Self-amplifying mRNA (saRNA) Part 5, Episode VIII: The Last Spinoff
"In order to build your immunity, we need to destroy it"
Reading time:
short story - novelette - novella - novel - PhD thesis - War and Peace - U.S. Tax Code
Any extracts used in the following article are for non-commercial research and educational purposes only and may be subject to copyright from their respective owners.
Contents
Introduction
The story so far:
“Self-amplifying mRNA (saRNA) Part 1: The Japanese registration documents” translated and annotated Dr Stebel’s excellent walkthrough of the Japanese (pre-) registration documents for ARCT-154 (“Kostaive”).
“Self-amplifying mRNA (saRNA) Part 2, Episode V: The Pharma Empire Strikes Back” discussed how vaccinia virus immune evasion proteins E3, K3, and B18 act together synergistically as “a highly potent blocker of PKR activation and of interferon (IFN)-β upregulation.”
“Self-amplifying mRNA (saRNA) Part 3, Episode VI: Return of the Cancer” did a deep dive into how IIPs E3, K3 and MERS-CoV ORF4a may promote cancer progression or recurrence through disruption of innate immunity signalling pathways. It also discussed a secondary mechanism, whereby endogenous retroviruses could be inhibited or activated, and how this is linked to cancers and diseases such as glioblastoma, heart disease, lupus, and arthritis.
"Self-amplifying mRNA (saRNA) Part 4, Episode VII: The Cancer Awakens" discussed how saRNA-essential non-structural proteins (nsPs) also cause immunosuppression, how they are linked to progressive neurological diseases, and gave an update on the latest reports of Kostaive side effects, and its pending final approval in the EU.
This Substack will review some of the literature discussing pathologies linked to double-stranded RNA (dsRNA) contamination, and the saRNA vaccine patent landscape, with its buried landmines.
Discussion
dsRNA, a problem that won’t go away
What is dsRNA?
dsRNA is found in most viral infections. It either makes up the viral genome of dsRNA viruses, or is generated in cells of the host as part of the viral replication process.

As part of our immune system, vertebrates have a set of innate immune receptors for dsRNA that set in motion a multitude of both cell-intrinsic and extrinsic immune responses.
If these are triggered in the absence of infection, then this may lead to a range of pathogenic auto-immune disorders, such as Aicardi–Goutières syndrome.1
Replicon vaccines and dsRNA
Replication of saRNA produces high amounts of dsRNA, which is a problem for those choosing to manufacture and administer these2. You may use expensive cellulose or affinity chromatography to remove as much as possible from the manufactured product. They failed miserably at removing dsRNA at scale when manufacturing mRNA COVID vaccines.
A paper from 2024 by Vanluchene et al. discusses how dsRNA creates a barrier to the use of successful gene therapies for retinal diseases.
They had to use both cellulose chromatography and a powerful IIP from a Vaccinia virus to significantly increase target protein expression. Even then, they failed to totally eliminate significant levels of innate immune signalling, and had to use very low doses of saRNA (0.05µg) to get it to work at all.
This is a complicated way of saying that the current generation of replicon vaccines are wholly unsuitable for administration to the general public without:
Accurate patient-centric dose titration.
Very high efficiency filtration.
Co-administration of immune-inhibiting proteins. (This is of course a hard pass, as far as I’m concerned.)
Key takes from “Less is more: Self-amplifying mRNA becomes self-killing upon dose escalation in immune-competent retinal cells”3:

… while the therapeutic potential of non-viral mRNA delivery was demonstrated recently in the worldwide vaccination efforts against the COVID-19 pandemic, mRNA delivery to the eye remains relatively unexplored.
Up to date, conventional (non-amplifying) mRNA is mostly used, containing modified nucleotides like N1-methylpseudo-UTP (m1ΨTP) to enhance mRNA stability and lower immunogenicity.
In previous work, we and others have demonstrated that although mRNA delivery is simple in concept, successful delivery of mRNA to retinal cells is hard to achieve.
An interesting strategy to increase the duration and level of protein expression when only a few mRNA molecules can reach their target, is the use of self-amplifying mRNA (saRNA).
Our results indicated that saRNA delivery elicited a strong dose-dependent innate immune response blocking its own translation.
To counter this, we attempted to maximize the in vitro transfection efficiency of saRNA complexes by tempering its immunogenicity through double-stranded (ds)RNA removal by cellulose-based purification and addition of B18R, a vaccinia-virus encoded protein acting as a decoy receptor for type I IFNs [15], [16], [17]. Both were found to strongly improve protein expression from saRNA.
It’s very easy to overdose replicon vaccines:
Importantly, comparisons between mRNA and saRNA were done using the same doses for both, but it should be noted that for a given dose, saRNA contained 10 times less molecules than mRNA.
As seen in the dose titration experiment, this “self-destructive” innate immune response was more pronounced when higher doses of saRNA were applied.
Even at very low doses, they were still getting those troublesome innate immune responses in non-immunogenic BHK-21 cells:
Although lower doses of saRNA revealed to be the most potent ones in ARPE-19 and MIO-M1 cells, these doses still induced significantly more eGFP expression in non-immunogenic BHK-21 cells, indicating that also these lower doses still evoke an innate immune response.
“When they go low, we go lower”:
Based on these observations, we conclude that below a certain saRNA concentration, the threshold for innate immune activation is not reached, allowing the translation machinery for massive protein production.
However, when innate immune activation is triggered above this threshold, the IFN response is too strong, thus inhibiting protein translation.
This is in line with Blakney et al., who reported on the nonlinearity of increasing the dose of saRNA delivered using pABOL polymers in human skin explants and after intramuscular injection in C57BL6/J mice.
Indeed, they stated that saRNA is inducing a binary state in a cell, either giving maximal protein production or strong inhibition of protein translation [25], [26]. This was confirmed by co-delivery of modified mRNA and saRNA, revealing that a low dose of saRNA maintained mRNA and saRNA translation. saRNA at a high dose, in contrast, induced a strong innate immune response blocking the translation of both mRNA and saRNA.
As mentioned above, we were able to show that cellulose-based purification of saRNA and B18R addition were highly efficient in reducing the innate immune response to higher doses of saRNA and consequently boosting protein expression.
It just won’t go away, regardless of how hard you try:
Despite this reduced immunogenicity, a simple comparison between the transfection efficiency of cellulose-based purified saRNA combined with B18R in BHK-21 cells and both retinal cell types clearly illustrated that still some innate immunogenicity is present in the retinal cell types, affecting protein expression.
The old tricks can’t be used:
This can most likely be attributed to two particular saRNA characteristics. First, nucleoside base modification, which is typically used to silence mRNA recognition by PRRs, is not an option for saRNA IVT mRNA, because it is expected to impair its self-replicating capacity and nucleoside modifications would be lost already after the first round of amplification [11], [34], [35].
The fatal flaw in a flawed technology, which no amount of filtration can reach:
Second, during saRNA’s replication, dsRNA amplification intermediates are formed that are known to be highly inflammatory [36].
Removal is easier said than done, is impractical, and not commercially viable at scale.
This isn’t conjecture. It’s why the mRNA/LNP agents were disastrously contaminated with dsRNA, frameshifted mRNA, fragmented mRNA, fragmented DNA, plasmid DNA, endotoxin, SV40 promoter, metal particles, etc …
Based on our data set, we recommend in general to remove the dsRNA contaminants from saRNA by cellulose-based purification to minimize its immunogenicity and maintain its functionality.
I don’t think this is possible to do during your ten minute vax appointment, if it’s possible at all in vivo:
We demonstrated that to keep the delicate balance between the efficacy and safety of saRNA, dose titration to identify the threshold for detrimental innate immune activation and the measures to limit saRNA’s immunogenicity will be crucial.
Another approach was by Gong et al. They used serial passage to generate saRNA mutants with less dsRNA as a byproduct4. They know that this is a serious problem. I discussed this in the “In vitro adaptive passaging” section of Self-amplifying mRNA (saRNA) Part 2, Episode V: The Pharma Empire Strikes Back
However, this conflicted paper from 2024 was also far too late to have any influence on ARCT-154 development, and the discussion is full of cautions, unanswered questions, weasel words and disclaimers.
Referring to Kostaive, “absence of evidence isn’t evidence of absence”:
… Our results confirmed that the dsRNA sensors TLR3, RIG-I, and MDA5 are substantially upregulated by saRNA transfection at 24 h compared to nrmRNA. However, in addition to the dsRNA sensors, other innate immune response genes (IFN-beta, IFIT2, CXCL10, and CCL5) were also upregulated as early as 6 h post-transfection for wt saRNA but not for the mutants (Figure 4G).
Therefore, reduced dsRNA levels may not be the only mechanism for the attenuation of the innate immune response by mutations in the MD of saRNA. The mutants may also delay the onset of other innate immune responses, contributing to the reduced immune response in the transfected cells.
… The strong innate immune response triggered by wt saRNA may not pose a major safety concern, as demonstrated by the recently approved COVID-19 vaccine ARCT-154, which has shown good clinical tolerability.[50]
… the potential impact of the cytotoxicity induced by saRNAs on in vivo or clinical applications also needs to be carefully investigated in future studies.
… Conflict of Interest
All authors are current employees of Virogin Biotech Co. Ltd., and the patent application related to this study is currently pending.
From: “A Novel Self-Amplifying mRNA with Decreased Cytotoxicity and Enhanced Protein Expression by Macrodomain Mutations” (2024)
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/advs.202402936
Grinsted et al. also discussed the problem of contamination.
Hat tip to @VaccineMole on X for finding this review paper from 2022.
Note: These techniques don’t help if the dsRNA is being produced in vivo, after vaccination:
“Purification of therapeutic & prophylactic mRNA by affinity chromatography”5.
Despite the prompt advancement of mRNA from trials to market, purification challenges remain. The cell-free synthesis of mRNA is responsible for the generation of product and process-related impurities, creating the potential for immunogenic effects and decreased translatability into the clinic.
Double-stranded RNA
dsRNA removal to very low levels from feed material is necessary because the molecule is highly immunogenic [3]. This is illustrated by the molecules ability to induce a cytokine storm in some cases [27,28].
Despite its immunogenicity, dsRNA holds natural biological purposes within human cellular nuclei [29]. However, the entry of dsRNA into the cytosol may induce apoptosis due to its association with viral material [30].
dsRNA works against mRNA expression due to the induction of RNase L, which is not good when you play the antibodies/schmantibodies game:
RNase L release within cells is induced by the activation of oligoadenylate synthetase in the presence of dsRNA.
Degradation of mRNA may occur in the presence of RNase L. This degradation leads to an inhibition in the translation of mRNA. This mechanism suggests that the removal of dsRNA may contribute to increased levels of mRNA expression.
Even the breakdown products of dsRNA itself may trigger PRRs:
RNase L is also able to cleave dsRNA. The resulting double-stranded fragments may activate intracellular receptors, Melanoma Differentiation Associated Protein 5 (MDA5) and Retinoic Acid Inducible Gene 1 (RIG1) [31].
dsRNA and major neurodegenerative disorders
From 2005, a paper by Scumpia et al. discussed using in vitro brain astroglial cultures and a rodent model to demonstrate how dsRNA contributes to neurological pathologies.
Key takes from “Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes“6.
Astrocyte inflammation, reactive oxygen species (ROS) formation, and dysfunction form a common denominator shared by all the major neurodegenerative disorders.
Viral infections are emerging as important events in the etiology of CNS damage involving astrocytes, but molecular understanding is incomplete.
