SARS-CoV-2 and LNP-mRNA platform induced neurological pathologies and associated autoimmune antibodies
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
TL;DR: Research into the most significant autoantibodies associated with various viral and vax related sequalae are discussed, along with broad spectrum therapeutics. Molecular mimicry is not necessarily the biggest trigger, the LNP platform is implicated too. Diagnosis of autoimmune disorders may be challenging, and one size doesn’t fit all - its a spectrum of multiple root causes and symptoms we try to label. Western medicine is stuck in the 70’s in some ways, find yourself a good TCM practitioner if needed and make sure you aren’t nutritionally deficient, especially in B vitamins & D3.
Also available in:
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Thank you!
Firstly I would like to extend a huge thank you to all my subscribers for your support as this Substack reaches the 2 year milestone at the end of next month.
I may post again before then, but its a very busy time for all of us and this is another Doorless biggie, its not a two minute read.
Without your backing and donations it wouldn’t be possible to sustain this work, and the subscriber count has grown just shy of 2,500 at the time of writing.
I trust this review breaks new ground by joining a lot of dots, but the paradox is that the more you demystify the mechanisms of disease the more complex the overall picture becomes. This is partly because so many factors can contribute to disease pathologies and a recurrent theme is cross-reactivity. It can make diagnosis a nightmare too, let alone finding effective treatments.
Introduction
In 2021 an 85 year old man presented to a hospital in Japan. He had vertigo and was vomiting one day after receiving his second dose of BNT162b2 LNP-mRNA.
A PCR test was performed, but this was negative and he had no fever or symptoms to suggest an infection. Neurological examination revealed involuntary movements of his right eye, but no weakness or sensory disturbance in his lower limbs and no hematologic abnormalities.
His vertigo and vomiting improved gradually and he was discharged from hospital on the 3rd day after receiving the booster shot.
15 days after the shot he was readmitted due to progressive gait disturbance and urinary retention. Again, no evidence of infection was found, but by now neurological examination revealed numbness in both sides of his lower extremities, and weakness in the muscles of his upper legs.
On day 16 a thoracic MRI revealed a longitudinal hyperintense lesion in his spinal cord.
By day 25 further tests were conducted and again he was infection free, but by now he was bedridden, disorientated and with right-dominant paraplegia. His numbness had spread to both lower limbs, together with a loss of normal reflex action.
Fortunately his consciousness improved quickly the next day. Blood tests were taken and these revealed elevated levels of glycated haemoglobin (6.9% HbA1c, a marker for diabetes) and C-reactive protein (3.26 mg/dL CRP, a marker for inflammation).
Cerebrospinal fluid (CSF) analysis demonstrated an immune response to neurovascular inflammation called “monomorphonuclear pleocytosis” (11 cells/μL), with elevated protein levels (120 mg/dL). Monomorphonuclear pleocytosis occurs when lymphocytes and monocytic B-cells produces antibodies against neuronal and vascular antigens.
Other readings were unremarkable, the patient was diagnosed with acute transverse myelitis (ATM) and high-dose intravenous steroid treatment was administered for 3 days, followed by oral steroids from day 30+.
However, his condition didn’t improve so a second course of IV methylprednisolone (0.5 g) was administered for three days from the 42nd day.
This also failed to resolve his neurological symptoms and by day 52 he presented with fever and oxygen desaturation. Blood tests revealed elevated CRP (15.72 mg/dL) and pancytopenia - in which there is significant reduction in the number of almost all blood cells.
Chest radiography revealed bilateral pneumonia. High dose IV antibiotics were administered but his condition didn’t improve, and he died on the 58th day after being boosted1.
This Substack was prompted by a question by Christie Grace2:
What are the odds you don't have spike protein expressing in nerves on the peripheral after vaccination, but any sort of neuropathy, be it AIDP, CIDP, or SFN, is due to molecular mimicry and auto immune from the CpG motif? Go ahead. Test it. I know what you'll be looking for in the tissue.
Hey Fredy and @DoorlessCarp
You two are on the ball here. Anti dsDNA antibodies are not what you would look for because it's molecular mimicry. You're looking for antibodies against the tissue. TPO is one set that could do this in a rare occurrence. Those can induce not just hashimoto's thyroiditis but hashimoto's encephalopathy as well and the TPO antibodies are also known to attack other tissues. And these are the wildest of times. Besides the TPO antibody that could attack the nerve tissue which would mean it is autoimmune demyelinating however it is in response to the TPO antibodies attacking.... Got thoughts on other tests you could do? Also when you biopsy the tissue, you'd look for a few things there as well that the typical medical path lab isn't.
In the following thread I suggested testing for two myelin-disrupting autoimmune antibodies associated with a range of disorders: anti-myelin oligodendrocyte glycoprotein (MOG-IgG) and anti-aquaporin-4 antibodies (AQP4-IgG).
As a more detailed follow-up to this is warranted I will review some of the research into autoimmune antibodies associated with ATM and other neurological disorders. Indeed, many VAERS case reports similar to the one above have been filed after LNP-mRNA administration or due to infection by SARS-CoV-2.
It is important to stress that there is no single autoimmune antibody (Abs) that is canonically associated with many of these disorders. You may test positive for abnormally elevated levels of an antibody without presenting symptoms of disease, or you may have normal levels yet still be diagnosed with the condition.
Autoimmune disorders may be seen as a spectrum of medical conditions, the presence of one doesn’t exclude the presence of another. Symptoms depend on what types of antibody are involved and which cells or organs they disrupt.
Multiple types of antibody may be detected and studying the subject is challenging at times for conditions like fibromyalgia as its still early days for much of the relevant research into it - there aren’t many papers available to review yet. And I’m only reviewing a few of the main autoantibodies involved with neurological pathways, meta-analysis revealed 77 common autoantibodies in healthy individuals.3
As for vaccine-induced autoimmunity there are two principle aetiologies:
Molecular mimicry.
Autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome).
A third type is secondary in nature. Vaccine mediated immunosuppression can lead to reactivation of latent viruses such as Epstein-Barr virus (EBV). EBV is associated with systemic autoimmune diseases (SADs) including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome (SS), via the development of diverse autoreactivities in genetically susceptible individuals4.
Non-autoimmune in nature, there is evidence that homologous spike protein S1-gp120, cleaved at the cathepsin cleavage site is also neurotoxic56 and can pass the blood-brain barrier.7
I will also discuss therapeutics - both conventional (allopathic) and alternate or repurposed (see disclaimer).
Transverse myelitis (TM)
TM may be either acute (developing over hours or days) or subacute (developing over one to four weeks). TM is a neurological disorder caused by inflammation of the spinal cord. “Myelitis” refers to damage to the myelin sheath that protects nerve cells. “Transverse” refers to the pattern of changes in sensation, which are often band-like across the trunk of the body, with sensory changes below that. Myelin damage most often occurs in the nerves in the upper back.
Some people recover quickly with few sequalae, most recover at least partially within three months but for some impairments to perform everyday tasks may be permanent.
Four of the classic features include:
Weakness in the legs and arms, particularly if the upper spinal cord is affected.
Pain, which at first includes the lower back or sharp, shooting sensations radiating down the legs, arms or around the torso.
Sensory alterations such as paraesthesias including burning, tickling, pricking, numbness, coldness, or tingling in the legs and sensory loss.
Bowel and bladder dysfunction. Symptoms include an increased frequency or urge to use the toilet, incontinence and constipation.
TM occurrence is associated with several different conditions:
Multiple sclerosis (MS).
Aquaporin-4 autoantibody associated neuromyelitis optica, a disorder that affects the eye nerves and spinal cord. The “aqua” in aquaporin-4 refers to a channel on the cell membrane that lets water enter a cell and helps maintain ionic and metabolite balance in the brain and spinal cord. Its extremely important for neurological functioning that AQP4 remains closely regulated - deletion of aquaporin-4 can inhibit meningeal lymphatic drainage. Hat tip to Jennifer Depew R.D. for mentioning its role in circadian rhythms. Lymphatic drainage occurs particularly whilst we sleep and helps to maintain amyloid beta, Tau protein and water homeostasis in the brain8.
Post-infectious or post-vaccine autoimmune phenomenon.
Abnormal immune response to an underlying cancer that leads to damage to the nervous system.
Other antibody-mediated conditions that remain undiscovered9.
Associated autoimmune antibodies
Aquaporin-4 IgG (AQP4) antibody, as above10.
Neuromyelitis optica spectrum disorder (NMOSD), mostly presenting as TM, has also been reported after both COVID-19 infection and vaccination, but particularly after the latter11121314:
Neuromyelitis optica spectrum disorder is a rare inflammatory disease that most often affects the optic nerves and spinal cord. Less often, it affects the brain. It often leads to sudden vision loss, paralysis or both. Symptoms after a first attack usually improve.
NMOSD is sometimes mistaken for MS because both have symptoms that come and go. It is also known as Devic disease or Devic’s disease.
From: “Neuromyelitis Optica Spectrum Disorder (NMOSD)”
https://www.ohsu.edu/brain-institute/neuromyelitis-optica-spectrum-disorder-nmosd
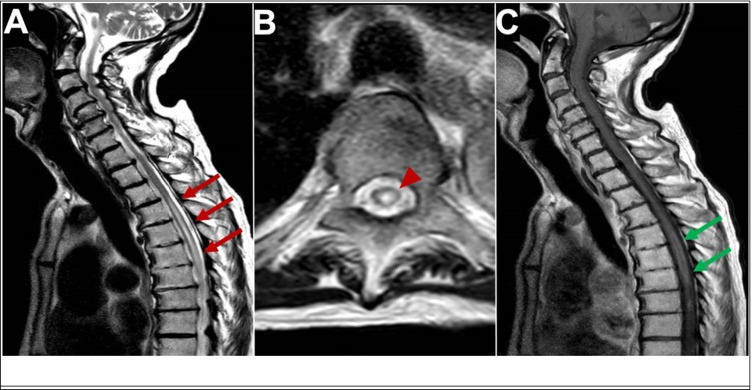
“MRI of longitudinal spinal cord lesions: T2-weighted sagittal view of the cervical and upper thoracic cord showing a longitudinally extensive cord signal abnormality (A, red arrows), predominantly involving the central aspect (B, red arrowhead) of the cord from T3–T4 down to T9–T10. T1-weighted sagittal view with gadolinium showing a peripheral enhancement pattern (C, green arrows). 338 × 160 mm (125 × 125 DPI)” from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9219391/ In one case of AQP4-positive NMOSD the patient’s serum also tested positive for antibodies for ANA, SSA, SSB, Ro-52, and p-ANCA, which tends to rule out molecular mimicry as the major reason for the production of AQP4-IgG.
This is probably indicative of a loss of function of FOXP3+ regulatory T (Treg) cells, or immune tolerance15:
It is possible that in the context of preexisting systemic immunity, the vaccine overactivated the immune system, exacerbated the production of AQP4-IgG, and eventually provoked NMOSD. As NMOSD is seriously harmful and curative, it is important to be aware of the NMOSD symptoms after vaccination. Cautions should also be given for those with preexisting systemic autoimmune abnormalities in vaccination for COVID-19.
From: “Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19“ (2021)
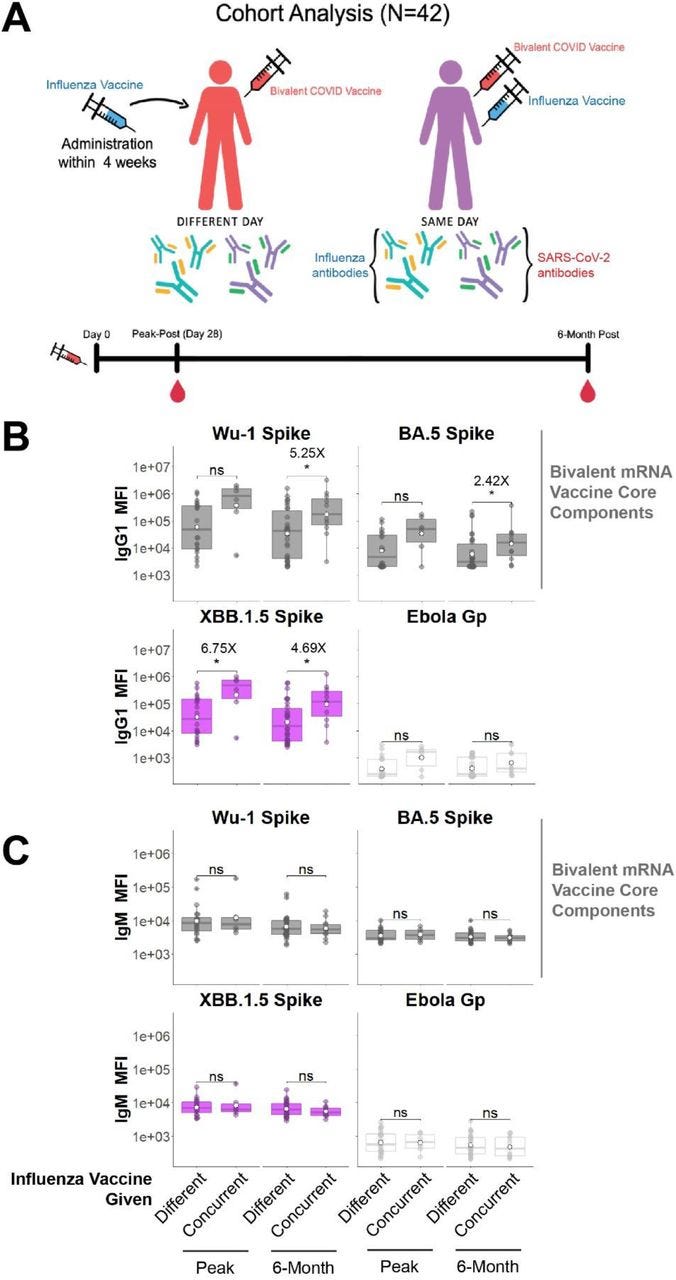
In light of the above, a preprint has just been published that studied concurrent administration of bivalent COVID-19 mRNA and influenza vaccines. They recommend this as Spike-specific antibody responses were greater than when they were given separately. However, antibodies are not a correlate of immunity, there was no measurement of viral load or T cell responses, and very low assays of mucosal antibodies IgA or IgM.
Restricted in follow-up to 6 months, they do mention that “…Safety profiles of concurrent COVID-19 and influenza vaccination have been reported”.
There are consequences to using antibodies as the only metric of your product. Escape variants, original antigenic sin (OAS), T-cell anergy, long term IgG4 class switching or loss of tolerance leading to autoimmune disorders are a natural consequence of a repeatedly overactivated immune system. Note the log scale on the y-axis:
One of the authors declared that they received financial support from AbbVie, Pfizer, GSK, the Bill and Melinda Gates Foundation and the Wellcome Trust.
Next, another case of post-vax neuromyelitis optica spectrum disorder, which unfortunately proved fatal as she didn’t respond to treatment. Again, the high dose immunosuppressants administered led to lymphopenia and then a fatal infection.
Along with high dose corticosteroids, cyclophosphamide is a chemotherapeutic used to suppress the immune system:
…Our patient was a 70-year-old woman admitted to our clinic with a history of numbness and weakness in her left limbs, 7 days after receiving the third dose of a COVID-19 vaccine (Sinovac: CoronaVac, a whole inactivated virus). Her left-sided hypoesthesia and hemiparesis progressed rapidly to paraplegia. Her upper limbs paresis worsened in the next 3 days. Spinal cord magnetic resonance imaging (MRI) determined a high T2- and a low T1- weighted long segment hemorrhagic lesion in the cervical cord (C1- C7) with a peripheral rim-shaped enhancement in the post-Gadolinium T1 image (Fig. 1 , A-C). A thoracic cord lesion (T1-T3) was also evident on MR image (Fig. 1D). Brain MRI was unremarkable.
…The patient did not benefit from pulse therapy, so we started a therapeutic plasma exchange (TPE). As she was non-responsive to the treatment, she developed respiratory insufficiency, and her symptoms escalated to quadriplegia, cyclophosphamide (600 mg on days 1, 2, and 9) (Awad and Stüve, 2009 Nov 28) was administered. The patient was non-responsive to the treatment and developed lymphopenia and fever following cyclophosphamide treatment without any clinical improvement. The patient died after 2 months of hospitalization.
…Considering the very late-onset of NMOSD in our patient following 7 days postvaccination, it is plausible that AQP4-Ab was triggered by vaccination. However, a previously asymptomatic seropositivity for AQP-4-Ab could not be excluded. Under such assumption, vaccination may provoke an NMOSD relapse even in the elderly. Anamnart et al. reported a favorable response to corticosteroids or corticosteroids and TPE in all post-COVID-19 vaccination NMOSD. Our patient had a fatal course of disease without any response to corticosteroids, TPE, and cyclophosphamide, which was in line with other reports about severe complications of the late-onset NMOSD (Krumbholz et al., 2015 May 10).
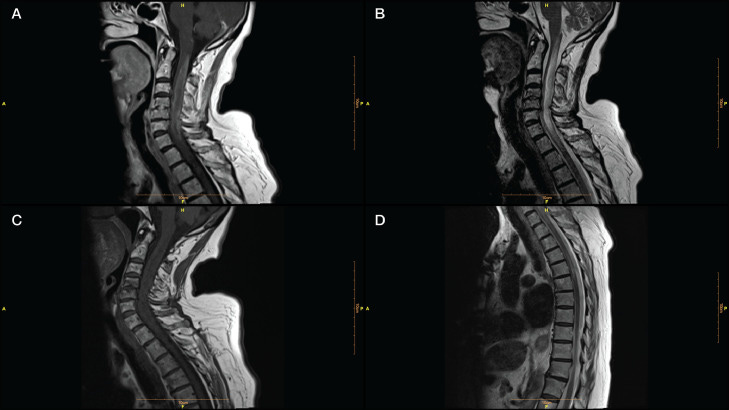
From: “Fulminant neuromyelitis optica spectrum disorder (NMOSD) following COVID-19 vaccination: A need for reconsideration?” (2022)
There are case reports of MS progressing to NMOSD after vaccination, with severe disease exacerbation16 and COVID-19 induced anti-aquaporin-4 antibodies has been causally linked to encephalomyeloradiculitis (inflammation of the brain, spinal cord and spinal nerve roots)17:

Parainfectious triggering can be explained by bystander activation or molecular mimicry hypothesis. Bystander activation involves inflammatory reactions that damage AQP4, triggering an immune cascade leading to the activation of AQP4-specific B cells. Molecular mimicry relies on antigenic similarity between structural epitopes or peptide sequences of an infectious agent and host-proteins.
From: “A recurrent longitudinally extensive transverse myelitis with Aquaporin-4(AQP4) antibody after herpes zoster” (2013)
https://www.sciencedirect.com/science/article/pii/S0022510X13028219?via%3Dihub#bb0030
A case control study of 13 patients with recurrent TM found that ten of them had anti-Ro (SSA) autoantibodies18. The name is derived from anti–Sjögren's-syndrome-related antigen A. Sjögren's syndrome (pronounced Show-grin's) is a chronic autoimmune disorder that happens when the immune system attacks the glands that make moisture in the eyes, mouth, and other parts of the body.
Relevance to SARS-CoV-2 and LNP/mRNA transfection
In one study from 2022, ACE2-transgenic mice were infected with SARS-CoV-2. and minor salivary gland (MSG) biopsies were collected from convalescent COVID-19 subjects.
