows.
The Australian study was published in the January 2002 issue of the peer-reviewed journal Annals of Allergy, Asthma and Immunology, a publication of the American College of Allergy, Asthma and Immunology.
Rob McCaleb, founder and president, Herb Research Foundation, said he doesn't expect a lot of fallout from the study. "I don't think anyone is promoting echinacea for allergy use. Allergies are caused by hypersensitivity of the immune system, so something that stimulates the immune system is probaby not a good idea. It seems counterintuitive."
Although prior analysis suggests some herbal regimens for asthma and allergies are scientifically justified, their increased use raises concerns about potentially serious risks and the need for more research, wrote Leonard Bielory, M.D., of the New Jersey Medical School, Newark, in an editorial accompanying the study. "To protect the common good, there is a need to know not only what CAM can do for us, but also what it can do to us," he wrote.
Normally, individuals need direct exposure to a substance before experiencing an allergic reaction. But the Australian researchers advised that "given the popularity of echinacea, even rare adverse events become inevitable when a large proportion of the population uses it mostly unsupervised. Because these reactions may occur even with their first ever known exposure, atopic patients should be cautioned appropriately."
Atopic patients are hypersensitive, possibly from hereditary causes, to hayfever, asthma, eczema and other allergies of the nose, airways and skin, explains Marianne Frieri, M.D., director of allergy and immunology at Nassau University Medical Center, East Meadow, N.Y. They are more likely to develop cross-reactive allergic responses to compounds that are structurally or botanically related.
Hypersensitivity reactions have been triggered by royal jelly, pollen preparations, chamomile, birch pollen and other plant compounds.
Echinacea is a member of the Asteraceae (Compositae) family, other members include species of Ambrosia (ragweed), Artemisia (mugwort and sagebrush), as well as chrysanthemums, dahlias, daisies, marigolds and sunflowers.
"Anyone taking echinacea for allergies should be tested first for ragweed, mugwort and echinacea sensitivity," said Frieri. "They can have a life-threatening reaction."
"The plant family that echinacea belongs to is one of the largest and thus [echinacea] has many brothers and sisters that are cross-allergens," said Anthony Almada, president and chief scientific officer, Imaginutrition, based in Laguna Niguel, Calif. "This could sensitize people to echinacea when they are echinacea naive. However, systematic research needs to be undertaken to examine this association more closely."
The echinacea study was based on observed reactions in five patients referred to the researchers for evaluation, and 51 adverse reaction reports submitted to the Australian Adverse Drug Reactions Advisory Committee. They involved at least six different brands of echinacea in the form of tea, tablets and liquid.
The researchers included echinacea in the routine panel of allergens when testing 100 consecutive patients referred for evaluation of asthma or allergic rhinitis. Although only three had taken echinacea previously, 20 percent reacted to skin prick testing with a significant visible, physical flare and raised wheal.
In the five observed cases, patients (including a health professional) reacted within five minutes to two days after they first or subsequently took echinacea. They experienced acute asthma attacks, shock, skin rash lasting up to six weeks, burning throat, chest pains, hives, diarrhea, facial and airway swelling, dizziness and disorientation. One teen had an acute asthma attack 10 minutes after her first exposure to echinacea, taken as a tea.
The researchers interviewed health providers on 51 cases, who determined echinacea was the sole trigger in 41 of those cases. More than 50 percent of the patients had pre-existing asthma or allergy symptoms affecting their eyes, noses or skin.
"This study is provocative and puzzling," Almada said. "The adverse event reports in the United States are far less abundant and do not mirror those described in this [Australian] paper."
"It is important to point out that this study includes only a few cases and this is an herb used by millions of people," McCaleb said.
