Reading time:
short story - novelette - novella - novel - PhD thesis - Trump’s tariff list - War and Peace - U.S. Tax Code
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
1.0 Introduction
1.1 A nerve agent attack? Kind of
A relatively high incidence of chronic limb pain, frequently complicated by violent, tremulous involuntary movements, has been noted in Japanese girls following human papillomavirus vaccination.
The average incubation period after the first dose of the vaccine was 5.47 ± 5.00 months. Frequent manifestations included headaches, general fatigue, coldness of the feet, limb pain, and weakness.
The skin temperature of the girls with limb symptoms was slightly lower in the fingers and moderately lower in the toes. Digital plethysmograms revealed a reduced peak of the waves, especially in the toes.
Limb symptoms of the affected girls were compatible with the diagnostic criteria for complex regional pain syndrome.
The Schellong test identified a significant number of patients with orthostatic hypotension and a few with postural orthostatic tachycardia syndrome.
Electron-microscopic examinations of the intradermal nerves showed an abnormal pathology in the unmyelinated fibers in two of the three girls examined.
The symptoms observed in this study can be explained by abnormal peripheral sympathetic responses. The most common previous diagnosis in the patients was psychosomatic disease.
Recently, delayed manifestation of cognitive dysfunction in the post-vaccinated girls has attracted attention. The symptoms include memory loss and difficulty in reading textbooks and/or calculation.
From: “Neurologic Complications in HPV Vaccination” (2015)
1.2 Human Papillomavirus (HPV)
HPV belongs to the Papillomaviridae family of DNA viruses. It commonly infects the skin and mucous membranes of the body. There are more than a hundred types of HPV. Whilst most of these infections clear up harmlessly, some types of genital HPV may lead to neoplastic transformation, leading to cancer of the cervix. HPV has also been linked to cancers of the anus, penis, vagina, vulva, and the back of the throat (oropharyngeal cancer)1.

From Wiki:
“Sexually transmitted HPV is divided into two categories: low-risk and high-risk. Low-risk HPVs cause warts on or around the genitals. Types 6 and 11 cause 90% of all genital warts and recurrent respiratory papillomatosis, which causes benign tumors in the air passages. High-risk HPVs cause cancer and consist of about twelve identified types.[11] Types 16 and 18 are responsible for causing most of the HPV-caused cancers. These high-risk HPVs cause 5% of the cancers in the world. In the United States, high-risk HPVs cause 3% of all cancer cases in women and 2% in men.[92]
Risk factors for persistent genital HPV infections, which increase the risk of developing cancer, include early age of first sexual intercourse, multiple partners, smoking, and immunosuppression.[1]”
This Substack will briefly discuss HPV-induced neoplastic transformation and the more commonly known HPV vaccine-related adverse events, but the main discussion is in support of a hypothesis — that both the virus and vaccinal proteins are associated with a significantly increased risk of pathologies, including osteoporosis, due to a sustained increased expression of pro-inflammatory cytokines. Immune priming helps to mediate the long-term effects observed.
I was prompted to investigate the pathology after citizen journalist The Underdog shared this anonymous post:
This work does not exclude the contribution of other vaccine-induced pathologies, such as premature ovarian insufficiency (POI)2, which has been subject to various court cases and class actions. These included claims for osteoporosis, a heart condition, and POTS.
The burden of proof is high, and not in your favour.
(For information only, not an endorsement):
Our lawyers are helping victims who want to bring a Gardasil HVP vaccine lawsuit throughout the United States. Our law firm is particularly focused on ovarian failure cases that lead to infertility in women who have taken Gardasil in the last few years.
Gardasil is a vaccine intended to prevent human papillomavirus (HPV), which can sometimes lead to cervical cancer in women. Gardasil was developed by the embattled pharmaceutical company Merck & Co.
Merck obtained FDA approval for Gardasil in 2006 based on deceptive research and clinical trials that misrepresented the vaccine’s efficacy while concealing its safety risks and side effects. Merck then launched an aggressive and highly misleading marketing campaign to include millions of parents vaccinating their pre-teen daughters with Gardasil.
Filing a Gardasil lawsuit is not an anti-vax statement. A Gardasil vaccine lawsuit is a statement that this specific vaccine that we all assumed was safe might not be.
Gardasil settlement projections for different types of injuries
Gardasil Class Action Lawsuit Updates in 2025
April 25, 2025 – Appeal
Bellwether plaintiffs in the Gardasil vaccine MDL appealed multiple adverse rulings to the Fourth Circuit after Judge Bell dismissed most of their claims. The plaintiffs’ opening appeal brief is due May 27, 2025.
March 12, 2025 – Tough Ruling in MDL
The MDL judge in the Western District of North Carolina has ruled in favor of Merck, granting summary judgment on the grounds of implied preemption. The decision effectively blocks failure-to-warn claims brought by plaintiffs who allege that Merck should have included warnings about Postural Orthostatic Tachycardia Syndrome (POTS) and Primary Ovarian Insufficiency (POI) on the Gardasil label. There are other claims, but the reality is this is a failure to warn case. So it is a crushing blow in the MDL.
The court found that under federal law, Merck could not have independently added these warnings without FDA approval. Since the FDA has never required such warnings—and has consistently rejected claims that Gardasil causes POTS or POI—the court determined that state law failure-to-warn claims conflict with federal regulation and are therefore preempted. The ruling is a major victory for Merck in the federal litigation, as it eliminates one of the plaintiffs’ central legal claims.
The decision does not apply to state court cases. So while this ruling is significant, it does not end Gardasil litigation altogether. This decision applies only to cases consolidated in the MDL in federal court. State court lawsuits remain unaffected, and plaintiffs can still pursue claims under state law, particularly in jurisdictions where failure-to-warn claims are treated differently or where alternative legal theories—such as fraud, negligence, or design defect—are in play.
The contrast between state and federal litigation is already becoming apparent. A state court trial in Los Angeles, brought by a woman who alleges Gardasil caused a heart condition that left her in a wheelchair, was recently postponed due to concerns over potential jury bias. The delay stemmed from the confirmation of Robert F. Kennedy Jr. as U.S. Secretary of Health and Human Services. Kennedy, a long-time critic of vaccines, referred cases to a law firm handling Gardasil litigation and was set to financially benefit from some of these lawsuits before transferring his stake to his son. His highly publicized confirmation process led to concerns that jurors might be influenced by his vaccine-related advocacy.
So despite this federal preemption ruling, the broader fight over Gardasil is not over. State courts will continue to hear cases, and the legal battle over whether Merck adequately disclosed Gardasil’s risks will play out in those jurisdictions. Merck’s victory in the MDL means federal plaintiffs may now focus on other claims, such as fraud or design defect, while state court litigation remains active.
March 8, 2025 – Why Is Gardasil Not in Vaccine Court
Gardasil is covered by the VICP, which means individuals alleging harm from Gardasil must file claims in Vaccine Court before they can sue the manufacturer. However, Gardasil is not listed in the Vaccine Injury Table, which outlines specific injuries presumed to be caused by particular vaccines within a defined timeframe.
Because Gardasil is not on the table:
There are no presumptive injuries associated with it, according to the federal government (maybe Kennedy does something about this)
Claimants do not get the benefit of the legal presumption of causation.
They must prove, using medical records and often expert testimony, that the vaccine caused the injury.
Without the benefit of a table injury:
The burden of proof is higher.
Claimants must show that the vaccine “more likely than not” caused the injury (preponderance of evidence).
This often requires retaining expensive medical experts and assembling detailed scientific arguments.n.
More: https://www.lawsuit-information-center.com/gardasil-hpv-vaccine-lawsuit.html
2.0 Discussion
2.1 Viral oncogenic mechanisms
This is from a paper from 2002 by Hwang et al. “Human Papillomavirus Type 16 E7 Binds to E2F1 and Activates E2F1-driven Transcription in a Retinoblastoma Protein-independent Manner”3.
Lower-risk HPV types bind E7 protein less strongly to E2F1, a tumor suppressor. Strong binding helps higher-risk HPV to stop E2F1 from restricting cancer cell division:
The human papillomavirus (HPV) E7 oncoprotein can immortalize primary human cells and induce tumor formation. These properties of E7 depend on its ability to inhibit the activity of retinoblastoma protein (pRB), which in turn affects E2F function.
E2F proteins control the expression of genes involved in differentiation, development, cell proliferation, and apoptosis. By using genetic and biochemical approaches, the present study shows that E7 binds to E2F1in vivo and in vitro and that both proteins co-localize in the nucleus. Importantly, the binding of the high risk group HPV E7 to E2F1 is tighter than the binding of the low risk group HPV E7 to E2F1.
Several DNA tumour viruses target E2F proteins. These include Adenovirus, Epstein-Barr Virus (EBV), Simian Virus 40 (SV40), Hepatitis B virus (HBV), and Human Cytomegalovirus (HCMV):
Summary
The protein encoded by this gene is a member of the E2F family of transcription factors. The E2F family plays a crucial role in the control of cell cycle and action of tumor suppressor proteins and is also a target of the transforming proteins of small DNA tumor viruses. The E2F proteins contain several evolutionally conserved domains found in most members of the family. These domains include a DNA binding domain, a dimerization domain which determines interaction with the differentiation regulated transcription factor proteins (DP), a transactivation domain enriched in acidic amino acids, and a tumor suppressor protein association domain which is embedded within the transactivation domain. This protein and another 2 members, E2F2 and E2F3, have an additional cyclin binding domain. This protein binds preferentially to retinoblastoma protein pRB in a cell-cycle dependent manner. It can mediate both cell proliferation and p53-dependent/independent apoptosis. [provided by RefSeq, Jul 2008]
Expression
Broad expression in bone marrow (RPKM 12.7), testis (RPKM 5.6) and 17 other tissues"
From: “E2F1 E2F transcription factor 1 [ Homo sapiens (human) ]”
2.2 Osteoporosis
I was simply curious to see what signalling pathways there may be, if any, to link HPV and/or a vaccine to the anonymous case report of osteoporosis in a teenage boy. I quickly ruled out premature ovarian insufficiency…
Aside from the sex of the patient, POI-associated osteoporosis usually develops over several years. It is probably a secondary effect, more akin to bone loss associated with premature menopause. Whereas in the case report, we may have a more acute disease occurrence, but we don’t know his vaccination history or timescales.
I expected there to be an association, though, as many vaccines promote systemic inflammation as part of the immune response. As we shall see later, cytokine levels may remain elevated for several weeks or months, and again after each booster dose or infection-associated antigen exposure.
Pro-inflammatory cytokines have a well-researched effect on the bones of the human skeleton, and can disrupt a delicate balance. They tend to promote bone-resorbing osteoclasts over bone-forming osteoblasts. This is a necessary part of maintaining a healthy bone structure, but taken to excess may lead to weakness and impaired healing ability.
I’ve written about this before, with respect to SARS-CoV-2 and engineered spike glycoprotein.
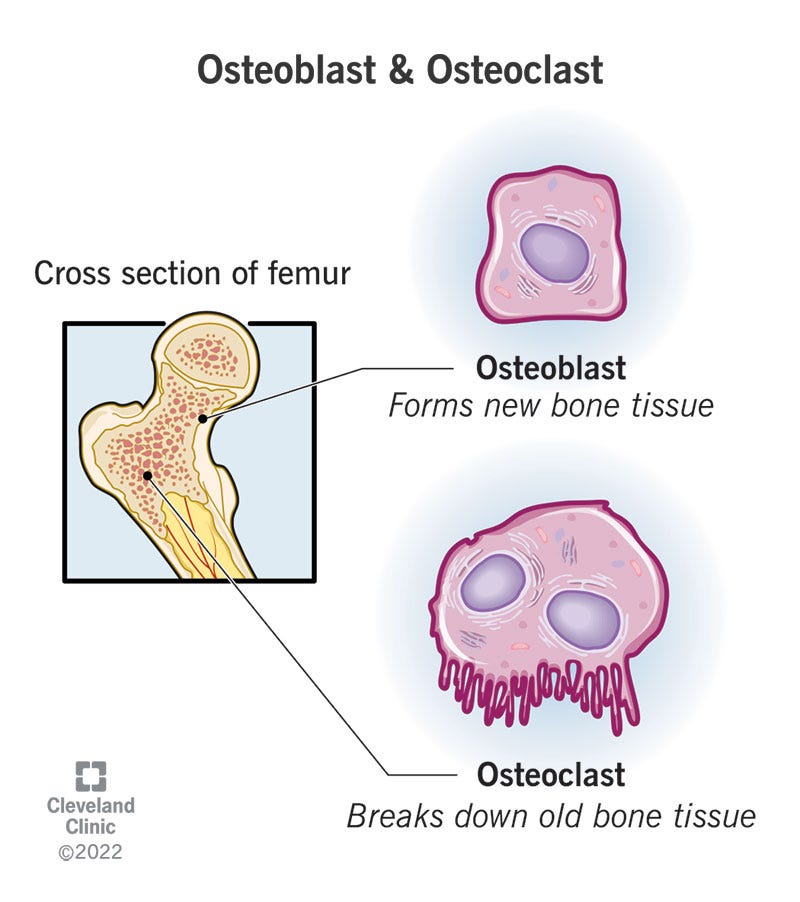
A way to remember which does which:
“Think clasts for cleaners and blasts for builders”.
