Therapeutic properties of Icariin and its metabolites - Part II: Cancer (continued)
A literature review
Reading time:
short story - novelette - novella - novel - PhD thesis - Trump’s tariff list - War and Peace - U.S. Tax Code
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.


Contents
2.1.1 Icaritin
2.1.2 Icariside II
2.2.1 Cancer staging
2.2.2 The role of Epimedium in TCM cancer treatments
2.3 Icariin as a treatment for long COVID and gene therapy poisoning
1 Introduction
Part I introduced the source of icariin and its metabolites, the genus Epimedium, and the anti-cancer therapeutic mechanisms of icariin.
Part II extends the review to include the anti-cancer properties of icaritin and icariside II, discusses clinical trials, TCM cancer treatments, and icariin for treating long COVID and vaccine poisoning.
Parting Shots will, as ever, include some late-breaking stories and articles that don’t quite justify their own Substack or fit in anywhere else.
2 Discussion
2.1 Anti-cancer properties

2.1.1 Icaritin
Icaritin is a prenylated flavonoid (or prenylflavonoid), a derivative of icariin which lacks the glycosidic bonded sugar groups. These are known to have potential anti-fungal and anti-microbial properties1, but studies have also demonstrated anti-cancer efficacy too at non-cytotoxic concentrations.

As with icariin, it’s harder for cancer stem cells and tumours to become resistant to icaritin because it interacts with several distinct cancer mechanisms and signaling pathways.
The bonus with taking extracts from the plant is that you get all these versatile therapeutic compounds in one dose.
Cell lines and pathways studied:
Generalised anti-tumour effects in mice, through the promotion of T-cell infiltration2; in mice and human cell lines “… via their effects on multiple biological pathways, including cell cycle regulation, apoptosis, angiogenesis, and metastasis, and a variety of signaling pathways including JAK2-STAT3, MAPK-ERK, and PI3k-Akt-mTOR.”3
Even a small increase in T-cell and NK cell numbers may have a disproportionately beneficial effect on the prognosis, or on reducing the likelihood of developing cancer in the first place.
Here, I present eight types of evidence in support of the cancer immunosurveillance hypothesis.
First, primary immunodeficiency in mice and humans is associated with increased cancer risk.
Second, organ transplant recipients, who are treated with immunosuppressive drugs, are more prone to cancer development.
Third, acquired immunodeficiency due to infection by human immunodeficiency virus (HIV-1) leads to elevated risk of cancer.
Fourth, the quantity and quality of the immune cell infiltrate found in human primary tumors represent an independent prognostic factor for patient survival.
Fifth, cancer cells harbor mutations in protein-coding genes that are specifically recognized by the adaptive immune system.
Sixth, cancer cells selectively accumulate mutations to evade immune destruction (“immunoediting”).
Seventh, lymphocytes bearing the NKG2D receptor are able to recognize and eliminate stressed premalignant cells.
Eighth, a promising strategy to treat cancer consists in potentiating the naturally occurring immune response of the patient, through blockade of the immune checkpoint molecules CTLA-4, PD-1, or PD-L1.
Thus, there are compelling pieces of evidence that a primary function of the immune system is to confer protection against cancer.
From: “Does the Immune System Naturally Protect Against Cancer?“ (2014)
Hepatocellular carcinoma, through the IL-6/Jak2/Stat3 inflammatory pathway.4

From: “Figure 1 Production source and main signaling pathway of IL-6. IL-6 transduction is mainly produced by monocytes, macrophages, T cells, B cells, fibroblasts, etc. IL-6R binds to the surface of the cellular membrane through the classical pathway. In the signaling transduction pathway, IL-6 binds to sIL-6R and then initiates signaling transduction.” https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2021.76097 Hepatocellular carcinoma, through the inhibition of splenic myeloid-derived suppressor cell (MDSC) generation5.
Hepatocellular carcinoma, through immunomodulation of myeloid-derived suppressor cells (MDSCs)6.
Hepatocellular carcinoma, through the use of nanomedicine‐boosting icaritin-based immunotherapy7.
Breast cancer cells, through sustained activation of the extracellular signal-regulated kinase (ERK) pathway8, and it beat tamoxifen in this study!
Here, we report that icaritin strongly inhibited the growth of breast cancer MDA-MB-453 and MCF7 cells. At concentrations of 2–3 μM, icaritin induced cell cycle arrest at the G2/M phase accompanied by a down-regulation of the expression levels of the G2/M regulatory proteins such as cyclinB, cdc2 and cdc25C.
Icaritin at concentrations of 4–5 μM, however, induced apoptotic cell death characterized by the accumulation of the annexin V- and propidium iodide-positive cells, cleavage of poly ADP-ribose polymerase (PARP) and down-regulation of the Bcl-2 expression.
In addition, icaritin also induced a sustained phosphorylation of extracellular signal-regulated kinase (ERK) in these breast cancer cells. U0126, a specific ERK activation inhibitor, abrogated icaritin-induced G2/M cell cycle arrest and cell apoptosis.
Icaritin more potently inhibited growth of the breast cancer stem/progenitor cells compared to anti-estrogen tamoxifen.
Our results indicate that icaritin is a potent growth inhibitor for breast cancer cells and provide a rationale for preclinical and clinical evaluations of icaritin for breast cancer therapy.
From: “An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells” (2011)
https://pubmed.ncbi.nlm.nih.gov/21376032/

Prostate cancer cells, via the regulation of miR-381-3p and its target gene UBE2C9; via the inhibition of high-fat diet-induced serum adipokine, in a TRAMP mice model.
Adipokines are proteins produced by fat cells. From these, leptin acts as a prostate cancer-promoting signalling molecules10.Glioblastoma cells, by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling, and inhibition of metastatic epithelial mesenchymal transition (EMT)11.
Note that a hypoxic environment induces hypervascularization and necrosis, which are the hallmarks of GB. The positive feedback of the hypoxic pathway helps to drive tumor growth:
Glioblastoma, through tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis12.

Graphical abstract. “TNF-related apoptosis-inducing ligand (TRAIL or Apo2 or TNFSF10) belongs to the TNF superfamily. When bound to its agonistic receptors, TRAIL can induce apoptosis in tumour cells, while sparing healthy cells.” https://www.mdpi.com/2073-4409/13/6/521# Glioblastoma, by positively modulating estrogen receptor β13.

From: “Figure 2. Genomic and non-genomic pathways of estrogen receptors (ERs). ERs can act through genomic pathways and nongenomic pathways. The nongenomic pathways may also result in the transcriptional activation of genes. (1) Classic pathway: dimerization of ERs triggered by the binding of E2, followed by nuclear translocation to regulate the transcriptional activity of genes bearing the estrogen response element (ERE). (2) ER activity independent of ERE: the recognition of promoters result from the protein−protein interaction of ERs with other transcription factors (TF). (3) Ligand-independent activity: the binding of growth factors to membrane receptors triggers protein kinase cascades that activate ERs by phosphorylation, promoting their nuclear translocation and recognition of the ERE in target genes. (4) Nongenomic pathway: the binding of the ligand causes mER and GPER1 to initiate signaling pathways, which promote the activation of enzymes and transcriptional factors that can regulate the transcription of diverse genes. ER, estrogen receptors; mER, membrane estrogen receptors; TF, transcription factor; ERE, estrogen response element; non-ERE, other transcription factors distinct from ERE; P, phosphorylated receptor; G, G protein-coupled receptor; GPER, G protein-coupled estrogen receptor; cav, caveolin.” https://www.mdpi.com/1422-0067/22/22/12404 Colorectal cancer cells, through targeting HSP90-TXNDC9 interaction via the sensitisation of low-temperature photothermal therapy14.
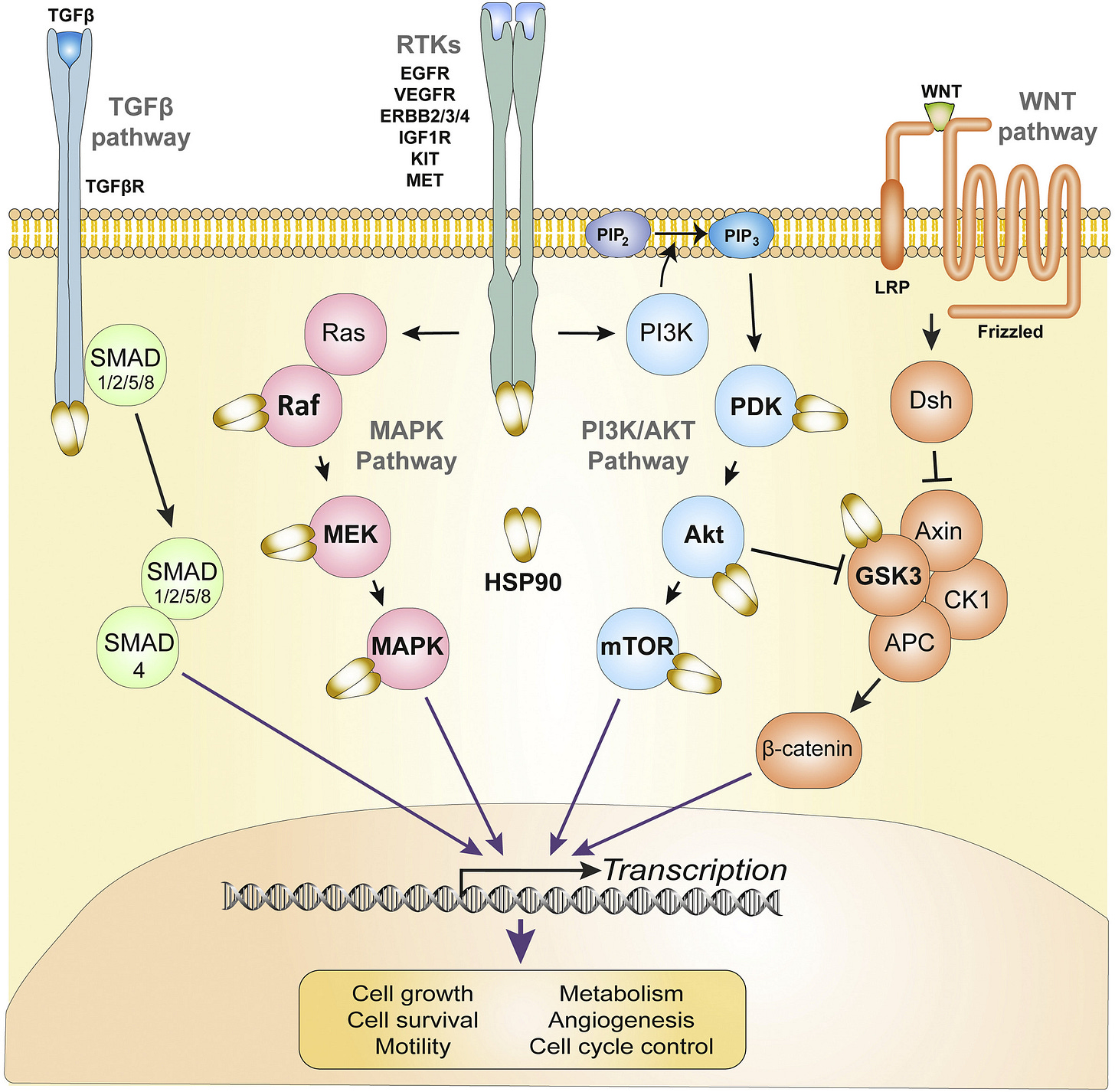
From: “Fig. 1. HSP90 chaperonage of oncogenic pathways in CRC. Four major oncogenic pathways that drive growth, proliferation and invasiveness of CRC are simplified and depicted in distinct colors. Receptor and downstream proteins marked in bold followed by an HSP90 molecule indicate signaling mediators that are known HSP90 clients in CRC. HSP90 – Heat shock protein 90; TGF-β – Transforming growth factor beta; TGFβR – TGF-β receptor; SMAD – Mothers against decapentaplegic homolog; EGFR – epidermal growth factor receptor; VEGFR – vascular endothelial growth factor receptor; ERBB – erythroblastic oncogene B; IGF1R – Insulin-like growth factor 1 receptor; KIT – KIT Proto-oncogene receptor tyrosine kinase; MET – MET proto-oncogene receptor tyrosine kinase; Ras – Kirsten rat sarcoma viral proto-oncogene; Raf – Raf proto-oncogene serine/threonine protein kinase; MEK – Mitogen-activated protein kinase kinase 1; MAPK – Mitogen-activated protein kinase; PI3K – Phosphoinositide 3-kinase; PIP2 – Phosphatidylinositol 4,5-bisphosphate; PIP3 – Phosphatidylinositol (3,4,5)-trisphosphate; PDK – Pyruvate dehydrogenase kinase; Akt – Protein kinase B; mTOR – Mechanistic target of rapamycin; Wnt – Proto-oncogene Wnt; LRP – Lipoprotein receptor-related protein; Dsh – Dishevelled protein; GSK-3 – Glycogen synthase kinase 3; CK1 – Casein kinase 1; APC – Adenomatosis polyposis coli.” Lung cancer tumor-bearing mice, through the enhancement of T lymphocyte responses15.
2.1.2 Icariside II
Icariside II (Chinese name: Baohuoside I) is the major pharmacological metabolite of Icariin:

Icariside II, an active flavonoid, is extracted from the traditional Chinese medicinal herb Epimedii. It possesses multiple biological and pharmacological properties, including anti-inflammatory, anticancer, and anti-osteoporotic properties.
In recent years, apoptosis has become the hot spot in anticancer therapies. Icariside II exerts positive effects on inducing apoptosis and inhibiting proliferation in various cancers.
The antitumorigenic activity of Icariside II was also proven through cell cycle arrest, triggering autophagy, reducing cellular metabolism, and inhibiting cancer metastasis and tumor-associated angiogenesis.
Additionally, Icariside II, as a natural product, contributed to a synergistic effect alongside chemotherapeutic drugs. Due to its poor aqueous solubility and permeability, more strategies were developed to improve its therapeutic effects.
From: “Icariside II: Anticancer Potential and Molecular Targets in Solid Cancers” (2021)
https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.663776/full
Cell lines and pathways studied:
Osteosarcoma, by blockade of the epidermal growth factor receptor/mammalian target of rapamycin (EGFR/mTOR) signaling pathway, via reduced cell proliferation16.
Prostate cancer, through suppression of both constitutive and arachidonic acid (AA)-induced cyclooxygenase-2 (COX-2) expression as well as reduced prostaglandin E2 (PGE2) levels, via the induction of apoptosis17.
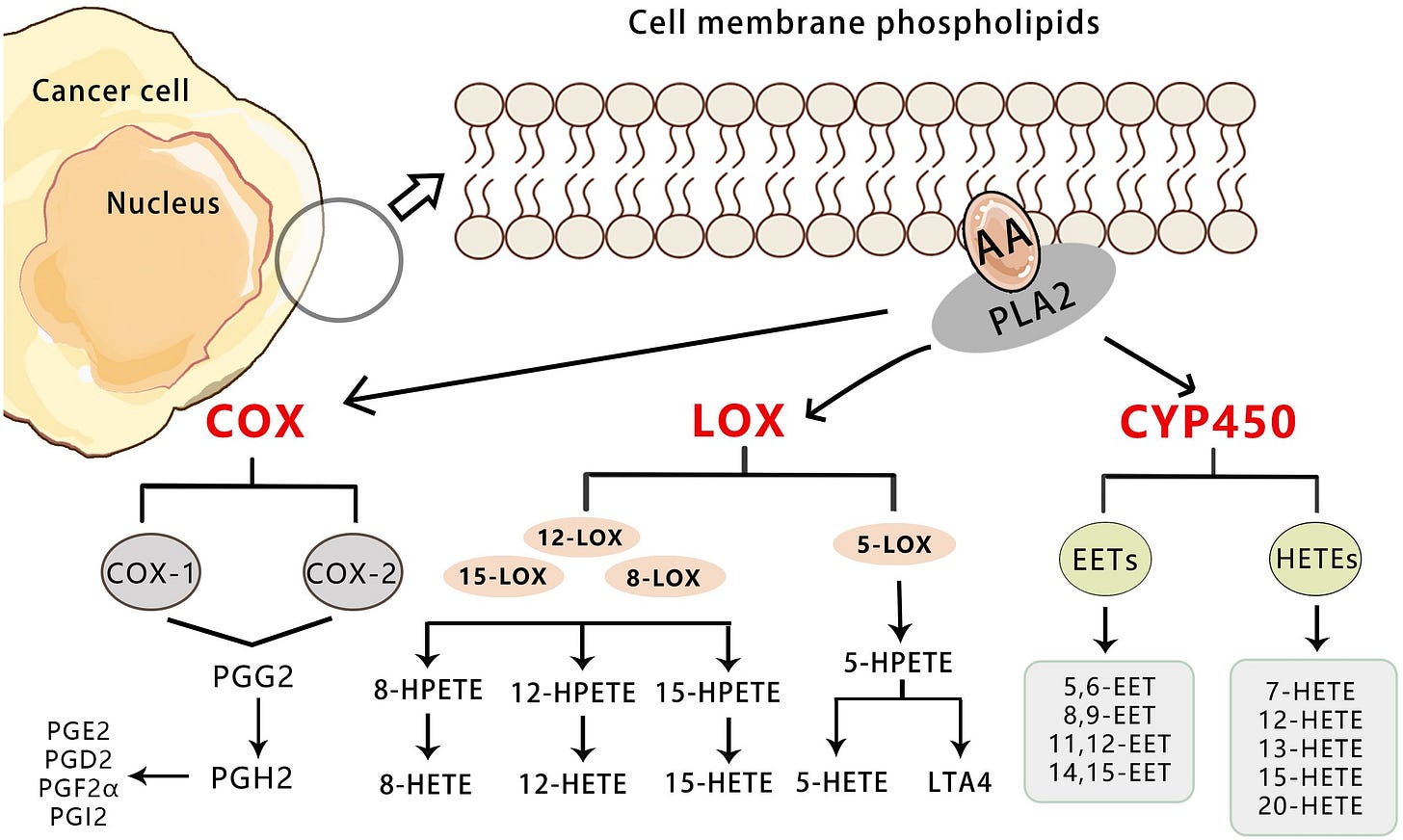
From: “Figure 1 Overview of the three pathways for AA metabolism in cancer. Upon cell stimulation, phospholipase A2 induces FFAs, primarily AA derived from the lipid bilayer. The released AA is metabolized into bioactive lipid signaling molecules by three different enzymatic pathways. COX-1/2 metabolize AA to a series of prostaglandins (PGD2, PGE2, PGF2, PGH2, and PGI2).5-LO metabolizes AA to 5-HETE, LTB4, and cysteine leukotrienes, and 12-LO metabolizes AA to 12-HETE. Both subtypes of 15-LO metabolize AA to 15-HETE and, to a lesser extent, 12-HETE. Cytochrome P450 metabolizes AA to 19-/20-HETEand so on.” https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2024.1381894/full Prostate cancer via the PI3K-AKT-mTOR signaling pathway18;
Lung cancer cells, through the induction of S phase cell cycle arrest and apoptosis19; non-small cell lung cancer cells, through the enhancement of cisplatin-induced apoptosis, by promoting endoplasmic reticulum (ER) stress signalling20; through the enhancement of anti-PD-1 immune checkpoint blockade by reducing chemotactic infiltration of myeloid-derived suppressor cells (MDSCs) into the tumor microenvironment via ROS-mediated inactivation of the SRC/ERK/STAT3 signaling pathways21;22
Ovarian cancer, by regulating the miR-144-3p/IGF2R axis.
“Icariside II markedly increased the level of miR‐144‐3p in ovarian cancer cells. Moreover, IGF2R was targeted by miR‐144‐3p directly. Icariside II significantly decreased the expression of IGF2R and the phosphorylation level of AKT and mTOR in ovarian cancer cells, which were partially reversed by miR‐144‐3p inhibitor.“23.
IGF2: Insulin-like growth factor 2. There is an interesting link between obesity, diabetes, and the risk of ovarian cancer through IGF2.
Gastric cancer cell invasion and migration, by vasodilator-stimulated phosphoprotein via the Rac1-dependent VASP pathway24;
Renal cell carcinoma, through the inhibition of JAK/STAT3 signalling25;
Acute myeloid leukemia (AML) cells, through the inactivation of STAT-3 signalling26;
Melanoma cell-bearing mice, through the downregulation of the JAK-STAT3 and MAPK survival pathways27;
U87 and A172 glioblastoma cells, through the suppression of Akt activation and by potentiation of FOXO3a activity: “ICA II could inhibit cell proliferation by blocking Akt-dependent FOXO3a phosphorylation and promoting FOXO3a transportation into the nucleus.”28.

