Magnesium deficiency and associated pathologies: Part 3
"Magnesium is no trace element: it is an essential giant mineral"
Reading time:
short story - novelette - novella 1- novel - PhD thesis - War and Peace - U.S. Tax Code
TL;DR:
Also available with the translator, 🇫🇷 🇪🇸 🇩🇪 🇯🇵 etc
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
To keep within email limits and to make content that is engaging and easy to read, the general advice is to keep Substacks to within about 1,500 words, or 7 minutes of reading.1
This presents a challenge when writing narrative literature reviews about magnesium, as this series would extend to over 20 weeks, which is longer than some soap operas run. So I have been pragmatic and limited it to 4 parts.
If you are a new follower or subscriber I recommend reading at least part 1, for the introduction to magnesium:
Part 1 looked into how severe magnesium depletion (hypomagnesemia) can almost double your risk of dying from coronary heart disease (CHD), stroke, thrombosis, atrial fibrillation (AF), sudden cardiac death (SCD), or a ruptured aneurysm.
Part 2 focused on Crohn’s disease, neurological disorders, osteoporosis, aging, muscle cramps, tinnitus, cancer, and diabetes.
In Part 3 we will look into the research linking magnesium to the prognosis of conditions including arthritis, kidney failure recovery, an underactive thyroid, peripheral neuropathy, learning and memory function, insomnia, depression, and fibromyalgia.
I also include a late-breaking story of a drug approval by NICE (National Institute for Health and Care Excellence) for the NHS, which has some potentially NASTY side effects.
Discussion
I will continue to include data indicating the profit motive for not effectively treating these conditions, including expected growth trends. There may be an inverse relationship, - the larger the market size, the less incentive to promote cheaper or more effective alternatives.
- A statin factoid
Magnesium supplements can work better than statins. In fact, much better overall:
…Mg deficiency is associated with increased activity of HMG-CoA reductase [125]. The ratio of saturated to unsaturated fatty acids increases and the levels of TG, LDL, HDL, and VLDL decrease when the activity of this enzyme is impaired [157]. Modulation of the gene expression of LDLR (LDL receptor) and other transcription factors, such as sterol regulatory element-binding proteins SREBP-1a and SREBP-2, may also contribute to the hypercholesterolemic effect of an inadequate intake of Mg, although the increase in LDL concentration is mediated by increased LDLR and SREBP expression [158].
…magnesium supplements work better than atorvastatin alone at improving all-lipid profiles and controlling dyslipidemia.
From: “The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients“ (2023)
- Arthritis
The global rheumatoid arthritis drugs market size is calculated at USD 18.27 billion for 2024 and is estimated to be worth around USD 27.38 billion by 2033, growing at a CAGR of 4.6% from 2023 to 2033.
From: “Rheumatoid Arthritis Drugs Market Size to Worth USD 27.38 Bn by 2033” (2024)
https://finance.yahoo.com/news/rheumatoid-arthritis-drugs-market-size-150500218.html
Our first review paper is by Kuang et al (2021): “Magnesium in joint health and osteoarthritis“2:
Chondrocyte = “The cartilage is solely composed of cells known as chondrocytes. Chondrocytes maintain the extracellular matrix (ECM) and produce the cartilage matrix. Surrounded by collagenous fibers, chondrocytes release substances to make cartilage strong yet flexible…Chondrocytes are also responsible for chondral repair; due to their reconstructive nature, they respond to outside trauma in case of tissue damage.”3
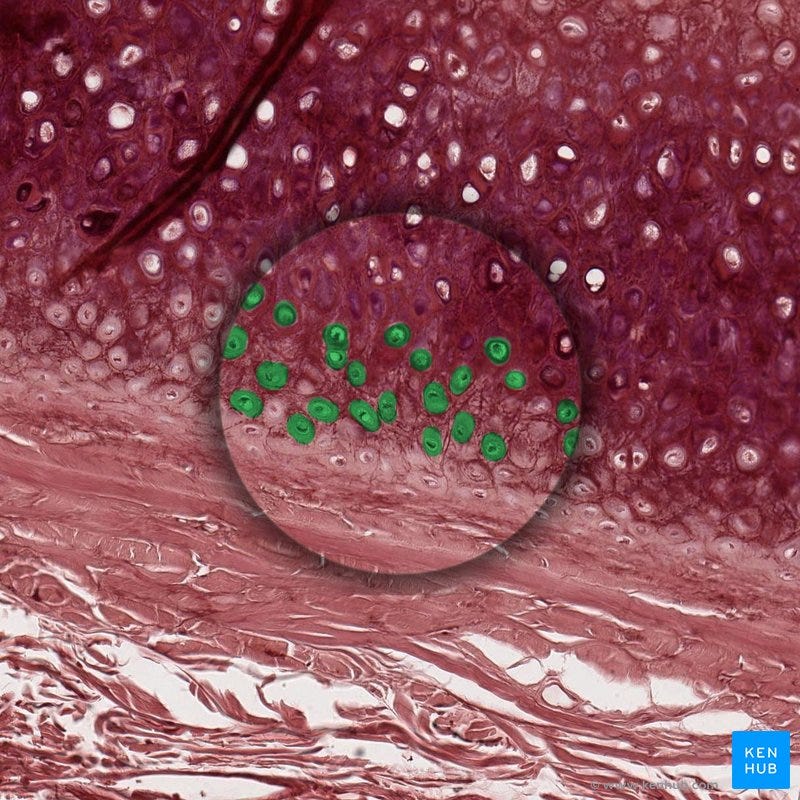
Magnesium helps to slow the loss of cells that are needed to repair and maintain healthy joints:
Osteoarthritis (OA) is a prevalent debilitating age-related skeletal disease. The hallmark of OA is the degradation of articular cartilage that cushions the joint during movement. It is characterized by chronic pain and disability. Magnesium, a critical trace element in the human body, plays a pivotal role in metabolism homeostasis and the energy balance. Humans obtain magnesium mainly from the diet. However, inadequate magnesium intake is not uncommon. Moreover, the magnesium status deteriorates with ageing. There has been a growing body of clinical studies pointing to an intimate relationship between dietary magnesium and OA although the conclusion remains controversial. As reported, the magnesium ion concentration is essential to determine cell fate. Firstly, the low-concentration magnesium ions induced human fibroblasts senescence. Magnesium supplementation was also able to mitigate chondrocyte apoptosis, and to facilitate chondrocyte proliferation and differentiation.
I would add glucosamine to the list too:
Dietary nutrition can be used as an important non-pharmacological treatment for OA. A diet supplemented with vitamin D has a positive effect on the thickness of the joint cartilage and joint lubrication [3]. Olive oil reduces the release of pro-inflammatory cytokines and increases lubricin synthesis, suggesting a positive protective effect on the joints [4,5]. Vitamin E supplementation can significantly increase the level of circulating antioxidant enzymes and relieve the pain of knee OA [6]. Fat-soluble vitamin K can affect the mineralization of bones and cartilage, which is associated with OA [7]. Obesity induced by a high-fat and high-sugar diet can cause inflammation and promote the development of OA. Supplementing prebiotic fiber can prevent the increase of serum endotoxin and microbial dysbiosis, so that it can improve knee joint damage [8,9]. Increasing intake of dietary fiber can reduce the risk of OA as well [10].
Moreover, the circadian rhythm of Mg2+ influx plays a role in controlling the daily metabolic activities at the cellular level [25].
MSCs = Mesenchymal stem cells
3.1. Magnesium and MSCs
Mg is essential for MSCs interaction with extracellular matrix. Mesenchymal stem cells (MSCs) can divide multiple times, and their progeny can differentiate into skeletal tissues such as bone and cartilage [50]. As these tissues play a major role in OA, it is important to evaluate the effect of Mg on MSCs. Mg has been shown to enhance the adhesion of synovial MSCs and then promote cartilaginous matrix assembly (ref). The adhesion of human synovial MSCs to collagen-coated slides in the presence of magnesium showed that Mg can enhance the adhesion to collagen (ref). Moreover, this effect is inhibited by the neutralizing antibodies of integrin α3 and β1. This points to an important function of integrin in the adhesion process. Additionally, Mg promoted the synthesis of cartilage matrix during the chondrogenesis of the synovial MSCs in vitro, which was related to the neutralizing antibodies of integrin β1. Finally, an in vitro experiment revealed that Mg enhanced the adhesion of human synovial MSCs to osteochondral defects [51]. (Fig. 1, Table 1)

Mg also plays a role in MSCs proliferation and differentiation.
…It was found that high concentrations of extracellular Mg could inhibit the mineralization process during MSCs osteogenic differentiation. Moreover, this study revealed that the Mg transporter SLC41A1 could regulate osteogenic differentiation [52]. Magnesium chloride was used as a Mg supplement to treat rat bone marrow MSCs. It could enhance MSC proliferation through Notch1 signal activation and induces osteogenic differentiation [53]. In conclusion Mg has a significant effect on the proliferation and differentiation of MSCs. Moreover, high concentration of magnesium can promote the treatment effect of human synovial MSCs on osteochondral defects. (Fig. 1,Table 1)
Mg ions induce osteoblast activity by enhancing gap junction intercellular communication between osteoblasts, which can help bone formation. This effect is proportional to the magnesium ion concentration and contact time [56]. (Fig. 1,Table 1)
5.1. Circulating magnesium and OA
The serum magnesium concentration is inversely proportional to OA. A study showed that patients with severe osteoarthritis had significantly lower serum magnesium levels than patients with mild osteoarthritis, but there was no association between serum magnesium concentration and the two inflammatory biomarkers [61].
A section on magnesium synergy with probiotics prompted these Substacks as follow-ups.
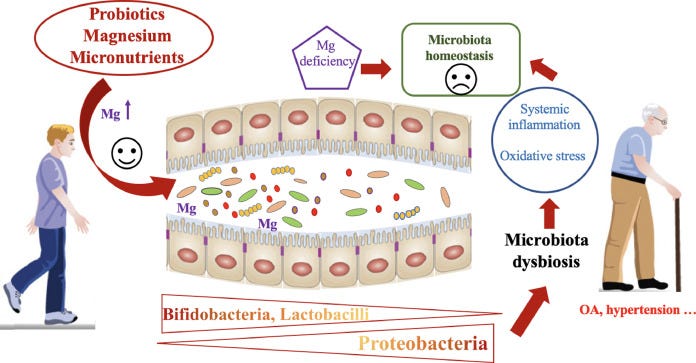
https://www.sciencedirect.com/science/article/abs/pii/S0271531721000142?via%3Dihub
Correlation does not necessarily equal causation but a rare caution against taking too much magnesium was between Mg intake and risk of rheumatoid arthritis (RA), as signaled in the retrospective analysis: “Relationship between dietary magnesium intake and rheumatoid arthritis in US women: a cross-sectional study”4 (2020).
It is difficult to overdose on Mg in practice as your kidneys and gut quickly excrete any excess, but it is worth knowing:
Objectives
Diet has been shown to be associated with rheumatoid arthritis (RA), and magnesium has been shown to inhibit inflammatory responses, but research on the relationship between dietary magnesium and RA is limited and controversial. In this study, we aimed to explore the non-linear relationship between dietary magnesium intake and RA in US women.
Design
Cross-sectional survey.
Setting
National Health and Nutrition Examination Survey (NHANES).
Primary and secondary outcome measures
Non-linear relationship between dietary magnesium intake and prevalence of RA.
Participants
A total of 13 324 women aged 18–80 years (RA n=12 306, non-RA n=1018) were included in this study.
Results
Overall, the absolute risk (AR) of RA was 7.24% in all participants. In the multivariable logistic regression analysis, we found a negative correlation between dietary magnesium intake and RA (OR=0.84, 95% CI 0.75 to 0.95, p=0.006). When we converted dietary magnesium intake into a categorical variable (tertiles), the ARs of the low group, the middle group and the high group were 9%, 7.1% and 4.9%, respectively. We noticed that the ORs between the three groups were not equidistant; then, we detected a U-shaped linking by smooth curve fitting and obtained inflection points at 181 and 446 mg/day. The prevalence of RA decreased when dietary magnesium intake was <181 mg/day (OR=0.7, 95% CI 0.5 to 0.8, p<0.001) and increased when it was >446 mg/day (OR=2.8, 95% CI 1.2 to 6.6, p=0.020), remaining at a minimum when it was between 181 and 446 mg/day (OR=1.0, 95% CI 0.7 to 1.2, p=0.700).
Conclusion
There was a U-shaped relationship between dietary magnesium and RA in women, and our study highlights the importance of moderate dietary magnesium intake in possibly exerting a protective role in women with RA.
Oestrogen may be the reason for this:
Solid evidence indicates that magnesium has a crucial regulatory role in nuclear factor kappa-B (NF-κB) activation, proinflammatory cytokine production and systemic inflammation, which have been proven to be strongly associated with the pathogenesis of RA.9 10 Furthermore, other studies showed that high magnesium levels are associated with low oestrogen levels in women,11 12 which is well known to be linked with RA.13 14 Therefore, there are reasons to suspect the role of dietary magnesium intake in women with RA.
They did attempt to adjust for covariates that may have skewed results, including sex, age, body mass index, race/ethnicity, poverty, smoking, drinking, other medical conditions, and oral contraceptives.
