M1U, a FOIA request, multiple COIs, why saRNAs are DOA, and a big GFY from the FCC...
"May the Farce be with you"
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Background
I was writing a Substack about heat shock proteins, to appear next as a spin-off in the important long-running IgG4-RA pathology series. But that will have to wait as life intervenes.
I learned yesterday that our work on a paper called “Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?” had been the subject of a Freedom of Information Act request, but I had no further details.
Intrigued, I did some “fact-checking” of my own, and quickly found that it concerned email communications between a pharma-funded “fact-checker” called Newsguard and a super senior researcher at pharma-funded Imperial College.
I won’t name names here and will redact the individual’s name to spare his blushes, just as mine was blanked out. I don’t need to do this though, as everything in this Substack is from the public domain, which is the whole point of a FOIA request.
He is a respected and outspoken supporter of vaccines. He had the foresight that only very few leaders in the field possess, such as Ralf Baric: “New variant human coronaviruses and antivirals: how Ralph Baric of UNC was ahead of his time”
We were being fact-checked by none other than a WEF apparatchik. Stand by your beds and salute:

This Substack is a follow-up to “Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?” - Further reading (Part I)”, and I recommend reading that for context.
In short, our paper appeared to send big pharma into a spin, as it wasn’t the approved narrative. Ironically, the team at Imperial also discuss the value of using N1-methyl-pseudouridine (m1Ψ) in vaccines, as it suppresses immune responses, just as we said along with many others, including the “mother of m1u” - Dr. Katalin Karikó:
… Modified nucleosides can also dampen activation of various dsRNA sensors, including TLR3, retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), protein kinase R (PKR), and 2’-5’-oligoadenylate synthetase (OAS) (Anderson et al., 2010, 2011; Karikó et al., 2005; Mu et al., 2018).
… Recognition of uridine-containing RNA is associated with increased expression of pro-inflammatory cytokines, particularly type I IFN (Karikó et al., 2005, 2011), which promotes the expression of RNA sensors even further and can lead to inhibition of antigen expression from the mRNA via the activities of PKR and OAS.
From “Innate immune mechanisms of mRNA vaccines” (2022)
The problem with suppressing innate immune responses, pattern recognition receptors and interferon signalling pathways is that this is not a vaccinal mRNA-specific attenuation. It inhibits all such signalling from the cell for as long as the mRNA persists, regardless of cell type (e.g. tumour cells, stem cells, macrophages, dendritic cells). We know this from proteome studies of transfected cells.
And we know from multiple studies that this can be for months, if not years, due to integration effects:
Discussion
The FOIA emails
Names redacted in black, out of professional courtesy.
White-out was used on the originals, by others.
And yes, Newsguard tried to discredit me.
I’m happy to debate my findings and let the conclusions of the peer review panels and the thoroughness of my work speak for itself:
“I would expect Elsevier to either with draw [sic] the review or have it corrected to remove the factually incorrect statements.“
Since when was he on the peer review panel?
What “factually incorrect statements”?
He never presented examples of the “misinformation” so that we could discuss the pathology research supporting it. This usually amounts to just pulling up reference X or Y, or perhaps newly published paper Z.
He also never contacted any of the other authors for clarification on these alleged points, as far as I’m aware.
Note that they tried the “ethics” route here, presumably because there was, of course, no evidence of fraud or plagiarism. I think this is disgusting, given the cancer case stats that keep getting worse. And more and more oncologists are speaking out.
Where is “Newsguard” now? If it is not caused by the DeathVax™ or the old chestnut of “missed appointments” then what is causing this?
We have a cancer emergency underway
Elevated:
8.9% Mortality (Novel 2.8% CAGR)
19.5% Treatment Expenditures (Oct 2024)
31% Ages 0-54 Mortality
15-21% Surgical & Medical Equip Mfg Beware of PhD's paid by pharma to lie to you and smear dissent... These were crimes, and they should face prison time.
As for it being a “false narrative”, what does that even mean? Is it an alleged ethics violation, or false, as in alleged academic fraud?
What we have here is a lot of panicky arm-waving, and a cry out to someone to come sort this for them and make it go away by strong-arming the nice ethics team at the publishers.
This is where pharma-funded “science” is now: use bully-boy tactics to get papers you don’t like taken down, instead of conducting further clinical trials or encouraging a dialogue between parties. And I did defend our paper to a different Professor.
Note that we were quite explicit in that we didn’t say the technology can “cause cancer” (i.e. initiate cancer) vs multiple experimental sources demonstrating promotion. Not the same thing:
… Considering the body of published research works, we suggest that until it is demonstrated that mRNA vaccines do not promote the development of cancer, clinical trials using 100 % modified mRNA vaccines with m1Ψ should not be carried out. We propose a moratorium on use with human subjects due to the precautionary principle that the potential benefit of modified mRNA vaccines achieving desired immunogenic humoral response might not outweigh the benefit of inducing innate immune responses seen with non-modified mRNA vaccines.