A systematic review published in 2012 by Huntley et al concluded Echinacea products have a good safety profile when taken in the short term:27
Abstract
Echinacea spp. are native to North America and were traditionally used by the Indian tribes for a variety of ailments, including mouth sores, colds and snakebites. The three most commonly used Echinacea spp. are E. angustifolia, E. pallida and E. purpurea. Systematic literature searches were conducted in six electronic databases and the reference lists of all of the papers located were checked for further relevant publications. Information was also sought from the spontaneous reporting programmes of the WHO and national drug safety bodies. Twenty-three manufacturers of echinacea were contacted and asked for data held on file. Finally our own departmental files were searched. No language restrictions were imposed. Combination products and homeopathic preparations were excluded.
Data from clinical studies and spontaneous reporting programmes suggest that adverse events with echinacea are not commonly reported. Gastrointestinal upsets and rashes occur most frequently. However, in rare cases, echinacea can be associated with allergic reactions that may be severe. Although there is a large amount of data that investigates the efficacy of echinacea, safety issues and the monitoring of adverse events have not been focused on.
Short-term use of echinacea is associated with a relatively good safety profile, with a slight risk of transient, reversible, adverse events. The association of echinacea with allergic reactions is supported by the present evaluation. While these reactions are likely to be rare, patients with allergy or asthma should carefully consider their use of echinacea. The use of echinacea products during pregnancy and lactation would appear to be ill-advised in light of the paucity of data in this area.
Interaction with chemotherapeutic drugs
In 2019, Fasinu and Rapp published the results of this investigation into 6 herbal products: echinacea, garlic, ginseng, grapefruit juice, milk thistle and St John's wort.28
Abstract
One of the most consequential risks associated with the concomitant use of herbal products and chemotherapeutic agents is herb-drug interactions. The risk is higher in patients with chronic conditions taking multiple medications. Herb-drug interaction is particularly undesirable in cancer management because of the precipitous dose-effect relationship and toxicity of chemotherapeutic agents. The most common mechanism of herb-drug interaction is the herbal-mediated inhibition and/or induction of drug-metabolizing enzymes (DME) and/or transport proteins leading to the alteration in the pharmacokinetic disposition of the victim drug. Most mechanistic research has focused on laboratory-based studies, determining the effects of herbal products on DMEs and extrapolating findings to predict clinical relevance; however, not all DME/transporter protein inhibition/induction results in clinical herb-drug interaction. This study reviews relevant literature and identified six herbal products namely echinacea, garlic, ginseng, grapefruit juice, milk thistle, and St John's wort, which have shown interactions with chemotherapeutic agents in humans. This focus on clinically significant herb-drug interaction, should be of interest to the public including practitioners, researchers, and consumers of cancer chemotherapy.
Keywords: cancer, chemotherapy, complementary and alternative medicine, drug interaction, herb-drug interaction, pharmacokinetics
Echinacea
Formulations of echinacea are globally popular for complementary treatment of respiratory infections and common cold. Among cancer patients, echinacea is popular as an immunomodulatory supplement (35, 36). Recent studies in animals have suggested that echinacea may have beneficial effect in abating some forms of cancer, like leukemia (37). The active constituents and the pharmacological mechanism of any beneficial effect is poorly understood. Echinacea is ranked one of the top widely sold herbal preparations in the United States (38). Most of the preparations of echinacea in the United States are made from one out of the nine common species—Echinacea purpurea. Several pre-clinical studies have suggested herb-drug interaction between echinacea and anti-cancer drugs. For example, extracts of echinacea induce P-gp and CYP3A4, two major enzyme/transporter combination that play major roles in the biotransformation and pharmacokinetics of anticancer drugs [Table 1; (39)]. Echinacea is also an inhibitor of CYP3A4 (40). This dual ability to inhibit and induce drug-metabolizing enzymes makes it difficult to predict clinically significant herb-drug interaction with the various CYP/P-gp drug substrates. In human studies, echinacea caused significant increase (34%) in the systemic clearance of midazolam, a CYP3A4 substrate (41). Therefore, there is a potential for herb-drug interaction between echinacea and anti-cancer drugs.