What do osteoblasts do?
Osteoblasts are like construction crews that build new bone cells. You might see them called osteogenic cells. They strengthen your existing bones and help form new bone tissue.
Osteoblasts have three main functions:
Growing new bones (bone formation).
Reshaping bones to help them change as you age (remodeling).
Healing damaged or broken bones.
Osteoblasts are triggered by chemical reactions or hormones when a bone grows or changes. They create and release (secrete) a mix of proteins called bone matrix. Bone matrix is made of proteins like collagen mixed with calcium, phosphate and other minerals.
After they’re activated, osteoblasts move into place and deposit bone matrix in spaces on a bone that needs to grow or be strengthened or repaired. After it’s in place, the bone matrix solidifies and hardens into new, healthy bone. Picture a worker pouring concrete. They might create a whole new sidewalk. But they can also use that same concrete to patch cracks or broken chunks in an existing path. In this example, osteoblasts are the worker, and bone matrix minerals are the concrete they use to create new bone.
Once the osteoblasts have completed their job of laying down new bone tissue, they can then become part of the bone by transforming (differentiating) into osteocytes, or they die (if they’re no longer needed).
Osteocytes act like a security system inside your bones. They’re the most common type of cell in your bones. They monitor changes in pressure and stress that affect your bones. They respond to everything from normal movement and the force of you using your body to more intense changes like injuries. They can send signals to the osteoclasts and osteoblasts to repair damaged bone tissue. For example, if a bone is cracked, damaged or broken, osteocytes trigger a reaction that attracts osteoclasts to dissolve the area around the break (to resorb damaged bone tissue) and osteoblasts to lay down new bone tissue, so it can begin to heal.
What do osteoclasts do?
Osteoclasts dissolve and break down old or damaged bone cells. They make space for osteoblasts to create new bone tissue in areas that are growing or need repair.
If osteoblasts are builders, osteoclasts are your bones’ demolition crew. Think clasts for cleaners and blasts for builders. Osteoclasts release enzymes that break down old bone. They trigger chemical reactions on the surface of old bone tissue that dissolves it and creates space for newer, stronger tissue to form in its place.
Osteoclasts dissolve bone tissue, but it’s not as violent or aggressive as acid eating a hole in metal in a cartoon. The process of breaking down areas of old tissue is tightly regulated or controlled and specific. Osteoclasts only target specific areas that have been tagged by osteocytes.
The enzyme osteoclasts release breaks down hardened bone matrix and reabsorbs it into your body. This leaves microscopic pits and divots on the surface of your bone. Once the targeted tissue in those places is dissolved, osteoblasts move in and deposit new bone in the same spot.
Anatomy
What do osteoblasts and osteoclasts look like?
Osteoblasts and osteoclasts are tiny cells found along the bone lining and in the bone itself.
Osteoblasts are shaped like cubes — they’re slightly box-shaped.
Osteoclasts are bigger than osteoblasts. They’re shaped like rounded domes.
More: https://my.clevelandclinic.org/health/body/24871-osteoblasts-and-osteoclasts
Our health represents the sum total of multiple factors. These include our familial genetic lottery, lifestyle and epigenetics, infection history, and the effects of a cocktail of different allopathic meds and vaccine programs.
Inflammation goes hand-in-hand with a robust immune response in response to vaccination.
If multiple vaccines and drugs are being administered, e.g. for HPV, SARS-CoV-2, influenza, shingles, corticosteroids, etc., then the risk of systemic immune-inflammation could be disproportionately increased further than just through HPV vaccines alone. Very few to no clinical trials will have been conducted to look at this type of interaction.
Unfortunately, even a 2% reduction in bone mineral density can lead to a significantly increased risk of fracture.
Purpose
This study aims to investigate the associations of the systemic immune-inflammation index (SII) with bone mineral density (BMD) and osteoporosis in adult females from a nationally representative sample.
… Considering osteoporosis as a risk factor for fracture, it is possible that SII could be a risk factor for fracture. Our results showed that the reduction of BMD could reach 2% with a doubling of SII change. A previous study using a meta-regression of published trials found a 2% improvement in femoral neck BMD was associated with a 28% reduction in vertebral fracture, a 15% reduction in hip fracture, and a 11% reduction in nonvertebral fracture (30). Thus, these about 2% reductions of BMD with a doubling of SII could be large enough to affect fracture risks.
From: “Systemic immune-inflammation index is associated with decreased bone mass density and osteoporosis in postmenopausal women but not in premenopausal women“ (2023)
To investigate the pathways, as with any complex task, it’s useful to break the process down into logical steps.
Research step 1:
HPV is associated with a significantly increased risk of osteoporosis, according to at least two studies:
“Human Papillomavirus Infections and Increased Risk of Incident Osteoporosis: A Nationwide Population-Based Cohort Study” is from 2023, by Sheng-Kai Ma et al4:
… The cumulative incidence of osteoporosis in patients with HPV was significantly higher than that in non-HPV controls (log-rank test p-value = 0.01) (Figure 1). The Cox proportional hazards regression models revealed that patients with HPV infections presented with significantly greater risk of osteoporosis (aHR = 1.32, 95% CI = 1.06–1.65) (Table 2). Consistent with previous studies [1,35,36], the risk of osteoporosis was lower in men (aHR = 0.23; 95% CI = 0.18–0.3), and in populations of high socioeconomic status [37] (aHR = 0.30; 95% CI = 0.14–0.64); while the risk of osteoporosis was higher in frequent out-patient department (OPD) visitors (aHR = 1.01; 95% CI = 1.01–1.01) and in patients with comorbidities including COPD (aHR = 1.35; 95% CI = 1.08–1.68), vitamin D deficiency (aHR = 18.3; 95% CI = 2.56–131.09), long term glucocorticoid use (aHR = 2.05; 95% CI = 1.44–2.92), and TCAs/SSRIs use (aHR = 1.60; 95% CI = 1.05–2.43). The risk of osteoporosis was also higher in the older age groups [38], in which patients aged between 60–70 (aHR = 4.29; 95% CI = 3.10–5.93), aged between 70–80 (aHR = 7.32; 95% CI = 5.23–10.26) and aged beyond 80 (aHR = 9.63; 95% CI = 6.22–14.88) were associated with a significantly high risk of osteoporosis (Table 2).
Inflammation is a common factor:
Studies have shown that virus infections, such as HIV, HBV, HCV, and herpes zoster infections, were independently associated with a higher risk of osteoporosis [10,11]. Collectively, evidence from these studies provided the association between viral infections and the risk of osteoporosis. For instance, it was demonstrated that patients with herpes zoster infections presented with a 4.55-fold greater risk of osteoporosis [12], as associated with significantly high levels of interleukin (IL)-1b, IL-6, IL-8, IL-10, and tumor necrosis factor-alpha (TNF-α) [39]. Among those inflammatory biomarkers, IL-6 was suggested as a potent stimulator of osteoclast-induced bone resorption and thus was central to the pathogenesis of bone loss in the context of chronic inflammation [40].
The exact mechanism of how HPV infections undermined bone loss or osteoporosis has not been studied. However, highly expressed RANKL has been observed during the progression of HPV infection-associated cervical cancer, which was secreted by HPV-infected cells [22]. The excessive production of RANKL released by tumor cells was reported to trigger osteoclast-mediated bone destruction and to increase tumor burden [24]. Subsequently, binding of RANKL to RANK receptors on osteoclasts was found to activate signals for bone resorption [22]. On the other hand, high levels of inflammatory mediators such as TNF-α in patients with HPV infections [46] could place those individuals in an inflammatory microenvironment that may intensify osteoclastic resorption [47] by promoting RANKL production [48], transducing RANKL-induced signal pathways, and amplifying osteo-clastogenesis [47,49]. Specifically, TNF-α, as triggered by infections, promotes osteoblasts apoptosis and reduces osteo-blastogenesis by stimulating DKK-1 and Sost expression [9]. Moreover, TNF-α could suppress osteoblast differentiation by inhibiting Smad signaling through an NF-κB-mediated process [50]. Collectively, osteo-clastogenesis in response to high concentrations of RANKL and TNF-α may explain bone resorption and osteoporosis in patients with HPV infections.
And from this year, “A case–control study based on the National Health and Nutrition Examination Survey to evaluate the effects of human papilloma virus on bone health in women” (2025) by Li et al5. further confirms the association:
Methods
This case–control study utilized data from the National Health and Nutrition Examination Survey (NHANES). Comparable datasets were created using nearest neighbor propensity score matching (PSM) at a ratio of 1:1. The association between HPV infection and bone mineral density (BMD) was analyzed using the Welch two-sample t-test. Furthermore, linear mixed models were employed for validation purposes. Restricted cubic spline (RCS) analysis and Kendall’s tau-b tests were performed to explore the effect of different types of HPV infection on BMD.
Results
Individuals with HPV infection (mean age 38.11 ± 11.32 years) had lower BMD in the femur and lumbar spine compared to uninfected individuals (mean age 37.92 ± 11.42 years). RCS analysis revealed that an increasing number of cooccurring HPV types in women was associated with lower BMD. Specifically, four HPV types were negatively associated with femur BMD, while 14 HPV types were negatively associated with lumbar spine BMD. Additionally, HPV types 53, 59, and 89 exhibited effects on both femur and lumbar spine BMD.
Studies investigating the effects of HPV vaccination on BMD haven’t been done yet. Now, why would that be?
Conclusions
HPV infection is associated with a decrease in BMD, and co-infection with multiple types of HPV implies even lower BMD. Appropriately designed trials are needed to determine if interventions targeted at preventing HPV infection can have a protective effect on BMD.
The more HPV coinfections you have, the greater the risk of BMD, and vaccination is specific to only a few types:
… RCS analysis was performed to gain a clearer understanding of the association between HPV infection and BMD. Figure 2 demonstrates a nonlinear association (P < 0.001) between the number of cooccurring HPV types and BMD. As the number of cooccurring HPV types increased, BMD decreased.
Gardasil 9 targets HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58. It would therefore only be protective against one of the BMD-decreasing HPV types: 06, 53, 59, 89.
The type Gardasil targets, 06, had the weakest impact on BMD:
Subsequently, we performed an analysis on individual HPV types to identify those that had the most significant impact on BMD. The data revealed that four HPV types (06, 53, 59, and 89) exhibited a negative correlation with lower limb BMD (P < 0.05). Notably, type 89 exhibited the strongest correlation (r = − 0.060). Fourteen HPV types demonstrated a negative correlation with lumbar spine BMD, with type 53 showing the strongest correlation (r = − 0.085). Specifically, three HPV types (53, 59, and 89) had a significant effect on both lower limb and lumbar spine BMDs. In our analysis, the effects of specific HPV types could not be evaluated individually because there is no participant data in the NHANES database for those who are solely infected with HPV types 26, 64, and 82. Therefore, these types of HPV were not included in this study.
Little research has been conducted on the association of viral infections and osteoporosis (OP):
… The current literature regarding the association between viral infection and OP is limited. Viruses such as HIV, hepatitis B virus, hepatitis C virus, and severe acute respiratory syndrome coronavirus 2 have been found to be associated with OP [40,41,42,43,44]. By integrating the findings from the PSM, linear mixed models, and RCS analyses, we established a negative correlation between HPV infection and BMD. Specifically, individuals infected with HPV (average age 38.11 ± 11.32 years) exhibited lower bone density in the femur and lumbar vertebrae compared to uninfected individuals (average age 37.92 ± 11.42 years). Furthermore, a higher number of co-occurring HPV types was associated with decreased BMD.
HPV infection is immediate (acute), yet the decline in BMD leading to OP usually takes a long time (chronic). The authors suggest that persistent HPV infection may lead to systemic bone damage, but their main interest is in inflammation.
Note that experiments have shown that HPV vaccines also trigger systemic inflammation with the proinflammatory cytokines stated: TNF, IL-8, and IL-12:
… As mentioned earlier, we confirmed the association between HPV and BMD. However, it is more important to determine the causal relationship between them. The decline in BMD and its progression to OP often occur over an extended period of time. In contrast, HPV infection can be regarded as an immediate occurrence. We posit that the decline in BMD is more likely caused by HPV infection rather than the other way around.