…When we converted dietary magnesium intake into tertile groups, the OR value showed non-equidistant changes, suggesting the existence of a non-linear relationship, so we conducted smooth curve fitting (penalised spline method) to detect the non-linear relationship. As shown in figure 2, there was an interesting U-shaped relationship between dietary magnesium intake and RA after fully adjusting the covariates in table 1. Then, we conducted a recursive algorithm to calculate the inflection point, as shown in table 3: if a woman’s dietary magnesium intake (log2) was below 7.5 (ie, <181 mg), a one-unit increase in magnesium intake (log2) resulted in a decrease of the relative odds of RA by 0.7 times with respect to the second, reference tertile (OR=0.7, 95% CI 0.5 to 0.8, p<0.001); when magnesium intake (log2) was between 7.5 and 8.8 (ie, 181–446 mg), the relative odds of RA did not change as dietary magnesium intake increased (OR=1.0, 95% CI 0.7 to 1.2, p=0.7). When magnesium intake (log2) was more than 8.8 (ie, >446 mg), the relative odds of RA increased by 2.8 times for a one-unit additional intake of magnesium (log2) compared with the second, reference tertile (OR=2.8, 95% CI 1.2 to 7.2, p=0.02).
In other words, the splines represent changes in the risk of RA when increasing Mg intake from <181 mg; 181-446 mg or >446 mg.
The authors noted that there were limited studies into this. But from their analysis, as long as you don’t overdo it (and for a long time), increasing intake is beneficial for decreasing the risk of oestrogen-associated RA:
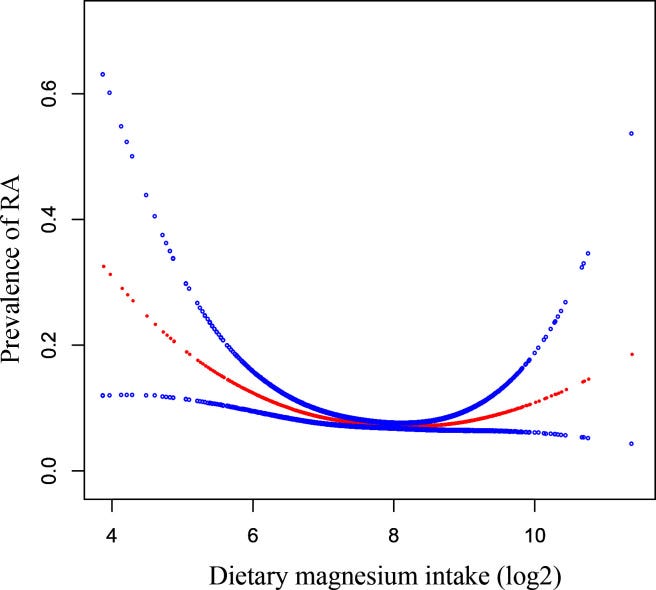
Our study explored that when dietary magnesium intake is below 181 mg/day, increased dietary magnesium intake was associated with a reduced prevalence of RA, which may be due to the anti-inflammatory effect of magnesium inhibiting proinflammatory gene expression.40–42
Our research found that dietary magnesium intake was 181 –446 mg/day, the relative odds of RA did not change as dietary magnesium intake increased. However, Mendelian randomisation analyses revealed that each SD increase in magnesium (0.16 mmol/L) is associated with an 8.94-fold increase in the risk of RA (p=0.044).18 Additionally, the joints of RA rats fed a high magnesium diet showed erosive changes in an in vivo experiment.15
The underlying mechanism may be the antagonism of high magnesium intake on oestrogen. This action triggers important secondary effects on RA, as oestrogen could inhibit the release of inflammatory cytokines related to RA through multiple mechanisms. Previous studies have proven that the lower serum oestrogen level among females was associated with a higher magnesium level11 12 48; an oestrogen deficiency may increase the risk of RA through its effect on immune dysregulation, exacerbate inflammatory conditions and cause massive bone destruction.13 14
Caution is warranted:
However, due to the lack of oestrogen data in NHANES, we were unable to investigate whether oestrogen acted as an intermediate factor between dietary magnesium and RA, so more prospective studies are needed to prove this hypothesis.
As with all studies, potential limitations should be noted. First, since the research data were derived from a cross-sectional study, the relationship was not necessarily identified as causal, indicating a potential temporality bias.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7654130/
Guess what you find when you visit the website of the “largest charity dedicated to supporting people with arthritis” to look for guidance about magnesium:
Magnesium contributes positively to the prognosis of many conditions and at minimal cost, too. You can understand why they don’t want you to know too much about it.
If the information is there they don’t make it easy to find!

The story of discovering and developing a new drug from bench to bedside is an extraordinary one. On average, it takes over 12 years and costs over £1 billion to develop a new medicine. And crucial to this process is the role of doctors, nurses and other NHS professionals – working with industry – to deliver these breakthroughs.
That’s why I’m proud of these relationships and why I feel passionately that they are critical to the success of the medical innovations of the future. But for these relationships to thrive, transparency about them is crucial.
From: “Managing conflicts of interest in the NHS“ (2016)
- Renal failure
Oh will it never end? Put your foot around the bend. Drive me crazy to an early grave. Tell me what is there to save tonight. Both ends burning.
Roxy Music
Pharma has both ends burning - by inducing acute kidney injury (AKI) and treating it too.
These cases may be understated by an under-reporting factor (URF) of between 30 and 100:5
Background
Acute kidney injury (AKI), a rare adverse event, cannot be ignored as millions of doses of coronavirus disease 2019 (COVID-19) vaccinations. We aimed to investigate the occurrence of post-vaccine AKI reported to the Vaccine Adverse Event Reporting System (VAERS).
Methods
After data mapping from December 2020 to June 2021, we summarized demographic and clinical features and outcomes of reported cases from three vaccines (Pfizer-BNT, MODERNA, and JANSSEN). The Bayesian and nonproportional analyses explored the correlations between COVID-19 vaccines and AKI.
We identified 1133 AKI cases. Pfizer-BNT appeared to have a stronger AKI correlation than MODERNA and JANSSEN, based on the highest reporting odds ratio (ROR = 2.15, 95% confidence interval = 1.97, 2.36). We observed the differences in ages, comorbidities, current illnesses, post-vaccine AKI causes, and time to AKI onset (all p<.05) among three vaccines. Most patients are elderly, with the highest age in MODERNA (68.41 years) and lowest in JANSSEN (59.75 years). Comorbidities were noticed in 58.83% of the cases and active infections in over 20% of cases. The leading cause of post-vaccine AKI was volume depletion (40.78%), followed by sepsis (11.74%). Patients in Pfizer-BNT had the worst outcome with 19.78% deaths, following 17.78% in MODERNA and 12.36% in JANSSEN (p = .217). The proportion of patients on dialysis was higher in JANSSEN than in Pfizer-BNT and MODERNA (14.61% vs. 6.54%, 10.62%, p = .008).
Conclusion
AKI could occur after the COVID-19 vaccines, predominantly in elderly patients. However, the causality needs further identification.
From: “Acute kidney injury after COVID-19 vaccines: a real-world study“ (2022)
I can add a little more as to the how, and the keyword is “autoimmune”.
Rhabdomyolysis, the breakdown of skeletal muscle cells, can develop due to many causes such as autoimmune, toxin-related, infectious, endocrine disorders, or trauma-like events10. In a comprehensive study in which patients with rhabdomyolysis were evaluated, the most common etiological reasons were trauma, immobilization, and sepsis, respectively11. In another study evaluating 475 patients, exogenous toxin exposure was the leading cause12. Underlying myopathy or metabolic defects were observed in the minority of the patients12. The release of cellular constituents (myoglobin, electrolytes, and cellular enzymes like creatine kinase) can lead to life-threatening consequences including acute kidney injury and disseminated intravascular coagulation13. The presentation of rhabdomyolysis includes muscle weakness, myalgias, myoglobinuria related to red to brown urine, and laboratory evidence of cellular damage like increased serum muscle enzymes, hyperkalemia, hyperphosphatemia, and/or hypocalcemia and acute renal injury. Removal of the underlying cause and maintaining fluid and electrolyte balance is the mainstay of treatment14.
From: “Rhabdomyolysis after BNT162b2 mRNA Covid-19 vaccine in an adolescent male“ (2022)
The patient, in this case, was young and quickly recovered, but for the elderly, it can be much more serious:
Many people recover after rhabdomyolysis treatment. But most people have lingering muscle weakness for a few weeks after the injury. In up to 50% of rhabdomyolysis cases, people experience acute kidney injury. Some people need dialysis for an extended time if their kidneys can't function.
From: “Rhabdomyolysis”
https://my.clevelandclinic.org/health/diseases/21184-rhabdomyolysis
Hence treating it may be even more profitable than even the following projections imply.
There is a caveat here: The money pot isn’t bottomless, and I hardly need to elucidate what rationing means, if you are the one being rationed:
The chronic kidney disease treatment market size was valued at $32 billion in 2022, and is estimated to reach $47.9 billion by 2032, growing at a CAGR of 4% from 2023 to 2032.
From: “Chronic Kidney Disease Treatment Market Sets New Record, Predicted to Hit USD 47.9 Billion by 2032, at 4% CAGR: Claims AMR“ (2023)
#Baffled as ever, the BHF posted this in March ‘24:
“Kidney failure patients more likely to die from heart attacks and strokes, British Heart Foundation finds”6
It’s worth discussing due to what they don’t say.
After reading many papers on the subject you can’t escape the likelihood that many of the kidney failure patients are also probably suffering from hypomagnesemia. Classic symptoms for this are in the headline, and we know that renal failure significantly limits magnesium reabsorption.
With that in mind, I looked forward to seeing the BHF finally acknowledging the importance of magnesium to help mitigate cardiovascular risks.
Unfortunately not. Magnesium doesn't exist in the Periodic Table that these pharma shills use.
They allegedly don’t know the cause, but the dangers are “unacceptably high”, and they now have the perfect excuse to throw millions at the “problem”, going around in circles looking for the elusive cure they can never find due to ignoring a key contributor:
One professor described the dangers as "unacceptably high".
People who have suffered kidney failure are more likely to also have a heart attack, new research has shown.
An analysis of patient data over 20 years by the British Heart Foundation Scotland indicated that people with kidney failure have a higher risk of dying to either cardiac arrest or a stroke. The study, which has been published in the European Heart Journal, also suggested women are at a greater risk than men.
The researchers used anonymous healthcare data from more than 16,000 patients from 1996-2016. Heart attack and stroke rates halved in kidney failure patients over the 20 years and the number of deaths because of them also fell.
David McColgan, head of British Heart Foundation Scotland, which funded the research, said: "The data shows that, compared to other groups, kidney failure patients’ risk of having a heart attack or stroke remains exceptionally high. It is crucial that further research is done to learn more about the link between the two and try to lower this risk."
This is better, do they mean by recommending magnesium here?
As part of the study, researchers concluded that treatments could improve survival rates - treatments which are already commonplace for patients.
Ah…no! Of course not.
Magnesium means illness and death - of your cash flow model.
This isn’t addressing the root cause, not least because these allopathic meds are given AFTER you have become ill and symptomatic with the cardiac-related problem:
The research revealed that over 40 per cent of patients who were not prescribed dual anti-platelet drugs died of a heart-related problem within a year whereas the figure dropped to nearly 14 per cent for those who were given the medications.
Anti-platelet drugs are commonly prescribed to the general population after a heart attack or stroke to prevent blood clotting.
I even checked out a featured article from their website called “The heart-kidney link”, featuring an interview with Dr Paul Kalra, consultant cardiologist at Queen Alexandra Hospital, Portsmouth.
You can read it yourself, but I wouldn’t bother. The TL;DR is that the M word doesn’t get a mention but a long list of prescription meds do. Pharma shills are just gonna’ shill.
If the BHF bothered to read reviews they could save themselves from wasting millions on unicorn-chasing research, as well as informing kidney patients about magnesium before they became a statistic.
Even better they could conduct further research and clinical trials to close the knowledge gap a little more, just as the “research agenda” recommended earlier.
It’s not like it is new research, this was from 2008, and it took me all of about 30 seconds to find:
“Serum magnesium in recovering acute renal failure”:7
Abstract
We studied the manifestations of hypomagnesemia in 50 patients with acute renal failure who had been admitted in our hospital over a period of ten months. All patients with serum creatine ≥ 2 mg/dL and normal baseline levels of serum calcium, magnesium, and potassium as well as normal ECG were included in the study. Patients with multi-organ failure, drug-induced acute renal failure, obstructive uropathy, and alcohol addiction were excluded. The mean age of our study population was 40 ± 15 years, 37 of the patients were male and 13 were female. Hypomagnesemia was observed in 31 patients out of 50 during the recovery period of acute renal failure with symptomatic hypomagnesemia being seen in 23 patients. Serum magnesium levels on the day of admission and during the recovery phase were 2.11 ± 0.38 mg/dL and 1.64 ± 0.41 mg/dL respectively. Paresthesia, irritability, agitation, dysartharia, vertigo, and associated hypokalemia and hypocalcemia were noted in symptomatic hypomagnesemic patients. Treatment of hypomagnesaemia and hypokalemia ameliorated the symptoms. We conclude that these abnormalities produce clinically significant manifestations in recovery phase of acute renal failure and clinicians should pay attention to these.