Double-stranded RNA (dsRNA) is a byproduct of viral replication and serves as the signature molecule for viral infection via Toll-like receptor 3 (TLR3) largely restricted to circulating peripheral dendritic cells.
However, astrocytes are strategically located at the blood-brain barrier (BBB) and throughout brain tissues, making these cells ideal candidates as innate immunity sentinels within the CNS.
We hypothesized that extracellular dsRNA, mimicked by polyinosinic-polycytidylic acid (Poly(I:C); PIC), initiates signaling of the double-edged sword of antiviral plus pathophysiological events in astrocytes.
The important thing to note is that “glutamate is responsible for most of the excitatory synaptic activity and oxidative stress induction in the mammalian brain”7, via oxidative stress, and that the dsRNA byproduct promotes glutamate cytotoxicity:
… these data provide evidence that dsRNA/TLR3-activated astrocytes initiate a battery of rapid innate pathogen-associated molecular pattern (PAMP) immune responses that are important for mounting antiviral defense in the CNS, yet also lead to pathophysiological events associated with the glutamate neurotoxicity of neurodegenerative diseases.
The full paper is accessible from here but is copy-protected.
The next-best option is to screenshot relevant sections of interest:
dsRNA is detected by innate-signalling PRR receptors, but as with LNP-mRNA agents, this may lead to neurodegeneration if it has crossed the blood-brain barrier:

A conference paper from 2007 by Chang et al. also adds more light. The implication is that the dsRNA byproducts of mRNA and saRNA agents such as ARCT-154 may contribute to, or lead to the accelerated progression of conditions such as Alzheimer’s disease, just as the previous paper discussed.
Key takes from “Direct neurotoxic effects of dsRNA: Implication of virus-induced neurodegeneration“8.
Neurodegeneration occurs in the brain infected by RNA-virus such as Japanese Encephalitis virus or Herpes Simplex virus. Degeneration of neurons may cause cognitive impairment and even loss of memory. Therefore, it has been considered that virus infection is a risk factor leading to the development of Alzheimer’s disease (AD).
Our laboratory is among the first to demonstrate that the double-stranded RNA-dependent protein kinase (PKR) plays important roles in mediating neuronal apoptosis in the pathogenesis of AD.
Boom!
We hypothesize that formation of double-stranded RNA released by RNA virus induces neurotoxicity. To test this hypothesis, polyinosine-polycytidylic acid (pIpC) was used as the analog of dsRNA in this study. Application of 20 ug/ml pIpC into primary cultured neurons resulted in an increase in 23% cytotoxicity determined by LDH assay.
The results were similar if human neuroblastoma SH-SHY5Y was used instead of primary cortical neurons.
Western-blot analysis detected an increase in the phosphorylation of PKR and its substrate, eukaryotic initiation factor 2α (eIF2α).
This is an area for another Substack, but there are therapeutics that can help mitigate the damage:
Furthermore, we investigate whether some well-known neuroprotective agents can protect neurons against pIpC toxicity. Studies were done to investigate whether memantine (a non-competitive NMDA receptor antagonist), minocyclin (a tetracycline) and anti-aging Chinese medicine Lycium barbarum exhibits neuroprotective effects against pIpC toxicity.
Taken together, dsRNA produced by RNA virus in the brain exhibit direct toxicity towards neurons.
Traditionally used neuroprotective agents, memantine, minocyclin or Lycium barbarum may be useful to reduce dsRNA neurotoxicity by infected RNA virus.
Viral infection OR other agents that produce dsRNA in the brain put you at risk of AD:
As viral infection in the brain during life time may be a pre-disposed risk factor leading to neurodegeneration in aging, the results have high implication in AD.
Unfortunately, this hypothesis is supported by experimental evidence. A paper from 2023 by Ochoa et al. confirmed a direct correlation between dsRNA and receptors, and AD with supranuclear palsy.
Key takes from “Pathogenic tau–induced transposable element–derived dsRNA drives neuroinflammation”9:
Deposition of tau protein aggregates in the brain of affected individuals is a defining feature of “tauopathies,” including Alzheimer’s disease.
Studies of human brain tissue and various model systems of tauopathy report that toxic forms of tau negatively affect nuclear and genomic architecture, identifying pathogenic tau–induced heterochromatin decondensation and consequent retrotransposon activation as a causal mediator of neurodegeneration.
We find that dsRNA and dsRNA sensing machinery are elevated in astrocytes of postmortem brain tissue from patients with Alzheimer’s disease and progressive supranuclear palsy and in brains of tau transgenic mice.
Using a Drosophila model of tauopathy, we identify specific tau-induced retrotransposons that form dsRNA and find that pathogenic tau and heterochromatin decondensation causally drive dsRNA-mediated neurodegeneration and neuroinflammation.
Our study suggests that pathogenic tau–induced heterochromatin decondensation and retrotransposon activation cause elevation of inflammatory, transposable element–derived dsRNA in the adult brain.
As dsRNA are a potent pathogen-associated molecular pattern sufficient to induce an interferon response, we next determined whether melanoma differentiation–associated protein 5 (MDA5) is elevated in tau-affected human brains.
MDA5 is a dsRNA helicase in the retinoic acid–inducible gene I–like (RIG-I) receptor family that detects long dsRNA via its DEAD box domain as part of the antiviral innate immune response (14, 15).
Costaining of postmortem human brain with antibodies detecting MDA5 and GFAP reveals a significant elevation of MDA5 in astrocytes of tau-affected brains (Fig. 2, E and FOpens in image viewer).
Together, these findings point toward an astrocytic increase in dsRNA and dsRNA surveillance machinery in the context of primary and secondary tauopathy.
dsRNA and other pathologies
dsRNA-dependent protein kinase R (PKR) is a ubiquitously expressed enzyme well known for its roles in immune response.
Upon binding to viral dsRNA, PKR undergoes autophosphorylation, and the phosphorylated PKR (pPKR) regulates translation and multiple signaling pathways in infected cells.
Here, we found that PKR is activated in uninfected cells, specifically during mitosis, by binding to dsRNAs formed by inverted Alu repeats (IRAlus).
From “PKR is activated by cellular dsRNAs during mitosis and acts as a mitotic regulator“ (2014)
In the context of alphaviruses, Ventoso (2012) discussed how protein kinase R (PKR) expression is promoted and activated by interferons induced by dsRNA10. PKR was first identified as a protector against viral infections. But it does much more than that. Its role and effects vary with age and according to which cells are affected.
Key takes from the aptly titled “PKR: A Kinase to Remember“ (2019) by Gal-Ben-Ari et al.11.
Aging puts you at greater risk of getting ill due from dsRNA-contaminated gene shots:
Aging is a major risk factor for many diseases including metabolic syndrome, cancer, inflammation, and neurodegeneration. Identifying mechanistic common denominators underlying the impact of aging is essential for our fundamental understanding of age-related diseases and the possibility to propose new ways to fight them.
One can define aging biochemically as prolonged metabolic stress, the innate cellular and molecular programs responding to it, and the new stable or unstable state of equilibrium between the two.
A candidate to play a role in the process is protein kinase R (PKR), first identified as a cellular protector against viral infection and today known as a major regulator of central cellular processes including mRNA translation, transcriptional control, regulation of apoptosis, and cell proliferation.
So I guess that 12 months of prolonged PKR activation probably isn’t the best idea:
Prolonged imbalance in PKR activation is both affected by biochemical and metabolic parameters and affects them in turn to create a feedforward loop.
As with “A Night to Remember”, your health will sink like the Titanic:
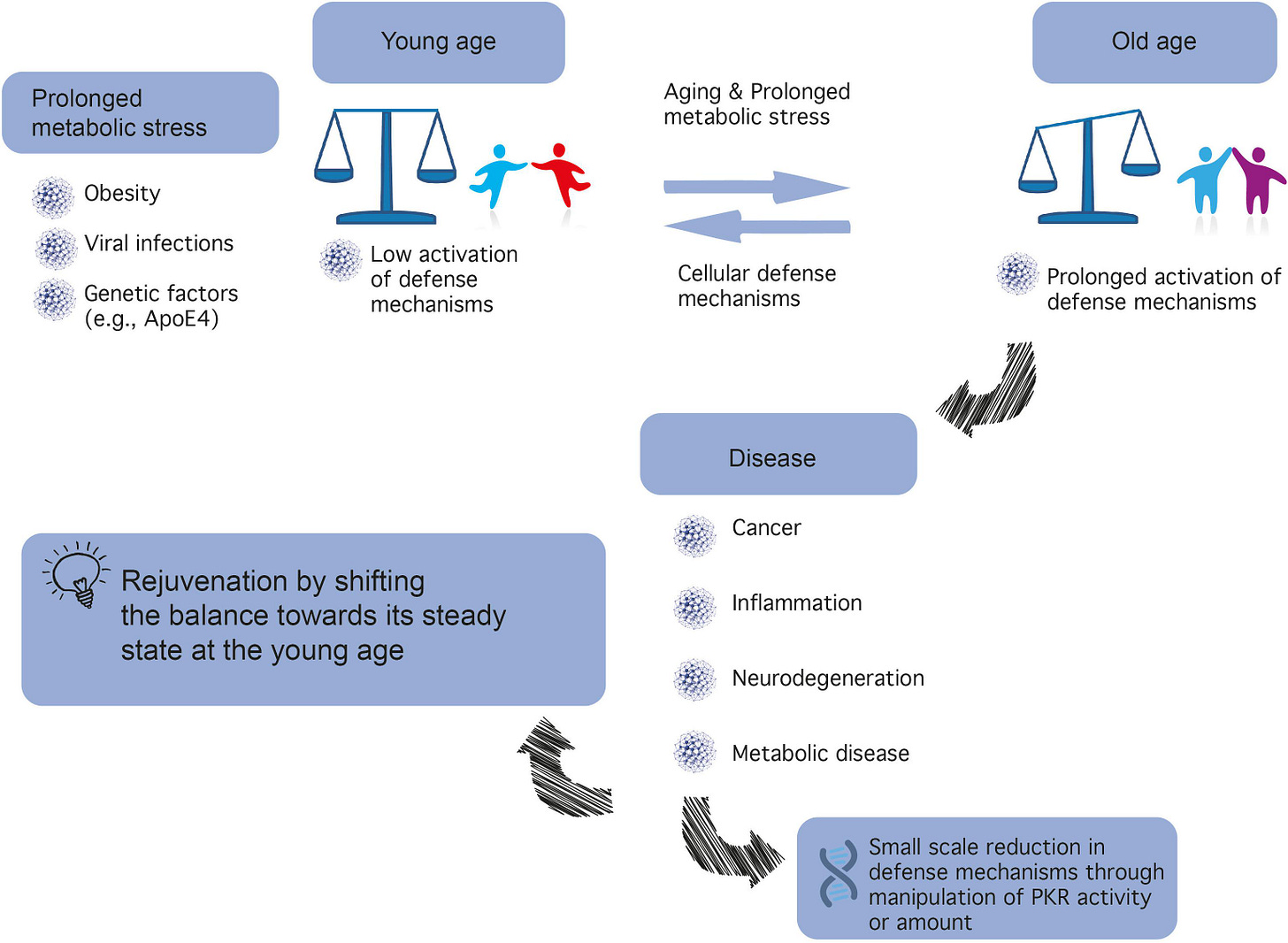
Figure 3 illustrates how dsRNA interacts with PKR in the cytoplasm and sets off an inflammatory cascade. All you need to is to substitute replicon vaccine dsRNA for the viral source shown top-left.