… the biological mechanisms responsible for the decline in BMD due to HPV infection are still unclear. Persistent HPV infection is known to elicit various responses, such as immunosuppression and abnormal cell destruction [45]. In a prospective study, Scott et al. discovered elevated IL-12 concentrations in cervicovaginal lavage fluid specimens from women with persistent HPV infection [46]. This might indicate the activation of macrophages induced by IL-12 and the ensuing chronic inflammation [47]. The tissue damage resulting from inflammation is self-evident. Furthermore, cervical HPV infection leads to the infiltration of macrophages and dendritic cells. Activation of these cells also results in the release of TNF, IL-8, and IL-12 [ [48, 49]. Additionally, HPV infection-induced oxidative stress and lipid peroxidation contribute to cellular damage that may result in cell death [50]. While these effects are primarily observed in locally infected tissues, prolonged effects can lead to systemic damage, including bone damage.
We also noted a larger decline in BMD in the lumbar spine than in the lower limbs in HPV-infected individuals. Fourteen HPV types showed an inverse association with lumbar spine BMD, a significantly higher number compared to the lower limbs (four types). This finding indicates that lumbar spine BMD is more responsive than lower limb BMD. This finding aligns partially with the results of previous studies.
HPV types 53, 59, and 89 should also be added to the shots, according to the authors.
I’m not sure that will help, though. Read on…
… Our findings revealed a negative association between three types of HPV (53, 59, and 89) and BMDs in both the lumbar spine and lower limbs. Therefore, public HPV preventive measures and the detection of HPV infection should prioritize these three types. Further research focusing on these types could help elucidate the biological mechanisms underlying the association between HPV and BMD.
More: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-025-03909-2
Research Step 2:
HPV vaccines
What protein(s) does, for example, “Gardasil”, use to trigger the antibodies/schmantibodies response?
I can then review the research into these, but with respect to viral pathologies, and see if the Venn diagrams of symptoms and side effects overlap.

Gardasil 9 contains L1 capsid proteins from nine different HPV types. If adopted, the recommendations of the previous study would increase that to 12.
It’s the old pharma trick of trying to avoid vaccine immune escape by putting all the ingredients in the bowl of pasta, throwing it at the wall, and hoping some of it sticks.
Note that it also contains aluminium as an adjuvant. This is a huge red flag, and it probably works synergistically with other pathways linked to OP. You would need to conduct controlled experiments to assess the relative contribution of all the vaccine components.
What is Gardasil 9 and what is it used for?
Gardasil 9 is a vaccine used in males and females from the age of nine years to protect against the following conditions caused by nine types of the human papillomavirus (HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58):
precancerous lesions (growths) and cancers in the cervix, vulva or vagina and anus;
genital warts.
Gardasil 9 is given according to official recommendations. It contains purified proteins from the nine types of HPV listed above.
How is Gardasil 9 used?
Gardasil 9 is a suspension for injection available in vials or prefilled syringes. Gardasil 9 is normally given according to a either a two-dose schedule or a three-dose schedule for males and females from 9 to 14 years old and a three-dose schedule for males and females 15 years old and over. For a two-dose schedule, the second dose should be given between five and thirteen months after the first dose. For a three-dose schedule, the second dose should be given two months after the first and the third given four months after the second. There should always be at least one month between the first and the second doses, and at least three months between the second and the third, and all doses should be given within a year.
It is recommended that individuals who receive the first dose of Gardasil 9 should complete the dosing regimen using this medicine. The vaccine is given as an injection into a muscle, preferably in the shoulder or the thigh.
The vaccine can only be obtained with a prescription.
How does Gardasil 9 work?
Human papillomaviruses are viruses that cause warts and abnormal tissue growth. There are more than 100 types of papillomavirus, some of which are associated with anogenital cancers in both men and women. Nearly 100% of cervical cancers are caused by HPV infection. In Europe, approximately 90% of anal cancers, 15% of vulvar cancers, 70% of vaginal cancers, and 30 to 40% of penile cancers are estimated to be caused by HPV infection. HPV types 16 and 18 cause a large majority of cervical and anal cancers, while HPV types 6 and 11 cause most of genital warts. A further 5 HPV types (31, 33, 45, 52, and 58) also carry a high risk for developing cancer (they cause around 20% of cervical cancers).
All papillomaviruses have a shell, or ‘capsid’, that is made up of proteins called ‘L1 proteins’. Gardasil 9 contains the purified L1 proteins for the nine HPV types above, produced by a method known as ‘recombinant DNA technology’. The proteins are assembled in ‘virus-like particles’ (structures that look like HPV, so that the body can recognise them easily). These virus-like particles are not capable of causing infection or disease.
When a patient is given the vaccine, the immune system makes antibodies against the L1 proteins. After vaccination, the immune system is able to produce antibodies more quickly when it is exposed to the real viruses. This will help to protect against the diseases caused by these viruses.
The vaccine also contains an ‘adjuvant’, a compound containing aluminium to stimulate a better response.
More: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil-9
… Aluminum is an experimentally demonstrated neurotoxin and the most commonly used vaccine adjuvant. Despite almost 90 years of widespread use of aluminum adjuvants, medical science's understanding about their mechanisms of action is still remarkably poor.
There is also a concerning scarcity of data on toxicology and pharmacokinetics of these compounds. In spite of this, the notion that aluminum in vaccines is safe appears to be widely accepted. Experimental research, however, clearly shows that aluminum adjuvants have a potential to induce serious immunological disorders in humans.
In particular, aluminum in adjuvant form carries a risk for autoimmunity, long-term brain inflammation and associated neurological complications and may thus have profound and widespread adverse health consequences.
In our opinion, the possibility that vaccine benefits may have been overrated and the risk of potential adverse effects underestimated, has not been rigorously evaluated in the medical and scientific community.
From: “Aluminum vaccine adjuvants: are they safe?“ (2011)
HPV vaccination is also used to stimulate anti-tumor responses, using cytotoxic T lymphocytes (CTLs):

From the “Patient information leaflet”, we see at worst, an autoimmune condition involving muscle weakness, tingling, or paralysis, called Guillain-Barré syndrome (GBS), and acute disseminated encephalomyelitis.
No mention of osteoporosis, premature ovarian insufficiency, heart conditions, or POTS.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them.
The following side effects can be seen after the use of Gardasil 9:
Very common (may affect more than 1 in 10 people): side effects found at the injection site (pain, swelling, and redness) and headache.
Common (may affect up to 1 in 10 people): side effects found at the injection site (bruising, and itching), fever, tiredness, dizziness and nausea.
Uncommon (may affect up to 1 in 100 people): swollen glands (neck, armpit, or groin), hives (urticaria), fainting sometimes accompanied by shaking or stiffening, vomiting; joint pain, aching muscles, unusual tiredness or weakness, chills, generally feeling unwell, lump (nodule) at the injection site.
Rare (may affect up to 1 in 1,000 people): allergic reactions.
Unknown (frequency cannot be estimated from the available data): serious allergic reactions (anaphylactic reaction).
When Gardasil 9 was given with a combined diphtheria, tetanus, pertussis [acellular, component] and poliomyelitis [inactivated] booster vaccine during the same visit, there was more injection-site swelling.
Fainting, sometimes accompanied by shaking or stiffening, has been reported. Although fainting episodes are uncommon, patients should be observed for 15 minutes after they receive HPV vaccine.
The following side effects have been reported with GARDASIL or SILGARD and may also be seen after getting GARDASIL 9:
Allergic reactions have been reported. Some of these reactions have been severe. Symptoms may include difficulty breathing and wheezing.
As with other vaccines, side effects that have been reported during general use include: muscle weakness, abnormal sensations, tingling in the arms, legs and upper body, or confusion (Guillain-Barré syndrome, acute disseminated encephalomyelitis); bleeding or bruising more easily than normal and skin infection at the injection site.
More: https://www.medicines.org.uk/emc/product/7330/pil#gref
Research Step 3:
L1 protein binding to immune cells
First, a little about L1.
L1 proteins naturally clump together in groups of 5 (pentamers), and these then form the viral envelope (capsid) by assembling groups of larger icosahedral structures (T=7).
For making a vaccine, the handy thing is that L1 readily forms immunogenic virus-like particles (VLPs) without needing viral DNA6:
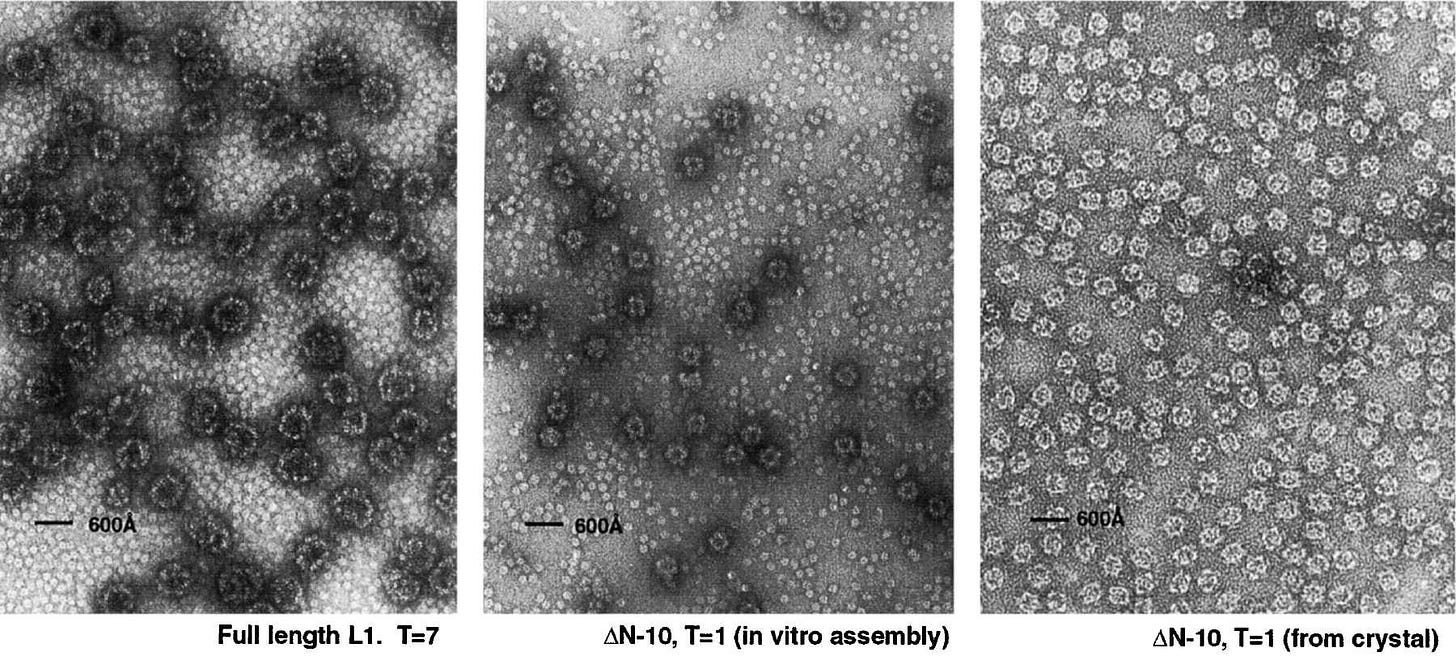
Papilloma virions contain two virally encoded proteins, L1 and L2, synthesized late in the infectious cycle. These two proteins encapsidate a histone-associated, closed circular, double-stranded DNA minichromosome. The outer shell of the virion contains 72 pentamers of L1, centered on the vertices of a T = 7 icosahedral lattice (1, 50). L2, a largely internal protein, is present at about 1/30 the abundance of L1 (Kirnbauer et al. 1993).
The overall surface organization of the papillomavirus shell resembles that of its close relatives, murine polyomavirus and SV40, which also contain 72 pentamers of a principal capsid protein, VP1 (Rayment et al. 1982).
From: “Structure of Small Virus-like Particles Assembled from the L1 Protein of Human Papillomavirus 16“ (2000)
https://www.sciencedirect.com/science/article/pii/S1097276500804499

Unfortunately, as with SARS-CoV-2 and engineered spike, just because it’s a “protein” does not mean that it isn’t bioactive or pathogenic.
From a 2008 paper by de Witte et al., “Binding of human papilloma virus L1 virus-like particles to dendritic cells is mediated through heparan sulfates and induces immune activation“7, we learn that HPV-L1 VLPs readily bind to two types of immune cell subsets: subepithelial dendritic cells (DCs) and Langerhans cells (LCs) via a receptor that is expressed on both cell types.
Its long name is “heparan sulfate proteoglycan syndecan-3”, and it is of relevance to both HPV vaccines and viral pathogenicity.
Key takes:
Immunization using human papilloma virus (HPV)-L1 virus-like particles (VLPs) induces a robust and effective immune response, which has recently resulted in the implementation of the HPV-L1 VLP vaccination in health programs.