ANA = anti-nuclear antibodies.
SjD = Sjögren’s Disease.
…The male patients showed elevated levels of anti-SSA/Ro52 compared to female patients, and female patients had more diverse ANA patterns.
Conclusion: Overall, our study shows a direct association between SARS-CoV-2 and SjD. Hallmark features of SjD salivary glands were histologically indistinguishable from convalescent COVID-19 subjects. The results potentially implicate that SARS-CoV-2 could be an environmental trigger for SjD.
From: “Evidence of a Sjögren's disease-like phenotype following COVID-19 in mice and human“ (2022)
Another case report of TM after COVID-19 vaccination was reviewed by Hsiao et al in 2021 and involved a 41 year old male who first became symptomatic 2 weeks after receiving his first dose of AZD1222. Free of infection, he tested negative for Aquaporin4 antibodies, which likely excluded neuromyelitis optica:
The proposed mechanism of the post-infection neurological disorder is the concept of “Molecular Mimicry”, which means that the microorganism epitope shares a similar structure to the host’s antigen. The cross-reaction between the epitope and self-antigen activates B lymphocyte and the bystander activation of T cells, which induces the immune response. These mechanism appears to be the explanation for the vaccine with viral antigen adjuvants, which might mediate immune responses targeting the spinal cords [1,6,7,15]. This could be somehow explained by the pleocytosis found in patients’ CSF considering that the blood–brain barrier might have been broken down within a focal area of the spinal cord [6,15]. It is noticeable that both the AZD1222 and Johnson & Johnson COVID-19 vaccines contain adenovirus antigens, and they might induce ATM by the same pathogenesis [1,7]. As other vaccines are without a viral vector, a similar hypothesis was proposed that immune dysregulation secondary to vaccination might trigger ATM [6]. However, the clear causal relation between the SARS-CoV-2 vaccine and ATM is still an issue for further investigation.
From: “Acute Transverse Myelitis after COVID-19 Vaccination“ (2021)
In 2021, Francis et al used live cell–based assays to quantify immunofluorescence detection of antibodies to AQP49 and MOG protein for patients presenting with new onset optic neuritis (ON) and/or transverse myelitis (TM), with or without other CNS inflammation.
All cases occurred within 8 weeks of vaccination with either ChAdOx1S (AZ) or BNT162b2. Their results are quite enlightening:
Of 25 patients identified (median age 38 years, 14 female), 12 (48%) had MOG antibodies (MOGIgG+), 2 (8%) had aquaporin 4 antibodies (AQP4IgG+), and 11 (44%) had neither. Twelve of 14 (86%) antibody-positive patients received the ChAdOx1S vaccine. MOGIgG+ patients presented most commonly with TM (10/12, 83%), frequently in combination with ADEM-like brain/brainstem lesions (6/12, 50%). Transverse myelitis was longitudinally extensive in 7 of the 10 patients. A peak in new MOGAD cases in Spring 2021 was attributable to postvaccine cases. Both AQP4IgG+ patients presented with brain lesions and TM. Four of 6 (67%) seronegative ChAdOx1S recipients experienced longitudinally extensive TM (LETM) compared with 1 of 5 (20%) of the BNT162b2 group, and facial nerve inflammation was reported only in ChAdOx1S recipients (2/5, 40%). Guillain-Barre syndrome was confirmed in 1 seronegative ChAdOx1S recipient and suspected in another.
From: “Acute Inflammatory Diseases of the Central Nervous System After SARS-CoV-2 Vaccination“ (2022)
As with MS and NMOSD, myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a demyelinating disease of the CNS.
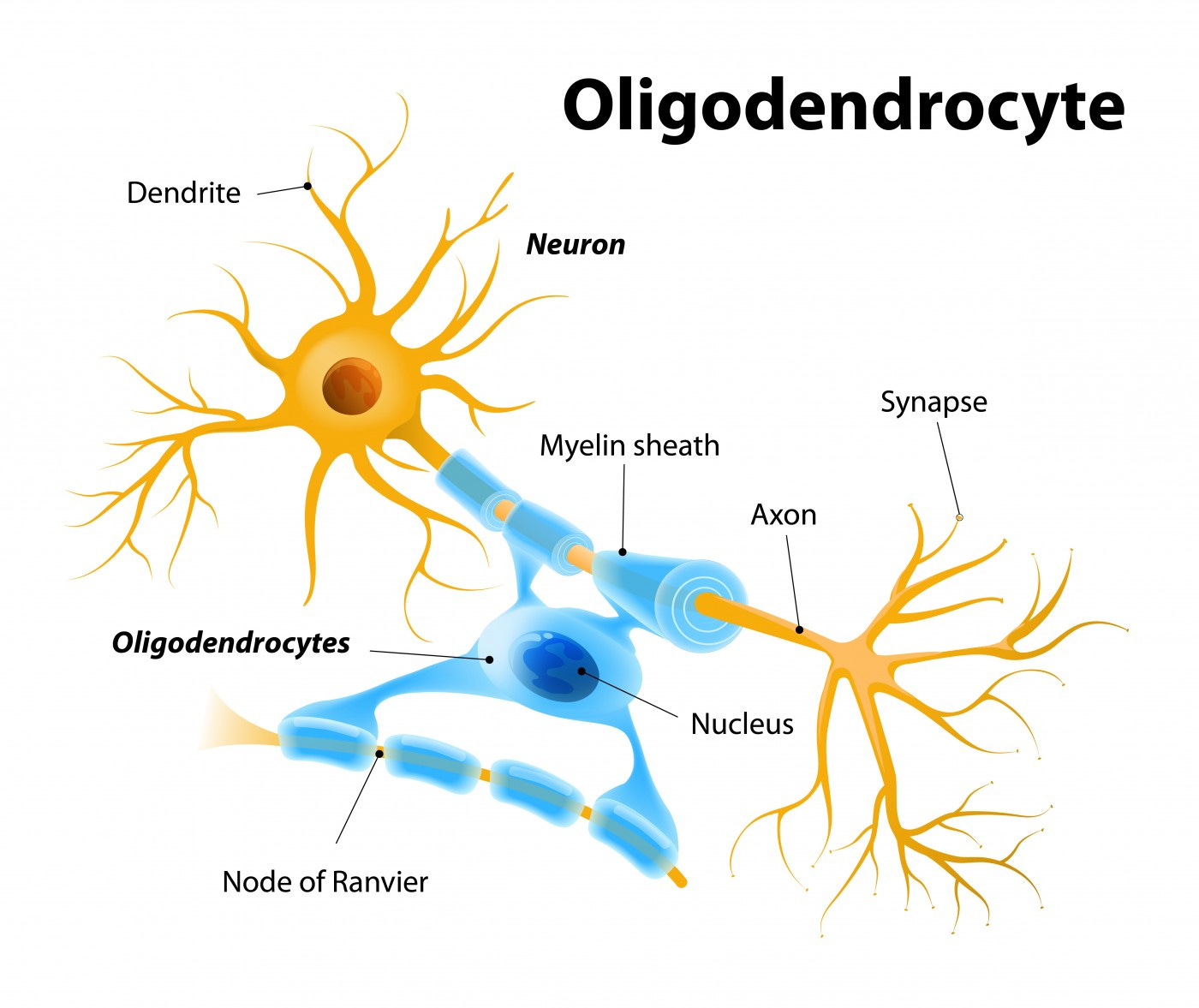
Oligodendrocytes are the myelinating cells of the central nervous system (CNS). They are the end product of a cell lineage which has to undergo a complex and precisely timed program of proliferation, migration, differentiation, and myelination to finally produce the insulating sheath of axons.
From: “Oligodendrocytes: biology and pathology” (2010)
It also frequently affects both eyes, causing more severe visual loss compared to MS. But there is often a better chance of recovery of vision in MOGAD than in NMOSD.
Along with optic neuritis, TM is also a common symptom of MOGAD.
Symptoms caused by optic neuritis include:19
Loss or blurring of vision in one or both eyes.
Loss of colour vision.
Eye pain.
MOGAD presents differently in adults and in children:
In children, MOGAD more commonly causes attacks on the brain, resulting in symptoms like confusion, incoordination, double vision, nausea and vomiting.
In adults, MOGAD often causes damage to the eyes (optic neuritis) and/or spinal cord (transverse myelitis).
MOGAD may be triggered by HIV. Unlike with spike protein exposure sequalae, at least with HIV the risk of death through infections due to immunosuppression is known in advance:
Case descriptions
The first patient, a 44-year-old black African man, presented with acute disseminated encephalomyelitis (ADEM) with positive serum MOG antibodies. He made a significant recovery with corticosteroids but had a quick relapse and died from sepsis. The second patient, an 18-year-old black woman, presented with paraplegia and imaging revealed a longitudinally extensive transverse myelitis and had positive serum MOG antibodies. She remained paraplegic after methylprednisone and plasmapheresis treatments. Her rehabilitation was complicated by development of pulmonary embolism and tuberculosis. The third patient, a 43-year-old mixed-race woman, presented with bilateral painless visual loss. Her investigations were notable for positive MOG antibodies, positive Varicella Zoster Virus on cerebral spinal fluid (CSF) and hyperintense optic nerves on magnetic resonance imaging (MRI). Her vision did not improve with immunosuppression and eventually died from sepsis.
…The pathophysiology of MOGAD is not completely understood; however, there are hypotheses that complement activation and CD4-positive T cell inflammation (unlike CD8 T cells in multiple sclerosis) predominate its pathogenesis [26].
…Although the pathogenicity of MOG antibodies and their utility in prognostication of MOGAD is unresolved, it is becoming evident that the positive predictive value (PPV) of MOG antibodies is higher with clear positive MOG antibodies (PPV of 100% for titer 1:1000, 82% for titre 1:100 and 51% for low positive titres < 1:40) [1, 28].
…When managing autoimmune disorders in immunosuppressed patients, balancing the benefits of (long-term) immunosuppression with the increased risk of infections, particularly opportunistic infections such as tuberculosis, is an additional important consideration [29, 30]. Indeed, two of our patients re-presented with infections. Unfortunately, there are no clear guidelines on how to approach this clinical conundrum; nonetheless, it may be prudent to initiate isoniazid preventative therapy prior to long-term immunosuppression.
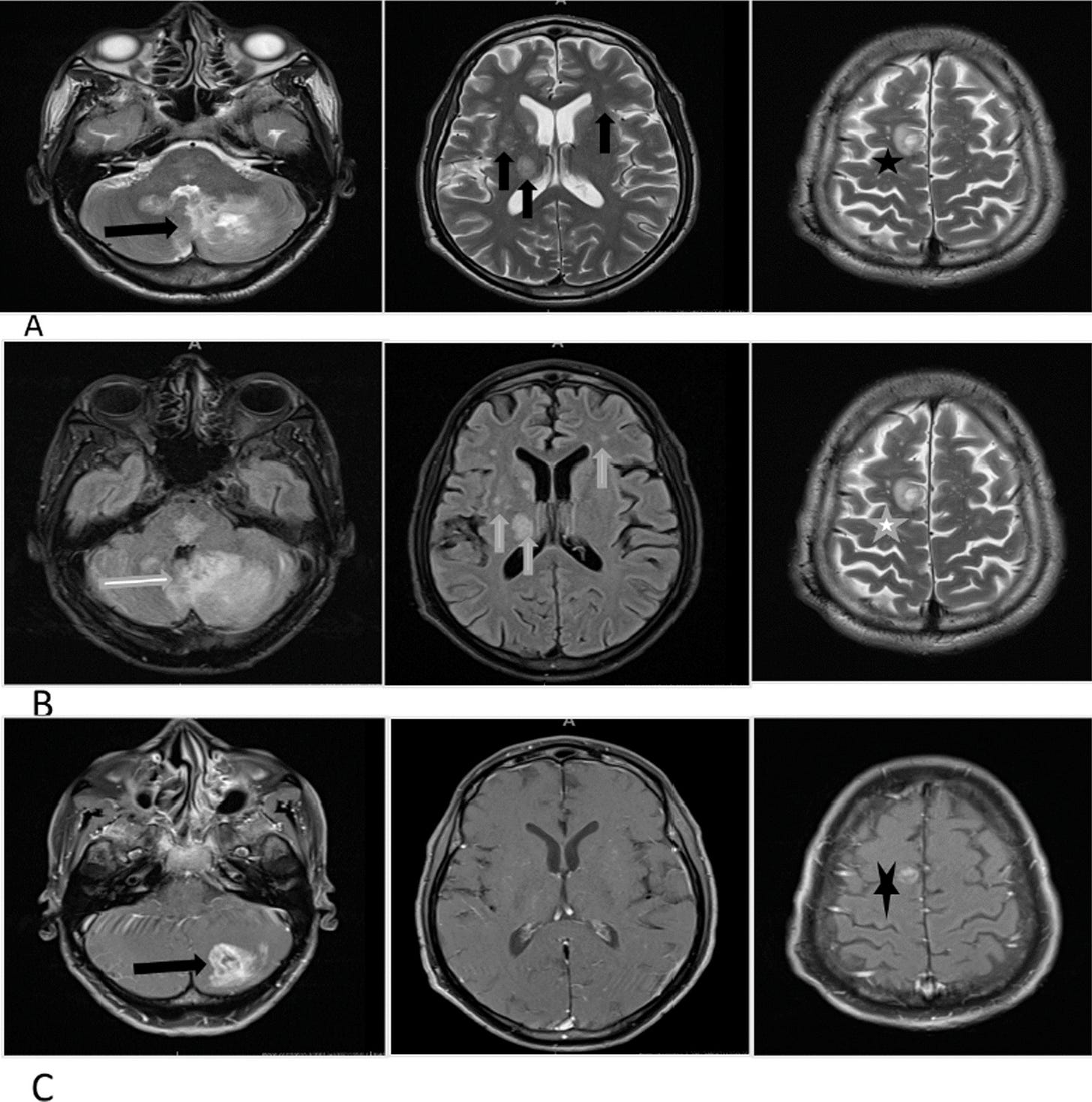
From: “Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and Human Immunodeficiency virus infection: dilemmas in diagnosis and management: a case series“ (2023)
The next paper was from 2022 and includes 9 case reports of MOGAD after SARS-CoV-2 infections. Vaccination status was not given, and 1 of the 9 died of pneumonia.
Abstract
This case series describes 9 patients diagnosed with myelin oligodendrocyte glycoprotein (MOG)-IgG associated disorder (MOGAD) following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Patients developed neurological symptoms between 4 days and 5 weeks following SARS-CoV-2 infection. Myelitis was observed in 4 patients; 4 presented with optic neuritis; and encephalopathy was observed in 3. Serum MOG-IgG cell-based assay was medium or high positive in each case. The majority of patients had near-complete recovery following acute immunosuppression. This series adds to the growing number of cases of central nervous system demyelination following SARS-CoV-2 infection and highlights a potential role of infection in the immunopathogenesis of MOGAD.
…The median latency from SARS-CoV-2 infection to neurological symptom onset was 2 weeks (range 4 days-5 weeks). SARS-CoV-2 infection was non-severe in 8/9 patients; one had severe SARS-CoV-2-related pneumonia.
…Optic neuritis (ON) was observed in 4 patients (unilateral [2], bilateral [2]). Myelitis was observed in 4. Encephalopathy/encephalitis was observed in 3, of whom 1 developed status epilepticus. One patient experienced a brainstem attack with nausea, vertigo, and multidirectional nystagmus.
…Cerebrospinal fluid analyses were performed in 8/9 patients. White blood cell count was elevated among 5/8 with a median of 65.5 (elevated range 63–144) cells/mm3. Oligoclonal bands were absent (7/8) or matched (1). Serum MOG-IgG titers ranged from 1:100-1:1000; FACS [6]), and 1:2560-1:5120; live CBA [3]). All patients were negative for serum aquaporin-4-IgG.
…The majority (7/9) of patients exhibited complete or near-complete recovery at a median follow-up of 5 months (range 23 days-18 months). No patients had a relapsing course. One patient with seizures died of severe SARS-CoV-2 pneumonia.

From: “Myelin oligodendrocyte glycoprotein-IgG associated disorders (MOGAD) following SARS-CoV-2 infection: A case series” (2022)
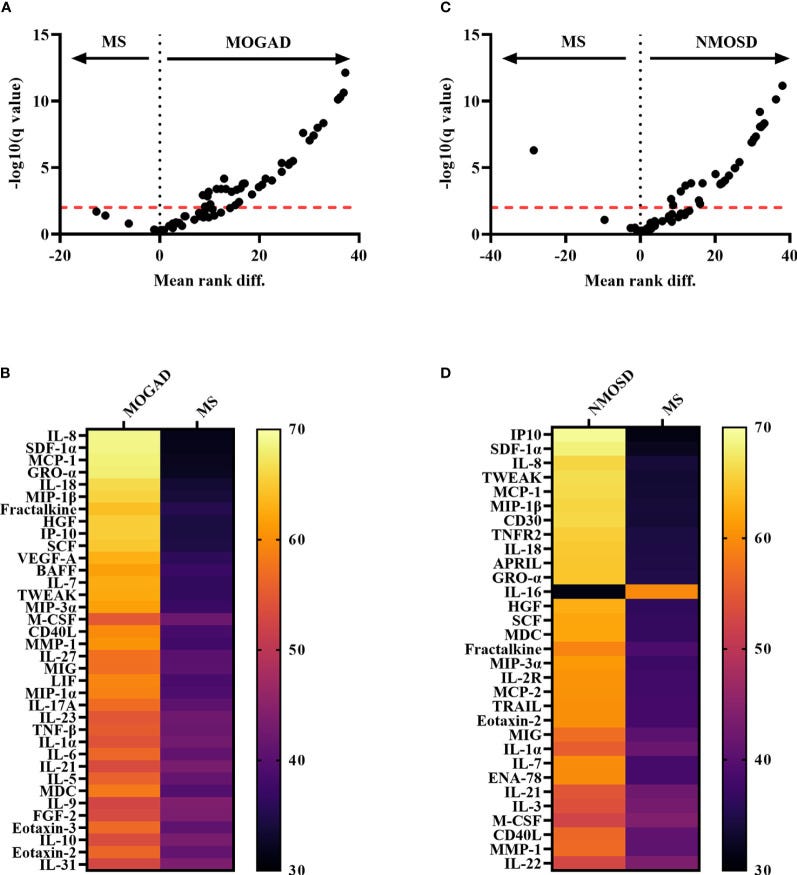
Pro-inflammatory cytokine changes associated with (MOGAD) and (AQP4+ NMOSD):
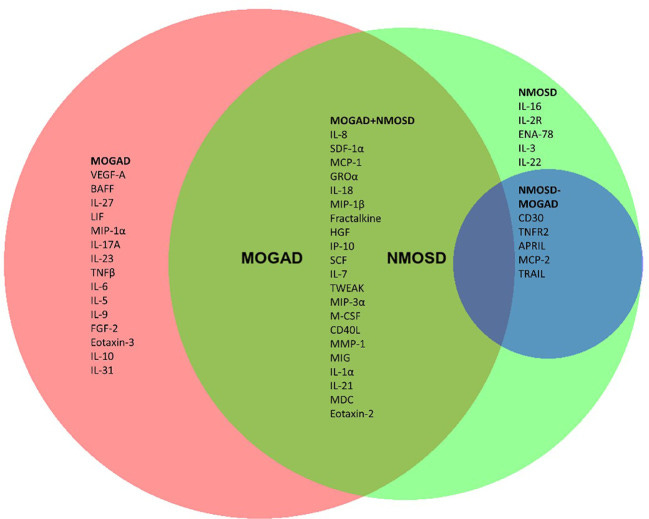
Marker levels for both conditions are multifold higher than with MS, note the log scale:
Our final case report of MOGAD occurred after a previously healthy 23-year-old woman had her second dose of BNT162b2…
The patient was released from hospital on day+120, but relapsed, was readmitted and a brain MRI on D+173 revealed multiple hyperintense lesions on the right side of the insular cortex. After restarting oral steroids she was finally released on D+187:
A healthy 23-year-old woman presented with vertigo, vomiting, and headache from the 33rd day after receiving the second dose of the BNT162b2 vaccine (Figure A). On the 35th day after vaccination, she presented with vertigo, vomiting, and mild headache on a physical examination after walking into the hospital. Neurological findings were unremarkable, and no abnormalities were observed on either otorhinolaryngological examinations or endoscopic examinations of the upper gastrointestinal tract in the outpatient department.