Also note the involvement of the tumour-suppressor/promotor phosphorylated eIF2a, as discussed in the last Substack, and blocking of insulin regulation:

PKR is one of four kinases that regulate protein synthesis via the eIF2α pathway. These kinases include, apart from PKR, the (PKR)-like endoplasmic reticulum kinase (PERK); general control non-derepressible 2 kinase (GCN2), and heme-regulated eIF2α kinase (HRI).
All four kinases regulate the phosphorylation of eukaryotic initiation factor 2 on its α subunit (eIF2α), a major regulator of the initiation phase of mRNA translation, the rate limiting step of protein synthesis.
A link between dsRNA and antiviral immunity:
Phosphorylation of eIF2α on Ser 51 by any of the four kinases leads to its inhibition and a consequent transient suppression of general protein synthesis, up to its complete blockade, concomitant with translation of mRNAs that encode for antiviral factors and/or mediate the integrated stress response (Hoang et al., 2018).
Of interest here is that most viruses express inhibitors that prevent PKR activation in infected cells, as this favours viral replication12:
Regulation of PKR activity is done by expression of proteins that disrupt PKR RNA binding sites by dsRNA sequestration, direct obstruction of these sites (e.g., Vaccinia virus, Influenza virus), or interference with the phosphorylation of eIF2α (e.g., Human Immunodeficiency Virus 1) (Dzananovic et al., 2018).
In the brain, PKR can lead to neurodegeneration, affect memory formation and learning, and lead to neuroinflammatory processes.
Other roles discussed in depth include how PKR may affect whole-body metabolism. It provides a link between metabolic stress, obesity, diabetes, and inflammation.
As PKR also helps to regulate insulin, an imbalance affects metabolism in the brain too:
In recent years, it has become increasingly clear that metabolic dysregulation in the brain underlies cognitive disorders, including AD, now considered type III diabetes (de la Monte and Wands, 2008). Such metabolic dysregulation or metabolic stress may result from aging, particularly when combined with high caloric intake and lack of physical exercise, which may lead to health problems spanning obesity, cardiovascular diseases, and diabetes (see Figure 1).
Insufficiency in amyloid beta clearance in AD is linked to an imbalance in PKR:
Metabolic stress also plays a role in AD, inter alia, through the Apolipoprotein E (ApoE) protein, which plays a role in lipid metabolism and transport in the liver and the brain, including clearance of Aβ peptide from the synapse (Li et al., 1988).
The ApoE4 ε4 allele (ApoE4) is currently the best studied risk factor for late-onset, sporadic AD, with a prevalence of 20% in the general population, compared to 50% in AD patients, although estimates vary between different sources (Ward et al., 2012).
… and poor memory too:
In another study, ApoE4 mice were shown to have poorer long-term memory compared to ApoE3 mice, as measured by freezing in the fear conditioning paradigm.
However, a single-dose treatment with the PKR inhibitor C16 (0.335 μg/g body weight, 1 h before conditioning) resulted in restoration of long term memory in ApoE4 mice, with freezing levels similar to ApoE3 mice in the fear conditioning paradigm.
PKR knockout or inhibitors have been shown to help with amyloid beta clearance:
Recent studies have shown that while i.c.v. administration of Aβ1-42 oligomers to mice resulted in long term memory impairment, this impairment was prevented both in PKR-/- mice and in TNFR-/- mice, and mice treated with either PKR inhibitor C16 or TNF-α neutralizing antibody, infliximab (Lourenco et al., 2013; Hwang et al., 2017).
Furthermore, treatment of hippocampal cultures with insulin prevented Aβ1-42 oligomer-induced phosphorylation of PKR (Lourenco et al., 2013).
Much like eIFa, PKR wears two hats with cancer. Its no surprise that these two pathways are linked:
While the role of PKR in metabolic stress and brain function is well established and described above, the role of PKR in cancer biology remains a subject of debate, as both tumor-suppressive and tumor-stimulatory functions have been attributed to this enzyme.
The attribution of different and even contradictory roles for PKR in tumorigenesis reflect its involvement in the regulation of diverse cellular processes which may differentially affect the cancer cell and its interaction with the tumor microenvironment.
Such processes include cell autonomous events such as the negative regulation of protein synthesis through eIF2α phosphorylation or signal transduction through different pathways including NF-κB, which alter the susceptibility of the cell to apoptosis and modulate the expression of inflammatory cytokines.
No informed consent for any of this for those being offered replicon gene shots:
Thus, variations in PKR expression and activity are predicted to affect both cancer-cell-autonomous and non-cell-autonomous aspects of the developing tumor.
p53, the guardian of the genome, is a significant tumour suppressor, and it interacts with PKR in feedback loops:
Furthermore, PKR and p53 physically interact, and PKR positively regulates p53 transcriptional activity (Cuddihy et al., 1999a,b), while p53 positively regulates gene induction by dsRNA (Hummer et al., 2001).
Together, these data suggest that PKR and p53 are intertwined in a positive feedback loop.
However, other studies show that dsRNA stimulates p53 degradation (Marques et al., 2005; Baltzis et al., 2007), suggesting a negative feedback loop involving p53 and PKR, and underscoring the complexity of their functional interactions. (iv)
In vivo experiments demonstrating an inverse correlation between PKR expression and/or activity and tumorigenicity.
Together, these data support the notions of a pro-tumorigenic association of PKR expression and cancer, and of the regulation of its expression by JAK-STAT signaling in cancer cells.
Once you have a tumour, the pendulum swings more towards tumour promotion:
Of note, JAK-STAT signaling pathway is intimately associated with the transduction of signals from inflammatory cytokines (e.g., interferon gamma), suggesting that the pro-tumorigenic role of PKR occurs within the context of tumor-related inflammation.
I would expect this to overlay nicely on the TNBC breast cancer/eIFa graphs from the last Substack. This is more confirmation of the deadly link between dsRNA contamination and cancer risk.
We can now add pancreatic cancer to the affected tumour types.
2000 days = 5 1/2 years …

In summary, and it’s quite dark:
(1) PKR level and post-translation modifications are excellent biomarkers for neurodegenerative diseases (e.g., AD, dementia, Parkinson’s disease, Huntington’s disease, and prion disease) and cancer (Figure 4, based on open source data).
(2) Inhibition of PKR is predicted to be highly beneficial in age-related neurodegenerative diseases. PKR is positioned in the center of metabolic syndrome disease, including glucose or Aβ load and inflammation and its inactivation reduces the insult (Figure 1).
(3) PKR inhibition contributes positively and directly to cognitive function in young and old mice.
(4) Inhibition of PKR is beneficial in certain cases of cancer. However, here, the situation is more complex as the role of PKR in tumors (pro- or anti-tumorigenic) may differ according to tumor type and/or stage.
(5) PKR inhibition or deletion is not essential for an organism response to viral infection as detected in PKR KO mice or prolonged treatments with the best-known PKR inhibitor, C16, and thus has the potential to serve as medical treatment.
(6) Treatment with C16 following different stimulations in most cases does not affect eIF2α phosphorylation levels, although many publications are trying to explain the phenotypes of PKR inhibition via regulation of mRNA translation (Table 1). Moreover, brains of PKR KO mice do not show significant change in eIF2α phosphorylation. On the other hand, most papers do show a clear effect of PKR inhibition on the NF-κB pathway (Table 1).
(7) The recent findings that PKR detects not only exogenous, viral dsRNA but also endogenous dsRNA, such as mitochondrial RNA, point to it as a new target for reducing oxidative stress and apoptosis in disease states and specifically in neurodegenerative diseases.
It’s not just brain, metabolism and cancer risk that are elevated by dsRNA-induced PKR. You also risk an early death through cardiovascular diseases and heart failure.
In 2017, Dhar published a literature review: “The Role of PKR as a Potential Target for Treating Cardiovascular Diseases“13.
Key takes:
It is well known that inflammation plays a key role in the pathogenesis of cardiovascular diseases and controlling this inflammatory pathway may inhibit the progression of this chronic disease.
Protein Kinase R (PKR), a serine threonine kinase is activated during various pathological conditions. Activation of PKR can induce apoptosis, inflammation and oxidative stress. Since PKR has multidimensional roles, thus PKR is an attractive target for treating cardiovascular and metabolic disorders.
PKR is a ubiquitously expressed serine threonine kinase and is activated by a number of signals, such as interferons, viral infection, high cholesterol diet, cytokines, pathogens, irradiation, heme limitation [7-15] as well as endoplasmic reticulum (ER) stress [16].
PKR contributes to inflammation and immune regulation through activation of mitogen-activated protein kinases (MAPKs) [11, 12], inhibitor of κB (IκB) kinase (IKK) [13, 14], and IFN-β-promoter simulator 1 (IPS-1) signaling [14] and, thus affects various transcriptional factors, including interferon regulatory factor 3 (IRF3) [15], nuclear factor κB (NF-κB) [13, 14], c-Jun, and activating transcription factor 2 (ATF2) [16, 17], which are required for the expression of genes encoding proinflammatory cytokines and interferons [8, 17-21].
PKR blocks translation initiation under stress conditions by phosphorylating eIF2D at Ser 51 [22-24].
PKR is also activated or induced by numerous other conditions such as oxidative stress, metabolic stress and mechanical stress [8, 25-28].
The link between PKR and congestive heart failure:
Recently Wang et al and group reported that PKR deficiency protected the human heart from systolic overload-induced congestive heart failure [34]. Thus, PKR inhibition is recognized as an attractive therapeutic target for diseases such as hypertension, diabetes, cancer, and novel pharmacological PKR inhibitors are under development.
Kostaive post-marketing surveillance isn’t looking for this:
Epidemiological and clinical studies have shown strong and consistent relationships between markers of inflammation and risk of future cardiovascular events.
From reported literature, it is evident that PKR is not only an anti-viral factor activated by interferons but also induced or activated under various pathological conditions [25-27, 38, 39].
Activated PKR may induce cellular stress in the heart leading to significant increase in inflammation and apoptosis and ultimately leading to chronic pathological conditions such as hypertension, atherosclerosis, CHF and stroke [42].
Wang et al. and group reported PKR expression in human patients suffering from CHF. There was a significant increase in myocardial expression and translocation of PKR in human patients and mice suffering from CHF [34].
Moreover, its ability to induce apoptosis of cardiomyocytes in response to a variety of stresses including hyperglycemia [45] makes this protein a therapeutic target that needs to be investigated at the clinical level as a potential biomarker and as a pharmacological target.
What to take from all this? The more elderly you are, and the more ill you are with cancers or comorbidities, and the worse the effects of dsRNA contamination are likely to be.
Introduction to the saRNA patent landscape
Interest in saRNA technology began over thirty years ago, but progress stalled until 2012. That year marked the first opportunity taken by scientists to use lipid nanoparticles (LNPs) instead of viral particles to encapsulate large saRNAs.
Progress slowed again from 2012 until the COVID-19 scamdemic led to the reckless “warp speed” fast-tracking of the first experimental mRNA COVID-19 gene therapy agents.
SaRNA technology development and associated patents exploded soon after, in the Wild West grab for cash:

Arcturus Therapeutics and its intellectual property
ARCT-154 (Kostaive) developers Arcturus Therapeutics (AT) or its subsidiary CSL Seqirus (CSL) didn’t even register in the saRNA tech timeline before 2022:

In addition to its mRNA platform, Arcturus has indicated that is has developed its own proprietary LNP delivery system called LUNAR. The company has stated that “LUNAR lipid-mediated delivery technology includes a diverse, growing library of over 200 proprietary lipids that we are rationally designing to be versatile, maximizing potential efficacy and improving tolerability of a diverse selection of nucleic acids, target cell types and routes of administration.”