By “immune modulation” they are referring to tolerance mechanisms:
… Initiated by foreign signals, iDCs are transferred into mature DCs (mDCs), which are capable of launching T-cell mediated immune responses against foreign attacks.11
iDCs encountering self-antigens or in the absence of foreign signals differentiate into tolerogenic DCs (tolDCs), which promote immune tolerance.12 The double-edged role of DCs seems to have been extensively studied for orchestrating immune responses,1 of which plasticity is generated from their unique role as sentinels, consecutively signaling their peripheral cues and exerting context-dependent properties.13,14
From: “The role of dendritic cells in the immunomodulation to implanted biomaterials“ (2022)
We learned before how this can go disastrously wrong due to engineered spike protein epitopes promoting tolerance and cancer progression via elevation of igG4 antibodies.
However, during infection, HPV can escape immune surveillance leading to latency and disease. Dendritic cells (DCs) induce effective immune responses after vaccination, but might also induce immune modulation during infection.
The interaction of HPV-L1 VLPs with mucosal DCs determines the immune response. However, little is known about the receptors on mucosal DC subsets involved in HPV-L1 VLP binding. Therefore, we set out to investigate the interaction of HPV-L1 VLPs with the different mucosal DC subsets; the subepithelial DCs and Langerhans cells (LCs).
We observed strong binding of HPV-L1 VLPs to both DCs and LCs. We did not observe an involvement for C-type lectins such as dendritic cell-specific ICAM-3 grabbing non-integrin (DC-SIGN) and langerin.
The HPV-L1 VLP binding to DCs was mediated through heparan sulfates, since it was abrogated by heparinase-II treatment. The heparan sulfate proteoglycan syndecan-3 binds VLPs and is expressed on both DCs and LCs.
Syndecan-3 (SDC3) is a transmembrane proteoglycan. It has a protein core with attached glycosaminoglycan (GAG) chains, composed of the linear polysaccharide heparan sulfate (HS):

Binding of VLPs to DCs, but not to LCs, strongly correlated with the levels of heparan sulfates and syndecan-3, suggesting that syndecan-3 is the main receptor for HPV-L1 VLPs on DCs.
This is where the RANK/RANKL/OPG osteoporosis pathway enters the picture:
VLP interaction with DCs resulted in the up-regulation of co-stimulatory molecules and the production of the cytokines IL-6, IL-8, IL-10 and IL-12p40.
Our results support an important role for syndecan-3 as a HPV receptor on DCs, which could be important for both vaccine development and understanding HPV pathogenesis.
Research Step 4:
Cytokine responses to HPV vaccines and cancer pathways
Three studies. The first, “Human Papillomavirus Immunization Is Associated with Increased Expression of Different Innate Immune Regulatory Receptors” by Colmenares et al8, was from 2012 and used the older Gardasil quadrivalent formulation. 23 healthy women were immunized and tested before and after.
Although they didn’t analyze cytokines, their findings are quite alarming:
We have studied the expression and function of ILT2 and the expression of other NK cell receptors in 23 healthy women before and after immunization with the quadrivalent HPV (type 6/11/16/18) vaccine (Gardasil).
Receptor expression was analyzed by flow cytometry in freshly isolated peripheral blood mononuclear cells as well as after in vitro stimulation with the quadrivalent HPV (type 6/11/16/18) vaccine. In addition, the regulatory function of ILT2 on cell proliferation and IFN-γ production was analyzed.
This is not good at all, as ILT2 is immunosuppressive:
We found a significant increase in the expression of ILT2 by NK and CD3+ CD56+ lymphocytes and monocytes after quadrivalent HPV (type 6/11/16/18) vaccine immunization.
In addition, the in vitro stimulation with the quadrivalent HPV (type 6/11/16/18) vaccine also increased the proportion of CD3− CD56+ ILT2+ NK cells.
But it’s OK, they say, as they put the telescope to their blind eyes:
Although the inhibitory function of ILT2 on cell proliferation was enhanced after HPV immunization, the in vitro engagement of this receptor did not affect the synthesis of IFN-γ induced by HPV.
Interferon-gamma doesn’t get you off the hook:
… Some researchers believe it has anti-tumorigenic properties, while others suggest that it contributes to tumor growth and progression. In our recent work, we have shown that concentration of IFN-γ in the TME determines its function.
Further, it was reported that tumors treated with low-dose IFN-γ acquired metastatic properties while those infused with high dose led to tumor regression.
Pro-tumorigenic role may be described through IFN-γ signaling insensitivity, downregulation of major histocompatibility complexes, upregulation of indoleamine 2,3-dioxygenase, and checkpoint inhibitors such as programmed cell death ligand 1.
From: “Roles of IFN-γ in tumor progression and regression: a review“ (2020)
https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-020-00228-x
Weighed and found wanting:
… Attempts at exploiting the potency of IFN-γ have yielded conflicting results, with both anti- and pro-tumorigenic effects being observed. Unfortunately, intravenous or sub-cutaneous administration of IFN-γ has not improved the outcome of melanoma [17], renal cell carcinoma [18], leukemia [19], pancreatic carcinoma [20], breast cancer [21], and colon cancer [22].
From: “IFN-γ: A cytokine at the right time, is in the right place“ (2020)
Upregulation of human inhibitory receptors Ig-like transcript 2 (ILT2) is positively associated with tumour size, cancer progression, and poor prognosis. Quite the opposite of what you would expect of a “HPV cancer-preventing” vaccine:
Background:
Ig-Like Transcript 2 (ILT2, LILRB1) is an inhibitory receptor prominently expressed on resting macrophages, monocytes, and dendritic cells (DCs), with variable expression on CD4+ T cells, CD8+ T cells, B cells, NK cells, and NKT cells in the tumor microenvironment.
Interaction of ILT2 with its ligands, HLA-G and classical MHC class I molecules, is reported to impair cytotoxic activity of NK and effector T cells, attenuate B cell function, inhibit antigen-presentation by dendritic cells, and promote immunosuppressive activity of myeloid cells.
Further, high ILT2 expression has been associated with poor prognosis in several human malignancies. As a result, ILT2 has been proposed as a therapeutic target to promote antitumor immunity and to overcome resistance to checkpoint blockade.
From: “Abstract 2906: AGEN1571 is a novel high-affinity ILT2 antagonist antibody that promotes adaptive and innate immune responses“ (2022)
IL2 is associated with a poor prognosis of gastric cancer and is itself a target for chemotherapy. You couldn’t make it up!
… The results suggest that ILT2 and ILT3 are expressed with diverse degrees in gastric cancer cells and tissues, and the expression of ILT2 is related with differentiation and size of tumors. Furthermore, the cytotoxic activity of NK92MI against the MKNI cell line was stronger than that against HGC-27.
This study indicates that ILT2 and ILT3 play a key role in gastric cancer immune escape, and ILT2 may be a new target in the clinical diagnosis and treatment of gastric cancer.
From: “Expression of immunoglobulin-like transcript (ILT)2 and ILT3 in human gastric cancer and its clinical significance“ (2012)
It gets better.
Another study even links upregulated ILT2 to cervical cancer progression:
… A previous study showed that the overexpression of HLA-G molecules on the surface of tumour cells affects tumour-specific T cell immunity, and upregulate the expression of ILT2 on tumour-infiltrating CD8+ T cells in the cancer microenvironment (10).
Therefore, blocking checkpoint molecule HLA-G could be used as a promising immunotherapeutic strategy for the treatment of cervical cancer.
From: “A Novel Prognostic Risk Model for Cervical Cancer Based on Immune Checkpoint HLA-G-Driven Differentially Expressed Genes“ (2022)
Our second investigation into immune responses was from 2014 and studied the effects of two major brands of HPV vax.
Key takes from “Comparison of adaptive and innate immune responses induced by licensed vaccines for human papillomavirus“ (2014) by Herrin et al.9:
Two HPV virus-like particle (VLP) vaccines, HPV-16/18 (GlaxoSmithKline, Cervarix®) and HPV-6/11/16/18 (Merck, Gardasil®), are currently licensed in the United States. Given the similar antigenic content but different adjuvant formulations in the 2 vaccines, they provide an efficient method for evaluating adjuvants and comparing the kinetics of the innate and adaptive immune responses.
We randomized women to receive either Cervarix® or Gardasil®, followed 6 month vaccination delivery schedules per manufacturer's recommendations, and analyzed the humoral immune response, T cell response, and circulating plasma cytokine levels in response to vaccination.
Comparing CD4+ T cell cytokine responses at month 12, there was a trend of increased levels of IL-2 and TNF-α in the Cervarix® groups versus the Gardasil® groups that was consistent across all 4 tested HPV types (16/18/33/45).
Elevated levels of circulating plasma cytokine/chemokines were observed post first vaccination in Gardasil® recipients and proinflammatory cytokines were elevated following 1st and 3rd Cervarix® vaccinations.
Eugh:
The Gardasil® vaccine is adjuvanted with aluminum hydroxyphosphate sulfate. The Cervarix® vaccine is formulated with AS04, which contains aluminum hydroxide salts and the TLR4 agonist MPL (3-O-desacyl-4’-monophosphoryl lipid A). The AS04 adjuvant is likely to play a role in the higher immunogenicity observed in Cervarix® recipients but because of other differences in production, it is difficult to attribute these results solely to the adjuvant.9–11
Gardasil promoted IL-12p40, IL-8, IP-10, MCP-1 (an immune cell-attracting chemokine) and MIP-1B (Macrophage inflammatory protein-1 beta), whereas Cervarix upregulated IL-6, IL-1a and IL-1B:
No statistically significant trends were observed for IFN-γ or TNF-α and data is not presented. The two vaccines induced significantly different responses at varying time points for the remaining cytokines, which are shown in Figure 6. Gardasil® induced significantly higher early cytokine/chemokine responses than Cervarix® (Figs. 6A-E) which peaked at day 5 for the following cytokines; IL-12p40: 1.70 fold greater (p = 0 .046), IL-8: 1.98 fold greater (p = 0 .045), IP-10: 2.03 fold greater (p = 0 .001), MCP-1: 2.00 fold greater (p = .006), and MIP-1β: 1.65 fold greater (p = .035).
All of these cytokines/chemokines remained significantly elevated in Gardasil® recipients compared to Cervarix® recipients at the day 14 time point. Conversely, median levels of inflammatory markers, IL-6, IL-1α, and IL-1β, were significantly higher in Cervarix® recipients following the first, and in some cases third, dose of vaccine (Figs. 6F-H).
The RHS represents the second booster, at 6 months. Gardasil immune training is evidenced by IL-8 and, to a lesser extent, by IL-6.
Cervarix priming is demonstrated by higher peaks in IL-1a/b. Again, the IL-6 response after boosting remains robust:
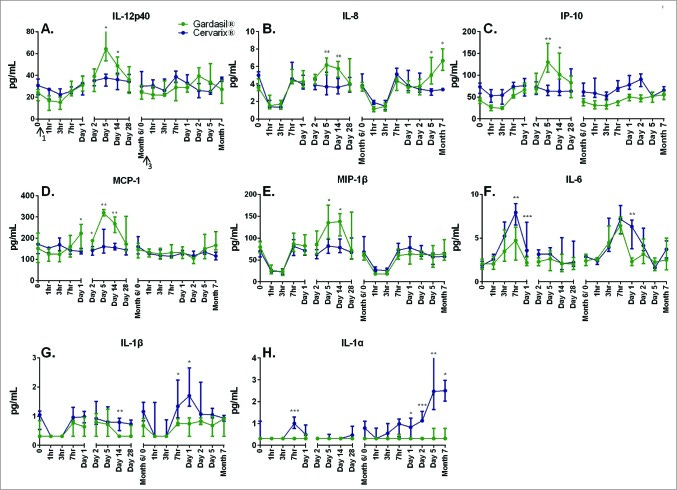
“… correlates of immune protection have not yet been determined”.
They pushed these on the market without knowing if or how they worked, and therefore without understanding pathogenicity risks.
Clownworld!
Both vaccines are efficacious and are known to produce a robust antibody response against oncogenic HPV vaccine types (HPV-16 and HPV-18).
Although correlates of immune protection have not yet been determined,16,17 partially due to the lack of unambiguous vaccine failures, preclinical data has demonstrated that neutralizing antibodies can mediate type-specific protection against infection in the absence of other immune effectors.18,19
The thresholds for B and T cell responses associated with protective immunity are not currently known. Understanding the kinetics, breadth, and magnitude of the innate and adaptive immune response can contribute to the identification of candidates for correlates of protection against infection and aid in building predictive models of vaccine immunogenicity and efficacy.
This is the first study that evaluated and compared circulating cytokine responses within hours and days after vaccination with the HPV vaccines. The most notable difference observed is an increased chemokine and cytokine (IL-12p40, IL-8, IP-10, MCP-1, and MIP-1β) response in Gardasil® recipients following first vaccination which peaks at day 5 and extends through day 14.
With the exception of IL-12p40, a T cell stimulating factor, Gardasil® induced mostly increases in chemokines. Conversely, Cervarix® recipients showed elevated circulating pro-inflammatory markers (IL-6, IL-1α, IL-1β) following first (3–7 hrs) and third vaccination (7 hrs through month 7) when compared to Gardasil®.