From: “Fig. 2 The functions and regulation of FOXO3a. The non-phosphorylated form of FOXO3a located in nucleus actively mediates multiple cellular processes, including cell apoptosis, proliferation, cell cycle, survival and DNA damage by inducing transcription of its target genes depends on the upstream stimuli. The growth factor signaling induced activation of protein kinases such as PKB, ERK, SGK, IKKΒ terminate FOXO3a activity by phosphorylation (in active form). The phosphorylated FOXO3a binds with 14–3-3 protein, which consequently leads to nuclear export of FOXO3a. In the cytoplasm, FOXO3a is ubiquitinated and degraded in a proteasome-dependent manner”. Critical role of FOXO3a in carcinogenesis Colorectal cancer, by epigenetically regulating the Circβ-Catenin-Wnt/β-Catenin axis29.
Wnt ON state: Gene transcription and multiple cancer pathways are activated:
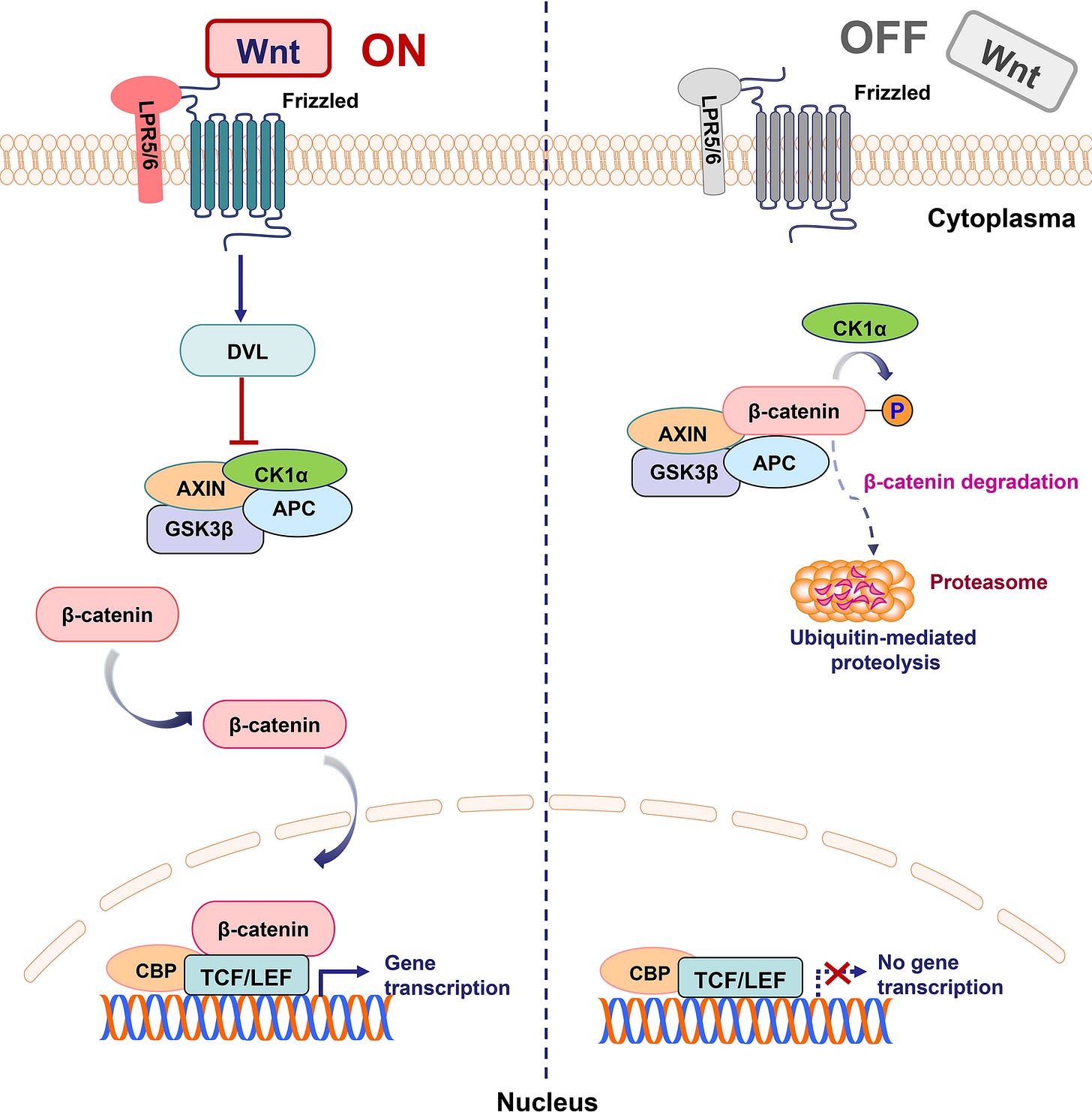
From: “Schematic representation of activated and inhibited Wnt/β-catenin pathway. “WNT ON state”: Upon ligation of Wnts to their receptors composed of frizzled proteins and LRP5/6, the cytoplasmic protein DVL is activated and induces the suppression of GSK3β. Subsequently, stabilized β-catenin translocates into the nucleus and binds to TCF/LEF transcription factors to lead to target gene transcription. “WNT OFF state”: In the absence of WNT ligand, the destruction complex of β-catenin, a tertiary complex formed by AXIN, CK1α, GSK3β and APC, phosphorylates β-catenin, which subsequently undergoes the ubiquitin-proteasomal degradation”. Targeting the Wnt/β-catenin signaling pathway in cancer
2.2 Clinical trials
OK, so we know from the hundreds of cell-line and in vivo research papers that icariin, icaritin, icariside II, and other metabolites can positively inhibit many different types of cancer through multiple pathways.
We also know that these mechanisms should help first-line allopathic treatments to work more effectively by overcoming resistance and by reducing the risk of recurrence via cancer stem cells.
But before you rush off to place an order, we need to pause here.
What do all these mean in practice, to the patient with cancer or the undiagnosed who may otherwise succumb to the disease? After all, “clinical beats lab”.
Most of the new cancer drugs that looked promising in the lab fail later at the trial stage. There is a saying that even a bullet can kill cancer cells in a petri dish!
There are many reasons why lab results may not apply equally to the patient. For example:
Humans may react differently to cell line studies or animal models, despite being relatively close genetically, or bred for the purpose.
The form of the drug may be different in the clinical setting from the lab setting. For example, ivermectin working more effectively because it forms a precipitate in vivo.30
The lowest experimental dose, measured in Moles (M), may have been much greater than the human plasma concentration when taken as a standard dose.
In humans, despite its theoretical efficacy, the drug may not be able to reach the tumour sites at a high enough concentration to be effective. The tumour may also inhibit penetration due to hypoxic conditions, ischemic conditions, or a low pH. This is also why you cannot expect a miracle from any drug if the cancer is too advanced. Don’t blame the fenbendazole!
It may be difficult to prove a negative (disease prevention) in small-scale, short-term experiments where the control groups may not provide much of a signal. It may take decades to fully evaluate with any degree of statistical significance, and other factors need to be considered too, such as genetic profile, lifestyle, comorbidities, health history, and other treatment regimes.
Despite this, although not many clinical trials have been funded, the outcomes are encouraging. There is an in-joke here:
The Chinese have been doing this informally for not just a year or two, but for thousands of years, and passing on the knowledge down the centuries.
Trial phases explained. Extracted from “CLINICAL TRIAL ENDPOINTS“ (emphasis mine):
ENDPOINTS NEED TO MATCH THE PURPOSE OF THE TRIAL
Phase 1:
Evaluate toxicity
Study drug disposition (pharmacokinetics, PK)
Proof of concept that drug inhibits its target (pharmacodynamics, PD)
Determine dose and schedule for Phase 2
Phase 2:
Estimate anti-tumour efficacy
Further define toxicity
Further PD studies
Phase 3:
Compare outcomes reflecting patient benefit with usual standard of care
ENDPOINTS APPROPRIATE FOR OTHER TYPES OF TRIAL
Phase 0:
Trials in which a (usually) low dose of a drug is given. Appropriate endpoints are measures of drug disposition and target inhibition
Phase 4:
Post-marketing studies. Appropriate endpoints are those of efficacy and toxicity under real-life conditions
Trials of local therapy:
In addition to endpoints used in trials of systemic therapy, other appropriate endpoints may include:
Local relapse-free survival
Functional effects
Completeness of resection
ENDPOINTS IN PHASE I AND PHASE II TRIALS
While the primary goal of phase I trials is to evaluate toxicity and tolerance (and PK and PD) agents that show no signs of activity rarely succeed in later trials.
The primary goal in phase II is to determine if there is sufficient evidence of antitumour activity to undertake further studies in phase III (very expensive in terms of human and €€€ resources).
Appropriate endpoints for phase II include measures of anti-tumour activity such as Overall Response Rate (ORR) or reduction of a tumour marker (e.g. PSA response rate).
Progression-free survival (PFS) or percent without progression at a given time are also appropriate endpoints in phase II trials, especially if they are randomised.
Identification of biomarkers is important in early phase trials. New endpoints such as reduction in circulating tumour cells (CTCs) are under investigation
TUMOUR RESPONSE AS AN ENDPOINT
Evaluation of Tumour response has been standardised using (modified) “Response Evaluation Criteria in Solid Tumours” (RECIST).
Stable Disease (SD) is reported frequently as an endpoint with the implication that it reflects an anti-tumour effect of a drug rather than a criterion to continue treatment.
While long-term SD (e.g. > 6 months) might imply anti-tumour effects, a tumour growing steadily with a volume doubling time of ≥2 months (typical for human tumours) will satisfy SD ≥1 month.
Endpoints used in Phase II trials do not measure benefit to patients. Tumour shrinkage is rarely correlated with endpoints of patient benefit such as Overall Survival (OS) or Quality of Life (QoL)
“Clinical benefit rate” (CR+PR+SD) should not “clinical benefit”.
ENDPOINTS IN PHASE III TRIALS
The goal of Phase 3 trials is to compare outcomes reflecting patient benefit with the usual standard of care.
There are essentially only 2 ways in which patients may benefit from treatment:
They either live longer or they live better.
Thus the most appropriate endpoints of phase III trials are:
Overall Survival (OS)
Quality of Life (QoL)
Any other endpoint is a surrogate endpoint, and should be shown to predict OS or QoL.
SURROGATE ENDPOINTS IN PHASE III TRIALS
While OS is a preferred endpoint and not subject to bias, the survival time for patients with many types of cancer is (fortunately) quite long. This is especially true for trials of adjuvant therapy.
Disease-Free Survival (DFS), also known as Relapse-Free Survival (RFS), is often used as a primary endpoint in phase III trials of adjuvant therapy.
Progression-Free Survival (PFS) is used commonly as a primary endpoint in phase III trials evaluating treatment of metastatic cancer.
Since the size of a trial is determined by the number of “events”, and recurrence or progression of cancer usually occurs before death, trials with DFS or PFS as the primary endpoint can be evaluated earlier, and require a smaller sample.
Some investigators also prefer these endpoints because they are not influenced by subsequent therapies.
ADVANTAGES AND DISADVANTAGES OF DFS OR PFS AS ENDPOINTS IN PHASE III TRIALS
Advantages
“Events” occur earlier, so trials can be reported earlier, potentially accelerating availability of active new treatments
Greater number of “events” allows smaller trials
Not subject to effects of subsequent treatment
Disadvantages
Not measures of patient benefit
Delayed tumour progression, but with toxicity causing detriment in QoL, and no improvement in OS, does not convey clinical benefit
Subject to bias
Uncertainty of time of relapse or progression
Censoring bias – imbalanced withdrawal of patients who are censored prior to relapse or progression
More: https://dam.esmo.org/image/upload/Tips-Tricks-Clinical-Trial-Endpoints.pdf
2.2.1 Cancer staging
Cancer staging provides a guide to the extent of your cancer, such as how large a tumour is, and how far it may have spread.
The stage referred to is always the stage it was given at diagnosis, even if it later gets worse or spreads. New information is then added to the original stage, so that its state and progression is easily discerned at a glance.
Various x-rays, lab tests, other tests, and procedures may be used to diagnose the cancer and for staging.
There is no single staging system. Some are particular to a type of cancer, whereas others may be used against many different types. An example of this is the TNM staging system.
Regardless of the system used, most include information for:
Tumour location.
Tumour size.
Has the cancer spread to nearby lymph nodes?
Has the cancer spread to any other parts of the body?
The TNM system is named after its components: Primary tumour (T), Regional lymph nodes (N), and Distant metastasis (M), and these are combined, as in “T1N0MX” or “T2N1M0”.
Primary tumour (T):
TX: Main tumour cannot be measured.
T0: Main tumour cannot be found.
T1, T2, T3, T4: Refers to the size and/or extent of the main tumour. A higher number indicates a larger tumour, or one that has spread into nearby tissues. Additional detail may be added by adding a letter code, i.e. T1a, T1b etc.
Regional lymph nodes (N):
NX: Cancer in nearby lymph nodes cannot be measured.
N0: There is no cancer in nearby lymph nodes.
N1, N2, N3: Refers to the number and location of lymph nodes that contain cancer. The higher the number, the more lymph nodes that have been invaded.
Distant metastasis (M):
MX: Metastasis cannot be measured.
M0: Cancer has not spread to other parts of the body.
M1: Cancer has spread to other parts of the body.
Staging may also be described using numbers, such as Stage 4 or Stage IV, and a letter may be added for more detail, such as Stage IVB.
Higher numbers denote a more advanced cancer:
Stage 0: Abnormal cells are present that haven’t spread.
Also called “carcinoma in situ” or “CIS”, which denotes a group of abnormal, pre-cancerous cells or lesion.
Stage 1-3 or Stage I-III: The higher the number, the larger the tumour and the more neighbouring tissues or organs have been invaded.
Stage 4 or Stage IV: This means that the cancer has spread (metastasised) to distant parts of the body.
The other type of staging frequently used is more descriptive:
In situ: Abnormal cells are present but have not spread to nearby tissue.
Localized: Cancer is limited to the place where it started, with no sign that it has spread.
Regional: Cancer has spread to nearby lymph nodes, tissues, or organs.
Distant: Cancer has spread to distant parts of the body.
Unknown: There is insufficient information to assign a stage.31
Liu et al summarised clinical trials data in “Icariin as a potential anticancer agent: a review of its biological effects on various cancers” (2023)32.
One of these is still underway, therefore, no results are available.
Key takes, extracted from TABLE 1.
I will include the dosing information, recorded from the trial records at https://clinicaltrials.gov/
Cancer: Non-small cell lung cancer
Clinical trial: -
Date: 2017-2018
Phase: -
Drug: Icaritin combined with Paclitaxel + Cisplatin or (Nivolumab/Pembrolizumab) + bevacizumab
Drug: Paclitaxel + Cisplatin or (Nivolumab/Pembrolizumab) + bevacizumab
Actual Enrollment: 33 participants
Intervention Model: Parallel Assignment
Primary Outcome Measures: median progression-free survival (mPFS)
Conclusions: The combination of icaritin with standard regimens has shown certain clinical efficacy and good safety tolerability in the treatment of advanced non-small cell lung cancer Zhou et al. (2019)
Cancer: Advanced hepatocellular carcinoma
Clinical trial: NCT02496949
Date: 2015
Phase: 1b
Drug: Icaritin
Actual Enrollment: 28 participants
Intervention Model: Single Group Assignment
Primary Outcome Measures: To assess safety of icaritin in advanced hepatocellular carcinoma patients (Time Frame: 1–2 years)
Conclusions: Icaritin was generally well-tolerated without dose limited toxicity (DLT) across tested dose levels. Preliminary durable survival benefits observed in patients with advanced HCC patients. Effect correlated with the drug immune-modulation activity Fan et al. (2019)
Cancer: Advanced hepatocellular carcinoma
Clinical trial: NCT01972672
Date: 2015
Phase: II
Drug: Icaritin
Actual Enrollment: 70 participants
Intervention Model: Single Group Assignment
Primary Outcome Measures: time to progress(TTP) (Time Frame: 1–2 years)
Conclusions: Icaritin has demonstrated satisfactory clinical safety and immunomodulatory clinical efficacy. Improved OS was observed in the subgroups of advanced HCC patients including PD-L1-positive immune cell expression Sun et al. (2018)
Cancer: Advanced hepatocellular carcinoma
Clinical trial: NCT03236636
Date: 2017-2019
Phase: III
Drug: Icaritin
Drug: Huachansu
Actual Enrollment: 312 participants
Intervention Model: Parallel Assignment
Primary Outcome Measures: Overall survival (OS) (Time Frame: 2–4 years)
Condition: Studies that have been completed but not published
From Wiki:
“HuaChanSu (bufo bufo gargarizans) is a traditional Chinese medicine extracted from the skin of toads from the genus Bufo that is believed by some [who?] to slow the spread of cancerous cells.[1] The parotoid gland of toads of the Bufo genus secrete a venom, which is dried and dissolved in water. This solution, HuaChanSu, is injected into a cancerous area and targets specific cancer cells.[1][2] HuaChanSu is undergoing further trials, and its effect is not completely understood.”
Cancer: HCC
Clinical trial: NCT03236649
Date: 2017-2022
Phase: III
Drug: Icaritin
Drug: Sorafenib Tosylate Tablets
Actual Enrollment: 89 participants
Intervention Model: Parallel Assignment
Primary Outcome Measures: OS (Time Frame: 1–2 years)
Condition: Not yet completed
From Wiki:
“Sorafenib, sold under the brand name Nexavar,[3] is a kinase inhibitor drug approved for the treatment of primary kidney cancer (advanced renal cell carcinoma), advanced primary liver cancer (hepatocellular carcinoma), FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.”
Cancer: Poor prognosis HCC
Clinical trial: NCT05594927
Date: 2022-2025
Phase: III
Drug: Icaritin
Drug: Huachansu
Actual Enrollment: 261 participants
Intervention Model: Parallel Assignment
Primary Outcome Measures: OS (Time Frame: From randomization to death from any cause, assessed up to approximately 24 months)
Condition: Not yet completed
Expanding on the above, some of the trials have been written up.
I will parse the abstracts for clarity.
From “A multicenter, single arm phase II trial of a small molecule immune-modulator icaritin: Safety, overall survival, immune dynamics, and PD-L1 expression in advanced hepatocellular carcinoma.” (2013), by Sun et al33:
OS: Overall survival.
DCR: Disease control rate; “the percentage of patients with advanced cancer whose therapeutic intervention has led to a complete response, partial response, or stable disease.”34
Background: HCC was characterized with high heterogeneity and immune “tolerogenic”. Despite of immune checkpoint inhibitors have demonstrated promising results with improved overall survival, a significant fraction of advanced HCC patients are still left with limited treatment options and poor survival.
The purpose of this study is to determine the safety, clinical activity and immune dynamic biomarkers of a small molecule icaritin, IL-6/STAT3 immune-modulator in advanced HCC.
Methods: Major eligibility criteria include histologically confirmed unresectable HCC patients with Child-Pugh Class A or B liver function. Total of 70 advanced HCC patients were enrolled and administrated with 600 mg b.i.d.
Primary endpoints were TTP, secondary endpoints were safety, OS, and DCR.
Local disease control was defined as no progressive disease (PD) by RECIST.
Kaplan-Meier analysis was utilized for OS assessment. Immune dynamic of NLR, IL-6 and baseline PD-L1 expression of immune cells was evaluated, retrospectively.
Results:
There was no ≥grade III drug related AE observed in all enrolled 70 advanced HCC patients.
Objective response evaluation in per-protocol population showed PR (1.6%), SD (32.8%) and PD (59.0%) and median overall survival (OS) 254day (95% CI, 172-296).
DCR was achieved 34.4% (95% CI, 22.7-47.7%); Median OS for the PD-L1-positive (n = 9) and negative (n = 24) subgroups were 389 (95% CI, 80-522) vs. 286.5days (95%CI, 135-482), for IL-6-advantage (n = 24) and disadvantage (n = 16) subgroups were 366.5 (95% CI, 277-566) vs. 157days (95% CI, 125-254), for NLR-advantage (n = 27) and disadvantage (n = 23) subgroups were 295(95% CI, 235-509) vs. 178days (95% CI, 135-296), respectively.
Conclusions: Icaritin has demonstrated its favorable clinical safety and immune-modulation clinical efficacy. The improved OS was implicated in the subgroups of advanced HCC patients including PD-L1-positive immune cell expression. Both safety and immune-response efficacy are warranted for the phase III trial. Clinical trial information: NCT01972672.
From “A first-in-human phase I study of ER-α36 modifier icaritin in patients with advanced solid tumors” (2013), by Fan et al35.
Background: ER-α36 was recently identified to be expressed in varieties of cancers and may play important roles in carcinogenesis and tumor progression.
Icaritin, a natural prenylflavonoid derived from the Chinese herb Epimedium, is a first of its kind ER-α36 modifier, which demonstrated potent anti-tumor effect in multiple cancer cell lines and their xenograft models.
This study aims to determine its safety, tolerability, pharmacokinetics (PK), and potential antitumor activity.
Methods: This phase I study comprises phase Ia and Ib.
In phase Ia part, patients with advanced breast cancer (ABC) were treated with escalating doses of Icaritin orally once daily on a continuous 28-day dosing schedule.
In phase Ib part, dosing was fixed to 600 or 800mg twice daily and expansion was made to other selected malignancies including hepatocellular cancer (HCC), colorectal cancer (CRC) and intrahepatic cholangiocarcinoma (ICC) to further explore PK parameters and efficacy.
Results: 24 patients were enrolled to receive Icaritin at six dose levels ranging from 50mg to 1600mg per day in phase Ia.
No dose limited toxicity (DLT) was found even in the highest dose defined in the protocol, thus the maximum tolerated dose (MTD) was not reached.
Only grade 1 drug-related adverse events were observed including neutropenia, ALT elevation, hypercholesteremia, fatigue, anorexia, hypertriglyceridemia, proteinuria, myalgia, hot flash and rash.
PK data from the fed dosing showed 3-fold increase of Cmax and AUC compared with the fast dosing. Half life was around 2-7 hours.
Among 22 evaluable subjects, no complete or partial response (CR or PR) was detected, 5 patients had stable disease(SD)for 3 months or longer.
For phase Ib study, 24 patients had been enrolled. One ABC, 2 CRC and 3 ICC patients progressed after 2 months of medication.
Among 7 HCC patients already evaluated, 1 obtained PR and progressed after one year of treatment and 2 remained in the study, stable for 5 months.
Similar drug related toxicity profile was noted in phase Ib.
Conclusions: Icaritin was generally well-tolerated without DLT across tested dose levels. Evidence of promising antitumor activity was observed in ABC and HCC. Final results will be presented at the meeting. Clinical trial information: NCT01278810.
From 2021, by Sun et al: “A randomized, double-blinded, phase III study of icaritin versus huachashu as the first-line therapy in biomarker-enriched HBV-related advanced hepatocellular carcinoma with poor conditions: Interim analysis result.”36