Acute renal failure (ARF), a syndrome with multiple etiologies, affects approximately 5.7% of all hospitalized patients.1,2 Approximately 7.2% of all general ward patients and 15% ofall ICU patients develop ARF.4
ARF is associated with significant morbidity and mortality because of the serious nature of the underlying illness and the high incidence of complications.4 Complications include metabolic, cardiovascular, gastrointestinal, neurological, and hematological infections. Electrolyte disturbances in the form of hypocalcemia, hypokalemia, hypophosphatemia, and hypomagnesemia are commonly seen in recovery phase of ARF.5
Although magnesium deficiency is a common clinical problem, serum magnesium levels are overlooked in recovering ARF cases. Manifestations of hypomagnesemia are reported to be similar to those of hypokalemia and hypocalcemia, which generally coexist.1–12 The overall reported prevalence of hypomagnesemia in hospitalized patients is variable and ranges from 4.6–47%, reflecting the type of the patient population studied and different cut-off levels used to define low serum magnesium in these studies.10–12 Hypomagnesemia produces diverse neuromuscular manifestations such as myclonic jerks, paresthesia, dysartharia, and neuropsychiatric manifestations such as agitation, anxiety, and depression. It also produces ventricular arrythmias and electrolyte abnormalities.13
In this study we observed hypomagnesemia in 62% of the patients (31/50) with recovering ARF, 74% of whom were symptomatic. A common cause of hypomagnesemia is the loss of magnesium from the gastrointestinal tract or the kidney. Urinary magnesium loss is often the basis for magnesium depletion, either because of sodium reabsorption in the same tubular segments (magnesium transport passively follows that of sodium), or because of a primary defect in renal tubular magnesium absorption.14
Hypomagnesemia can persist for a long time after acute tubular damage has been reversed. We therefore excluded alcoholics and drug-induced ARF from our study and none of our patients received diuretics at any time.
From 2008. It’s a shame this never happened:
In conclusion, hypokalemia and hypocalcemia were commonly seen with hypomagnesemia in recovering ARF patients in our study. Treating hypomagnesemia and associated electrolyte abnormalities ameliorated the symptoms. Our findings highlight the need for a large-scale study, including larger numbers of patients and close monitoring for symptoms of hypomagnesemia. We need to rule out other causes which produce similar symptoms and determine whether correction of serum magnesium levels in recovering ARF patients will benefit these patients and also, whether monitoring serum magnesium in recovering ARF patients is mandatory.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813135/
Hypomagnesemia has been investigated in non-CKD, CKD, and end stage renal disease (ESRD) patient populations and has been found to be associated with increased mortality [1, 7, 8], notably increased cardiovascular mortality [9]
From: “Serum magnesium, mortality and disease progression in chronic kidney disease” (2020)
https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-020-1713-3
“Abundant experimental evidence”, but you would never know it from official sources:
Cardiovascular complications are the leading cause of death in patients with chronic kidney disease (CKD). Abundant experimental evidence suggests a physiological role of magnesium in cardiovascular function, and clinical evidence suggests a role of the cation in cardiovascular disease in the general population.
…Experimental studies have shown that magnesium inhibits vascular calcification, both by direct effects on the vessel wall and by indirect, systemic effects. Moreover, an increasing number of epidemiologic studies in patients with CKD have shown associations of serum magnesium levels with intermediate and hard outcomes, including vascular calcification, cardiovascular events and mortality.
From: “Magnesium and cardiovascular complications of chronic kidney disease” (2015)
Another story just broke from the BHF. They don’t want to ask why this is happening, which is astonishing:
The British Heart Foundation has been forced to triple the number of nurses working on its helpline amid soaring NHS waiting lists.
The charity said that it has increased the number of cardiac nurses working on its Heart Helpline to 14 after a 75 per cent surge in callers in February compared with the same month last year.
It comes as the NHS waiting list for heart care rose to more than 400,000 at the end of January, according to analysis of the latest data.
The number waiting for heart tests, operations or procedures is now double the 203,893 it was in February 2021.
More than 3,550 people were in touch with its information and support service in February, the charity said, with most people concerned about being unable to get a diagnosis or appointment for a heart problem, or worried about new and changing symptoms.
As well as people waiting for appointments with heart specialists, the charity estimates that there are tens of thousands more waiting for a GP referral, regular check-up with a specialist, or for aftercare such as cardiac rehabilitation.
From: “British Heart Foundation triples helpline nurses as NHS waiting lists rise“ (26th March ‘23)
https://www.telegraph.co.uk/news/2024/03/26/british-heart-foundation-triples-helpline-nurses/
If you aren’t speaking out at this point then you are complicit in the crime, especially as the drug companies themselves warned you, eventually:
To be fair they wanted the warnings to be stronger, but their shareholders made them take it out:
- Hypothyroidism
Not as large a market as expected, but even so it’s yet another growing market:
What is the global market size of Thyroid Gland Disorders Treatment Market? Global Thyroid Gland Disorders Treatment Market size was valued at USD 2.37 billion in 2022 and is poised to grow from USD 2.45 billion in 2023 to USD 3.14 billion by 2031, growing at a CAGR of 3.17% in the forecast period (2024-2031).
The global thyroid gland disorders treatment market has grown significantly in the last several years. This is explained by the fact that thyroid conditions such as hyperthyroidism are becoming more commonplace throughout the world. This market has grown as a result of expanding aging populations, improved medical technology, and growing awareness of the value of early diagnosis and treatment.
From: “Global Thyroid Gland Disorders Treatment Market Insights” (2024)
https://www.skyquestt.com/report/thyroid-gland-disorders-treatment-market
“Long-”conditions, an underactive thyroid, and many LNP/spike protein autoimmune-related diseases could benefit from extra magnesium.
Anti-thyroid peroxidase (TPO) antibodies may also be inhibited. By association, this should be beneficial for some cases of fibromyalgia too.
“Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study”8 (2018)
Abstract
Trace elements, such as iodine and selenium, are closely related to autoimmune thyroiditis and thyroid function. Low serum magnesium is associated with several chronic diseases; however, its associations with autoimmune thyroiditis and thyroid function are unclear. We investigated the relationships between low serum magnesium, autoimmune thyroiditis, and thyroid function in 1,257 Chinese participants. Demographic data were collected via questionnaires, and levels of serum thyroid stimulating hormone, anti-thyroid peroxidase antibody, anti-thyroglobulin antibody (TGAb), free thyroxine, serum magnesium, serum iodine, and urinary iodine concentration were measured. Participants were divided into serum magnesium level quartiles (≤0.55, 0.551–0.85, 0.851–1.15, and >1.15 mmol/L). The median serum magnesium level was 0.89 (0.73–1.06) mmol/L; levels ≤0.55 mmol/L were considered severely low (5.9% of participants). The risks of TGAb positivity and Hashimoto thyroiditis (HT) diagnosed using ultrasonography in the lowest quartile group were higher than those in the adequate magnesium group (0.851–1.15 mmol/L) (p < 0.01, odds ratios [ORs] = 2.748–3.236). The risks of total and subclinical-only hypothyroidism in the lowest quartile group were higher than those in the adequate magnesium group (0.851–1.15 mmol/L) (p < 0.01, ORs = 4.482–4.971). Severely low serum magnesium levels are associated with an increased rate of TGAb positivity, HT, and hypothyroidism.
Recent evaluations of serum magnesium as an indicator of magnesium status have indicated that individuals with serum magnesium values >0.85 mmol/L most likely have adequate magnesium levels20,28,29. Therefore, in our study, serum magnesium levels ≤0.85 mmol/L were considered low. Additionally, serum magnesium levels ≤0.55 mmol/L were considered severely low in our study, as previously described in the literature30,31.
Make sure that you aren’t deficient in iodine too, (thanks Jennifer):
Magnesium, as an enzyme cofactor, plays a critical role in mitochondrial oxidative phosphorylation and ATP synthesis, and its deficiency can affect these functions and lead to decreased iodine uptake by thyroid cells and a subsequent drop in thyroid hormone synthesis, thereby causing the secretion of thyroid-stimulating hormone (TSH). Animal experiments have shown that magnesium supplementation can significantly increase radioactive iodine uptake by thyroid cells, while its deficiency does the opposite34. Notably, the majority of participants with hypothyroidism in this study exhibited subclinical hypothyroidism;
Our study found that serum magnesium levels ≤0.55 mmol/L were related to the risk of TGAb positivity and prevalence of HT. There are at least two explanations for this: first, severely low serum magnesium can increase TGAb via the abnormal activation of immune cells and induction of an autoimmune response. A study on patients with Graves’ disease found that their serum magnesium concentrations were lower than in normal individuals, and that the serum magnesium concentration was inversely related to the activation levels of CD3+, CD4+, CD8+T, and CD19+B cells. It was speculated that low serum magnesium might lead to decreased immune tolerance and abnormal activation of immune cells26. Second, given its function as a coenzyme, magnesium is involved in a variety of antioxidant metabolism pathways, such as glutathione synthesis; low serum magnesium could therefore reduce the antioxidant response capacity in cells and allow the accumulation of free radicals, resulting in oxidative stress and tissue damage21,38,39. Epidemiological studies have shown that insufficient magnesium intake is associated with a variety of chronic inflammatory diseases and elevated serum C-reactive protein levels6,22,23,40.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6028657/
A clinical trial including sportspersons in Tae-Kwon-Do found that supplementation increased their thyroid-stimulating hormone (TSH) levels after training hard. In other words, training to exhaustion can seriously deplete your serum magnesium levels and lead to all the inflammatory, endocrine, and immunosuppressive effects associated with that, for a time.
“The effects of magnesium supplementation on thyroid hormones of sedentars and Tae-Kwon-Do sportsperson at resting and exhaustion”9 (2007).
TSH, FT3, FT4, TT3 ve TT4 = thyroid hormone types.
Abstract
The effect of magnesium on thyroid hormones of sedentars and sportsperson in Tae-Kwon-Do, has been investigated in a 4-weeks training program. Group 1 consisted of sedentars receiving 10 mg/kg/day Mg for 4 weeks. Group 2 consisted of subjects receiving magnesium (Mg) supplement and practicing Tae-Kwon-Do for 90-120 min/day, for five days a week. Group 3 consisted of subjects practicing Tae-Kwon-Do but receiving Mg supplements. TSH levels increased with training and Mg supplementation (p<0.05). Mg increased FT3 values. (p<0.05). TT3 values of groups reduced in all groups (p<005). After supplementation, group 1 had higher TT4 values than groups 1 and 3 and the group 2 had higher TT4 values than the third group (p<005). Results of this research show that training until exhaustion causes reduction in thyroid hormone activity in sedentars and sportsperson. It has been established that Mg supplementation however, prevents reduction in thyroid hormone activity in sedentars and sportsperson.
https://www.nel.edu/userfiles/articlesnew/NEL280507A05.pdf
- Peripheral neuropathy
The global peripheral neuropathy market size was USD 3.61 Billion in 2022 and is expected to register a revenue CAGR of 9.0% during the forecast period. Rise in peripheral neuropathy cases, the increased prevalence of chronic illnesses, and increasing number of pipeline drugs are some of the key factors driving market revenue growth.
From: “Peripheral Neuropathy Market Size Worth USD 8.54 Billion in 2032 | Emergen Research“ (2024)
https://finance.yahoo.com/news/peripheral-neuropathy-market-size-worth-155700155.html
I reviewed the pathophysiology and therapeutics at length. See here and here.
Background and Objectives:
Various peripheral neuropathies, particularly those with sensory and autonomic dysfunction may occur during or shortly after acute COVID-19 illnesses. These appear most likely to reflect immune dysregulation. If similar manifestations can occur with the vaccination remains unknown.
Results:
…Together, 52% (12/23) of patients had objective evidence of small-fiber peripheral neuropathy. 58% patients (7/12) treated with oral corticosteroids had complete or near-complete improvement after two weeks as compared to 9% (1/11) of patients who did not receive immunotherapy having full recovery at 12 weeks. At 5–9 months post-symptom onset, 3 non-recovering patients received intravenous immunoglobulin with symptom resolution within two weeks.
Conclusions:
This observational study suggests that a variety of neuropathic symptoms may manifest after SARS-CoV-2 vaccinations and in some patients might be an immune-mediated process.
From: “Neuropathic symptoms with SARS-CoV-2 vaccination“ (2022)
If anything these cases are too optimistic and not representative - more of a short-term acute reaction to Spike/LNP/LPS toxins. Lupus-like disorders and fibromyalgia are usually not eliminated just with steroids, and instead, we expect case numbers to keep rising for years. Autoimmune antibodies in chronic conditions tend to increase gradually over time, asymptomatic at first.
ANA antibodies are a marker for systemic lupus erythematosus (SLE). 98% of SLE sufferers have these abs, and there is no cure:10
The aim of this single centre prospective follow-up study was to evaluate whether healthcare workers (HCWs) vaccinated with BNT162b2 mRNA and mRNA-1273 will show a development and/or a persistence of autoantibodies, focussing on the detection of antibodies against nuclear antigens (antinuclear antibodies, ANA). We enrolled 155 HCWs, however only 108 of them received the third dose and were considered for further analysis. Blood samples were collected before vaccine inoculation (T0), at 3 (T1) and 12 months (T2) after the first dose. All samples were analysed for the presence of a) ANA using indirect Immunofluorescence [IIF] (dilutions of 1:80, 1:160. 1:320 and 1:640), and anti-smooth muscle antibodies (ASMA); b) anti-myeloperoxidase (anti-MPO), anti-proteinase 3 (anti-PR3) and anti-citrullinated peptide antibodies (aCCP) [FEIA]; c) anti-phospholipid antibodies (anticardiolipin [aCL], anti-beta-2- glycoprotein I [anti-ß-2GPI] (Chemiluminescence). Line-blot technology was performed using the following kit: EUROLINE ANA profile 3 plus DFS70 (IgG). Our research suggests that mRNA based anti-SARSCoV-2 vaccines can induce the production of de novo ANA in 22/77(28,57%) of subjects and that the percentage of positivity seems to be directly correlated to the number of vaccine expositions: 6/77 (7,79%) after 2 doses; 16/77 (20,78%) after 3 doses. Since it is known that hyperstimulation of the immune system could lead to autoimmunity, these preliminary results seem to further sustain the idea that the hyperstimulation of the immune system might lead to an autoinflammatory mechanism and eventually to autoimmune disorders.