While preclinical studies have shown strong evidence of echinacea interacting with CYP and transport proteins, there is insufficient clinical data on herb-drug interaction with anti-cancer drugs. In a study in 10 cancer patients, echinacea did not cause significant alterations in the pharmacokinetics of docetaxel, which is a substrate of CYP3A4 and P-gp (14). The patients received an intravenous dose of docetaxel on day 1, and were then treated with echinacea supplementation (20 oral drops three times daily of a commercially available product) on days 7–21. They were then administered with another dose of docetaxel on day 22. No significant changes were observed in the pharmacokinetics of docetaxel with or without echinacea supplementation. However, with darunavir, an antiretroviral drug, echinacea caused a general decrease in concentration in the HIV/AIDS patient participants (42).
In a case report, echinacea caused a significant interaction in a cancer patient taking etoposide (13). The adult patient, who was newly diagnosed with squamous cell carcinoma of the lung received cisplatin and etoposide on the first day of treatment with a recorded normal bloodwork. However, by day 8 of this first cycle chemotherapy, his platelet count had dropped by over two-thirds, necessitating platelet transfusion. The discontinuation of echinacea in the cycle 2 chemotherapy helped the patient avoid any further need for platelet transfusion. No further incidence was reported until discharge after 20 days in the hospital. Patient was instructed to avoid taking any more herbal supplements. Etoposide, a cytotoxic agent, is a CYP substrate, whose dose-limiting toxicity is myelosuppression. This interaction is understood to have been as a result of echinacea-induced CYP inhibition, leading to etoposide accumulation and the resultant thrombocytopenia.
Conclusion
While the beneficial effects of the commonly consumed herbal products by cancer patients is uncertain, data from human studies suggest that some of these supplements are capable of interacting with chemotherapeutic agents. It is therefore prudent and advisable to avoid the concomitant use of anti-cancer drugs and herbal products, especially echinacea, garlic, ginseng, grapefruit juice, milk thistle, and St John's wort. Clinicians and practitioners need to be vigilant in monitoring for any herb-anticancer combination.
No conflict of interest was declared.
Bioavailability
This study from 2006 by Woelkart et al found no significant difference in TNF-alpha or IL-8 endpoints whether taken as tincture or as a tablet, and no significant changes in the concentration of IL-6 were observed:29
Abstract
Echinacea is a widely used herbal remedy for the prevention and treatment of the common cold. Recently, many new insights concerning the molecular mode of action of the main lipophilic constituents, the alkamides, have renewed interest in this plant. In order to compare the bioavailability of alkamides from liquid and tablet preparations of E. purpurea (Echinaforce) in humans and to study the effects on ex vivo stimulated blood cells, a randomized, single-dose, crossover study with 10 (8 test, 2 placebo) volunteers has been performed. They received either 4 ml of the standardized E. purpurea (Echinaforce) tincture or 12 E. purpurea (Echinaforce) tablets or placebo. Both doses contained the same amount (0.07 mg) of the major alkamides, dodeca-2E,4E,8Z, 10E/Z-tetraenoic acid isobutylamides. Liquid chromatography electrospray ionization ion-trap mass spectrometry was used to determine the content of alkamides in serum. It was found that the arithmetic mean C(max) of dodeca-2E,4E, 8Z,10E/Z-tetraenoic acid isobutylamides absorbed after oral application of the Echinaforce tincture appeared after 30 min (0.40 ng/ml serum). In comparison, the t(max) of tablets was 45 min with a C(max) of 0.12 ng/ml. An ex vivo stimulation of blood by LPS was carried out to measure the influence of E. purpurea on the innate and adaptive immune system. Both E. purpurea preparations led to the same effects on the immune system according to the concentration of pro-inflammatory cytokines TNF-alpha and IL-8. 23 hours after oral application a significant down-regulation of TNF-alpha and IL-8 in LPS pre-stimulated whole blood was found. However, no significant changes in the concentration of IL-6 were observed. Although a quarter of the dodeca-2E,4E,8Z, 10E/Z-tetraenoic acid isobutylamides was absorbed from the tablets, the study shows that the formulations trigger the same effects on the measured immune parameters.