From: “Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer?” (2024)
On the last point, whilst making my own enquiries about the author, I discovered that their team is working hard to develop self-amplifying (saRNA) vaccines.
Multiple conflicts of interest
In my opinion, the last point, and the large sums received for vaccine development using RNA technology, amount to multiple conflicts of interest, and all such conflicted members of the team at Imperial should have recused themselves from acting as a consultant to Newsguard.
This applies not least to any patent holders of related technology.

I cannot say how much they have actually received to date, but either way a potential $195 million is no small beer. From 23rd September 2021, we are talking serious amounts of lolly here:
Development work by Imperial on self-amplifying RNA technology
I was intrigued to see they were developing this, with combined phase I and II clinical trials:
Imperial vaccine tech to target COVID mutations and booster doses
by Andrew Scheuber
26 January 2021
Imperial is focusing its RNA vaccine technology to target SARS-CoV-2 mutations, boosters and thermostability rather than an immediate efficacy trial.
As other COVID-19 vaccines are being licensed and rolled out, Professor XXXXXX’s team is working to maximise the impact of their groundbreaking self-amplifying RNA technology.
This includes developing the ability to respond rapidly if vaccine-resistant strains emerge, or the need for booster vaccines arises.
It also offers the possibility of more thermostable RNA vaccines that can be stored in ordinary refrigerators.
The saRNA technology’s development accelerated during the COVID-19 pandemic, with a combined phase I and II clinical trial involving over 400 participants. The results of these studies are still pending and will inform the next steps of development.
I was intrigued to see this bombshell:
Professor Fiona Watt, Executive Chair of the Medical Research Council, which helped to fund the research, said: “The Imperial team has worked incredibly hard over the past year to develop a new self-amplifying RNA vaccine for COVID-19, which is currently in early-stage human trials. Although the outcome of those trials is unknown, the decision has been taken not to progress to Phase III trials in the UK. This is because several COVID-19 vaccines have already been approved. Rather than conducting immediate further clinical trials, the Imperial team can now focus their research on how their exciting new technology can be used to develop new or improved vaccines.”
https://www.imperial.ac.uk/news/213313/imperial-vaccine-tech-target-covid-mutations/
In my experience, you would never invest such sums in the research and then just pull the rug “because several COVID-19 vaccines have already been approved”.
Now, what was the real reason they pulled the pin? Normally it’s a failure to meet its nominated endpoints for efficacy, safety, or both. And it has to be pretty bad, as the regulators will pass anything put in front of them, due to regulatory capture.
Mode of action
I won’t go into great detail here, as that could either:
Fill several Substacks.
Be very lightweight, because the research hasn’t been done, or the findings have not been released.
But the underlying principle is that the lipid nanoparticle contains both the machinery to tell the ribosomes to make lots of the desired immunogenic protein, and the framework to amplify the numbers of these mRNA replicates in the cell.
Dr Rose has recently been writing about this:
Amplification
mRNA vaccines use either non-amplifying (conventional) mRNA or self-amplifying mRNA.[101] Pfizer–BioNTech and Moderna vaccines use non-amplifying mRNA. Both mRNA types continue to be investigated as vaccine methods against other potential pathogens and cancer.[32]
Non-amplifying
The initial mRNA vaccines use a non-amplifying mRNA construct.[69] Non-amplifying mRNA has only one open reading frame that codes for the antigen of interest.[101] The total amount of mRNA available to the cell is equal to the amount delivered by the vaccine. Dosage strength is limited by the amount of mRNA that can be delivered by the vaccine.[102] Non-amplifying vaccines replace uridine with N1-Methylpseudouridine in an attempt to reduce toxicity.[103]
Self-amplifying
Self-amplifying mRNA (saRNA) vaccines replicate their mRNA after transfection.[104] Self-amplifying mRNA has two open reading frames. The first frame, like conventional mRNA, codes for the antigen of interest. The second frame codes for an RNA-dependent RNA polymerase (and its helper proteins) which replicates the mRNA construct in the cell. This allows smaller vaccine doses.[104] The mechanisms and consequently the evaluation of self-amplifying mRNA may be different, as self-amplifying mRNA is a much bigger molecule.[3]
SaRNA vaccines being researched include a malaria vaccine.[105] Gritstone bio started in 2021 a phase 1 trial of an saRNA COVID-19 vaccine, used as a booster vaccine. The vaccine is designed to target both the spike protein of the SARS‑CoV‑2 virus, and viral proteins that may be less prone to genetic variation, to provide greater protection against SARS‑CoV‑2 variants.[106][107] saRNA vaccines must use uridine, which is required for reproduction to occur.[103]
From: “mRNA vaccine“
The last point about needing to use uridine, instead of m1u, gives us a clue as to why these may fail.