Our third study is from 2023, used mice, echoed the previous findings, and found that Gardasil also upregulated both IL-6 and TNF-α after a second “stimulation”, due to immune training.
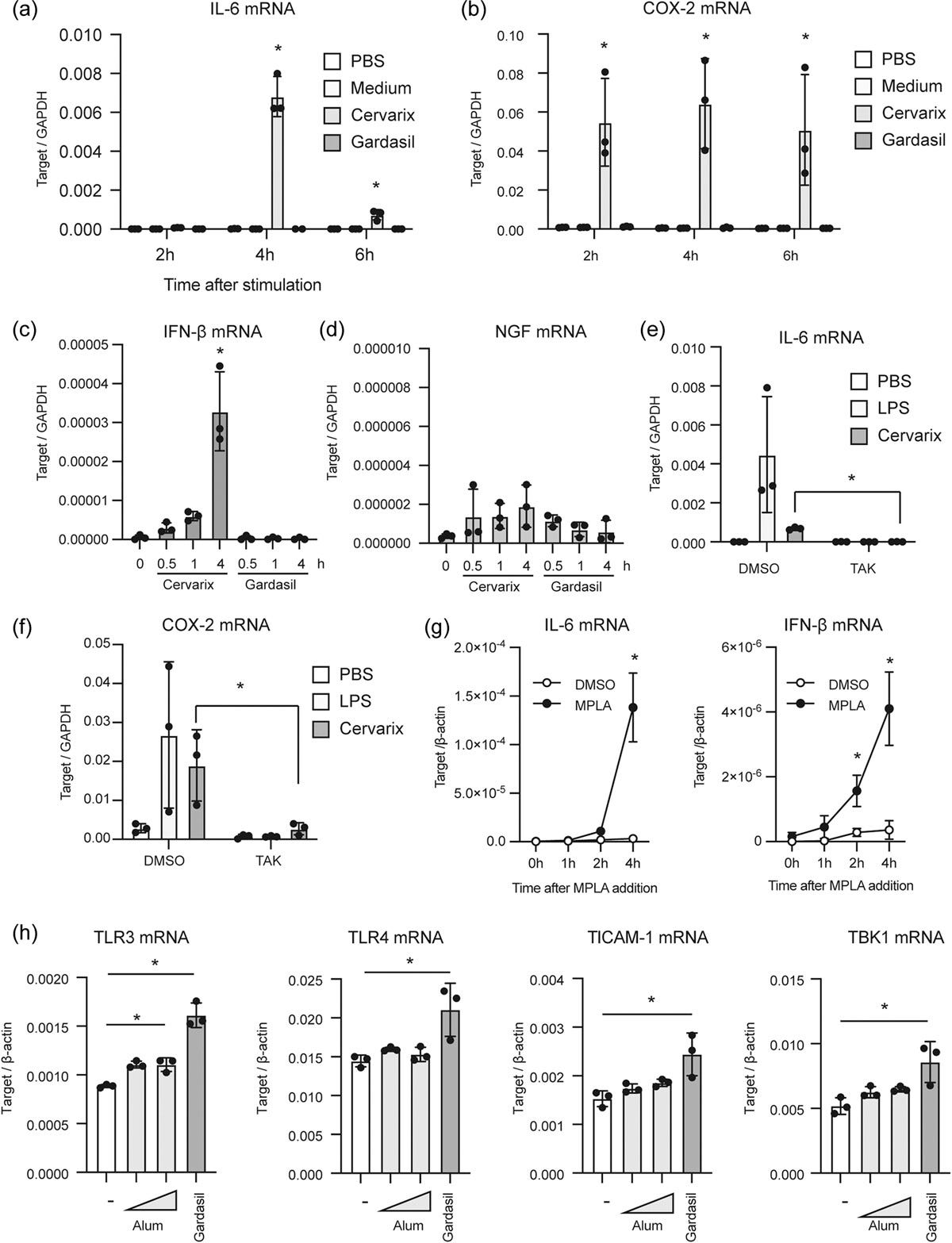
Key takes from “HPV vaccines induce trained immunity and modulate pro-inflammatory cytokine expression in response to secondary Toll-like receptor stimulations“ (2023) by Yamaguchi et al.10:
In this work, we explored the innate immune responses and trained immunity caused by two HPV vaccines, Cervarix and Gardasil. Cervarix includes monophosphoryl lipid A and an aluminum adjuvant, and it significantly increased the expression of IL-6 and IFN-β mRNAs in RAW264.7 cells.
On the contrary, Gardasil, which only includes an aluminum adjuvant, exhibited little cytokine expression but increased the expression of TLRs. Furthermore, Cervarix significantly increased IL-1β secretion from mouse macrophages, while Gardasil only mildly induced IL-1β secretion.
Interestingly, initial stimulation with Gardasil enhanced the expression of IL-6 and TNF-α mRNAs upon secondary stimulation with TLR ligands, indicating that Gardasil induced trained immunity in macrophages.
Moreover, Gardasil injection into mice resulted in enhanced TNF-α production in sera following secondary TLR stimulation. Our findings suggest that HPV vaccinations have the ability to induce trained immunity that modulate TLR ligand responses.
Note that they only analysed cytokine changes over the first 4-6 hours, and that the previous study found that levels increased for days, weeks, or months after this:
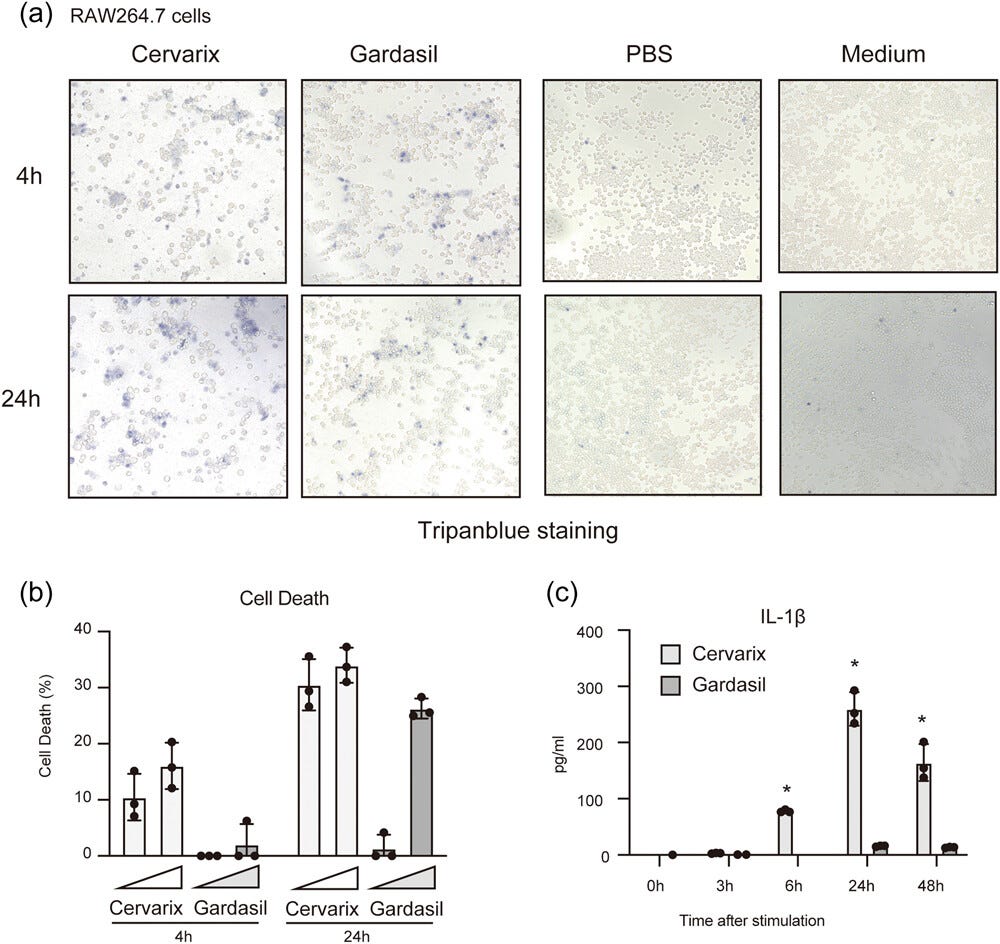
Both vaccines induced cell death by the 24h time point, even at lower concentrations.
This is notable, because dying cells passively release pro-inflammatory HMGB1, which is part of the osteoporosis pathway11. I discussed it in the last Substack, in relation to nueropathologies, via the p53/SIRT1/HMGB1/RAGE signalling pathway.
The adjuvant alum content also led to a significant increase of the pro-inflammatory cytokine IL-1β:
Next, we quantitated percentages of dead cells using the LDH assay. The percentage of dead cells was increased by treatment with Cervarix compared with Gardasil at earlier time points. However, the levels of dead cells were comparable between Gardasil and Cervarix-treated cells at 24 h, particularly at a high concentration of the vaccines.
The high concentration was slightly above those used for cytokine expression observation in Figure 1 (final conc. 80 μL/mL vs. 60 μL/mL). Notably, cell death was detected even at the lower concentration of Gardasil (20 μL/mL) (Figure 2b).
IL-1β and HMGB1 can promote each other, in a positive feedback loop12:
It is worth noting that alum not only induces cell death but also activates the inflammasome, leading to the secretion of IL-1β.15 Hence, we measured the concentration of the IL-1β protein in the cell culture medium of RAW264.7 cells after treatment with the vaccines.
Gardasil moderately induced IL-1β production by RAW264.7 cells, whereas Cervarix significantly induced IL-1β production (Figure 2c). These findings indicate that the vaccines can elicit various types of innate immune responses, including TLRs- and inflammasome-mediated responses.
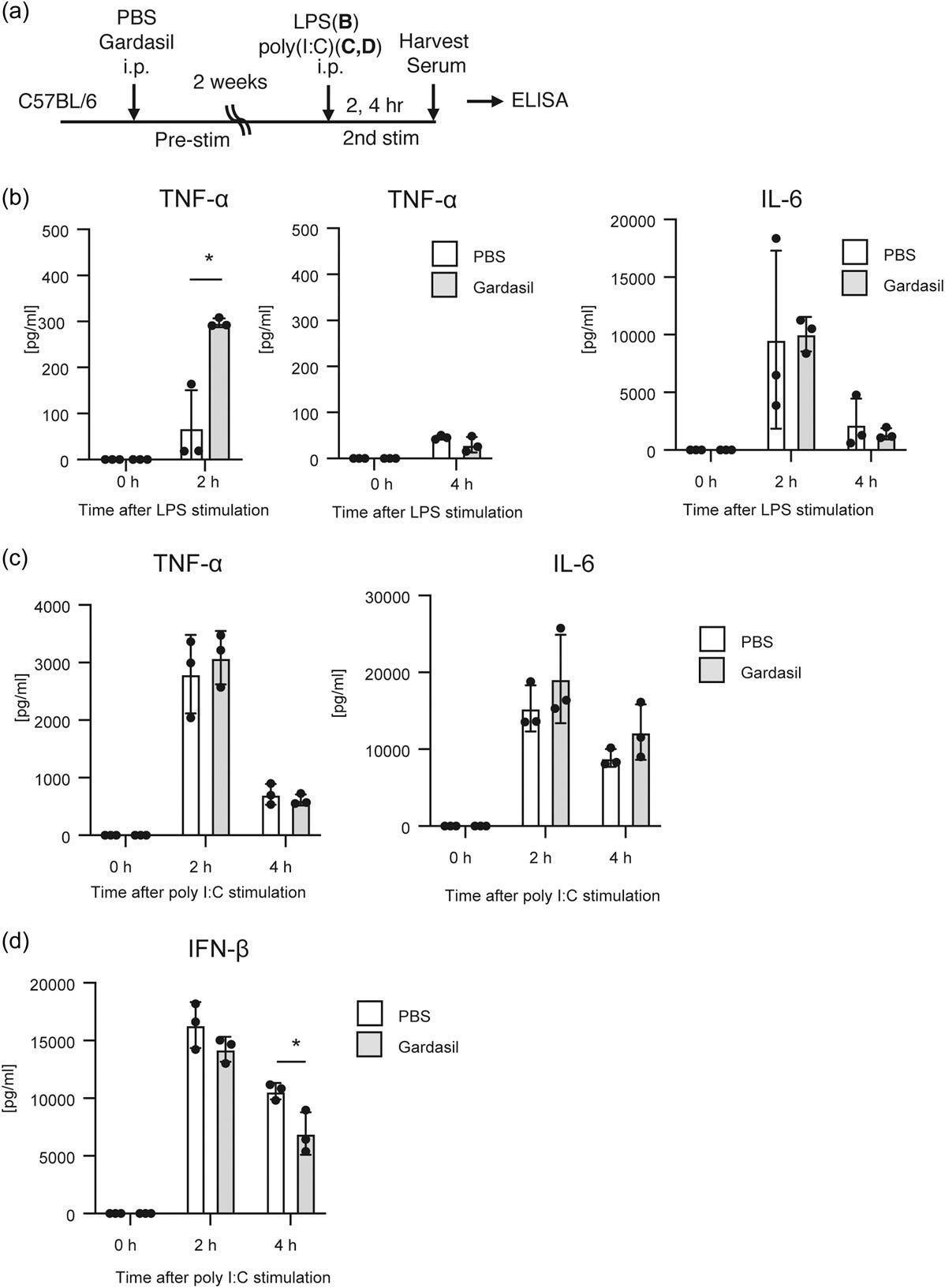
Their study of the effects of HPV vaccines on trained immunity in mice is also enlightening, in that they led to impaired IFN-β responses when later exposed to immunogens.