Dosage recommendations
From healthline (2018):30
There is currently no official dosage recommendation for echinacea.
One reason being that findings from echinacea research are highly variable.
In addition, echinacea products often may not contain what is written on the label. One study found that 10% of echinacea products samples did not contain any echinacea (32Trusted Source).
This is why you should purchase echinacea products from trusted brands.
That said, research has found the following doses to be effective in aiding immunity (11Trusted Source):
Dry powdered extract: 300–500 mg of Echinacea purpurea, three times daily.
Liquid extract tinctures: 2.5 ml, three times daily, or up to 10 ml daily.
However, it’s best to follow the instructions that come with your specific supplement.
Keep in mind that these recommendations are for short-term use, as echinacea’s long-term effects on the body are still relatively unknown.
From the Botanical Institute:31
Echinacea Safety:
Safety Class: 1 (can be safely used when consumed properly)
Interaction Class: A (no clinically relevant reactions are expected)
Echinacea is generally considered as being safe when taken within the recommended dosages.
There are no drug contraindications. However, it is theorized that taking Echinacea may be harmful to individuals with autoimmune diseases. As with all herbs, it’s best to talk with one’s doctor prior to ingestion.
Pregnancy & Lactation:
There are no studies on the safety of Echinacea for women who are pregnant or nursing. While no concerns have been identified, safety has not been conclusively established.
Dosing:
Tincture (1:5): 1–4 mL up to three times per day.
Decoction: Add 1-2 teaspoons of root in 1 cup of water and bring slowly to a boil. Let simmer for 10-15 minutes, then steep for 1 hour. Drink three times a day.
Capsules (Dried Whole Root): 1 gram (roughly 4 capsules), three times daily.
Capsules (Extract 1:1): 500-1,000mg per day.
Conclusion
Research into the three Echinacea species E. purpurea, E. angustifolia and E. pallida has provided ample experimental evidence to support the claims of many health benefits, such as reduced inflammation, improved immunity to bacteria and many viruses, anti-cancer, anti-aging activity and lower blood sugar levels, to name just a few.
Bioavailability is excellent in all forms of preparation, but tincture should have the longest shelf life.
The safety profile is excellent for taking in the short term, although tests for allergic reactions are advised and patients on chemotherapy need to exercise caution due to the risk of unintended drug interactions.
Taking these into consideration I would therefore recommend adding Echinacea products to the list of therapeutics to take in response to various infections or conditions.
Disclaimer
This site is strictly an information website about potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References
Nicolussi S, Ardjomand-Woelkart K, Stange R, Gancitano G, Klein P, Ogal M. Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms. 2022 Jan 19;10(2):211. doi: 10.3390/microorganisms10020211. PMID: 35208665; PMCID: PMC8879308.
Karsch-Völk M, Barrett B, Kiefer D, Bauer R, Ardjomand-Woelkart K, Linde K. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev. 2014 Feb 20;2(2):CD000530. doi: 10.1002/14651858.CD000530.pub3. PMID: 24554461; PMCID: PMC4068831.
Hudson J, Vimalanathan S. Echinacea—A Source of Potent Antivirals for Respiratory Virus Infections. Pharmaceuticals (Basel). 2011 Jul 13;4(7):1019–31. doi: 10.3390/ph4071019. PMCID: PMC4058675.
Fusco D, Liu X, Savage C, Taur Y, Xiao W, Kennelly E, Yuan J, Cassileth B, Salvatore M, Papanicolaou GA. Echinacea purpurea aerial extract alters course of influenza infection in mice. Vaccine. 2010 May 21;28(23):3956-62. doi: 10.1016/j.vaccine.2010.03.047. Epub 2010 Apr 9. PMID: 20382242; PMCID: PMC3016056.