Without N1-methyl-pseudouridine to suppress the immune system it suffers from all the drawbacks of both more rapid destruction of the saRNA components themselves, and the risk of a dangerously hyper-stimulated immune response.
This is doubly dangerous as there is no automatic off-switch or antidote to reverse the injection. You can’t change your mind if you get a bad reaction.
The Imperial phase II/III trial
It didn’t take long to find the paper of interest.
Key takes from “Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial”1 (2022) by Pollock et al.
Summary
Background
Lipid nanoparticle (LNP) encapsulated self-amplifying RNA (saRNA) is a novel technology formulated as a low dose vaccine against COVID-19.
Methods
A phase I first-in-human dose-ranging trial of a saRNA COVID-19 vaccine candidate LNP-nCoVsaRNA, was conducted at Imperial Clinical Research Facility, and participating centres in London, UK, between 19th June to 28th October 2020. Participants received two intramuscular (IM) injections of LNP-nCoVsaRNA at six different dose levels, 0.1-10.0μg, given four weeks apart. An open-label dose escalation was followed by a dose evaluation. Solicited adverse events (AEs) were collected for one week from enrolment, with follow-up at regular intervals (1-8 weeks). The binding and neutralisation capacity of anti-SARS-CoV-2 antibody raised in participant sera was measured by means of an anti-Spike (S) IgG ELISA, immunoblot, SARS-CoV-2 pseudoneutralisation and wild type neutralisation assays. (The trial is registered: ISRCTN17072692, EudraCT 2020-001646-20).
Findings
192 healthy individuals with no history or serological evidence of COVID-19, aged 18-45 years were enrolled. The vaccine was well tolerated with no serious adverse events related to vaccination. Seroconversion at week six whether measured by ELISA or immunoblot was related to dose (both p<0.001), ranging from 8% (3/39; 0.1μg) to 61% (14/23; 10.0μg) in ELISA and 46% (18/39; 0.3μg) to 87% (20/23; 5.0μg and 10.0μg) in a post-hoc immunoblot assay. Geometric mean (GM) anti-S IgG concentrations ranged from 74 (95% CI, 45-119) at 0.1μg to 1023 (468-2236) ng/mL at 5.0μg (p<0.001) and was not higher at 10.0μg. Neutralisation of SARS-CoV-2 by participant sera was measurable in 15% (6/39; 0.1μg) to 48% (11/23; 5.0μg) depending on dose level received.
Interpretation
Encapsulated saRNA is safe for clinical development, is immunogenic at low dose levels but failed to induce 100% seroconversion. Modifications to optimise humoral responses are required to realise its potential as an effective vaccine against SARS-CoV-2.
Funding
This study was co-funded by grants and gifts from the Medical Research Council UKRI (MC_PC_19076), and the National Institute Health Research/Vaccine Task Force, Partners of Citadel and Citadel Securities, Sir Joseph Hotung Charitable Settlement, Jon Moulton Charity Trust, Pierre Andurand, Restore the Earth.
So what went wrong? Several things.
A grade 3 adverse event is a severe, medically significant side effect that, although not immediately life-threatening, means that you require hospitalisation or an extension to an existing stay. It can really spoil your day, as it may be disabling and limit your ability to perform your usual day-to-day activities.
For context, Pfizer’s BNT162b2 uses 30μg doses, and Moderna’s Spikevax uses 100μg:
Reactogenicity
The proportion of participants reporting a systemic reaction increased significantly with dose reaching 100% of those receiving 5.0 or 10.0μg (p=0.0001).
Severity also increased with dose and four (17%) of 24 participants who received 10.0μg reported a grade 3 (severe) systemic reaction after the first or second vaccine (see Appendix 2, Table 5.4).
Common reactions reported were fatigue (108/192; 56%), headache (99; 52%), myalgia (68; 35%), arthralgia (46; 24%), chills (45; 23%) and nausea (34; 18%) (Figure 2B).
I find it extremely disturbing that any participant had a fever in response to such as low dose.
But then again, what is the measured final dose, including all the amplified copies - the area under the curve? I couldn’t find an answer to that in this study, and it would be very challenging to measure accurately over time (eg radioactive markers, antibody counts):
Only one (1%) of 144 participants in dose groups 0.1-2.5μg had a fever (≥38°C), whereas eight (17%) of 48 in the two highest dose groups recorded a fever, and in three participants in the two highest dose groups this was 39.0-40.0°C (grade 3).
AE’s of this severity and magnitude would likely contribute to the decision not to proceed with the Imperial clinical trials:
No-one reported a severe local reaction, and 53/192 (28%) reported no local reaction at all, but the frequency also increased significantly with dose (p<0.0001) and 47 (98%) of 48 participants receiving 5.0 or 10.0μg reported a local reaction with one in three grading this as moderately severe.
Erythema is the medical term best likened to painful swelling associated with a bee sting. Eugh:
Overall, tenderness/discomfort and pain were reported by 133/192 (69%) and 73 (38%) respectively; erythema (5; 3%) and swelling (2; 1%) were uncommon (Figure 2A).