From: “The onset of de novo autoantibodies in healthcare workers after mRNA based anti-SARS-CoV-2 vaccines: a single centre prospective follow-up study“ (2023)
I need to update Therapeutics for Multiple Sclerosis and Peripheral Neuropathy with magnesium.
A review from 2021 discussed some of the mechanisms, including protection against nerve damage by free radicals and inflammation.
“Magnesium Promotes the Regeneration of the Peripheral Nerve” (2021)
The protective effect of magnesium ions on the central nervous system has been studied. Clinically, magnesium sulfate has been used to prevent cerebral palsy in preterm infants. Wolf et al. (2020) has revealed that in the case of imminent preterm birth, prenatal treatment with magnesium sulfate during 24–32 weeks of gestation reduced the risk of moderate to severe cerebral palsy and had a neuroprotective effect. In addition, magnesium has been suggested to improve functional neurological outcomes in patients with global cerebral ischemia associated with cardiac surgery and cardiac arrest (Pearce et al., 2017). Identically magnesium also exerts neuroprotective effects on peripheral nerves.
NMDA again:
It is generally believed that magnesium ions can play a neuroprotective role by inhibiting the secondary injury after nerve injury through regulating cell function, antagonizing of N-methyl-D-aspartic acid receptor (NMDA receptor) and calcium ions (Koltka et al., 2011). In addition to the degeneration of the damaged nerve stump, the uninjured nerve might also suffer from degeneration. Under the stress state, the magnesium ion level decreases. Then the calcium ion channel opens massively, and the calcium ion flows inside, leading to cell swelling and apoptosis. Thus, a supplementary magnesium ion can block the ion channel of NMDA receptor through the action of electric charge, thereby inhibiting the entry of calcium ion into cells and antagonizing the changes in cell permeability caused by injury and the neurotoxic effect of calcium ion (Jia et al., 2016).
Lambuk et al. (2017) found that the excitatory toxicity of glutamate plays a crucial role in the loss of retinal ganglion cells (RGCs) in glaucoma. The toxic effect of glutamate on RGCs was mediated by overstimulation of NMDA receptors. At the same time, intravitreal injection of magnesium Acetyltaurate (MgAT) could prevent NMDA-induced retinal and optic nerve damage (Lambuk et al., 2017).
Promote Schwann Cell Proliferation and Axon Regeneration
Schwann cells are neuro-gliocytes that constitute the peripheral nervous system. It not only participates in the formation of the myelin sheath but also maintains the growth environment of the axons through its rapid proliferation, division and secretion of various protein molecules after peripheral nerve injury and also promotes the self-repair and regeneration of nerves.
Excess may be inhibitory, but left unsaid is that deficiency will be too:
NGF = nerve growth factor.
Appropriate concentrations of magnesium ions in the tissue microenvironment can promote SCs proliferation (Monfared et al., 2018a), as well as SCs synthesis and NGF secretion…However, the effect of magnesium ions on the promotion of SCs proliferation is not positively correlated with its concentration. Excessive magnesium ion concentration had been reported to inhibit SCs proliferation (Monfared et al., 2018a).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8385315/
“Low serum phosphate and magnesium levels are associated with peripheral neuropathy in patients with type 2 diabetes mellitus”11 (2018, paywalled).
Results
Serum phosphate and serum magnesium levels were significantly lower in patients with diabetic peripheral neuropathy (DPN) (P < 0.01). And the percentages of DPN patients were lower in high tertile of serum phosphate and serum magnesium (P < 0.05). Furthermore, composite z score of conduction velocity (CV) (P = 0.012) were positively associated with serum phosphate levels and the composite z score of amplitude (P < 0.001) and CV (P = 0.041) were positively associated with serum magnesium levels. After adjusting potential related factors (age, gender, smoking, diabetes duration, body mass index, systolic blood pressure, glycated hemoglobin, total cholesterol, estimated glomerular filtration rate), serum levels of phosphate and magnesium were still related to status of DPN in logistic regression (P < 0.05).
Conclusion
Lower serum phosphate and magnesium significantly correlated with parameters of nerve conduction in T2DM patients. Serum phosphate and magnesium might underlie the pathophysiologic features of DPN.
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes [1], occurs in approximately 50% of patients with diabetes [2]. DPN is also a significant independent risk factor for diabetic foot ulcers, which is the major course of lower extremity amputation in patients with diabetes [3].
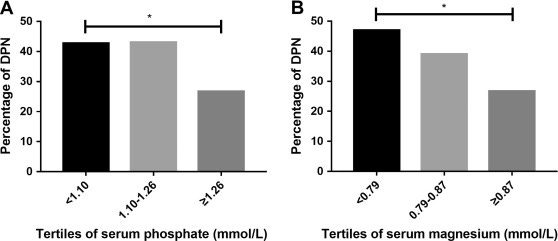
In our study, we found that lower serum magnesium levels were significantly correlated with lower amplitude which indicated axonal function in peripheral neurons. Our result was consistent with another study [23]. Here are some possible mechanisms: Magnesium is an essential biological element acting as an important regulator of energy metabolism. It preserves the efficiency of mitochondrial ATP formation by maintenance of transmembrane ion gradients at the inner mitochondrial membrane [31]. Magnesium supplementation had beneficial effect on glucose control, oxidative stress, inflammation in diabetes [32]. A recent RCT showed that 12 week magnesium supplementation among subjects with diabetic foot ulcer had improved the parameters of ulcer size, glucose metabolism and serum hsCRP [33]. Animal study showed that magnesium acetyltaurate had protective effect against axonal degeneration by reducing oxidative stress with caspase-3 inactivation [34].
https://www.sciencedirect.com/science/article/abs/pii/S0168822718311173
- Learning and memory
In 2011 Hoane wrote a comprehensive review, discussing its importance:
“The role of magnesium therapy in learning and memory”12
Abstract
The old saying “you are what you eat” is becoming increasingly important in the field of neuroscience these days. There is mounting evidence that nutritional factors are beginning to play a major role in cognitive status, or cognitive wellbeing. One of these emerging factors is magnesium (Mg2+). Although the physiological investigation of Mg2+ has a long history, its role in cognitive function is just starting to emerge. The focus of this chapter is to review the available literature on the effects of Mg2+ on cognitive function in the healthy and diseased/injured brain. In addition, data from our laboratory will be presented that has investigated the effects of Mg2+ manipulation on learning and memory tasks in rodents, as well as the ability of Mg2+ therapy to improve cognitive performance in the damaged brain.
Although the main focus of this chapter is on animal models, there are some interesting human studies that have examined the role of Mg2+ in cognitive ability. A recent study examined the correlation between levels of several trace minerals (iron, Mg2+, potassium and zinc) in the hair of adolescent girls and their academic record. Although care must be taken with the interpretation of correlational studies of this nature, there was evidence that some trace minerals correlated more highly with increased academic performance. Specifically, it was found that Mg2+ and zinc demonstrated a strong positive correlation with academic performance (Wang et al., 2008).
Wernicke-Korsakoff syndrome = a neurological disorder caused by the lack of thiamine (vitamin B1).
Furthermore, a recent case report of a patient presenting with anorexia nervosa and Wernicke-Korsakoff syndrome was found to have a low serum Mg2+ level (Saad et al., 2010). Although the main cause of this condition was thiamine deficiency, a major adjunct factor was believed to be Mg2+ deficiency. The results from this case study are supported by a review article on micronutrients and cognitive performance that details the inter- relationships between Mg2+ and other micronutrients such as the B-group vitamins and how deficiencies in these nutrients interact to produce cognitive deficits (Huskisson et al., 2007). Thus, there is an expanding literature that suggests that Mg2+ status plays an important role in cognitive performance.
TBI = Traumatic brain injury.
A unique condition in which Mg2+ has been implicated is TBI. McIntosh and colleagues have shown that Mg2+ homeostasis is disrupted following CNS injury (McIntosh et al., 1988; Vink et al., 1988). Fluid percussion injury (FPI) produced a rapid and severe decline in intra- and extracellular Mg2+ levels, which correlated significantly with the severity of the behavioural deficits observed following injury (McIntosh et al., 1988; Vink et al., 1988).
We have also recently investigated the effect of dietary Mg2+ deficiency on learning acquisition in the MWM. Rats were placed on either a standard laboratory diet or a commercially available Mg2+- deficient diet for 14 days. Peripheral blood collect- ions were performed for determination of serum Mg2+ levels and all animals were placed back on to the standard diet. The serum analysis indicated a significant loss in serum Mg2+ in the deficiency group (p < 0.05). One week later, animals were tested for the acquisition of a reference memory task over 4 days (4 trials per day, 15 min ITI). As can be seen in Figure 2A, the 14 days of Mg2+ deficiency significantly impaired the initial acquisition of the task on the first day (p<0.05).

https://www.ncbi.nlm.nih.gov/books/NBK507270/
A self-reported dietary intake report from 2012 by Ozawa et al revealed the link between potassium, calcium, magnesium, and the risk of dementia. A long-term study, a statistically significant link between intake and reduced risk of dementia was found, especially with vascular dementia, but not with Alzheimer’s Disease.
There were 1081 community-dwelling Japanese individuals without dementia, aged 60 and older. Risk reductions were between 36% and 48% for all-cause dementia, and between 74% and 80% for vascular dementia:
“Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the Japanese: The Hisayama Study”13
Measurements
A 70-item semiquantitative food frequency questionnaire was used to assess potassium, calcium, and magnesium intakes. Hazard ratios (HRs) for the development of all-cause dementia and its subtypes were estimated using Cox proportional hazards model.
Results
During a 17-year follow-up, 303 participants experienced all-cause dementia; of these, 98 had vascular dementia (VaD), and 166 had Alzheimer's disease (AD). The multivariable-adjusted HRs for the development of all-cause dementia were 0.52 (95% confidence interval [CI] = 0.30–0.91), 0.64 (95% CI = 0.41–1.00), and 0.63 (95% CI = 0.40–1.01) for the highest quartiles of potassium, calcium, and magnesium intake, respectively, compared with the corresponding lowest quartiles. Similarly, the HRs for the development of VaD were 0.20 (95% CI = 0.07–0.56), 0.24 (95% CI = 0.11–0.53), and 0.26 (95% CI = 0.11–0.61) for the highest quartiles of potassium, calcium, and magnesium intake, respectively. There was no evidence of a linear association between these mineral intakes and the risk of AD.
Conclusion
Higher self-reported dietary intakes of potassium, calcium, and magnesium reduce the risk of all-cause dementia, especially VaD, in the general Japanese population.
Recent evidence has emerged to indicate that dietary modification has an important role in preventing life style-related diseases.1 In several prospective studies, higher intake of potassium, calcium, and magnesium reduced the risk of developing hypertension and stroke.2-4 These findings raise the possibility that these mineral intakes may be effective at reducing the burden of cardiovascular risk factors and subsequent vascular diseases.
Several longitudinal studies have reported the preventive effects of dietary intakes of these minerals on the risk of stroke,3, 4 but to the best of the knowledge of the authors of the current study, this is the first prospective cohort study showing that higher self-reported dietary intakes of potassium, calcium, and magnesium are associated with a lower risk of dementia. The separate effects of each mineral on dementia were not distinguished because these minerals were strongly correlated with one another. Furthermore, the possibility that some other factors contained in the foods than the minerals themselves caused the favorable effects on dementia cannot be excluded. Nevertheless, these findings may provide intriguing information on the beneficial effects of a diet rich in these minerals against dementia in Japanese.
The mechanism through which the risk of VaD decreased with higher intakes of these minerals is unclear. Hypertension has been recognized as a strong risk factor for vascular diseases, including VaD.26 There is some evidence of the antihypertensive effects of these mineral intakes,2 but the adjustment for hypertension had little effect on the association between each mineral intake and the risk of VaD in the present study. As alternative mechanisms, it has been reported that these minerals may have some favorable effects against vascular diseases through inhibition of free radical formation and platelet aggregation, improvement of dyslipidemia, and an increase in insulin sensitivity.27-29 Further investigation will be needed to clarify this issue.
https://agsjournals.onlinelibrary.wiley.com/doi/10.1111/j.1532-5415.2012.04061.x

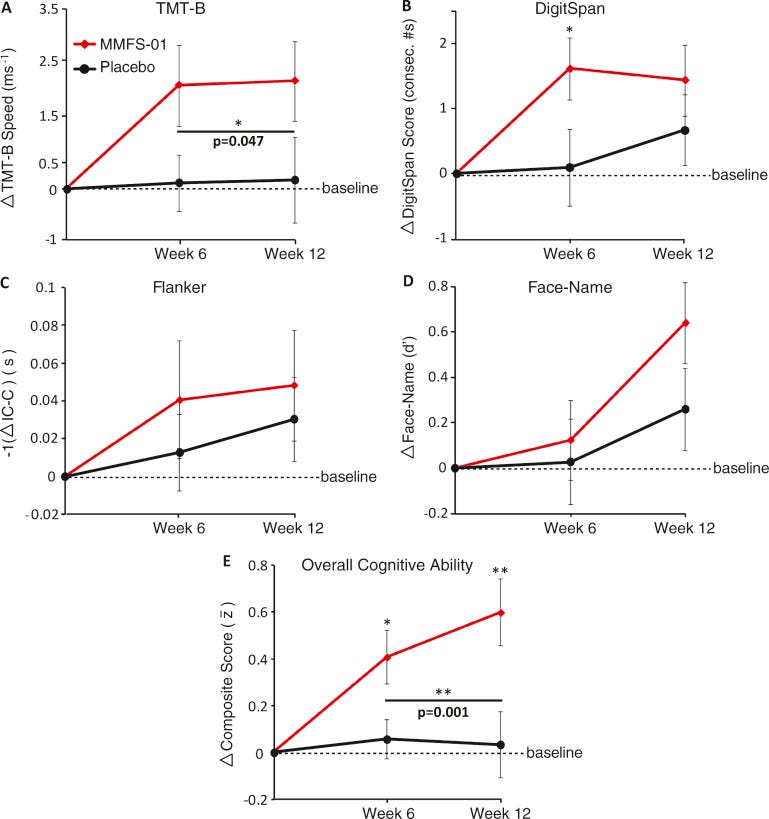
In 2016, Liu et al conducted a randomized, double-blind, placebo-controlled, parallel-designed trial of 44 subjects aged 50-70.