A reverse-vaccine effect, which also lasts indefinitely because you have trained your immune system. If you also have cancer, then there is also a risk that this could promote progression:
Subsequently, we investigated the trained immune responses in vivo. C57BL/7 mice were initially injected with Gardasil or PBS. After a 2-week period, poly I:C or LPS was injected into the mice, and the cytokine levels in the sera were determined by ELISA (Figure 5a).
We confirmed that cytokine expression was not detectable two weeks later after the first stimulation (Figure 5b–d, 0 h). Interestingly, pre-stimulation with Gardasil enhanced TNF-α production in response to secondary LPS stimulation (Figure 5b), while IL-6 production in response to LPS and poly I:C was not affected by Gardasil pre-stimulation (Figure 5b,c).
Furthermore, we observed that serum IFN-β levels at 4 h postinjection with poly I:C were significantly reduced in mice pre-stimulated with Gardasil (Figure 5d).
This reduction in IFN-β production in the mouse sera aligned with the observation that Gardasil pre-treatment attenuated IFN-β mRNA expression in response to secondary TLR stimulation in RAW264.7 cells (Figure 3d).
These observations provide evidence for trained immunity induced by Gardasil in vivo.
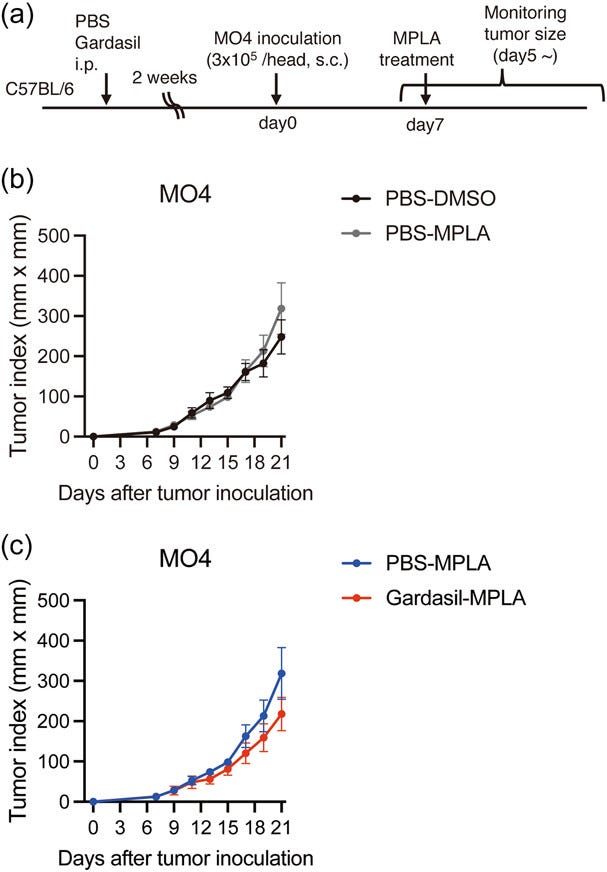
They also investigated whether Gardasil-induced TNF-α could exert an anti-tumour effect on injected melanoma cells. Perhaps unsurprisingly, it didn’t cause a statistically significant reduction in tumor growth in the mice:
Since HPV vaccines are known to prevent cervical cancer onset, it was hypothesized that trained immunity induced by the vaccines might also prevent other types of cancer in an antigen-independent manner.
Given that Gardasil enhanced TNF-α production in response to secondary stimulation with the TLR4 ligand (Figure 5b), we investigated whether pre-treatment with Gardasil could impact tumor growth in mice treated with the TLR4 ligand. MPLA was utilized for TLR4 stimulation instead of LPS, as LPS is considered too harmful to the host.
Mice were injected with Gardasil or PBS, and two weeks later, MO4 melanoma cells were subcutaneously inoculated into the mice. Seven days after inoculation, mice were treated with MPLA to induce antitumor immune responses, and tumor growth was monitored for 21 days following the schedule shown in Figure 6a.
In this experimental condition, treatment with MPLA did not affect tumor growth in this experimental condition (Figure 6b). Gardasil treatment showed a trend of decreased tumor growth compared to the PBS control, but the difference was not statistically significant (Figure 6c).
Although our data suggested that HPV vaccines could induce trained immunity, affecting cytokine expression in response to secondary stimulation in vitro, the trained immunity induced by Gardasil in vivo did not exhibit a significant effect on tumor growth, at least in this experimental condition.
In conclusion, for over ten years, they pushed these as cancer-preventing vaccines without doing the cancer-related immunological studies. This is lamentable, as cancer promotion couldn’t be excluded either, such as through disruption of ILT2 and IFN-β expression.
Considering that HPV vaccination started over a decade ago, clinical studies exploring the relationship between HPV vaccination and the prevention of other cancers will be reported in the future. The regulation of epigenomic and metabolic states plays crucial roles in trained immunity. Therefore, further investigation into the changes in epigenetic and metabolic states is also necessary to fully understand the trained immunity induced by HPV vaccines and its potential implications.
An analysis of HPV-associated cancer rates was published in August last year, and it’s a bombshell.
“Update on Effects of the Prophylactic HPV Vaccines on HPV Type Prevalence and Cervical Pathology” (2024), by Hamson & Oliver13.
Synopsis:
Despite widely adopted and successful HPV vaccination programs, HPV-associated cancer rates are back where they were, or worse than in the late 1970’s — long before the vaccination programs started.
The authors suggest that this may be because carcinogenic types of HPV that are not vaccinated against may have become prevalent. Its called “type replacement”.
Another factor is that many of these may lead to cancers with latencies of 30 years, vs vaccinal types, which have shorter latencies of around 20 years. Thus, it is only now that the lack of vaccine efficacy is being exposed, as cancer cases continue to climb.
The immunosuppressive effects of SARS-CoV-2 experimental gene therapies can only serve to steepen the curve.
Abstract
Most national prophylactic HPV vaccination programs started in approximately 2008, with either the bivalent Cervarix HPV16/18 or quadrivalent Gardasil (HPV6/11/16/18) vaccines, which were then followed by introduction of the nonavalent Gardasil 9 (HPV6/11/16/18/ 31/33/45/52/58) vaccine from 2015.
Since that time, these products have demonstrated their ability to prevent infection with vaccine-covered HPV types and subsequent development of HPV-related cervical and genital pathologies. The data indicate that vaccination of young girls prior to sexual debut is more effective than vaccination of older HPV+ve women.
Although some studies have shown a decline in the prevalence of vaccine-covered HPV types, there are national and regional differences in overall vaccine efficacy. Furthermore, several recently published studies show an increase in the prevalence of non-vaccine-covered HPV types in vaccinated populations, which is indicative of HPV type-replacement.
It is also notable that vaccine-related changes in HPV type prevalence spread between vaccinated and unvaccinated women at the same geographical location—presumably via sexual transmission.
In conclusion, it is not yet clear what effect dissemination of vaccine-associated changes in HPV type prevalence will have on vaccine efficacy and cervical pathology, particularly in mixed populations of vaccinated and unvaccinated women.
However, it is very clear these observations do underscore the need for long-term continuation of cervical screening combined with regular reassessment of testing practices.
Keywords: HPV; vaccines; prophylactic; invasive cervical cancer; HPV type-replacement; clinical unmasking; cervical intraepithelial neoplasia; CIN; superinfection exclusion
Key takes:
Although there have been some concerns over vaccine-related adverse events [2,3], these have not established causality and both Cervarix and Gardasil are now judged to be well tolerated and safe [4]. Moreover, there are now many studies which have shown that prophylactic vaccination with either product is effective when given to young women prior to sexual debut and HPV infection [5].
This was demonstrated for Cervarix in a trial carried out in Costa Rica [6] although non-HPV16/18 types were still present in vaccinated women at the same location [7]. Indeed, all the current vaccines are effective against specific HPV types and they have the potential to reduce the global incidence of cervical cancer, albeit this will be over a period of 30–40 years from the onset of vaccination.
Moreover, the overall long-term impact of vaccination will depend to what extent this is influenced by other factors such as HPV type-replacement [9,10] and superinfection exclusion [11].
To clarify these last two points, it is important to realise there are >50 different types of HPV known to infect the genital epithelium. These consist of ~14 well established high-risk (HR) types in addition to ~36 types which are either probable high-risk or low-risk (LR) types.
Most significantly, it is well known that HPV infections of one type can affect susceptibility to infection with others by either stimulating cross-type immunity [9] or by superinfection exclusion [11]. In this way, the cervix may play host to a changing population of different HPV types.
Given this level of complexity, it is not clear what effect the HPV vaccines will have on the prevalence of non-vaccine-covered HPV types and, in the longer term, any associated dysplasia. In order to address this issue, there are important questions which remain to be answered.
Boom!
Are infections with vaccine-covered high-risk HPV types replaced by HPV types which are not covered by the vaccines and how does this affect the incidence of cervical intraepithelial neoplasia (CIN) and invasive cervical cancer (ICC)?
Are vaccine-induced changes in HPV types that are observed in vaccinated women then transferred to unvaccinated women in the same population?
As with SARS-CoV-2, you can never outrun a mutating virus with vaccines. And if you try, you risk causing immune exhaustion, immunotolerance, autoimmune disorders, and original antigenic sin (OAS) due to priming:
Evidence supporting vaccine-related HPV type-replacement is provided by a cross sectional study of two Spanish communities vaccinated with either Cervarix or Gardasil [12].
Analysis of pre and post-vaccination prevalence of 35 HPV types showed that LR HPV6/11 and HPV16 infections declined, although the latter was not statistically significant and there was no change in the prevalence of HPV18.
Furthermore, Gardasil does not cover HR types 31, 52 and 45, which all showed a significant post-vaccination increase in prevalence.
Most notably, Gardasil 9 does not cover HR HPV types 35, 39, 56, 59 and 68, which also increased post-vaccination (see Supplementary Material, additional data file in [12]).
These findings are consistent with studies carried out in New York (see Figures 1 and 2 in [13]) and Stockholm (see Figure 1 in [14]), which shows a post-vaccination increase in non-vaccine-covered high-risk HPV types in both vaccinated and unvaccinated women.
Further studies confirmed that vaccine failure was occurring. Has it all been for nothing, just another flop? It looks that way.
In 2010, the prevalence of HR and LR HPV types was evaluated in cervical smears taken from 3817 women aged between 25 and 64 from multiple screening centres in Central and Southern Italy [15]. This was followed by a larger Italian multi-centre study in 2013 which analysed smear samples from 37,077 unvaccinated women aged 25–60 years, for the prevalence of multiple HR HPV types in order to provide baseline data for vaccine efficacy [16].
In 2023, following a 14-year vaccination period, analysis of multiple HR HPV types was carried out on smear samples taken from 30,445 from women aged 30–64 years from the Lazio region of Italy [17].
Comparison of these three studies showed the number of women positive for any of 13 HR HPV types was 9% in 2010 [15], 8% in 2013) [16] and 13.9% (2023) [17], which equates to an average increase of ~65% between 2010 and 2023, as illustrated in Figure 1A.
Analysis of the prevalence of individual HR HPV types found by these three studies is shown in Figure 1B.
It is surprising that, in spite of 14 years post-vaccination, there was no overall decrease in the percentage of HPV 16- or 18-positive women, and all the other HR HPV types analysed actually showed a marked increase in the 2023 population.
Moreover, it is also very clear that the observed increase in prevalence of non-vaccine-covered HPV types is supportive of vaccine-related type replacement.
There were confounding factors, such as HPV detection sensitivities, and that a 2023 study only enrolled women aged ≥30 years. But this should have had, if anything, a negative effect on the observed rates.
This is what vaccine failure looks like:

And it’s not due to lack of uptake:
Even though HPV vaccine coverage in Italy has been below the desired target, particularly during the COVID pandemic, for many years it still achieved >70% [19]. Thus, the lack of any post-vaccination reduction in the prevalence of HPV16 and 18 in 2023, combined with an increase in non-vaccine-covered HPV types, is counterintuitive.