And you may get this every time you get boosted…
Solicited reactions following the first and second vaccine were similar overall with no significant differences in maximum grade of systemic or local reactions (p=0.06 Table 5.7 and p=0.52 Table 5.9, Appendix 2 respectively).
With hindsight (and foresight), the decision to combine phase I and II trials caused some headaches:
This was also the case when the test for marginal homogeneity was applied to each unique reaction other than headache, where higher grades of headache were more frequently observed following the second dose compared to the first (p=0.01, Appendix 2, Table 5.8).
Its OK bro, you probably only have a week or so of this. Probably.
Seven days after each vaccination, laboratory safety parameters remained largely within normal limits (Appendix 2 p15-16, Figures 3-13).
I found the same kind of AEs in this assessment of Pfizers saRNA candidate called BNT162c2. It had been redacted, but I found a copy that had been missed.
Note the low levels of doses used, and that 2 of the 12 young, healthy volunteers had “severe local reactions”:
In the Imperial study, immunogenicity (seroconversion) targets were not met. This may have been due to a combination of factors, including:
Lack of N1-methyl-pseudouridine modifications. Nucleotidases and nucleases cause the rapid degradation of the saRNA framework or mRNA copies, if PRR-associated signalling is not attenuated.
Premature apoptosis of cells containing the saRNA machinery.
Immune exhaustion, such as the lymphopenia seen with the Pfizer gene agents.
Antiinflammatory interleukin damping mechanisms. IL-10 is frequently upregulated in a feedback cycle. IL-4 or IL-21 would indicate the risk of inducing IgG4-related tolerance. I cannot find any reference to these in the paper.
They measured anti-Spike antibody levels, and the highest dose of 10.0μg was less effective than the 5μg dose.
Again, it’s the edge cases who responded well to the lowest doses who would be at risk of a hyper-immune response, autoimmune disorders, myocarditis, aneurysm etc. You may not know this until your product is approved commercially and the participant count and cohorts greatly increased:
Low rates of seroconversion to anti-S IgG were observed by ELISA in participants who received 0.1 or 0.3μg, only 3/39 (8%) and 10/39 (26%) at week six (two weeks after the second vaccination), respectively (Table 2).
The highest seroconversion rates occurred in the 10.0μg group, 8/23 (35%) four weeks after the first vaccination, increasing to 14/23 (61%) two weeks after the second vaccination. Intermediate rates were observed in the 1.0, 2.5 and 5.0μg groups.
Among those who seroconverted, anti-S IgG titres appeared to be higher at six weeks in the 5.0μg group (GM=1023ng/mL) and 10.0μg group (GM=500ng/mL) compared with the lower dose groups. Most individual antibody concentrations ranged from approximately 100ng/mL to 2000ng/mL, broadly consistent with values derived from convalescent sera (Figure 3A).
5 of the 32 participants who responded to the prime dose had no response to the booster. Immune responses of the participants were unpredictable and all over the shop.
If you cannot dose accurately and appropriately then every shot is a Russian Roulette spin of the barrel. Wholly unsuitable for commercial release, and I suspect that by its very nature this will plague all such attempts to force this stuff on us:
Interestingly there were different patterns of response in the binding antibody (ELISA) according to time after the first and second injections. Thirty-two of 190 (17%) participants responded to a single prime dose and this included participants in every dose group from 0.1-10.0µg (Figure 4A).
Among the 32 participants who did respond to the prime dose, five had no detectable response following the booster dose, 13 showed no or a marginal (<0.5 log10) increase in antibody titre, while 13 had evidence of a boosting effect (>0.5 log10 increase); one sample was not collected at week six.
A boosting effect was more commonly observed in those receiving 5.0 or 10.0µg but was also observed in those receiving a lower dose.
Note the log scales on the x and y axis. In other words, it’s worse than it looks, and this looks ugly as it is.