Background: Many advanced hepatocellular carcinoma (aHCC) patients (pts) are often with more complicated clinical conditions such as damaged liver or blood function, poor physical conditions. Those aHCC pts are not suitable for molecular target drug like sorafenib or systemic chemotherapy and no standard or generally accepted treatment.
Icaritin, a single molecule ( > 98% purity) derived from Epimedii herba (Traditional Chinese herbal medicine), is a novel immune-modulation anti-tumor agent.
Preclinical studies demonstrated that Icaritin induced anti-HCC activities through targeting IL-6/JAK//STAT3 pathways and modulating inflammation-immune systems including Th1 cytokines, and down-regulation of alpha-fetoprotein (AFP).
Prior phase II study demonstrated favorable overall survival (OS) improvement in aHCC pts with poor conditions and correlated with the combined serum biomarkers.
The current phase III study was designed to confirm above clinical benefits and safety of Icaritin in those patients.
Methods: An adaptive enrichment design was used in a multicenter randomized, double-blinded study of comparing Icaritin with Huachashu (a TCM formula commonly used in China) as first line therapy for those aHCC pts (NCT03236636).
The primary endpoint was overall survival (OS) and secondary endpoints included time-to-progression (TTP), progression-free-survival (PFS), disease control rate (DCR), and safety.
The pts were randomized (1:1) to receive either Icaritin at 600mg or Huachashu.
Based on prior studies, a composite biomarker score (CBS) of AFP(≥400 ng/mL), TNF-a( < 2.5 pg/mL) and IFN-g(≥7.0 pg/mL) was used for pts selection and a CBS score of 2/3 was predefined positive.
Patients with CBS-positive were applied in interim analysis according to the protocol and statistical analysis plan (SAP).
Results: A total of 283 aHCC pts were enrolled and randomized from Sept. 2017, and 71 enriched pts was CBS-positive with combined risk/poor prognosis factors such as BCLC stage C, HBV infection, and thrombocytopenia etc.
Thirty-three and 38 CBS-positive aHCC pts were treated with Icaritin or Huachashu, respectively.
With a median follow-up of 8.1 mo (cutoff date, Dec.30,2020), the treatment outcomes for Icaritin and Huachashu arm showed following, that is mOS, 13.54 vs. 7.06 mo (HR = 0.40, 95%CI 0.21-0.77, p = 0.0046), mTTP, 3.65 vs. 1.84 mo (HR = 0.67, 95%CI, 0.36-1.22), mPFS, 2.79 vs. 1.84 mo (HR = 0.75, 95%CI, 0.43-1.33), and DCR, 48.5% vs. 26.3, respectively.
Treatment-related adverse event (AE≥3 grades) observed were 15.2% vs. 31.6%, respectively.
Conclusions: Small molecule immunomodulation agent Icaritin could significantly improve the overall survival with favorable safety in a prospectively CBS-enriched HBV-related advanced HCC pts with poor conditions. Clinical trial information: NCT03236636.
From 2014, by Fan et al: “Icaritin efficacy and tolerability in advanced hepatocellular carcinoma: Final phase Ib result.”37
Background: Hepatocellular carcinoma (HCC) is a highly malignant tumor with poor prognosis and limited treatment options. ER-α36 was recently discovered in several tumors and may play important roles in tumor progression.
Icaritin, a prenylflavonoid derived from Chinese herb Epimedium, is an ER-α36 modifier, which demonstrated potent anti-tumor effects in preclinical studies.
Icaritin also inhibits growth of hepatic cancer stem cells through down-regulating STAT activation, which promotes hepatic cancer stem cell growth.
A phase Ib study was carried out to determine safety and potential efficacy of Icaritin, in advanced solid tumors with majority of HCCs.
Methods: In this phase Ib study, patients with advanced solid tumors (18 HCCs, 3 intrahepatic cholangiocarcinoma, 2 colorectal and 2 breast cancers) were given either 600 or 800 mg icaritin twice daily (BID).
The results of efficacy and toxicity in HCC patients are analyzed and reported. Biopsy samples were collected for exploratory analyses of genomic expression and cytokine such as IL-6, IL-6 receptor, HGF.
Results: Out of 18 HCC patients (average age = 55 yrs old ranging 31-73), 12 received icaritin 600 mg BID and 6 received 800 mg BID. 16 patients had prior hepatic B virus infection. 13 patients were evaluable for objective response.
1 got partial response lasted 12 months and 6 had stable disease (clinical benefit rate = 53.8%). The median time to tumor progression was 168 days.
Patients were allowed to receive icaritin after disease progression under physician’s discretion. Patients were also followed up for overall survival data.
Only grade 1 drug-related adverse events were observed which includes neutropenia, ALT elevation, hypercholesteremia, fatigue, anorexia, hypertriglyceridemia, proteinuria, myalgia, hot flash and rash.
SAEs such as jaundice, gastrocintestinal bleeding were reported but determined to be non-drug related.
Conclusions: Icaritin was generally well-tolerated without much safety concerns. Promising antitumor activities were observed in advanced HCC patients. Further study in advanced HCC patients is warranted. Final results including overall survival, genomic and biomarker examination will be presented. Clinical trial information: NCT01278810.
Our final study is from chapter 13 of the “Annals of Traditional Chinese Medicine - Vol. 3: Alternative Treatment for Cancer” (2007), edited by Ping-Chung Leung and Harry Fong.
Key takes from “Clinical Evaluation of Herbal Formula Decoction in Treating Non-Small Cell Lung Cancer by Various Rating Scales”, by Jie You & Zhi-Ming Shi.
Decoction: “a concentrated liquor resulting from heating or boiling a substance, especially a medicinal preparation made from a plant.”
Objective:
To comprehensively evaluate the clinical effect of Herbal Formula decoction in treating patients with non-small cell lung cancer by various rating scales.
Methods:
One hundred and two cases of non-small lung cancer were randomly divided into two groups, namely the combination group (which consisted of 61 cases and treated by both Herbal Formula decoction and chemotherapy) and the control group (41 cases receiving chemotherapy only).
A total of two therapeutic courses were observed. The efficacy was evaluated by using various scales, including two international quality of life scales — the European Organization for Research and Treatment of Cancer Lung Cancer Quality of Life Questionnaire (EORTC QLQ-LC43) and the Functional Assessment of Cancer Therapy General and Lung (FACT-L); and Karnofsky’s index of Performance Status (KPS) and the Eastern Cooperative Oncology Group (ECOG) performance status scales; and the Primary Lung Cancer Symptoms Scale of Traditional Chinese Medicine (LCSL-TCM).
Phase III trials may classify a chemotherapeutic as meeting its endpoint and consequently be licensed, even though the effects on your quality of life may cause a high drop-out rate, or make you feel that your life is not worth living.
They found that combination therapy with a herbal formula can change this.
Result:
The quality of life scores in the combination group were significantly improved after treatment in various fields, including the functional symptomatic sub-fields, whereas those of the chemotherapy group significantly decreased.
The difference between them was statistically significant (P < 0.05). For the combination group patients, most symptoms were alleviated after treatment, whereas most symptoms were aggravated in the control group, and their quality of life scores decreased in many fields.
ECOG and KPS scoring showed similar results. The gastrointestinal reactions and bone marrow suppression of the combination group were less than those of the control group (P < 0.05).
The clinical remission rates were not significantly different between the two groups (P > 0.05).
Conclusion:
Herbal Formula decoction can improve the quality of life in non-small cell lung cancer patients, alleviate the disease or therapy-related symptoms, reduce the side-effects of chemotherapy, and improve the performance status.
Keywords: Herbal Formula Decoction; Non-Small Cell Lung Cancer; Quality of Life; Rating Scales; Efficacy Assessment.
13.2.3 Treatment methods
Both groups of patients received general and symptomatic treatments. For example, a painkiller (acesodyne) was given to patients based on the principle of the World Health Organization’s (WHO) three-step method of relieving pain; federal cough syrup was given to treat dry cough; licorice mixture was given to treat expectoration; codeine was given to treat serious cough; G-CSF was used when the bone marrow was suppressed to level II; and anti-emetic was given prior to chemotherapy.
The combination group was treated by both Herbal Formula decoction and chemotherapy while the chemotherapy group received chemotherapy only. Both groups received NP chemo-therapy regimen, on which vinorebine was given by intraveneous injection at 25 mg/m2 on days 1 and 8, cisplatin was given by intraveneous infusion at 80 mg/m2 on day 1.
One cycle was 28 days and patients received two cycles of treatment in total.
The herbal formula decoction contained 15g of Epimedium:
Herbal Formula decoction was composed of the following Chinese medicinal herbs: Sheng Huang Qi (Astragalus membranacens) 30 g, Sheng Bai Zhu (Atractylodes Rhizome) 15 g, Bei Sha Sen (Radix glehniae) 15g, Shi Shang Bai (Selaginella doederleinii) 30 g, Qi Ye Yi Zhi Hua (Paris polyphylla var. chinensis) 24 g, Bing Qiu Zi (Oreorchis patens) 30 g, Shan Yu Rou (Determining) 12 g, Xian Ling Pi (Epimedium) 15 g, etc.
It was continuously given from the third day of the first cycle of chemotherapy to the 28th day of the second cycle of chemotherapy.
This is highly encouraging. Apart from a lack of efficacy and recurrences, the side effects of conventional allopathic medicine would certainly dissuade me from trying them:
As shown in Table 13.3, after treatment the EORTC QLQ-LC43 scores of the combination group increased in the following fields, including role, emotional, and cognitive function, general health, and social function, whereas it decreased in physical field.
This indicated the quality of life had improved in the above fields after treatment. In the symptom subscales, scores on fatigue, pain, dyspnoea, insomnia, and appetite loss were significantly lower than those of pre-treatment (P < 0.05), while nausea and vomiting, constipation, and diarrhea did not change after treatment.
In contrast, chemotherapy alone produced symptoms consistent with “poison”:
After treatment, EORTC QLQ-LC43 scores of the chemotherapy group increased in body field, while they decreased in the fields of role, emotional, and cognitive function, general health, and social function (P < 0.05).
In the symptom sub-scales, scores on nausea and vomiting, fatigue, pain, appetite loss, and constipation were significantly higher than those of pre-treatment (P < 0.05), while dyspnoea, diarrhea, insomnia did not change after treatment.
Total symptom sub-scales score was significantly higher (P < 0.05), which suggested the symptoms tended to get worse.
The combination treatment improved many of the QoL scores.
Higher is better:
As listed in Table 13.4, for the combination group, except the score on the relationship with doctor did not change obviously after treatment, scores on all other fields increased significantly compared to pre-treatment (P < 0.05).
The scores on all fields in the chemotherapy group were significantly lower after treatment compared to those of pre-treatment. The scores on the fields of relationship with doctor, society, and emotion did not show any significant change after treatment.
The correlation extended to physical symptoms, too.
Lower is better:
After treatment, in the combination group except the scores on constipation, diarrhea, dysphagia, peripheral neuropathy, alopecia, other site’s pain, scores on all other symptoms were significantly lower than those of pre-treatment.
In the chemotherapy group after treatment, scores on fatigue, nausea and vomiting, pain, appetite loss, arm or shoulder pain were significantly higher, which indicated the symptoms were getting worse, with the exception was that the scores on the fields of diarrhea, hemoptysis, oral ulcer, and other site’s pain between the combination and chemotherapy group showed no difference, scores on all other fields in the combination group were significantly lower than those of the chemotherapy group after treatment.
This is important, as you may be less likely to “throw in the towel” and resign to your fate:
Lung cancer is a chronic disease with poor prognosis (Frost et al., 2002). Especially in the advanced stage, due to the serious disease-related symptoms such as cough, pain, fatigue, and dyspnoea, etc. patients with lung cancer always suffer from psychological distress and physical pain and their quality of life is affected.
Although chemotherapy is one of the main therapeutic approaches for advanced lung cancer, many patients cannot endure chemotherapy due to various reasons.
Even for those who can tolerate chemotherapy, the median survival time only increased two to four months (Breathnach et al., 2001; Cella, 2003); furthermore, the pain caused by the toxicity and side-effect of chemotherapy may offset the clinical advantages of tumor shrinkage or stability.
Codey et al. (2003) found that in 177 lung cancer patients who received chemotherapy, the symptoms of fatigue, pain, constipation, loss of appetite, and vomiting were aggravated and might last for six months.
Quality of life is one of the independent factors for prognosis and survival expectation. Due to the limited survival period of lung cancer patients, alleviating of disease-related or therapeutic-related symptoms is the main aim of treatment for lung cancer, especially in advanced stages.
In the second cycle of chemotherapy, the level and duration of gastrointestinal reaction and bone marrow suppression of the combination group were less than those of the chemotherapy group, which objectively further demonstrated Herbal Formula decoction could alleviate the disease and chemotherapy-related symptoms in patients with non-small cell lung cancer and improved their quality of life.
Although the short-term therapeutic effect was not significantly different between the two groups, the remission rate of the combination group was higher than that of the chemotherapy group.
Clinical studies have demonstrated that TCM can alleviate patients’ symptoms and improve their quality of life. However, well-recognized normalized and quantitative measures have been lacking for a long time. The application of the symptom sub-scale of the international quality of life scale provided a new approach for research in this field.
In this study, we evaluated the effect of Herbal Formula decoction on alleviating the symptoms in patients with non-small cell lung cancer using the symptoms sub-scale, lung cancer specific scale, and TCM primary lung cancer symptoms scale. We evaluated this decoction by normalized and quantitative measures. The results indicated the data measured via the above scales were consistent.
2.2.2 The role of Epimedium in TCM cancer treatments
I will discuss side effects, precautions, interactions, and dosing more in a later Substack, but I will include a recipe for a materia medica-based soup for cancer patients.
From page 380 of “Management of Cancer with Traditional Chinese Medicine” by Li Peiwen:
YIN YANG HUO MIAN TANG (EPIMEDIUM NOODI .F SOUP)
Epimedium (Yin Yang Huo, Herba Epimedii), 15g
Longan fruit (Long Yan Rou, Arillus Euphoriae Longanae), 15g
Chinese yam (Shan Yao, Rhizoma Dioscoreae Oppositae), 20g
Thin noodles, 50-100g (depending on appetite)
Spinach, 50g
Chicken stock cube
Soy sauce, 1 teaspoon
Coriander, 10g
Sesame oil, 5 drops
Preparation: Decoct the epimedium in 300ml of water for 30 minutes. Strain off the liquid and discard the residue. Peel the Chinese yam, cut into cubes, add 100 ml of water and boil down until there is a paste . Put the longan fruit in another pot with 500m1 of water and bring to the boil . Add the noodles, the epimedium decoction, the Chinese yam paste and the spinach . Bring to the boil, add the chicken stock cube, soy sauce, coriander and sesame oil, and serve .
Properties: Salty and warm.
Channels entered: Spleen, Stomach, Lung, and Heart .
Functions: Supplements the Middle .; Burner and augments Qi, quiets the Spirit and nourishes the Blood, has anti-cancer properties and safeguards general health.
Indications: Convalescent stage for patients with brain tumors, since the soup activates the function of the brain cells; anemia and reduced appetite due to radiotherapy and chemotherapy or other causes.
2.3 Icariin as a treatment for long COVID and gene therapy poisoning
A hat tip to Dr Kevin “Obi-Wan” McKernan for sharing a link to a paper from 2024:
Key takes from “Transfected SARS-CoV-2 spike DNA for mammalian cell expression inhibits p53 activation of p21(WAF1), TRAIL Death Receptor DR5 and MDM2 proteins in cancer cells and increases cancer cell viability after chemotherapy exposure” by Zhang & El-Deiry (3 May 2024)38.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 infection has led to worsened outcomes for patients with cancer. SARS-CoV-2 spike protein mediates host cell infection and cell-cell fusion that causes stabilization of tumor suppressor p53 protein.
In-silico analysis previously suggested that SARS-CoV-2 spike interacts with p53 directly but this putative interaction has not been demonstrated in cells. We examined the interaction between SARS-CoV-2 spike, p53 and MDM2 (E3 ligase, which mediates p53 degradation) in cancer cells using an immunoprecipitation assay.
We observed that SARS-CoV-2 spike protein interrupts p53-MDM2 protein interaction but did not detect SARS-CoV-2 spike bound with p53 protein in the cancer cells.
We further observed that SARS-CoV-2 spike suppresses p53 transcriptional activity in cancer cells including after nutlin exposure of wild-type p53-, spike-expressing tumor cells and inhibits chemotherapy-induced p53 gene activation of p21(WAF1), TRAIL Death Receptor DR5 and MDM2.
The suppressive effect of SARS-CoV-2 spike on p53-dependent gene activation provides a potential molecular mechanism by which SARS-CoV-2 infection may impact tumorigenesis, tumor progression and chemotherapy sensitivity.
In fact, cisplatin-treated tumor cells expressing spike were found to have increased cell viability as compared to control cells. Further observations on γ-H2AX expression in spike-expressing cells treated with cisplatin may indicate altered DNA damage sensing in the DNA damage response pathway.
The preliminary observations reported here warrant further studies to unravel the impact of SARS-CoV-2 and its various encoded proteins including spike on pathways of tumorigenesis and response to cancer therapeutics.
More efforts should be directed at studying the effects of the SARS-CoV-2 spike and other viral proteins on host DNA damage sensing, response and repair mechanisms.
A goal would be to understand the structural basis for maximal anti-viral immunity while minimizing suppression of host defenses including the p53 DNA damage response and tumor suppression pathway.
Such directions are relevant and important including not only in the context of viral infection and mRNA vaccines in general but also for patients with cancer who may be receiving cytotoxic or other cancer treatments.
Keywords: SARS-COV2 spike, p53, MDM2, chemotherapy, cancer
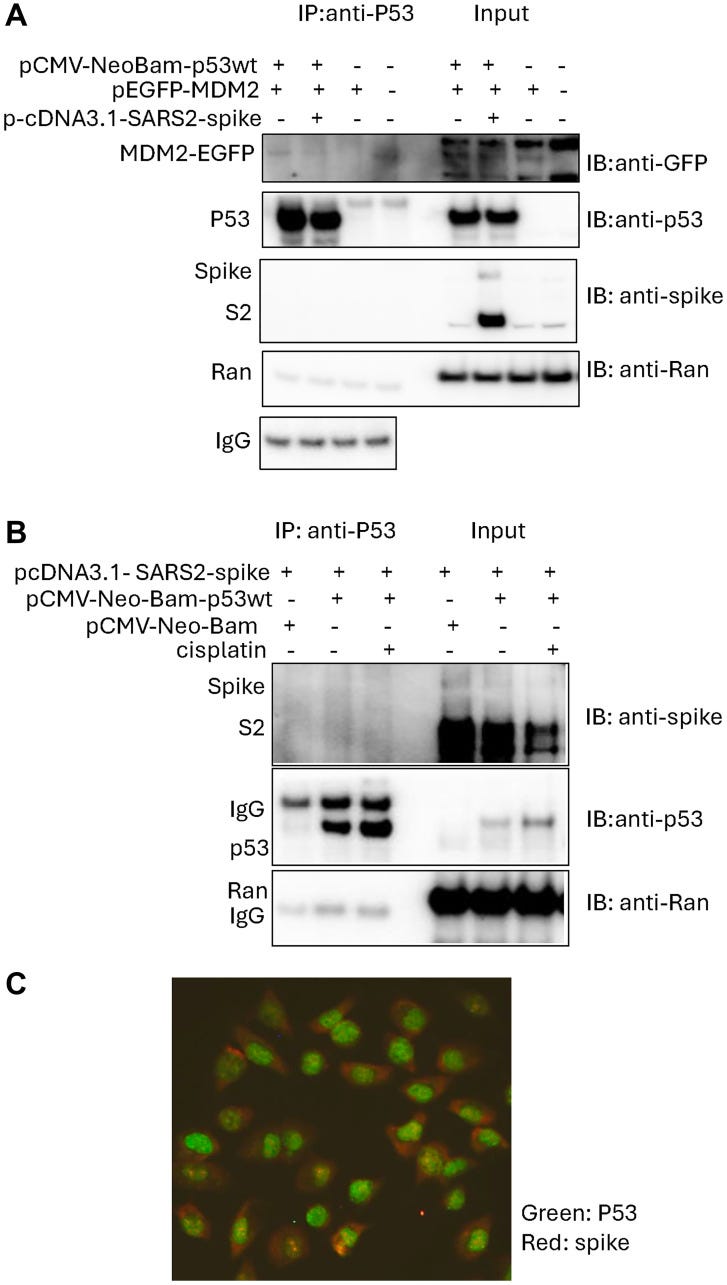
It can help cause your course of chemotherapy to fail, and it must not be given to cancer patients.
Enough already!
We further investigated if the SARS-CoV-2 spike protein can inhibit chemotherapy-induced p53 transcriptional activity in cancer cells.
p53 wild-type breast cancer cells MCF7 and lung cancer cells H460 were transiently transfected with the pcDNA3.1-SARS2-spike and PG13-luc, followed by etoposide or 5-Fluorouracil (5-FU) treatment.
The PG13-luciferase reporter assay showed a reduction of the p53-responsive bioluminescence in the cells transfected with pcDNA3.1-SARS2-spike, as compared to the pcDNA3.1 transfection control (Figure 3A).
A similar reduction of p53-responsive transcription-mediated bioluminescence was also observed in p53 wild-type HCT116-PG13-luc cells which have been stably transduced with PG13-luc (Figure 3B).
These results suggest that SARS-CoV-2 spike reduces chemotherapy-induced p53 transcriptional activity in cancer cells.