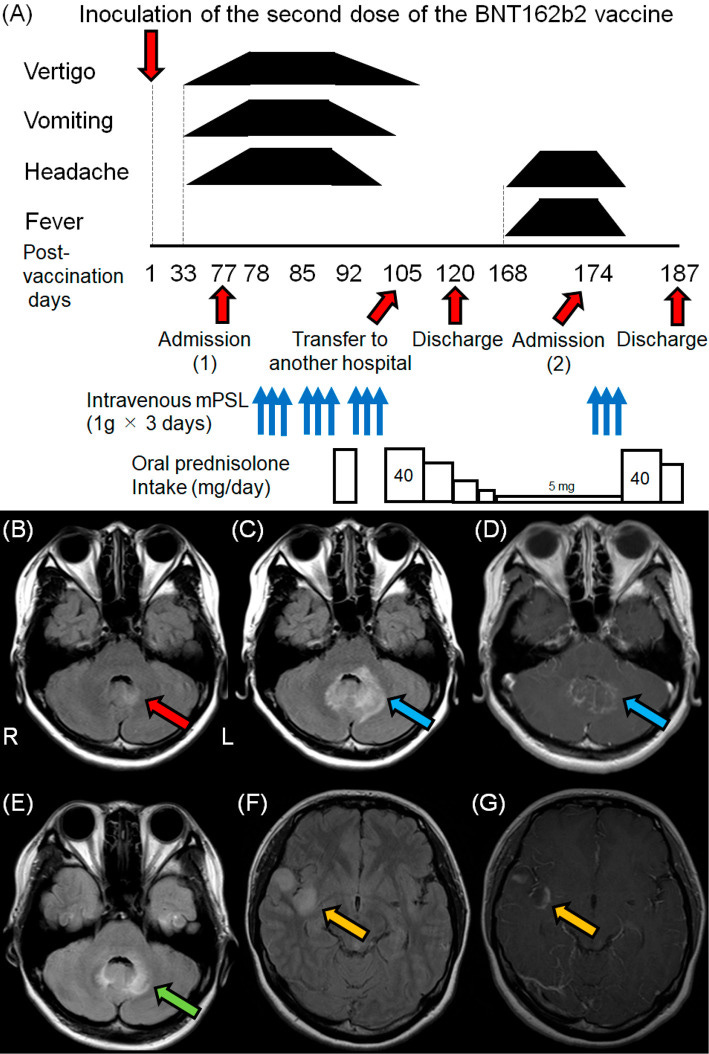
Brain magnetic resonance imaging (MRI) on the 57th day after vaccination revealed mild hyperintensity around the fourth ventricle on fluid-attenuated inversion recovery (FLAIR) imaging (Figure B). She was unable to walk independently due to severe vertigo on the 58th day after vaccination. Therefore, she was admitted to a hospital in a bedridden state on the 77th day after vaccination.
…On the 98th day after vaccination, serological tests confirmed the presence of anti-MOG antibodies in a live cell-based assay with titers of more than 1:16, using anti-IgG1 Fc as the secondary antibody; therefore, she was diagnosed with MOGAD.
From: “Relapsing Anti-MOG Antibody-associated Disease following COVID-19 Vaccination: A Rare Case Report and Review of the Literature“ (2023)
Fatigue
Inflammation of the brain and the CNS due to cytokine signalling is particularly associated with fatigue. One of the pathways involves cytokine activation of indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme that degrades tryptophan along the kynurenine pathway.
Activation of GTP-CH1 and IDO reduces the synthesis of dopamine and serotonin and cytokines also modulate dopamine and serotonin transporter activity, which reduces their synaptic availability for nerve signalling20.
Pro-inflammatory cytokines also contribute to the initiation and propagation of autoimmune inflammation, which links COVID-19 and transfection induced inflammation of the brain and CNS to central fatigue21. Other factors are involved too, but beyond the scope of this narrative review.
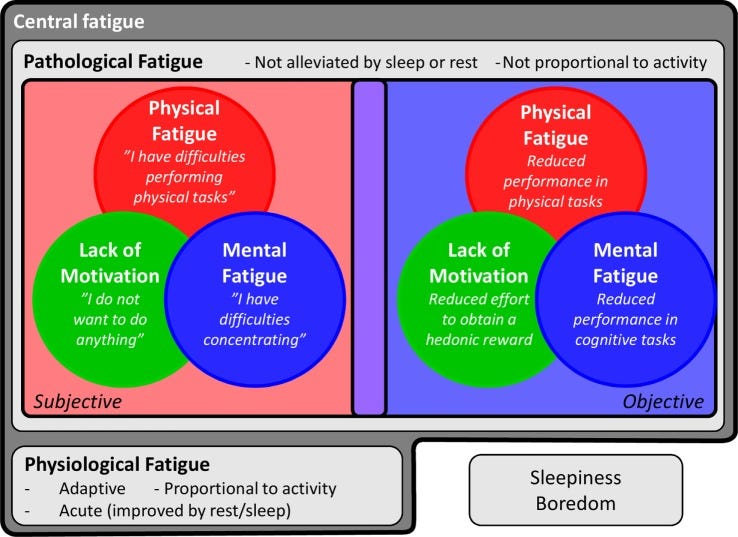
…The extreme clinical form of fatigue in CFS/ME has been the subject of extensive study and provides a good model for assessing the potential role of inflammation in the development of fatigue (6). CFS/ME is a debilitating multisystem condition primarily defined by a disabling fatigue for more than 6 months, along with several other symptoms, including pain and cognitive changes.
…One of the key symptoms is “postexertional fatigue” (64), which, interestingly, appears to be somewhat unique for this patient group (65). Although the underlying mechanisms of this disease are complex, a clear immunological component stands out; CFS/ME often appears following an infection, and some of the most promising treatments are immunomodulatory (64, 66). Furthermore, an extensive literature indicates that patients suffering from CFS/ME exhibit increased systemic production of pro-inflammatory cytokines [e.g., IL-6 or tumor necrosis factor (TNF)-α] and higher CRP at baseline as well as after immune stimulation, compared to non-fatigued individuals (67–72).
From: “Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms” (2017)
A newly published preprint by Krumholz et al described the reported symptoms from 241 individuals who self-reported post-vaccination syndrome (PVS) after Covid-19 vaccination. The pattern of neurological pathologies is striking, with similarities to MS:
…Among these participants with PVS, 127 (55%) had received the BNT162b2 [Pfizer-BioNTech] vaccine, and 86 (37%) received the mRNA-1273 [Moderna] vaccine. The median time from the day of index vaccination to symptom onset was three days (IQR: 1 day to 8 days).
…The five most common symptoms were exercise intolerance (71%), excessive fatigue (69%), numbness (63%), brain fog (63%), and neuropathy (63%). In the week before survey completion, participants reported feeling unease (93%), fearfulness (82%), and overwhelmed by worries (81%), as well as feelings of helplessness (80%), anxiety (76%), depression (76%), hopelessness (72%), and worthlessness (49%) at least once.

From: “Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization“ (Nov. ‘23)
https://www.medrxiv.org/content/10.1101/2023.11.09.23298266v1.full-text
Guillain-Barré syndrome (GBS)
“Guillain-Barré syndrome (GBS) is also called acute inflammatory demyelinating polyradiculoneuropathy (AIDP). It is a neurological disorder in which the body's immune system attacks the peripheral nervous system, the part of the nervous system outside the brain and spinal cord.”22
GBS mainly affects the feet, hands and limbs, causing problems such as numbness, weakness and pain. It can affect all ages, but is more common in adults and males.
Classic features:23
Symptoms often start in feet and hands before spreading to your arms and legs.
Numbness.
Pins and needles.
Muscle weakness.
Pain
Problems with balance and co-ordination.’
Symptoms may continue to get worse over the first few days or weeks after first becoming apparent before slowly starting to improve.
In severe cases, a difficulty in moving, walking, breathing and/or swallowing. These are considered medical emergencies.
Recovery from most symptoms usually takes 6 to 12 months, but full recovery from the nerve damage may take years.
Blood clots or severe breathing difficulties may lead to fatalities in around 5% of cases.
A Japanese study by Uchibori & Chiba from 2015 discusses autoantibodies associated with GBS. Their results were that these were mainly antibodies to gangliosides:
“Gangliosides are sialic acid-containing glycosphingolipids. They occur especially on the cellular surfaces of neuronal cells, where they form a complex pattern, but are also found in many other cell types.”24
(Translated, paywalled):
In Guillain-Barré syndrome, antibodies to glycolipids, mainly gangliosides, are detected in about 60%. Antibody titers are highest in the acute phase and decrease with the course of time. There is a certain correspondence between the clinical disease type and the type of antibody detected. The mechanism of complement-mediated neuronal tissue damage by glycolipid antibodies has been inferred by the localization of gangliosides in peripheral nerve tissues, the verification of molecular homologous glycan structures in pre-infectious pathogens, and the preparation of ganglioside-sensitized animal models.
From: “All About Guillain-Barré Syndrome: A 100-Year Trail. Autoantibodies for Guillain-Barré syndrome” (2015)
https://webview.isho.jp/journal/detail/abs/10.11477/mf.1416200305
A case report of GBS in an Italian 82-year-old woman after the second dose of BNT162b2 confirmed the presence of ganglioside autoantibodies:
…We present the case of an Italian 82-year-old woman, nonsmoker, suffering from permanent atrial fibrillation and arterial hypertension, on pharmacological treatment with rivaroxaban and with a preexisting walking disorder due to bilateral hip prosthesis for which she walked with a walker.
…Two weeks later she presented a progressive worsening of walking, so she went to the emergency department. The patient began to experience a sudden worsening of walking associated with a lack of strength and sensitivity in the lower limbs. In the following days the appearance of similar symptoms in the upper limbs was associated with greater involvement of the proximal arm than the distal. The walking disturbance had become so severe that the patient was completely bedridden.
…Standard laboratory tests and special blood tests (HbA1c, ANA, ENA, anti‐DNA, c‐ANCA, p‐ANCA, HIV, HBV, HCV, serum vitamin B12‐level, and serum protein electrophoresis) were also within the normal range. Campylobacter jejuni antibodies were tested as negative. Two nasopharyngeal swabs for SARS-COV2 were negative. A lumbar puncture was performed and cerebrospinal fluid (CSF) analysis showed albumin cytologic dissociation (protein of 5,7 gr/l and 2 cells), consistent with the diagnosis of GBS. CSF cytology was negative and the analysis of all neurotropic viruses gave negative results in the same fluid.
…Taking the medical history, the patient had denied any symptoms (including respiratory and gastrointestinal ones) in the 2–4 weeks prior to the onset of neurological symptoms. The only trigger identified was the vaccine given 15 days earlier.
To complete the diagnostic framework, anti-ganglioside antibodies tests and electromyoneurography were carried out (Tables 2 and 3)
…Electroneurography results (performed on the eleven day of hospitalization) showed acute sensory-motor neuropathy of the demyelinating type (Table 2). Electromyography showed decreased recruitment to the analysis of voluntary muscle activity, with signs of spontaneous activity (Table 3). Anti-ganglioside antibodies, in particular anti-sulfatide IgG (+) and IgM (++), anti-GM2 IgM (+) and anti-GM4 IgM (+) antibodies, were positive.
From: “Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine“ (2021)
In contrast, a retrospective analysis of 60 case reports from 2023 revealed that post-COVID-19 vaccination GBS had lower positive rates of anti-ganglioside antibodies. This paradox may be explained by plasmid DNA or other truncated peptide contamination and batch variability25.
AIDP: acute inflammatory demyelinating polyradiculoneuropathy.
Retrospective analysis of 60 case reports revealed that post-COVID-19 vaccination GBS occurred mostly after the first dose of the vaccination (54 cases, 90%) and was common for DNA vaccination (38 cases, 63%), common in middle-aged and elderly people (mean age: 54.5 years), and also common in men (36 cases, 60%). The mean time from vaccination to onset was 12.3 days. The classical GBS (31 cases, 52%) was the major clinical classification and the AIDP subtype (37 cases, 71%) was the major neurophysiological subtype, but the positive rate of anti-ganglioside antibodies was low (7 cases, 20%). Bilateral facial nerve palsy (76% vs 18%) and facial palsy with distal paresthesia (38% vs 5%) were more common for DNA vaccination than for RNA vaccination.
…The association between a vaccine and the increased incidence of GBS has been confirmed only for influenza vaccine so far, with 1–2 cases of GBS per 1 million doses of influenza vaccine (43–45). Molecular mimicry is often the primary pathogenic mechanism for vaccine-associated GBS. Specifically, the vaccine contains the same structure as gangliosides, and thus vaccinated individuals produce anti-ganglioside antibodies that attack neural autoantigens, thereby causing neurological damage and associated clinical symptoms.
…With the widespread use of COVID-19 vaccines worldwide, many cases of post-vaccination GBS have been reported (7–41). Hill proposed that the criteria for assessing the causal relationship between clinical outcome and possible pathological injury consist of the following nine characteristics: strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, experimental evidence, and analogy (46). The only evidence for the association of these cases with COVID-19 vaccines was temporality. The mean time from vaccination to symptom onset was 12.3 days in our review, which was consistent with the expected period of maximal immune response to the vaccine, while there was no evidence that the patient was subject to other infectious or autoimmune factors.
Antibodies against the spike protein may cross-react with peripheral nerve components (gangliosides) to cause GBS. However, contrary to expectations, GBS cases that occur after COVID-19 vaccination have a low positive rate of anti-ganglioside antibodies (20%), which is significantly lower than that (80%–90%) in other reported GBS cases, a discrepancy suggesting that gangliosides may not be the true antigenic target for GBS that emerges after COVID-19 vaccination (51). Molecular mimicry antigens may be structurally related to adenoviral vectors, which explains the relative safety of RNA vaccines (47). In addition, abnormal splice variants, contaminated proteins, or other components of the vaccine may all be sources of immune response in GBS, but its true antigenic targets remain to be further investigated.
…Of note, the present study observed that bilateral facial nerve palsy was generally the initial symptom of post-COVID-19 vaccination GBS and that the frequency of facial palsy with distal paresthesia was much higher than expected, with both bilateral facial nerve palsy and facial palsy with distal paresthesia being common in the DNA vaccine group. Pegat et al. analyzed all cases reported in the French pharmacovigilance database (June 29, 2021) and found that 23 (33%) of GBS cases following COVID-19 vaccination presented with bilateral facial nerve palsy, 21 (91%) of which occurred after vaccination with DNA vaccines—namely ChAdOx1-S (46% [20/44]) and Ad26.COV2S (33% [1/3])—accounting for 45% of all GBS cases following DNA vaccination (54). For GBS following DNA vaccination, facial involvement is more frequent and facial palsy with distal paresthesia is more common, suggesting that GBS may appear as a specific clinical subtype following DNA vaccination. This supports a possible causal relationship between COVID-19 vaccines and the syndrome, while it is still necessary to further elucidate this possible causal relationship and the underlying immunopathologic mechanism through prospective studies.
…However, the negative serology in the majority of the cases suggested that the dominant neurophysiological subtype of post-COVID-19 vaccination GBS is likely to be AIDP; this is because (1) anti-ganglioside antibodies are highly selective in attacking axons and are considered to be a biomarker of axonal injury rather than demyelination and (2) anti-ganglioside antibodies (GM1, GM1b, GD1a) are common in the serum of cases with the AMAN subtype of GBS, while the AIDP subtype was largely serologically negative for these antibodies, consistent with our expectation (55, 56). In short, the present results suggest that the neurophysiological subtypes of GBS following COVID-19 vaccination are indeed dominated by AIDP (55, 56).
From: “Guillain-Barre syndrome following COVID-19 vaccines: A review of literature“ (2023)
Another case study of GBS, this time after BNT162b2 was published in 2022. Again, serum results were unremarkable and more research is needed. At this stage it is more likely to be retrospective due to confounding factors, and only if anyone is prepared to fund it:
…Herein, we report, for the first time in South Korea, the case of a 21-year-old man who presented with facial diplegia and mild ataxia that later progressed to mild motor weakness after the first dose of BNT162b2 (Pfizer) vaccination.
…Autoimmune-related laboratory tests such as rheumatoid factor, thyroid function test, SSA, SSB, antinuclear antibodies, antineutrophil cytoplasmic antibodies, lupus anticoagulant antibody, and anti-dsDNA were all unremarkable. Antiganglioside antibodies encompassing anti-GM1 IgG, anti-GQ1b IgG, anti-GT1a, and anti-GD1b IgG were also unremarkable. The patient received SARS-CoV-2 serology test, which was negative for IgM, IgG, and IgA antibodies to nucleocapsid protein.
From: “Guillain–Barré syndrome associated with BNT162b2 COVID vaccination: a first case report from South Korea“ (2022)
OpenVAERS and GBS/TM
OpenVAERS provides ready access to US adverse events reports. 2021 represented a dramatic inflection point, and although flu vaccination rates don’t match that for COVID-19 they have been at 43-53% since 2010, but with a proportionally far lower number of GBS/TM reports26:
Bell’s palsy (BP)
Bell's palsy is an idiopathic peripheral nerve palsy involving the facial nerve. It accounts for 60 to 75% of all cases of 1-sided facial paralysis. Facial droop can also be the symptom of a stroke, which is a medical emergency.
Classic features:27
Weakness on 1 side of your face, or not being able to move 1 side of your face. This usually happens over a few days.
A drooping eyelid or corner of your mouth.
Drooling.
A dry mouth.
Loss of taste.
A dry or watering eye.
It many be difficult to close the eye on the weak side of the face.
Rarely, paralysis may affect both sides of the face.
Steroids, again, sometimes with an antiviral drug are the usual treatment. Recovery may take 6 months or longer, and long term recurrences are a risk.
In 2012, Greco et al discussed BP and autoimmunity. It accounts for 60 to 75% of all cases of unilateral facial paralysis. Peak incidence occurs in the age range 15 to 45, the median age is 40 and equal numbers of males and females are affected.
In terms of autoimmunity it parallels GBS in many ways:
…Bell's palsy is diagnosed upon the abrupt onset of unilateral facial weakness or complete paralysis of all the muscles on one side of the face, dry eye, pain around the ear, an altered sense of taste, hyperacusis (hypersensitivity to sounds), or decreased tearing [11]. In patients with Bell's palsy, on attempted closure, the eye rolls upward (Bell's phenomenon). The disease usually progresses from the onset of symptoms to maximal weakness within three days.