Arcturus claims it is the sole owner of over 200 patents and pending applications in the U.S. and abroad, including patents and patent applications directed to its Lipid Particle Delivery System (LUNAR), UNA oligomer and LNA oligomer technologies utilized in certain RNA medicines.
From: “The mRNA IP and Competitive Landscape: Translate BIO; Arcturus; eTheRNA and Other Startups; and LNP Technology (Part II)“ (2021)
https://www.jdsupra.com/legalnews/the-mrna-ip-and-competitive-landscape-8652492
In December 2022 they closed a licensing agreement with the Australian biotech company CSL Seqirus, which granted CSL access to Arcturus Therapeutics’ saRNA technology. The upfront payment to AT was $200 million, along with conditional royalty payment rights.
The AT patent portfolio, as at 2023, amounted to some 46 patent families. Of these the company owns four, published between 2020 - 2022:
(WO2020191103, WO2022056413, WO2022036170, and WO2021183563).
The first three patents discuss technology related to encapsulating RNA nanoparticles in LNPs. The last patent “WO2021183563 CORONAVIRUS VACCINE COMPOSITIONS AND METHODS” is of interest to us as it applies to ARCT-154 (“Kostaive”).
This patent grants the holder exclusive rights to use a wide range of different alphavirus proteins, nSPs, and immunogenic target payloads.
There is a lot to this work - the full document amounts to some 333 pages. By all means read it yourself as a cure for insomnia, but to save you the trouble I’ve picked out some areas of interest relating to potential pathologies that the regulators may have skipped over, not least being the use of original, “native” Spike.
This is now of little immunological value, given the demonstrated high risk of original antigenic sin and breakthrough infections by a myriad of newer viral strains.
Emphasis mine:
[0128] In some embodiments, the composition is administered at a dosage of about 1, 2, 5, 7.5, or 10 μg of nucleic acid.
[0129] In yet another aspect, the disclosure provides a method of inducing an immune response in a subject comprising administering to the subject an effective amount of any of the nucleic acid molecules described herein.
The patent covers all means of administration, including by aerosol:
[0130] In some embodiments, the nucleic acid molecule may be administered intramuscularly, subcutaneously, intradermally, transdermally, intranasally, orally, sublingually, intravenously, intraperitoneally, topically, by aerosol, or by a pulmonary route.
On page 158 they discuss using dsRNA to induce an immune response.
In other words, it’s inclusion in the platform is not accidental, they know exactly what they are doing:
[0133] In some embodiments, the nucleic acid molecules described herein may be used in inducing an immune response to the first antigenic protein or fragment thereof.
[0134] In some embodiments, the nucleic acid molecules described herein may be used in the manufacture of a medicament for inducing an immune response to the first antigenic protein or fragment thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
[0135] FIGs. lA-lD show design and expression of a SARS-Co V-2 vaccine in mRNA and self-replicating RNA (STARR™) platforms. (IA) Schematic diagram of the SARS-CoV-2 self-replicating STARR™ RNA and mRNA vaccine constructs. The STARR™ construct encodes for the four non-structural proteins, nsl-ns4, from Venezuelan equine encephalitis virus (VEEV) and the SARS-CoV-2 full length spike (S) protein. The mRNA construct codes for the SARS-CoV-2 full length spike S protein.
A transgene is a piece of DNA from one organism that is introduced into another organism by gene therapy. Arcturus allow for ten or more of these being used in their COVID vaccines:
… [0237] ...Any number of transgenes can be included in second polynucleotides of nucleic acid molecules provided herein, such as one, two, three, four, five, six, seven, eight, nine, ten, or more transgenes.
It is not clear how many transgenes Kostaive includes, or whether these are encoding a second antigenic protein, in addition to Spike. This may be proprietary information:
In one aspect, the second polynucleotide of nucleic acid molecules provided herein includes a second transgene encoding a second antigenic protein or a fragment thereof or an immunomodulatory protein.
… [0239] A second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, etc., transgene included in second polynucleotides of nucleic acid molecules provided herein can encode an immunomodulatory protein or a functional fragment or functional variant thereof.
It shouldn't be possible to patent any natural proteins, but they are being non-specific here, and this is where the technology gets dark. Dark as is in being vague as to which Kostaive uses, as well as in potential long-term clinical outcomes:
Any immunomodulatory protein or a functional fragment or functional variant thereof can be encoded by a transgene included in second polynucleotides.
I will conclude this review by citing studies linking most of these cytokines to cancer, if they are expressing them in Kostaive.
Pfizer appeared to do the same, which may also be contributing to the sharp increase in cancer cases. Three years ago I published a Substack that discussed the expression of a potent oncogenic microRNA (miRNA) called miR-21 in BNT162b2, and the use of miRNAs in drugs goes back at least 40 years.
Its big business, if they can get it to work:
… Biopharmaceutical industries are investing in the development of therapeutic miRNAs and siRNAs. Several biopharmaceutical industries, such as Alnylam Pharmaceuticals, Rosetta Genomics, and Regulus Therapeutics, were established during the last 25 years (Figure 2).

These biopharma companies are investing in the development of miRNA- and siRNA-based therapeutic molecules. However, there is a challenge for small biotechnology companies because there is some financial volatility in this area.25, 26
Big Pharma is using small companies to develop molecules for R&D to clinical trials. Big Pharma is investing in this new area to enter into the market with new therapeutic miRNA and siRNA molecules as early as possible.
… The development of therapeutic miRNAs and siRNAs is progressing at a quick pace. However, pharmaceutical companies working with therapeutic miRNAs and siRNAs are somewhat different from those focusing on NCE molecules. This is primarily due to the technical differences between these two kinds of molecules. RNA-based drug molecules are more similar to traditional gene therapy. Soon, there will be no specific applications or efficacy guidelines for miRNAs and siRNAs, which will allow researchers and companies to utilize this important technology to solve real-world problems.
It is likely that there may be product failure during the development of drug molecules, which can be attributed to a number of factors including safety, efficacy, target selection, and delivery technologies. These and other factors, such as clinical trial design and commercial considerations, will require optimization to produce successful drugs.
Nevertheless, in the near future, RNA-based therapeutics will overcome these obstacles, and therapeutic miRNAs and siRNAs will enter into the clinic as next generation drugs.
From: “Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine“ (2017)
https://www.sciencedirect.com/science/article/pii/S2162253117301907
And now we have another patent further supporting their inclusion in vaccines.
Section [0163] protects vaccinal miRNA expression, because they define miRNA as an “exemplary nucleic acid”:
When you get injected with gene therapy agents such as Kostaive, there is no telling what proteins or nucleic acids it may be encoding. The disclosed ones, such as Spike and the nsPs, are bad enough:
… [0241] In one aspect, a second transgene included in second polynucleotides of nucleic acid molecules provided herein encodes a cytokine, a chemokine, or an interleukin. Exemplary cytokines include interferons, TNF- α , TGF-β, G-CSF, and GM-CSF. Exemplary chemokines include CCL3, CCL26, and CXCL7. Exemplary interleukins include IL-I, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-18, IL-21, and IL-23.
Anything else they wish to include is protected under patent and may be proprietary:
Any transgene or combination of transgenes encoding any cytokine, chemokine, interleukin, or combinations thereof, can be included in second polynucleotides of nucleic acid molecules provided herein.
From page 158, and how dsRNA inclusion is no accident:
… [0452] The extent to which STARR™ vaccines reproduce the features of live vaccines remain to be experimentally defined. Without being limited by theory, the superior quality of immune responses elicited by STARR™ SARS-CoV-2 RNA over the mRNA vaccine construct could be attributable to multiple factors, all of which have been found to be associated with live vaccination. For example, higher and longer expression of immunogens produce better immunity, likely through better engagement of T follicular helper cells and thereby leading to more diverse antibody targets and more neutralizing antibody responses. Replication of STARR™ SARS-CoV-2 RNA would result in the formation of a negative- strand template for production of more positive-strand mRNA and sub-genomic mRNA expressing the S transgene. Interaction between the negative- and positive-strands would form double stranded RNA (dsRNA), which would interact with TLR3 and RIG-I-like receptors to stimulate interferon responses, which has been shown to correlate with superior adaptive immune responses. Production of IFNy can then stimulate development of cytotoxic CD8+ T cells.
They are being deliberately vague here, and not supporting their words with evidence to support that their patented technology does the same:
Importantly, the S protein does contain human CD8+ T cell epitopes. Without being limited by theory, the development of T cell memory could be important for long-term immunity, as suggested by recent findings on T cell responses to SARS-CoV-2 and other coronavirus infections.
As for the huge role that nSPs play in replicon technology and pathology, it is curious that I could only find one further mention after section [135]. Even so, it is disturbingly vague, considering the dual role they play in the live virus (i.e. RNA replication and immune suppression), and what we already know about potential neurotoxicity (see Part 4).
[0453] It is unclear whether the VEEV nsP1-4 forming the replication complex contains any immunogenic properties, although mutations in the nsP proteins have been shown to affect induction of type I IFN.
Parenteral administration (e.g. injection) bypasses the mucosa. Aerolised saRNA would be a different matter:
VEEV replicons have also been shown to adjuvant immune responses at mucosal sites, further illustrating the advantages of using the STARR™ platform to develop a COVID-19 vaccine.
This is being disingenuous, considering the studies reviewed in Part 4, which showed how replicons NSPs may cause paralysis and death. Note their use of weasel words and how they contradict themselves:
Without being limited by theory, there does not appear to be an immune response to replicon non-structural proteins, as indicated by an increase in antigen-specific IgG production upon a second administration of replicon to animals. In the presence of an immune response to non-structural proteins, a limited or no increase in antigen-specific IgG production may have resulted following a second administration. The RNA is encapsulated in lipid nanoparticles (LNP), which together can form potent adjuvants leading to robust immune responses. In addition, using the genetic sequence of an antigen, including a viral antigen such as the spike protein from SARS-CoV-2, for example, STARR™ vaccines can be rapidly generated and manufactured using cell-free and rapidly scalable techniques.
[0454] In conclusion, a STARR™ vaccine as exemplified by STARR™ SARS-CoV-2 RNA offers an approach to simulate several of the properties of live vaccination and offers a potential for single-dose vaccination against COVID-19.
Source:
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021183563
Image file:
https://patentimages.storage.googleapis.com/a7/ae/2d/7ca9e10d3a0e03/WO2021183563A1.pdf
Second polynucleotides to encode cytokines, chemokines, or interleukins
Definitions:
Cytokine (SY-toh-kine): “A type of protein that is made by certain immune and non-immune cells and has an effect on the immune system. Some cytokines stimulate the immune system and others slow it down. They can also be made in the laboratory and used to help the body fight cancer, infections, and other diseases. Examples of cytokines are interleukins, interferons, and colony-stimulating factors (filgrastim, sargramostim).”
Chemokine (KEE-moh-kine): “One of a large group of proteins that is made by certain immune cells and other cells in the body. Chemokines play an important role in the body’s immune response. They stimulate the movement of certain types of white blood cells and attract them to areas of inflammation to help the body fight infections, inflammatory conditions, and other diseases. They also help keep the immune system working the way it should. A chemokine is a type of cytokine.”
Interleukin (in-ter-LOO-kin): “One of a group of related proteins made by leukocytes (white blood cells) and other cells in the body. Interleukins regulate immune responses. Interleukins made in the laboratory are used as biological response modifiers to boost the immune system in cancer therapy. An interleukin is a type of cytokine. Also called IL.”