Vimalanathan S, Schoop R, Suter A, Hudson J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017 Apr 2;233:51-59. doi: 10.1016/j.virusres.2017.03.006. Epub 2017 Mar 7. PMID: 28279802.
Ghaemi A, Soleimanjahi H, Gill P, Arefian E, Soudi S, Hassan Z: Echinacea purpurea Polysaccharide Reduces the Latency Rate in Herpes Simplex Virus Type-1 Infections. Intervirology 2009;52:29-34. doi: 10.1159/000212988
Alkhalifah MI, Alsobki HE, Alwael HM, Al Fawaz AM, Al-Mezaine HS. Herpes Simplex Virus Keratitis Reactivation after SARS-CoV-2 BNT162b2 mRNA Vaccination: A Report of Two Cases. Ocul Immunol Inflamm. 2021 Aug 18;29(6):1238-1240. doi: 10.1080/09273948.2021.1986548. Epub 2021 Oct 12. PMID: 34637667.
Chapter 19 - Delayed-Type Hypersensitivity Reactions, Immunology for Pharmacy
2012, Pages 152-161.
https://www.sciencedirect.com/science/article/pii/B9780323069472100197
Pillai S, Pillai C, Mitscher LA, Cooper R. Use of quantitative flow cytometry to measure ex vivo immunostimulant activity of echinacea: the case for polysaccharides. J Altern Complement Med. 2007 Jul-Aug;13(6):625-34. doi: 10.1089/acm.2006.6361. PMID: 17718645.
Ashmore LM, Shopp GM, Edwards BS. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J Immunol Methods. 1989 Mar 31;118(2):209-15. doi: 10.1016/0022-1759(89)90008-2. PMID: 2466904.
NF-κB - Wikipedia
Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008 Jan;79(1):53-8. doi: 10.1016/j.fitote.2007.07.012. Epub 2007 Aug 11. PMID: 17855021.
Sharma M, Schoop R, Hudson JB. Echinacea as an antiinflammatory agent: the influence of physiologically relevant parameters. Phytother Res. 2009 Jun;23(6):863-7. doi: 10.1002/ptr.2714. PMID: 19107735.
Chonov DC, Ignatova MMK, Ananiev JR, Gulubova MV. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced J Med Sci. 2019 Jul 20;7(14):2391-2398. doi: 10.3889/oamjms.2019.589. PMID: 31592285; PMCID: PMC6765074.
Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009 Dec 1;84(3):353-60. doi: 10.1093/cvr/cvp241. Epub 2009 Jul 18. PMID: 19617600.
Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, Hall E, Herrera M, Parikh P, Guevara-Coto J, Triche TJ, Scott P, Hekmati S, Maglinte D, Chang X, Mora-Rodríguez RA, Mora J. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front Immunol. 2022 Jan 10;12:746021. doi: 10.3389/fimmu.2021.746021. PMID: 35082777; PMCID: PMC8784688.
Doorlesscarp, Therapeutic properties of Berberine - A literature review, (2022),
https://doorlesscarp953.substack.com/p/therapeutic-properties-of-berberine
Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, Kohut ML, Cunnick JE. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J Med Food. 2007 Sep;10(3):423-34. doi: 10.1089/jmf.2006.257. PMID: 17887935; PMCID: PMC2362099.
Paula D. Johnson, David G. Besselsen, Practical Aspects of Experimental Design in Animal Research, ILAR Journal, Volume 43, Issue 4, 2002, Pages 202–206, https://doi.org/10.1093/ilar.43.4.202
https://academic.oup.com/ilarjournal/article/43/4/202/981687
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999 Nov;5(4):285-94. doi: 10.1097/00054725-199911000-00009. PMID: 10579123.
Chiou SY, Sung JM, Huang PW, Lin SD. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea purpurea Flower Extract and Caffeic Acid Derivatives Using In Vitro Models. J Med Food. 2017 Feb;20(2):171-179. doi: 10.1089/jmf.2016.3790. Epub 2017 Jan 6. PMID: 28061036.