The perfect med would put these dots on top of each other, in a dose-dependent line:
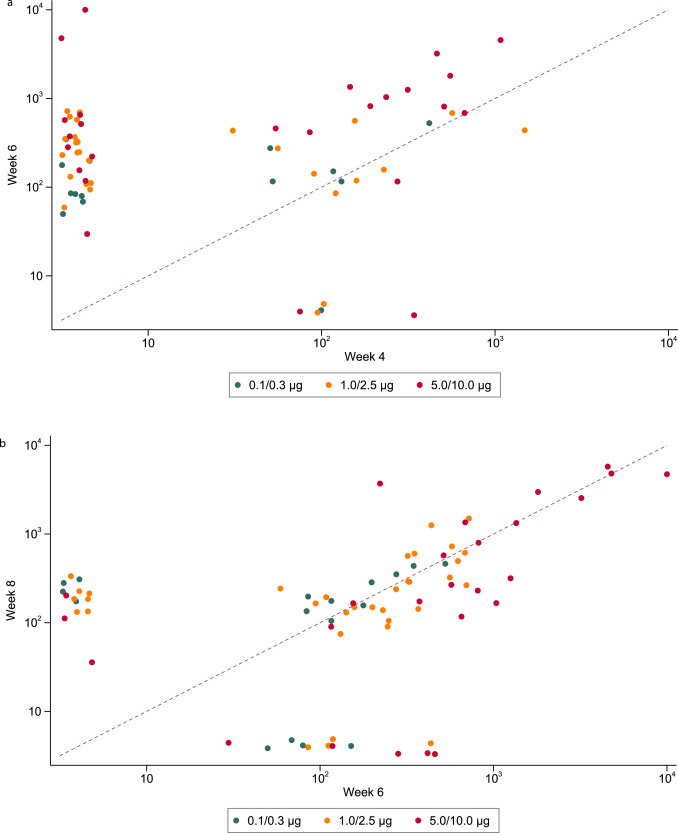
From the discussion, we learn that two of the young, healthy eager volunteers had such bad reactions (sustained mRNA amplification and expression?) that they had to delay the booster. They were also self-reporting any AE’s:
There were no unexpected safety issues in this short period following two IM injections of LNP-nCoVsaRNA up to 10.0μg in this cohort of 192 adults aged 18-45 years.
Two participants had adverse events that led to a delay in their second vaccines, which were administered with no recurrence. Reactogenicity was dose dependent, with the highest proportion of grade 3 reactions in those receiving 10.0μg.
We saw no evidence of clinically significant potentiation after the second dose, beyond a slight increase in grade 2 headaches.
If you don’t look, you won’t find:
No allergic events were considered related to the saRNA vaccine,16 although this may reflect exclusion of subjects with a significant allergy history.
Doses of 1.0μg and below were associated with very low levels of reactogenicity. Seroconversion by ELISA was sensitive to dose level, with maximum rates (14/23; 61%) at 10.0μg.
This can only be considered a flop, when you make allowances for impaired immune responses from less-healthy cohorts in a wider trial or roll-out, such as the elderly or frail:
However, none of the groups reached 100% seroconversion with a range of 39% (9/23; 2.5μg and 5.0μg) to 61 % (14/23; 10.0μg) for the 1.0-10.0μg groups.
The highest titre of anti-S IgG antibody was in those receiving 5.0μg and was not further augmented in those receiving double this dose.
Why were there differences between test readings?
Differences between seroconversion by ELISA and immunoblot reflect differences in sensitivity (see Appendix 2, Figure 18) between the two assays. This may reflect differences in the presentation of binding epitopes on immobilisation of the spike glycoprotein to ELISA plates versus nitrocellulose membrane.17
However, 16/59 participants positive by pseudovirus neutralizing assay were negative by both ELISA and immunoblot. Although observed by others,18 the reason for such discordance is unclear but may reflect exposure of epitopes on pseudotyped virions not accessible or represented by the purified recombinant protein.
As with BNT162c2:
Interestingly, for on-going development, immune responses were observed at low dose levels (1.0μg) and even at ultralow dose levels (0.1μg, 20/39 (51%) by immunoblot).
Nevertheless, the proportion of individuals generating neutralizing antibodies (14/42 (33%) and 6/39 (15%) respectively) would not be sufficient for an effective vaccine.
We present here an interim report of data obtained, the study is on-going, and participants will be followed for 1 year.
If I can find these other papers I will share them:
Data on cellular responses, epitope mapping and persistence of antibody will be reported elsewhere.
This is concerning. Get it wrong and you could create an alphavirus with gain-of-function or autoimmune disorders. Or IgG4-RD:
In this saRNA vaccine design, only non-structural proteins are derived from VEEV preventing potentially infectious genetic reversion, reactogenicity from immune response to structural alphavirus proteins, or off-target immunity.
Impossible to dose this product safely, if that is the case:
Pre-clinical studies of our LNP-nCoVsaRNA vaccine demonstrated high levels of neutralising anti-S IgG even when given at 0.01μg, with a dose response across a three-log range.5
Due to constraints with vaccine preparation, the volume of administration for the 0.1μg dose level was 0.2mL. It is conceivable that this may have affected this very low dose. However, immunogenicity was also low at 0.3μg, and the most plausible explanation is that below 1.0μg there was insufficient RNA payload within target cells leading to suboptimal antigen expression.
It failed equally at all doses at 1.0μg or above, and the first dose may have impeded the second unless you leave a longer interval. They also recommended longer gaps with some of the HIV vaccine trials that flopped.
It’s effectively game over by this point, and I think they knew this too:
At 1.0μg and above, the dose response by ELISA was more complex and hints at a potential mechanism for the level of non-responders. Whilst increasing dose increased the reactogenicity, concentrations of anti-S IgG were not linearly affected, likely indicating persistently low antigen expression.