The researchers are trying to sound the alarm, in quiet desperation:
Our results have implications for the biological effects of spike subunits in human cells whether spike is present due to primary COVID-19 infection or due to mRNA vaccines where its expression is used to promote anti-viral immunity.
A perturbed p53 pathway is concerning but also complicated in sorting out since cellular transformation and cancer are a multi-step process that evolves over time.
I would like to see lncRNA-interaction analysis too:
Our experiments highlight an issue that should have earlier consideration more widely in vaccine development. This is about testing numerous natural-variants as well as synthetic-variants of proteins such as spike to maximize anti-viral immunity while minimizing suppression of innate host pathways that respond to invading viruses or their gene products.
Such work can be done using recombinant DNA technologies and standard molecular and cellular biology techniques with proteins such as spike or other viral antigens in isolation, in simpler screening systems, and away from viral infection that may have risks from gain-of-function research (or confounding effects due to the stress of viral infection or cell fusion that occurs with SARS-CoV-2).
The pathways include the host innate and adaptive immune response as well as host proteins that may be targeted for inactivation including the protective p53 tumor suppressor protein.
Manufacturers should be expected to provide such evidence in their development process and this should be publicly available in the future.
It would be helpful to know more about some other risks including long-term risks from foreign antigens that may target host tumor suppressive pathways or that may impact on the efficacy of chemotherapy.
Such information would be helpful to patients and physicians as they weigh the risks and benefits of certain medical interventions.
This is the anti-cancer pathway that Spike inhibits, taken from a paper discussing breast cancer.
Five years later …crickets.
Nothing to see here, move along:
The good news is that a study using human colon carcinoma HCT116 cells and normal colon epithelial FHC cells demonstrated that icariin can reverse these effects, and I linked to this paper earlier.
Key takes from “Icariin reduces human colon carcinoma cell growth and metastasis by enhancing p53 activities” (2018) by Tian et al.39
Icariin significantly suppressed colon carcinoma HCT116 cells by decreasing migration and viability, and simultaneously promoting apoptosis.
Icariin exerted the anti-tumor effect in a dose-dependent manner by up-regulating p53.
During treatment of icariin, p-p53, p21, and Bax levels increased, and Bcl-2 level decreased.
Short time treatment with icariin induced DNA damage in HCT116 cells.
Furthermore, the cytotoxicity of icariin was decreased after p53 knockdown or by using caspase inhibitors. p53 was involved in activities of caspase-9 and caspase-3.
Icariin repressed colon carcinoma cell line HCT116 by enhancing p53 expression and activating p53 functions possibly through Bcl-2/Bax imbalance and caspase-9 and -3 regulation. Icariin treatment also induced DNA damage in HCT116 cells.
Some studies reported that the transcriptional factor p53 played an indispensable role in active function of icariin (7,8). p53 is one of the most important tumor suppressors in cells, which can protect normal cell growth and initiate malignant cell death.
In unstressed cells, the level and activity of p53 is strictly controlled especially by the ubiquitin E3 ligase mdm2 (9). Blocking the mdm2-p53 interaction and reactivating p53 function is a promising therapeutic strategy for the treatment of cancers (10).
p53 can be activated when cells suffer toxic stresses, inducing cell growth arrest, cell senescence, and apoptosis (11,12). Thus the functions of p53 in icariin-treated cells were analyzed.
p53 was up-regulated in icariin-treated cells
The anti-colon carcinoma effect of icariin was verified, but the underlying mechanism was unclear. HCT116 cells express p53, an important tumor suppressor, the overexpression of which can lead to cell cycle arrest and apoptosis. Therefore, we speculated that p53 might be involved in the carcinostatic role of icariin.
According to the results, p53 was indeed up-regulated, and mRNA and protein levels of p53 were enhanced along with the increase in icariin concentration (Figure 4A and B).
Phosphorylation of the NH2-terminal residues of p53 could mediate its stabilization and nuclear accumulation after treatment with anticancer drugs (13). Thus, p-p53 level gradually increased when treated with equally increasing concentrations of icariin.
In normal, non-transformed cells, p53 levels are kept low through activities of negative regulators, such as mdm2 (14). Some studies reported that under stress and with the inhibition of mdm2/p53 interaction, p53 protein levels rapidly increased (15,16).
In this study, we found that mdm2 was down-regulated after icariin treatment, which was accordingly disproportionate to p53 level. Therefore, p53 might be targeted by mdm2 in icariin-treated cells.
Effect of icariin on cell growth-related proteins in colon carcinoma cells
p21 was the key p53 target protein, which was enhanced after icariin treatment (Figure 5). Next, levels of important apoptosis-related proteins Bcl-2 and Bax were also evaluated.
The anti-apoptosis protein Bcl-2 was down-regulated by icariin in a dose-dependent manner, while the pro-apoptosis protein Bax level was increased (Figure 5).
These data suggested that icariin not only enhanced p53 expression, but possibly also regulated other important genes interfering with cell growth.
Again, they recommend using icariin as a combination therapy:
Our results showed that the combination of icariin with cisplatin provided more effective anti-tumor activity compared to treatment with icariin or cisplatin alone.
Previous studies demonstrated that icariin enhanced the in vitro antitumor activity of arsenic trioxide against acute promyelocytic leukemia (18), as well as the cytotoxicity of doxorubicin in human multidrug-resistant osteosarcoma cells (19). These findings suggest that a combination of icariin with other anticarcinogens might offer a therapeutic benefit to patients with cancer.
Based on our results, icariin exerted its anti-tumor effect by regulating caspase-9 and caspase-3, but the caspases could not be activated by icariin after p53 knockdown. Therefore, caspase-9 and caspase-3 might be directly regulated by p53, and p53 by icariin.
Some studies reported that icariin inhibited colorectal cancer cell growth by mainly suppressing NF-κB activity (28,29). However, the pharmacological effects of anti-cancer drugs are particularly complex and their effects might occur in more than one way. Therefore, more studies are needed in future.
In summary, our study showed that icariin effectively inhibited migration and viability of the colon carcinoma cell line HCT116 and promoted apoptosis.
Icariin had an anti-tumor effect in a p53-dependent manner and p53 might be regulated by mdm2. Icariin may represent a promising therapeutic strategy for the treatment of human colon carcinoma.
3 Parting shots
… As I have long warned, “Society in Highly C-19–Vaccinated Countries Will Be Caught by Surprise,” as the rise of increasingly immune-subversive SC-2 variants diverts the attention of the medical community toward all kinds of other infectious and non-infectious diseases—many of which are increasing in prevalence due to vaccine breakthrough infection (VBTI)-associated immune dysregulation.
- Geert Vanden Bossche
https://voiceforscienceandsolidarity.substack.com/p/please-dont-call-me-a-visionary
This is awful. OK, so correlation isn’t causation and we don’t know his full medical history, but THIS medical history isn’t going to help, given our understanding of how these death shots cause ongoing or worsening immunosuppression.
2022:

Present day:
Tributes have been paid to Ben Askren after he was hospitalised and ‘unresponsive’ with pneumonia.
The former UFC star and MMA fighter’s wife, Amy Askren, shared an update with her followers about the news.
She wrote: "You may have heard that my husband Ben is going through something.
“He developed severe pneumonia which came on very suddenly. He's currently in the hospital and unable to respond to anything at this time. We welcome all prayers for healing and peace."
Amy also requested privacy for her and her family during an incredibly difficult time.
Who is Ben Askren?
Askren is a former MMA fighter, who retired in 2019. After his retirement from MMA, he moved to UFC and beat Robbie Lawler, as well as suffering a defeat to Jake Paul in 2021.
During his career, Askren also represented Team USA at the 2008 Olympics in wrestling. He became an MMA professional in 2009.
Tributes paid for Ben Askren
The MMA world has sent messages of support for Askren and his family since the news broke.
Henry Cejudo wrote: “Pray for Ben Askren.”
Many similar stories are being posted daily. These are just two examples.
Cause is unknown, but it’s definitely not The Thing™, according to Big Pharma’s AI Overview, which conveniently ignores clinical cases and research confirming multiple neurotoxicity mechanisms:

Of course, the BHF is #Baffled, chasing unicorns, and self-censoring so as not to upset anyone:
Warning over 'worrying' surge in health problem killing 420 people a week
People’s heart health in the UK has declined more quickly at the start of the 2020s than any other decade for more than 50 years, figures suggest. Analysis by the British Heart Foundation (BHF) found rising deaths among working-age adults from cardiovascular disease, increasing heart failure and growing risks from obesity and diabetes.
Cardiovascular deaths in working age adults have risen by 18% since 2019, from 18,693 to 21,975 in 2023, averaging 420 a week. Since 2020, the BHF has found a “worrying trend”, including:
A 21% rise in the number of people diagnosed with heart failure in the UK, to a record high of 785,000 in March 2024 from 650,000 in March 2020;
A 10% rise in the number of people diagnosed with atrial fibrillation, up to a record high of 1.62 million up from 1.48 million over the same time period;
A 12% rise in the number of adults diagnosed with diabetes, a major risk factor for cardiovascular disease, up to a record high of 4.6 million from 4.1 million;
Rising rates of obesity across the board.
The new analysis also shows an 83% increase in people waiting for planned heart hospital treatment in England, from 232,082 at the start of the decade to 425,372 in March 2025. Cardiac waiting lists have also grown in Scotland, Wales and Northern Ireland.
The BHF said the shift follows decades of progress to nearly halve annual deaths from conditions such as heart attack and stroke since the 1960s. It said issues such as an increasingly unhealthy population, widening health inequalities, the impact of Covid, pressure on the NHS and a lack of action over the last decade have all had an effect.
Launching a new strategy, the BHF said focusing investment in areas such as artificial intelligence (AI), data science and genomics could help revolutionise how the UK prevents and treats cardiovascular disease. Dr Charmaine Griffiths, chief executive of the charity, said: “It’s been the worst start to a decade for heart health for half a century, but we’re entering an era of immense scientific opportunity that can turn this tide.
“By driving a research revolution, we can reverse this worrying trend and save more lives than ever before. The BHF’s new strategy will be key to this, and the next step for us as we aim to save many more families the heartbreak of losing loved ones far too soon.”
A commercial with this sick scene is getting aired several times a day even now, as they constantly have the begging bowls out.
But they never, ever, link outcomes to heart-damaging gene therapy drugs administered to millions under phony EUAs.