Why would Arcturus want to do this? To be generous, one reason might be to protect their intellectual property, if used in a future cancer vaccine. However, this patent does not concern cancer therapies. It’s for “CORONAVIRUS VACCINE COMPOSITIONS AND METHODS”, i.e. to stimulate anti-Spike/anti-viral immune responses.
But surely, the expression of immunogenic Spike or dsRNA would suffice, especially when administered in an LNP carrier which may have adjuvant properties? Well perhaps not, as you suppressed the innate immune signalling pathways by inhibiting pattern recognition receptors and other mechanisms.
You might also wish to “paint the charts” with immunogenic markers to indicate efficacy, when later studies analysing your product get published in a pro-vaccine journal, such as Nature.
MHCII presentation of Spike
Major Histocompatibility Complex II (MHCII) epitope presentation occurs when short MHCII peptides on the surface of antigen-presenting cells (APCs) are recognised by CD4+ (helper) T cells.
Typical APCs include dendritic cells (DCs), macrophages and B cells. The relevance to innate immune signalling is that MHCs work synergistically with mesenchymal stromal cells, fibroblasts, endothelial cells, epithelial cells and enteric glial cells when stimulated by interferon gamma (IFNγ).

Major histocompatibility complex (MHC) class II molecules play crucial roles in immune activation by presenting foreign peptides to antigen-specific T helper cells and thereby inducing adaptive immune responses.
Although adaptive immunity is a highly effective defense system, it takes several days to become fully operational and needs to be triggered by danger-signals generated during the preceding innate immune response.
Here we show that MHC class II molecules synergize with Toll-like receptor (TLR) 2 and TLR4 in inducing an innate immune response.
From: “MHC Class II Molecules Enhance Toll-Like Receptor Mediated Innate Immune Responses“ (2010)
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0008808
Thus, because replicon vaccines suppress innate signalling, CD4+ responses are also inhibited. Is Arcturus trying to repair what they broke?
The processes still aren’t fully understood. But what we know is that levels of immune signalling proteins are only elevated as much as needed, for as short a duration as required, to overcome the invading pathogen.
Not quite “Gone in 60 seconds”, but in this study of mice, IL-6 was only elevated for as long as was necessary to survive the viral infection:

By inducing what amounts to artificial inflammation of extended duration (not days but weeks, months, years?) you are also at risk of inducing conditions in the vaccinee that are known to be favorable for tumour growth, relapse, and metastasis, i.e. progression, with a poor prognosis.
I’m not sure I agree that “most human malignancies are attributed to exposure to infectious organisms“, certainly not after what happened from December 2020, but agree with the point about inflammation:
Most human malignancies are attributed to exposure to infectious organisms such as viruses. Certain infections that can induce cancer can evade the immune system, leading to persistent inflammation that facilitates uncontrolled cell growth. Moreover, these pathogens can increase the likelihood of oncogenic transformation, leading to cancer development.
From: “The role of viruses in cancer progression versus cancer treatment: A dual paradigm“ (2024)
https://www.sciencedirect.com/science/article/abs/pii/S002432052400095X
No drug-approving regulator or vaccine manufacturer will tell you that their products are designed to do the same, due to envelope-protein and viral-mimicry.
DCC: dormant disseminated cancer cells. Exactly as it sounds. Your cancer is lying dormant - perhaps you have been in remission for decades. And then something awakens the cancer. This is a story we are hearing far too often:
Infection with respiratory viruses (e.g. influenza or SARS-CoV-2) is common and triggers an inflammatory response locally and systemically2,3.
Here we show that influenza virus infection leads to loss of the pro-dormancy mesenchymal phenotype in breast DCC in the lung, causing DCC proliferation within days of infection, and a greater than 100-fold expansion of carcinoma cells into metastatic lesions within two weeks.
Such DCC phenotypic change and expansion is interleukin-6 (IL-6)-dependent.
We further show that CD4 T cells are required for the maintenance of pulmonary metastatic burden post-influenza virus infection, in part through attenuation of CD8 cell responses in the lungs.
Single-cell RNA-seq analyses reveal DCC-dependent impairment of T-cell activation in the lungs of infected mice. SARS-CoV-2 infected mice also showed increased breast DCC expansion in lungs post-infection.
Expanding our findings to human observational data, we observed that cancer survivors contracting a SARS-CoV-2 infection have substantially increased risks of lung metastatic progression and cancer-related death compared to cancer survivors who did not.
From: “Respiratory viral infection promotes the awakening and outgrowth of dormant metastatic breast cancer cells in lungs“ (2024)
Oncogenic, pro-inflammatory Interleukin-6 was implicated in this study. It’s normally elevated for just a few days in response to a SARS-CoV-2 infection, but persistent Spike expression in lymph node germinal centres has been confirmed for at least 60 days after a BNT162b2 shot, in one study.
I discussed this and sustained post-booster IL-6 levels in a Substack three years ago. IL-6 was elevated for 23+ days [C]:
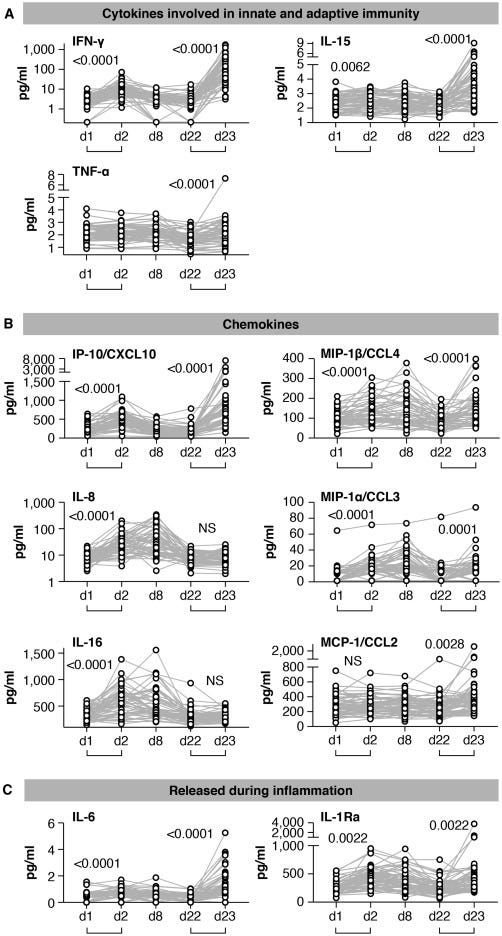
Kostaive extends expression further still.
Another risk caused by encoding pro-inflammatory cytokines is a fatal cytokine storm, due to positive feedback loops (e.g apoptosis of transfected cells):
… Replicase-based DNA vaccines stimulate TH1-biased immune responses at ultralow doses and induce self-removal of transfected cells through apoptosis.
… One of the mechanisms underlying the superior immunogenicity of self-replicating vaccines seems to be activation of professional antigen-presenting cells through enhanced uptake of antigen from transfected cells26,27 that die apoptotically20,21 because of antiviral defense mechanisms.25
Two features clearly distinguish replicon DNA vaccines from conventional DNA vaccines: the extraordinary immunogenicity and the short-term expression of the plasmid resulting from apoptotic death of transfected cells.
… Replicon vaccines gain immunogenicity through double-stranded RNA intermediates recognized by TLR341,42 and through induction of immune apoptosis.43 The latter leads to reduced availability of soluble antigen accessible to B cells, thereby accounting for the observed lower antibody titers.
In contrast, death by apoptosis increases the uptake of antigen by professional antigen-presenting cells for processing and presentation to T cells.26
From: “Immunization with a low-dose replicon DNA vaccine encoding Phl p 5 effectively prevents allergic sensitization” (2006)
https://www.jacionline.org/article/S0091-6749(06)00943-2/fulltext
One of the reasons for the failure of replicon vaccines is that our baseline level of immunity cannot practically be considered for dosing the drug. You may be less reactive to the immunogen. It fails at the one-size-fits-all dose (at least 30% of patients). saRNA vaccines elevate the risk by orders of magnitude, and their selling point is duration of action.
Or you may have a hyper-immune response, leading to multisystem inflammatory syndrome (MIS), anaphylaxis, or worse, especially if you later contract a breakthrough infection.
Even for Big Pharma, what sort of cretin would ever consider tagging on encoding for powerful, oncogenic signalling proteins to the replicon machinery? How can this ever be safe and predictable enough for mass administration? Even if repurposed to treat a late stage cancer patient, it’s a bit of a stretch to make the risk worthwhile. Talk about “kill or cure”.
Nevertheless, I searched for other studies investigating their use clinically with replicon vaccines, but drew a blank. Please comment below if you have anything. Regardless, this didn’t stop the regulators from approving products built to this patent families’ IP.
Let’s do a literature search to see how the other Arcturus-specified immunogenic peptides are also linked to cancer promotion. The reality is that very few cytokines are not linked to cancer promotion, albeit in a context-sensitive manner.
Even anti-inflammatory cytokines such as IL-4 may result in this because they suppress anti-tumour immune responses by skewing the immune response from Th1 to Th2 (T helper 2).
“Heads they win, tails you lose.”
It’s as if they want to induce as much cancer and disease as possible. Not right away, mind you, but over a long enough timescale to make product recall requests or legal action unlikely to succeed.
The NHS and cancer charities are all equally guilty, leaving a trail of early deaths behind them. And they are still pushing these snake oils. They show no signs of learning any lessons, or changing their advice.
If you need this many doses, it’s not a vaccine:

To recap:
… [0241] In one aspect, a second transgene included in second polynucleotides of nucleic acid molecules provided herein encodes a cytokine, a chemokine, or an interleukin. Exemplary cytokines include interferons, TNF- α , TGF-β, G-CSF, and GM-CSF. Exemplary chemokines include CCL3, CCL26, and CXCL7. Exemplary interleukins include IL-I, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-18, IL-21, and IL-23.
“TNF-alpha in promotion and progression of cancer” (2006)
… Tumour necrosis factor alpha is a member of the TNF/TNFR cytokine superfamily. In common with other family members, TNF-alpha is involved in maintenance and homeostasis of the immune system, inflammation and host defence. However, there is a 'dark side' to this powerful cytokine; it is now clear that, especially in middle and old age, TNF-alpha is involved in pathological processes such as chronic inflammation, autoimmunity and, in apparent contradiction to its name, malignant disease.
“TGF-β Signaling in Cancer” (2016)
… The transforming growth factor-β (TGF-β) is a family of structurally related proteins that comprises of TGF-β, activins/inhibins, and bone morphogenic proteins (BMPs).
Members of the TGF-β family control numerous cellular functions including proliferation, apoptosis, differentiation, epithelial-mesenchymal transition (EMT), and migration.
The first identified member, TGF-β is implicated in several human diseases, such as vascular diseases, autoimmune disorders, and carcinogenesis. Activation of the TGF-β receptor by its ligands induces the phosphorylation of serine/threonine residues and triggers phosphorylation of the intracellular effectors, SMADs. Upon activation, SMAD proteins translocate to the nucleus and induce transcription of their target genes, regulating several cellular functions.
TGF-β dysregulation has been implicated in carcinogenesis. In early stages of cancer, TGF-β exhibits tumor suppressive effects by inhibiting cell cycle progression and promoting apoptosis. However, in late stages TGF-β exerts tumor promoting effects, increasing tumor invasiveness, and metastasis.
Furthermore, the TGF-β signaling pathway communicates with other signaling pathways in a synergistic or antagonistic manner and regulates cellular functions. Elevated TGF-β activity has been associated with poor clinical outcome.