They could not use m1u-modified saRNA, as it will not amplify. This means they couldn’t suppress innate and adaptive immune response signalling caused by those pesky PRRs.
Is this “misinformation”? Asking for a friend:
It is possible that recognition of both lipid nanoparticle and/or saRNA exposure by endosomal and cytoplasmic pathogen-associated molecular pattern receptors (e.g. TLRs, RIG-I & MDA5) may restrict saRNA amplification and antigen expression.19,20
And you cannot predict this in the clinical setting:
Differences in threshold for triggering such pathways at an individual level may have influenced initiation of self-amplification and determined whether antigen expression levels were sufficient for seroconversion by ELISA and/or immunoblot.
As the threshold for innate sensing is influenced by the basal state of immune activation21,22 and given the different patterns of response to the first or second injection irrespective of dose, it is conceivable that innate activation from the first immunisation may have impacted on the second.
21 days! And that’s just from the base case studies:
Indeed, the replication kinetics of saRNA, with antigen expression occurring for up to 21 days,10,11 suggests a four-week interval between doses may not be optimal where a longer interval may be required to return to a more quiescent state.
As I said earlier, they just don’t know how this stuff works in the clinical setting, let alone at the sub-cellular level:
Understanding the impact of different 5’ UTRs on variability of immune response to saRNA and the attenuated VEEV TC-83 strain used for vaccination25 could provide further insight to the development of this platform. Indeed, clinically, very little is currently known about how different saRNA designs perform.
Yes, but with the same unacceptable levels of AEs and lack of dose control:
The two mRNA COVID-19 vaccines (Moderna and Pfizer/BioNTech) now in widespread clinical use26,27 performed better than our LNP-saRNA, demonstrating 100% seroconversion.
Both mRNA vaccines use a similar, if not identical 2P-S presentation of the S glycoprotein as used in this study, formulated in LNPs, the Pfizer vaccine using identical lipids.27
If the Spike conformation and LNPs were the same, why did the mRNA vaccines work as well as they did, for better or worse? I think Newsguard need to step in here, with all this discussion of N1-methyl-pseudouridine-induced immune suppression:
These mRNA vaccines, incorporate the modified nucleobase methyl-pseudouridine to minimise innate recognition of the mRNA using higher doses (100μg and 30μg for Moderna and Pfizer respectively) than those in this study.
However, the compatibility of modified bases with saRNA has not been established and these would be rapidly lost on amplification.
Furthermore, induction of innate restriction pathways triggered within hours may have a greater impact on saRNA than mRNA given differences in kinetics of expression.10,11
Those flippin’ mice:
Importantly the observed immunogenicity in humans was inferior to that observed in mice. This likely reflects differences in innate restriction of exogenous RNA sensing.28, 29, 30, 31
Agreed - there are huge gaps in our/their understanding:
Understanding species specific differences with respect to innate restriction of saRNA expression may prove key to unlocking the true potential of this technology for humans and development of more predictive animal models.
You mean you need to suppress the immune response somehow, with the logical risk of promoting tumour growth. But I thought the FOIA expert confirmed this was all “misinformation”?
Is he going to censor himself?
It is probable that these issues can be resolved through further refinement of saRNA vaccine design such as the inclusion of encoded modulators of human pattern recognition receptors (now undergoing clinical evaluation),32 use of alternative UTRs and a wider range of potential modifications.33
In conclusion:
This is the first human study to report on the response to LNP encapsulated saRNA based on an alphavirus replicon in humans. These data provide preliminary data into this novel RNA platform technology.
Nevertheless, responses with this construct were insufficient to meet the target for translation in Phase 3 trials and further work is needed to refine the platform.
If modified to improve antibody titres, encapsulated saRNA could provide potential advantages for vaccine development including scalability, tolerability, and flexibility in antigen design, to meet requirements for the ongoing response to the COVID-19 pandemic.
Perhaps unsurprisingly, we have a very interesting, and relevant, COI declaration:
Declaration of interests
P.F.M. and R.J.S. are co-inventors on a patent application covering this SARS-CoV-2 saRNA vaccine. All the other authors have nothing to report.
A cited study
Reference #10 from their study caught my eye, and again we have to ask why Newsguard didn’t send in the brown shirts to visit these authors too.
Chillingly, although from 2019, its discussion about potential vaccine side effects could almost be written about the Pfizer product.
Key takes from “Expression Kinetics and Innate Immune Response after Electroporation and LNP-Mediated Delivery of a Self-Amplifying mRNA in the Skin”2 (2019) by Huysmans et al.:
And remember, the FOIA expert co-wrote the paper citing this. The hypocrisy hits you in the face.