Tsai YL, Chiu CC, Yi-Fu Chen J, Chan KC, Lin SD. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J Ethnopharmacol. 2012 Oct 11;143(3):914-9. doi: 10.1016/j.jep.2012.08.032. Epub 2012 Sep 1. PMID: 22971663.
Yotsawimonwat, S., Rattanadechsakul, J., Rattanadechsakul, P. and Okonogi, S. (2010), Skin improvement and stability of Echinacea purpurea dermatological formulations. International Journal of Cosmetic Science, 32: 340-346. https://doi.org/10.1111/j.1468-2494.2009.00559.x
https://onlinelibrary.wiley.com/doi/10.1111/j.1468-2494.2009.00559.x
Echinacea Linked to Allergies, (2002).
https://www.webmd.com/allergies/news/20020122/echinacea-linked-to-allergies
If You Get Skin Testing for Allergies, 2020.
Wendy Bonifazi, Echinacea May Cause Allergic Reaction, (2008).
https://www.newhope.com/science/echinacea-may-cause-allergic-reaction
Huntley, A.L., Coon, J.T. & Ernst, E. The Safety of Herbal Medicinal Products Derived from Echinacea Species. Drug-Safety 28, 387–400 (2005). https://doi.org/10.2165/00002018-200528050-00003
https://link.springer.com/article/10.2165/00002018-200528050-00003
Fasinu PS, Rapp GK. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front Oncol. 2019 Dec 3;9:1356. doi: 10.3389/fonc.2019.01356. PMID: 31850232; PMCID: PMC6901834.
Woelkart K, Marth E, Suter A, Schoop R, Raggam RB, Koidl C, Kleinhappl B, Bauer R. Bioavailability and pharmacokinetics of Echinacea purpurea preparations and their interaction with the immune system. Int J Clin Pharmacol Ther. 2006 Sep;44(9):401-8. doi: 10.5414/cpp44401. PMID: 16995328.