“G-CSF Is a Novel Mediator of T-Cell Suppression and an Immunotherapeutic Target for Women with Colon Cancer” (2023)
… G-CSF enhances colon cancer development. This study defines the prevalence and effects of increased G-CSF signaling in human colon cancers and investigates G-CSF inhibition as an immunotherapeutic strategy against metastatic colon cancer.
… In human colon cancer samples, the levels of G-CSF and G-CSFR are higher compared to normal colon tissues from the same patient. High patient serum G-CSF is associated with increases in markers of poor prognosis, (e.g., VEGF, IL6). Circulating T cells from patients express G-CSFR at double the rate of T cells from controls. Prolonged G-CSF exposure decreases T cell IFNγ production.
“GM-CSF: A Double-Edged Sword in Cancer Immunotherapy” (2022)
… Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that drives the generation of myeloid cell subsets including neutrophils, monocytes, macrophages, and dendritic cells in response to stress, infections, and cancers.
… As with other soluble mediators of immunity, too much or too little GM-CSF has been found to promote cancer aggressiveness. While too little GM-CSF prevents the appropriate production of innate immune cells and subsequent activation of adaptive anti-cancer immune responses, too much of GM-CSF can exhaust immune cells and promote cancer growth.
The consequences of GM-CSF signaling in cancer progression are a function of the levels of GM-CSF, the cancer type, and the tumor microenvironment.
“CCL3 Signaling in the Tumor Microenvironment” (2020)
… Within the tumor microenvironment, chemokines play a key role in immune cell trafficking regulation and immune landscape formulation. CCL3 or macrophage inflammatory protein-1α (MIP-1α), an important chemokine implicated in both immune surveillance and tolerance, has emerged as a prognostic biomarker in both solid and hematological malignancies.
CCL3 exerts both antitumor and pro-tumor behavior which is context dependent highlighting the complexity of the underlying interrelated signaling cascades.
“Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells” (2012)
… Impact of different cancer-associated fibroblast (CAF) cell lines on proliferation, migration, invasion and differential expressions of genes in different hepatocellular carcinoma (HCC) cell lines was investigated.
… Ten up-regulated genes (APLN, CCL2, CCL26, CXCR4, IL6, MUC1, LOXL2, PDGFA, PGK1, VEGFA) related to proliferation, migration, invasion and angiogenesis of HCC detected by microarray were selected for quantitative reverse transcriptase-polymerase chain reaction analysis.
… Only CCL2, CCL26, IL6 and LOXL2 genes were consistently up-regulated in both HCC cell lines. In conclusion, the effects of CAFs to promote proliferation, migration and invasion of HCC cells are influenced by the characteristics of both CAFs and HCC cells. Up-regulations of CCL2, CCL26, IL6 and LOXL2 genes in cancer cells are part of the common effects of CAFs on HCC cells.
“Monocytes secrete CXCL7 to promote breast cancer progression” (2021)
… Certain immune cells and inflammatory cytokines are essential components in the tumor microenvironment to promote breast cancer progression. To identify key immune players in the tumor microenvironment, we applied highly invasive MDA-MB-231 breast cancer cell lines to co-culture with human monocyte THP-1 cells and identified CXCL7 by cytokine array as one of the increasingly secreted cytokines by THP-1 cells.
… Further investigations indicated that upon co-culturing, breast cancer cells secreted CSF1 to induce expression and release of CXCL7 from monocytes, which in turn acted on cancer cells to promote FAK activation, MMP13 expression, migration, and invasion.
… Clinical investigation further suggested that high CXCL7 expression is correlated with breast cancer progression and poor overall survival of patients.
Overall, our study unveils an important immune cytokine, CXCL7, which is secreted by tumor infiltrating monocytes, to stimulate cancer cell migration, invasion, and metastasis, contributing to the promotion of breast cancer progression.
Note: The reverse is true, and the following studies highlight the importance of having a probiotic diet full of antioxidants and anti-inflammatories, together with a healthy lifestyle. Although anti-inflammatory, the difference is that your immune system is not being suppressed in a way that promotes cancer progression:
“Interleukin-1 in tumor progression, therapy, and prevention” (2021)
Interleukin-1 (IL-1) is a key orchestrator of inflammation and plays an important role in tumor progression. Based on preclinical models and human genetic associations, we surmise that targeting IL-1 should be considered in treating selected human tumors as well as in a prevention and/or interception setting.
https://pubmed.ncbi.nlm.nih.gov/33989512/
“Interleukin-2 therapy of cancer-clinical perspectives” (2021)
Interleukin (IL)-2 is a pleiotropic cytokine that displays opposing activities on immune system acting either in favor of or against cancer progression. Advanced/metastatic melanoma and renal cell carcinoma (RCC) are the two types of cancers that included most studies implemented for assessing the role of high-dose IL-2 therapy.
The use of high-dose IL-2 therapy can, however, increase the rate of toxicities and interferes with the activity of endothelial cells (ECs) and effector T cells in tumor microenvironment (TME).
“Interleukin-3 production by basal-like breast cancer cells is associated with poor prognosis” (2024)
… Breast cancer represents a collection of pathologies with different molecular subtypes, histopathology, risk factors, clinical behavior, and responses to treatment.
"Basal-like" breast cancers predominantly lack the receptors for estrogen and progesterone (ER/PR), lack amplification of human epidermal growth factor receptor 2 (HER2) but account for 10-15% of all breast cancers, are largely insensitive to targeted treatment and represent a disproportionate number of metastatic cases and deaths.
Analysis of interleukin (IL)-3 and the IL-3 receptor subunits (IL-3RA + CSF2RB) reveals elevated expression in predominantly the basal-like group. Further analysis suggests that IL-3 itself, but not the IL-3 receptor subunits, associates with poor patient outcome.
Note: Related to the next Arcturus cytokine, IL-4, we just published a review paper that’s available as a preprint, pending peer review14:
Abstract
An increase in IgG4 levels is typically associated with immunological tolerance states and develops after prolonged exposure to antigens. Accordingly, IgG4 is considered an anti-inflammatory antibody with a limited ability to trigger efficient immune responses.
Additionally, IgG4 reduces allergic reactions by blocking immunoglobulin E (IgE) activity. In the case of COVID-19, it has been reported that the repeated administration of some vaccines induces high IgG4 levels.
The latest research data has revealed a surprising IgE anti-receptor binding domain response after both natural infection and several SARS-CoV-2 vaccines.
The presence of IgG4 and IgE in COVID-19 disease suggests that the virus may induce an “allergic-like” response to evade immune surveillance, leading to a shift from Th1 to Th2, which promotes tolerance to the virus and potentially contributes to chronic infection.
The spike protein from vaccines could also induce such a response. Interestingly, "allergen-like" epitopes and IgE responses have been reported for other viruses, such as HIV and respiratory syncytial virus (RSV). The impact of this viral-induced tolerance will be discussed, concerning the protective efficacy of vaccines.
From: “Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?” (2025)
IL-4 is highly associated with IgG4 class switching of the mRNA-transfected, mostly due to the multiple allergen-like epitopes of Spike.
It’s one of the reasons why IgG4-RD is associated with a multifold increase in the risk of contracting many cancers - especially lymphoma and pancreatic.
Targeting of IL-4 and IL-13 receptors for cancer therapy” (2015)
… The Th2 cytokines, interleukin (IL)-4 and -13, are structurally and functionally related. They regulate immune responses and the immune microenvironment, not only under normal physiological conditions, but also in cancer.
Both cytokines bind to their high-affinity receptors and form various configurations of receptor subtypes. We and others have reported that IL-4 and IL-13 bind to IL-4Rα and IL-13Rα1 chains, forming functional receptors in cancer cells.
IL-13 also binds with high affinity to a private chain IL-13Rα2. After forming ligand-receptor complexes, both cytokines initiate signal transduction and mediate biological effects, such as tumor proliferation, cell survival, cell adhesion and metastasis.
In certain cancers, the presence of these cytokine receptors may serve as biomarkers of cancer aggressiveness. In a series of studies, we reported that overexpression of IL-4 and IL-13 receptors on cancer cells provides targets for therapeutic agents for cancer therapy. In addition, both of these cytokines and their receptors have been shown to play important roles in modulating the immune system for tumor growth. IL-4, IL-13 and their receptors seem to play a role in cancer stem cells and provide unique targets to eradicate these cells.
“Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment” (2015)
… Using heterotopic and intravenous injection models of lung metastasis in mice, we found that IL5, a cytokine involved in allergic and infectious diseases, facilitates metastatic colonization through recruitment of sentinel eosinophils and regulation of other inflammatory/immune cells in the microenvironment of the distal lung. G
More on IL-6, the motherload of cancer pathogenesis.
This review opens with a sobering reality check - that allopathic drugs don’t work that well:
“Role of interleukin-6 in cancer progression and therapeutic resistance” (2016)
In the last several decades, the number of people dying from cancer-related deaths has not reduced significantly despite phenomenal advances in the technologies related to diagnosis and therapeutic modalities.
The principal cause behind limitations in the curability of this disease is the reducing sensitivity of the cancer cells towards conventional anticancer therapeutic modalities, particularly in advance stages of the disease.
… Interleukin-6 (IL-6), one of the major cytokines in the tumour microenvironment, is an important factor which is found at high concentrations and known to be deregulated in cancer.
Its overexpression has been reported in almost all types of tumours. The strong association between inflammation and cancer is reflected by the high IL-6 levels in the tumour microenvironment, where it promotes tumorigenesis by regulating all hallmarks of cancer and multiple signalling pathways, including apoptosis, survival, proliferation, angiogenesis, invasiveness and metastasis, and, most importantly, the metabolism.
Moreover, IL-6 protects the cancer cells from therapy-induced DNA damage, oxidative stress and apoptosis by facilitating the repair and induction of countersignalling (antioxidant and anti-apoptotic/pro-survival) pathways.
Both IL-6 and IL-8 act as chemokines too15:
“Interleukin-8 in cancer pathogenesis, treatment and follow-up” (2017)
Interleukin-8 (CXCL8) was originally described as a chemokine whose main function is the attraction of a polymorphonuclear inflammatory leukocyte infiltrate acting on CXCR1/2.
Recently, it has been found that tumors very frequently coopt the production of this chemokine, which in this malignant context exerts different pro-tumoral functions.
Reportedly, these include angiogenesis, survival signaling for cancer stem cells and attraction of myeloid cells endowed with the ability to immunosuppress and locally provide growth factors. Given the fact that in cancer patients IL-8 is mainly produced by tumor cells themselves, its serum concentration has been shown to correlate with tumor burden.
The next two cytokines both promote tumours through similar mechanisms, involving upregulation of immunosuppressive programmed cell death protein 1 (PD-1).
Note: Vaccinal Spike also promotes PD-1, and a Substack is pending.
Twice as much bang per buck:
“IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction” (2024)
Conclusions: Treg-derived IL-10 upregulates PD-L1 expression in monocytes, which in turn reduces CD8+ T-cell infiltration and related antitumor immunity in the context of colorectal cancer-derived liver metastases. These findings provide the basis for future monitoring and targeting of IL-10 in colorectal cancer-derived liver metastases.
… Our data show that IL-10 is a pro-metastatic factor involved in liver metastasis formation and that it acts as a regulator of PD-L1.
Low levels of IL-18 suffice:
“IL-18 induces PD-1-dependent immunosuppression in cancer” (2011)
Immunosuppressive cytokines subvert innate and adaptive immune responses during cancer progression. The inflammatory cytokine interleukin-18 (IL-18) is known to accumulate in cancer patients, but its pathophysiological role remains unclear.