…Approximately 10% of patients with Bell's palsy experience one or more recurrences after a mean latency of 10 years.
…Some evidence implicates the involvement of immune mechanisms in Bell's palsy. Many reports have indicated the association between facial paralysis and Guillain–Barré syndrome (GBS) [53], a condition that was recently shown to be a cell mediated, autoimmune neuritis [54].
…Abramsky et al. [47] demonstrated a defined in vitro response to a human basic protein (P1L) of peripheral nerve myelin in patients with Bell's palsy. They suggested that cell-mediated autoimmune mechanisms may be of importance in the pathogenesis of Bell's palsy.
…Approximately the same in vitro transformation in the presence of P1L protein was found in cases with GBS [55]. The specific in vitro stimulation of lymphocytes from Bell's palsy and GBS patients using peripheral P1L basic protein suggests that an in vivo sensitisation to such self protein may occur in these two conditions, and that cellmediated autoimmune mechanisms may be an important factor in the pathogenesis of the paralysis.
…In Bell's palsy patients, the percentage of T suppressor cells was significantly reduced, whereas the percentage of T helper cells was normal [56]. This is in line with the findings in patients with acute Guillain–Barré syndrome [58].
…An examination of the serum samples of patients with Bell's palsy shows elevated concentrations of the cytokines interleukin-1 (IL-1), IL-6, and tumour necrosis factor-alpha (TNF-alpha) compared with control populations [64], suggesting an activation of cell-mediated effectors.
From: “Bell's palsy and autoimmunity” (2012)
https://www.sciencedirect.com/science/article/pii/S1568997212001152
Searches revealed that most of the papers investigating BP were published in the 1970’s or 80’s282930, which is a great for background reading but not so good for reviewing interactions with modern gene therapy agents.
Again, few papers investigate antibody subclasses but we can get an indication that LNP assisted breaking of immune tolerance by overstimulation is a factor. Its interesting that this was raised by a Pfizer representative, and interferon production (IFN) is implicated:
…A meningococcal conjugate vaccine showed a significant association with Bell's palsy when administered simultaneously with other vaccines such as influenza, human papillomavirus, or diphtheria-tetanus-pertussis vaccines.5
…One theory suggests vaccines could be associated with autoimmune phenomenon, which is thought to occur via either mimicry of host molecules by the vaccinal antigen or bystander activation of dormant autoreactive T-cells.7 Such theorised associations have not withstood close scrutiny. The SARS-CoV-2 vaccines do not contain an exogenous adjuvant, but discussion between members of the FDA's Vaccines and Related Biologic Products Advisory Committee and a sponsor (Pfizer) raised the possibility that the vaccine might induce innate immune activation from a combined effect of mRNA and lipids, potentially including interferon production. Such interferon production could transiently break peripheral tolerance, a hypothetical phenomenon invoked in several case reports.8, 9
From: “Bell's palsy and SARS-CoV-2 vaccines” (2021)
To put it another way they knew the risk of this prior to the rollout, and worse still so did the regulators, yet they failed to act.
Incidentally I got sent to Twitter jail for 7 days in 2021 after posting about Yellow Card reports of Bell’s - i.e. for stating facts that didn’t fit the narrative of the day:

This recently published paper goes into detail to explain the pathophysiology of LNP>TLR7,8,9>IFN1 mediated autoimmune disease:
…Several studies have indicated that the key response of autoimmune diseases is the type 1 interferon response. Increased type 1 IFN levels provoke peripheral tolerance breakdown through the activation of immature myeloid dendritic cells (mDCs), and IFN-matured mDCs activate autoreactive T cells. These cells, together with plasmacytoid DCs, expand autoreactive B cells. IFN-mature DCs also promote apoptosis by activating cytotoxic CD8 + T cells. Capture of apoptotic cells by mDCs and capture of nucleic acid-containing immune complexes by plasmacytoid DCs and B cells enhance the autoimmune response69. Furthermore, the process of identifying autoantigens is necessary for the onset of autoimmunity, and endosomal TLR7, TLR8, and TLR9 mostly recognize RNA-related immune complexes. TLR7 and TLR9 recognize viral nucleic acids and nucleic acid-containing immune complexes and stimulate IFN-1 expression to cause systemic lupus erythematosus69,70,71,72. In addition, the B-cell receptor/TLR7 of autoreactive B cells is activated by RNA and RNA-associated autoantigens, which leads to lupus73. In fact, when PBMCs from healthy people were exposed to autoantibodies specific to RNA-binding proteins and anti-double-stranded DNA autoantibodies in the serum of patients with autoimmune disorders, IFN-1 was generated74.

From: “Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics” (Oct. ‘23)
The following year, as with related GBS, Khurschid et al suggest the involvement of anti-ganglioside antibodies. Of interest to me from other work I’ve been doing is the reference to binding to sialic acid-containing glycoproteins. Both the spike protein S1-RBD and NTD sites (the “galectin fold” or pocket) can bind (sialo)glycans and mediate potentially autoimmune antibodies31:
Over half, 53.45%, of our recorded patients had bilateral facial palsy. Bell's Palsy is usually unilateral with an idiopathic aetiology whereas, bilateral is exceedingly rare, and secondary to systemic diseases like GBS [42]. This association must be credited as 67.24% of our patients were primarily diagnosed with GBS. Post-vaccination GBS has been analysed by several authors. The SARS-CoV 2 spike protein, in the vaccine, increases its transmission by binding to sialic acid-containing glycoprotein and gangliosides present on the neuronal cells' surface. After adequate exposure to the nerve components, antiganglioside antibodies are generated, ensuing in an autoimmune reaction. Thus, demyelination occurs after inflammatory changes, presenting with the afore-mentioned polyradiculopathy [26,27]. This could include the Facial Nerve (CN VII) of both sides, defining bilateral Bell's Palsy.
From: “Development of facial palsy following COVID-19 vaccination: A systematic review” (2022)
OpenVAERS and Bell’s palsy
Peripheral Neuropathy (PN)
PN develops when nerves at the periphery of the CNS are damaged, such as in the hands, feet and arms. Specific symptoms depend on which nerves have been affected.
The peripheral nervous system lies outside of the brain and spinal cord and consist of three types of nerve, each with their own function:
Sensory nerves – responsible for transmitting sensations, such as pain and touch.
Motor nerves – responsible for controlling muscles.
Autonomic nerves – responsible for regulating automatic functions of the body, such as blood pressure and bladder function.
Classic features:32
Numbness and tingling in the feet or hands.
Burning, stabbing or shooting pain in affected areas.
Loss of balance and co-ordination.
Muscle weakness, especially in the feet.
T1 & T2 diabetes is the most common cause of PN.
The prognosis varies according to the underlying cause.
If left untreated can progress to a foot ulcer, for example, or even gangrene.
If the nerves controlling the automatic functions of the heart and circulation system have been affected (ie cardiovascular autonomic neuropathy) then hypertensive drugs or, in rare cases, a pacemaker may need to be fitted.
PN may affect:
only 1 nerve (mononeuropathy).
several nerves (mononeuritis multiplex).
all the nerves in the body (polyneuropathy).
As we are still at the periphery of the CNS, away from the brain and spinal cord, its not unexpected that anti-ganglioside autoantibodies are involved again:
Anti-ganglioside antibodies are principally associated with autoimmune peripheral neuropathies. In these disorders, immune attack is inadvertently directed at peripheral nerve by autoantibodies that target glycan structures borne by glycolipids, particularly gangliosides concentrated in nerve myelin and axons. The most thoroughly studied disorder is the acute paralytic disease, Guillain–Barré syndrome (GBS) in which IgG autoantibodies against gangliosides arise following acute infections, notably Campylobacter jejuni enteritis. Additionally, chronic autoimmune neuropathies are associated with IgM antibodies directed against many glycolipids including gangliosides.
From: “Anti-ganglioside Antibodies in Peripheral Nerve Pathology“ (2018)
https://link.springer.com/protocol/10.1007/978-1-4939-8552-4_7
This article further details specific autoantibodies, with MAG glycoprotein particularly targeted.
ALS: amyotrophic lateral sclerosis (also called motor neurone disease or Lou Gehrig's disease). “ALS is a fatal motor neuron disease. It is characterized by progressive degeneration of nerve cells in the spinal cord and brain. ALS affects voluntary control of arms and legs, and leads to trouble breathing. ALS does not affect intelligence, thinking, seeing, or hearing.”33
…The gangliosides most commonly recognized by neuropathy associated autoantibodies are GM1, asialo-GM1, GD1a, GD1b, and GQ1b.
…Myelin-associated glycoprotein (MAG) is a constituent of peripheral and central nervous system myelin. High titer IgM antibodies to MAG are associated with sensorimotor demyelinating peripheral neuropathy. Sensory symptoms tend to dominate early in disease, with motor symptoms occurring later. MAG antibodies are usually associated with the presence of an IgM monoclonal protein; approximately 50% of patients with IgM monoclonal gammopathies and associated peripheral neuropathies have detectable MAG antibodies.
…Sulfatide is a glycolipid found in greatest quantity in the CNS and peripheral nerve myelin. Antibodies against sulfatide are associated with Guillain-Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), sensory neuropathy and sensorimotor neuropathy.

From: “Neuropathy Associated Antibodies“
http://www.clinlabnavigator.com/neuropathy-associated-antibodies.html
Ganglioside autoantibodies appear to be particularly associated with loss of motor function in PN:
Ganglioside antibodies are associated with diverse peripheral neuropathies. Elevated antibody levels to ganglioside-monosialic acid (GM1) and the neutral glycolipid, asialo-GM1 are associated with motor or sensorimotor neuropathies, particularly multifocal motor neuropathy. Anti-GM1 may occur as IgM (polyclonal or monoclonal) or IgG antibodies. These antibodies may also be found in patients with diverse connective tissue diseases as well as normal individuals. GD1a antibodies are associated with different variants of Guillain-Barre syndrome (GBS) particularly acute motor axonal neuropathy while GD1b antibodies are predominantly found in sensory ataxic neuropathy syndrome. Anti-GQ1b antibodies are seen in more than 80% of patients with Miller-Fisher syndrome and may be elevated in GBS patients with ophthalmoplegia. The role of isolated anti-GM2 antibodies is unknown.
From: “Ganglioside (Asialo-GM1, GM1, GM2, GD1a, GD1b, and GQ1b) Antibodies“
There is a high prevalence of post COVID-19 neuropathy & myopathy (muscle weakness), according to this study from 2022:
Methods: The study involved 400 patients, divided into 2 groups, with a history of COVID-19 infection with or without symptoms of neuromuscular affection, and 30 gender- and age-matched healthy volunteers were involved as controls. They were referred to the Department of Rheumatology and Rehabilitation for electro-diagnosis. All participants performed complete clinical examination and laboratory measures with an electrophysiological study.
Results: The prevalence of peripheral neuropathy and myopathy in post-COVID-19 patients was 56.3% among all patients. A significant difference was detected among patients of both groups regarding serum creatine phosphokinase level, clinical signs, and electrophysiologic findings of neuropathy and myopathy compared to the control group, with more prominent features among the symptomatic group. Histories of hospitalization, severe and long-lasting respiratory symptoms were risk factors for developing neuromuscular complications.
Conclusions: The present study could indicate that muscle involvement and peripheral nerve affection are common problems even among asymptomatic patients after COVID-19 infection, especially in the presence of any risk factors.
From: “Prevalence of peripheral neuropathy and myopathy in patients post-COVID-19 infection“ (2022)
Looking at case reports post-vax, again molecular mimicry leading to pathogenic levels of a particular autoantibody appears to be less contributory than LNP mediated loss of immunotolerance.
9 cases were discussed in this paper from 2021, although the authors at the time didn’t discuss the adjuvant properties of PEGylated LNPs34:
…Facial nerve palsy has been described as an adverse event following immunization against other pathogens including influenza, hepatitis B, polio, diphtheria-tetanus-pertussis, and acellular pertussis, as well as the measles-mumps-rubella vaccine [1]. Although the mechanism is not fully elucidated, it is thought to involve the additive adjuvants that stimulate an immunomodulatory reaction [2]. The BNT162b2 vaccine creates an immune reaction using a different method, without adjuvants, based on viral spike protein being translated and expressed based on mRNA.
…To the best of our knowledge, this is the first report describing several cases of peripheral facial nerve palsy following administration of the BNT162b2 SARS-CoV-2 vaccine in real world data.
From: “Peripheral Facial Nerve Palsy Following BNT162b2 (COVID-19) Vaccination“ (pdf, 2021)
The Moderna LNP-mRNA product is a potential trigger too:
…We report a case of chronic inflammatory demyelinating polyneuropathy (CIDP) post-mRNA-1273 (Moderna) COVID-19 vaccine.
…We present a case of an elderly female who developed lower extremities weakness gradually over five months. Clinical, pathognomonic, lab, and electromyography (EMG) findings were consistent with CIDP. Outside a few reports in VAERS, this is the first case of CIDP documented following immunization with the Moderna COVID-19 (mRNA-1273) vaccine.
…The distinction between GBS and CIDP is important. GBS is the most prevalent cause of acute flaccid paralysis, characterized by autonomic dysfunction, sensory abnormalities, and varying degrees of weakness. Although the specific pathophysiology is unknown, this disorder is believed to result from an autoimmune response. GBS is often preceded by a respiratory or gastrointestinal infection. CIDP is a chronic disorder and the most common autoimmune polyneuropathy in adults.
From: “Chronic Inflammatory Demyelinating Polyneuropathy Post-mRNA-1273 Vaccination” (2022)
As discussed earlier, a secondary trigger for peripheral neuropathy is latent viral reactivation and new onset infections due to Spike induced immunosuppression. Multiple mechanisms contribute to this and were detailed in earlier Substacks. Notable amongst these include impairment of DNA damage repair which is required for effective V(D)J recombination in adaptive immunity35; inflammaging36; igG4 class switching37 and induction of T-cell anergy38:
…Any discussion of the PNS complications of infectious disease would be incomplete without comment about human immunodeficiency virus (HIV), arguably the most well-described viral etiology of PNS dysfunction.
…Human immunodeficiency virus commonly affects both the central nervous system (CNS) and the PNS (Table 1). Distal symmetric polyneuropathy (DSP) associated with HIV is the most common PNS complaint, affecting up to 30% to 50% of patients with advanced infection.1,2 Typical symptoms of the DSP include paresthesias or numbness in a stocking-glove distribution, though up to 71% of patients may be asymptomatic.1
…The herpes viruses are double-stranded DNA viruses that can produce symptoms after years of lying dormant. The ability of these viruses to cause CNS disease and transverse myelitis is well documented,11 but they also impact the PNS and have been implicated in radiculopathies and cranial neuropathies. Three of these viruses, varicella-zoster virus (VZV), and herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), are neurotropic and reside in neural ganglia.
…Epstein-Barr virus (EBV) is also a lymphotropic herpes virus. Infection of the PNS is rare but has been associated with GBS, acute myeloradiculitis, and encephalomyeloradiculitis. In a serologic study of 100 patients with GBS, 8 had EBV-specific IgM.25 Rare case reports of more systemic neurologic involvement of EBV in the form of a myeloradiculitis or an encephalomyeloradiculitis are also documented in the literature. Majid et al reported on 4 such cases, all of which had a CSF mononuclear predominant pleocytosis, elevated CSF protein, and EBV DNA in the CSF.26 All 4 cases received antiviral treatment with either acyclovir or ganciclovir, and 2 of the 4 cases received steroids.
From: “Peripheral Nervous System Manifestations of Infectious Diseases“ (2014)
VAERS and peripheral neuropathy
Written reamarks in response to the Advisory Committee on Immunization Practices (ACIP) meeting of April 19 2023 included the estimated underreporting factor (URF) for VAERS. Even so, this must vastly underestimate case numbers, especially over the long term.
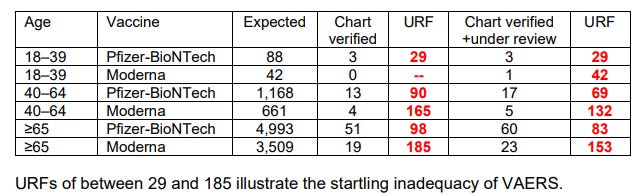


Hashimoto's thyroiditis
What relation has the thyroid gland to autoimmune neuropathies?
The answer is cross-reactivity of at least two kinds of autoantibodies.
The first are antinuclear antibodies (ANAs), which strongly correlate to COVID-19 and systemic autoimmune disorders such as lupus (SLE):
…The antinuclear antibody (ANA) is a defining feature of autoimmune connective tissue disease. ANAs are a class of antibodies that bind to cellular components in the nucleus, including proteins, DNA, RNA, and nucleic acid-protein complexes.[1] First described in 1948, ANA identification has been the foundation of diagnosis for autoimmune connective tissue disease, including systemic lupus erythematosus (SLE), Sjogren's syndrome, and polymyositis/dermatomyositis.[2] Although 20 to 30% of the average population has detectable levels of ANAs, increased titers are characteristic of individuals with connective tissue disorders.[3]
From: “StatPearls: Biochemistry, Antinuclear Antibodies (ANA)“ (2022)
…Extending the evaluation of ANA in COVID‐19 to other experiences, we can observe a similar prevalence to ours in a Greek study, 4 where the authors found an ANA positivity in 10 patients out of 29 (34%), and, interestingly, 7 of them exhibited a nucleolar ANA pattern. Moreover, two recent studies from Nanjing (China) and from Seattle (United States) reported a frequency of ANA of 50% and 25% respectively, even though the detection of these reactivities was not carried out by indirect immunofluorescence but by immunochemical method. 4 , 5
From: “Antinuclear antibodies in COVID 19“ (2021)
Classic features of Hashimoto’s thyroiditis:39
An underactive thyroid (hypothyroidism) is when your thyroid gland does not produce enough of the hormone thyroxine (also called T4).
A condition called Hashimoto's disease is the most common type of autoimmune reaction that causes an underactive thyroid.
Common signs of an underactive thyroid are tiredness, weight gain and feeling depressed.
Other signs include being sensitive to the cold, muscle aches and having dry skin and hair.
Daily hormone replacement tablets called called levothyroxine can be taken as a treatment.
Hashimoto’s thyroiditis and ANAs (translated):
Results:
Forty-seven percent of the patients were ANA positive. Of them 60% showed a titre of 1:40. The most frequent sonography picture was pseudo-nodular (66%) with predominance in ANA negative subgroups (71%) than in ANA positive one (60%). On the basis of all the autoimmune parameters evaluated in every subgroups, 72% of our patients were positive to at least one autoimmunity parameter and/or have an autoimmune disease besides Hashimoto's thyroiditis.
Conclusion:
The conclusion is drawn that the patient with Hashimoto's thyroiditis should be considered as an autoimmune patient. Thus, it is necessary to carry out an autoimmune screening with ANA in every new diagnosed patients and to study in depth the familiar and pathological history. Periodical checking of the autoimmune parameters should not be underestimated in these patients.