It could be argued that any effects of vaccination on HPV type prevalence may be diluted by the inclusion of older unvaccinated women although, as previously discussed, it has been shown that vaccine-related changes in HPV type prevalence spread between vaccinated and unvaccinated individuals at the same geographical location [13,14].
It is notable that Pisani and Cenci 2024 also analysed the prevalence of infections with multiple HPV types in individual women [20] using the same patient cohort as their earlier study [17].
“HPV type replacement”:
Comparison of cervical smears from 2005 to those taken in 2019 found that the incidence of LR HPV types showed a ~50% reduction whereas Gardasil 9-covered HR HPV types showed little change over the study period. However, the prevalence of non-vaccine-covered HR HPV types and untypable HPVs had both increased >100% in 2019 (see Table 2 in [21]).
A reduction in the incidence of HPV16 and HPV31 was found in biopsy-confirmed CIN1 but only in younger women aged 21–29 years. This was not seen in women aged ≥30 years and no reduction in HPV16 incidence was observed for women with CIN2.
Most notably, an increase in HPV-negative lesions, those positive for non-vaccine-covered HPV, and unknown HPV types was also seen across all age groups.
“Cause for concern”.
It gets worse.
Most of the Cervical Intraepithelial Neoplasia Grade 2 (CIN2) precancerous lesions still tested positive for HPV types the vaccines were meant to be protecting you from:
These results support vaccine-related HPV type replacement and the observation that the majority of lesions remained positive for all HR HPVs targeted by the vaccines, irrespective of age or severity of cervical lesion, is cause for concern.
As with polio14 and SARS-CoV-2, a further study showed that the vaccine appeared to be working against its target types, but non-vaccine-covered HPV types were quickly taking their place:
Gardasil was the main vaccine used in the previously discussed observational Italian study [21]. A more recent report examined long-term follow-up of women treated with Cervarix in the double-blinded Costa Rican HPV Vaccine Trial (CVT) [22]. Initially 7466 women aged 18–25 were randomly assigned either Cervarix or a hepatitis A control vaccine and both arms followed up for a period of 1–4 years. After the follow-up period, women in the hepatitis A control arm were then offered vaccination with Cervarix.
At this time an observational unvaccinated control group (UCG) of 2836 women from the same birth cohort and geographical location as the previous vaccine arms was recruited. The incidence of cervical dysplasia and HPV type in the original Cervarix arm and the more recent UCG arm was then evaluated over a further period of 7 years, providing a total of 11 years of follow-up.
Most notably, this study specifically addressed the incidence of CIN related to infection with non-vaccine-covered HPV types. At years 1–4, a ~50% reduction in HPV16/18-related CIN2 and CIN3 was found in the Cervarix arm compared to the hepatitis A vaccine control arm.
Furthermore, when compared to the UCG at years 7–11, this had reduced by 90% for CIN2 and 87% for CIN3. Significant cross-protection against HPV31/33/45-related CIN2/3 was also observed for both time periods, whereas protection against strain types covered by Cervarix vaccination was as expected.
And it was all going so well.
CIN3 is a high-grade precancerous condition of the cervix:
However, a marked increase in the incidence of CIN positivity for non-vaccine-covered HPV types 35/39/51/52/56/58/59 was found in Cervarix-vaccinated women. For CIN2 at years 1–4, there was no change, whereas at years 7–11 this had increased by 71.2%. For CIN3 at years 1–4, there was an increase of 17.4%, but at years 7–11 this had increased to 135%.
Other studies found the same, and the authors summarised the findings in figures 2 and 3.
… Comparison of vaccine cross-protected HPV types in CIN2 showed vaccination-associated reduction in the prevalence of types 31/45, although type 33 had increased by 100%.
For CIN3, vaccine-associated reduction of type 16/18-positive lesions was less pronounced and type 33 lesions had also increased by >100% and, unlike CIN2, there was no reduction in prevalence of type 31 lesions.
Furthermore, there was also a marked increase in non-vaccine-covered HPV types 52/51/33/58/39/82/66 in CIN2/3 from vaccinated versus unvaccinated women (see Figure 2 in [23]).
In order to clarify the overall impact of vaccination on the prevalence of HPV types found in CIN2/3, Figure 2 and Figure 3 show these results reformatted to illustrate the percentage of vaccine-related change in lesions positive for vaccine-covered versus non-vaccine-covered HPV types.
Overall vaccination induced a significant reduction in the prevalence of HPV16/18-positive CIN2/3, although there was clearly a marked increase in lesions positive for other HR HPV types.
Vaccinal types down, non-vaccinal types up:

High-grade precancerous lesions are now dominated by non-HPV-vaccine types:

A US study found the same despite low vaccine uptake, but they didn’t analyse the lesion types:
The previously discussed findings are also consistent with an American study which investigated changes in CIN prevalence across five states between 2008 and 2015 [24].
HPV vaccination was approved in the USA in 2006 although uptake was slow, achieving only 40% in 2014 [25]. Analysis of the incidence of 16,572 CIN2 lesions in American women aged 18–39 years demonstrated a reduction in the prevalence of CIN2 for women aged 18–24.
However, a marked increase was seen in women aged 25–39 years with the same trend observed for CIN3, although HPV types per lesion were not specified [24].
A study of age-related changes in the incidence of squamous cell carcinoma (SCC) of the cervix, using stats from the United States Cancer Statistics (USCS) database showed that the vaccine program is failing for many age groups:
Given the initial older age of vaccine recipients, combined with low uptake in the USA [25], it is very unlikely that the observed drop in SCC incidence rate/year for women aged 21–29 years between 1999 and 2011 [26] has any relation to vaccination and is probably due to changes in screening practice (see Figure 1 in [26]).
It is much more likely that the increased drop in incidence rate/year seen for women aged 15–24 years from 2012 to 2017 could be related to the effects of vaccination. However, what is the explanation for the observed increase in incidence rate/year in women aged 25–29 during the same study period?
Since vaccination uptake in the USA was initially greatest in 16–19-year-olds in 2007 [25], it is very clear that a significant proportion of these will be captured in the 2012–2017 USCS cohort of women aged 25–29 years [26]. As a consequence, it is highly likely this cohort will also contain the largest number of women who were HPV-positive at the time of vaccination when it proves less effective.
However, this still does not explain the increase in SCC incidence rate/year between 2012 and 2017 and it is also notable that these data do not include the age group with the highest incidence of SCC in the USA, which is 30–44 years.
Print this out, frame it, and hang it above your desk:
From a 2020 Scottish study, with an uptake of over 80%, we learn that the incidence of cervical cancer (ICC) rate in the unvaccinated is 8.4 cases per 100,000 women/year. However, after a promising start, the ICC rates in the ≥18 years vaccinated now exceed those of the unvaccinated:
Compared to an incidence rate of 8.4 cases/100,000 women/year in the control unvaccinated cohort, no cases of ICC were detected in girls vaccinated when aged 12–13 years, although this group was tenfold smaller than the unvaccinated control group.
Nevertheless, a much larger group of girls vaccinated at ≥14 years also had a reduced incidence of 3.2 cases per 100,000 women/year. However, the incidence rates/100,000 women per year for age groups 14–16, 17–18 and >18 are not shown, although the results required to calculate this are included (see Table 2, footnote “d” in [27]).
Notably, 3601 women were vaccinated at >18 years, of whom <5 developed ICC. If the same type of calculation is performed, as for the younger age groups, these data do indicate a higher incidence rate for these women than the 8.4 cases/100,000 women/year in the unvaccinated control group, which is consistent with many of the preceding cited studies.
Get your flares out of the cupboard and put your ABBA records on, because in Sweden we can party like it’s 1977:
These aforementioned studies demonstrate a clear underlying trend of age group-specific changes in the incidence rate of ICC in HPV vaccinated communities as a general phenomenon which is not confined to a single country.
For example, in 2021 Swedish women aged 15–24 years had an all-time low incidence of ICC at 0.52/100,000 women/year whereas women aged 25–39 had an incidence rate of ~15/100,000 women/year, which showed little change from 1977–2007.
Indeed, from 2008, the rate increased for women aged 25–44, peaking at 20.5/100,000 in 2014, which then reduced to 16.1/100,000 in 2021 (see hyperlink reference [28], accessed 17 June 2024).
However, this rate is higher than that found for the same age group in 1977, despite intervening improvements in screening technology combined with the onset of vaccination.
The Finnish data excludes the effects of the SARS-CoV-2 gene therapy shots, as the 2021 cutoff wouldn’t provide a long enough latency period for ICC growth:
Arguably, out of all the Nordic countries, Finland has seen the most profound changes in ICC incidence over time. In 1965, prior to the onset of cervical screening, the incidence of ICC in Finnish women aged 25–44 was 17.2/100,000. By 1985, this reduced to 2.9/100,000 which was one of the lowest incidence rates in the world at that time.
Finland introduced a national HPV vaccination program for young girls in 2013 and yet, irrespective of screening and vaccination, from 1990 onwards the rate of ICC has steadily increased until the current peak rate of 15.8/100,000 in 2021 (see hyperlink reference [29], accessed 17 June 2024).
UK rates have also gone UP for those aged 25-34 since mass vaccination was introduced:
Furthermore, it is significant that a similar trend of increased incidence rates in women aged 25–34 years has also been observed in the UK, where this has increased from 13/100,000 in 2004 to 19/100,000 by 2018 (see hyperlink reference [30], accessed 17 June 2024).
Are older women being infected with non-HPV-vax cancer types, due to selective pressure from the younger vaccinated groups, or due to infections acquired prior to vaccination? Otherwise, their rates would have stayed at around 8-13/100,000.
These UK data also highlight the very low incidence rate of 1 case of ICC per 100,000 for women <24 years old, and which has remained the same since 1993.
In conclusion:
… at this stage it is not clear to what extent these alterations in HPV type prevalence will disseminate between mixed populations of vaccinated and unvaccinated women.
Most importantly, the oncogenic potential of these changes has not yet been explored in these populations and it has been speculated they may take significantly longer to cause ICC than HPV16. Indeed, it has been shown that women develop ICC, positive for HPV16, 18 or 45, 10.5 years younger than women with tumours positive for other HPV types [33].
Thus, moving forward, it is very clear that extensive long-term surveillance of at least >30 years from the present day will be essential in order to monitor vaccine-related changes in HPV type prevalence and how this affects the subsequent development of neoplasia.
Furthermore, it will also be very important to reassess both HPV and cytology testing practices in order to accommodate the impact of vaccine-related changes on the repertoire of HR cervical HPV infections over time [34].
They did try to warn the medical profession before:
Notably, these and other practices have been previously advocated in our earlier review on the effects of the prophylactic HPV vaccines [11].
Is it “paradoxical”, given that prior infection or vaccination primes your immune response to those types, and it does this at the expense of a greater risk of contracting and transmitting infections by future HPV types?
As time progresses, can we expect the younger vaccinated cohorts to also experience higher incidence rates of CIN1>CIN2>CIN3 and then ICC due to vaccine failure, immune priming, and HPV type replacement?
The authors do a great job of discussing vaccine escape, without using that expression:
The previously discussed assessment of national changes in the incidence of ICC, before and after the introduction of vaccination, highlights some interesting observations. For example, the recent increase in the incidence of ICC for women aged >25 in the Nordic countries [28,29] and the UK [30] is paradoxical considering their extensive vaccination programs, with a high uptake >80%.
I have to disagree:
Obviously, it may be too early for vaccination to have impacted on these statistics, although these findings clearly warrant further investigation, as indicated in our previous review [11].
The last line of the paper hints that cancers may be due to vaccine escape, with high-grade CIN types not expressing vaccinal-type L1 proteins:
As a final point, it is has [sic] been shown that Gardasil has efficacy as an adjuvant therapy against benign HPV6/11-related recurrent respiratory papillomatosis (RRP) which, given its prophylactic mode of action, may seem counterintuitive [37].
However, the most likely explanation is that, unlike high-grade CIN, RRP is a productive HPV infection which still expresses the L1 vaccine target protein, which promotes post-surgical immune surveillance of regrowing RRP lesions.
There is a COI declaration:
A.W.O. is employed by Ravan Bio Ltd. I.N.H. is CEO and director of Ravan Bio Ltd.
3.0 Parting shots
3.1 RSV deathshots™
Dr. Kevin Stillwagon does a fantastic job of explaining the madness:
Follow
No drug on earth should be associated with a 2% infant mortality when used as a preventative against a disease that has a death rate less than 1 in a thousand. Who ever heard of 1 in 50 babies dying?