23 were treated with MMFS-01 (magnesium‐L‐threonate) and 21 placebo for 12 weeks. Cognitive ability, sleep quality, and emotion were evaluated.
“Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial”14:
Objective:
Since brain atrophy during aging is strongly associated with both cognitive decline and sleep disorder, we evaluated the efficacy of MMFS-01 in its ability to reverse cognitive impairment and improve sleep.
“Cohens d is a standardized effect size for measuring the difference between two group means. Frequently, you'll use it when you're comparing a treatment to a control group….Cohen’s d characterizes the effect size by relating the mean difference to variability, similar to a signal-to-noise ratio. A large Cohen’s d indicates the mean difference (effect size = signal) is large compared to the variability (noise).”15
Results:
With MMFS-01 treatment, overall cognitive ability improved significantly relative to placebo (p = 0.003; Cohen’s d = 0.91). Cognitive fluctuation was also reduced. The study population had more severe executive function deficits than age-matched controls from normative data and MMFS-01 treatment nearly restored their impaired executive function, demonstrating that MMFS-01 may be clinically significant. Due to the strong placebo effects on sleep and anxiety, the effects of MMFS-01 on sleep and anxiety could not be determined.
Conclusions:
The current study demonstrates the potential of MMFS-01 for treating cognitive impairment in older adults.
They needed to use large doses of magnesium-L-threonate. With a low 7% elemental Mg this is at most about 140mg:
Dosage
Dosage was set to correspond to approximately 25 mg/kg/day. To accomplish this, subjects between 50 and 70 kg took 1.5 g/day, and subjects between 70 and 100 kg took 2 g/day of MMFS-01. At conclusion of the study, 8 subjects (35 percent) were taking 1.5 g of MMFS-01 per day, and 15 subjects (65 percent) were taking 2 g of MMFS-01 per day.
- Insomnia
The global insomnia industry was worth US$ 3.8 billion in 2022. From 2023 to 2031, it is expected to increase at a CAGR of 6.0%, reaching US$ 6.4 billion.
From: “Insomnia Market Size to be Worth USD 6.4 billion by 2031, with Notable CAGR of 6.0%| Transparency Market Research, Inc.“ (2023)
https://finance.yahoo.com/news/insomnia-market-size-worth-usd-160000992.html
A double-blind placebo-controlled clinical trial of 46 elderly subjects investigated the effects of 500mg of magnesium or placebo daily for 8 weeks on their questionnaire-based insomnia severity index (ISI).
A significantly decreased ISI score was associated with the treatment arm. Insomnia is also linked to NMDA.
“The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial”16 (2012)
Background:
Nearly 50% of older adults have insomnia, with difficulty in getting to sleep, early awakening, or feeling unrefreshed on waking. With aging, several changes occur that can place one at risk for insomnia, including age-related changes in various circadian rhythms, environmental and lifestyle changes, and decreased nutrients intake, absorption, retention, and utilization. The natural N-methyl-D-aspartic acid (NMDA) antagonist and GABA agonist, Mg2+, seems to play a key role in the regulation of sleep. The objective of this study was to determine the efficacy of magnesium supplementation to improve insomnia in elderly.
Results:
No significant differences were observed in assessed variables between the two groups at the baseline. As compared to the placebo group, in the experimental group, dietary magnesium supplementation brought about statistically significant increases in sleep time (P = 0.002), sleep efficiency (P = 0.03), concentration of serum renin (P < 0.001), and melatonin (P = 0.007), and also resulted in significant decrease of ISI score (P = 0.006), sleep onset latency (P = 0.02) and serum cortisol concentration (P = 0.008). Supplementation also resulted in marginally between-group significant reduction in early morning awakening (P = 0.08) and serum magnesium concentration (P = 0.06). Although total sleep time (P = 0.37) did not show any significant between-group differences.
Conclusion:
Supplementation of magnesium appears to improve subjective measures of insomnia such as ISI score, sleep efficiency, sleep time and sleep onset latency, early morning awakening, and likewise, insomnia objective measures such as concentration of serum renin, melatonin, and serum cortisol, in elderly people.
NHANCES III analysis shows that elders’ magnesium intake in United States is much less than recommended dietary allowance (RDA) and compared to recommended levels of 420 and 320 mg/day, respectively for men and women, is equal to 225 mg/day for men and 166 mg/day for women. Yet, little is known about the magnesium status in Iran, especially in elderly population. The only well-designed population-based survey in Iran of dietary magnesium intake was conducted from 1999 through 2001 (within the framework of the Tehran Lipid and Glucose Study). A striking result of an analysis of that survey was the lower mean magnesium intake among participants. In aforementioned study, 95% of subjects failed to meet magnesium requirements (137 ± 28 mg/day).[27]
Poor bioavailability, yet still effective:
Magnesium was administered as magnesium oxide tablets twice a day (each tablet containing 414 mg magnesium oxide as 250 mg elemental magnesium) for 8 weeks.
Why supplement with melatonin if you can address the root cause?
We know from other studies that serum Mg tends to lag total Mg during repletion:
As compared to the placebo group, in the experimental group, the serum renin (P < 0.001) and melatonin (P = 0.007) levels showed a significant increase and serum cortisol level (P = 0.008) showed a significant decrease [Table 3]. Despite favorable increase of serum magnesium level during this study, the related changes in its level were just marginally significant (P = 0.06).
Table 3
Comparing serum magnesium and biochemical indices of circadian cycle in magnesium supplementation and placebo groups before and after intervention
The results of our study showed that as compared to the placebo group, in the experimental group dietary magnesium supplementation brought about statistically significant increase in serum melatonin concentration (P = 0.007). Our result is consistent with the study of Zhao et al. which suggested that magnesium sulfate injection to experimental rats caused the stimulation and significant increase of melatonin secretion from pineal gland.[51] Also the results of this study are consistent with the results of Billyard's study which stated that magnesium deficiency led to plasma melatonin reduction in rats.
However, since only plasma melatonin was measured in this study, it is not clear that the melatonin reduction was due to reduced synthesis or increased destruction of melatonin.[55] On the other hand, the results of Murck and Steiger study showed that no change was observed in cortisol, growth hormone, prolactin, and melatonin secretions due to magnesium supplementation.[30]
In general, studies show that magnesium deficiency affects circadian cycle, melatonin reduction, and sleep disorders.[56,57] Morton and James suggested that the N-acetyltransferase (NAT) activity in rat is increased after magnesium injection. Moreover, magnesium increases NAT activity in pineal gland in vitro, suggesting that the pineal gland, not another place of the body, is the affect site.[58] The mentioned findings implicates on possible magnesium deficiency role in reduced NAT activity and reduced melatonin production.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3703169/
- Major depression (MD)
The global depression drugs market reached a valuation of around US$ 13 Bn in 2020, which amounts to close to 50% share of the psychotropic drugs market. Sales of depression drugs are slated to accelerate at a CAGR of 4% to top US$ 20 Bn by 2031.
From: “Depression Drugs Market“ (2021)
A review from 2011 by Eby et al discusses how Mg supplementation can rival allopathic drugs for efficacy but without the side effects, tolerance, or risk of dependency.
You could easily fill a book with chapters discussing magnesium and MD.
“Magnesium and major depression”17
Abstract
The treatment of major depression (MD) is still a major unmet medical need in the majority of patients. Sixty percent of cases of MD are treatment-resistant depression (TRD), showing that classical treatments for MD are poorly effective to non-effective. Magnesium has been largely removed from processed foods, especially refined grains, in the Western world, harming the brain and causing mood disorders. Magnesium deficiency causes N-methyl-D-aspartate (NMDA) coupled calcium channels to be biased towards opening which causes neuronal injury and neurological dysfunction, which we believe results in MD. Oral administration of Mg to animals produced antidepressant-like effects that were comparable to those of antidepressant drugs. Cerebral spinal fluid (CSF) Mg has been found to be low in suicidal TRD. The first report of Mg treatment for agitated depression was published in 1921 showing success in 220 out of 250 cases. One 2008 randomized clinical trial showed that Mg was as effective as the tricyclic antidepressant imipramine in treating MD. Intravenous and oral Mg protocols have been reported to rapidly terminate MD safely and without side effects. Brain Mg deficiency reduces serotonin levels, and antidepressant drugs have been shown to have the action of raising brain Mg. Excessive calcium, glutamate and aspartate intake can greatly worsen MD. We believe that, when taken together, there is more than sufficient evidence to implicate inadequate dietary Mg as contributing to the cause of MD, and we suggest that physicians prescribe Mg for its prevention and treatment.
Conclusions
Lack of definitive, large-scale, double-blind, placebo-controlled clinical trials is the limiting factor for making strong treatment recommend- ations using Mg. Countering that point of view is the good safety of Mg compared to side-effect prone antidepressant and anti-anxiety medications.
You can tell that the authors want to pick some people up and shake them…
From evidence reviewed here and from the 2010 review by Eby and Eby, the relationship between low Mg intake as a risk factor for MD appears to have extremely important preventative and treatment implications. A dietary supplement of 600 to 800 mg/day Mg (other than magnesium oxide) should be a universal prevention strategy.
Mechanisms, which lead to CNS Mg depletion, should be further studied in an effort to discover new targets and medication for MD. Evidence for a Mg regulation effect of psychoptropic drugs already exists, however the exact mechanism is unclear. Reasonable candidates go beyond monoaminergic mechanisms and may include manipulation of the renin-angiotensin- aldosterone system and, less studied, specific Mg transport mechanisms. The best direct measurement of brain Mg is by phosphorus NMR spectroscopy, but this will probably be reserved for research purposes.
Although more research is clearly needed, we suggest that it is past time to give Mg in appropriate doses and to reduce intake of calcium, glutamate and aspartate for the prevention and treatment of MD, especially TRD, and anxiety, since we can expect rapid improvements in patient health and major reductions in patient expenses without side effects. We did not pay attention to those early Mg and brain pioneers Meltzer and Auer in 1905 and other magnesium researchers over the last 100 years and we may pay the price today.
https://www.ncbi.nlm.nih.gov/books/NBK507265/
- A charitable view of magnesium as a treatment for MD
This looks encouraging:
Mind is the leading mental health charity in England and Wales. We work hard to understand the needs and experiences of people with mental health problems.
https://www.mind.org.uk/workplace/influence-and-participation-toolkit/what/what/
They mention some alternatives such as St. John’s Wort (Hypericum perforatum), but not the silver elephant in the room:
If it is allopathic poisons that you want to know about, then you have come to the right place:
- Fibromyalgia
A recent report from Allied Market Research projected that the Global Fibromyalgia Treatment Market size, which was valued at $3.1 billion in 2022, is estimated to reach $4.6 billion by 2032, growing at a compound annual growth rate (CAGR) of 4.0% from 2023 to 2032.
From: “Global Fibromyalgia Treatment Market Expected to Reach $4.6 billion by 2032“ (2022)
I’ve selected five papers that studied or discussed how magnesium compounds might benefit sufferers. I discussed underlying autoimmune factors here.
A literature review from 2021 by Boulis et al provides a great overview:
“Magnesium and Fibromyalgia: A Literature Review”18
Abstract
Fibromyalgia, a widespread chronic pain disorder, imposes a multitude of hardships on patients and their communities. Supplements, specifically magnesium supplements, have been widely used by fibromyalgia patients in an attempt to control their symptoms. The aim of this work is to investigate if the widespread use of magnesium in fibromyalgia is supported by evidence in the literature. This review provides a layout of the studies examining the correlation between body magnesium levels and fibromyalgia. Furthermore, it elaborates on the trials testing the effectiveness of magnesium in treating different clinical parameters of fibromyalgia.