Echinacea: Benefits, Uses, Side Effects and Dosage, (2018).
5 Echinacea Benefits: Dosage & Safety, (2021).








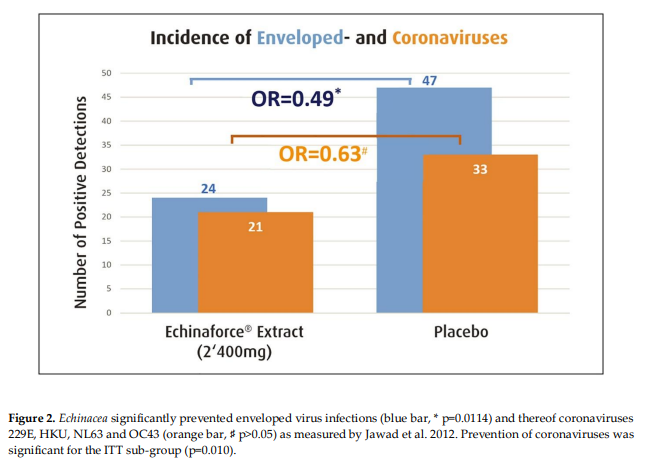





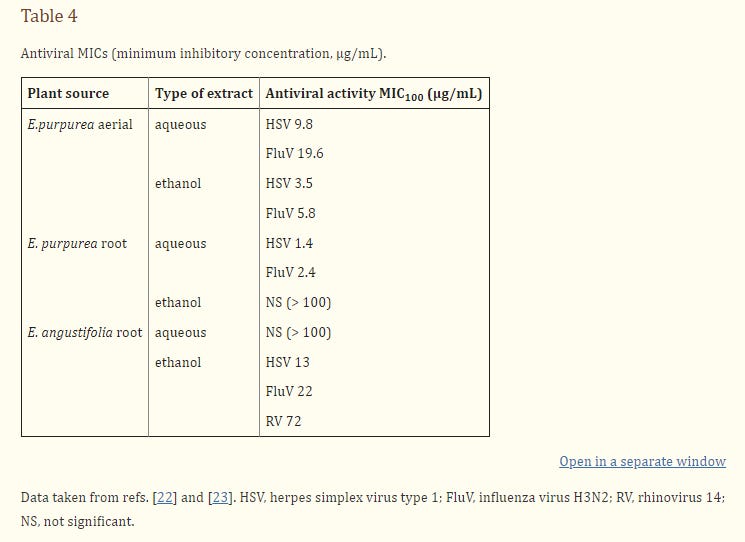
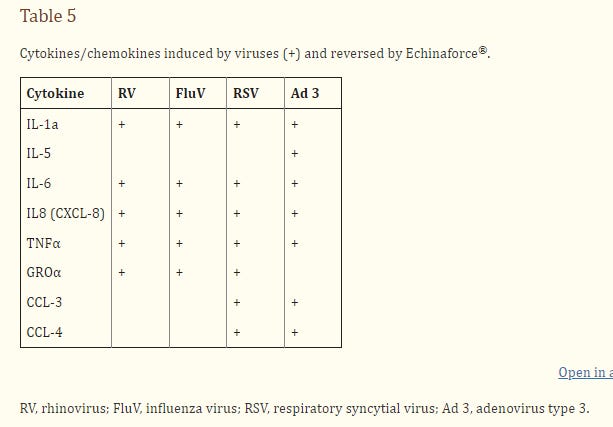






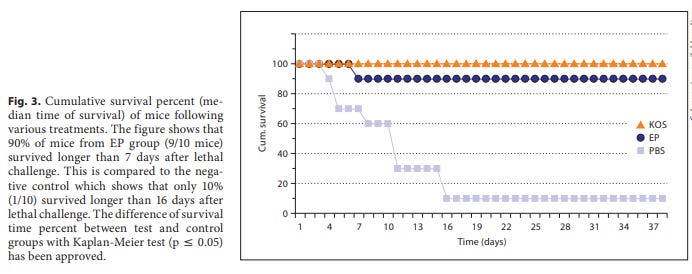





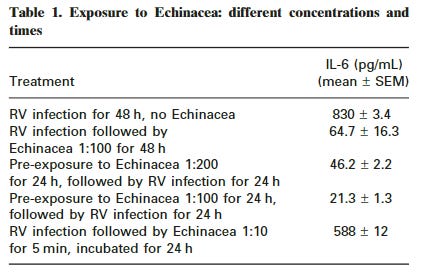
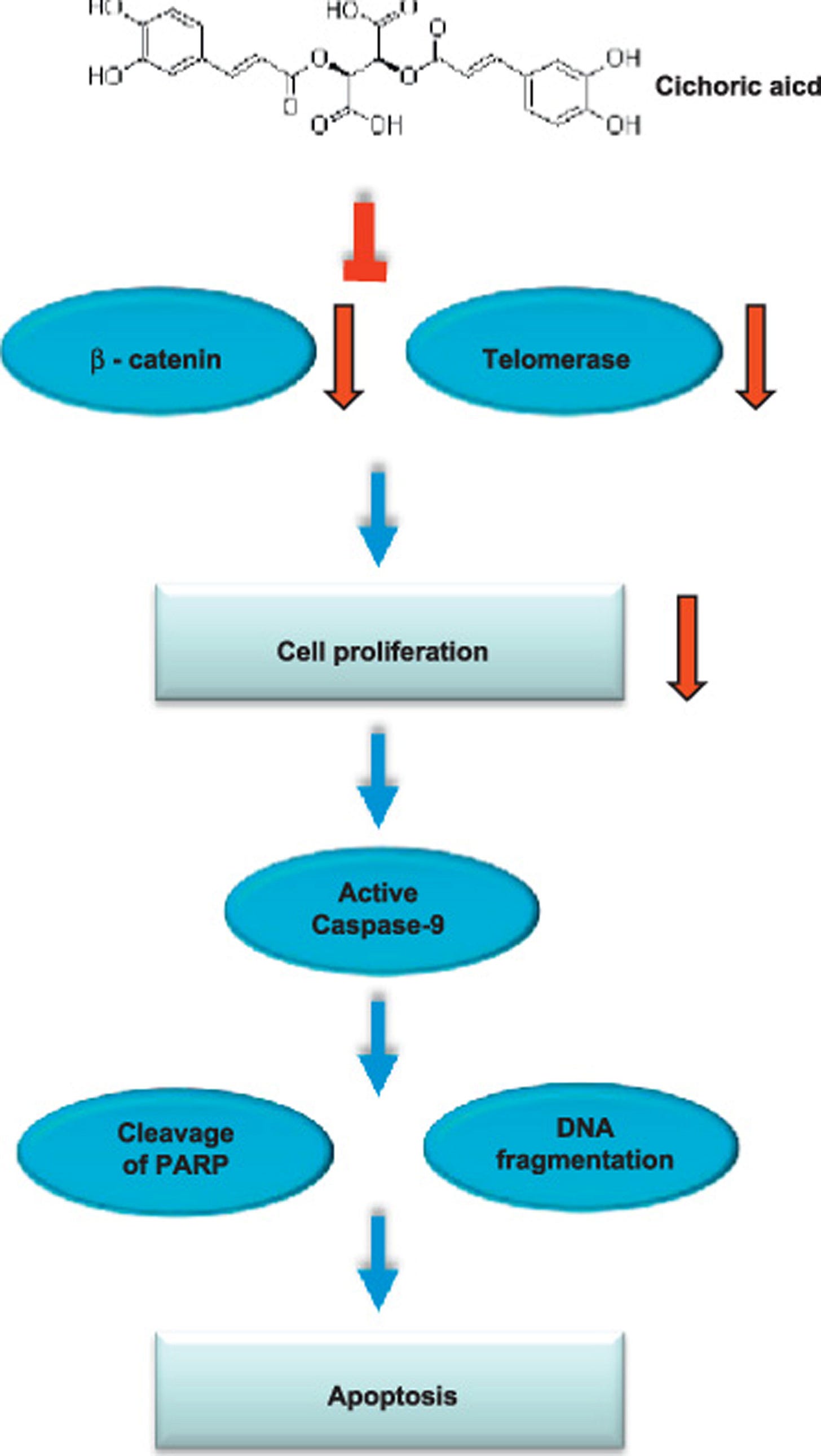



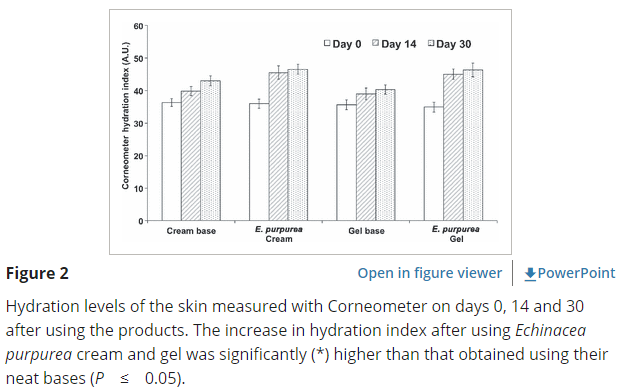



I've used echinacea for years. I used it for one really bad strain of flu that was going around and was able to keep working while the rest of them were suffering badly. I think the trick is to take a lot of it. Farmers used to feed it to their horses. I took a lot of it in the winter, and it was like I could feel the heat from it.
Thank you for this! Echinacea had kind of dropped off my radar