Japanese saRNA gene therapy agents incorporate GOF viral proteins developed from military bioweapons programmes, and then supercharge them, as discussed here, here, here, here, here, and here.
The manufacturer of the replicon mRNA Covid “vaccine” in Japan, Meiji Seika Pharma, has brought a lawsuit against a member of the Japanese parliament, Kazuhiro Haraguchi. Haraguchi had commented that the Covid injections are “akin to a biological weapon,” a statement which the Meiji Pharma president claimed was beyond the bounds of acceptable expression.
However, statements like Haraguchi’s about the dangers of the Covid mRNA injections are now commonplace in many nations, and drug companies do not seem to be suing people for making them, at least in the US. Instead, state attorneys general in Kansas and Texas have been suing Pfizer for misrepresenting its Covid injections.
In general, Japan has been gradually evolving into a place where it is difficult to publicly express ideas unapproved by powerful business interests and officialdom. In addition to government and mainstream news media collusion to keep Covid medical realities from the Japanese public, the government passed a law to squelch nonconforming messaging online.
The intentions behind this measure are clear: Prominent government figures have openly declared their conviction that “misinformation” is a major problem in Japan. In December 2024, Prime Minister Ishiba stated that he was considering more regulations concerning Internet discourse that he considers problematic, and a prominent LDP (Liberal Democratic Party) politician named Noda commented recently that Japan was being influenced more and more by “fake” information.
More: https://brownstone.org/articles/japan-rides-the-censorship-bandwagon/
Shi Rubbish in - Rubbish out:
More: https://x.com/itsalexvacca/status/1927393691267690922
He may be batsh!t crazy in some respects, but he is right about this.
Let’s see if this order makes any difference at all?
And whilst you are at it, for Pete’s sake, Stop the Shots !
Activist Scientists Rage About Trump’s Executive Order, Comparing Him to Hitler For Insisting That Federally-Funded Science Projects Are More Scientifically Rigorous
Activist scientists on the Federal payroll in the United States are reeling from President Trump’s recent executive order designed to promote openness and integrity in an often corrupted and politicised scientific process. The order mandates transparency, objectivity and it provides a protection for dissenting views and safeguards against political interference. Scientific results must be falsifiable, computer models must be explainable and negative results available. Needless to say, not everyone is happy with this return to the “gold standard” with a group of scientists including Michael ‘Hockey Stick’ Mann writing in the Guardian – seemingly without irony – that it will “destroy American science as we know it”. A group called Stand Up for Science, whose executive director also helped write the Guardian article, is collecting signatures noting that “state sponsored” scientific programmes in Nazi Germany led to the deaths of millions of Jews, people with disabilities and people identifying as LGBTQ+.
Of course, the Hitler trope is often deployed when political activists are circling the wagons to defend a way of doing business “as we know it”. In fact, the Trump executive order does no more than provide guidance as to how science should be conducted. It is patently necessary because much of the science produced during the recent Covid panic and in the current fake climate emergency is biased towards promoting the political agenda of an influential, moneyed elite. Even the Guardian finds it hard to quarrel with the new requirement that science produced by Federal employees should be informed by “the most credible, reliable, and impartial scientific evidence available”. Quite how the newspaper’s writers believe “science is under siege” with an order enshrining such basic scientific principles is not immediately clear.
4 Concluding remarks
I would certainly put Epimedium up there with baicalin for therapeutic potential, as both interact with so many different signalling pathways and biological mechanisms. It also manages all this with low toxicity and a high therapeutic index (ratio).
Epimedium punches above its weight due to a secondary benefit: by making allopathic cancer treatments more bearable when taken as an adjunct, both in terms of the highly important QoL, but also the symptom scores.
It may lack the “knockdown punch” of ivermectin or fenbendazole, but it’s still useful as these aren’t efficacious against all cancer types, and icariin targets different cancer pathways. An ability to overcome resistance and to fill treatment gaps may lend a role in combination with, or as a substitute for Fen or IVM, but more research is needed.
I will go into more depth about self-medicating next time, as well as review the literature investigating treatment potential for other diseases.
Thank you for reading.
5 Disclaimer
This site is strictly an information website reviewing research into potential therapeutic agents. It does not advertise anything, or provide medical advice, diagnosis, or treatment. This site does not promote any of these as potential treatments or offer any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses, or pharmacists. This content is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Any extracts quoted in the previous article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
6 References
Osorio M, Carvajal M, Vergara A, et al. Prenylated Flavonoids with Potential Antimicrobial Activity: Synthesis, Biological Activity, and In Silico Study. Int J Mol Sci. 2021;22(11):5472. doi:10.3390/ijms22115472
Hao, H., Q. Zhang, H. Zhu, Y. Wen, D. Qiu, J. Xiong, X. Fu, Y. Wu, K. Meng, and J. Li. ‘Icaritin Promotes Tumor T-Cell Infiltration and Induces Antitumor Immunity in Mice’. Eur J Immunol 49, no. 12 (December 2019): 2235–44. https://doi.org/10.1002/eji.201948225.
Chen, M., J. Wu, Q. Luo, S. Mo, Y. Lyu, Y. Wei, and J. Dong. ‘The Anticancer Properties of Herba Epimedii and Its Main Bioactive Componentsicariin and Icariside II’. Nutrients 8, no. 9 (13 September 2016). https://doi.org/10.3390/nu8090563.
Zhao H, Guo Y, Li S, et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 2015;6(31):31927-31943. doi:10.18632/oncotarget.5578
Tao H, Liu M, Wang Y, et al. Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation. Front Immunol. 2021;12:609295. doi:10.3389/fimmu.2021.609295
Qin, S. K., Q. Li, J. Ming Xu, J. Liang, Y. Cheng, Y. Fan, J. Jiang, et al. ‘Icaritin-Induced Immunomodulatory Efficacy in Advanced Hepatitis B Virus-Related Hepatocellular Carcinoma: Immunodynamic Biomarkers and Overall Survival’. Cancer Sci 111, no. 11 (November 2020): 4218–31. https://doi.org/10.1111/cas.14641.
Lu, Yi, Yue Gao, Huan Yang, Yong Hu, and Xin Li. ‘Nanomedicine‐boosting Icaritin-Based Immunotherapy of Advanced Hepatocellular Carcinoma’. Military Medical Research 9, no. 1 (12 December 2022): 69. https://doi.org/10.1186/s40779-022-00433-9.
Guo Y, Zhang X, Meng J, Wang ZY. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol. 2011;658(2-3):114-122. doi:10.1016/j.ejphar.2011.02.005
Hu J, Wu X, Yang C, et al. Anticancer effect of icaritin on prostate cancer via regulating miR-381-3p and its target gene UBE2C. Cancer Med. 2019;8(18):7833-7845. doi:10.1002/cam4.2630
Wu, X., X. Long, C. Yang, H. Chen, C. Sharkey, K. Rashid, M. Hu, et al. ‘Icaritin Reduces Prostate Cancer Progression via Inhibiting High-Fat Diet-Induced Serum Adipokine in TRAMP Mice Model’. J Cancer 11, no. 22 (2020): 6556–64. https://doi.org/10.7150/jca.48413.
Xu B, Jiang C, Han H, et al. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin Exp Pharmacol Physiol. 2015;42(12):1296-1307. doi:10.1111/1440-1681.12488
Han, H., B. Xu, P. Hou, C. Jiang, L. Liu, M. Tang, X. Yang, Y. Zhang, and Y. Liu. ‘Icaritin Sensitizes Human Glioblastoma Cells to TRAIL-Induced Apoptosis’. Cell Biochem Biophys 72, no. 2 (June 2015): 533–42. https://doi.org/10.1007/s12013-014-0499-y.
Li, X., W. Zhang, L. Liang, X. Duan, J. Deng, and Y. Zhou. ‘Natural Product-Derived Icaritin Exerts Anti-Glioblastoma Effects by Positively Modulating Estrogen Receptor β’. Exp Ther Med 19, no. 4 (April 2020): 2841–50. https://doi.org/10.3892/etm.2020.8571.
He, Dan, Siliang Chen, Xiaoyun Wang, Xiang Wen, Changyang Gong, Lei Liu, and Gu He. ‘Icaritin Represses Autophagy to Promote Colorectal Cancer Cell Apoptosis and Sensitized Low-Temperature Photothermal Therapy via Targeting HSP90-TXNDC9 Interactions’. Advanced Science (Weinheim, Baden-Wurttemberg, Germany), 4 April 2025, e2412953. https://doi.org/10.1002/advs.202412953.
Huang, C., Z. Li, J. Zhu, X. Chen, Y. Hao, R. Yang, R. Huang, et al. ‘Systems Pharmacology Dissection of Epimedium Targeting Tumor Microenvironment to Enhance Cytotoxic T Lymphocyte Responses in Lung Cancer’. Aging (Albany NY) 13, no. 2 (17 January 2021): 2912–40. https://doi.org/10.18632/aging.202410.
Geng Y di, Yang L, Zhang C, Kong LY. Blockade of epidermal growth factor receptor/mammalian target of rapamycin pathway by Icariside II results in reduced cell proliferation of osteosarcoma cells. Food Chem Toxicol. 2014;73:7-16. doi:10.1016/j.fct.2014.08.002
Lee KS, Lee HJ, Ahn KS, et al. Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett. 2009;280(1):93-100. doi:10.1016/j.canlet.2009.02.024
Li, Shuang, Yunlu Zhan, Yingwei Xie, Yonghui Wang, and Yuexin Liu. ‘The Impact of Icariside II on Human Prostate Cancer Cell Proliferation, Mobility, and Autophagy via PI3K-AKT-mTOR Signaling Pathway’. Drug Design, Development and Therapy 14 (31 December 2020): 4169–78. https://doi.org/10.2147/DDDT.S268524.
Zheng Q, Liu WW, Li B, et al. Anticancer effect of icaritin on human lung cancer cells through inducing S phase cell cycle arrest and apoptosis. J Huazhong Univ Sci Technolog Med Sci. 2014;34(4):497-503. doi:10.1007/s11596-014-1305-1
Tang, Z., W. Du, F. Xu, X. Sun, W. Chen, J. Cui, W. Tang, et al. ‘Icariside II Enhances Cisplatin-Induced Apoptosis by Promoting Endoplasmic Reticulum Stress Signalling in Non-Small Cell Lung Cancer Cells’. Int J Biol Sci 18, no. 5 (2022): 2060–74. https://doi.org/10.7150/ijbs.66630.
Icariside II potentiates the anti-PD-1 antitumor effect by reducing chemotactic infiltration of myeloid-derived suppressor cells into the tumor microenvironment via ROS-mediated inactivation of the SRC/ERK/STAT3 signaling pathways. Phytomedicine. 2023;110:154638. doi:10.1016/j.phymed.2022.154638
Tan, H. L., K. G. Chan, P. Pusparajah, S. Saokaew, A. Duangjai, L. H. Lee, and B. H. Goh. ‘Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives’. Front Pharmacol 7 (2016): 191. https://doi.org/10.3389/fphar.2016.00191.
Yuan, D., T. Guo, H. Qian, H. Ge, Y. Zhao, A. Huang, X. Wang, et al. ‘Icariside II Suppresses the Tumorigenesis and Development of Ovarian Cancer by Regulating miR-144-3p/IGF2R Axis’. Drug Dev Res 83, no. 6 (September 2022): 1383–93. https://doi.org/10.1002/ddr.21967.
Wang Y, Dong H, Zhu M, et al. Icariin exterts negative effects on human gastric cancer cell invasion and migration by vasodilator-stimulated phosphoprotein via Rac1 pathway. Eur J Pharmacol. 2010;635(1-3):40-48. doi:10.1016/j.ejphar.2010.03.017
Li S, Priceman SJ, Xin H, et al. Icaritin Inhibits JAK/STAT3 Signaling and Growth of Renal Cell Carcinoma. PLOS ONE. 2013;8(12):e81657. doi:10.1371/journal.pone.0081657
Kang, S. H., S. J. Jeong, S. H. Kim, J. H. Kim, J. H. Jung, W. Koh, J. H. Kim, D. K. Kim, C. Y. Chen, and S. H. Kim. ‘Icariside II Induces Apoptosis in U937 Acute Myeloid Leukemia Cells: Role of Inactivation of STAT3-Related Signaling’. PLoS One 7, no. 4 (2012): e28706. https://doi.org/10.1371/journal.pone.0028706.
Wu, J., J. Xu, E. A. Eksioglu, X. Chen, J. Zhou, N. Fortenbery, S. Wei, and J. Dong. ‘Icariside II Induces Apoptosis of Melanoma Cells through the Downregulation of Survival Pathways’. Nutr Cancer 65, no. 1 (2013): 110–17. https://doi.org/10.1080/01635581.2013.741745.
Quan, K., X. Zhang, K. Fan, P. Liu, Q. Yue, B. Li, J. Wu, et al. ‘Icariside II Induces Cell Cycle Arrest and Apoptosis in Human Glioblastoma Cells through Suppressing Akt Activation and Potentiating FOXO3a Activity’. Am J Transl Res 9, no. 5 (2017): 2508–19. https://pmc.ncbi.nlm.nih.gov/articles/PMC5446533/
Shi, C. J., S. Y. Li, C. H. Shen, F. F. Pan, L. Q. Deng, W. M. Fu, J. Y. Wang, and J. F. Zhang. ‘Icariside II Suppressed Tumorigenesis by Epigenetically Regulating the Circβ-Catenin-Wnt/β-Catenin Axis in Colorectal Cancer’. Bioorg Chem 124 (July 2022): 105800. https://doi.org/10.1016/j.bioorg.2022.105800.
Qabbus, Manal Bin, Katey S. Hunt, Joshua Dynka, Craig D. Woodworth, Shantanu Sur, and Damien S. K. Samways. ‘Ivermectin-Induced Cell Death of Cervical Cancer Cells in Vitro a Consequence of Precipitate Formation in Culture Media’. Toxicology and Applied Pharmacology 449 (15 August 2022): 116073. https://doi.org/10.1016/j.taap.2022.116073.
‘Cancer Staging - NCI’. cgvArticle, 9 March 2015. Nciglobal,ncienterprise. https://www.cancer.gov/about-cancer/diagnosis-staging/staging.
Liu FY, Ding DN, Wang YR, et al. Icariin as a potential anticancer agent: a review of its biological effects on various cancers. Front Pharmacol. 2023;14:1216363. doi:10.3389/fphar.2023.1216363
Sun Y, Li Q, Xu JM, et al. A multicenter, single arm phase II trial of a small molecule immune-modulator icaritin: Safety, overall survival, immune dynamics, and PD-L1 expression in advanced hepatocellular carcinoma. JCO. 2018;36(15_suppl):4077-4077. doi:10.1200/JCO.2018.36.15_suppl.4077
Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. Am J Cancer Res. 2021;11(4):1121-1131.
Fan Y, Xu B, Ding X, et al. A first-in-human phase I study of ER-α36 modifier icaritin in patients with advanced solid tumors. JCO. 2013;31(15_suppl):2614-2614. doi:10.1200/jco.2013.31.15_suppl.2614
Sun Y, Qin S, Li W, et al. A randomized, double-blinded, phase III study of icaritin versus huachashu as the first-line therapy in biomarker-enriched HBV-related advanced hepatocellular carcinoma with poor conditions: Interim analysis result. JCO. 2021;39(15_suppl):4077-4077. doi:10.1200/JCO.2021.39.15_suppl.4077
Fan Y, Xu B, Ding X, et al. Icaritin efficacy and tolerability in advanced hepatocellular carcinoma: Final phase Ib result. JCO. 2014;32(15_suppl):e15107-e15107. doi:10.1200/jco.2014.32.15_suppl.e15107
Zhang S, El-Deiry WS. Transfected SARS-CoV-2 spike DNA for mammalian cell expression inhibits p53 activation of p21(WAF1), TRAIL Death Receptor DR5 and MDM2 proteins in cancer cells and increases cancer cell viability after chemotherapy exposure. Oncotarget. 2024;15:275-284. doi:10.18632/oncotarget.28582
Tian M, Yang S, Yan X. Icariin reduces human colon carcinoma cell growth and metastasis by enhancing p53 activities. Braz J Med Biol Res. 2018;51(10):e7151. doi:10.1590/1414-431X20187151