The term fibromyalgia is derived from the Latin “fibra,” meaning fibrous tissues; the Greek “mys,” meaning muscles; and “algia,” meaning pain. It is a complex widespread pain disorder.1 Pain is considered widespread when it involves both sides of the body, above and below the waist, and along the axial skeleton.2 Fibromyalgia is often associated with sleep difficulties, memory impairment, mood disturbance, irritable bowel syndrome, and fatigue.3
“Central sensitization corresponds to an enhancement in the functional status of neurons and circuits in nociceptive pathways throughout the neuraxis caused by increases in membrane excitability, synaptic efficacy, or a reduced inhibition…central sensitization produces pain hypersensitivity by changing the sensory response elicited by normal inputs, including those that usually evoke innocuous sensations.”19
The underlying etiology for fibromyalgia remains obscure.3 While genetic and hormonal factors among others are thought to play a role in fibromyalgia patients, central sensitization is considered to be the main mechanism.10 Magnesium is known to play an important role in the prevention of central sensitization by blocking N-methyl-D-aspartate (NMDA) receptors in a voltage-dependent manner.11 Magnesium deficiency has been largely associated with muscle pain along with fatigue, sleep difficulties, and anxiety; all of which are common symptoms of fibromyalgia.12,13 In some studies, it is thought that magnesium deficiency, through reductions in muscle ATP levels, may play a role in the development of fibromyalgia.14 Other studies correlate increased levels of substance P (a neurotransmitter known for its role in pain perception) with magnesium deficiency as well as pain intensity in fibromyalgia, raising the question of a possible correlation between magnesium deficiency and fibromyalgia.15-17 While some estimates suggest that about half of the population in the United States consume inadequate amounts of magnesium, studies have shown that magnesium is one of the most widely used supplements by fibromyalgia patients.18-23
Some studies showed decreased magnesium levels in several tissue compartments in fibromyalgia patients along with a negative correlation between magnesium levels and the clinical parameters of fibromyalgia.24-27 Bagis el al24 reported significantly lower serum and erythrocyte magnesium levels in patients with fibromyalgia compared to controls, along with a negative correlation between the magnesium levels and fibromyalgia symptoms.
Results between different studies were mixed, with some finding no association. Measuring total magnesium levels is challenging due to its distribution; randomized placebo-controlled trials were not conducted and Mg was rarely administered in isolation. Indeed, synergies are seen with the coadministration of other macronutrients, which complicates the process of demonstrating therapeutic value.
Romano et al13 measured plasma and red blood cell magnesium levels in fibromyalgia patients and compared them with those of osteoarthritis control subjects. They found that fibromyalgia patients, compared to reference laboratory and osteoarthritis controls, had significantly lower red blood cell magnesium levels but no difference in plasma magnesium levels.
In a review, Gaby37 describes his clinical experience with the therapeutic use of intravenous nutrients in fibromyalgia. He gave the modified Myers’ cocktail to about 30 patients with fibromyalgia. The modified Myers’ cocktail consists of magnesium, calcium, B vitamins, and vitamin C. Half of the patients have experienced a significant improvement mostly after 3 or 4 treatments.
In most of the trials that explored the effect of magnesium on fibromyalgia, magnesium was given along with other interventions.14,36,37,39,40 Only a few studies used magnesium as a single intervention.24,35 Nevertheless, none of these few studies was designed as a blinded randomized controlled study. This adds to the uncertainty about the role of magnesium in fibromyalgia.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8371721/
“Effects of transdermal magnesium chloride on quality of life for patients with fibromyalgia: A feasibility study”20 (2015)
…Forty female patients with the diagnosis of fibromyalgia were enrolled. Each participant was provided a spray bottle containing a transdermal magnesium chloride solution and asked to apply 4 sprays per limb twice daily for 4 weeks. Participants were asked to complete the Revised Fibromyalgia Impact Questionnaire, SF-36v2 Health Survey, and a quality-of-life analog scale at baseline, week 2, and week 4.
Main outcome measure
Questionnaire and survey scores, evaluated through intent-to-treat and per-protocol analyses.
Results
Twenty-four patients completed the study (mean [SD] age, 57.2 [7.6] years; white, 95%; mean body mass index, 31.3 kg/m2). With intention-to-treat analysis, Revised Fibromyalgia Impact Questionnaire subscale and total scores were significantly improved at week 2 and week 4 (total score, P = 0.001). Per-protocol analysis results were similar: all subscales of the Revised Fibromyalgia Impact Questionnaire were significantly improved at week 2 and week 4 (total score, P = 0.001).
Conclusion
This pilot study suggests that transdermal magnesium chloride applied on upper and lower limbs may be beneficial to patients with fibromyalgia. Trial registration ClinicalTrials.gov. ldentifier NCT01968772.
“Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia?”21 (2012)
The aims of this study were to investigate the relationship between magnesium levels and fibromyalgia symptoms and to determine the effect of magnesium citrate treatment on these symptoms.
Sixty premenopausal women diagnosed with fibromyalgia according to the ACR criteria and 20 healthy women whose age and weight matched the premenopausal women were evaluated.
Pain intensity, pain threshold, the number of tender points, the tender point index, the fibromyalgia impact questionnaire (FIQ), the Beck depression and Beck anxiety scores and patient symptoms were evaluated in all the women. Serum and erythrocyte magnesium levels were also measured.
Amitriptyline hydrochloride is a tricyclic antidepressant. An analgesic, it is used to treat neuralgia, migraines, insomnia, and depression, but not as a first-line treatment.
The patients were divided into three groups. The magnesium citrate (300 mg/day) was given to the first group (n = 20), amitriptyline (10 mg/day) was given to the second group (n = 20), and magnesium citrate (300 mg/day) + amitriptyline (10 mg/day) treatment was given to the third group (n = 20). All parameters were reevaluated after the 8 weeks of treatment.
The serum and erythrocyte magnesium levels were significantly lower in patients with fibromyalgia than in the controls. Also there was a negative correlation between the magnesium levels and fibromyalgia symptoms. The number of tender points, tender point index, FIQ and Beck depression scores decreased significantly with the magnesium citrate treatment.
The combined amitriptyline + magnesium citrate treatment proved effective on all parameters except numbness. Low magnesium levels in the erythrocyte might be an etiologic factor on fibromyalgia symptoms.
The magnesium citrate treatment was only effective tender points and the intensity of fibromyalgia. However, it was effective on all parameters when used in combination with amitriptyline.
https://pubmed.ncbi.nlm.nih.gov/22271372/
“Magnesium and Pain” (2020)
In terms of antinociceptive action, the main mode of action of magnesium involves its antagonist action at the N-methyl-d-aspartate (NMDA) receptor, which prevents central sensitization and attenuates preexisting pain hypersensitivity. Given the pivotal function of NMDA receptors in pain transduction, magnesium has been investigated in a variety of pain conditions. The oral and parenteral administration of magnesium via the intravenous, intrathecal, or epidural route may alleviate pain and perioperative anesthetic and analgesic requirements.
These beneficial effects of magnesium therapy have also been reported in patients with neuropathic pain, such as malignancy-related neurologic symptoms, diabetic neuropathy, postherpetic neuralgia, and chemotherapy-induced peripheral neuropathy. In addition, magnesium treatment is reportedly able to alleviate fibromyalgia, dysmenorrhea, headaches, and acute migraine attacks.
https://pubmed.ncbi.nlm.nih.gov/32718032/
A comment was raised in 2021 about the lack of randomized double-blind clinical trials, giving the authors some doubt as to the benefits of magnesium itself.
The following year Macian et al helped to firm up the case for using it in standard practice. There were 75 patients at inclusion.
“Short-Term Magnesium Therapy Alleviates Moderate Stress in Patients with Fibromyalgia: A Randomized Double-Blind Clinical Trial”22 (2022)
Patients suffering from fibromyalgia often report stress and pain, with both often refractory to usual drug treatment. Magnesium supplementation seems to improve fibromyalgia symptoms, but the level of evidence is still poor.
This study is a randomized, controlled, double-blind trial in fibromyalgia patients that compared once a day oral magnesium 100 mg (Chronomag®, magnesium chloride technology formula) to placebo, for 1 month.
Only patients scoring over 18 were included:
“The primary outcome was the stress score at D28, assessed by the DASS-42. This questionnaire is a self-report instrument designed to measure the three related negative emotional states of depression, anxiety, and stress, and we only considered the dimension of stress [38] for inclusion. The stress class is scored as follows: 0–14 (normal), 15–18 (mild, (m)), 19–25 (moderate (M)), 26–33 (severe (S)), and 34 and more (extremely severe (S+)). DASS-42 measures were carried out at every visit.”
The primary endpoint was the level of stress on the DASS-42 scale, and secondary endpoints were pain, sleep, quality of life, fatigue, catastrophism, social vulnerability, and magnesium blood concentrations.
After 1 month of treatment, the DASS-42 score decreased in the magnesium and placebo groups but not significantly (21.8 ± 9.6 vs. 21.6 ± 10.8, respectively, p = 0.930).
Magnesium supplementation significantly reduced the mild/moderate stress subgroup (DASS-42 stress score: 22.1 ± 2.8 to 12.3 ± 7.0 in magnesium vs. 21.9 ± 11.9 to 22.9 ± 11.9 in placebo, p = 0.003).
Pain severity diminished significantly (p = 0.029) with magnesium while the other parameters were not significantly different between both groups.
These findings show, for the first time, that magnesium improves mild/moderate stress and reduces the pain experience in fibromyalgia patients. This suggests that daily magnesium could be a useful treatment to improve the burden of disease of fibromyalgia patients and calls for a larger clinical trial.
This dose is quite low:
Chronomag® magnesium technology is an advanced patented low-dose formulation in a specific matrix specifically designed to provide a single daily intake of 100 mg of Mg element (vs. the current recommended dose of 300 mg/day) by providing a unique continuous low-dose Mg release. This continuous ‘low-dose’ Mg release throughout the gastrointestinal tract is in line with the natural physiological absorption process. Thus, this technology improves bioavailability and gastrointestinal tolerance.


This was avoidable by using a different magnesium supplement with high bioavailability and low gut irritability, such as glycinate:
Concerning drug tolerance, 30% of the patients in the Mg group and 31% of the patients in the placebo group reported minor gastrointestinal disorders (abdominal pain, diarrhea, etc.) during the study.
The mechanism of action of Mg in general and on stress in FM is complex [32]. The Mg formula, Chronomag®, was chosen as it is a low dose of Mg chloride, with excellent absorption and bioavailability [48], which means that Mg is readily available to cross the cell membranes [48].
FM patients had baseline Mg serum and erythrocyte concentrations (0.92 ± 0.08/2.86 ± 0.32 mmol/L for Mg group vs. 0.91 ± 0.08/2.97 ± 0.31 mmol/L for placebo group) that were within the reference range (0.66–1.07/2.22–3.51 mmol/L), and they were not hypomagnesemic.
After the intervention, Mg serum concentrations were 0.87 ± 0.07/2.87 ± 0.37 mmol/L for the Mg group vs. 0.87 ± 0.08/2.91 ± 0.37 mmol/L for the placebo group (p = 0.422/0.875). This finding is different from some publications, in which FM patients were reported to have hypomagnesemia and lower erythrocyte Mg concentrations than controls [23,27,49,50].
In practice, an Mg deficiency is difficult to identify because serum levels are often compensated by the release of Mg from the bone reservoir [51]. The literature has also reported nutritional deficits/disorders/adaptations in FM persons with changes in their dietary Mg intake [28,52]. In fact, people may have a chronic negative Mg balance without obvious measured hypomagnesemia [51,53], and may still benefit from Mg supplementation [54].
The authors recognize that the dose may have been “far too low”!
In our study, the dosage of 100 mg per day may have been far too low to compensate an Mg deficit in severely stressed persons while it was adequate in moderately stressed FM persons.
The pain diminution from 5.7 ± 1.3 to 5.1 ± 1.7 on the BPI pain severity scale observed in our study, although statistically significant, appears marginal compared to studies with pharmacological therapies, such as pregabalin [82,83,84], duloxetine [85,86], or amitriptyline [87].
These studies have reported, for example, the following impacts on the WPI [86] or pain scores: using the Visual Analogic Scale, an effect size of 0.38 at week 6; using the BPI pain severity scale, an effect size of 0.36 at week 6 [82] with pregabalin; and a difference of −0.43 with amitriptyline [87].
Allopathic drugs had adverse drug reactions:
Furthermore, these drugs had adverse drug reactions [82], such as dizziness, lightheadedness, and dry mouth, with patient withdrawals, and the treatment duration was much longer.
Agreed 100%, along with currently non-allopathic supplements:
Moreover, in its recommendations [88], the European League Against Rheumatism stresses that first-line treatment of FM should involve exercise and patient education and should focus on non-pharmacological therapies, and not drugs.
In conclusion:
We believe that the alleviation of stress and pain, in moderately impaired persons suffering from FM, achieved from using a non-drug treatment for one month may improve patients’ daily life. These findings represent a good basis for further clinical studies, with a possible demonstration of benefits not only on stress and pain but also fatigue and quality of life, including more patients and with a longer duration of treatment in FM and other complex clinical situations.
https://pubmed.ncbi.nlm.nih.gov/35631229/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9145501/
- NICE approval of Atogepant for migraine
The UK’s NHS, helping pharma again. It’s the gift that keeps on giving.
As you probably guessed, “Uncle Magnesium” is the embarrassing relative they didn’t want to invite to this party:
From “Atogepant: New migraine drug recommended for NHS use in England” (11th April ‘24)
The first oral treatment for preventing both chronic and episodic migraines could soon be available on the NHS.
Health experts said up to 170,000 people in England could benefit from taking atogepant to prevent severe head pain, which can be debilitating.
It has been recommended for those who have not responded well to other medications or cannot have injections.
One migraine charity described it as a positive step and said it hoped access to the drug would be "swift".
The National Institute for Health and Care Excellence (NICE) has recommended the drug, which comes in tablet form, after clinical trials suggested it was effective in some adults.
In its final draft guidance, NICE said atogepant should be offered to people who had unsuccessfully tried three other medications, many of which need to be taken by injection or infusion.
Atogepant is designed to be taken daily to prevent both chronic migraines (occurring more than 15 times a month) and episodic migraines (occurring between four and 15 times a month).
At first, it will only be available from specialist doctors in secondary care settings, rather than from GPs.
Rob Music, chief executive of the Migraine Trust, said it was good news as migraines could be very "debilitating".
"It is positive to see even more therapies emerging for people with migraine, as many still rely on treatments developed for other conditions."