Abstract
In this work, we studied the expression kinetics and innate immune response of a self-amplifying mRNA (sa-RNA) after electroporation and lipid-nanoparticle (LNP)-mediated delivery in the skin of mice. Intradermal electroporation of the sa-RNA resulted in a plateau-shaped expression, with the plateau between day 3 and day 10. The overall protein expression of sa-RNA was significantly higher than that obtained after electroporation of plasmid DNA (pDNA) or non-replication mRNAs. Moreover, using IFN-β reporter mice, we elucidated that intradermal electroporation of sa-RNA induced a short-lived moderate innate immune response, which did not affect the expression of the sa-RNA. A completely different expression profile and innate immune response were observed when LNPs were used. The expression peaked 24 h after intradermal injection of sa-RNA-LNPs and subsequently showed a sharp drop. This drop might be explained by a translational blockage caused by the strong innate immune response that we observed in IFN-β reporter mice shortly (4 h) after intradermal injection of sa-RNA-LNPs. A final interesting observation was the capacity of sa-RNA-LNPs to transfect the draining lymph nodes after intradermal injection.
To overcome activation of the RNA sensors, the mRNA can be rendered less immunogenic through incorporation of modified nucleotides like pseudouridine, 5-methylcytidine, and N1-methylpseudouridine.44, 45, 46, 47
However, incorporation of N1-methylpseudouridine-modified nucleosides in our non-replicating mRNA did not reduce the innate immune response; hence, no significant increases in protein production were observed after electroporation of nucleoside-modified mRNAs.
Similar results have been reported by Kauffman et al.,48 who found that incorporation of pseudouridine-modified nucleosides had no significant effect on either immunogenicity or protein expression of mRNA-LNPs after systemic injection.
The “happy accident” of dsRNA contamination, as I discussed here. There is currently no easy, economic way of removing this from the commercial quantities required:
In our work and in that of Kauffman et al., the mRNA was not purified by high-pressure liquid chromatography (HPLC); hence, double-stranded mRNA or short aborted mRNA species that are known to be highly immunogenic were probably not completely removed, causing the innate immune response.
On LPS (i.e. endotoxin) contamination, plasmid risks (e.g. the SV40 promotor) and cancer risk. This paper was, again, ahead of its time:
Although the expression of pDNA is lower than that of sa-RNA during the first weeks, pDNA is still an interesting vector for protein (replacement) therapy, as its expression lasts longer than that of sa-RNA (up to 5 months after intradermal injection).49
“CpG motifs are considered pathogen-associated molecular patterns (PAMPs) due to their abundance in microbial genomes but their rarity in vertebrate genomes.”
CpG motifs in the pDNA as well as LPS contamination during pDNA preparation can result in, respectively, TLR9 and TLR4 activation, leading to IFN-β induction.
However, in our hands, the innate immune response after intradermal pDNA electroporation was very low and short-lived compared to mRNA vectors (Figure 4A), which further supports the use of pDNA for protein therapy.
They used expensive lab processes to remove as many contaminants as they could, but the plasmid DNA still acts to trigger bacterial PAMPs. We are talking cGAS-STING pathways here. Big hat-tip to Christie Grace:
Since we use an endotoxin-removing purification kit for the preparation of the pDNA, most of the IFN-β response observed in Figure 4A is probably due to the CpG motifs in the pDNA.
Boom!
Nevertheless, the use of pDNA has some drawbacks like, e.g., the theoretical risk of genomic integration and oncogenic mutagenesis; the presence of antibiotic-resistance genes; and the fact that, for certain therapeutic proteins, an uncontrolled expression during several months is not warranted.
You really don’t want this stuff transfecting immune cells in your lymph nodes either:
A very interesting observation was the presence of a short-lived expression (up to 48 h after administration; data not shown) in the draining subiliac lymph node after intradermal delivery of sa-RNA-LNPs (Figures 6 and 7).
Expression of luciferase in the lymph nodes can be the result of the transport of the sa-RNA-LNPs toward the draining lymph nodes or due to migration of transfected immune cells at the injection site toward the draining lymph nodes. The luciferase protein expressed at the site of injection can also be transported to the lymph nodes. Further research is necessary to determine how the lymph nodes get luciferase positive and which types of cells are transfected.
In conclusion, not being able to use m1u-modified mRNA renders this line of vaccine almost uncontrollably dangerous, as you cannot predict outcomes at the level of the individual:
The expression rapidly peaked 24 h after intradermal injection of sa-RNA-LNPs and subsequently showed a sharp drop that can be attributed to a massive induction of the innate immune system.
Interestingly, intradermal injection of sa-RNA-LNPs also resulted in a protein expression in the lymph nodes, which supports the potential use of sa-RNA-LNPs as vaccines.
Almost no pharmacokinetic control of amounts, distribution, duration or immunogenicity at the level of the individual. This is unacceptable:
However, it needs to be examined whether the induced innate immune response after administration of the sa-RNA-LNPs is balanced enough to potentiate adaptive immune responses. Indeed, it has been shown that a too-high innate immune response can also be detrimental for the adaptive immune response.52, 53
Late-breaking news
Real-world updates relevant to this Substack are coming in almost faster than I can type.