In this study, we show that low levels of circulating IL-18, either exogenous or tumor derived, act to suppress the NK cell arm of tumor immunosurveillance. IL-18 produced by tumor cells promotes the development of NK-controlled metastases in a PD-1-dependent manner. Accordingly, PD-1 is expressed by activated mature NK cells in lymphoid organs of tumor bearers and is upregulated by IL-18.
The final two interleukins that Arcturus may use have context-sensitive effects on cancer cells:
“IL-21 Signaling in the Tumor Microenvironment” (2020)
IL-21 is an immunomodulatory cytokine produced by natural killer (NK) cells and T cells that has pleiotropic roles in immune and nonimmune cells. IL-21 can modulate innate and specific immunity activities.
It is a potent stimulator of T and natural killer cell-mediated antitumor immunity but also has pro-inflammatory functions in many tissues and is involved in oncogenesis.
“Interleukin 23 regulates proliferation of lung cancer cells in a concentration-dependent way in association with the interleukin-23 receptor” (2013)
A proinflammatory cytokine, interleukin 23 (IL-23), plays a role in tumor progression by inducing inflammation in the tumor microenvironment, although there is debate about its role in carcinogenesis.
Direct effects of IL-23 on tumor cells have been reported rarely, and contradictory effects have been observed. Here, we studied such effects of IL-23 in lung cancer cells in vitro and in vivo and explored the underlying mechanism.
… Low concentrations of IL-23 promoted the proliferation of IL-23 receptor-positive A549 and SPCA-1 lung cancer cells by binding to IL-23r, whereas high concentrations of IL-23 inhibited proliferation of these cells by binding to both IL-23r and IL-12Rβ1.
In contrast, IL-23 had no effect on IL-23 receptor-negative SK-MES-1 cells. IL-23 regulated the growth of human lung cancer cells through its effects on STAT3 expression and phosphorylation in a concentration-dependent way; the Ki-67 gene was involved in these processes.
This review discusses how tumour-suppressive IL-12 opposes the actions, or balances the effects of pro-tumour IL-23:
“Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer” (2018)
The balance of proinflammatory cytokines interleukin (IL)-12 and IL-23 plays a key role in shaping the development of antitumor or protumor immunity.
… In cancer, inflammation has been shown to play a critical role in tumor initiation, growth, and metastasis (Grivennikov et al. 2010; Elinav et al. 2013).
It is now appreciated that the balance between the proinflammatory cytokine IL-12 and IL-23 in tumors can shape the development of antitumor or protumor immunity.
Given that the importance of IL-12 in promoting antitumor immunity is well recognized and recently reviewed (Tugues et al. 2015), this review will particularly focus on discussing the role of IL-23 in tumor biology and its mechanism of action in promoting tumor growth and metastases.
Finally, we discuss how IL-12 and IL-23 are cross-regulated and how the IL-12/IL-23 axis of inflammation can be targeted for cancer therapy.
IL-12 signalling of anti-tumour Th1 CD4+ T cells is dependent on IFN type II (IFN-γ) pathways:
APCs, such as DCs and macrophages, are thought to be the predominant source of IL-12 and IL-23 (Hunter 2005). A major role of IL-12 is to promote differentiation of Th1 cells and to induce type II interferon (IFN)-γ production.
The requirement for host IL-12 and its downstream cytokine in activating antitumor immunity is well recognized and has been previously reviewed extensively (Colombo and Trinchieri 2002; Dunn et al. 2006; Tugues et al. 2015).
A number of studies have clearly illustrated the importance of endogenous IL-12 and IFN-γ in preventing cancer initiation, growth, and metastasis.
IL-23 promotes expression of the oncogenic, auto-immunogenic IL-17 family cytokines.
And, in turn, IL-17 enhances IL-6 signalling cascades16. IL-23 is certainly not to be played around with.
Although Arcturus don’t specify direct encoding of IL-17 itself, they do specify IL-23!
Nice one.
In contrast, IL-23 plays an important role in promoting the proliferation and effector function of Th17 cells, which are characterized by expression of the IL-17 family cytokines (Aggarwal et al. 2003; Langrish et al. 2004; Langrish et al. 2005).
Patent WO2020254804A1: IIP use in RNA constructs
This was filed in June 2020 by Imperial College Innovations Limited, with our friend Dr. Shattock, Blakney, and Mackay as co-authors.
I won’t go into great depth here as the contents have already been discussed in detail in earlier Substacks.
Its sister patent, BR112021024786A2, was filed in Brazil at the same time and was cited by Arcturus in the same two patents:
What is new is that both patents were cited by Arcturus in two of their patent applications.
WO2020254804A1:
Rna construct
Abstract
The invention relates to RNA constructs encoding (i) at least one therapeutic biomolecule; and (ii) at least one innate inhibitor protein (IIP). The constructs are RNA replicons and saRNA molecules, and the invention includes genetic constructs or vectors encoding such RNA replicons. The invention extends to the use of such RNA constructs and replicons in therapy, for example in treating diseases and/or in vaccine delivery. The invention extends to pharmaceutical compositions comprising such RNA constructs, and methods and uses thereof.
… Accordingly, there is a need in the art to produce new means by which RNA therapeutics may be delivered and expressed in patients, such that they are able to overcome innate immune system sensing of RNA. The inventors have developed novel self-amplifying RNAs (saRNA) that advantageously overcome innate immune system sensing of RNA, by expressing innate inhibitor proteins that block or reduce the innate immune system machinery, resulting in improved protein expression and self-amplification. Accordingly, in a first aspect of the invention, there is provided an RNA construct encoding (i) at least one therapeutic biomolecule; and (ii) at least one innate inhibitor protein (IIP).
… Statement of Financial Support
The project leaving to this application has received funding from European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 794059.
… Cited By (18)
… US11744887B2 2020-03-09 2023-09-05 Arcturus Therapeutics, Inc. Coronavirus vaccine compositions and methods
… US12178921B2 2020-08-14 2024-12-31 Arcturus Therapeutics, Inc. Method of lyophilizing lipid nanoparticles
More:
The timing is interesting. This is what I have uncovered about the EU’s well-funded “Horizon 2020” programme:
What was Horizon 2020?
Horizon 2020 was the EU's research and innovation funding programme from 2014-2020 with a budget of nearly €80 billion.
The programme has been succeeded by Horizon Europe.
All news, events, programme details, project lists and more are available on the archived Horizon 2020 website.
Horizon 2020 website (no longer updated)
More:
The website linked to above is light on objectives for all this expenditure, but its successor programme “Horizon Europe” reveals a huge taxpayer funded slush fund that appears to exist to advance science and technology WEF-style goals:
What is Horizon Europe?
Horizon Europe is the EU’s key funding programme for research and innovation. Following the Multiannual Financial Framework Midterm Review (MTR) decision, the indicative funding amount for Horizon Europe for the period 2021-2027 is EUR 93.5 billion.
It tackles climate change, helps to achieve the UN’s Sustainable Development Goals and boosts the EU’s competitiveness and growth.
The programme facilitates collaboration and strengthens the impact of research and innovation in developing, supporting and implementing EU policies while tackling global challenges.
More:
You can see where Marie Sklodowska-Curie fits into the big picture, under Pillar 1 “Excellent Science”.
Someone has a sense of humour.
Breaking News
It wouldn’t be a saRNA Substack without another fact-check on the fact-chump clowns that prompted this mini-series in the first place, due to them bringing in their big guns to try to get our paper retracted.
What had they got to hide? Why the flak barrage?
This time, Reuters Fact Check” had the tables turned on them by Paul D. Thacker.
Enjoy!
Reuters Fake Fact-Check Fails Again
I had the pleasure of being contacted by Reuters Fact Check last week, with a demand that I explain and justify a headline on a story I wrote about COVID vaccines. I immediately started laughing.
Why?
Well, I should probably explain Reuters Fact Check first.
Reuters Fact Check is one of the many disreputable fact-check organizations that Meta’s Mark Zuckerberg cut ties with in January because, in his words, “The fact checkers have been too politically biased and destroyed more trust than they created, especially in the US.” At the time, Zuckerberg cut financial links with Reuters Fact Check and about two-thirds of the entire fact checking industry.
Zuckerberg began to change his tune about fact checking last May after House Republicans released Meta’s internal communications. Zuckerberg and other Meta executive were exposed complaining in texts and emails that the Biden White House pressured them to censor COVID topics, and they discussed fact checkers falsely labeling some posts as false.
Reuters launched their fact checking initiative in partnership with Meta back in 2020, claiming in a press release, “Reuters has a superior track record in sourcing, verifying and clearing user-generated content for distribution to thousands of clients globally and we are best placed in using our in-house expertise to fact check social media content.”
Well, not really. Like all the fact check organizations, Reuters fact checks narratives, not facts. But before getting into any details, let’s wind the clock back to 2022 when Dr. Kerryn Phelps testified before the Australian Parliament about her COVID vaccine injury.
“Regulators of the medical profession have censored public discussion about adverse events following immunisation,” Phelps testified, “with threats to doctors not to make any public statements about anything that ‘might undermine the government’s vaccine rollout’ or risk suspension or loss of their registration.”
Laying out the details of how the COVID vaccine harmed her and her wife, Phelps explained that medical professionals dismissed vaccine injury, a point driven home by the venue for her testimony: an Australian government inquiry, not on vaccine injury, but on Long COVID. Phelps’ credentials made it hard for fact checkers and pharma friendly reporters to shoot her down. She is a conjoint professor at the NICM Health Research Institute, the first woman elected president of the Australian Medical Association (AMA), and a former member of the Australian Parliament.
What became clear at the time, however, is that people injured by the COVID vaccine would only be taken seriously if they discussed their symptoms as a type of Long COVID. A group of Australian patients injured by the COVID vaccine called COVERSE submitted testimony to Australian Parliament that adverse reactions to COVID-19 vaccines could be mistaken for Long COVID, given significant overlap in symptoms.
This second point was further underlined in Brianne Dressen’s book Worth a Shot?: Secrets of the Clinical Trial Participant Who Inspired a Global Movement. Dressen recounts participating in a U.S. COVID vaccine clinical trial only to be vaccine injured. Some of her story was documented in a Science Magazine article on the NIH’s investigation of COVID vaccine injuries.
Dressen later founded the advocacy group, React 19, for patients harmed by COVID vaccine, and describes in her book how many vaccine injured patients are misdiagnosed with Long COVID.
Dressen is also one of the 28 authors on the preprint I covered that discussed patients who had been harmed by the COVID vaccine: “Yale Researchers Find COVID Spike Protein in Blood 709 Days After Vaccination, Positing Millions of Long COVID Patients May Actually Be Vaccine Injured.”
My headline triggered an anonymous writer for Reuters Fact Check to contact me demanding an explanation:
The senior authors of the paper and independent experts we consulted all refuted that claim, saying there is no such evidence or conclusion in the study.
We wanted to offer you the opportunity to comment on whether you stand by your article and, if possible, to point us to any evidence in the paper that supports your assertion.
I started laughing at first, as Reuters Fact Check has a tattered history with numerous fact checks undermined by scientific research as well as the very Yale study they were citing and that I had reported on. Here’s how I responded to Reuters in email:
MY EMAIL TO REUTERS:
Reuters Fact Check falsely asserted, “The mRNA instructs cells to make the [spike] protein and is broken down by the body shortly thereafter” although the new study found “spike in circulation up to 709 days after vaccination.” When does Reuters Fact Check plan to correct this falsehood in accordance with what is reported in this new study?