However, the charity warned that many people had struggled to access similar new drugs, because of a lack of knowledge among doctors, and long waiting lists for specialists.
"We now need to ensure access is swift, so that migraine patients can benefit from them as quickly as possible," Mr Music said.
https://www.bbc.co.uk/news/health-68780240
What is this wonder drug?
Atogepant impedes migraine-associated pain by acting as an antagonist to calcitonin gene-related peptide (CGRP).
CGRP is a nerve-related or neuropeptide consisting of 37 amino acids. A CGRP antagonist can impede nociceptive signaling pathways by blocking the receptors to CGRP.23
The pharmacoeconomic review
We shouldn’t need to be “fact-checking” NICE, NHSScotland24 or the NHS, but that’s not the world we inhabit. They don’t deserve any trust, not least due to what happened in the last four years, so every recommendation has to be taken as suspect by default.
In 2023 the Canadian equivalent of NICE published a Pharmacoeconomic Review and came to quite a different conclusion to NICE.
The TL;DR is that there are lots of unknowns:
Head-to-head trials with other anti–calcitonin gene–related peptides (CGRPs) weren’t conducted and one of these was shown to be more effective at just 2% of the price; no one knows if atogepant is equally effective in the long term or what the long-term consequences may be.
Key takes from “Atogepant (Qulipta): CADTH Reimbursement Review: Therapeutic area: Migraine, prevention”25 (August 2023).
Some parts of the review were redacted.
Conclusions
Based on the CADTH clinical review, atogepant may reduce migraine frequency and improve quality of life among patients with episodic migraine (EM) compared to placebo.
There are no direct head-to-head trials comparing atogepant to anti–calcitonin gene–related peptides (CGRPs) (i.e., eptinezumab, fremanezumab, and galcanezumab) or to oral preventive migraine medications.
MMD: monthly migraine days.
The cost-effectiveness of atogepant in the full Health Canada–indicated and reimbursement populations, which are not restricted based on having at least 4 MMDs, is thus unknown.
CADTH was also unable to address uncertainty in the health state utility values, limitations related to the sponsor’s modelling approach (i.e., model structure, subsequent treatments, and transparency), uncertainty in the long term effectiveness of atogepant, and the lack of clinical data for patients with fewer than 4 MMDs.
NMA: network meta-analysis
While the findings of CADTH’s reanalysis were generally aligned with those submitted by the sponsor (i.e., atogepant is not a cost-effective treatment option for EM in adults), the sponsor-submitted NMAs suggests that there may be | || || || || || between atogepant and relevant comparators in terms of reducing MMDs. As such, there is insufficient evidence to suggest that atogepant should be priced higher than other treatments for EM.
Clinician input was received from the Canadian Headache Society. The group noted that currently available treatment options are not effective for all patients, that they may lose effectiveness over time, that some options have significant side effects or contraindications, that currently available oral medications are not disease specific, and that injectables are not ideal for all patients.
Clinicians also noted that, while monoclonal antibodies may be seen as a specialist option, primary care physicians may feel more confident prescribing atogepant, which would be beneficial owing to limited access to migraine specialists in Canada.
The drug plans noted the absence of safety and efficacy information in the pediatric population and an insufficient number of study participants older than 65 years.
The brand name of Atogepant is Qulipta, and it is eye-wateringly expensive at a current price of just under $40 per tablet, or over $1,159.99 for 30 (i.e. per month).26
Atogepant is available as 10 mg, 30 mg, and 60 mg oral tablets, with a recommended dose of 10 mg, 30 mg, or 60 mg once daily. The submitted price of atogepant is $18.44 per tablet, regardless of strength, which corresponds to an annual per-patient cost of $6,735.5
Propranolol is a “cheap-as-chips” beta-blocker. Magnesium doesn’t get a look in:
The annual per-patient cost of fremanezumab (225 mg monthly or 675 mg every 3 months) was $6,429, the annual per-patient cost of eptinezumab was $7,240 for 100 mg every 12 weeks and $21,719 for 300 mg every 12 weeks, and the annual per-patient cost of galcanezumab was $7,213 in the first year and $6,659 each year thereafter. The sponsor’s estimated costs for oral preventive treatments were amitriptyline at $112, propranolol at $149, and topiramate at $167.
The baseline population characteristics used to inform the model were based on the ADVANCE trial, which included patients aged 18 years to 80 years with 4 MMDs to 14 MMDs.4 BSC was represented by the placebo group of the ADVANCE trial. Patients in all groups within the trial were permitted to take acute treatments for migraine attacks including triptans, ergot derivatives, opioids, analgesics, nonsteroidal anti-inflammatory drugs, and antiemetic drugs, but received no preventive treatments with the exception of atogepant for those randomized to receive it.4
The fox is guarding the hen house:
The primary measures of efficacy in the model were the probability of a treatment response at 12 weeks (i.e., at least a 50% reduction in MMDs) and the mean change from baseline in the number of MMDs per 28-day period. The efficacy estimates were derived from the sponsor-conducted NMAs.
CADTH identified several key limitations to the sponsor’s analysis that have notable implications for the economic analysis.
Full Health Canada–indicated population was not modelled: The sponsor submitted 2 base-case analyses — 1 intended to reflect the Health Canada indication (EM patients regardless of the number of previous treatments) and 1 intended to reflect the reimbursement request (EM and ≥ 2 prior therapies).
The clinical evidence used to inform the pharmacoeconomic model for both populations was obtained from the ADVANCE trial, which included patients with 4 MMDs to 14 MMDs.4,7
Given that the Health Canada indication and reimbursement request are not restricted to patients with at least 4 MMDs, the modelled populations do not reflect the full Health Canada–indicated population or the full reimbursement population. As such, the sponsor’s analyses reflect the cost-effectiveness of atogepant in a subset of the indicated population and the requested reimbursement population (i.e., those with 4 MMDs to 14 MMDs).
CADTH was unable to address this limitation, owing to a lack of clinical data. The cost-effectiveness of atogepant in patients with fewer than 4 MMDs is unknown, as is the cost-effectiveness of atogepant for the full Health Canada and reimbursement populations. CADTH reanalyses thus reflect patients with 4 MMDs to 14 MMDs.
Other comparisons were not provided either, including head-to-head evidence comparing other treatments, cost of lost productive days, effectiveness of other treatments at the end of the course (ie the patient doesn’t just stop, take nothing, and have a recurrence).
There is still no long-term efficacy or safety data:
Uncertainty in long-term treatment efficacy of atogepant: In the sponsor’s model, patients who remained on treatment were assumed to maintain the improved frequency of MMDs achieved in the first 12 weeks for the remainder of the time horizon (5 years). This assumption was not explicitly justified. The ADVANCE trial assessed the effectiveness of atogepant over a 12-week treatment period, and it is uncertain whether the short-term results are maintained indefinitely.
As noted in the CADTH clinical review, although 2 long-term extension studies of atogepant have been undertaken (Study 309 and Study 302), neither has reported on the effectiveness of atogepant (i.e., these studies were undertaken to assess the long-term safety and tolerability only).
While the sponsor’s model includes an option to allow MMDs for all patients to increase over time, this does not consider the impact of waning treatment effect relative to BSC or potentially different waning effects among active comparators. As noted in a prior CADTH review, up to 20% of patients may stop benefiting from prophylactic treatment over time.21
CADTH was unable to address this limitation. Should patients receiving atogepant experience a reduction in efficacy over time, the ICERs associated with atogepant relative to BSC would be higher than estimated. The direction and magnitude of the impact of this assumption is unknown when comparing atogepant to anti-CGRPs and oral preventive migraine treatments, to which patients may also experience a reduction in efficacy over time.
Model lacked transparency: The sponsor’s submitted model included numerous IFERROR statements, which lead to situations in which the parameter value is overwritten with an alternative value without alerting the user to the automated overwriting. The systematic use of IFERROR statements makes thorough auditing of the sponsor’s model impractical, as it remains unclear whether the model is running inappropriately by overriding errors.
CADTH was unable to address this limitation and notes that a thorough validation of the sponsor’s model was not possible.
It’s so expensive that the price would have to fall substantially to make it worthwhile. And that’s without considering magnesium:
Among patients with EM (4 MMDs to 14 MMDs) and 2 or more prior therapies, a 61% price reduction would be required for atogepant 60 mg to be considered cost-effective compared to BSC at a WTP threshold of $50,000 per QALY gained.
It wasn’t even worth their while to do a cost-benefit analysis:
No formal price reduction analyses were undertaken for the Health Canada–indicated population, as all doses of atogepant were less effective than propranolol, and at least a 98% price reduction would be required for the drug cost of atogepant to be equivalent to that of propranolol.
A bombshell of a conclusion. I guess NICE or NHSScotland didn’t make these comparisons, and they will be crying out for more taxpayer money soon:
The findings of CADTH’s reanalysis were generally aligned with those submitted by the sponsor: atogepant was not a cost-effective treatment option for EM in adults.
In the Health Canada–indicated population, atogepant (all doses) was dominated by propranolol (i.e., atogepant was less effective and more costly than propranolol) and in the reimbursement population, atogepant was dominated (atogepant 10 mg and atogepant 30 mg) by fremanezumab or extendedly dominated (atogepant 60 mg) by fremanezumab and BSC, such that it would not be the optimal treatment choice. In both populations, a price reduction would be required for atogepant to be considered cost-effective relative to currently available treatments.
What about side effects?
More common side effects include27:
Constipation.
Nausea.
Sleepiness or unusual drowsiness.
Unusual tiredness or weakness.
Less common:
Decreased appetite.
Decreased weight.
Incidence not known:
Chest tightness.
Cough.
Difficulty swallowing.
Dizziness.
Fast heartbeat.
Hives, itching, skin rash.
Puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue.
Trouble breathing.
We also have cases of optic neuritis (ON), a type of neuropathy (inflammation-induced nerve disease) that can cause eye pain and loss of vision. This sounds like an autoimmune response. That, and liver damage markers.
“Don’t go there” would be my advice.
Key takes from “Novel Oral CGRP Receptor Antagonist Atogepant in the Prevention of Migraine“28 (2023):
Atogepant's safety has been demonstrated in clinical trials, with the majority of TRAEs being minimal to moderate in severity.
AE’s increased with dose. Of course, they were never related to the experimental drug:
The rate of TRAEs was higher with atogepant than with placebo, and it was related to dosage escalation. The serious adverse events (SAE) reported by Goadsby et al. included urethritis, cholecystitis, migraine, and significant depression; however, none of these were found to be related to atogepant10.
An acute asthmatic attack, post-surgical laryngospasm, and optic neuritis were among the SAEs reported by Ailani et al13.
Optic neuritis, a significant TRAE, was observed in the atogepant 10 mg group. The study found optic neuritis as an SAE with 10 mg of atogepant, which began on day 23 after randomization and resulted in reduced visual acuity. The SAE was found to be associated with atogepant. It resolved on its own, leaving no lasting vision impairment.
Markers for liver damage? It’s all OK, nobody died.
At least not yet…
Although the studies reported ≥ 3 times elevation in ALT/AST levels in some participants, no patient reported having a drug-induced liver injury by either study. Apart from the studies listed in the review, atogepant is currently being evaluated for long-term safety and tolerability.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10411686/
What does high AST and ALT mean?
Abnormal liver function test with raised alanine aminotransferase (ALT) and raised aspartate aminotransferase (AST) are commonly seen in primary care setting. Chronic alcohol consumption, drugs, non-alcoholic steatohepatitis (NASH) and chronic viral hepatitis are common causes associated with raised ALT and AST.
…Persistent mild elevation of ALT and AST in asymptomatic persons should be followed up and if the levels go above 2 times the normal range, further evaluation is necessary. After excluding drug-induced hepatitis or hepatic injury, recent hepatobiliary surgery and obesity, further investigations should be performed to look for underlying cause such as alcohol abuse, chronic phase of hepatitis B and hepatitis C infection, and undiagnosed liver cirrhosis.
From: “Elevated Alt and Ast in an Asymptomatic Person: What the primary care doctor should do?“ (2009)
What about Magnesium?
Atogepant blocks the CGRP receptors. Calcitonin is a hormone that is secreted by the C-cells of the thyroid gland. It opposes the actions of the parathyroid hormone and helps to regulate the calcium levels in your blood. Both CGRP and calcitonin are in the calcitonin family of peptides.
We touched on the effects of magnesium on inflammatory proteins in part 2, including CGRP, vasoactive peptide, and Substance P.
Magnesium works differently to Atogepant. Instead of blocking the receptors it significantly reduces the levels of both circulating CGRP and calcitonin.
They knew of the calcitonin proteins and effects of propranolol at least 50 years ago:29
Propranolol, a beta-adrenergic blocking agent, lowers serum calcium and enhances the hypocalcaemic action of calcitonin in young rats.
This effect is not due to an inhibition of parathyroid hormone, since propranolol also produced a fall in serum calcium in thyroparathyroidectomized animals.
No changes could be found in serum phosphorus following propranolol administration, which argues against a calcitonin-like mechanism.
Propranolol also lowered the serum calcium level in nephrectomized rats, which rules out the kidney as a primary mediator of the hypocalcaemic action.
The changes in serum calcium induced by propranolol are most likely due to its membrane stabilizing properties.
From: “Hypocalcaemic Effect of Propranolol in Rats“ (1975)
https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0028-1093773
CGRP is just one of the migraine pain-related pathways that magnesium beneficially affects:

Key takes from “Magnesium sulphate infusion decreases circulating calcitonin gene-related peptide (CGRP) in women with primary Raynaud's phenomenon“30 (1994, paywalled).