Follow
Dear @Jikkyleaks and @RWMaloneMD
The situation is even more alarming. In an additional trial conducted by MERCK (protocol oo7), the Clesrovimab vs. Palivizumab study (see slide 16 in MERCK’s presentation to the CDC), the findings are deeply troubling: there were eight deaths in the Clesrovimab group (1.8%) compared to four in the Palivizumab group (0.9%) – that’s double the mortality rate! This discrepancy warrants further investigation and discussion (Presentation: https://cdc.gov/acip/downloads/slides-2024-10-23-24/02-RSV-Mat-Peds-Sinha-508.pdf…)
I wish they put as much effort into improving their sh!tty products as they did into hunting doctors down.
Nasty.
The Merck precedent Back in the early 2000s, pharmaceutical giant Merck faced growing criticism of its blockbuster painkiller, Vioxx. Internal emails uncovered during litigation revealed that Merck kept a literal “hit list” of doctors and academics who spoke out about the drug’s cardiovascular risks. “We may need to seek them out and destroy them where they live.”
3.2 Pharma share-apocalypse
A Note From QTR
Coming into 2025, I was openly skeptical of Big Pharma’s direction, which is why I included a short position on PPH (Invesco Pharmaceutical ETF) in my “25 Stocks to Watch for 2025” list. YTD the ETF is underperforming the SPY by about 3.5%.
My conviction stemmed from the belief that much of the sector’s valuation was built not on true efficacy or transparency, but on regulatory capture, lobbying power, and narratives shaped more by marketing than data.
More: https://quoththeraven.substack.com/p/fda-exposed-hundreds-of-drugs-approved
3.3 Baffled health insurers
Jeff did some excellent, but chilling analysis, saying the quiet part out loud.
I’ve been discussing the looming disaster for over five years, if you include Twitter (before my account got nuked). But it doesn’t make this any less horrifying —watching the story unfold. We would rather have been wrong in our analysis and predictions.
(Extract):
☕️ SUDDEN AND UNEXPECTED ☙ Thursday, July 3, 2025 ☙ C&C NEWS 🦠
From a politically seismic swamp reunion to a deep dive into the sudden and unexpectedly bad swamp of bright-red danger signals of a pending iatrogrenic catastrophe.
Jul 03, 2025
💉💉💉
… I will now depart from normal C&C practice, and make a calculated leap of speculation with potentially vast implications. But recent evidence demands it. Yesterday, ZeroHedge ran a story headlined, “Centene Crashes Most On Record, Sparks Selloff In Managed Care Stocks.” This is not good news. It could be awful.
The managed care industry got carpet-bombed yesterday, after Centene Corporation, one of the largest health insurers in the U.S., suffered the worst single-day stock drop in its history—crashing up to 40% after yanking its 2025 guidance. The crash was caused by devastating new actuarial data showing that Centene’s Affordable Care Act (Obamacare) enrollees are sicker, costlier, and fewer than expected, especially in 22 states where Centene holds significant market share.
The company now faces an unexpected $1.8 billion hit to its 2025 earnings, triggering immediate Wall Street downgrades and a sector-wide investor panic. UnitedHealth had already slashed its forecast weeks earlier and replaced its CEO. Now, analysts warn that the Obamacare risk pool is unraveling, with spiking Medicaid costs and mispriced premiums dragging down the entire industry. Bluntly, insurers had bet on healthy growth— but have hemorrhaging patients instead.
Centene Corporation is one of the largest health insurance providers in the United States, specializing in government-sponsored programs like Medicaid, Medicare Advantage, and Affordable Care Act (ACA) marketplace plans. Headquartered in St. Louis, Centene serves over 28 million members (about a tenth of the entire country), primarily low-income and vulnerable populations. Its rapid rise came from aggressively expanding into public health contracts across dozens of states, making it a bellwether for the broader managed care industry. In short, Centene is a key pillar of the federalized U.S. healthcare safety net.
But the company’s explanations made the hair on the back of my neck stand up. They cited two “unexpected” developments. First, morbidity (sickness and permanent disability) is rocketing upwards. Seon, at the same time, their insured pools are shriveling. The loss of enrollees is, presumably, because of excess death. Why else would very sick people drop off free or heavily subsidized insurance rolls?
Centene’s clients are not typical MAHA folks who might be fleeing ACA for non-traditional healthcare. They are folks captured by government healthcare.
💉 Centene’s customers are possibly the most heavily jab-propagandized populations on planet Earth. Its core customer base includes Medicaid recipients, ACA exchange enrollees, and Medicare Advantage members. I.e., seniors and low-income, working-age folks. These are precisely the populations that faced the most aggressive vaccine outreach, were most subject to institutional mandates and incentives, and had the fewest options to resist or opt out.
More: https://www.coffeeandcovid.com/p/sudden-and-unexpected-thursday-july?
They are either #Baffled and incompetent, or they are #Lying.
Pick one.
ABC article cited: https://www.abc.net.au/news/2025-07-07/cancer-diagnosis-rates-under-50s-rising-causes-four-corners/105495620
Denial of this mRNA vaccine cancer effect is killing people right now.
We need intervention research, and it is being blocked by dissonance and stupidity. I wish we had this data for the USA.
To a systems analyst, what happened here is OBVIOUS...
4.0 Concluding remarks
I have a shortlist of Substack literature reviews to author throughout the year. I was planning to write more about spike protein pathologies induced via the p53/SIRT1/HMGB1/RAGE pathway, associated miRNAs, and more about the link to ALS. But a day is a long time in public health, and recent developments and research papers tell us that HPV vaccination is another Big Pharma house of cards that is collapsing.
Thanks to Annelise’s collaboration, I’ve learned that the link between HPV vaccination and the RANKL/HMGB1/RAGE signalling pathway also needs reviewing. This may explain some of the observed cases of osteoporosis and neuropathologies.
Part II will continue this review, including the links between aluminium adjuvants and OP. This review was only meant to discuss OP. The bombshell link to cancer was unexpected, discussed in some of the same papers, and almost certainly the bigger story to follow up. I have more papers discussing the paradoxical ILT2 cervical and other cancers pathway.
If you are personally affected by anything discussed, and are concerned about vaccine escape I do have some good news. Many of the therapeutics discussed previously also act to suppress both the HPV infection and cancer risk. I’m building up a list to share next time, and so far, cervical-cancer specific research has demonstrated positive results for treatments using vitamin D, aspirin, or quercetin.
5.0 Disclaimer
This site is strictly an information website reviewing research into potential therapeutic agents. It does not advertise anything, or provide medical advice, diagnosis, or treatment. This site does not promote any of these as potential treatments or offer any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses, or pharmacists. This content is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Any extracts quoted in the previous article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
6.0 References
Mayo Clinic. ‘HPV Infection: Vaccine May Prevent Most Common Types-HPV Infection - Symptoms & Causes’. Accessed 30 June 2025. https://www.mayoclinic.org/diseases-conditions/hpv-infection/symptoms-causes/syc-20351596.
Tatang, Collins, Teigna Arredondo Bisonó, Aurore Bergamasco, Francesco Salvo, Sue Ann Costa Clemens, and Yola Moride. ‘Human Papillomavirus Vaccination and Premature Ovarian Failure: A Disproportionality Analysis Using the Vaccine Adverse Event Reporting System’. Drugs - Real World Outcomes 9, no. 1 (12 September 2021): 79–90. https://doi.org/10.1007/s40801-021-00271-6.
‘Human Papillomavirus Type 16 E7 Binds to E2F1 and Activates E2F1-Driven Transcription in a Retinoblastoma Protein-Independent Manner’. Journal of Biological Chemistry 277, no. 4 (25 January 2002): 2923–30. https://doi.org/10.1074/jbc.M109113200.
Ma, Kevin Sheng-Kai, Ning-Chien Chin, Ting-Yu Tu, Yao-Cheng Wu, Hei-Tung Yip, James Cheng-Chung Wei, and Ren-in Chang. ‘Human Papillomavirus Infections and Increased Risk of Incident Osteoporosis: A Nationwide Population-Based Cohort Study’. Viruses 15, no. 4 (April 2023): 1021. https://doi.org/10.3390/v15041021.
Li, Xiang, Guangjun Jiao, and Yunzhen Chen. ‘A Case–Control Study Based on the National Health and Nutrition Examination Survey to Evaluate the Effects of Human Papilloma Virus on Bone Health in Women’. BMC Medicine 23, no. 1 (7 February 2025): 75. https://doi.org/10.1186/s12916-025-03909-2.
Chen, Xiaojiang S., Robert L. Garcea, Ilya Goldberg, Gregory Casini, and Stephen C. Harrison. ‘Structure of Small Virus-like Particles Assembled from the L1 Protein of Human Papillomavirus 16’. Molecular Cell 5, no. 3 (1 March 2000): 557–67. https://doi.org/10.1016/S1097-2765(00)80449-9.
Witte, Lot de, Younes Zoughlami, Birgit Aengeneyndt, Guido David, Yvette van Kooyk, Lutz Gissmann, and Teunis B. H. Geijtenbeek. ‘Binding of Human Papilloma Virus L1 Virus-like Particles to Dendritic Cells Is Mediated through Heparan Sulfates and Induces Immune Activation’. Immunobiology, Macrophage and Dendritic Cells, 212, no. 9 (18 January 2008): 679–91. https://doi.org/10.1016/j.imbio.2007.09.006.
Colmenares, V., D. E. Noyola, A. Monsiváis-Urenda, M. Salgado-Bustamante, L. Estrada-Capetillo, R. González-Amaro, and L. Baranda. ‘Human Papillomavirus Immunization Is Associated with Increased Expression of Different Innate Immune Regulatory Receptors’. Clinical and Vaccine Immunology : CVI 19, no. 7 (July 2012): 1005–11. https://doi.org/10.1128/CVI.00043-12.
Herrin, Douglas M, Emily E Coates, Pamela J Costner, Troy J Kemp, Martha C Nason, Kapil K Saharia, Yuanji Pan, et al. ‘Comparison of Adaptive and Innate Immune Responses Induced by Licensed Vaccines for Human Papillomavirus’. Human Vaccines & Immunotherapeutics 10, no. 12 (1 November 2014): 3446–54. https://doi.org/10.4161/hv.34408.
Yamaguchi, Mako, Yohana S. Mtali, Hitomi Sonokawa, Ken Takashima, Yoshimi Fukushima, Takahisa Kouwaki, and Hiroyuki Oshiumi. ‘HPV Vaccines Induce Trained Immunity and Modulate Pro-Inflammatory Cytokine Expression in Response to Secondary Toll-like Receptor Stimulations’. Microbiology and Immunology 68, no. 2 (February 2024): 65–74. https://doi.org/10.1111/1348-0421.13108.
Zhou, Zheng, Jun-Yan Han, Cai-Xia Xi, Jian-Xin Xie, Xu Feng, Cong-Yi Wang, Lin Mei, and Wen-Cheng Xiong. ‘HMGB1 Regulates RANKL-Induced Osteoclastogenesis in a Manner Dependent on RAGE’. Journal of Bone and Mineral Research 23, no. 7 (July 2008): 1084–96. https://doi.org/10.1359/JBMR.080234.
Fang, Fang, and Dianming Jiang. ‘IL-1β/HMGB1 Signalling Promotes the Inflammatory Cytokines Release via TLR Signalling in Human Intervertebral Disc Cells’. Bioscience Reports 36, no. 5 (16 September 2016): e00379. https://doi.org/10.1042/BSR20160118.
Hampson, Ian N., and Anthony W. Oliver. ‘Update on Effects of the Prophylactic HPV Vaccines on HPV Type Prevalence and Cervical Pathology’. Viruses 16, no. 8 (August 2024): 1245. https://doi.org/10.3390/v16081245.
Modlin, John F., Ananda S. Bandyopadhyay, and Roland Sutter. ‘Immunization Against Poliomyelitis and the Challenges to Worldwide Poliomyelitis Eradication’. The Journal of Infectious Diseases 224, no. 12 Suppl 2 (30 September 2021): S398–404. https://doi.org/10.1093/infdis/jiaa622.






















"The court found that under federal law, Merck could not have independently added these warnings without FDA approval."
This is the most disgusting immoral technicality I have ever seen in a while.
Merck does zero investigation into the harms.
FDA claims there's no evidence of the harms.
Merck claims because the FDA claims there's no evidence of the harms (because Merck didn't do any research into the harms) they can't add warnings that the harms (that obviously exist) exist on their label.
The victims got fucked over and the court said 'Merck can't warn you because we've made it so the FDA prohibits warnings'. A regulatory agency that prohibits warnings. Man, burn that nonsense to the ground.
Very informative. I recall when they released that toxic shot. Having already been traveling the road of revelation regarding the harmaceutics it was nonetheless concerning. When they then introduced the push to also give young boys this toxic mixture, the depth of being appalled only went deeper. My children were early/preteen at the time and had not gone through the schedule. I knew my son would not submit himself to it... but wasn't sure about my daughter. The clinics in the area we lived in would take in young people and subject them to all manner of harmaceuticals without parental notification or consent.