Reuters Fact Check asserted, “There is no proof that spike proteins created in response to mRNA vaccines are harmful to the body.” However, the new study reported:
Interaction with full-length S, its subunits (S1, S2), and/or peptide fragments with host molecules may result in prolonged symptoms in certain individuals. Recently, a subset of non-classical monocytes has been shown to harbor S protein in patients with PVS. Further, biodistribution studies on mRNA–LNP platforms in animal models indicate its ability to cross the blood-brain barrier, and the local S expression could result in neurocognitive symptoms.
When does Reuters Fact Check plan to correct this falsehood in accordance with what is reported in this new study?
I spoke for several hours on background with one of the paper's 28 authors, so who is Reuters Fact Check speaking with?
Did you notice that each time I caught Reuters with a fake fact check, their mistakes always favored the pharmaceutical industry? Well, Reuters Fact Check never responded to me. Instead, Reuters posted a fake fact check that never bothered to quote anything I sent them. Nor did Reuters go back and correct their fake fact checks from the past. And their fake headline attacked something that was never claimed.
Nobody I’m aware of, nor anyone I have spoken with thinks “Long COVID is a vaccine injury” as Reuters falsely contends. But with tens of millions of Americans reported to have Long COVID, some subset of these patients are likely vaccine injured.
Reuters Fact Check simply made up a claim, debunked it, and proclaimed victory. This is exactly how another former Meta fact checker, Leadstories.com, behaved when they did a fake fact check of a BMJ investigation that exposed problems with Pfizer’s COVID clinical trial.
I spoke to Dr. Danice Hertz, another of the study authors, to get her views on fake fact checks and why so many medical professionals have politicized the issue of vaccines. “Up until this moment, vaccine injury was not acknowledged,” Hertz told me. “And there’s been a concerted effort to cover it up.”
A couple weeks after Zuckerberg cut ties in January with biased fact checkers like Reuters, he sat for an interview with Joe Rogan and complained that Meta was pressured to censor anything on vaccine side effects.
“Who’s pressuring you take down things that talk about vaccine side effects,” Rogan asked.
“It was people in the Biden administration,” Zuckerberg said.
https://twitter.com/i/status/1877791346297336037
https://www.zerohedge.com/political/reuters-fake-fact-check-fails-again
Our next late breaking story won’t be a revelation to anyone who has been paying attention, but it is further confirmation:
'They Knew... & Covered It Up': German Intel Told Govt In 2020 That COVID Originated In Chinese Lab
The American media is doing its best to ignore the biggest news this week.
As Alex Berenson notes, while every legacy media outlet is reminding you it’s been exactly five years since the Little Epidemic That Could started its long chug into your lungs, or at least your upper respiratory tract, two big German newspapers - Zeit and Süddeutsche Zeitung -broke some actual news.
Turns out, Germany’s Federal Intelligence Service (BND) has long had evidence that the Covid-19 virus originally came from a lab in Wuhan, China.
It was kept classified, however.
… When the BND submitted their report, their conclusion was that there was an 80 to 95 percent probability that Covid had originated in a Chinese lab and was then leaked accidentally.
Upon the report’s completion in 2020, Bruno Kahl, who has been the BND’s president since 2016, personally briefed then-Chancellor Angela Merkel on its findings.
And finally, we have another scoop from Dr Kevin “Obi Wan” McKernan:
More:
Concluding remarks
And with that, we have reached the end of this mini-series of investigations into the hidden, and not so hidden dangers to your health from these products.
When Sabine and I started reviewing the Japanese registration documents back in December, I thought that one more post would suffice to discuss the pathologies. After all, what more could there be to add than has been covered with reference to the mRNA platforms, over the last five years? With the benefit of hindsight I had underestimated this by about 50%, and even then my focus has been on immune suppression, not other adverse events.
The “Pharma Empire Strikes Back” opening credits are being borne out though, as the rollout of replicon vaccines heads West to Europe, with the US, Australia and the rest of the World to follow within months.
And we mustn’t take our eyes off the disastrous mRNA products too. A hat tip to James Roguski for listing all these:
More:
Clearly, nothing is going to change any time soon, regardless of who is in power. The goldrush continues.
You need to be your own drugs “regulator”, and Just Say No if you aren’t happy with anything. Your body, your choice.
I hope these Substacks have moved you closer to having informed consent, even if the takeaway from this series is you cannot give full informed consent yet.
Either the research and clinical trials were never completed, or the manufacturers don’t need to disclose them, or we are meddling with areas of immune system function and biochem that are leading edge. Either way, the papers make clear that we have gaping holes in our understanding and lack long-term clinical data.
Buyer beware.
References
Chen, Y. Grace, and Sun Hur. ‘Cellular Origins of dsRNA, Their Recognition and Consequences’. Nature Reviews Molecular Cell Biology 23, no. 4 (April 2022): 286–301. https://doi.org/10.1038/s41580-021-00430-1.
Gong Y, Yong D, Liu G, Xu J, Ding J, Jia W. A Novel Self-Amplifying mRNA with Decreased Cytotoxicity and Enhanced Protein Expression by Macrodomain Mutations. Advanced Science. 2024;11(43):2402936. doi:10.1002/advs.202402936
Vanluchene, Helena, Oriane Gillon, Karen Peynshaert, Stefaan C. De Smedt, Niek Sanders, Koen Raemdonck, and Katrien Remaut. ‘Less Is More: Self-Amplifying mRNA Becomes Self-Killing upon Dose Escalation in Immune-Competent Retinal Cells’. European Journal of Pharmaceutics and Biopharmaceutics 196 (1 March 2024): 114204. https://doi.org/10.1016/j.ejpb.2024.114204.
Gong Y, Yong D, Liu G, Xu J, Ding J, Jia W. A Novel Self-Amplifying mRNA with Decreased Cytotoxicity and Enhanced Protein Expression by Macrodomain Mutations. Advanced Science. 2024;11(43):2402936. doi:10.1002/advs.202402936
Grinsted, Julian, John Liddell, Emir Bouleghlimat, Ka Yan Kwok, Georgia Taylor, Marco P. C. Marques, and Daniel G. Bracewell. ‘Purification of Therapeutic & Prophylactic mRNA by Affinity Chromatography’. Accessed 19 February 2025. https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/2408/Purification-of-therapeutic-prophylactic-mRNA-by-affinity-chromatography.
Scumpia PO, Kelly KM, Reeves WH, Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52(2):153-162. doi:10.1002/glia.20234
Herrera F, Sainz RM, Mayo JC, Martín V, Antolín I, Rodriguez C. Glutamate induces oxidative stress not mediated by glutamate receptors or cystine transporters: protective effect of melatonin and other antioxidants. J Pineal Res. 2001;31(4):356-362. doi:10.1034/j.1600-079x.2001.310411.x
Chang RCC, Yik SY, Ho YS, Lai SW, So KF. Direct neurotoxic effects of dsRNA: Implication of virus-induced neurodegeneration. Published online 2007. Accessed February 16, 2025. http://hub.hku.hk/handle/10722/95697
Ochoa, Elizabeth, Paulino Ramirez, Elias Gonzalez, Jasmine De Mange, William J. Ray, Kevin F. Bieniek, and Bess Frost. ‘Pathogenic Tau–Induced Transposable Element–Derived dsRNA Drives Neuroinflammation’. Science Advances 9, no. 1 (6 January 2023): eabq5423. https://doi.org/10.1126/sciadv.abq5423.
Ventoso, Iván. ‘Adaptive Changes in Alphavirus mRNA Translation Allowed Colonization of Vertebrate Hosts’. Journal of Virology 86, no. 17 (September 2012): 9484–94. https://doi.org/10.1128/jvi.01114-12.
Gal-Ben-Ari, Shunit, Iliana Barrera, Marcelo Ehrlich, and Kobi Rosenblum. ‘PKR: A Kinase to Remember’. Frontiers in Molecular Neuroscience 11 (9 January 2019). https://doi.org/10.3389/fnmol.2018.00480.
Ventoso, Iván, Miguel Angel Sanz, Susana Molina, Juan José Berlanga, Luis Carrasco, and Mariano Esteban. ‘Translational Resistance of Late Alphavirus mRNA to eIF2α Phosphorylation: A Strategy to Overcome the Antiviral Effect of Protein Kinase PKR’. Genes & Development 20, no. 1 (1 January 2006): 87–100. https://doi.org/10.1101/gad.357006.
Dhar, Arti. ‘The Role of PKR as a Potential Target for Treating Cardiovascular Diseases’. Current Cardiology Reviews 13, no. 1 (February 2017): 28–31. https://doi.org/10.2174/1573403X12666160526122600.
Piscopo, Marina, David Cowley, Vladimir N. Uversky, Elrashdy M. Redwan, Carlo Brogna, and Marina Piscopo. ‘Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?’ Preprints, 7 March 2025. https://doi.org/10.20944/preprints202503.0461.v1.
Clahsen, Thomas, and Fred Schaper. ‘Interleukin-6 Acts in the Fashion of a Classical Chemokine on Monocytic Cells by Inducing Integrin Activation, Cell Adhesion, Actin Polymerization, Chemotaxis, and Transmigration’. Journal of Leukocyte Biology 84, no. 6 (1 December 2008): 1521–29. https://doi.org/10.1189/jlb.0308178.
Ma, Xiangyu, Stephanie L. Reynolds, Brandi J. Baker, Xingang Li, Etty N. Benveniste, and Hongwei Qin. ‘Interleukin-17 Enhancement of the Interleukin-6 Signaling Cascade in Astrocytes’. Journal of Immunology (Baltimore, Md. : 1950) 184, no. 9 (1 May 2010): 4898–4906. https://doi.org/10.4049/jimmunol.1000142.






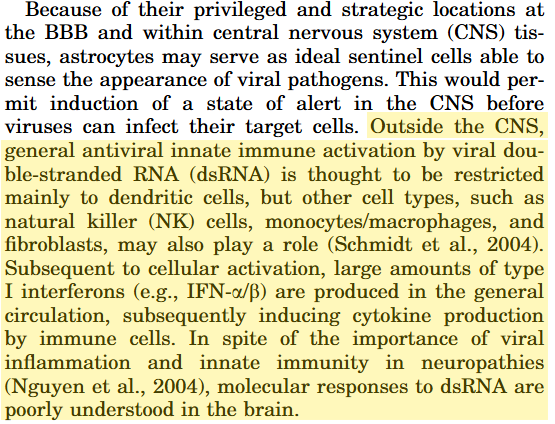

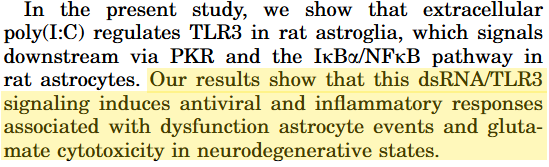


























I miss you !! Best regards from France
A. B.
PS : I tried to send you some papers about anti-HSP70.
We should not be interfering with healthy immune systems by breaching the immune barriers. Dr Couey the biologist of GigoaholmBiological firmly states that "intramuscular injection of any combination of substances with the intent to augment the immune system is dumb". It is short and to the point but for me it makes sense, why breach a barrier whose sole purpose is to protect us? The transfections that they have criminally injected into more than half of the world's population without knowing the longterm consequences of this action is for me terrifying. Instead of reflecting on this they are doubling down and engineering even more transfections and trying to introduce it into all vaccines. The vaccine scientists constructed products based on lies, exaggerations and excess hubris while taking full advantage of the fake pandemic terror campaign perpetrated by governments, this was not science it was pure experimentation "buyer beware" should be stamped on every product they produce. I have no hope in fully understanding the complexity of your article but I truly appreciate and thank you for the effort you have made to try and break the information down even though my knowledge of such things is woeful.