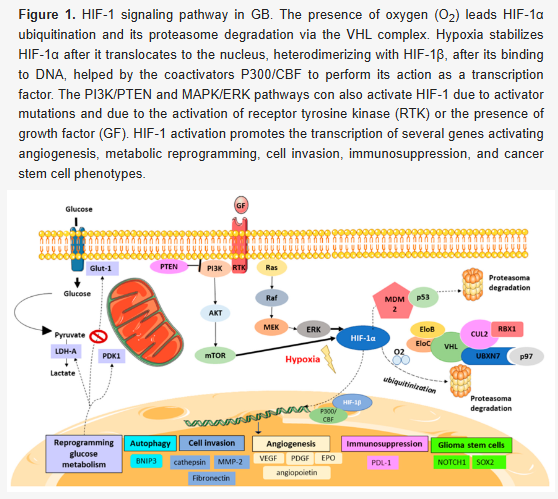
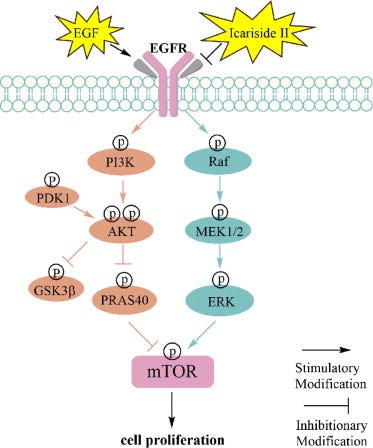







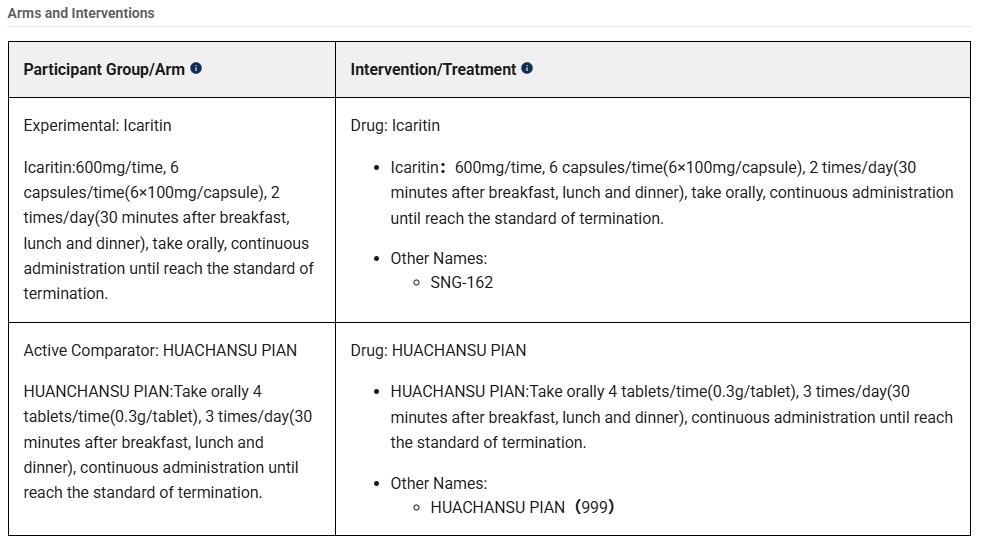













I love learning about new, potentially anti-cancer drugs. My disappointment is usually what the evil pharma industry do is see an idea, copy the ingredient, *slap another ingredient with it* (say, mix in some potassium chloride or some worthless shit), and then claim it is patented, then give it a stupid sounding unpronounceable name, like Itzshoveduptheazzalacone. No credit given to original researchers, of course.
I prefer the strategy of not looking at the benefits a drug does (everyone wants to do that), but actively looking for studies that look at the toxicity of the drug, especially in chronic, continuous, or large doses. It is better for the patient to receive a non-toxic, harmless drug that doesn't do anything, than a horribly toxic drug whose toxicity is how the "benefits" of killing cancer cells emerge (namely in that it kills everything else, too).
Everyone forgets the toxicity studies.
Cyprinus carpio, as I commented in Part 1: "Kaempferol is one of my favorites"... as its anti cancer therapeutic value is immense, and yet its highly underrated and ignored by allopathic medicine. For obvious reasons as cures are not the goal. As for your latest opus, yet once again as predicted - brilliant work from a brilliant mind, replete with recipe. Shine on you crazy diamond.