From: “[Hashimoto's thyroiditis and autoimmunity parameters: descriptive study]“ (2007)
Postural tachycardia syndrome (POTS)
“Postural tachycardia syndrome (PoTS) is when your heart rate increases very quickly after getting up from sitting or lying down. It can get better with changes to your lifestyle, but some people may need treatment with medicines.”
Classic features.40 You will recognise many of these in both postacute sequelae of SARS-CoV-2 infection (PASC) and in post-vax adverse event case reports:
Dizziness or light-headedness.
Fainting or almost fainting.
Noticeable heartbeats (heart palpitations).
Chest pain.
Shortness of breath (dyspnoea).
Shaking and sweating.
Problems with your stomach or digestion, such as feeling sick, being sick, diarrhoea, constipation, bloating and stomach pain.
Headaches and problems with your sight, such as blurred vision or tunnel vision.
Your hands and feet looking purple.
Weakness and extreme tiredness (fatigue), not being able to do much exercise, and sleeping badly.
Problems with thinking, memory and concentration (brain fog).
ANA’s are associated with POTS:
Methods and results
Medical records of 100 consecutive patients with POTS evaluated at our clinic were reviewed. In this cohort (90% females, mean age 32, range 13–54 years), 25% had positive ANA, 7% had at least one positive aPL antibody and 31% had markers of autoimmunity. When compared to the general population, patients with POTS had a higher prevalence of ANA (25% vs. 16%, OR 1.8, CI 1.1–2.8, p < 0.05), aPL antibody (7% vs. 1%, OR 7.5, CI 3.4–16.1, p < 0.001) and co-morbid autoimmune disorders (20% vs. highest estimated 9.4%, OR 2.4, CI 1.5–3.9, p < 0.001). The most prevalent autoimmune disorder was Hashimoto’s thyroiditis (11% vs. up to 2%, OR 6.1, CI 3.2–11.3, p < 0.001), followed by RA (4% vs. up to 1%, OR 4.1, CI 1.5–11.2, p < 0.01) and SLE (2% vs. up to 0.12%, OR 17, CI 4.1–69.7, p < 0.001). The prevalence of CVID was very high (2% vs. 0.004%, OR 510.2, CI 92.4–2817.8, p < 0.001), while celiac disease showed a nonsignificant trend toward increased prevalence.
Conclusion
Patients with POTS have a higher prevalence of autoimmune markers and co-morbid autoimmune disorders than the general population. One in four patients have positive ANA, almost one in three have some type of autoimmune marker, one in five have a co-morbid autoimmune disorder, and one in nine have Hashimoto’s thyroiditis.
From: “Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS)“ (2015)
Ganglionic synaptic transmission is affected by ANA and other autoantibodies:
…An autoimmune basis has been suggested as a causal mechanism of POTS, and several autoreactive IgGs have been identified, including ganglionic acetylcholine receptor (gAChR), voltage‐gated potassium channel complex, cardiac lipid raft‐associated proteins, α1‐adrenergic receptor, as well as β1‐ and β2‐adrenergic receptors.5, 6, 7
…elevated expression of antibodies to gAChR subunits may contribute to secondary autoimmune responses to ganglionic neuron damage in seropositive patients. The prevalence of anti‐gAChR antibodies in this study was higher than that in previous study.17 Anti‐gAChR antibodies may impair autonomic ganglionic synaptic transmission, and antibodies that interfere with ganglionic transmission may contribute to dysautonomia in patients with POTS.18
From: “Autoimmune postural orthostatic tachycardia syndrome“ (2018)
Thyroid peroxidase antibodies (TPOab)
The other autoantibodies associated with Hashimoto’s are anti-TPO, just as Christie Grace discussed.
TPO is the key enzyme involved in thyroid hormone synthesis:
…The pathophysiology of Hashimoto thyroiditis involves the formation of antithyroid antibodies that attack the thyroid tissue, causing progressive fibrosis. The diagnosis can be challenging, and consequently, the condition is sometimes not diagnosed until late in the disease process. The most common laboratory findings demonstrate elevated thyroid-stimulating hormone (TSH) and low thyroxine (T4) levels, coupled with increased antithyroid peroxidase (anti-TPO) antibodies.
…This disease is also known as chronic autoimmune thyroiditis and chronic lymphocytic thyroiditis. The pathology of the disease involves the formation of antithyroid antibodies that attack the thyroid tissue, causing progressive fibrosis. The diagnosis is often challenging and may take time until later in the disease process.
…earlier on in the course of the disease, patients may exhibit signs, symptoms, and laboratory findings of hyperthyroidism or normal values. This is because the destruction of the thyroid gland cells may be intermittent.
…Fatigue, exertional dyspnea, and exercise intolerance are likely associated with a combination of limited pulmonary and cardiac reserve in addition to decreased muscle strength or increased muscle fatigue. Hypothyroid rats have been shown to have decreased endurance. Biochemical changes in this population have shown decreased muscle oxidation of pyruvate and palmitate, increased utilization of glycogen stores, and diminished fatty acid mobilization. Muscle weakness and myopathy are important features.
The presentation may also be subclinical. Early symptoms may include constipation, fatigue, dry skin, and weight gain. More advanced symptoms may include cold intolerance, decreased sweating, nerve deafness, peripheral neuropathy, decreased energy, depression, dementia, memory loss, muscle cramps, joint pain, hair loss, apnea, menorrhagia, and pressure symptoms in the neck from goiter enlargement such as voice hoarseness.
From: “StatPearls: Hashimoto Thyroiditis“ (2023)
With TPOab we can more directly implicate spike protein molecular mimicry as the trigger, rather than the LNP>IFN1 pathway:
…Looking at the reaction between SARS-CoV-2 spike protein antibody and tissue proteins (Fig. 1A), we found that the strongest reactions were with transglutaminase 3 (tTG3), transglutaminase 2 (tTG2), ENA, myelin basic protein (MBP), mitochondria, nuclear antigen (NA), α-myosin, thyroid peroxidase (TPO), collagen, claudin 5+6, and S100B. The reaction of this antibody was not as strong with several other antigens (Fig. 1A).


The nucleoprotein antibody showed some overlap in immune cross-reactivity with anti-spike protein antibody. As shown in Fig. 1B, nucleoprotein antibody reacted strongly with mitochondria, tTG6, NA, TPO, ENA, TG, actin, and MBP. Similar to spike protein, the nucleoprotein antibody reaction was not as strong with several other antigens as shown in Fig. 1A and B.
From: “Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases“ (2020)
Fibromyalgia (FM)
…we have demonstrated similarities between pathophysiological mechanisms and cardinal symptoms of FM and COVID-19, speculating that SARS-CoV-2 might represent a critical mediator of FM or an exacerbator of its symptoms once both syndromes share similar mechanisms and complaints. Therefore, pharmacologic and non-pharmacological approaches commonly used to treat FM could serve as strategic therapies to attenuate painful and neurological manifestations of post-COVID syndrome.
From: “Could the fibromyalgia syndrome be triggered or enhanced by COVID-19?“ (2023)
“Fibromyalgia, also called fibromyalgia syndrome (FMS), is a long-term condition that causes pain all over the body.”
Classic features:41
Increased sensitivity to pain.
Muscle stiffness.
Difficulty getting to sleep or staying asleep, leading to fatigue.
Problems with mental processes (known as "fibro-fog"), affecting concentration and memory.
Headaches.
Irritable bowel syndrome (IBS).
Feelings of frustration, worry or low mood.
Symptoms aren’t consistent and may suddenly get worse or improve.
The NHS outdoes itself with the treatment protocols, akin to being told to “deal with it” and being sent packing by the army doc with an aspirin.
They aren’t necessarily wrong but just have nothing behind them that shows recognition of potential root causes. Either that or they are holding back information, neither of which does much to instil confidence:
Although there's currently no cure for fibromyalgia, there are treatments to help relieve some of the symptoms and make the condition easier to live with.
Treatment tends to be a combination of:
lifestyle changes, such as exercise programmes and relaxation techniques
talking therapies, such as cognitive behavioural therapy (CBT) and acceptance and commitment therapy (ACT)
medicine, such as antidepressants
In particular, exercise has a number of important benefits for people with fibromyalgia, including helping to reduce pain.
What causes fibromyalgia?
The exact cause of fibromyalgia is unknown, but it's thought to be related to abnormal levels of certain chemicals in the brain and changes in the way the central nervous system (the brain, spinal cord and nerves) processes pain messages carried around the body.
It's also suggested that some people are more likely to develop fibromyalgia because of genes inherited from their parents.
In many cases, the condition appears to be triggered by things that are physical or emotional like an injury, an infection or stress.
From: “Fibromyalgia” (2023)
I think we can do better than that, and for several years now researchers have been testing the hypothesis that FM has autoimmunity as a key contributory factor to symptomology and disease progression.
In 2021 Goebel et al published the findings from an elegant study involving mice.
They transferred immunoglobulin (igG, antibodies) from FMS to mice and recorded any neurological changes, especially with respect to sensitivity and responsiveness to cold and mechanical stimulation. If autoimmune igG is a causative factor then it should be transferrable to mice, which have genomes 85-99% identical to humans.
This is indeed what they found, and they recommend a more credible treatment path on the back of it:
Abstract
Fibromyalgia syndrome (FMS) is characterized by widespread pain and tenderness, and patients typically experience fatigue and emotional distress. The etiology and pathophysiology of fibromyalgia are not fully explained and there are no effective drug treatments. Here we show that IgG from FMS patients produced sensory hypersensitivity by sensitizing nociceptive neurons. Mice treated with IgG from FMS patients displayed increased sensitivity to noxious mechanical and cold stimulation, and nociceptive fibers in skin-nerve preparations from mice treated with FMS IgG displayed an increased responsiveness to cold and mechanical stimulation. These mice also displayed reduced locomotor activity, reduced paw grip strength, and a loss of intraepidermal innervation. In contrast, transfer of IgG-depleted serum from FMS patients or IgG from healthy control subjects had no effect. Patient IgG did not activate naive sensory neurons directly. IgG from FMS patients labeled satellite glial cells and neurons in vivo and in vitro, as well as myelinated fiber tracts and a small number of macrophages and endothelial cells in mouse dorsal root ganglia (DRG), but no cells in the spinal cord. Furthermore, FMS IgG bound to human DRG. Our results demonstrate that IgG from FMS patients produces painful sensory hypersensitivities by sensitizing peripheral nociceptive afferents and suggest that therapies reducing patient IgG titers may be effective for fibromyalgia.
…The prevalence of reactivities against human peptides in our preliminary assessment of patient sera is nevertheless consistent with autoreactive IgG being responsible for our findings. Intriguingly, sera from COVID-19 patients contain a wide range of functional autoantibodies, which have been proposed to influence the symptomatic profile in patients (59).
…The identification of a pivotal role for autoreactive IgG in the pathophysiology of FMS may transform future research and facilitate development of mechanism-based therapeutic interventions. Our results suggest that therapies which reduce the total IgG titer, such as plasmapheresis or immunoadsorption (e.g., with protein A columns), or which specifically reduce autoreactive IgG (using antigen-specific adsorption) may be effective for FMS (60). Alternatively, symptomatic therapies that interfere with the binding of autoreactive antibodies or prevent their functional consequences may also provide effective treatment approaches.
From: “Passive transfer of fibromyalgia symptoms from patients to mice“ (2021)
In the light of these findings, last year (2022) the authors convened a working group to further develop and trial revised treatment protocols:
…Until recently, no clear pathophysiological mechanism for fibromyalgia had been established, resulting in management challenges. Recent research has indicated that serum immunoglobulin Gs (IgGs) may play a role in FMS. We undertook a research prioritisation exercise to identify the most pertinent research approaches that may lead to clinically implementable outputs.
Methods
Research priority setting was conducted in five phases: situation analysis; design; expert group consultation; interim recommendations; consultation and revision. A dialogue model was used, and an international multi-stakeholder expert group was invited. Clinical, patient, industry, funder, and scientific expertise was represented throughout. Recommendation-consensus was determined via a voluntary closed eSurvey. Reporting guideline for priority setting of health research were employed to support implementation and maximise impact.
Results
Arising from the expert group consultation (n = 29 participants), 39 interim recommendations were defined. A response rate of 81.5% was achieved in the consensus survey. Six recommendations were identified as high priority- and 15 as medium level priority. The recommendations range from aspects of fibromyalgia features that should be considered in future autoantibody research, to specific immunological investigations, suggestions for trial design in FMS, and therapeutic interventions that should be assessed in trials.
…There was strong agreement that immune parameters should be assessed during FMS flares versus non-flare periods; the dominant antibody subclasses and their titres should be studied [15].
…Stakeholders also agreed that the mechanisms underpinning the production of non-inflammatory Aab should be investigated.
In clinical trials, Aab serum levels—alongside the above immunological parameters—should be ascertained both at baseline and post-intervention; this includes both pharmaceutical, and non-pharmaceutical intervention (e.g., behavioural) trials.
From: “Research Recommendations Following the Discovery of Pain Sensitizing IgG Autoantibodies in Fibromyalgia Syndrome“ (2022)
To be fair to the NHS its still early days to process these recommendations, although in private practice and TCM its old news in terms of treatment approaches:
In Nanjing University Hospital, fibromyalgia is treated primarily with herbal medicine and only secondarily with acupuncture. Herbal medicine includes over 2000 different medicinal substances with 80-100 commonly used for the treatment of fibromyalgia. As practiced in the US, herbal formulas are generally given in decoction, powdered or pill form. The formulas tend to be 6-12 herbs with a balanced approach that include a focus on ameliorating the side effects that one has from single herbs. For example, it is very common that formulas have a small amount of ginger added to make the formulas easier on the digestion.
…Herbal decoctions tend to focus on reducing stress with formulas such as jiawei xiaoyao san or reducing blood stagnation and cold with formulas such as shugan jieyu huoxue tongluo wan. The only single herbs studied for the treatment of fibromyalgia was rhizome drynariae, which showed no significant effect on VAS. (9) It should be noted that there are a plethora of Chinese herbs that fibromyalgia patients might be taking to treat the symptoms of fibromyalgia such as hypericum (Guan Ye Lian Qiao) for depression, American ginseng (Xi Yang Shen) for lethargy and valerian root (Xie Cao) for insomnia.
From: “Traditional Chinese Medicine for Fibromyalgia“ (2010)
I will cross reference some of these in the “alternative therapeutics” section.
Hepatitis B vaccine and FMS
The NHS doesn’t do literature reviews it would appear, which is curious as it should be a core component of evidence based medicine (EBM)42.
This study from 2014 hypothesised that Hep B adjuvants may mediate chronic fatigue syndrome (CFS) and FMS due to ASIA syndrome:
The objectives of this study were to gather information regarding demographic and clinical characteristics of patients diagnosed with either fibromyalgia (FM) or chronic fatigue (CFS) following hepatitis B vaccination (HBVv) and furthermore to apply the recently suggested criteria of autoimmune (auto-inflammatory) syndromes induced by adjuvants (ASIA), in the aim of identifying common characteristics that may suggest an association between fibromyalgia, chronic fatigue and HBV vaccination.
…The mean latency period from the last dose of HBVv to onset of symptoms was 38.6 ± 79.4 days, ranging from days to a year. Eight (42.1 %) patients continued with the immunization program despite experiencing adverse events. Manifestations that were commonly reported included neurological manifestations (84.2 %), musculoskeletal (78.9 %), psychiatric (63.1 %), fatigue (63.1 %), gastrointestinal complains (58 %) and mucocutaneous manifestations (36.8 %). Autoantibodies were detected in 71 % of patients tested. All patients fulfilled the ASIA criteria. This study suggests that in some cases CFS and FM can be temporally related to immunization, as part of ASIA syndrome.
From: “Chronic fatigue syndrome and fibromyalgia following immunization with the hepatitis B vaccine: another angle of the ‘autoimmune (auto-inflammatory) syndrome induced by adjuvants’ (ASIA)“ (2014)
ANA’s and FMS
A study demonstrating a significant association between ANA’s and CFS or FMS was published by Nishikai et al back in 2001:
Objective. To identify antinuclear antibodies (ANA) specific for chronic fatigue syndrome (CFS), and in related conditions such as fibromyalgia (FM) or psychiatric disorders.
…Results. Autoantibodies to a 68/48 kDa protein were present in 13.2 and 15.6% of patients with CFS and primary FM, respectively. In addition, autoantibodies to a 45 kDa protein were found in 37.1 and 21.6% of the patients with secondary FM and psychiatric disorders, respectively. Meanwhile, these two autoantibodies were not found at all in connective tissue disease patients without FM, nor in healthy subjects (P<0.05). As a group, the anti‐68/48 kDa‐positive CFS patients presented more frequently with hypersomnia (P<0.005), short‐term amnesia (P<0.07) or difficulty in concentration (P<0.05) than those CFS patients without the antibodies.
Conclusions. The presence of the anti‐68/48 kDa protein antibodies in a portion of both CFS and primary FM patients suggests the existence of a common immunological background. These antibodies may find utility as possible markers for a clinicoserological subset of CFS/FM patients with hypersomnia and cognitive complaints.
From: “Autoantibodies to a 68/48 kDa protein in chronic fatigue syndrome and primary fibromyalgia: a possible marker for hypersomnia and cognitive disorders“ (2001)
https://academic.oup.com/rheumatology/article/40/7/806/1787923?login=false
TPO autoantibodies and FMS
Apart from the two studies showing an immunological connection between FMS and CFS, our story has a twist here, the hypothesis being that not only can both viral and synthetic Spike induce Hashimoto’s thyroiditis by both ANAs and TPO autoantibodies, but that TPOab can also trigger fibromyalgia.
Therefore it would not be unexpected to see PASC patients including both ANA and TPO autoantibodies, together with a spectrum of symptoms resembling CFS, FMS and Hashimoto’s.
Indeed, studies are starting to make the connection and develop diagnostic procedures, - are cases of “fibromyalgia” actually complex post-viral or post-vax autoimmune disorders?
…Fibromyalgia (FM) is a chronic musculoskeletal pain disorder that often leads to functional disability and severe impairment of quality of life. LC and FM share several clinical features, including pain that often makes them indistinguishable.
…Patients with LC often suffer from widespread pain, fatigue, post-exertional malaise, physical and cognitive dysfunction, psychological factors, sleep disturbance, and autonomic dysregulation, amongst many others [7,8,9,10,11]; LC can be seen in up to 10–30% of individuals following infection and has also been recognized to develop in a small portion of individuals following SARS-CoV-2 vaccination [7]. With over 65 million individuals affected by LC worldwide, as a number that continues to grow, the treatment of LC is a global health crisis. Moreover, the mechanism of chronic pain in LC has not been definitively defined [12,13].
From: “Metabolic Fingerprinting for the Diagnosis of Clinically Similar Long COVID and Fibromyalgia Using a Portable FT-MIR Spectroscopic Combined with Chemometrics“ (2023)
TPO autoantibodies and/or anti-thyroglobulin antibodies (αTG) are also associated with FMS and rheumatic manifestations of lymphocytic thyroiditis (CLT).
Key takes from a study by Tagoe et al (2013):43
Arthralgias (joint pain) were a presenting complaint in 98% of patients.
FMS was found in 59% of patients.
Raynaud’s phenomenon (limited blood flow in smaller arteries) occurred in 28% and sicca symptoms (dry eyes and mouth) in 26% of patients.
2/46 patients had seronegative arthritis resembling rheumatoid arthritis.
Arthritis was radiographically present in 88 %, affecting the spine in 45% of patients.