ZeroHedge just reported how the Federal Communications Commission (FCC) has put no other than NewsGuard on notice. Yes, the very same “fact-checkers” who keep causing me trouble, didn’t like our review paper and tried to have it taken down by all means possible.
X Sees Return Of Major Advertisers As NewsGuard & "Boycott Cartel" Come Under Fire From FCC
While Mark Cuban and other sore losers are leaving X to shout into the void, several major advertisers have returned to the platform.
Advertising Cartel Under Fire
Speaking of the tide turning, the woke cabal of advertisers trying to starve conservative platforms out of a voice is now coming under fire (have we mentioned lately that we really appreciate our premium subscribers?).
In a Wednesday letter to Microsoft, Alphabet (Google), Apple, and Meta, FCC Commissioner Brendan Carr accused them of having "participated in a censorship cartel that included not only technology and social media companies but advertising, marketing, and so-called "fact-checking" organizations as well as the Biden-Harris Administration itself."
"The relevant conduct extended from removing or blocking social media posts to suppress their information and viewpoints, including through efforts to delist them, lower their rankings, or harm their profitability."
Carr then suggested that their protection from liability under Section 230 may be on the line.
"As you know, Big Tech's prized liability shield, Section 230, is codified in the Communications Act, which the FCC administers. As relevant here, Section 230 only confers benefits on Big Tech companies when they operate, in the words of the statute, "in good faith."
Wow...
Carr then set his sights on NewsGuard - which Jonathan Turley notes has been long accused by conservatives "of targeting conservative and libertarian sites and carrying out the agenda of its co-founder Steven Brill. Conversely, many media outlets have heralded his efforts to identify disinformation sites for advertisers and agencies."
Basically, NewsGuard bombards conservative sites with struggle-session questionnaire emails demanding explanations for the slightest of indiscretions, after which they issue a "report card" that advertisers use to justify pulling ad spend.
As Carr notes in the letter; "It is in this context that I am writing to obtain information about your work with the one specific organization - the Orwellian named NewsGuard. As exposed by the Twitter Files, NewsGuard is a for-profit company that operates as part of the broader censorship cartel. Indeed, NewsGuard bills itself as the Internet's arbiter of truth or, as its co-founder put it, a "Vaccine Against Misinformation." Newsguard purports to rate the credibility of news and information outlets and tells readers and advertisers which outlets they can trust."
Carr suggests following NewsGuard's ratings may constitute a violation of Section 230 (this is huge).
"NewsGuard's own track record raises questions about whether relying on the organization's products would constitute "good faith" actions within the meaning of Section 230. For one, reports indicate that NewsGuard has consistently rated official propaganda from the Communist Party of China as more credible than American publications."
"For another, NewsGuard aggressively fact checked and penalized websites that reported on the COVID-19 lab leak theory."
Carr then demands the following information:
A list of every one of your products or services (if any, including advertising) that use or rely on any NewsGuard product, service, or ranking.
A list of every one of your products or services (if any) that enables any of your users or customers to use or rely on NewsGuard product, service, or ranking.
If you offer an advertising service, provide details on the use of any media monitor or fact checking service, including NewsGuard, that you may utilize.
Full article:
This is a very encouraging development, as I keep coming under personal attack or questioning because of their BS-muckraking censorship attempts.
DoorlessCarp’s Scientific Literature Reviews is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
References
Pollock KM, Cheeseman HM, Szubert AJ, et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. eClinicalMedicine. 2022;44:101262. doi:10.1016/j.eclinm.2021.101262
Huysmans H, Zhong Z, De Temmerman J, et al. Expression Kinetics and Innate Immune Response after Electroporation and LNP-Mediated Delivery of a Self-Amplifying mRNA in the Skin. Molecular Therapy - Nucleic Acids. 2019;17:867-878. doi:10.1016/j.omtn.2019.08.001


























Thank you for your dedication and research. What is so insidious about this technology is that it is a wolf dressed in sheep’s clothing. By simply calling it a vaccine people put it into their minds as a preventative therapy, when it neither prevents infection or transmission, yet I see talking heads on YouTube laughing at RFK jnr as an antivaxer, when he is just an anti gene therapy anti Vaxer. It’s very disturbing to be caught inside this Fauci psy-ops machine, with no way out, so I wrote and published a book, Pandoras Box!
Strange you should mention that FOIA. I stumbled across it whilst eyeballing FOIAs for write-ups. The FOIA is incomplete, despite claims to the contrary. There's reference to a prior message/contact but it's not included in the FOIA. Maybe the UCL researcher in question kept it off the record books in another medium? WhatsApp, DM, text message, or some other backhanded deal.
Newsguard colluding to censor rather than "verify". More like Legacyguard. Guarding the old deceitful decrepit pieces of shit.