Raynaud's phenomenon is a disorder that causes decreased blood flow to the fingers and toes; occasionally to blood vessels in the ears, toes, nipples, knees, or nose.31
Summary. The effects of two different vasodilating agents (MgSO4 infusion and the calcium antagonist nifedipine) on circulating levels of calcitonin gene-related peptide (CGRP) were studied in 12 women with pronounced primary Raynaud's phenomenon (PRP) and in 12 healthy females. There were no significant differences with regard to basal levels of circulating CGRP between women with PRP and the control group; median 15–5 (range 10–48) vs. 14 (range 10–69) pmol 1-1, respectively.
However, treatment with MgSO4 infusion significantly decreased circulating CGRP in women with PRP only from median 15–5 (range 10–48) to 10 (range 10–110) pmol l-1) (P<0–05). On the other hand 14 days of treatment with nifedipine did not affect circulating CGRP in either of the investigated groups.
Erythrocyte magnesium (ery-Mg) levels increased significantly after MgSO4 infusion in women with PRP (2–43± 0–13 vs. 2–52 ± 0–15mmol 1-1, P<0–05) but not in the controls (2–51 ± 0–24 vs. 2–57 ± 0–28 mmol l-1, ns).
In conclusion, the decrease of circulating CGRP after MgSO4 infusion in women with PRP provides further evidence that magnesium plays a significant role in the pathophysiology of PRP.
https://onlinelibrary.wiley.com/doi/10.1111/j.1475-097X.1994.tb00412.x
If you are a migraine sufferer, I also came across multiple studies showing that an extract of chili peppers called capsaicin also significantly reduces CGRP - not only in the brain but in the heart, indicating cardiovascular benefits too.
All patients treated with capsaicin reported an improvement in their migraine, which was judged to be between 50 and 80%. One patient treated with placebo reported a 20% improvement. Statistical evaluation (χ2, P<0.01) showed that capsaicin was significantly efficacious compared with placebo.
From: “Repeated intranasal capsaicin applications to treat chronic migraine” (2003)
https://www.bjanaesthesia.org/article/S0007-0912(17)37540-2/fulltext
There is a close relationship between capsaicin, CGRP, and Substance P.
CGRP-IR: CGRP-immunoreactive.
Substance P-LI: Substance P-like.
…The distribution of CGRP-IR nerves in the dorsal horn of the spinal cord, of positive cell bodies in thoracic spinal and nodose ganglia and nerves in peripheral organs was closely related to that of substance P-LI. Double staining experiments revealed that in most cases peripheral CGRP-IR nerve terminals also contained SP-LI.
...HPLC analysis revealed that the major peak of CGRP-LI in the heart of rat and man had the same retention times as the synthetic equivalents. Systemic capsaicin pretreatment and adult guinea-pigs caused a loss of CGRP-IR terminals in the dorsal horn of the spinal cord as well as in peripheral organs including the heart. After capsaicin treatment, the content of CGRP-IR was reduced by 70% in the heart and by 60% in the dorsal part of the spinal cord.
From: “Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin“ (1987)
If you want to know how “effective” a new drug is you only need to look at who is on the take:
Patient groups are essential to understanding the value of medicines, but NICE must improve its disclosure practices for conflicts of interest to ensure credibility and impartiality, write Iva Parvanova, Arianna Gentilini, and colleagues.
…An investigation by The Guardian reported on the financial connections between Novo Nordisk and UK based patient and medical experts who supported the funding of the company’s new obesity drug, semaglutide (Wegovy).6 Payments from the manufacturer were declared in accordance with NICE’s conflict of interest (COI) policies; however, these may be insufficient to prevent industry sponsorship bias in patient contributions.6 A separate investigation regarding serious breaches of the Association of British Pharmaceutical Industry code of conduct in connection with a weight management programme culminated in Novo Nordisk’s suspension from the association for two years.7
From: “Safeguarding NICE from patient groups’ conflicts of interest“ (2023)
Conclusion
Addressing magnesium deficiencies may decrease the likelihood of developing many conditions, and improve the prognosis if you are a sufferer. Supplementation can often reduce the need for other allopathic medications, sometimes entirely. These are frequently more expensive, rationed by price, less effective, and have a worse side effects profile than magnesium compounds.
In Part 4, which will conclude this mini-series, I will discuss endocrine effects (hormones), dietary sources, the magnesium content of different foods, supplements, and bioavailability.
And finally…
I am privileged to have been invited to co-write with an excellent team, and I will post more about this in due course. None of this would have been possible without your ongoing support - I thought my research paper writing days had ended long ago.
I have no plans to change this Substack, other than for the better, with new material.
Disclaimer
This site is strictly an information website reviewing research into potential therapeutic agents. It does not advertise anything, or provide medical advice, diagnosis, or treatment. This site does not promote any of these as potential treatments or offer any claims for efficacy. Its content is aimed at researchers, registered medical practitioners, nurses, or pharmacists. This content is not a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. Always consult a qualified health provider before introducing or stopping any medications as any possible drug interactions or effects will need to be considered.
Any extracts quoted in the previous article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
References
Botticello C. Substack Word Limit. Substack Course. Published December 8, 2023. Accessed April 12, 2024. https://substackcourse.com/substack-word-limit/
Kuang X, Chiou J, Lo K, Wen C. Magnesium in joint health and osteoarthritis. Nutr Res. 2021;90:24-35. doi:10.1016/j.nutres.2021.03.002
Nahian A, Sapra A. Histology, Chondrocytes. In: StatPearls. StatPearls Publishing; 2024. Accessed March 20, 2024. http://www.ncbi.nlm.nih.gov/books/NBK557576/
Hu C, Zhu F, Liu L, Zhang M, Chen G. Relationship between dietary magnesium intake and rheumatoid arthritis in US women: a cross-sectional study. BMJ Open. 2020;10(11):e039640. doi:10.1136/bmjopen-2020-039640
Why Won’t the CDC or FDA Reveal the VAERS Vaccine Underreporting Factor. Global Research. Published November 4, 2021. Accessed April 10, 2024. https://www.globalresearch.ca/why-wont-cdc-fda-reveal-vaers-vaccine-underreporting-factor/5760672
Kidney failure patients more likely to die from heart attacks and strokes, British Heart Foundation finds. Accessed March 19, 2024. https://www.msn.com/en-gb/health/other/kidney-failure-patients-more-likely-to-die-from-heart-attacks-and-strokes-british-heart-foundation-finds/ar-BB1ja17N
Satish R, Gokulnath G. Serum magnesium in recovering acute renal failure. Indian J Nephrol. 2008;18(3):101-104. doi:10.4103/0971-4065.43688
Wang K, Wei H, Zhang W, et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci Rep. 2018;8(1):9904. doi:10.1038/s41598-018-28362-5
Cinar V. The effects of magnesium supplementation on thyroid hormones of sedentars and Tae-Kwon-Do sportsperson at resting and exhaustion. Neuro Endocrinol Lett. 2007;28(5):708-712.
CDC. Systemic lupuserythematosus (SLE). Centers for Disease Control and Prevention. Published January 31, 2023. Accessed April 10, 2024. https://www.cdc.gov/lupus/facts/detailed.html
Zhang Q, Ji L, Zheng H, et al. Low serum phosphate and magnesium levels are associated with peripheral neuropathy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;146:1-7. doi:10.1016/j.diabres.2018.09.015
Hoane MR. The role of magnesium therapy in learning and memory. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011. Accessed March 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK507270/
Ozawa M, Ninomiya T, Ohara T, et al. Self-Reported Dietary Intake of Potassium, Calcium, and Magnesium and Risk of Dementia in the Japanese: The Hisayama Study. Journal of the American Geriatrics Society. 2012;60(8):1515-1520. doi:10.1111/j.1532-5415.2012.04061.x
Liu G, Weinger JG, Lu ZL, Xue F, Sadeghpour S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J Alzheimers Dis. 49(4):971-990. doi:10.3233/JAD-150538
Frost J. Cohens D: Definition, Using & Examples. Statistics By Jim. Published June 27, 2022. Accessed March 26, 2024. https://statisticsbyjim.com/basics/cohens-d/
Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J Res Med Sci. 2012;17(12):1161-1169.v
Eby GA, Eby KL, Murk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011. Accessed March 22, 2024. http://www.ncbi.nlm.nih.gov/books/NBK507265/
Boulis M, Boulis M, Clauw D. Magnesium and Fibromyalgia: A Literature Review. J Prim Care Community Health. 2021;12:21501327211038433. doi:10.1177/21501327211038433
Latremoliere A, Woolf CJ. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain. 2009;10(9):895-926. doi:10.1016/j.jpain.2009.06.012
Engen DJ, McAllister SJ, Whipple MO, et al. Effects of transdermal magnesium chloride on quality of life for patients with fibromyalgia: A feasibility study. Journal of Integrative Medicine. 2015;13(5):306-313. doi:10.1016/S2095-4964(15)60195-9
Bagis S, Karabiber M, As I, Tamer L, Erdogan C, Atalay A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol Int. 2013;33(1):167-172. doi:10.1007/s00296-011-2334-8
Macian N, Dualé C, Voute M, et al. Short-Term Magnesium Therapy Alleviates Moderate Stress in Patients with Fibromyalgia: A Randomized Double-Blind Clinical Trial. Nutrients. 2022;14(10):2088. doi:10.3390/nu14102088
Chowdhury S, Dave T. Novel Oral CGRP Receptor Antagonist Atogepant in the Prevention of Migraine. Discoveries (Craiova). 2023;11(2):e167. doi:10.15190/d.2023.6
atogepant (Aquipta). Scottish Medicines Consortium. Accessed April 12, 2024. https://www.scottishmedicines.org.uk/medicines-advice/atogepant-aquipta-abbreviated-smc2599/
Qulipta Prices, Coupons, Copay & Patient Assistance. Drugs.com. Accessed April 11, 2024. https://www.drugs.com/price-guide/qulipta
Pharmacoeconomic Review. In: Atogepant (Qulipta): CADTH Reimbursement Review: Therapeutic Area: Migraine, Prevention [Internet]. Canadian Agency for Drugs and Technologies in Health; 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK596767/
Atogepant (Oral Route) Side Effects - Mayo Clinic. Accessed April 13, 2024. https://www.mayoclinic.org/drugs-supplements/atogepant-oral-route/side-effects/drg-20523241
Chowdhury S, Dave T. Novel Oral CGRP Receptor Antagonist Atogepant in the Prevention of Migraine. Discoveries (Craiova). 2023;11(2):e167. doi:10.15190/d.2023.6
Anast C, David L, Winnacker J, et al. Serum calcitonin-lowering effect of magnesium in patients with medullary carcinoma of the thyroid. J Clin Invest. 1975;56(6):1615-1621. doi:10.1172/JCI108244
Myrdal U, Leppert J, Edvinsson L, et al. Magnesium sulphate infusion decreases circulating calcitonin gene-related peptide (CGRP) in women with primary Raynaud’s phenomenon. Clinical Physiology. 1994;14(5):539-546. doi:10.1111/j.1475-097X.1994.tb00412.x
Raynaud’s Phenomenon. Published August 8, 2021. Accessed April 11, 2024. https://www.hopkinsmedicine.org/health/conditions-and-diseases/raynauds-phenomenon


















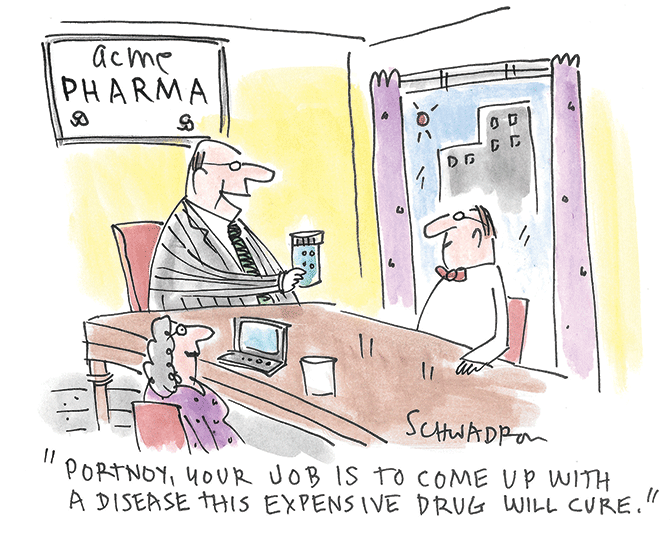
Well... I've sent this to my endocrinologist!!
She sees me for acromegaly, however I thought the sections on hypothyroid and peripheral neuropathy alone worth her time and attention!!
Another banner, carp!!
I do get particularly enthused when a simple, natural, adjustment to one's diet or lifestyle can be the salve of so much misery!!
Thank you. I need to present this all to my doc, who is about as good as they get.
I have a question for the science folks (I am a cellular-mag deficient 'client'). Hypomagnesemia is the word for low serum mag. What is the word...IS there a word?..for low cellular mag?
I need to make sure my understanding is as thorough as it can be.
Some of these studies make me wonder. "...obesity caused by high fat... diet..." I thought that one had been laid to rest.
I wonder about the aging/OA caused by mag deficiency. I had both hips replaced, due to OA, they tell me, at 45. I, now at 60, have no gray hair, and little in the way of wrinkles. As they were replacing my hips, the doctor noted that I had no sign of osteoporosis, despite very little physical activity (recall the 2 rotten hips), for the previous 1.5 years.
Anyway, I will be getting my rbc mag tested again soon. Always interesting
Am I just weird?