Thyroid-stimulating hormone (TSH) levels positively correlated with levels of αTPO, but not with erythrocyte sedimentation rate (ESR) or αTG levels.
A positive ANA was found in 24% of patients.
1/46 developed subclinical hypothyroidism during the study.
A literature review and meta-analysis by Park et al from 2022 further investigated studies linking FMS to thyroid autoimmunity (abstract only):44
Thyroid autoantibody positivity was more common in fibromyalgia patients compared to healthy controls (thyroid peroxidase antibody: OR 3.41, 95% CI 1.97-5.90; thyroglobulin antibody: OR 2.23, 95% CI 1.23-4.01).
The frequency of postmenopausal status was significantly higher in fibromyalgia patients with thyroid autoantibodies (OR 1.95, 95% CI 1.23-3.08).
The severity of FMS and prevalence of depression did not show a statistically significant difference according to thyroid autoantibody positivity.
A systematic review from 2023 confirmed how common FMS criteria were fulfilled after COVID-19 infection, but vaccination status wasn’t always reported:
3.3. Fibromyalgia in Post-COVID-19 Patients
Two studies evaluated the prevalence of fibromyalgia in post-COVID-19 patients. The results of an Italian web-based cross-sectional survey including 616 patients showed that 31% fulfilled the classification criteria for fibromyalgia after an average of 6 months from the diagnosis of COVID-19, whose strongest predictors were obesity and male gender [68]. Using a similar online survey methodology in Sweden, based on self-reported data collected after a mean of 47 weeks from infection, 40% of the participants fulfilled the 2016 criteria for fibromyalgia and 22% reported to be heathy before COVID-19 [32].
…Several limitations of our review must be considered.
…the vaccination status of these patients was not always reported. Information about COVID-19 vaccination might be relevant when assessing the development of rheumatic musculoskeletal conditions because, for instance, cases of new-onset inflammatory arthritis and PMR after COVID-19 vaccine administration have been described [96,97,98,99].
From: “Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review“ (2023)
IgG4-Related Neuropathy
Significantly elevated serum igG4 antibodies due to isotype class switching, many months after SARS-CoV-2 “vaccination”, has been discussed in at least 9 studies.454647 48 49 50 51 52 53
IgG4 may also be a factor in cases of post-vax autoimmune neuropathy. I must give credit here to Dr. Alberto Rubio-Casillas for highlighting the possibility.
The full study pdf is in Japanese, but I can translate the abstract of a paper published in 2014 by Oyama et al, “Clinical and Pathology of IgG4-Related Neuropathy”54
Key takes:
In recent years, a group of diseases associated with elevated IgG4, one of the subclasses of IgG, has been reported as IgG4-related diseases (IgG4-RD) and has attracted attention.
IgG4-RD is characterized by swelling and enlargement of organs, IgG4-positive plasma cell infiltration with fibrosis in tissues, and elevated serum IgG4 levels.
Mononeuropathy multiplex (or mononeuritis multiplex) is a type of peripheral neuropathy that involves damage to at least two different areas of the peripheral nervous system. It presents with sensory and motor deficits.55
IgG4-related neuropathy was found in the form of multiple mononeuritis with kinesthetic impairment with a predominance of the lower extremities. Sural nerve biopsy showed IgG4-positive plasma cell infiltration and fibrosis of the epineural membrane, and decreased myelinated fiber density.
Axonal degeneration was observed. It was suggested that IgG4-RD may also be one of the differential diseases of neuropathy.
Following this work, a case report of igG4-related peripheral neuropathy was published by Tarisawa et al in 2022. A 64 y/o male presented with left upper limb weakness and dysesthesia (an unpleasant, abnormal sense of touch) for 4 months.
Key takes from “IgG4-Related Peripheral Neuropathy with Unilateral Cervical Nerve Root and Brachial Plexus Swelling: A Case Report“:56
The serum IgG4 level was elevated (762.7 mg/dL). <sup>18</sup>F-FDG-PET showed high uptake in the mediastinal lymph nodes, and biopsy revealed infiltration of IgG4-positive plasma cells.
We diagnosed IgG4-related neuropathy, and steroid therapy administration improved the symptoms. IgG4-related disease should be considered in the differential diagnosis of peripheral nerve swellings.
If biopsy of the disordered nerves is difficult, lymph nodes or other organs should be considered.
Although reports of peripheral neuropathy associated with IgG4-RD are limited, the disease may be overlooked and remain undiagnosed in some asymptomatic cases.
A 64-year-old man presented to our department with complaints of progressive weakness of the distal muscles in the left upper limb and a tingling sensation from the left 1st–3rd digits to the left forearm, which started 4 months before admission.
Erythrocyte sedimentation rate (ESR or sed rate) is a non-specific measure of inflammation:
There were no signs of ataxia or autonomic disturbance and no abnormalities in standing or walking. Laboratory tests showed no abnormalities in blood cells, liver or renal function, and CRP levels; however, the erythrocyte sedimentation rate was 53 mm/h, which was considered high.
The vitamin B1 level was low at 23 ng/mL (reference range, 24–66 ng/mL), and the soluble interleukin-2 receptor level was mildly elevated at 805 U/mL (reference range, 122–496 U/mL).
Immunoglobulin G (IgG) level was high at 2,205 mg/dL (reference range 870–1,700) as well as IgG4, 762.7 mg/dL (reference range 11–121), and IgE levels, 1,540 IU/mL (reference range 0–250).
ANA titres of less than or equal to 1:40 are considered negative, anything above 1:80 may be significant, but age is a consideration.57 1:320 is a positive result:
The antinuclear antibody titer was 1:320, while those of anti-SS-A/SS-B antibody, MPO-ANCA, PR3-ANCA, and anti-ganglioside antibody were negative.
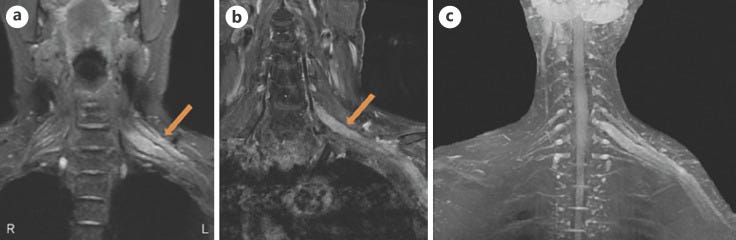
Diagnosis was quite challenging:
Initially, we suspected focal chronic inflammatory demyelinating polyneuropathy (CIDP), vasculitis, or malignant lymphoma; however, because of the lack of systemic symptoms, such as fever, night sweats, and weight loss, and negative laboratory findings of other organ damage and monoclonal immunoglobulins, none of these diagnoses were subsequently considered.
Neuralgic amyotrophy was also not considered because of the lack of preceding pain. Thiamine was supplemented from the 13th day for vitamin B1 deficiency, but the symptoms did not improve.
Because of the high levels of serum IgG4 and IgE, both of which might have been induced by Th2 cytokines, IgG4-RDs were finally considered; thus, needle biopsy was performed on the mediastinal lymph nodes in which 18F-FDG-PET showed high uptake; however, no histologically significant findings were observed.
It took until day 42 to get a positive diagnosis, and it was partly a process of elimination:
On the 42nd day, thoracoscopic mediastinal lymph node biopsy was performed. Histopathological examination of a subcarinal lymph node revealed infiltration of lymphocytes and plasma cells into the paracortical area, and immunostaining revealed CD138-positive plasma cell infiltration. The IgG4/IgG ratio was 0.8, and the number of IgG4-positive cells was 200 cells/HPF (shown in Fig. Fig.3).3). IgG4-RD associated with peripheral neuropathy (IgG4-related peripheral neuropathy) was diagnosed based on the comprehensive diagnostic criteria of 2020.
In addition, it is important to exclude malignant tumors of each organ (solid cancer, malignant lymphoma, etc.) and similar inflammatory diseases (Sjögren's syndrome, primary/secondary sclerosing cholangitis, Castleman disease, secondary retroperitoneal fibrosis, granulomatosis with polyangiitis, sarcoidosis, and eosinophilic granulomatosis with polyangiitis).
The epineurium is the outermost layer of dense irregular connective tissue surrounding a peripheral nerve:
The patient's clinical symptoms showed multiple mononeuropathy patterns, which may be due to the infiltration of inflammatory cells and fibrosis of the epineurium, thereby obstructing the vessels and causing ischemia of the peripheral nerves, resulting in axonal neuropathy.
I suspect igG4 is contributing to the pathology of other autoimmune diseases, and may be elevated in response to immune-damping cytokines like Il-4, Il-10 or IL-13:
Moreover, in 2015, Ohyama et al. [6] retrospectively examined 149 cases of patients with inflammatory peripheral neuropathy who underwent sural nerve biopsy; serum IgG4 level >135 mg/dL was observed in 35 cases, and 29 cases had IgG4-positive cell infiltration.
These cases were classified as microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, Sjögren's syndrome, and rheumatoid arthritis. These findings suggest that IgG4-positive cell infiltration is detected even in diseases that need to be differentiated from IgG4-RD.
However, even after excluding these related diseases, five cases that were not diagnosed before and after the biopsy still met the criteria for IgG4-RD. IgG4-related peripheral neuropathy may have been overlooked in inflammatory peripheral neuropathy, whose pathology was previously unknown.
IgG4-subclass antibody-positive autoimmune nodopathies have recently been established as a disease concept independent of CIDP because their clinical presentations differ from those of typical CIDP.
Anti-neurofascin-155 (NF155) IgG4 antibodies:58
…histopathological examination revealed detachment of the terminal Schwann cell loops from the axons at the paranodes, resulting in the inhibition of the interaction between NF155 and contactin-1/Caspr1, thereby causing demyelination.
Therefore, IgG4-related peripheral neuropathy and IgG4-subclass antibody-positive autoimmune nodopathies have different pathophysiologies.
To go into further detail, igG4 can use FAE to become more pathogenic:
“In blood, IgG4 have the potency to exchange their moiety with other unrelated IgG4 through a process called Fab-arm exchange (FAE). This process results in functionally monovalent antibodies and may affect the pathogenicity of autoantibodies.”59
As discussed in my galectin Substack, IgG4-specific anti-galectin-3 antibodies are strongly associated with igG4-related disease, but more research is needed to investigate this further and to confirm the pathophysiology of anti-Spike S1-NTD-Gal-3 antibodies.60 Cleaved S1-NTD-Gal-3 itself may also mediate disease progression in neurological disorders such as Alzheimer’s Disease.61
“…We tested our IgG4-RD cohort for anti-galectin-3 autoantibody responses using plasma from each subject’s initial clinical encounter as a means of ensuring the highest frequency of untreated and active patients. Thus, it is not possible for us to determine which alteration occurs first, the over-expression of galectin-3 or the development of anti-galectin-3 autoantibodies. This answer must await further studies.”62
Damaged Schwann cells residing in the PNS upregulate Gal-3 expression following traumatic injury, and experimental knock down (KD) of Gal-3 has a striking effect on microglia.63 Could this mediate a positive feedback loop with Gal-3 binding igG4 autoantibodies and Schwann cell damage?
Key takes from “Anti-Neurofascin 155 Antibody-Positive Chronic Inflammatory Demyelinating Polyneuropathy/Combined Central and Peripheral Demyelination: Strategies for Diagnosis and Treatment Based on the Disease Mechanism” by Jun-ichi Kira (2021):64
IgG4 subclass anti-NF155 antibodies predominate in NF155+ CIDP (2, 4, 5). It is essential to understand the structure and function of IgG4 to elucidate the mechanism of IgG4 autoantibody-mediated nodopathy/paranodopathy.
IgG4 has a compact structure arising from a trans heavy-chain CH1–CH2 domain interaction, which makes the CH2 domain inaccessible for complement fixation [Figure 2A; (12)]. Therefore, IgG4 cannot activate the complement cascade because it is unable to bind C1q.
In addition, IgG4 exists in vivo in a bispecific form that is monovalent to its target. This is because of the half-molecule exchange following interchain disulfide bond cleavage by the protein disulfide isomerase expressed on immunocytes and endothelial cells [Figure 2B; (12)].
Consequently, IgG4 cannot internalize target antigens. In physiological conditions, IgG4 produced by chronic antigenic stimulation blocks allergen-specific IgE binding to allergens, thereby mitigating allergic inflammation (12). Therefore, IgG4 autoantibodies can merely block protein–protein interactions without causing full-blown inflammation.
In our study of CIDP cases, all had predominantly IgG4 subclass anti-NF155 antibodies, while one anti-NF155 antibody-positive case with Guillain–Barré syndrome (GBS) had IgG1 subclass (5). Another study also reported that, in acute-onset neuropathies, including GBS, the anti-paranodal antibody subclass was IgG2/3 but not IgG4, which suggests that an IgG class switch from IgG2/3 to IgG4 may correlate with a progressive course following GBS-like onset (30). It seems likely that IgG4 subclass anti-NF155 antibodies are specific for a subset of CIDP patients.
Alternative therapeutics
A Substack devoted to therapeutics for MS and PN is linked to in “Further Reading” and I can add a few more here from some of the reviews.
I can’t get into dosing, toxicity or contraindications as this Substack is long enough already, this is strictly for signposting.
Microdosed psilocybin for chronic pain
This has been suggested as particularly useful for helping to manage chronic FMS symptoms when other treatments have failed.
Key takes from a 2023 paper:65
Psychedelic serotonergic agonists such as psilocybin have recently been shown to produce sustained benefit in refractory depression, end of life anxiety, and addiction when administered in hallucinogenic doses and coupled with psychotherapy.
Although it has been suggested that similar high-dose protocols may help chronic pain conditions, there are few published clinical trials of psychedelics for pain. The use of these agents in subpsychedelic doses for chronic pain management has received even less attention.
This case series details the experiences of 3 individuals who have used low-dose psilocybin to manage chronic neuropathic pain. Although the nature and etiology of each patient's pain vary, they share a common experience, including inefficacy of current therapeutics and decreased quality of life. Through self-administration of psilocybin, these patients have achieved robust pain relief with decreased reliance on traditional analgesic medications.
Despite varying preparations and uncertain potencies, the analgesic effects for all 3 patients occurred at doses without a psychedelic experience and with minimal cognitive or somatic adverse effects.
Furthermore, the efficacy of pain relief and, in some cases, the duration of the effect were magnified when coupled with functional exercise. In addition, in 1 case, repeated dosing seemed to produce increased relief, suggesting a possible long-term plasticity-mediated effect.
These commonalities highlight psilocybin's therapeutic potential in the treatment of chronic pain that warrants further investigation.
Lion’s Main mushroom (Hericium erinaceus) for neurotropic effects
Native to North America, Europe and Asia, H. erinaceus is also known as the mountain-priest mushroom, bearded tooth fungus, and bearded hedgehog.
It has a long history of use in TCM66 and you can see why it acquired its common names:

Key takes from a 2012 study that used a rat model to investigate nerve recovery after crush injury.67 H. erinaceus extracts can stimulate nerve regrowth i.e. its neurotrophic:
We present a model case study of the activity of aqueous extract of Hericium erinaceus fresh fruit bodies in promoting functional recovery following crush injury to the peroneal nerve in adult female Sprague-Dawley rats. The aim was to explore the possible use of this mushroom in nerve repair. The activities of aqueous extract were compared to activities exhibited by mecobalamin (vitamin B12), which has been widely used in the treatment of peripheral nerve disorders.
Analysis of walking track indicated that return of hind limb function and normal toe spreading occurred earlier in treated groups than in the negative control (non-treated) group.
Regeneration of axons and reinnervation of motor endplates/neuromuscular junction in extensor digitorum longus muscle of rats in treated groups developed better than in the negative control group.
Akt cascade plays a major role in mediating neurotrophin-promoted cell survival, while MAPK cascade is involved in mediating neurite outgrowth. Immediate early gene expression was also involved in the cascade of events leading to regeneration.
Local axonal protein synthetic machinery was also enhanced in the distal segments of crushed nerves in treated groups. Therefore, daily oral administration of H. erinaceus could promote the regeneration of injured rat peroneal nerve in the early stage of recovery.
Key takes from an in vitro study using human cell lines from 2013.68 H. erinaceus extracts can induce nerve growth factor (NGF) synthesis:
Neurotrophic factors are important in promoting the growth and differentiation of neurons. Nerve growth factor (NGF) is essential for the maintenance of the basal forebrain cholinergic system. Hericenones and erinacines isolated from the medicinal mushroom Hericium erinaceus can induce NGF synthesis in nerve cells.
In this study, we evaluated the synergistic interaction between H. erinaceus aqueous extract and exogenous NGF on the neurite outgrowth stimulation of neuroblastoma-glioma cell NG108-15. The neuroprotective effect of the mushroom extract toward oxidative stress was also studied. Aqueous extract of H. erinaceus was shown to be non-cytotoxic to human lung fibroblast MRC-5 and NG108-15 cells.
The combination of 10 ng/mL NGF with 1 μg/mL mushroom extract yielded the highest percentage increase of 60.6% neurite outgrowth. The extract contained neuroactive compounds that induced the secretion of extracellular NGF in NG108-15 cells, thereby promoting neurite outgrowth activity.
However, the H. erinaceus extract failed to protect NG108-15 cells subjected to oxidative stress when applied in pre-treatment and co-treatment modes.
In conclusion, the aqueous extract of H. erinaceus contained neuroactive compounds which induced NGF-synthesis and promoted neurite outgrowth in NG108-15 cells. The extract also enhanced the neurite outgrowth stimulation activity of NGF when applied in combination. The aqueous preparation of H. erinaceus had neurotrophic but not neuroprotective activities.
From the TCM study: Rhizoma Drynariae
Rhizoma Drynariae (Gusuibu or Gu-Sui-Bu) is the dried rhizome of the perennial pteridophyte fern Aglaomorpha fortunei (syn. Drynaria fortunei). It is native to Eastern Asia and eastern China and has been used in TCM for thousands of years as a treatment for joint pain.69
A study by Wang et al (2016) using rats identified eriodictyol as the putative active compound of R. drynariae extract that protected them from induced traumatic brain injury (TBI).70
Gu-Sui-Bu has also has been demonstrated experimentally to have bone healing properties too.7172
You can obtain Gu-Sui-Bu both as dried root powder or as tincture.

Proteomics and bioinformatics investigations into the drug value of Rhizoma Drynariae by Cao et al (2022) revealed that root derived extracellular vesicles could have value for treating various neurological disorders.73
They analysed EV’s from freshly ground roots, not dried but even so its use as a therapeutic in TCM long predates this work.
Key takes:
Seventy-seven proteins were identified from Drynariae Rhizoma root-derived EVs, with enzymes accounting for 47% of the proteins. All of the enzymes were involved in important biological processes in plants.
Most of them, including NAD(P)H-quinone oxidoreductase, were enriched in the oxidative phosphorylation pathway in plants and humans, and Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease, which are associated with oxidative stress in humans.
These findings suggested that EVs from Drynariae Rhizoma roots could alleviate such neurological diseases and that enzymes, especially NAD(P)H-quinone oxidoreductase, might play an important role in the process.
Natural medicines are an important source of future new drugs. However, the multi-component and multi-target properties of herbal medicines should be considered, which is a challenge in scientific research of traditional Chinese medicine. Studying plant EVs may advance the development of natural medicines. Drynariae Rhizoma is a commonly used medicine in orthopedics.
A few recent studies have reported that it can also improve memory deficits.74
Plant EVs, like animal EVs, are involved in cell-to-cell communication and have been speculated to be important members of the innate immune system.12,13 A small number of studies have demonstrated the effects of plant EVs on animal cells and disease models.
Citrus limon-derived EVs could inhibit the proliferation of tumor cells in vitro and suppress CML tumor growth in vivo both locally and via intraperitoneal injection.8
Mice pre-fed with EVs derived from grapefruit and grape exhibited a significantly lower rate of intestinal inflammatory diseases.31,32
In addition, the potential negative immune stimulation of edible plant EVs has not been encountered. Instead, edible plant EVs have been shown to stabilize and strengthen anti-inflammatory responses, protecting the cells from inflammatory damage.32 However, these studies have all focused on edible plants, while herbs have rarely been studied.
An in vivo experiment from 2011 by Rossato et al used mice and rats to demonstrate the antinociceptive effects of a compound extracted from R. drynariae: eriodictyol. This helps to explain why Drynariae Rhizoma is a useful therapeutic for conditions involving chronic pain such as PN or FMS. Currently used allopathic drugs like AMG517 present several clinical limitations.
Key takes from “Eriodictyol: A flavonoid antagonist of the TRPV1 receptor with antioxidant activity“:75
The transient potential vanilloid 1 receptor (TRPV1) is a calcium-permeable channel responsible for the transduction and modulation of acute and chronic pain signaling. As such, this receptor is a potential target for the treatment of a number of pain disorders. However, AMG517, a TRPV1 antagonist, presents several clinical limitations that include the induction of severe hyperthermia.
Eriodictyol was able to displace [3H]-resiniferatoxin binding (IC50 = 47; 21–119 nM) and to inhibit calcium influx mediated by capsaicin (IC50 = 44; 16–125 nM), suggesting that eriodictyol acts as a TRPV1 antagonist.
Oral administration led to pharmacokinetically active doses:
Moreover, eriodictyol induced antinociception in the intraplantar capsaicin test, with maximal inhibition of 49 ± 10 and 64 ± 4% for oral (ID50 = 2.3; 1.1–5.7 mg/kg) and intrathecal (ID50 = 2.2; 1.7–2.9 nmol/site) administration, respectively.
Eriodictyol did not induce any change in body temperature or locomotor activity. Orally administered eriodictyol (4.5 mg/kg) prevented the nociception induced by intrathecal injections of capsaicin, as well as the non-protein thiol loss and 3-nitrotyrosine (3-NT) formation induced by capsaicin in spinal cord.
Eriodictyol also reduced the thermal hyperalgesia and mechanical allodynia elicited by complete Freund's adjuvant (CFA) paw injection. In conclusion, eriodictyol acts as an antagonist of the TRPV1 receptor and as an antioxidant; it induces antinociception without some of the side effects and limitations such as hyperthermia that are expected for TRPV1 antagonists.
Vitamin B12
B12, or cobalamin, is one of 8 B vitamins. Its especially important for maintaining healthy nerve cells and it helps with the synthesis of DNA and RNA. It works closely with with other B vitamins, eg B9 (folic acid) in erythropoiesis (red cell generation) and helps avoid iron deficiency anaemia and with B6 to regulate blood levels of the amino acid homocysteine.
In 2022, Gharibpoor at al published a study into the effects of B12 on FM patients. Results were very encouraging - Fibromyalgia Impact Questionnaire (FIQR) scores in all areas were significantly improved after patients took 1000mcg B12 for fifty days.
Nociplastic pain: “…The symptoms observed in nociplastic pain include multifocal pain that is more widespread or intense, or both, than would be expected given the amount of identifiable tissue or nerve damage, as well as other CNS-derived symptoms, such as fatigue, sleep, memory, and mood problems. This type of pain can occur in isolation, as often occurs in conditions such as fibromyalgia or tension-type headache, or as part of a mixed-pain state in combination with ongoing nociceptive or neuropathic pain, as might occur in chronic low back pain.”76
Key takes from “Effect of vitamin B12 on the symptom severity and psychological profile of fibromyalgia patients; a prospective pre-post study“:77
Background: Fibromyalgia (FM) as a prototypical nociplastic pain condition displays a difficult therapeutic situation in many cases. Given the promising data on the effect of vitamin B12 in improving pain and cognitive functions in various nociplastic pain conditions, we aimed to determine the efficacy of 1000 mcg daily dose of oral vitamin B12 on the symptom severity and psychological profile of FM patients.
Methods: This open-label, pre-post study was performed on FM patients whose diagnoses were confirmed by a rheumatologist based on the 2016 American College of Rheumatology (ACR). Patients were instructed to take a daily dose of 1000mcg vitamin B12 for fifty days.
Results: Of 30 eligible patients, 28 patients completed the study protocol. Patients were female with a mean age of 47.50 ± 8.47 years. FIQR scores in all domains improved significantly after treatment (total FIQR: 49.8 ± 21.86 vs 40.00 ± 18.36, p value < 0.01; function: 13.17 ± 7.33 vs 10.30 ± 5.84, p value: 0.01; overall: 10.32 ± 6.22 vs 8.25 ± 6.22, p value: 0.03; symptoms: 26.30 ± 10.39 vs 21.44 ± 8.58, p value < 0.01).
Vitamin B12 also improved anxiety scores from 9.33 ± 4.30 to 7.70 ± 3.60, p value: 0.01. Depression, pain-VAS, and SF-12 didn't improve following the treatment. The Generalized estimating equations (GEE) analysis showed the improvement in total FIQR score is not cofounded by the improvement of anxiety and patients' baseline characteristics.
Conclusions: This study showed a short course of sublingual vitamin B12, 1000 mcg daily, significantly improves the severity of FM and anxiety score. We postulate that vitamin B12 has a strong potential to consider, at least, as adjunctive therapy of FM.
Vitamin D and autoimmune disease
The findings of a study was published in 2022 by Hahn et al:
25,871 participants (12,786 men ≥50 years and 13 085 women ≥55 years) took vitamin D (2000 IU/day) or matched placebo, and omega 3 fatty acids (1000 mg/day) or matched placebo.
Participants self-reported all incident autoimmune diseases from baseline to a median of 5.3 years of follow-up and these diseases were confirmed by extensive medical record review.
Key takes from: “Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial”:78
For the vitamin D arm, 123 participants in the treatment group and 155 in the placebo group had a confirmed autoimmune disease (hazard ratio 0.78, 95% confidence interval 0.61 to 0.99, P=0.05). In the omega 3 fatty acids arm, 130 participants in the treatment group and 148 in the placebo group had a confirmed autoimmune disease (0.85, 0.67 to 1.08, P=0.19).
Compared with the reference arm (vitamin D placebo and omega 3 fatty acid placebo; 88 with confirmed autoimmune disease), 63 participants who received vitamin D and omega 3 fatty acids (0.69, 0.49 to 0.96), 60 who received only vitamin D (0.68, 0.48 to 0.94), and 67 who received only omega 3 fatty acids (0.74, 0.54 to 1.03) had confirmed autoimmune disease.
Conclusions: Vitamin D supplementation for five years, with or without omega 3 fatty acids, reduced autoimmune disease by 22%, while omega 3 fatty acid supplementation with or without vitamin D reduced the autoimmune disease rate by 15% (not statistically significant). Both treatment arms showed larger effects than the reference arm (vitamin D placebo and omega 3 fatty acid placebo).
Vitamin D positively regulates multiple pathways involved with autoimmune disorders.
If lacking sufficient UV exposure we need about 1000–2000 IU (25–50 μg) per day to avoid deficiency, which The Clinical Guidelines Subcommittee of The Endocrine Society defines as less than 20 ng/mL (50 nmol/L).
Key takes from “An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases“ by Sirbe et al (2022):79
Considering these values, a study based on 6275 American children and adolescents revealed that 61% presented 25(OH)D insufficiency and 9% deficiency [40]. A high prevalence (up to 40%) of vitamin D insufficiency and 6% deficiency is described in adults [41,42,43].
There is a direct relationship between the dose of vitamin D and serum 25(OH)D with a linear curve at a daily dose of 1000–2000 IU (25–50 μg), but this curve flattens at higher doses [48]. A daily intake of 1040 IU (26 μg) in vitamin D deficiency and 400 IU (10 μg) in vitamin D insufficiency is needed to obtain a concentration above 20 ng/mL (50 nmol/L) in 97.5% of the population [49].
Low levels of serum 25-hydroxyvitamin D (25(OH)D) are associated with an increased risk of developing immune-related diseases such as psoriasis, type 1 diabetes, multiple sclerosis, and autoimmune diseases.
Various clinical trials describe the efficacy of supplementation of vitamin D and its metabolites for treating these diseases that result in variable outcomes. Different disease outcomes are observed in treatment with vitamin D as high inter-individual difference is present with complex gene expression in human peripheral blood mononuclear cells.
However, it is still not fully known what level of serum 25(OH)D is needed. The current recommendation is to increase vitamin D intake and have enough sunlight exposure to have serum 25(OH)D at a level of 30 ng/mL (75 nmol/L) and better at 40–60 ng/mL (100–150 nmol/L) to obtain the optimal health benefits of vitamin D.
CYP11A1 can convert vitamin D into the non-calcemic analog 20S-hydroxyvitamin D3 [20S(OH)D3]. This analog could be used as a potential treatment for rheumatoid arthritis (RA) and other autoimmune disorders by suppressing clinical signs of arthritis and repairing joint damage in a mouse model. 20S(OH)D3 can also decrease the number of CD4 and CD19 cells, reducing inflammatory cytokines. Arthritis can be attenuated by 20S(OH)D3 by reducing pro-inflammatory cytokines and antibodies against type II collagen [13].
Vitamin D has pleiotropic effects suggested by the expression of VDR in lymphocytes and dendritic cells. Many studies are based on vitamin D’s role in various diseases such as autoimmune disorders, cardiovascular diseases and tumors. In autoimmune and inflammatory diseases, vitamin D intervenes in the innate and adaptive immune systems [3,8,83,85,88,91,92,93,94]. Animal studies have demonstrated that administering vitamin D or its analogs can influence the occurrence and progression of many immune-related disorders [5,29].
The hormonal form of vitamin D(3), 1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)) or calcitriol, is an immune system modulator and induces expression of the TLR coreceptor CD14, i.e. it can mimic bacterial peptides and induce antimicrobial peptide gene expression in isolated human keratinocytes, monocytes and neutrophils and human cell lines.80
“The recent discovery that vitamin D induces antimicrobial peptide gene expression explains, in part, the 'antibiotic' effect of vitamin D and has greatly renewed interest in the ability of vitamin D to improve immune function.”81
Its also a galectin-3 inhibitor, which is a very useful property for resisting infections and cancer cell proliferation.82
If anyone ever screams “don’t take vitamin D, its immunosuppressive!” just point them to studies like these.
Many studies reported that low vitamin D levels are associated with an increased risk of infections and autoimmune diseases due to molecular mimicry [104,105].
1,25-dihydroxivitamin D regulates NK cell activity, degranulation process, cytokine secretion, and TLR4 expression. This could demonstrate the benefit of vitamin D supplementation in patients with cancer [109].
Vitamin D regulates the intracellular TLRs differently, down-regulating TLR9, whereas TLR3 is unaffected. The decreased expression of TLR9 results in less IL-6 secreted. This highlights the association between vitamin D deficiency and the risk of developing autoimmune diseases [110].
…reduced expression of TLR might inhibit inflammatory T lymphocyte responses that could generate T-helper 1 (Th1) lymphocyte autoimmunity. Feedback regulation of vitamin D is controlled by CYP24A1, the enzyme that catalyzes the synthesis of less active vitamin D metabolites [128].
Vitamin D inhibits the synthesis of various pro-inflammatory Th1, Th9 and Th22 cytokines and stimulates the synthesis of anti-inflammatory Th2 cytokines. These findings could demonstrate the beneficial effect of vitamin D in minimizing the risk of developing autoimmune diseases [144].
FoxP3 from T follicular helper cells (TFH) helps to prevent autoimmune disorders.83
Calcitriol stimulates the expression of CTLA-4 and FoxP3, requiring the presence of IL-2 [159]. Calcitriol promotes Th1 cellular immunity more than Th2 humoral immunity. This is an essential key factor regarding the beneficial effects of vitamin D in autoimmune diseases [160].
Calcitriol or its synthetic analogs can cause induction of Treg cells offering protection against autoimmune disorders and immune tolerance in organ transplantation [167,168].
Increased 25(OH)D concentration is associated with a decreased risk of developing MS. The authors found limited evidence supporting the protective effect of 25(OH)D on obesity, breast and prostate cancer, and autoimmune, metabolic, cardiovascular, and neuro-psychiatric diseases [244].
One meta-analysis identified 9 RCTs of vitamin D treatment for at least 3 months in autoimmune diseases, which included five studies of RA patients, but with no statistical significance. These patients presented a decrease in the number of flares, patient global visual analog scale score (VAS) and disease activity score 28 (DAS-28) with vitamin D supplementation [214,266,282].
Bai Shao and FMS
From 2020 and Yao et al cite studies for both Rhizoma drynariae and Baishao zongdai as demonstrating significant efficacy in terms of safety in the management of FM. Unfortunately I was unable to source the full paper.84
Baishao (or Bai Shao) in TCM is more commonly known as the White Peony Root Paeonia lactiflora Pall.
However, a study by Du et al from 2020 investigated the underlying mechanisms as a treatment for Parkinson’s disease.85
Its very encouraging to see broad spectrum efficacy through targeting some of the key pathways involved in the pathophysiology of disease.
Key takes:
Results: Six active ingredients of P. lactiflora (kaempferol, ß-sitosterol, betulinic acid, palbinone, paeoniflorin and (+)-catechin) as well as six core targets strongly related to PD treatment [AKT1, interleukin-6, CAT, Tumor necrosis factor (TNF), CASP3, and PTGS2] were identified.
The main pathways were shown to involve neuroactive ligand-receptor interaction, Calcium signaling pathway, PI3-Akt signaling pathway, TNF signaling pathway, and apoptosis signaling pathway. The main biological process included the regulation of neurotransmitter levels.
Conclusion: P. lactiflora may retard neurodegeneration by reducing neuroinflammation, inhibiting intrinsic and extrinsic apoptosis, and may improve motor and non-motor symptoms by regulating the levels of neurotransmitters. Our study has revealed the mechanism of P. lactiflora in the treatment of PD and may contribute to novel drug development for PD.
The top 20 KEGG pathways (Kyoto Encyclopaedia of Genes and Genomes) were identified:
The main pathways involved were neuroactive ligand-receptor interaction, Calcium signaling pathway, P450 Drug metabolism-cytochrome P450, TNF signaling pathway, IL-17 signaling pathway, HIF-1 signaling pathway, cholinergic synapse, serotonergic synapse, PI3K-Akt signaling pathway, and apoptosis. Details were listed in Table 2.
Moreover, the PD signaling pathway was also observed, with five targets namely ADORA2A, CASP3, CASP9, DRD1, and SLC6A3.
Fisetin and FMS
The full study by Yao et al used a rat model to investigate the effects of the polyphenol fisetin on FMS, artificially induced by the antiadrenergic agent reserpine.
Again I would expect this to be a broad spectrum therapeutic for treating disorders of the both the CNS and PNS, based on the signalling pathways being affected and ability to cross the blood brain barrier.86
Key takes from “Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol“87:
Fisetin, a plant flavonoid polyphenol, has been reported to possess potent antioxidant, antinociceptive and neuroprotective activities.
Dose conversion to humans (allometric scaling) always requires careful consideration and safety testing but an approximate guide is to divide the effective dose in a rat by 6. This is derived from a normalization of dose to body surface area and consideration of the animals metabolic rate:
Administration of reserpine induced allodynia, hyperalgesia and depression, which were significantly ameliorated (P<0.05) by fisetin (10 and 25 mg/kg), as reflected by an increase in paw and tail withdrawal latency, increased paw withdrawal threshold, and decreased immobility time.
Reserpine led to decreased biogenic amine levels [5-hydroxytryptamine (5-HT), noradrenaline (NA) and dopamine (DA)] and increased the ratio to their metabolite 3,4-dihydroxyphenylacetic acid. 5-hydroxyindoleacetic acid in the spinal cord, thalamus and prefrontal cortex was significantly decreased (P<0.05) by fisetin.
Immunohistological analysis of brain tissue revealed that fisetin significantly inhibited (P<0.05) reserpine-induced depletion of 5-HT. It also significantly inhibited (P<0.05) elevated oxido-nitrosative stress and reactive oxygen species (ROS) levels, as analyzed by flow cytometry in RIF rats. Fisetin exerts its antinociceptive and anti-depressive potential via modulation of decreased levels of biogenic amines (5-HT, NA and DA), elevated oxido-nitrosative stress and ROS to ameliorate allodynia, hyperalgesia, and depression in experimental RIF.
A study estimated that 3–6% of the world population is affected by FM and that it causes substantial morbidity and disability, predominantly in women.
The estimated cost of treatment for FM is between $12-14 billion per year, whereas the mean annual cost per patient is $2,274-9,573, worldwide (6). Thus, FM imposes a significant economic burden on healthcare systems.
Conclusion
I would like to review more herbs as used in TCM as treatments for various neurological disorders but that would require a book long Substack…
Nevertheless I’ve listed the key ones that studies have raised as treatments for FMS, neuropathies and other degenerative disorders and trust these will be of interest.
As we’ve seen its a huge, expensive, quality-of-life impairing problem that’s only likely to get worse. Meanwhile the medical profession in the West appears to be stuck in the 1970’s. I would seriously consider finding a good TCM practitioner if the current protocols aren’t working for you.
Further Reading
Hat tip to Walter for his hypothesis linking autoimmune spike induced denervation to cases of sudden cardiac deaths:
A narrative review and hypothesis about cleaved S1-gp120 induced neurotoxicity as a contributory factor to “brain fog” symptomology:
Disclaimer
This site is strictly an information website about pathology, potential therapeutic agents and a review of the current state of research. It does not advertise anything, provide medical advice, diagnosis or treatment. This site is not promoting any of these as potential treatments or offers any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses or pharmacists. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
References
Nakano H, Yamaguchi K, Kawabata K, Asakawa M, Matsumoto Y. Acute transverse myelitis after BNT162b2 vaccination against COVID-19: Report of a fatal case and review of the literature. J Neurol Sci. 2022;434:120102. doi:10.1016/j.jns.2021.120102
Shome M, Chung Y, Chavan R, Park JG, Qiu J, LaBaer J. Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep. 2022;39(9):110873. doi:10.1016/j.celrep.2022.110873
Draborg AH, Duus K, Houen G. Epstein-Barr Virus in Systemic Autoimmune Diseases. Clin Dev Immunol. 2013;2013:535738. doi:10.1155/2013/535738
Zhao MM, Zhu Y, Zhang L, et al. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Discov. 2022;8(1):1-18. doi:10.1038/s41421-022-00419-w
Fantini J, Chahinian H, Yahi N. Convergent Evolution Dynamics of SARS-CoV-2 and HIV Surface Envelope Glycoproteins Driven by Host Cell Surface Receptors and Lipid Rafts: Lessons for the Future. International Journal of Molecular Sciences. 2023;24(3). doi:10.3390/ijms24031923
Rhea EM, Logsdon AF, Hansen KM, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24(3):368-378. doi:10.1038/s41593-020-00771-8
Cao X, Xu H, Feng W, Su D, Xiao M. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res Bull. 2018;143:83-96. doi:10.1016/j.brainresbull.2018.10.007
Transverse Myelitis | National Institute of Neurological Disorders and Stroke. Accessed November 16, 2023. https://www.ninds.nih.gov/health-information/disorders/transverse-myelitis
Park JS, Hwang SJ, Shin JH, Kim DS. A recurrent longitudinally extensive transverse myelitis with Aquaporin-4(AQP4) antibody after herpes zoster. Journal of the Neurological Sciences. 2013;334(1):69-71. doi:10.1016/j.jns.2013.07.2510
Harahsheh E, Callister M, Hasan S, Gritsch D, Valencia-Sanchez C. Aquaporin-4 IgG neuromyelitis optica spectrum disorder onset after Covid-19 vaccination: Systematic review. J Neuroimmunol. 2022;373:577994. doi:10.1016/j.jneuroim.2022.577994
1.Umezawa S, Ioka K, Aizawa S, Tashiro Y, Yoshizawa K. First manifestation of AQP4-IgG-positive neuromyelitis optica spectrum disorder following the COVID-19 mRNA vaccine BNT162b2. Neurol Sci. 2023;44(2):451-455. doi:10.1007/s10072-022-06465-2
1.Jentzer A, Carra-Dallière C, Lozano C, et al. Neuromyelitis optica spectrum disorder following COVID-19 infection with increase in pre-existing anti-aquaporin-4 antibodies. J Neurol. 2022;269(6):2850-2853. doi:10.1007/s00415-022-10972-9
Kuntz S, Saab G, Schneider R. Antibody-Positive Neuromyelitis Optica Spectrum Disorder After Second COVID-19 Vaccination: a Case Report. SN Compr Clin Med. 2022;4(1):130. doi:10.1007/s42399-022-01213-1
Li Z, Li D, Tsun A, Li B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol Immunol. 2015;12(5):558-565. doi:10.1038/cmi.2015.10
Lohmann L, Glaser F, Möddel G, Lünemann JD, Wiendl H, Klotz L. Severe disease exacerbation after mRNA COVID-19 vaccination unmasks suspected multiple sclerosis as neuromyelitis optica spectrum disorder: a case report. BMC Neurol. 2022;22(1):185. doi:10.1186/s12883-022-02698-y
Corrêa DG, de Souza Lima FC, da Cruz Bezerra D, Coutinho AC, Hygino da Cruz LC. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: Cause or coincidence? Mult Scler. 2021;27(6):973-976. doi:10.1177/1352458520949988
Melikyan G, Abdelrahman MH, D’Suoza A, Akhtar N, Elzouki AN, Hammoudeh M. Transverse Myelitis Associated with Anti-Ro (SSA) Autoantibodies: A Record of Two Cases. Case Rep Rheumatol. 2012;2012:515768. doi:10.1155/2012/515768
Myelin Oligodendrocyte Glycoprotein Antibody Disease (MOGAD). National Multiple Sclerosis Society. Accessed November 18, 2023. https://www.nationalmssociety.org/What-is-MS/Related-Conditions/MOGAD
Karshikoff B, Sundelin T, Lasselin J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front Immunol. 2017;8:21. doi:10.3389/fimmu.2017.00021
O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2(1):37-45. doi:10.1038/nri702
Flu Vaccination Coverage, United States, 2021–22 Influenza Season | FluVaxView | Seasonal Influenza (Flu) | CDC. Published November 14, 2022. Accessed November 18, 2023. https://www.cdc.gov/flu/fluvaxview/coverage-2022estimates.htm
Guillain-Barré Syndrome. Published November 19, 2019. Accessed November 19, 2023. https://www.hopkinsmedicine.org/health/conditions-and-diseases/guillainbarr-syndrome
Guillain-Barré syndrome. nhs.uk. Published October 23, 2017. Accessed November 18, 2023. https://www.nhs.uk/conditions/guillain-barre-syndrome/
Kolter T. Ganglioside Biochemistry. ISRN Biochem. 2012;2012:506160. doi:10.5402/2012/506160
McKernan K, Helbert Y, Kane LT, McLaughlin S. Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose. Published online April 10, 2023. doi:10.31219/osf.io/b9t7m
Bell’s palsy. nhs.uk. Published October 24, 2017. Accessed November 19, 2023. https://www.nhs.uk/conditions/bells-palsy/
Abramsky O, Webb C, Teitelbaum D, Arnon R. Cellular immune response to peripheral nerve basic protein in idiopathic facial paralysis (Bell’s palsy). Journal of the Neurological Sciences. 1975;26(1):13-20. doi:10.1016/0022-510X(75)90109-4
McGovern FH, Estevez J, Jackson R. Immunological Concept for Bell’s Palsy: Further Experimental Study. Ann Otol Rhinol Laryngol. 1977;86(3):300-305. doi:10.1177/000348947708600304
Aviel A, Ostfeld E, Marshak G, Burstein R, Bentwich Z. Peripheral Blood T and B Lymphocyte Subpopulations in Bell’s Palsy. Ann Otol Rhinol Laryngol. 1983;92(2):187-191. doi:10.1177/000348948309200218
Tomris I, Unione L, Nguyen L, et al. SARS-CoV-2 Spike N-Terminal Domain Engages 9-O-Acetylated α2–8-Linked Sialic Acids. ACS Chem Biol. 2023;18(5):1180-1191. doi:10.1021/acschembio.3c00066
Peripheral neuropathy. nhs.uk. Published October 23, 2017. Accessed November 20, 2023. https://www.nhs.uk/conditions/peripheral-neuropathy/
Amyotrophic Lateral Sclerosis (ALS). Published August 8, 2021. Accessed November 25, 2023. https://www.hopkinsmedicine.org/health/conditions-and-diseases/amyotrophic-lateral-sclerosis-als
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi:10.1016/j.addr.2022.114416
Gioia U, Tavella S, Martínez-Orellana P, et al. SARS-CoV-2 infection induces DNA damage, through CHK1 degradation and impaired 53BP1 recruitment, and cellular senescence. Nat Cell Biol. 2023;25(4):550-564. doi:10.1038/s41556-023-01096-x
Domingues R, Lippi A, Setz C, Outeiro TF, Krisko A. SARS-CoV-2, immunosenescence and inflammaging: partners in the COVID-19 crime. Aging (Albany NY). 2020;12(18):18778-18789. doi:10.18632/aging.103989
Uversky VN, Redwan EM, Makis W, Rubio-Casillas A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines (Basel). 2023;11(5):991. doi:10.3390/vaccines11050991
Visvabharathy L, Hanson BA, Orban ZS, et al. T cell responses to SARS-CoV-2 in people with and without neurologic symptoms of long COVID. medRxiv. Published online October 21, 2022:2021.08.08.21261763. doi:10.1101/2021.08.08.21261763
Underactive thyroid (hypothyroidism). nhs.uk. Published October 24, 2017. Accessed November 21, 2023. https://www.nhs.uk/conditions/underactive-thyroid-hypothyroidism/
Postural tachycardia syndrome (PoTS). nhs.uk. Published October 19, 2017. Accessed November 21, 2023. https://www.nhs.uk/conditions/postural-tachycardia-syndrome/
Fibromyalgia. nhs.uk. Published October 20, 2017. Accessed November 22, 2023. https://www.nhs.uk/conditions/fibromyalgia/
Boren SA, Moxley D. Systematically Reviewing the Literature: Building the Evidence for Health Care Quality. Mo Med. 2015;112(1):58-62. Accessed November 22, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6170102/
Tagoe CE, Zezon A, Khattri S, Castellanos P. Rheumatic manifestations of euthyroid, anti-thyroid antibody-positive patients. Rheumatol Int. 2013;33(7):1745-1752. doi:10.1007/s00296-012-2616-9
Park S, Kwon JS, Park YB, Park JW. Is thyroid autoimmunity a predisposing factor for fibromyalgia? A systematic review and meta-analysis. Clin Exp Rheumatol. 2022;40(6):1210-1220. doi:10.55563/clinexprheumatol/y3gfva
Irrgang P, Gerling J, Kocher K, et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol. 2023;8(79):eade2798. doi:10.1126/sciimmunol.ade2798
Buhre JS, Pongracz T, Künsting I, et al. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front Immunol. 2022;13:1020844. doi:10.3389/fimmu.2022.1020844
Kiszel P, Sík P, Miklós J, et al. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci Rep. 2023;13(1):13166. doi:10.1038/s41598-023-40103-x
Emmenegger M, Fiedler S, Brugger SD, et al. Both COVID-19 infection and vaccination induce high-affinity cross-clade responses to SARS-CoV-2 variants. iScience. 2022;25(8). doi:10.1016/j.isci.2022.104766
Selva KJ, Ramanathan P, Haycroft ER, et al. Preexisting immunity restricts mucosal antibody recognition of SARS-CoV-2 and Fc profiles during breakthrough infections. JCI Insight. 2023;8(18). doi:10.1172/jci.insight.172470
Valk AM, Keijser JBD, Dam KPJ van, et al. Suppressed IgG4 class switching in dupilumab- and TNF inhibitor-treated patients after repeated SARS-CoV-2 mRNA vaccination. Published online October 2, 2023:2023.09.29.23296354. doi:10.1101/2023.09.29.23296354
Hartley GE, Fryer HA, Gill PA, et al. Third dose COVID-19 mRNA vaccine enhances IgG4 isotype switching and recognition of Omicron subvariants by memory B cells after mRNA but not adenovirus priming. Published online September 19, 2023:2023.09.15.557929. doi:10.1101/2023.09.15.557929
Farkash I, Feferman T, Cohen-Saban N, et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Reports. 2021;37(11):110114. doi:10.1016/j.celrep.2021.110114
Sheehan J, Ardizzone CM, Khanna M, Trauth AJ, Hagensee ME, Ramsay AJ. Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine. Vaccines. 2023;11(11):1720. doi:10.3390/vaccines11111720
Ohyama K, Koike H, Takahashi M, Kawagashira Y, Iijima M, Sobue G. [Clinicopathological features of neuropathy associated with IgG4-related disease]. Rinsho Shinkeigaku. 2014;54(12):1047-1049. doi:10.5692/clinicalneurol.54.1047
Mononeuritis multiplex - Symptoms, diagnosis and treatment | BMJ Best Practice. Accessed November 24, 2023. https://bestpractice.bmj.com/topics/en-gb/799
Tarisawa M, Kano T, Tanaka D, Yoshino M, Houzen H. IgG4-Related Peripheral Neuropathy with Unilateral Cervical Nerve Root and Brachial Plexus Swelling: A Case Report. Case Rep Neurol. 2022;14(2):326-333. doi:10.1159/000525908
Antinuclear Antibody (ANA) Test Results & Causes of Elevated ANAs. MedicineNet. Accessed November 24, 2023. https://www.medicinenet.com/antinuclear_antibody/article.htm
Devaux JJ, Miura Y, Fukami Y, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology. 2016;86(9):800-807. doi:10.1212/WNL.0000000000002418
Jentzer A, Attal A, Roué C, et al. IgG4 Valency Modulates the Pathogenicity of Anti–Neurofascin-155 IgG4 in Autoimmune Nodopathy. Neurol Neuroimmunol Neuroinflamm. 2022;9(5):e200014. doi:10.1212/NXI.0000000000200014
Schroeder JT, Bieneman AP. The S1 Subunit of the SARS-CoV-2 Spike Protein Activates Human Monocytes to Produce Cytokines Linked to COVID-19: Relevance to Galectin-3. Frontiers in Immunology. 2022;13. Accessed October 17, 2023. https://www.frontiersin.org/articles/10.3389/fimmu.2022.831763
Tan Y, Zheng Y, Xu D, Sun Z, Yang H, Yin Q. Galectin-3: a key player in microglia-mediated neuroinflammation and Alzheimer’s disease. Cell & Bioscience. 2021;11(1):78. doi:10.1186/s13578-021-00592-7
1.Perugino CA, AlSalem SB, Mattoo H, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. Journal of Allergy and Clinical Immunology. 2019;143(2):736-745.e6. doi:10.1016/j.jaci.2018.05.011
Rotshenker S. Galectin-3 (MAC-2) controls phagocytosis and macropinocytosis through intracellular and extracellular mechanisms. Front Cell Neurosci. 2022;16:949079. doi:10.3389/fncel.2022.949079
Kira J ichi. Anti-Neurofascin 155 Antibody-Positive Chronic Inflammatory Demyelinating Polyneuropathy/Combined Central and Peripheral Demyelination: Strategies for Diagnosis and Treatment Based on the Disease Mechanism. Frontiers in Neurology. 2021;12. Accessed November 24, 2023. https://www.frontiersin.org/articles/10.3389/fneur.2021.665136
Lyes M, Yang KH, Castellanos J, Furnish T. Microdosing psilocybin for chronic pain: a case series. Pain. 2023;164(4):698-702. doi:10.1097/j.pain.0000000000002778
Spelman K, Sutherland E, Bagade A. Neurological Activity of Lion’s Mane (Hericium erinaceus). Journal of Restorative Medicine. 2017;6(1):19. doi:10.14200/jrm.2017.6.0108
Wong KH, Naidu M, David RP, Bakar R, Sabaratnam V. Neuroregenerative potential of lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (higher Basidiomycetes), in the treatment of peripheral nerve injury (review). Int J Med Mushrooms. 2012;14(5):427-446. doi:10.1615/intjmedmushr.v14.i5.10
Lai PL, Naidu M, Sabaratnam V, et al. Neurotrophic properties of the Lion’s mane medicinal mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int J Med Mushrooms. 2013;15(6):539-554. doi:10.1615/intjmedmushr.v15.i6.30
Chen GY, Liu XY, Yan XE, et al. Total Flavonoids of Rhizoma Drynariae Treat Osteoarthritis by Inhibiting Arachidonic Acid Metabolites Through AMPK/NFκB Pathway. JIR. 2023;16:4123-4140. doi:10.2147/JIR.S418345
Wang W, Li H, Yu J, et al. Protective Effects of Chinese Herbal Medicine Rhizoma drynariae in Rats After Traumatic Brain Injury and Identification of Active Compound. Mol Neurobiol. 2016;53(7):4809-4820. doi:10.1007/s12035-015-9385-x
Sun JS, Lin CY, Dong GC, et al. The effect of Gu-Sui-Bu (Drynaria fortunei J. Sm) on bone cell activities. Biomaterials. 2002;23(16):3377-3385. doi:10.1016/s0142-9612(02)00038-8
Wong RW, Rabie B, Bendeus M, Hägg U. The effects of Rhizoma Curculiginis and Rhizoma Drynariae extracts on bones. Chin Med. 2007;2:13. doi:10.1186/1749-8546-2-13
Cao Y, Zhao Q, Liu F, et al. Drug Value of Drynariae Rhizoma Root-Derived Extracellular Vesicles for Neurodegenerative Diseases Based on Proteomics and Bioinformatics. Plant Signal Behav. 17(1):2129290. doi:10.1080/15592324.2022.2129290
Yang Z, Kuboyama T, Tohda C. A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer’s Disease and Its Target Molecule. Front Pharmacol. 2017;8:340. doi:10.3389/fphar.2017.00340
Rossato MF, Trevisan G, Walker CIB, et al. Eriodictyol: a flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem Pharmacol. 2011;81(4):544-551. doi:10.1016/j.bcp.2010.11.004
1. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. The Lancet. 2021;397(10289):2098-2110. doi:10.1016/S0140-6736(21)00392-5
Gharibpoor F, Ghavidel-Parsa B, Sattari N, Bidari A, Nejatifar F, Montazeri A. Effect of vitamin B12 on the symptom severity and psychological profile of fibromyalgia patients; a prospective pre-post study. BMC Rheumatol. 2022;6(1):51. doi:10.1186/s41927-022-00282-y
Hahn J, Cook NR, Alexander EK, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376:e066452. doi:10.1136/bmj-2021-066452
Sîrbe C, Rednic S, Grama A, Pop TL. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int J Mol Sci. 2022;23(17):9784. doi:10.3390/ijms23179784
Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-2912. doi:10.4049/jimmunol.173.5.2909
Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151-1165. doi:10.2217/fmb.09.87
Ji J, Cheng X, Wang W, Zhang J. Vitamin D regulates cell viability, migration and proliferation by suppressing galectin-3 (Gal-3) gene in ovarian cancer cells. J Biosci. 2020;45:69.
Mercer F, Unutmaz D. The Biology of FoxP3: A Key Player in Immune Suppression during Infections, Autoimmune Diseases and Cancer. Adv Exp Med Biol. 2009;665:47-59. Accessed November 25, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3732823/
Gao: A randomized and controlled study of clinical efficacy for total flavone of Rhizoma Drynariae on fibromyalgia syndrome - Google Scholar. Accessed November 24, 2023. https://scholar.google.com/scholar_lookup?journal=Chin+J+New+Drug+Clin+Remedies&title=A+randomized+and+controlled+study+of+clinical+efficacy+for+total+flavone+of+Rhizoma+Drynariae+on+fibromyalgia+syndrome&author=GM+Gao&author=L+Jiang&author=SY+Liu&author=ZH+Zheng&author=ZS+Liu&volume=26&publication_year=2007&pages=837-840&
Du W, Liang X, Wang S, Lee P, Zhang Y. The Underlying Mechanism of Paeonia lactiflora Pall. in Parkinson’s Disease Based on a Network Pharmacology Approach. Front Pharmacol. 2020;11:581984. doi:10.3389/fphar.2020.581984
He WB, Abe K, Akaishi T. Oral administration of fisetin promotes the induction of hippocampal long-term potentiation in vivo. J Pharmacol Sci. 2018;136(1):42-45. doi:10.1016/j.jphs.2017.12.008
Yao X, Li L, Kandhare AD, Mukherjee-Kandhare AA, Bodhankar SL. Attenuation of reserpine-induced fibromyalgia via ROS and serotonergic pathway modulation by fisetin, a plant flavonoid polyphenol. Exp Ther Med. 2020;19(2):1343-1355. doi:10.3892/etm.2019.8328

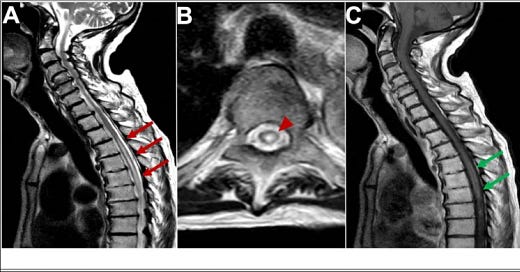












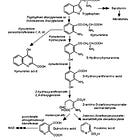


Thanks VERY MUCH, again. You offer huge work. That said, I wish that there could be a compact, less-than-100-words explanation as to how the Spikes in both the 'COVID' diseases and the consequent 'vaccines' combine as maladies to human beings. It seems: the more research, the more manifestations and complexities. Are there root pathogens? Can they be remedied? On the question of intentions behind these poisonous inventions, these Labs-made bio-weapons, answers may be a lot simpler. Evil unto Mania ... May we overthrow the Criminal Lunatics soon!
I just saw this... My notifications aren't coming through and I was scrolling through your stacks. Amazing work. High five.